Abstract
Zoonoses have rapidly spread globally, necessitating the implementation of vaccination strategies as a control measure. Emerging and re-emerging vector-borne diseases are among the major global public health concerns. Dengue, a zoonotic viral infection transmitted to humans by a vector, the Aedes mosquito, is a severe global health problem. Dengue is a serious tropical infectious disease, second only to malaria, causing around 25,000 deaths each year. The resurgence of Dengue is mainly due to climate change, demographic transitions and evolving social dynamics. The development of an effective vaccine against Dengue has proven to be a complex undertaking due to four different viral serotypes with distinct antigenic profiles. This review highlights the urgent need to address the dengue threat by exploring the application of biotechnological and -OMICS sciences.
Keywords: Dengue, One Health, Zoonoses, Global health, Vector-borne diseases, -OMICS sciences
Graphical abstract
Highlights
-
•
This review article analyses and explores the current dengue challenge in the One Health contest.
-
•
This review provides an overview of the utility of -OMICS science in the dengue challenge.
-
•
This review article analyses and explores the current challenges associated with the spread of the dengue vector.
1. Introduction
Dengue is one of the most widespread mosquito-borne re-emerging diseases in the world. Currently, Dengue is endemic in 128 countries. In 2022, 2,809,818 cases of dengue fever were reported (including 1290 deaths) [1]. Between 1 January 2023 and 4 March 2023, a total of 342,243 cases of Dengue were reported in the Region of the Americas, including 86 deaths [2]. Dengue is an emerging zoonotic disease caused by a virus of the family Flaviviridae. Dengue virus (DENV) has four main serotypes: DENV-1, DENV-2, DENV-3, and DENV-4 [3]. Flaviviruses are viruses with a positive single-stranded RNA genome [4,5]. “One Health” is a collaborative global approach to achieving optimal health for people, animals, and the environment. It is essential to address emerging zoonotic infections and vector-borne diseases, such as Dengue, which are becoming major public health concerns [6]. Dengue fever, caused by DENV and transmitted mainly by Aedes mosquitoes, is increasing in incidence and geographical spread. Factors contributing to its emergence include urbanization, climate change, globalization, inadequate vector control measures, lack of population immunity and the continuous evolution of the virus. The “One Health” approach is valuable in the management of Dengue because it promotes system surveillance of human populations and mosquito vectors and the study of potential reservoirs in a collaborative framework of professionals and researchers from different disciplines [7,8]. The emergence of Dengue and its increasing geographical spread requires a multidisciplinary response involving public health, ecology, climatology, and other disciplines. In addition, effective vector control measures, preventive educational interventions, targeted research on vaccination, and ongoing epidemiological surveillance are essential to address this complex health challenge and reduce its health impact. This review aims to highlight how Dengue is an emerging global health problem and how the “One Health” approach, combined with biotechnology and omics sciences, offers a promising strategy to effectively address this disease before it potentially becomes a pandemic similar to the Coronavirus disease-19 (COVID-19).
2. Influence of climate change
In the coming years, mosquito survival, reproduction rates, viral incubation rates, and distribution of DENV and its vectors increase [9]. These observations have been attributed in part to rising global temperatures but also to globalization, air travel, urbanization and inadequate vector control [[10], [11], [12]]. Today, Aedes aegypti, the main vector of Dengue, is showing a global expansion due to climate change, threatening almost half of the world's population [13,14]. Climate change can affect pathogens both directly and indirectly by affecting reproduction, survival, and habitat and by altering patterns of human-pathogen and human-vector contact [[15], [16], [17], [18], [19]]. In particular, the identification of the environmental factors essential for the evolution and dynamics of the virus, host and vector is a crucial element for the management of Dengue in the context of future climate change [20]. In this regard, it has been observed that the survival and development of the pathogen are strongly influenced by temperature [21]. Climate change, combined with human impact on nature, negatively affects biodiversity by acting indirectly as an adjuvant in the transmission of infectious diseases [22,23]. In addition, several non-climate factors have been implicated in the emergence of vector-borne diseases, including urbanization, trade, international travel, agricultural systems, the spread of water basis, and the use of antimicrobial drugs [24,25]. Rising temperatures could increase the risk of Dengue by increasing the rate of mosquito development and reducing the incubation period [26,27]. Extremely warm temperatures can also increase mosquito mortality rates and thus reduce the risk of Dengue [28]. In addition, heavy rainfall can act as a detergent by washing eggs, larvae and pupae out of containers, but at the same time, the residual water can provide the ideal breeding habitat [29,30]. Finally, a dry climate can affect normal human activities by causing people to store water in containers that could become breeding sites for vectors. [31].
3. Prediction of the geographic distribution of Aedes mosquitoes in Europe
The rapid geographical spread of Dengue is a major global public health concern. The distribution of the vectors, Aedes aegypti and Aedes albopictus, is a crucial element in analyzing dengue expansion and is an essential tool for public health planning [32]. In a study by Oliveira et al., a prediction was made to identify the geographical areas in Europe where there will be a future expansion of vector distribution [33]. This analysis based on current and future climatic conditions was carried out for the entire European geographical area and in 65 large urban areas. In particular, the study showed that the north-west of the Iberian Peninsula, Italy, the southern areas of France and the Balkan and Greek coasts are currently suitable geographical areas for carrier development. Around 83% of urban areas are expected to become suitable in the future, compared to around 49% today. A recent editorial by Brem in 2023 reported on the transmission of Dengue in European areas [34]. The authors confirmed observations reported by previous studies indicating that territories within the European geographical area are becoming an ideal breeding ground for the spread of Aedes vectors. Moreover, they examined the situation of transmitted infections in Europe in the years 2022 and 2023. In particular, it highlighted how annual rainfall of over 500 mm, annual temperatures of over 11 °C, and temperatures in January of over 0 °C can create ideal conditions for the distribution of virus vectors. It was pointed out in detail that in Italy, the two cities most affected are Milan and Rome, which are characterized by a high tourist vocation. In contrast, in France and Spain, most cases were recorded along the coast, and outbreaks were also reported in cities such as Paris and Madrid.
