Abstract
Retinitis pigmentosa (RP) is a group of genetic disorders characterized by progressive degeneration of photoreceptors and retinal pigment epithelium (RPE) cells. Its main clinical manifestations include night blindness and progressive loss of peripheral vision, making it a prevalent debilitating eye disease that significantly impacts patients' quality of life. RP exhibits significant phenotypic and genetic heterogeneity. For instance, numerous abnormal genes are implicated in RP, resulting in varying clinical presentations, disease progression rates, and pathological characteristics among different patients. Consequently, gene therapy for RP poses challenges due to these complexities. However, stem cells have garnered considerable attention in the field of RPE therapy since both RPE cells and photoreceptors can be derived from stem cells. In recent years, a large number of animal experiments and clinical trials based on stem cell transplantation attempts, especially cord blood mesenchymal stem cell (MSC) transplantation and bone marrow-derived MSC transplantation, have confirmed that stem cell therapy can effectively and safely improve the outer retinal function of the RP-affected eye. However, stem cell therapy also has certain limitations, such as the fact that RP patients may involve multiple types of retinal cytopathia, which brings great challenges to stem cell transplantation therapy, and further research is needed to solve various problems faced by this approach in the clinic. Through comprehensive analysis of the etiology and histopathological changes associated with RP, this study substantiates the efficacy and safety of stem cell therapy based on rigorous animal experimentation and clinical trials, while also highlighting the existing limitations that warrant further investigation.
Keywords: retinitis pigmentosa, photoreceptor, stem cell therapy
INTRODUCTION
Retinitis pigmentosa (RP) is a group of hereditary retinal disease characterized by damage to retinal photoreceptor cells and retinal pigment epithelial (RPE) cells. Clinically, it is characterized by night blindness, progressive reduced visual field, pigmentary retinopathy, and poor photoreceptor function. The disease usually occurs in both eyes, and in rare cases, it occurs in one eye. The prevalence of RP ranges from 1/7000 to 1/3000 worldwide and about 1/4000 in China[1]. It usually begins before the age of 30, most commonly in childhood or adolescence, and becomes worse in adolescence until middle age or old age due to macular involvement and severe visual impairment and blindness. One of the most well-known treatments for this disease is tissue therapy. However, with the development of medicine, various therapeutic methods such as gene therapy, retinal prosthesis, optogenetic therapy, drug therapy, complication therapy, and stem cell therapy have been proposed.
PATHOGENESIS OF RETINITIS PIGMENTOSA
A genetic abnormality is currently considered to be the main cause of RP. Abnormal genes affect retinal photoreceptor or RPE cells, weakening intracellular material transport, molecular movement, and light conduction between photoreceptor and RPE cells, ultimately leading to visual impairment[2]. The genetic basis and mutations that cause RP are complex and highly heterogeneous. More than 70 genes have been identified as associated with RP, typically each with a different disease-causing mutation[3]–[4]. According to the latest findings, there are three types of autosomal recessive, dominant, and X-linked recessive inheritance, with autosomal recessive inheritance being the most common. Numerous studies have shown that mutations in seven known splicing factors are associated with autosomal dominant RP, including pre-mRNA processing factor 3 (PRPF3), PRPF4, PRPF6, PRPF8, PRPF31, small nuclear ribonucleoprotein particle 200 (SNRNP200), and pre-mRNA 9 (RP9; Table 1)[5]–[15]. Mutations in PRPF31, are the most common among these, accounting for 6%-11.1% of autosomal dominant RP[10]. PRPF31 is a highly conserved gene. Studies have found that homozygous PRPF31 knockout mice and zebrafish are embryonically lethal, indicating the importance of PRPF31. The present studies reveal that PRPF31-RP demonstrates incomplete exodominance due to haploinsufficiency, wherein the disease is attributed to reduced gene expression levels of the mutated allele[11]. Xi et al[12] found that carriers of the PRPF31 heterozygous mutation may be asymptomatic if the wild-type (WT) allele produces enough PRPF31 to maintain normal retinal function or may progress to blindness if the remaining PRPF31 level falls below a critical threshold.
Table 1. Causes of RP from different splicing factors.
| Types of splicing factors | Causes of RP | References |
| PRPF3 | Altered PRPF3 amino acids are highly conserved in all known PRPF3 orthologues | [5] |
| PRPF4 | Haploinsufficiency and dominant-negative effects | [6] |
| PRPF6 | Abnormal localization of endogenous PRPF6 within the nucleus | [7] |
| PRPF8 | C-terminus exhibit a high degree of conservation | [8] |
| PRPF31 | Incomplete penetrance; reduced levels of gene expression from the mutated allele | [9]–[12] |
| PR9 | Proliferation and migration were decreased; FSCN2 and BBS2 were down-regulated in the mutated cells; pre-mRNA splicing of the FSCN2 gene was markedly affected | [13]–[14] |
| SNRNP200 | A defect in hBrr2-dependent RNA unwinding and a consequent defect in spliceosome activation | [15] |
PRPF3: Pre-mRNA processing factor 3; PR9: Pre-mRNA 9; SNRNP200: Small nuclear ribonucleoprotein particle 200; FSCN2: Fascin actin-bunding protein 2; BBS2: Bardet-biedl syndrome 2; RP: Retinitis pigmentosa.
