Abstract
The baculovirus expression vector system is considered to be a safe, powerful, but cell-lytic heterologous protein expression system in insect cells. We show here that there is a new baculovirus system for efficient gene transfer and expression using the popular and genetically well-understood Drosophila S2 cells. The recombinant baculovirus was constructed to carry an enhanced green fluorescent protein under the control of polyhedrin promoter as a fluorescent selection marker in the Sf21 cell line. Recombinant baculoviruses were then used to transduce S2 cells with target gene expression cassettes containing a Drosophila heat shock protein 70, an actin 5C, or a metallothionein promoter. Nearly 100% of the S2 cells showed evidence of gene expression after infection. The time course for the optimal protein expression peaked at 24 to 36 h postinfection, which is significantly earlier than a polyhedrin-driven protein expression in Sf21 cells. Importantly, S2 cells did not appear to be lysed after infection, and the protein expression levels are comparable to those of proteins under the control of polyhedrin promoter in several lepidopteran cell lines. Most surprisingly, S2 cells permit repetitive infections of multiple baculoviruses over time. These findings clearly suggest that this baculovirus-S2 system may effect the efficient gene transfer and expression system of the well-characterized Drosophila S2 cells.
Drosophila S2 cells have been proven to be a useful experimental system for a high level of protein expression (23). In particular, previous studies have shown that protein processing, such as glycosylation (2, 25, 36, 41) and amidation (27), in S2 cells is basically similar to that in mammalian cells. Thus, S2 cells provide an attractive alternative for mammalian recombinant protein expression. S2 cells have also been used to express and study recombinant proteins, including cell adhesion molecules (3), oncogenes (14, 21), antibodies (22), receptors (13, 20, 29, 41), transcription factors (10, 38), and viral antigens (6, 7). In most cases, the test proteins were reliably processed and biologically active. Up until now, there has been a major restraint in using S2 cells for protein expression, i.e., the low transfection efficiency attained by various standard DNA transfection methods (17, 18). Therefore, it is gratifying to introduce a far more efficient and expeditious method that delivers target genes into S2 cells.
Baculovirus is one of the most powerful vehicles for foreign gene expression at extremely high levels. In nature, baculovirus only replicates in insect (lepidopteran) host cells and was considered to be nonpermissive in other insect cells (such as Drosophila cell lines) (35). Nevertheless, Miller and colleagues demonstrated that baculovirus could in deed be used to transduce Drosophila DL-1 and DM cells under well-specified laboratory conditions (8, 30, 35, 37). It was also found that foreign gene expression in other nonpermissive cells (including insect and mammalian cells) is promoter dependent and that the polyhedrin (PH) promoter of baculovirus has little or no activity in these cell lines (4, 8, 19, 30, 31). Of special note was the application of recombinant baculovirus with mammalian expression cassettes in the delivery and expression of foreign genes in such mammalian cell lines as HeLa, CHO, BHK, and COS-7 (4, 9, 19, 39, 42). Accordingly, we explored the possibility of utilizing recombinant baculovirus as a gene delivery vehicle for target gene expression in the widely employed Drosophila S2 cells, whose genetic background is well understood.
Here we describe a baculovirus-mediated gene expression system in Drosophila S2 cells which we call the “baculovirus-S2 system”. This system provides several advantages over the classical baculovirus system in lepidopteran host cells. First, the baculovirus-S2 system does not cause cell lysis after S2 cells are infected with virus, and the protein expression level in S2 cells is comparable with that in Sf9, Sf21, and High Five cells. Thus, the baculovirus-S2 system provides a valuable alternative for large-scale protein production. Second, baculovirus can be successively used to deliver multiple exogenous genes into S2 cells. Third, this system can be incorporated into the method (12) used in viral plaque screening and viral titer determination using enhanced green fluorescent protein (EGFP) as a visible marker. Our findings significantly expand the applications of Drosophila S2 cells for protein expression and genetic studies.
MATERIALS AND METHODS
Construction and production of recombinant baculoviruses.
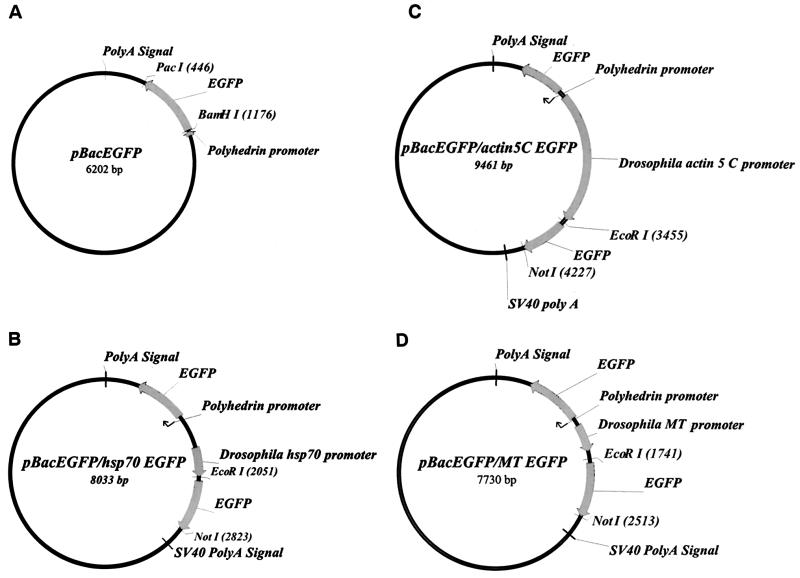
All viruses utilized were constructed by using shuttle vectors derived from pBacPAK8 (Clontech). pBacEGFP (Fig. 1A) was constructed by cloning an EGFP PCR product into pBacPAK8 using BamHI and PacI sites. The EGFP fragment was PCR amplified from pEGFP-1 (Clontech) with primers 5′-CAGGATCCGCCACCATGGTGAGCAAGGGCG-3′ and 5′-AGCAATTAATTAATGAACATGTCGAGCAGGTAC-3′. The constructs of pBacEGFP/hsp70, pBacEGFP/actin 5C, and pBacEGFP/MT were derived from pBacEGFP through cloning PCR fragments of Drosophila heat shock protein 70 (hsp70), actin 5C, and metallotheionein (MT) promoters in combination with simian virus 40 polyadenylation sequences from pUAST (5), pAc5.1/V5/HisA, and pMT/V5/HisA (Invitrogen) into the EcoRV site. These promoters were located next to the baculovirus PH promoter, but in the opposite direction. For the convenience of assay, the second EGFP was cloned into pBacEGFP/hsp70, pBacEGFP/actin 5C, and pBacEGFP/MT at EcoRI and NotI sites as the foreign target gene. The resulting constructs were named pBacEGFP/hsp70 EGFP, pBacEGFP/actin 5C EGFP, and pBacEGFP/MT EGFP, respectively (Fig. 1B, C, and D). EGFP under the control of the PH promoter was used as a selection marker for plaque selection and virus titer assay. The vector pBacEGFP/hsp70 ena, which contains ena (encoding an Abl kinase substrate) as the target gene was cut from pPAC ena (21) and inserted into the NotI site of pBac EGFP/hsp70. Another target gene, HA-dok (another kinase substrate of Abl), was also constructed into NotI site of pBacEGFP/hsp70. The pBacEGFP/hsp70 His-EGFP was constructed by cloning His-EGFP PCR product into pBacEGFP/hsp70 using EcoRI and NotI sites. The His-EGFP fragment was PCR amplified from pEGFP-1 with primers 5′-CAGGAAT TCCCACCATGCATCATCACCATCACCATGTGAGCAAGGGCG - 3′ and 5′-AGCGCGGCCGCAATGAACATGTCGAGCAGGTAC-3′. The vector pBacEGFP/hsp70 HA-dcdc2 was constructed by cutting dcdc2 (Drosophila cdc2) from pPAC HA-dcdc2 and inserted into the XbaI site of pBacEGFP/MT. The vector pBacEGFP/hsp70 DsRed was engineered to carry a red fluorescent target gene DsRed from pDsRed-N1 (Clontech). Recombinant viruses were generated by using standard protocols of the BacPAK system (Clontech).
