Abstract
Type 1 diabetes (T1D) is a complex, chronic autoimmune disease that affects over 1.6 million people in the United States. It is now understood that T1D may be undetected for many years while the disease progresses quietly without producing symptoms. T1D can be identified through diabetes-related autoantibody screening and staged accordingly, enabling healthcare providers to identify high-risk individuals in the early stages of the disease and either provide a stage-specific intervention or offer clinical trial opportunities to preserve beta cell function and anticipate the onset of clinical T1D. Evidence-based clinical practice guidelines currently do not exist for routine diabetes-related autoantibody screening of individuals at risk of developing T1D or of the general population. The purpose of this article is to help clinicians acquire an understanding of the rationale and protocols recommended for identifying patients at risk of developing T1D and monitoring such patients for autoimmune markers and progression of disease from Stage 1 to Stage 3 (clinical disease).
Keywords: Type 1 Diabetes, autoantibodies, screening, immunotherapy
Plain Language Summary
Type 1 diabetes (T1D) is a life-long condition where the body’s immune system (which normally fights infection) mistakenly attacks cells in the pancreas that make insulin. Insulin allows one to use energy from food and controls blood sugar levels. Without early recognition and treatment, high blood sugar can cause serious symptoms and life-threatening complications, such as diabetic ketoacidosis (DKA). DKA happens when there is very low insulin, and if not spotted early, it can cause coma and death. T1D can occur at any age. The chance of getting T1D is higher if another family member has it. T1D progresses silently for months or years before symptoms appear such as increased thirst, frequent urination, and unintentional weight loss. Healthcare providers can now screen and identify people who are at early stages of T1D (without symptoms) with blood tests called autoantibodies. Early detection through screening allows people to 1) learn about the disease before symptoms start and insulin is needed, 2) potentially receive treatments that delay T1D progression, and 3) participate in research trials. By detecting T1D at early stages, people can connect with the right care team and develop the skills needed to manage later stage T1D. Early detection has been shown to prevent hospitalization and life-threatening conditions. Screening for T1D will help people maximize their opportunities to delay T1D onset while preparing for diabetes care.
Graphical Abstract
Introduction
The year 2021 marked the centennial celebration of insulin’s discovery, which is recognized as one of the greatest breakthroughs in diabetes treatment history.1 While there have been many advances in the field of type 1 diabetes (T1D) since that first discovery, screening and monitoring for T1D have been limited to research programs, primarily because of the lack of approved treatments for the prevention of T1D.2,3 With the advent of teplizumab, a US Food and Drug Administration (FDA)-approved treatment to delay the progression from Stage 2 to Stage 3 T1D, demand for screening may increase.4
Globally, the number of people living with T1D was estimated to be approximately 8.4 million in 2021.5 By 2040, the prevalence is expected to increase to 13.5–17.4 million (60–107% higher than in 2021).5 The prevalence and incidence vary greatly from country to country. There are gaps in reported incidence data worldwide, with only 45% of the countries reporting their own incidence data.6 The age-standardized incidence of T1D is highest in Northern European countries (including Finland and Sweden) and in a few countries in the Middle East and North Africa (including Kuwait and Algeria). India and the United States have the most estimated incidence cases of T1D.6 In the United States, over 1.8 million people (1.6 million adults as well as 244,000 children and adolescents <20 years of age) have T1D, with an approximated annual increase of 1.9% (although rates vary across regions) and greater increases in younger and minority populations.7–9
According to the Centers for Disease Control and Prevention, primary care visits accounted for 50.3% of all medical office visits in the US in 2019. Often the first point of contact for patients, primary care practitioners provide critical aspects of care, such as disease monitoring and secondary complication management. As such, general practitioners are uniquely positioned to contribute to the care of patients at risk of T1D by assisting in appropriate screening, monitoring, and education and suggesting strategies to prevent disease progression.
Extensive research has proven that T1D is a complex chronic autoimmune disease with both genetic and environmental influences.10,11 It is now known that T1D may remain undetected for some time as the disease progresses insidiously and without overt symptoms, leading to the eventual autoimmune-driven destruction of insulin-producing beta cells with resultant insulin deficiency.10,11 While the long-term complications of T1D pose a significant threat to both the health and quality of life of affected individuals, of more immediate concern is the increased risk of diabetic ketoacidosis (DKA), particularly among the large percentage of individuals unaware of their diabetes until DKA occurs.10,11
It is well-recognized that the detection and monitoring of early T1D significantly increase the number of individuals diagnosed before progressing to a DKA event.11–15 T1D can be identified through diabetes-related autoantibody screening and staged according to the number of diabetes-related autoantibodies, level of dysglycemia, and presence of symptoms. As discussed below, this enables primary healthcare providers (HCPs) in collaboration with specialists to identify high-risk individuals in the early stages of the disease and either provide a stage-specific intervention or offer clinical trial opportunities.11,16 However, evidence-based clinical practice guidelines do not currently exist for routine diabetes-related autoantibody screening of individuals at risk of developing T1D (eg, family members of those with clinical T1D) or of the general population.16
Stages of T1D
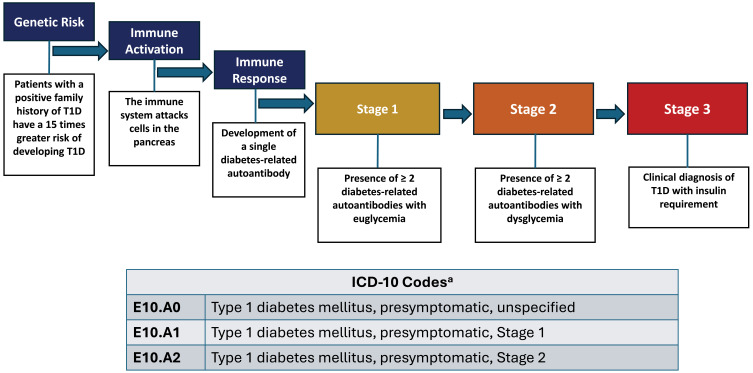
Islet autoimmunity is defined as the presence of serum antibodies (diabetes-related autoantibodies) against islet antigens. T1D can be subdivided into three distinct stages characterized by the number of diabetes-related autoantibodies present and the degree of dysglycemia (Figure 1).11,17,18
Figure 1.
