Abstract
Changes in physiological factors may result in large pharmacokinetic variability of vancomycin in pediatric patients, thereby leading to either supratherapeutic or subtherapeutic exposure and potentially affecting clinical outcomes. This study set out to characterize the disposition of vancomycin, quantify the exposure target and establish an optimal dosage regimen among the Southern Chinese pediatric population. Routine therapeutic drug monitoring data of 453 patients were available. We performed a retrospective population pharmacokinetic analysis of hospitalized children prescribed intravenous vancomycin using NONMEM® software. A one‐compartment PPK model of vancomycin with body weight and renal functions as covariates based on a cutoff of 2 years old children was proposed in this study. Both internal and external validation showing acceptable and robust predictive performance of the model to estimate PK parameters. The value of area under the curve over 24 h to minimum inhibitory concentration ratio (AUC0‐24/MIC) ≥ 260 was a significant predictor for therapeutic efficacy. Monte Carlo simulations served as a model‐informed precision dosing approach and suggested that different optimal dose regimens in various scenarios should be considered rather than flat dosing. The evaluation of vancomycin exposure‐efficacy relationship indicated that lower target level of AUC0‐24/MIC may be needed to achieve clinical effectiveness in children, which was used to derive the recommended dosing regimen. Further prospective studies will be needed to corroborate and elucidate these results.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Children are constantly growing, and changes in physiological factors may result in large pharmacokinetic variability of vancomycin, thereby leading to either supratherapeutic or subtherapeutic exposure.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study characterized the disposition of vancomycin, quantified the exposure target and established an optimal dosage regimen among the Southern Chinese pediatric population.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This is the first study to comprehensively explain the PK disposition of vancomycin in Chinese southern pediatric patients using an age (2 years) cutoff separated PPK model. The evaluation of the vancomycin exposure–efficacy relationship indicated that a target level of AUC0–24/MIC ≥ 260 is clinically effective in children.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
Monte Carlo simulations were used to propose dose recommendations for different subgroup pediatric patients, which paved the way for potential use in personalized medicine and individualized prediction.
INTRODUCTION
As an antibiotic extensively prescribed for the treatment of serious infections caused by Gram‐positive bacteria, vancomycin has remained the first‐line agent for five decades. 1 However, its clinical application is complicated due to the characteristics of high variability in pharmacokinetics (PK) parameters. 2 It is worth mentioning that the vancomycin inter‐individual variability (IIV) of clearance (CL) was reported to reach as high as 99.2%. 3 Vancomycin is a hydrophilic drug, with approximately 90% eliminated by the renal system and excreted in the urine as a prototype after intravenous administration. 4
Children, as a heterogeneous population susceptible to irrational medicine use, are constantly growing and have an immature hemodynamic response, large variability in body size and organ function. 5 Children are distinct from adults, namely, they have a higher volume of distribution (V d), lower CL on vancomycin, and greater variability caused by patient‐specific factors. 6 Consequently, it is challenging to ensure that treatment in pediatric patients is delivered in a reasonable way.
Vancomycin trough concentration (C min) used as a therapeutic target may not be appropriate for pediatrics, as some studies have demonstrated that the C min was not associated with the success of infection treatment. 7 , 8 , 9 Thus, C min‐guided dosing may lead to subtherapeutic or excessive vancomycin exposure. 10 , 11 , 12 The value of area under the curve over 24 h to minimum inhibitory concentration ratio (AUC0–24/MIC) has been reported as the better indicator for favorable clinical effects. 13 This is owing to vancomycin exhibiting a time‐dependent antimicrobial killing mechanism. 14
According to the guidelines, vancomycin AUC0–24/MIC >400 for suspected methicillin‐resistant Staphylococcus aureus (MRSA) infection was a suitable therapeutic drug monitoring (TDM) target to attain successful clinical efficacy. 15 , 16 The AUC‐guided strategy based on the Bayesian approach was recommended for individualized vancomycin therapy as well. However, it is controversial whether the recommended target threshold supported by data from adults can be extrapolated to children due to inadequate evidences for efficacy and safety. 17 , 18 , 19
Population pharmacokinetic (PPK) modeling is an analytical approach quantifying sources of PK variability. The advantage is the ability to derive the effect of covariates on PK parameters from sparse sampling to reduce bloodwork in children particularly. 20 While a myriad of PPK model has been developed to describe vancomycin disposition and utilized for model‐informed precision dosing (MIPD), there are limited data available on the evaluation of relationship between PK/PD parameters and clinical outcome. 21 , 22
Moreover, poor attainment of PK/PD parameters has been commonly observed in current empiric dosing regimens, and dosage adjustment based on the dynamic physiological variables of patients is frequently neglected clinically. These challenges encountered illustrate the importance of constructing appropriate dosages for different subgroups of pediatric patients. The present study aimed to (1) establish and evaluate externally a comprehensive vancomycin PPK model in Southern Chinese pediatric population, (2) quantitatively evaluate the relationship between PK/PD variables and clinical efficacy, and identify the optimal exposure target of interest, and (3) perform model‐based simulations to optimize dosing recommendations.
MATERIALS AND METHODS
Patients and data collection
A retrospective study was conducted between 2016 and 2022 at the Baoan Women's and Children's Hospital and Shenzhen Children's Hospital. Children with Gram‐positive infections who received vancomycin treatment more than 3 days were selected. Patients who fulfilled the following exclusion criteria were not enrolled in this study: age under 1 month; hypersensitivity to vancomycin or its excipients; dialysis required or blood purification at the time of vancomycin treatment; Gram‐positive bacterial colonization. The evaluation of models was conducted on a randomly selected external validation set from 15% samples of enrolled patients.
Clinical data, including basic demographic information, diagnoses, clinical symptoms, as well as laboratory data including pathogenic bacteria and their sensitivity, infection sites, serum creatinine (Scr), creatinine clearance rate (CLcr), blood urea nitrogen (Bun), and albumin (Alb), were collected based on electronic medical records.
Bioassay
Vancomycin, with the trade name of Vancocin from Vianex S.A.‐PlantC (Athens, Greece), was administered intravenously for at least 60 min. TDM samples were collected and obtained as C min with sampling within 30 min prior to fourth‐fifth dose or as peak concentrations (C max) with sampling 0.5–1 h after the intravenous infusion. 15 Serum concentrations of vancomycin were measured by homogeneous enzyme immune methods (Viva‐E, Siemens, Erlangen, Germany). The within and between assays coefficients of variation were <10%. Patient bacterial isolates from patients were collected and used to determine the MIC value of vancomycin (broth microdilution).