HISTOPATHOLOGICAL FEATURES
The most important changes in RP visible under the light microscope are the progressive degeneration of the upper cortex of the retinal nerve, especially the rod cells[16]. Early studies showed that the lesions spread from the outer retinal layer inward, extending from the equator to the macula, eventually penetrating the nerve cell layer and leading to atrophy of the entire retina. In the early stage of the disease, the rods and pigment epithelial cells in the equatorial region, as well as the photoreceptor cells, simultaneously underwent degeneration and proliferation, and the proliferative epithelial cells and macrophages migrated to the vascular layer in the retina and near the veins. Degeneration and hyperplasia occur in the pigment epithelium, as the loss or accumulation of pigment that migrates to the inner retina[17].
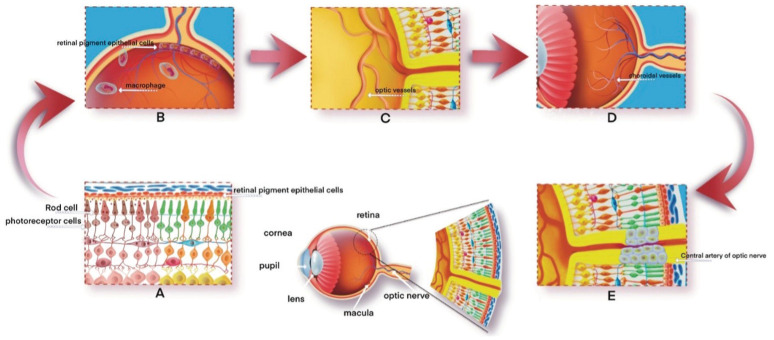
Proliferative epithelial cells, macrophages, and free pigment particles accumulated around the retinal blood vessels, and the outer membrane of the retinal blood vessels experienced hyaloid degeneration and thickening; the tube diameter was significantly reduced; and the lumen was narrow or even completely blocked. The choroidal blood vessels can then be sclerotized to varying degrees, and the late choroidal capillaries disappear completely or partially. Occlusion of the central artery of the optic nerve can result in optic nerve atrophy and often gliosis, where a membranous mass forms that is connected to the glial membrane in the retina (Figure 1). Recent studies have shown that the proliferating glia cells are mainly derived from Müller cells (MC), which have the properties of neural stem cells. After retinal injury, MC can proliferate and differentiate into retinal neurons to promote retinal repair[18].
Figure 1. Process of pathological change.
A: The rod cells, retinal pigment epithelial cells, and photoreceptors undergo denaturation and proliferation; B: The proliferating retinal pigment epithelial cells and macrophages migrate towards the vascular layer within the retina, in close proximity to the veins; C: The optic vessel's outer membrane exhibited hyalinoid degeneration and thickening; D: Choroidal vascular sclerosis of different degrees; E: Occlusion of the central artery of the optic nerve can result in optic neuropathy.
STEM CELL THERAPY OF RETINITIS PIGMENTOSA
Photoreceptor and Retinal Pigment Epithelium Cells
The retina is responsible for the conversion of light signals into electrical signals. Visual signals originate in photoreceptor cells, which mainly express photosensitive proteins in the outer segments. The human retina has two types of light-sensitive cells, rods and cones, which are responsible for dim vision and daylight vision (including color), respectively[19]. The differentiation and proper functioning of photoreceptor cells and RPE cells are mutually dependent. During development, the neuroepithelial cells consist of two layers, which will give rise to the RPE cells and the neuronal retina[20]. The absence of synaptic input and nutritional factors has been observed to inevitably result in transneuronal degeneration of retinal neurons due to photoreceptor death. However, even in cases of severe RP, approximately 30% of ganglion cells and around 80% of inner layer neurons remain unaffected[21]. As a result, treatments for transplanting photoreceptors into the subretinal space and integrating them into the host retina by establishing synapses with the host bipolar cells began to emerge[22].
Stem Cell Therapy
Stem cells are pluripotent cells with the ability for targeted differentiation and proliferation. When the internal environment of the body changes, the differentiation potential of stem cells can be activated through various signaling pathways in the body and targeted differentiation into different cells and tissues, which is why stem cells are called universal cells[23]. Because stem cells have the potential to replace damaged or missing retinal cells, they can be stimulated to differentiate into functional retinal photoreceptor cells and then injected into the retina to replace the damaged cells and repair the normal retinal tissue structure. Therefore, recent studies have shown that stem cell therapy is more commonly used and effective in RP because it can effectively promote retinal cell survival and inhibit the inflammatory response[24].