Stages of Type 1 Diabetes Progression.16,17,19 Adapted with permission from https://www.trialnet.org/t1d-facts. aEffective October 1, 2024.
Abbreviations: ICD-10, International Classification of Diseases, Tenth Revision; T1D, type 1 diabetes.
In individuals with a genetic risk, the initial presence of diabetes-related autoantibodies peaks between 9 months and 2 years of age.20 Seroconversion from one to two of these diabetes-related autoantibodies is almost certainly prognostic of clinical (Stage 3) diabetes.10,11 Risk of progression from single to multiple diabetes-related autoantibodies depends on several factors, including age at first autoantibody detection, number of autoantibodies, autoantibody specificity, and autoantibody titer.21 The risk of progressing from Stage 1 (euglycemia and ≥2 diabetes-related autoantibodies) to Stage 3, which is clinical, symptomatic T1D, is 44% at 5 years, 70% at 10 years, and 84% at 15 years from the time of seroconversion, and lifetime risk approaches 100%.10 For individuals whose disease has progressed to Stage 2 (dysglycemia and ≥2 diabetes-related autoantibodies), the risk of developing Stage 3 T1D is 60% at 2 years and 75% at 4–5 years from the time of incident dysglycemia.10
Screening individuals for diabetes-related autoantibodies and monitoring blood glucose levels in those found to be positive provide clinicians with a valuable opportunity to stage the progression of T1D in its presymptomatic phases prior to clinical disease onset. Clinicians can then provide education, monitor for progression, and potentially offer disease-modifying interventions, such as teplizumab for Stage 2 T1D, or other investigational therapy options.10,11 Recently, the Centers for Medicare and Medicaid Services released its Medicare hospital rule to include the Centers for Disease Control’s new International Classification of Diseases, Tenth Revision (ICD-10) diagnosis codes to capture early-stage, presymptomatic T1D.19 The new ICD-10 codes, which will be effective October 1, 2024, include: E10.A0 (Type 1 diabetes mellitus, presymptomatic, unspecified), E10.A1 (Type 1 diabetes mellitus, presymptomatic, Stage 1), and E10.A2 (Type 1 diabetes mellitus, presymptomatic, Stage 2).
The purpose of this article is to help clinicians acquire an understanding of the rationale and protocols recommended for identifying patients at risk of developing T1D and monitoring such patients for autoimmune markers and disease progression from Stage 1 to Stage 3 (clinical disease).
Screening for Diabetes-Related Autoantibodies
Who and When to Screen
National and international consensus guidelines recognize the importance of identifying T1D before symptom onset.10,11 Current guidance suggests diabetes-related autoantibody screening for presymptomatic T1D can be considered in relatives of patients with T1D and offered to appropriate candidates in the setting of a research study.10,11,22,23 The lifetime risk of developing T1D is increased approximately 15-fold in family members of individuals with T1D compared with the general population;16 the prevalence of developing Stage 3 T1D by age 20 is significantly higher (~5%) in those with a first-degree relative with diabetes than in the general population (~0.3%).24 Because of the heightened risk in individuals with a family history, screening programs and clinical trials have historically focused on this group.16 However, it is important to recognize that 80–90% of the individuals diagnosed with Stage 3 T1D do not have a family history of the disease16,25 and thus identify the need to ultimately move towards general population screening.
Although the optimal ages for screening in the general population have not been conclusively determined, recent consensus guidelines recommend screening at ages 2 and 6 for optimal predictive value for the development of clinical T1D by the age of 15 years in public health settings.26 Admittedly, this approach misses a small subset of younger infants and toddlers who rapidly progress with T1D in the first 2 years of life. These individuals have the highest rates of DKA and greatest risk of its associated co-morbidities.27,28 This screening window also does not include adolescents (ages 10–18 years) who may be at risk of developing T1D. A recent study determined that single screening adolescents with an increased risk of developing T1D for diabetes-related autoantibodies was effective, as the positive predictive value for detecting clinical diabetes by the age of 18 was 66% (95% CI, 60–72%).29 Therefore, one can conclude that if the suggested initial screening window of 2 to 6 years is missed, any time thereafter is a good time to initially screen children who providers feel are appropriate candidates.
