Abstract
Brucellosis, caused by Brucella spp., is a re-emerging One Health disease with increased prevalence and incidence in Chinese dairy cattle and humans, severely affecting animal productivity and public health. In dairy cattle, B. abortus is the primary causative agent although infections with other Brucella species occur occasionally. However, the epidemiological and comparative importance of B. abortus in dairy cattle and humans remains inadequately understood throughout China due to the heterogeneity in locations, quality, and study methods. This scoping review aims to describe the changing status of B. abortus infection in dairy cattle and humans, investigate the circulating Brucella species and biovars, and identify factors driving the disease transmission by retrieving publicly accessible literature from four databases. After passing the prespecified inclusion criteria, 60 original articles were included in the final synthesis. Although the reported animal-level and farm-level prevalence of brucellosis in dairy cattle was lower compared to other endemic countries (e.g. Iran and India), it has been reported to increase over the last decade. The incidence of brucellosis in humans displayed seasonal increases. The Rose Bengal Test and Serum Agglutination Test, interpreted in series, were the most used serological test to diagnose Brucella spp. in dairy cattle and humans. B. abortus biovar 3 was the predominant species (81.9%) and biovar (70.3%) in dairy cattle, and B. melitensis biovar 3 was identified as the most commonly detected strain in human brucellosis cases. These strains were mainly clustered in Inner Mongolia and Shannxi Province (75.7%), limiting the generalizability of the results to other provinces. Live cattle movement or trade was identified as the key factor driving brucellosis transmission, but its transmission pattern remains unknown within the Chinese dairy sector. These knowledge gaps require a more effective One Health approach to be bridged. A coordinated and evidence-based research program is essential to inform regional or national control strategies that are both feasible and economical in the Chinese context.
Keywords: Dairy cattle, Brucella abortus, Diagnosis, Epidemiology, One health, Control strategy
Highlights
-
•
A comprehensive scoping review examined the seroprevalence of brucellosis in dairy cattle and humans.
-
•
B. abortus biovar 3 occupied major brucellosis in dairy cattle, while B. melitensis biovar 3 dominated in humans in China.
-
•
The review identified heterogeneous research quality and knowledge gaps to refine the future research in China.
-
•
A “One Health” approach is required to guide effective control schemes for B. abortus in dairy cattle in China.
1. Introduction
Brucellosis, mainly caused by Brucella species (Brucella spp.), is globally recognized as a significant zoonotic and One Health disease [1]. Brucella spp. contains more than twelve species of intracellular bacteria; among them four zoonotic species infect multiple hosts, such as cattle and small ruminants [2]. Brucella spp. exhibits host tropism but is not restricted to an exclusive host; B. abortus mainly infects cattle, whereas B. melitensis primarily affects small ruminants [2]. Characteristic clinical signs of B. abortus infection in cattle and small ruminants are abortion, retained placenta, orchitis, infertility, and reduced milk yield [1,3,4]. In humans, the symptoms of this disease include muscle pain, arthritis, rising and falling “undulant” fever, hyperhidrosis, fatigue, and night sweats [5]. Given the profound impact of B. abortus on humans and animals, the World Health Organisation (WHO) and World Organisation for Animal Health (WOAH, founded as OIE) have classified B. abortus as a notifiable or listed disease affecting the international trade in animals and their products [6,7].
B. abortus is a highly contagious pathogen that can spread across multiple hosts. The primary transmission routes of B. abortus for cattle are by direct contact with aborted products or vaginal secretions of infected animals or consumption of unpasteurized milk [8,9]. Contacting wild animals (e.g., rodents and deer) and ticks may also provoke the re-emergence of B. abortus infection in domestic animals [10,11]. Direct contact with infected animals and their aborted products without wearing personal protection equipment (PPE, e.g., gloves and masks) is considered the riskiest way to acquire brucellosis in humans [12,13]. Consumption of raw or unpasteurized dairy products and inhaling Brucella aerosols are common routes of Brucella spp. acquisition in humans [1,[14], [15], [16]]. Therefore, B. abortus is regarded as a One Health hazard to animals, occupation-related workers, and the public.
Since almost all cases of brucellosis in humans are of animal origin, prevention of brucellosis in humans could be achieved through either human hygiene measures or control measures in infected animal populations. Controlling the spread of B. abortus on farms is often the most efficient and cost-effective approach to mitigate public health and food safety risks compared to traditional hygiene measures at the processing stage [[17], [18], [19]]. Intensified surveillance in animal host species, test-and-slaughter, restriction of live animal movement, and mass vaccination are effective brucellosis control strategies [20]. Australia and New Zealand have successfully eradicated B. abortus from domestic farms by implementing extensive mandatory vaccination, followed by a rigorous test-and-culling approach [21,22]. In areas where B. abortus is endemic, vaccinating susceptible animals is highly recommended by WOAH to provide protection against virulent Brucella spp. strains and reduce disease impacts on reproduction and production [6].
B. abortus is still endemic in many countries, with a particularly high prevalence in Africa and Asia, including China [23,24]. Given China's substantial population of 3.09 million dairy cows and 11 million livestock workers, addressing this production-limiting and zoonotic disease at the population level is crucial for economic, food security, and One Health [25]. However, B. melitensis is widely considered as primary concern in humans [26,27], indirectly resulting in B. abortus being overlooked in both dairy cows and humans for a long time. There is still controversy over the the relative importance of B. abortus to B. melitensis in cows and humans and whether to adopt more intense control measures in cows in China. A comprehensive understanding of the current epidemiology of B. abortus in dairy cattle and humans is a prerequisite for making evidence-based decisions about this pathogen and facilitating the comparative opportunities in facilitating brucellosis One Health management. However, existing literature reports are heterogeneous in geographical location, study design, and reporting quality, making it difficult to assess the overall B. abortus status in China. A scoping review is a type of literature review that transparently and reproducibly integrates the current literature on a related topic with little or no research done and can also be a precursor to a subsequent systematic review [28]. Although many works of literature report brucellosis in China, there has not been a formal scoping review on B. abortus in dairy cows and humans. This scoping review aimed to: (1) provide an overview of B. abortus status in Chinese dairy cattle and human populations between January 2004 and December 2022; and (2) identify the key factors driving the spread of B. abortus. The insights gleaned from this review can be used to tailor evidence-based control strategies to reduce the disease burden both in dairy farms and humans in China.
2. Methods
2.1. Literature search strategy
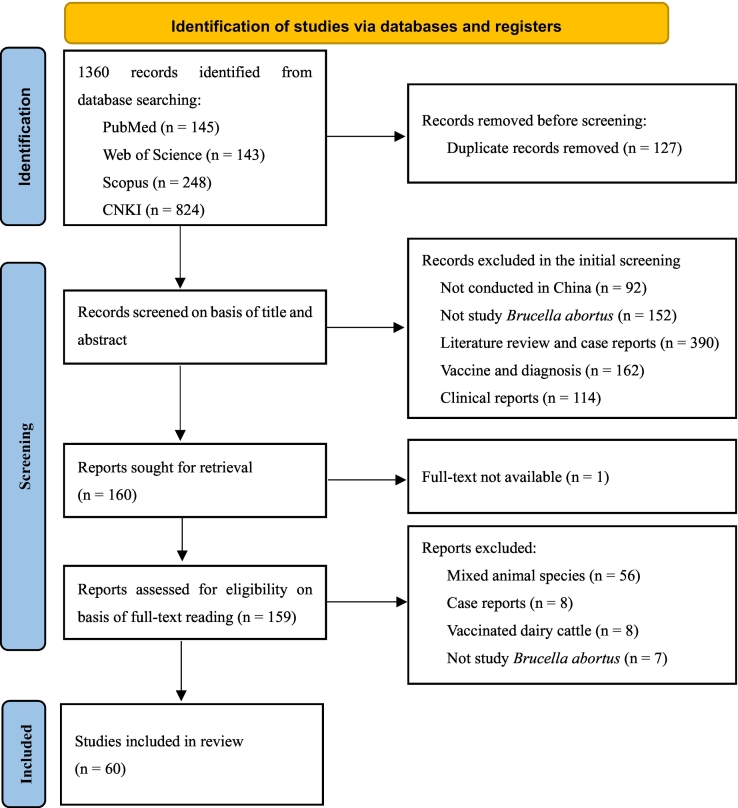
Following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [29], a comprehensive literature search was conducted to identify scientific articles published from 1st January 2004 to 31st December 2022 using four databases (PubMed, Web of Science, Scopus, China National Knowledge Infrastructure - CNKI) as shown in Fig. 1. A complete list of the search terms and their combinations used for each database is available in Supplemental Table S1. The reference management software program EndNote X9 (Clarivate Analytics, Philadelphia, PA) was used to organize and remove duplicate publications retrieved from the four databases.
Fig. 1.
