Abstract
Objective
Aortoesophageal fistula is a rare, life-threatening condition. There is no consensus regarding the surgical management of the esophagus in this condition.
Methods
We retrospectively evaluated 13 patients diagnosed with aortoesophageal fistulas at a single institution from 2003 to 2021. Descriptive statistics were used to analyze patient characteristics, operative characteristics, and patient outcomes. Kaplan–Meier survival analysis was performed.
Results
Patients’ mean age was 63.5 years, and 6 (46.2%) were female. The most common presenting symptoms were hemoptysis/hematemesis (69.2%), chest/back pain (46.2%), and fever (38.5%). Twelve patients (92.3%) had a history of aortic procedures. The median time between the index operation and repair of the secondary aortoesophageal fistula in the 12 patients was 5 months. The index operation was a thoracic endovascular aortic repair in 10 of 12 patients (83.3%). Eleven patients (84.6%) underwent primary esophageal repair with flap coverage (omentum or muscle). One of these patients needed an esophagectomy within 1 year. The primary surgical management of the aorta was graft excision and replacement, aside from 1 patient who underwent primary repair. The 30-day survival was 69.2%, and 1-year and 5-year survivals were 31.7%. There were no recurrent infections at the esophageal fistula site.
Conclusions
Aortoesophageal fistula remains a rare condition, but its case numbers have increased with thoracic endovascular aortic repair. It continues to be a difficult condition to manage and has a high fatality rate. Esophageal-preserving surgery may be a safe and less-invasive option for patients with a small defect.
Key Words: AEF, aortoesophageal fistula, esophageal-preserving surgery
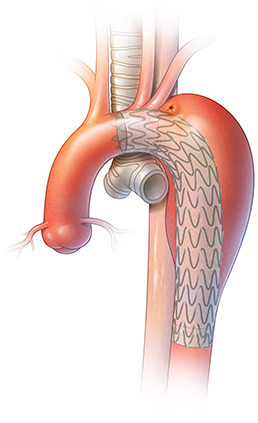
AEF with endovascular stent graft.
Central Message.
AEF is a rare yet life-threatening condition. Aortic graft replacement with concomitant primary repair of the esophagus may be a safe and less-invasive option when the defect is smaller than 3.0 cm.
Perspective.
AEF is a rare, life-threatening condition. We retrospectively evaluated 13 patients diagnosed with AEF at a single institution from 2003 to 2021. We found that AEF continues to be a difficult condition to manage and has a high fatality rate. Esophageal-preserving surgery may be a safe and less-invasive option for patients with a small defect.
Aortoesophageal fistula (AEF) is a rare but deadly condition that consists of a communication between the aorta and esophagus (Figure 1). Primary AEF occurs in the setting of aortic aneurysmal disease. Secondary AEF can be a complication after aortic surgery, after either open aortic graft placement or thoracic endovascular aortic repair (TEVAR). With the increase in use of TEVAR, secondary AEF has become a more common condition.1
Figure 1.