T1D is not solely a childhood disease, as more than half of all new T1D cases occur in adults.30 A longitudinal study demonstrated that of the 64,000 individuals diagnosed annually with T1D in the US, 58% (n=37,000) are 20–64 years old.9 Because not all adults require insulin at diagnosis, new-onset T1D may be misdiagnosed in approximately 40% of the adults; this risk of misdiagnosis increases with age.30 To preserve endogenous insulin production for as long as possible and enable individuals to access appropriate modern therapies including immunotherapy, proper diagnosis and staging must be accomplished promptly. Consequently, adults should be screened whenever an indication for testing or heightened risk is present, including family history of T1D and personal or family history of another autoimmune disease, such as autoimmune thyroiditis, Addison’s disease, celiac disease, autoimmune gastritis, and pernicious anemia.14,31
Screening Options
Measurement of diabetes-related autoantibodies is available and accessible to individuals through a HCP order at commercial laboratories and as part of regional and national research studies (eg, TrialNet, ASK, CASCADE, PLEDGE). Through these channels, different assays for measuring diabetes-related autoantibodies are used, including radiobinding assays (RBA), enzyme-linked immunosorbent assays (ELISA), electrochemiluminescence (ECL), and antibody detection by agglutination-polymerase chain reaction (ADAP).16 Many of these assays (along with several in development) are evolving, and future improvements may refine the ability to identify and predict the progression of autoimmune T1D. These methodologies are not comparable—results may require confirmation and interpretation by a specialist in early T1D. Providers are encouraged to determine which tests are available to order in their institution/area. As shown in Table 1, age and eligibility criteria can vary across clinical research studies and commercial laboratories.
Table 1.
| Location | Ages Screened | Screening Material | Requires Order from Clinician | |
| Research-Based Screening Programs | ||||
| ASK | Hospital specialty clinics and in-home test kits | All children/adolescents 1 to <18 years of age | Serum or capillary sample | No |
| CASCADE | Newborn screens and elementary schools in Washington State | All newborns to 8 months OR children 4 to 8 years old | Serum | No |
| PLEDGE |
Sanford Health System clinics and labs in North Dakota, South Dakota, Minnesota, Iowa, and Nebraska (Sanford Health patients only) |
Newborns to <6 years of age or ages 9 to <17 years Children ages 6 to 17 who have a sibling with T1D or T1D antibodies may also join the study |
Serum or capillary sample | No |
| TrialNetb | TrialNet centers and affiliates in the US or in-home test kits |
2.5 to 45 years of age and first-degree relative with T1D or 2.5 to 20 years of age and second-degree relative with T1D OR 2.5 to 45 years of age and tested positive for at least one T1D-related autoantibody outside TrialNet |
Serum or capillary sample | No |
|
Clinical Laboratorya Most commercial laboratories will test for glutamic acid decarboxylase (GAD)-65 antibody, IA-2 antibody, insulin autoantibody, and zinc transporter 8 antibody | ||||
| LabCorp | US | All ages | Serum | Yes |
| Mayo Laboratories | US | All ages | Serum | Yes |
| Quest | US | All ages | Serum | Yes |
| Enable Biosciences | US in participating clinics | >1 year of age | Capillary | No |
Notes: aAvailability of screening programs, laboratories, and associated tests as of March 2024; details are likely to change as the criteria are constantly being re-evaluated and updated. Many individuals will not fit the criteria for screening used by research programs. Criteria used by research programs for screening are not necessarily recommended criteria that practitioners should use in clinical practice. bAt the time of publication, TrialNet was actively re-evaluating screening criteria and may potentially broaden age groups and screening regimens.
Abbreviation: T1D, type 1 diabetes.
Screening Results and Next Steps
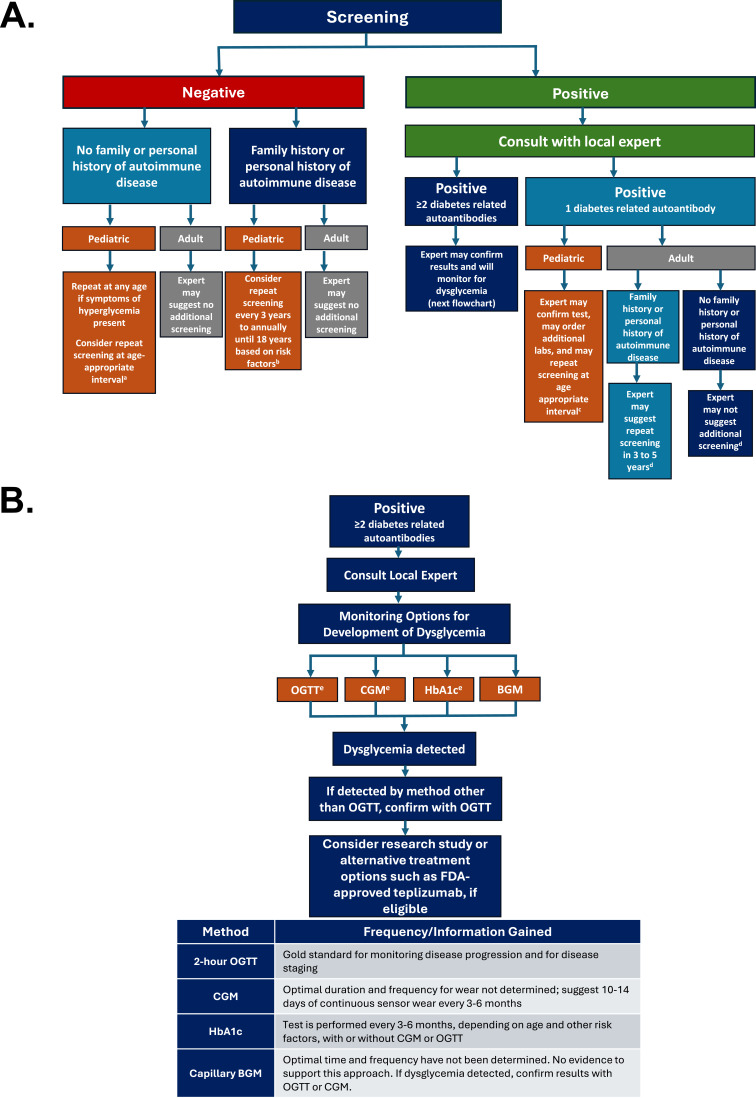
Figure 2A presents a pathway for screening that clinicians may want to consider.