Population pharmacokinetic model development
Using NONMEM® software (version 7.5, ICON Development Solutions, Ellicott City, MD, USA), the evolution of vancomycin serum concentrations–time in children was fitted to a PPK model. The graphical user interface Pirana® (version 3.0, http://www.pirana‐software.com) and the R programming environment (version 4.1, http://www.r‐project.org) and the software packages Perl‐speaks‐NONMEM (version 5.3, https://uupharmacometrics.github.io/PsN/index.html) were used for visual diagnosis. The First Order Conditional Estimation with interaction (FOCE‐I) method was used for the estimation of PK parameters.
The initial base model was selected on the basis of objective function value (OFV) and visual inspection of diagnostic plots. In this study, an exponential error model was adopted to evaluate IIV of PK parameters, and the residual variability (RV) was evaluated using an additive error model. Since the majority of data were trough vancomycin concentration, the IIV of V d was estimated to be very small (<1 × 10−3) and removed from the model. Furthermore, body weight (BW) was incorporated into V d using the Equation (1) according to the Holford et al. 6
| (1) |
The covariate analysis included demographic variables (sex, age, postnatal age [PNA], body weight), hepatic and renal functions (blood urea nitrogen, albumin, serum creatinine, and creatinine clearance rate), and concomitant drugs (more than 10% of the total patients: meropenem, cefoperazone, ceftriaxone, and mannitol), which were investigated for their influence only on the CL. The CLcr was computed using the Cockroft‐Gault formula. 23 Linear, exponential, and power models' functions were tested to describe the covariate effects, respectively.
It is widely known that size and maturation appeared to be the main factors influencing PK in pediatrics. 24 Therefore, age and BW were screened first. As BW was found to have a greater impact on CL than age in the present study, six different models based on BW allometric scaling were tested using Equation (2). 6
| (2) |
where F mat is a factor for maturation which defines the maturing process, and it was fixed to 1 unless Model III was used.
Model I: 3/4 Allometric model, k was fixed to 0.75.
Model II: Simple allometric model, k was estimated.
Model III: 3/4 Allometric and age maturation function model, F mat was calculated according to Equation (3):
| (3) |
where TM50 is maturation half‐time, and γ is the Hill coefficient which controls the slope of sigmoid function.
Model IV: Age‐cutoff model, different values for the k were evaluated based on model II for two sub‐populations.
Model V: BW‐dependent exponent model (BDE), k was estimated according to Equation (4):
| (4) |
Model VI: Age‐dependent exponent model (ADE), k was estimated according to Equation (5):
| (5) |
where k 0 is the value of the exponent at a theoretical BW of 0 kg (Model V) or 0 years (Model VI), k max is the maximum decrease of the exponent, k 50 is the BW (Model V) or Age (Model VI) at which a 50% decrease in the maximum decrease is attained. 25 The selection criteria was the same as detailed in our previous study, 26 the final model was obtained by the stepwise forward inclusion (∆OFV > 6.64) and backward elimination (∆OFV > 10.8).
Model evaluation
The established final model was internally evaluated by graphical tools such as the goodness‐of‐fit (GOF) plots for diagnostic purposes, along with visual predictive check (VPC) and bootstrap analysis. In addition, a dataset from additional patients was used to externally validate the final model. The mean relative prediction error (MPE%) and mean relative absolute prediction error (MAPE%) were used to evaluate the model predictions. 27 The model with MPE% and MAPE% within the range of ±20% and 30%, respectively, was considered to be acceptable.
Efficacy analysis
The dataset included in the analysis of efficacy comprised patients who had clinical symptoms of infection and Gram‐positive bacteria cultured in sterile body fluid samples (i.e., blood and cerebrospinal fluid). Investigation of clinical efficacy was double‐evaluated by two independent investigators, and divided into treatment success or failure. The clinical outcomes were analyzed in terms of both curative effect and bacterial clearance to obtain reliable determination. We define treatment success if the patients have improvement of the original symptoms or resolution of infection, negativization of bacterial cultures at the site of the primary infection after the end of the vancomycin treatment. Patients with bacteria eradication failure after vancomycin initiation, requirement for adding or switching to another anti‐gram‐positive bacterial drug, deterioration of clinical features, and laboratory examinations or even mortality, were defined as treatment failure.
Individual PK parameter (CL over the first 24 h) was estimated using an empirical Bayesian method in NONMEM® based on the final PPK model with covariates. Vancomycin AUC0–24 was calculated by the Equation (6):
| (6) |
The ratio of AUC0–24/MIC was calculated in all eligible patients and then used to evaluate the relationship with clinical response, as it was considered to be the gold standard for determining efficacy.
Simulation of dosing regimen
A Monte Carlo simulation was performed on virtual patients (n = 1000), which were divided into subgroups based on the incorporated covariates. We sampled body weight and age from China National Survey Data for children, 28 while CLcr was additionally classified into four degrees of renal function between 60 and 150 mL/min. Dosage schedules covering a range of 30–70 mg/kg administered every 8 h were considered as the initial vancomycin dose. The probability of target attainment (PTA) of achieving the optimal exposure range for each candidate dosing regimens was calculated. Subsequently, the dose regimen selected were compared with those from other established vancomycin PPK studies in children.
Statistical analysis
Statistical tests were performed using SPSS® 27.0 (IBM, Armonk, New York, NY, USA). Multivariate analysis was carried out using binary logistic regression to quantify the relationship between vancomycin exposure (C min, AUC0–24, and AUC0–24/MIC) and clinical response. Receiver operating characteristic (ROC) was applied to assess the predictive accuracy of PK/PD indices and find their medical margin level for vancomycin antibiotic efficacy. 9 A p value of <0.05 was considered significant.
Ethics statement
The studies involving human participants were reviewed and approved by the Baoan Women's and Children's Hospital Ethics Committee (Appr. Number LLSC 2021‐3‐12‐KS‐02, date of approval: March 12, 2021).
