In this issue of Cell Reports, Xu et al. reveal the transient wave of monocyte-derived macrophage subpopulation (Bhlhe41+ Mφs) post myocardial infarction (MI) dominated in the developing infarct zone endowed with cardiac repair function [1].
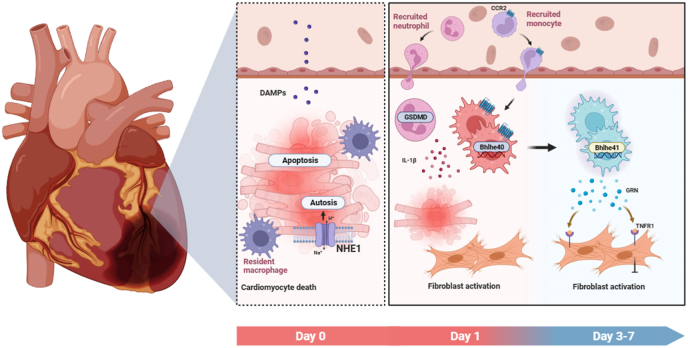
Apoptosis, necrosis, autosis and other cell death patterns in cardiomyocytes are interwoven in response to the ischemic injury. Previous work conducted in the same laboratory demonstrated that the inhibition of the activity of the Na+/H+ exchanger 1 (NHE1) in the cardiomyocytes by sodium–glucose cotransporter 2 (SGLT2) inhibitors can rescue excessive autophagy and reverse ventricular remodeling after MI (see Fig. 1) [2]. The generated cellular corpses from inflammatory cell death initiate the intense sterile inflammation. Neutrophils are the first immune cell type to migrate and infiltrate the infarcted myocardium, the process of which aggravates the myocardial injury by releasing the pro-inflammatory mediators including reactive oxygen species and interleukin-1β (IL-1β). Jiang et al. identified GSDMD, the key component of the NACHT, LRR, and PYD domains-containing protein 3 (NLRP3) inflammasome, mediates the IL-1β release independently of plasma membrane pores and pyroptosis in neutrophils (see Fig. 1) [3].
Fig. 1.
The immune response post myocardial infarction.
Followed by neutrophils, monocytes arrive within the infarcted heart and differentiate into macrophages that replace the cardiac resident macrophages and play an active role in the early initiation of acute inflammation and later in the reparative phase [4]. Given the capacity to sense the various inputs, macrophages are highly plastic in response to complex cardiac microenvironment post MI. In the infarcted heart, cardiac macrophages form a large group of cells with different origins, temporal dynamics and states. In addition, myocardial infarcted zone and areas are also highly heterogeneous, eliciting immune microenvironment that may in turn contribute to reconstructing the macrophage subsets. Thus, delineating the contribution of specific macrophage subtype to cardiac remodeling post-MI in their spatial context will be key to develop novel therapeutics. In the present study, Xu et al. used a combination of single-cell RNA sequencing (scRNA-Seq) and spatially resolved transcriptomics to study the cell-specific changes in gene regulation and signaling pathway, providing an integrated temporal-spatial transcriptomics map of cardiac remodeling after MI. Notably, Xu et al. identified a temporary appearance of monocyte-derived macrophage subpopulation (Bhlhe41+ Mφs) dominated in the developing infarct zone and peaked on the Day 7 post MI, with the capability in suppression of myofibroblast activation and myocardial fibrosis (see Fig. 1). This study reveals that both timing and location shape macrophage phenotype and plasticity, and transient resident macrophage subpopulation involved in the developing infarction cardiac remodeling.
Dynamics of the immune response post MI. The complex immune response is finely orchestrated after the cardiomyocyte death. The acute inflammation (1–3 d) involves a massive influx of myeloid cells, including neutrophils and monocytes. Bhlhe41 is then activated in the monocytes derived macrophages under the exposure of cardiac microenvironment in the reparative phase (3–7 d), serving to suppress the excessive myofibroblast activation and the expand of infarcted region.
CRediT authorship contribution statement
Peihui Zhou: Supervision, Writing – original draft, Writing – review & editing. Suzhen Chen: Supervision, Writing – original draft, Writing – review & editing. Junli Liu: Supervision, Writing – original draft, Writing – review & editing.
Contributor Information
Suzhen Chen, Email: cszdream@163.com.
Junli Liu, Email: liujunli@sjtu.edu.cn.
References
- 1.Xu Y., et al. A transient wave of Bhlhe41(+) resident macrophages enables remodeling of the developing infarcted myocardium. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.113174. [DOI] [PubMed] [Google Scholar]
- 2.Jiang K., et al. Cardioprotective mechanism of SGLT2 inhibitor against myocardial infarction is through reduction of autosis. Protein Cell. 2022;13:336–359. doi: 10.1007/s13238-020-00809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang K., et al. Gasdermin D inhibition confers antineutrophil-mediated cardioprotection in acute myocardial infarction. J Clin Invest. 2022;132 doi: 10.1172/jci151268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prabhu S.D., Frangogiannis N.G. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res. 2016;119:91–112. doi: 10.1161/circresaha.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]