PRISMA flow diagram of the studies identified, screened, assessed, and included in a scoping review of brucellosis in dairy cattle and humans in China.
2.2. Study inclusion and exclusion criteria
The inclusion and exclusion criteria for literature retrieval were detailed in Table 1. Studies that reported the prevalence of antibodies against B. abortus in dairy cows in China with explicit diagnostic tests were included. Reviews and clinical reports that only described the clinical symptoms and treatment were excluded because they were not relevant to the scope of this review. An initial screening for inclusion was made according to the titles and abstracts, and publications that reported on other countries, species, and diseases were removed immediately. The full-text manuscripts were then read before the final decision was made to include or exclude them in this review according to the criteria in Table 1. Details of the specific reasons for exclusion during the two-step screening process can be seen in Fig. 1. YW initially conducted the literature selection and data extraction and discussed with other authors to reach a consensus if there were any uncertainties. However, given the language barriers for some authors regarding literature written in Chinese, the main work for retrieved Chinese literature was completed by YW.
Table 1.
Inclusion and exclusion criteria used for literature screening in this study.
| Inclusion | Exclusion |
|---|---|
| Study conducted in China | Study conducted elsewhere |
| Study dairy cows or humans | Study other hosts |
| Clearly defined study year and province | Unclear study time and place |
| Tested for Brucella abortus using explicit tests | Study not investigating Brucella abortus |
| Original observational studies | Review and clinical reports |
| Full text available | Full text unavailable |
2.3. Data extraction
A template was created to record information about the methodology and results of each publication. The data set documented general study characteristics, including author, publication year, investigation time, province, diagnostic tests used, criteria for positivity, number of units and group (i.e., animals and farms), animal-level and farm-level prevalence, and study type. Sampling times, province, species and biovars of strains, and identification methods were recorded for studies that reported molecular characterization. Only confirmed isolated strains were counted with relevant species and biovars identification methods. Sample size, study population, study type, and statistical methods used were documented for risk factor analysis studies. Data on the monthly number of notified human cases and annual incidence rates from January 2004 to December 2022 were retrieved from the Data Center of China Public Health Science (https://www.phsciencedata.cn/Share/en/index.jsp). Human brucellosis diagnosis and notification protocols have been described elsewhere [13,27].
3. Results
3.1. Characteristics of included studies
The initial literature searching identified 1360 records from four databases, of which 127 were removed for duplication. After screening the titles and abstracts, 1073 of them were rejected for one or more reasons, including mixing dairy cows with other species, ambiguous study places or times, not declaring diagnostic tests used, and others, as detailed in Fig. 1. Literature reviews and case reports (390/1073) accounted for about a third of the reasons for exclusion. Full-text assessment of the articles was made on 159, and 60 studies were finally enrolled for data extraction (Fig. 1). Articles written in either Chinese or English were retrieved, of which forty-four (44/60) were in Chinese.
3.2. Brucellosis prevalence estimation studies in dairy cattle
Thirty-two reports investigated the prevalence of antibodies against B. abortus in dairy cattle. Table 2 summarizes the main characteristics of these studies. Major studies were conducted in a single province, covering 17 of 32 provinces or autonomous regions in China (Fig. 2). The cross-sectional design was employed in 72% of the studies, followed by cohort studies. A combined Rose Bengal Test (RBT) and Serum Agglutination Test (SAT) interpreted in series was used in 84% of the studies (27/32) to judge B. abortus status. About half of the studies investigated mixed-type (comprising both large-scale and smallholder) dairy farms, while 30 % of studies did not report herd size. Sampling frame and method were unavailable in 47% of the studies (15/32), and census (6/32) and stratified random sampling (6/32) were the usual methods in the remaining studies. The age of sampled animals was not reported in two-thirds of the studies (21/32), and other studies sampled groups with varying minimum ages, ranging from 3 months to >2 years of age. The median of all reported animal-level prevalences was 2.1% (range: 0.0% – 13.5%), while at the farm level its median prevalence increased to 10.2% (range: 0.0%–100%). However, only half of the studies reported farm-level prevalence of brucellosis.
Table 2.
Characteristics of retrieved studies that investigated the seroprevalence of brucellosis in Chinese dairy cows, including study design, geographical location, diagnostic tests, population selection and age, and sample size.
| Study period | Study type | Province | Sample type | Diagnostic tests | Herd size | Population selection | Age group | Sample size⁎ | Animal-level prevalence (%) | Herd-level prevalence (%) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2000–2009 | Cohort | Beijing | Blood | RBT + SAT | Mixed | Census | Census | 173,024 | 0.1 | – | [30] |
| 2003–2013 | Cohort | Guangxi | Blood | RBT + SAT | Not stated | Not stated | Not stated | 1039 | 2.7 | – | [31] |
| 2004–2010 | Cohort | Zhejiang | Blood | RBT + SAT | Mixed | Census | Census | 110,220 | 1.5 | – | [32] |
| 2007 | Cross-sectional | Zhejiang | Blood | RBT + SAT | Not stated | Not stated | Not stated | 1071 | 2.4 | – | [33] |
| 2007–2011 | Cohort | Qinghai | Blood | RBT + SAT | Not stated | Not stated | Not stated | 138,350 | 0.3 | – | [34] |
| 2009–2011 | Cross-sectional | 15 provinces | Milk | PCR | Large-scale | Stratified randomized | > 2 years | 5211 | 1.1 | – | [35] |
| 2009–2011 | Cross-sectional | Inner Mongolia | Blood | RBT + SAT | Mixed | Not stated | Not stated | 5875 (196) | 6.2 | 26.5 | [36] |
| 2009–2013 | Cohort | Gansu | Blood | RBT + SAT | Not stated | Not stated | Not stated | 10,742 | 2.0 | – | [37] |
| 2012 | Cross-sectional | Shandong | Blood | RBT + SAT | Mixed | Census | > 3 months | 30,119 (1803) | 0.4 | 2.6 | [38] |
| 2012 | Cross-sectional | Shandong | Blood | RBT + SAT | Not stated | Not stated | Not stated | 333 | 1.5 | – | [39] |
| 2012 | Cross-sectional | Qinghai | Blood | RBT + SAT | Not stated | Stratified randomized | Not stated | 18,282 | 0.2 | – | [40] |
| 2012–2013 | Cross-sectional | Shandong | Blood | RBT + SAT | Mixed | Simple randomized | Not stated | 485 (13) | 4.9 | 15.4 | [41] |
| 2012–2013 | Cross-sectional | Heilongjiang | Blood | RBT + SAT | Mixed | Not stated | Not stated | 1590 | 1.1 | – | [42] |
| 2013 | Cross-sectional | Liaoning | Blood | RBT + SAT | Mixed | Census | Census | 37,888 (187) | 0.2 | 10.2 | [43] |
| 2013 | Cross-sectional | Hebei | Blood | RBT + SAT | Large-scale | Not stated | > 6 months | 4279 (109) | 0.1 | 5.5 | [44] |
| 2013–2014 | Cross-sectional | Heilongjiang | Blood | I-ELISA + C-ELISA | Large-scale | Not stated | Not stated | 4131 (22) | 12.3 | 77.3 | [45] |
| 2013–2017 | Cohort | Sichuan | Blood | RBT + SAT | Not stated | Stratified randomized | > 12 month | 5598 | 0.2 | – | [46] |
| 2013–2017 | Cohort | Gansu | Blood | RBT + SAT | Not stated | Not stated | > 8 months | 1431 (160) | 4.5 | 7.5 | [47] |
| 2014 | Cross-sectional | Xinjiang | Blood | RBT + SAT | Large-scale | Not stated | > 6 months | 987 (3) | 6.5 | 100.0 | [48] |
| 2015 | Cross-sectional | Henan | Blood | RBT | Mixed | Census | Census | 218 (5) | 7.8 | 100.0 | [49] |
| 2015 | Cross-sectional | Yunan | Blood | RBT + SAT | Large-scale | Not stated | Not stated | 223 | 13.5 | – | [50] |
| 2015–2017 | Cohort | Sichuan | Blood | RBT + SAT | Mixed | Census | Census | 159,071 (1157) | 0.7 | 19.5 | [51] |
| 2015–2017 | Cross-sectional | Guizhou | Blood | RBT + SAT | Mixed | Simple randomized | Not stated | 25,910 (148) | 0.1 | 4.1 | [52] |
| 2017–2018 | Cross-sectional | Xinjiang | Blood | RBT + C-ELISA | Smallholder | Stratified randomized | > 2 years | 1406 | 6.8 | – | [53] |
| 2017–2019 | Cross-sectional | Inner Mongolia | Blood | RBT + SAT | Mixed | Stratified randomized | Not stated | 1758 | 2.7 | – | [54] |
| 2018 | Cross-sectional | Xinjiang | Blood | RBT + SAT | Mixed | Simple randomized | Not stated | 1203 | 4.7 | – | [55] |
| 2018 | Cross-sectional | Henan | Blood | RBT + SAT | Mixed | Simple randomized | Not stated | 25,088 (581) | 0.8 | 7.2 | [56] |
| 2018 | Cross-sectional | Shannxi | Blood | RBT + SAT | Not stated | Stratified randomized | Not stated | 92 (3) | 0.0 | 0.0 | [57] |
| 2019 | Cross-sectional | Henan | Blood | RBT + SAT | Mixed | Census | Census | 12,755 (68) | 2.2 | 33.8 | [58] |
| 2020 | Cross-sectional | Hainan | Blood | RBT + SAT | Large-scale | Simple randomized | Census | 1690 (2) | 0.0 | 0.0 | [59] |
| 2021 | Cross-sectional | Shandong | Blood | RBT + SAT | Mixed | Not stated | Not stated | 49,080 (1079) | 0.5 | 2.9 | [60] |
| 2021 | Cross-sectional | Xinjiang | Blood | RBT + SAT | Not stated | Not stated | Not stated | 7102 | 0.6 | – | [61] |
⁎: Number of animals tested (Number of farms tested); −: not available; RBT: Rose Bengal Test; SAT: Serum Agglutination Test; C-ELISA: competitive Enzyme-linked Immunosorbent Assay.