4. Use of pesticides
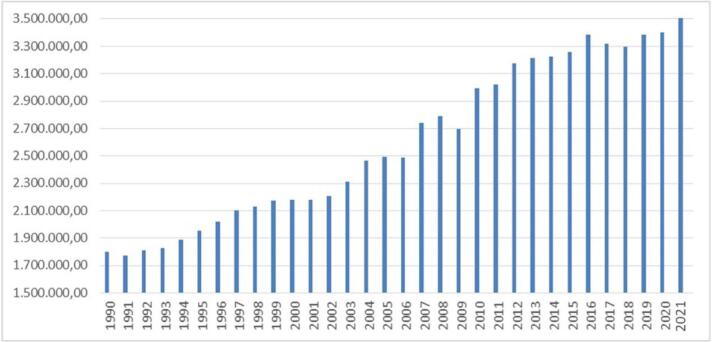
Pesticides are an essential tool in agriculture to protect crops and seeds from insects, bacteria, fungi and rodents, but their use is associated with contamination of soil, water, flora and fauna and a reduction in biodiversity. In recent years, the total use of pesticides has increased by almost 50% since the 1990s. Over the past three decades, annual pesticide use has averaged 1.58 kg per hectare and 0.37 kg per person [35]. Pyrethroids are an important group of pesticides used in the management of Dengue. Resistance to the use of these insecticides by the Aedes mosquito has already been demonstrated. The increasing use of pyrethroids and pesticides coupled with the development of resistance phenomena in combination with expected climate change could contribute to the spread of the Aedes vector with the implementation of Dengue. This observation should be evaluated throughout Europe, particularly in regions with large water basins, which represent the ideal habitat for the potential proliferation of vector mosquitoes [36]. Food and Agriculture Organization corporate statistical database (FAOSTAT) constitutes a database containing information regarding pesticide trade internationally. Since 1990, FAOSTAT has been reporting information regarding the physical quantity of pesticide use. Fig. 1 shows the use of pesticides for agricultural use from 1990 to 2020. This image, created using data provided by FOASTAT, shows an exponential increase in the use of pesticides worldwide. (https://www.fao.org/faostat/en/#search/Insecticides%20%E2%80%93%20Pyrethroids).
Fig. 1.
Pesticide use tons worldwide from 1990 to 2021.
The picture shows the development of pesticide consumption worldwide by showing the years considered on the x-axis and the consumption per tonne on the y-axis.
5. Pesticide resistance
A further threat, and confirmation of the need for a “One Health” approach, is the development of pesticide resistance in the vector, which seriously threatens the effectiveness of current dengue control programmes. The use of chemical insecticides is currently the most widely used method of controlling Aedes aegypti. Pyrethroid insecticides are neurotoxins that alter the normal function of the insect's nervous system by disrupting the Vssc gene by depolarising neurons, paralyzing and eventually killing the insect [37]. The main pyrethroids include deltamethrin, bifenthrin, cypermethrin, cyfluthrin, bifenthrin, and etofenprox [38]. Insecticide-based control plays an important role in dengue control, but as vector resistance to insecticides needs to be managed, it is necessary to clarify the underlying causes of resistance [39]. Detoxification by metabolic enzymes and mutations in insecticide target receptors are the main routes of resistance to pesticide use [40,41]. Physiological resistance is the survival of mosquitoes after exposure to chemical insecticides that would normally kill them. Mechanisms conferring resistance to pyrethroid insecticides in Aedes aegypti include metabolic detoxification by oxidases (P450-mediated monooxygenases), esterases and glutathione S-transferase [42,43]. Cuticle resistance is also responsible for insecticide resistance in Aedes aegypti [44,45]. Thickening of the cuticle reduces insecticide absorption.
6. Vector
DENV is transmitted to humans by the female mosquito of the Aedes genus. There are several species, including Aedes aegypti, which is considered the primary vector because of its importance in tropical and subtropical regions, and secondary vectors such as Aedes albopictus, and Aedes polynesiensis [46]. DENV is characterized by two modes of transmission with different characteristics: sylvan transmission/diffusion to non-human primates with occasional spillover into human populations and urban transmission where the host is human [47]. Aedes aegypti and Aedes albopictus are the main vectors for urban transmission. In contrast, Aedes luteocephalus, Aedes furcifer and Aedes taylor are the main vectors for sylvatic transmission [48]. Infected mosquitoes transmit the virus vertically to their offspring via transovarial transmission, which is an essential factor in the maintenance of transmission cycles in both humans and wildlife [49]. Non-vector routes of transmission have also been reported, including blood transfusion, bone marrow transplantation, and intrapartum and perinatal transmission [50]. The life cycle of Aedes mosquitoes is characterized by two phases: an aquatic phase (larvae, pupae) and a terrestrial phase (eggs, adults), which is articulated and lasts approximately 8–10 days. Aedes albopictus is emerging as a particularly important vector due to its ability to adapt to temperate climates, which has allowed it to spread rapidly across Europe. This type of mosquito feeds mainly on human blood during the day, but Aedes mosquitoes are also active at dusk and at night. Humans act as amplifying hosts for the spread of the virus [51]. Humans are the main host reservoir for dengue, but a meta-analysis by Gwee et al., considers how different animals may be involved in maintaining the virus in an enzootic cycle [52]. The results of the meta-analysis showed that dengue virus is present in 10.1% of bats, 34.1% of pigs and 27.3% of non-human primates.
7. Pathogenesis
The pathogenesis of DENV is influenced by many factors, including virulence, immune status, host genetics, and possible pre-existing diseases. DENV infection is characterized by a spectrum of diseases ranging from mild asymptomatic dengue fever (DF) to severe dengue haemorrhagic fever (DHF) and life-threatening dengue shock syndrome (DSS) [53]. The gateway for DENV is the skin, where the virus enters with the saliva of the mosquito [54]. In the subcutaneous and dermal layers, the virus adheres to the surface of the leukocytes.
Inside the cell, the virus replicates in microvesicles associated with the endoplasmic reticulum. The viral RNA genome is copied, and protein production begins, eventually leading to the assembly of new virions in the Golgi apparatus. These virions leave the cell by exocytosis and are released to infect other cells. Infected white blood cells produce interferon, initiating processes that cause symptoms such as fever, aches, chills and sweating, similar to flu symptoms [55]. Severe infections with high viral loads can also affect the liver and bone marrow, causing damage to parenchymal cells and the endothelium of capillary vessels. Viral replication in bone marrow cells disrupts haematopoiesis, altering the maturation of blood cells and leading to plateletopenia. This condition is responsible for the haemorrhages typical of Dengue [56]. In addition, viral mechanisms can cause cellular damage, particularly to the vascular endothelium, leading to increased permeability. After the initial febrile period, pleural effusion or the presence of ascites in the abdomen may occur. This leads to a decrease in intravascular fluid, resulting in hypovolemia and poor perfusion of vital organs. Severe manifestations of Dengue, such as shock or haemorrhagic fever, occur in less than 5% of patients, mainly in those infected with a different DENV serotype from a previous infection.
