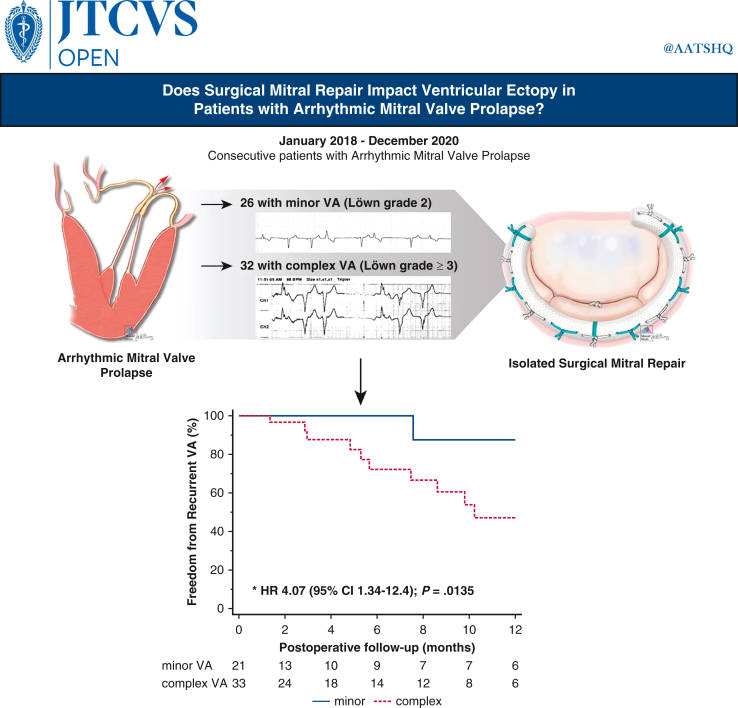
Abstract
Objective
The effect of mitral valve (MV) surgery on the natural history of ventricular arrhythmia (VA) in patients with arrhythmic MV prolapse remains unknown. We sought to evaluate the cumulative incidence of VA at 1 year after surgical mitral repair.
Methods
A retrospective review of progressively captured data identified 204 consecutive patients who underwent elective MV repair for significant degenerative mitral regurgitation as a first-time cardiovascular intervention in a quaternary reference center between January 2018 and December 2020. A subset of 62 consecutive patients with diagnosed arrhythmic MV prolapse was further evaluated for recurrent VA after MV repair.
Results
The median age was 62 years (range, 27-77 years) and 26 of 62 (41.9%) were female. The median time from initial mitral regurgitation/MV prolaspe diagnosis-to-referral was 13.8 years (interquartile range [IQR], 5.4-25) and from VA diagnosis-to-referral was 8 years (IQR, 3-10.6). Using the Lown-Wolf classification, complex VA (Lown grade ≥3) was identified in 36 of 62 patients (58%) at baseline, whereas 8 of 62 (13%) had a cardioverter/defibrillator implanted for primary (4/8) or secondary (4/8) prevention. Left ventricular myocardial scar was confirmed in 23 of 34 (68%) of patients scanned at baseline. The prevailing valve phenotype was bileaflet Barlow (59/62; 95.2%). All patients underwent surgical MV repair by the same team. Surgical repair was stabilized with an annuloplasty prosthesis (median size 36 mm [IQR, 34-38]). Concomitant procedures included tricuspid valve repair (51/62; 82.3%), cryo-maze ± left atrial appendage exclusion (14/62, 23%), and endocardial cryoablation of VA ectopy (4/62; 6.5%). The 30-day and 1-year freedom from recurrent VA were 98.4% and 75.9%, respectively. Absent VA after mitral repair was uniformly observed in patients with minor VA at baseline. Absent VA after mitral repair was uniformly observed in patients with minor VA preoperatively. Complex baseline VA was the strongest predictor of recurrent VA (hazard ratio, 10.8; 95% confidence interval, 1.4-84.2; P = .024), irrespective of myocardial fibrosis.
Conclusions
In a series of 62 consecutive patients operated electively for arrhythmic mitral prolapse, VA remained undetected in 75.9% of patients at 1 year. Freedom from recurrent VA was greater among patients without complex VA preoperatively, whereas baseline Lown grade ≥3 was the strongest independent risk factor for recurrent VA at 1 year. These findings attest to the importance of early recognition and prompt referral of patients with mitral prolapse and progressive VA to specialty interdisciplinary care.
Key Words: arrhythmic mitral valve prolapse, complex ventricular, degenerative mitral valve disease, malignant mitral prolapse, mitral regurgitation, mitral repair outcomes, recurrent ventricular arrhythmia, sudden cardiac death, surgical mitral repair, ventricular ectopy
Graphical Abstract
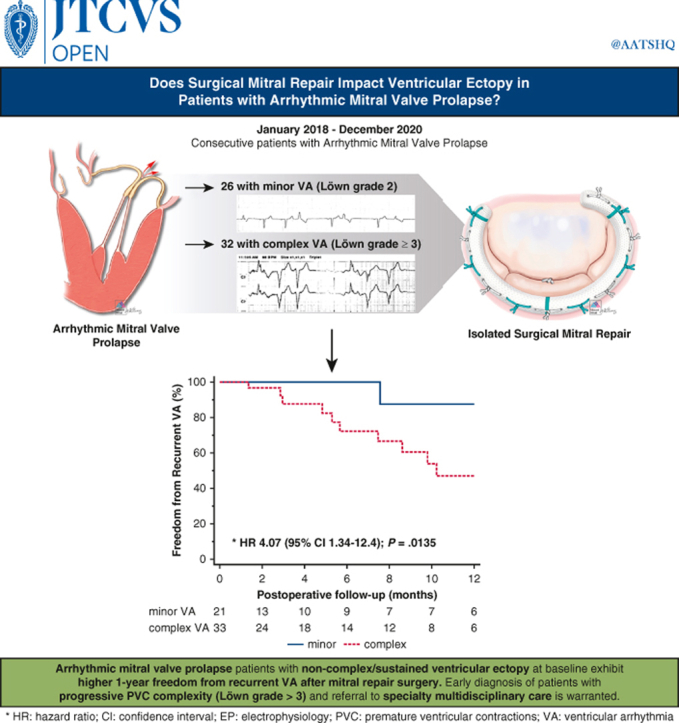
Greater freedom from recurrent VA after mitral repair with less severe VA at baseline.
Central Message.
Arrhythmic mitral valve prolapse patients with noncomplex/sustained ventricular ectopy at baseline exhibit greater 1-year freedom from recurrent ventricular arrhythmia after mitral repair surgery.
Perspective.
The effect of mitral valve repair on ventricular arrhythmia (VA) in arrhythmic mitral valve prolapse patients remains unclear. Our study suggests that complex baseline VA (Lown grade ≥3) is an important independent risk factor for recurrent VA, with a more uniform postoperative effect in patients without complex/sustained VA. Careful follow-up of mitral prolapse patients with complex VA is warranted.
See Discussion on page 114.
The evolving narrative for evaluating the quality of mitral repair has recently involved arrhythmic mitral valve prolapse (AMVP),1 a term used to describe the subset of patients with degenerative mitral prolapse and concomitant ventricular arrhythmia (VA). Although frequent premature ventricular contractions (PVCs) can be observed in up to one-third of patients with mitral prolapse2 irrespective of the degree of regurgitation, between 0.2% and 1.9% may develop sustained VAs and sudden cardiac arrest (SCA).1 The small event rate in addition to incongruent operative outcomes from this patient subset2 hinders the detection of potentially at-risk individuals and limits the generalizability of the reported outcomes to the greater patient demographic with degenerative mitral valve disease. As such, whether corrective mitral surgery may improve the burden of VA and lower the hazard from sudden cardiac death remains unknown,2 mainly because of the contrasting results in relevant literature3, 4, 5, 6, 7 confounded by patient selection, referral, and/or treatment allocation biases observed in the respective studies. Additional limitations arise from variations in VA origin, complexity, and underlying proarrhythmic substrate, which may prevent a uniform result after surgical correction of mitral valve prolapse (MVP).
As evidence from the Society of Thoracic Surgeons Adult Cardiac Surgery Database8 suggests, the outstanding outcomes of mitral repair are a result of standardized operative strategies, selective referral of patients to centers of excellence, and careful patient management by a dedicated heart team.9, 10, 11 We therefore sought to report our experience in patients undergoing mitral valve repair for chronic severe primary mitral regurgitation (MR) who have history of VA.
Methods
Patient Selection
Electronic health records (EHRs) were queried for consecutive index surgical mitral repairs (isolated mitral repair ± tricuspid repair)12 performed electively in our institution as first-time cardiovascular intervention for severe degenerative MR between January 1, 2018, and December 31, 2020. Identified mitral repairs were further ascertained for preoperative diagnosis of PVCs and whether they met criteria for AMVP diagnosis at the time of surgical referral. The study was approved by the Mount Sinai Institutional Review Board (STUDY-22-00800, August 29, 2022) and included a waiver of consent.
Definition of AMVP
The mechanism, disease etiology, and severity of degenerative mitral valve prolapse were confirmed on preoperative transthoracic echocardiography as previously defined.13 VA was stratified as minor (m-VA) or complex (c-VA) according to the Lown-Wolf classification.14 To summarize, c-VA = Lown grade ≥3 (ie, pleiomorphic PVCs, couplets/triplets, ventricular tachycardia [VT]) and m-VA = Lown grade 2 (ie, frequent, isolated unifocal PVCs). Patients with none or rare (<1/min or 30/h) isolated, monomorphic PVCs (Lown grade <2) were classified as non-VA and excluded. AMVP was defined as degenerative mitral prolapse with frequent or c-VA (grade ≥2).
Preoperative Screening
Eligible patients with guideline-recommended indications for mitral surgery15 and documented history of PVCs were routinely referred for formal arrhythmia evaluation as part of our mitral repair reference center’s practice standards for preoperative screening. Select patients with frequent/complex VAs undergo simultaneous 18-labeled fluorodeoxyglucose cardiac positron emission tomography (PET) and magnetic resonance imaging (MRI) with late gadolinium enhancement (LGE) on a hybrid PET/MRI system (Biograph mMR; Siemens Healthineers), as previously described.16 This imaging modality allows for the detection and quantification of myocardial inflammation (PET+) and fibrosis (LGE+), which have been linked to the development of a proarrhythmic substrate for VA in myxomatous mitral prolapse, as recently reported by our group.17,18
Arrhythmia Evaluation and Risk Stratification
Baseline 12-lead electrocardiograms (ECGs; n = 62/62) and ambulatory rhythm monitoring (n = 48/62) were analyzed for the presence, morphology, and ectopic PVC burden. Patients without a baseline rhythm monitoring or if a previous one was not recent (<6 months) underwent a 24-hour Holter monitor followed by mobile cardiac outpatient event monitoring (7-14 days) as previously described.17 New Holter requirement was ascertained according to the severity of baseline VA (ie, multifocal PVCs, couplets, triplets, nonsustained VT). Patients with very high PVC burden (>15%) with a predominant PVC origin morphology on ECG and positive hybrid cardiac PET/MRI uptake signal underwent direct endocardial PVC cryoablation at the time of mitral repair, as previously described.6
Perioperative and Recovery Considerations
Mitral repair was performed according to Carpentier’s principles19 using a combination of reconstructive techniques. The repair was true sized to the anterior leaflet height and stabilized with a mitral annuloplasty prosthesis. Care was taken to minimize endogenous catecholamine circulation and blunt sympathetic surge with very deep narcotic-based intravenous induction and avoidance of potentially arrhythmogenic agents (ie, succinylcholine, epi-/norepinephrine, milrinone) where possible. Rigorous blood preservation, avoidance of pulmonary artery catheterization, and transfusion of blood/products were employed as per standard practice for elective mitral repair.
