Summary
Background
Chronic kidney disease (CKD) is a global concern that presents significant challenges for disease management. Several factors drive CKD prevalence, including primary risk factors, such as type 2 diabetes and hypertension, and an ageing population. Inside CKD is an international initiative that aims to raise awareness of the substantial burden incurred by CKD.
Methods
Using a peer-reviewed microsimulation method, the clinical burden of CKD was estimated from 2022 to 2027. Demographic data from the Americas, Europe, and Asia–Pacific/Middle East were used to generate virtual populations and to project the prevalence of CKD, kidney replacement therapy, associated cardiovascular complications, comorbid conditions, and all-cause mortality in the CKD population over the modelled time frame.
Findings
Across the 31 participating countries/regions, the total prevalence of CKD was projected to rise to 436.6 million cases by 2027 (an increase of 5.8% from 2022), with most cases (∼80%) undiagnosed. Inside CKD projected a mean of 8859 cases of heart failure, 10,244 of myocardial infarction, and 7797 of stroke per 100,000 patients with CKD by 2027.
Interpretation
The clinical impact of CKD is substantial and likely to increase; the high prevalence of undiagnosed cases and associated complications may benefit from the implementation of health policy interventions that promote screening, earlier diagnosis, and interventions to improve outcomes.
Funding
AstraZeneca.
Keywords: Burden of disease, Chronic kidney disease, Epidemiology, Microsimulation, Policy
Research in context.
Evidence before this study
A pragmatic literature review was independently performed by local agencies in 31 countries/regions. Local language search terms and exact databases were not recorded, but data were retrieved from available national registries, published literature, and healthcare databases at country and regional levels, which were then assessed by a Scientific Steering Committee. Data were gathered on the prevalence and/or incidence of chronic kidney disease (CKD); the rate of diagnosis; kidney replacement therapy (KRT)—haemodialysis, peritoneal dialysis, and kidney transplantation initiation and maintenance; disease-associated comorbidities of hypertension and type 2 diabetes; cardiovascular complications of heart failure, myocardial infarction, and stroke. Previous studies demonstrate that in addition to complications related to CKD itself and the development of kidney failure, CKD is associated with a substantial risk of cardiovascular complications. CKD has a high prevalence that affects ∼10–15% of the global population and its impact on healthcare are well known, yet a culture of inertia and under-diagnosis undermine effective interventions. There is a lack of available epidemiological data, which undermines the development of sustainable, effective healthcare strategies to improve patient outcomes, which in turn limits the ability of clinicians and policy makers aiming to reduce the burden of disease.
Added value of this study
The Inside CKD programme produced a simulation across 31 countries/regions, which modelled a virtual population, predicting an estimated 436.6 million cases across the full data set by 2027. This was associated with a projected substantial clinical burden, as evidenced by the cumulative incidence of cardiovascular complications, comorbidities, KRT, and all-cause mortality within the CKD population. The plethora of variables modelled and the global perspective are currently unprecedented in CKD epidemiological research and offer scope for healthcare policy makers to explore projected data specific to their locality.
Implications of all the available evidence
Inside CKD provides country-specific projections of clinical burden, with the aim of raising awareness of CKD and informing evidence-based policy making. The microsimulation can be programmed to examine the utility and potential impact of various interventions, such as systematic CKD screening programmes.
Introduction
Chronic kidney disease (CKD) is a debilitating condition that affects 10–15% of the global adult population.1 CKD is usually diagnosed using two critical observations, albuminuria (>30 mg/g creatinine) and impaired kidney function, as defined by a glomerular filtration rate (GFR) < 60 mL/min/1.73 m2. CKD is further classified by the level of albuminuria (stages A1–3), and the reduction of GFR (stages G3a–5).2 As kidney function declines with age, older patients may be vulnerable to developing CKD,3 with type 2 diabetes, obesity,4,5 and hypertension6 also acting as risk factors. Once diagnosed, CKD progression is associated with adverse cardiovascular events, including stroke,7 myocardial infarction,8 and heart failure9; these conditions may also accelerate the onset of kidney failure. In patients with CKD, comorbid conditions, complications, and major health events all contribute to a lower health-related quality of life, and a higher risk of premature death.10,11
Given the current trajectory of risk factors, CKD is predicted to become an even more common cause of death by 2040 than it is now12 with low rates of diagnosis and a general lack of awareness exacerbating the implications of the condition.13,14 Despite a clear need for proactive disease management, there is a paucity of data regarding disease burden that limits optimisation of management strategies. Complementary to real-world data studies, microsimulations create virtual, heterogeneous populations through which clinical outcomes can be modelled. By using a broad range of real-world inputs (including hospital records and national statistics), the course of a disease and associated patient outcomes can be projected.15 A particular strength of microsimulation modelling is that the granularity of inputs enables the full complexity of a disease to be considered. Ideally, microsimulations should incorporate multiple inputs with a high level of granularity, and model interventions that can be explored in virtual scenarios.
Inside CKD uses a microsimulation methodology to quantify the projected global impact of CKD from 2022 to 2027. Here, we outline changes in the prevalence and distribution of disease stages and kidney replacement therapy (KRT) modalities, assessing the clinical burden for 31 countries/regions in the Americas, Europe, and Asia–Pacific/Middle East.
Methods
Study design
Inside CKD uses input variables from a range of demographic, epidemiological, and clinical data sets to assess the impact of various health states on disease progression and to project the clinical burden of CKD. A detailed methodology, including sensitivity analyses and validation, has been previously published and the microsimulation has not changed in structure since this description.16 The economic burden of CKD, derived from the same microsimulation, is described by Chadban and colleagues in this volume.17
This study was conducted in accordance with ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines. This study did not require informed consent or institutional/ethical review board approval because this is a non-interventional study based on secondary data use.
Overview
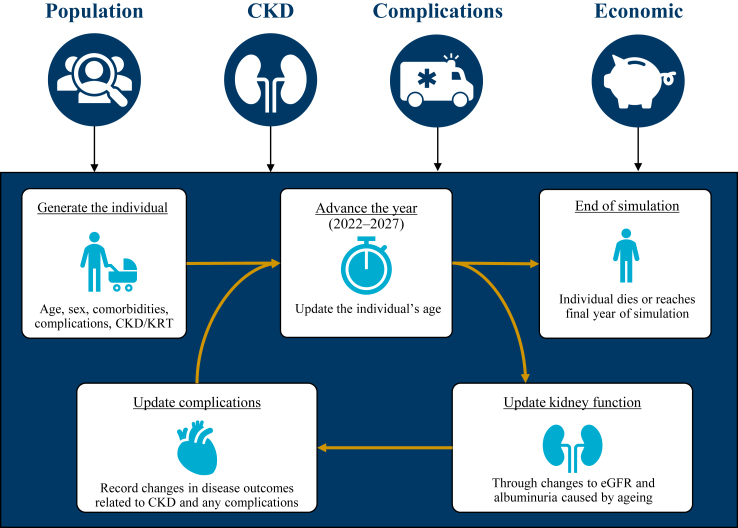
We generated a population of 20 million virtual individuals for each country or region (620 million for the full data set) to allow a starting point for a Monte Carlo procedure. To ensure that the populations were reflective of a given population, we used local demographic data, online databases, published literature, and input from local clinical experts. These virtual individuals then progressed through the microsimulation in 1-year increments from 2022 to 2027. This relatively short time frame allowed for a greater degree of confidence than longer periods, which are associated with increased uncertainty. The time frame also reflected a typical election cycle period, which may be of relevance in the context of healthcare policy, and the slight overlap between present and future data allowed corroboration of the projected baseline findings when the Scientific Steering Committee considered outputs for face validity.
At the beginning of each year, the simulation updated the age, estimated glomerular filtration rate (eGFR), and albuminuria values, the related risk of having a cardiovascular complication, and the likelihood of having a comorbidity related to CKD (type 2 diabetes and/or hypertension). These characteristics were also modelled to influence all-cause mortality. Ageing (on a year-to-year basis) increased the risk of complications and all-cause mortality, as well as the likelihood of a new CKD diagnosis or progression of existing disease; in turn, reduced level of GFR and increased level of albuminuria (according to Kidney Disease: Improving Global Outcomes [KDIGO] risk categories)18 influenced the relative risk of cardiovascular complications (heart failure, myocardial infarction, and stroke) and all-cause mortality. Accordingly, we updated the health status of each individual each year until estimated death or the study endpoint (Fig. 1). A detailed description of model development, calibration, and statistical drivers has been previously published.16
Fig. 1.
Overview of the microsimulation modules. CKD = chronic kidney disease. CV = cardiovascular. eGFR = estimated glomerular filtration rate. KRT = kidney replacement therapy.
Here, we report the results from the analysis of 31 countries/regions: Australia, Belgium, Brazil, Canada, China, Colombia, Denmark, France, Germany, Greece, Hungary, India, Israel, Italy, Japan, Mexico, Netherlands, Philippines, Poland, Romania, Saudi Arabia, Singapore, South Korea, Spain, Sweden, Taiwan, Thailand, Türkiye, UAE (Emirati population), UK, and USA. These countries were selected based on the availability of epidemiological data (albeit with some variation of completeness) and the agreement of local experts who expressed an interest in participation.
A Scientific Steering Committee comprising local clinical experts provided advice on the modelling approach, conceptualisation, clinical assumptions, validation of data inputs, addressing of data gaps, model calibrations, and interpretation of results from a global and local perspective (Supplementary Table S1).