RESULTS
Demographic characteristics
Out of 538 patients, 85 patients were excluded. The patient enrollment process from two hospitals is detailed in Figure 1. The demographic and clinical characteristics of patients eligible for this study are summarized in Table 1. The age range was 1 month to 17.45 years with a mean body weight of 13.55 kg (range: 0.88–49.5 kg). There were 182 patients (47%) younger than 2 years old. A median daily dose of vancomycin was 43 mg/kg/day.
FIGURE 1.
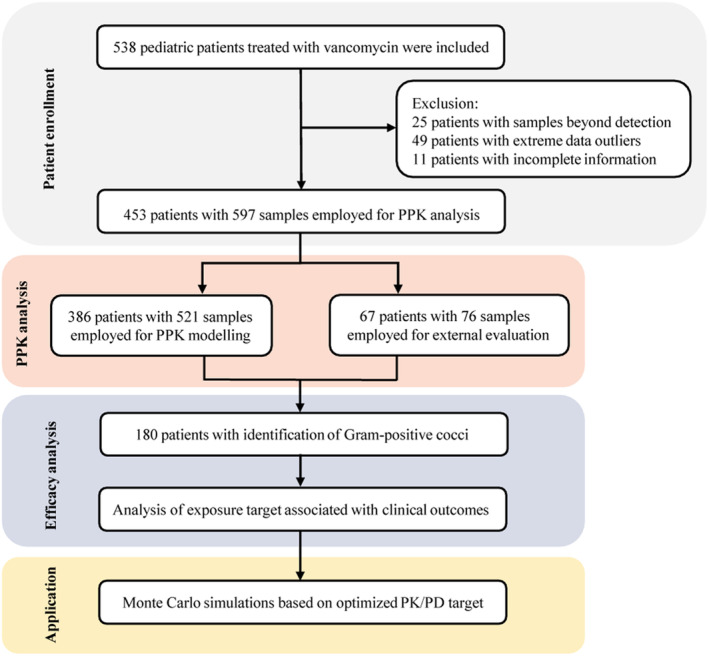
Study flow diagram. The overview of the study population enrollment process and study protocol.
TABLE 1.
Demographics and clinical characteristics of the model‐building and validation datasets.
| Dataset for model building | Dataset for model validation | |||
|---|---|---|---|---|
| N 1 = 210 | N 2 = 176 | Total = 386 | N = 67 | |
| No. of concentrations | 272 | 249 | 521 | 76 |
| Gender (male/female) | 134/76 | 105/71 | 239/147 | 41/26 |
| Age (years) | 0.95 (0.08–13.14) [2.08 ± 2.47] | 4.32 (0.10–17.45) [5.0 ± 3.82] | 2.22 (0.08–17.45) [3.42 ± 3.48] | 3.8 (0.10–15.51) [4.82 ± 4.53] |
| Postnatal age (weeks) | 49.5 (4.43–685) [108.5 ± 128.79] | 225.3 (5.14–909.9) [260.67 ± 199.34] | 116.05 (4.43–909.9) [178.13 ± 181.51] | 198.29 (5–808.6) [251.21 ± 236.42] |
| Body weight (kg) | 9.9 (0.88–45) [10.75 ± 6.97] | 15.4 (1.56–49.5) [16.87 ± 9.60] | 11.95 (0.88–49.5) [13.55 ± 8.81] | 13 (1.62–49.9) [17.73 ± 12.65] |
| Serum creatinine (μmol/L) | 26 (13–111) [29.17 ± 13.40] | 25.3 (7.6–73.6) [26.74 ± 10.51] | 26 (7.6–111) [28.08 ± 12.22] | 26 (12–110.1) [29.16 ± 14.73] |
| Creatinine clearance (mL/min) | 60.92 (10.5–212.85) [62.83 ± 33.62] | 97.19 (10.74–265.6) [100.25 ± 44.85] | 74.16 (10.5–265.6) [79.95 ± 43.33] | 87.99 (10.38–217.85) [91.61 ± 45.85] |
| Albumin (g/L) | 36.05 (20–59.3) [35.55 ± 5.89] | 34.2 (0.61–44.5) [33.43 ± 5.89] | 35.15 (0.61–59.3) [34.61 ± 5.96] | 35.5 (22.6–49.5) [35.58 ± 5.85] |
| Blood urea nitrogen (mmol/L) | 2.97 (0.7–25.5) [3.57 ± 2.77] | 3.4 (0.7–15.8) [3.74 ± 2.10] | 3.18 (0.7–25.5) [3.66 ± 2.48] | 3.3 (1.12–13.88) [4.11 ± 2.59] |
| Vancomycin dose (mg/kg) | 45 (16.5–62.5) [45.63 ± 8.25] | 40.50 (12.3–79.37) [41.74 ± 9.28] | 43.24 (12.3–79.4) [43.76 ± 8.96] | 41.32 (18.18–60) [41.79 ± 7.39] |
| Vancomycin concentration (mg/L) | 7 (2.1–30.4) [8.7 ± 6.0] | 6.4 (0.7–40.5) [8.27 ± 5.85] | 6.8 (0.7–40.5) [8.52 ± 5.93] | 6.35 (2.8–17.7) [6.89 ± 2.63] |
| Co‐administered drugs during vancomycin therapy (n/%) | ||||
| Meropenem | 84 (30.88%) | 144 (57.83%) | 228 (43.76%) | 28 (36.84%) |
| Cefoperazone | 81 (29.78%) | 54 (21.69%) | 135 (25.91%) | 17 (22.37%) |
| Ceftriaxone | 115 (42.28%) | 19 (7.63%) | 134 (25.72%) | 19 (25%) |
| Mannitol | 61 (22.43%) | 5 (2.01%) | 66 (12.67%) | 2 (2.63%) |
| Amoxicillin | 27 (9.93%) | 0 (0%) | 27 (5.18%) | 5 (6.58%) |
| Furosemide | 9 (3.31%) | 20 (8.03%) | 29 (5.57%) | 2 (2.63%) |
Note: Data are expressed as median (range) [mean ± standard deviation] unless specified otherwise.
Population pharmacokinetic model development
A one‐compartment model with first‐order elimination (ADVAN1 TRANS2) was used as the structural model. RV was best characterized by an additive error model for each of the two study centers.