Follow-Up and Arrhythmia Monitoring
Clinical data from postoperative follow-up visits including physical examination, imaging, 12-lead ECG, Holter monitoring, and/or remote ambulatory telemetry reports were recorded from the cohort patients’ EHRs and analyzed for adverse events including recurrent VA. To capture events from patient follow-up visits recorded in other institutions, we leveraged the health information exchange platform Care Everywhere, provided by Epic (our institutional EHR provider). As of October 2017, more than 1700 hospitals and 34,000 clinics are live on Care Everywhere and connected to more than 70,000 provider sites using other EHRs and health information exchanges.20
Statistical Analysis
Continuous variables are presented as medians with minimum-maximum or interquartile range (IQR). Assessment of between-group event rates was performed using a 2-sample t test or Wilcoxon rank-sum test, as appropriate, for continuous outcomes. Between-group differences were assessed using the χ2 or Fisher exact test for categorical values, as appropriate. Paired sampled t test was used to compare continuous variables with different follow-up intervals. The cumulative probability of postoperative recurrent VA was estimated with Kaplan-Meier analysis. Predictors of recurrent VA were identified using Cox proportional hazards regression analysis. Statistically significant risk factors with P < .15 in univariate analysis were entered in the final multivariable model for backward regression analysis. The results are presented as hazard ratios with corresponding 95% confidence intervals (CIs). All tests are 2-tailed, and a P value < .05 is considered statistically significant. Statistical analysis was performed using Statistics Package for Social Sciences Statistics for Windows (SPSS), version 25 (IBM Corp) and MedCalc, version 22.007 for Windows (MedCalc Software Ltd).
Results
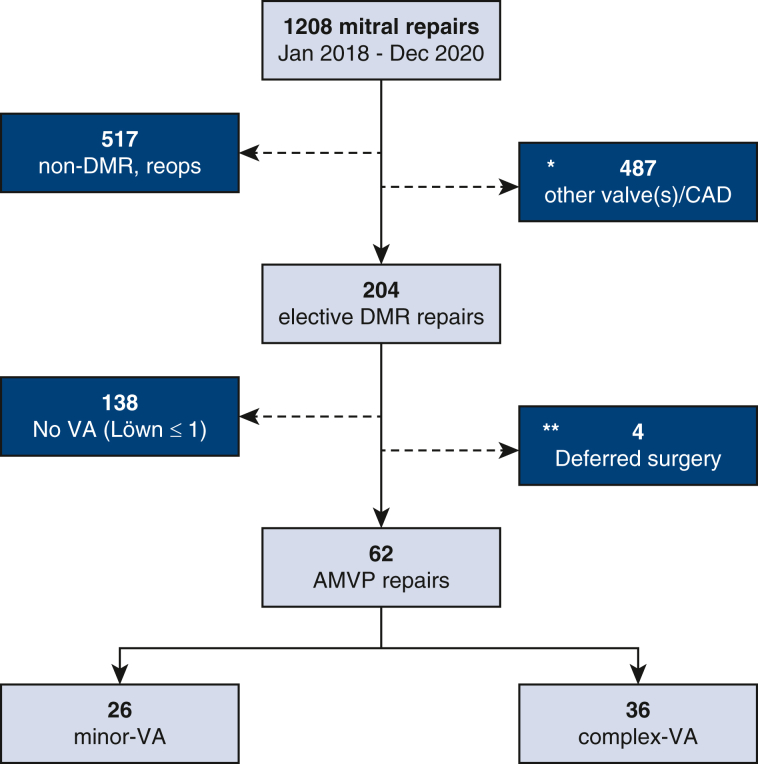
From a cohort of 204 consecutive, unselected surgical mitral repair cases operated for severe degenerative MR as a first-time cardiovascular procedure, 62 patients met criteria for AMVP and were included for analysis. The final cohort (N = 62) comprised 36 patients with c-VA (58%) and 26 patients with m-VA (42%) (Figure E1).
Figure E1.
Study population flow gram. ∗Excluded cases with concomitant moderate or greater aortic/pulmonary valve disease, significant CAD (including previous coronary intervention), or need for concomitant CABG. ∗∗Patients with AMVP deferred mitral surgery because of absent clinical symptoms. CAD, Coronary artery disease; DMR, degenerative mitral regurgitation; AMVP, arrhythmic mitral valve prolapse; VA, ventricular arrhythmia; CABG, coronary artery bypass graft surgery.
Patient Characteristics
Baseline demographics, comorbidities, imaging, arrhythmia, and ECG-derived data are summarized in Table 1. MVP or MR (whichever came first) was diagnosed between the third and fourth decade of life (40.3 ± 17.2 years; 95% CI for the mean: 35.9-44.6). Diagnosis of VA was established on average 9 years after initial diagnosis of MVP/MR prolapse (95% CI for Δ: 3.95-14.5; P = .0008). Patients with c-VA were relatively younger at the time of surgery (55 vs 63.5 years old; P = .029) and diagnosed with VA at a younger age (47 vs 57.4 years old; P = .007). Self-reported familial mitral prolapse clustering, atrial fibrillation, and preoperative antiarrhythmic therapy (27.4%, 21%, and 37.1%, respectively) showed a trend for greater frequency in patients with c-VA. Of note, 75% of survivors of SCA endorsed a positive family history of mitral prolapse. Cardiac chamber dimensions and function were similar between VA subsets, including left ventricular (LV) strain and mechanical dispersion. Bileaflet prolapse was more common in patients with c-VA compared with m-VA (75% vs 50%; P = .04). Stratified by gender, bileaflet distribution was skewed toward male subject (67.7%; P = .005) with a trend favoring the c-VA subset (41.7% vs 19.2%; P = .064).
Table 1.
Patient characteristics
| n (%)/median (IQR) | All patients (N = 62) | m-VA (n = 26) | c-VA (n = 36) | P value |
|---|---|---|---|---|
| Demographics and comorbidities | ||||
| Age, y | 59.5 (51-65) | 63.5 (56-67) | 55 (49.5-62.5) | .029 |
| Age at MR/MVP diagnosis, y | 42.4 (27.5-53) | 50.5 (31-58) | 41.2 (23-51) | .098 |
| Age at VA diagnosis, y | 50.8 (38-58.4) | 57.4 (47.5-63.7) | 46.7 (37.8-54.9) | .007 |
| Symptoms functional class | .145 | |||
| NYHA I | 29 (46.8) | 15 (57.7) | 14 (38.9) | |
| NYHA II | 28 (45.2) | 8 (30.8) | 20 (55.6) | |
| NYHA III | 5 (8.1) | 3 (11.5) | 2 (5.6) | |
| Female | 26 (41.9) | 10 (38.5) | 16 (44.4) | .64 |
| Familial MVP | 17 (27.4) | 5 (19.2) | 12 (33.3) | .223 |
| Hypertension | 28 (45.2) | 11 (42.3) | 17 (47.2) | .704 |
| Diabetes mellitus | 2 (3.2) | 1 (3.8) | 1 (2.8) | .816 |
| Chronic lung disease | 3 (4.8) | 2 (7.7) | 1 (2.8) | .377 |
| Smoking | 15 (24.2) | 7 (26.9) | 8 (22.2) | .902 |
| Obstructive sleep apnea | 3 (4.8) | 0 (0) | 3 (8.3) | .134 |
| Secondary PHT | 22 (35.5) | 10 (38.5) | 12 (33.3) | .679 |
| Atrial fibrillation/flutter | 13 (21) | 3 (11.5) | 10 (27.8) | .124 |
| BP systolic, mm Hg | 130 (118-137) | 130 (120-142) | 127 (112.5-132.5) | .157 |
| BP diastolic, mm Hg | 73.9 (68-81) | 78.5 (68-83) | 74 (68-80) | .461 |
| Pulse Pressure, mm Hg | 53 (44-63) | 58 (50-64) | 50 (44-60) | .139 |
| Antiarrhythmic therapy | 23 (37.1) | 7 (26.9) | 16 (44.4) | .162 |
| Diuretic therapy | 7 (11.3) | 3 (11.5) | 4 (11.1) | .958 |
| Serum K+, mEq/L | 4.2 (4.1-4.4) | 4.2 (4-4.4) | 4.3 (4.1-4.5) | .271 |
| BNP, pg/mL | 59 (26.9-108.2) | 57.6 (23.8-123) | 59 (29.8-105) | .825 |
| Multimodality imaging | ||||
| Resting echocardiography | 62 (100) | 26 (100) | 36 (100) | – |
| LV EF, % | 63 (59-65) | 64 (60-68) | 62 (57.8-65) | .167 |
| LVEDDi, cm/m2 | 2.92 (2.7-3.2) | 2.87 (2.6-3.1) | 2.95 (2.67-3.2) | .617 |
| LVESDi, cm/m2 | 1.87 (1.67-2) | 1.85 (1.7-2.06) | 1.87 (1.66-2.05) | .96 |
| LV mass(i), g/m2 (range) | 117.5 (100-131.6) | 118.6 (97.7-131.7) | 116.9 (102.4-131.4) | .765 |
| LV wall stress (×10³), dyn/cm2 | 69.7 (51.2-83.3) | 74.6 (56.5-81.2) | 67.6 (48.4-88.3) | .485 |
| Peak GLS, % | −25.5 (−27 to −23.5) | −25.9 (−26.8 to −24.3) | −25 (−27.3 to −23.1) | .315 |
| LV mechanical dispersion, ms | 50 (33.7-80.2) | 40.9 (30.7-81) | 52.2 (36.9-78.7) | .253 |
| LAVi, mL/m2 | 61.7 (54.2-89.6) | 67.6 (54.3-91.4) | 60.4 (54-83) | .406 |
| PASP, mm Hg | 28 (24-40) | 27.3 (25-43) | 28 (23.4-40) | .196 |
| MV E/A | 1.5 (1.13-1.77) | 1.43 (1.15-1.66) | 1.47 (1.13-1.87) | .523 |
| MV E/e' | 9.9 (7.3-12.9) | 10.1 (8.9-14.1) | 8.8 (7.3-12.5) | .28 |
| Diastolic filling time, ms | 512 (423-602) | 488.7 (400.3-572.5) | 513.5 (433-624) | .330 |
| Bileaflet prolapse | 40 (64.5) | 13 (50) | 27 (75) | .04 |
| Bileaflet prolapse—female patients | 20 (32.3) | 5 (19.2) | 15 (41.7) | .064 |
| Cardiac MRI | 36 (58.1) | 13 (50) | 23 (63.9) | .278 |
| LV scar/fibrosis (LGE+) | 24 (68.6) | 9 (69.2) | 15 (68.2) | .739 |
| Preoperative hybrid PET/MRI | 22 (35.5) | 10 (38.5) | 12 (33.3) | .679 |
| LV inflammation (FDG+) | 19 (86.4) | 7 (70) | 12 (100) | .046 |
| Coronary anatomy | .168 | |||
| Normal coronaries | 44 (71) | 16 (61.5) | 28 (77.8) | |
| Nonobstructive CAD | 18 (29) | 10 (38.5) | 8 (22.2) | |
| Baseline ventricular arrhythmia profile, complexity, and ECG characteristics | ||||
| MR diagnosis to VA, y | 9 (4.4-22); (n = 45) | 8 (3-20); (n = 22) | 13 (6-27); (n = 23) | .146 |
| VA diagnosis to MR, y | 2 (1.9-3.5); (n = 17) | 2.5 (1.9-3); (n = 4) | 2 (1.8-6); (n = 13) | .817 |
| Ambulatory rhythm monitor | 48 (77.4) | 13 (50) | 35 (97.2) | <.0001 |
| VA burden, % (range) | 0.97 (0.01-47) | 0.2 (0.01-10) | 1.53 (0.1-47) | .006 |
| Bigeminy | 24 (39.3) | 2 (7.7) | 22 (62.9) | – |
| Pleiomorphic PVCs | 21 (33.9) | 21 (58.3) | ||
| Ventricular couplets | 26 (41.9) | 26 (72.2) | ||
| Ventricular tachycardia | 30 (48.4) | 30 (83.3) | ||
| VA-related syncope | 10 (16.1) | 10 (27.8) | – | |
| Sudden cardiac arrest | 4 (6.5) | 4 (11.1) | – | |
| ICD | 8 (12.9) | 8 (22.2) | – | |
| Heart rate, beats/min | 66.5 (59-79) | 68.5 (63-80) | 65 (58.5-74) | .288 |
