Abstract
Sofosbuvir/Velpatasvir (SOF/VEL) is a combination drug used for chronic hepatitis C (HCV) infection. However, limited information exists regarding the pharmacokinetics of SOF/VEL and its metabolites in hemodialysis patients. We conducted a prospective investigation of the pharmacokinetic parameters of SOF/VEL after a single dose of SOF/VEL (400/100 mg) on days with and without dialysis in 12 Thai hemodialysis patients with chronic HCV infection, who had been undergoing hemodialysis for a duration of 0.5–20 years. Blood samples were collected before dose (0) and 0.5, 1.0, 2.0, 2.5, 3.0, 3.5, 4.0, 5.0, 6.0, 8.0, and 12.0 h after dose. Dialysate samples were also collected before dose (0) and 1.0, 2.0, 3.0, and 4.0 h after dose. Plasma and dialysate samples were quantified for SOF and its metabolite, GS‐331007, and VEL concentrations using a fully validated LCMS technique. In addition, a preliminary efficacy study was conducted using the proposed SOF/VEL dose reduction regimen in all patients. No differences in SOF/VEL PK parameters between on‐ and off‐dialysis studies. On the contrary, GS‐331007 exhibited a 30% reduction in the area under the plasma concentration–time curve from time 0 to 24 h (AUC0‐24h) on dialysis days compared with non‐dialysis days (AUC0‐24h ratio 0.68 vs. 1.04, respectively). The dialysis clearance of SOF and GS‐331007 was 9.35 (8.72–15.11) and 8.89 (8.52–14.07) mL/min, respectively. Subsequently, an alternate‐day regimen of SOF/VEL (400/100 mg) was administered for 12 weeks, resulting in an undetectable plasma HCV viral load without side effects. Further clinical studies are warranted to validate the efficacy and safety of our proposed dose reduction regimen.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
SOF/VEL has shown efficacy in treating chronic HCV infection in CKD patients, regardless of HCV genotypes. Studies indicate that in individuals with severe kidney impairment, a SOF metabolite, GS‐331007, can accumulate 5–10 times. Despite this, no dose adjustment is currently recommended for the SOF/VEL regimen in patients with renal impairment. However, caution is warranted, as CKD patients often receive multiple medications that pose the risk of drug–drug interactions and toxicities.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study investigated the pharmacokinetic profiles of SOF/VEL in hemodialysis patients with HCV infection, with the goal of establishing an appropriate dose regimen of SOF/VEL based on the obtained pharmacokinetic data. Subsequently, the efficacy of the SOF/VEL dosage regimen determined was also evaluated.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Hemodialysis did not affect plasma levels of SOF and VEL. However, GS‐331007 had a 30% reduction in the extent of the drug in the body on dialysis days compared with non‐dialysis days. Based on pharmacokinetic data, the SOF/VEL dose was determined to be a 50% reduction from the standard dose [SOF/VEL (400/100 mg) administration after each hemodialysis session for a duration of 12 weeks]. The preliminary efficacy study demonstrated an undetectable plasma HCV viral load without side effects with this proposed regimen.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
This study sheds light on the pharmacokinetics of SOF/VEL and GS‐331007 in hemodialysis patients with chronic HCV infection. The proposed dose reduction regimen holds promise in minimizing the risk of drug toxicities and related complications. Additionally, its implementation could offer significant benefits to low‐ and middle‐income countries struggling with limited healthcare budgets.
INTRODUCTION
Hepatitis C virus (HCV) infection poses a significant global health challenge, especially in low‐ and middle‐income countries. 1 , 2 Left untreated, ~ 50% of individuals infected with HCV will develop chronic hepatitis, cirrhosis, or hepatocellular carcinoma. 3 Patients with end‐stage renal disease (ESRD) requiring hemodialysis or peritoneal dialysis face a higher risk of HCV infection and transmission within dialysis units. 4 , 5 , 6 In Thailand, the incidence of HCV infection among dialysis patients stands at ~ 4.2%, amplifying the likelihood of progressive liver disease in those chronically infected with HCV. 7 Furthermore, HCV infection accelerates the decline in kidney function and increases morbidity and mortality rates among hemodialysis patients. 8
Various highly effective direct‐acting antivirals (DAA) are employed in treating HCV infection. 8 , 9 , 10 , 11 Sofosbuvir (SOF), an inhibitor of HCV NS5B polymerase, and Velpatasvir (VEL), an inhibitor of HCV NS5A polymerase, constitute a DAA regimen that demonstrates effectiveness and good tolerability in chronic kidney disease (CKD) patients, regardless of HCV genotypes. 8 , 12 , 13 , 14 , 15 SOF is a prodrug that undergoes metabolism to its metabolite, GS‐331007, which is primarily excreted through the kidneys. 15 Conversely, clearance of VEL occurs primarily through hepatic clearance. 11
According to the product prescribing information, in HCV‐negative subjects with mild (estimated glomerular filtration (eGFR) 50–80 mL/min/1.73 m2), moderate (eGFR 30–50 mL/min/1.73 m2), and severe renal impairment (eGFR < 30 mL/min/1.73 m2) following a single 400 mg dose of SOF, the area under the concentration–time curve from time 0 to infinity (AUC0‐inf) of GS‐331007 was 55%, 88%, and 451% higher in mild, moderate, and severe renal impairment, respectively, compared with those with eGFR >80 mL/min/1.73 m2. In ESRD, compared with individuals with normal renal function, AUC0inf of GS‐331007 was 1280% higher when SOF was administered 1 h before hemodialysis. 15 Despite these data, no dosage adjustment is required for patients with renal impairment, especially mild‐to‐moderate renal impairment. 9 Recent guidance also suggests that no dosage adjustment is required for SOF in patients with severe renal impairment or ESRD with a relatively low level of recommendation and less strength of evidence. 9
Several concerns should also be noted, as CKD patients often receive multiple medications that pose a risk of drug–drug interactions. For instance, administration of SOF with amiodarone has been associated with life‐threatening bradycardia. 8 Co‐administration of SOF/VEL with dabigatran can increase dabigatran exposure by 161% due to inhibition of drug transporters. 16 Recently, a pharmacokinetic drug–drug interaction study between SOF/VEL and crizotinib revealed severe cardiac toxicity, with potential implications for other tyrosine kinase inhibitors. 17 Furthermore, previous reports have highlighted potential SOF toxicity in patients with renal failure. 18 , 19 Therefore, further investigation of alterations in SOF/VEL pharmacokinetics in ESRD patients with hemodialysis, appropriate dosage regimen for CKD stage V as per pharmacokinetic study, and the efficacy of SOF/VEL dose reduction remains an area requiring study. Moreover, a dose reduction regimen could prove beneficial for low and middle‐income countries facing financial constraints in healthcare budget allocation.
The objective of this study was to comprehensively analyze the pharmacokinetic profiles of SOF/VEL in hemodialysis patients with chronic HCV infection. Furthermore, the study aimed to establish a suitable SOF/VEL dose regimen based on the pharmacokinetic data obtained, which was subsequently administered to a cohort of Thai hemodialysis patients with chronic HCV infection to evaluate the efficacy of the proposed regimen.