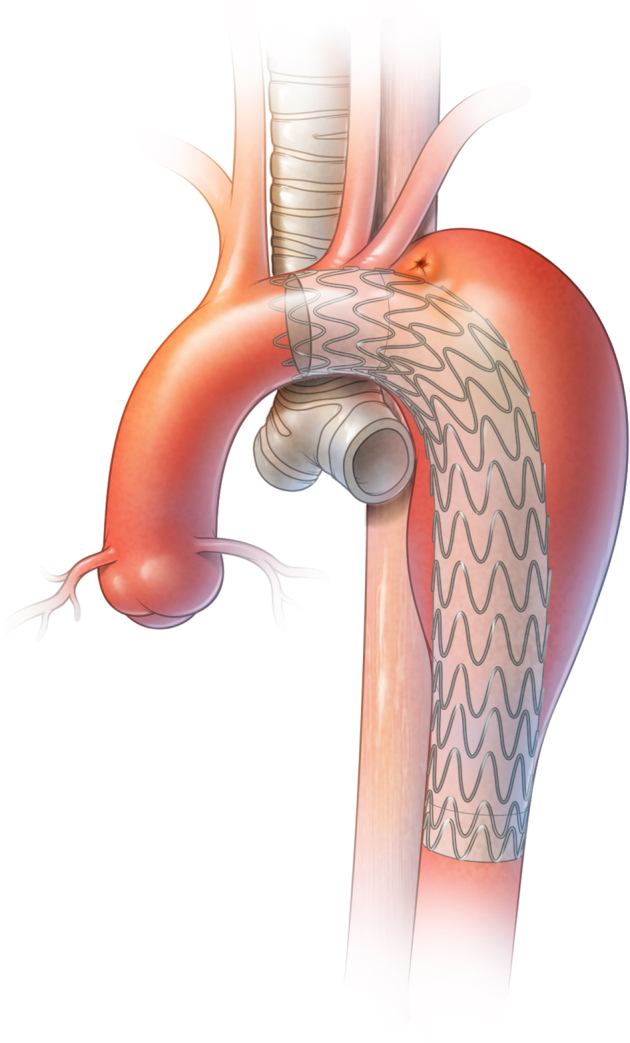
AEF in a patient with an endovascular stent graft.
The management of this condition consists of antimicrobial coverage, repair of the aorta, and repair of the esophagus. Surgical management of the aorta traditionally has been performed through an open approach, but the use of bridging TEVAR for stabilization has been shown as a reasonable adjunct.2 For the esophagus, certain centers prefer esophagectomy because of the ability to completely remove the infectious source as an attempt to prevent postoperative sepsis.3,4 At our institution, we have primarily used an esophageal-sparing approach by repairing the esophagus to minimize surgical insults. There is no consensus on how the esophagus should be surgically managed.
We sought to retrospectively evaluate our institutional experience with AEF management from 2003 to 2021. The focus was on the management of the esophagus to compare our esophageal-preserving strategy with an esophagectomy approach performed at other institutions. We hypothesized that an esophageal-preserving approach can be a safe alternative to esophagectomy.
Material and Methods
Patient Selection and Data Collection
Patients were identified through our prospective registry that includes all patients undergoing open aortic surgery at Memorial Hermann Hospital–Texas Medical Center. Patient consent was obtained to be entered into the registry, which was approved by the Institutional Review Board (Registry Number: HSC-MS-03-077; approval date October 10, 2014). A retrospective review of these patients was then performed. Inclusion criterion was a diagnosis of AEF, either primary or secondary, in patients who underwent subsequent open surgery. Preoperative data consisted of presenting symptoms, medical and surgical history, vital signs, relevant laboratory values (ie, white blood cell count, lactic acid levels), imaging results (ie, chest radiograph, computed tomography [CT] scans), and endoscopic findings, if performed. Operative data consisted of operative approach, surgical management of the aorta and esophagus, and operative complications. Postoperative data consisted of recurrent infection rates, postoperative complications (ie, pneumonia, prolonged intubation, tracheostomy), need for additional surgical interventions, length of stay, and discharge disposition. Follow-up information was collected via chart review, telephone encounters, or the National Death Index. The telephone encounter consisted of following up on any additional surgical interventions if performed outside of our hospital system.