| Sinus bradycardia | 16 (25.8) | 5 (19.2) | 11 (30.6) | .318 |
| QTc, ms | 434.5 (420-446) | 434.5 (421-453) | 434.5 (420-446) | .643 |
| QTc (age-adj), ms | 433.4 (431-435) | 434.6 (432.4-435.6) | 432.1 (430.5-434.3) | .029 |
| Prolonged QTc | 22 (35.5) | 11 (42.3) | 11 (30.6) | .343 |
| Prolonged QTc, ms | 451.5 (446-457) | 454 (442.8-466.3) | 448 (446.3-455.8) | .645 |
| Prolonged QTc (age-adj), ms | 434.1 (432.7-434.7) | 434.7 (434.1-435.7) | 433.6 (428.9-434.4) | .012 |
| QRS, ms (range) | 97 (88-104) | 98 (90-102) | 95 (86-104) | .943 |
| Nonspecific ST-T | 15 (24.2) | 7 (26.9) | 8 (22.2) | .672 |
| Repolarization abnormalities | 9 (14.5) | 4 (15.4) | 5 (13.9) | .870 |
| Inverted/biphasic T-waves | 31 (50) | 13 (50) | 18 (50) | – |
| Infarct pattern | 23 (37.1) | 8 (30.8) | 15 (41.7) | .385 |
| T-wave inversion—inferior | 7 (30.4) | 1 (12.5) | 6 (40) | .182 |
| T-wave inversion—anterior | 16 (69.6) | 7 (87.5) | 9 (60) | |
| Conduction delay | 21 (33.9) | 5 (19.2) | 16 (44.4) | .040 |
| 1° AV block | 8 (12.9) | 2 (7.7) | 6 (16.7) | .302 |
| Fascicular block | 13 (21) | 3 (11.5) | 10 (27.8) | .124 |
| LBBB | 1 (7.7) | 1 (33.3) | – | .067 |
| RBBB | 12 (92.3) | 2 (66.7) | 10 (100) | |
| LVH-ECG voltage criteria | 17 (27.4) | 6 (23.1) | 11 (30.6) | .518 |
IQR, Interquartile range; m-VA, minor ventricular arrhythmia; c-VA, complex ventricular arrhythmia; MR, mitral regurgitation; MVP, mitral valve prolapse; VA, ventricular arrhythmia; NYHA, New York Heart Association; PHT, pulmonary hypertension; BP, blood pressure; BNP, brain natriuretic peptide; LV, left ventricle/-cular; EF, ejection fraction; EDDi, end-diastolic diameter index; ESDi, end-systolic diameter index; GLS, global longitudinal strain; LAVi, left atrial volume index; PASP, pulmonary arterial systolic pressure; MV, mitral valve; MRI, magnetic resonance imaging; LGE, late gadolinium enhancement; PET, positron emission tomography; FDG, fluorodeoxyglucose; CAD, coronary artery disease; ECG, electrocardiogram; MR, mitral regurgitation; PVC, premature ventricular contraction; ICD, implantable cardioverter/defibrillator; AV, atrioventricular; LBBB, left bundle branch block; RBBB, right bundle branch block; LVH, left ventricular hypertrophy.
Myocardial fibrosis was identified in 68.6% of scanned patients and similar between VA subgroups. Focal or focal-on-diffuse inflammation (PET+) was detected in 86.4% of scanned patients and was concordant with areas of myocardial fibrosis (LGE+) in 84.2% of patients. Preoperative ambulatory rhythm monitoring (Holter/implantable cardiac defibrillator [ICD] interrogation) was completed in 35 of 36 patients with c-VA (97.2%) and 13 of 26 (50%) m-VA. The median baseline VA burden was overall low (0.97%) but significantly greater in c-VA, which consisted of 83.3% (30/36) patients with a history of VT and greater prevalence of conduction delays, predominantly right bundle branch block pattern.
Operative Characteristics
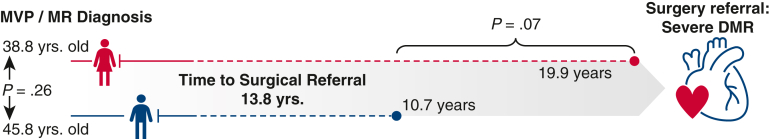
Patients were referred for mitral repair within 13.8 years (IQR, 5.4-24.8) after initial MVP/MR diagnosis. Of note, referrals of women tended to be delayed compared with referrals of men (19.9 vs 10.7 years; P = .07) despite their relatively earlier MVP diagnosis (Figure E2). Surgical referral was triggered primarily by functional deterioration (45.2%) followed by onset of symptomatic palpitations and/or progression of MR severity (21% for both). The operative risk profile was balanced between VA subsets with 0.42% median predicted risk of mortality. The predominant MVP phenotype was bileaflet Barlow disease with at least one flail leaflet segment identified in more than half of the study cohort, notably similar between VA subsets, whereas fibroelastic deficiency and isolated anterior leaflet prolapse were noted in a minority of patients with c-VA. Tricuspid valve repair was the most frequent concomitant procedure (82.3%), followed by atrial fibrillation cryoablation ± left atrial appendage exclusion (22.6%), and endocardial cryoablation of VA in 4 patients (6.5%), respectively (Table 2).
Figure E2.
Surgical referral practice patterns. The median time to surgical referral was 13.8 years from initial diagnosis of mitral prolapse or regurgitation (whichever came first). Female patients were diagnosed relatively earlier than male patients but were referred later for surgical mitral repair. MVP, Mitral valve prolapse; MR, mitral regurgitation; DMR, degenerative mitral regurgitation.
Table 2.
Surgical referral and operative characteristics
| n (%)/median (IQR) | All patients (N = 62) | m-VA (n = 26) | c-VA (n = 36) | P |
|---|---|---|---|---|
| MR diagnosis to surgery, y (range) | 13.8 (5.4-24.8) | 10.8 (5.4-20.4) | 16.4 (5.4-27.6) | .339 |
| Surgical referral trigger | ||||
| Dyspnea/exercise tolerance | 28 (45.2) | 9 (34.6) | 19 (52.8) | .301 |
| MR severity progression | 13 (21) | 8 (30.8) | 5 (13.9) | |
| Palpitations | 13 (21) | 5 (19.2) | 8 (22.2) | |
| Worsening LV dimensions/EF | 8 (12.9) | 4 (15.4) | 4 (11.1) | |
| Operative indication | ||||
| ACC/AHA class I | 43 (69.4) | 16 (61.5) | 27 (75) | .260 |
| ACC/AHA class IIa | 19 (30.6) | 10 (38.5) | 9 (25) | |
| STS PROM, % | 0.42 (0.28-0.72) | 0.39 (0.24-0.74) | 0.43 (0.3-0.68) | .804 |
| DMR etiology | ||||
| Barlow—bileaflet | 42 (67.7) | 17 (65.4) | 25 (69.4) | .674 |
| Barlow—forme-fruste | 16 (25.8) | 7 (26.9) | 9 (25) | |
| Fibroelastic deficiency | 3 (4.8) | 2 (7.7) | 1 (2.8) | |
| Anterior leaflet prolapse | 1 (1.6) | 1 (2.8) | ||
| Flail (chordal rupture/elongation) | 36 (58.1) | 17 (65.4) | 19 (52.8) | .325 |
| Segments repaired, n (range) | 2 (1-6) | 3 (1-6) | 2 (1-5) | .526 |
| Repair complexity score | 3 (1-5) | 3 (1-6) | 2 (1-5) | .285 |
| Simple | 19 (30.6) | 7 (26.9) | 12 (33.3) | .824 |
| Intermediate | 26 (41.9) | 11 (42.3) | 15 (41.7) | |
| Complex | 17 (27.4) | 8 (30.8) | 9 (25) | |
| Mitral ring size, mm | 36 (31-34) | 36 (32-38) | 36 (34-38) | .514 |
| Mitral annuloplasty prosthesis | ||||
| Flexible band | 31 (50) | 13 (50) | 18 (50) | – |
| Remodeling ring | 31 (50) | 13 (50) | 18 (50) | |
| Concomitant procedures | ||||
| Tricuspid valve repair | 51 (82.3) | 23 (88.5) | 28 (77.8) | .281 |
| LAA exclusion | 16 (25.8) | 4 (15.4) | 12 (33.3) | .114 |
| AF cryoablation | 14 (22.6) | 3 (11.5) | 11 (30.6) | .079 |
| Cox-maze IV | 9 (64.3) | 2 (22.2) | 7 (77.8) | .926 |
| Box PVI | 5 (35.7) | 1 (20) | 4 (80) | |
| PFO closure | 9 (14.5) | 3 (11.5) | 6 (16.7) | .575 |
| AICD leads exchange | 2 (3.2) | – | 2 (5.6) | .226 |
| CPB, min | 118.5 (94-132) | 109 (87-131) | 120 (101-136.5) | .095 |
| Crossclamp, min | 88.5 (72-103) | 83 (67.8-104) | 95 (80.3-102.8) | .245 |
| ICU stay, d | 1.1 (0.93-2.08) | 1.02 (0.9-1.9) | 1.45 (0.93-2.9) | .166 |
| Hospital stay, d | 6.5 (5-9) | 7 (5-9) | 6 (5-8.5) | .629 |
| Discharge antiarrhythmic | ||||
| Beta-blocker | 36 (58.1) | 17 (65.4) | 19 (52.8) | .212 |
| Beta-blocker + amiodarone | 15 (24.2) | 7 (26.9) | 8 (22.2) | |
| Beta-blocker CI | 11 (17.7) | 2 (7.7) | 9 (25) |
IQR, Interquartile range; m-VA, minor ventricular arrhythmia; c-VA, complex ventricular arrhythmia; MR, mitral regurgitation; LV, left ventricle/-cular; EF, ejection fraction; ACC, American College of Cardiology; AHA, American Heart Association; STS PROM, Society of Thoracic Surgeons Predicted Risk Of Mortality; DMR, degenerative mitral regurgitation LAA, left atrial appendage; AF, atrial fibrillation; PVI, pulmonary vein isolation. PFO, patent foramen ovale; AICD, automated implantable cardioverter defibrillator; CPB, cardiopulmonary bypass; ICU, intensive care unit; CI, contraindication.
Procedural Outcomes
Patients were discharged home within 6.5 days (IQR, 5-9). Residual MR was trace/none in 61 of 62 patients (98.4%) and mild in one, at predischarge echocardiography. No operative death, myocardial infarction, recurrent MR, cardiac reintervention, or new pacemaker implantation were observed at 30-day and 1-year follow-up (Table 3). One patient was reported with asymptomatic mild-moderate MR at 6-month surveillance echocardiography, which remained stable without symptoms and LV ejection fraction of 70% at 1 year.
Table 3.