Clinical data inputs
Details of the sources for each data input can be found in Supplementary Table S2. These were identified through a robust and extensive literature search within each country and based on local language search terms. Literature search dates varied by country based on data availability but broadly captured publications from the last 10 years, with a cap at 20 years if more recent input sources were scarce.16 In brief, we assigned each virtual individual a profile based on a series of input variables that can be broadly grouped as follows: (1) the population module, including baseline demographics such as age and sex; (2) the CKD status module, in which each individual was assigned a CKD status based on eGFR using the CKD-Epidemiology Collaboration equation and on albuminuria as defined by the urine albumin:creatinine ratio, all based on distributions from national and/or regional databases or published literature; the version of the source was appropriate to the data derived from the country or region, and can be assessed in the Supplementary materials detailing input variables; (3) the disease burden module, including the prevalence and/or incidence of comorbid conditions (type 2 diabetes and hypertension), cardiovascular complications (heart failure, myocardial infarction, and stroke), KRT use and the probability of death from all causes; and (4) the health economics module, which included direct CKD costs.
The health economics module was pivotal in determining the financial burden of CKD, and a detailed description of inputs related to costs, as well as the findings from a parallel microsimulation run using the Inside CKD model are described in detail by Chadban and colleagues17 in this volume.
Because inputs were selected to reflect the specific disease demographics and healthcare systems for a given country or region, there was considerable variation in these parameters, including the threshold of clinical indicators for KRT initiation, the availability of KRT modalities, and the proportion of patients who were able to access treatment. Disease calibrations have been previously described,16 and for all countries/regions, KRT prevalence was calibrated against the input data via a regression analysis that extrapolated historical datapoints and compared them with the projected outputs. When country- or region-specific input data were limited, we identified a suitable proxy either from recommendations of key experts or by using a project-specific algorithm (Supplementary Table S2).
To determine disease progression rates, we used annual slopes of eGFR decline for various CKD patient subtypes based on regression analyses from the DISCOVER CKD database.19 We assigned disease progression rates to individuals based on clinical characteristics, including the presence of comorbid conditions and cardiovascular complications. The eGFR slopes were assumed to be consistent across modelled countries/regions (see Supplementary Table S2 for input parameters).
Clinical outputs
We obtained outputs specific to our 31 countries/regions, including the projected prevalence of CKD by GFR stages (G1–G5), age, diagnosis status (diagnosed and undiagnosed), and comorbidity status, as well as that of KRT modality (haemodialysis, peritoneal dialysis, and kidney transplantation), cardiovascular events, and all-cause mortality. These outputs were generated for the end of each year. To account for differences in population size between countries/regions, prevalence data were presented as per 100,000 and/or as percentage change over time, from 2022 to 2027, as appropriate. Prevalence was representative of the total, age-standardised population and was calculated separately for each country. Cumulative incidence was calculated from baseline (2022) up to and including 2027 (i.e. the sum of annual incidences that occurred between 2022 and 2027). Total values for the data set were generated as a straightforward sum of all data. If prevalence was described for the whole data set, weighted mean values for the total data set were used to account for differences in population size.
Model validation and sensitivity analyses
Although the basic microsimulation model has been previously substantiated,15 we undertook a comprehensive evaluation of validity specific to these Inside CKD outputs. This approach included validation from key clinical experts and internal checks. A summary of the model validation has been previously published.16 In brief, the validation strategy was conducted in accordance with the International Society for Pharmacoeconomics and Outcomes Research and the Society for Medical Decision Making (ISPOR-SMDM) task force, and comprised four different aspects of validation: external validation, internal validation, cross-validation, and face validity.20 Here, we report external validation for the specific countries/regions for which data were available (Supplementary Table S3). The Scientific Steering Committee provided face validity assessments of all included input data and endpoints reported here, including the input data and outputs for type 2 diabetes and hypertension.
A sensitivity analysis was conducted for each country/region on diagnosis rates for each CKD stage in which prevalence of CKD was projected using a 10% increase and a 10% decrease in the total weighted diagnosis rate. The data inputs used for diagnosis rates are provided in Supplementary Table S2. Diagnosis rate input data were not available for many countries/regions, in these cases, proxy values were utilised, and the sensitivity analysis was performed to assess the robustness of the model against changes in diagnostic rates.
Role of the funding source
The funder of the study was involved in the study design, data analysis, data interpretation and writing of the report. The funder had no role in data collection, which were conducted by HealthLumen. Authors (LR and LW) checked and corroborated the data. All authors had full access to all data and the corresponding author had final responsibility for the decision to submit for publication.
Results
Baseline demographics of the virtual population
Each virtual population was scaled to its given regional population, with baseline demographic characteristics shown in Table 1 and Table 2. Baseline drivers of CKD are provided in Supplementary Table S4. Sex distribution was consistent across regions, with the exception of the UAE, where the proportion of men was higher (Table 1). The proportion of persons aged 65 years and older varied widely (range 3.6–28.9%) (Table 1). The overall proportion of persons with CKD (diagnosed and undiagnosed) in the virtual population varied between 5.5% and 18.8% (Table 2). The proportion of persons with diagnosed CKD also varied between 0.8% and 5.9% (Table 2). Among those with CKD, an overall weighted mean of 26.3% were diagnosed with type 2 diabetes, and 60.0% were diagnosed with hypertension (Table 2). At baseline, in 2022, all participating countries/regions showed a high prevalence of diagnosed CKD (range 846–5927 per 100,000), but an even greater prevalence of undiagnosed CKD (range 4137–12,860) (Supplementary Fig. S1, Table 3).
Table 1.
Demographic summary for each virtual population.
| Region | Country | Population size (number of people) |
Sex, % (2022) |
Age, % (2022) |
Percentage increase in population ≥65 years old (2022–2027) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2022 | 2027 | Female | Male | 0–17 years | 18–34 years | 35–64 years | ≥65 years | |||
| Americas | Brazil | 215,353,588 | 221,142,806 | 50.9 | 49.1 | 24.5 | 26.6 | 38.6 | 10.3 | 22.5 |
| Canada | 38,388,416 | 39,939,039 | 50.3 | 49.7 | 18.9 | 22.2 | 39.9 | 19.0 | 17.7 | |
| Colombia | 51,512,766 | 52,530,216 | 50.9 | 49.1 | 26.2 | 28.3 | 35.8 | 9.7 | 22.6 | |
| Mexico | 131,562,775 | 137,608,574 | 51.1 | 48.9 | 30.2 | 27.8 | 33.9 | 8.1 | 20.9 | |
| USA | 334,805,268 | 344,100,699 | 50.5 | 49.5 | 22.0 | 23.3 | 37.3 | 17.4 | 14.0 | |
| Europe | Belgium | 11,668,276 | 11,817,128 | 50.4 | 49.6 | 20.3 | 20.3 | 39.5 | 19.9 | 11.0 |
| Denmark | 5,834,952 | 5,942,672 | 50.3 | 49.7 | 19.7 | 22.0 | 37.8 | 20.6 | 7.9 | |
| France | 65,584,514 | 66,323,948 | 51.6 | 48.4 | 21.0 | 19.5 | 38.1 | 21.4 | 8.9 | |
| Germany | 83,883,587 | 83,346,306 | 50.5 | 49.5 | 16.9 | 19.2 | 41.6 | 22.3 | 9.3 | |
| Greece | 10,316,641 | 10,054,798 | 50.9 | 49.1 | 16.3 | 17.5 | 43.2 | 23.0 | 6.5 | |
| Hungary | 9,606,252 | 9,447,725 | 52.4 | 47.6 | 17.5 | 19.9 | 41.6 | 21.0 | 2.7 | |
| Italy | 60,262,779 | 59,555,936 | 51.2 | 48.8 | 15.5 | 17.5 | 43.1 | 23.9 | 7.9 | |
| Netherlands | 17,211,449 | 17,380,060 | 50.1 | 49.9 | 18.8 | 20.9 | 39.4 | 20.9 | 12.0 | |
| Poland | 37,739,779 | 37,314,983 | 51.6 | 48.4 | 18.1 | 19.7 | 42.2 | 20.0 | 11.0 | |
| Romania | 19,031,330 | 18,590,731 | 51.4 | 48.6 | 18.7 | 19.4 | 41.9 | 20.1 | 2.1 | |
| Spain | 47,865,714 | 48,494,124 | 51.0 | 49.0 | 17.3 | 18.3 | 44.3 | 20.2 | 11.9 | |
| Sweden | 10,218,972 | 10,488,223 | 49.9 | 50.1 | 21.0 | 21.2 | 37.2 | 20.6 | 7.0 | |
| Türkiye | 85,561,976 | 87,612,953 | 50.6 | 49.4 | 28.0 | 26.1 | 36.4 | 9.6 | 20.2 | |
| UK | 68,497,913 | 69,771,961 | 50.5 | 49.5 | 21.0 | 21.2 | 38.8 | 19.0 | 9.3 | |
| Asia–Pacific/Middle East | Australia | 26,068,793 | 27,406,327 | 50.2 | 49.8 | 23.0 | 22.3 | 37.9 | 16.8 | 15.2 |
| China | 1,448,471,404 | 1,461,797,638 | 48.7 | 51.3 | 20.9 | 22.7 | 43.6 | 12.8 | 18.4 | |
| India | 1,406,631,781 | 1,469,338,564 | 48.0 | 52.0 | 30.8 | 28.9 | 33.3 | 7.0 | 19.4 | |
| Israel | 8,922,893 | 9,582,849 | 50.2 | 49.8 | 32.4 | 23.3 | 31.6 | 12.7 | 12.2 | |
| Japan | 125,584,839 | 122,754,999 | 51.2 | 48.8 | 14.9 | 16.5 | 39.7 | 28.9 | 1.5 | |
| Philippines | 112,508,991 | 119,632,644 | 49.8 | 50.2 | 34.8 | 29.2 | 30.0 | 5.9 | 24.9 | |
| Saudi Arabia | 35,844,913 | 38,115,319 | 42.2 | 57.8 | 28.5 | 26.5 | 41.2 | 3.8 | 39.4 | |
| Singapore | 5,943,551 | 6,158,533 | 47.7 | 52.3 | 14.9 | 22.5 | 47.4 | 15.2 | 35.8 | |
| South Korea | 51,329,905 | 51,293,519 | 50.0 | 50.0 | 14.8 | 20.8 | 47.0 | 17.4 | 26.2 | |
| Taiwan | 23,888,600 | 23,997,588 | 50.4 | 49.6 | 15.2 | 21.3 | 46.2 | 17.3 | 22.4 | |
| Thailand | 70,078,198 | 70,394,102 | 51.4 | 48.6 | 19.6 | 22.6 | 43.6 | 14.2 | 24.2 | |
| UAE Emiratia | 1,604,749 | 1,749,670 | 23.3 | 76.7 | 31.0 | 29.8 | 35.7 | 3.6 | 122.4 | |
CKD = chronic kidney disease.