The emergence of pluripotent stem cells, from the initial embryonic stem cells (ESCs) to the human induced pluripotent stem cells (hiPSCs) currently being studied, provides a new idea for the treatment of RP[25]. Numerous studies have shown that these cells can differentiate into several major retinal cell types, including photoreceptor cells[26]–[28]. The emergence of pluripotent stem cells, initially ESCs and more recently hiPSCs, has provided a promising alternative source for attempting photoreceptor regeneration through cell transplantation. In addition, adult retinal pigment epithelial stem cells (RPESCs) offer a distinct lineage source for deriving RPE cells compared to ESCs and hiPSCs[29]. Recent studies have demonstrated the presence of RPESCs in RPE cell cultures isolated from deceased human eyes[30]. Under appropriate culture conditions, these stem cells can differentiate into functional RPE cells once again[31]–[36]. Singh et al's[29] research suggests that adult RPESC-derived RPE cells may be a safer option than those derived from ESCs and hiPSCs; however, they may present other challenges as well. For instance, recent evidence indicates that cadaveric RPE cells may retain intracellular phenotypes associated with aging and disease, which could potentially limit their effectiveness as therapeutic substrates[37]. The latest endeavor in this field aims to target the genetic form of RP by utilizing a specific type of cell derived from the eye, known as retinal progenitor cells (RPCs), which are isolated from fetal human eyes[38]–[41]. In comparison to stem cells derived from bone marrow or neural stem cells, RPC transplantation may offer a more targeted treatment mechanism for retinal degeneration[29]. Current clinical evidence suggests that transplanted RPCs retain the potential to differentiate into certain types of retinal cells, albeit with limited efficiency[42]–[44]. Therefore, subretinal RPC transplantation has the potential to achieve permanent tissue repair through the actual regeneration of photoreceptor cells[29].
Animal Experiment
Animal studies have found that pluripotent stem cells, when used in mouse models of RP, have the potential not only to survive but also to differentiate into and function as photoreceptor cells. For example, the study conducted by Zhang et al[45] revealed a significant increase in the survival rate of photoreceptors and a notable enhancement in visual function following the intravitreal injection of mesystimal stem cell (MSCs) in mice. Liang et al[46] conducted animal experiments with umbilical cord MSCs (UCMSCs) and concluded that intravenous injection of UCMSCs could delay retinal degeneration and protect vision in rats. Brown et al[47] employed an innovative approach to investigate the therapeutic efficacy of original MSCs-derived RPCs in rd12 mouse models with retinal degeneration. Their findings demonstrated that transplanted RPCs effectively attenuated inflammation, provided retinal protection, and facilitated neurogenesis, ultimately leading to improvements in both retinal structure and physiological function in rd12 mice. Dezfuly et al[48] established a progressive acute retinal injury model by intravitreal injection of sodium iodate in rats to compare the therapeutic effects of human adipose-derived stem cells (hADSCs) and their secretome on in vivo models of sodium iodate-induced retinal neurodegeneration. The results demonstrated that hADSCs effectively facilitated photoreceptor regeneration and restoration of retinal function. By comparing the effects of isogenic bone marrow mononuclear stem cell (BM-MNC) transplantation on two animal models with different etiologies, namely RCS and P23H-1 rats, Di Pierdomenico et al[49] observed that intravitreous and subretinal homologous BM-MNC transplantation mitigated photoreceptor degeneration and exhibited anti-glioma properties; however, it did not enhance retinal function. Furthermore, isogenic BM-MNCs transplantation demonstrated superior efficacy compared to xenotransplantation of these cells. Given the potential risk of blood vessel obstruction from intravenous infusion cells becoming blocked in the lung, Liang et al[46] utilized a 10 µm filter to obtain small-cell UCMSCs (S-UCMSCs) and found that, compared to UCMSCs, intravenous infusion of S-UCMSCs is a safer option. In fact, it has been shown to delay retinal degeneration and protect visual function in RCS rats, making it a promising treatment method for RP. Liu et al[50] investigated various types of stem cells, including hADSCs, human amniotic fluid stem cells (hAFSCs), human bone marrow stem cells (hBMSCs), human dental pulp stem cells (hDPSCs), hiPSCs, and hiPSC-derived RPE cells, in their study on the protective effects and therapeutic potential of subretinal transplantation in rats with RP disease. They discovered that both adult and fetal stem cell injections resulted in improved visual function within 4wk, primarily attributed to the paracrine activity of multiple growth factors secreted by these stem cells.