Figure 2.
(A) Pathways for Screening, Monitoring and Treatment.11,14,18,21,29,31–36 (B) Glycemic Monitoring and Treatment Options.4,11,18,37–39
Notes: (A) aPediatric patients initially screened between the ages of 2 to 6 years may benefit from additional screening at 10 years of age. bRisk factors include the age of the patient at the time of initial screening, family history, and the presence of autoimmunity. Identical twins are at higher risk of development of T1D than fraternal twins and may require more frequent monitoring. cExperts may recommend repeat screening in 6 months to 2 years, depending on the age of the patient at the time of initial screening, family history, and presence of autoimmunity. A twin (fraternal or identical) with one positive antibody should be screened annually due to increased risk with the presence of diabetes autoantibodies. dAt this time, rescreening is not recommended for adults with 1 positive autoantibody and no family history of disease; however, there are limited data, and guidance is currently lacking. Experts recommend that adults with one positive autoantibody and a positive family history of autoimmunity repeat screening every 3 to 5 years. (B) eBecause individuals can stay at Stage 1 for decades, HbA1c testing every 3–6 months, depending on age and other risk factors, with or without use of CGM or OGTT is reasonable for glycemic monitoring.36,38 However, individuals at Stage 2 (dysglycemia) should be monitored every 3 months with HbA1c and intermittent CGM.
Abbreviations: BGM, blood glucose monitoring; CGM, continuous glucose monitoring; HbA1c, glycated hemoglobin; OGTT, oral glucose tolerance test.
Negative Test Result
A negative test result indicates limited immediate risk of progression to T1D, regardless of which method/program was initially used to screen an individual. However, rescreening for diabetes-related autoantibodies in individuals with a family history of T1D (or other potential risk factors, including family/personal history of another autoimmune diseases) is advised because diabetes-related autoantibodies can develop later in life, especially when the initial screening is done as a young child.29 If pediatric patients without family history of T1D or a history of other autoimmune diseases are initially screened between the ages of 2 and 6 years and test negative, it is reasonable to repeat screening at 10 years to capture a potential diagnosis within the peripubertal years.11,29,32 Rescreening should be performed at any time if symptoms of hyperglycemia develop. Practitioners should consider patient age (eg, younger with higher risk) and family and/or personal history of autoimmune conditions when assessing risk of developing T1D. A heightened concern may be present for individuals with a first- or second-degree relative with T1D, those with a family history of autoimmune diseases (eg, celiac, autoimmune thyroid disease, Addison’s, autoimmune gastritis, pernicious anemia), or those with a high genetic risk of developing T1D because of the presence of specific HLA genes identified by other testing.14,23,31–33 While data are unclear, diabetes-related autoantibody rescreening may be recommended from every 3 years to annually up to the age of 18 years for these pediatric patients because of their higher risk compared to the general population. Although the data vary, identical twins have a higher risk of progression of diabetes-related autoantibodies and T1D after one twin is diagnosed, with rates ranging from 30–70%; in fraternal twins, the rate is believed to be comparable to that in non-twin siblings (risk of 6–10% for T1D).34 Due to their increased risk, management of an identical twin with a positive family history should be discussed with a local expert. Currently, adults with no family history or history of autoimmune conditions along with a negative screening result do not warrant further testing or repeat screening.
Positive Test Result
If a positive screening result is obtained, further consultation with an expert in early T1D should be considered. The expert may then recommend confirmation testing and/or additional labs based on the patient’s age, antibody results, and method/program utilized. Pediatric patients who test positive for one diabetes-related autoantibody may be rescreened in 6 months to 2 years, depending on the age of the patient at the time of initial screening, family and/or personal history of T1D, and presence of other autoimmune diseases. Families should be aware that although less likely, a child may develop T1D with only one positive diabetes-related antibody.35 The risk of T1D at 3 years is high for identical twins who initially have single or multiple diabetes-related autoantibodies and for fraternal twins with multiple positive diabetes-related autoantibodies. As such, a twin (fraternal or identical) with one positive diabetes-related antibody should be screened at least annually.
Currently, it is reasonable to consider rescreening adults (>18 years) with familial risk of T1D who test positive for one diabetes-related autoantibody every 3 to 5 years. However, there are currently insufficient data to recommend repeat testing for adults without familial risk of T1D who test positive for one diabetes-related autoantibody. Research suggests that the risk of progressing to multiple diabetes-related antibodies varies according to age and the type of diabetes-related autoantibodies present; adults appear to be at a lower risk of progressing to multiple diabetes-related autoantibodies than children.21,39 As this field is rapidly evolving with increased research, recommendations are likely to change, and expert opinion may vary.