8. Vaccines
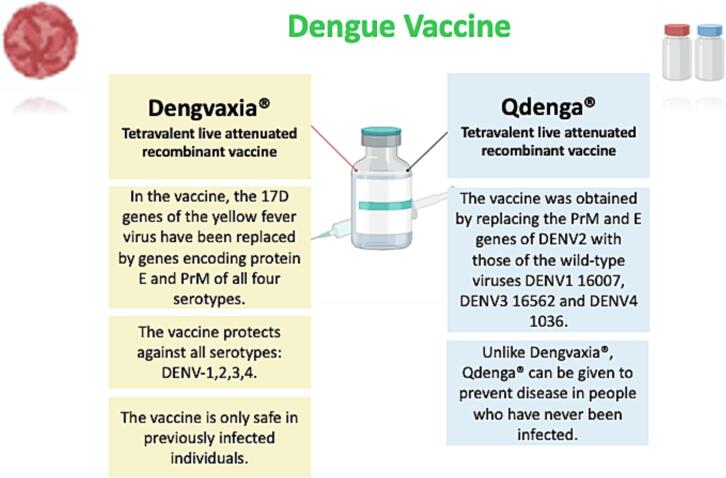
The only two licensed vaccines against the DENV are Dengvaxia® and Qdenga®, both of which are tetravalent, live-attenuated vaccines targeting DENV1–4 serotypes (Fig. 2). The World Health Organization (WHO) identified the development of a safe and effective vaccine against Dengue as an urgent priority in its 2013 guidelines. In recent years, researchers have been engaged in applying various research strategies to construct new vaccines, including inactivated, live attenuated, DNA, mRNA, subunit and viral vector vaccines. Seven promising dengue vaccines are currently being developed. Dengvaxia® (CYD-TDV) is a live attenuated vaccine in which the yellow fever virus 17D genes have been replaced by genes encoding protein E and PrM of all four serotypes [57]. It was approved by the FDA in 2015, but with strict restrictions on its use, taking into account the age and serological status of patients. Dengvaxia® is used in children aged 9–16 years with a history of laboratory-confirmed dengue infection and living in endemic areas. This vaccine is effective against all serotypes, but it is only safe in a person who has had a previous dengue infection. In fact, the vaccine increases the risk of developing severe infection during spontaneous disease in a previously uninfected individual, even up to 3 years after injection. Qdenga® (TAK-003) is a live attenuated vaccine obtained by replacing the PrM and E genes of DENV2 with those of the wild-type viruses DENV1 16,007, DENV3 16,562 and DENV4 1036 [58]. Qdenga® can be given to adults, adolescents and children from 4 years of age [59]. Unlike Dengvaxia®, Qdenga® can be given to prevent disease in people who have never been infected. An ideal dengue vaccine should be able to provide effective protection against all serotypes of the virus and also protect against antibody-dependent enhancement. In the first case, tetravalent vaccine formulations are often hampered by antigenic competition, while in the second case, the vaccine-induced protection could increase the risk of developing severe infection over time.
Fig. 2.
The approved dengue vaccines are Dengvaxia® and Qdenga®.
The column in dark pink shows some of the characteristics of the Dengvaxia® vaccine, while the column in light blue shows the characteristics of the Qdenga® vaccine.
9. Vector control
Effective management of the DENV requires a multifaceted approach that includes vector reduction and population control. In addition, mosquito habitat reduction is a key element in the fight against Dengue. This strategy aims to eliminate or reduce the places where mosquitoes lay their eggs and develop. This can be achieved through interventions such as removing stagnant water containers, cleaning drains and managing waste. Currently, strategies to reduce DENV infections are based on vector control, such as public awareness campaigns, the use of insecticides and vector surveillance systems. Responsible use of insecticides is essential to avoid potential risks to human health and the environment [60]. Biological control of mosquitoes offers a sustainable alternative to chemical insecticides. This strategy involves the use of organisms or biological agents that prey on or compete with mosquitoes. For example, releasing fish larvae that feed on mosquito larvae into water tanks can help reduce the mosquito population. Other biological control methods include the use of bacteria such as Bacillus thuringiensis israelensis, which kills mosquito larvae [61,62]. New strategies are also being developed and tested, such as interfering with the vitality of vectors, e.g. transgenic mosquitoes [63]. Continuous monitoring of the mosquito population and evaluation of the effectiveness of control strategies are essential to adapt control measures to changing local conditions.
10. Diagnosis
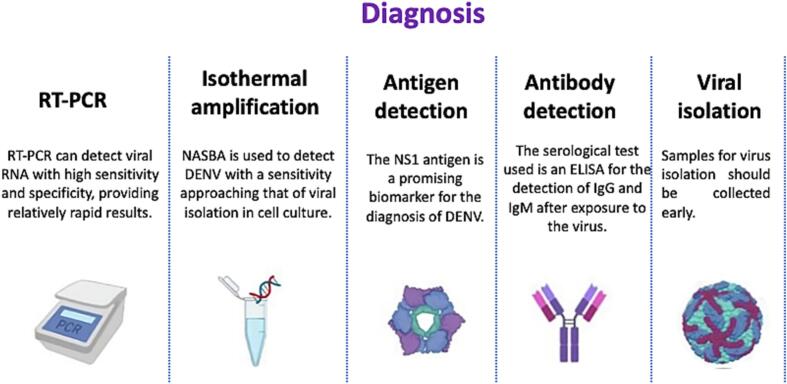
The choice of diagnostic method depends on the objective of the test, the type of laboratory equipment, the experience of the staff, the cost and the time required for sample collection. Molecular and serological tests are used to diagnose Dengue (Fig. 3);
Fig. 3.
Molecular and serological tests for the diagnosis of dengue.
The figure shows the main techniques for dengue diagnosing in the five sections presented.