The virtual population comprised 20 million individuals, and was scaled to the given country or region population. Note that the increase in population is given as the percentage of projected change.
These demographic data were generated for both the Emirati and non-Emirati populations, but it should be noted that the relatively high proportion of males reflects the predominantly male expatriate subgroup in the non-Emirati population.
Table 2.
CKD demographics for each virtual population at baseline (2022).
| Region | Country | CKD stage, % (diagnosed and undiagnosed) |
CKD, all stages, % |
Comorbidity prevalence in the CKD population, % |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3a | 3b | 4 | 5 | Diagnosed and undiagnosed | Diagnosed | Type 2 diabetes | Hyper-tension | ||
| Americas | Brazil | 1.57 | 1.73 | 4.70 | 1.55 | 0.16 | 0.02 | 9.73 | 2.96 | 36.0 | 78.6 |
| Canada | 3.10 | 2.39 | 5.59 | 1.87 | 0.35 | 0.05 | 13.34 | 3.88 | 32.2 | 56.9 | |
| Colombia | 1.74 | 1.19 | 3.11 | 0.53 | 0.10 | 0.01 | 6.68 | 1.71 | 33.1 | 77.4 | |
| Mexico | 4.46 | 3.19 | 2.24 | 0.73 | 0.18 | 0.14 | 10.93 | 2.43 | 43.1 | 79.8 | |
| USA | 5.33 | 3.81 | 4.31 | 1.32 | 0.11 | 0.02 | 14.89 | 3.40 | 29.5 | 62.9 | |
| Europe | Belgium | 2.97 | 2.42 | 5.65 | 2.20 | 0.81 | 0.13 | 14.19 | 4.28 | 19.9 | 55.8 |
| Denmark | 3.05 | 2.48 | 6.06 | 1.94 | 0.33 | 0.04 | 13.89 | 4.05 | 19.4 | 55.4 | |
| France | 3.58 | 2.33 | 3.38 | 1.09 | 0.33 | 0.11 | 10.82 | 3.06 | 19.9 | 52.7 | |
| Germany | 8.33 | 1.64 | 2.01 | 0.61 | 0.11 | 0.01 | 12.71 | 2.08 | 20.0 | 48.1 | |
| Greece | 1.63 | 1.98 | 6.32 | 1.37 | 0.25 | 0.03 | 11.58 | 3.63 | 19.6 | 57.8 | |
| Hungary | 3.11 | 2.50 | 5.95 | 1.77 | 0.27 | 0.03 | 13.63 | 3.53 | 27.8 | 72.6 | |
| Italy | 2.06 | 1.28 | 2.46 | 0.63 | 0.19 | 0.09 | 6.72 | 1.78 | 35.1 | 77.8 | |
| Netherlands | 3.09 | 2.78 | 4.67 | 1.39 | 0.25 | 0.03 | 12.19 | 3.37 | 19.7 | 55.4 | |
| Poland | 2.05 | 2.54 | 4.57 | 1.33 | 0.22 | 0.02 | 10.73 | 3.17 | 20.0 | 57.3 | |
| Romania | 1.39 | 1.22 | 3.75 | 0.59 | 0.07 | 0.01 | 7.02 | 2.06 | 27.0 | 72.0 | |
| Spain | 1.65 | 1.82 | 5.65 | 1.32 | 0.24 | 0.04 | 10.73 | 3.39 | 31.9 | 77.2 | |
| Sweden | 2.29 | 1.92 | 3.51 | 1.10 | 0.28 | 0.17 | 9.26 | 2.49 | 33.8 | 62.8 | |
| Türkiye | 5.32 | 2.72 | 1.98 | 1.40 | 0.23 | 0.08 | 11.74 | 2.50 | 20.9 | 52.8 | |
| UK | 3.00 | 2.33 | 5.68 | 1.96 | 0.36 | 0.05 | 13.38 | 3.94 | 19.8 | 55.3 | |
| Asia–Pacific/Middle East | Australia | 3.53 | 2.22 | 2.43 | 0.54 | 0.14 | 0.06 | 8.92 | 2.03 | 20.6 | 52.5 |
| China | 6.13 | 0.96 | 1.17 | 0.21 | 0.05 | 0.02 | 8.54 | 1.10 | 20.8 | 49.9 | |
| India | 3.54 | 0.70 | 0.84 | 0.28 | 0.05 | 0.05 | 5.46 | 0.85 | 30.0 | 65.1 | |
| Israel | 7.19 | 2.47 | 2.24 | 0.80 | 0.36 | 0.07 | 13.13 | 2.32 | 37.7 | 44.7 | |
| Japan | 2.18 | 6.33 | 5.43 | 4.31 | 0.50 | 0.04 | 18.79 | 5.93 | 19.9 | 54.6 | |
| Philippines | 1.88 | 0.93 | 2.15 | 0.38 | 0.09 | 0.03 | 5.47 | 1.33 | 22.3 | 66.6 | |
| Saudi Arabia | 4.13 | 2.63 | 3.86 | 1.03 | 0.34 | 0.30 | 12.28 | 3.21 | 28.2 | 60.6 | |
| Singapore | 5.86 | 3.84 | 4.89 | 0.94 | 0.27 | 0.09 | 15.89 | 3.58 | 20.7 | 53.6 | |
| South Korea | 4.59 | 2.83 | 2.58 | 0.62 | 0.16 | 0.02 | 10.81 | 2.31 | 20.7 | 52.5 | |
| Taiwan | 4.54 | 2.75 | 2.54 | 0.61 | 0.16 | 0.02 | 10.62 | 2.26 | 42.0 | 66.2 | |
| Thailand | 2.43 | 3.56 | 8.64 | 2.24 | 0.39 | 0.21 | 17.46 | 4.90 | 20.0 | 57.5 | |
| UAE Emirati | 3.94 | 2.51 | 3.53 | 0.81 | 0.31 | 0.25 | 11.35 | 2.89 | 28.0 | 60.8 | |
CKD = chronic kidney disease.
The UAE comprised the Emirati population and the expatriate population. Only the Emirati populations are considered in the results. The virtual population comprised 20 million individuals, and was scaled to the given country or region population.
Table 3.
Prevalence of diagnosed and undiagnosed CKD cases, per 100,000 of the national population.