Diagnosis and Initial Management
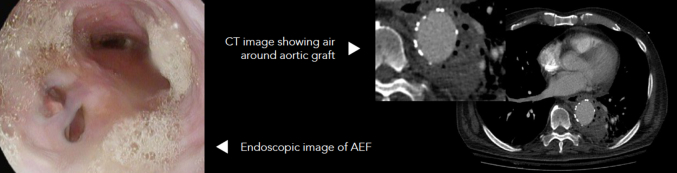
The diagnosis of AEF is multifaceted. It consists of a combination of presenting symptoms, medical and surgical history, laboratory data, imaging findings, and sometimes endoscopic evaluation. Presenting symptoms usually consist of chest/back/abdominal pain, hematemesis or hemoptysis, or nonspecific symptoms, such as fever or malaise. Relevant history is a history of aortic aneurysm, prior aortic graft placement, and prior TEVAR. Laboratory data can show leukocytosis with a left-shift, elevated lactic acid levels, and anemia. Some concerning imaging findings would be pneumomediastinum on chest radiograph or CT scans, or periaortic air seen on CT, especially around an aortic graft. If diagnostic uncertainty remains and the patient is stable, endoscopic evaluation can be performed to look for fistulation. An example of positive findings on a CT scan and endoscopy evaluation are shown in Figure 2. Patients are admitted to the intensive care unit and started on intravenous resuscitation and broad-spectrum antimicrobials for sepsis treatment. Their overall stability determines the urgency of operative intervention. For these patients, preoperative nutritional optimization is not usually available, given the urgency of the intervention, and we did not obtain data of the patients’ preoperative nutritional status.
Figure 2.
Left: Endoscopic evaluation demonstrating a fistula at the 7 o'clock position. Right: CT of AEF fistula. CT, Computed tomography; AEF, aortoesophageal fistula.
Operative Management
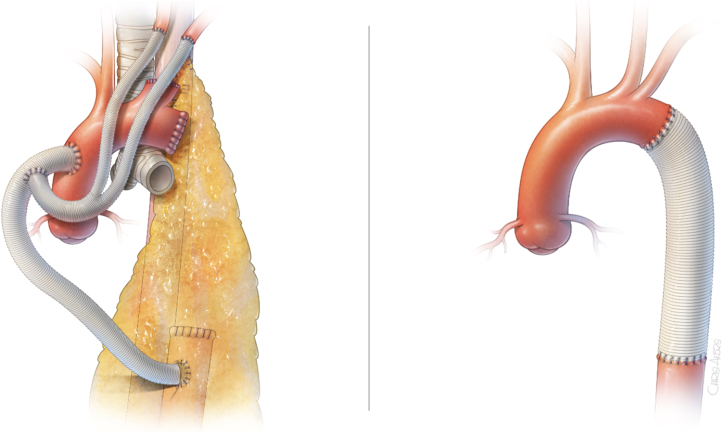
The operation for AEF is performed in a single-stage or 2-stage manner depending on the location of the AEF on the aorta (Figures 3 and 4). Determination between in situ aortic repair and extra-anatomic bypass repair was determined preoperatively based on the area of the aorta involved. If it was isolated to the descending thoracic aorta, in situ repair was feasible, and this was performed in a single-stage manner through a thoracotomy or thoracoabdominal incision (aorta and esophagus). If the AEF involved the proximal descending aorta or the aortic arch, extra-anatomic bypass would be performed through a median sternotomy, and the esophageal repair would follow through a thoracotomy in a 2-stage manner.
Figure 3.
Left: Primary repair of esophagus with omental buttress. Right: partial esophagectomy.
Figure 4.
Left: Extra-anatomic aorta bypass. Right: In situ aorta graft replacement.
Once in the operating room, the patient is placed on cardiopulmonary bypass. For the aorta, the affected area is debrided, the prior graft/endograft is resected, and an antibiotic-soaked graft is placed. For the esophagus, the area involved is then further exposed, and the area is copiously irrigated and debrided. For extra-anatomic reconstruction, a median sternotomy was performed and the bypass was performed via a posterior pericardial approach. The esophageal repair was done in a 2-stage manner, given inadequate exposure via median sternotomy. For in situ reconstruction, a thoracotomy or thoracoabdominal approach was performed, and the esophagus was repaired during that operation. For smaller defects (typically ≤3.0 cm), the esophagus is repaired in 2 layers and buttressed with omental or muscle pedicled flaps to protect the esophageal defect and aortic stent graft. Chest tubes are then placed in the mediastinal space. For large defects (>3.0 cm), a partial esophagectomy was performed, with margins of 3 to 5 cm to the lesion (Figure 3).