FIG. 1.
Maps of the transfer vectors for constructing recombinant baculoviruses (detailed construct information described in text). (A) pBacEGFP. (B) pBacEGFP/hsp70 EGFP. (C) pBacEGFP/actin 5C EGFP. (D) pBacEGFP/MT EGFP. The PH promoter-driven EGFP selection marker was used to isolate a single virus plaque for amplification and to determine the virus titer.
Cell culture.
S2 cells were maintained at 23.5°C in a modified M3 medium supplemented with 10% fetal bovine serum (Life Technologies). Spodoptera frugiperda (fall armyworm) Sf21 and Sf9 cells were maintained at 27°C in Grace's medium (Life Technologies) with 10% fetal bovine serum (Life Technologies). High Five cells were maintained at 27°C in High Five cell culture medium (Invitrogen).
Infection of S2 cells by recombinant baculovirus.
S2 cells were seeded in 24-well culture dishes at 106 cells per well. Culture medium was removed and replaced with virus inoculum at the indicated multiplicities of infection (MOIs), and S2 cells were incubated with 40 rpm shaking for 1 h at room temperature. After removal of the virus, fresh medium was added, and S2 cells were incubated at 23.5°C. For Western blot analysis, cell pellets were added with sample buffer, boiled, and analyzed by polyacrylamide gels under denaturing and reducing conditions. Proteins were transferred to Immobilon-P transfer membranes (Millipore) by Semidrier (Bio-Rad) and immunoblotted by standard protocols. EGFP was detected by the Living Colors Peptide Antibody (Clontech). Hemagglutinin (HA) epitope was detected by HA monoclonal antibody (BAbCO). His tag was detected by His tag monoclonal antibody (Clontech). Ena was detected by Ena monoclonal antibody (21).
Flow cytometry.
Infected S2 cells were collected, washed, and resuspended in phosphate-buffered saline (PBS). Data collection was performed on a Becton Dickinson FACSCalibur flow cytometer. EGFP was used as a reporter gene to assess transfection efficiencies and relative protein expression levels. To quantitate the relative EGFP expressions of infected S2 cells, the LinearFlow Green Flow Cytometry Intensity Calibration Kit (Molecular Probes, Ltd.) was used to calibrate the EGFP intensity. The fluorescence intensity standard was generated by six precisely determined intensity level of fluorescent microspheres. Six suspensions of fluorescent polystyrene microspheres were mixed in PBS and excited in a flow cytometer with a 488-nm excitation. These microspheres were used for calibrating the FL1 channel of a flow cytometer and as reference standards. Relative EGFP expression values were estimated by intrapolation from the linear fluorescence intensity standard.
Confocal microscopy.
S2 cells were infected with BacEGFP/hsp70 EGFP at MOIs of 1, 10, 100, and 200. At 36 h postinfection, cells were harvested and plated on polylysin-coated glass slides for 30 min. Cells were washed twice with PBS, covered with cover slips, and sealed by using mounting medium. EGFP expressions were detected by a Leica TCS NT confocal microscope.
Relative protein quantification assay.
Cells were seeded at different densities in six-well dishes with 1 × 106 S2 cells and 2 × 105 cells of Sf9, Sf21, and High Five to make up for the cell size difference (S2 cells were about one-fifth of the volume of Sf or High Five cells) for the optimal virus infection and culturing conditions. S2 cells were infected by BacEGFP/hsp70 EGFP with an MOI of 100 PFU per cell and incubated for 36 h until assay. The same titer of BacEGFP was used to infect Sf9, Sf21, and High Five cells and then incubated for 72 h. At the indicated postinfection times, cells were harvested, washed twice with PBS, and lysed on ice for 30 min in 1 ml of cold lysis buffer. Protease inhibitors (100 μM N-acetyl-l-leucinyl-l-leucinyl-methioninal, 100 μM N-acetyl-l-leucinyl-l-leucinyl-norleucinal, 100 μM ALLN, 1 mM pefabloc, and 1 μg of pepstatin, 1 μg of aprotinin, and 1 μg of leupeptin per ml) were added into the lysis buffer. Total proteins of individual cell types were quantified by DC Protein Assay (Bio-Rad). Cell lysates of equal amount of total protein from different cell lines were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis. Proteins were transferred to nitrocellulose membranes and immunoblotted by using Living Colors Peptide Antibody against EGFP. Relative EGFP protein of each cell extract was quantified by using a FLA-2000 phosphorimager (Fujifilm). The experimental means were obtained by averaging data from three independent experiments.
RESULTS AND DISCUSSION
Baculovirus-mediated gene expression in S2 cells.
