Abstract
In his beautiful book, Consilience: The Unity of Knowledge, the eminent biologist Edward O Wilson, advocates the need for integration and reconciliation across the sciences. He defines consilience as “literally a ‘jumping together’ of knowledge with a linking of facts ... to create a common groundwork of explanation”. It is the premise of this paper that as much as basic biomedical research is in need of data generation using the latest available techniques– unifying available knowledge is just as critical. This involves the necessity to resolve contradictory findings, reduce silos, and acknowledge complexity. We take the cornea and the lens as case studies of our premise. Specifically, in this perspective, we discuss the conflicting and fragmented information on protein aggregation, oxidative damage, and fibrosis. These are fields of study that are integrally tied to anterior segment research. Our goal is to highlight the vital need for Wilson's consilience and unity of knowledge which in turn should lead to enhanced rigor and reproducibility, and most importantly, to greater understanding and not simply knowing.
Keywords: anterior segment, ocular surface, cornea, lens, cataract, posterior capsular opacification, protein aggregation, oxidative damage, antioxidants, fibrosis, wound healing, consilience
INTRODUCTION
In July 1844, 35-year-old Charles Darwin finished the first report of his new theory: evolution by natural selection. As narrated by Darwin's great-great-grandson, the author Randal Keynes in his book, Darwin: His Daughter and Human Evolution, Charles next relayed to his wife Emma, “I write this in case of my sudden death as my most solemn and last request, that you will devote £400 to (the essay's) publication”. Mrs. Darwin read the 230-page essay carefully. She was supportive but skeptical. She hit at the heart of the theory by questioning how it could explain a structure as complex as the eye. Finding his explanation unconvincing, Charles Darwin set aside his essay and went on to study his barnacles, until 14y later, when Alfred Wallace's letter and manuscript arrived, jolting Darwin to write his book, On the Origin of Species. But even then, Charles could not really answer Emma's question. Charles wrote in his book, “To suppose that the eye with all its inimitable contrivances ... could have been formed by natural selection, seems, I freely confess, absurd in the highest degree”. Over a 150y later, the awe and the beauty of the eye have not diminished.
Whether you are looking for complexity or for simplicity, the eye has it. If you are into complexity, take the retina: five types of neurons, multiple subtypes of support cells, blood vessels, immune cells, and that is just at the anatomical level. But if you are into simplicity, take the lens: two types of cells–epithelial cells at the front and fiber cells in the rest. No nerves, no blood vessels, not even nuclei in most cells. Even organelles like ribosomes in the core lens fiber cells which may scatter light–gone. This means no translation and not even transcription. What could be simpler than that? Yet this simple structure is responsible for the number 1 cause of blindness worldwide[1]–[2]. Globally, there are approximately 20 million people who are bilaterally blind because of cataract. Even if we look at just the United States, over 24 million Americans are affected by cataract, and given an aging population, the numbers are expected to rise significantly (https://bit.ly/46NhV2C). Millions of surgeries are performed each year with considerable cost. And it gets worse. Cataract has a twin –presbyopia which is the loss of accommodative ability. And just like a cloudy lens is no good; a lens that cannot focus is no good either. Presbyopia is essentially universal. Practically every US adult over the age of 50 has it[3]–[4].
A core mission of the National Eye Institute (NEI), a component of the U.S. National Institutes of Health (NIH), is to “understand the eye and visual system”. Related to this mission, the NEI has the long-standing Cornea Program as well as Lens and Cataract Program. The aim of the former program is to support research projects directed at understanding the normal and diseased cornea (e.g., keratoconus and Fuchs' endothelial dystrophy) as well as tear-secreting glands and their dysfunction (e.g., dry eye disease). The aim of the latter program is to support research directed at understanding normal and diseased ocular lens (especially cataract and presbyopia). This understanding is essential to be able to reduce the burden of visual disorders in the United States and worldwide. Over the years, these NEI programs have supported many research projects which can be searched via the publicly accessible RePORTER database going all the way to 1985: https://reporter.nih.gov/advanced-search.
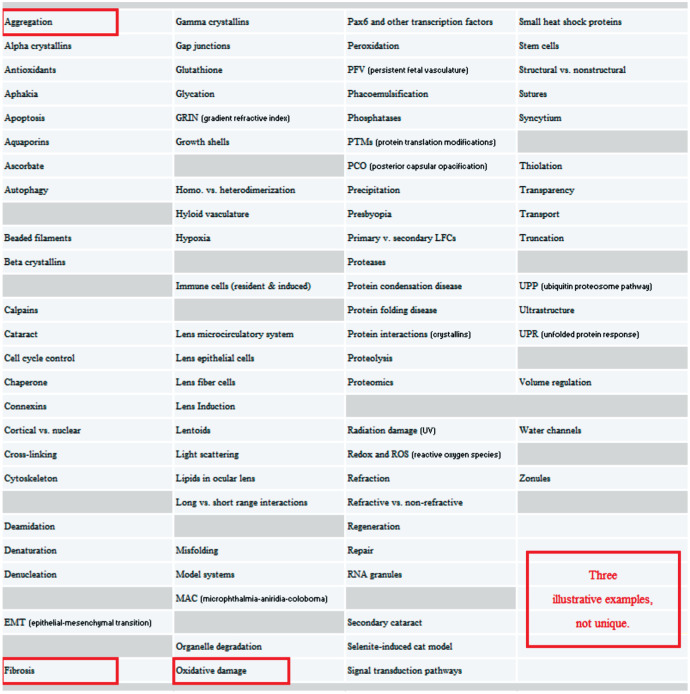
A large number of ideas have been proposed to understand biology and disease of the anterior segment. Figure 1 lists a subset of these terms directed at funded lens and cataract projects. This list can easily be doubled. Each entry addresses a relatively-isolated part of the lens biology and pathobiology puzzle. The usual formula is: The key to lens function is understanding_and you fill in the blank. Be it aquaporins, connexins, crystallins, etc. It has been proposed. Now we can take essentially any of these stories to illustrate a key barrier in translating basic findings to clinical insights but let us take these three: protein aggregation/oxysterols, oxidative damage/antioxidants, and fibrosis/wound healing. It is critical to emphasize that these are just illustrative examples and are not unique. We can take many others. It is also critical to emphasize that the barriers we describe not only affect lens and cornea research areas but other fields of inquiry too. Relating to the anterior segment and beyond. Relating to the eye and beyond. Replace our three stories with research topics X, Y, or Z and you are likely to reach similar conclusions. The need for consilience is broad.
Figure 1. Subset of targets proposed in lens and cataract research projects.
PROTEIN AGGREGATION AND OXYSTEROLS
Let us take the story of oxysterols and reversing cataract. A few years ago, two reports came out: the first in Nature titled, “Lanosterol reverses protein aggregation in cataracts”[5]. A couple of months later, the second report came out in Science titled, “Pharmacological chaperone for alpha-crystallin partially restores transparency in cataract models”[6]. Using Mendelian genetics (reverse approach) in the Nature work and high-throughput screening (forward approach) in the Science work, both papers hit upon an oxysterol, lanosterol in the Nature paper and a lanosterol-like molecule in the Science paper, as being able to reverse cataract in vivo. What is lanosterol? As the name implies, it is a sterol that is a key metabolite in the synthesis of cholesterol, and sterols are known to modulate membrane lipid domains in the lens[7].