Fig. 2.
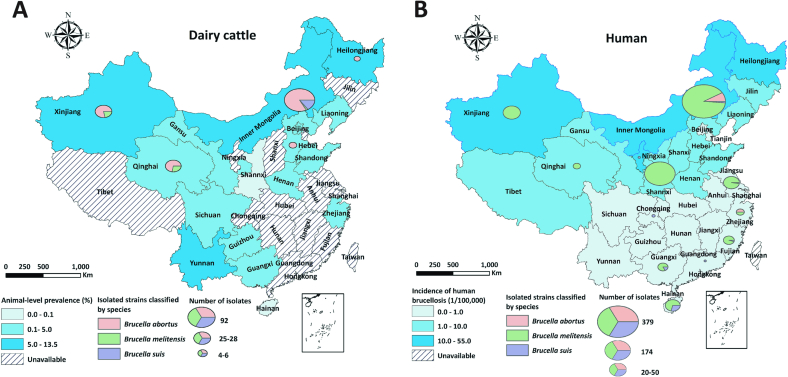
Geographical distribution of reported animal-level prevalence of brucellosis in dairy cattle (Panel A) and incidence of human brucellosis in 2019 (Panel B) with respective count of strain isolation and species identification, in China from 60 studies identified from a scoping review.
3.3. Circulating Brucella spp. strains in dairy cattle and humans
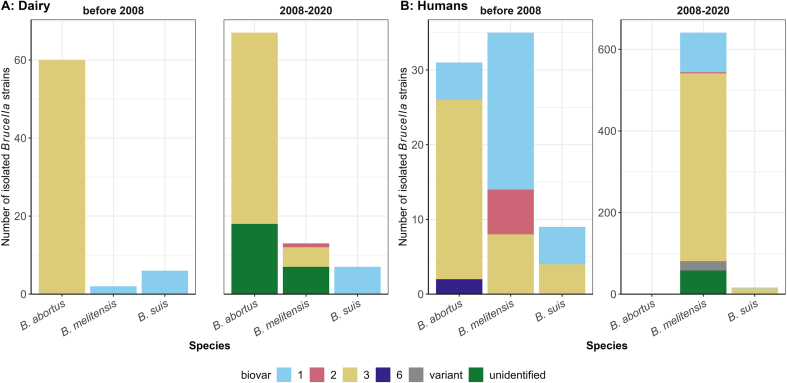
A total of 888 Brucella spp. strains were isolated from dairy cattle and humans in 14 provinces of China during 2004–2022 (Table 3, Fig. 2). 733 strains were from humans (across 15 studies) and 155 from dairy cattle (across 8 studies). More Brucella strains were isolated after 2008 than before 2008 (745 vs 143, Fig. 3), aligning with the number of studies conducted during each period (16 studies vs 5 studies). Nearly half of the molecular studies (9/21) were conducted in Inner Mongolia, where about half of the Brucella isolates (441/888) were acquired. Notably, one study isolated 174 Brucella strains in Shannxi Province accounting for 19.6% of the total [62]. B. melitensis represented about three-quarters of the total isolates from both humans and dairy cattle, followed by B. abortus (18.8%). Specifically, 84.3% of B. abortus strains were identified as B. abortus biovar 3, while 11.3% were not subjected to specific biovar identification. Over 60% of these B. abortus isolates (77/127) were from Inner Mongolia. The proportion of brucellosis attributable to B. melitensis in dairy cattle increased markedly from 2.9% before 2008 to 14.9% in 2008–2020 (Fig. 3 Panel A). In humans, B. melitensis accounted for over 90% of all Brucella isolates, followed by B. abortus (4.6%). Before 2008, B. abortus and B. melitensis were the main etiologies of human brucellosis, and B. melitensis biovar 3 stood out after 2008 (Fig. 3 Panel B).
Table 3.
Characteristics of 846 Brucella strains isolated from cattle and humans in China.
| Variables |
Host |
||
|---|---|---|---|
| Human (%) | Dairy cattle (%) | Total (%) | |
| Total | 733 (82.5) | 155 (17.5) | 888 |
| Species | |||
| B. abortus | 32 (4.4) | 127 (81.9) | 159 (17.9) |
| B. melitensis | 676 (92.2) | 15 (9.7) | 691 (77.8) |
| B. suis | 25 (3.4) | 13 (8.4) | 38 (4.3) |
| B. abortus biovars | |||
| Biovar 1 | 5 (15.6) | 0 (0.0) | 5 (3.1) |
| Biovar 3 | 25 (78.1) | 109 (85.8) | 134 (84.3) |
| Biovar 6 | 2 (6.3) | 0 (0.0) | 2 (1.3) |
| Unidentified | 0 (0.0) | 18 (14.2) | 18 (11.3) |
| B. melitensis biovars | |||
| Biovar 1 | 118 (17.5) | 2 (13.3) | 120 (17.4) |
| Biovar 2 | 9 (1.3) | 1 (6.7) | 10 (1.4) |
| Biovar 3 | 468 (69.2) | 5 (33.3) | 473 (68.5) |
| Variant | 23 (3.4) | 7 (46.7) | 30 (4.3) |
| Unidentified | 58 (8.6) | 0 (0.0) | 58 (8.4) |
| B. suis | |||
| Biovar 1 | 7 (28.0) | 13 (100.0) | 20 (52.6) |
| Biovar 3 | 17 (68.0) | 0 (0.0) | 17 (44.7) |
| Unidentified | 1 (4.0) | 0 (0.0) | 1 (2.6) |
| Region | |||
| Inner Mongolia | 349 (47.6) | 92 (59.4) | 441 (49.7) |
| Shannxi | 174 (23.7) | 0 (0.0) | 174 (19.6) |
| Other provinces | 168 (28.6) | 63 (40.6) | 231 (30.7) |
Fig. 3.
Species and biovars of Brucella spp. strains isolated from dairy cattle (Panel A) and humans (Panel B) during 1984–2020, bv represents biovar.
3.4. Risk factors for Brucella abortus in dairy cattle
Five studies have identified several risk factors associated with B. abortus infection in dairy cattle, including a history of abortion, the purchase of new animals, commingling with small ruminants, presence of dogs within a herd, and small herd size [35,38,43,58,63]. The lack of exclusive animal transport vehicles and workers entering sheds without wearing specific boots resulted in a higher prevalence of brucellosis [43]. In all studies, multivariate logistic regression analyses were employed to estimate the strength of associations between Brucella infection and potential risk factors. The estimated regional risk of B. abortus seroprevalence displayed considerable variation, with distinct geographical patterns identified in disease prevalence among Chinese dairy herds. The prevalence of brucellosis in nothern provinces was higher than that in southern provinces (Fig. 2). Some Brucella isolates from southern provinces were genetically associated with strains from multiple northern provinces in China [64], suggesting cross-province B. abortus transmission via animal movements.
3.5. Public health relevance
Eight studies estimated the prevalence of antibodies against Brucella spp. in humans in seven provinces of China. All studies targeted occupation-associated populations, such as livestock workers or hospitalized patients. In more than half of the studies (5/8), individuals who tested positive for both RBT and SAT were classified as brucellosis-positive. Half of the studies utilized a non-random, convenience sampling method to enroll participants. The median seroprevalence of brucellosis among these investigated people was 3.7% (Range: 1.8–16.4) (Table 4).
Table 4.