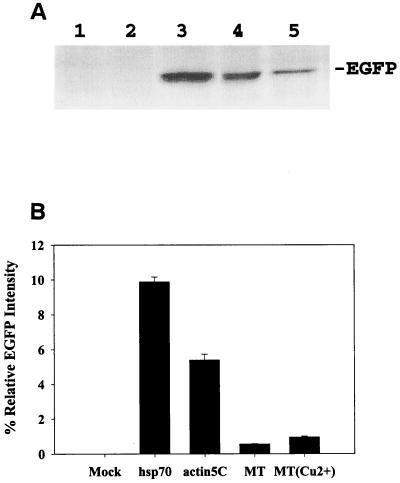
Autographa californica nuclear polyhedrosis virus (AcNPV) is currently the most commonly used baculovirus for foreign gene expression. To examine if AcNPV is able to deliver an effective gene expression cassette into S2 cells, we constructed recombinant baculoviruses coding for EGFP under the control of four different promoters. One (pBacEGFP) was derived from the baculovirus PH promoter and three (pBacEGFP/hsp70, pBacEGFP/actin 5C, and pBacEGFP/MT) were obtained from the Drosophila hsp70, actin 5C, and MT promoters, respectively (Fig. 1). Gene expression levels by the four recombinant baculoviruses were examined at 36 h postinfection. Western blots of cell extracts were probed with antibody directed against EGFP. When S2 cells were mock infected or infected with BacEGFP, no EGFP expression could be detected (Fig. 2A, lanes 1 and 2). Expression of EGFP could only be observed in S2 cells infected with baculovirus containing Drosophila promoters (hsp70, actin 5C, and MT) (Fig. 2A, lanes 3, 4, and 5). These results support previous studies that the virus PH promoter is functionally restrained in nonpermissive cells (8, 37). Thus, baculovirus-mediated gene expression in S2 cells is also promoter dependent. Our data suggest that modified recombinant baculovirus containing a Drosophila expression cassette can transduce gene expression in S2 cells.
FIG. 2.
Susceptibility of Drosophila S2 cells to baculovirus-mediated foreign gene transduction. (A) Western blot analysis of EGFP expression in S2 cells at 36 h postinfection. Lane 1, uninfected cells; lane 2, cells infected with BacEGFP (with PH promoter), showing no EGFP expression; lanes 3 and lane 4, cells infected with BacEGFP/hsp70 EGFP and BacEGFP/actin 5C EGFP, respectively: lane 5, S2 cells infected with recombinant virus BacEGFP/MT EGFP, and EGFP expression was induced by 1 mM CuSO4 at 24 h postinfection. (B) Efficacy of EGFP expression by these promoters as analyzed by flow cytometry (the EGFP quantification method is described in detail in the text). Results are the averages of three independent infections.
To further assess the efficacy of these promoters in S2 cells, flow cytometry analysis was conducted to determine the expression levels of EGFP. As shown in Fig. 2B, EGFP expression by recombinant virus containing hsp70 promoter gave the highest level among three different Drosophila expression cassettes. We then investigated whether we could manipulate EGFP expression by heat shock treatments since the Drosophila hsp70 promoter is known as a temperature-inducible promoter in various systems (11, 40). With various heat shock treatments, the EGFP expression levels in S2 cells were examined by Western blot analysis. No significant difference of EGFP expression levels among the various treatments was noted (data not shown). Therefore, hsp70 may be a temperature-independent and constitutively active promoter in the baculovirus-S2 system. Similarly, the inducibility of the MT promoter in the baculovirus-S2 system was also tested with the recombinant baculovirus of BacEGFP/MT EGFP containing a Cu2+-inducible MT promoter. The results show that the MT promoter is weak and leaky in the baculovirus-S2 system (Fig. 2B). Collectively, these data suggest that the baculovirus-S2 system can serve as an efficient gene delivery vehicle for the target gene expression in Drosophila S2 cells. Among various Drosophila promoters tested, hsp70 functioned as the most potent and constitutively active promoter.
Plaque selection by a fluorescent marker.
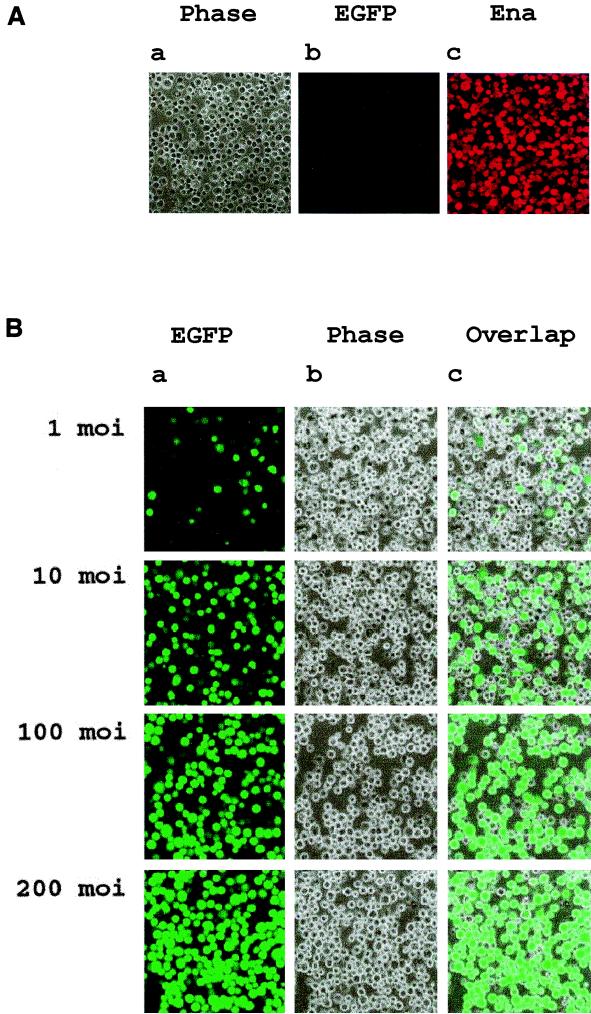
GFP has been used as a tool for screening recombinant baculoviruses in Sf9 cells (12, 33). Taking advantage of the promoter-dependent phenomenon that virus PH promoter is active in Sf21 cells but not in S2 cells, we constructed EGFP under the control of the PH promoter as a visible selection marker for virus plaque assay in Sf21 cells. To investigate the use of EGFP for recombinant virus selection, a gene called ena that encodes a kinase substrate that is phosphorylated by the Abl proto-oncogene found in mammalian and Drosophila cells (14, 21) was cloned under the hsp70 promoter into a vector with EGFP under the PH promoter. After cotransfection of Sf21 cells, fluorescent plaques were selected and characterized to identify those expressing Ena. The resulting recombinant baculovirus was then used to infect S2 cells for exogenous gene expression. While the autofluorescent signal of the EGFP marker was below the detection level in S2 cells (Fig. 3Ab), the exogenous gene expression of Ena was detected by antibody directed against Ena (Fig. 3Ac). The finding that the selection marker was only expressed in Sf9 and Sf21 cells but not in S2 cells eliminates the possible competition and interference with the target gene expression in S2 cells. This design also excludes the possibility of target gene mutation from the traditional chemical plaque selection procedure by neutral red in Sf21 cells (26).