Clinic Trial
As for clinical trials, a variety of experiments have shown that stem cell therapy can alleviate clinical symptoms in patients with RP (Table 2)[51]–[57]. Kahraman and Oner[51] conducted a clinical trial in which UCMSCs suspension was injected into transplanted fatty tissue between the choroid and sclera in 124 eyes of 82 patients during a six-month follow-up. Suprachoroidal administration of stem cells has beneficial effects on best corrected vision acuity (BCVA), visual field (VF) testing, and multifocal electroretinogram (mfERG) recording measurements. Zhao et al[52] conducted a comparative study to assess the safety and efficacy of modified subtenon capsule injection of triamcinolone alone (TA) and intravenous infusion of UCMSCs in the treatment of RP combined with macular edema (ME) (RP-ME). No serious adverse reactions were observed in all patients, indicating that both the modified sub-Tenon capsule injection of TA and the intravenous infusion of UCMSCs are safe for RP-ME patients. In terms of effectiveness, TA injections demonstrated superior short-term outcomes in alleviating ME and improving visual function, while the effect of intravenous UCMSC on ME relief was slower but sustained over a longer period, leading to improved visual function[52]. The Mer tyrosine kinase (MERTK) gene encodes the Tyro3/Axl/Mer family of receptors, which are implicated in RP. Tagawa et al[53] successfully generated hiPSCs from RP patients and healthy individuals carrying homozygous or complex heterozygous mutations in MERTK. These hiPSCs were then differentiated into RPE cells. Although there were no significant morphological differences observed between diseased and normal RPE cells, cytoskeletal staining indicated potential minor interference with cell polarity. Notably, the internalization of photoreceptor outer segments was significantly reduced in diseased hiPSC-RPE cells compared to normal hiPSC-RPE cells. This in vitro disease model holds promise for elucidating disease progression mechanisms and screening potential treatments. In 2009, a kind of original stem cell, a Wharton's Jelly-derived MSC (WJ-MSC), was extracted and cultured from the umbilical cord Wharton's Jelly of the fetus after birth. Özmert and Arslan[54] injected its suspension into the subtendon space of each patient's eye. The research results showed that this transplant method can be considered an effective and safe option to slow or stop the progression of the disease, regardless of the gene mutation. By conducting suprachoroidal implantation of bone MSCs (BMSCs) in patients, Özkan et al[55] observed that the implementation of spherical MSCs as a stem cell therapy in RP patients with relatively good vision led to improvements in BCVA, 10-2 and 30-2 VF examination, and mfERG records during the follow-up period. This suggests that utilizing globular MSCs with enhanced effects may be more effective in preventing apoptosis and promoting retinal tissue healing in RP patients. Hirami et al[56] conducted a study where they performed allogeneic hiPSC-derived retinal organoid transplantation in two patients with advanced RP. The results showed that the grafts remained viable for 2y, leading to increased retinal thickness at the graft site and no occurrence of serious adverse events in either patient. Moreover, during follow-up, visual function deterioration was observed to progress at a slower rate compared to untreated eyes. These findings highlight the potential therapeutic value of allogeneic hiPSC-derived retinal organoid transplantation; however, further investigation is required to assess its safety and efficacy in treating visual function[56]. The study conducted by Ozmert and Arslan[57] demonstrated that the combination therapy of WJ-MSC and Magnovision can effectively decelerate the progression of RP in patients for a duration of up to 3y.
Table 2. Advantages and disadvantages of different stem cell therapies.
| Types of stem cells | Methods | Advantages | Disadvantages | References |
| UCMSCs | Mesenchymal stem cell suspension was injected into transplanted fatty tissue between the choroid and sclera | Beneficial effects on BCVA, VF, and mfERG | Follow-up time was short; results do not apply to patients with early-stage disease | [51] |
| UCMSCs | Subtenon capsule injection of TA and intravenous infusion of UCMSCs | TA injections demonstrated superior short-term outcomes in improving visual function | The effect of intravenous UCMSC on ME relief was slower but sustained over a longer period | [52] |
| iPSCs | Differentiated iPSCs carrying homozygous or complex heterozygous mutations of MERTK into RPE | The internalization of photoreceptor outer segments was significantly reduced | Minor interference with cell polarity | [53] |
| WJ-MSC | Injected its suspension into the subtendon space of each patient's eye | An effective and safe option to slow or stop the progression of the disease | Intracellular mutant protein deposits could be detected by FAF; changes in deposits; FAF-field and visual field are not correlated in some cases | [54],[57] |
| BMSCs | Conduct suprachoroidal implantation of MSCs in RP patients with relatively good vision | Effective in preventing apoptosis and promoting retinal tissue healing | Small sample size and short duration of follow-up; genetic factors and response to treatment were ignored | [55] |
| Allogeneic iPSCs | Performed allogeneic iPSC-derived retinal organoid transplantation in two patients with advanced RP | Visual function deterioration was observed to progress at a slower rate | Further investigation is required to assess its safety and efficacy in treating visual function | [56] |
UCMSCs: Umbilical cord mesenchymal stem cells; BCVA: Best corrected visual acuity; VF: Visual field; mfERG: Multifocal electroretinogram; FAF: Fundus autofluorescence; TA: Triamcinolone alone; ME: Macular edema; iPSCs: Induced pluripotent stem cells; MERTK: Mer tyrosine kinase; RPE: Retinal pigment epithelium; BMSCs: Bone mesenchymal stem cells; WJ-MSC: Wharton's Jelly-derived mesenchymal stem cells.
