Abstract
The European Commission asked EFSA to update its 2011 risk assessment on tetrabromobisphenol A (TBBPA) and five derivatives in food. Neurotoxicity and carcinogenicity were considered as the critical effects of TBBPA in rodent studies. The available evidence indicates that the carcinogenicity of TBBPA occurs via non‐genotoxic mechanisms. Taking into account the new data, the CONTAM Panel considered it appropriate to set a tolerable daily intake (TDI). Based on decreased interest in social interaction in male mice, a lowest observed adverse effect level (LOAEL) of 0.2 mg/kg body weight (bw) per day was identified and selected as the reference point for the risk characterisation. Applying the default uncertainty factor of 100 for inter‐ and intraspecies variability, and a factor of 3 to extrapolate from the LOAEL to NOAEL, a TDI for TBBPA of 0.7 μg/kg bw per day was established. Around 2100 analytical results for TBBPA in food were used to estimate dietary exposure for the European population. The most important contributors to the chronic dietary LB exposure to TBBPA were fish and seafood, meat and meat products and milk and dairy products. The exposure estimates to TBBPA were all below the TDI, including those estimated for breastfed and formula‐fed infants. Accounting for the uncertainties affecting the assessment, the CONTAM Panel concluded with 90%–95% certainty that the current dietary exposure to TBBPA does not raise a health concern for any of the population groups considered. There were insufficient data on the toxicity of any of the TBBPA derivatives to derive reference points, or to allow a comparison with TBBPA that would support assignment to an assessment group for the purposes of combined risk assessment.
Keywords: food, human exposure, occurrence, risk assessment, TBBPA, tetrabromobisphenol A, toxicology
Summary
Brominated flame retardants (BFRs) are anthropogenic chemicals, which are used in a wide variety of consumer/commercial products to improve their resistance to fire. Concern has been raised because of the occurrence of several chemical compounds from the group of BFRs in the environment, food and in humans. This has led to bans on the production and use of certain formulations.
The European Commission asked the European Food Safety Authority (EFSA) to update its 2010–2012 risk assessments on the different families of BFRs, i.e. hexabromocyclododecanes (HBCDDs), polybrominated diphenyl ethers (PBDEs), tetrabromobisphenol A (TBBPA) and its derivatives, brominated phenols and their derivatives and novel and emerging BFRs. The CONTAM Panel is updating the risk assessments of different classes of BFRs in a series of separate Opinions.
The similarities in chemical properties and effects seen in the previous EFSA assessments for the different BFR families warrant the consideration of a mixture approach. The Panel on Contaminants in the Food Chain (CONTAM Panel) will evaluate the appropriateness of applying a mixture approach in an additional opinion once the risk assessment for each BFR family has been updated. It will be based on the EFSA Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals.
The first and second opinions in the current series updated the risk assessments of HBCDDs in food and PBDEs in food. This third opinion updates the risk assessment of TBBPA and its derivatives in food previously performed by EFSA and published in 2011. The current assessment focusses on TBBPA and five TBBPA derivatives, i.e. TBBPA bismethyl ether (TBBPA‐bMeE), TBBPA bis(2‐hydroxyethyl) ether (TBBPA‐bOHEtE), TBBPA bisallyl ether (TBBPA‐bAE), TBBPA bis(glycidyl ether) (TBBPA‐bGE), TBBPA bis(2,3‐dibromopropyl) ether (TBBPA‐bDiBPrE), as requested in the terms of reference by the European Commission.
TBBPA and the derivatives included in this Opinion have been used commercially as flame retardants. TBBPA is used as both an additive and a reactive flame retardant, i.e. both where it is simply mixed with the product and where it is chemically bonded to the plastics where it is used.
The present assessment takes into account the occurrence data in food and biological samples submitted to EFSA after the publication of its previous Opinion on TBBPA and its derivatives, as well as the newly available scientific information of relevance to hazard identification and characterisation.
The draft scientific Opinion underwent a public consultation from 26 March 2024 to 7 May 2024. The comments received were taken into account when finalising the scientific Opinion and are presented and addressed in Annex E.
The analytical determination of TBBPA is primarily performed by liquid chromatography‐mass spectrometry (LC–MS)‐based methods, although analysis by gas chromatography‐MS (GC–MS) is also used. The analysis of volatile derivatives such as TBBPA‐bDiBPrE and TBBPA‐bAE is primarily done by GC–MS methods, sometimes using derivatisation techniques prior to GC–MS analysis.
Hazard Identification and characterisation
In rodents, TBBPA is well absorbed but due to extensive metabolism, the oral bioavailability in rats is in the range of 2%–5%. TBBPA is metabolised by UDP‐glucuronosyltransferases and sulfotransferases to form glucuronide and sulfate conjugates. After oral administration, TBBPA is found in different tissues, but it does not accumulate in tissues due to rapid elimination, e.g. of the conjugates. TBBPA is principally eliminated in the faeces, within 24 h after oral administration, mainly through biliary excretion. The maximum half‐life reported in tissues was less than 3 days. In rodents, there is evidence of transplacental transfer and transfer via lactation of TBBPA.
Limited data on toxicokinetics in rodents are available for TBBPA derivatives. Following oral administration, TBBPA‐bDiBPrE is eliminated in the faeces (95% of dose by 36 h). One study on TBBPA‐bDiBPrE suggested a low rate of metabolism but did not identify metabolites.
In humans, no data were identified on the absorption of TBBPA, whereas limited data are available on the metabolism and elimination. Following oral administration of TBBPA, TBBPA‐mono‐glucuronide, TBBPA‐diglucuronide, TBBPA‐mono‐sulfate and TBBPA‐di‐sulfate metabolites have been detected in urine. No data were identified on the toxicokinetics of TBBPA derivatives in humans.
Several studies have measured concentrations of TBBPA in human samples, as a conjugate mostly in serum and partly in human milk. The TBBPA mean concentrations in human milk samples collected from European countries ranged from 0.05 to 3.5 ng/g lipid. No data were available on concentrations of TBBPA derivatives in human samples.
No studies on the transfer from feed to food of animal origin of TBBPA or the derivates included in this opinion were identified.
In the previous Opinion, it was concluded that the main targets of TBBPA in subchronic and chronic toxicity studies in rats and mice were liver, kidney, serum thyroid hormones levels, immune, nervous and reproductive systems. No data were available on the toxicity of any of the TBBPA derivatives.
Since then, the newly identified toxicity studies in experimental animals tested TBBPA and only one of the TBBPA derivatives, i.e. TBBPA‐bDiBPrE.
The acute toxicity of TBBPA and TBBPA‐bDiBPrE was low.
Repeated exposure to TBBPA in mice and rats showed some evidence of effects on the liver, i.e. small increases in liver weight (< 12%) at doses of 500 mg/kg bw per day. Histopathological changes were reported in some studies, without a clear dose–response relationship. Studies with TBBPA‐bDiBPrE in rats and mice at doses up to about 1000 mg/kg bw per day showed no effects on the liver.
A significant reduction of serum TT4 levels was reported in rats exposed to TBBPA by gavage at 100 mg/kg bw per day. The only observed effects in mice were an increased height of thyroid follicular epithelial cells in males exposed by gavage at 20 mg/kg bw per day.
Kidney lesions have been observed in neonatal mice or rats following exposure of dams (during gestation and lactation) by gavage to high doses (> 100 mg/kg bw per day) of TBBPA as well as in adult mice. No effects on kidneys were reported in studies with TBBPA‐bDiBPrE in rats (doses up to 714 mg/kg bw per day) and in mice (doses up to 1429 mg/kg bw per day).
In rats, two‐generation reproductive toxicity studies by gavage showed no effects on reproduction or development up to doses of 1000 mg TBBPA/kg bw per day. In another study, a delay in the time to preputial separation was observed in F1 male pups of rat dams exposed by gavage (GD6–PND21) to TBBPA at 250 mg/kg bw per day. Studies conducted on adult rats and mice exposed to TBBPA‐bDiBPrE by gavage for 3 months showed no reproductive toxicity at doses up to 1000 and 2000 mg/kg bw, respectively.
Exposure to TBBPA (200 mg/kg bw per day) directly from the diet or in utero can have effects on immune system in mice with associated changes in serum cytokine concentrations in offspring from TBBPA exposed dams.
TBBPA was shown to induce behavioural disturbances later in life after an early exposure of the animals by gavage in utero, during lactation or postnatally in rats and mice. A reduction in the interest for social novelty of adult male mice exposed to TBBPA at 0.2 mg/kg bw per day (GD8–PND21) was reported, as well as a memory retention impairment in a passive‐avoidance test performed in adult mice previously exposed for 2 weeks (PND42–56) to TBBPA by gavage with a no observed adverse effect level (NOAEL) of 20 mg/kg bw per day. An increase in the spontaneous locomotor activity in adult female rats perinatally exposed to 0.1 mg/kg bw per day, as well as an increase in the level of anxiety in adult rats orally exposed to TBBPA (GD6–PND21) at 25 mg/kg bw per day was reported.
In rats, TBBPA has been found to induce testicular adenoma and uterine epithelial tumours (predominantly uterine adenocarcinoma). The lowest dose of TBBPA reported to cause a significant increase in tumours was 500 mg/kg bw (357 mg/kg bw per day), for adenocarcinomas of the uterus in rats. A significant increase in atypical endometrial hyperplasia of the uterus (which is considered to be a preneoplastic lesion) was observed at 250 mg/kg bw (179 mg/kg bw per day), the lowest dose tested. In mice, there was some evidence of an increased incidence of hepatoblastoma in males, with no carcinogenic effects in females at the assessed doses, which were not considered to provide a robust basis for risk assessment. No carcinogenicity studies were available for any of the TBBPA derivatives.
TBBPA was not mutagenic in vitro in bacterial, yeast and mammalian mutagenicity assays in the presence or absence of metabolic activation. It did not cause structural chromosome aberrations in Chinese hamster lung and human peripheral lymphocytes. In in vitro Comet assays, TBBPA induced single strand breaks (SSB) and double strand breaks (DSB), as well as oxidised DNA bases in human peripheral blood mononuclear cells. It also induced SSB in THP‐1 cells. Due to the lack of validation and appropriate OECD guidelines for the in vitro Comet assay, these results were regarded of limited relevance. Moreover, in the absence of other positive results, these in vitro Comet assay results were not considered a sign of mutagenic potential. In vivo, no increase in micronuclei was observed in a peripheral blood micronucleus test in mice. The results of this test were of low relevance with no convincing evidence for target organ exposure. In view of the lack of effect in vitro, according to the 2011 EFSA Opinion on genotoxicity testing strategies applicable to food and feed safety assessment, it is not necessary to test in vivo. Based on the available evidence, TBBPA is not genotoxic.
TBBPA‐bDiBPrE was not mutagenic in vitro in Salmonella Typhimurium. In vivo, no increase in micronuclei was observed in a peripheral blood micronucleus test in mice exposed at very high dose. However, there was no convincing evidence for target organ exposure. Based on these very limited data, no conclusion could be drawn regarding the genotoxic potential of TBBPA‐bDiBPrE.
Since the previous Opinion, nine epidemiological studies have been identified assessing associations between TBBPA and several endpoints including thyroid function, neurotoxicity, cancer, lipid and sugar metabolism and birth outcome. Sporadic findings reported in these epidemiological studies included associations of TBBPA with decreased levels of TT3, reduced risk of gestational diabetes mellitus and decrease in birth weight and size. The cumulative evidence is non‐longitudinal consisting of a small number of small studies. Exposure assessment for other contaminants was rarely reported and in the few studies in which it was done, adjustment for these contaminants was rarely incorporated into the TBBPA analysis. The lack of prospective epidemiological evidence, the small number of studies, the small study sample sizes, the lack of consistency and replication of the associations under study render this body of evidence insufficient.
Regarding the mode of action, based on in vitro studies in several cell types, it was shown that generation of reactive oxygen species (ROS), and the resulting oxidative damage, apoptosis and mitochondrial dysfunction may be mechanisms whereby TBBPA exerts toxic effects: carcinogenicity, kidney and testis toxicity, impaired oocyte maturation, neurotoxicity and immunotoxicity. Moreover, increasing cytosolic Ca2+ concentration may be a primary event triggering oxidative damage and neurotoxicity.
As a correlate with the increasing cytosolic Ca2+ concentration, TBBPA induced some electrophysiology and glutamate neurotransmission disturbances in various neural cell types. Recent studies using new alternative in vitro neurotoxicity models indicated the potentiality of TBBPA to be a developmental neurotoxicant that affects both neuronal and glial cell types.
There is evidence that the carcinogenicity of TBBPA occurs via non‐genotoxic mechanisms, which are likely to have thresholds for effects due to the multiple biochemical events involved.
The CONTAM Panel noted four studies that administered TBBPA to mice via drinking water that investigated effects in the thyroid, neurotoxicity or reproductive toxicity and reported effects at exceptionally low levels (150 and 1500 ng/mL, reported to be about 0.05 and 0.5 mg/kg bw per day). The Panel noted that these studies were generally well conducted; however, the concentrations in the drinking water were not confirmed by analysis of TBBPA, which may be important, e.g. because of the low solubility of TBBPA in water. The CONTAM Panel considered that there is a high level of uncertainty regarding the doses received by the animals, and therefore, no NOAELs/LOAELs were identified from these studies, and no dose–response assessment was performed. The potential relevance of these studies was considered further in the uncertainty analysis.
The CONTAM Panel concluded that the evidence from the available human data did not provide a sufficient basis for the risk assessment. Thus, the CONTAM Panel considered the data from studies in experimental animals to identify reference points for the human risk characterisation.
Neurotoxicity and carcinogenicity were identified as the critical effects for the hazard characterisation. Since TBBPA is carcinogenic, but via non‐genotoxic mechanisms, and considering the new data that had become available since the previous Opinion, the CONTAM Panel considered it appropriate to set a tolerable daily intake (TDI). Dose–response modelling of the data from the carcinogenicity study in rats resulted in a BMDL10 of 42 mg/kg bw per day, based on the incidence of uterine atypical endometrial hyperplasia, a preneoplastic lesion. Effects on neurodevelopment were reported at lower doses than this BMDL10 in studies with one dose level, and the data could not be modelled. An LOAEL of 0.2 mg/kg bw per day for decreased interest in social interaction in adult male mice exposed from GD8–PND21 via the dams was identified as the most appropriate reference point for TBBPA risk characterisation. Applying the default uncertainty factor of 100 for inter‐ and intraspecies variability, and a factor of 3 to extrapolate from LOAEL to NOAEL, a TDI for TBBPA of 0.7 μg/kg bw per day was established.
There were insufficient or lack of data on the toxicity of the five TBBPA derivatives included in the TORs to derive reference points for any of the derivatives, and there were insufficient data on the mode of action of any of the TBBPA derivatives included in the TORs to allow a comparison with TBBPA that would support assignment to an assessment group for the purposes of combined risk assessment.
Occurrence and dietary exposure assessment for the European population
A total of 2090 analytical results on TBBPA in food fulfilled the quality criteria applied and were used in the assessment of dietary exposure to TBBPA. The left‐censored data accounted for 64%–82% of all analytical results. The highest quantified results for TBBPA were found in the food category ‘Fish and seafood’ with the highest level found in ‘Fish liver’ (13 μg/kg ww) followed by ‘Ocean perch’ and ‘Pollack’ (2.9 and 1.7 μg/kg ww, respectively). The next highest quantified results were found in ‘Animal fresh fat tissue’ (0.1 μg/kg ww) within the food category ‘Meat and meat products’.
Occurrence data on two derivates were submitted to EFSA; 359 analytical results on TBBPA‐bME and 346 analytical results on TBBPA‐bDiBPrE. The left‐censored data accounted for 60%–98% and 85%–97% all analytical results, respectively. For TBBPA‐bDiBPrE, the highest quantified results were found in ‘Fish and seafood’ (highest value in clams 0.7 μg/kg ww). For TBBPA‐bME, the highest quantified results were found in ‘Mussels’ (0.01 μg/kg ww). Occurrence data on these two derivatives were not further used as the CONTAM Panel deemed it not possible to identify a reference point or perform a risk assessment for them due to lack of, or limited, toxicological studies.
No occurrence data were submitted to EFSA for food for infants. Thus, the CONTAM Panel decided to use for the dietary exposure assessment to TBBPA, LB and UB mean concentrations of TBBPA in infant and follow‐on formula identified in two studies from the literature from European countries.
Mean dietary exposure to TBBPA ranged across surveys and LB and UB estimates, from < 0.01 ng/kg bw per day in adolescents, adults, elderly and very elderly to 30 ng/kg bw day in infants. P95 dietary exposure to TBBPA ranged across surveys and LB and UB estimates, from 0.01 ng/kg bw per day in very elderly to 85 ng/kg bw day in Infants.
The food categories with the highest number of surveys in which the contribution was higher than 10% are ‘Fish and seafood’, ‘Meat and meat products’ and ‘Milk and dairy products’ for Other children, Adolescents, Adults, Elderly and Very elderly. For the age groups Infants and Toddlers, ‘Foods for young populations’ had the highest number of surveys in which the contribution was greater than 10%.
The highest percentage contribution was found in Infants and Toddlers for ‘Foods for the young population’ (> 99%) and in the Elderly for ‘Fish and seafood’ (80.3%). ‘Meat and meat products’ have contributed up to 48.3% in Adults and ‘Milk and milk products’ up to 42.5% in other children, across surveys and age groups.
An exposure scenario for breastfed infants using the range of TBBPA mean concentrations in human milk samples from European countries reported in the literature (range: 0.05–3.5 ng/g lipid), resulted in daily exposure estimates for average human milk consumption between 0.23 and 16.1 ng/kg bw per day. For infants with high human milk consumption, this resulted in an exposure between 0.34 and 24.1 ng/kg bw per day. Considering the highest mean TBBPA concentration in human milk reported in a study in which a hydrolysis step was applied to cleave conjugates, exposure estimates were 85.8 and 129 ng/kg bw per day, for mean and high consumption, respectively.
An exposure scenario for formula fed infants below 16 weeks of age considering mean TBBPA occurrence data at the LB, resulted in daily exposure estimates of 9.4 and 12.2 ng/kg bw per day, respectively, for mean and P95 infant formula consumption. At the UB, it resulted in estimates of 116 and 151 ng/kg bw per day, respectively. Considering P95 TBBPA occurrence data at the LB, it resulted in daily exposure estimates of 36.2 and 47.1 ng/kg bw per day, for mean and P95 infant formula consumption. At the UB, it resulted in estimates of 200 and 260 ng/kg bw per day, respectively.
The available data suggest that for most of the population, diet represents the largest source of exposure to TBBPA.
No suitable data were identified in the scientific literature with respect to the effects of cooking and processing on levels of TBBPA and the TBBPA derivatives considered.
Risk characterisation
The exposure estimates to TBBPA for the European population, including breastfed and formula‐fed infants, are all below the TDI of 0.7 μg/kg bw per day.
The CONTAM Panel concluded that the chronic dietary exposure to TBBPA in the European population does not raise a health concern.