Study characteristics of seroprevalence of Brucella infection in Chinese humans.
| Study period | Study type | Province | Tests | Source population | Population selection method | Sample size | Prevalence (%) | References |
|---|---|---|---|---|---|---|---|---|
| 2004–2010 | Cohort | Zhejiang | RBT + SAT | Occupation-associated | Convenient sampling | 10,430 | 2.0 | [32] |
| 2005 | Cross-sectional | Fujian | SAT | Occupation-associated | Convenient sampling | 1321 | 3.2 | [67] |
| 2008–2020 | Cohort | Shannxi | RBT + SAT | Occupation-associated | Convenient sampling | 179,907 | 4.3 | [62] |
| 2010–2012 | Cohort | Sichuan | RBT + SAT | Occupation-associated | Convenient sampling | 450 | 4.4 | [68] |
| 2010–2014 | Cohort | Inner Mongolia | SAT | Occupation-associated | Stratified randomized sampling | 838,956 | 3.6 | [69] |
| 2012–2016 | Cohort | Inner Mongolia | RBT + SAT | Suspected populations | Convenient sampling | 1,102,304 | 3.8 | [70] |
| 2013 | Cross-sectional | Inner Mongolia | RBT + SAT | Occupation-associated | Census sampling | 13,098 | 1.8 | [71] |
| 2014–2021 | Cohort | Shannxi | RBT + SAT | Occupation-associated | Convenient sampling | 4263 | 1.4 | [72] |
| 2016 | Cross-sectional | Jiangsu | RBT + SAT | Occupation-associated | Convenient sampling | 895 | 16.4 | [73] |
| 2016–2020 | Cohort | Fujian | SAT | Occupation-associated | Not available | 4934 | 1.7 | [74] |
| 2019–2020 | Cross-sectional | Shanxi and Xinjiang | SAT | Occupation-associated | Simple randomized sampling | 2384 | 2.6 | [75] |
RBT: Rose Bengal Test; SAT: Serum Agglutination Test.
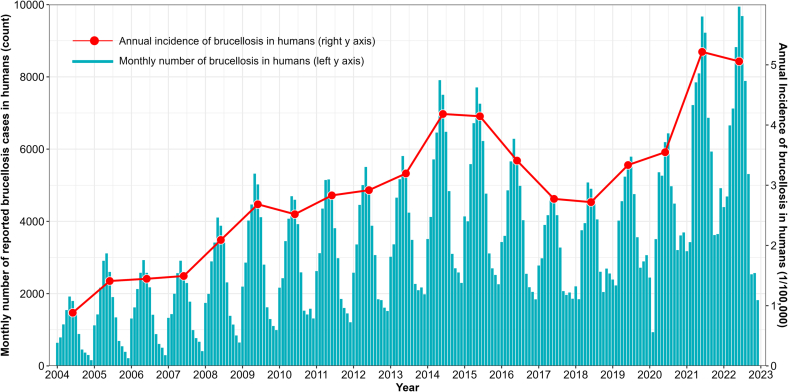
The incidence of notified human brucellosis cases displayed a typical seasonal increase with peaks in June–July (summer) and troughs in December–January (winter) each year (Fig. 4), followed by a decline during 2015–2018, and another rise through the present (Fig. 4). The historical peak occurred in 2021, with 73,645 human brucellosis cases notified, equivalent to approximately 5.22 cases per 100,000 person-years (Fig. 4). Although the average incidence was lower than that in other countries, such as Iraq and Jordan with >25 cases /100,000 person-years [4], in some regions of China (e.g. Inner Mongolia, 40.9 cases/100,000 person-years) it was comparable to severely affected countries [13]. A higher incidence of human brucellosis was found among livestock-related practitioners, males aged 35–54 and those residing in specific geographical areas (e.g. Inner Mongolia) [13,27,65]. For occupationally at-risk groups, behaviors such as consuming raw milk, assisting in calving, and handling aborted products without appropriate PPE were risk factors for Brucella infection [63]. In contrast, frequent disinfection of calving sites and proper disposal of aborted calves were identified as protective factors. Additionally, some meteorological and geographic factors, such as moderate altitude, grassland, medium temperatures, and reduced sunshine, were significantly associated with the incidence of human brucellosis [65,66].
Fig. 4.
Monthly number of human brucellosis notifications in China between January 2004 and December 2022 (cyan bar: monthly number of cases by the left y-axis, red line: annual incidence of brucellosis in humans by the right y-axis, 1/100,000 people). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
This scoping review provides a comprehensive overview of studies investigating the prevalence of antibodies against B. abortus and species and biovars of circulating Brucella spp. strains in dairy cattle and humans from literature published between 1st January 2004 and 31st December 2022. Out of the 1360 studies retrieved, only 60 were eligible for inclusion in this review. The exclusion of such a large number of studies may be due to unrelated research topics, heterogeneous quality of study reporting, and inexplicit or missing information (Fig. 1). Most of the studies were published in local journals and not possibly in English, making these results less accessible to international researchers. Therefore, this review aggregated the retrieved data that were less accessible by international colleagues to provide a more thorough understanding of B. abortus infection in dairy cattle and humans in China. Brucella spp. poses a significant global One Health threat to both bovines and humans [76]. In China, while B. melitensis has acquired attention and stringent control measures in sheep and goat flocks, B. abortus has often been neglected as a pathogen in cows and humans, particularly when compared to B. melitensis [77,78]. Justifying a focus on B. abortus becomes essential within a One Health framework of brucellosis control in China, especially considering the prevailing gaps in understanding the relative importance of different Brucella species in cows and humans within China. The increasing dairy population, booming milk demand, and comparative opportunities in facilitating One Health management of brucellosis underline the significant role of B. abortus in China. Recognizing the indispensable role of B. abortus in One Health management of brucellosis, this scoping review has integrated available reports to refine the current understanding of prevalence and distribution of B. abortus in dairy cows and humans relative to other Brucella species nationwide and lay the foundation for future evidence-based disease management strategies in China.
Brucellosis has been recognized as a priority disease by the Ministry of Agriculture and Rural Affairs (MARA) of China, given the notable increase in notifications among humans and reported prevalence in livestock [79]. Since 2004, the notified brucellosis cases in humans and animals exhibited key characteristics: (1) a substantial increase in incidence and prevalence; (2) seasonal fluctuations with high incidence in summer and low incidence in winter; (3) spatial expansion from northern to southern provinces [13,27,65]. The underlying causes were likely multifaceted and may be attributed to several factors, such as: (1) increased stocking density and associated higher contact frequency between animals, accelerating within-herd transmission of infectious diseases [26,65]; (2) thriving inter- and intra-provincial live animal trade and movement, which likely facilitated disease transmission between herds [13]; (3) low vaccination coverage, failing to establish sufficient herd immunity to resist B. abortus (re-)invasion [80]; (4) inadequate financial resources, making test-and-slaughter strategies impractical and unaffordable [80]; (5) vulnerable on-farm biosecurity measures, struggling to prevent pathogen (re-)invasion [81]; (6) limited farmer awareness about the public health and economic significance of B. abortus, compromising the adoption of disease control measures [82]. However, these factors may not fully explain the observed fluctuations in brucellosis notifications in humans between 2015 and 2022. Financial limitations and competing priorities, such as the current COVID-19 pandemic, may exacerbate the challenges associated with controlling and preventing brucellosis in China. The limited financial resources available to implement effective control measures, coupled with a focus on the COVID-19 response, may further hinder the prevention and control of brucellosis after January 2020.
The median reported animal-level prevalence of antibodies against Brucella in dairy cattle in China was 1.5%, comparable to the 1.9% reported in a previous meta-analysis [83]. However, the median reported farm-level prevalence reached 10.2%, indicating that a considerable proportion of dairy populations were affected by Brucella spp. Although these prevalences were lower than those reported in endemic countries such as India (animal-level: 15.1%, herd-level: 32.9%) [84] and Ethiopia (2.6% and 16.3%) [85], or other countries in the early eradication stage like New Zealand (15% and 59%) [22] and Australia [86], China still confronts a significant challenge in controlling brucellosis because of its sizable dairy population and complex conditions. Furthermore, prevalence is influenced by the incidence, average disease duration, and increased culling rate of test-positive animals. From a production standpoint, brucellosis-positive cows can cause a 30%–80% pregnancy loss, and 10%–30% milk losses [3,87], thereby having significant economic implications on the dairy industry. The magnitude of economic impacts depends on various parameters, including disease prevalence, increased abortion rate, decreased milk production, milk, meat and animal prices, labor costs, and vaccination [88]. Unfortunately, these parameters are not readily available or tailored to the Chinese unique epidemiological and socioeconomic context.
Another knowledge gap is the underreporting of farm-level prevalence of B. abortus infection in the dairy sector. The reasons for the underreporting are unclear, and we hypothesize that some researchers may undervalue the importance of farm-level prevalence or may be concerned that reporting a high farm-level prevalence could raise public concern. The lack of farm-level prevalence data made epidemiological information incomplete and hindered a thorough and transparent understanding of disease status at the farm level, as most control measures were designed and implemented on a farm-level basis [20]. Selective reporting could cause adverse effects and mislead policymakers and stakeholders in their decision-making. Therefore, transparent reporting of farm-level prevalence and detailed information on the investigated farms should be strengthened to acquire a robust understanding of the disease status in the population.