METHODS
We conducted a prospective pharmacokinetic study involving hemodialysis patients with chronic HCV infection. The study adhered to the principles outlined in the Declaration of Helsinki of the World Medical Association and followed the guidelines for Good Clinical Practice established by the International Conference on Harmonization.
Participants
We enrolled adult hemodialysis patients who tested positive for HCV antibodies. Exclusion criteria included a history of hypersensitivity to SOF/VEL or current use of drugs considered moderate to strong inducers of cytochrome P450‐2B6 (CYP2B6), CYP2C8, or CYP3A4, such as antacids, carbamazepine, efavirenz, famotidine, omeprazole, phenytoin, phenobarbital, rifampicin, rifapentine, and St. John's wort. In addition, participants who currently receive treatment with amiodarone, atorvastatin, digoxin, rosuvastatin, or tenofovir were excluded. Pregnant or breastfeeding individuals, those currently undergoing SOF/VEL treatment, and those with systemic infections that require antibiotics, antifungals, or antivirals other than anti‐HIV drugs were also excluded. All participants underwent both the pharmacokinetic study and the subsequent efficacy evaluation of the proposed SOF/VEL regimen.
Sample size calculation
The sample size calculation for the pharmacokinetic study was performed using SAS® version 9.4. We used the paired means procedure, which evaluates the sample size using a lognormal distribution and an exact (asymptotic) method, based on previous data reported by Borgia et al. 20 Assuming a correlation within‐patient correlation between paired observations on‐ and off‐dialysis ranging from 0.5 to 0.7, and considering a maximum coefficient of variation of 0.51 (51%), 20 enrolling 10 patients would provide 80% power to detect a reduction of 30% or more in the maximum plasma concentration (C max) and the area under the concentration–time curve from time 0 to 24 h (AUC0–24h) between on‐ and off‐dialysis. This analysis was carried out at a two‐sided significance level of 5%.
Sample collection for pharmacokinetic study
All participants underwent two single‐dose pharmacokinetic studies (on‐ and off‐dialysis), with a washout period of at least 2 weeks between each study. During both studies, participants received a single dose of SOF/VEL (400/100 mg), and venous blood samples (3 mL) were collected before dose (0) and 0.5, 1.0, 2.0, 2.5, 3.0, 3.5, 4.0, 5.0, 6.0, 8.0, and 12.0 h after dose, in EDTA tubes. Plasma concentrations of SOF, GS‐331007, and VEL were measured.
On the day of dialysis, dialysate samples (20 mL) were collected before dose (0) and 1.0, 2.0, 3.0, and 4.0 h after dose to measure SOF, GS‐331007, and VEL concentrations. Additionally, blood flow, dialysate flow, and dialyzer models were recorded. All participants underwent dialysis sessions using a polysulfone dialyzer with a surface area of 1.8 m2 and an ultrafiltration coefficient (Kuf) of 55 mL/h/mmHg.
Bioanalysis of SOF, GS‐331007, and VEL in plasma and dialysate
The concentrations of SOF, GS‐331007, and VEL in plasma and dialysate were quantified with a fully validated liquid chromatography mass spectrometry technique (LC–MS/MS) according to previous reports with modifications. 21 , 22 Chromatographic separation was performed with a Luna C18 column (100 × 2 mm, 3 μm, Phenomenex, USA). The column oven was set at 40°C. The detector was MS/MS with an electrospray ionization probe in the positive mode for the precursor to product ion transition (m/z) of 530.10–243.05, 261.25–113.05, 883.25–709.15, and 390.10–268.10, for SOF, GS‐331007, VEL, and tadalafil (internal standard), respectively.
For the preparation of the plasma sample, 200 μL of plasma sample and 10 μL of 1 μg/mL tadalafil (Sigma‐Aldrich, Germany), and 1 mL of ethyl acetate (Merck, Germany) were mixed and centrifuged at 1940g, 10°C, for 10 min. The organic layer was then evaporated to dryness using a vacuum evaporator. Subsequently, the residue was later reconstituted with 200 μL of the mobile phase composed of 50/50 v/v 0.1% formic acid in water (Merck, Germany) and acetonitrile (Merck, Germany) and was filtrated through a 0.22 μm nylon syringe in a vial and placed on an autosampler tray at 15°C. Finally, 2.5 μL of the extracted solution was injected into the LC–MS/MS system (LCMS‐8060NX, Lab Solution Software version 5.99 SP2, Shimadzu, Kyoto, Japan). For the preparation of dialysate samples, 2 mL of dialysate sample, 10 μL of 1 μg/mL tadalafil, and 3 mL of ethyl acetate were mixed and centrifuged at 1940g, 10°C, for 10 min. The rest of the sample preparation process was similar to that of plasma sample preparation. The final volume of 1 μL of the extracted dialysate solution was injected into the LC–MS/MS system.
Validation of the analytical method validation was consistent with the European Medicines Agency 23 and the US Food and Drug Administration 24 guidelines. Briefly, the technique showed good selectivity since there was no interference peak at the retention time of all the analytes (SOF, GS‐331007, and VEL) in blank plasma and blank dialysate from 6 different sources. The calibration curve was performed using seven standard points of data with good linearity (r 2 > 0.99), covering concentrations of 0.202–4039.469 ng/mL, 10.110–3017.933 ng/mL, and 2.513–1522.962 ng/mL for plasma SOF, GS‐331007, and VEL, respectively, and 0.203–1016.383 ng/mL, 9.995–1031.772 ng/mL, and 3.057–185.294 ng/mL for dialysate SOF, GS‐331007, and VEL, respectively.
The accuracy and precision were determined by analyzing five replicates run for three different days at plasma and dialysate concentrations of the lower limit of detection (LLOQ) and quality control (QC) concentrations including low (LQC), medium (MQC), and high (HQC). Acceptance criteria for accuracy and precision were ± 15% from the actual values and coefficient variation (CV) of <15%, except for the concentration at LLOQ where the acceptance criteria were accuracy of 80%–120% from the actual values and precision of <20% CV. Our method was carried out with good accuracy and precision as given in Table S1.
The carryover test was assessed by continuously injecting a series of blank samples after the upper limit of detection (ULOQ) concentration of the calibration curve in plasma and dialysate to see if there was any carryover concentration appearing (sequence: blank sample, ULOQ, blank sample, ULOQ, blank sample, blank sample, and LLOQ). Here, the peak responses of SOF, GS331007, VEL, and internal standard were undetected in blank plasma and blank dialysate confirming no residual carryover of all analytes.
Additionally, the dilution integrity of the plasma samples was assessed for a dilution factor of 1:1. The %accuracy and precision (%CV) of the dilution integrity were 105.943% (0.450%CV), 105.789% (0.650%CV), and 100.432% (0.934%CV) for SOF, GS‐331007, and VEL, respectively, and these were in the acceptance criteria showing good dilution integrity in plasma samples.
The post‐preparative stability for plasma was also tested using three processed samples of LQC and HQC. The samples were kept in an autosampler at 15°C for 48 h. Subsequently, the samples were analyzed at 48 h and the concentrations obtained were compared with the concentrations of freshly prepared QC samples. Our method demonstrated good stability of the samples at 15°C for 48 h in the autosampler (Table S2).