Operative outcomes
| n (%) | All patients (N = 62) | m-VA (n = 26) | c-VA (n = 36) | P |
|---|---|---|---|---|
| MAE (at 30 d) | 5 (8.1%) | 3 (11.5%) | 2 (5.6%) | .397 |
| Death | – | – | – | – |
| MI | – | – | – | – |
| Stroke | 1 (1.6%) | 1 (3.8%) | – | .239 |
| Acute kidney injury | 1 (1.6%) | – | 1 (2.8%) | .395 |
| Respiratory failure | 3 (4.8%) | 2 (7.7%) | 1 (2.8%) | .377 |
| Major bleeding | – | – | – | – |
| New PPM | – | – | – | – |
| Cardiac reintervention (any) | – | – | – | – |
| Residual MR >1+ | – | – | – | – |
| Recurrent VA | 1 (1.6%) | – | 1 (2.8%) | .395 |
| MAE (at 1 y) | All (N = 54) | m-VA (n = 21) | c-VA (n = 33) | |
| Recurrent MR >1+ | – | – | – | – |
| Cardiac-related hospital admission | – | – | – | – |
| Cardiac reintervention (any) | – | – | – | – |
| Recurrent VA | 13 (24.1%) | 1 (4.8%) | 12 (36.4%) | .009 |
m-VA, Minor ventricular arrhythmia; c-VA, complex ventricular arrhythmia; MAE, major adverse events; MI, myocardial infarction; PPM, permanent pacemaker; MR, mitral regurgitation; VA, ventricular arrhythmia.
Cryoablation of Ventricular Ectopy Foci
Endocardial cryoablation of ventricular arrhythmia foci was performed in 4 patients with c-VA: 2 bilateral papillary muscle cryoablation, 1 posteromedial papillary muscle only, and 1 LV summit cryoablation at the base of the left coronary aortic leaflet and into the interventricular septum, respectively. At 1 year, PVC burden decreased significantly in the former 2 (1.9% from 42%, and 11.4% from 20%, respectively) and was fully abated in the latter 2 cases, on pairwise comparison of Holter and/or remote ambulatory rhythm telemetry data at baseline and follow-up.
Postoperative Antiarrhythmic Regime
Unless contraindicated (ie, bradycardia [4/62] or hypotension [3/62]; atrioventricular block [4/62]), patients were discharged on low-dose metoprolol (n = 51; 82.3%). A tapered dose of oral amiodarone was added (n = 15; 24.2%) for the management of supraventricular tachyarrhythmias (12/15; 80%) and nonsustained ventricular ectopy (3/15; 20%) during postoperative recovery (Table 2). After hospital discharge, titration of antiarrhythmic therapy was managed by the respective patient’s referring cardiology service.
Follow-up Data Collection
One-year data including clinical events, echocardiography, and rhythm monitoring were available for 54 of 62 patients (87%); 8 patients were lost to follow-up. Medication prescription data were available for 50 of 54 (92.6%) of patients at 1 year.
VA Recurrence
The median follow-up was 11.2 months (IQR, 1.6-30.7). All patients were discharged in normal sinus rhythm and 61 of 62 (98.4%) remained in normal sinus rhythm without recurrent VA at 30 days based on continuous rhythm monitoring (including serial 12-lead ECG, Holter, and/or interrogation of remote ambulatory telemetry recordings, accordingly). One patient developed recurrent, low-burden VA (2%) at postoperative day 27, after discontinuing their antiarrhythmic because of fatigue. Stratified by baseline VA profile, 30-day freedom from recurrent VA was 100% for m-VA and 97.2% for c-VA patient subsets (P = .395). At 1 year, antiarrhythmic therapy was maintained in 70% of patients with comparable rates between m-VA and c-VA subsets (77.8% vs 65.6%; P = .408).
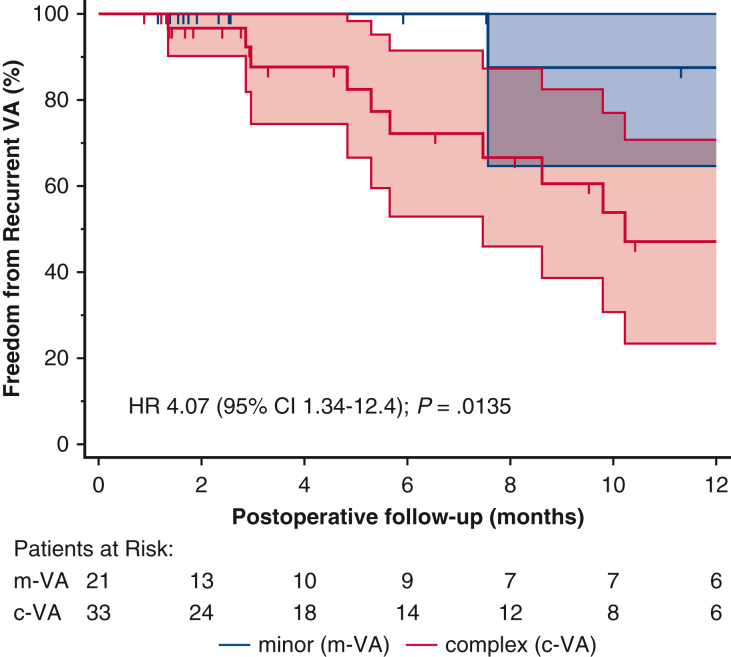
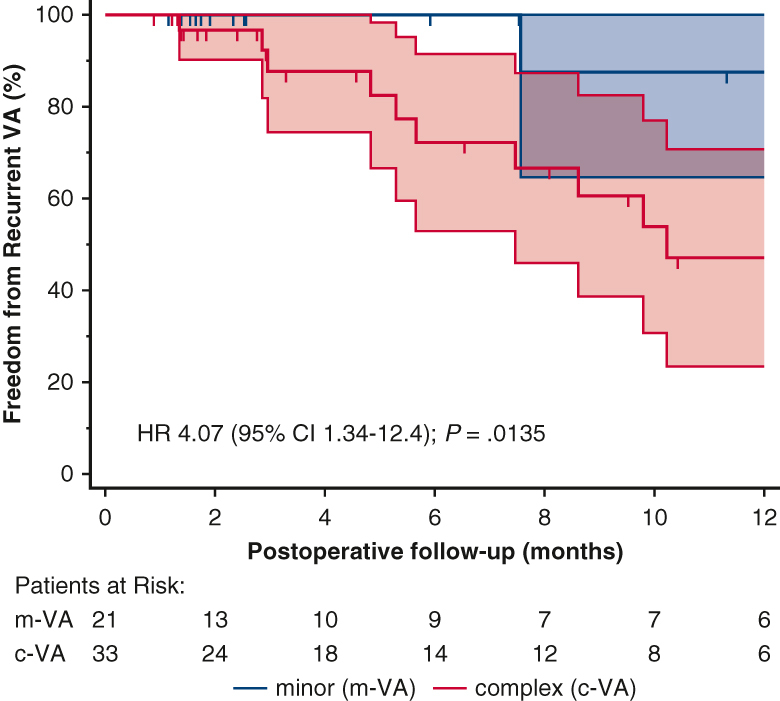
The cumulative incidence of recurrent VA at 1 year was 24.1% (n = 13/54; Table 3) of whom 12 (92.3%; P = .009) were c-VA with preexisting VT. Remarkably, no further VT events were recorded postoperatively in 8 of those 12 patients (66.7%) on follow-up Holter and/or remote ambulatory telemetry. Four patients, including the survivor of SCA, had rare (1, 3, 2, and 1 event, respectively), asymptomatic nonsustained VT events on 1-year follow-up Holter (n = 3) and ICD telemetry (n = 1) and were maintained on antiarrhythmic therapy. Of note, 92.3% of patients with recurrent VA were on active antiarrhythmic therapy, considerably more compared with patients on antiarrhythmic medication but no VA recurrence at 1 year (62.2%; P = .043). The overall freedom from recurrent VA at 1 year was 75.9% and greater for m-VA compared with the c-VA subset (95.2% vs 63.6%; P = .009, Table 3). Patients with c-VA were 4 times more likely to experience postoperative VA recurrence (95% CI 1.3-12.4; P = .014, Figure 1). Furthermore, the median postoperative VA burden was reduced significantly in 7 of 13 (53.8%) patients with recurrent VA compared with baseline (1.9% from 20%; P = .016 for the median Δ: −18.9%) and remained stable at a low grade (0.9%) in the remaining 5 recurrent VA cases on pairwise comparison.
Figure 1.
Arrhythmic mitral valve prolapse patients with non-complex/sustained ventricular ectopy at baseline exhibit higher 1-year freedom from recurrent VA after mitral repair surgery. Early diagnosis of patients with progressive PVC complexity (Lown grade >3) and referral to specialty multidisciplinary care is warranted. VA, Ventricular arrhythmia; HR, hazard ratio; CI, confidence interval; PVC, premature ventricular contraction.
Predictors of Recurrent VA After Mitral Valve Repair
Table E1 summarizes the univariate prognostic value of all patient, imaging, ECG, and operative characteristics for VA recurrence after surgical mitral repair. Preexisting c-VA was the predominant independent predictor, increasing the hazard for postoperative VA recurrence by a factor of 10.8 (95% CI, 1.4-84.2; P = .024; C-index 0.80) (Table 4).
Table 4.
Risk factors associated with recurrent VA after mitral valve repair
| Variable | Univariate log regression |
Cox regression |
||
|---|---|---|---|---|
| OR (95% CI) | P | HR (95% CI) | P | |
| Preoperative complex VA (Lown ≥3) | 15.91 (1.9-131) | .01 | 10.8 (1.4-84.2) | .024 |
| Arterial hypertension | 4.85 (1.3-17.6) | .016 | 3.1 (0.8-11.4) | .089 |
| QRS, ms | 1.06 (1-1.13) | .04 | 1.04 (0.99-1.09) | .090 |
| ECG infarct pattern | 3.54 (1.1-11.8) | .04 | – | – |
| Conduction delay | 6.55 (1.84-23.3) | .004 | – | – |
OR, Odds ratio, CI, confidence interval; VA, ventricular arrhythmia; HR, hazard ratio; ECG, electrocardiogram.
Discussion
We assessed the 1-year outcomes of VA after elective, isolated mitral valve repair surgery in patients with VA and severe, chronic degenerative MR (Figure 2). The principal findings were (1) VA was absent in all patients in the immediate postoperative period (postoperative day 0 to discharge) and remained undetected at 30 days; (2) recurrent VA occurred in 24% of patients at 1-year follow-up, almost exclusively (92.3%) in those with previous VT and/or SCA; and (3) patients with complex VAs are more likely to experience recurrent VA after mitral repair.
Figure 2.
Freedom from recurrent VA. Kaplan-Meier curves with 95% CIs for postoperative VA-free probability stratified by baseline VA complexity. Presented % values represent the cumulative VA-free rates for each subset of preoperative VA over the postoperative follow-up period. Preoperative VA complexity has a significant influence on the time to recurrent VA (log-rank P = .0135), with preexisting complex VA associated with an incremental risk for postoperative recurrence compared with simple baseline VA (HR, 4.07; 95% CI, 1.34-12.4). VA, Ventricular arrhythmia; HR, hazard ratio; CI, confidence interval; m-VA, minor ventricular arrhythmia; c-VA, complex ventricular arrhythmia.
The Natural History of AMVP Remains Unclear
Although all cohort subjects endorsed an innocuous onset of PVCs with sporadically perceived palpitations up to 3 years before being formally documented, some patients subsequently developed c-VA (Lown grade ≥3) whereas others did not. The reason for this divergence in the natural history of VA in patients with mitral prolapse is unclear. Furthermore, the inflection point from what one can assume to have been isolated unifocal PVCs to more sustained, complex VAs could not be identified. Patients with c-VA were generally diagnosed with mitral prolapse at a younger age compared with m-VA, and a syncopal or SCA episode was often the first documented event. Most female patients with c-VA in our cohort endorsed such events at a younger age and during conditions of adrenergic surge (ie, pre-labor; physical stress or emotional duress), whereas male patients with c-VA developed more sustained or severe VA relatively later in life and unrelated to physical/emotional stress. The etiology and timing of this unique electromechanical pattern variance remains unknown and warrants further study.