As presented in Table 2, Model V and Model VI had highly variable parameters. Model IV with a cutoff value of 2 years showed a better fit of the model to the data (Figure S1). Another variable related to vancomycin CL was renal function. Interestingly, the addition of either CLcr or Bun led to a significant variation in OFV. However, the graphical diagnostics of the covariate‐parameter relationship by the CLcr‐adjusted model performed superior to that of the Bun. Table S1 outlines the details of the model development process.
TABLE 2.
Parameter estimates of the six body weight‐dependent clearance candidate models.
| Parameters | Model I | Model II | Model III | Model IV | Model V | Model VI |
|---|---|---|---|---|---|---|
| 3/4 Allometric model | Allometric model | 3/4 Allometric and age maturation function model | Age‐cutoff model | BW‐dependent exponent model (BDE) | Age‐dependent exponent model (ADE) | |
| Model description | CLp∙(BW/BWmedian)0.75 | CLp∙(BW/BWmedian)k | CLp∙(BW/BWmedian)0.75 F mat | CLp∙(BW/BWmedian)k | CLp∙(BW/BWmedian)k1 | CLp∙(BW/BWmedian)k1 |
| OFV | 2417.412 | 2343.071 | 2414.845 | 2320.382 | 2320.595 | 2323.046 |
| AIC | 2427.412 | 2355.071 | 2428.845 | 2336.382 | 2338.595 | 2341.046 |
| BIC | 2448.691 | 2380.606 | 2458.635 | 2370.428 | 2376.897 | 2379.348 |
| CLp (L/h) | 2.09 (4.3) | 2.10 (4.0) | 2.11 (4.3) | 2.70 (7.1) a /2.18 (7.1) b | 2.30 (5.0) | 2.42 (5.5) |
| k | / | 1.22 (4.6) | / | 0.679 a /1.33 b | / | / |
| F mat = 1/[1 + (age/TM50)−γ ] | ||||||
| TM50 | / | / | 17.4 (0.5) | / | / | / |
| γ | / | / | 22.6 (13.6) | / | / | / |
| k 1 = k 0 − k max∙BW γ /(k 50 γ + BW γ ) or k 1 = k 0 − k max∙age γ /(k 50 γ + age γ ) | ||||||
| k 0 | / | / | / | / | 1.38 (5.2) | 1.43 (4.9) |
| k max | / | / | / | / | 0.755 (37.6) | 0.630 (22.1) |
| k 50 (years or kg) | / | / | / | / | 25.0 (24.1) | 3.63 (27.4) |
| γ | / | / | / | / | 4.73 (75.1) | 13.4 (76.5) |
| V p (L) | 40.9 (14.6) | 23.8 (28.2) | 40.8 (14.5) | 23.0 (25.5) | 23.3 (27.1) | 22.7 (25.5) |
| IIV of CLp | 0.443 (13.5) | 0.378 (9.2) | 0.434 (14.7) | 0.359 (9.5) | 0.355 (9.9) | 0.361 (9.7) |
| η‐shrinkage (%) | 46.5 | 39.1 | 47.2 | 40.0 | 40.7 | 39.5 |
| RV1 (mg/L) | 5.52 (7.6) | 4.72 (10.4) | 5.54 (7.6) | 4.71 (9.9) | 4.74 (10.2) | 4.70 (10.2) |
| ε 1‐shrinkage (%) | 10.5 | 15.5 | 10.1 | 14.9 | 14.5 | 15.1 |
| RV2 (mg/L) | 5.14 (10.3) | 4.79 (9.4) | 5.15 (10.3) | 4.67 (9.1) | 4.69 (9.1) | 4.66 (9.2) |
| ε 2‐shrinkage (%) | 12.1 | 14.3 | 11.8 | 14.0 | 13.6 | 14.3 |
Note: Estimates are expressed as estimate (% standard error).
Abbreviations: BW, body weight; CLp, typical value of apparent clearance; F mat, the fraction of the adult value of clearance; TM50, the value of age when 50% of the adult clearance is attained through maturation; γ, Hill coefficient determining the steepness of the sigmoidal decline; k 0, value of the exponent at a theoretical BW of 0 kg or age of o year; k 1, exponent coefficient of BW; k max, maximum decrease; k 50, the BW or age at which there is a 50% decrease in the k max; V p, typical value of apparent volume of distribution; IIV, inter‐subject variability of apparent clearance; RV, residual variability.
Estimates for children >2 year of age.
Estimates for children ≤2 year of age.
For the final model, OFV was obviously lower compared to the base model and exhibited acceptable RSE of the parameter estimates (Table 3). The condition numbers no greater than 100 demonstrated the stability of the model. The established final PPK model is represented as follows:
| (7) |
| (8) |
| (9) |
TABLE 3.
Pharmacokinetic parameter estimates for the final model including the bootstrap (996/1000 runs successful) analysis.
| Description | Parameter | Final model | Bootstrap | Relative error (%) | ||
|---|---|---|---|---|---|---|
| Estimate | RSE (%) | Median | 95%CI | |||
| CLage>2 (L/h) | θ (CLage>2) | 2.59 | 6.7 | 2.56 | 2.30–2.88 | −1.2 |
| CLage≤2 (L/h) | θ (CLage≤2) | 1.98 | 6.8 | 1.97 | 1.75–2.21 | −0.5 |
| V d (L) | θ (V d) | 22.4 | 23.5 | 22.2 | 15.05–32.02 | −0.9 |
| BWage>2 on CL | θ (BWage>2) | 0.38 | 29.8 | 0.39 | 0.193–0.571 | 2.6 |
| BWage≤2 on CL | θ (BWage≤2) | 0.739 | 16.7 | 0.747 | 0.542–0.948 | 1.1 |
| CLcr on CL | θ (CLcr) | 0.517 | 15.1 | 0.512 | 0.385–0.635 | −1.0 |
| IIV on CL | ω (CL) | 0.319 | 11.7 | 0.316 | 0.240–0.373 | −0.9 |
| η‐shrinkage (%) | η (shrinkage) | 43.1 | ||||
| RV1 (mg/L) | σ (additive1) | 4.64 | 9.4 | 4.56 | 3.80–5.22 | −1.7 |
| ε 1‐shrinkage (%) | ε (shrinkage1) | 13.3 | ||||
| RV2 (mg/L) | σ (additive2) | 4.53 | 9.0 | 4.56 | 3.81–5.22 | 0.7 |
| ε 2‐shrinkage (%) | ε (shrinkage2) | 12.4 | ||||
Note: Relative error% = (Bootstrap median – estimate in final model)/estimate in final model × 100%.