These findings were really exciting because as the accompanying news and views essays made clear–this insight presented a way to chemically “dissolve” cataract[8]. No surgery needed, no intraocular lenses, and no risk of secondary cataract. Beyond the clear clinical significance and the wide-utility of such intervention, there were lots of other reasons to be excited about such discovery. Most importantly, there is biological plausibility. Alpha crystallins are small heat shock proteins that act as chaperones and prevent proteins from forming light-diffracting aggregates. During aging, this chaperone function is overwhelmed, thereby allowing light-scattering protein aggregates to form which in turn results in cataract. Moreover, human subjects with a homozygous mutation in the lanosterol synthase gene have congenital cataract[5]. It also turns out that a lanosterol-analogue docks nicely into a groove formed at the alpha-crystallin-A and alpha-crystallin-B dimer interface which stabilizes the native state[6]. Also critically, the nice overlap between the two sets of findings, obtained independently, means there is impressive reproducibility of the conclusions. In sum, the studies had high clinical relevance, wide-utility, biological plausibility, and independent confirmation. No wonder it was called a “new dawn for cataracts”[9].
Unfortunately, a subsequent report found neither lanosterol reported in the Nature paper nor the other oxysterol reported in the Science paper successfully reversed protein aggregation[10]. This report came on the heels of another paper that reported similar failure of oxysterol (lanosterol solution) to restore lens clarity from cataract[11]. It is worthwhile to note these are just two publications that overcame the difficulty of publishing negative findings. One can only guess if other groups encountered the same outcome but did not report their findings in the published literature.
So, what is going on? Could it be that the original two reports were just a flash in the pan? Perhaps the new era of cataract treatment is just the same as the old? Not so fast. Because following the two reports of negative findings, there were at least eight publications, representing six new research teams, with positive findings consistent with the original Nature and Science papers[12]–[19]. The dilemma is that the groups are using essentially different models. Indeed, a crowded set of model systems are involved in these published reports including human lenses[10]–[11], human lens progenitor cells[5], human induced pluripotent stem cells (iPSC)-derived lentoid bodies[15], zebrafish[17], mouse[6],[18], Sprague Dawley rat[10], Shumiya cataract rat (SCR)[12], selenite-induced cataract rat[14], rabbit[5],[19], dog[5], and cynomolgus monkey[16], not to mention in vitro[13], and in silico[6],[10] approaches. Needless to say, these are confusing sets of results. To quote the author Tom Peters, “If you're not confused, you're not paying attention”.
Of course the issue is not about this mouse, rat, or model system. It is about us. It is about answering the important clinical question: Can we have a “nonsurgical treatment for cataract” in the form of an oxysterol? The answer is: Maybe, maybe not. We do not know for sure. And beyond the lens, protein aggregation is known to play a role in some corneal dystrophies such as the link between transforming growth factor-β-induced protein (TGFBIp), an extracellular matrix protein that is the second most abundant protein in the corneal stroma, and granular corneal dystrophy (GCD)[20]. This kind of situation of seemingly oppositional reports in the literature that are not reconciled into a cohesive whole may present a significant barrier to move findings from the lab to the clinic.
OXIDATIVE DAMAGE AND ANTIOXIDANTS
The barrier is not limited to oxysterols and cataract nor the broader topic of protein aggregation in disease (whether in the anterior segment, the rest of the eye, or the rest of the body). The use of antioxidants to mitigate oxidative damage is another example where there is high clinical significance, broad-utility, biological plausibility, and independent confirmation, yet many questions remain unanswered. The idea of oxidative damage leading to disease is so attractive and powerful that it has been put forward to explain a wide range of diseases throughout the body, including eye diseases beyond cataract and Fuchs endothelial corneal dystrophy. The concept looks great and explains all sorts of observations. Environmental insults (e.g., smoking, pollutants, UV radiation, etc.) generate highly reactive species that damage cellular proteins, lipids and DNA which in turn lead to homeostatic dysregulation and ultimately to a disease state once the normal protective and cellular repair mechanisms are overwhelmed. Indeed, several cellular defense mechanisms are known to be involved, including superoxide dismutase, catalase, thioredoxin, peroxiredoxin, glutathione peroxidase, and glutathione S-transferase. Moreover, aging, the defining feature of age-related cataract (ARC), age-related macular degeneration (AMD), and age-related changes to the corneal surface[21], can be subsumed under the theme of oxidative damage/stress. Fundamentally, the paradigm suggests an unmistakable way to treat the resulting disease, namely, either reduce the oxidative stress or increase the antioxidant mechanisms. A beautiful cause-effect link with a clear linear relationship. A sharp dichotomy. A balance between oxidants and reductants. In a way, it can be thought of as a central dogma of age-related disease research. So, what is the issue?
Take a look at this headline: “The Myth of Antioxidants” by Moyer[22]. The author tells us that, “The oxidative damage, or free radical, theory of aging can be traced back to Denham Harman, who found his true calling in December 1945, thanks to the Ladies' Home Journal ”. Moyer concludes, “aging is far more intricate and complex than Harman imagined it to be nearly 60 years ago”. This is even more so a decade after Moyer's 2013 article. Here is another headline: “The Science Myths That Will Not Die”[23]. The article quotes the Canadian biologist Siegfried Hekimi, that it is “one of the few scientific theories to have reached the public: gravity, relativity and that free radicals cause ageing, so one needs to have antioxidants”. Yet, the British biologist David Gems concludes, “There's a question mark about whether really the whole thing (molecular damage causing ageing) should be chucked out”.
Could it be that antioxidants are more effective when we focus on the eye? Let us take a look. A Cochrane review looked at a popular antioxidant–N-acetylcarnosine or NAC–and concluded that, “There is currently no convincing evidence that NAC reverses cataract, nor prevents progression of cataract”[24]. Maybe that is just for one specific antioxidant? Let us look more broadly. Another Cochrane review focused on the evidence for antioxidant vitamin supplementation (beta-carotene, vitamin C and vitamin E) for prevention or slowing of cataract and found no evidence of benefit[25]. In fact, the authors concluded, “We do not recommend any further studies to examine the role of antioxidants against ARC”[25]. Results from other more-recent clinical trials seemingly lend further support to these conclusions. For example, a report from the Antioxidants for the Prevention of Cataracts study found “no difference in the risk of cataract extraction between the antioxidant vitamin group (vitamins A, C, and E) and the placebo group”[26].
Here is another example. Resveratrol, a polyphenol enriched in grapes, is also thought to counteract oxidative stress. In fact, besides its purported antioxidant ability, it has been proposed to have anti-angiogenic, anti-inflammatory, anti-platelet, anti-proliferative and a Janus-faced pro-proliferative ability[27]. Published literature reports evidence of resveratrol efficacy in various ocular models, including a selenite-induced cataract mouse model[28]–[29]; a streptozotocin-induced diabetic cataract rat model[30]–[31]; a naphthalene-induced cataract rat model[32]; in high glucose-induced oxidative damage in human lens epithelial cells[33], and in a human lens capsular bag model[34]. Yet the same literature currently reports zero publications for actual resveratrol efficacy against ARC in humans. Admittedly, this does not preclude that future clinical trials may yet show resveratrol efficacy. In fact, Clinicaltrials.gov shows 205 hits for resveratrol. But as things currently stand, MIT researcher Leonard Guarente's quip comes to mind, “Resveratrol is very, very good (at activating SIRT1 and extending lifespan) if you're a mouse” (https://bit.ly/46vaKwh).
The challenge of course is broader than NAC, resveratrol or vitamins. There is a zoo of so-called phytochemicals–both flavonoids and non-flavonoids; carotenes and xanthophylls and other agents with promise, plausibility, and publications and yet a lot of varying views on their efficacy not to mention effectiveness[35]–[36]. And this is before we take into account the complexity that arises from systems biology and the various omics approaches used to do read outs of cell and tissue function and dysfunction[37]. In sum, there is a cataract of information and fragmentation. We have a lot of information on anterior segment biology but a lot less understanding of anterior segment biology.