The recovery in plasma was determined by comparing five replicates of the detector response at pre‐extraction of all analytes and internal standards in LQC and HQC with the detector response obtained from post‐extraction. Our method demonstrated that recovery was consistent, reproducible, and precise at % CV of ≤3.415%, 7.311%, 10.304%, and 9.063% for SOF, GS‐331007, VEL, and internal standard, respectively. The % recovery is shown in Table S3.
Efficacy study
Based on the C max and AUC0‐24h obtained in the pharmacokinetic study, we determined the SOF/VEL dosage regimen for chronic hemodialysis participants by comparing our SOF, GS‐331007, and VEL data with reported data from healthy subjects 20 , 25 and patients with HCV infection with normal renal function. 20 The objective of designing these dosing regimens was to ensure adequate plasma drug exposure while minimizing potential side effects. Furthermore, we considered the nature of SOF/VEL drug formulation and the impact of hemodialysis on the elimination of SOF, GS‐331007, and VEL when determining the dose. The duration of the treatment period was based on previously reported data. 13 , 20 , 25 , 26
All participants in the pharmacokinetic study underwent quantitative HCV RNA tests. Those with active viral replication of HCV received SOF/VEL according to the proposed dose schedule derived from the pharmacokinetic study, for a duration of 12 weeks. Adverse events were monitored and recorded throughout the treatment period. Following completion of the 12‐week course of SOF/VEL treatment, quantitative HCV RNA tests were performed to evaluate the efficacy of the treatment.
Pharmacokinetics and statistical analyzes
Statistical analysis were performed using SPSS for Windows (version 22). Pharmacokinetic parameters were calculated using noncompartmental analysis (Phoenix WinNonlin, version 8.3). The reported pharmacokinetic parameters included AUC0–24h, C max, time to reach C max (T max), elimination rate constant (Kel), half‐life (T 1/2), and dialysis clearance (CL‐dialysis). Unless otherwise specified, continuous data were presented as mean ± standard deviation or median (range). The Wilcoxon signed‐rank test was used to assess differences in these pharmacokinetic parameters between on‐ and off‐dialysis, with the statistical significance set at p < 0.05.
Ethics statement
The approval for the study protocol was obtained from the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB number 997/64). Prior to participating in the study, all participants provided written informed consent.
RESULTS
Characteristics of the participants
A total of 12 participants (7 men and 5 women, aged between 35 and 61 years) were enrolled in the study. Their body weight ranged from 39.0 to 85.0 kg, with a mean of 66.1 ± 15.3 kg. Their body mass index (BMI) ranged from 17.3 to 34.0 kg/m2, with a mean of 23.3 ± 5.2 kg/m2. Participants had been on hemodialysis for a duration of 0.5–20 years, with a mean dialysis duration of 7.9 ± 6.6 years. The causes of ESRD included Alport syndrome (n = 1), autosomal dominant polycystic kidney disease (n = 1), chronic urate nephropathy (n = 1), diabetic kidney disease (n = 1), and hypertensive nephrosclerosis (n = 8), (Table 1).
TABLE 1.
Characteristics of the participants (n = 12).
| Characteristics | |
|---|---|
| Male/female (n) | 5/7 |
| Age (years) | 49.9 ± 11.7 |
| Body weight (kg) | 66.1 ± 15.3 |
| Height (cm) | 170 ± 10 |
| Body mass index (kg/m2) | 23.3 ± 5.2 |
| Dialysis vintage (years) | 7.9 ± 6.6 |
| Cause of end stage renal disease | |
| Alport syndrome | 1 |
| Autosomal dominant polycystic kidney disease | 1 |
| Chronic urate nephropathy | 1 |
| Diabetic kidney disease | 1 |
| Hypertensive nephrosclerosis | 8 |
| Clinical parameters on pharmacokinetic study | |
| Dialysis day | |
| Systolic blood pressure (mmHg) | 143.5 ± 20.3 |
| Diastolic blood pressure (mmHg) | 72.0 ± 11.4 |
| Mean arterial pressure (mmHg) | 87.9 ± 30.1 |
| Pulse (bpm) | 72 ± 13 |
| Body temperature (°C) | 36.3 ± 0.3 |
| Blood flow (mL/min) | 320.8 ± 33.4 |
| Dialysate flow (mL/min) | 550.0 ± 100.0 |
| Ultrafiltration volume (mL) | 3.054 ± 828 |
| Hemoglobin (g/dL) | 11.2 ± 0.5 |
| Blood urea nitrogen (mg/dL) | 79.4 ± 6.9 |
| Creatinine (mg/dL) | 7.7 ± 0.8 |
| Aspartate aminotransferase (U/L) | 28.2 ± 3.3 |
| Alanine transaminase (U/L) | 28.4 ± 1.8 |
| Albumin (g/dL) | 3.8 ± 0.2 |
| Non‐dialysis day | |
| Systolic blood pressure (mmHg) | 141.7 ± 18.4 |
| Diastolic blood pressure (mmHg) | 78.2 ± 12.0 |
| Mean arterial pressure (mmHg) | 99.4 ± 12.1 |
| Pulse (bpm) | 70 ± 10 |
| Body temperature (°C) | 36.6 ± 0.2 |
Note: Data are presented as mean ± standard deviation unless otherwise stated.
SOF/VEL pharmacokinetic parameters and the effect of hemodialysis
For the pharmacokinetic study conducted on dialysis days, participants received a single dose of SOF/VEL (400/100 mg) before undergoing a hemodialysis session while fasting. The mean arterial pressure (MAP) of the participants was 87.9 ± 30.1 mmHg and the heart rate was 72 ± 13 beats per minute. None of the participants had a fever, with oral body temperatures recorded at 36.3 ± 0.3°C. The average blood flow during hemodialysis was 320.8 ± 33.4 mL/min (range: 300–400 mL/min), and the dialysate flow was 550 ± 100 mL/min (range: 500–800 mL/min). The ultrafiltration rate during the 4‐h hemodialysis session was 3054 ± 828 mL (range: 1800–4200 mL). All participants completed the 4‐h hemodialysis session without intradialytic events. For the pharmacokinetic study conducted on non‐dialysis days, participants also received a single dose of SOF/VEL (400/100 mg) in a fasting state. The mean MAP was 99.4 ± 12.1 mmHg and the heart rate was 70 ± 10 beats per minute. None of the participants had a fever, with oral body temperatures recorded at 36.6 ± 0.2°C. All participants successfully completed the pharmacokinetic studies on dialysis and non‐dialysis days without experiencing adverse events.
No differences were observed in plasma SOF concentrations between on‐ and off‐dialysis conditions (Table 2, Figure 1a, Figure S1). However, plasma concentrations of GS‐331007 showed a significant decrease of approximately 30% from C2.5 on a dialysis day compared with a non‐dialysis day (Table 2, Figure 1b, Figure S2). Furthermore, significant differences in plasma VEL concentrations were observed at C5 and C6 between on‐ and off‐dialysis days (Table 2, Figure 1c, Figure S3). During the 4‐h hemodialysis session, SOF and GS‐331007, but not VEL, were excreted by dialysis (Table 3, Figure 2).