1) Molecular tests: a) Nucleic acid tests (NAT) diagnose and quantify viral RNA/DNA with high precision, high specificity, high sensitivity, high speed and low cost, allowing detection in the acute phase; b) reverse transcriptase-polymerase chain reaction (RT-PCR): This is the gold standard in dengue diagnosis [64]. There are multiple tests that can distinguish between Dengue, Zika virus and chikungunya; c)ISOTHERMAL AMPLIFICATION: these do not require a thermal cycler and can be less complex and less expensive. Examples include reverse transcription-loop mediated isothermal amplification (RT-LAMP), reverse-transcription recombinase polymerase amplification (RT-RPA) and nucleic acid sequence-based amplification (NASBA) [65,66].
2)Serological tests; a)ANTIGEN DETECTION: NS1 antigen plays a key role in DENV replication and is detected by immunochromatographic or immunofluorescence tests on serum [67]. b)ANTIBODY DETECTION: Detection of IgG and IgM in serum and cerebrospinal fluid by ELISA [68]. A limitation of serological tests is certainly the cross-reactivity between DENV, Zika virus, and West Nile virus serotypes due to similarities in viral structure, which also induces clinical symptoms in areas of endemicity of these infectious diseases. Currently, the gold standard for differentiating these flaviviruses is the neutralization test, which is the most reliable and specific among flaviviruses.
11. Perspectives of -OMICS sciences in Dengue control
The -OMICS sciences represent a wide range of disciplines that aim to identify the structural and functional characteristics of the biological molecules of the organisms under study. The omics sciences, and in particular the multi-omics approach, can solve complex biological problems through systematic methodology and analytical completeness and allow the characterization of molecular targets and immunoreactive proteins, which are fundamental for the development of new diagnostic techniques and new prophylactic interventions [[69], [70], [71]]. Early diagnosis is a crucial step in the management of infectious diseases, especially those infections characterized by a complex biological cycle that falls under the One Health management model [72]. In recent years, several studies have been conducted on the evolution of Dengue from a genomic perspective, with particular emphasis on the identification of genotypes and serotypes involved in epidemics in different areas of the world [[73], [74], [75]]. Genomic information on different genotypes present on a global scale is crucial for understanding genes associated with virulence and differential gene expression [76]. One of the most interesting and comprehensive comparative genomics studies was performed by Letizia et al., in Burkina Faso in 2017 through a retrospective genomic characterization of a dengue outbreak [77]. The researchers sequenced 29 DENV genomes and confirmed the co-circulation of DENV-1, DENV-2 and DENV-3 serotypes. Phylogenetic analysis showed that DENV-2 is endemic in Burkina Faso, with two complete genomes and one partial genome obtained from PCR-negative samples. Among the omics sciences, proteomics provides data directly applicable to the development of rapid diagnostic tests and innovative vaccines. In recent years, proteomics has been successfully applied to the study of Dengue to identify specific immunoreactive epitopes, detectable antigens and antibody responses. Testa et al., showed that dengue virus-infected cells have epitopes derived from capsid protein C and non-structural protein NS4B and NS5, which are conserved across DENV serotypes [78]. These epitopes could be used to characterize T cell-mediated immunity for the development of new vaccines or to complement current vaccines in development. The most widely used serological tests are those based on the detection of IgM and IgG antibodies, but cross-reactivity between flaviviruses and the inability to diagnose disease in the acute phase are important limitations of these tests [79,80]. The non-structural protein NS1 may be an attractive biomarker for the detection of acute dengue infection and may also provide a serological alternative to viral RNA by RT-PCR. In a study by Allonso et al., an ELISA was developed to detect and quantify NS1 protein from the four DENV serotypes [81]. Interestingly, NS1 positivity for DENV4 samples was lower than for DENV1, and circulating NS1 concentrations ranged from 7 to 284 ng/mL. In addition to their dynamic significance, proteins have important logistical advantages over nucleic acids because of their greater stability. In fact, proteins are less susceptible to sample degradation during transport than viral RNA, thus reducing the likelihood of false negatives [82]. Advances in proteomics technologies are revolutionizing the field of microbiology through the development of analysis algorithms, but above all, through the application of new knowledge about host-pathogen interactions. In order to get a defined picture of the complex interactions underlying systems biology in host-pathogen interactions, proteomics must be combined with other -OMICS sciences (genomics, transcriptomics, metabolomics) [83]. In the approach to vaccine design, it is always necessary to have a deep understanding of these interactions to ensure durable and effective protection. An ideal vaccine should be able to induce a complete immune response, but to ensure this, it would be necessary to understand the interactions of the components of innate immunity, antibodies, and adaptive cell-mediated immunity at a systems level. This approach, called ‘systems biology’, comprehensively assesses the complexity of biological systems using omics methods. Statistical analyses and computational models are then used to understand which of these differences contribute to the initiation, development, kinetics, maintenance and durability of immune responses to vaccines. Alsaiari et al., conducted an interesting study based on the technique of reverse vaccinology to design a vaccine against all four serotypes of dengue [84]. In particular, the researchers attempted to design a multi-epitope vaccine in silico from immunogenic B- and T-cell epitopes capable of eliciting humoral and cell-mediated immune responses. From all four serotypes, only five epitopes were effective for vaccine construction. After molecular docking and molecular dynamics simulations against HLA and TRL receptors 2 and 4 and transcription of the vaccine sequence into cDNA to generate an expression vector in Escherichia coli K12, Alsaiari et al., classified the potential vaccine construct ‘V5’ as antigenic, non-allergenic and stable. However, experimental analysis is required as this is still an in silico experiment. In addition, a study by Bondhon et al., evaluated forty-three phytochemicals as potential inhibitors of the DENV-2 serotype NS2B-NS3 protease [85]. The results of the study indicated that catechin gallate, luteolin-7-O-glucoside and eriodictyol had the most promising data as therapeutic agents against DENV-2. Finally, metagenomics has recently been used to analyze the microbiota of the vector in order to identify strategies to manipulate the same microbiota that may be useful in the control of Dengue. In this regard, a study by Wu et al., showed that the symbiont Serratia marcescens promotes susceptibility to infection in vectors due to its ability to degrade the mucins of the intestinal membrane, causing DENV to spread in the intestine and becoming a potential target [86].