FIBROSIS AND WOUND HEALING
The idea of too much of this process or factor; too little of that is rather entrenched in multiple areas of investigation. Take the concept of fibrosis. Just like oxidative stress is thought to result from an overload of the natural cellular repairs mechanism, fibrosis is thought to result from an overload of the tissue's wound healing ability. But rather than subcellular damage seen in oxidative stress, fibrosis involves excessive accumulation of extracellular matrix components that results in a fibrotic scar. This is another example where there is high clinical significance, broad-utility, and biological plausibility. Yet questions remain.
Liver, heart, kidney, lung, and eye can all be affected by fibrosis. Let us take the anterior segment of the eye. We will start with the lens. Posterior capsular opacification (PCO) is an unfortunate and fairly common side effect of cataract surgery. PCO is thought to result from residual epithelial lens cells after capsulorhexis that proliferate, migrate to the posterior capsule and differentiate. These cells then clump and obstruct the light on its way to the posterior segment of the eye. The paradigm that gained the most traction to explain this pathologic process is the so-called epithelial-mesenchymal transition (EMT). Lots of work has gone into the molecular dissection of this pathway involving many proposed transcription factors and effectors. Multiple signaling pathways were also suggested with the transforming growth factor beta (TGFβ) in particular repeatedly implicated in PCO pathogenesis[38]–[42]. The attractiveness of the paradigm of EMT involvement in PCO goes beyond the identification of plausible transcription factors, effectors and signaling pathways. An intriguing and attractive idea is the potential role of TGFβ pathway in the so-called regenerative repair versus fibrotic repair[43]. Yet while the link between TGF and PCO was recognized over 3 decades ago[44]–[46], as of now, there are no approved drugs against PCO whether targeting the TGF/smad pathway or another. This is despite many promising reports. Here are 30 examples[47]–[76]. In fact, a recent Nature paper observed, “Approved antifibrotics have proven modest in efficacy”[77]. This is for all tissues and not just the anterior segment.
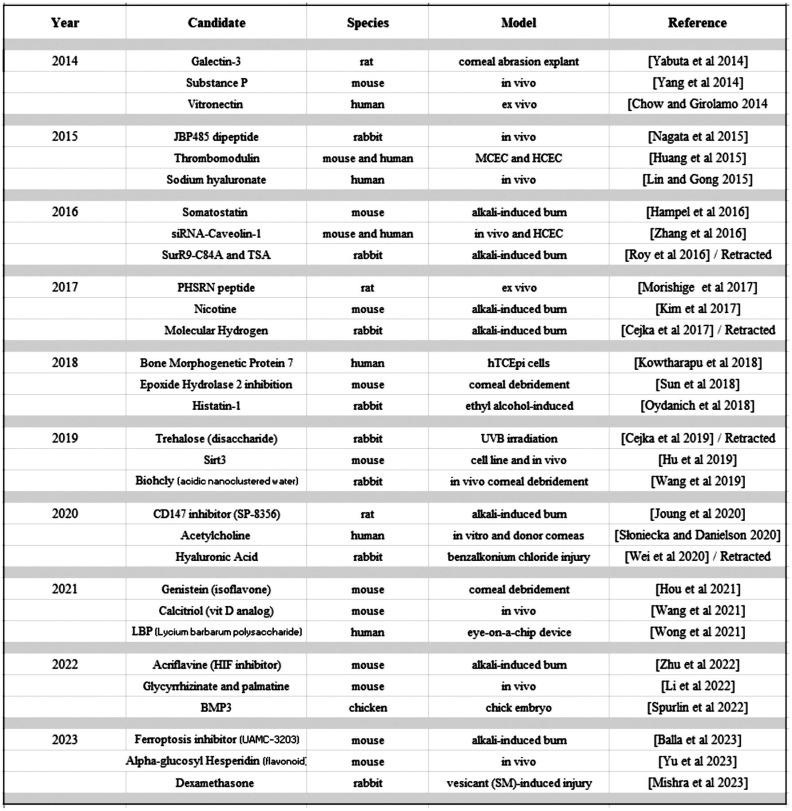
Let us now take a closer look at the cornea. Here the canvas is more vivid, yet interestingly, the dominant EMT and TGFβ paradigms seen in PCO of the lens are crowded out by a wider array of potential players in corneal wound healing. Figure 2 shows the results of an Embase search we performed to illustrate this richness. Importantly, this is not an exhaustive list. We simply pick 30 representative examples–3 for each year of the last decade[78]–[107]. We limited ourselves to corneal epithelial wound healing while setting aside corneal neovascularization and endothelial pathology, and we focused on small molecule or protein candidates while setting aside gene or cell-based therapies. We can easily compile numerous candidates proposed to be involved in corneal wound healing. One sees a labyrinth of potential hits and an abundance of proposals. Yet underneath this web of ideas, as with the PCO situation, again there is no clear synthesis. We find ourselves with an assortment of potential leads but little consilience. The classic Buddhist parable of the elephant comes to mind. A group of blindfolded fellows is told about something called an elephant, but none aware of its shape or form. One touches the trunk and thinks it must be a snake. Another touches the tail and is confident it must be a rope. And it goes. Leg being mistaken for a tree trunk, ear mistaken for a fan, tusk mistaken for a spear. Each confident they have the solution to the puzzle. Yet each focusing on only a piece of the puzzle while having little awareness of how the other pieces may fit together into a cohesive whole. Now this is just a parable of course. The worry, however, is that we may be seeing hints of it in the different approaches that arise in anterior segment research. So perhaps it is time to unify the clues and put the elephant together.
Figure 2. Representative examples of candidate effectors involved in corneal wound healing.
CONCLUSION
George Orwell tells us that, “To see what is in front of one's nose requires a constant struggle”. The fragmentation in knowledge is not limited to aggregation, oxidative damage or fibrosis. These are just illustrative examples. The issue certainly transcends the anterior segment with multiple domains and research areas that are beckoning to be further developed and untangled. This fragmentation is a challenge although it is also an opportunity. But how can we reconcile discrepant reports and chaotic models of disease and more meaningfully position basic science findings to better inform translational research and ultimately better patient care?
The challenges are formidable. Peer reviewers for many granting agencies and foundations tend to favor relatively safe projects supported with an abundance of preliminary data acting essentially as a promissory note for future publication(s). The expedient measure of success is the output in terms of publications. There is little recognition that perhaps some outcomes can be essentially immeasurable, at least in real time or in short term. Thus, a project whose team worked really hard and imaginatively but came up with negative findings is not viewed as a successful project and its continuation is in serious question. It is as if the underlying biological complexity that a lab happens to encounter becomes a problem for the lab and principal investigator rather than being a problem for the field. Ironically, such expediency seems to also underly the unequivocal reliance on P-value in statistical significance testing[108]–[110]. Aside from the misplaced burden of biological complexity, such incremental process tends to select for projects proposing bite-size advancement in knowledge at the expense of longer-term, higher-risk but higher-reward and broader investment. This can lead to fragmentary information; “splitting rather than lumping”[111]; silos[112]–[113]. So what can be done?
Let us start with a deeper and more humble recognition and acknowledgement of robustness in biological systems. In his book, Arrival of the Fittest, the evolutionary biologist Andreas Wagner points out that half the genes in our genome may have duplicates. But even more intriguing is the case of single-copy genes that are still dispensable. Are they purposeless? Take the example of connexin 23 which is enriched in the lens. Yet when it was deleted, Cx23-null mouse lenses were found to have transparency and refractive properties similar to wild type lenses[114]. To illustrate his point about robustness, Wagner writes that even an organism as simple as an E. coli can use >80 different molecules as its only source of energy be it an amino acid, a sugar, or a fatty acid. The cell is robust and this robustness can frustrate the typical reductionist approaches of one gene at-a-time or one protein at-a-time or one molecule at-a-time.