Recurrent Ventricular Arrhythmia Is Not Contingent on Mitral Surgery Itself
Although observational data show a uniform protective benefit of mitral repair from cardiovascular events across a wide range of surgeon volumes, expertise, and disease complexity,21, 22, 23 outcome variability still persists.24 This observation is attributed at least in part to patient and/or treatment variance although unaccounted factors have been implicated in confounding the observed outcomes.25,26 The effect of mitral repair in minimizing recurrent VA is equally exposed to similar biases, which may explain the disparities in reported series.3, 4, 5,27 Systematic differences in the identification mode of mitral prolapse or VA (patients selected from heart rhythm clinic registries vs from mitral prolapse surgical lists), differences in local referral patterns and treatment selection practices (patients allocated to intervention earlier are subject to different risk exposure to arrhythmia and MR from those operated later), have direct implications on the treatment effect of mitral repair in reducing the morbidity hazard from VA. Assuming a uniform effect from mitral repair mitigating the prolapse-related stretch/stress injury to the underlying myocardium, one should expect an equally uniform result in postoperative VA improvement with the proviso that the intervention is performed in comparable patient cohorts using comparable reconstructive techniques. All patients in our study had similar risk profiles and were operated on by the same surgical team over 3 recent consecutive years using similar reconstructive principles. However, 1 in 5 patients developed recurrent VA within 1 year. It stands therefore to reason that additional factors beyond stretch/stress forces normalized by a successful mitral repair may be implicated in the etiopathogenesis and maintenance of ventricular electrical instability in the absence of additional acquired or congenital structural pathology and warrants further investigation.
Complex VA Begets Complex VA Regardless of Substrate or Surgery
Our data show that VA recurrence occurred almost exclusively in patients with preexisting c-VA (12/13; 92.3%), which was also the strongest predictor for postoperative VA, increasing the recurrence hazard by a factor of 10.8. Remarkably, most of the known markers related to the degree of LV adaptation to chronic volume loading and incremental MR severity or factors related to operative complexity and duration of ischemic arrest, were not associated with postoperative VA recurrence (Table E1). However, this patient subset had a greater incidence of hypertension (77% vs 37%, P = 012), conduction delays (left bundle branch block: 46% vs 17%, P = .034), and pleiomorphic PVCs (62% vs 24%, P = .014) at baseline (Table E2). Remarkably, we found that patients with minor VAs at baseline as well as those with complex PVCs (ie, couplets, triplets, pleiomorphic PVCs) but not sustained VAs (VT/SCA; n = 12/35) regardless of LGE status on preoperative MRI, did not experience recurrent VA after mitral repair. It appears that contrary to the outcome variability observed after mitral repair in patients with complex VAs, mitral repair had a more uniform favorable effect on patients with minor or nonsustained VAs, associated with absent ventricular ectopy up to 1 year postrepair. These findings emphasize the importance of early diagnosis before the development of more complex/sustained VAs, when corrective mitral valve surgery may have a more predictable protective effect against recurrent ectopy.
Focal Myocardial Fibrosis Does Not Equate to Ventricular Arrhythmia Risk
One half of our patients with preoperative VT/SCA (n = 11/22) did not develop recurrent VA within 1 year despite a positive baseline LGE in 46.2% (6/11), which raises the question of prognostic value when LGE is used in isolation. Recent evidence from a large countywide postmortem analysis of more than 1000 consecutive adult sudden cardiac deaths28 found that only 31% of MVP-related cases exhibited focal myocardial fibrosis and mitral annular disjunction, another marker linked to regional myocardial fibrosis,29,30 and considered by some investigators to increase the risk of VA.31,32 Kitkungvan and colleagues33 corroborated similar prevalence of focal myocardial scar (LGE+) in 34.1% of 229 patients with MVP; however, the authors found that diffuse (instead of focal) interstitial fibrosis, inferred by extracellular volume, was independently associated with MR-related symptoms and clinical events. Similar observations were reported by Bui and colleagues,34 where replacement fibrosis was only observed in a minority of patients with mitral prolapse (n = 11/41) with moderate or greater chronic primary MR, and LGE was not the major predictor of arrhythmic risk. These findings place the previously asserted hypothesis of focal myocardial scar as requisite substrate for arrhythmogenesis—and thus impervious to the benefit of reverse LV remodeling after corrective mitral surgery—in further uncertainty and shift the focus towards alternative mechanisms unrelated to traction forces35 such as interstitial fibrosis. This may help generate impetus for larger-scale work to understand the mechanistic relationship between mitral prolapse and the adaptive myocardial ultrastructural alterations leading to electrical instability and manifest VAs.
Myocardial Inflammation—a Novel Marker of Arrhythmic Potential
Preoperative inflammation was present in 70% of scanned patients with m-VA and in 100% of patients with c-VA (Table 1). Importantly, PET+ inflammation was present in all asymptomatic patients with AMVP with preserved LV at baseline. Although PET/MRI readings were available from only 36% of the evaluated cohort, PET+ uptake suggests that this occult ultrastructural process may have a wider role in arrhythmogenesis and natural history of VA regardless of the degree of LV compensation and may be associated with a variety of PVC morphologies. Further evidence has recently emerged to describe the presence of myocardial inflammation during the early stages of degenerative mitral prolapse,36 reinforcing our hypothesis and findings. As myocardial inflammation was present in both VA subsets with successful suppression of PVCs at 1 year (82.4%) and in 100% of cases with recurrent VAs (Table E2), this noninvasive method may provide important insight in the evolution and temporal expression of ectopic activity in patients with mitral prolapse and warrants further evaluation from larger case series.
AMVP Involves a Wide Spectrum of Valve Phenotypes
Although bileaflet Barlow was the predominant prolapse phenotype, the presence of a flail segment was not biased to patients with c-VA as previously suggested,37 and it was equally present in patients without VA (grade <2). The presence of fibroelastic deficiency and forme-fruste anterior leaflet prolapse noted in a minority of patients with c-VA in our study further highlights the underappreciated diversity of the pathoanatomic spectrum encompassed by the so-called arrhythmic mitral prolapse syndrome, as the commonly postulated origins of ventricular arrhythmogenesis are incongruent when applied to differing degrees of MR severity, LV remodeling, and degenerative stigmata of mitral prolapse.2
Our cohort’s risk profile and operative characteristics were similar between patients with/-out VA recurrence except for their baseline VA signature. In a series of 32 consecutive patients with AMVP and significant MR, although VA was not successfully suppressed after mitral surgery, Naksuk and colleagues3 found that postoperative VA burden was decreased (>10%) in the younger subset (n = 53.1%; P = .04) of their cohort. Our findings are in contradistinction with this observation, as there was no age difference between VA recurrence subgroups or among patients with refractory VA with/-out postoperative ectopic burden reduction in our study (Table E2). Although not readily clear, a smaller sample size and a male prevalence in their nonreduction subset may have contributed in this disparity.
Implications for Practice
Early Recognition: The Role of the Community Doctor
Identification of the AMVP patient phenotype should ideally begin at the primary care level. Early recognition of at-risk individuals should be followed with a thorough evaluation and surveillance protocol, establishment of appropriate risk modifiers (antiarrhythmic therapy, avoidance of adrenergic surge triggers, preventive ICD), and timely referral to specialty interdisciplinary team.
Does Concomitant VA Warrant Earlier Mitral Intervention?
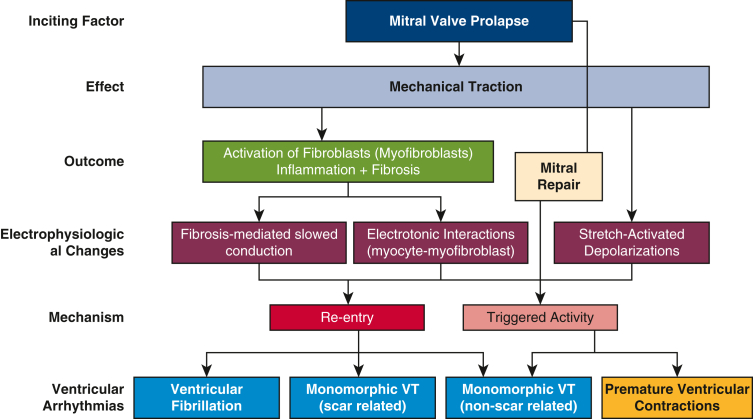
Although MVP-related VA seems to follow a progressive course in its temporal distribution of severity (or complexity), we do not know when this inflection point actually happens in the course of mitral valve disease. In addition, although some patients with MVP exhibit slow progression in VA burden and grade of severity (Lown ≤2), others may show evidence of c-VAs (Lown ≥3) including VT and SCA as early as the second decade of life when MR is not yet significant, LV dimensions are still small, and mitral surgery has no therapeutic role. However, patients with advanced myxomatous mitral prolapse (ie, Barlow disease) and at least moderate MR with complex VA (Lown grade ≥2) may benefit from the effect of mitral repair to alleviate prolapse-related traction forces on the papillary muscles and adjacent myocardium, thereby disrupting the stretch-activated pathway of arrhythmogenesis (Figure E3). When mitral prolapse and/or MR severity are less than significant and mitral intervention is not contemplated, complex VA should trigger a formal electrophysiologic evaluation and follow-up and prompt the physician to restratify such patients based on the incremental risk related to the expected progression of the arrhythmic substrate38 and not solely on the advent of guideline-defined symptomatic and/or structural operative triggers.39
Figure E3.
Mechanistic role of mitral repair in VA suppression. Suggested downstream effect of stretch-stress activation of myocardial inflammatory response due to traction from excess leaflet motion in mitral valve prolapse.E1 The resulting electrophysiologic alterations in myocardial steady-state favor an environment of electrical instability (ie, stretch-activated depolarizations; altered electrotonic interactions) and the development of ventricular arrhythmias. Corrective mitral valve repair surgery is thought to abolish the VA mechanism related to triggered activity by alleviating the prolapse excess traction and associated stretch-activated inflammatory myocardial response by restoring physiologic mitral valve anatomy and function, thereby normalizing the intracavitary forces. VT, Ventricular tachycardia; VA, ventricular arrhythmia.
Limitations
Our analysis is vulnerable to bias inherent to the retrospective nature of our study and study sample selection from a quaternary mitral repair reference center. Furthermore, our patient sample does not include a comparator group with moderate or less MR, which introduces additional lead-time bias by excluding patients with AMVP without significant MR or survivors of SCA with existing MVP and less than moderate MR. The potential role of individual antiarrhythmic strategy during the follow-up period was not evaluated because of the limited sample size of the cohort’s subgroups. However, the proportion of patients on antiarrhythmic regimes were comparable at discharge (Table 1) and greater among patients with recurrent VA at 1 year (Table E2), which could suggest a limited therapeutic advantage in influencing ectopic recurrence were it not confounded by sampling restrictions.
Determination of postoperative VA was based on Holter and/or ICD interrogation in all 13 patients with recurrent VA. For the remaining 41, recurrent VA was determined by Holter/ICD interrogation in 12 (30%) and by absent PVCs on serial ECGs plus a negative report of patient-perceived palpitations in the remaining 29 of 41 patients, which may have underestimated the incidence of recurrent VA. However, short ECG rhythm recording (up to 2-minute strip) is a validated alternative to longer ambulatory rhythm monitoring for ventricular ectopy.40
Conclusions
In a series of 62 consecutive patients operated electively for AMVP, VA remained undetected in 75.9% of patients at 1 year (Figure 1). Freedom from recurrent VA was greater among patients without c-VA preoperatively, whereas baseline Lown grade ≥3 was the strongest independent risk factor for recurrent VA at 1 year. These findings attest to the importance of early recognition and prompt referral of patients with mitral prolapse and progressive VA to specialty interdisciplinary care.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://www.aats.org/resources/surgical-mitral-valve-repair-improves-ventricular-ectopy-burden-in-arrhythmic-mvp-patients.