| Diagnosed CKD prevalence per 100,000 people (including KRT) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Year | Country/region | CKD stage |
Total | % Change 2022–2027 | |||||
| 1 | 2 | 3a | 3b | 4 | 5 | ||||
| Americas | |||||||||
| 2022 | Brazil | 67 | 469 | 1379 | 888 | 139 | 20 | 2963 | 7.8 |
| 2027 | 74 | 430 | 1677 | 783 | 176 | 55 | 3193 | ||
| 2022 | Canada | 133 | 652 | 2057 | 741 | 256 | 46 | 3885 | 1.1 |
| 2027 | 144 | 598 | 2246 | 646 | 203 | 88 | 3926 | ||
| 2022 | Colombia | 76 | 322 | 915 | 304 | 85 | 11 | 1713 | 22.3 |
| 2027 | 88 | 295 | 1365 | 249 | 68 | 31 | 2095 | ||
| 2022 | Mexico | 190 | 870 | 658 | 418 | 158 | 131 | 2425 | 0.8 |
| 2027 | 215 | 745 | 924 | 364 | 136 | 63 | 2446 | ||
| 2022 | USA | 229 | 1036 | 1268 | 756 | 98 | 17 | 3404 | −0.3 |
| 2027 | 252 | 896 | 1373 | 663 | 162 | 48 | 3394 | ||
| Europe | |||||||||
| 2022 | Belgium | 128 | 657 | 2081 | 871 | 595 | 131 | 4463 | −5.3 |
| 2027 | 141 | 592 | 2280 | 711 | 340 | 164 | 4228 | ||
| 2022 | Denmark | 134 | 673 | 2233 | 766 | 243 | 37 | 4087 | −2.6 |
| 2027 | 151 | 601 | 2494 | 529 | 137 | 69 | 3981 | ||
| 2022 | France | 155 | 635 | 1244 | 431 | 241 | 111 | 2817 | 7.3 |
| 2027 | 173 | 528 | 1647 | 356 | 217 | 102 | 3023 | ||
| 2022 | Germany | 359 | 445 | 739 | 243 | 83 | 14 | 1884 | 11.1 |
| 2027 | 349 | 448 | 991 | 209 | 73 | 24 | 2093 | ||
| 2022 | Greece | 92 | 163 | 2328 | 544 | 186 | 32 | 3345 | 11.3 |
| 2027 | 106 | 148 | 2865 | 431 | 114 | 60 | 3723 | ||
| 2022 | Hungary | 136 | 683 | 1749 | 1021 | 242 | 28 | 3858 | −7.1 |
| 2027 | 153 | 599 | 2021 | 640 | 119 | 54 | 3585 | ||
| 2022 | Italy | 88 | 349 | 724 | 362 | 166 | 89 | 1778 | 6.3 |
| 2027 | 94 | 288 | 976 | 352 | 118 | 62 | 1890 | ||
| 2022 | Netherlands | 134 | 754 | 1720 | 551 | 181 | 28 | 3369 | 7.7 |
| 2027 | 151 | 676 | 2242 | 392 | 116 | 50 | 3628 | ||
| 2022 | Poland | 89 | 688 | 1682 | 523 | 159 | 24 | 3165 | 7.5 |
| 2027 | 107 | 641 | 2177 | 345 | 89 | 42 | 3401 | ||
| 2022 | Romania | 61 | 332 | 1384 | 231 | 49 | 6 | 2063 | 33.2 |
| 2027 | 69 | 262 | 2160 | 170 | 48 | 39 | 2748 | ||
| 2022 | Spain | 71 | 493 | 2084 | 522 | 180 | 37 | 3387 | 9.5 |
| 2027 | 79 | 439 | 2456 | 525 | 141 | 69 | 3709 | ||
| 2022 | Sweden | 458 | 385 | 702 | 554 | 222 | 165 | 2486 | −3.6 |
| 2027 | 517 | 321 | 911 | 381 | 141 | 126 | 2398 | ||
| 2022 | Türkiye | 230 | 740 | 729 | 555 | 173 | 75 | 2502 | 0.4 |
| 2027 | 253 | 667 | 1034 | 348 | 161 | 49 | 2512 | ||
| 2022 | UK | 128 | 634 | 2086 | 776 | 267 | 48 | 3940 | −3.2 |
| 2027 | 140 | 567 | 2202 | 641 | 188 | 75 | 3814 | ||
| Asia–Pacific/Middle East | |||||||||
| 2022 | Australia | 152 | 604 | 896 | 216 | 103 | 58 | 2030 | 7.0 |
| 2027 | 168 | 516 | 1141 | 219 | 82 | 46 | 2171 | ||
| 2022 | China | 267 | 263 | 432 | 84 | 37 | 16 | 1098 | −1.6 |
| 2027 | 270 | 248 | 455 | 80 | 19 | 8 | 1081 | ||
| 2022 | India | 151 | 190 | 310 | 111 | 35 | 48 | 846 | −2.5 |
| 2027 | 157 | 172 | 401 | 67 | 19 | 8 | 825 | ||
| 2022 | Israel | 401 | 202 | 792 | 527 | 329 | 67 | 2319 | −9.4 |
| 2027 | 420 | 185 | 902 | 375 | 160 | 60 | 2101 | ||
| 2022 | Japan | 94 | 1717 | 1999 | 1710 | 370 | 36 | 5927 | 9.9 |
| 2027 | 111 | 1518 | 3179 | 1122 | 487 | 97 | 6514 | ||
| 2022 | Philippines | 276 | 137 | 713 | 126 | 48 | 30 | 1331 | 6.4 |
| 2027 | 295 | 117 | 820 | 134 | 28 | 22 | 1417 | ||
| 2022 | Saudi Arabia | 179 | 720 | 1131 | 593 | 302 | 284 | 3210 | 15.0 |
| 2027 | 202 | 673 | 1441 | 687 | 333 | 355 | 3692 | ||
| 2022 | Singapore | 859 | 564 | 1617 | 312 | 141 | 89 | 3583 | 10.6 |
| 2027 | 920 | 546 | 1888 | 401 | 113 | 95 | 3964 | ||
| 2022 | South Korea | 197 | 771 | 951 | 246 | 119 | 22 | 2305 | 32.3 |
| 2027 | 212 | 699 | 1628 | 286 | 162 | 64 | 3051 | ||
| 2022 | Taiwan | 196 | 749 | 935 | 242 | 114 | 21 | 2258 | 28.8 |
| 2027 | 208 | 658 | 1574 | 266 | 146 | 56 | 2908 | ||
| 2022 | Thailand | 356 | 523 | 2864 | 743 | 205 | 206 | 4897 | 11.2 |
| 2027 | 407 | 472 | 3142 | 895 | 263 | 269 | 5448 | ||
| 2022 | UAE (Emirati) | 173 | 678 | 1051 | 471 | 279 | 235 | 2888 | 20.1 |
| 2027 | 205 | 724 | 1563 | 457 | 303 | 217 | 3469 | ||
| 2022 | UAE (Expatriate) | 192 | 100 | 91 | 24 | 5 | 7 | 419 | 28.8 |
| 2027 | 195 | 102 | 209 | 22 | 9 | 3 | 540 | ||
| Undiagnosed CKD prevalence per 100,000 people (including KRT) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Year | Country/region | CKD stage |
Total | % Change 2022–2027 | |||||
| 1 | 2 | 3a | 3b | 4 | 5 | ||||
| Americas | |||||||||
| 2022 | Brazil | 1503 | 1263 | 3318 | 661 | 17 | 1 | 6763 | 10.3 |
| 2027 | 1664 | 1149 | 4042 | 581 | 21 | 2 | 7459 | ||
| 2022 | Canada | 2963 | 1743 | 3535 | 1125 | 94 | 0 | 9460 | 2.8 |
| 2027 | 3216 | 1598 | 3852 | 983 | 74 | 0 | 9723 | ||
| 2022 | Colombia | 1668 | 867 | 2194 | 227 | 10 | 0 | 4966 | 12.0 |
| 2027 | 1859 | 793 | 2714 | 184 | 8 | 1 | 5560 | ||
| 2022 | Mexico | 4265 | 2322 | 1581 | 309 | 19 | 6 | 8502 | 7.9 |
| 2027 | 4725 | 1947 | 2212 | 268 | 16 | 3 | 9171 | ||
| 2022 | USA | 5098 | 2774 | 3043 | 561 | 12 | 1 | 11,488 | 1.9 |
| 2027 | 5526 | 2373 | 3294 | 493 | 19 | 2 | 11,708 | ||
| Europe | |||||||||
| 2022 | Belgium | 2847 | 1758 | 3571 | 1332 | 215 | 0 | 9723 | 1.1 |
| 2027 | 3128 | 1577 | 3917 | 1082 | 123 | 0 | 9827 | ||
| 2022 | Denmark | 2913 | 1803 | 3824 | 1172 | 89 | 0 | 9801 | −2.0 |
| 2027 | 3228 | 1605 | 3910 | 812 | 49 | 0 | 9605 | ||
| 2022 | France | 3429 | 1692 | 2134 | 657 | 85 | 2 | 7999 | 4.9 |
| 2027 | 3795 | 1407 | 2578 | 535 | 72 | 2 | 8389 | ||
| 2022 | Germany | 7967 | 1193 | 1269 | 366 | 30 | 0 | 10,825 | 1.4 |
| 2027 | 7719 | 1206 | 1707 | 315 | 26 | 0 | 10,972 | ||
| 2022 | Greece | 1535 | 1820 | 3989 | 825 | 67 | 0 | 8236 | 3.7 |
| 2027 | 1766 | 1642 | 4443 | 652 | 41 | 0 | 8544 | ||
| 2022 | Hungary | 2971 | 1819 | 4198 | 753 | 29 | 1 | 9771 | −1.0 |
| 2027 | 3252 | 1590 | 4341 | 472 | 14 | 2 | 9671 | ||
| 2022 | Italy | 1974 | 933 | 1740 | 267 | 20 | 4 | 4938 | 11.6 |
| 2027 | 2118 | 771 | 2345 | 259 | 14 | 3 | 5509 | ||
| 2022 | Netherlands | 2952 | 2021 | 2947 | 840 | 65 | 0 | 8825 | 4.20 |
| 2027 | 3263 | 1812 | 3476 | 599 | 42 | 0 | 9192 | ||
| 2022 | Poland | 1961 | 1856 | 2889 | 802 | 57 | 0 | 7565 | 4.6 |
| 2027 | 2348 | 1724 | 3285 | 526 | 32 | 0 | 7916 | ||
| 2022 | Romania | 1326 | 889 | 2361 | 358 | 19 | 0 | 4952 | 14.6 |
| 2027 | 1468 | 703 | 3225 | 262 | 18 | 0 | 5676 | ||
| 2022 | Spain | 1582 | 1329 | 3569 | 797 | 65 | 0 | 7342 | 9.0 |
| 2027 | 1764 | 1177 | 4214 | 794 | 52 | 0 | 8001 | ||
| 2022 | Sweden | 1830 | 1537 | 2803 | 548 | 54 | 0 | 6772 | 3.4 |
| 2027 | 2060 | 1277 | 3256 | 377 | 32 | 0 | 7002 | ||
| 2022 | Türkiye | 5094 | 1982 | 1254 | 847 | 62 | 0 | 9239 | 5.7 |
| 2027 | 5604 | 1789 | 1778 | 534 | 56 | 0 | 9761 | ||
| 2022 | UK | 2872 | 1693 | 3589 | 1184 | 98 | 0 | 9437 | 0.3 |
| 2027 | 3125 | 1513 | 3788 | 974 | 69 | 0 | 9468 | ||
| Asia–Pacific/Middle East | |||||||||
| 2022 | Australia | 3374 | 1614 | 1535 | 327 | 37 | 1 | 6888 | 6.5 |
| 2027 | 3659 | 1364 | 1953 | 331 | 29 | 1 | 7338 | ||
| 2022 | China | 5862 | 701 | 741 | 128 | 13 | 0 | 7446 | 0.9 |
| 2027 | 5950 | 661 | 775 | 123 | 7 | 0 | 7516 | ||
| 2022 | India | 3389 | 509 | 531 | 168 | 12 | 0 | 4610 | 3.4 |
| 2027 | 3518 | 455 | 684 | 102 | 7 | 0 | 4766 | ||
| 2022 | Israel | 6792 | 2263 | 1450 | 275 | 31 | 0 | 10,811 | 1.5 |
| 2027 | 7047 | 2074 | 1644 | 197 | 16 | 0 | 10,978 | ||
| 2022 | Japan | 2086 | 4609 | 3434 | 2599 | 133 | 0 | 12,860 | 7.9 |
| 2027 | 2452 | 4071 | 5464 | 1714 | 174 | 0 | 13,875 | ||
| 2022 | Philippines | 1608 | 792 | 1440 | 254 | 44 | 0 | 4137 | 5.3 |
| 2027 | 1721 | 681 | 1660 | 270 | 25 | 0 | 4356 | ||
| 2022 | Saudi Arabia | 3955 | 1907 | 2725 | 437 | 37 | 12 | 9074 | 12.2 |
| 2027 | 4404 | 1754 | 3455 | 510 | 40 | 16 | 10,179 | ||
| 2022 | Singapore | 5005 | 3279 | 3271 | 628 | 126 | 0 | 12,309 | 7.7 |
| 2027 | 5358 | 3172 | 3812 | 811 | 102 | 0 | 13,255 | ||
| 2022 | South Korea | 4394 | 2056 | 1633 | 376 | 44 | 0 | 8503 | 16.2 |
| 2027 | 4726 | 1867 | 2796 | 431 | 59 | 0 | 9879 | ||
| 2022 | Taiwan | 4345 | 2003 | 1604 | 370 | 41 | 0 | 8362 | 14.0 |
| 2027 | 4606 | 1762 | 2703 | 408 | 54 | 0 | 9532 | ||
| 2022 | Thailand | 2071 | 3039 | 5777 | 1497 | 183 | 0 | 12,568 | 7.3 |
| 2027 | 2367 | 2745 | 6337 | 1802 | 235 | 0 | 13,486 | ||
| 2022 | UAE (Emirati) | 3764 | 1827 | 2482 | 343 | 32 | 10 | 8457 | 11.5 |
| 2027 | 4169 | 1890 | 3012 | 318 | 31 | 9 | 9429 | ||
| 2022 | UAE (Expatriate) | 4199 | 271 | 222 | 18 | 1 | 0 | 4711 | 4.8 |
| 2027 | 4272 | 268 | 377 | 16 | 1 | 0 | 4934 | ||
CKD = chronic kidney disease. KRT = kidney replacement therapy.