Let us also recognize the importance of negative data despite the disincentives to share and publish such outcomes[115]. Equally important is the need to acknowledge potentially conflicting data. Such discordance in findings does not necessarily mean that one set of findings is correct while the other false. Rather, it could be a result of the underlying biological complexity. Perhaps it is a reflection in the differences of the models used, definitions of a disease (e.g., “dry-eye”), biological readouts, “biomarkers” used, endpoints, or a myriad of other known and unknown factors. Here are a couple of recent cautionary tales. A comprehensive review of 162 published biomarkers for major mental disorders found that only 2 estimates met a priori defined criteria for convincing evidence, leading the authors to conclude, “This literature is fraught with several biases and is underpowered”[116]. While in another “reproducibility trial”, 246 biologists analyzed the same datasets and got widely divergent results (https://go.nature.com/3tFvfYU). Add to that, typical pathway figures/cartoons in the various published reviews which tend to give a false sense of order and simplicity to whatever biological pathway under discussion. Arrows giving a clear sense of direction. Labels giving a sense of conciseness and precision. One is reminded of Lewis Carroll's Through the Looking Glass (“When I use a word,” Humpty Dumpty said, “it means just what I choose it to mean—neither more nor less”).
The goal to simplify complex biological processes is certainly understandable. But it comes at a price of giving the illusion of knowing more than we actually do. Important caveats vanishing. Incongruent details fading. Contrary to myth, we tend to assume our ability to hold in our heads the cumulative knowledge we read in the past, when in fact we are unlikely to recall even a fraction of it. Just because such material is accessible via PubMed or an Embase search, does not mean we have incorporated it into our mental models of whatever biological mechanism that happens to be under consideration. Not that everyone is affected to the same degree. But there is an illusion of mastery. Take the jargon commonly used to report on this biological process or that. “TGFβ pathway”, “PCO”, “PTMs”(post-translational modifications), “oxidative damage”, “epigenetic”, “fibrotic”, “anti-inflammatory”, “pro-inflammatory”, “neuroprotective”, and many others. Each designation clearly carrying some meaning at its core yet in reality obscuring a lot of assumptions. The proverbial advice to making profit is an apt analogy: “Buy low; Sell high”. Works great on paper yet one belatedly comes to find out that in actuality this “insight/precision” is obscuring almost as much as it is informing. It seems to explain, and to a certain degree it does, but not nearly to the level at face value. And it is with many of our cherished buzzwords and lingo. As the American writer Walter Lippmann noted, we are all captives of the pictures in our heads, and we treat “these pictures as if they were the reality”. The author Derek Leebaert was even more astute when he wrote, “We live in a world to be labelled not understood.” There is a false sense of familiarity: if we label something, somehow, we understand it. But the comprehension is not merely labeling. Knowledge is not synonymous with understanding. Admittedly, the two are easy to conflate. It is a subtle trap to fall into, like the fellows examining the elephant each becoming attached to their assumptions and explanations.
This is where consilience comes in. Perhaps there could be incentives to motivate people to work together to resolve these seemingly discrepant reports and observations. To bridge the gulf between plausible explanations and real explanations. This is not about your typical “hypothesis-driven” projects. Those are here to stay. More data generation projects will always be needed. But instead of each project or team pushing their own model or favorite hypothesis in relative isolation–a “consilience-driven” project may be warranted. Perhaps even a hypothesis-free project. Minimal prior assumptions. All that is needed is a child-like sense of wonder. Darwin's bulldog, Thomas Huxley said it best, when he wrote, “Sit down before fact like a little child, and be prepared to give up every preconceived notion, follow humbly wherever and to whatever abyss Nature leads or you shall learn nothing”. Certainly, rigor and reproducibility are necessary and need to be enhanced. But more than that. It is about consilience. It is about dealing with the information overload and reducing complexity, resolving contradictory findings, reducing fragmentation, and reducing silos of basic versus clinical research. It is about understanding and not simply knowing. It is about Wilson's Unity of Knowledge. Perhaps we can start with the Unity of Anterior Segment. The Darwins would likely approve. Afterall, unity of type (descent with modification), was at the center of Charles's thesis to Emma.
Footnotes
The author thanks Dr. Tony Gover for insightful feedback, Dr. George McKie for comments on the concept, Dr. David Yeung for an in-depth review of the manuscript, and Dr. Martha Flanders for suggesting the title of the paper.
Disclaimer: The content is the sole responsibility of the author and does not necessarily represent the official view of the National Eye Institute (NEI), the National Institutes of Health (NIH), the Department of Health and Human Services (HHS), or any part of the U. S. Federal Government.
Conflicts of Interest: Araj H, None.
REFERENCES
- 1.Flaxman SR, Bourne RRA, Resnikoff S, et al. Vision Loss Expert Group of the Global Burden of Disease Study Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Data on Visual Impairments 2010 (WHO/NMH/PBD/12.01) Geneva: WHO; 2012. https://www.iapb.org/wp-content/uploads/GLOBALDATAFINALforweb.pdf. Accessed on April 2, 2024. [Google Scholar]
- 3.World Health Organization (WHO) World Report on Vision. 2019. https://cdn.who.int/media/docs/defaultsource/documents/world-vision-report-post-launch-accessible.pdf?sfvrsn=1b29f0e7_2. Accessed on: April 2, 2024.
- 4.Fricke TR, Tahhan N, Resnikoff S, Papas E, Burnett A, Ho SM, Naduvilath T, Naidoo KS. Global prevalence of presbyopia and vision impairment from uncorrected presbyopia: systematic review, meta-analysis, and modelling. Ophthalmology. 2018;125(10):1492–1499. doi: 10.1016/j.ophtha.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Zhao L, Chen XJ, Zhu J, et al. Corrigendum: Lanosterol reverses protein aggregation in cataracts. Nature. 2015;526(7574):595. doi: 10.1038/nature15253. [DOI] [PubMed] [Google Scholar]