12. Conclusions
The threat of Dengue, the impact of climate change on the virus, the difficulty of managing the emergency using traditional methods, and the fragility of vaccination underline the need for a One Health approach to zoonotic disease management. The review highlighted the urgent need to address the threat posed by the dengue virus through the use of biotechnology and -OMICS sciences to identify new vaccination and prophylactic strategies in managing a virus that is increasingly emerging as a severe threat to global health. -OMICS sciences, using a multidisciplinary approach, can solve complex biological problems through systematic methodology and analytical completeness and allow the characterization of molecular targets and immunoreactive proteins, which are fundamental for developing new diagnostic techniques and new prophylactic interventions. In addition, the use of bioinformatic analysis and big data is emerging as a tool with extraordinary potential for the design of future effective vaccines. In conclusion, the review highlights how advances in the understanding of the dengue virus are needed to provide information on immunogenic dynamics and pathogen-host interactions and how -OMICS sciences and the One-Health approach are essential elements in managing this threat.
Funding
None.
CRediT authorship contribution statement
Anna Caterina Procopio: Conceptualization, Data curation, Visualization, Writing – original draft. Simona Colletta: Conceptualization, Visualization, Writing – original draft. Emanuela Laratta: Conceptualization, Visualization, Writing – original draft. Matteo Mellace: Conceptualization, Visualization, Writing – original draft. Bruno Tilocca: Methodology, Supervision, Writing – review & editing. Carlotta Ceniti: Supervision, Writing – review & editing. Andrea Urbani: Methodology, Supervision, Writing – review & editing. Paola Roncada: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
Fig. 2, Fig. 3 and graphical abstract created with BioRender.com.
Data availability
No data was used for the research described in the article.
References
- 1.WHO Dengue and Severe Dengue. Mar. 2023. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue
- 2.Dengue Virus Disease Cases Reported April 2022–March 2023. https://www.ecdc.europa.eu/en/publications-data/dengue-virus-disease-cases-reported-april-2022-march-2023
- 3.Gubler D.J. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 1998;11:480–496. doi: 10.1128/CMR.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuhn R.J., Zhang W., Rossmann M.G., Pletnev S.V., Corver J., Lenches E., Jones C.T., Mukhopadhyay S., Chipman P.R., Strauss E.G., Baker T.S., Strauss J.H. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108:717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischl W., Bartenschlager R. Exploitation of cellular pathways by Dengue virus. Curr. Opin. Microbiol. 2011;14:470–475. doi: 10.1016/j.mib.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Stadtländer C.T. One health: people, animals, and the environment. Infect. Ecol. Epidemiol. 2015;5:30514. doi: 10.3402/iee.v5.30514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chala B., Hamde F. Emerging and re-emerging vector-borne infectious diseases and the challenges for control: a review. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.715759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morens D.M., Folkers G.K., Fauci A.S. Emerging infections: a perpetual challenge. Lancet Infect. Dis. 2008;8:710–719. doi: 10.1016/S1473-3099(08)70256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrington L.B., Armijos M.V., Lambrechts L., Barker C.M., Scott T.W. Effects of fluctuating daily temperatures at critical thermal extremes on Aedes aegypti life-history traits. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S.C., Hsieh M.H. Modeling the transmission dynamics of dengue fever: implications of temperature effects. Sci. Total Environ. 2012;431:385–391. doi: 10.1016/j.scitotenv.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Descloux E., Mangeas M., Menkes C.E., Lengaigne M., Leroy A., Tehei T., Guillaumot L., Teurlai M., Gourinat A.C., Benzler J., Pfannstiel A., Grangeon J.P., Degallier N., De Lamballerie X. Climate-based models for understanding and forecasting dengue epidemics. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Earnest A., Tan S.B., Wilder-Smith A. Meteorological factors and El Niño Southern Oscillation are independently associated with dengue infections. Epidemiol. Infect. 2012;140:1244–1251. doi: 10.1017/S095026881100183X. [DOI] [PubMed] [Google Scholar]
- 13.Caminade C., Medlock J.M., Ducheyne E., McIntyre K.M., Leach S., Baylis M., Morse A.P. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. J. R. Soc. Interface. 2012;9:2708–2717. doi: 10.1098/rsif.2012.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sly P.D. Health impacts of climate change and biosecurity in the Asian Pacific region. Rev. Environ. Health. 2011;26:7–12. doi: 10.1515/reveh.2011.002. [DOI] [PubMed] [Google Scholar]
- 15.Hales S., de Wet N., Maindonald J., Woodward A. Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet. 2002;360:830–834. doi: 10.1016/S0140-6736(02)09964-6. [DOI] [PubMed] [Google Scholar]
- 16.Chakravarti A., Kumaria R. Eco-epidemiological analysis of dengue infection during an outbreak of dengue fever, India. Virol. J. 2005;2:32. doi: 10.1186/1743-422X-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gubler D.J. The economic burden of dengue. Am. J. Trop. Med. Hyg. 2012;86:743–744. doi: 10.4269/ajtmh.2012.12-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halasa Y.A., Shepard D.S., Zeng W. Economic cost of dengue in Puerto Rico. Am. J. Trop. Med. Hyg. 2012;86:745–752. doi: 10.4269/ajtmh.2012.11-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaves L.F., Koenraadt C.J. Climate change and highland malaria: fresh air for a hot debate. Q. Rev. Biol. 2010;85:27–55. doi: 10.1086/650284. [DOI] [PubMed] [Google Scholar]