Prevalence estimates are often influenced by various factors such as sampling methods, target populations, testing assays, and case definitions. Small herd size has been reported to be a risk factor for Brucella positivity at the farm level in some studies [43,58] but not in other international studies showing increased risk with large herd size [89]. Differences in livestock management practices and levels of farm biosecurity may explain this discrepancy [80]. The age of animals sampled is essential since older animals are expected to have a longer exposure time, making them more likely to be positive to B. abortus [9,53,63,65]. Unfortunately, many prevalence estimate studies have not mentioned the age of animals, which may introduce biases that cannot be accounted for. Reporting as much detail as possible about the study population is of great help in interpreting results and adjusting for potential biases. Additionally, provinces with a high disease prevalence, like Inner Mongolia and Shannxi province, may be overrepresented in research reports, while the lack of brucellosis highlights the need for greater transparency in reporting true Brucella spp. status in these unreported regions.
Using RBT and SAT in series to judge the serological status of Brucella spp. exposure in dairy cattle is common in China (Table 2) [13,27]. However, this testing strategy compromises overall sensitivity while improving specificity, potentially leading to false-negative results and underestimated prevalence [90]. False-negative results may result in infected cattle being retained on the farm as Brucella spp. reservoirs, perpetuating disease endemicity and transmission to other susceptible animals or farms [91,92]. In cases of low prevalence, the serial testing strategy is economically justified to avoid false positives at the animal level, particularly for test-and-slaughter policies. Conversely, prioritizing the identification of positive farms may favor parallel testing to enhance the possibility of detecting positive farms at the early stage of a control program. Testing strategies should be adjusted based on the real-time prevalence of Brucella spp. and the control aims rather than remaining fixed. Furthermore, incorporating polymerase chain reaction (PCR) or bacterial culture techniques when serology suggests a positive result can help identify species and biovar of Brucella [93].
Our findings indicated that B. abortus, specifically B. abortus biovar 3, was the most commonly identified strain in Chinese dairy farms over the two decades. Although this aligns with the general consensus, the research on Brucella spp isolation and identification for Chinese dairy populations is still limited compared to humans as shown in Fig. 2. The isolation of Brucella spp in dairy cattle has only been reported in five provinces, possibly due to the lack of biosafety level 3 laboratories and trained professionals required to work with this organism in other provinces. It is crucial to update our knowledge of the species and biovars of currently circulating Brucella strains in unreported prevalent provinces (Fig. 2A). Molecular epidemiology is essential for enhancing our understanding of brucellosis transmission and contact patterns in endemic regions that maintain molecular databanks of circulating Brucella spp. strains [70]. Researchers can infer transmission timing and direction by examining the molecular features and evolutionary relationships of these isolates from different farms or regions [64,78,94]. Investigating these epidemiological links will allow for more informed inferences about the most critical transmission routes in endemic areas, enabling evidence-based biosecurity recommendations tailored to individual farms to help mitigate the risk of disease introduction.
B. melitensis has been identified as the predominant Brucella spp. strain causing human brucellosis in China (Figs. 2B & 3B), aligning with previous research findings [77]. The higher virulence and infectivity of B. melitensis and more potential contacts with B. melitensis hosts (e.g., sheep and goats) may account for this dominance [6,95]. The seasonal grazing and crop-grazing patterns of small ruminants allow farmers more contact with animals relative to intensively raised dairy cows. For the general public, foodborne infection is the most common route, with infections from the consumption of raw milk frequently reported [96]. Occasional Brucella vaccine leaks have also been reported in China [15,16], highlighting the need to improve laboratory biosafety management. Among ten provinces, 691 strains were isolated from humans, with most of these isolates clustered in Inner Mongolia and Shannxi Provinces (Fig. 2B). Consequently, caution should be exercised in generalizing the current findings to other provinces, as the heterogeneous distribution of Brucella isolates across provinces may impact the broader applicability of the results (Fig. 2).
This review identified live cattle movement, trade, and shared milk tankers as potential risks for the between-farms transmission of Brucella spp. and other infectious diseases [43,58,97]. Understanding these factors is essential when tailoring a risk-based control scheme in China. Even if a farm has achieved brucellosis-free status, the risk of transmission between farms remains if farms are still connected via other routes (e.g., animal movements) [43]. The imbalance of meat and milk consumption, cattle population, and animal market price differences across Chinese provinces accelerate inter- and intra-provincial movement or trade of live animals and animal products. Although studies have investigated animal movement and trade patterns between Chinese pig farms and markets regarding other infectious diseases [98,99], the impact of cattle movements on B. abortus transmission remains unexplored in China. Assessing the effectiveness of the current measures to prevent B. abortus transmission through live cattle movements should be a priority to develop more targeted and effective control measures [98,100].
In March 2022, the MARA of China launched a five-year action plan for the prevention and control of animal brucellosis (2022–2026) [79]. The plan encompasses five key principles for controlling brucellosis in dairy herds: (1) identifying and eliminating Brucella-infected animals with appropriate compensation to disrupt the within-herd transmission cycle, (2) mandating vaccination for brucellosis-positive dairy farms to reduce disease prevalence, (3) implementing pre-movement testing of animals and preventing live animal movements from vaccinated areas (high-risk) to non-vaccinated areas (low risk), (4) maintaining continuous surveillance to maintain brucellosis-free status in farms that have achieved freedom, and (5) disseminating disease knowledge to improve farmers' awareness towards the disease. These measures align with the findings of this review and are anticipated to mitigate the impact of B. abortus on dairy farms and public health. Moreover, a One Health framework for brucellosis and other zoonoses should be prioritized and coordinate multifaceted efforts from farmers, veterinary, public health, and government departments to combat the current brucellosis epidemic in China [76]. Available links between human brucellosis and animal brucellosis strains are still limited, which makes it difficult to track and eliminate the source of infection in aniamls. Only joint, scientific, evidence-based contributions should be conducive to effective and resonant control strategies. Given the available evidence, B. abortus is the principal causative agent of brucellosis in dairy cows and should be included as an integral part in the One Health management of brucellosis in China.
Despite the countermeasures adopted by the Chinese government over the past decades, brucellosis remains endemic in China, posing ongoing challenges for public health, veterinary authorities, and farm stakeholders. A successful disease control and prevention program requires a One Health approach with collaborative efforts from public health, veterinary departments, and stakeholders. Adequate financial resources are critical, particularly for the test-and-slaughter approach, and appropriate compensation for culled animals serves as a key motivator for stakeholders to report cases and comply with control measures. Furthermore, as mandated by government policy, transparent reporting of vaccination information and effective tracking of vaccinated animals is essential to prevent animal movements from vaccinated areas to non-vaccinated areas. The Chinese government is already promoting the use of electronic tracking systems to replace the original paper documents to record animal movements. More data in the future will allow effective monitoring of animal movements and successful establishment of epidemiological links between animals and humans. However, the limited number of veterinary professionals and the large population of China constrain the capacity of surveillance and control programs to conduct farm censuses, farm registration, vaccination records, animal movement tracking, data collection, and stakeholder communication. The heterogeneity across regions and the lack of transparent and high-quality reporting further hinder stakeholders' judgment of the priority and importance of the disease. Addressing these challenges requires joint efforts, financial resources, and professional expertise to effectively tackle the B. abortus epidemic in China. As new policies are implemented, the self-determination to adopt either vaccination or test-and-culling measures will depend strongly on farm decision-makers' awareness and understanding of brucellosis, and further scientific evidence is required to guide farmers in making evidence-based decisions.
5. Conclusion
In conclusion, this scoping review provides a comprehensive summary of the current status of B. abortus for dairy cattle and humans in China, highlighting the need for effective and efficient control strategies to mitigate the disease's impact on public health, animal welfare and economics. The study identified B. abortus biovar 3 as the strain currently prevalent in dairy cattle and still a non-negligible One Health threat to humans although B. melitensis biovar 3 is currently the dominant strain in human infections. The key risk factors that drove disease transmission between dairy herds include cattle movement or trade, introduction of new animals, and shared facilities with other farms. While China has the financial resources and capacity to achieve control of B. abortus in dairy cows within certain areas, several challenges need to be addressed, such as limited veterinary professionals, inadequate reporting, and lack of transparent vaccination information. Addressing these challenges will require a One Health approach with joint efforts, increased financial and professional resources, and a high level of disease awareness. Ultimately, successful control of B. abortus in dairy cattle can lead to reduced disease burden in humans, improved animal welfare, increased economic sustainability in the dairy sector, and provide comparative opportunities in facilitating One Health management of brucellosis in China.
Ethical approval
Not applicable.