FIG. 3.
Confocal microscopic analysis of the gene expression and the infection efficiency. (A) Confocal microscopic analysis of EGFP selection marker and target gene (ena) expression in the baculovirus-S2 system. Mid-exponentially grown S2 cells (106 cells/well) were seeded into a 24-well petri dish and exposed to an MOI for BacEGFP/hsp70 ena of 100. At 36 h postinfection, cells were harvested, attached to poly-l-lysine coated slides, stained with anti-Ena polyclonal antibody, and then analyzed by confocal microscopy (Leica TCS NT). (Aa) Phase image of BacEGFP/hsp70 ena-infected S2 cells. (Ab). No detectable EGFP signal in S2 cells. (Ac) Ena protein detected in S2 cells by immunocytochemistry with polyclonal antibody directed against Ena. (B) Confocal microscopy analysis of the dose-response infection efficiency. S2 cells transduced with BacEGFP/hsp70 EGFP at the indicated MOIs were examined by confocal microscopy to determine infection efficiencies.
High infection efficiency and gene expression levels.
The results described above show the capability of baculovirus as a vehicle for gene delivery and expression in Drosophila S2 cells. Previous researchers observed that the MOI is critical in achieving baculovirus-mediated protein expression in mammalian cells (9). The optimal virus MOI for the highest infection efficiency in S2 cells was examined. S2 cells were transduced with an increasing titer of BacEGFP/hsp70 EGFP viruses. At 36 h postinfection, the infection efficiency was examined by confocal microscopy (Fig. 3B). For accurate calculation, infection efficiency was determined by flow cytometry studies. At an MOI of 1 PFU per cell, approximately 17% of the cells showed EGFP expression. The “infection efficiency” increased steadily with higher MOIs (39, 51, 73, and 85% for MOIs of 5, 10, 25, and 50, respectively) and reached close to 100% at an MOI of 100. Therefore, the infection efficiency of S2 cell is virus dose dependent at from 1 to 100 MOI. However, at an MOI of from 100 to 800, the infection efficiency remained optimal.
Using the PH promoter, protein expression by the conventional baculovirus expression system usually reaches its maximum expression level by 48 to 72 h postinfection. To explore the optimal timing for baculovirus-mediated protein expression in S2 cells, a time course study of virus-mediated gene expression was done. S2 cells were transduced with BacEGFP/hsp70 EGFP at an MOI of 100. Infection efficiencies and total gene expression levels were examined at 12, 24, 36, 48, 60, and 72 h postinfection by flow cytometry analysis. The relative EGFP expressions were calibrated to relative fluorescence intensities by using the LinearFlow Green Flow Cytometry Intensity Calibration Kit (Molecular Probes, Inc.). The optimal efficiency was achieved by as early as 24 to 36 h postinfection at an MOI of 100 (Table 1). The total EGFP level of the infected cells increased steadily over the 36 h of baculovirus transduction and started to decline by 36 to 72 h postinfection (Table 1). These results suggest that the optimal protein expression driven by the hsp70 promoter in the baculovirus-S2 system is about 12 to 36 h earlier than that by the PH promoter in lepidopteran cells.
TABLE 1.
Time course of infection efficiency and total EGFP expression of S2 cell transduced with a BacEGFP/hsp70 EGFP MOI of 100a
| Time elapsed (h) | Mean infection efficiency ± SEM | Total EGFP expression (106) ± SEM |
|---|---|---|
| 12 | 90.1 ± 0.9 | 3.8 ± 0.1 |
| 24 | 96.6 ± 0.3 | 9.9 ± 0.3 |
| 36 | 95.4 ± 0.1 | 12.1 ± 0.2 |
| 48 | 94.8 ± 0.4 | 9.9 ± 0.5 |
| 60 | 91.1 ± 1.4 | 10.8 ± 0.3 |
| 72 | 82.2 ± 1.1 | 11.2 ± 0.5 |
Total protein expression levels at the indicated time points were calculated by multiplying the relative EGFP intensity by the total cell number. Results from the flow cytometry analysis are the averages of three independent experiments.
Nonlytic system for S2 cells.
In the classical baculovirus system, lepidopteran cells such as Sf9, Sf21, and High Five cells undergo a lytic cycle after virus infection. Such a lethal viral infection in cells might trigger the cellular protein processing machinery to deteriorate several days postinfection. This event might lead to improper posttranscriptional and/or posttranslational modifications of gene products. To investigate whether S2 cells are destined for the same lytic pathway, S2 cells were exposed to the BacEGFP/hsp70 EGFP virus at various MOIs, and viabilities were determined by trypan blue exclusion assay. The percent viabilities of S2 cells transduced with BacEGFP/hsp70 EGFP baculovirus at various MOIs were as follows (MOI, percent viability [mean ± standard deviation]): 1, 99.2 ± 0.4; 5, 98.6 ± 0.2; 10, 96.2 ± 0.5; 25, 95.2 ± 0.7; 50, 95.5 ± 0.3; 100, 93.1 ± 0.7; 200, 94.6 ± 0.5; 400, 93.2 ± 0.6; and 800, 95.8 ± 0.4. The viability of the infected S2 cells was determined by staining the cells with 0.4% trypan blue solution (Sigma) at 72 h postinfection. These results are the averages of three independent experiments. The result indicates that there are no apparent cytopathic effects on S2 cells by 72-h postinfection, even at an MOI of 800. Thus, this expression system does not include the lytic cycle of baculovirus.