Uncertainty analysis
An uncertainty analysis was performed. Based on the weight of evidence, the CONTAM Panel concluded that TBBPA is carcinogenic but almost certainly (≥ 99% probability) via non‐genotoxic mechanisms. Considering that all the exposure estimates were far below the TDI for TBBPA, and taking account of all associated uncertainties, the CONTAM Panel concluded with 90%–95% certainty 1 that current dietary exposure to TBBPA would not raise a health concern for any of the surveys and population groups considered.
Recommendations
The CONTAM Panel made the following recommendations: More data on occurrence of TBBPA in human milk and food for infants, with more sensitive analytical methods, are needed to enable a more robust exposure assessment for infants. Data on the occurrence of TBBPA in food of plant origin are needed. More toxicokinetic data on TBBPA in humans and rodents are needed. More reproductive studies are needed, allowing a comparison between rats and mice, both in male and females. Developmental neurotoxicity studies on TBBPA are needed to better characterise the dose–response relationship and explore sensitive endpoints and species differences. More information on the mode of action of developmental neurotoxicity is needed. Information is needed that would allow understanding of the large differences between the doses inducing effects in studies performed by gavage or drinking water administration.
In order to conduct a risk assessment for the TBBPA derivatives, the CONTAM Panel made the following recommendations: Data on the occurrence of the TBBPA derivatives in food are needed, with sensitive analytical methods. Occurrence of TBBPA derivatives in human milk and food for infants, with sensitive analytical methods, is needed to enable an exposure assessment for infants. Information is needed that would allow hazard identification and characterisation for the TBBPA derivatives.
1. INTRODUCTION
1.1. Background and terms of reference as provided by the requestor
BACKGROUND
Brominated flame retardants (BFRs) are anthropogenic chemicals, which are added to a wide variety of consumer/commercial products in order to improve their fire resistance. The major classes of BFRs are brominated bisphenols, diphenyl ethers, cyclododecanes, phenols, biphenyl derivatives and the emerging and novel BFRs.
Concern has been raised because of the occurrence of several chemical compounds from the group of BFRs in the environment, including feed and food, and in humans. This has led to bans on the production and use of certain formulations of polybrominated diphenyl ethers (PBDEs).
Between September 2010 and September 2012, the Scientific Panel on Contaminants in the Food of EFSA adopted six scientific Opinions on different classes of brominated flame retardants. Because in its Opinion EFSA highlighted several data gaps, hampering the consumer risk assessment for these substances, by means of Commission Recommendation 2014/118/EU on the monitoring of traces of brominated flame retardants in food, Member States were recommended to collect in 2014 and 2015 occurrence data for specific substances in specific foodstuffs.
The newly available occurrence data would enable an updated consumer exposure assessment. Furthermore, since the publication of the EFSA scientific Opinions between 2010 and 2012, new scientific information has become available, therefore it would be necessary to verify whether an update of these scientific Opinions would be appropriate, including an update of the consumer risk assessment.
TERMS OF REFERENCE
In accordance with Art. 29 (1) of Regulation (EC) No 178/2002, the European Commission asks the European Food Safety Authority for an updated exposure assessment for the brominated flame retardants, covered by Recommendation 2014/118/EU, taking into account the occurrence data in food, submitted after the publication of the 2010–2012 EFSA scientific Opinions, and an updated consumer risk assessment, taking into account newly available scientific information.
1.2. Interpretation of the terms of reference
Following the request from the European Commission, the CONTAM Panel will update its 2010–2012 risk assessments on the different classes of BFRs: hexabromocyclododecanes (HBCDDs), polybrominated diphenyl ethers (PBDEs), tetrabromobisphenol A (TBBPA) and its derivatives, brominated phenols and their derivatives and novel and emerging BFRs (EFSA CONTAM Panel, 2011a, 2011b, 2011c, 2012a, 2012b).
The first two opinions in the series updated the risk assessments of HBCDDs in food (EFSA CONTAM Panel, 2021) and PBDEs in food (EFSA CONTAM Panel, 2024). This third opinion is an update of the risk assessment of TBBPA and its derivatives in food previously performed by EFSA (EFSA CONTAM Panel, 2011c). In Commission Recommendation 2014/118/EU, the following derivatives were listed and will be considered in the current update:
TBBPA bismethyl ether (TBBPA‐bMeE, CAS No 37853‐61‐5),
TBBPA bis(2‐hydroxyethyl) ether (TBBPA‐bOHEtE, CAS No 4162‐45‐2),
TBBPA bisallyl ether (TBBPA‐bAE, CAS No 25327‐89‐3),
TBBPA bis(glycidyl ether) (TBBPA‐bGE, CAS No 3072‐84‐2),
TBBPA bis(2,3‐dibromopropyl) ether (TBBPA‐bDiBPrE, CAS No 21850‐44‐2).
The similarities in chemical properties and effects seen in the previous EFSA assessments for the different BFR classes warrant the consideration of a mixture approach. The CONTAM Panel will evaluate the appropriateness of applying a mixture approach for the different classes of BFRs in an additional Opinion once the risk assessment for the each BFR class has been updated. It will be based on the EFSA Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals (EFSA Scientific Committee, 2019).
1.3. Supporting information for the assessment
1.3.1. Physicochemical properties
The physicochemical properties of 3,3′,5,5′‐tetrabromobisphenol A (TBBPA) and its derivatives were described in Section 1.3 (Chemical characteristics) of the previous EFSA Opinion on TBBPA and its derivatives in food (EFSA CONTAM Panel, 2011c). These are summarised in Table 1 below for the compounds included in the current TORs. The basic structure of TBBPA consists of two hydroxyphenyl rings linked by a carbon bridge with bromine substitution at the 3, 3′, 5 and 5′‐position, as shown in Figure 1 together with some of its derivatives.
TABLE 1.
Physicochemical characteristics of TBBPA and the derivatives considered in this Opinion (from Bergman et al., 2012).
| Compounds | Structured abbreviation (practical abbreviation) | CAS | Molecular weight (g/mol) | Log K ow | pKa | Vapour pressure (Torr) |
|---|---|---|---|---|---|---|
| TBBPA | TBBPA | 79‐94‐7 | 543.9 | 6.5 a | 7.5/8.5 b | 1.88 × 10−5 |
| TBBPA bismethyl ether | TBBPA‐bMeE (TBBPA‐BME) | 37853‐61‐5 | 571.9 | 10.6 | N c | 2.25 × 10−6 |
| TBBPA bis(2‐hydroxyethyl) ether | TBBPA‐bOHEtE (TBBPA‐BHEE) | 4162‐45‐2 | 632.0 | 8.5 | 13.76 | 2.89 × 10−12 |
| TBBPA bisallyl ether | TBBPA‐bAE (TBBPA‐BAE) | 25327‐89‐3 | 642.0 | 11.4 | N c | 1.83 × 10−8 |
| TBBPA bis(glycidyl ether) | TBBPA‐bGE (TBBPA‐BGE) | 3072‐84‐2 | 656.0 | 8.9 | N c | 1.64 × 10−10 |
| TBBPA bis(2,3‐dibromopropyl) ether | TBBPA‐bDiBPrE (TBBPA‐BDBPE) | 21850‐44‐2 | 943.6 | 13.0 | N c | 2.85 × 10−15 |
Abbreviations: CAS, Chemical Abstract Service; log Kow, n‐octanol–water partition coefficient; pKa, logarithm of the acid dissociation constant.
Experimental log K ow of the non‐ionic form at pH = 3.0 (Kuramochi et al., 2008; USEPA, 2020). Experimental log K ow show lower values, such as 3.2–6.4 at neutral pH (ECB, 2006).
Since TBBPA has two phenol groups, the compound has two pka values.
Neutral.
FIGURE 1.
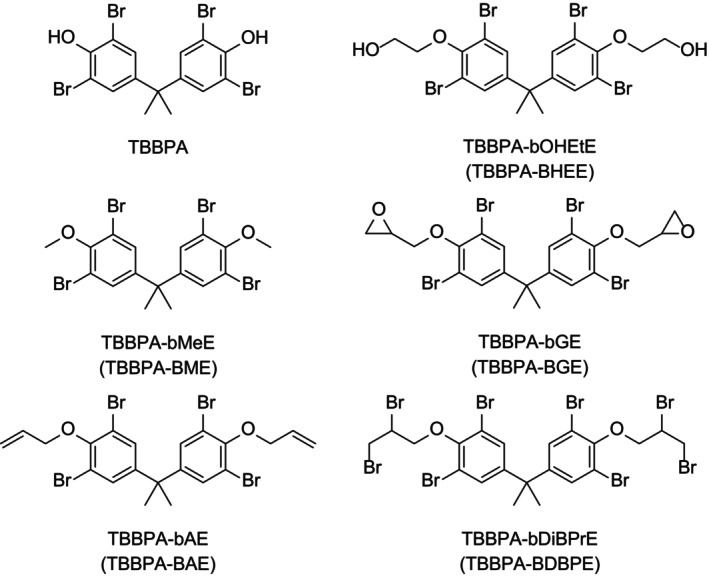
Structure of TBBPA and the derivatives considered in this Opinion.
1.3.2. Production and industrial use
TBBPA is manufactured by bromination of bisphenol A leading to the formation of the tetrabrominated form of bisphenol A. Traces of other isomers of TBBPA and tribromobisphenol A (TriBBPA) may be present in commercial TBBPA (ECB, 2006; EFSA CONTAM Panel, 2011c).
TBBPA and the derivatives included in this Opinion have been used commercially as flame retardants (EFSA CONTAM Panel, 2011c). Each of these derivatives (Figure 1) is produced as an individual chemical, and some derivatives (TBBPA‐bOHEtE and TBBPA‐bGE) are also produced as mixtures with other derivatives which are not included in this Opinion. 2 The purity of the compound is related to the technical quality of the production, and traces of by‐products may be present in the commercial products. When methanol is used as the solvent during production, methyl bromide is formed as a by‐product (EFSA CONTAM Panel, 2011c).
TBBPA is used as both an additive and as a reactive BFR, i.e. both where it is simply mixed with the product and where it is chemically bonded to the plastics where it is used. Reactive BFRs are generally more stable and are not as easily released into the environment as additive BFRs (Guerra et al., 2011).
1.3.3. Environmental levels and fate
TBBPA can enter the environment as a result of releases from production sites but probably more importantly via migration from products, especially where it has been used as an additive flame retardant. The use could be either during normal use of the product or at end of life, e.g. at landfill or e‐waste sites (e.g. Bhattacharya & Khare, 2016; Saha et al., 2021).
The sections below are not a comprehensive review of the literature but rather give an overview of some aspects related to the environmental fate and levels of TBBPA and the TBBPA derivatives covered in the TORs.
1.3.3.1. Biodegradation/transformation
When TBBPA enters the environment, it may undergo degradation under both aerobic and anaerobic conditions to form lower brominated analogues, and eventually be completely dehalogenated to bisphenol A (BPA) (Liu et al., 2019). BPA is used in combination with other chemicals to manufacture certain plastics and resins and was the subject of a recent EFSA Opinion in the context of its potential to migrate from food packaging into food (EFSA CEP Panel, 2023).
Under aerobic experimental conditions, TBBPA may undergo photolysis (Eriksson et al., 2004) and oxidative transformations (Moreira Bastos et al., 2008) to yield the debromination products, dibromohydroquinone, dibromo‐isopropylphenol and to produce brominated alkylphenols. The anaerobic degradation of TBBPA was confirmed by Gerecke et al. (2006). TBBPA is not hydrolysed and does not undergo substitution reactions. It is easily polymerised or co‐polymerised, due to the reactivity of the phenol groups.
Liu et al. (2019) conducted a critical review of transformation and degradation products of TBBPA and its derivatives. It was recognised that it is a challenge to analyse trace levels of both unknown and known transformation and degradation products due to the complexity of environmental matrices and the lack of commercial/artificial standard chemicals. Frequent detection of high levels of TBBPA and its derivatives such as TBBPA‐bAE and TBBPA‐bDiBPrE in water, soil, atmosphere, dust, biological samples, etc., were said to correspond to decomposition products associated with transformation and degradation reactions. The most frequently reported metabolites include lower brominated products and brominated phenol analogues, such as TriBBPA, dibromobisphenol A (DBBPA), monobromobisphenol A (MBBPA) and BPA. This degradation commonly takes place under anaerobic or reductive conditions. Brominated phenol analogues, such as 2,6‐dibromo‐4‐ isopropylphenol, isopropylene‐2,6‐dibromophenol, 2,6‐dibromo‐4‐(propan‐2‐ylidene)cyclohexa‐2,5‐dienone, 4‐(2‐hydroxyisopropyl)‐2,6‐dibromophenol, 4‐(2‐methoxylisopropyl)‐2,6‐dibromophenol, are compounds that are often formed via beta‐scission at the centre C atom under aerobic or strongly oxidative conditions. Additionally, biotransformation processes in algae have been shown to lead to the formation of TBBPA‐sulfate and TBBPA‐glucuronide (Liu et al., 2018, 2019).
Liu et al. (2019) stated that TBBPA derivatives account for 18% of the total commercial TBBPA products, and that the complex BFRs structurally related to TBBPA containing more bromine atoms are likely to be one of the most important sources of TBBPA, BPA and other brominated congeners.
1.3.3.2. Occurrence in the environment
Soil, vegetation and the terrestrial environment
There are very few data in the literature on concentrations of TBBPA in soils, vegetation and the terrestrial environment, although there are a few papers that discuss factors that may influence its adsorption, such as the contribution of various soil components and other properties of the soil (e.g. Han et al., 2013). Most of the available data are from Asia and are associated with known contamination sites. Concentrations of TBBPA in soil rarely exceed a few tens of ng/g in soil (Jin et al., 2006; Peng et al., 2007) although concentrations of up to 7758 ng/g dry weight have been reported in Shouguang, Shandong at a location close to a TBBPA manufacturing site where it may be assumed that there was an impact resulting from industrial releases (Liu, Li, et al., 2016).
TBBPA and BPA in soils from a typical e‐waste recycling area in South China were investigated by Huang et al. (2014), and were found at concentrations up to 220 and 325 ng/g dry weight, respectively. Both compounds increased by the same approximate proportion in the following order: pond sediments < paddy soils = vegetable soils < wasteland < dismantling sites < former open burning sites. BPA concentrations were higher than TBBPA concentrations in all six land‐use soils, and they correlated significantly. TBBPA and BPA were transported through the soil profiles, being found at relatively high concentrations in soil 0–40 cm deep, but only at low concentrations in soil 40–80 cm deep.
Jeon et al. (2021) investigated the spatial distribution, source identification and anthropogenic effects of brominated flame retardants including TBBPA in soils collected from South Korea. The mean concentration was 17.2 ng/g. Industrial sites had statistically higher BFR concentrations when compared to suburban sites but no significant difference when compared with urban sites and were significantly correlated with population density, gross domestic product and the number of companies (p < 0.01), indicating a direct impact of anthropogenic activities.
Sediments
TBBPA has rarely been found in sediments that are taken from sites distant from where they were produced or away from places associated with their disposal.
Viganò et al. (2023) examined the temporal profiles of several organic micropollutants including TBBPA from a sediment core sampled from an industrial tributary of the Po River, the Lambro River in Northern Italy. TBBPA was detected only in three deep sections of the Lambro sediment core and was below the LOQ (< 0.11 ng/g), despite the high production volumes of this chemical (> 100,000 t/year).
Viganò et al. (2015) had previously measured TBBPA and TBBPA‐bDiBPrE in sediments from the same river system in a study on contaminants with endocrine active potentials in sediments and fish. TBBPA had the lowest concentrations of any of the flame retardants measured at 1.2 ng/g dry weight. TBBPA‐bDiBPrE and decabromodiphyenyl ethane (DBDPE) were only detected in the sediment of the River Lambro, with concentrations of 16.3 and 27 ng/g, respectively.
Gil‐Solsona et al. (2022) made an assessment of the sources, prevalence and distribution of BPA and analogues including TBBPA in water, sediments, biota and plastic litter of the Ebro Delta (Spain). The aim was to investigate possible sources of BPA into watercourses from sources such as waste water treatment plants and leachates from plastic litter; no specific industrial use in the region was mentioned. Concentrations of TBBPA ranging from 54.0 to 67.5 ng/g were found in river sediments.
Higher concentrations of tens or hundreds ng TBBPA/g have been determined in sewage sludges or in sediments collected downstream of plastic factories, plants manufacturing flame retardants or e‐waste dismantling plants (Covaci et al., 2009; Feng et al., 2012; He et al., 2013).
Hloušková et al. (2014) reported on various groups of halogenated chemicals, including TBBPA, in 31 sediment samples collected in different localities of the Czech Republic. TBBPA was determined in 36% of samples with concentrations ranging from 3.18 to 17.7 μg/kg dry weight.
Sühring et al. (2015) measured 53 brominated and chlorinated flame retardants in sediment samples from the German rivers Elbe and Weser, the German Bight, Jadebusen, East Frisian Coast as well as the UK East coast with an aim to investigate specific congener patterns that could help identify sources. The Northern UK coastal fingerprint (Mablethorpe to the Scottish border) was dominated by TBBPA with 56% contribution to total flame retardant content, whereas TBBPA contributed only 25% to the Southern UK coast fingerprint (Lowestoft and South). Concentrations were a few thousand pg/g in the samples from the UK coastal areas but ranged from < LOD to a few hundred pg/g in samples from the other areas.
TBBPA was found in all but one sample of sediment taken from the river Thames in London, with a maximum concentration of 2.6 μg/kg dry weight and an average of 0.6 μg/kg dry weight (Ganci et al., 2019). Sediments from locations within the industrial area of London had significantly higher concentrations of many BFRs, including TBBPA when compared to more rural locations.
Sediment samples from other global regions including North America and Asia showed concentrations of TBBPA of a similar order of magnitude with higher levels found in industrial areas and close to production sites and other sources when compared to more rural areas (Feng et al., 2012; Hu et al., 2019; Li et al., 2016; Lu et al., 2015; Pan et al., 2022).
Aquatic environment
A review of historical aquatic toxicity and bioconcentration data for TBBPA and its effects on fish, invertebrates, algae and microbial communities was reported by Pittinger and Pecquet (2018). Molluscan shell growth was found to be uniquely sensitive to TBBPA, in particular it was more sensitive than chronic fish or crustacean toxicity endpoints. The review detailed historical studies that had not been previously published on TBBPA that were pivotal in regulatory assessments by the European Union, Canada and the USA and compared these with more recent research. The review did not report environmental levels, but did collate bioconcentration factors that have been reported, for example, the review stated that bioconcentration factors calculated by the EU (assuming 87 and 79% metabolite contributions to 14C tissue residues) were 156 for the fathead minnow, and 148 in oysters.
Gil‐Solsona et al. (2022) reported data for BPA analogues including TBBPA in samples of fish from the delta of the river Ebro (Spain). TBBPA concentrations in fish plasma had a median value of 28.3 ng/mL (range < LOD–44.8 ng/mL; 35% detected), for fish liver TBBPA was detected in 26% of samples but always < LOQ, and TBBPA was found in fish muscle at concentrations with a median value of 1.45 ng/mL (range < LOD–5.9; 30% detected).