Funding
This work was supported by the China Agriculture Research System of MOF and MARA (Grant Number: CARS-37).
CRediT authorship contribution statement
Emilie Vallée: Methodology, Resources, Software, Supervision, Writing – review & editing. Cord Heuer: Conceptualization, Methodology, Resources, Supervision, Writing – review & editing. Youming Wang: Conceptualization, Methodology, Resources, Supervision. Yu Wang: Conceptualization, Methodology, Software, Writing - review & editing. Aizhen Guo: Conceptualization, Funding acquisition, Methodology, Resources, Software, Supervision, Writing – review & editing. Zhen Zhang: Methodology, Resources, Supervision. Chris Compton: Conceptualization, Funding acquisition, Methodology, Supervision, Validation, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no conflicting interests.
Acknowledgments
The authors thank EpiCentre, School of Veterinary Science, Massey University, for providing financial support for the doctoral tuition and stipend of Yu Wang.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2024.100683.
Contributor Information
Yu Wang, Email: y.wang22@massey.ac.nz.
Aizhen Guo, Email: aizhen@mail.hzau.edu.cn.
Appendix A. Supplementary data
Supplementary material
Data availability
We have shared the data in supplementary material.
References
- 1.Seleem M.N., Boyle S.M., Sriranganathan N. Brucellosis: a re-emerging zoonosis. Vet. Microbiol. 2010;140:392–398. doi: 10.1016/j.vetmic.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Whatmore A.M., Foster J.T. Emerging diversity and ongoing expansion of the genus Brucella. Infect. Genet. Evol. 2021;92 doi: 10.1016/j.meegid.2021.104865. [DOI] [PubMed] [Google Scholar]
- 3.Bernués A., Manrique E., Maza M.T. Economic evaluation of bovine brucellosis and tuberculosis eradication programmes in a mountain area of Spain. Prev. Vet. Med. 1997;30:137–149. doi: 10.1016/S0167-5877(96)01103-8. [DOI] [PubMed] [Google Scholar]
- 4.Dean A.S., Crump L., Greter H., Schelling E., Zinsstag J. Global burden of human brucellosis: a systematic review of disease frequency. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dean A.S., Crump L., Greter H., Hattendorf J., Schelling E., Zinsstag J. Clinical manifestations of human brucellosis: a systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WOAH . 2019. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2019 Chapter 3.1.4. Brucellosis (Brucella abortus, B. melitensis and B. suis) (Infection with B. abortus, B. melitensis and B. suis) [Google Scholar]
- 7.WHO . World Health Organization; Geneva (Switzerland): 2006. Brucellosis in Humans and Animals: WHO Guideline. [Google Scholar]
- 8.Abd El-Wahab E.W., Hegazy Y.M., El-Tras W.F., Mikheal A., Kabapy A.F., Abdelfatah M., et al. A multifaceted risk model of brucellosis at the human–animal interface in Egypt. Transbound. Emerg. Dis. 2019;66:2383–2401. doi: 10.1111/tbed.13295. [DOI] [PubMed] [Google Scholar]
- 9.Zeng J., Duoji C., Yuan Z., Yuzhen S., Fan W., Tian L., et al. Seroprevalence and risk factors for bovine brucellosis in domestic yaks (Bos grunniens) in Tibet, China. Trop. Anim. Health Prod. 2017;49:1339–1344. doi: 10.1007/s11250-017-1331-7. [DOI] [PubMed] [Google Scholar]
- 10.Godfroid J., Garin-Bastuji B., Saegerman C., Blasco J.M. Brucellosis in terrestrial wildlife. Rev. Sci. Tech. 2013;32:27–42. doi: 10.20506/rst.32.1.2180. [DOI] [PubMed] [Google Scholar]
- 11.Kamath P.L., Foster J.T., Drees K.P., Luikart G., Quance C., Anderson N.J., et al. Genomics reveals historic and contemporary transmission dynamics of a bacterial disease among wildlife and livestock. Nat. Commun. 2016;7:11448. doi: 10.1038/ncomms11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franco M.P., Mulder M., Gilman R.H., Smits H.L. Human brucellosis. Lancet Infect. Dis. 2007;7:775–786. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y., Wang Y., Zhang L., Wang A., Yan Y., Chen Y., et al. An epidemiological study of brucellosis on mainland China during 2004-2018. Transbound. Emerg. Dis. 2021;68:2353–2363. doi: 10.1111/tbed.13896. [DOI] [PubMed] [Google Scholar]
- 14.Kiambi S.G., Fèvre E.M., Omolo J., Oundo J., de Glanville W.A. Risk factors for acute human brucellosis in Ijara, North-Eastern Kenya. PLoS Negl. Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008108. e0008108-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baoshan L., Yinbo Y., Jingbo Z., Yi Z., Jianghua Y., Dawei C., et al. Combined nucleic acid assays for diagnosis of A19 vaccine-caused human brucellosis. Transbound. Emerg. Dis. 2021;68:368–374. doi: 10.1111/tbed.13685. [DOI] [PubMed] [Google Scholar]
- 16.Pappas G. The Lanzhou Brucella leak: the largest laboratory accident in the history of infectious diseases? Clin. Infect. Dis. 2022 doi: 10.1093/cid/ciac463. ciac463. [DOI] [PubMed] [Google Scholar]
- 17.Roth F., Zinsstag J., Dontor O., Chimed-Ochir G., Guy H., Ottorino C., et al. Human health benefits from livestock vaccination for brucellosis: case study. Bull. World Health Organ. 2003;81:867–876. https://apps.who.int/iris/handle/10665/268863 [PMC free article] [PubMed] [Google Scholar]
- 18.Singh B.B., Kostoulas P., Gill J.P.S., Dhand N.K. Cost-benefit analysis of intervention policies for prevention and control of brucellosis in India. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng J., Robertson I.D., Ji Q.-M., Dawa Y.-L., Bruce M. Evaluation of the economic impact of brucellosis in domestic yaks of Tibet. Transbound. Emerg. Dis. 2019;66:476–487. doi: 10.1111/tbed.13049. [DOI] [PubMed] [Google Scholar]
- 20.Zhang N., Huang D., Wu W., Liu J., Liang F., Zhou B., et al. Animal brucellosis control or eradication programs worldwide: a systematic review of experiences and lessons learned. Prev. Vet. Med. 2018;160:105–115. doi: 10.1016/j.prevetmed.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Davidson R.M. Control and eradication of animal diseases in New Zealand. N. Z. Vet. J. 2002;50:6–12. doi: 10.1080/00480169.2002.36259. [DOI] [PubMed] [Google Scholar]
- 22.Adlam G.H. The eradication of bovine brucellosis in New Zealand: history and objectives. N. Z. Vet. J. 1978;26:42–43. doi: 10.1080/00480169.1978.34492. [DOI] [PubMed] [Google Scholar]
- 23.Pappas G., Papadimitriou P., Akritidis N., Christou L., Tsianos E.V. The new global map of human brucellosis. Lancet Infect. Dis. 2006;6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 24.Laine C., Johnson V., Scott H.M., Arenas-Gamboa A. Global estimate of human brucellosis incidence. Emerg. Infect. Dis. J. 2023;29:1789. doi: 10.3201/eid2909.230052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng C., Zhou H., Guan P., Wu W., Huang D.-S. An estimate of the incidence and quantitative risk assessment of human brucellosis in mainland China. Transbound. Emerg. Dis. 2020;67:1898–1908. doi: 10.1111/tbed.13518. [DOI] [PubMed] [Google Scholar]
- 26.Deqiu S., Donglou X., Jiming Y. Epidemiology and control of brucellosis in China. Vet. Microbiol. 2002;90:165–182. doi: 10.1016/S0378-1135(02)00252-3. [DOI] [PubMed] [Google Scholar]
- 27.Lai S., Zhou H., Xiong W., Yu H., Huang Z., Yu J., et al. Changing epidemiology of human brucellosis, China, 1955–2014. Emerg. Infect. Dis. J. 2017;23:184. doi: 10.3201/eid2302.151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munn Z., Peters M.D.J., Stern C., Tufanaru C., McArthur A., Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018;18:143. doi: 10.1186/s12874-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Huang D. Chinese Academy of Agricultural Sciences; 2012. Research on Epidemiological Survey of Cow Brucellosis in Changping District, Beijing from 2000 to 2009 [Master] [Google Scholar]
- 31.Jiang J., Dai G., Wei B., Xie L., Liang Z., Li M., et al. Epidemiological survey of major diseases of dairy cattle in Wuzhou from 2003 to 2013. J. Anim. Sci. Vet. Med. 2014;33:99–101. [Google Scholar]