- 6.Makley LN, McMenimen KA, DeVree BT, Goldman JW, McGlasson BN, Rajagopal P, Dunyak BM, McQuade TJ, Thompson AD, Sunahara R, Klevit RE, Andley UP, Gestwicki JE. Pharmacological chaperone for α-crystallin partially restores transparency in cataract models. Science. 2015;350(6261):674–677. doi: 10.1126/science.aac9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timsina R, Wellisch S, Haemmerle D, Mainali L. Binding of alpha-crystallin to cortical and nuclear lens lipid membranes derived from a single lens. Int J Mol Sci. 2022;23(19):11295. doi: 10.3390/ijms231911295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hejtmancik JF. Ophthalmology: cataracts dissolved. Nature. 2015;523(7562):540–541. doi: 10.1038/nature14629. [DOI] [PubMed] [Google Scholar]
- 9.Quinlan RA. Drug discovery. A new dawn for cataracts. Science. 2015;350(6261):636–637. doi: 10.1126/science.aad6303. [DOI] [PubMed] [Google Scholar]
- 10.Daszynski DM, Santhoshkumar P, Phadte AS, Sharma KK, Zhong HA, Lou MF, Kador PF. Failure of oxysterols such as lanosterol to restore lens clarity from cataracts. Sci Rep. 2019;9(1):8459. doi: 10.1038/s41598-019-44676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shanmugam PM, Barigali A, Kadaskar J, Borgohain S, Mishra DK, Ramanjulu R, Minija CK. Effect of lanosterol on human cataract nucleus. Indian J Ophthalmol. 2015;63(12):888–890. doi: 10.4103/0301-4738.176040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagai N, Umachi K, Otake H, Oka M, Hiramatsu N, Sasaki H, Yamamoto N. Ophthalmic in situ gelling system containing lanosterol nanoparticles delays collapse of lens structure in shumiya cataract rats. Pharmaceutics. 2020;12(7):629. doi: 10.3390/pharmaceutics12070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou H, Li YY, Yang Y, Liu ST, Yang ZX. Lanosterol reduces the aggregation propensity of ultraviolet-damaged human γD-crystallins: a molecular dynamics study. Phys Chem Chem Phys. 2021;23(24):13696–13704. doi: 10.1039/d1cp00132a. [DOI] [PubMed] [Google Scholar]
- 14.Deguchi S, Kadowaki R, Otake H, Taga A, Nakazawa Y, Misra M, Yamamoto N, Sasaki H, Nagai N. Combination of lanosterol and nilvadipine nanosuspensions rescues lens opacification in selenite-induced cataractic rats. Pharmaceutics. 2022;14(7):1520. doi: 10.3390/pharmaceutics14071520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang LF, Qin ZW, Lyu DN, Lu B, Chen ZJ, Fu QL, Yao K. Postponement of the opacification of lentoid bodies derived from human induced pluripotent stem cells after lanosterol treatment-the first use of the lens aging model in vitro in cataract drug screening. Front Pharmacol. 2022;13:959978. doi: 10.3389/fphar.2022.959978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang KK, He WW, Du Y, Zhou YG, Wu XK, Zhu J, Zhu XJ, Zhang K, Lu Y. Inhibitory effect of lanosterol on cataractous lens of cynomolgus monkeys using a subconjunctival drug release system. Precis Clin Med. 2022;5(3):pbac021. doi: 10.1093/pcmedi/pbac021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu CF, Ou-Yang Y, Huang CY, Jao SW, Kuo YK, Chen HC, Cheng SC, Wang NK, Chuang LH, Chen YH, Chen WY. Zebrafish (danio rerio) is an economical and efficient animal model for screening potential anti-cataract compounds. Transl Vis Sci Technol. 2022;11(8):21. doi: 10.1167/tvst.11.8.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang KH, Hoshino M, Uesugi K, Yagi N, Pierscionek BK, Andley UP. Oxysterol compounds in mouse mutant αA- and αB-crystallin lenses can improve the optical properties of the lens. Invest Ophthalmol Vis Sci. 2022;63(5):15. doi: 10.1167/iovs.63.5.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo C, Xu J, Fu C, Yao K, Chen X. New insights into change of lens proteins' stability with ageing under physiological conditions. Br J Ophthalmol. 2023;107(3):442–446. doi: 10.1136/bjophthalmol-2021-319834. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen NS, Gadeberg TAF, Poulsen ET, Harwood SL, Weberskov CE, Pedersen JS, Andersen GR, Enghild JJ. Mutation-induced dimerization of transforming growth factor-β-induced protein may drive protein aggregation in granular corneal dystrophy. J Biol Chem. 2021;297(1):100858. doi: 10.1016/j.jbc.2021.100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vitályos G, Kolozsvári BL, Németh G, Losonczy G, Hassan Z, Pásztor D, Fodor M. Effects of aging on corneal parameters measured with Pentacam in healthy subjects. Sci Rep. 2019;9:3419. doi: 10.1038/s41598-019-39234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moyer MW. The myth of antioxidants. Sci Am. 2013;308(2):62–67. doi: 10.1038/scientificamerican0213-62. [DOI] [PubMed] [Google Scholar]
- 23.Scudellari M. The science myths that will not die. Nature. 2015;528(7582):322–325. doi: 10.1038/528322a. [DOI] [PubMed] [Google Scholar]
- 24.Dubois VD, Bastawrous A. N-acetylcarnosine (NAC) drops for age-related cataract. Cochrane Database Syst Rev. 2017;2(2):CD009493. doi: 10.1002/14651858.CD009493.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathew MC, Ervin AM, Tao J, Davis RM. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012;6(6):CD004567. doi: 10.1002/14651858.CD004567.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srinivasan M, Ravindran RD, O'Brien KS, Kim UR, Wilkins JH, Whitcher JP, Lietman TM, Gritz DC, Keenan JD. Antioxidant vitamins for cataracts: 15-year follow-up of a randomized trial. Ophthalmology. 2020;127(7):986–987. doi: 10.1016/j.ophtha.2020.01.050. [DOI] [PubMed] [Google Scholar]
- 27.Abu-Amero KK, Kondkar AA, Chalam KV. Resveratrol and ophthalmic diseases. Nutrients. 2016;8(4):200. doi: 10.3390/nu8040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doganay S, Borazan M, Iraz M, Cigremis Y. The effect of resveratrol in experimental cataract model formed by sodium selenite. Curr Eye Res. 2006;31(2):147–153. doi: 10.1080/02713680500514685. [DOI] [PubMed] [Google Scholar]
- 29.Lin CH, Shih CC. Potential protective activities of extracts of phellinus linteus and the altered expressions of GSTM3 on age-related cataract. Evid Based Complement Alternat Med. 2021;2021:4313805. doi: 10.1155/2021/4313805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang HM, Li GX, Zheng HS, Wu XZ. Protective effect of resveratrol on lens epithelial cell apoptosis in diabetic cataract rat. Asian Pac J Trop Med. 2015;8(2):153–156. doi: 10.1016/S1995-7645(14)60307-2. [DOI] [PubMed] [Google Scholar]
- 31.Higashi Y, Higashi K, Mori A, Sakamoto K, Ishii K, Nakahara T. Anti-cataract effect of resveratrol in high-glucose-treated streptozotocin-induced diabetic rats. Biol Pharm Bull. 2018;41(10):1586–1592. doi: 10.1248/bpb.b18-00328. [DOI] [PubMed] [Google Scholar]
- 32.Singh A, Bodakhe SH. Resveratrol delay the cataract formation against naphthalene-induced experimental cataract in the albino rats. J Biochem Mol Toxicol. 2020;34(1):e22420. doi: 10.1002/jbt.22420. [DOI] [PubMed] [Google Scholar]
- 33.Chen PZ, Yao ZY, He ZH. Resveratrol protects against high glucose-induced oxidative damage in human lens epithelial cells by activating autophagy. Exp Ther Med. 2021;21(5):440. doi: 10.3892/etm.2021.9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith AJO, Eldred JA, Wormstone IM. Resveratrol inhibits wound healing and lens fibrosis: a putative candidate for posterior capsule opacification prevention. Invest Ophthalmol Vis Sci. 2019;60(12):3863–3877. doi: 10.1167/iovs.18-26248. [DOI] [PubMed] [Google Scholar]