Viganò et al. (2015) reported concentrations of contaminants with endocrine active potential in sediments and fish from the River Po (Italy). No evidence of contamination by TBBPA‐bDiBPrE was found. Trichlorobisphenol A (TCBPA) and TBBPA were found in slightly higher contents in bream (Abramis brama) (up to about 15 ng/g fat), but only low levels were found in Common carp (Cyprinus carpio), sanders (Sander lucioperca) and sheatfishes (Silurus glanis), with no apparent relationships with fish age or niche.
Gu et al. (2017) reported TBBPA concentrations of ND–158 ng/g fat in bivalves in a study on the contamination status of many endocrine‐disrupting compounds in the River Po and its tributary, the River Lambro (Italy).
Choo et al. (2019) found that TBBPA concentrations in fish from the southern part of the Republic of Korea were generally significantly higher in pelagic fish (1.31–11.35 ng/g fat) than in demersal fish (ND–4.45 ng/g fat) and benthic invertebrates (ND–8.11 ng/g fat), reflecting a dependence on habitat.
Yang et al. (2012) examined the distribution of TBBPA in fish tissues, and the impact of seasonal variation in water and sediment of Lake Chaohu (China). Tissue distributions of TBBPA in four fish species were similar, and the mean concentrations in the fish were in the range of 28.5–39.4 ng/g, which is much higher than those reported in Japan, Europe and USA. The source was from industrial sites that contaminated the lake water.
Air
Vasiljevic and Harner (2021) conducted a review on BPA and its analogues in outdoor and indoor air, and found that microbial metabolism of TBBPA under anaerobic conditions in soil and sediment can sequentially degrade TBBPA to TriBBPA to DBBPA and on to form MBBPA and eventually BPA. Because BPA has a greater tendency to partition to soil and sediment, it was concluded that it was unlikely that BPA degraded from TBBPA is a major emission source for atmospheric BPA.
de Wit et al. (2010) reported that indoor air concentrations of TBBPA and its derivatives are generally higher than outdoor air concentrations, indicating that there are emissions from flame‐retarded products. Their presence in outdoor air and in the Arctic was said to indicate that these compounds are capable of long‐range atmospheric transport.
In a review by Liu, Li, et al. (2016), it was found that the most serious case of TBBPA air pollution in China was in Guiyu, Guangdong, at a primitive e‐waste dismantling site where concentrations of TBBPA were as high as 66,010–95,040 pg/m3.
In a study on a global atmospheric passive sampling network (GAPS‐Megacities), Saini et al. (2020) reported on a range of organic pollutants in the air of 20 megacities/major cities across the globe. The goal was to gain a better understanding and comparison of ambient air levels of persistent organic pollutants and other chemicals of emerging concern. TBBPA was detected in 79% of samples with concentrations ranging from 0.54 to 118 pg/m3. Tokyo (Japan) had the highest concentration of TBBPA (118 pg/m3) followed by New Delhi (India, 41.0 pg/m3). The concentrations at other sites were less than 30 pg/m3.
Dust
Dust can be an important source of exposure to BFRs (EFSA CONTAM Panel, 2024; Kefeni et al., 2011) due to their use in furniture and domestic and office appliances. It can be especially important for infants and toddlers because of their behaviour with respect to crawling and also due to high hand to mouth contact.
Since the previous Opinion, several studies have reported the concentrations of TBBPA in dust from European countries. Only one study was identified reporting levels of the TBBPA derivative TBBPA‐bDiBPrE. These studies are summarised in Table 2, including data on the estimate of exposure via dust if reported by the authors (see Section 3.3.3). Data from non‐European countries are reported in Appendix A, A1).
TABLE 2.
Concentrations of TBBPA and TBBPA‐bDiBPrE in dust samples from European countries, and estimate of dust exposure when reported by the authors.
| Country sampling year | Number of samples | Concentration (ng/g) | Exposure estimate | Reference |
|---|---|---|---|---|
| TBBPA | ||||
| Germany NR | N = 24 homes | Median: 48 | NR | Abb et al. (2011) |
| Germany NR | N = 5 houses | Range: 2.9–232.8 | NR | Kopp et al. (2012) |
| Germany NR | N = 20 houses | Median, Mean (range): 28.0, 44.1 (2.96–233) |
Intake based on average dust intake and median concentrations in dust: Adults: 0.012 ng/kg bw per day Toddlers: 0.140 ng/kg bw per day Intake based on average dust intake and P95 concentrations in dust: Adults: 0.045 ng/kg bw per day Toddlers: 0.525 ng/kg bw per day |
Fromme et al. (2014) a |
| France 2008 |
N = 9 homes N = 11 offices N = 7 cars |
Median (range): Homes: 44 (7–165) Offices: 79 (32–1155) Cars: 47 (9–66) |
Intake based on average dust intake and median concentrations in dust: Adults: 2.2 ng per day Toddlers: 5.6 ng per day Intake based on high dust ingestion and P95 concentrations in dust: Adults: 16.8 ng per day Toddlers: 67.3 ng per day |
Abdallah et al. (2016) b |
| Italy 2019 |
N = 3 homes N = 2 workplaces |
Median: Homes: < 124.1 Workplaces: 32.32 |
NR | Simonetti et al. (2020) |
| Greece 1997–2015 | N = 25 cars |
Mean (range): 44.8 (< 10–1064) |
Intake based on average dust intake and median concentrations in dust: Adults = 0.00063 ng/kg bw per day Toddlers = 0.00441 ng/kg bw per day |
Besis et al. (2017) c |
| Norway 2013–2014 | N = 60 settled dust |
Median (range): 62 (< 0.92–10,000) |
Intake based on average dust intake and median concentrations in dust: 28 pg/kg bw per day |
Tay et al. (2017) d |
| Czech Republic 2013 | N = 18 houses |
Range: < 2.5–269 Only 2 samples > LOQ |
NR | Lankova et al. (2015) |
| UK 2009 | N = 14 cars (trunk and cabin) |
Median: Trunk: < 0.2 Cabin: 4.5 |
Intake based on average dust intake and median concentrations in dust: Adults: 4.4 ng per day Toddlers: 3.8 ng per day |
Harrad and Abdallah (2011) e |
| TBBPA‐bDiBPrE | ||||
| UK 2013–2015 |
N = 30 homes N = 42 offices |
Median (range): Homes: 1000 (71–49,000) Offices: 2300 (310–14,000) |
Intake based on average dust intake and median concentrations in dust: Adults: 15 ng per day Toddlers: 23 ng per day |
Tao et al. (2016) f |
Average daily intake of house dust assumed to be 30 mg for adults and 60 mg for toddlers. Body weight of toddlers assumed to be 12 kg, and that the adults 70 kg (Fromme et al., 2014).
Average daily intake of house dust assumed to be 20 mg for adults and 50 mg for toddlers. High dust ingestion assumed to be 50 mg for adults and 200 mg for toddlers (Abdallah et al., 2016).
Average daily ingestion of dust assumed to be 50 mg per day for adults and 60 mg per day for toddlers. Body weight of toddler assumed to be 12 kg and that the adults 70 kg (Besis et al., 2017).
Average daily ingestion of dust assumed to be 30 mg per day for adults (Tay et al., 2017).
Daily ingestion of dust assumed to be 20 mg per day for adults and 50 mg for toddlers. Assuming 4.1% of time spent in vehicle (Harrad & Abdallah, 2011).
Daily ingestion of dust assumed to be 20 mg per day for adults and 50 mg for toddlers (Tao et al., 2016).
Concentrations were typically found in the range from below 10 ng/g up to around 1000 ng/g although in one study from Norway a maximum concentration of 10,000 ng/g was found (Tay et al., 2017). The single study reporting TBBPA‐bDiBPrE in dust at concentrations up to 49,000 ng/g (Tao et al., 2016).
Wildlife
There is very little information in the literature on the presence of TBBPA and its derivatives in wildlife. Some of the data that have been published since the previous Opinion (EFSA CONTAM Panel, 2011c) are summarised below.
In a study conducted in Belgium to investigate legacy and current‐use brominated flame retardants in the Barn Owl (Tyto alba), Eulaers et al. (2014) found that, in contrast to PBDEs, TBBPA bioaccumulated poorly (2.3%) in tissues, but was present in 96% of all body feather samples (0.36–7.07 ng/g dw).
Reindl and Falkowska (2015) reported concentrations of TBBPA and other BFRs in African penguins (Spheniscus demersus) from Gdansk Zoo and in their sole food, Baltic herring (Clupea harengus), from Gdansk Bay, Poland. The average concentration of TBBPA in whole herring was approximately 2.3 ± 1.3 ng/g lipid weight. The highest concentration of TBBPA in the birds was 14.8 ng/g lipid and was found in the brain of an adult penguin. The strongest accumulation factor for BFRs was also established for brain tissue, but it showed stronger magnification in muscle than in liver. TBBPA was found in penguin guano and eggs, with a BFR content in yolk approximately 10 times greater than in albumen due to the lipophilic property of these compounds.
TBBPA was analysed in 12 tissues of prey (mud carp) and predator (northern snakehead) fish from an e‐waste area, South China (Tang et al., 2015). The TBBPA concentrations in different tissues ranged from 0.03 to 2.85 ng/g ww in mud carp and 0.04 to 1.30 ng/g ww in northern snakehead.
Tissue distributions of TBBPA in four fish species from Southern China were found to be similar in a study by Yang et al. (2012) where fish from Lake Chaohu (China) were analysed. The mean concentrations in the fish were in the range of 28.5–39.4 ng/g dry weight, which was said to be much higher than those reported in Japan, Europe and the USA. The maximum concentrations of TBBPA were found in kidneys, where TBBPA concentration was positively correlated with fish size of Cyprinus carpio.
The occurrence of TBBPA‐BDBPE is generally unknown in wildlife. A highly sensitive, gas chromatography–mass spectrometry‐based method was developed by Gauthier et al. (2019) to report on temporal and spatial trends in pooled samples of herring gull eggs and individuals from 14 colony sites across the Laurentian Great Lakes of North America. TBBPA‐BDBPE was quantifiable in 95% of egg pools from all colonies sampled in 2013–2017, and retrospective analysis of archived eggs (2001–2017) at three of the 14 colonies indicated that TBBPA‐BDBPE concentrations were greater in pools from eggs collected in more recent years (< LOD to 42.8 ng/g wet weight). Selected colonies with known dietary differences (i.e. terrestrial vs. aquatic) indicated that TBBPA‐BDBPE exposure was associated with terrestrial origin.
While toxicokinetic studies show that the elimination half‐lives of TBBPA are considerably lower than for other POPs (2–3 days in humans; see Section 3.1.1.2.5), it nevertheless bioaccumulates to some extent and is found in fatty tissues especially at higher trophic levels of the food chain. TBBPA is not on the list of persistent organic pollutants (POPs) under the Stockholm Convention (www.pops.int), although it meets many of the criteria for being classified as a POP.
1.3.4. Sampling and methods of analysis
There has been little significant development in methods of sampling and analysis for TBBPA in foods since the last EFSA Opinion (EFSA CONTAM Panel, 2011c). There are no specific guidelines for the sampling, and so basic rules for sampling of organic contaminants or pesticides should be followed. Methods using mass spectrometry (MS) as a detection technique are generally considered most suitable for generating occurrence data for official control and for use in risk assessment.
Extraction of solid materials is often carried out by Soxhlet or pressurised liquid extraction (PLE). Column extraction after blending with mixtures of organic solvents (e.g. hexane‐acetone or hexane‐dichloromethane) are often used for extraction. For liquids (milk, blood), liquid–liquid extraction and solid‐phase extraction (SPE) are often used. TBBPA has pKa values of 7.5 and 8.5, meaning that the pH should be carefully controlled in order to avoid losses of TBBPA in the analytical procedure (EFSA CONTAM Panel, 2011c).
Clean‐up methods include gel permeation chromatography, use of neutral or acidified silica or sulfuric acid treatment followed by fractionation, which may be needed to isolate TBBPA from other pollutants (such as PBDEs and HBCDDs) and potentially interfering compounds. This is typically done by silica column fractionation or reversed‐phase fractionation (EFSA CONTAM Panel, 2011c).
TBBPA is analysed mostly by liquid chromatography‐mass spectrometry (LC–MS) methods, although analysis by gas chromatography‐MS (GC–MS) is also used. The analysis of volatile derivatives such as TBBPA‐bDiBPrE and TBBPA‐bAE is primarily done by GC–MS methods, sometimes using derivatisation of the hydroxyl groups within the molecule (Covaci et al., 2009; EFSA CONTAM Panel, 2011c).
Cunha et al. (2017) and Li et al. (2017) reported on using the Quick, Easy, Cheap, Effective, Rugged and Safe (QuEChERS)‐based extraction method for TBBPA in seafood including fish, bivalves and seaweeds. This is a quick and simple method with multiple applications that is very commonly used for pesticides, mycotoxins and several other classes of residues and contaminants.
Okeke et al. (2022) conducted a recent review of analytical methods for TBBPA and its derivatives in a variety of environmental and biological samples including food. TBBPA‐bOHEtE, 3 TBBPA brominated epoxy oligomer, TBBPA‐bDiBPrE, 4 TBBPA‐bAE and TBBPA carbonate oligomers were discussed, but the review focussed on the three main derivatives TBBPA‐bOHEtE, TBBPA‐bAE and TBBPA‐bDiBPrE. The review identified recent improvements in methodology using atmospheric pressure chemical ionisation–mass spectrometry (APCI‐MS) (Yu et al., 2011), and atmospheric pressure photoionisation‐mass‐spectrometry (APPI‐MS) (Wang et al., 2012) for the accurate detection of TBBPA derivatives (Letcher & Chu, 2010). The APCI‐MS method recorded the best sensitivity when compared to other methods with an absolute detection limit of 12–112 pg for three derivatives of TBBPA.
Methods using a real‐time immuno‐PCR assay (Bu et al., 2015) and a method using molecularly imprinted polymer‐capped wrinkled silica‐quantum dot hybrid particles for fluorescent determination of TBBPA (Chao et al., 2021) have been reported, but not widely used for the determination of TBBPA in biological samples and not used in food control.
The review by Abdallah (2016) on environmental occurrence, analysis and human exposure to TBBPA describes the use of five TBBPA derivatives (i.e. tetrabromobisphenol‐A dibromopropyl ether, TBBPA‐bAE, TBBPA‐bOHEtE, TBBPA brominated epoxy oligomer and TBBPA carbonate oligomer), but does not include any discussion of analytical methods or occurrence data of these compounds, probably due to lack of availability.
Hajeb et al. (2022) made a critical review of analytical methods for the determination of flame retardants in human matrices on an extensive range of halogenated flame retardants, but reported very little information on derivatives of TBBPA.
A review that focussed on the analysis of TBBPA/TBBPS, TBBPA/TBBPS derivatives and their transformation products by Qu et al. (2016), gave probably the most in depth information on TBBPA derivatives. It was shown that for environmental matrices, LC–MS is most commonly used, although GC–MS can be used for some of the compounds. While the focus of the review was on inorganic environmental samples including waters (e.g. river water, waste water, tap water), air, soil, sediment and sludge, it did cover methods for the analysis of bird eggs which can be analysed by LC–MS methods and plants which can be analysed using GC–MS methodology.
Zhang, Dong, et al. (2018) produced a polyclonal antibody capable of recognising TBBPA‐bOHEtE 5 and tetrabromobisphenol A mono(hydroxyethyl) ether (TBBPA‐OHEtE6) (cross‐reactivity, 100% for TBBPA‐bOHEtE; 98.7% for TBBPA‐OHEtE), a derivative and by‐product of TBBPA, respectively. The antibody was used to develop a novel ultrasensitive competitive immunosensor using an electrochemical impedimetric strategy for the simultaneous detection of both chemicals. While the method was suitable for environmental water samples, it was not used for food or biological samples.
The EURL for Halogenated POPs in Feed and Food published in 2022 a guidance on the essential analytical parameters to be used for organobromine contaminant analysis in food and feed intended for laboratories involved in the official control of these contaminants in food and feed. 6 While the focus of this document is on PBDEs and HBCDDs, many of the parameters described are applicable to TBBPA.
Proficiency testing results do not indicate any specific difficulties with the analysis of TBBPA and results are in line with those obtained for exercises conducted with other POPs. 7 Dvorakova et al. (2021) investigated the analytical comparability and accuracy of laboratories analysing TBBPA and other flame retardants in serum and urine by a quality assurance and control (QA/QC) scheme comprising interlaboratory comparison investigations and external quality assurance schemes performed as part of the European Human Biomonitoring Initiative (HBM4EU). The small number of participants made it not possible to formally evaluate the results for TBBPA.
The EURL for Halogenated POPs in Feed and Food also conducts proficiency testing exercises aimed at the network of National Reference laboratories and official control labs within Europe (https://eurl‐pops.eu), but these exercises include only TBBPA and not derivatives (e.g. the EURL Interlaboratory Study on the Determination of Brominated Contaminants and PCNs in Cod Liver Oil 2021 included TBBPA 8 ).
1.3.5. Previous assessments
In 2011, the EFSA CONTAM Panel published its first risk assessment on TBBPA and its derivatives in food (EFSA CONTAM Panel, 2011c). The Panel identified at that time thyroid hormone homeostasis as the main target for TBBPA toxicity and identified a reference point (lower confidence limit for a benchmark response of 10%, BMDL10) of 16 mg/kg bw per day for a 10% decrease in circulating T4 (van der Ven et al., 2008, BMDL as reported by the authors). Due to limitations and uncertainties in the database, the CONTAM Panel did not find it appropriate to establish a health‐based guidance value (HBGV) for TBBPA and instead used a margin of exposure (MOE) approach for the risk characterisation by comparing the BMDL10 with the dietary exposure based on TBBPA levels in food submitted to EFSA at that time.
At the time of the previous Opinion, a total of 652 food samples on TBBPA covering the period from 2003 to 2010 were submitted to EFSA. All analytical results were left‐censored 9 (< LOQ, i.e. in general ≤ 1 ng/g wet weight). Thus, a meaningful exposure assessment for the general population was not possible. In order to provide some indication of whether there could be a possible health concern with respect to dietary exposure to TBBPA, a worst‐case intake estimate for two specific groups of the population was done: adult high fish consumers and high cow's milk consumers (toddlers). The highest LOQs reported for those food categories of 1 and 0.65 ng/g wet weight were used for the exposure estimates, resulting in ‘upper bound’ intake estimates of 2.6 and 55.7 ng/kg bw per day, respectively.
Comparison of the ‘upper bound’ dietary exposure estimate with the BMDL10 of 16 mg/kg bw resulted in MOEs of 6 × 106 and of 3 × 105 for adult high fish consumers and for high cow's milk consumers (i.e. toddlers), respectively. The Panel noted that usually an MOE of 100 is sufficient to cover uncertainties and variability with respect to kinetic and dynamic differences between animal species and humans and within the human population, and to conclude that there is no health concern. In the case of TBBPA, the CONTAM Panel noted that an additional factor would be needed to cover deficiencies in the toxicological database. The Panel considered that the MOEs were sufficiently large and concluded that the dietary exposure to TBBPA for these specific population groups with potential high exposure did not raise a health concern.