TABLE 2.
Plasma concentrations of Sofosbuvir, GS‐331007, and Velpatasvir (n = 12) from time 0 to 24 h of drug administration.
| Timepoint | Sofosbuvir (ng/mL) | GS‐331007 (ng/mL) | Velpatasvir (ng/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Off‐dialysis | On‐dialysis | p value | Off‐dialysis | On‐dialysis | p value | Off‐dialysis | On‐dialysis | p value | |
| C0 | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 1.000 | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 1.000 | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 1.000 |
| C0.5 | 1944.00 (1.66–3562.98) | 1310.25 (11.24–3478.32) | 0.182 | 244.81 (81.90–542.23) | 147.57 (26.23–876.07) | 0.401 | 67.83 (7.04–221.28) | 28.53 (12.43–102.95) | 0.173 |
| C1 | 1542.52 (2.43–3291.32) | 1182.58 (11.45–3880.99) | 0.814 | 496.77 (9.88–908.45) | 514.55 (171.82–1329.26) | 0.953 | 193.24 (44.45–516.81) | 175.57 (4.26–307.95) | 0.241 |
| C2 | 432.12 (4.04–2434.16) | 466.94 (52.40–2671.36) | 0.530 | 874.98 (243.40–2845.94) | 612.32 (34.71–1701.98) | 0.110 | 465.94 (26.19–913.85) | 368.04 (6.12–633.37) | 0.594 |
| C2.5 | 227.50 (5.57–889.37) | 226.51 (24.48–2169‐27) | 0.814 | 878.37 (407.57–3352.16) | 646.64 (35.63–1566.29) | 0.003 | 425.72 (85.94–677.86) | 455.68 (3.58–916.95) | 0.722 |
| C3 | 129.81 (8.99–384.51) | 153.21 (14.38–914.22) | 0.638 | 996.21 (503.62–3666.62) | 780.04 (117.53–1534.36) | 0.003 | 495.88 (146.37–972.87) | 458.27 (28.75–914.48) | 0.859 |
| C3.5 | 75.94 (14.31–200.46) | 80.34 (6.25–367.31) | 0.695 | 1091.64 (576.53–3795.40) | 782.98 (107.69–1532.69) | 0.003 | 545.26 (173.30–1093.85) | 464.34 (37.27–982.85) | 0.594 |
| C4 | 41.17 (9.04–109.58) | 44.54 (4.09–367.81) | 0.530 | 1179.81 (610.55–3660.82) | 804.99 (215.70–1259.72) | 0.003 | 440.74 (167.94–1076.23) | 455.40 (92.58–1007.96) | 0.929 |
| C5 | 14.39 (3.42–479.37) | 15.52 (1.59–967.03) | 0.695 | 1296.19 (45.55–3529.06) | 704.48 (378.67–1115.63) | 0.012 | 317.20 (3.30–821.96) | 441.56 (158.51–964.54) | 0.028 |
| C6 | 7.39 (1.11–1157.58) | 7.09 (0.64–616.41) | 1.000 | 1235.60 (290.17–3610.27) | 826.97 (521.83–1329.14) | 0.012 | 218.77 (33.65–712.29) | 414.19 (164.31–981.10) | 0.041 |
| C8 | 1.76 (0.35–239.04) | 2.19 (0.69–214.01) | 0.929 | 1307.30 (506.77–3194.72) | 906.70 (578.83–1241.41) | 0.008 | 131.10 (52.80–565.12) | 255.91 (134.84–505.64) | 0.099 |
| C12 | 0.41 (0.25–149.64) | 0.31 (0.23–19.00) | 0.345 | 1489.93 (652.99–2810.26) | 993.59 (571.75–1263.53) | 0.005 | 99.35 (35.96–281.09) | 112.57 (63.01–255.73) | 0.638 |
| C24 | nd | nd | nd | 1533.57 (921.15–2686.62) | 1123.66 (658.14–1431.87) | 0.005 | 41.80 (13.86–132.24) | 42.18 (19.55–88.74) | 0.937 |
Note: Data are presented as median (range).
Abbreviation: nd, not detected.
FIGURE 1.
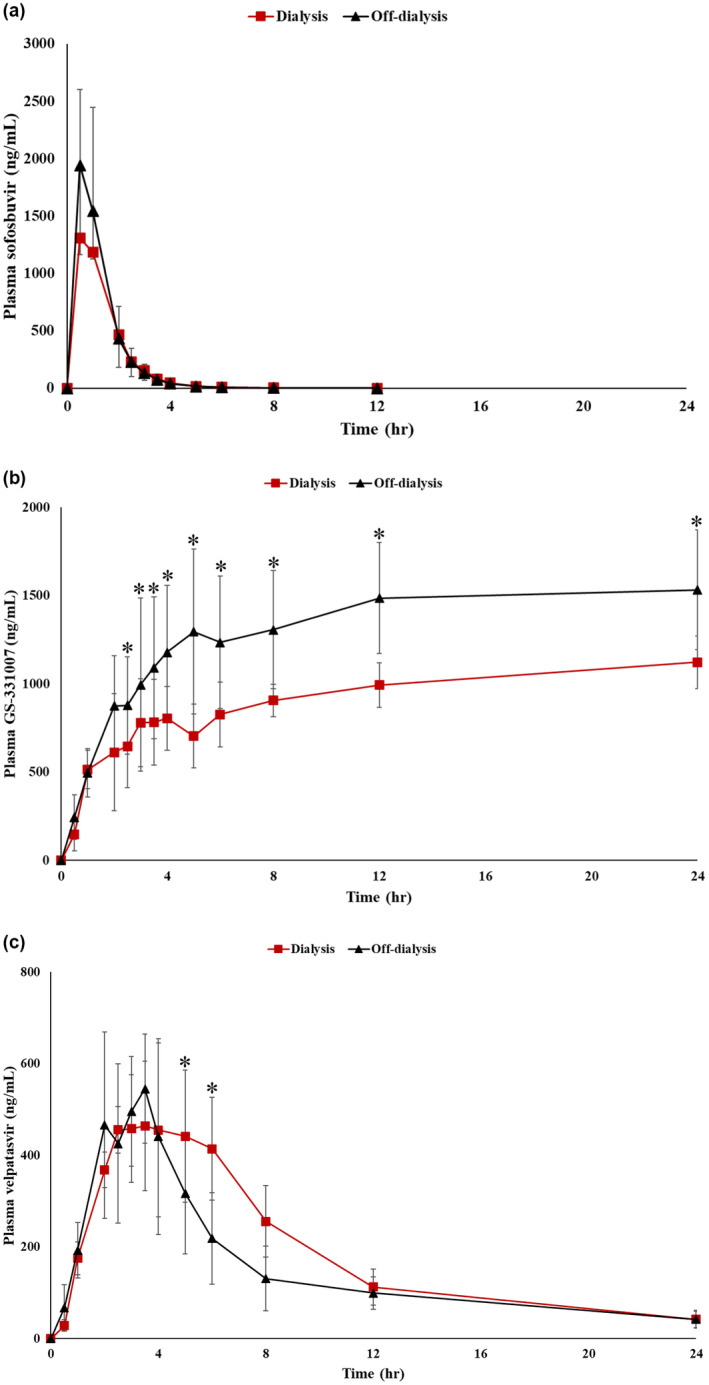
Plasma concentration–time profile of Sofosbuvir (a), GS‐331007 (b), and Velpatasvir (c) during on‐ and off‐dialysis (n = 12). *p < 0.05.