Conflict of Interest Statement
D.P. received nonfinancial support from TOMTEC Imaging Systems GmbH (PI - research license for myocardial strain analysis software). M.M. has served as a consultant to Boston Scientific. D.A. is the National co-Principal Investigator for the Triluminate-II US Pivotal Trials, the Medtronic Apollo and CoreValve US Pivotal Trials, and ReChord US Pivotal Trial, respectively. The Icahn School of Medicine at Mount Sinai receives royalty payments from Edwards Lifesciences and Medtronic for intellectual property related to D.A.’s involvement in the development of 3 mitral valve repair rings and a tricuspid valve repair ring. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
This work was partly supported by National Institutes of Health grant R01 HL071021 (to Dr Fayad) and KL2 TR001435 and R01HL166720 (to Dr Trivieri).
Role of the funder/sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Drs Pandis and David contributed equally to this article.
Appendix E1
Table E1.
Risk factors related to recurrent VA at 1 year after mitral repair surgery
| R2 | OR (95% CI) | AUC | P | |
|---|---|---|---|---|
| Demographics and comorbidities | ||||
| Age, y | 0.01 | 0.98 (0.93-1) | 0.58 | .43 |
| Age at MR/MVP diagnosis, y | 0.043 | 0.97 (0.94-1) | 0.64 | .11 |
| Age at VA diagnosis, y | 0.026 | 0.97 (0.92-1.02) | 0.61 | .21 |
| Symptoms (NYHA II-III) | 0.005 | 0.71 (0.22-2.27) | 0.54 | .56 |
| Female | 0.003 | 1.29 (0.4-4.15) | 0.53 | .67 |
| Familial MVP | 0.001 | 0.95 (2.56-3.5) | 0.51 | .94 |
| Hypertension | 0.1 | 4.85 (1.3-17.6) | 0.69 | .016 |
| Diabetes mellitus | 0.01 | 3.29 (0.19-56) | 0.52 | .42 |
| Smoking | 0.0006 | 1.23 (0.12-13.2) | 0.51 | .86 |
| Obstructive sleep apnea | 0.002 | 1.6 (0.14-19.1) | 0.51 | .71 |
| Secondary PHT | 0.01 | 0.59 (0.16-2.1) | 0.56 | .42 |
| Atrial fibrillation/flutter | 0.0002 | 0.93 (0.22-3.9) | 0.51 | .92 |
| BP systolic | 0.044 | 1.03 (0.99-1.06) | 0.61 | .09 |
| BP diastolic | 0.023 | 1.04 (0.98-1.11) | 0.57 | .24 |
| Pulse pressure, mm Hg | 0.03 | 1.03 (0.99-1.07) | 0.57 | .17 |
| Antiarrhythmic therapy | 0.03 | 2.44 (0.75-7.98) | 0.61 | .15 |
| Serum K+, mEq/L | 0.018 | 3.27 (0.37-28.6) | 0.56 | .29 |
| BNP, pg/mL | 0.01 | 0.99 (0.98-1) | 0.56 | .49 |
| Rest echocardiography | ||||
| LV EF, % | 0.022 | 0.95 (0.86-0.24) | 0.58 | .24 |
| LVEDDi, cm/m2 | 0.0005 | 1.15 (0.23-5.8) | 0.51 | .86 |
| LVESDi, cm/m2 | 0.001 | 0.77 (0.1-5.9) | 0.54 | .79 |
| LV mass(i), g/m2 (range) | 0.00008 | 0.99 (0.97-1.02) | 0.50 | .94 |
| RWT | 0.0008 | 0.06 (0-194) | 0.57 | .50 |
| LV wall stress (×10³), dyn/cm2 (SD) | 0.002 | 0.99 (0.97-1.02) | 0.56 | .73 |
| Peak GLS, % | 0.01 | 0.93 (0.78-1.1) | 0.52 | .39 |
| LV mechanical dispersion, ms | 0.005 | 0.99 (0.98-1.01) | 0.55 | .59 |
| LAVi, mL/m2 | 0.021 | 0.98 (0.95-1.01) | 0.59 | .31 |
| PASP, mm Hg | 0.052 | 0.94 (0.87-1.01) | 0.66 | .10 |
| MV E/A | 0.00009 | 1.03 (0.49-2.15) | 0.53 | .94 |
| MV E/e' | 0.022 | 0.92 (0.79-1.08) | 0.60 | .30 |
| Bileaflet prolapse | 0.0006 | 1.13 (0.33-3.87) | 0.51 | .84 |
| Cardiac MRI | ||||
| LV scar/fibrosis (LGE+) | 0.01 | 1.6 (0.34-7.64) | 0.55 | .56 |
| Hybrid PET/MRI | ||||
| LV inflammation (FDG+) | 0.11 | – | 0.6 | 1 |
| Ventricular arrhythmia and ECG characteristics | ||||
| MR diagnosis to VA, y | 0.01 | 1.02 (0.97-1.7) | 0.61 | .51 |
| VA diagnosis to MR, y | 0.006 | 1.07 (0.72-1.6) | 0.54 | .73 |
| Preoperative VA burden, % (range) | 0.06 | 1.05 (0.99-1.11) | 0.70 | .11 |
| Preoperative VA complex (Lown ≥3) | 0.18 | 15.91 (1.9-131) | 0.73 | .01 |
| VA-related syncope | 0.06 | 4.2 (1.01-17.5) | 0.61 | .047 |
| History of sudden death | 0.08 | 11.5 (1.1-121) | 0.59 | .04 |
| Heart rate, beats/min | 0.003 | 0.99 (0.95-1.03) | 0.52 | .68 |
| QTc, ms | 0.002 | 1 (0.98-1.03) | 0.52 | .74 |
| Prolonged QTc | 0.011 | 0.59 (0.16-2.12) | 0.56 | .42 |
| QRS, ms (range) | 0.073 | 1.06 (1-1.13) | 0.66 | .041 |
| Nonspecific ST-T | 0.014 | 1.9 (0.51-6.66) | 0.56 | .35 |
| Repolarization abnormalities | 0.007 | 1.71 (0.37-7.87) | 0.54 | .49 |
| Inverted/biphasic T-waves | 0.068 | 3.54 (1.1-11.8) | 0.65 | .04 |
| Conduction delay (LV or AV) | 0.14 | 6.55 (1.84-23.3) | 0.72 | .004 |
| Fascicular block | 0.11 | 5.98 (1.6-22.6) | 0.67 | .008 |
| LVH-ECG voltage criteria | 0.005 | 0.46 (0.42-5.13) | 0.54 | .56 |
| Operative characteristics | ||||
| MR diagnosis to surgery, y | 0.03 | 1.03 (0.99-1.07) | 0.64 | .17 |
| STS PROM, % | 0.0016 | 1.34 (0.21-8.48) | 0.56 | .75 |
| Flail (chordal rupture/elongation) | 0.003 | 0.78 (0.24-2.5) | 0.53 | .67 |
| Segments repaired, n (range) | 0.0001 | 0.98 (0.62-1.6) | 0.51 | .93 |
| Repair complexity score | 0.001 | 0.97 (0.78-1.19) | 0.51 | .78 |
| Mitral ring size, mm (IQR) | 0.0035 | 0.96 (0.81-1.14) | 0.56 | .64 |
| CPB, min | 0.008 | 0.99 (0.97-1.01) | 0.53 | .488 |
| Crossclamp | 0.0036 | 0.99 (0.97-1.02) | 0.52 | .648 |
| ICU stay, d | 0.065 | 0.54 (0.26-1.15) | 0.62 | .112 |
| Hospital stay, d | 0.0001 | 0.86 (0.02-39) | 0.66 | .937 |
| MAE | 0.0009 | 0.77 (0.08-7.45) | 0.51 | .819 |
| Postoperative transient VT/PVC’s | 0.0034 | 1.42 (0.32-6.4) | 0.53 | .641 |
OR, Odds ratio; CI, confidence interval; AUC, area under the curve; MR, mitral regurgitation; MVP, mitral valve prolapse; NYHA, New York Heart Association; MVP, mitral valve prolapse; PHT, pulmonary hypertension; BP, blood pressure; BNP, brain natriuretic peptide; LV, left ventricle/-cular; EF, ejection fraction; EDDi, end-diastolic diameter index; ESDi, end-systolic diameter index; SD, standard deviation; GLS, global longitudinal strain; LAVi, left atrial volume index; PASP, pulmonary arterial systolic pressure; MV, mitral valve; MRI, magnetic resonance imaging; LGE, late gadolinium enhancement; PET, positron emission tomography; FDG, fluorodeoxyglucose; ECG, electrocardiogram; MR, mitral regurgitation; VA, ventricular arrhythmia; AV, atrioventricular; CAD, coronary artery disease; LVH, left ventricular hypertrophy; STS PROM, Society of Thoracic Surgeons Predicted Risk Of Mortality; IQR, interquartile range; CPB, cardiopulmonary bypass; ICU, intensive care unit; MAE, major adverse events; VT, ventricular tachycardia; PVC, premature ventricular contraction.
Table E2.