Since then, several bodies have performed risk/hazard assessments for TBBPA.
In Canada, in 2013, Environment and Health Canada released a Screening Assessment Report for TBBPA and two derivatives (TBBPA‐bOHEtE and TBBPA‐bAE) (Health Canada, 2013). Estimated exposure to TBBPA and the two derivatives was calculated based on the TBBPA UB occurrence levels for breastfed infants (1.95 × 10−4 mg/kg bw per day). Environment and Health Canada considered that overestimation of the TBBPA intake levels (based on TBBPA UB occurrence levels) used in the exposure assessment is expected to compensate for the contribution of additional levels from the other two derivatives. For the risk characterisation, two reference points were considered from studies conducted with TBBPA, i.e. a LOAEL of 140.5 mg/kg bw per day, based on liver toxicity in female offspring identified in a reproductive study in mice (Tada et al., 2006), and a NOAEL of 40 mg/kg bw per day for effects on the kidney from a developmental toxicity study in new‐born rats (Fukuda et al., 2004). The critical effects observed in studies conducted with TBBPA were considered representative of potential hazard from TBBPA and two other derivatives on the basis of structural similarity of the derivatives with TBBPA. The MOE values calculated (MOE of 7.2 × 105 based on the LOAEL for liver toxicity and MOE of 2.1 × 105 based on the NOAEL for kidney toxicity) were considered adequate to cover uncertainties in the toxicological and exposure databases for TBBPA and two derivatives. Based on the information presented in the report, Environment and Health Canada concluded that exposure of humans to TBBPA and two derivatives is not expected to ‘constitute a danger’ for humans in Canada.
In 2017, the French Agency for Food, Environmental and Occupational Health and Safety (ANSES) published a three‐volume report on the current state of knowledge regarding uses, exposure sources and toxicity for several polybrominated compounds (ANSES, 2017a, 2017b, 2017c). Occurrence levels of TBBPA in food for the French population were only available for non‐breastfed children between ages 0 and 3 years via the infant Total Dietary Study (ANSES, 2016). ANSES concluded that dietary exposure to TBBPA in infants is considered ‘tolerable’ (90th percentile daily exposure for the UB ranged from 1.80 ng/kg bw per day for children aged 13–36 months to 31.3 ng/kg bw per day for children aged 1–4 months). However, it is not clear how this conclusion was reached. Nevertheless, ANSES highlighted the evidence of carcinogenicity for TBBPA based on experimental data in animals and concluded that, among other polybrominated compounds, TBBPA warrants a health risk assessment.
In 2018, the International Agency for Research on Cancer (IARC) published a monograph on the evaluation of the carcinogenicity of several industrial chemicals, including TBBPA (IARC, 2018). Overall, TBBPA was classified as probably carcinogenic to humans (Group 2A) by IARC. The majority of the IARC Working Group supported the classification of TBBPA as Group 2A based on the sufficient evidence in experimental animals for the carcinogenicity of TBBPA (2‐year studies in rodents, incidence of tumours reported in several organs, NTP, 2014) and strong mechanistic evidence. In particular, the key mechanistic characteristics of carcinogenicity of TBBPA highlighted in the monograph which are also reported in humans were modulation of receptor‐mediated effects (direct interaction with human nuclear receptors relevant to human cancers including thyroid hormone and peroxisome proliferator‐activated receptor‐γ, modulation of enzymes relevant for the endocrine system, inhibition of aromatase and sulfotransferase), induction of oxidative stress and immunosuppression. A minority of the IARC Working Group considered the mechanistic evidence insufficient for the classification of TBBPA as Group 2A.
The UK Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) published a statement on toxicological data of TBBPA (COT, 2004), recommending a tolerable daily intake (TDI) of 1 mg/kg bw per day. This statement was reported in the previous EFSA Opinion; therefore, no details on the derivation of the TDI have been added in this paragraph. In 2020, the COT published an addendum to the overarching statement on the potential risks from contaminants diet of infants and young children (COT, 2020). The COT considered previous risk assessments of TBBPA including the previous Opinion from EFSA (EFSA CONTAM Panel, 2011c). For the characterisation of the risk, in the light of the previous Opinion from EFSA, the COT followed the MOE approach, using as the reference point the BMDL10 of 16 mg/kg bw per day and the estimated chronic dietary exposures to TBBPA in the UK population. The COT noted that the MOEs were all greater than 1000,000. The COT noted the National Toxicology Program (NTP) report on the toxicology and long‐term carcinogenicity studies of TBBPA (NTP, 2014) and concluded that the available scientific data indicated that the carcinogenicity of TBBPA is not mediated through a genotoxic mechanism and an MOE of 100 was considered to be sufficiently protective for human health. Therefore, the UK MOE values were considered not to be a cause for concern.
In Australia, in 2005, TBBPA was classified as a Priority Existing Chemical (PEC) under the Industrial Chemicals (Notification and Assessment) Act 1989 (the ICNA Act). In May 2020, the Australian Department of Health (Office of Chemical Safety) published the Priority Existing Chemical Assessment Report on TBBPA in accordance with the ICNA Act (NICNAS, 2020). Based on this assessment, TBBPA was classified according to the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) as a Category 2 carcinogen, with the Health Hazard statement ‘Suspected of causing cancer – H351’. A NOAEL of 42 mg/kg bw per day was identified from a postnatal developmental study in ICR mice (Tada et al., 2006) based on histopathological changes observed in liver and kidneys and used for the risk assessment. Public (non‐occupational) exposure to TBBPA was estimated using a ‘reasonable worst‐case’ approach and combining indoor, outdoor exposure, exposure from food and human milk for the different population groups (31.81 ng/kg bw per day for infants, 16.17 ng/kg bw per day for toddlers, 4.98 ng/kg bw per day for children and 2.05 ng/kg bw per day for adults). Public exposure estimates were considered to be of very low levels under normal conditions of consumer use. These exposure estimates as compared to the identified NOAEL resulted in MOEs of > 1000 for all population groups. Therefore, in its report, the Australian Department of Health concluded that the risk of exposure to TBBPA in levels that would lead to health effects is very low for all population groups.
The ECHA's Committee for Risk Assessment (RAC) adopted its opinion on TBBPA 10 in September 2021 based on the proposal submitted in September 2020, by the Norwegian and Danish Competent authorities for harmonised classification and labelling (CLH) of TBBPA 11 as carcinogenic. Specifically, the Norwegian and Danish Competent authorities, based on recent scientific data and largely on findings reported by NTP (2014), suggested to add the classification of TBBPA as carcinogenic, category 1B, which applies to ‘presumed human carcinogens’ and RAC concluded positively by consensus. RAC evaluated several human health hazards that were assessed by the Norwegian and Danish authorities, i.e. specific target organ toxicity after repeated exposure to the substance, germ cell mutagenicity, carcinogenicity and reproductive toxicity of TBBPA, based on data included in the REACH registration dossiers, a study on TBBPA from the NTP (2014) and literature data until early 2020. RAC concluded in agreement with the proposal that classification of TBBPA for hazard classes other than carcinogenicity are not warranted.
In the United States, TBBPA is identified as a high priority chemical to undergo risk evaluation under the Toxic Substances Control Act (TSCA) by the United States Environmental Protection Agency (US‐EPA) since December 2019. In August 2020, US‐EPA released a scope document providing information on the hazards, exposures, conditions of use and the potentially exposed or susceptible subpopulations the agency foresees to consider in its risk evaluation, which is currently ongoing. 12
1.3.6. Legislation
In this Opinion, where reference is made to European legislation (Regulations, Directives, Recommendations, Decisions), the reference should be understood as relating to the most recent amendment at the time of publication of this Opinion, unless otherwise stated.
In order to protect public health, Article 2 of Council Regulation (EEC) No 315/93 13 of 8 February 1993 laying down Community procedures for contaminants in food stipulates that, where necessary, maximum tolerances for specific contaminants shall be established. A number of maximum levels (MLs) are currently laid down in Commission Regulation (EU) 2023/915 of 25 April 2023 that repeals Commission Regulation (EC) No. 1881/2006. 14 TBBPA and the TBBPA derivatives considered in this Opinion are not regulated under this Regulation or under any other specific European Union (EU) regulation for food.
Council Directive 2002/32/EC regulates undesirable substances in animal feed. TBBPA and the derivatives considered in this Opinion are not regulated under this Directive or any other specific EU regulation for feed.
TBBPA is registered under Regulation (EC) No 1907/2006 15 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH Regulation), and is manufactured in and/or imported to the European Economic Area, at 10,000–100,000 tonnes per annum. It is included in the Community Rolling Action Plan (CoRAP) currently under assessment (by the Danish Competent authority) as persistent, bioaccumulative and toxic (PBT) or very persistent and very bioaccumulative (vPvB) and as endocrine disruptors (ED). TBBPA has a harmonised classification as Aquatic Acute 1 and Aquatic Chronic 1 under Regulation (EC) No 1272/2008.
Moreover, as of January 2023, TBBPA is identified as a substance of very high concern (SVHC) 16 meeting the criteria of the Article 57 (a) of the Regulation (EC) No 1907/2006 (REACH), owing to its classification in the hazard class carcinogenicity, category 1B.
As regards the TBBPA derivatives listed in the ToR, TBBPA‐bMeE and TBBPA‐bOHEtE are not registered under REACH, whereas TBBPA‐bAE and TBBPA‐bGE are registered. TBBPA‐bDiBPrE is registered under REACH and it has been assessed under substance evaluation. In November 2012, Germany after evaluation of TBBPA‐bDiBPrE concluded that no‐regulatory follow‐up actions are needed. 17 Moreover, other TBBPA derivatives and mixtures thereof are registered under REACH Regulation.
In December 2021, ECHA published an assessment of regulatory needs for TBBPA and its derivatives 18 suggesting further restriction of TBBPA and all its derivatives in this group. 19 In March 2023, ECHA released its regulatory strategy for flame retardants, 20 in which the need to minimise release of aromatic brominated flame retardants is highlighted. The strategy suggests a wide and generic restriction for all aromatic brominated flame retardants, including TBBPA and its derivatives, that are confirmed or will be confirmed to be PBT/vPvB through harmonised classification or identification as SVHCs.
Currently, TBBPA is not listed under the restricted substances included in Annex II of the Directive 2011/65/EU of the European Parliament and of the Council of 8 June 2011 on the restriction of the use of certain hazardous substances in electrical and electronic equipment (recast). However, European Commission is considering adding TBBPA to the list of restricted substances, following a proposal by the Expert Group for RoHS 2 adaptation and enforcement. 21
With Commission Recommendation 2014/118/EU, the European Commission recommended that Member States should perform monitoring on the presence of BFRs in food. Besides various other BFRs, the Recommendation included TBBPA and some derivatives: TBBPA‐bMeE (CAS No 70156‐79‐5), TBBPA‐bOHEtE (CAS No 4162‐45‐2), TBBPA‐bAE (CAS No 25327‐89‐3), TBBPA‐bGE (CAS No 3072‐84‐2) and TBBPA‐bDiBPrE (CAS No 21850‐44‐2). The aim of the monitoring was to include a wide variety of individual foodstuffs reflecting consumption habits to give an accurate estimation of exposure. Regarding TBBPA and the derivatives listed, it was recommended to analyse fish and other seafood, meat and meat products, milk and dairy products and eggs and egg products, using analytical methods with an LOQ of 0.1 ng/g wet weight or lower.
2. DATA AND METHODOLOGIES
The current updates of the EFSA risk assessments on BFRs, including this one on TBBPA and TBBPA derivatives, were developed applying a structured methodological approach, which involved developing a priori the protocol or strategy of the full risk assessments and performing each step of the risk assessment in line with the strategy and documenting the process. The protocol in Annex A of this Opinion contains the method that was used for all the steps of the risk assessment process, including any subsequent refinements/changes made.
The CONTAM Panel used its previous risk assessment on TBBPA and its derivatives in food (EFSA CONTAM Panel, 2011c) as a starting point for drafting the current Opinion.
2.1. Supporting information for the assessment
Information on physicochemical properties, production and industrial use, environmental fate and levels, analytical methods, previous assessments and legislation was gathered from the previous EFSA Opinion on TBBPA and its derivatives (EFSA CONTAM Panel, 2011c), assessment by international bodies (by checking the original websites of the relevant organisations), and from current EU legislation. Literature searches were conducted to identify new information in reviews and other peer‐reviewed publications. Details about the literature searches are given in Appendix B. The information was summarised in a narrative way based on expert knowledge and judgement.
The draft scientific Opinion underwent a public consultation from 26 March 2024 to 7 May 2024. The comments received were taken into account when finalising the scientific Opinion and are presented and addressed in Annex E.
2.2. Hazard identification and characterisation
Information relevant for the sections under hazard identification and characterisation was identified by an outsourced literature search. EFSA outsourced a call for ‘Identifying and collecting relevant literature related to the toxicity of polybrominated diphenyl ethers (PBDEs), Tetrabromobisphenol A (TBBPA) and brominated phenols’. The call was launched as a reopening competition for a specific contract under multiple framework contract CT/EFSA/AMU/2014/01 Lot 2. The technical University of Denmark (DTU) was awarded the contract, and a final project report was delivered in October 2019. The aim of the assignment was to identify and collect all relevant literature related to the toxicity of TBBPA and its derivatives (as well as PBDEs and Brominated Phenols) to support the preparatory work for the hazard identification and characterisation steps in the human health risk assessment of these substances. Literature searches were designed and performed to retrieve all potentially relevant studies within the following four areas: Area 1: Data on toxicokinetics in experimental animals and humans and from in vitro studies, Area 2: Data on toxicity in experimental animals, Area 3: Data on in vitro and in vivo genotoxicity and mode of action and Area 4: Data on observations in humans (including epidemiological studies, case reports, biomarkers of exposure). Details of the methodology and the results are reported in Bredsdorff et al. (2023).
Additional literature searches to identify studies published since October 2019 were made as reported in Appendix B.
The selection of the scientific papers for inclusion or exclusion was based on consideration of the extent to which the study was relevant to the assessment or on general study quality considerations (e.g. sufficient details on the methodology, performance and outcome of the study, on dosing, substance studied and route of administration and on statistical description of the results), irrespective of the results. Limitations in the information used are documented in this Scientific Opinion.
Benchmark dose (BMD) analysis was carried out according to the latest EFSA guidance (EFSA Scientific Committee, 2022). To perform the BMD modelling, EFSA used the Bayesian BMD Modelling web‐app (https://zenodo.org/record/7334435#.Y5osYXbMLD4) available at the EFSA R4EU platform (https://efsa.openanalytics.eu/). All analyses were performed using Bridge sampling because of the higher level of accuracy with respect to Laplace approximation set as default (EFSA Scientific Committee, 2022; Hoeting et al., 1999; Morales et al., 2006).
2.3. Occurrence data submitted to EFSA
2.3.1. Data collection
Following a mandate from the European Commission to EFSA, a call for annual collection of chemical contaminant occurrence data in food was issued by the former EFSA Dietary and Chemical Monitoring Unit (now iDATA Unit) in December 2010. Since then, data have been submitted every year by a deadline agreed with the EFSA Scientific Network on Chemical Monitoring Data collection. 22
The data submission to EFSA follows the requirements of the EFSA Guidance on Standard Sample Description for Food and Feed (EFSA, 2010a) and the EFSA Guidance on Standard Sample Description 2 (EFSA, 2013). Occurrence data are managed following the EFSA standard operational procedures (SOPs) on ‘Data collection and validation’ and on ‘Data analysis of food consumption and occurrence data’.
By the 1st of December 2022, a total of 2958 analytical results were available for TBBPA (n = 2213), TBBPA‐bDiBPrE (n = 366) and TBBPA‐bME (n = 379) in the EFSA database between year 2011 and 2021. No data were available for other TBBPA derivatives.
2.3.2. Data validation and analysis
Following EFSA's Technical report on handling of occurrence data for dietary exposure assessment (EFSA, 2021) to guarantee an appropriate quality of the data used in the exposure assessment, the initial data set was carefully evaluated by applying several data cleaning and validation steps. Special attention was paid to the identification of duplicates and to the accuracy of different parameters, such as ‘Sampling strategy’, ‘Sampling year’, ‘Sampling country’, ‘Analytical methods’, ‘Result express’ (expression of results, e.g. fat weight), ‘Reporting unit’, ‘Limit of detection/quantification’ and the codification of analytical results under FoodEx classification (EFSA, 2011a, 2011b, 2015).
Left‐censored data were treated using the substitution method as recommended in the ‘Principles and Methods for the Risk Assessment of Chemicals in Food’ (WHO/IPCS, 2009). This is the same method as indicated in the EFSA scientific report ‘Management of left‐censored data in dietary exposure assessment of chemical substances’ (EFSA, 2010b). The guidance suggests that the lower bound (LB) and upper bound (UB) approach should be used for chemicals likely to be present in the food (e.g. naturally occurring contaminants, nutrients and mycotoxins). The LB is obtained by assigning a value of zero (minimum possible value) to all samples reported as lower than the LOD (< LOD) or LOQ (< LOQ). The UB is obtained by assigning the numerical value of LOD to values reported as < LOD and LOQ to values reported as < LOQ (maximum possible value), depending on whether LOD or LOQ is reported by the laboratory.
A mean LB and UB occurrence value was then calculated at each level of the FoodEx2 classification.
Means for specific food categories calculated on less than six analytical results were not used in the dietary exposure assessment. However, these analytical results were included in the calculation of averages for categories at higher levels of the Foodex2 classification in case at least six were available.
Specific food subcategories with 100% left‐censored results were included with mean LB and UB concentration of parent FoodEx2 categories.
Specific food subcategories for which there were no occurrence data available were attributed with the concentration of the parent FoodEx2 category where TBBPA, TBBPA‐bDiBPrE and TBBPA‐bME contamination could not be excluded, and this was the case for all subcategories of the Foodex2 Level 1 for which quantified data were available, e.g. the mean concentration for ‘Mammals liver’ was attributed to ‘Pig liver’, subcategory for which there were no specific occurrence data.
In the cases in which there were no analytical results available for food derivatives, the TBBPA LB and UB mean concentration for the food derivative was obtained from TBBPA LB and UB mean concentration in the raw primary commodity if available, e.g. TBBPA mean concentration in egg powder was calculated from TBBPA mean concentration in whole egg, applying the reverse yield factor available in EFSA raw primary commodity (RPC) model (EFSA, 2019). For food derivatives obtained through the process ‘separation’ it was assumed that TBBPA was concentrating into the fatty part (e.g. milk to butter or to cream conversion).
Composite foods documented to contain ingredients belonging to the food categories for which data were available (e.g. foods belonging to the Fine bakery wares category reported to contain butter, milk and/or eggs) were also included in the dietary exposure assessment of TBBPA, with LB and UB mean concentration calculated using the recipe information available in EFSA's RPC model and the available LB and UB mean concentration in the ingredients.