Monitoring for Dysglycemia and Treatment Options
To preserve endogenous insulin production for as long as possible and potentially prevent detrimental long-term consequences, T1D must be appropriately staged; each stage differs according to glycemic status, and treatment or research opportunities may be available. Consensus guidelines recommend offering glycemic testing and ongoing monitoring to individuals who test positive for ≥2 diabetes-related autoantibodies.37 However, family wishes, personal preferences, and available resources should be key considerations in determining the intensity of these efforts. Once 2 or more diabetes-related autoantibodies are detected, further consultation with an expert in early T1D may be warranted. Figure 2B highlights the various modalities for glycemic monitoring and the potential treatment options an expert may recommend.
Glycemic testing can be performed in several ways with varying levels of time and clinical commitment, including continuous glucose monitoring (CGM), oral glucose tolerance test (OGTT), and glycosylated hemoglobin (HbA1c) testing. The 2-hour OGTT is widely accepted as the gold standard for disease staging in patients with multiple diabetes-related autoantibodies and should be used for glycemic monitoring when practical and available.11,37 Compared to the OGTT, HbA1c testing may be more acceptable to the patient and advantageous, as fasting is not required and there are fewer daily fluctuations when patients might be experiencing illness, excess stress, or changes in nutrition.11 However, the HbA1c test is generally considered insensitive in new-onset Stage 3 T1D.11,37 HbA1c testing may also fail to detect dysglycemia in young children, who often progress rapidly.37 Emerging evidence supports the use of continuous glucose monitoring (CGM) to detect glycemic abnormalities in individuals with Stage 2 T1D, as it may accurately predict stage progression.38 Although the use of self-blood glucose monitoring for detecting impaired fasting glucose or impaired glucose tolerance in conjunction with other methods may be considered, there is no evidence to support this approach.37
Because individuals can remain in Stage 1 (normoglycemia) for decades, HbA1c testing every 3–6 months, depending on age and other risk factors, with or without use of CGM or OGTT is reasonable.18,36,38 However, individuals in Stage 2 (dysglycemia) should be monitored every 3 months with HbA1c and intermittent CGM.36,38
Considerations for Treatment
If dysglycemia and autoimmunity (≥2 diabetes-related autoantibodies) are confirmed, experts in T1D may provide patients/caregivers with options including research or therapy for potentially delaying the onset of Stage 3 with teplizumab.18 Teplizumab is an FDA-approved therapy indicated to delay the onset of Stage 3 T1D in adult and pediatric patients aged 8 years and older with Stage 2 T1D.4 As this field continues to evolve, the current treatment landscape is likely to change.
HCPs and patients/caregivers must weigh several factors when determining whether teplizumab therapy should be considered. Teplizumab treatment is given intravenously and requires a 14-day course of daily infusions, which may not be economically or logistically feasible for many families. Other factors to consider include personal/professional tolerance for the potential side effects of therapy and the burden of navigating insurance coverage.
Counseling and Education for Patients/Caregivers
Early detection of T1D creates the need for HCPs to provide follow-up education and counseling to individuals and their families to address stress, anxiety, and other psychological issues during each phase of screening, monitoring, and potential treatment. Research has shown that when families with children diagnosed with screening in the early presymptomatic stages of T1D (Stage 1 or 2) are provided education, quality of life is improved and parental stress is lowered at diagnosis of clinical or Stage 3 T1D.40
Post-Screening Counseling
Table 2 presents useful discussion points that may help address individual/caregiver concerns when screening and confirmatory tests indicate the presence of diabetes-related autoantibodies. Imminent counseling and support are important given the psychological impact on families with children who test positive for diabetes-related autoantibodies.12,40–42 When counseling patients and their families who have received a confirmed positive test for ≥2 diabetes-related autoantibodies, it is important to be culturally sensitive and to ensure that common misperceptions regarding potential causes of T1D (eg, eating sweets, emotional stress, something a parent did or did not do) are clarified. Moreover, when discussing the initiation of new medications (eg, insulin), clinicians should be sensitive to the often large racial/ethnic disparities in an individual’s or a caregiver’s willingness to follow such advice, frequently due to different cultural beliefs, limited financial resources, and/or mistrust of one’s HCPs. Recent studies reported that many minorities (especially Black participants) had a lower rate of diabetes medication initiation and CGM and insulin delivery device use.43–46 Much work remains to ensure all patients, despite any socioeconomic or racial/ethnic disparities, are given the opportunity to be screened and monitored for T1D. Given such disparities, it would be beneficial for HCPs to continue to advocate for these patients and encourage appropriate candidates to enroll in research-based screening programs (Table 1).
Table 2.
Counseling for Positive Test Result
| Value of early detection |
|
| Benefit of monitoring for T1D progression |
|
Abbreviations: DKA, diabetic ketoacidosis; T1D, type 1 diabetes.
Although having a negative diabetes-related autoantibody screen is reassuring, informing patients and their families about the need for periodic repeat screening may cause anxiety. This challenge highlights the importance of adequate counseling on negative screening results and the value in teaching patients to recognize any future development of symptoms or dysglycemia. Consistent communication with patients in Stage 1 and Stage 2 is essential for preventing DKA; the benefit of monitoring is significantly lower if adequate follow-up is not maintained.47 Box 1 presents discussion points that may help address individual/caregiver concerns when the screening/confirmatory test is negative.
Box 1.
Key Points to Communicate When Counseling About Negative Test Result
|
|
|
|
Abbreviation: HCP, healthcare provider.