Abbreviations: θ, factor describing the relationship between the covariate and the clearance; ω, coefficient variation of inter‐individual variability; σ, coefficient variation of residual variability; RSE (%), relative standard error (standard error/estimate × 100%); 95%CI, 95% confidence interval.
Model evaluation
Figure 2 showed that the final model had greatly improved accuracy and less structural bias compared with the base model, and the VPC plot exhibits the good predictive performance of the final model (Figure S2). The results of the bootstrap analysis matched the final model well with relative error < ±2.6% (Table 3). In addition, extra 67 pediatric patients were included in the external evaluation. MPE, MPE%, MAPE, and MAPE% corresponding to the final model were −1.27, −14.08%, 1.76, and 24%, respectively.
FIGURE 2.
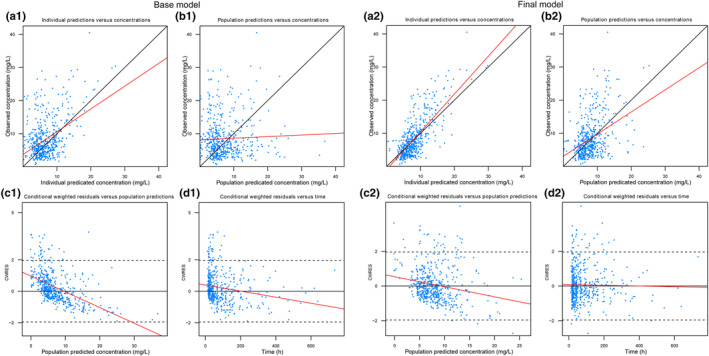
Diagnostic goodness‐of‐fit (GOF) plots of the base model (1) and final model (2). (a) Observed concentration (DV) versus individual predicted concentration (IPRED); (b) DV versus population predicted concentration (PRED); (c) conditional weighted residuals (CWRES) versus PRED; and (d) CWRES versus time. The black solid lines are the reference lines, and red solid lines represent linear fit lines.
Clinical outcomes
The number of cases that detected Gram‐positive isolates was 180, treatment failure was considered in 29 of them (16%). The main pathogenic organisms cultured in this study were MRSA, methicillin‐resistant coagulase‐negative Staphylococci aureus (MRCNS), and other Enterococcus species. It should be noted that the majority of MIC values of clinical isolates was 1 mg/L or less in our two study institutions, so the default MIC value was considered to be 1 mg/L for those undetected pathogens as previous studies. 29 , 30 Furthermore, The European Committee on Antimicrobial Susceptibility Testing show that 75% of Staphylococcus aureus isolates have a vancomycin MIC of 1 mg/L. 31
The results in Table 4 showed that the AUC0–24/MIC was statistically different (p < 0.05) between the treatment success and failure group. The relationship between C min and clinical effective rate is weaker than AUC0–24/MIC (Figure S3), which seemed to reach a plateau around 200–300. In ROC curve (Figure S4), the area under the AUC0–24/MIC (value = 0.782, 95%CI: 0.695–0.870) was greater than that of C min. The result supported that AUC0–24/MIC could be a better predictor for vancomycin‐related efficacy, and 260 mg·h/L (according to the highest Youden index) might be an applicable threshold.
TABLE 4.
Multivariable logistic regression analyses on clinical efficacy of vancomycin therapy.
| Variables | Clinical efficacy | |
|---|---|---|
| Wald χ 2 | p Value | |
| C min | 0.796 | 0.372 |
| AUC0–24 | 2.297 | 0.130 |
| AUC0–24/MIC | 6.181 | 0.013 |
Model‐based simulation
The simulation results are provided in Figure 3. The dosing recommendations from the other established model based on Monte Carlo simulations are displayed in Table S2. It can be seen that when a dose of 40 mg/kg/day was given in the group of renal impairment (CLcr = 60 mL/min), over 90% of simulated subjects could achieve the PD target (AUC0–24/MIC ≥260). In the normal renal function group, the weight of 20 kg (5.5‐year‐old age) was used as a cutoff point to divide patients into two cohorts, and the exposure was lower in the high‐weight compared to the low‐weight population with the same vancomycin dose. Patients with augmented renal function (ARC, CLcr = 150 mL/min) resulted in a 43% increase in CL, presenting a higher dose of 60 mg/kg/day as a more appropriate choice.
FIGURE 3.
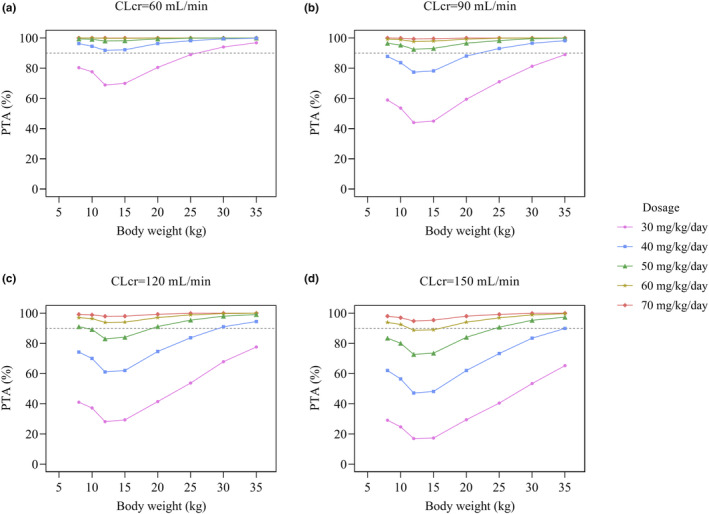
Simulated probability of target attainment (AUC ≥260) of vancomycin at each CLcr level for different dosage in patients with various body weights.
DISCUSSION
As far as we know, this is the first study to comprehensively explain the PK disposition of vancomycin in Chinese southern pediatric patients using an age (2 years) cutoff separated PPK model. Of note, the evaluation of vancomycin exposure‐efficacy relationship indicated that there may be no need to obtain a target level of AUC0–24/MIC (≥400 required by the guidelines) to achieve clinical effectiveness in children. Finally, dosing recommendations for subgroups were derived from Monte Carlo simulation based on the optimized exposure target.