Dilution factors suggested in EFSA guidelines (EFSA, 2018) were also used to calculate LB and UB TBBPA mean concentration for ready‐to‐eat foods or ready‐to‐drink beverages from the available concentrations in the dry ingredients, and vice versa. All calculations made are available in Annex B (Table B2).
No occurrence data were submitted to EFSA for food for infants. Thus, the CONTAM Panel decided to use for the dietary exposure assessment to TBBPA, LB and UB mean concentrations of TBBPA in infant and follow‐on formula identified in two studies from the literature, i.e. Martínez et al. (2019) and Rivière et al. (2019). The raw data from the two studies were made available to EFSA. The number of analytical results, % of left‐censored data and range of quantified results available in the two studies are provided in Table 3. Rivière et al. (2019) reported varying LODs close to 0.01 μg/L while all results from Martínez et al. (2019) were reported with LODs of 1 μg/L.
TABLE 3.
Number of analytical results, % of left‐censored data, number of quantified results and range of quantified values from Rivière et al. (2019) and Martínez et al. (2019) and the combination of the two studies for infant formula and follow‐on formula (μg/kg ww).
| Reference | FOODEX2_L7_ID | N | %LC | Quant | Min | Max | MEAN LB | MEAN UB | P95 LB | P95 UB |
|---|---|---|---|---|---|---|---|---|---|---|
| Rivière et al. (2019) | Follow‐on formulae, liquid | 42 | 21% | 33 | 0.0068 | 0.5805 | 0.050 | 0.052 | ||
| Infant formulae, liquid | 28 | 39% | 17 | 0.0113 | 0.3212 | 0.045 | 0.049 | |||
| Martínez et al. (2019) | Follow‐on formulae, liquid | 14 | 79% | 3 | 0.9100 | 1.9000 | 0.315 | 1.100 | ||
| Infant formulae, liquid | 36 | 94% | 2 | 0.6000 | 1.1500 | 0.049 | 0.993 | |||
| Combined | Follow‐on formulae, liquid | 56 | 36% | 36 | 0.0068 | 1.9 | 0.116 | 0.314 | ||
| Combined | Infant formulae, liquid | 64 | 70% | 19 | 0.0113 | 1.15 | 0.047 | 0.58 | 0.181 | 1.000 |
The CONTAM Panel considered it likely that the actual occurrence of TBBPA in milk formula in France and Spain would be similar, given the expected similarity of milk formula products available across the EU, and the data from Rivière et al. (2019) and Martínez et al. (2019) are compatible with this. Considering the high LOD of the Martínez et al. (2019) study and the high % of left‐censored data, the UB estimates of the mean concentrations will be an overestimation of up to about an order of magnitude. This is because the many left‐censored measurements from Martínez et al. (2019) are replaced by their LOD of 1 μg/L when calculating the UB means, whereas it is likely that most of them should follow a similar distribution to the quantified values of Rivière et al. (2019), which have means of 0.07 and 0.06 μg/L for infant and follow‐on formulae, respectively. On the other hand, the LB means (which replace left‐censored data with zeroes rather than the LOD) will underestimate the true values, but to much a smaller degree, since 0.06–0.07 is much closer to 0 than to 1. These considerations are taken into account when interpreting exposure and risk estimates for age groups which consume significant amounts of milk formula (infants and toddlers).
The outcome of the data cleaning, analysis and of the calculations is presented in Section 3.2.1.
2.4. Food consumption data
Food consumption data from the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) were used for the dietary exposure assessment. This database contains national data on food consumption at the individual level, which are the most complete and detailed data currently available in the EU.
The food consumption data gathered in the Comprehensive Database were collected using repeated 24‐h or 48‐h dietary recalls or dietary records covering 3 or 7 days per individual. Owing to the differences in the methods used for data collection, direct country‐to‐country comparisons of the exposure estimates should be avoided.
Details of how the Comprehensive Database are used to assess the dietary exposure to food chemicals are published in a 2011 EFSA Guidance (EFSA, 2011b). The latest version of the Comprehensive Database was published in December 2022 and contains results from 53 dietary surveys carried out in 24 Member States covering 95,410 individuals. Six surveys provide information on ‘Pregnant women’, two on ‘Lactating women’ and one on Vegetarians. When two different dietary surveys are available for one country and age class, the most recent one is used in the dietary exposure assessment.
A chronic dietary exposure assessment is relevant in the context of the terms of reference. For such an assessment, surveys in which food consumption data were collected over only 1 day are not considered appropriate. Exclusion of these surveys resulted in a total of 49 dietary surveys carried out in 22 Member States covering 84,676 individuals. Table 4 provides an overview of the population groups and countries included in the dietary exposure assessment.
TABLE 4.
Population groups and countries included in the chronic dietary exposure assessment.
| Population group | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | > 12 weeks to < 12 months | Bulgaria, Cyprus, Denmark, Estonia, Finland, France, Germany, Italy, Latvia, Portugal, Slovenia, Spain |
| Toddlers | ≥ 12 months to < 36 months | Belgium, Bulgaria, Cyprus, Denmark, Estonia, Finland, France, Germany, Hungary, Italy, Latvia, Netherlands, Portugal, Slovenia, Spain |
| Other children | ≥ 36 months to < 10 years | Austria, Belgium, Bulgaria, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Italy, Latvia, Netherlands, Portugal, Spain, Sweden |
| Adolescents | ≥ 10 years to < 18 years | Austria, Belgium, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Italy, Latvia, Netherlands, Portugal, Romania, Slovenia, Spain, Sweden |
| Adults | ≥ 18 years to < 65 years | Austria, Belgium, Croatia, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Netherlands, Portugal, Romania, Slovenia, Spain, Sweden |
| Elderly | ≥ 65 years to < 75 years | Austria, Belgium, Cyprus, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Netherlands, Portugal, Romania, Slovenia, Spain, Sweden |
| Very elderly | ≥ 75 years | Austria, Belgium, Denmark, France, Germany, Hungary, Ireland, Italy, Latvia, Netherlands, Portugal, Romania, Sweden |
According to the EFSA Scientific Committee Guidance on the risk assessment of substances present in food intended for infants under 16 weeks of age, the exposure assessment for these infants should be carried out separately from that for older infants, following the procedure described in the guidance (EFSA Scientific Committee, 2017). Based on this guidance, infants under 16 weeks of age should be excluded from the dietary exposure estimation of the infants age group. However, due to uncertainty in the reported individual ages of infants in the Comprehensive Database, the cut‐off age was based on a validated existing age group in this database corresponding to 12 weeks of age. Thus, food consumption data of infants between 12 and 16 weeks of age were also included in the exposure assessment. As the number of children within this age range in the database is limited, it is not expected that this will have affected the exposure estimate for infants of 16 weeks up to 12 months of age.
Annex B (Table B1) provides details on the dietary surveys included in the dietary exposure assessment.
2.5. Food classification
Consumption and occurrence data were codified according to the FoodEx2 classification system (EFSA, 2011a, 2011b). Since 2018, all consumption records in the Comprehensive Database as well as all occurrence data submitted to EFSA have been codified according to the FoodEx2 classification system (EFSA, 2015). The FoodEx2 classification system consists of a large number of standardised basic food items aggregated into broader food categories in a hierarchical parent–child relationship. Additional descriptors, called facets, are used to provide additional information about the codified foods (e.g. information on food processing and packaging material).
2.6. Exposure assessment
For calculating the chronic dietary exposure to TBBPA, food consumption and body weight data at the individual level were retrieved from the Comprehensive Database. Occurrence data and consumption data were linked at the relevant FoodEx2 level.
Chronic dietary exposures were calculated by combining mean TBBPA occurrence values for food samples collected in different countries (pooled European occurrence data) with the average daily consumption for each food at the individual level in each dietary survey and age class. Consequently, individual average exposures per day and body weight were obtained for all individuals. The following formula describes the calculation made:
where
is the average exposure of individual i.
is the mean TBBPA concentration in each food or food group f (belonging to set of foods F i for individual i).
is the consumed amount of food f by individual i on day d.
bw i is individual body weight of individual i.
d is the survey day (belonging to the set of survey days D i for individual i).
|D i | represents the number of survey days of individual i.
The distributions of individual exposures were then used to calculate the mean and high (95th percentile) exposure per survey and per age class. These exposure estimates were obtained using the LB and UB mean concentration of TBBPA.
All analyses were run using the SAS Statistical Software (SAS enterprise guide 8.3 Update 5).
2.7. Risk characterisation
The general principles of the risk characterisation for chemicals in food as described by the WHO/IPCS (2009) were applied as well as the different EFSA guidance documents relevant to this step of the risk assessment (see Annex A).
3. ASSESSMENT
3.1. Hazard identification and characterisation
3.1.1. Toxicokinetics
3.1.1.1. Toxicokinetics studies in experimental animals
3.1.1.1.1. Absorption
The previous Opinion on TBBPA and its derivatives in food described some toxicokinetic studies in rats and mice but only a few addressed the absorption rate/bioavailability of TBBPA and TBBPA‐bDiBPrE (EFSA CONTAM Panel, 2011c). These studies are summarised in Table 5.
TABLE 5.
Summary of toxicokinetic studies on TBBPA and TBBPA‐bDiBPrE addressing absorption rate/bioavailability reported in the previous Opinion (EFSA CONTAM Panel, 2011c) and studies identified since then.
| Dose(s) tested | Route of exposure | Absorption rate/bioavailability | Species | Reference |
|---|---|---|---|---|
| TBBPA | ||||
| 2 mg/kg bw of [14C]‐TBBPA | Gavage | 70% a | Cannulated and conventional male Sprague–Dawley rats | Hakk et al. (2000) |
| 2, 20, 200 mg/kg bw [14C]‐TBBPA | Oral/iv | 1.6% b | Male Fischer‐344 rats | Kuester et al. (2007) |
| 25, 250, 1000 mg/kg bw [14C]‐TBBPA | Oral/iv | 4.8% b | Female Wistar Han IGS rats | Knudsen et al. (2014) |
| TBBPA derivatives | ||||
| 20 mg/kg bw of [14C]‐TBBPA‐bDiBPrE | Oral/iv | 2.2% b | Male Fischer‐344 rats | Knudsen et al. (2007) |
Abbreviation: iv, intravenous.
These values estimated the % of absorption, based on the recovery rate after single oral exposure only.
These values correspond to the bioavailability (comparison oral vs. iv route).
Since the previous Opinion, two additional studies have been identified:
Kuester et al. (2007) 23 performed a toxicokinetic study on male Fisher rats dosed orally by gavage (at 2, 20 or 200 mg/kg bw) and iv route (at 20 mg/kg bw) of [14C]‐TBBPA. The authors reported an oral bioavailability of 1.6%, based on the area under the curve (AUC) (comparison oral vs. iv route, calculated after 10 days).
In female Wistar Han rats dosed orally by gavage (25, 250 or 1000 mg/kg bw) and iv route (at 250 mg/kg bw) of [14C]‐TBBPA, Knudsen et al. (2014) reported a rapid absorption of TBBPA from the gastrointestinal tract with an observed C max at 1.5 h (at 250 mg/kg bw) and calculated an oral bioavailability of 4.8% (value calculated at 72 h).
In summary, following administration of [14C]‐TBBPA in male and female rat, the available studies indicate that the oral bioavailability is in the range 1.6%–4.8%. It seems that TBBPA is very well absorbed, but it undergoes biotransformation leading to a low oral bioavailability.
3.1.1.1.2. Distribution
The previous Opinion on TBBPA and its derivatives in food (EFSA CONTAM Panel, 2011c) described some toxicokinetic studies in rodents that addressed the distribution of TBBPA and TBBPA‐bDiBPrE. These studies are summarised in Table 6.
TABLE 6.
Summary of toxicokinetic studies on TBBPA and TBBPA‐bDiBPrE addressing distribution reported in the previous Opinion (EFSA CONTAM Panel, 2011c) and studies identified since then.
| Dose(s) tested | Route of exposure | Tissues distribution | Species | Reference |
|---|---|---|---|---|
| TBBPA | ||||
| 7 mg/kg bw [14C]‐TBBPA | Gavage | Liver, gonads, adipose tissue (no quantitative data reported) | Sprague–Dawley rats | Brady (1979, as cited in WHO/IPCS, 1995) |
| 2 mg/kg bw [14C]‐TBBPA | Gavage | Lung, liver, kidneys (with 0.2%, 0.06% and 0.003% of the dose in non‐cannulated rat, respectively) | Cannulated and conventional male Sprague–Dawley rats | Hakk et al. (2000) |
| 250, 1000 mg/kg bw [14C]‐TBBPA | ip | Muscle, fat, blood, liver (with 4.27%, 3.72%, 1.78% and 0.38% of the dose at 24 h, respectively) | Wistar rats | Szymańska et al. (2001) |
| 5 mg/kg bw [14C]‐TBBPA | Gavage (GD10–GD16) | Liver (maternal tissue = 0.26% of the dose). Liver (fetus = 0.06% of the maternal dose) | Pregnant Wistar rats | Meerts et al. (1999) |
| 0.192 mg/kg bw [14C]‐TBBPA | Gavage (GD16–GD19) | Intestine (1.75% of the dose), Liver (0.038% of the dose) | Pregnant Wistar rats | Riu (2006) (PhD, in French) |
| 300 mg/kg bw TBBPA | Gavage | Not measured | Sprague–Dawley rats | Schauer et al. (2006) |
| 2, 20, 200 mg/kg bw [14C]‐TBBPA | Gavage/iv | Tissues (0.2%–0.9% of the highest dose after 72 h) | Male Fischer‐344 rats | Kuester et al. (2007) |
| 200, 500, 1000 mg/kg bw [14C]‐TBBPA | Gavage | Blood, urine and kidney (no accumulation in kidney) | Male Sprague–Dawley rats | Kang et al. (2009) |
| 25, 250, 1000 mg/kg bw [14C]‐TBBPA | Oral/iv | Adrenals, brain, heart, ovaries, spleen, thymus, thyroid, urinary bladder and uterus (< 0.01% of the dose) | Female Wistar Han IGS rats | Knudsen et al. (2014) |
| 50, 100, 250, 500, 1000 mg/kg bw [14C]‐TBBPA | Gavage | Liver, plasma and uterus | Female Wistar Han IGS rats | Borghoff et al. (2016) |
| 25 mg/kg bw [14C]‐TBBPA | Gavage | Mammary tissues (< 0.02% of the dose) | Wistar Han IGS rats dams and pups | Knudsen et al. (2018) |
| 100 μg dissolved in 0.1 mL of corn oil | Gavage | Liver, intestine, kidney, fat, heart and brain and accounted for < 0.1% of the administered dose | C57BL/6 mice dams | Nakao et al. (2022) |
| TBBPA derivatives | ||||
| 20 mg/kg bw of [14C]‐ TBBPA‐bDiBPrE | Oral/iv | Liver (6.7% of the dose), adipose tissue (0.3% of the dose) at 24 h post oral dose | Male Fischer‐344 rats | Knudsen et al. (2007) |
Abbreviation: ip, intraperitoneal.
Since the previous Opinion, additional studies have been identified:
Kuester et al. (2007) 24 performed a toxicokinetic study on male Fisher rats dosed orally by gavage (at 2, 20 or 200 mg/kg bw) and iv route (at 20 mg/kg bw) of [14C]‐TBBPA. The authors showed that 72 h after administration of 200 mg/kg bw, the amounts of radioactivity were between 0.2% and 0.9% in tissues. In their repeated dose experiment (daily oral dose of 20 mg/kg of TBBPA during 5 and 10 days), Kuester et al. (2007) did not find radioactivity in tissues.
Kang et al. (2009) administered by gavage 0, 200, 500 or 1000 mg/kg of TBBPA to male Sprague–Dawley for 14 days and measured TBBPA concentrations in blood, urine and kidney. The authors found that TBBPA did not accumulate in kidney of male Sprague–Dawley rats receiving daily doses of 0‐1000 mg/kg of TBBPA up to 14 days.
Knudsen et al. (2014) reported that, after 72 h following oral doses of 25, 250 or 1000 mg [14C]‐TBBPA/kg in female Wistar Han rats, the radioactivity measured in a number of tissues was less than 0.01% of administered doses (adrenals, brain, heart, ovaries, spleen, thymus, thyroid, urinary bladder and uterus).
Knudsen et al. (2018) administered [14C]‐TBBPA in a single dose (25 mg/kg bw) in pregnant and nursing Wistar Han IGS rats at GD20, PND12 or PND20. The following tissues were collected at 0.5 to 24 h after dosing: adipose, adrenals, brain, heart, kidneys, large intestine, liver, lung, mammary, muscle, pancreas, ovaries, skin, small intestine, spleen, stomach, thymus, thyroid, urinary, bladder and uterus. The authors found a very low radioactivity in mammary tissue (< 0.02% of the fraction of the total dose), but no radioactivity was detected in other tissues. In fetuses and placentas (collected at GD20), TBBPA and its metabolites (glucuronide and sulfate) accounted for less than 1% of the dose. The authors detected a low level of TBBPA in the PND20 pup livers suggesting that pups were exposed to TBBPA from mother's milk.
Borghoff et al. (2016) performed a toxicokinetic study in female Wistar Han rats. The authors measured TBBPA and its metabolites (glucuronide and sulfate conjugates) in plasma, liver and uterine tissues following repeated administration (28 days) by gavage at doses of 0, 50, 100, 250, 500 and 1000 mg/kg bw per day. The authors found that concentrations of TBBPA and its metabolites in liver and uterus tissue increased linearly according to the dose. The authors found that TBBPA did not accumulate in plasma, liver or uterus of rats receiving daily doses of 0–1000 mg/kg of TBBPA for 28 days.
As described in the previous Opinion and in Table 6, the radioactivity measured in rats following administration of [14C]‐TBBPA (oral or iv route) accounted for less than 0.5% of the administered dose, the highest levels being found in intestine, adipose tissue and liver (Meerts et al., 1999; Riu, 2006). Meerts et al. (1999) measured radioactivity in pregnant rats at GD20 after 5 mg/kg bw [14C]‐TBBPA administration by gavage during GD10–GD16. The authors found that the amount of radioactivity in fetuses represents less than 0.01% of the dose. Riu (2006) found similar results when pregnant rats were treated by gavage (GD16–GD19) to 0.192 mg/kg bw [14C]‐TBBPA. The level of radioactivity in fetus was less than 0.01% at GD20.
Nakao et al. (2022) administered by gavage a single dose of 100 μg (in corn oil) of TBBPA in female C57BL/6 mice after delivery. The authors collected faecal, blood sample and tissues of dams (gastrointestinal content, liver, kidney, heart, fat and brain) at different times (0–72 h after administration). At the same time point, milk sample was collected from the stomach of pups. The authors detected concentrations of TBBPA in the following order: liver, intestine, kidneys, fat, heart and brain, and accounted for < 0.1% of the administered dose. Conjugated metabolites were also found in liver and kidneys. In milk samples collected from the stomach of the pups, the authors detected TBBPA (< 0.1% of the administered dose), and two debrominated metabolites (TriBBA and DBBPA, with less than of 0.01% of administered dose).