- 35.Castro-Castaneda CR, Altamirano-Lamarque F, Ortega-Macías AG, Santa Cruz-Pavlovich FJ, Gonzalez-De la Rosa A, Armendariz-Borunda J, Santos A, Navarro-Partida J. Nutraceuticals: a promising therapeutic approach in ophthalmology. Nutrients. 2022;14(23):5014. doi: 10.3390/nu14235014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imelda E, Idroes R, Khairan K, Lubis RR, Abas AH, Nursalim AJ, Rafi M, Tallei TE. Natural antioxidant activities of plants in preventing cataractogenesis. Antioxidants (Basel) 2022;11(7):1285. doi: 10.3390/antiox11071285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anand D, Lachke SA. Systems biology of lens development: a paradigm for disease gene discovery in the eye. Exp Eye Res. 2017;156:22–33. doi: 10.1016/j.exer.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wormstone IM, Tamiya S, Anderson I, Duncan G. TGF-beta2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. Invest Ophthalmol Vis Sci. 2002;43(7):2301–2308. [PubMed] [Google Scholar]
- 39.Sponer U, Pieh S, Soleiman A, Skorpik C. Upregulation of alphavbeta6 integrin, a potent TGF-beta1 activator, and posterior capsule opacification. J Cataract Refract Surg. 2005;31(3):595–606. doi: 10.1016/j.jcrs.2004.05.058. [DOI] [PubMed] [Google Scholar]
- 40.Hosler MR, Wang-Su ST, Wagner BJ. Role of the proteasome in TGF-beta signaling in lens epithelial cells. Invest Ophthalmol Vis Sci. 2006;47(5):2045–2052. doi: 10.1167/iovs.05-0650. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Tang X, Chen X. Comparative effects of TGF-β2/Smad2 and TGF-β2/Smad3 signaling pathways on proliferation, migration, and extracellular matrix production in a human lens cell line. Exp Eye Res. 2011;92(3):173–179. doi: 10.1016/j.exer.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Kubo, Shibata T, Singh DP, Sasaki H. Roles of TGF β and FGF signals in the lens: tropomyosin regulation for posterior capsule opacity. Int J Mol Sci. 2018;19(10):3093. doi: 10.3390/ijms19103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lovicu FJ, Shin EH, McAvoy JW. Fibrosis in the lens. Sprouty regulation of TGFβ-signaling prevents lens EMT leading to cataract. Exp Eye Res. 2016;142:92–101. doi: 10.1016/j.exer.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jampel HD, Roche N, Stark WJ, Roberts AB. Transforming growth factor-beta in human aqueous humor. Curr Eye Res. 1990;9(10):963–969. doi: 10.3109/02713689009069932. [DOI] [PubMed] [Google Scholar]
- 45.Wickström K, Madsen K. The effect of transforming growth factor-alpha (TGF$aL) on rabbit and primate lens epithelial cells in vitro. Curr Eye Res. 1993;12(12):1123–1128. doi: 10.3109/02713689309033510. [DOI] [PubMed] [Google Scholar]
- 46.Liu J, Hales AM, Chamberlain CG, McAvoy JW. Induction of cataract-like changes in rat lens epithelial explants by transforming growth factor beta. Invest Ophthalmol Vis Sci. 1994;35(2):388–401. [PubMed] [Google Scholar]
- 47.Mansfield KJ, Cerra A, Chamberlain CG. FGF-2 counteracts loss of TGFbeta affected cells from rat lens explants: implications for PCO (after cataract) Mol Vis. 2004;10:521–532. [PubMed] [Google Scholar]
- 48.Awasthi N, Wagner BJ. Suppression of human lens epithelial cell proliferation by proteasome inhibition, a potential defense against posterior capsular opacification. Invest Ophthalmol Vis Sci. 2006;47(10):4482–4489. doi: 10.1167/iovs.06-0139. [DOI] [PubMed] [Google Scholar]
- 49.Kitano A, Saika S, Yamanaka O, Reinach PS, Ikeda K, Okada Y, Shirai K, Ohnishi Y. Genipin suppression of fibrogenic behaviors of the alpha-TN4 lens epithelial cell line. J Cataract Refract Surg. 2006;32(10):1727–1735. doi: 10.1016/j.jcrs.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Gotoh N, Perdue NR, Matsushima H, Sage EH, Yan Q, Clark JI. An in vitro model of posterior capsular opacity: SPARC and TGF-beta2 minimize epithelial-to-mesenchymal transition in lens epithelium. Invest Ophthalmol Vis Sci. 2007;48(10):4679–4687. doi: 10.1167/iovs.07-0091. [DOI] [PubMed] [Google Scholar]
- 51.Zhou P, Lu Y, Sun XH. Zebularine suppresses TGF-beta-induced lens epithelial cell-myofibroblast transdifferentiation by inhibiting MeCP2. Mol Vis. 2011;17:2717–2723. [PMC free article] [PubMed] [Google Scholar]
- 52.Yao J, Yang W, Liu Y, Sun YX, Jiang Q. Dexamethasone inhibits TGF-β2-induced migration of human lens epithelial cells: implications for posterior capsule opacification prevention. Mol Med Rep. 2012;5(6):1509–1513. doi: 10.3892/mmr.2012.827. [DOI] [PubMed] [Google Scholar]
- 53.Yang YF, Ye YM, Lin XC, Wu KL, Yu MB. Inhibition of pirfenidone on TGF-beta2 induced proliferation, migration and epithlial-mesenchymal transition of human lens epithelial cells line SRA01/04. PLoS One. 2013;8(2):e56837. doi: 10.1371/journal.pone.0056837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li P, Jing JN, Hu JY, Li TJ, Sun YC, Guan HJ. RNA interference targeting snail inhibits the transforming growth factor β2-induced epithelial-mesenchymal transition in human lens epithelial cells. J Ophthalmol. 2013;2013:869101. doi: 10.1155/2013/869101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wertheimer C, Liegl R, Kernt M, Docheva D, Kampik A, Eibl-Lindner KH. EGFR-blockade with erlotinib reduces EGF and TGF-β2 expression and the actin-cytoskeleton which influences different aspects of cellular migration in lens epithelial cells. Curr Eye Res. 2014;39(10):1000–1012. doi: 10.3109/02713683.2014.888453. [DOI] [PubMed] [Google Scholar]
- 56.Dong N, Xu B, Benya SR, Tang X. Retraction Note: MiRNA-26b inhibits the proliferation, migration, and epithelial–mesenchymal transition of lens epithelial cells. Mol Cell Biochem. 2024. [DOI] [PubMed]
- 57.Kayastha F, Madhu H, Vasavada A, Johar K. Andrographolide reduces proliferation and migration of lens epithelial cells by modulating PI3K/Akt pathway. Exp Eye Res. 2014;128:23–26. doi: 10.1016/j.exer.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 58.Chang KC, Petrash JM. Aldose reductase mediates transforming growth factor β2 (TGF-β2)-induced migration and epithelial-to-mesenchymal transition of lens-derived epithelial cells. Invest Ophthalmol Vis Sci. 2015;56(8):4198–4210. doi: 10.1167/iovs.15-16557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tiwari A, Kumar R, Ram J, Sharma M, Luthra-Guptasarma M. Control of fibrotic changes through the synergistic effects of anti-fibronectin antibody and an RGDS-tagged form of the same antibody. Sci Rep. 2016;6:30872. doi: 10.1038/srep30872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang CM, Liu JJ, Jin N, Zhang GM, Xi YH, Liu HL. SiRNA targeting mTOR effectively prevents the proliferation and migration of human lens epithelial cells. PLoS One. 2016;11(12):e0167349. doi: 10.1371/journal.pone.0167349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kubo, Shibata S, Shibata T, Kiyokawa E, Sasaki H, Singh DP. FGF2 antagonizes aberrant TGFβ regulation of tropomyosin: role for posterior capsule opacity. J Cell Mol Med. 2017;21(5):916–928. doi: 10.1111/jcmm.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu B, Sun JH, Lei XQ, Zhu ZQ, Pei C, Qin L. MicroRNA-486-5p suppresses TGF-β2-induced proliferation, invasion and epithelial-mesenchymal transition of lens epithelial cells by targeting Smad2. J Biosci. 2017;42(4):575–584. doi: 10.1007/s12038-017-9709-2. [DOI] [PubMed] [Google Scholar]