There are two putative explanations for this nonlytic virus cycle in S2 cells. First, protein expression by this baculovirus system might be a persistent infection in S2 cells. However, data from our aforementioned time course studies do not reveal a persistent expression of the exogenous genes in S2 cells. The total EGFP level of the entire infected cells started to decline by 36 to 72 h postinfection. Thus, it is likely that the transduced viral DNA might be degraded or diluted upon cellular replication over time. To test the second hypothesis, we isolated total DNA from the infected cells at 30 days postinfection and tested for the virus genomic DNA by PCR analysis. Neither EGFP cDNA nor baculovirus genomic DNAs could be detected in the cells (data not shown). These data suggest that the S2 cell line is not a persistent infection target for baculovirus. In nature, baculovirus DNA replicates by 6 h postinfection and virions bud from the infected cell's surface to infect other host cells by 10 to 12 h postinfection (15). Studies by others (8) also showed that baculovirus-mediated foreign gene expression in Drosophila DL-1 cells has limited viral DNA replication and undergoes DNA degradation sometime after infection.
Comparable protein expression levels in S2 versus that in lepidopteran cells.
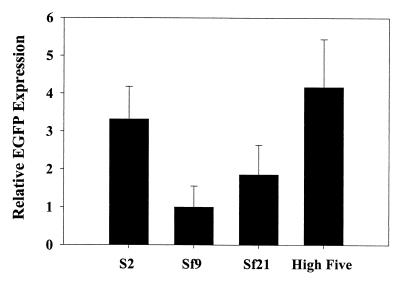
Baculovirus has been proven to be a powerful tool for evoking a high level of heterologous protein production in eukaryotic cells. To compare the relative potency of protein expressions by different baculovirus systems, EGFP protein expression from S2, Sf9, Sf21, and High Five cells were examined by Western blot analysis. EGFP proteins were quantified by phosphorimager analysis, and the relative yields of EGFP protein from the same amount of total protein in different cell lines were calculated. The relative EGFP protein (3.32 U) expressed from S2 cells is substantially higher than that from Sf9 (1 U) and Sf21 (1.85 U) cells and is comparable with that in High Five cells (4.17 U) (Fig. 4). The baculovirus expression system is known to express exogenous proteins at levels ranging from 1 to 500 mg/liter (34). To determine the absolute level of the EGFP expression by the baculovirus-S2 system, 100 ml of mid-exponentially grown S2 cells were cultured in a spin flask with 8 × 106 cells/ml and infected with MOI 100 BacEGFP/hsp70 EGFP for 1 h. At 36 h postinfection, cells were lysed for Western blot analysis. The absolute EGFP level was quantified with recombinant EGFP standard (Clontech) by using a phosphorimager. The predicted absolute level of EGFP expressed by S2 cells is approximately 3.2 mg/liter. Our data suggest that the baculovirus-mediated gene expressions in S2 cells might be an alternative for large-scale protein expressions.
FIG. 4.
Comparison of the baculovirus-mediated EGFP expression levels in S2 cells and in other host cells. S2 cells were transduced with BacEGFP/hsp70 EGFP at an MOI of 100 PFU/cell, and cells were harvested at 36 h postinfection for Western blot analysis. The same titer of BacEGFP (EGFP under the control of the PH promoter) was transduced into Sf9, Sf21, and High Five cells, and cells were harvested at 72 h postinfection for Western blot analysis. Total proteins in the cell lysate were measured by using the DC Protein Assay (Bio-Rad). The relative yields of EGFP expression from equal amounts of total protein were determined by using a phosphorimager. Results are the averages of three independent infections.
Superinfection in S2 cells.
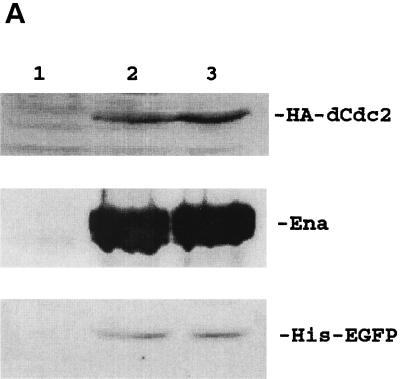
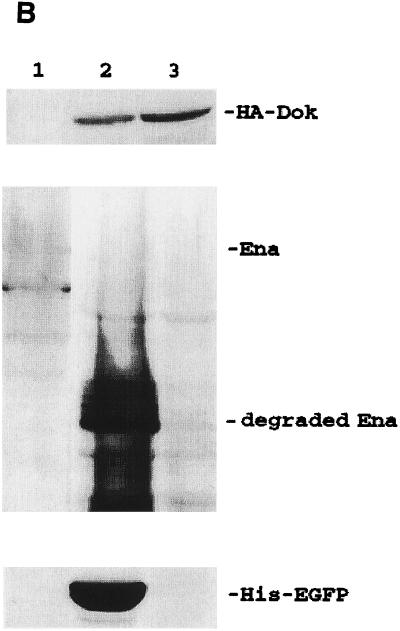
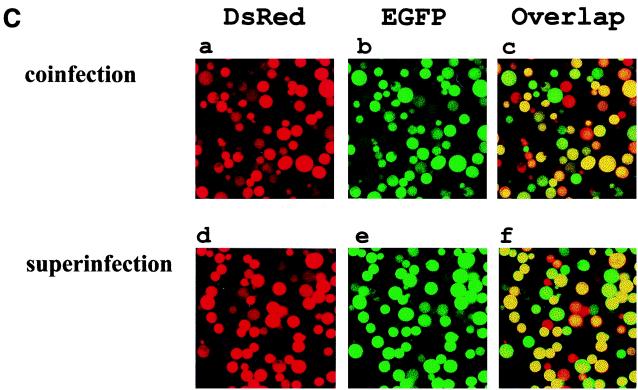
Superinfection is defined as an infected cell that is capable of being infected by another virus at subsequent time points. For the classical baculovirus expression system, multiple recombinant baculoviruses are capable of coinfecting into the same cell concurrently. However, the same host cell becomes nonpermissive to another baculovirus infection once it is first infected by a homologous (the same species) recombinant baculovirus (16, 24) at an earlier time point. Others have shown that it is possible for an infected Sf cell to be infected with another heterologous virus (24). However, there is no report describing the prospect of homologous baculovirus superinfection in any applied cell lines. To explore the feasibility of utilizing baculovirus to express two or more genes in one cell at different infection times, S2 cells were either concurrently coinfected with 100-MOI virus doses of BacEGFP/hsp70 HA-dcdc2, BacEGFP/hsp70 ena, and BacEGFP/hsp70 His6-EGFP in combination or were individually infected with these three viruses sequentially at intervals of 4 h. At 36 h postinfection, the three Western blots of cell extract were probed, respectively, with antibodies directed against HA-tagged dCdc2, Ena, and His-tagged EGFP and subsequently detected with alkaline phosphatase-conjugated goat anti-mouse secondary antibody. As shown in Fig. 5A, three proteins, which were either transduced concurrently or sequentially, were expressed equally well in the S2 cells. The same superinfection studies were also done with High Five cells. Three different recombinant baculoviruses (BacEGFP/hsp70 HA-dok, BacEGFP/hsp70 ena, and BacEGFP/hsp70 His6-EGFP) were used for this experiment. In contrast to the S2 cells being repeatedly transduced with multiple recombinant baculovirus, lepidopteran High Five cells can only be infected once by baculovirus (Fig. 5B). To confirm that the coexpression of multiple target genes by superinfection is not due to different