In summary, after oral administration by gavage, TBBPA is rapidly distributed among different tissues (liver, uterus and adipose tissue…), but within 0.5–72 h less than 1% of the administered dose remained due to rapid elimination. The results of repeated exposure in rats (up to 28 days) demonstrate that TBBPA will not accumulate in tissues following oral exposure.
3.1.1.1.3. Metabolism
Hakk et al. (2000) measured in the bile of cannulated male Sprague–Dawley rats after oral exposure to 2 mg/kg bw [14C]‐TBBPA, a monoglucuronide and a diglucuronide metabolite, and also a glucuronic acid/sulfate diconjugate of TBBPA. No quantification of the metabolites was provided.
Schauer et al. (2006) measured in plasma from rats (after a single oral dose of TBBPA of 300 mg/kg bw) a glucuronide and a sulfate TBBPA metabolite, and also TBBPA‐diglucuronide, TBBPA‐glucuronide/sulfate, TriBBPA and TriBBPA‐glucuronide. The level in plasma of TBBPA‐sulfate was higher than that of TBBPA and TBBPA‐glucuronide. The measured AUCs were 9255, 1028 and 1028 nmol/mL per hour, respectively.
TBBPA‐glucuronide and TBBPA‐diglucuronide were also identified by Kuester et al. (2007) in male Fisher rats dosed orally by gavage (at 2, 20 or 200 mg/kg bw). No quantification of the metabolite was provided.
Knudsen et al. (2014, 2018), after administration of TBBPA (see descriptions in previous subsection) detected TBBPA‐glucuronide and TBBPA‐sulfate metabolites at GD20 in dam blood. The monoglucuronide and monosulfate metabolites were eliminated in the bile. No quantification of the metabolites was reported.
Borghoff et al. (2016) found that TBBPA was metabolised through glucuronidation (TBBPA‐glucuronide) and sulfation (TBBPA‐sulfate) after oral administration, and identified these metabolites in plasma, liver and uterus. The authors showed an increased plasma concentration of these metabolites with repeated administration. The authors showed a decrease in the TBBPA‐sulfate/TBBPA‐glucuronide ratio in a dose‐dependent manner, suggesting that this decrease in ratio reflects a saturation of the sulfation pathway at dose levels of 250 mg/kg and above.
Knudsen et al. (2014) observed a delayed elimination after an oral administration of TBBPA at 1000 mg/kg bw, which according to the authors would be due to saturation of conjugation reactions, since no saturation or delay was detected at lower doses. Borghoff et al. (2016) reported saturation of elimination pathways from 250 mg/kg bw.
In rats orally exposed to TBBPA, Kuester et al. (2007) showed that 50% of the TBBPA was excreted via the bile in the form of diglucuronide (24%), glucuronic acid/sulfate diconjugate (14%) and monoglucuronic acid conjugate (24%).
In their review, Liu et al. (2018) suggested a debromination of TBBPA leading to the formation of TriBBPA, DBBPA and MBBPA, since these products have been detected in human milk. Szymańska et al. (2001) detected in rat (ip route) a metabolite (TriBBPA, suggesting also a debromination) eliminated in faeces.
The suggested metabolic pathway of TBBPA is shown in Figure 2.
FIGURE 2.
Suggested metabolism pathway of TBBPA (from CLH Report, 2020; Colnot et al., 2014).
After oral administration of 20 mg/kg bw of [14C]‐TBBPA‐bDiBPrE, Knudsen et al. (2007) found a metabolite (not identified) in bile which represented less than 2% of the total dose.
Nakao et al. (2022) found that the main metabolites of TBBPA in mice were conjugated metabolite (sulfate and glucuronide), and also detected debrominated metabolites, including TriBBPA, DiBBPA and MBBPA, in dam blood.
In summary, TBBPA is metabolised to glucuronide and sulfate conjugates. TBBPA‐diglucuronide, TBBPA‐glucuronide/sulfate, TriBBPA and TriBBPA‐glucuronide have been also detected in plasma, urine and faeces. Saturation of sulfation pathway at high doses (from 250 mg/kg bw) has been described. There is evidence for partial, but not total, debromination of TBBPA in rats. Debrominated metabolites have been detected in mice.
One study on TBBPA‐bDiBPrE suggested a low rate of metabolism but did not identify metabolites.
3.1.1.1.4. Elimination
The EFSA CONTAM Panel (2011c) described some toxicokinetic studies in rodents that addressed the elimination of TBBPA and TBBPA‐bDiBPrE. These studies are summarised in Table 7.
TABLE 7.
Summary of toxicokinetic studies on TBBPA and TBBPA‐bDiBPrE addressing elimination reported in the previous Opinion (EFSA CONTAM Panel, 2011c) and studies identified since then.
| Dose(s) tested | Route of exposure | Excretion route | % of retention/half‐life | Species | Reference |
|---|---|---|---|---|---|
| TBBPA | |||||
| 7 mg/kg bw of [14C]‐TBBPA | Gavage | Faeces (99%) at 72 h | 19.9 h in the blood, 70.8 h in fat, 17.1 h in kidneys, 10.8 h in the liver, 39.3 h in the spleen, 48.0 h in muscle, 60.5 h in the gonads | Sprague–Dawley rats | Brady (1979) (as cited in WHO/IPCS, 1995) |
| 2 mg/kg bw of [14C]‐TBBPA | Gavage | Faeces (99%) Urine (1%) | 90% of the dose excreted after 72 h | Cannulated male Sprague–Dawley rats | Hakk et al. (2000) |
| 250 or 1000 mg/kg bw of [14C]‐TBBPA | ip | Faeces (51%–65%) at 72 h | 230 h in blood | Wistar rats | Szymańska et al. (2001) |
| 5 mg/kg bw of [14C]‐TBBPA | Gavage (GD10‐GD16) |
Faeces (99%) Urine (1%) |
90% of the dose excreted after 72 h | Pregnant Wistar rats | Meerts et al. (1999) |
| 300 mg/kg bw TBBPA | Gavage | Only urine was measured | 13 h | Male Sprague–Dawley rats | Schauer et al. (2006) |
| 2, 20, 200 mg/kg bw [14C]‐TBBPA | Oral/iv |
Faeces (> 99%) Urine (< 1%) |
983 min | Male Fischer‐344 rats | Kuester et al. (2007) |
| 25, 250, 1000 mg/kg bw [14C]‐TBBPA | Oral/iv |
Faeces (> 99%) Urine (< 1%) |
155 min | Female Crl:WI (Han) rats | Knudsen et al. (2014) |
| 25 mg/kg bw [14C]‐TBBPA | Gavage | NR | 17 h | Wistar Han IGS rats dams and pups | Knudsen et al. (2018) |
| 100 μg dissolved in 0.1 mL of corn oil | Gavage | Faeces (71%) at 24 h | 12h | C57BL/6 mice dams | Nakao et al. (2022) |
| TBBPA derivatives | |||||
| 20 mg/kg bw of [14C]‐TBBPA‐bDiBPrE | Oral/iv | Faeces (95% of dose by 96 h) | 13.9 h | Male Fischer‐344 rats | Knudsen et al. (2007) |
Abbreviations: ip, intraperitoneal; iv, intravenous.
Since the previous Opinion, additional studies have been identified:
Kuester et al. (2007) 25 showed that TBBPA was mainly excreted in faeces after oral or iv administration, and less than 2% was eliminated in urine. After oral dosing of 2, 20 or 200 mg TBBPA/kg bw, 90% of administered doses were recovered in faeces within 72 h, with most of the dose eliminated within the first 24 h after administration. The authors calculated an elimination half‐life of 83 min.
Knudsen et al. (2014) previously reported that the main route of elimination of [14C]‐TBBPA was in faeces where the dose recoveries in 72 h were more than 95% (at doses 25, 250 and 1000 mg/kg). The authors noted a delay in faecal elimination at the highest dose. In rats receiving TBBPA by iv administration, TBBPA was found in the intestine supporting the conclusion that TBBPA is eliminated via bile. The authors calculated an elimination half‐life of 155 min.
After a single dose (25 mg/kg) in pregnant rats, Knudsen et al. (2018) reported an elimination half‐life of 17 h.
Nakao et al. (2022) found that in mice, approximately 71%, 11% and 6.8% of TBBPA, TBBPA‐glucuronide, TBBPA‐sulfate, respectively, were excreted in faeces at 24 h.
In summary, after oral administration to rats, TBBPA is mainly eliminated in the faeces (mainly through biliary excretion). In all studies identified, most of the dose was eliminated in the first 24 h. According to Brady (1979), the maximum half‐life reported in tissues was less than 3 days.
3.1.1.1.5. Summary of toxicokinetic studies in rodents
Studies in rats showed that TBBPA is well absorbed (> 70%) but has a low bioavailability (< 5%) due to extensive metabolism in the liver. In rats and mice, TBBPA is metabolised by UDP glucuronosyltransferases and sulfotransferases to form glucuronide and sulfate conjugates. Saturation of sulfation pathways at high doses (from 250 mg/kg bw) has been described.
There is no tissue accumulation, since most of the TBBPA and/or its metabolites (TBBPA‐sulfate and TBBPA‐glucuronide) are rapidly eliminated in the faeces, mainly through biliary excretion. The maximum half‐life reported in tissue was less than 3 days. TBBPA and its debrominated metabolites are transferred to pups via milk.
In rodents, there is evidence of transplacental transfer and transfer via lactation of TBBPA.
Following a single oral administration, TBBPA‐bDiBPrE was eliminated in the faeces (95% of dose by 36 h). One study on TBBPA‐bDiBPrE suggested a low rate of metabolism, but did not identify metabolites.
3.1.1.2. Toxicokinetics studies in humans
3.1.1.2.1. Absorption
No data were identified on the absorption of TBBPA in humans.
3.1.1.2.2. Distribution
Limited data are available on the distribution of TBBPA in humans.
Schauer et al. (2006) administered to human volunteers a single oral dose of 0.1 mg TBBPA/kg bw, the authors measured a peak plasma concentration of TBBPA‐glucuronide at 4 h; the amount of parent compound was < LOD.
Some studies have demonstrated the transfer of TBBPA from the mother to the infant via breastfeeding (see Section 3.1.1.3).
3.1.1.2.3. Metabolism
Limited data are available on the metabolism of TBBPA in humans.
Schauer et al. (2006) found after a single oral dose of 0.1 mg TBBPA/kg bw TBBPA‐glucuronide in urine corresponding to < 0.1% of the administered dose of TBBPA.
Ho et al. (2017) measured TBBPA conjugates in urine samples from 140 voluntary donors in Hong Kong. The authors quantified the following conjugates: TBBPA‐mono‐glucuronide, TBBPA‐diglucuronide, TBBPA‐mono‐sulfate and TBBPA‐di‐sulfate (see Section 3.1.1.3).
TBBPA is metabolised by UDP glucuronosyltransferase (UGT) and sulfotransferase isozymes to form glucuronide and sulfate conjugates. The expression and activities of these two enzymes have been studied in different age groups by several authors (Badée et al., 2019; Dubaisi et al., 2019; Ladumor et al., 2019; Miyagi & Collier, 2011; Neumann et al., 2016). The comparison in expression and activity of UGTs from paediatric and adult liver microsomes showed that some isoforms matured after 6 months to 1 year. For sulfotransferase, data showed that protein expression was at higher abundance during early childhood (1 to < 6 years), than in adult. Assuming that TBBPA could be predominantly metabolised by glucuronoconjugation rather than sulfoconjugation in humans, these data suggest that children at young age could be more vulnerable to TBBPA exposure.
Hanioka et al. (2024) performed a comparative study to assess the TBBPA glucuronidation abilities between laboratory animals and humans. By using animal and human liver microsomes, the authors found that the TBBPA glucuronidation abilities in rats were equal to 62% of that measured in humans. In mice, the activity was 1.1‐fold compared to humans. These data suggest that TBBPA glucuronidation abilities in experimental animals and humans differ. By using recombinant human UGT (n = 10), the authors found that UGT1A1 and UGT1A9 were the main contributors to TBBPA glucuronidation abilities.
3.1.1.2.4. Elimination
Limited data are available on the elimination of TBBPA in humans.
Geyer et al. (2004, extended abstract) estimated the terminal elimination half‐life of TBBPA in humans, but based on half‐lives determined in rats. The authors calculated half‐lives of 6.6 and 23.6 days (blood and adipose tissue) for males and 76.7 and 21.0 days (blood and adipose tissue) for females.
Schauer et al. (2006) showed that after a single oral dose of 0.1 mg TBBPA/kg bw administered in gel capsules to three healthy male volunteers, the half‐life of TBBPA‐glucuronide in plasma appeared to be between 48 and 72 h. This was confirmed by Hagmar et al. (2000); the authors estimated a half‐life of 2.2 days, based on concentrations of TBBPA in blood serum from four employees working in an electronic plant.
3.1.1.2.5. Summary of toxicokinetic studies in humans
No data are available on the absorption of TBBPA.
TBBPA‐mono‐glucuronide, TBBPA‐diglucuronide, TBBPA‐mono‐sulfate and TBBPA‐di‐sulfate have been detected in urine.
The elimination half‐lives of TBBPA were 2–3 days in humans.
3.1.1.3. Levels in human samples
The previous Opinion on TBBPA and its derivatives (EFSA CONTAM Panel, 2011c) summarised the occurrence data in human milk and other human samples from European countries published in the literature until 2011. The number of studies overall was limited, with three studies reporting on the occurrence of TBBPA in human milk samples (Abdallah & Harrad, 2011; Cariou et al., 2008; Thomsen et al., 2002), four studies on serum samples from the general European population (Cariou et al., 2008; Dirtu et al., 2008, 2010; Thomsen et al., 2007) and two studies on serum samples from workers at an electronic dismantling plant or from computer technicians (Hagmar et al., 2000; Jakobsson et al., 2002, respectively). One study reported on the levels of TBBPA in adipose tissue (Cariou et al., 2008).
Since then, a limited number of studies in European countries have become available, mainly on the occurrence of TBBPA in human milk and serum samples. As for the previous Opinion, no data on any of the TBBPA derivatives considered in this Opinion were identified. The studies identified that reported on TBBPA are summarised in Table 8.
TABLE 8.
Concentration of TBBPA in human milk, serum and adipose tissue samples from European countries.
| Country year | Number of samples | Concentration | Detection frequency | Reference |
|---|---|---|---|---|
| Human milk | ||||
| Spain NR | n = 53 |
MB Mean (SD): Total TBBPA: 18.7 (16.7) ng/g lipid |
Detected in 3 out of the 53 samples analysed (LOD = 1 μg/L) |
Martínez et al. (2019) Hydrolysis step in the sample preparation to cleave conjugates |
|
Breastfeeding period: < 1 month: n = 18 1–6 months: n = 20 > 6 months: n = 5 |
According to breastfeeding period: < 1 month: 0.6 ± 0.42 μg/L 1–6 months: 0.61 ± 0.39 μg/L > 6 months: < 1.00 μg/L |
|||
| Spain 2016–2019 |
n = 40 Breastfeeding period: < 1 month: n = 22 1–6 months: n = 29 > 6 months: n = 9 |
MB Mean, median (range): Free TBBPA: 1.1, < 0.7 (< 0.7–7.8) ng/g lipid Total TBBPA: 2.8, 13 (< 0.7–14) ng/g lipid |
Detected in 20 out of the 40 samples analysed (LOD = 0.7 ng/g lipid) |
Rovira et al. (2022) Hydrolysis step in the sample preparation to cleave conjugates |
| France 2005 | n = 23 mother/new born pairs |
Median (range): 0.172 (0.034–9.4) ng/g lipid |
Substitution method for values < LOD/LOQ NR | Antignac et al. (2008) a |
| France 2010 | n = 106 |
Mean (range): LB: 3.2 (0–15) ng/g lipid UB: 3.5 (0.5–15) ng/g lipid |
Detected in 44 out of the 106 samples analysed (LOD = 0.5 ng/g lipid, LOQ = 2.5. ng/g lipid) | Inthavong et al. (2017) |
| Ireland NR | n = 11 pools from 109 first‐time mothers |
Mean (range): LB: 0.05 ng/g lipid UB: 0.33 (< 0.29–0.17) ng/g lipid |
Detected in 2 out of the 11 pools analysed (LOD NR) | Pratt et al. (2013) |
| Czech Republic 2010 | n = 50 |
MB Range: < 2–688 ng/g lipid |
Detected in 15 out of the 50 samples analysed (LOQ = 2 ng/g lipid) | Lankova et al. (2013) |
| Czech Republic 2019–2021 | n = 231 | ND | (LOD = 0.15 ng/mL) | Parizek et al. (2023) |
| UK NR | n = 34 |
Average, median (range): 0.06, < 0.04 (< 0.04–0.65) ng/g lipid |
Detected in 36% of the samples analysed (LOQ = 0.04 ng/g lipid) Substitution method for values < LOD/LOQ NR |
Abdallah and Harrad (2011) |
| UK 2010–2011 | n = 120 |
Average, median (P5–P95): 0.06, 0.04 (0.03–0.17) ng/g lipid |
Detected in 61% of the samples analysed (LOQ = 0.050 ng/g lipid) Concentrations < LOQ were substituted by f × LOQ, where f is the fraction of samples in which TBBPA was present > LOQ |
Harrad and Abdallah (2015) |
| Serum | ||||
| France 2005 |
n = 26 maternal serum n = 26 umbilical cord serum |
Average, median (range): 0.054, 0.007 (0.002–0.783) ng/g ww 0.152, 0.010 (0.002–1.012) ng/g ww |
‐ | Antignac et al. (2008) a |
| Norway 2013–2014 |
n = 61 Adults |
MB Mean, median (range): 9.4, < 0.28 (< 0.28–74) ng/g lipid |
Detected in 36% of the samples analysed (method LOD = 120 pg/serum sample, method LOQ = 170 pg/serum sample) | Tay et al. (2019) |
| Belgium 2015 |
n = 274 Adults |
MB Mean, median (range): < 4.1, < 4.1 (< 4.1–43.6) pg/mL |
Detected in 31% of the samples analysed (LOQ = 4.1 pg/mL) Concentrations < LOQ were substituted by LOQ × detection frequency |
Dufour et al. (2017) |
| Adipose tissue | ||||
| France 2005 |
n = 26 Women |
ND | (LOD NR) | Antignac et al. (2008) a |
| Portugal 2009–2011 |
n = 188 female patients undergoing bariatric surgery for weight reduction |
ND | (Method LOD = 0.003–0.009 μg/L, method LOQ = 0.008–0.03 μg/L) | Sousa et al. (2023) |
Abbreviations: LB, lower bound; LOD, limit of detection; LOQ, limit of quantification; MB, medium bound; ND, not detected; NR, not reported; UB, upper bound.
The study was not captured in the previous opinion, but it is reported in the current update for completeness.