Note: individual values have been rounded to whole numbers. Slight discrepancies may occur between the total sum reported as a result.
Prevalence of CKD
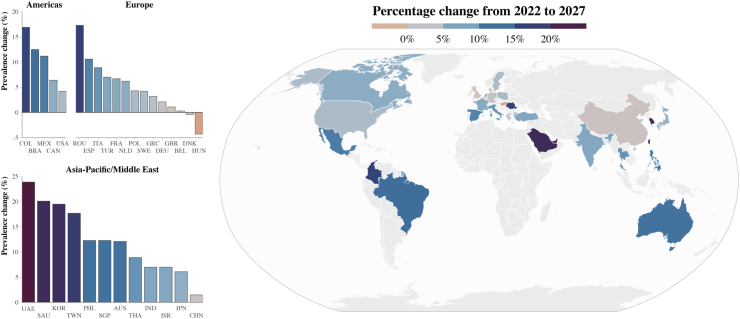
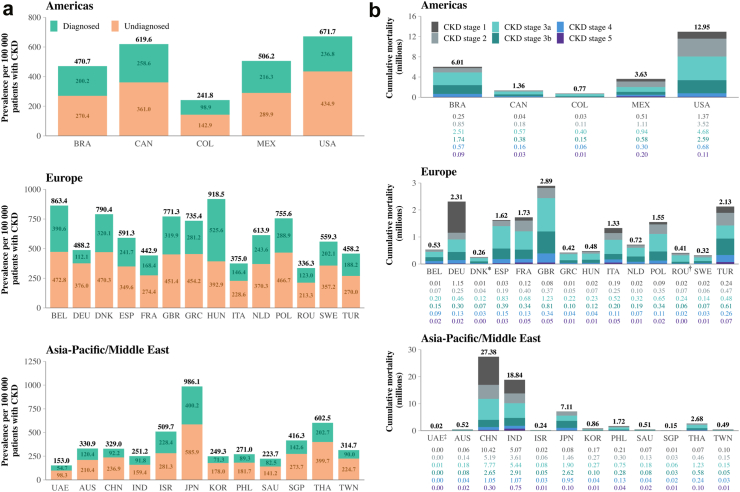
Across the complete data set, a total of 436.6 million cases were projected by 2027 (an increase of 5.8% from 2022) with all countries showing an increase in absolute numbers except for Hungary and Denmark (Fig. 2, Supplementary Table S5). Assuming no changes to diagnostic techniques and screening, undiagnosed individuals were projected to constitute 80.0% of the CKD population across data sets in 2027 (range: 68.1–87.4%) (Table 3).
Fig. 2.
Projected percentage change of CKD prevalence in each population 2022–2027. Notes: The UAE has a large and diverse Expatriate population with a different CKD profile; only the Emirati population has been presented here. CKD = chronic kidney disease.
Cardiovascular complications in the CKD population
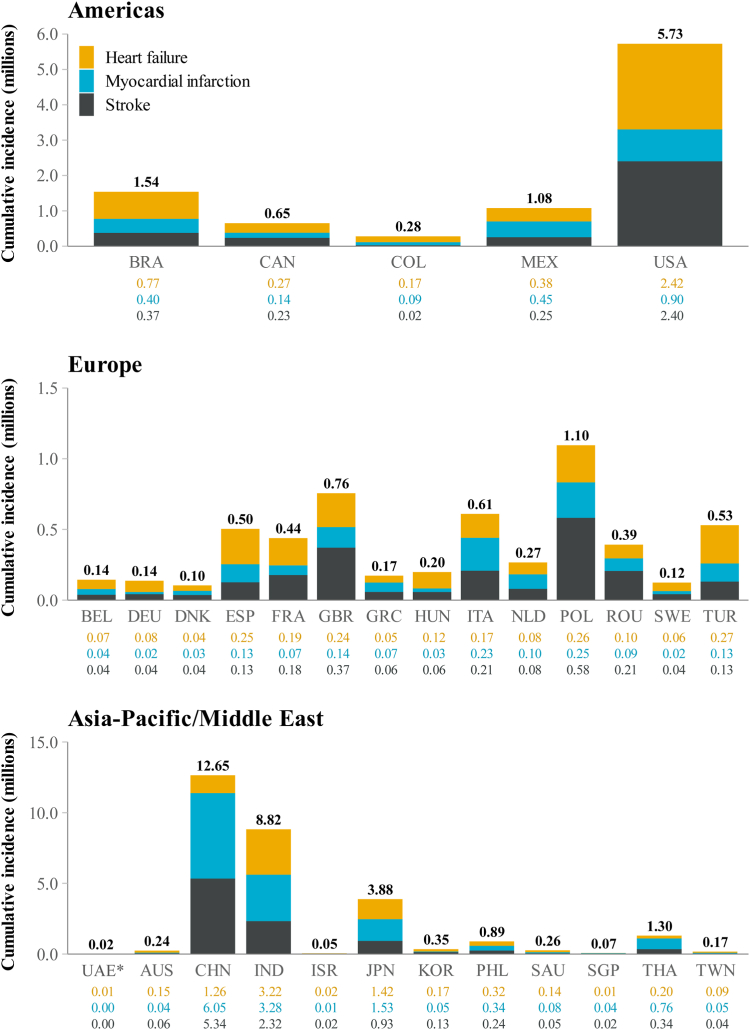
Fig. 3 illustrates the cumulative incidence of cardiovascular complications in patients diagnosed with CKD by 2027. Across the data set, per 100,000 patients and expressed as a weighted mean, Inside CKD projected 8859 cases of heart failure, 10,244 of myocardial infarction, and 7797 of stroke per 100,000 patients with CKD by 2027 (Table 4).
Fig. 3.
Projected cumulative incidence (2022–2027) of cardiovascular complications in patients with diagnosed CKD. Note: The UAE has a large and diverse Expatriate population with a different CKD profile; only the Emirati population has been provided here. Individual values have been rounded to two decimal places. Slight discrepancies may occur between the total sum reported as a result. CKD = chronic kidney disease. ∗UAE cumulative incidence where values shown are <0.01 million: Heart failure, 7328; Myocardial infarction, 4151; Stroke, 4183.
Table 4.
Projected prevalence of cardiovascular complications per 100,000 patients with CKD in 2027.
| Region | Country/region | Heart failure | Myocardial infraction | Stroke | Total |
|---|---|---|---|---|---|
| Americas | Brazil | 7595 | 3107 | 2825 | 13,527 |
| Canada | 9852 | 6874 | 9276 | 26,001 | |
| Colombia | 9678 | 8132 | 1709 | 19,519 | |
| Mexico | 4947 | 6703 | 3962 | 15,612 | |
| USA | 13,114 | 6505 | 12,876 | 32,494 | |
| Europe | Belgium | 6272 | 6181 | 4498 | 16,951 |
| Denmark | 11,979 | 10,753 | 9710 | 32,442 | |
| France | 4666 | 3662 | 5923 | 14,252 | |
| Germany | 1307 | 474 | 954 | 2735 | |
| Greece | 13,243 | 11,611 | 7686 | 32,540 | |
| Hungary | 25,826 | 5524 | 9591 | 40,941 | |
| Italy | 13,496 | 11,758 | 8641 | 33,895 | |
| Netherlands | 6744 | 14,469 | 8059 | 29,273 | |
| Poland | 14,501 | 17,762 | 44,838 | 77,101 | |
| Romania | 13,560 | 15,608 | 38,915 | 68,083 | |
| Spain | 10,555 | 4691 | 4196 | 19,441 | |
| Sweden | 11,569 | 5508 | 8011 | 25,090 | |
| Türkiye | 5536 | 2052 | 2279 | 9868 | |
| UK | 4707 | 4267 | 7827 | 16,800 | |
| Asia–Pacific/Middle East | Australia | 20,621 | 5288 | 5005 | 30,914 |
| China | 4987 | 13,676 | 9253 | 27,916 | |
| India | 11,259 | 10,878 | 5228 | 27,365 | |
| Israel | 3074 | 2628 | 2856 | 8559 | |
| Japan | 19,769 | 21,231 | 7365 | 48,366 | |
| Philippines | 13,100 | 13,275 | 8838 | 35,213 | |
| Saudi Arabia | 6150 | 3989 | 1880 | 12,020 | |
| Singapore | 5829 | 9499 | 5914 | 21,243 | |
| South Korea | 5795 | 1995 | 7251 | 15,041 | |
| Taiwan | 10,759 | 4687 | 2949 | 18,395 | |
| Thailand | 6071 | 16,662 | 6929 | 29,661 | |
| UAE Emirati | 8017 | 5816 | 5068 | 18,902 |
CKD = chronic kidney disease.