TABLE 3.
Dialysate concentrations of Sofosbuvir, GS‐331007, and Velpatasvir (n = 12) during 4 h of dialysis session.
| Timepoint | Concentration (ng/mL) | Accumulative concentration (ng/mL) | ||||
|---|---|---|---|---|---|---|
| Sofosbuvir | GS‐331007 | Velpatasvir | Sofosbuvir | GS‐331007 | Velpatasvir | |
| C0 | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | nd | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | nd |
| C1 | 38.29 (2.51–236.61) | 185.67 (83.73–394.11) | nd | 38.29 (2.51–236.61) | 185.67 (83.73–394.11) | nd |
| C2 | 10.35 (2.75–91.76) | 175.51 (17.40–313.13) | nd | 52.93 (7.49–251.22) | 347.06 (14.27–634.11) | nd |
| C3 | 2.06 (0.62–56.53) | 158.80 (17.40–313.13) | nd | 54.92 (8.21–257.87) | 558.56 (31.67–805.19) | nd |
| C4 | 0.69 (0.27–33.79) | 119.17 (44.77–207.05) | nd | 62.81 (9.77–260.05) | 716.34 (76.44–920.51) | nd |
Note: Data are presented as median (range).
Abbreviation: nd, not detected.
FIGURE 2.
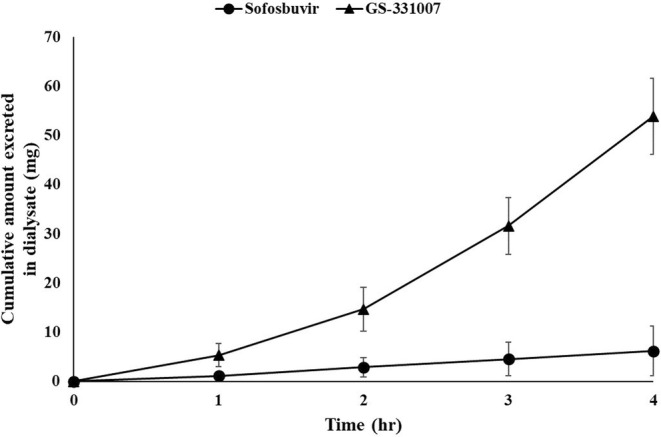
Cumulative amount of Sofosbuvir and GS‐331007 in dialysate (n = 12).
Plasma pharmacokinetic parameters of SOF, GS‐331007, and VEL were determined (Table 4). No differences were observed in SOF pharmacokinetic parameters (T max, C max, AUC0‐24h, T 1/2, and Kel) between on‐ and off‐dialysis studies (Table 4). Calculations of T max, C max, T 1/2, and Kel for GS‐331007 were not feasible since the plasma concentration–time profiles did not show a terminal elimination phase (Figure 1b). However, GS‐331007 exhibited a significant reduction in AUC0‐24h on a dialysis day (~30% reduction, AUC0‐24h ratio 0.68) compared with non‐dialysis (AUC0‐24h ratio 1.04, Table 4). The CL‐dialysis of SOF and GS‐331007 was 9.35 (8.72–15.11) and 8.89 (8.52–14.07) mL/min, respectively. As expected, no significant changes were observed in the pharmacokinetic parameters of VEL (Table 4).
TABLE 4.
Pharmacokinetic parameters of Sofosbuvir, GS‐331007, and Velpatasvir during on‐ and off‐dialysis (n = 12).
| Pharmacokinetic parameters | Sofosbuvir | GS‐331007 | Velpatasvir | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Off‐dialysis | On‐dialysis | p value | Off‐dialysis | On‐dialysis | p value | Off‐dialysis | On‐dialysis | p value | |
| T max (h) | 0.5 (0.5–6.0) | 0.8 (0.5–5.0) | 0.786 | na | na | na | 3.5 (2.0–12.0) | 4.5 (2.5–6.0) | 0.153 |
| C max (ng/mL) | 2349.64 (1157.58–3562.98) | 2280.57 (967.03–3880.99) | 0.754 | na | na | na | 534.40 (173.30–1093.85) | 497.75 (201.73–1007.96) | 0.480 |
| AUC0‐24h (ng.h/mL) | 2881 (1645‐,5084) | 2773 (1029‐4681) | 0.937 | 32,330 (13,198‐67,911) | 21,400 (13,694‐31,382) | 0.006 | 3742 (1249‐9351) | 4613 (1732‐8405) | 0.347 |
| T 1/2 (h) | 1.21 (0.62–1.77) | 1.18 (0.75–1.11) | 0.286 | na | na | na | 7.26 (4.83–10.74) | 6.18 (4.97–8.17) | 0.050 |
| Kel (1/h) | 0.574 (0.392–1.112) | 0.599 (0.264–0.930) | 0.213 | na | na | na | 0.095 (0.065–0.143) | 0.113 (0.085–0.139) | 0.062 |
| CL‐dialysis (mL/min) | na | 9.35 (8.72–15.11) | na | na | 8.89 (8.52–14.07) | na | na | na | na |
| AUC0‐24h ‐on/AUC0‐24h ‐off‐dialysis ratio | na | 1.04 (0.42–1.64) | na | na | 0.68 (0.38–1.47) | na | na | 1.41 (0.36–3.20) | na |
Note: Data are presented as median (range).
Abbreviations: AUC0‐24h, area under the concentration–time curve from time 0 to 24 h; C max, maximum plasma concentration; CL, clearance; Kel, elimination rate constant; na, not applicable; T max, time to maximum plasma concentration; T 1/2, half‐life.
SoF/VEL dose reduction regimen for chronic hemodialysis patients
Although no dose adjustment is recommended for the SOF/VEL regimen in patients with renal impairment, a lower level of recommendation and less strength of evidence were stated for ESRD patients requiring hemodialysis. 9 Furthermore, it is important to note that CKD patients often receive multiple medications that pose a risk of drug–drug interactions and toxicity, 8 , 16 , 17 , 18 , 19 affecting quality of life and expenses due to the amount of drug used and other costs related to toxicity. Therefore, the optimal dose reduction regimen was proposed for ESRD patients with hemodialysis using the pharmacokinetic data from our study. Here, we observed that at least 30% of GS‐331007 was eliminated by dialysis (Tables 2, 3, 4, Figure 1b). Considering the constraints posed by the combined formulation of SOF/VEL tablets (400/100 mg), which cannot be divided, we established a dose reduction regimen for SOF/VEL. This regimen entails the administration of one tablet of SOF/VEL every other day, following the conclusion of the hemodialysis sessions, both on dialysis and non‐dialysis days, regardless of its alignment with the scheduled administration day.