Outcomes and Analysis
The primary outcomes for this study focused on mortality and reinfection after primary repair of the esophagus. Additional outcomes were length of stay, need for tracheostomy, need for surgical enteral access, and unplanned surgical reintervention. Descriptive statistics were performed in RStudio (RStudio version 4.2.0, PBC), and Microsoft Excel (Microsoft Corp) was used for creating tables. A time-to-event survival analysis was performed using the Kaplan–Meier method. All data gathered and reported, as well as outcomes, are consistent with the STROBE Statement checklist.
Results
The mean age of patients was 63.5 14.2 years, and 6 of 13 (46.2%) were female. The most common presenting symptoms were hemoptysis/hematemesis (69.2%), chest/back pain (46.2%), and fever (38.5%). One of the patients had a primary AEF from a descending thoracic aortic aneurysm, and the other 12 patients developed secondary AEF after aortic surgery. The index operation was a TEVAR in 10 of the 12 patients (83.3%) with secondary AEF, and the remaining 2 patients (17.7%) had open repair of descending thoracic aortic aneurysms. For the patients undergoing TEVAR, 7 were for a descending thoracic aortic aneurysm, 2 were for an aortic arch aneurysm, and 1 was for an aortic dissection. The median time between the index operation and repair of the AEF was 5 months (interquartile range, 1-33 months). At the time of the operation, all but 1 of the patients had an associated descending thoracic aortic aneurysm. Table 1 displays the patient demographics, surgery strategies, and outcomes.
Table 1.
Patient demographics, surgical strategies, and outcomes
| Patient | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Age, y | 77 | 77 | 41 | 33 | 51 | 68 |
| Gender | M | F | M | M | M | F |
| Previous aortic surgery | TEVAR | TEVAR | TEVAR | TEVAR | TEVAR | -- |
| Presenting symptom(s) | Chest/back pain, fever | Back pain | Chest pain, fever | Fever, hemoptysis | Back pain, fever | Hematemesis |
| Operative approach | ||||||
| Aortic repair | Graft excision and in situ replacement | Graft excision and in situ replacement | Graft excision and extra-anatomic replacement | Graft excision and in situ replacement | Graft excision and extra-anatomic replacement | Graft excision and in situ replacement |
| Esophageal repair | Primary repair, omental flap | Primary repair, intercostal muscle flap | Primary repair, serratus anterior flap | Primary repair, omental flap | Primary repair, omental flap | Primary 3 |
| 30-day outcomes (cause of death) | Alive | Dead (poor intraoperative oxygenation and respiratory failure leading to cardiac arrest shortly after the operation) | Alive | Alive | Alive | Dead (profound coagulopathy and metabolic acidosis postoperatively) |
| Length of stay (days) | 24 | 0 | 31 | 49 | 34 | 1 |
| Late result (1 year) | Dead (postoperative hypoxic respiratory failure leading to cardiac arrest at LTAC) | -- | Alive | Dead (drug overdose) | Dead (upper gastrointestinal bleed—gastric ulcers on endoscopy) | -- |
| Patient | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|
| Age, y | 60 | 72 | 68 | 79 | 74 | 63 | 63 |
| Gender | F | M | M | F | F | M | F |
| Previous aortic surgery | TEVAR | TEVAR | TAR | TEVAR | TEVAR | TEVAR | TAR |
| Presenting symptom(s) | Chest pain fever hematemesis | Hemoptysis hematemesis | Hemoptysis | Fever | -- | Hemoptysis | Chest pain hemoptysis |
| Operative approach | |||||||
| Aortic repair | Graft excision and in situ replacement | Graft excision and in situ replacement | -- | Graft excision and in situ replacement | Graft excision and in situ replacement | Graft excision and in situ replacement | Primary repair |
| Esophageal repair | Esophagectomy (defect = 5.0 cm) |
Primary repair, omental flap | -- | Primary repair, intercostal muscle flap | Primary repair, latissimus dorsi flap | Primary repair, omental flap | Primary repair, omental flap |
| 30-day outcomes (cause of death) | Alive | Alive | Dead (intraoperative cardiac arrest) | Alive | Dead (upper gastrointestinal bleed due to gastric ulcers) | Alive | Alive |
| Length of stay (days) | 29 | 46 | 0 | 36 | 27 | 32 | 7 |
| Late result (1 year) | Lost to follow-up | Alive | -- | Lost to follow-up | -- | Lost to follow-up | Alive |
TEVAR, Thoracic endovascular aortic repair; LTAC, long-term acute care; TAR, total arch replacement.