- 20.Scott T.W., Morrison A.C., Lorenz L.H., Clark G.G., Strickman D., Kittayapong P., Zhou H., Edman J.D. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: population dynamics. J. Med. Entomol. 2000;37:77–88. doi: 10.1603/0022-2585-37.1.77. [DOI] [PubMed] [Google Scholar]
- 21.Brady O.J., Johansson M.A., Guerra C.A., Bhatt S., Golding N., Pigott D.M., Delatte H., Grech M.G., Leisnham P.T., Maciel-de-Freitas R., Styer L.M., Smith D.L., Scott T.W., Gething P.W., Hay S.I. Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasit. Vectors. 2013;6:351. doi: 10.1186/1756-3305-6-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keesing F., Belden L.K., Daszak P., Dobson A., Harvell C.D., Holt R.D., Hudson P., Jolles A., Jones K.E., Mitchell C.E., Myers S.S., Bogich T., Ostfeld R.S. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468:647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swei A., Couper L.I., Coffey L.L., Kapan D., Bennett S. Patterns, drivers, and challenges of vector-borne disease emergence. Vector Borne Zoonotic Dis. 2020;20:159–170. doi: 10.1089/vbz.2018.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry B.D., Grace D., Sones K. Current drivers and future directions of global livestock disease dynamics. Proc. Natl. Acad. Sci. U. S. A. 2013;110:20871–20877. doi: 10.1073/pnas.1012953108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong M.X., Hansen A., Hanson-Easey S., Cameron S., Xiang J., Liu Q., Sun Y., Weinstein P., Han G.S., Williams C., Bi P. Infectious diseases, urbanization and climate change: challenges in future China. Int. J. Environ. Res. Public Health. 2015;12:11025–11036. doi: 10.3390/ijerph120911025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Focks D.A., Daniels E., Haile D.G., Keesling J.E. A simulation model of the epidemiology of urban dengue fever: literature analysis, model development, preliminary validation, and samples of simulation results. Am. J. Trop. Med. Hyg. 1995;53:489–506. doi: 10.4269/ajtmh.1995.53.489. [DOI] [PubMed] [Google Scholar]
- 27.Patz J.A., Epstein P.R., Burke T.A., Balbus J.M. Global climate change and emerging infectious diseases. JAMA. 1996;275:217–223. doi: 10.1001/jama.1996.03530270057032. [DOI] [PubMed] [Google Scholar]
- 28.Hii Y.L., Rocklöv J., Ng N., Tang C.S., Pang F.Y., Sauerborn R. Climate variability and increase in intensity and magnitude of dengue incidence in Singapore. Glob. Health Action. 2009:2. doi: 10.3402/gha.v2i0.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Githeko A.K. Advances in developing a climate based dengue outbreak models in Dhaka, Bangladesh: challenges & opportunities. Indian J. Med. Res. 2012;136:7–9. [PMC free article] [PubMed] [Google Scholar]
- 30.Lundgren-Nilsson Å., Jonsdottir I.H., Pallant J., Jr G., Ahlborg. Internal construct validity of the Shirom-Melamed Burnout Questionnaire (SMBQ) BMC Public Health. 2012;12:1. doi: 10.1186/1471-2458-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aziz A.T., Al-Shami S.A., Mahyoub J.A., Hatabbi M., Ahmad A.H., Rawi C.S. An update on the incidence of dengue gaining strength in Saudi Arabia and current control approaches for its vector mosquito. Parasit. Vectors. 2014;7:258. doi: 10.1186/1756-3305-7-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kraemer M.U., Sinka M.E., Duda K.A., Mylne A.Q., Shearer F.M., Barker C.M., Moore C.G., Carvalho R.G., Coelho G.E., Van Bortel W., Hendrickx G., Schaffner F., Elyazar I.R., Teng H.J., Brady O.J., Messina J.P., Pigott D.M., Scott T.W., Smith D.L., Wint G.R., Golding N., Hay S.I. The global distribution of the arbovirus vectors Aedes aegypti and Ae. Albopictus. Elife. 2015;4 doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliveira S., Rocha J., Sousa C.A., Capinha C. Wide and increasing suitability for Aedes albopictus in Europe is congruent across distribution models. Sci. Rep. 2021;11:9916. doi: 10.1038/s41598-021-89096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brem J., Elankeswaran B., Erne D., Hedrich N., Lovey T., Marzetta V., Salvado L.T., Züger C., Schlagenhauf P. Dengue "homegrown" in Europe (2022 to 2023) New Microbes New Infect. 2023;56 doi: 10.1016/j.nmni.2023.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pesticides Use, Pesticides Trade and Pesticides Indicators. Global, Regional and Country Trends, 1990–2020. https://www.fao.org/documents/card/en?details=cc0918en
- 36.Ahamad A., Kumar J. Pyrethroid pesticides: an overview on classification, toxicological assessment and monitoring. J. Hazard. Mater. Adv. 2023;10:100284. doi: 10.1016/j.hazadv.2023.100284. [DOI] [Google Scholar]
- 37.Mazzoni E., Battilani P. Pyrethroid Scientific Forum. 2009. Pyrethroids and the food chain–mycotoxin management. [Google Scholar]
- 38.Ping L.T., Yatiman R., Gek L.P. Susceptibility of adult field strains of Aedes aegypti and Aedes albopictus in Singapore to pirimiphos-methyl and permethrin. J. Am. Mosq. Control Assoc. 2001;17:144–146. [PubMed] [Google Scholar]
- 39.Narahashi T. Nerve membrane ion channels as the target site of environmental toxicants. Environ. Health Perspect. 1987;71:25–29. doi: 10.1289/ehp.877125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith L.B., Kasai S., Scott J.G. Pyrethroid resistance in Aedes aegypti and Aedes albopictus: important mosquito vectors of human diseases. Pestic. Biochem. Physiol. 2016;133:1–12. doi: 10.1016/j.pestbp.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Ishak I.H., Jaal Z., Ranson H., Wondji C.S. Contrasting patterns of insecticide resistance and knockdown resistance (kdr) in the dengue vectors Aedes aegypti and Aedes albopictus from Malaysia. Parasit. Vectors. 2015;8:181. doi: 10.1186/s13071-015-0797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brito L.P., Linss J.G., Lima-Camara T.N., Belinato T.A., Peixoto A.A., Lima J.B., Valle D., Martins A.J. Assessing the effects of Aedes aegypti kdr mutations on pyrethroid resistance and its fitness cost. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chareonviriyaphap T., Bangs M.J., Suwonkerd W., Kongmee M., Corbel V., Ngoen-Klan R. Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasit. Vectors. 2013;6:280. doi: 10.1186/1756-3305-6-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kasai S., Komagata O., Itokawa K., Shono T., Ng L.C., Kobayashi M., Tomita T. Mechanisms of pyrethroid resistance in the dengue mosquito vector, Aedes aegypti: target site insensitivity, penetration, and metabolism. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nkya T.E., Akhouayri I., Kisinza W., David J.P. Impact of environment on mosquito response to pyrethroid insecticides: facts, evidences and prospects. Insect Biochem. Mol. Biol. 2013;43:407–416. doi: 10.1016/j.ibmb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 46.Malavige G.N., Fernando S., Fernando D.J., Seneviratne S.L. Dengue viral infections. Postgrad. Med. J. 2004;80:588–601. doi: 10.1136/pgmj.2004.019638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simmons C.P., Farrar J.J., Nguyen V., Wills B. Dengue. N. Engl. J. Med. 2012;366:1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 48.Chen R., Vasilakis N. Dengue--quo tu et quo vadis? Viruses. 2011;3:1562–1608. doi: 10.3390/v3091562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holmes E.C., Twiddy S.S. The origin, emergence and evolutionary genetics of dengue virus. Infect. Genet. Evol. 2003;3:19–28. doi: 10.1016/s1567-1348(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 50.Chen L.H., Wilson M.E. Update on non-vector transmission of dengue: relevant studies with Zika and other flaviviruses. Trop. Dis. Travel. Med. Vaccines. 2016;2:15. doi: 10.1186/s40794-016-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choy M.M., Sessions O.M., Gubler D.J., Ooi E.E. Production of infectious Dengue virus in Aedes aegypti is dependent on the ubiquitin proteasome pathway. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gwee S.X.W., St John A.L., Gray G.C., Pang J. Animals as potential reservoirs for dengue transmission: a systematic review. One Health. 2021;12 doi: 10.1016/j.onehlt.2021.100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martina B.E., Koraka P., Osterhaus A.D. Dengue virus pathogenesis: an integrated view. Clin. Microbiol. Rev. 2009;22:564–581. doi: 10.1128/CMR.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tjaden N.B., Thomas S.M., Fischer D., Beierkuhnlein C. Extrinsic incubation period of Dengue: knowledge, backlog, and applications of temperature dependence. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halstead S.B. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 56.Rigau-Pérez J.G., Clark G.G., Gubler D.J., Reiter P., Sanders E.J., Vorndam A.V. Dengue and dengue haemorrhagic fever. Lancet. 1998;352:971–977. doi: 10.1016/s0140-6736(97)12483-7. [DOI] [PubMed] [Google Scholar]
- 57.Shukla R., Ramasamy V., Shanmugam R.K., Ahuja R., Khanna N. Antibody-dependent enhancement: a challenge for developing a safe Dengue vaccine. Front. Cell. Infect. Microbiol. 2020;10 doi: 10.3389/fcimb.2020.572681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sirivichayakul C., Barranco-Santana E.A., Esquilin-Rivera I., Oh H.M., Raanan M., Sariol C.A., Shek L.P., Simasathien S., Smith M.K., Velez I.D., Wallace D., Gordon G.S., Stinchcomb D.T. Safety and immunogenicity of a tetravalent Dengue vaccine candidate in healthy children and adults in Dengue-endemic regions: a randomized, placebo-controlled phase 2 study. J. Infect. Dis. 2016;213:1562–1572. doi: 10.1093/infdis/jiv762. [DOI] [PubMed] [Google Scholar]
- 59.Rivera L., Biswal S., Sáez-Llorens X., Reynales H., López-Medina E., Borja-Tabora C., Bravo L., Sirivichayakul C., Kosalaraksa P., Martinez Vargas L., Yu D., Watanaveeradej V., Espinoza F., Dietze R., Fernando L., Wickramasinghe P., Duarte MoreiraJr E., Fernando A.D., Gunasekera D., Luz K., Venâncioda Cunha R., Rauscher M., Zent O., Liu M., Hoffman E., LeFevre I., Tricou V., Wallace D., Alera M., Borkowski A. Three-year efficacy and safety of Takeda’s Dengue vaccine candidate (TAK-003) Clin. Infect. Dis. 2022;75:107–117. doi: 10.1093/cid/ciab864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fitzpatrick C., Haines A., Bangert M., Farlow A., Hemingway J., Velayudhan R. An economic evaluation of vector control in the age of a dengue vaccine. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bohari R., Jin Hin C., Matusop A., Abdullah M.R., Ney T.G., Benjamin S., Lim L.H. Wide area spray of bacterial larvicide, Bacillus thuringiensis israelensis strain AM65-52, integrated in the national vector control program impacts dengue transmission in an urban township in Sibu district, Sarawak, Malaysia. PLoS One. 2020;15 doi: 10.1371/journal.pone.0230910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Utarini A., Indriani C., Ahmad R.A., Tantowijoyo W., Arguni E., Ansari M.R., Supriyati E., Wardana D.S., Meitika Y., Ernesia I., Nurhayati I., Prabowo E., Andari B., Green B.R., Hodgson L., Cutcher Z., Rancès E., Ryan P.A., O’Neill S.L., Dufault S.M., Tanamas S.K., Jewell N.P., Anders K.L., Simmons C.P., AWED Study Group Efficacy of Wolbachia-infected mosquito deployments for the control of Dengue. N. Engl. J. Med. 2021;384:2177–2186. doi: 10.1056/NEJMoa2030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evans B.R., Kotsakiozi P., Costa-da-Silva A.L., Ioshino R.S., Garziera L., Pedrosa M.C., Malavasi A., Virginio J.F., Capurro M.L., Powell J.R. Transgenic Aedes aegypti mosquitoes transfer genes into a natural population. Sci. Rep. 2019;9:13047. doi: 10.1038/s41598-019-49660-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bachman J. Reverse-transcription PCR (RT-PCR) Methods Enzymol. 2013;530:67–74. doi: 10.1016/B978-0-12-420037-1.00002-6. [DOI] [PubMed] [Google Scholar]
- 65.Diagne C.T., Faye M., Lopez-Jimena B., Abd El Wahed A., Loucoubar C., Fall C., Mencatelli G., Faye O., Faye O., Weidmann M., Sall A.A. Comparative analysis of Zika virus detection by RT-qPCR, RT-LAMP, and RT-RPA. Methods Mol. Biol. 2020;2142:165–179. doi: 10.1007/978-1-0716-0581-3_14. [DOI] [PubMed] [Google Scholar]