For children, maturation and size are significant predictors to explain some of the IIV in PPK models. Although the ¾ allometric exponent approach on CL has been extensively used, this value remains controversial due to over‐ and under‐prediction of CL in neonates and infants, respectively. 32 , 33 In contrast, the age‐cutoff model scales CL well throughout the whole lifetime in our study population (Table 2). Typical CL estimate appears to vary between age groups: children ≤2 years old and children >2 years old (Figure S1). It is because hemodynamic variations in children might result in faster glomerular filtration rate (GFR) rates compared with the adults, 34 and the maturation effect on PK behavior can be accentuated in children younger than 2 years old, 6 so the effect of age should be considered altogether as well.
We selected the CLcr‐adjusted model to scale vancomycin CL, Since Scr and Bun are known to be influenced by age, sex, muscle mass, and diet—limiting their utility as a marker of the GFR. 35 Other variables that produced no significant impact during the modeling process were Alb, sex, and concomitant therapies. This may be explained by the fact that the range and proportion of covariates being tested are not large enough to have a statistical effect.
In the current PPK model, the weight‐adjusted typical CL were 0.216 L/h/kg (age >2 years old) and 0.165 L/h/kg (age ≤2 years old), which fell within the known range (0.0155–0.255 L/h/kg) summarized in a systematic review. 36 Both internal and external evaluation supported the precise predictive performance of the final model. 37 However, there was a bit large error in population prediction from a new set of data, this can be explained by the unexplained IIV of CL. Indeed, the extent of renal clearance should also involve possible protein binding and tubular transport. 38 , 39 For another, the combination with compounds undergoing renal elimination could cause a drug–drug interaction.
Logistic regression analysis indicated that AUC0–24/MIC has significant correlations with clinical outcomes (Table 4). The cutoff point (Figure S4) in predicting effectiveness (AUC0–24/MIC ≥260) is far below the recommended target of adults (AUC0–24/MIC ≥400). Research by Shen et al. showed that the median value of AUC/MIC was between 200 and 300 with an overall clinical effective rate of 92.6%, indicating that a relatively low exposure is effective for pediatric patients. 40 Another research in children with MRSA bacteremia showed that the relationship between vancomycin AUC0–24/MIC <400 and treatment failure could not be established. 41 Additionally, targeting the value above 400 in pediatrics would expose them to unnecessary adverse events. 10 Taken together, these results suggested that a target level (≥400) may not be required. This discrepancy may be attributed to the higher sensitivity of bacteria in our population and a higher level of unbound vancomycin fraction in children. 42
In a multicenter study performed on Chinese children, most received relatively low vancomycin dosages (37.79 ± 11.31 mg/kg/day), but the microbiological eradication rate was >80% and comparable to studies with higher dosage levels. 9 Furthermore, other studies also found that the uniform or empirical vancomycin recommended dose did not achieve ideal PD targets in most pediatric patients. 43 , 44 Here, we developed a patient‐tailored dosing regimen according to the PTA achievement.
On the basis of the AUC0–24/MIC target value (above 260, assuming MIC = 1 mg/L), it is suggested that for children with CLcr of 60 and 150 mL/min, the suitable vancomycin dose should be 40 and 60 mg/kg/day, respectively. For children with normal renal function, those weighing less than 20 kg (equal to 5.5‐year‐old age) need to have a relatively high dose of 50–60 mg/kg/day versus 40–50 mg/kg/day in high‐weight population. This is consistent with the previous cohort studies reporting that the risk of suboptimal exposure might be higher in pediatric patients 1–6 years of age, so the initial dose could be increased. 15
The present study also provided a summary of dosing recommendations of vancomycin in pediatrics. 23 As listed in Table S2, the variability between studies was attributed to the different covariates considered as well as various target values. Recommended dosage regimens based on our model are similar to study in Chinese children 22 and marginally lower compared with that of western people 10 and IDSA guideline. 16 It is of note that most studies used a AUC0‐24/MIC threshold of 400 as a PD target which differs from our study, and seldom of them considered the effect of renal function. It has been suggested that AUC0‐24 < 537 and 480 mg·h/L may be appropriate thresholds for predicting vancomycin‐associated nephrotoxicity in Chinese children and neonates, respectively. 19 , 45
The primary limitation of this study is its retrospective nature, and the imbalanced number of treatment success and failure groups resulted in wide CIs around odds ratios in logistic regression analyses, these can affect the ROC‐derived threshold. Secondly, a study of more sample size and a larger range of covariates characteristics could be conducted to improve the precision and accuracy of the PPK model in depth, which has the potential to explain the remaining variation that is not yet identified. Finally, the proposed dosing schedule has not been applied in clinical practice and cannot replace the need for routine TDM. Therefore, these results should be extrapolated to other populations with caution and confirmed prospectively in further studies to ensure their generalizability.
CONCLUSIONS
A “one‐dose‐fits‐all” approach to managing patients no longer seems reasonable, and model‐based approaches are emerging as an alternative promising option. A PPK model of vancomycin for Chinese children with body weight and renal functions as covariates based on a cutoff of 2 years old was proposed in this study, showing overall acceptable and robust predictive performance. The clinical outcomes as measured by PK/PD parameters were further explored to determine appropriate target thresholds assuring clinical efficacy. The developed optimal dosing strategies based on the final model pave the way for potential use in personalized medicine and individualized prediction.
AUTHOR CONTRIBUTIONS
X.H.S., X.M.F., and W.Z.L. wrote the manuscript, X.M.F., and W.Z.L. designed the research, X.H.S., X.J.L., Z.Z., Z.B.C., and J.P.Z. performed the research, J.L.L., Y.D.H., and J.H.Z. analyzed the data.
FUNDING INFORMATION
This work was supported by the Research Foundation of Shenzhen Baoan Women's and Children's Hospital, Jinan University (BAFY2023001), the Shenzhen Science and Technology Planning Program (JCYJ20230807145859002), the Natural Science Foundation of Guangdong province (2024A1515030299) and Shenzhen Baoan medical health research project (2022JD110).
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests for this work.