Of the 13 patients, 12 underwent successful repair, and 1 died intraoperatively. For the esophagus, 11 underwent primary esophageal pair, and 1 underwent upfront esophagectomy. During primary repair, the repair was usually buttressed with omentum, intercostal muscle, or latissimus dorsi muscle pedicled flaps. The 1 patient who required up-front esophagectomy had a larger esophageal defect of 5.0 cm. The primary surgical management of the aorta was graft excision and replacement (in situ N = 9 vs extra-anatomic bypass N = 2), aside from 1 patient who underwent primary repair. Of the patients undergoing primary esophageal repair, 1 (patient No. 3) underwent a partial esophagectomy within 1 year at an outside facility. Patient 4 required an unplanned intervention of the aorta due to false aneurysm formation, which was treated with graft excision and extra-anatomic bypass. Additionally, the median defect size for the esophageal repair patients was 2.0 cm (interquartile range, 1.2-3.0). As described, all patients except 1 survived the AEF operation. The intraoperative death was due to coagulopathy/massive bleeding from a thoracic aortic aneurysm rupture in a patient who presented with an AEF and required emergency repair due to ongoing hemorrhage. Only 1 patient underwent a bridging TEVAR, which was performed at an outside facility.
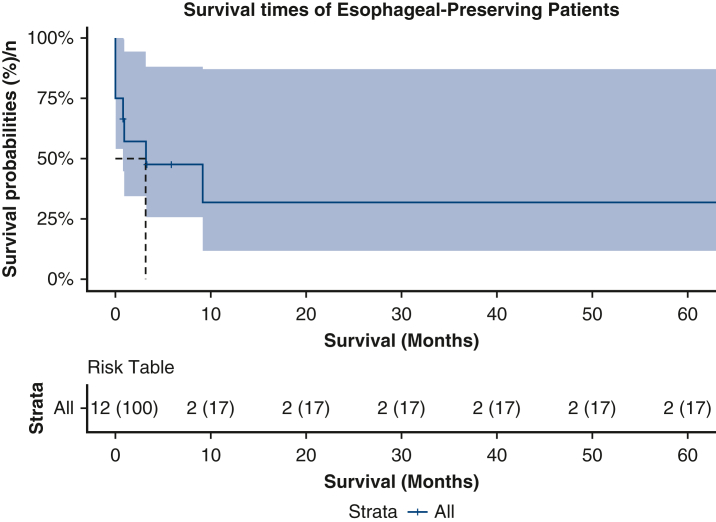
Median hospital length of stay was 31.0 days (interquartile range, 7.0-36.0 days). In terms of postoperative complications, the rate of postoperative pneumonia was 53.8%, tracheostomy was 38.5%, renal failure was 38.5%, stroke was 15.4%, paraplegia was 0%, and sepsis was 53.8%. Persistent sepsis (>7 days postoperatively) was seen in only 1 patient who underwent primary esophageal repair, which eventually resolved with prolonged antibiotic therapy. There were no reinfections of the esophageal fistula site or aortic grafts associated with the primary esophageal repair, although 2 patients had positive blood cultures postoperatively. These cultures were cleared with antibiotic therapy. Of the 8 patients who were successfully discharged (all patients who survived for 30 days besides patient No. 5), 5 (62.5%) were discharged on antimicrobial agents, with 1 (patient No. 13) being discharged on lifelong antimicrobial suppression. Survival at 30 days was 69.2%. The patients who survived were all discharged to a long-term, acute care hospital. Estimated survivals at 1- and 5-year intervals were both 31.7% (95% CI, 11.6%-87.1%) (Figure 5). The patient who underwent esophagectomy was lost to follow-up.