Two studies applied an analytical method that included a hydrolysis step in the sample preparation to cleave conjugates (Martínez et al., 2019; Rovira et al., 2022; J. Rovira Solano, 2024, personal communication). The results were reported as ‘total TBBPA’ (conjugated + unconjugated) (Martínez et al., 2019) or as both ‘total TBBPA’ and ‘free TBBPA’ (unconjugated) (Rovira et al., 2022). None of the remaining studies identified applied this hydrolysis step; thus, the concentrations reported correspond to free TBBPA.
3.1.1.3.1. Human milk
Nine studies were identified reporting levels of TBBPA in human milk from European countries.
The study by Martínez et al. (2019) analysed 53 human milk samples from Spanish mothers (sampling year not reported) in different times of breastfeeding, i.e. first month, 1–6 months and > 6 months. ‘Total TBBPA’ was detected in only three samples, corresponding to mothers aged > 35 years, with a mean level of 18.7 ± 16.7 ng/g lipid. TBBPA was not detected in samples from mothers with more than 6 months of breastfeeding (LOD < 0.04 μg/L).
In the study by Rovira et al. (2022), the authors analysed 40 human milk samples collected in Spain during the years 2016–2019. ‘Total TBBPA’ was detected in half of the samples, with a median concentration of 13 ng/g lipid, while for ‘free (unconjugated) TBBPA’ the median was < 0.7 ng/g lipid.
Levels of TBBPA in human milk samples from France have been reported by Antignac et al. (2008) 26 and Inthavong et al. (2017), collected in 2005 and 2010, respectively. In the samples collected in 2005, levels ranged from 0.034 to 9.4 ng/g lipid (Antignac et al., 2008), while in the samples from 2010, the levels (LB) ranged from 0 to 15 ng/g lipid (Inthavong et al., 2017).
Pratt et al. (2013) analysed pooled human milk samples from Ireland. TBBPA was detected in two out of the 11 pools analysed, with levels (UB) ranging from < 0.29 to 0.17 ng/g lipid (mean LB = 0.05, mean UB = 0.33 ng/g lipid).
Lankova et al. (2013) analysed TBBPA in Czech human milk samples collected in 2010. TBBPA was detected in 30% of the samples, with concentrations ranging from < 2 to a maximum of 688 ng/g lipid. In a later study by Parizek et al. (2023), TBBPA was not detected in any of the 231 samples of human milk collected between 2019 and 2021 in two cities from the Czech Republic, one with a history of coal mining and heavy industry.
Two studies reporting levels in human milk samples collected in the United Kingdom were identified. Abdallah and Harrad (2011) analysed 34 samples (sampling year not reported). TBBPA was detected in 36% of the samples with a mean concentration of 0.06 ng/g lipid (range < 0.04–0.65 ng/g lipid). In another study by the same authors, human milk samples from first‐time mothers were collected between 2010 and 2011 (Harrad & Abdallah, 2015). To evaluate whether concentrations varied significantly over the first 12 months postpartum, the authors collected one sample per month from each of the 10 participant mothers during 12 months, amounting to a total of 120 samples. Similar mean levels as in the previous study were reported, i.e. 0.06 ng/g lipid (P5–P95 0.03–0.17 ng/g lipid). No significant change in the concentration of TBBPA over the 12 months of lactation was observed.
In the previous CONTAM Panel Opinion, the TBBPA concentrations in human milk from the studies identified at that time ranged from < 0.04 to 37.34 ng/g lipid, with average levels ranging between 0.06 and 4.11 ng/g lipid. The highest maximum and average levels corresponded to the study in which hydrolysis was applied in the sample preparation (Cariou et al., 2008).
In the studies identified from European countries in the literature since then, the TBBPA mean concentrations ranged from 0.05 to 3.5 ng/g lipid. A higher mean value of 18.7 ng/g lipid was reported in a study in which a hydrolysis step was introduced (Martínez et al., 2019).
The CONTAM Panel noted a maximum concentration of 688 ng/g lipid reported in samples collected in 2010 in the Czech Republic (Lankova et al., 2013). However, in a later study in the same country, TBBPA was not detected in any of the 231 samples collected between 2019 and 2021 in two Czech cities, one with a history of coal mining and heavy industry (Parizek et al., 2023). This maximum value was not considered further in the exposure scenario for breastfed infants reported in Section 3.3.1.3.
3.1.1.3.2. Serum
Three studies have been identified on the presence of TBBPA in serum samples.
Antignac et al. (2008) analysed maternal and umbilical cord serum from 23 French mother/newborn pairs. Concentrations ranged from 2 to 783 pg/g ww in maternal serum and from 2 to 1012 pg/g ww in cord serum. The authors reported a poor correlation between the TBBPA levels in maternal and umbilical serum samples.
The other two studies reported the levels in serum samples from adults in Norway (n = 61, collected in 2013–2014, Tay et al., 2019) and Belgium (n = 274, collected in 2015, Dufour et al., 2017). The levels ranged from < 0.28 to 74 ng/g lipid and < LOQ–43,6 pg/mL, respectively.
3.1.1.3.3. Adipose tissue
TBBPA was not detected in any of the 26 samples of adipose tissue analysed by Antignac et al. (2008). The authors concluded that TBBPA was expected to be less prone to bioaccumulation in fat tissues than other persistent POPs due to its relatively low lipophilic properties because of the presence of two hydroxyl groups on the molecule.
3.1.1.3.4. Levels of TBBPA and derivatives in human samples from non‐European countries
Several publications have reported TBBPA concentrations in human samples collected from non‐European countries (see Appendix C, C1).
In a review, Shi et al. (2018) reported on studies on TBBPA in human milk from China that showed an eightfold increase in the levels from 2007 (mean levels: 0.93 ng/g lipid for urban samples and 0.961 ng/g lipid for rural samples) to 2011 (mean levels: 7.58 ng/g lipid, Shi et al., 2017a) in certain areas. Later studies also found an increase in the levels from 2011 to 2014 and 2018, whereas that of PBDEs had decreased during this period. The authors suggested a shift in the production and use of BFRs in China from PBDEs to other flame retardants, such as TBBPA. In Japan, samples collected in 2008–2010 (median 3.2 ng/g lipid, Fujii et al., 2018) showed higher levels than those from 2005 to 2006 (median 0.72 ng/g lipid, Fujii, Nishimura, et al., 2014). Samples collected in 2012–2013 showed lower mean levels (1.9 ng/g lipid, Nakao et al., 2015). Only one study from the USA was identified that reported levels between < 0.03 and 0.550 ng/g lipid from samples collected in 2004–2005.
Besides some additional studies in serum samples, one study has reported the occurrence of TBBPA in samples of adipose tissue and liver collected post‐mortem, and several studies have reported the presence of TBBPA in urine samples. In most of these studies, TBBPA was analysed together with a set of other bisphenols, including BPA. In several studies, TBBPA was not detected in any of the samples, while in those that it was detected, detection frequency was generally low (below 7%) except in two cases where detection frequencies were higher (up to 80%).
3.1.1.4. Toxicokinetic modelling
Different toxicokinetic/PBK models for TBBPA have been published since the previous Opinion (Abdallah & Harrad, 2011; Kamiya et al., 2019; Miura et al., 2021; Zhang, Bartels, et al., 2018).
For most of these models, the authors do not describe how the model was calibrated, nor how it was validated from independent data. This makes it difficult to use these models for a risk assessment. The following was noted:
– Abdallah and Harrad (2011) developed a simple pharmacokinetic model (one compartment). From estimated intakes of UK adults via inhalation, diet and dust ingestion, body burdens were estimated and compared to 34 human milk samples. The authors found that the observed body burdens of TBBPA exceeded the predictions via the model.
– Kamiya et al. (2019) developed a rat PBK model for TBBPA based on reported toxicokinetics determined after oral administration to rats. The authors modelled the plasma and hepatic pharmacokinetics of TBBPA after virtual oral administrations in rats. The authors suggested that oral intake of TBBPA could result in liver accumulation.
– Miura et al. (2021) developed a human PBPK model for TBBPA using a mouse humanised‐liver model (immunodeficient TK‐NOG mice). The authors performed a reverse dosimetry estimations based on plasma data from human biomonitoring data, and calculated a daily intake.
– Zhang, Bartels, et al. (2018) evaluated the toxicokinetic parameters of TBBPA with GastroPlusTM software. Toxicokinetic data of TBBPA were compared to model predictions. This study was designed to assess the predictivity of existing PBPK models.
3.1.1.5. Transfer from feed to food of animal origin
No studies on the transfer of TBBPA or TBBPA derivates included in the TORs were identified.
Two studies reported on the levels of TBBPA in cattle and three game species (Shin et al., 2020; Zacs et al., 2018) with no information on the transfer from feed to food of animal origin.
3.1.2. Toxicity in experimental animals
This section provides an overview of the toxicity data in experimental animals described in the previous EFSA Opinion on TBBPA and its derivatives in food (EFSA CONTAM Panel, 2011c), plus the new data published in the open literature since then. The new studies identified tested TBBPA and only one of the TBBPA derivatives included in the TORs, i.e. TBBPA‐bDiBPrE.
The sections below provide, by toxicological endpoint, a brief summary of the effects reported in the previous Opinion, a summary of the effects reported in the new studies identified in the open literature since then, and an overall summary of all the evidence available. The details of the studies considered in the previous Opinion can be found in EFSA CONTAM Panel (2011c). The details of the new studies published since then are provided in Appendix D (Table D1 for TBBPA, and Table D2 for TBBPA‐bDiBPrE), the only TBBPA derivative considered in the TORs for which toxicity data have been identified.
3.1.2.1. Acute toxicity studies
In the previous Opinion, TBBPA was reported to have a very low acute toxicity in rodents with an oral LD50 > 50,000 mg/kg bw in rat, and > 10,000 mg/kg bw in mice (see Table 9) (ECB, 2006; EFSA CONTAM Panel, 2011c).
TABLE 9.
Oral LD50 of TBBPA in rats or mice reported by the IPCS/WHO.
| Mouse (M, B6C3F1) | LD50 = 4.4 g/kg bw | Sekizawa (1994, as cited by IPCS/WHO, 1995) |
| Mouse (F, B6C3F1) | LD50 = 4.5 g/kg bw | |
| Mouse (sex and strain NR) | LD50 = 10 g/kg bw | IPCS/WHO (1995) |
| Mouse (sex and strain NR) | LD50 = 3.2 g/kg bw | Eastman Kodak Co. (1973); Gustafsson and Wallen (1988, as cited by IPCS/WHO, 1995) |
| Rat (sex and strain NR) | LD50 > 2 g/kg bw | |
| Rat (sex and strain NR) | LD50 > 5 g/kg bw | Hardy (1994, as cited by IPCS/WHO, 1995) |
TBBPA induced oxidative stress in the kidney at high doses. Male rats (8 weeks of age) were treated orally at 0, 200, 500 or 1000 mg/kg in a single administration study, after which transient (at 5 h) elevation of renal lipid peroxidation levels (TBARS) was noted at the highest dose and increased superoxide dismutase activity (SOD) was observed at all doses (Kang et al., 2009).
For the TBBPA derivatives, the rat oral LD50 of TBBPA‐bDiBPrE is > 2000 mg/kg bw (ECHA, 2013).
In summary, TBBPA and TBBPA‐bDiBPrE have a low acute oral toxicity in rodents.
3.1.2.2. Repeated dose toxicity studies
In the previous Opinion (EFSA CONTAM Panel, 2011c), it was concluded that the main targets in subchronic and chronic toxicity studies in rats and mice for TBBPA were liver, kidney, thyroid hormones, immune, nervous and reproductive systems. No data were available on the toxicity of the TBBPA derivatives.
3.1.2.2.1. Effects on the liver
Studies considered in the previous EFSA assessment
In the previous Opinion, the CONTAM Panel noted that TBBPA exhibits some signs of hepatotoxicity in rats and mice, particularly in juvenile mice. This was based primarily on observations of increased liver weight and histopathological changes in dams and offspring of pregnant mice administered TBBPA in the diet from GD0 to PND27 (Tada et al., 2006) and in male mice given TBBPA by gavage for 14 days (Tada et al., 2007). The Opinion noted that no signs of hepatic changes, including effects on hepatic drug metabolism, were found at doses below 350 mg/kg bw per day. However, the current CONTAM Panel noted that in the reproductive study of Tada et al. (2006), the dietary doses of the dams during gestation were equal to 16, 141 and 1640 mg/kg bw per day. Statistically significantly increased relative liver weights were reported at the highest dose (increase of 23%, 7% and 2% in dams, male pups and female pups, respectively) and histopathological changes (slight enlargement of hepatocytes, inflammatory cell infiltration and focal necrosis of hepatocytes) were reported in dams and pups. Similarly, in the study of Tada et al. (2007) increased absolute and relative liver weight (by 22% and 17%, respectively) was reported at the top dose of 1400 mg/kg bw per day, but not at 350 or 700 mg/kg bw per day. Histopathological changes (slight enlargement of hepatocytes, inflammatory cell infiltration and focal necrosis of hepatocytes) were reported in some dose groups, but not in a dose‐dependent manner. In the absence of statistical analysis of the histopathology findings in Tada et al. (2006), and in view of the lack of dose–response for most of the observations, the current Panel concluded that it is not possible to determine a NOAEL or LOAEL from these studies.
Studies published since the previous EFSA assessment
TBBPA
Two new studies were identified.
Choi et al. (2011) dosed pre‐pubertal Sprague–Dawley male rats with TBBPA at 0, 125, 250 or 500 mg/kg bw per day by gavage from PND18–PND48. There were no differences in body weight gain between control and TBBPA‐treated groups. In the high‐dose group animals, absolute and relative liver weights were significantly increased (by 11.5% and 10.6%, respectively). There were no treatment‐related histopathological findings in the liver.
In a study designed to investigate the mechanisms leading to TBBPA‐induced uterine carcinogenicity, Dunnick et al. (2017) administered TBBPA at 0, 25, 250 and 1000 mg/kg bw 5 days per week, (equivalent to 0, 18, 179 and 714 mg/kg bw per day) by gavage to female rats for 13 weeks. There were no treatment‐related effects on body weight. A small (6%) statistically significant increase in relative liver weight was reported at the top dose. This was not associated with histopathological changes in the liver.
TBBPA derivatives
Three studies were identified on TBBPA‐bDiBPrE.
In studies conducted by the NTP (2017), TBBPA‐bDiBPrE 27 was orally administered by gavage to groups of 10 F344/NTac rats/sex/group at doses of 0, 62.5, 125, 250, 500 or 1000 mg/kg bw per day for 14 weeks (core study). Additional groups of 10 F344/Ntac rats were administered the same TBBPA‐bDiBPrE doses for 23 days (special study). In the mouse study, TBBPA‐bDiBPrE was administered by gavage to groups of 10 B6C3F1/N mice/sex/group at doses of 0, 125, 250, 500, 1000, 2000 mg/kg bw per day (5 days per week) for 14 weeks. There were no treatment‐related clinical findings, changes in clinical chemistry parameters, haematology or effects on body weights or organ weights. There were no treatment‐related gross or histopathological lesions and no treatment‐related changes in the number of spermatozoa or spermatids, sperm motility or oestrus cycle in either species (see Section 3.1.2.3). There was an increased microsomal protein content but no evidence of induction of various drug metabolising enzymes (acetanilide‐4‐hydroxylase, 7‐ethoxyresorufin‐O‐deethylase, 7‐pentoxyresorufin‐O‐dealkylase and UDP‐glucuronosyltransferase towards T4) in both rats and mice, commencing at 125 mg/kg bw per day. This change was considered not to be biologically relevant.
Shockley et al. (2020a) found no effects on liver weight or histopathology in rats dosed with TBBPA‐bDiBPrE 28 by gavage at 0, 0.1, 1, 10, 100, 1000 μmol/kg bw per day (corresponding to 0, 0.09, 0.94, 9.4, 94, 944 mg/kg bw per day of TBBPA‐bDiBPrE) for 5 days.
Yao et al. (2021) dosed male mice by oral gavage with 30 μg/kg bw per day TBBPA or 50 μg/kg bw per day TBBPA‐bDiBPrE25 either once or daily for 7 days. There were no histopathological changes in the liver, and no alterations in ALT or AST in the serum.
Summary of liver effects
In summary, TBBPA has shown some evidence of effects on the liver in mice and rats. Increases in liver weight were generally small (< 12%) and occurred at doses of 500 mg/kg bw per day or higher. Histopathological changes were reported in some studies, without a clear dose–response relationship.
No effects on liver weight or histopathology were reported in studies on TBBPA‐bDiBPrE in rats and mice at doses up to about 1000 mg/kg bw per day. No studies are available on the other TBBPA derivatives.
3.1.2.2.2. Effects on the thyroid hormone system
Studies considered in the previous EFSA assessment
In the previous Opinion (EFSA CONTAM Panel, 2011c), it was concluded that TBBPA can affect thyroid hormone homeostasis. Most of the studies indicated a decrease in serum TT4. The effects on other parameters, such as levels of TT3 and TSH, as well as histopathological effects on the thyroid, were not consistent throughout the different studies.
In a 28‐day toxicity study, Wistar rats were dosed with 0, 30, 100 or 300 mg TBBPA/kg bw per day via the diet (Van der Ven et al., 2008). The only effects observed were decreased serum TT4 and increased TT3 levels in males (BMDL10 = 48 and 124 mg/kg bw per day, respectively, as calculated by the authors), and non‐significant trends for these parameters in females (Van der Ven et al., 2008).
In a one‐generation (F1) reproduction study in Wistar rats (10 parental rats per group), TBBPA was administered in the diet at doses of 0, 3, 10, 30, 100, 300, 1000 or 3000 mg/kg bw per day (Van der Ven et al., 2008). Exposure of parental rats started 10 days or 2 weeks before mating for males and females, respectively, and was continued throughout mating, gestation and lactation. After weaning, offspring received continued exposure throughout their lives. Plasma TT4 level was decreased in male and female pups (BMDL10 = 31 and 16 mg/kg bw per day, respectively, as calculated by the study authors) and TT3 was increased in plasma (measured only in female pups BMDL10 = 2.3 mg/kg bw per day). The hypothyroxinaemia correlated with a cluster of developmental parameters (see Section 3.1.2.3) (Van der Ven et al., 2008).
The ECB (2006) reported results of a two‐generation Sprague–Dawley rat study in which TBBPA was administered daily by oral gavage (10, 100 or 1000 mg/kg bw per day) (MPI Research, 2002a, 2003). In the F0 generation, decreases in TT4 levels were found at the high dose in males and females and at 100 mg/kg bw per day in males. In the F1 generation, lower serum TT4 concentrations were observed in both sexes at 100 and 1000 mg/kg bw per day. TT3 serum levels were significantly lower only in F0 males of the 1000 mg/kg bw group. No changes in serum TSH levels, compared to vehicle control animals, were observed in any of the treated groups. No treatment‐related histopathologic changes were observed. The NOAEL was 10 mg/kg bw per day.