populations of infected cells each infected with a different virus, confocal microscopic analysis of the coexpression of two fluorescent target genes in S2 cells was conducted. BacEGFP/hsp70 DsRed (a red fluorescent protein from sea anemone) (28) and BacEGFP/hsp70 EGFP were either coinfected or sequentially infected at 4-h intervals. Figure 5C shows that more than 96% of the cells coexpressed both fluorescent proteins either by coinfection or by superinfection of recombinant viruses. It is evident that the initial entry of the recombinant baculovirus did not prohibit the transduction of another homologous virus into the same S2 cells over time. We have also noted that the expression levels of two heterologous genes in the same cell were not always equivalent. The expression level of the second transduced target gene is not necessarily lower than that of the first target gene. Together, these data strongly suggest that the baculovirus-S2 system may be an alternative approach to coinfect two or more recombinant baculoviruses concurrently or sequentially over time for the production of biologically active proteins.
FIG. 5.
Superinfection of multiple homologous baculovirus in S2 and High Five cells. (A) Western blot of S2 cell lysates. Lane 1, uninfected S2 cells; lane 2, S2 cells coinfected with BacEGFP/hsp70 dcdc2 (HA tagged), BacEGFP/hsp70 ena, and BacEGFP/hsp70 EGFP (His tagged) at MOIs of 100; lane 3, S2 cells were sequentially infected by the aforementioned three viruses at 4-h intervals. Western blot analysis of HA-tagged dcdc2, Ena, and His-tagged EGFP expressions were detected with antibodies against HA tag, Ena, and His tag. The results are the averages of three independent infections. (B) Western blot analysis of High Five cell lysates. Lane 1, uninfected Sf21 cells; lane 2, High Five cells coinfected with BacEGFP/hsp70 dok (HA tagged), BacEGFP/hsp70 ena, and BacEGFP/hsp70 EGFP (His tagged) at an MOI of 100; lane 3, Sf21 cells sequentially infected by the aforementioned three viruses with 4-h intervals. Western blot analysis of HA-tagged Dok, Ena, and His-tagged EGFP expressions were detected with antibodies against HA tag, Ena, and His tag, respectively. (C) Confocal microscopy of fluorescent target gene coexpressions in S2 cells. BacEGFP/hsp70 DsRed and BacEGFP/hsp70 EGFP were either coinfected (upper panels) or sequentially infected (lower panels) at 4-h intervals. More than 96% of the cells demonstrated coexpression of two proteins either by coinfection or by superinfection of recombinant viruses. Nonetheless, expression levels of two target genes were not always comparable.
Cell lysis-associated proteolysis is also considered as the major drawback for the baculovirus expression system (32). For instance, if the protein of interest is a secreted protein, proteinases from the lysed cells could severely compromise the yield of protein production. In the present study we also noted that Ena was degraded in High Five cells but not in S2 cells by the baculovirus expression system (Fig. 5B, lane 2). According to our previous studies (21), Ena is a relatively stable protein while it was transiently expressed in S2 cells and some mammalian cells by the liposome transfection method. In addition, we know of no report that suggests Ena could interact with the other two proteins (EGFP and Dok) used in the study. Thus, ena was considered to be an appropriate independent target gene for the study of superinfection in our system. However, we were surprised that Ena was actually relatively degradable while it was expressed by the conventional baculovirus system in the High Five cell line, which permits the lytic cycle of baculovirus. In contrast, the putative cell lysis associated proteolysis of Ena had not occurred in our nonlytic baculovirus-S2 system. Consequently, the protein expression level and quality might be severely compromised in the conventional baculovirus protein expression system. The baculovirus-S2 system may be considered as an alternative to solve such a problem. Thus, this baculovirus-S2 system provides a very useful and flexible superinfection vehicle in order to analyze protein-protein or protein-drug interactions in intact cells.
Among currently known viral expression systems in eukaryotic cells, baculovirus is a relatively safe expression system with optimal infection efficiency and high expression levels for heterologous protein production. Nonetheless, some features of baculovirus expression are considered to be inconvenient for many common applications. Restricted host range and cell lysis occurring after infection are the two major limiting factors for the application of the conventional baculovirus system in modern biomedical research. We report here a baculovirus-S2 system that mediates protein expression in Drosophila S2 cells without the major drawbacks of the classical baculovirus expression. In addition, this alternative system may also provide a desirable device for the superinfection of multiple target genes at the appropriate stoichiometric ratios over an experimental time course. In particular, S2 cells share several general features of baculovirus AcNPV host cells that are favorable for the mass production of proteins. For example, the cells adapt easily to the spinner flask and can achieve high densities in an inexpensive media cultured at room temperature without CO2 supplementation. In addition, Drosophila S2 cells with a completely sequenced genome (1) could be treated as a known background for the expression and purification of heterologous mammalian proteins without the interference of the native protein in cells.