- 63.Shao JZ, Qi Y, Du SS, Du WW, Li FZ, Zhang FY. In vitro inhibition of proliferation, migration and epithelial-mesenchymal transition of human lens epithelial cells by fasudil. Int J Ophthalmol. 2018;11(8):1253–1257. doi: 10.18240/ijo.2018.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li H, Song H, Yuan XY, Li J, Tang H. MiR-30a reverses TGF-β2-induced migration and EMT in posterior capsular opacification by targeting Smad2. Mol Biol Rep. 2019;46(4):3899–3907. doi: 10.1007/s11033-019-04833-4. [DOI] [PubMed] [Google Scholar]
- 65.Ma B, Jing R, Liu J, Qi T, Pei C. Gremlin is a potential target for posterior capsular opacification. Cell Cycle. 2019;18(15):1714–1726. doi: 10.1080/15384101.2019.1632125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J, Xue W, Wang X, Huang W, Wang XX, Li H, Cui X, Li M, Mu H, Ren Y, Zhang F, Hu Y. HSP90 as a novel therapeutic target for posterior capsule opacification. Exp Eye Res. 2019;189:107821. doi: 10.1016/j.exer.2019.107821. [DOI] [PubMed] [Google Scholar]
- 67.Liu H, Jiang B. Let-7a-5p represses proliferation, migration, invasion and epithelial-mesenchymal transition by targeting Smad2 in TGF-b2-induced human lens epithelial cells. J Biosci. 2020;45:59. [PubMed] [Google Scholar]
- 68.Huang XB, Wang YL, Zhang P, Zou HD. A HGF-derived peptide suppresses EMT in human lens epithelial cells via the TGF-β/Smad and Akt/mTOR signaling pathways. Mol Med Rep. 2020;22(1):551–558. doi: 10.3892/mmr.2020.11097. [DOI] [PubMed] [Google Scholar]
- 69.Wang X, Wang L, Sun Y, Chen B, Xiong L, Chen J, Huang M, Wu J, Tan X, Zheng Y, Huang S, Liu Y. MiR-22-3p inhibits fibrotic cataract through inactivation of HDAC6 and increase of α-tubulin acetylation. Cell Prolif. 2020;53(11):e12911. doi: 10.1111/cpr.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun Y, Xiong L, Wang XR, Wang LP, Chen BX, Huang JQ, Huang M, Chen JP, Wu J, Huang S, Liu YZ. Autophagy inhibition attenuates TGF-β2-induced epithelial–mesenchymal transition in lens epithelial cells. Life Sci. 2021;265:118741. doi: 10.1016/j.lfs.2020.118741. [DOI] [PubMed] [Google Scholar]
- 71.Wang L, Tian Y, Shang ZQ, Zhang BY, Hua X, Yuan XY. Metformin attenuates the epithelial-mesenchymal transition of lens epithelial cells through the AMPK/TGF-β/Smad2/3 signalling pathway. Exp Eye Res. 2021;212:108763. doi: 10.1016/j.exer.2021.108763. [DOI] [PubMed] [Google Scholar]
- 72.Shihan MH, Novo SG, Wang Y, Sheppard D, Atakilit A, Arnold TD, Rossi NM, Faranda AP, Duncan MK. αVβ8 integrin targeting to prevent posterior capsular opacification. JCI Insight. 2021;6(21):e145715. doi: 10.1172/jci.insight.145715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huai B, Huang C, Hu L. Curcumin suppresses TGF-β2-induced proliferation, migration, and invasion in lens epithelial cells by targeting KCNQ1OT1/miR-377-3p/COL1A2 axis in posterior capsule opacification. Curr Eye Res. 2022;47(5):715–726. doi: 10.1080/02713683.2021.2021537. [DOI] [PubMed] [Google Scholar]
- 74.Guo M, Su FF, Chen Y, Su B. Interfering Hsa_circRNA_0060640 suppresses TGF-β2-induced proliferation, motility and emt in human lens epithelium cells by targeting miR-214-3p and collagen type i alpha2 chain. Curr Eye Res. 2022;47(5):735–746. doi: 10.1080/02713683.2022.2053724. [DOI] [PubMed] [Google Scholar]
- 75.Xiong L, Sun Y, Huang J, Ma P, Wang X, Wang J, Chen B, Chen J, Huang M, Huang S, Liu Y. Long non-coding RNA H19 prevents lens fibrosis through maintaining lens epithelial cell phenotypes. Cells. 2022;11(16):2559. doi: 10.3390/cells11162559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shih CC, Lee CY, Wong FF, Lin CH. Protective effects of one 2, 4-dihydro-3H-pyrazol-3-one derivative against posterior capsular opacification by regulation of TGF-β2/SMADs and non-SMAD signaling, collagen I, and fibronectin proteins. Curr Issues Mol Biol. 2022;44(10):5048–5066. doi: 10.3390/cimb44100343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhattacharya M, Ramachandran P. Immunology of human fibrosis. Nat Immunol. 2023;24(9):1423–1433. doi: 10.1038/s41590-023-01551-9. [DOI] [PubMed] [Google Scholar]
- 78.Yabuta C, Yano F, Fujii A, Shearer TR, Azuma M. Galectin-3 enhances epithelial cell adhesion and wound healing in rat cornea. Ophthalmic Res. 2014;51(2):96–103. doi: 10.1159/000355846. [DOI] [PubMed] [Google Scholar]
- 79.Yang LL, Di GH, Qi X, Qu ML, Wang Y, Duan HY, Danielson P, Xie LX, Zhou QJ. Substance P promotes diabetic corneal epithelial wound healing through molecular mechanisms mediated via the neurokinin-1 receptor. Diabetes. 2014;63(12):4262–4274. doi: 10.2337/db14-0163. [DOI] [PubMed] [Google Scholar]
- 80.Chow S, di Girolamo N. Vitronectin: a migration and wound healing factor for human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2014;55(10):6590–6600. doi: 10.1167/iovs.14-15054. [DOI] [PubMed] [Google Scholar]
- 81.Nagata M, Nakamura T, Hata Y, Yamaguchi S, Kaku T, Kinoshita S. JBP485 promotes corneal epithelial wound healing. Sci Rep. 2015;5:14776. doi: 10.1038/srep14776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang YH, I CC, Kuo CH, Hsu YY, Lee FT, Shi GY, Tseng SH, Wu HL. Thrombomodulin promotes corneal epithelial wound healing. PLoS One. 2015;10(3):e0122491. doi: 10.1371/journal.pone.0122491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin T, Gong L. Sodium hyaluronate eye drops treatment for superficial corneal abrasion caused by mechanical damage: a randomized clinical trial in the People's Republic of China. Drug Des Dev Ther. 2015:687. doi: 10.2147/DDDT.S77270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hampel U, Frömmling P, Bräuer L, Schaefer I, Sel S, Holland D, Paulsen F. Somatostatin supports corneal wound healing in vivo. Ann Anat Anat Anz. 2016;205:1–8. doi: 10.1016/j.aanat.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 85.Zhang C, Su X, Bellner L, Lin DH. Caveolin-1 regulates corneal wound healing by modulating Kir4.1 activity. Am J Physiol Cell Physiol. 2016;310(11):C993–C1000. doi: 10.1152/ajpcell.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roy K, Sriramoju B, Kanwar RK, Kanwar JR. Ophthalmic combination of SurR9-C84A and trichostatin-a targeting molecular pathogenesis of alkali burn. Front Pharmacol. 2016;7:226. doi: 10.3389/fphar.2016.00226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Morishige N, Uemura A, Morita Y, Nishida T. Promotion of corneal epithelial wound healing in diabetic rats by the fibronectin-derived peptide PHSRN. Cornea. 2017;36(12):1544–1548. doi: 10.1097/ICO.0000000000001344. [DOI] [PubMed] [Google Scholar]