These results suggest that stem cell therapy can significantly improve visual impairment in patients with RP and its safety has been confirmed by a variety of experiments. Therefore, stem cell therapy is widely used in clinical practice.
CONCLUSIONS
The treatment of RP primarily focuses on symptomatic management and retarding the degeneration of retinal cells. This includes drug therapy, nutritional support therapy, and the application of nerve growth factors. With advancements in medicine, gene therapy, tissue therapy, stem cell therapy, and other approaches have been proposed. Unfortunately, gene therapy can only target a single gene and is effective for a limited number of patients. Tissue therapy may lead to immune rejection and potential tumorigenic risks.
Due to their self-renewal ability and multiple differentiation potentials, stem cells offer hope for curing RP. Extensive innovative animal and clinical studies provide a strong foundation for the feasibility and safety of stem cell therapy in RP patients, thus making it one of the most effective treatment options available[58]. Stem cells can fully regenerate RPE cells to replace those that have lost function. Furthermore, stem cell transplantation offers several advantages, such as protecting retinal blood vessels, promoting the repair of damaged nerve cells, nourishing nerves, inhibiting inflammatory responses, etc.
However, it should be noted that stem cell therapy also has certain limitations, including variability in animal models and human mutations, immature gene transfer techniques, surgical complications, and local or systemic immunosuppression complications. Additionally, its long-term efficacy remains uncertain. At present, placental MSCs show broad prospects in the treatment of RP, but they are still poorly understood and need further research[59].
Footnotes
Conflicts of Interest: Qi XY, None; Mi CH, None; Cao DR, None; Chen XQ, None; Zhang P, None.
REFERENCES
- 1.Tsang SH, Sharma T. Retinitis pigmentosa (non-syndromic) Adv Exp Med Biol. 2018;1085:125–130. doi: 10.1007/978-3-319-95046-4_25. [DOI] [PubMed] [Google Scholar]
- 2.Xu J, Peng QH. Retinitis pigmentosa treatment with western medicine and traditional Chinese medicine therapies. J Ophthalmol. 2015;2015:421269. doi: 10.1155/2015/421269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez-Fernandez de la Camara C, Cehajic-Kapetanovic J, MacLaren RE. Emerging gene therapy products for RPGR-associated X-linked retinitis pigmentosa. Expert Opin Emerg Drugs. 2022;27(4):431–443. doi: 10.1080/14728214.2022.2152003. [DOI] [PubMed] [Google Scholar]
- 4.Daiger SP, Bowne SJ, Sullivan LS. Genes and mutations causing autosomal dominant retinitis pigmentosa. Cold Spring Harb Perspect Med. 2015;5(10):a017129. doi: 10.1101/cshperspect.a017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakarova CF, Hims MM, Bolz H, et al. Mutations in HPRP3, a third member of pre-mRNA splicing factor genes, implicated in autosomal dominant retinitis pigmentosa. Hum Mol Genet. 2002;11(1):87–92. doi: 10.1093/hmg/11.1.87. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Liu Y, Sheng XL, et al. PRPF4 mutations cause autosomal dominant retinitis pigmentosa. Hum Mol Genet. 2014;23(11):2926–2939. doi: 10.1093/hmg/ddu005. [DOI] [PubMed] [Google Scholar]
- 7.Tanackovic G, Ransijn A, Ayuso C, Harper S, Berson EL, Rivolta C. A missense mutation in PRPF6 causes impairment of pre-mRNA splicing and autosomal-dominant retinitis pigmentosa. Am J Hum Genet. 2011;88(5):643–649. doi: 10.1016/j.ajhg.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKie AB, McHale JC, Keen TJ, et al. Mutations in the pre-mRNA splicing factor gene PRPC8 in autosomal dominant retinitis pigmentosa (RP13) Hum Mol Genet. 2001;10(15):1555–1562. doi: 10.1093/hmg/10.15.1555. [DOI] [PubMed] [Google Scholar]
- 9.Vithana EN, Abu-Safieh L, Allen MJ, et al. A human homolog of yeast pre-mRNA splicing gene, PRP31, underlies autosomal dominant retinitis pigmentosa on chromosome 19q13.4 (RP11) Mol Cell. 2001;8(2):375–381. doi: 10.1016/s1097-2765(01)00305-7. [DOI] [PubMed] [Google Scholar]