- 32.Yu X., Shentu P., Chen L. Monitoring data analysis of brucellosis in Jinhua from 2004 to 2010. Chin. Rural Health Serv. Adm. 2011;31:1060–1061. [Google Scholar]
- 33.Yang R., Lv L., Lv Y. Surveillance report on brucellosis in 2007 in Quzhou municipality. Chin. Prim. Health Care. 2009;23:64–65. [Google Scholar]
- 34.Fu Y., Wang S., Liu W., Lin Y., Li X., Qi Q. Surveillance and analysis of brucellosis in cattle and sheep in Qinghai Province from 2007 to 2011. China Herbivore Sci. 2012;32:50–51. [Google Scholar]
- 35.Ning P., Guo M., Guo K., Xu L., Ren M., Cheng Y., et al. Identification and effect decomposition of risk factors for Brucella contamination of raw whole milk in China. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X. Jilin University; 2013. Epidemiolody Survey and Preliminary Study on the Infection Mechanism of Cow Brucellosis in Tongliao District of Inner Mongolia [PhD] [Google Scholar]
- 37.Lu W., He F., Li S. Epidemiological survey of brucellosis in Qingyang from 2009 to 2013. J. Anim. Sci. Vet. Med. 2014;33 73–4+6. [Google Scholar]
- 38.Ma Q., Shen C., Liu J. Serological investigation and risk analysis of dairy cow brucellosis in Laixi of Qingdao. China Anim. Health Inspect. 2014;31:55–59. [Google Scholar]
- 39.Meng W., Chen P., Xu R., Liu S., Qiu Z., Zhu R., et al. Epidemiological investigation of brucellosis in cattle and sheep in Shizhong District, Zaozhuang. Shandong J. Anim. Sci. Vet. Med. 2013;34:71–72. [Google Scholar]
- 40.Qi Q., Cao Y. Surveillance and epidemiological survey of brucellosis in cattle and sheep in Qinghai Province in 2012. Shandong J. Anim. Sci. Vet. Med. 2013;34:57–58. [Google Scholar]
- 41.Hao Z., Xing H., Zhang J., Wang L., Kong L., Ying H., et al. Surveillance and elimination of tuberculosis and brucellosis in cattle and sheep in Qufu city. Shandong J. Anim. Sci. Vet. Med. 2014;35:43–45. [Google Scholar]
- 42.Zhang J., Xu J., Fang L., Yu S., Sun G. Investigation and analysis of epidemic situation of brucellosis in livestock in Heilongjiang province. Heilongjiang Anim. Sci. Vet. Med. 2016:104–106. [Google Scholar]
- 43.Cui J., Wang J., Gu Z. Investigation of prevalence and risk factors of brucellosis in dairy cows in X City of Liaoning Province. China Anim. Health Inspect. 2014;31:51–54. [Google Scholar]
- 44.Liu T., Xue Z., Li L., Xu H., Wang L., Zhang X., et al. Epidemiological investigation of paratuberculosis and brucellosis in dairy cows in Hebei Province. Heilongjiang Anim. Sci. Vet. Med. 2016:180–183. [Google Scholar]
- 45.Liu G. Northeast Agricultural University; 2019. Incidence and Control Effect of Mastitis and Quarantine Purification of Brucellosis and Tuberculosis in Large-Scale Dairy Farm in Reclamation Area [Master] [Google Scholar]
- 46.Ran X. Sichuan Agricultural University; 2018. Epidemiological Survey of Brucellosis and Tuberculosis in Dairy Cattle in Gongjing District, Zigong [Master] [Google Scholar]
- 47.Wu Z., Cao X., Lin X., Zhang J., Zhang L., Jin S., et al. Surveillance, isolation and identification of Brucella Surveillence in cows in Yongjing County of Gansu Province. China Anim. Health Inspect. 2018;35 83–6+9. [Google Scholar]
- 48.Huang C. Shihezi University; 2019. Epidemiological Investigation and Pathogen Isolation and Biotype Identification of Brucellosis in some Large-Scale Cattle Farms in Shihezi City [Master] [Google Scholar]
- 49.Wan J. Investigation on infection of brucellosis in dairy cows in an area of Henan Province. China Herbivore Sci. 2017;37:75–76. [Google Scholar]
- 50.Tao X., Qiu L., Chen N., Xie M., Wang W. Epidemiological survey of animal brucellosis in Chuxiong prefecture in 2015. Shanghai J. Anim. Husb. Vet. Med. 2016:19. [Google Scholar]
- 51.Chen D., Zhou M., Tang C., Yang A., Chen B., Chen D., et al. A seroepidemiological survey of bovine brucellosis in Sichuan Province from 2015 to 2017. Chin. J. Vet. Med. 2019;55:66–67. [Google Scholar]
- 52.Li Z., Liu X., Xu C., Tang D. Sero-epidemiological investigation on brucellosis in cows in Guizhou Province during 2015 to 2017. China Anim. Health Inspect. 2019;36:19–21. [Google Scholar]
- 53.Zhang H., Deng X., Cui B., Shao Z., Zhao X., Yang Q., et al. Abortion and various associated risk factors in dairy cow and sheep in Ili, China. PLoS One. 2020;15 doi: 10.1371/journal.pone.0232568. e0232568-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu S. Inner Mongolia Agricultural University; 2021. Epidemiological Investigation and Analysis of Brucellosis in Ulanqab City of Inner Mongolia [Master] [Google Scholar]
- 55.Qi S. Grass-Feeding Livestock (In Chinese) 2019. Epidemiological survey of brucellosis in dairy cows in Urumqi County; pp. 33–37. [DOI] [Google Scholar]
- 56.Zhao S., Wang S., Zhao P., Yuan S., Fang D., Guo L., et al. Investigation on brucellosis infection of dairy cows in Henan Province in 2018. Chin. J. Vet. Med. 2020;56:42–44. [Google Scholar]
- 57.Zhu X., Wang M., Zheng X., Zhang P. Epidemiological investigation of livestock brucellosis in Gaoling District. J. Anim. Sci. Vet. Med. 2019;38:42–44. [Google Scholar]
- 58.Liu Y., Ban F., Hu W., Cheng Y., Zhang H., Xie C., et al. Cross-sectional study on the prevalence and risks of spreading brucellosis among cow farms in Pingdingshan City of Henan Province. China Anim. Health Inspect. 2020;37 22–6+38. [Google Scholar]
- 59.Ren R. Xinjiang Agricultural University; 2021. Epidemic Situation of Main Diseases in Two Dairy Farms in Hainan [Master] [Google Scholar]
- 60.Geng H. Shandong Agricultural University; 2022. Establishment of Indirect ELISA Method for Brucella VirB9 Protein and Epidemiological Investigation of Bovine Brucellosis in Shandong Province in 2021 [Master] [Google Scholar]
- 61.Wu X. Xinjiang Agricultural University; 2022. Epidemiological Investigation and Prevention Measures of Bovine Brucellosis in Urumqi [Master] [Google Scholar]
- 62.An C.-H., Nie S.-M., Sun Y.-X., Fan S.-P., Luo B.-Y., Li Z., et al. Seroprevalence trend of human brucellosis and MLVA genotyping characteristics of Brucella melitensis in Shaanxi Province, China, during 2008–2020. Transbound. Emerg. Dis. 2022;69:e423–e434. doi: 10.1111/tbed.14320. [DOI] [PubMed] [Google Scholar]
- 63.Wang X., Xu K., Zhang J., Feng P., Gao X., Zhao K. Risk factors related to Brucella infection in occupational exposure population at high risk in cattle/sheep farms in Xi’an City of Shaanxi Province. China Anim. Health Inspect. 2021;38:5–9. [Google Scholar]
- 64.Wang H., Xu W.-M., Zhu K.-J., Zhu S.-J., Zhang H.-F., Wang J., et al. Molecular investigation of infection sources and transmission chains of brucellosis in Zhejiang, China. Emerg. Microbes Infect. 2020;9:889–899. doi: 10.1080/22221751.2020.1754137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y.-J., Li X.-L., Liang S., Fang L.-Q., Cao W.-C. Epidemiological features and risk factors associated with the spatial and temporal distribution of human brucellosis in China. BMC Infect. Dis. 2013;13:547. doi: 10.1186/1471-2334-13-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peng C., Li Y.-J., Huang D.-S., Guan P. Spatial-temporal distribution of human brucellosis in mainland China from 2004 to 2017 and an analysis of social and environmental factors. Environ. Health Prev. Med. 2020;25:1. doi: 10.1186/s12199-019-0839-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhuo M., Zhang Z., Huang J., Zhu H., Wang L., Lin D., et al. Investigation report on human brucellosis in Nanping, Fujian province. Dis. Surveill. 2007:498–499. [Google Scholar]
- 68.Zhou X., Jiang G., Liu H., Hu Y., Ren S., Li X. Seropepidemiological survey of brucellosis among dairy farmers in Xindu District from 2010 to 2012. J. Prev. Med. Inf. 2014;30:580–582. [Google Scholar]