- 88.Kim JW, Lim CW, Kim B. Effects of nicotine on corneal wound healing following acute alkali burn. PLoS One. 2017;12(6):e0179982. doi: 10.1371/journal.pone.0179982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cejka C, Kossl J, Hermankova B, Holan V, Cejkova J. Molecular hydrogen effectively heals alkali-injured cornea via suppression of oxidative stress. Oxid Med Cell Longev. 2017;2017:8906027. doi: 10.1155/2017/8906027. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Kowtharapu B, Prakasam R, Murín R, Koczan D, Stahnke T, Wree A, Jünemann A, Stachs O. Role of bone morphogenetic protein 7 (BMP7) in the modulation of corneal stromal and epithelial cell functions. Int J Mol Sci. 2018;19(5):1415. doi: 10.3390/ijms19051415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun HJ, Lee P, Yan CX, Gao N, Wang JM, Fan XQ, Yu FS. Inhibition of soluble epoxide hydrolase 2 ameliorates diabetic keratopathy and impaired wound healing in mouse corneas. Diabetes. 2018;67(6):1162–1172. doi: 10.2337/db17-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oydanich M, Epstein SP, Gadaria-Rathod N, Guers JJ, Fernandez KB, Asbell PA. In vivo efficacy of histatin-1 in a rabbit animal model. Curr Eye Res. 2018;43(10):1215–1220. doi: 10.1080/02713683.2018.1490772. [DOI] [PubMed] [Google Scholar]
- 93.Cejka C, Kossl J, Hermankova B, Holan V, Kubinova S, Olmiere C, Cejkova J. The healing of oxidative injuries with trehalose in UVB-irradiated rabbit corneas. Oxid Med Cell Longev. 2019;2019:1857086. doi: 10.1155/2019/1857086. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Hu J, Kan T, Hu X. Sirt3 regulates mitophagy level to promote diabetic corneal epithelial wound healing. Exp Eye Res. 2019;181:223–231. doi: 10.1016/j.exer.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 95.Wang T, Li WH, Zhong L, Ye H, Deng J, Chen YG, Wang T, Ling SQ. Evaluation of the effects of biohcly in an in vivo model of mechanical wounds in the rabbit cornea. J Ocul Pharmacol Ther. 2019;35(3):189–199. doi: 10.1089/jop.2018.0098. [DOI] [PubMed] [Google Scholar]
- 96.Joung C, Noh H, Jung J, Song HY, Bae H, Pahk K, Kim WK. A novel CD147 inhibitor, SP-8356, attenuates pathological fibrosis in alkali-burned rat cornea. Int J Mol Sci. 2020;21(8):2990. doi: 10.3390/ijms21082990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Słoniecka M, Danielson P. Acetylcholine decreases formation of myofibroblasts and excessive extracellular matrix production in an in vitro human corneal fibrosis model. J Cellular Molecular Medi. 2020;24(8):4850–4862. doi: 10.1111/jcmm.15168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wei N, Xu X, Huang C, Cao L. Retraction note: hyaluronic acid-Pluronic®F127-laden soft contact lenses for corneal epithelial healing: in vitro and in vivo studies. AAPS PharmSciTech. 2022;23(7):242. doi: 10.1208/s12249-022-02400-y. [DOI] [PubMed] [Google Scholar]
- 99.Hou YZ, Xin M, Li QQ, Wu XG. Glycyrrhizin micelle as a genistein nanocarrier: Synergistically promoting corneal epithelial wound healing through blockage of the HMGB1 signaling pathway in diabetic mice. Exp Eye Res. 2021;204:108454. doi: 10.1016/j.exer.2021.108454. [DOI] [PubMed] [Google Scholar]
- 100.Wang YD, Wan LQ, Zhang ZZ, Li J, Qu ML, Zhou QJ. Topical calcitriol application promotes diabetic corneal wound healing and reinnervation through inhibiting NLRP3 inflammasome activation. Exp Eye Res. 2021;209:108668. doi: 10.1016/j.exer.2021.108668. [DOI] [PubMed] [Google Scholar]
- 101.Wong HL, Hung LT, Kwok SS, Bu Y, Lin Y, Shum HC, Wang H, Lo ACY, Yam GHF, Jhanji V, Shih KC, Chan YK. The anti-scarring role of Lycium barbarum polysaccharide on cornea epithelial-stromal injury. Exp Eye Res. 2021;211:108747. doi: 10.1016/j.exer.2021.108747. [DOI] [PubMed] [Google Scholar]
- 102.Zhu S, Shan H, Li J, Pan L, Wang S, Zhu J, Guo H, Mi F, Wu X, Yin J, Pang K. Therapeutic potential of topical administration of acriflavine against hypoxia-inducible factors for corneal fibrosis. Front Pharmacol. 2022;13:996635. doi: 10.3389/fphar.2022.996635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Q, Xin M, Wu X, Lei B. A nano-phytochemical ophthalmic solution for marked improvement of corneal wound healing in healthy or diabetic mice. Nanomedicine (Lond) 2022;17(3):151–165. doi: 10.2217/nnm-2021-0417. [DOI] [PubMed] [Google Scholar]
- 104.Spurlin JW, 3rd, Garis MR, Lwigale PY. BMP3 inhibits TGFβ2-mediated myofibroblast differentiation during wound healing of the embryonic cornea. NPJ Regen Med. 2022;7(1):36. doi: 10.1038/s41536-022-00232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Balla A, Tran B, Valtari A, Steven P, Scarpellini C, Augustyns K, Urtti A, Vellonen KS, Ruponen M. A novel ferroptosis inhibitor UAMC-3203,a potential treatment for corneal epithelial wound. Pharmaceutics. 2022;15(1):118. doi: 10.3390/pharmaceutics15010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu LR, Zhang QL, Zhou LP, Wei YJ, Li MS, Wu XG, Xin M. Ocular topical application of alpha-glucosyl hesperidin as an active pharmaceutical excipient: in vitro and in vivo experimental evaluation. Drug Deliv Transl Res. 2024;14(2):373–385. doi: 10.1007/s13346-023-01403-x. [DOI] [PubMed] [Google Scholar]
- 107.Mishra N, Kant R, Kandhari K, Tewari-Singh N, Anantharam P, Croutch CR, Pantcheva MB, Petrash JM, Araj H, Agarwal C, Agarwal R. Establishing a dexamethasone treatment regimen to alleviate sulfur mustard-induced corneal injuries in a rabbit model. J Pharmacol Exp Ther. 2024;388(2):469–483. doi: 10.1124/jpet.123.001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.How the strange idea of ‘statistical significance’ was born. https://www.sciencenews.org/article/statistical-significance-p-value-null-hypothesis-origins. Accessed on: April 2, 2024.
- 109.Odds Are, It's Wrong. https://www.sciencenews.org/article/odds-are-its-wrong. Accessed on: April 2, 2024.
- 110.Null Science. https://sciencenews.org/archive/null-science. Accessed on: April 2, 2024.
- 111.Yeung DT, Araj H, Harper JR, Platoff GE. Considerations in developing medical countermeasures against chemical ocular toxicity. Toxicol Lett. 2020;334:1–3. doi: 10.1016/j.toxlet.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Araj H, Tumminia SJ, Yeung DT. Ocular surface - merging challenges and opportunities. Transl Vis Sci Technol. 2020;9(12):3. doi: 10.1167/tvst.9.12.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Araj H, Tseng H, Yeung DT. Supporting discovery and development of medical countermeasures for chemical injury to eye and skin. Exp Eye Res. 2022;221:109156. doi: 10.1016/j.exer.2022.109156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Berthoud VM, Minogue PJ, Snabb JI, Dzhashiashvili Y, Novak LA, Zoltoski RK, Popko B, Beyer EC. Connexin23 deletion does not affect lens transparency. Exp Eye Res. 2016;146:283–288. doi: 10.1016/j.exer.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nimpf S, Keays DA. Why (and how) we should publish negative data. EMBO Rep. 2020;21(1):e49775. doi: 10.15252/embr.201949775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carvalho AF, Solmi M, Sanches M, et al. Evidence-based umbrella review of 162 peripheral biomarkers for major mental disorders. Transl Psychiatry. 2020;10(1):152. doi: 10.1038/s41398-020-0835-5. [DOI] [PMC free article] [PubMed] [Google Scholar]