Education on Staging
After receiving confirmation of Stage 1 or 2 T1D, patients/caregivers will need diabetes education and adequate support. Referral to a specialized diabetes center is recommended. HCPs should explain the probabilities of progressing to Stage 3 based on the number of diabetes-related antibodies present and glycemic status to help patients/caregivers understand and accept the risk of progressing to clinical T1D. HCPs should discuss the rationale for glucose monitoring, make patients/caregivers aware of the symptoms that may indicate emergent hyperglycemia, and provide a plan to avoid potential DKA. Providers should also collaborate with patients/caregivers to develop a strategy for monitoring disease progression, including review of the various options for monitoring blood glucose to assist in determining the method that best meets their individual needs and circumstances (eg, insurance coverage, logistical issues). The plan should also include clear guidance for clinical follow-up and access to ongoing counseling as needed.
A Call to Action: The Need for Universal Screening Guidelines and Best Practices
The development of evidence-based screening guidelines is of paramount importance, given that early diagnosis of T1D has the potential to prevent serious morbidity and mortality. Now that there is an approved immunotherapy for treatment, T1D meets the World Health Organization (WHO) criteria for establishing a universal screening program.48 There is a need for more research and guidelines that provide refined recommendations on frequency of testing based on age/risk, how results are communicated to patients/caregivers, blood glucose monitoring recommendations if indicated, and pre- and post-teplizumab treatment guidance for those who receive treatment. Guidelines should be concordant among the various medical groups and societies to provide clear and consistent recommendations to clinicians and education to patients. T1D screening may be less cost effective in less-resourced countries with a lower T1D incidence; therefore, these countries may focus on providing correct etiological diagnosis and increasing access to and the quality of clinical care for Stage 3 T1D.37 One recent survey of HCPs involved in diabetes care from 76 countries indicated higher support for screening in low-income countries, possibly due to the increased occurrence of DKA and the mortality associated with undiagnosed T1D.49
Acknowledgment
The authors are grateful to Jill Weissberg-Benchell, PhD, CDCES, for contributions to the development of the manuscript. Medical writing and editorial assistance were provided by Cynthia D. Gioiello, PharmD, and Anthony DiLauro PhD, ELS, of PharmaWrite Scientific Information Services, LLC (funded by Sanofi), and prior medical writing support was provided by Christopher G. Parkin, MS, of CGParkin Communications, Inc., and was funded by Provention Bio, Inc., a Sanofi Company. The authors wish to thank Charlotte Singh, MD, CMPPTM (Sanofi) for coordinating the development, facilitating author discussions, and providing a critical review of this manuscript, and Jacquelyn Brown, PhD (formerly of Provention Bio, Inc., a Sanofi Company) for coordinating the development of this manuscript. This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company-Sponsored Medical Research: GPP 2022 update”.
Summary
Ongoing research programs continue to expand our understanding of T1D and its progression. With the FDA approval of a new medication to delay the progression of Stage 2 to Stage 3 T1D, practical guidelines for screening and monitoring should be developed. The transition from clinical research protocols to community-based care will require further research to support the development and acceptance of clinical practice guidelines. General practitioners are often the first line of defense for patients, and as such, they are in an optimal position to counsel patients and family members on the risks of T1D and the benefits of early screening in conjunction with local experts. HCPs should continue to advocate for equal opportunities for screening and monitoring of patients with racial/ethnic or socioeconomic disparities; patients should be encouraged to enroll in research-based programs whenever possible and appropriate. Although ongoing research will continue to broaden our understanding of the root cause(s) and possible interventions to delay and potentially cure T1D, it is imperative that the healthcare community informs patients about the benefits of diagnosing T1D early. Early T1D detection (prior to symptom onset and need for exogenous insulin) is essential to preserve beta cell function, prepare for disease progression, and apply evidence-based guidance for the monitoring and management of individuals at risk of developing T1D.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
DM is a consultant for Provention Bio. NL is a consultant for Sanofi. WP reports research support from Abbot and Dexcom; is a consultant for Bigfoot, Eli Lilly, Embecta, Mankind, Novo Nordisk, Provention Bio and Sanofi. HR reports research support from Dexcom, Eli Lilly, MannKind, Medtronic, Novo Nordisk, and Provention Bio; serves on an advisory board for Provention Bio; serves on a Data and Safety Monitoring Board for Merck and Provention Bio; participated in Speaker’s Bureau for Provention Bio. He also reports personal fees from Sanofi for Disease State Presentations DSMB and TrialNet & EDIC funding from NIH, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Powers AC. Type 1 diabetes mellitus: much progress, many opportunities. J Clin Invest. 2021;131(8):e142242. doi: 10.1172/JCI142242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenbaum CJ. A key to T1D prevention: screening and monitoring relatives as part of clinical care. Diabetes. 2021;70(5):1029–1037. doi: 10.2337/db20-1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiang JL, Maahs DM, Garvey KC, et al. Type 1 diabetes in children and adolescents: a position statement by the American Diabetes Association. Diabetes Care. 2018;41(9):2026–2044. doi: 10.2337/dci18-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James MM. TZIELD Injection, for Intravenous Use [Prescribing Information]. Red Bank, NJ: Provention Bio, Inc.; 2022. [Google Scholar]
- 5.Gregory GA, Robinson TIG, Linklater SE, et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. 2022;10(10):741–760. doi: 10.1016/S2213-8587(22)00218-2 [DOI] [PubMed] [Google Scholar]
- 6.Ogle GD, James S, Dabelea D, et al. Global estimates of incidence of type 1 diabetes in children and adolescents: results from the International Diabetes Federation Atlas, 10th edition. Diabet Res Clin Pract. 2022;183:109083. doi: 10.1016/j.diabres.2021.109083 [DOI] [PubMed] [Google Scholar]
- 7.Divers J, Mayer-Davis EJ, Lawrence JM, et al. Trends in incidence of type 1 and type 2 diabetes among youths - selected counties and Indian reservations, United States, 2002–2015. MMWR Morb Mortal Wkly Rep. 2020;69(6):161–165. doi: 10.15585/mmwr.mm6906a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States; 2020. Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed July 26, 2023.