Supporting information
Data S1:
ACKNOWLEDGMENTS
The authors would like to thank the patients who participated in this study, as well as all investigators and the medical, nursing and laboratory staff who were involved in the present work.
Shen X, Li X, Lu J, et al. Population pharmacokinetic analysis for dose regimen optimization of vancomycin in Southern Chinese children. CPT Pharmacometrics Syst Pharmacol. 2024;13:1201‐1213. doi: 10.1002/psp4.13151
Xianhuan Shen and Xuejuan Li contributed equally to this work.
Contributor Information
Xiaomei Fan, Email: xmfane@163.com.
Wenzhou Li, Email: wenzhoulee@foxmail.com.
DATA AVAILABILITY STATEMENT
The data presented in this study are available from the corresponding authors (Dr Xiaomei Fan and Dr Wenzhou Li) upon reasonable request.
REFERENCES
- 1. Levine DP. Vancomycin: a history. Clin Infect Dis. 2006;42(Suppl 1):S5‐S12. doi: 10.1086/491709 [DOI] [PubMed] [Google Scholar]
- 2. Jeffres MN. The whole Price of vancomycin: toxicities, troughs, and time. Drugs. 2017;77(11):1143‐1154. doi: 10.1007/s40265-017-0764-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bae SH, Yim DS, Lee H, et al. Application of Pharmacometrics in pharmacotherapy: open‐source software for vancomycin therapeutic drug management. Pharmaceutics. 2019;11(5):224. doi: 10.3390/pharmaceutics11050224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Estes KS, Derendorf H. Comparison of the pharmacokinetic properties of vancomycin, linezolid, tigecyclin, and daptomycin. Eur J Med Res. 2010;15(12):533‐543. doi: 10.1186/2047-783x-15-12-533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kearns GL, Abdel‐Rahman SM, Alander SW, et al. Developmental pharmacology – drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157‐1167. doi: 10.1056/NEJMra035092 [DOI] [PubMed] [Google Scholar]
- 6. Holford N, Heo YA, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013;102(9):2941‐2952. doi: 10.1002/jps.23574 [DOI] [PubMed] [Google Scholar]
- 7. Cao L, Li Z, Zhang P, Yong S. Relationship between vancomycin trough serum concentrations and clinical outcomes in children: a systematic review and meta‐analysis. Antimicrob Agents Chemother. 2022;66(8):e0013822. doi: 10.1128/aac.00138-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Le J, Bradley JS, Murray W, et al. Improved vancomycin dosing in children using area under the curve exposure. Pediatr Infect Dis J. 2013;32(4):e155‐e163. doi: 10.1097/INF.0b013e318286378e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liang X, Fan Y, Yang M, et al. A prospective multicenter clinical observational study on vancomycin efficiency and safety with therapeutic drug monitoring. Clin Infect Dis. 2018;67(suppl_2):S249‐S255. doi: 10.1093/cid/ciy680 [DOI] [PubMed] [Google Scholar]
- 10. Alsultan A, Abouelkheir M, Alqahtani S, et al. Optimizing vancomycin monitoring in pediatric patients. Pediatr Infect Dis J. 2018;37(9):880‐885. doi: 10.1097/inf.0000000000001943 [DOI] [PubMed] [Google Scholar]
- 11. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042‐17. doi: 10.1128/aac.02042-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsutsuura M, Moriyama H, Kojima N, et al. The monitoring of vancomycin: a systematic review and meta‐analyses of area under the concentration‐time curve‐guided dosing and trough‐guided dosing. BMC Infect Dis. 2021;21(1):153. doi: 10.1186/s12879-021-05858-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42(Suppl 1):S35‐S39. doi: 10.1086/491712 [DOI] [PubMed] [Google Scholar]
- 14. Moise‐Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43(13):925‐942. doi: 10.2165/00003088-200443130-00005 [DOI] [PubMed] [Google Scholar]
- 15. He N, Su S, Ye Z, et al. Evidence‐based guideline for therapeutic drug monitoring of vancomycin: 2020 update by the division of therapeutic drug monitoring, Chinese pharmacological society. Clin Infect Dis. 2020;71(Suppl 4):S363‐S371. doi: 10.1093/cid/ciaa1536 [DOI] [PubMed] [Google Scholar]
- 16. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin‐resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health‐System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835‐864. doi: 10.1093/ajhp/zxaa036 [DOI] [PubMed] [Google Scholar]
- 17. Tkachuk S, Collins K, Ensom MHH. The relationship between vancomycin trough concentrations and AUC/MIC ratios in pediatric patients: a qualitative systematic review. Paediatr Drugs. 2018;20(2):153‐164. doi: 10.1007/s40272-018-0282-4 [DOI] [PubMed] [Google Scholar]
- 18. Yoo R, So H, Seo E, Kim M, Lee J. Impact of initial vancomycin pharmacokinetic/pharmacodynamic parameters on the clinical and microbiological outcomes of methicillin‐resistant Staphylococcus aureus bacteremia in children. PLoS One. 2021;16(4):e0247714. doi: 10.1371/journal.pone.0247714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou B, Xiong W, Bai K, et al. Clinical application value of pharmacokinetic parameters of vancomycin in children treated in the pediatric intensive care unit. Front Pediatr. 2022;10:867712. doi: 10.3389/fped.2022.867712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duffull SB, Wright DF, Winter HR. Interpreting population pharmacokinetic‐pharmacodynamic analyses – a clinical viewpoint. Br J Clin Pharmacol. 2011;71(6):807‐814. doi: 10.1111/j.1365-2125.2010.03891.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chhim RF, Arnold SR, Lee KR. Vancomycin dosing practices, trough concentrations, and predicted area under the curve in children with suspected invasive staphylococcal infections. J Pediatric Infect Dis Soc. 2013;2(3):292. doi: 10.1093/jpids/pit032 [DOI] [PubMed] [Google Scholar]
- 22. Zhang H, Wang Y, Gao P, et al. Pharmacokinetic characteristics and clinical outcomes of vancomycin in young children with various degrees of renal function. J Clin Pharmacol. 2016;56(6):740‐748. doi: 10.1002/jcph.653 [DOI] [PubMed] [Google Scholar]