- 10.Wheway G, Douglas A, Baralle D, Guillot E. Mutation spectrum of PRPF31, genotype-phenotype correlation in retinitis pigmentosa, and opportunities for therapy. Exp Eye Res. 2020;192:107950. doi: 10.1016/j.exer.2020.107950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aweidah H, Xi ZH, Sahel JA, Byrne LC. PRPF31-retinitis pigmentosa: challenges and opportunities for clinical translation. Vision Res. 2023;213:108315. doi: 10.1016/j.visres.2023.108315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xi ZH, Vats A, Sahel JA, Chen YY, Byrne LC. Gene augmentation prevents retinal degeneration in a CRISPR/Cas9-based mouse model of PRPF31 retinitis pigmentosa. Nat Commun. 2022;13(1):7695. doi: 10.1038/s41467-022-35361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keen TJ, Hims MM, McKie AB, et al. Mutations in a protein target of the Pim-1 kinase associated with the RP9 form of autosomal dominant retinitis pigmentosa. Eur J Hum Genet. 2002;10(4):245–249. doi: 10.1038/sj.ejhg.5200797. [DOI] [PubMed] [Google Scholar]
- 14.Lv JN, Zhou GH, Chen XJ, Chen H, Wu KC, Xiang LE, Lei XL, Zhang X, Wu RH, Jin ZB. Targeted RP9 ablation and mutagenesis in mouse photoreceptor cells by CRISPR-Cas9. Sci Rep. 2017;7:43062. doi: 10.1038/srep43062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao C, Bellur DL, Lu S, et al. Autosomal-dominant retinitis pigmentosa caused by a mutation in SNRNP200, a gene required for unwinding of U4/U6 snRNAs. Am J Hum Genet. 2009;85(5):617–627. doi: 10.1016/j.ajhg.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Writing Group For Practice Guidelines for Diagnosis and Treatment of Genetic Diseases Medical Genetics Branch of Chinese Medical Association. Yang Z, Yang J, Zhang Q, Li Y. Clinical practice guidelines for retinitis pigmentosa. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2020;37(3):295–299. doi: 10.3760/cma.j.issn.1003-9406.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Jones BW, Pfeiffer RL, Ferrell WD, Watt CB, Marmor M, Marc RE. Retinal remodeling in human retinitis pigmentosa. Exp Eye Res. 2016;150:149–165. doi: 10.1016/j.exer.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song HP, Zeng MY, Peng J, et al. Effects of Salviae miltiorrhizae on characteristic gene changes and key protein expression of Müller cells in pathological process of retinitis pigmentosa. Chinese Traditional and Herbal Drugs. 2019;50(8):1863–1872. [Google Scholar]
- 19.Jin ZB, Gao ML, Deng WL, Wu KC, Sugita S, Mandai M, Takahashi M. Stemming retinal regeneration with pluripotent stem cells. Prog Retin Eye Res. 2019;69:38–56. doi: 10.1016/j.preteyeres.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85(3):845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 21.Santos A. Preservation of the inner retina in retinitis pigmentosa. Arch Ophthalmol. 1997;115(4):511. doi: 10.1001/archopht.1997.01100150513011. [DOI] [PubMed] [Google Scholar]
- 22.Tezel TH, Kaplan HJ, Klein J. Hemoglobin-based methods for prophylaxis, diagnosis and/or treatment of retinal disorders. U.S. Patent Application No. 12/294,409. https://patentimages.storage.googleapis.com/87/b1/12/0c75a6b3231803/US20090053816A1.pdf . [Google Scholar]
- 23.Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26(2):495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- 24.Jones MK, Lu B, Girman S, Wang SM. Cell-based therapeutic strategies for replacement and preservation in retinal degenerative diseases. Prog Retin Eye Res. 2017;58:1–27. doi: 10.1016/j.preteyeres.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jayakody SA, Gonzalez-Cordero A, Ali RR, Pearson RA. Cellular strategies for retinal repair by photoreceptor replacement. Prog Retin Eye Res. 2015;46:31–66. doi: 10.1016/j.preteyeres.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Hirami Y, Osakada F, Takahashi K, Okita K, Yamanaka S, Ikeda H, Yoshimura N, Takahashi M. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009;458(3):126–131. doi: 10.1016/j.neulet.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 27.Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103(34):12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, Akaike A, Sasai Y, Takahashi M. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26(2):215–224. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- 29.Singh MS, Park SS, Albini TA, Canto-Soler MV, Klassen H, MacLaren RE, Takahashi M, Nagiel A, Schwartz SD, Bharti K. Retinal stem cell transplantation: balancing safety and potential. Prog Retin Eye Res. 2020;75:100779. doi: 10.1016/j.preteyeres.2019.100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salero E, Blenkinsop TA, Corneo B, Harris A, Rabin D, Stern JH, Temple S. Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell. 2012;10(1):88–95. doi: 10.1016/j.stem.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Blenkinsop TA, Salero E, Stern JH, Temple S. The culture and maintenance of functional retinal pigment epithelial monolayers from adult human eye. Methods Mol Biol. 2013;945:45–65. doi: 10.1007/978-1-62703-125-7_4. [DOI] [PubMed] [Google Scholar]