- 9.Rogers MAM, Kim C, Banerjee T, Lee JM. Fluctuations in the incidence of type 1 diabetes in the United States from 2001 to 2015: a longitudinal study. BMC Med. 2017;15(1):199. doi: 10.1186/s12916-017-0958-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964–1974. doi: 10.2337/dc15-1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ElSayed NA, Aleppo G, Bannuru RR; American Diabetes Association Professional Practice Committee. 2. Diagnosis and classification of diabetes: standards of care in diabetes—2024. Diabetes Care. 2024;47(suppl 1):S20–S42. doi: 10.2337/dc24-S002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziegler AG, Kick K, Bonifacio E, et al. Yield of a public health screening of children for islet autoantibodies in Bavaria, Germany. JAMA. 2020;323(4):339–351. doi: 10.1001/jama.2019.21565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elding Larsson H, Vehik K, Bell R, et al. Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care. 2011;34(11):2347–2352. doi: 10.2337/dc11-1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker JM, Goehrig SH, Barriga K, et al. Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care. 2004;27(6):1399–1404. doi: 10.2337/diacare.27.6.1399 [DOI] [PubMed] [Google Scholar]
- 15.Winkler C, Schober E, Ziegler AG, Holl RW. Markedly reduced rate of diabetic ketoacidosis at onset of type 1 diabetes in relatives screened for islet autoantibodies. Pediatr Diabetes. 2012;13(4):308–313. doi: 10.1111/j.1399-5448.2011.00829.x [DOI] [PubMed] [Google Scholar]
- 16.Sims EK, Besser REJ, Dayan C, et al. Screening for type 1 diabetes in the general population: a status report and perspective. Diabetes. 2022;71(4):610–623. doi: 10.2337/dbi20-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Type 1 Diabetes TrialNet. T1D disease progression; 2017. Available from: https://beyondtype1.org/wp-content/uploads/2017/01/FINAL-TrialNet-Stages-of-Diabetes-graph-1.jpg. Accessed October 10, 2023.
- 18.ElSayed NA, Aleppo G, Bannuru RR; American Diabetes Association Professional Practice Committee. 3. Prevention or delay of diabetes and associated comorbidities: standards of care in diabetes—2024. Diabetes Care. 2024;47(suppl 1):S43–S51. doi: 10.2337/dc24-S003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Medicare and Medicaid Services. ICD-10-CM/PCS medicare severity diagnosis related group v42.0 definitions manual. Available from: https://www.cms.gov/icd10m/FY2025-NPRM-Version42-fullcode-cms/fullcode_cms/P0001.html. Accessed May 3, 2024.
- 20.Ziegler AG, Bonifacio E. Age-related islet autoantibody incidence in offspring of patients with type 1 diabetes. Diabetologia. 2012;55(7):1937–1943. doi: 10.1007/s00125-012-2472-x [DOI] [PubMed] [Google Scholar]
- 21.Bosi E, Boulware DC, Becker DJ, et al. Impact of age and antibody type on progression from single to multiple autoantibodies in type 1 diabetes relatives. J Clin Endocrinol Metab. 2017;102(8):2881–2886. doi: 10.1210/jc.2017-00569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ask the experts. T1D information for providers; 2023. Available from: https://www.askhealth.org/experts-for-providers. Accessed August 3, 2023.