In summary, our study describes a novel virus expression system in S2 cells with several favorable attributes. First, baculovirus can serve as an efficient gene transfer vehicle in the widely used Drosophila S2 cell line. Protein expression in this nonpermissive insect cell line is promoter dependent, and the Drosophila hsp70 promoter in the expression cassette functions as a constitutively active promoter in S2 cells. Second, a visible EGFP selection maker in this system can facilitate the operation of plaque selection and virus titer determination. Third, an infection efficiency of up to 100% can be reached, and this value is critical in some applications that require a homogeneous background (e.g., microarray analysis). Fourth, comparable transient protein expression levels with Sf9, Sf21, and High Five cells are not compromised by the proteolysis effect resulting from the viral lytic cycle. Last, and perhaps most importantly, our experimental results reveal the first characterized homologous superinfection system for sequential multiple target gene expressions in eukaryotic cells. We are now investigating whether the same superinfection phenomena might take place in mammalian cells as well.
ACKNOWLEDGMENTS
We thank Tsu-Fen Huang for assistance with the confocal microscopy. We also thank Stanley D. Carlson, Entomology Department and Neuroscience Training Program, University of Wisconsin–Madison, and Michael Betenbaugh, Department of Chemical Engineering, The Johns Hopkins University, for reviewing the manuscript.
REFERENCES
- 1.Adams M D, Celniker S E, Holt R A, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- 2.Aldecoa A, Gujer R, Fischer J A, Born W. Mammalian calcitonin receptor-like receptor/receptor activity modifying protein complexes define calcitonin gene-related peptide and adrenomedullin receptors in Drosophila Schneider 2 cells. FEBS Lett. 2000;471:156–160. doi: 10.1016/s0014-5793(00)01387-9. [DOI] [PubMed] [Google Scholar]
- 3.Bellosta P, Costa M, Lin D A, Basilico C. The receptor tyrosine kinase ARK mediates cell aggregation by homophilic binding. Mol Cell Biol. 1995;15:614–625. doi: 10.1128/mcb.15.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyce F M, Bucher N L. Baculovirus-mediated gene transfer into mammalian cells. Proc Natl Acad Sci USA. 1996;93:12348–12352. doi: 10.1073/pnas.93.6.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brand A H, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 6.Brighty D W, Rosenberg M, Chen I S, Ivey-Hoyle M. Envelope proteins from clinical isolates of human immunodeficiency virus type 1 that are refractory to neutralization by soluble CD4 possess high affinity for the CD4 receptor. Proc Natl Acad Sci USA. 1991;88:7802–7805. doi: 10.1073/pnas.88.17.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brighty D W, Rosenberg M. A cis-acting repressive sequence that overlaps the Rev-responsive element of human immunodeficiency virus type 1 regulates nuclear retention of env mRNAs independently of known splice signals. Proc Natl Acad Sci USA. 1994;91:8314–8318. doi: 10.1073/pnas.91.18.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carbonell L F, Klowden M J, Miller L K. Baculovirus-mediated expression of bacterial genes in dipteran and mammalian cells. J Virol. 1985;56:153–160. doi: 10.1128/jvi.56.1.153-160.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Condreay J P, Witherspoon S M, Clay W C, Kost T A. Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc Natl Acad Sci USA. 1999;96:127–132. doi: 10.1073/pnas.96.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courey A J, Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1998;55:887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 11.Elkins T, Hortsch M, Bieber A J, Snow P M, Goodman C S. Drosophila fasciclin I is a novel homophilic adhesion molecule that along with fasciclin III can mediate cell sorting. J Cell Biol. 1990;110:1825–1832. doi: 10.1083/jcb.110.5.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksson S, Raivio E, Kukkonen J P, Eriksson K, Lindqvist C. Green fluorescent protein as a tool for screening recombinant baculoviruses. J Virol Methods. 1996;59:127–133. doi: 10.1016/0166-0934(96)02032-0. [DOI] [PubMed] [Google Scholar]
- 13.Fehon R G, Kooh P J, Rebay I, Regan C L, Xu T, Muskavitch M A T, Artavanis-Tsakonas S. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell. 1990;61:523–534. doi: 10.1016/0092-8674(90)90534-l. [DOI] [PubMed] [Google Scholar]
- 14.Getler F B, Comer A R, Juang J L, Ahern S M, Clark M J, Liebl E C, Hoffmann F M. Enabled, a dosage-sensitive suppressor of mutations in the Drosophila Abl tyrosine kinase, encodes an Abl substrate with SH3 domain-binding properties. Genes Dev. 1995;9:521–533. doi: 10.1101/gad.9.5.521. [DOI] [PubMed] [Google Scholar]
- 15.Granados R R, Federici B A. In vivo infection and replication of baculoviruses. In: Granados R R, Williams K A, editors. The biology of baculovirus. I. Biological properties and molecular biology. Boca Raton, Fla: CRC Press; 1986. pp. 89–108. [Google Scholar]
- 16.Granados R R, Federici B A. Persistent baculovirus infection. In: Burand J P, Kawanishi C Y, Huang Y S, editors. The biology of baculovirus. I. Biological properties and molecular biology. Boca Raton, Fla: CRC Press; 1986. pp. 159–175. [Google Scholar]
- 17.Han K, Levine M S, Manley J L. Synergistic activation and repression of transcription by Drosophila homeobox proteins. Cell. 1989;56:573–583. doi: 10.1016/0092-8674(89)90580-1. [DOI] [PubMed] [Google Scholar]
- 18.Han K. An efficient DDAB-mediated transfection of Drosophila S2 cells. Nucleic Acids Res. 1996;24:4362–4363. doi: 10.1093/nar/24.21.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmann C, Sandig V, Jennings G, Rudolph M, Schlag P, Strauss M. Efficient gene transfer into human hepatocytes by baculovirus vectors. Proc Natl Acad Sci USA. 1995;92:10099–10103. doi: 10.1073/pnas.92.22.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johanson K, Appelbaum E, Doyle M, Hensley P, Zhao B, Abdel-Mequid S S, Young P, Cook R, Carr S, Matico R, Cusimano D, Dul E, Angelichio M, Brooks I, Winborne E, McDonnell P, Morton T, Bennett D, Sokolski T, McNulty D, Rosenberg M, Chaiken I. Binding interactions of human interleukin 5 with its receptor alpha subunit. J Biol Chem. 1995;270:9459–9471. doi: 10.1074/jbc.270.16.9459. [DOI] [PubMed] [Google Scholar]