The UAE comprised the Emirati population and the expatriate population. Only the Emirati populations are considered in the results. The virtual population comprised 20 million individuals, and was scaled to the given country or region population. All CKD stages, cases per 100,000 of the population with CKD.
Comorbid conditions in the CKD population
The prevalence of comorbidities varied between countries/regions but was generally high in the CKD population in 2027 for hypertension (range: 26,684.8–52,887.3), type 2 diabetes (range: 7072.2–19,445.6) and the co-occurrence of both (range: 9729.8–32,986.4) (Table 5).
Table 5.
Projected prevalence of comorbid conditions in the population with CKD in 2027.
| Region | Country | Hypertension only | Type 2 diabetes only | Hypertension and type 2 diabetes |
|---|---|---|---|---|
| Americas | Brazil | 50,767.4 | 7195.9 | 27,527.9 |
| Canada | 38,142.5 | 13,830.7 | 18,353.3 | |
| Colombia | 52,802.3 | 7119.0 | 24,411.4 | |
| Mexico | 46,301.8 | 8265.4 | 32,986.4 | |
| USA | 43,392.3 | 10,035.9 | 18,727.9 | |
| Europe | Belgium | 44,746.4 | 8832.9 | 11,001.7 |
| Denmark | 44,809.1 | 8645.8 | 10,549.4 | |
| France | 42,923.2 | 9244.0 | 10,355.8 | |
| Germany | 38,994.2 | 10,179.7 | 9729.8 | |
| Greece | 47,191.4 | 8073.0 | 11,057.5 | |
| Italy | 51,052.7 | 7494.9 | 26,539.5 | |
| Hungary | 52,459.1 | 7411.6 | 19,982.0 | |
| Netherlands | 43,924.0 | 8984.5 | 10,525.9 | |
| Poland | 45,876.8 | 8345.0 | 11,059.2 | |
| Romania | 52,878.1 | 7256.0 | 18,520.2 | |
| Spain | 52,887.3 | 7072.2 | 24,257.1 | |
| Sweden | 42,048.4 | 12,333.1 | 20,661.2 | |
| Türkiye | 41,552.4 | 9716.6 | 11,018.4 | |
| UK | 44,477.8 | 8880.0 | 10,901.8 | |
| Asia–Pacific/Middle East | Australia | 41,937.8 | 9607.5 | 10,830.4 |
| China | 38,898.2 | 10,392.9 | 10,255.8 | |
| India | 45,134.7 | 9831.3 | 19,519.1 | |
| Israel | 26,684.8 | 19,445.6 | 17,865.7 | |
| Japan | 44,477.8 | 8798.2 | 10,911.5 | |
| Philippines | 50,986.2 | 7227.2 | 14,694.8 | |
| Saudi Arabia | 42,248.3 | 10,153.2 | 17,284.0 | |
| Singapore | 42,511.7 | 9452.0 | 11,037.4 | |
| South Korea | 42,676.0 | 9402.1 | 11,045.5 | |
| Taiwan | 35,880.6 | 12,793.2 | 26,631.3 | |
| Thailand | 46,358.1 | 8354.1 | 11,472.9 | |
| UAE Emirati | 42,670.8 | 9854.0 | 17,205.7 |
CKD = chronic kidney disease.
The UAE comprised the Emirati population and the expatriate population. Only the Emirati populations are considered in the results. The virtual population comprised 20 million individuals, and was scaled to the given country or region population. All CKD stages, cases per 100,000 of the population with CKD.
Projected prevalence of KRT by modality
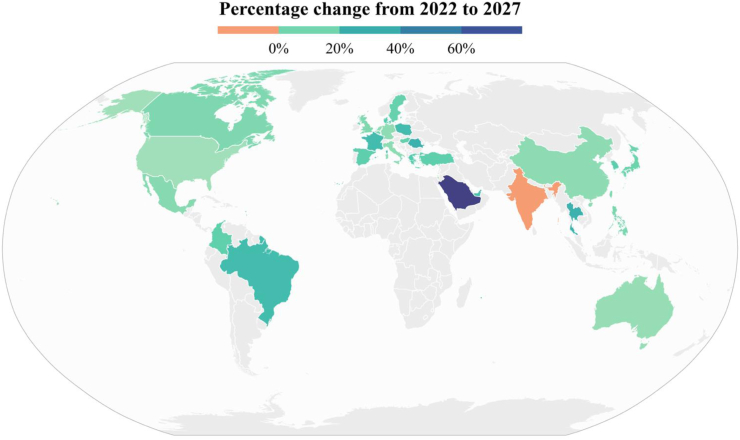
In diagnosed CKD cases, across the data set the number of patients with moderate to severe kidney disease (stages G3a–G5) was 155 million cases; assuming current treatment strategies remain unchanged over time, the total number of persons receiving KRT is also projected to increase by 7.7% between 2022 and 2027. The use of KRT and its modalities varied considerably across different healthcare systems; across the data set as a whole, haemodialysis was the most common modality at baseline (Supplementary Fig. S2) but trends in use were projected to change slightly with relative increases of 8.5%, 12.5%, and 4.1% for haemodialysis, peritoneal dialysis, and kidney transplantation, respectively between 2022 and 2027 (Fig. 4, Supplementary Table S6).
Fig. 4.
Projected percentage change in prevalence of kidney replacement therapy from 2022 to 2027. Notes: The UAE has a large and diverse Expatriate population with a different CKD profile; only the Emirati population has been provided here. CKD = chronic kidney disease.
All-cause mortality in the diagnosed and undiagnosed CKD population
Our simulation suggests that the effects of CKD are not limited to associated complications and comorbid conditions; in 2022, the prevalence of all-cause mortality in the CKD population varied across our data set, but was generally high (range: 153.0–986.1 cases per 100,000), with deaths in the undiagnosed population generally higher than those in the population diagnosed with CKD (Fig. 5). By 2027, the weighted mean average all-cause mortality rate in the undiagnosed CKD population was 242.5 per 100,000 (range 146.4–545.1), over double that of the diagnosed CKD group 111.4 per 100,000 (range 70.2–391.1).
Fig. 5.
a: Projected all-cause mortality in the diagnosed and undiagnosed CKD population in 2022, per 100,000 of the population with CKD. b: Projected cumulative incidence of all-cause mortality in people with CKD between 2022 and 2027, by CKD stage. Notes: The UAE comprised the Emirati population and also a population of those who had migrated to the country, with only the former considered in the results shown. Individual values have been rounded to one–two decimal places. Slight discrepancies may occur between the total sum reported as a result. Cumulative all-cause mortality where values are shown are <0.01 million: ∗Denmark: CKD stage 1, 7970; CKD stage 2, 36,374; CKD stage 3a, 116,474; CKD stage 3b, 69,083; CKD stage 4, 25,190; CKD stage 5, 3820. †Romania: CKD stage 1, 18,261; CKD stage 2, 70,336; CKD stage 3a, 236,637; CKD stage 3b, 61,983; CKD stage 4, 19,474; CKD stage 5, 3041. ‡UAE: CKD stage 1, 2760; CKD stage 2, 4977; CKD stage 3a, 6660; CKD stage 3b, 2427; CKD stage 4, 1439; CKD stage 5, 505.
Validation analyses and sensitivity analysis
Validation of outputs has been described previously, and a UK example is used to describe the process in detail, which comprised four aspects: external validation, internal validation, cross-validation, and face validity.16 This was conducted in accordance with the ISPOR-SMDM task force.20 Inside CKD outputs were externally validated against literature sources that were not used as data inputs (Supplementary Table S3), which additionally involved consideration of values by the Scientific Steering Committee (face validity). Alongside their expert clinical judgement, the Scientific Steering Committee considered the following factors: (i) the date of the external validation source; (ii) the use of proxy data; and (iii) any assumptions that were applied to Inside CKD input data. Combining these factors enabled the Scientific Steering Committee to validate the data set. It is notable that data sources for external validation varied greatly in terms of endpoints, data collection years, and methodology, hence direct comparisons are not provided or advised, but the exercise is included for transparency of reporting.
The sensitivity analysis that was performed on diagnosis rates indicated that the model is robust against minor fluctuations in diagnostic rate (Supplementary Data Appendix 1).
Discussion
The Inside CKD microsimulation is unprecedented in the breadth and granularity of its inputs, as well as the range of countries/regions that it includes. The plethora of factors can be tailored to the epidemiology of a given locality in order to predict future trends. The use of a microsimulation strategy was a core strength of this research because it enabled exploration of a wider remit of outcomes than are usually reported in real-world studies. Microsimulations also provide a future-orientated perspective and allow insight into countries or regions where data are sparse. The model itself can be further adjusted as new data become available, and interventions can be incorporated and tested.16 The simulation reported here generated a virtual population of 20 million for 31 countries/regions, each scaled to the corresponding national population. We predicted not only a consistently high prevalence of CKD until 2027, but also a substantial clinical burden, as evidenced by the cumulative incidence of cardiovascular complications, comorbidities, KRT, and all-cause mortality within the CKD population.