Efficacy study
Among the 12 participants, 10 showed active HCV viral replication (Table 5), with a median plasma HCV viral load of 142,819 IU/mL (range: 179–33,500,000 IU/mL). These 10 participants completed the proposed SOF/VEL dose reduction regimen for 12 weeks and achieved a sustained viral response, evidenced by an undetectable plasma HCV viral load (<15 IU/mL, Table 5). None of them reported any adverse effects during or after receiving SOF/VEL.
TABLE 5.
Efficacy of Sofosbuvir/Velpatasvir in the eradication of the hepatitis C virus in patients on chronic hemodialysis.
| Participants No | Plasma hepatitis C viral load (copies/mL) | |
|---|---|---|
| Pretreatment | Post 12‐week treatment | |
| 01 | 129,000 | <15 |
| 02 | 25,300 | <15 |
| 03 | <15 | na |
| 04 | <15 | na |
| 05 | 237,000 | <15 |
| 06 | 33,500,000 | <15 |
| 07 | 97,900 | <15 |
| 08 | 14,700,000 | <15 |
| 09 | 156,639 | <15 |
| 10 | 651,446 | <15 |
| 11 | 179 | <15 |
| 12 | 41,554 | <15 |
Abbreviation: na, not applicable.
DISCUSSION
This study aimed to characterize the pharmacokinetic profiles of a single dose of SOF/VEL in chronic hemodialysis patients with chronic HCV infection. The results revealed a 30% reduction in plasma concentrations of GS‐331007, the SOF metabolite, during hemodialysis, while hemodialysis did not affect plasma levels of SOF and VEL. Based on the pharmacokinetic study, a 50% reduction in the SOF/VEL dose (alternate‐day administration of SOF/VEL (400/100 mg) after hemodialysis session for 12 weeks) was implemented. The preliminary efficacy study of the proposed dose regimen demonstrated the effectiveness of the dose reduction regimen in hemodialysis patients with chronic HCV infection to eradicate HCV.
Our study indicates that the extent of SOF (represented by C max, AUC, and T 1/2) in chronic hemodialysis patients differs from those of non‐dialysis individuals, while the absorption rate (Tmax) was not affected. 25 , 27 Phase I pharmacokinetic data in 14 healthy Chinese subjects 25 (male/female = 9/5, mean age 29 years, receiving a 400 mg single dose of SOF, and a 400 mg once daily dose of SOF for 7 days) showed that, in healthy Chinese subjects, C max (single dose C max = 1001.7 ng/mL; multiple dose C max = 922.3 ng/mL) and AUC (single dose AUC0‐inf = 861 ng.h/mL; multiple dose AUC0‐Ʈ = 872.2 ng.h/mL) were lower than our data in hemodialysis patients, either on‐dialysis (single dose C max = 2280.57 ng/mL and AUC0‐24h = 2773 ng.h/mL) or off‐dialysis (single dose C max = 2349.64 ng/mL and AUC0‐24h = 2881 ng.h/mL). The T 1/2 in this group of healthy Chinese subjects was 0.38 h, less than our data (on‐dialysis T 1/2 = 1.18 h, off‐dialysis = 1.21 h). The same was found in another study of 14 healthy Chinese subjects (male/female = 7/7, mean age 29 years, receiving a single dose (day 1) and multiple dose (day 8–14) of SOF/VEL (400/100 mg)), C max, AUC0‐24h and T 1/2 of SOF in our chronic hemodialysis patients was higher compared with data in healthy Chinese subjects (single dose C max = 1551.4 ng/mL, AUC0‐24h = 1739.7 ng.h/mL, T 1/2 = 0.43 h; multiple dose C max = 1531.1 ng.mL, AUC0‐24h = 2019.7 ng.h/mL, T 1/2 = 0.44 h). 27 It must be taken into account that the subjects in these two previous studies were younger than our hemodialysis patients, the differences observed may be from a combination of age and renal insufficiency.
Our results are consistent with previous findings in CKD patients. Lawitz et al. 28 reported a 1.4–2 fold increase in SOF‐AUC0‐24h in CKD patients with GFR <30 mL/min without dialysis compared with those with GFR >60 mL/min. Additionally, for hemodialysis patients, a phase II study involving 59 patients of various ethnic groups (Asian, Black, and Caucasian) demonstrated that chronic hemodialysis patients with HCV infection exhibited a 1.8‐fold increase in both the C max and AUC0‐24h of SOF compared with individuals with normal renal function. 20
Similar to SOF, our CKD cohort also had a 2–3 folds increase in AUC0‐24h of GS‐331007 compared with healthy individuals. 25 , 27 The magnitude of the changes was greater than in dialysis patients in whom C max and AUC0‐24h of GS‐331007 increased by 1.8 and 1.2 times, respectively. 20 Tmax (2–4 h), C max (836–1283 ng/mL), and T 1/2 (19.5–30.5 h) were also reported in previous studies 25 , 27 ; however, we cannot determine these pharmacokinetic parameters of GS‐331007 in our study as the concentration–time profile did not show the terminal elimination phase (Table 2, Figure 1b). In fact, SOF is initially metabolized in the liver into the pharmacologically active nucleoside analog triphosphate GS‐461203. The metabolic activation pathway involves sequential hydrolysis of the carboxyl ester moiety catalyzed by human cathepsin A or carboxylesterase 1 and phosphoramidate cleavage by histidine triad nucleotide‐binding protein 1 followed by phosphorylation by the pyrimidine nucleotide biosynthesis pathway. This is followed by dephosphorylation to the main nucleoside metabolite GS‐331007, which accounts for more than 90% of systemic exposure. 11 , 15 The intermediate metabolite GS‐461203, which was not detected in our study, could explain the increase in GS‐331007 level after SOF has been eliminated from plasma.
Our indirect measurement of the hemodialysis extraction ratio was estimated by the ratio between the AUC0‐24h on‐dialysis and the AUC0‐24h off‐dialysis, resulting in the estimated hemodialysis extraction ratio of 1.04 and 0.68 for SOF and GS‐331007, respectively (Table 4). Desnoyer et al. conducted a pharmacokinetic study before and after hemodialysis in patients with chronic HCV infection who received 400 mg SOF once a day (n = 7) or three times a week (n = 5), after hemodialysis. 26 They showed that their hemodialysis extraction ratio of GS‐331007, which was left over from the previous dose, was ~ 50%, comparable to our data. However, after the hemodialysis session, the patients in that study received DAA and plasma concentrations of SOF and GS‐331007 were measured 1.5 h after dose (C1.5). The time of plasma SOF and GS‐331007 concentrations measured in that study (C1.5) was between our plasma SOF and GS‐331007 concentration measurement at 1 (C1) and 2 (C2) hours after dose on a non‐dialysis day. We observed that SOF concentrations were comparable between studies (our non‐dialysis SOF C1 = 1542.52 ng/mL, C2 = 432.12 ng/mL vs. Desnoyer et al. SOF C1.5 828 ng/mL), but plasma GS‐331007 concentrations on our non‐dialysis day were lower (our non‐dialysis GS‐331007 C1 = 496.77 ng/mL, C2 = 874.98 ng/mL vs. Desnoyer et al. GS‐331007 C1.5 4415 ng/mL). It should be noted that the study by Desnoyer et al. was conducted in Caucasians. Most of the patients studied had advanced‐stage liver disease (cirrhosis). Half of them were null responders from previous treatment with pegylated interferon and/or ribavirin and some were relapsed patients. These differences in ethnicity and clinical baseline characteristics of the patients may influence differences in the findings between studies.