- 21.Juang J L, Hoffmann F M. Drosophila Abelson interacting protein (dAbi) is a positive regulator of Abelson tyrosine kinase activity. Oncogene. 1999;37:5138–5147. doi: 10.1038/sj.onc.1202911. [DOI] [PubMed] [Google Scholar]
- 22.Kirkpatrick R B, Ganguly S, Angelichio M, Griego S, Shatzman A, Silverman C, Rosenberg M. Heavy chain dimers as well as complete antibodies are efficiently formed and secreted from Drosophila via a Bip-mediated pathway. J Biol Chem. 1995;270:19800–19805. doi: 10.1074/jbc.270.34.19800. [DOI] [PubMed] [Google Scholar]
- 23.Kirkpatrick R B, Shatzman A. Drosophila S2 system for heterologous gene expression. In: Fernandez J M, Hoeffler J P, editors. Gene expression systems: Drosophila S2 system for heterologous gene expression. San Diego, Calif: Academic Press; 1999. pp. 289–331. [Google Scholar]
- 24.Lee J-C, Chen H-H, Wei H-L, Chao Y-C. Superinfection-induced apoptosis and its correlation with the reduction of viral progeny in cells persistently infected with Hz-1 baculovirus. J Virol. 1993;67:6989–6994. doi: 10.1128/jvi.67.12.6989-6994.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li B, Tsing S, Kosaka A H, Nguyen B, Osen E G, Bach C, Chan H, Barnett J. Protein expression of human dopamine beta-hydroxylase in Drosophila Schneider 2 cells. Biochem J. 1996;69:125–133. doi: 10.1042/bj3130057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longnecker D S, Curphey T J, Daninel D S. Mutagenicity of neutral red. Mutat Res. 1977;48:109–111. doi: 10.1016/0027-5107(77)90195-6. [DOI] [PubMed] [Google Scholar]
- 27.Matsumura M, Saito Y, Jackson M R, Song E S, Peterson P A. In vitro peptide binding to soluble empty class I major histocompatibility complex molecules isolated from transfected Drosophila melanogaster cells. J Biol Chem. 1992;267:23589–23595. [PubMed] [Google Scholar]
- 28.Matz M V, Fradkov A F, Labas Y A, Savitsky A P, Zaraisky A G, Markelov M L. Fluorescent proteins from nonbioluminescent Anthozoa. Nat Biotechnol. 1999;17:969–973. doi: 10.1038/13657. [DOI] [PubMed] [Google Scholar]
- 29.Millar N, Buckingham S. Stable expression of a functional homo-oligomeric Drosophila GABA receptor in a Drosophila cell line. Proc R Soc London Ser B. 1994;258:307–314. doi: 10.1098/rspb.1994.0178. [DOI] [PubMed] [Google Scholar]
- 30.Morris T D, Miller L K. Promoter influence on baculovirus-mediated gene expression in permissive and nonpermissive insect cell lines. J Virol. 1992;66:7397–7405. doi: 10.1128/jvi.66.12.7397-7405.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris T D, Miller L K. Characterization of productive and non-productive AcMNPV infection in selected insect cell line. Virology. 1993;197:339–348. doi: 10.1006/viro.1993.1595. [DOI] [PubMed] [Google Scholar]
- 32.Naggie S, Bentley W E. Appearance of protease activities coincides with p10 and polyhedrin-driven protein production in the baculovirus expression system: effects on yield. Biotechnol Prog. 1998;14:227–232. doi: 10.1021/bp980002b. [DOI] [PubMed] [Google Scholar]
- 33.Nordstrom T, Senkas A, Eriksson S, Pontynen N, Nordstrom E, Lindqvist C. Generation of a new protein purification matrix by loading ceramic hydroxyapatite with metal ions—demonstration with polyhistidine-tagged green fluorescent protein. J Biotechnol. 1999;69:125–133. doi: 10.1016/s0168-1656(99)00038-3. [DOI] [PubMed] [Google Scholar]
- 34.O'Reilly D R, Miller L K, Luckow V A. Baculovirus expression vectors: a laboratory manual. New York, N.Y: W. H. Freeman and Company; 1992. p. 28. [Google Scholar]
- 35.Pennock G D, Shoemaker C, Miller L K. Strong and regulated expression of Escherichia coli β-galactosidase in insect cells with a baculovirus vector. Mol Cell Biol. 1984;4:399–406. doi: 10.1128/mcb.4.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Percival M D, Bastien L, Griffin P R, Kargman S, Ouellet M, O'Neill G P. Investigation of human cyclooxygenase-2 glycosylation heterogeneity and protein expression in insect and mammalian cell expression systems. Protein Expr Purif. 1997;9:388–398. doi: 10.1006/prep.1996.0685. [DOI] [PubMed] [Google Scholar]
- 37.Rice W C, Miller L K. Baculovirus transcription in the presence of inhibitors and in nonpermissive Drosophila cells. Virus Res. 1986;6:155–172. doi: 10.1016/0168-1702(86)90047-x. [DOI] [PubMed] [Google Scholar]
- 38.Santoro C, Mermod N, Andrews P C, Tjian R. A family of human CCAAT-box-binding proteins active in transcription and DNA replication: cloning and expression of multiple cDNAs. Nature. 1998;334:218–224. doi: 10.1038/334218a0. [DOI] [PubMed] [Google Scholar]
- 39.Shoji I, Aizaki H, Tani H, Ishii K, Chiba T, Saito I, Miyamura I, Matsuura Y. Efficient gene transfer into various mammalian cells, including non-hepatic cells, by baculovirus vectors. J Gen Virol. 1997;78:2657–2664. doi: 10.1099/0022-1317-78-10-2657. [DOI] [PubMed] [Google Scholar]
- 40.Snow P M, Bieber A J, Goodman C S. Fasciclin III: a novel homophilic adhesion molecule in Drosophila. Cell. 1989;59:313–323. doi: 10.1016/0092-8674(89)90293-6. [DOI] [PubMed] [Google Scholar]
- 41.Tota M R, Xu L, Sirotina A, Strader C D, Graziano M P. Interaction of [fluorescein-Trp]glucagon with the human glucagon receptor expressed in Drosophila Schneider 2 cells. J Biol Chem. 1995;270:26466–26472. doi: 10.1074/jbc.270.44.26466. [DOI] [PubMed] [Google Scholar]
- 42.Yap C-C, Ishii K, Aoki Y, Aizaki H, Tani H, Shimizu H, Ueno Y, Miyamura T, Matsuura Y. A hybrid baculovirus-T7 RNA polymerase system for recovery of an infectious virus from cDNA. Virology. 1997;231:192–200. doi: 10.1006/viro.1997.8537. [DOI] [PubMed] [Google Scholar]