- 23. Chung E, Sen J, Patel P, Seto W. Population pharmacokinetic models of vancomycin in Paediatric patients: a systematic review. Clin Pharmacokinet. 2021;60(8):985‐1001. doi: 10.1007/s40262-021-01027-9 [DOI] [PubMed] [Google Scholar]
- 24. Marsot A. Pharmacokinetic variability in pediatrics and intensive care: toward a personalized dosing approach. J Pharm Pharm Sci. 2018;21(1):354‐362. doi: 10.18433/jpps30082 [DOI] [PubMed] [Google Scholar]
- 25. Wang C, Peeters MY, Allegaert K, et al. A bodyweight‐dependent allometric exponent for scaling clearance across the human life‐span. Pharm Res. 2012;29(6):1570‐1581. doi: 10.1007/s11095-012-0668-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shen X, Chen X, Lu J, et al. Pharmacogenetics‐based population pharmacokinetic analysis and dose optimization of valproic acid in Chinese southern children with epilepsy: effect of ABCB1 gene polymorphism. Front Pharmacol. 2022;13:1037239. doi: 10.3389/fphar.2022.1037239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van der Meer AF, Marcus MA, Touw DJ, et al. Optimal sampling strategy development methodology using maximum a posteriori Bayesian estimation. Ther Drug Monit. 2011;33(2):133‐146. doi: 10.1097/FTD.0b013e31820f40f8 [DOI] [PubMed] [Google Scholar]
- 28. Li H, Ji CY, Zong XN, Zhang YQ. Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years. Zhonghua Er Ke Za Zhi. 2009;47(7):487‐492. [PubMed] [Google Scholar]
- 29. Dao K, Guidi M, André P, et al. Optimisation of vancomycin exposure in neonates based on the best level of evidence. Pharmacol Res. 2020;154:104278. doi: 10.1016/j.phrs.2019.104278 [DOI] [PubMed] [Google Scholar]
- 30. Issaranggoon Na Ayuthaya S, Katip W, Oberdorfer P, et al. Correlation of the vancomycin 24‐h area under the concentration‐time curve (AUC(24)) and trough serum concentration in children with severe infection: a clinical pharmacokinetic study. Int J Infect Dis. 2020;92:151‐159. doi: 10.1016/j.ijid.2019.12.036 [DOI] [PubMed] [Google Scholar]
- 31. EUCAST . Antimicrobial wild type distributions of microorganisms. Accessed March 01, 2023; https://mic.eucast.org/
- 32. Mahmood I. Prediction of drug clearance in children from adults: a comparison of several allometric methods. Br J Clin Pharmacol. 2006;61(5):545‐557. doi: 10.1111/j.1365-2125.2006.02622.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peeters MY, Allegaert K, Blussé van Oud‐Alblas HJ, et al. Prediction of propofol clearance in children from an allometric model developed in rats, children and adults versus a 0.75 fixed‐exponent allometric model. Clin Pharmacokinet. 2010;49(4):269‐275. doi: 10.2165/11319350-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 34. De Cock RF, Allegaert K, Brussee JM, et al. Simultaneous pharmacokinetic modeling of gentamicin, tobramycin and vancomycin clearance from neonates to adults: towards a semi‐physiological function for maturation in glomerular filtration. Pharm Res. 2014;31(10):2643‐2654. doi: 10.1007/s11095-014-1361-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baxmann AC, Ahmed MS, Marques NC, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3(2):348‐354. doi: 10.2215/cjn.02870707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aljutayli A, El‐Haffaf I, Marsot A, et al. An update on population pharmacokinetic analyses of vancomycin, part II: In pediatric patients. Clin Pharmacokinet. 2022;61(1):47‐70. doi: 10.1007/s40262-021-01050-w [DOI] [PubMed] [Google Scholar]
- 37. Holford NH, Buclin T. Safe and effective variability‐a criterion for dose individualization. Ther Drug Monit. 2012;34(5):565‐568. doi: 10.1097/FTD.0b013e31826aabc3 [DOI] [PubMed] [Google Scholar]
- 38. Golper TA, Noonan HM, Elzinga L, et al. Vancomycin pharmacokinetics, renal handling, and nonrenal clearances in normal human subjects. Clin Pharmacol Ther. 1988;43(5):565‐570. doi: 10.1038/clpt.1988.74 [DOI] [PubMed] [Google Scholar]
- 39. Marsot A, Boulamery A, Bruguerolle B, Simon N. Vancomycin: a review of population pharmacokinetic analyses. Clin Pharmacokinet. 2012;51(1):1‐13. doi: 10.2165/11596390-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 40. Shen K, Fan Y, Yang M, et al. Modeling approach to optimizing dose regimen of vancomycin for Chinese pediatric patients with gram‐positive bacterial infections. Front Pharmacol. 2021;12:648668. doi: 10.3389/fphar.2021.648668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hahn A, Frenck RW Jr, Allen‐Staat M, et al. Evaluation of target attainment of vancomycin area under the curve in children with methicillin‐resistant Staphylococcus aureus bacteremia. Ther Drug Monit. 2015;37(5):619‐625. doi: 10.1097/ftd.0000000000000190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smits A, Pauwels S, Oyaert M, et al. Factors impacting unbound vancomycin concentrations in neonates and young infants. Eur J Clin Microbiol Infect Dis. 2018;37(8):1503‐1510. doi: 10.1007/s10096-018-3277-8 [DOI] [PubMed] [Google Scholar]
- 43. Patel J, Lucas CJ, Ryan J, Jenkins M, Martin JH. Vancomycin therapeutic drug monitoring in paediatrics. J Paediatr Child Health. 2020;56(4):563‐570. doi: 10.1111/jpc.14683 [DOI] [PubMed] [Google Scholar]
- 44. Zhao W, Zhang D, Fakhoury M, et al. Population pharmacokinetics and dosing optimization of vancomycin in children with malignant hematological disease. Antimicrob Agents Chemother. 2014;58(6):3191‐3199. doi: 10.1128/aac.02564-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tang Z, Guan J, Li J, et al. Determination of vancomycin exposure target and individualised dosing recommendations for neonates: model‐informed precision dosing. Int J Antimicrob Agents. 2021;57(3):106300. doi: 10.1016/j.ijantimicag.2021.106300 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1:
Data Availability Statement
The data presented in this study are available from the corresponding authors (Dr Xiaomei Fan and Dr Wenzhou Li) upon reasonable request.