Patient, imaging, VA, and operative characteristics stratified by VA recurrence
| n (%)/median (IQR) | 1-y follow-up (N = 54) | No VA recurrence (n = 41) | Recurrent VA (n = 13) | P |
|---|---|---|---|---|
| Demographics and comorbidities | ||||
| Age, y (IQR) | 59 (51-64) | 59 (50.3-65) | 59 (50-63.3) | .785 |
| Age at MR/MVP diagnosis, y | 41.7 (27.5-52.5) | 41.4 (29.6-54.8) | 43 (19.5-47.3) | .379 |
| Age at VA diagnosis, y | 50.3 (37.5-57.8) | 51 (37.3-58.7) | 45.5 (37-55.6) | .407 |
| Symptoms functional class | .229 | |||
| NYHA I | 24 (44.4) | 17 (41.5) | 7 (53.8) | |
| NYHA II | 25 (46.3) | 19 (46.3) | 6 (46.2) | |
| NYHA III | 5 (9.3) | 5 (12.2) | - | |
| Female | 21 (38.9) | 15 (36.6) | 6 (46.2) | .541 |
| Familial MVP | 16 (29.6) | 5 (19.2) | 12 (33.3) | .223 |
| Hypertension | 25 (46.3) | 15 (36.6) | 10 (76.9) | .012 |
| Diabetes mellitus | 2 (3.7) | 1 (2.4) | 1 (2.7) | .387 |
| Chronic lung disease | 3 (5.6) | 3 (7.3) | – | .320 |
| Smoking | 13 (24.1) | 8 (19.5) | 5 (38.5) | .285 |
| Obstructive sleep apnea | 3 (5.6) | 2 (4.9) | 1 (7.7) | .702 |
| Secondary PHT | 19 (35.2) | 15 (36.6) | 4 (30.8) | .705 |
| Atrial fibrillation/flutter | 13 (24.1) | 10 (24.4) | 3 (23.1) | .924 |
| BP systolic | 130 (118-137) | 128 (116-133.3) | 132 (121-149.8) | .129 |
| BP diastolic | 73.9 (68-81) | 74 (64.8-80) | 74 (70-87.5) | .105 |
| Pulse Pressure, mm Hg | 53.5 (44-64) | 53 (46.3-60) | 60 (43-75) | .510 |
| Anti-arrhythmic therapy (n, 50) | 35/50 (70) | 23/37 (74) | 12/13 (92.3) | .043 |
| Diuretic therapy | 7 (13) | 4 (9.8) | 3 (23.1) | .217 |
| Serum K+, mEq/L | 4.2 (4.1-4.4) | 4.2 (4.1-4.4) | 4.3 (4.1-4.5) | .567 |
| BNP, pg/mL | 62.7 (32.6-119.5) | 64.7 (31.2-125.7) | 45.8 (29.6-106.2) | .504 |
| Imaging echo | ||||
| Resting echocardiography | 54 (100) | 41 (100) | 13 (100) | – |
| LV EF, % | 63 (60-65) | 63 (60-67) | 60 (57.3-63.3) | .116 |
| LVEDd, cm | 5.5 (5.1-5.8) | 5.5 (5-5.9) | 5.53 (5.3-5.8) | .641 |
| LVEDDi, cm/m2 | 2.92 (2.66-3.21) | 2.92 (2.66-3.21) | 3 (2.65-3.16) | .754 |
| LVEDs, cm | 3.5 (3.1-3.9) | 3.38 (3.1-3.9) | 3.6 (3.3-3.85) | .436 |
| LVESDi, cm/m2 | 1.85 (1.66-2.04) | 1.81 (1.67-2.02) | 1.85 (1.66-2.06) | .557 |
| LV mass(i), g/m2 (range) | 117.5 (100-131.6) | 118.6 (97.7-131.7) | 116.9 (102.4-131.4) | .765 |
| LV wall stress (×10³), dyn/cm2 (SD) | 68.98 (51.2-86.4) | 74.01 (50.4-86.7) | 66.3 (60.3-81.9) | .832 |
| Peak GLS, % | −25.5 (−26.8 to −23.5) | −25.8 (−26.7 to −22.8) | −24.9 (−27.8 to −23.9) | .895 |
| LV mechanical dispersion, ms | 52.2 (34.4-82.8) | 55.4 (33.2-91.2) | 51.4 (38.8-69) | .634 |
| LAVi, mL/m2 | 61.5 (54.2-91.1) | 64.6 (53.3-91.8) | 59.6 (57.3-72.9) | .585 |
| PASP, mm Hg | 27.5 (24-38.6) | 27 (24.9-39.3) | 28 (23.6-36.3) | .557 |
| MV E/A | 1.47 (1.13-1.79) | 1.44 (1.1-2) | 1.54 (1.2-1.7) | .764 |
| MV E/e' | 9.95 (7.3-13.5) | 10.8 (9.1-14.8) | 7.8 (7.2-10.1) | .055 |
| Diastolic filling time, ms | 515 (423-616.8) | 513.5 (404-620.5) | 526 (451.6-610.3) | .604 |
| Bileaflet prolapse | 37 (68.5) | 28 (68.3) | 9 (69.2) | .949 |
| Bileaflet prolapse—female | 18 (33.3) | 13 (31.7) | 5 (38.5) | .656 |
| Imaging-PET/MRI | ||||
| Cardiac MRI | 33 (61.1) | 24 (58.5) | 9 (69.2) | .495 |
| LV scar/fibrosis (LGE+) | 22 (68.7) | 16 (69.6) | 6 (66.7) | .876 |
| Preoperative hybrid PET/MRI | 22 (40.7) | 17 (41.5) | 5 (38.5) | .849 |
| LV inflammation (FDG+) | 19 (86.4) | 14 (82.4) | 5 (100) | .323 |
| VA and ECG | ||||
| MR diagnosis to VA, y | 9.9 (5-21)/(n = 38) | 1.7 (4.8-24)/(n = 27) | 8.4 (5-17.5)/(n = 11) | .847 |
| VA diagnosis to MR, y | 2 (1.95-4)/(n = 16) | 2 (2-3)/(n = 14) | 5 (1-9)/(n = 2) | .935 |
| Ambulatory rhythm monitor | 43 (79.6) | 32 (78) | 11 (84.6) | .612 |
| VA burden, % (range) | 20 (0.01-42)/(n = 11) | – | 20 (0.01-42)/(n = 11) | – |
| Bigeminy | 24 (39.3) | 13 (32.5) | 7 (53.8) | .172 |
| Pleiomorphic PVCs | 21 (33.9) | 10 (24.4) | 8 (61.5) | .014 |
| Ventricular couplets | 26 (41.9) | 17 (41.5) | 7 (53.8) | .438 |
| Ventricular tachycardia | 30 (48.4) | 18 (43.9) | 12 (92.3) | .002 |
| VA-related syncope | 10 (18.5) | 6 (14.6) | 4 (30.8) | .196 |
| Sudden cardiac arrest | 4 (7.4) | 1 (2.4) | 3 (23.1) | .014 |
| ICD in situ | 8 (14.8) | 5 (12.2) | 3 (23.1) | .340 |
| Heart rate, bpm | 65.5 (59-79) | 64 (59-79) | 70 (59.8-81.3) | .544 |
| Sinus bradycardia | 15 (27.8) | 12 (29.3) | 3 (23.1) | .667 |
| QTc, m | 434.5 (418-447) | 428 (417.3-447) | 438 (427.3-446.5) | .302 |
| QTc (age-adj), ms | 433.3 (431-434.7) | 433.3 (430.7-435) | 433.3 (430.66-434.5) | .785 |
| Prolonged QTc | 19 (35.2) | 15 (36.6) | 4 (30.78) | .705 |
| Prolonged QTc, ms | 453 (446.3-457) | 453 (446.3-456.5) | 459 (447-471.5) | .582 |
| Prolonged QTc (age-adj), ms | 434.1 (432.8-434.9) | 434.1 (432.8-434.9) | 433.9 (430.9-435.1) | .841 |
| QRS, ms (range) | 98 (88-104) | 94 (86-102) | 100 (93.5-107.5) | .043 |
| Nonspecific ST-T | 11 (20.4) | 7 (17.1) | 4 (30.8) | .289 |
| Repolarization abnormalities | 8 (14.8) | 6 (124.6) | 2 (15.4) | .948 |
| Inverted/biphasic T-waves | 23 (42.6) | 16 (39) | 7 (53.8) | .351 |
| Infarct pattern | 23 (37.1) | 8 (30.8) | 15 (41.7) | .385 |
| T-wave inversion—inferior | 7 (30.4) | 4 (25) | 3 (42.9) | .402 |
| T-wave inversion—anterior | 16 (69.6) | 12 (75) | 4 (57.1) | |
| Conduction delay | 21 (38.9) | 13 (31.7) | 8 (61.5) | .057 |
| 1° AV block | 8 (14.8) | 5 (12.2) | 3 (23.1) | .340 |
| Fascicular block | 13 (24.1) | 7 (17.1) | 6 (46.2) | .034 |
| LBBB | 1 (7.7) | 1 (14.3) | – | .354 |
| RBBB | 12 (92.3) | 6 (85.7) | 6 (100) | |
| LVH-ECG voltage criteria | 16 (29.6) | 13 (31.7) | 3 (23.1) | .556 |
| Surgical referral and intraoperative | ||||
| MR diagnosis to surgery, y | 14 (3.6-24.8) | 10.8 (2.7-23.9) | 18.9 (13-25.6) | .178 |
| Surgical referral trigger | ||||
| Dyspnea/exercise tolerance | 25 (46.3) | 19 (46.3) | 6 (46.2) | .503 |
| MR severity progression | 10 (18.5) | 7 (17.1) | 3 (23.1) | |
| Palpitations | 10 (18.5) | 7 (17.1) | 3 (23.1) | |
| Worsening LV dimensions/EF | 7 (13) | 7 (17.1) | – | |
| Operative indication | ||||
| ACC/AHA class I | 38 (70.4) | 31 (75.6) | 7 (53.8) | .138 |
| ACC/AHA class IIa | 16 (29.6) | 10 (24.4) | 6 (46.2) | |
| STS PROM, % | 0.43 (0.27-0.74) | 0.395 (0.25-0.74) | 0.52 (0.35-0.62) | .402 |
| Prolapse phenotype | ||||
| Barlow—bileaflet | 37 (68.5) | 27 (65.9) | 10 (76.9) | .758 |
| Barlow—forme-fruste | 14 (25.9) | 11 (26.8) | 3 (23.1) | |
| Fibroelastic deficiency | 2 (3.7) | 2 (4.9) | – | |
| Anterior leaflet prolapse | 1 (1.9) | 1 (2.4) | – | |
| Flail (chordal rupture/elongation) | 32 (59.3) | 25 (61) | 7 (53.8) | .652 |
| Segments repaired, n (range) | 2 (1-6) | 2 (1-6) | 2 (1-4) | .720 |
| Repair complexity score, n (range) | 3 (1-13) | 2 (1-13) | 3 (1-5) | .975 |
| Simple | 17 (31.5) | 13 (31.7) | 4 (30.8) | .893 |
| Intermediate | 23 (42.6) | 18 (43.9) | 5 (38.5) | |
| Complex | 14 (25.9) | 10 (24.4) | 4 (30.8) | |
| Mitral annuloplasty size, mm | 36 (34-38) | 36 (32-38) | 34 (34-36) | .466 |
| Mitral annuloplasty type | ||||
| Flexible band | 27 (50) | 18 (43.9) | 9 (69.2) | .195 |
| Remodeling ring | 27 (50) | 23 (56.1) | 3 (30.8) | |
| Concomitant procedures | ||||
| Tricuspid valve repair | 44 (81.5) | 35 (85.4) | 9 (69.2) | .196 |
| LAA exclusion | 16 (29.6) | 13 (31.7) | 3 (23.1) | .556 |
| AF cryo-maze | 14 (25.9) | 11 (26.8) | 3 (23.1) | .789 |
| PFO closure | 7 (13) | 5 (12.2) | 2 (15.4) | .768 |
| ICD leads exchange | 2 (3.7) | 1 (2.4) | 1 (7.7) | .387 |
| CPB, min (IQR) | 118.5 (95-132) | 120 (94.5-134.5) | 110 (96-125.5) | .357 |
| Crossclamp, min | 88.5 (70-103.5) | 95 (69.3-110) | 85 (79-96.8) | .459 |
| ICU stay, d | 1.1 (0.9-2.1) | 1.1 (0.9-2.8) | 0.9 (0.8-1.4) | .166 |
| Hospital stay, d | 7 (5-9) | 7 (5-9.3) | 6 (5-8) | .425 |
| Discharge antiarrhythmic | ||||
| Beta-blocker | 29 (53.7) | 19 (46.3) | 10 (76.9) | .072 |
| Beta-blocker+ amiodarone | 14 (25.9) | 11 (26.8) | 3 (23.1) | |
| Beta-blocker CI | 11 (20.4) | 11 (26.8) | – | |
| Operative outcomes | ||||
| MAE (at 30 d) | 4 (7.4) | 3 (7.3) | 1 (7.7) | .964 |
| Operative death | – | – | – | – |
| MI | – | – | – | – |
| Stroke | 1 (1.9) | 1 (2.4) | – | .573 |
| Acute kidney injury | – | – | – | – |
| Respiratory failure | 3 (5.6) | 2 (4.9) | 1 (7.7) | .702 |
| New PPM | – | – | – | – |
| MAE (at 1 y) | – | – | – | – |
| Recurrent MR >1+ | – | – | – | – |
| Cardiac-related readmission | – | – | – | – |
| Cardiac reintervention (any) | – | – | – | – |
IQR, Interquartile range; VA, ventricular arrhythmia; MR, mitral regurgitation; MVP, mitral valve prolapse; NYHA, New York Heart Association; PHT, pulmonary hypertension; BP, blood pressure; BNP, brain natriuretic peptide; LV, left ventricle/-cular; EF, ejection fraction; EDDi, end-diastolic diameter index; ESDi, end-systolic diameter index; SD, standard deviation; GLS, global longitudinal strain; LAVi, left atrial volume index; PASP, pulmonary arterial systolic pressure; MV, mitral valve; PET, positron emission tomography; MRI, magnetic resonance imaging; LGE, late gadolinium enhancement; FDG, fluorodeoxyglucose; ECG, electrocardiogram; MR, mitral regurgitation; PVCs, premature ventricular contractions; ICD, implantable cardioverter/defibrillator; AV, atrioventricular; LBBB, left bundle branch block; RBBB, right bundle branch block; LVH, left ventricular hypertrophy; ACC, American College of Cardiology; AHA, American Heart Association; STS PROM, Society of Thoracic Surgeons Predicted Risk Of Mortality; LAA, left atrial appendage; AF, atrial fibrillation; PFO, patent foramen ovale; CI, contraindication; MAE, major adverse events; MI, myocardial infarction; PPM, permanent pacemaker.