Figure 5.
Kaplan–Meier survival curve of patients undergoing esophageal-preserving surgery for AEF, which includes 95% CIs. AEF, Aortoesophageal fistula.
Discussion
In this retrospective review, we evaluated 13 consecutive patients who underwent surgery for AEF over a 19-year period. The purpose of this evaluation was to ascertain the safety of esophageal preservation in the setting of AEF management and compare our institutional outcomes with esophagectomy outcomes reported at other institutions. Eleven of the 13 patients who underwent open surgical management for AEF at our institution underwent primary repair of the esophagus along with aortic graft replacement. Most early deaths were related to noninfectious causes, such as respiratory failure. Of note, there were no esophageal reinfections or stent-graft infections after the fistula repair and only 1 patient needed a reintervention with esophagectomy at approximately 1 year after repair. AEF continues to be a difficult condition to manage and is relatively rare. The mainstay of managing this condition is early sepsis control through broad-spectrum antibiotic therapy and early surgery. Although recent studies have shown improvements with a “radical operative approach,” we contend that resection and graft placement of the aorta combined with primary repair of the esophagus can be used as an alternative approach in select patients with small esophageal defects.4 We demonstrated that although AEF remains a difficult condition to manage, with a high rate of morbidity and mortality, esophageal preservation can be safe in the right setting: Small esophageal defects, less than 3 cm in size, may be considered for a primary repair.
Initially described by Dubrueil in 1818, AEF has traditionally been associated with Chiari's triad, which is midthoracic chest pain/dysphagia, sentinel hemorrhage, and followed by fatal hemorrhage.5,6 This condition can be classified as primary or secondary AEF. Primary AEF is associated with thoracic aortic aneurysms, malignancy, or foreign body ingestion. Secondary AEF is observed after aortic surgery. The pathogenesis behind AEF formation is not fully understood. The working mechanistic theories involve esophageal ischemia and fistula formation from increased pressure in the posterior mediastinum, inflammation from clot resorption, and aneurysmal compression, among others.7 The rate of secondary AEF has seemingly increased with the increased use of TEVAR, as Czerny and colleauges7 showed a rate of 1.5% across multiple centers after TEVAR. This may be due to the inflammation more seen with TEVAR compared with open surgical repair, and the size of the aneurysm does not shrink immediately after the procedure, unlike in open repair, so the aneurysm continues to pressurize the esophagus.
All patients presenting with AEF require prompt diagnosis, medical management of the infection/sepsis, and surgical treatment. Increased availability and improvements in diagnostic capabilities, such as CT and endoscopy, have greatly improved the ability to detect AEF, which can expedite the initiation of appropriate management. The first successful surgical repair of primary and secondary AEF was described by Yonago and colleagues8 in 1969 followed by Snyder and Crawford in 1983.9 Since then, several case reports and small case series, as well as larger collective analyses, have been performed but the management of this condition remains heterogenous.