- 69.Ning C., Shuyi G., Tao Y., Hao Z., Zhang X. Epidemiological survey of human brucellosis in Inner Mongolia, China, 2010–2014: a high risk groups-based survey. J. Infect. Public Health. 2018;11:24–29. doi: 10.1016/j.jiph.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 70.Liu Z.-G., Wang M., Ta N., Fang M.-G., Mi J.-C., Yu R.-P., et al. Seroprevalence of human brucellosis and molecular characteristics of Brucella strains in Inner Mongolia Autonomous region of China, from 2012 to 2016. Emerg. Microbes Infect. 2020;9:263–274. doi: 10.1080/22221751.2020.1720528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang B. Serological investigation and analysis of zoonotic brucellosis in Wuyuan County. J. Inner Mongolia Med. Univ. 2014;36:116–117. [Google Scholar]
- 72.Zhao C., Liu K., Jiang C., Wei X., Song S., Wu X., et al. Epidemic characteristics and transmission risk prediction of brucellosis in Xi’an city, Northwest China. Front. Public Health. 2022:10. doi: 10.3389/fpubh.2022.926812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xiao Y., Zou G., Yin J., Tan W., Zhou J., Zhang H. Seroepidemiology of human Brucella infection in Yixing, China. Trop. Dr. 2016;47:165–167. doi: 10.1177/0049475516640191. [DOI] [PubMed] [Google Scholar]
- 74.Han T., Liu J., Liu W., Zeng Z., He F., Xiao F., et al. Epidemiological characteristics and control strategy of human brucellosis in Fujian, 2016-2020. Dis. Surveill. 2022;37:1442–1446. [Google Scholar]
- 75.Lin S., Wang Z., Liu X., Yu A., Hasan M., Bayidawulieti J., et al. Serological prevalence survey among the high-risk populations of brucellosis-endemic areas-China, 2019−2020. China CDC Weekly. 2021;3 doi: 10.46234/ccdcw2021.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghai R.R., Wallace R.M., Kile J.C., Shoemaker T.R., Vieira A.R., Negron M.E., et al. A generalizable one health framework for the control of zoonotic diseases. Sci. Rep. 2022;12:8588. doi: 10.1038/s41598-022-12619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu X., Zhao Z., Ma S., Guo Z., Wang M., Li Z., et al. Brucella melitensis, a latent “travel bacterium,” continual spread and expansion from Northern to Southern China and its relationship to worldwide lineages. Emerg. Microbes Infect. 2020;9:1618–1627. doi: 10.1080/22221751.2020.1788995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Z., Wang X.-M., Zhu X., Wang M., Cheng H., Li D., et al. Molecular characteristics of Brucella isolates collected from humans in Hainan Province, China. Front. Microbiol. 2020;11:452. doi: 10.3389/fmicb.2020.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ministry of Agriculture and Rural Affairs . Bureau of Animal Husbandry and Veterinary Medicine, Editor. Beijing. 2022. Five-year action plan for animal brucellosis prevention and control (2022–2026) [Google Scholar]
- 80.Chen Y., Wang Y., Robertson I.D., Hu C., Chen H., Guo A. Key issues affecting the current status of infectious diseases in Chinese cattle farms and their control through vaccination. Vaccine. 2021;39:4184–4189. doi: 10.1016/j.vaccine.2021.05.078. [DOI] [PubMed] [Google Scholar]
- 81.Robertson I.D. Disease control, prevention and on-farm biosecurity: the role of veterinary epidemiology. Engineering. 2020;6:20–25. doi: 10.1016/j.eng.2019.10.004. [DOI] [Google Scholar]
- 82.Zhang N., Zhou H., Huang D.S., Guan P. Brucellosis awareness and knowledge in communities worldwide: a systematic review and meta-analysis of 79 observational studies. PLoS Negl. Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ran X., Cheng J., Wang M., Chen X., Wang H., Ge Y., et al. Brucellosis seroprevalence in dairy cattle in China during 2008–2018: a systematic review and meta-analysis. Acta Trop. 2019;189:117–123. doi: 10.1016/j.actatropica.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 84.Holt H.R., Bedi J.S., Kaur P., Mangtani P., Sharma N.S., Gill J.P.S., et al. Epidemiology of brucellosis in cattle and dairy farmers of rural Ludhiana, Punjab. PLoS Negl. Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sibhat B., Tessema T.S., Nile E., Asmare K. Brucellosis in Ethiopia: a comprehensive review of literature from the year 2000–2020 and the way forward. Transbound. Emerg. Dis. 2022;69:e1231–e1252. doi: 10.1111/tbed.14495. [DOI] [PubMed] [Google Scholar]
- 86.Lehane R. CSIRO publishing; 1996. Beating the Odds in a Big Country: The Eradication of Bovine Brucellosis and Tuberculosis in Australia. [Google Scholar]
- 87.The Center for Food Security and Public Health Brucellosis: Brucella abortus, bovine brucellosis, undulant fever, contagious abortion. Bangs Dis. 2018:1–12. [Google Scholar]
- 88.Kiiza D., Denagamage T., Serra R., Maunsell F., Kiker G., Benavides B., et al. A systematic review of economic assessments for brucellosis control interventions in livestock populations. Prev. Vet. Med. 2023;213 doi: 10.1016/j.prevetmed.2023.105878. [DOI] [PubMed] [Google Scholar]
- 89.de Alencar Mota A.L.A., Ferreira F., Ferreira Neto J.S., Dias R.A., Amaku M., Hildebrand Grisi-Filho J.H., et al. Large-scale study of herd-level risk factors for bovine brucellosis in Brazil. Acta Trop. 2016;164:226–232. doi: 10.1016/j.actatropica.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 90.Ducrotoy M.J., Muñoz P.M., Conde-Álvarez R., Blasco J.M., Moriyón I. A systematic review of current immunological tests for the diagnosis of cattle brucellosis. Prev. Vet. Med. 2018;151:57–72. doi: 10.1016/j.prevetmed.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 91.Capparelli R., Parlato M., Iannaccone M., Roperto S., Marabelli R., Roperto F., et al. Heterogeneous shedding of Brucella abortus in milk and its effect on the control of animal brucellosis. J. Appl. Microbiol. 2009;106:2041–2047. doi: 10.1111/j.1365-2672.2009.04177.x. [DOI] [PubMed] [Google Scholar]
- 92.Bercovich Z. Maintenance of Brucella Abortus-free herds: a review with emphasis on the epidemiology and the problems in diagnosing brucellosis in areas of low prevalence. Vet. Q. 1998;20:81–88. doi: 10.1080/01652176.1998.9694845. [DOI] [PubMed] [Google Scholar]
- 93.Khurana S.K., Sehrawat A., Tiwari R., Prasad M., Gulati B., Shabbir M.Z., et al. Bovine brucellosis – a comprehensive review. Vet. Q. 2021;41:61–88. doi: 10.1080/01652176.2020.1868616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ma S.-Y., Liu Z.-G., Zhu X., Zhao Z.-Z., Guo Z.-W., Wang M., et al. Molecular epidemiology of Brucella abortus strains from cattle in Inner Mongolia, China. Prev. Vet. Med. 2020;183 doi: 10.1016/j.prevetmed.2020.105080. [DOI] [PubMed] [Google Scholar]
- 95.Pappas G., Akritidis N., Bosilkovski M., Tsianos E. Brucellosis. N. Engl. J. Med. 2005;352:2325–2336. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 96.Piao D., Li Z., Yang X., Tian G., Zhao H., Jiang H. Emerging brucellosis outbreaks associated with unpasteurized milk in China. China CDC Weekly. 2020;2:888–890. doi: 10.46234/ccdcw2020.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Y., Robertson I.D., Cheng S., Wang Y., Hou L., Wang G., et al. Evaluation of a milk ELISA as an alternative to a serum ELISA in the determination of the prevalence and incidence of brucellosis in dairy herds in Hubei Province, China. Prev. Vet. Med. 2020;182 doi: 10.1016/j.prevetmed.2020.105086. [DOI] [PubMed] [Google Scholar]
- 98.Li Y., Huang B., Shen C., Cai C., Wang Y., Edwards J., et al. Pig trade networks through live pig markets in Guangdong Province, China. Transbound. Emerg. Dis. 2020;67:1315–1329. doi: 10.1111/tbed.13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shi F., Huang B., Shen C., Liu Y., Liu X., Fan Z., et al. Characterization and influencing factors of the pig movement network in Hunan Province, China. Prev. Vet. Med. 2021;193 doi: 10.1016/j.prevetmed.2021.105396. [DOI] [PubMed] [Google Scholar]
- 100.Marquetoux N., Stevenson M.A., Wilson P., Ridler A., Heuer C. Using social network analysis to inform disease control interventions. Prev. Vet. Med. 2016;126:94–104. doi: 10.1016/j.prevetmed.2016.01.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
We have shared the data in supplementary material.