- 32.Mishra B, Wilson DR, Sripathi SR, Suprenant MP, Rui Y, Wahlin KJ, Berlinicke CA, Green JJ, Zack DJ. A combinatorial library of biodegradable polyesters enables non-viral gene delivery to post-mitotic human stem cell-derived polarized RPE monolayers. Regen Eng Transl Med. 2019;6(3):273–285. doi: 10.1007/s40883-019-00118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis RJ, Blenkinsop TA, Campbell M, et al. Human RPE stem cell-derived rpe preserves photoreceptors in the royal college of surgeons rat: method for quantifying the area of photoreceptor sparing. J Ocul Pharmacol Ther. 2016;32(5):304–309. doi: 10.1089/jop.2015.0162. [DOI] [PubMed] [Google Scholar]
- 34.Davis RJ, Alam NM, Zhao CP, et al. The developmental stage of adult human stem cell-derived retinal pigment epithelium cells influences transplant efficacy for vision rescue. Stem Cell Reports. 2017;9(1):42–49. doi: 10.1016/j.stemcr.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saini JS, Temple S, Stern JH. Human retinal pigment epithelium stem cell (RPESC) Adv Exp Med Biol. 2016;854:557–562. doi: 10.1007/978-3-319-17121-0_74. [DOI] [PubMed] [Google Scholar]
- 36.Stanzel BV, Liu Z, Somboonthanakij S, et al. Human RPE stem cells grown into polarized RPE monolayers on a polyester matrix are maintained after grafting into rabbit subretinal space. Stem Cell Reports. 2014;2(1):64–77. doi: 10.1016/j.stemcr.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golestaneh N, Chu Y, Xiao YY, Stoleru GL, Theos AC. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis. 2017;8(1):e2537. doi: 10.1038/cddis.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klassen H. Stem cells in clinical trials for treatment of retinal degeneration. Expert Opin Biol Ther. 2016;16(1):7–14. doi: 10.1517/14712598.2016.1093110. [DOI] [PubMed] [Google Scholar]
- 39.Luo J, Baranov P, Patel S, et al. Human retinal progenitor cell transplantation preserves vision. J Biol Chem. 2014;289(10):6362–6371. doi: 10.1074/jbc.M113.513713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitt S, Aftab U, Jiang CH, Redenti S, Klassen H, Miljan E, Sinden J, Young M. Molecular characterization of human retinal progenitor cells. Invest Ophthalmol Vis Sci. 2009;50(12):5901. doi: 10.1167/iovs.08-3067. [DOI] [PubMed] [Google Scholar]
- 41.Warfvinge K, Kiilgaard JF, Lavik EB, Scherfig E, Langer R, Klassen HJ, Young MJ. Retinal progenitor cell xenografts to the pig retina: morphologic integration and cytochemical differentiation. Arch Ophthalmol. 2005;123(10):1385–1393. doi: 10.1001/archopht.123.10.1385. [DOI] [PubMed] [Google Scholar]
- 42.Baranov PY, Tucker BA, Young MJ. Low-oxygen culture conditions extend the multipotent properties of human retinal progenitor cells. Tissue Eng Part A. 2014;20(9-10):1465–1475. doi: 10.1089/ten.tea.2013.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tucker BA, Redenti SM, Jiang CH, Swift JS, Klassen HJ, Smith ME, Wnek GE, Young MJ. The use of progenitor cell/biodegradable MMP2-PLGA polymer constructs to enhance cellular integration and retinal repopulation. Biomaterials. 2010;31(1):9–19. doi: 10.1016/j.biomaterials.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 44.Steedman M, Tao S, Klassen H, Desai T. Enhanced differentiation of retinal progenitor cells using microfabricated topographical cues. Biomed Microdevices. 2010;12:363–369. doi: 10.1007/s10544-009-9392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J, Li P, Zhao G, He S, Xu D, Jiang W, Peng Q, Li Z, Xie Z, Zhang H, Xu Y, Qi L. Mesenchymal stem cell-derived extracellular vesicles protect retina in a mouse model of retinitis pigmentosa by anti-inflammation through miR-146a-Nr4a3 axis. Stem Cell Res Ther. 2022;13(1):394. doi: 10.1186/s13287-022-03100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang Q, Li Q, Ren B, Yin ZQ. Intravenous infusion of small umbilical cord mesenchymal stem cells could enhance safety and delay retinal degeneration in RCS rats. BMC Ophthalmol. 