Unlike SOF and GS‐331007, VEL pharmacokinetics were not affected by reduced renal function and hemodialysis, as reported in previous studies. 20 , 25 , 29 However, our study revealed a significant increase in plasma VEL concentrations at 5 and 6 h after dose administration (Table 2), with concentrations on dialysis days being higher than on non‐dialysis days. This observation may be attributed to a rebound increase in plasma drug concentration, which can occur after hemodialysis, hemoperfusion, or plasma exchange. 30 The rebound is due to a redistribution of a drug from tissue to plasma that is slower than with a transfer of drug from plasma to the dialysate. Consequently, plasma samples collected at the end of dialysis can produce falsely high concentrations. 30
Various SOF‐based regimens with dose reduction have been investigated for the treatment of HCV infection in CKD patients and chronic hemodialysis patients. These regimens include 200 mg daily for 12–24 weeks, 13 400 mg every other day for 12 weeks, 13 400 mg daily for 12–24 weeks, 20 , 26 , 31 and 400 mg dose three times a week for 12–24 weeks. 31 In particular, these dose reduction regimens were not established based on pharmacokinetic studies and were conducted in hemodialysis patients with relatively more severe liver insufficiency compared with our cohort. Those studies also included patients with relapsed or failed previous anti‐HCV treatment. Adverse events reported with these regimens included anemia, 13 , 26 , 31 headache, 20 , 26 , 31 fatigue, 13 , 26 diarrhea, 13 , 31 rash, 13 , 20 , 26 nausea, 20 and vomiting, 20 which can be associated with the nature of severe liver insufficiency itself. Based on our SOF/VEL pharmacokinetic data and the non‐splitable combined tablet formulation of SOF/VEL, we propose that the administration of SOF/VEL (400/100 mg) every other day for 12 weeks would be an alternative option to treat HCV infection in chronic hemodialysis patients with mild liver insufficiency with no history of previous anti‐HCV treatment. Furthermore, considering that the SOF metabolite, GS‐331007, can be eliminated by dialysis, patients should take the medication after completing their hemodialysis sessions, regardless of whether it aligns with their regular medication schedule, both dialysis, and non‐dialysis days.
To validate our proposed dose reduction regimen, we conducted a preliminary efficacy study involving ten patients with active HCV viral replication, presenting with high plasma viral loads of up to 33,500,000 IU/mL. Following treatment with our proposed SOF/VEL dose reduction regimen, all patients achieved an undetectable viral load, and none reported side effects. These results offer a critical treatment option for hemodialysis patients with HCV infection, particularly those who concurrently receive multiple medications that can pose risks for pharmacokinetic drug–drug interactions 16 , 17 or severe adverse events. 18 , 19 Importantly, these findings hold significant implications for healthcare policymakers, as they facilitate access to appropriate treatment at a reduced dose for this patient population, thereby alleviating the burden on healthcare budgets in resource‐limited countries.
This study has several limitations that must be considered. First, the sample size was calculated specifically for the pharmacokinetic study, which may have limited power to demonstrate efficacy. Additional clinical studies with larger sample sizes are necessary to confirm the efficacy and safety of our proposed dose‐reduction regimen. Second, the pharmacokinetic study was conducted with a single‐dose administration; therefore, the extrapolation of these results to data from multiple doses is limited. Third, there were at least two types of hemodialysis schedules (twice or three times a week), but this study did not account for differences in the dialysis schedule, which could influence pharmacokinetic parameters. Furthermore, all hemodialysis participants enrolled in the study did not have urine production; therefore, our findings may not be applicable to chronic hemodialysis patients or patients with advanced‐stage CKD who still have significant urine production. Administration of SOF/VEL in our pharmacokinetic study may pose a risk of drug resistance; however, all patients were entered into our preliminary efficacy study and had an undetectable viral load after the 12‐week treatment. Therefore, no drug resistance was observed in our cohort.
In conclusion, in hemodialysis patients with chronic HCV infection, dialysis has no effect on SOF and VEL pharmacokinetics, but GS‐331007 can be 30% cleared during the hemodialysis session. Administration of the dose reduction regimen, an alternate day of SOF/VEL (400/100 mg) for 12 weeks, would be an alternative option for the treatment of chronic HCV infection in hemodialysis patients. Further clinical studies are warranted to validate the efficacy and safety of our proposed dose reduction regimen.
AUTHOR CONTRIBUTIONS
P.C., S.K., A.A., P.T., S.L., W.P., T.T., and K.P. wrote the manuscript; P.C., S.K., A.A., P.T., and K.P. designed the research; P.C., S.L., W.P., T.T., N.P., S.W., S.J., P.S., and K.P. performed the research; P.C. and S.K. analyzed the data.
FUNDING INFORMATION
This study was supported by the Thailand Science Research and Innovation Fund, Chulalongkorn University, Bangkok, Thailand (CUFRB65_hea(15)_022_30_03).
CONFLICT OF INTEREST STATEMENT
The authors declared no competing interests for this work.
Supporting information
Data S1.
ACKNOWLEDGMENTS
The authors thank all the staff of the Division of Nephrology, Department of Medicine, Chulalongkorn University, for supporting the collection of clinical data. The authors thank Chula Clinical Research Center under the Royal Patronage, Faculty of Medicine, Chulalongkorn University for supporting the study management and the collection of clinical samples. Additionally, we thank all patients who participated in the study.
Chariyavilaskul P, Prompila N, Wittayalertpanya S, et al. Pharmacokinetics of Sofosbuvir/Velpatasvir and efficacy of an alternate‐day treatment in hemodialysis patients with chronic hepatitis C infection. Clin Transl Sci. 2024;17:e13884. doi: 10.1111/cts.13884
DATA AVAILABILITY STATEMENT
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Mikolajczyk AE, Aronsohn AI. Current management of chronic hepatitis B and C in chronic kidney disease. Adv Chronic Kidney Dis. 2015;22:352‐360. [DOI] [PubMed] [Google Scholar]
- 2. Messina JP, Humphreys I, Flaxman A, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis. 2005;9:383‐398. [DOI] [PubMed] [Google Scholar]
- 4. Liu CH, Kao JH. Treatment of hepatitis C virus infection in patients with end‐stage renal disease. J Gastroenterol Hepatol. 2011;26:228‐239. [DOI] [PubMed] [Google Scholar]
- 5. Fabrizi F, Takkouche B, Lunghi G, Dixit V, Messa P, Martin P. The impact of hepatitis C virus infection on survival in dialysis patients: meta‐analysis of observational studies. J Viral Hepat. 2007;14:697‐703. [DOI] [PubMed] [Google Scholar]
- 6. Kalantar‐Zadeh K, Kilpatrick RD, McAllister CJ, et al. Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol. 2007;18:1584‐1593. [DOI] [PubMed] [Google Scholar]
- 7. Thanachartwet V, Phumratanaprapin W, Desakorn V, et al. Viral hepatitis infections among dialysis patients: Thailand registry report. Nephrology (Carlton). 2007;12:399‐405. [DOI] [PubMed] [Google Scholar]
- 8. Sise ME, McQuaid T, Martin P. Sofosbuvir‐based hepatitis C therapies in patients with chronic and end‐stage kidney disease. Nephrol Dial Transplant. 2022;37:2327‐2334. [DOI] [PubMed] [Google Scholar]
- 9. American Association for the Study of Liver Diseases and Infectious Diseases Society of America . HCV guidance: recommendations for testing, managing, and treating hepatitis Cม, American Association for the Study of Liver Diseases and Infectious Diseases Society of America.