AEF remains a condition that should be managed surgically, because prior studies have shown no survivors when managed nonoperatively.7,10 In terms of the aorta, in primary AEF, successful management has been shown to include graft replacement of the aortic aneurysm.11 For secondary AEF, a similar strategy has been shown to be successful with the addition of resecting the graft/endograft before reconstruction.1 With the increased use of endovascular strategies, when a patient is clinically deteriorating due to hemorrhage, TEVAR, with prolonged antibiotics, has been shown as safe for hemorrhage control and as a bridging therapy to open repair in patients who are poor operative candidates.2,12 In our case series, we only included patients who underwent open repair. All but 3 of these patients underwent in situ graft replacement. Extra-anatomic bypass was used in patients with severely infected aortic grafts, which resulted in no deaths within 30 days, but only 1 of those patients survived at 1 year.
Management of the esophagus for patients with AEF remains heterogenous and center specific. One of the first esophageal primary repairs for AEF was described by Sloop and Thompson in 1967.13 They described a repair performed in 2 layers alongside suture repair of the aortic defect. Unfortunately, this patient died of respiratory insufficiency on postoperative day 2. Since Sloop and Thompson's first description of this repair, the management of the esophagus in AEF has evolved to include primary repair, endoluminal stents, and esophagectomy. Over the last 5 years, there have been increased reports of improved outcomes with a “radical surgical approach,” which includes aortic graft replacement and esophagectomy.4,7,14 These reports showed 1-year survival ranging from 28.0% to 42.4%, and one report showed 5-year actuarial survival of 42.4% with this approach. Outside of AEF, esophageal defects are generally closed in 2 layers, as described above. They only require esophageal resection for a defect greater than 10.0 cm, diffuse necrosis, and malignancy. In our center, we prefer an esophageal-preserving approach, given this background information. Our approach has yielded comparable 30-day outcomes, but our 1-year and 5-year estimated survivals were slightly lower. Our hospital length of stay was also shorter than the above reports, which is likely due to early oral feeds that can be achieved with an esophageal-preserving approach.
The decision for the length of antimicrobial suppression postoperatively is patient specific and multidisciplinary. This depends on the level of contamination, the size of the defect, the quality of the surgical debridement, the results of postoperative cultures (if positive, bacterial vs fungal), and what kind of graft (in situ or extra) was used for reconstruction. Of the 8 patients who were discharged in our series, 5 were continued on antimicrobial agents, and 1 was continued on lifelong antimicrobial suppression. In general, this therapy is discontinued within 6 months of the repair. The 1 patient who required lifelong suppression therapy underwent primary repair of the aorta, so inadequate debridement likely facilitated that decision. This is in contrast to patients who undergo TEVAR for the treatment of AEF because they need lifelong antimicrobial suppression.2,12 The use of antimicrobial suppression after open surgery is poorly reported.4
Study Limitations
First, given the retrospective, observational design of our study from a single center, there are inherent biases, such as lack of external validity and reproducibility. Our registry also only contains patients who underwent open surgery, so patients with AEF managed endovascularly might have been missed. We also did not collect data for any patients who might have undergone nonoperative management for this condition. Last, we had limited imaging follow-up (outside of patient No. 3); therefore, the integrity of the repair was not adequately evaluated at intermediate follow-up. However, most other reports are case studies or case series smaller than ours. Thus, our number of patients should strengthen our study compared with the available literature.
Conclusions
AEF is a rare condition, but its case numbers may be increasing with TEVAR. It continues to be a difficult condition to manage and has a high fatality rate. Aortic graft replacement with concomitant primary repair of the esophagus may be a safe and less-invasive option when the esophageal defect is smaller than 3.0 cm.
Conflict of Interest Statement
Dr Estrera is a consultant for WL Gore, CryoLife, Edwards Lifesciences, and Terumo Aortic. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
Institutional Review Board: Registry #HSC-MS-03-077 (Approval Date October 10, 2014).