In terms of prevalence, our findings are consistent with existing epidemiological data; for example, the CaReMe CKD study used healthcare database analyses from 2020 to estimate a pooled CKD prevalence of 10% in an adult population of 2.4 million across 11 countries.21 Our modelled data substantiates the observation that CKD has reached global epidemic levels and predicts that cases are likely to remain high. Of particular concern was the proportion of undiagnosed patients; assuming current diagnostic protocols remain unchanged, most of the CKD population will not be eligible for treatment (unless therapeutic agents are prescribed for alternative reasons, such as angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for the treatment of hypertension). The implications of clinical and political inertia may be substantial: most deaths in the CKD population are likely to occur among those without a diagnosis. Because people with early-stage CKD are typically asymptomatic, they are unlikely to present at clinic unless seeking support for a different condition.
Diagnosis rates vary in the literature between regions, with current estimates suggesting between 62% and 96% of those with CKD stage 3 are undiagnosed.22 Delays in diagnosis can lead to silent disease progression and may accelerate the onset of kidney failure. At this stage, patients usually require KRT, yet the availability of KRT may be limited by costs; the literature suggests access to KRT varies regionally, and 47–73% of patients with kidney failure do not have receive these resource-intensive therapies.23,24 Proactive interventions, such as screening,25,26 earlier diagnosis,22 and earlier treatment27 have been shown to slow disease progression and to reduce clinical burden, including the need for KRT.18, 19, 20, 21, 22, 23, 24 In the absence of these strategies, complications arising from untreated CKD may incur substantial clinical and economic costs.
Investigating across different parameters allows Inside CKD to compare how the general trend of increased prevalence is mediated by demographic factors. Inside CKD used the World Health Organisation classifications and groupings for age, and found a consistent, direct correlation between prevalence of CKD and the proportion of individuals aged 65 years or older. Advanced age may increase vulnerability to comorbid conditions and eGFR decline,1 as well as diminished health-related quality of life.10 Future simulations may use age disaggregation to better understand diverse subpopulations of elderly people in different regions. For example, in the Japanese population, which has a relatively high proportion of older people and a long life-expectancy, stratification of individuals aged 65 years or older may help to refine outcome projections.
Type 2 diabetes and hypertension are common among patients with CKD, and can also accelerate CKD progression.4,6,28 The frequency of both conditions in our data set broadly aligned with age, albeit with some caveats: the highest proportion of type 2 diabetes in the CKD population was projected in Mexico (43.1%), which has a relatively young population. Healthcare interventions focusing on these and other comorbid conditions and their risk factors (e.g. obesity, insulin resistance) may decrease the prevalence and progression of not only CKD but also of related diseases. Exploring cardiovascular complications, we found a high cumulative incidence across the data set, with some variations. Germany had the lowest rate of cardiovascular complications, while unusually high rates were found in Hungary, Poland, Romania, and Japan. The interplay and variability of factors in the Inside CKD countries substantiates the need to consider data on a national level and avoid making direct comparisons between countries/regions.
Disease management is mediated by a complex interplay of socioeconomic and demographic factors. For example, gross domestic product and other income categories may influence healthcare systems and the availability of treatments; just as access to KRT was notably varied, so was the modality of approach. Generally, haemodialysis use was proportionately more prevalent in the Asia–Pacific/Middle East region, with a correspondingly lower overall proportion of kidney transplants than in the Americas and Europe. Our findings substantiate the concerns of global advocates, such as the Kidney Health Initiative, which observed that nearly three times as many people will need KRT as will receive it.29
In terms of limitations, the eGFR slopes used to determine CKD progression were deemed to be consistent across countries/regions, but this assumption merits some reflection: decline rates or CKD progression may be driven by genetic and or environmental factors. Notably, the literature shows differences for key outcomes (for example, mortality) by ethnicity even after adjustment for possible counfounders.30 These differences may indicate a more rapid decline of eGFR due to genetic and/or environmental factors.
The results provided here were contingent on the input data; accordingly, the variation observed among nations' results is attributable not only to distinct demographics (e.g. population size, population ageing, fertility rates) and healthcare infrastructures, but also to disparities in data collection methodologies, temporal differences in input data and nuances in the criteria employed across input sources. This underscores the importance of considering data at a national level and to avoid making direct country comparisons. Gaps and reporting differences in epidemiological data available for input are a common challenge in modelling. For the UAE, United Nations demographic data were utilised for the de facto population, that included Emirati and non-Emirati. A relatively high male:female sex ratio reflects a predominantly male expatriate subgroup within the non-Emirati population. Very little detailed population information is available from the official UAE statistics, and, because data for the whole population (including individuals with and without CKD) were needed, this use of combined statistics for Emirati and non-Emirati was necessary. Our sensitivity analysis suggested that the use of a skewed sex ratio for the Emirati population may not have significantly affected the overall findings, but future research specific to the UAE could explore a greater granularity in the individual Emirate states and their subpopulations (data not shown). It is notable that the UAE has the most rapidly ageing population compared with other countries/regions, which may contribute toward the high projected percentage change of CKD prevalence reported here. The UAE also has one of the smallest national populations that were modelled and outputs such cumulative incidence (i.e. the total sum of incidences that occurred over the analysis period) are directly impacted by population size, and therefore cross-country comparisons should be interpreted carefully. China, as well has having the highest national population, also has a large working age population and a low fertility rate. These unique demographics in addition to national policies aimed at improving health (e.g. Healthy China 2030) may contribute to its relatively low projected percentage change in CKD. Each country/region modelled holds its own unique set of input data, population demographics and country-specific nuances, which warrant careful interpretation.
To the best of our knowledge, we used the most up-to-date and representative epidemiological data for each country or region included in the microsimulation at the time of its first iteration. We acknowledge that new data may have been published in the interim between the first iteration and simulations run for data presented here, and future runs could utilise updated sources. When no suitable data were available for a specific endpoint, a proxy country or region was selected using a project-specific algorithm and informed by an expert panel. Despite our best efforts to ensure comparability, these proxies may not have been fully representative of the population in question. Furthermore, the diverse types of input data used, comprising hospital records, and national statistics, makes it challenging to achieve parity of input definitions for each region. Our literature search highlighted a crucial need for national epidemiological studies of CKD to improve understanding of how discrepancies in healthcare systems may influence patient outcomes. The lack of surveillance that we observed may reflect a low prioritisation in some regions; by promoting awareness of the clinical burden associated with CKD, we hope to encourage improved monitoring and data generation to inform subsequent policy changes. We also acknowledge that periodic updates of literature searches and subsequent microsimulation runs would likely provide useful new information.
Owing to the time of study initiation, the microsimulation did not account for the impact of the COVID-19 pandemic. It is probable that reduced healthcare resources and the interplay of the two diseases affected kidney health, with CKD having influenced COVID-19 disease outcomes and vice versa. The effects of climate change, and the possibility of future pandemics and broad-based geopolitical developments were also not considered; however, the flexibility of dynamic simulation allows future modules to be added that can be tailored to address these or other issues.
In terms of other potential modifications and future iterations, the microsimulation could be extended to quantify the role of other pathologies (e.g., infectious diseases) and the impact of specific interventions on cross-regional health, socioeconomic factors, and health equity. Inside CKD modelled medium- and high-income countries using the World Bank definitions, but low-income countries were not modelled; low-income countries may experience higher incidences of CKD drivers because of discrepancies in access to care and vulnerability to environmental factors. These countries could be included in future iterations of the model and, in general, it would be useful to broaden the participation of different nations; notably representation from Africa would be particularly welcome. Inside CKD could also be adjusted to investigate how specific at-risk populations are affected by low diagnosis rates. By changing key input parameters, such as the potential impact of screening programmes, we could identify the most appropriate interventions for different patient populations.
Informing healthcare policy may stimulate discussion of protective interventions. Our data strongly suggest that persons with type 2 diabetes, obesity, and/or hypertension represent an ‘at risk’ group who would benefit from initiatives to increase awareness of CKD and, ideally, prevent its development. The cumulative incidence of cardiovascular disease within the CKD population observed supports the benefits of holistic approaches to care across clinical disciplines to minimise the risk of complications. Reviewing demographic risk factors and anticipating changing trends may substantiate national-level decisions for lifestyle interventions, for example, anti-smoking or weight-loss campaigns.
In conclusion, Inside CKD projected a global increase in CKD prevalence from 2022 to 2027 that, without intervention, is likely to incur a substantial clinical burden, presenting a clear challenge for global healthcare. Earlier diagnosis combined with timely intervention has the potential to decrease the clinical burden of CKD, protract disease progression, and reduce the use of KRT associated with treating late-stage CKD. In turn, this is likely to lead to a reduced healthcare burden. Our research highlights the need to explore interventions, and ultimately healthcare policy change, to identify people with CKD at an earlier stage of their disease to support timely initiation of treatment aimed at preserving kidney function and preventing complications of CKD.
Contributors
TC, LW and LR contributed to study conceptualisation, data curation, formal analysis, investigation, methodology, visualisation, data interpretation, writing concept creation at outline, and writing review and editing of all drafts. GMC, RCR, K-U E, EK, AK, GL, CFC, PS, SGH and ECH contributed to study conceptualisation, data curation (review of model inputs and source data), resources, validation, data interpretation, writing concept creation at outline, and writing review and editing of all drafts. JJGS, SB, CC and SN contributed to overall study supervision, study conceptualisation, formal analysis, methodology, visualisation, data interpretation, writing concept creation at outline, and writing review and editing of all drafts. All authors have access to the underlying study data. Authors GC, TC, LW, LR and JJGS verify the underlying study data.