- 10. European Association for Study of, L . EASL recommendations on treatment of hepatitis C 2015. J Hepatol. 2015;63:199‐236. [DOI] [PubMed] [Google Scholar]
- 11. Smolders EJ, Jansen AME, ter Horst PGJ, Rockstroh J, Back DJ, Burger DM. Viral hepatitis C therapy: pharmacokinetic and pharmacodynamic considerations: a 2019 update. Clin Pharmacokinet. 2019;58:1237‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martin P, Awan AA, Berenguer MC, et al. Executive summary of the KDIGO 2022 clinical practice guideline for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int. 2022;102:1228‐1237. [DOI] [PubMed] [Google Scholar]
- 13. Ram BK, Frank C, Adam P, et al. Safety, efficacy and tolerability of half‐dose Sofosbuvir plus Simeprevir in treatment of hepatitis C in patients with end stage renal disease. J Hepatol. 2015;63:763‐765. [DOI] [PubMed] [Google Scholar]
- 14. Singer AW, Reddy KR, Telep LE, et al. Direct‐acting antiviral treatment for hepatitis C virus infection and risk of incident liver cancer: a retrospective cohort study. Aliment Pharmacol Ther. 2018;47:1278‐1287. [DOI] [PubMed] [Google Scholar]
- 15. Gilead Sciences . Full prescribing information: Sofosbuvir. Accessed May 30, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/204671s002lbl.pdf
- 16. Bellesini M, Bianchin M, Corradi C, Donadini MP, Raschi E, Squizzato A. Drug‐drug interactions between direct oral anticoagulants and hepatitis C direct‐acting antiviral agents: looking for evidence through a systematic review. Clin Drug Investig. 2020;40:1001‐1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Monribot A, Huillard O, Khoudour N, et al. Cardiac toxicity associated with pharmacokinetic drug‐drug interaction between crizotinib and Sofosbuvir/Velpatasvir: a case report. Br J Clin Pharmacol. 2023;89:1486‐1490. [DOI] [PubMed] [Google Scholar]
- 18. Welker MW, Luhne S, Lange CM, et al. Lactic acidosis in patients with hepatitis C virus cirrhosis and combined Ribavirin/Sofosbuvir treatment. J Hepatol. 2016;64:790‐799. [DOI] [PubMed] [Google Scholar]
- 19. Wanchoo R, Thakkar J, Schwartz D, Jhaveri KD. Harvoni (Ledipasvir with Sofosbuvir)‐induced renal injury. Am J Gastroenterol. 2016;111:148‐149. [DOI] [PubMed] [Google Scholar]
- 20. Borgia SM, Dearden J, Yoshida EM, et al. Sofosbuvir/Velpatasvir for 12 weeks in hepatitis C virus‐infected patients with end‐stage renal disease undergoing dialysis. J Hepatol. 2019;71:660‐665. [DOI] [PubMed] [Google Scholar]
- 21. Rezk MR, Basalious EB, Karim IA. Development of a sensitive UPLC‐ESI‐MS/MS method for quantification of Sofosbuvir and its metabolite, GS‐331007, in human plasma: application to a bioequivalence study. J Pharm Biomed Anal. 2015;114:97‐104. [DOI] [PubMed] [Google Scholar]
- 22. Konam K, Reddy S. Quantification of Sofosbuvir and Velpatasvir in human plasma using LCMS/MS technique ‐application to pharmacokinetic study. J Young Pharm. 2019;11:266‐273. [Google Scholar]
- 23. European Medicines Agency . Guideline on bioanalytical method validation. 2012. Accessed May 30, 2024. https://www.ema.europa.eu/en/bioanalytical‐method‐validation‐scientific‐guideline [DOI] [PubMed]
- 24. U.S. Department of Health and Human Services, F.a.D.A., Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM) . Bioanalytical method validation: guidance for industry. 2018. Accessed May 30, 2024. https://www.fda.gov/files/drugs/published/Bioanalytical‐Method‐Validation‐Guidance‐for‐Industry.pdf
- 25. Li C, Li X, Zhu X, et al. Pharmacokinetics, safety, and tolerability of Ledipasvir/Sofosbuvir and Sofosbuvir/Velpatasvir in healthy Chinese subjects. Clin Ther. 2020;42:448‐457. [DOI] [PubMed] [Google Scholar]
- 26. Desnoyer A, Pospai D, Lê MP, et al. Pharmacokinetics, safety and efficacy of a full dose Sofosbuvir‐based regimen given daily in hemodialysis patients with chronic hepatitis C. J Hepatol. 2016;65:40‐47. [DOI] [PubMed] [Google Scholar]
- 27. Li X, Chen H, Niu J, et al. Pharmacokinetics, safety, and tolerability of the direct‐acting hepatitis C antiviral Sofosbuvir in healthy Chinese subjects. Clin Ther. 2018;40:1556‐1566. [DOI] [PubMed] [Google Scholar]
- 28. Lawitz E, Landis CS, Flamm SL, et al. Sofosbuvir plus ribavirin and Sofosbuvir plus ledipasvir in patients with genotype 1 or 3 hepatitis C virus and severe renal impairment: a multicentre, phase 2b, non‐randomised, open‐label study. Lancet Gastroenterol Hepatol. 2020;5:918‐926. [DOI] [PubMed] [Google Scholar]
- 29. Mogalian E, German P, Kearney BP, et al. Preclinical pharmacokinetics and first‐in‐human pharmacokinetics, safety, and tolerability of Velpatasvir, a pangenotypic hepatitis C virus NS5A inhibitor, in healthy subjects. Antimicrob Agents Chemother. 2017;61:e02084‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keller F, Offermann G, Scholle J. Kinetics of the redistribution phenomenon after extracorporeal elimination. Int J Artif Organs. 1984;7:181‐188. [PubMed] [Google Scholar]
- 31. Lin T, Wang X, Gao H, et al. Effect of hemodialysis on efficacy and pharmacokinetics of Sofosbuvir Coformulated with either daclatasvir or ledipasvir in patients with end‐stage renal disease. Blood Purif. 2020;49:692‐699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.