- 23.American Diabetes Association Professional Practice Committee. 14. Children and adolescents: standards of care in diabetes—2024. Diabetes Care. 2024;47(suppl 1):S258–S281. doi: 10.2337/dc24-S014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang JL, Kirkman MS, Laffel LM, Peters AL. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37(7):2034–2054. doi: 10.2337/dc14-1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkkola A, Härkönen T, Ryhänen SJ, Ilonen J, Knip M. Extended family history of autoimmune diseases and phenotype and genotype of children with newly diagnosed type 1 diabetes. Eur J Endocrinol. 2013;169(2):171–178. doi: 10.1530/EJE-13-0089 [DOI] [PubMed] [Google Scholar]
- 26.Ghalwash M, Dunne JL, Lundgren M, et al. Two-age islet-autoantibody screening for childhood type 1 diabetes: a prospective cohort study. Lancet Diabetes Endocrinol. 2022;10(8):589–596. doi: 10.1016/S2213-8587(22)00141-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabbone I, Maltoni G, Tinti D, et al. Diabetic ketoacidosis at the onset of disease during a national awareness campaign: a 2-year observational study in children aged 0–18 years. Arch Dis Child. 2020;105(4):363–366. doi: 10.1136/archdischild-2019-316903 [DOI] [PubMed] [Google Scholar]
- 28.Kao KT, Islam N, Fox DA, Amed S. Incidence trends of diabetic ketoacidosis in children and adolescents with type 1 diabetes in British Columbia, Canada. J Pediatr. 2020;221:165–173.e2. doi: 10.1016/j.jpeds.2020.02.069 [DOI] [PubMed] [Google Scholar]
- 29.Ghalwash M, Anand V, Lou O, et al. Islet autoantibody screening in at-risk adolescents to predict type 1 diabetes until young adulthood: a prospective cohort study. Lancet Child Adolesc Health. 2023;7(4):261–268. doi: 10.1016/S2352-4642(22)00350-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leslie RD, Evans-Molina C, Freund-Brown J, et al. Adult-onset type 1 diabetes: current understanding and challenges. Diabetes Care. 2021;44(11):2449–2456. doi: 10.2337/dc21-0770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Block CE, De Leeuw IH, Van Gaal LF. Autoimmune gastritis in type 1 diabetes: a clinically oriented review. J Clin Endocrinol Metab. 2008;93(2):363–371. doi: 10.1210/jc.2007-2134 [DOI] [PubMed] [Google Scholar]
- 32.Barker JM. Clinical review: type 1 diabetes-associated autoimmunity: natural history, genetic associations, and screening. J Clin Endocrinol Metab. 2006;91(4):1210–1217. doi: 10.1210/jc.2005-1679 [DOI] [PubMed] [Google Scholar]
- 33.Cerolsaletti K, Hao W, Greenbaum CJ. Genetics coming of age in type 1 diabetes. Diabetes Care. 2019;42(2):189–191. doi: 10.2337/dci18-0039 [DOI] [PubMed] [Google Scholar]
- 34.Triolo TM, Fouts A, Pyle L, Yu L, Gottlieb PA, Steck AK. Identical and nonidentical twins: risk and factors involved in development of islet autoimmunity and type 1 diabetes. Diabetes Care. 2019;42(2):192–199. doi: 10.2337/dc18-0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309(23):2473–2479. doi: 10.1001/jama.2013.6285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steck AK, Dong F, Taki I, et al. Continuous glucose monitoring predicts progression to diabetes in autoantibody positive children. J Clin Endocrinol Metab. 2019;104(8):3337–3344. doi: 10.1210/jc.2018-02196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Besser REJ, Bell KJ, Couper JJ, et al. ISPAD Clinical Practice Consensus Guidelines 2022: stages of type 1 diabetes in children and adolescents. Pediatr Diabetes. 2022;23(8):1175–1187. doi: 10.1111/pedi.13410 [DOI] [PubMed] [Google Scholar]
- 38.Steck AK, Dong F, Geno Rasmussen C, et al. CGM metrics predict imminent progression to type 1 diabetes: Autoimmunity Screening for Kids (ASK) study. Diabetes Care. 2022;45(2):365–371. doi: 10.2337/dc21-0602 [DOI] [PubMed] [Google Scholar]
- 39.Jacobsen LM, Bocchino L, Evans-Molina C, et al. The risk of progression to type 1 diabetes is highly variable in individuals with multiple autoantibodies following screening. Diabetologia. 2020;63(3):588–596. doi: 10.1007/s00125-019-05047-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith LB, Liu X, Johnson SB, et al. Family adjustment to diabetes diagnosis in children: can participation in a study on type 1 diabetes genetic risk be helpful? Pediatr Diabetes. 2018;19(5):1025–1033. doi: 10.1111/pedi.12674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson SB. Psychological impact of screening and prediction in type 1 diabetes. Curr Diab Rep. 2011;11(5):454–459. doi: 10.1007/s11892-011-0208-9 [DOI] [PubMed] [Google Scholar]
- 42.Johnson SB, Lynch KF, Roth R, Schatz D. My child is islet autoantibody positive: impact on parental anxiety. Diabetes Care. 2017;40(9):1167–1172. doi: 10.2337/dc17-0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fantasia KL, Wirunsawanya K, Lee C, Rizo I. Racial disparities in diabetes technology use and outcomes in type 1 diabetes in a safety-net hospital. J Diabetes Sci Technol. 2021;15(5):1010–1017. doi: 10.1177/1932296821995810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agarwal S, Schechter C, Gonzalez J, Long JA. Racial-ethnic disparities in diabetes technology use among young adults with type 1 diabetes. Diabetes Technol Ther. 2021;23(4):306–313. doi: 10.1089/dia.2020.0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai CW, Lipman TH, Willi SM, Hawkes CP. Racial and ethnic disparities in rates of continuous glucose monitor initiation and continued use in children with type 1 diabetes. Diabetes Care. 2021;44(1):255–257. doi: 10.2337/dc20-1663 [DOI] [PubMed] [Google Scholar]
- 46.Willi SM, Miller KM, DiMeglio LA, et al. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135(3):424–434. doi: 10.1542/peds.2014-1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacobsen LM, Vehik K, Veijola R, et al. Heterogeneity of DKA incidence and age-specific clinical characteristics in children diagnosed with type 1 diabetes in the TEDDY study. Diabetes Care. 2022;45(3):624–633. doi: 10.2337/dc21-0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization. Screening: when is it appropriate and how can we get it right?; 2020. Available from: https://apps.who.int/iris/handle/10665/330810. Accessed July 31, 2023.
- 49.Neuman V, Piona C, Cudizio L, et al. Are we ready to screen for type 1 diabetes? A structured worldwide survey among healthcare providers involved in paediatric diabetes care. Diabet Med. 2024;41(6):e15329. doi: 10.1111/dme.15329 [DOI] [PubMed] [Google Scholar]