References
- 1.Miller M.A., Dukkipati S.R., Turagam M., Liao S.L., Adams D.H., Reddy V.Y. Arrhythmic mitral valve prolapse: JACC Review Topic of the Week. J Am Coll Cardiol. 2018;72(23):2904–2914. doi: 10.1016/j.jacc.2018.09.048. [DOI] [PubMed] [Google Scholar]
- 2.Delling F.N., Noseworthy P.A., Adams D.H., et al. Research opportunities in the treatment of mitral valve prolapse: JACC Expert Panel. J Am Coll Cardiol. 2022;80(24):2331–2347. doi: 10.1016/J.JACC.2022.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naksuk N., Syed F.F., Krittanawong C., et al. The effect of mitral valve surgery on ventricular arrhythmia in patients with bileaflet mitral valve prolapse. Indian Pacing Electrophysiol J. 2016;16(6):187. doi: 10.1016/J.IPEJ.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaidya V.R., DeSimone C.V., Damle N., et al. Reduction in malignant ventricular arrhythmia and appropriate shocks following surgical correction of bileaflet mitral valve prolapse. J Interv Card Electrophysiol. 2016;46(2):137–143. doi: 10.1007/s10840-015-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosseini S., Rezaei Y., Samiei N., et al. Effects of mitral valve repair on ventricular arrhythmia in patients with mitral valve prolapse syndrome: a report of two cases. Int J Cardiol. 2016;222:603–605. doi: 10.1016/J.IJCARD.2016.08.053. [DOI] [PubMed] [Google Scholar]
- 6.El-Eshmawi A., Pandis D., Miller M.A., et al. Surgical cryoablation of papillary muscle PVCs during mitral valve surgery: therapeutic consideration for malignant MVP. J Am Coll Cardiol. 2020;76(25):3061–3062. doi: 10.1016/J.JACC.2020.10.037. [DOI] [PubMed] [Google Scholar]
- 7.Vohra J., Morton J.B., Morgan J., Tatoulis J. Cryoablation of papillary muscles at surgery for malignant ventricular arrhythmias due to mitral valve prolapse. Heart Lung Circ. 2022;31(9):1285–1290. doi: 10.1016/J.HLC.2022.04.058. [DOI] [PubMed] [Google Scholar]
- 8.Badhwar V., Chikwe J., Gillinov A.M., et al. Risk of surgical mitral valve repair for primary mitral regurgitation. Ann Thorac Surg. 2023;115(3):600–610. doi: 10.1016/J.ATHORACSUR.2022.12.024. [DOI] [PubMed] [Google Scholar]
- 9.Adams D.H., Anyanwu A.C. Revisiting the long-term goals of degenerative mitral valve repair: beyond eliminating mitral regurgitation. J Am Coll Cardiol. 2019;74(8):1054–1056. doi: 10.1016/J.JACC.2019.07.023. [DOI] [PubMed] [Google Scholar]
- 10.Gillinov M., Mick S., Suri R.M. The specialty of mitral valve repair. J Am Coll Cardiol. 2017;69(19):2407–2409. doi: 10.1016/J.JACC.2017.01.059. [DOI] [PubMed] [Google Scholar]
- 11.Anyanwu A.C., Adams D.H. Benchmarking mitral valve repair: defining standards for surgical and percutaneous treatment of severe mitral regurgitation. J Am Coll Cardiol. 2023;81(7):649–652. doi: 10.1016/J.JACC.2023.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Badhwar V., Rankin J.S., He X., et al. The Society of Thoracic Surgeons Mitral Repair/Replacement Composite Score: a report of the Society of Thoracic Surgeons Quality Measurement Task Force. Ann Thorac Surg. 2016;101(6):2265–2271. doi: 10.1016/J.ATHORACSUR.2015.11.049. [DOI] [PubMed] [Google Scholar]
- 13.Zoghbi W.A., Adams D., Bonow R.O., et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30(4):303–371. doi: 10.1016/J.ECHO.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Lown B., Wolf M. Approaches to sudden death from coronary heart disease. Circulation. 1971;44(1):130–142. doi: 10.1161/01.CIR.44.1.130. [DOI] [PubMed] [Google Scholar]
- 15.Doherty J.U., Kort S., Mehran R., et al. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 appropriate use criteria for multimodality imaging in Valvular Heart Disease. J Am Soc Echocardiogr. 2018;31(4):381–404. doi: 10.1016/j.echo.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Dweck M.R., Abgral R., Trivieri M.G., et al. Hybrid magnetic resonance imaging and positron emission tomography with fluorodeoxyglucose to diagnose active cardiac sarcoidosis. JACC Cardiovasc Imaging. 2018;11(1):94–107. doi: 10.1016/J.JCMG.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller M.A., Adams D.H., Pandis D., et al. Hybrid positron emission tomography/magnetic resonance imaging in arrhythmic mitral valve prolapse. JAMA Cardiol. 2020;5(9):1000–1005. doi: 10.1001/jamacardio.2020.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller M.A., Devesa A., Robson P.M., et al. Arrhythmic mitral valve prolapse with only mild or moderate mitral regurgitation: characterization of myocardial substrate. JACC Clin Electrophysiol. 2023;9(8):1709–1716. doi: 10.1016/J.JACEP.2023.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Carpentier A., Adams D.H., Filsoufi F. 1st ed. Saunders/Elsevier; 2010. Carpentier’s Reconstructive Valve Surgery: From Valve Analysis to Valve Reconstruction. [Google Scholar]
- 20.Care everywhere | HIMSS. https://www.himss.org/resource-environmental-scan/care-everywhere
- 21.Badhwar V., Peterson E.D., Jacobs J.P., et al. Longitudinal outcome of isolated mitral repair in older patients: results from 14,604 procedures performed from 1991 to 2007. Ann Thorac Surg. 2012;94(6):1870–1879. doi: 10.1016/J.ATHORACSUR.2012.05.105. [DOI] [PubMed] [Google Scholar]
- 22.Gammie J.S., Chikwe J., Badhwar V., et al. Isolated mitral valve surgery: the Society of Thoracic Surgeons Adult Cardiac Surgery Database analysis. Ann Thorac Surg. 2018;106(3):716–727. doi: 10.1016/J.ATHORACSUR.2018.03.086. [DOI] [PubMed] [Google Scholar]
- 23.David T.E., David C.M., Tsang W., Lafreniere-Roula M., Manlhiot C. Long-term results of mitral valve repair for regurgitation due to leaflet prolapse. J Am Coll Cardiol. 2019;74(8):1044–1053. doi: 10.1016/J.JACC.2019.06.052. [DOI] [PubMed] [Google Scholar]
- 24.Chikwe J., Toyoda N., Anyanwu A.C., et al. Relation of mitral valve surgery volume to repair rate, durability, and survival. J Am Coll Cardiol. 2017;69(19):2397–2406. doi: 10.1016/J.JACC.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 25.Anyanwu A.C. The vagaries of patient selection in cardiovascular surgery. J Thorac Cardiovasc Surg. 2016;152(3):842–846. doi: 10.1016/J.JTCVS.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 26.Likosky D.S., Goldberg J.B., Discipio A.W., et al. Variability in surgeons’ perioperative practices may influence the incidence of low-output failure after coronary artery bypass grafting surgery. Circ Cardiovasc Qual Outcomes. 2012;5(5):638–644. doi: 10.1161/CIRCOUTCOMES.112.967091. [DOI] [PubMed] [Google Scholar]
- 27.Alqarawi W., Birnie D.H., Burwash I.G. Mitral valve repair results in suppression of ventricular arrhythmias and normalization of repolarization abnormalities in mitral valve prolapse. HeartRhythm Case Rep. 2018;4(5):191. doi: 10.1016/J.HRCR.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delling F.N., Aung S., Vittinghoff E., et al. Antemortem and post-mortem characteristics of lethal mitral valve prolapse among all countywide sudden deaths. JACC Clin Electrophysiol. 2021;7(8):1025–1034. doi: 10.1016/J.JACEP.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marra M.P., Basso C., De Lazzari M., et al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ Cardiovasc Imaging. 2016;9(8) doi: 10.1161/CIRCIMAGING.116.005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitkungvan D., Nabi F., Kim R.J., et al. Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J Am Coll Cardiol. 2018;72(8):823–834. doi: 10.1016/j.jacc.2018.06.048. [DOI] [PubMed] [Google Scholar]
- 31.Sabbag A., Essayagh B., Barrera J.D.R., et al. EHRA expert consensus statement on arrhythmic mitral valve prolapse and mitral annular disjunction complex in collaboration with the ESC Council on valvular heart disease and the European Association of Cardiovascular Imaging endorsed by the Heart Rhythm Society, by the Asia Pacific Heart Rhythm Society, and by the Latin American Heart Rhythm Society. Europace. 2022;24(12):1981–2003. doi: 10.1093/EUROPACE/EUAC125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Essayagh B., Mantovani F., Benfari G., et al. Mitral annular disjunction of degenerative mitral regurgitation: three-dimensional evaluation and implications for mitral repair. J Am Soc Echocardiogr. 2022;35(2):165–175. doi: 10.1016/J.ECHO.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Kitkungvan D., Yang E.Y., El Tallawi K.C., et al. Extracellular volume in primary mitral regurgitation. JACC Cardiovasc Imaging. 2021;14(6):1146–1160. doi: 10.1016/J.JCMG.2020.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Bui A.H., Roujol S., Foppa M., et al. Diffuse myocardial fibrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart. 2017;103(3):204–209. doi: 10.1136/HEARTJNL-2016-309303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delgado V., Podlesnikar T. Focal replacement and diffuse fibrosis in primary mitral regurgitation: a new piece to the puzzle. JACC Cardiovasc Imaging. 2021;14(6):1161–1163. doi: 10.1016/J.JCMG.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Yang L.T., Ahn S.W., Li Z., et al. Mitral valve prolapse patients with less than moderate mitral regurgitation exhibit early cardiac chamber remodeling. J Am Soc Echocardiogr. 2020;33(7):815–825.e2. doi: 10.1016/J.ECHO.2020.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grigioni F., Enriquez-Sarano M., Ling L.H., et al. Sudden death in mitral regurgitation due to flail leaflet. J Am Coll Cardiol. 1999;34(7):2078–2085. doi: 10.1016/s0735-1097(99)00474-x. [DOI] [PubMed] [Google Scholar]
- 38.Edwards N.C., Moody W.E., Yuan M., et al. Quantification of left ventricular interstitial fibrosis in asymptomatic chronic primary degenerative mitral regurgitation. Circ Cardiovasc Imaging. 2014;7(6):946–953. doi: 10.1161/CIRCIMAGING.114.002397. [DOI] [PubMed] [Google Scholar]
- 39.Otto C., Nishimura R., Bonow R., et al. 2020 ACC/AHA Guideline for the management of patients with Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol. 2021;77(4):e25–e197. doi: 10.1016/J.JACC.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 40.Evenson K.R., Lamar Welch V.L., Cascio W.E., Simpson R.J. Validation of a short rhythm strip compared to ambulatory ECG monitoring for ventricular ectopy. J Clin Epidemiol. 2000;53(5):491–497. doi: 10.1016/S0895-4356(99)00190-0. [DOI] [PubMed] [Google Scholar]
E-Reference
- Malyshev Y., Miller M.A. Catheter ablation of arrhythmic mitral valve prolapse: should we be MAD? No, quite the opposite. JACC Clin Electrophysiol. 2023;9(8):1276–1278. doi: 10.1016/J.JACEP.2023.05.008. [DOI] [PubMed] [Google Scholar]