References
- 1.Sugiyama K., Iwahashi T., Koizumi N., Nishibe T., Fujiyoshi T., Ogino H. Surgical treatment for secondary aortoesophageal fistula. J Cardiothorac Surg. 2020;15(1):251. doi: 10.1186/s13019-020-01293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kubota S., Shiiya N., Shingu Y., et al. Surgical strategy for aortoesophageal fistula in the endovascular era. Gen Thorac Cardiovasc Surg. 2013;61(10):560–564. doi: 10.1007/s11748-013-0280-y. [DOI] [PubMed] [Google Scholar]
- 3.Okita Y., Yamanaka K., Okada K., et al. Strategies for the treatment of aorto-oesophageal fistula. Eur J Cardiothorac Surg. 2014;46(5):894–900. doi: 10.1093/ejcts/ezu094. [DOI] [PubMed] [Google Scholar]
- 4.Takeno S., Ishii H., Nanashima A., Nakamura K. Aortoesophageal fistula: review of trends in the last decade. Surg Today. 2020;50(12):1551–1559. doi: 10.1007/s00595-019-01937-z. [DOI] [PubMed] [Google Scholar]
- 5.Dubrueil O. Observation sur la perforation de l’esophague et de l’aorte thoracique par une portion d’os oval: avec des réflexions. J Univ Sci Med. 1818;9:357–363. [Google Scholar]
- 6.Glodean A., Grobholz R., El-Hag K., Ziaka M., Schmid J.P. Midthoracic pain, sentinel arterial haemorrhage and exsanguination after a symptom-free interval (Chiari's triad) is diagnostic of arterio-oesophageal fistula: a life-threatening cause of gastrointestinal bleeding. Eur J Case Rep Intern Med. 2021;8(3) doi: 10.12890/2021_002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czerny M., Eggebrecht H., Sodeck G., et al. New insights regarding the incidence, presentation and treatment options of aorto-oesophageal fistulation after thoracic endovascular aortic repair: the European Registry of Endovascular Aortic Repair Complications. Eur J Cardiothorac Surg. 2014;45(3):452–457. doi: 10.1093/ejcts/ezt393. [DOI] [PubMed] [Google Scholar]
- 8.Yonago R.H., Iben A.B., Mark J.B. Aortic bypass in the management of aortoesophageal fistula. Ann Thorac Surg. 1969;7(3):235–237. doi: 10.1016/s0003-4975(10)66178-4. [DOI] [PubMed] [Google Scholar]
- 9.Snyder D.M., Crawford E.S. Successful treatment of primary aorta-esophageal fistula resulting from aortic aneurysm. J Thorac Cardiovasc Surg. 1983;85(3):457–463. [PubMed] [Google Scholar]
- 10.Hollander J.E., Quick G. Aortoesophageal fistula: a comprehensive review of the literature. Am J Med. 1991;91(3):279–287. doi: 10.1016/0002-9343(91)90129-l. [DOI] [PubMed] [Google Scholar]
- 11.Reardon M.J., Brewer R.J., LeMaire S.A., Baldwin J.C., Safi H.J. Surgical management of primary aortoesophageal fistula secondary to thoracic aneurysm. Ann Thorac Surg. 2000;69(3):967–970. doi: 10.1016/s0003-4975(99)01087-5. [DOI] [PubMed] [Google Scholar]
- 12.Seal K.S., Abu-Halimah S.J., Dyer B.W., Tobin E.C., Jadue-Tobar A. Staged repair of primary aortoesophageal fistula. Am Surg. 2023;89(9):3864–3866. doi: 10.1177/00031348231173949. [DOI] [PubMed] [Google Scholar]
- 13.Sloop R.D., Thompson J.C. Aorto-esophageal fistula: report of a case and review of literature. Gastroenterology. 1967;53(5):768–777. [PubMed] [Google Scholar]
- 14.Yamazato T., Nakamura T., Abe N., et al. Surgical strategy for the treatment of aortoesophageal fistula. J Thorac Cardiovasc Surg. 2018;155(1):32–40. doi: 10.1016/j.jtcvs.2017.09.047. [DOI] [PubMed] [Google Scholar]