2022;22(1):67. doi: 10.1186/s12886-021-02171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown C, Agosta P, McKee C, Walker K, Mazzella M, Alamri A, Svinarich D, Chaudhry GR. Human primitive mesenchymal stem cell-derived retinal progenitor cells improved neuroprotection, neurogenesis, and vision in rd12 mouse model of retinitis pigmentosa. Stem Cell Res Ther. 2022;13(1):148. doi: 10.1186/s13287-022-02828-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dezfuly AR, Safaee A, Amirpour N, Kazemi M, Ramezani A, Jafarinia M, Dehghani A, Salehi H. Therapeutic effects of human adipose mesenchymal stem cells and their paracrine agents on sodium iodate induced retinal degeneration in rats. Life Sci. 2022;300:120570. doi: 10.1016/j.lfs.2022.120570. [DOI] [PubMed] [Google Scholar]
- 49.Di Pierdomenico J, Gallego-Ortega A, Martínez-Vacas A, García-Bernal D, Vidal-Sanz M, Villegas-Pérez MP, García-Ayuso D. Intravitreal and subretinal syngeneic bone marrow mononuclear stem cell transplantation improves photoreceptor survival but does not ameliorate retinal function in two rat models of retinal degeneration. Acta Ophthalmol. 2022;100(6):e1313–e1331. doi: 10.1111/aos.15165. [DOI] [PubMed] [Google Scholar]
- 50.Liu Q, Liu J, Guo M, Sung TC, Wang T, Yu T, Tian Z, Fan G, Wu W, Higuchi A. Comparison of retinal degeneration treatment with four types of different mesenchymal stem cells, human induced pluripotent stem cells and RPE cells in a rat retinal degeneration model. J Transl Med. 2023;21(1):910. doi: 10.1186/s12967-023-04785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kahraman NS, Oner A. Umbilical cord derived mesenchymal stem cell implantation in retinitis pigmentosa: a 6-month follow-up results of a phase 3 trial. Int J Ophthalmol. 2020;13(9):1423–1429. doi: 10.18240/ijo.2020.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao TT, Lie HX, Wang F, Liu Y, Meng XH, Yin ZQ, Li SY. Comparative study of a modified sub-tenon's capsule injection of triamcinolone acetonide and the intravenous infusion of umbilical cord mesenchymal stem cells in retinitis pigmentosa combined with macular edema. Front Pharmacol. 2021;12:694225. doi: 10.3389/fphar.2021.694225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tagawa M, Ikeda HO, Inoue Y, Iwai S, Iida Y, Hata M, Asaka I, Tsujikawa A. Deterioration of phagocytosis in induced pluripotent stem cell-derived retinal pigment epithelial cells established from patients with retinitis pigmentosa carrying Mer tyrosine kinase mutations. Exp Eye Res. 2021;205:108503. doi: 10.1016/j.exer.2021.108503. [DOI] [PubMed] [Google Scholar]
- 54.Özmert E, Arslan U. Management of retinitis pigmentosa by Wharton's jelly-derived mesenchymal stem cells: prospective analysis of 1-year results. Stem Cell Res Ther. 2020;11(1):353. doi: 10.1186/s13287-020-01870-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Özkan B, Yılmaz Tuğan B, Hemşinlioğlu C, Sır Karakuş G, Şahin Ö, Ovalı E. Suprachoroidal spheroidal mesenchymal stem cell implantation in retinitis pigmentosa: clinical results of 6months follow-up. Stem Cell Res Ther. 2023;14(1):252. doi: 10.1186/s13287-023-03489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirami Y, Mandai M, Sugita S, et al. Safety and stable survival of stem-cell-derived retinal organoid for 2 years in patients with retinitis pigmentosa. Cell Stem Cell. 2023;30(12):1585–1596.e6. doi: 10.1016/j.stem.2023.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Ozmert E, Arslan U. Management of retinitis pigmentosa via wharton's jelly-derived mesenchymal stem cells or combination with magnovision: 3-year prospective results. Stem Cells Transl Med. 2023;12(10):631–650. doi: 10.1093/stcltm/szad051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma A, Jaganathan BG. Stem cell therapy for retinal degeneration: the evidence to date. Biologics. 2021;15:299–306. doi: 10.2147/BTT.S290331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vingolo EM, Mascolo S, Miccichè F, Manco G. Retinitis pigmentosa: from pathomolecular mechanisms to therapeutic strategies. Medicina (kaunas) 2024;60(1):189. doi: 10.3390/medicina60010189. [DOI] [PMC free article] [PubMed] [Google Scholar]