Data sharing statement
The data sets generated and/or analysed during the current study are available from the corresponding author on reasonable request. In particular, we note that the extensive portfolio of input data may be of interest.
Declaration of interests
AK has received honoraria for lectures from AstraZeneca. ECH, GL, EK, CFC and RCR have received support from AstraZeneca for their contributions to this work. Stanford University School of Medicine has received grants/contracts from NIDDK, NIAID and CSL Behring on behalf of work conducted by GMC. GMC has received consulting fees from AstraZeneca, Akebia, Ardelyx, Renibus, Miromatrix, Sanifit, Unicycive and Vertex. GMC has received royalties/licences from Elsevier. GMC has participated in advisory boards/data safety monitoring boards by Bayer, Mineralys and ReCor. GMC has a leadership/fiduciary role with Satellite Healthcare Board of Directors (which is non-profit). GMC has stock/stock options at Applaud, CloudCath, Durect, Eliaz Therapeutics, Miromatrix, Outset, Renibus and Unicycive. K-UE has received grants from AstraZeneca, Amgen, Bayer, Evotec and Vifor. K-UE has received consulting fees from Akebia, AstraZeneca, Bayer, Otsuka and Retrophin. K-UE has received honoraria/payment for lectures by AstraZeneca and Bayer. K-UE has participated in advisory boards/data safety monitoring boards by AstraZeneca. LR, LW and TC are employees of HealthLumen Ltd, and AstraZeneca provided funding to HealthLumen Ltd for their contributions to this work. HealThink received funding from AstraZeneca based on the contributions of PS to this work. RCR has received grants/contracts from AstraZeneca. Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán has received grants/contracts from Boehringer Ingelheim, Novo Nordisk, Roche and Baxter on behalf of work conducted by RCR. RCR has received consulting feeds from Chinook, Boehringer, Bayer and Medxl. RCR has received honoraria/payment for lectures by AstraZeneca, Amgen, Boehringer, Novo Nordisk and Bayer. SEHA Kidney Care received honoraria payments from AstraZeneca for the contributions of SGH to this work. JJGS, SB, CC and SN are employees and shareholders of AstraZeneca.
Acknowledgements
The authors would like to thank all investigators participating in the Inside CKD study programme, which was funded by AstraZeneca. The authors would also like to thank Laura Hudson PhD, Rachael Grime PhD and Charlotte Mulcare PhD, for providing medical writing and editorial support on behalf of Oxford PharmaGenesis Ltd, Oxford, UK, which has been funded by AstraZeneca, Cambridge, UK, in accordance with Good Publication Practice (GPP 2022) guidelines (https://www.ismpp.org/gpp-2022).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102614.
Appendix ASupplementary data
References
- 1.Bikbov B., Purcell C.A., Levey A.S., et al. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamb E.J., Levey A.S., Stevens P.E. The kidney disease improving global outcomes (KDIGO) guideline update for chronic kidney disease: evolution not revolution. Clin Chem. 2013;59(3):462–465. doi: 10.1373/clinchem.2012.184259. [DOI] [PubMed] [Google Scholar]
- 3.Ortiz A., Mattace-Raso F., Soler M.J., Fouque D. Ageing meets kidney disease. Clin Kidney J. 2022;15(10):1793–1796. doi: 10.1093/ckj/sfac151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang A., Kramer H. CKD progression: a risky business. Nephrol Dial Transplant. 2012;27(7):2607–2609. doi: 10.1093/ndt/gfs095. [DOI] [PubMed] [Google Scholar]
- 5.Vivante A., Golan E., Tzur D., et al. Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Arch Intern Med. 2012;172(21):1644–1650. doi: 10.1001/2013.jamainternmed.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ku E., Lee B.J., Wei J., Weir M.R. Hypertension in CKD: core curriculum 2019. Am J Kidney Dis. 2019;74(1):120–131. doi: 10.1053/j.ajkd.2018.12.044. [DOI] [PubMed] [Google Scholar]
- 7.Lee M., Saver J.L., Chang K.H., Liao H.W., Chang S.C., Ovbiagele B. Low glomerular filtration rate and risk of stroke: meta-analysis. BMJ. 2010;341 doi: 10.1136/bmj.c4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meisinger C., Döring A., Löwel H. Chronic kidney disease and risk of incident myocardial infarction and all-cause and cardiovascular disease mortality in middle-aged men and women from the general population. Eur Heart J. 2006;27(10):1245–1250. doi: 10.1093/eurheartj/ehi880. [DOI] [PubMed] [Google Scholar]
- 9.George L.K., Koshy S.K.G., Molnar M.Z., et al. Heart failure increases the risk of adverse renal outcomes in patients with normal kidney function. Circ Heart Fail. 2017;10(8) doi: 10.1161/CIRCHEARTFAILURE.116.003825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Artzi-Medvedik R., Kob R., Fabbietti P., et al. Impaired kidney function is associated with lower quality of life among community-dwelling older adults : the screening for CKD among older people across Europe (SCOPE) study. BMC Geriatr. 2020;20(Suppl 1):340. doi: 10.1186/s12877-020-01697-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper J.T., Lloyd A., Sanchez J.J.G., Sörstadius E., Briggs A., McFarlane P. Health related quality of life utility weights for economic evaluation through different stages of chronic kidney disease: a systematic literature review. Health Qual Life Outcome. 2020;18(1):310. doi: 10.1186/s12955-020-01559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foreman K.J., Marquez N., Dolgert A., et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392(10159):2052–2090. doi: 10.1016/S0140-6736(18)31694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carriazo S., Villalvazo P., Ortiz A. More on the invisibility of chronic kidney disease and counting. Clin Kidney J. 2022;15(3):388–392. doi: 10.1093/ckj/sfab240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi M., Shinkawa K., Yanagita M., Kawakami K. Prevalence, recognition and management of chronic kidney disease in Japan: population-based estimate using a healthcare database with routine health checkup data. Clin Kidney J. 2021;14(10):2197–2202. doi: 10.1093/ckj/sfab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Retat L., Pimpin L., Webber L., et al. Screening and brief intervention for obesity in primary care: cost-effectiveness analysis in the BWeL trial. Int J Obes. 2019;43(10):2066–2075. doi: 10.1038/s41366-018-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tangri N., Chadban S., Cabrera C., Retat L., Sánchez J.J.G. Projecting the epidemiological and economic impact of chronic kidney disease using patient-level microsimulation modelling: rationale and methods of inside CKD. Adv Ther. 2023;40(1):265–281. doi: 10.1007/s12325-022-02353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chadban S, Arıcı M, Power A, et al. Projecting the economic burden of chronic kidney disease at the patient level (Inside CKD): a microsimulation modelling study. eClinMed. 2024.
- 18.Levin A., Stevens P.E., Bilous R.W., et al. Kidney disease: improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150. [Google Scholar]
- 19.Pecoits-Filho R., James G., Carrero J.J., et al. Methods and rationale of the DISCOVER CKD global observational study. Clin Kidney J. 2021;14(6):1570–1578. doi: 10.1093/ckj/sfab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eddy D.M., Hollingworth W., Caro J.J., et al. Model transparency and validation: a report of the ISPOR-SMDM modeling Good research practices task force-7. Med Decis Making. 2012;32(5):733–743. doi: 10.1177/0272989X12454579. [DOI] [PubMed] [Google Scholar]
- 21.Sundström J., Bodegard J., Bollmann A., et al. Prevalence, outcomes, and cost of chronic kidney disease in a contemporary population of 2.4 million patients from 11 countries: the CaReMe CKD study. Lancet Reg Health Eur. 2022;20 doi: 10.1016/j.lanepe.2022.100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tangri N., Moriyama T., Schneider M.P., et al. Prevalence of undiagnosed stage 3 chronic kidney disease in France, Germany, Italy, Japan and the USA: results from the multinational observational REVEAL-CKD study. BMJ Open. 2023;13(5) doi: 10.1136/bmjopen-2022-067386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bharati J., Jha V., Levin A. The global kidney health atlas: burden and opportunities to improve kidney health worldwide. Ann Nutr Metabol. 2020;76(suppl 1):25–30. doi: 10.1159/000515329. [DOI] [PubMed] [Google Scholar]
- 24.Liyanage T., Ninomiya T., Jha V., et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385(9981):1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 25.McCullough P.A., Brown W.W., Gannon M.R., et al. Sustainable community-based CKD screening methods employed by the national kidney foundation’s kidney early evaluation program (KEEP) Am J Kidney Dis. 2011;57(3):S4–S8. doi: 10.1053/j.ajkd.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Brown W.W., Collins A., Chen S.-C., et al. Identification of persons at high risk for kidney disease via targeted screening: the NKF Kidney Early Evaluation Program. Kidney Int. 2003;63:S50–S55. doi: 10.1046/j.1523-1755.63.s83.11.x. [DOI] [PubMed] [Google Scholar]
- 27.Zelniker T.A., Wiviott S.D., Raz I., et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139(17):2022–2031. doi: 10.1161/CIRCULATIONAHA.118.038868. [DOI] [PubMed] [Google Scholar]
- 28.Khunti K., Charbonnel B., Chen H., et al. Prevalence and progression of chronic kidney disease among patients with type 2 diabetes: insights from the DISCOVER study. Diabetes Obes Metabol. 2021;23(8):1956–1960. doi: 10.1111/dom.14401. [DOI] [PubMed] [Google Scholar]
- 29.Kidney Health Initiative Kidney replacement therapy (KRT) roadmap. https://khi.asn-online.org/roadmap/
- 30.Jolly S.E., Burrows N.R., Chen S.C., et al. Racial and ethnic differences in mortality among individuals with chronic kidney disease: results from the Kidney Early Evaluation Program (KEEP) Clin J Am Soc Nephrol. 2011;6(8):1858–1865. doi: 10.2215/CJN.00500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.