- 66.Herrada C.A., Kabir M.A., Altamirano R., Asghar W. Advances in diagnostic methods for Zika virus infection. J. Med. Device. 2018;12:0408021–4080211. doi: 10.1115/1.4041086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jayathilaka D., Gomes L., Jeewandara C., Jayarathna G.S.B., Herath D., Perera P.A., Fernando S., Wijewickrama A., Hardman C.S., Ogg G.S., Malavige G.N. Role of NS1 antibodies in the pathogenesis of acute secondary dengue infection. Nat. Commun. 2018;9:5242. doi: 10.1038/s41467-018-07667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sirivichayakul C., Limkittikul K., Chanthavanich P., Yoksan S., Ratchatatat A., Lim J.K., Arunsodsai W., Sabchareon A. Monoclonal antibody-based capture ELISA in the diagnosis of previous dengue infection. Virol. J. 2019;16:125. doi: 10.1186/s12985-019-1222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hasin Y., Seldin M., Lusis A. Multi-omics approaches to disease. Genome Biol. 2017;18:83. doi: 10.1186/s13059-017-1215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wörheide M.A., Krumsiek J., Kastenmüller G., Arnold M. Multi-omics integration in biomedical research – a metabolomics-centric review. Anal. Chim. Acta. 2021;1141:144–162. doi: 10.1016/j.aca.2020.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patti G.J., Yanes O., Siuzdak G. Innovation: metabolomics: the apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012;13:263–269. doi: 10.1038/nrm3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sim S., Hibberd M.L. Genomic approaches for understanding dengue: insights from the virus, vector, and host. Genome Biol. 2016;17:38. doi: 10.1186/s13059-016-0907-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hwang E.H., Kim G., Chung H., Oh H., Park J.H., Hur G.H., Hong J., Koo B.S. Molecular evolution of dengue virus types 1 and 4 in Korean travellers. Arch. Virol. 2021;166:1103–1112. doi: 10.1007/s00705-021-04973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun B., Zhang X., Zhang H., Liu H., Sun L., Tan Q., Liang M., Wu D., Liu D. Genomic epidemiological characteristics of dengue fever in Guangdong province, China from 2013 to 2017. PLoS Negl. Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Inizan C., O’Connor O., Worwor G., Cabemaiwai T., Grignon J.C., Girault D., Minier M., Prot M., Ballan V., Pakoa G.J., Grangeon J.P., Guyant P., Lepers C., Faktaufon D., Sahukhan A., Jr Merilles O.E., Gourinat A.C., Simon-Lorière E., Dupont-Rouzeyrol M. Molecular characterization of Dengue type 2 outbreak in Pacific Islands countries and territories, 2017-2020. Viruses. 2020;12:1081. doi: 10.3390/v12101081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gardy J.L., Loman N.J. Towards a genomics-informed, real-time, global pathogen surveillance system. Nat. Rev. Genet. 2018;19:9–20. doi: 10.1038/nrg.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Letizia A.G., Pratt C.B., Wiley M.R., Fox A.T., Mosore M., Agbodzi B., Yeboah C., Kumordjie S., Di Paola N., Assana K.C., Coulidiaty D., Ouedraogo C., Bonney J.H.K., Ampofo W., Tarnagda Z., Sangaré L. Retrospective genomic characterization of a 2017 Dengue Virus Outbreak, Burkina Faso. Emerg. Infect. Dis. 2022;28:1198–1210. doi: 10.3201/eid2806.212491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Testa J.S., Shetty V., Sinnathamby G., Nickens Z., Hafner J., Kamal S., Zhang X., Jett M., Philip R. Conserved MHC class I-presented dengue virus epitopes identified by immunoproteomics analysis are targets for cross-serotype reactive T-cell response. J. Infect. Dis. 2012;205:647–655. doi: 10.1093/infdis/jir814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwartz E., Mileguir F., Grossman Z., Mendelson E. Evaluation of ELISA-based sero-diagnosis of dengue fever in travellers. J. Clin. Virol. 2000;19:169–173. doi: 10.1016/s1386-6532(00)00114-1. [DOI] [PubMed] [Google Scholar]
- 80.Oceguera L.F., 3rd, Patiris P.J., Chiles R.E., Busch M.P., Tobler L.H., Hanson C.V. Flavivirus serology by Western blot analysis. Am. J. Trop. Med. Hyg. 2007;77:159–163. doi: 10.4269/ajtmh.2007.77.159. [DOI] [PubMed] [Google Scholar]
- 81.Allonso D., Meneses M.D., Fernandes C.A., Ferreira D.F., Mohana-Borges R. Assessing positivity and circulating levels of NS1 in samples from a 2012 dengue outbreak in Rio de Janeiro, Brazil. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grossegesse M., Hartkopf F., Nitsche A., Schaade L., Doellinger J., Muth T. Perspective on proteomics for virus detection in clinical samples. J. Proteome Res. 2020;19:4380–4388. doi: 10.1021/acs.jproteome.0c00674. [DOI] [PubMed] [Google Scholar]
- 83.Schubert O.T., Gillet L.C., Collins B.C., Navarro P., Rosenberger G., Wolski W.E., Lam H., Amodei D., Mallick P., MacLean B., Aebersold R. Building high-quality assay libraries for targeted analysis of SWATH MS data. Nat. Protoc. 2015;10:426–441. doi: 10.1038/nprot.2015.015. [DOI] [PubMed] [Google Scholar]
- 84.Alsaiari A.A., Hakami M.A., Alotaibi B.S., Alkhalil S.S., Hazazi A., Alkhorayef N., Jalal K., Yasmin F. Rational design of multi-epitope-based vaccine by exploring all dengue virus serotypes proteome: an immunoinformatic approach. Immunol. Res. 2023 doi: 10.1007/s12026-023-09429-6. [DOI] [PubMed] [Google Scholar]
- 85.Bondhon T.A., Hasan A., Jannat K., Paul A., Jahan R., Mahboob T., Nissapatorn V., Dolma K., Pereira M.L., Wiart C., Rahmatullah M. Molecular docking study of Lens culinaris L. phytochemicals to NS3-NS2B protease of dengue virus serotype 2. Ger. J. Microbiol. 2021;1:26–37. doi: 10.51585/gjm.2021.0005. [DOI] [Google Scholar]
- 86.Wu P., Sun P., Nie K., Zhu Y., Shi M., Xiao C., Liu H., Liu Q., Zhao T., Chen X., Zhou H., Wang P., Cheng G. A gut commensal bacterium promotes mosquito permissiveness to arboviruses. Cell Host Microbe. 2019;25:101–112.e5. doi: 10.1016/j.chom.2018.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.