Studies published since the previous EFSA assessment
TBBPA
Wistar Han rats were exposed by gavage to 0 or 250 mg TBBPA/kg bw per day for five consecutive days (Sanders et al., 2016). TBBPA treatment did not affect body weight. There were no clinical signs of toxicity. TBBPA treatment resulted in a statistically significant decrease in TT4 compared to control rats. No significant differences were observed between control and TBBPA‐treated rats for TT3, or TSH concentrations in serum.
In an NTP study, male and female F344/NTac rats were administered 0, 10, 50, 100, 500 or 1000 mg TBBPA/kg bw by gavage, 5 days per week for up to 14 weeks (0, 7.1, 35.7, 71.4, 357 or 714 mg/kg bw per day) (NTP, 2014). Dose‐related decreases in TT4 concentrations were observed on day 4 and at week 14 in 500 and 1000 mg/kg bw per day males and females; decreases were also observed on week 14 in males and on day 4 in females in the 100 mg/kg bw per day groups. There was no effect on TT3 and TSH (NTP, 2014). The NOAEL was 50 mg/kg bw per day.
Male and female B6C3F1 mice were administered TBBPA in corn oil by gavage 5 days per week at doses of 0, 10, 50, 100, 500 or 1000 mg/kg bw (0, 7.1, 35.7, 71.4, 357 or 714 mg/kg bw per day), for 14 weeks. No effects on the thyroid were reported. The thyroid hormones were not analysed (NTP, 2014).
TBBPA was administered to CD rats by gavage at doses of 0, 100, 300 or 1000 mg/kg bw per day for 13 weeks (Osimitz et al., 2016). A 6‐week post‐treatment recovery period was included for additional 5 animals/group at 0 and 1000 mg/kg bw per day. There were no treatment‐related effects on mortality, clinical signs, body weight, absolute and relative‐to‐body thyroid/parathyroid weights, thyroid histopathology, serum TSH and TT3 measurements. Mean serum TT4 levels were decreased at all doses in TBBPA‐treated animals (on day 33 and at the termination of dosing in males, and on day 33 in females). These levels returned to baseline after the recovery period. As this change was not dose‐related, the CONTAM Panel did not consider this effect as adverse and identified a NOAEL of 1000 mg/kg bw per day (Osimitz et al., 2016).
In a two‐generation toxicity study, Sprague–Dawley rats were exposed by gavage to 0, 10, 100 or 1000 mg TBBPA/kg bw per day (Cope et al., 2015). Exposure to doses ≥ 100 mg/kg bw per day resulted in decreased serum TT4 levels in F0/F1 males and females. Decreased serum TT3 levels were only observed in F0 males at 1000 mg/kg bw per day at termination. There was no effect on TSH levels up to the highest dose. There were no histopathological alterations in the thyroid (Cope et al., 2015). The NOAEL was 10 mg/kg bw per day.
Yu et al. (2018) exposed young female Sprague–Dawley rat pups (PND21) by gavage for 20 days to 50 mg TBBPA/kg bw per day. TBBPA treatment had no effect on body weight gain nor on thyroid weight. TBBPA did not influence serum levels of thyroid hormones (TT3, TT4 and TSH) and no histopathological changes were observed in the thyroid gland.
In the Choi et al. (2011) paper in which male Sprague–Dawley rat pups were treated orally by gavage with TBBPA at 0, 125, 250 or 500 mg/kg bw per day for 30 days (PND18–PND48), no effect on body weight gain was noted. There were significant decreased serum TT4 levels at 250 mg/kg bw per day, and significant decreased absolute (by 24%) and relative‐to‐body (15%) thyroid weight at 500 mg/kg bw per day. There were no treatment‐related histological findings in the thyroid gland (Choi et al., 2011). The NOAEL was 125 mg/kg bw per day.
Pregnant Sprague–Dawley rats were exposed to 0, 100, 1000 or 10,000 mg/kg TBBPA in the diet from GD10 to PND20. Doses for dams corresponded to 0, 9.5, 87 or 819 mg/kg bw per day from GD10‐20, to 0, 18, 150 or 1466 mg/kg bw per day from PND1–PND9, and to 0, 23, 202 or 2129 mg/kg bw per day from PND9–PND20. Pups were exposed via dams during gestation and lactation until weaning (Saegusa et al., 2009, 2012). Litters were culled to four pups/sex/group on PND2. On PND20, 20 pups/sex/group were subjected to pre‐pubertal necropsy. Remaining animals were sacrificed and necropsied on PND77. As reported previously (Saegusa et al., 2009), there were no statistically significant changes in relative thyroid weight or histopathology in dams on PND20. Though not significant, the incidence of diffuse thyroid follicular cell hypertrophy on PND20 showed a marginal increase from 1000 mg/kg diet. There was no effect of TBBPA on body weight or relative thyroid weight in offspring on PND77. There were no changes in the serum concentrations of thyroid‐related hormone levels (TT4, TT3 and TSH) in male offspring on PND20 or PND77 (Saegusa et al., 2009, 2012). The NOAEL was at the highest concentration tested, i.e. 10,000 mg/kg in the diet of the dams. The actual intake for pups was not calculated by the authors.
In a study by Yang et al. (2016), male and female Sprague–Dawley rats were treated with 0, 5, 50, 250, 1000 mg TBBPA/kg bw per day. It is stated that the study was conducted in accordance with the US‐EPA Office of Chemical Safety and Pollution Prevention (OCSPP) Guideline 890.1450 and 890.1500 (Pubertal Development and Thyroid Function in Intact Juvenile/Peripubertal Male/Female Rats Assay) to assess potential interaction of TBBPA with the endocrine system in an in vivo mammalian system. No further details on study methodology were presented in the paper. The guidelines specify treatments as being daily by oral gavage. Juvenile male rats are exposed from PND23 through PND53. Juvenile female rats were exposed from PND22 through PND42. The levels of serum TT3, TT4 and TSH in rats tested in this study showed that all the indicators presented a non‐monotonic dose‐effect relationship clearly, except TSH in male rats. Serum TT4 and TSH levels of female rats were all higher than those of male rats, whereas serum TT3 showed the opposite trend. There were increased serum TT3 level in female rats between 0 and 50 mg/kg bw per day, decreased from 50 to 250 mg/kg bw per day and then increased from 250 to 1000 mg/kg bw per day. Serum TT4 levels of female rats decreased from 0 to 250 mg/kg bw per day, and then increased from 250 to 1000 mg/kg bw per day. Serum TSH levels of female rats increased from 0 to 5 mg/kg bw per day, decreased from 5 to 250 mg/kg bw per day, and then increased from 250 to 1000 mg/kg bw per day. Serum TT3 levels of male rats increased considerably from 0 to 250 mg/kg bw per day and decreased from 250 to 1000 mg/kg bw per day. Serum TT4 levels of male rats increased from 0 to 5 mg/kg bw per day, decreased from 5 to 50 mg/kg bw per day, increased from 50 to 250 mg/kg bw per day and finally decreased again from 250 to 1000 mg/kg bw per day (Yang et al., 2016).
Male and female C57BL/6 mice were exposed for 5 weeks by gavage to 0, 0.002, 0.02, 2 and 20 mg TBBPA/kg bw per day (Hu et al., 2023). An increased height of thyroid follicular epithelial cells was observed in male mice after exposure to 20 mg/kg bw per day. There was no effect on serum TT3, TT4 or TSH levels in male or female mice.
After parturition, CD‐1 mice dams were administered 0, 15, 150 or 1500 ng TBBPA/mL in drinking water. Based on estimated daily water consumption and body weight, the average daily intake of TBBPA by each dam was estimated by the authors to be about 5, 50 or 500 μg/kg bw per day. Male pups were exposed during lactation (PND0‐21) and after weaning directly (PND22‐56) via drinking water (Song et al., 2024). On PND15, the highest dose of TBBPA caused thyroid histological alteration in male pups: evidence of shrinkage in the follicle size and colloid. On PND35, the serum levels of TT3 and TT4 were lower in the 150 and 1500 ng/mL groups. When exposure to TBBPA continued to adulthood (PND56), the thyroid injury was more severe in male offspring than during lactation. In addition to the same effect as those observed on PND15, compensatory follicular hyperplasia and colloids loss were particularly pronounced in the two highest dose groups and blood sinuses between the thyroid follicles appeared to increase in these two groups. Significant decreases in the serum TT3 and TT4 levels were also observed at these two doses. During puberty and adulthood, the thyroid morphological alterations became more pronounced in the TBBPA‐treated animals. The Panel noted that the histopathological effects on the thyroid and the changes in TT3 and TT4 levels were reported at doses several orders of magnitude lower than in the studies in the preceding paragraphs. The authors noted that exposure through drinking water can allow the animals to receive TBBPA multiple times within 1 day thereby maintaining TBBPA at a relatively stable level in animals, and suggested this may be a reason for the differences observed between gavage and drinking water studies (Song et al., 2024). The authors indicated that the approximate average doses were 5, 50 or 500 μg/kg bw per day for both dams and pups, which is an estimate based on another study (Li et al., 2022). The CONTAM Panel noted that this drinking water study was well conducted; however, the concentrations in the drinking water were not confirmed by analysis of TBBPA, which may be important, e.g. because of the low solubility of TBBPA in water (see Section 1.3.1). The CONTAM Panel considered that there is a high level of uncertainty regarding the doses calculated from the concentrations in drinking water.
TBBPA derivatives
TBBPA‐bDiBPrE 29 was administered by gavage (5 days/week) to F344/NTac rats at 0, 62.5, 125, 250, 500 and 1000 mg/kg bw per day for 14 weeks (NTP, 2017). In the mouse study, TBBPA‐bDiBPrE was administered by gavage to B6C3F1/N mice at 0, 125, 250, 500, 1000 and 2000 mg/kg bw per day (5 days/week) for 14 weeks There were no treatment‐related changes in absolute or relative thyroid weights in male or female rats or mice. There were no gross or histologic lesions in the thyroid of rats or mice that were considered treatment related.
Summary of thyroid effects
In summary, the only notable thyroid effect in TBBPA‐treated rats by gavage was significant reduction of serum TT4 levels occurring at doses from 100 mg/kg bw per day. This change was not accompanied by changes in serum TT3 or TSH levels. In addition, there was no evidence of histopathologic damage in the thyroid (Cope et al., 2015; NTP, 2014).
There was no evidence of any effect on mouse thyroid morphology after repeated exposure by gavage to TBBPA (Cope et al., 2015; NTP, 2014; Osimitz et al., 2016) with the exception of an increased height of thyroid follicular epithelial cells in male mice after exposure by gavage to 20 mg/kg bw per day. There was no effect on serum TT3, TT4 or TSH levels (Hu et al., 2023).
In a study in male mice exposed through drinking water during lactation (PND0‐21) and after weaning (PND22–56) (Song et al., 2024), thyroid morphological alterations accompanied by decreased serum thyroid hormone levels were observed in the TBBPA‐treated animals at concentrations of 150 and 1500 ng/mL. During puberty and adulthood, the thyroid morphological alterations became more pronounced. The CONTAM Panel considered that there is a high level of uncertainty regarding the doses administered via drinking water.
No effects on thyroid weight or histopathology were reported in studies on TBBPA‐bDiBPrE in rats at doses up to about 1000 mg/kg bw (714 mg/kg bw per day), and in mice at doses up to about 2000 mg/kg bw (1429 mg/kg bw per day). No studies are available on the other TBBPA derivatives.
3.1.2.2.3. Effects on kidney
Studies considered in the previous EFSA assessment
Polycystic lesions of the kidney were reported in male and female newborn rats exposed by gavage to TBBPA from PND4‐PND21 to 200 or 600 mg/kg bw per day (Fukuda et al., 2004). The NOAEL was 40 mg/kg bw per day. However, no histopathological changes occurred in the kidneys of young rats (5 weeks old) exposed by gavage for 18 days to 2000 or 6000 mg TBBPA/kg bw per day. The authors concluded that the nephrotoxicity of TBBPA might be specific for newborn rats although the toxic dose level was relatively high (Fukuda et al., 2004).
TBBPA induced oxidative stress in the kidney at high doses. Male rats (8 weeks of age) were treated orally at 0, 200, 500 or 1000 mg/kg in a 14‐day repeated dose study. TBBPA was not toxic to the kidney (Kang et al., 2009).
In a study in ICR mice where TBBPA was administered in the diet (0%, 0.01%, 0.1% or 1%) to dams during gestation (GD0–GD17) and lactation (PND0–PND21), and then directly to offspring till PND27. The doses corresponded to 0, 15.7, 141 or 1640 mg/kg bw per day from GD0‐17, and to 0, 42, 380 or 4156 mg/kg bw per day from PND0‐21. Increases in dilation or atrophy of renal tubules and cysts in the kidney were observed in treated dams or offspring (Tada et al., 2006, 30 ).
Studies published since the previous EFSA assessment
TBBPA
After postnatal exposure (PND18–48) of male Sprague–Dawley rat pups by gavage to 0, 125, 250 or 500 mg TBBPA/kg bw per day, no effects on body weight gain or kidney weights were noted and there were no histological findings in the kidney (Choi et al., 2011).
Male and female B6C3F1/N mice were administered 0, 10, 50, 100, 500 or 1000 mg TBBPA/kg bw by gavage, 5 days per week for 14 weeks (corresponding to 0, 7.1, 35.7, 71.4, 357 or 714 mg/kg bw per day) (NTP, 2014). There were slight but statistically significant decreases in absolute and relative kidney weights in males at 1000 mg/kg bw. In the kidney, incidences of renal tubule cytoplasmic alteration were significantly increased in the 500 and 1000 mg/kg bw male mice dose groups. The severity of the lesion increased with the dose (NTP, 2014). The NOAEL was 71 mg/kg bw per day.
In the Yu et al. (2018) study in which young female rat pups (PND21) were exposed to TBBPA by gavage for 20 days at 50 mg/kg bw per day, TBBPA‐treatment had no effect on body weight gain, however, kidney weight was reduced and there were indications of oxidative stress (decrease of SOD and MDA content) in the kidney (Yu et al., 2018).
TBBPA derivatives
TBBPA‐bDiBPrE 31 was administered by gavage (5 days/week) to F344/NTac rats at doses of 0, 62.5, 125, 250, 500 or 1000 mg/kg bw (0, 45, 89, 179, 357, 714 mg/kg bw per day) for 14 weeks (NTP, 2017). In the mouse study, TBBPA‐bDiBPrE was administered by gavage (5 days/week) to B6C3F1/N mice at doses of 0, 125, 250, 500, 1000 or 2000 mg/kg bw (0, 89, 179, 357, 714, 1429 mg/kg bw per day) for 14 weeks. There were no treatment‐related changes in absolute or relative kidney weights in male or female rats or mice. There were no gross or histologic lesions in rats or mice that were considered treatment related.
Summary of kidney effects
Polycystic lesions of the kidney were reported in male and female neonatal rats exposed by gavage from PND4–21 to ≥ 200 mg TBBPA/kg bw per day. Increases in dilation or atrophy of renal tubules and cysts occurred in the kidney of mice exposed to TBBPA in utero and during lactation. Increases in renal tubule alterations were also seen in adult mice exposed for 14 weeks at doses ≥ 357 mg/kg bw per day.
No effects on kidney weight or histopathology were reported in studies on TBBPA‐bDiBPrE in rats at doses up to 714 mg/kg bw per day, and in mice at doses up to 1429 mg/kg bw per day. No studies are available on the other TBBPA derivatives.
3.1.2.3. Developmental and reproductive toxicity studies
Studies considered in the previous EFSA assessment
The available studies described in the previous Opinion indicated that no effect on fertility, reproductive performance or development was observed after exposure to TBBPA.
Reproductive toxicity of TBBPA was studied in a one‐generation reproduction study in Wistar rats exposed orally via the diet to 0, 3, 10, 30, 100, 300, 1000 or 3000 mg/kg bw per day (van der Ven et al., 2008). In F1 males, exposure to TBBPA caused increased weight of testes (BMDL5 of 0.5 mg/kg bw per day, as calculated by the authors); however, the dose response was unclear. There were no treatment‐related effects on fertility or fecundity or changes in sex ratios in F1 litters.
In a study in ICR mice where TBBPA was administered in the diet at 0%, 0.01%, 0.1% or 1% (corresponding to 0, 15.7, 140.5or 1639.7 mg/kg bw per day from GD0–17, and to 0, 42, 380 or 4156 mg/kg bw per day from PND0–21) to dams from GD0 to weaning at PND27, no effect was observed on the measured reproductive endpoints and there were no effects on reproductive organ weights or histopathological changes (Tada et al., 2006).
ECB (2006) reported results of a two‐generation Sprague–Dawley rat study in which TBBPA (0, 10, 100 or 1000 mg/kg bw per day) was administered daily by oral gavage (MPI Research, 2002a, 2003). No treatment‐related effects were observed in either the F0 or F1 generation based on evaluations of clinical signs of toxicity, oestrous cyclicity, reproductive performance, body weight gain, gestation/lactation body weights or food consumption, gestation length, litter data or on the macroscopic and microscopic evaluations, organ weights, sperm evaluations and primordial follicle counts. No treatment‐related effect was shown in either the F1 or F2 pups with regard to body weights, clinical findings, sex ratios, survival to weaning, macroscopic findings or organ weight data. For neurobehavioural/neuropathological effects, see Section 3.1.2.5.
Studies published since the previous EFSA assessment
TBBPA
No effect on oestrus cycle or on oestradiol concentrations were observed in Wistar Han rats exposed by gavage to TBBPA at 250 mg/kg bw per day for 5 days (Sanders et al., 2016).
In a two‐generation reproductive toxicity study in Sprague–Dawley rats exposed by gavage, no effect on reproduction was observed up to 1000 mg TBBPA/kg bw per day, the highest dose tested (Cope et al., 2015).
No maternal toxicity, embryotoxicity, fetotoxicity, variations or malformations were observed in a developmental toxicity study in Sprague–Dawley rats exposed by gavage from GD0–19 up to 1000 mg TBBPA/kg bw per day. The authors identified a NOAEL for maternal and developmental toxicity of 1000 mg/kg bw per day, the highest dose tested (Cope et al., 2015).
Pregnant Wistar Han rat dams and their progeny were exposed by gavage to 0, 0.1, 25 or 250 mg TBBPA/kg bw per day during gestation and after parturition till weaning of the pups (GD6–PND21). Offspring were exposed directly to the same doses after weaning (PND22–90) (Brown et al., 2020). No differences in the morphology of testes, sperm, prostates or secondary sex organs were observed in F1 males. TBBPA had no impact on the anogenital distance. A delay in the time to preputial separation was found in the 250 mg/kg bw per day group. Although the numbers of sperm collected from the cauda epididymis and vas deferens of the PND90 group was comparable to the control, a significant difference was observed in the 1‐year‐old 25 mg/kg bw per day treated males, which had fewer sperm than control animals, but spermatogenesis was not affected (Brown et al., 2020). The NOAEL was 25 mg/kg bw per day.
No effect on uterus weight or ovary weight was noted in young female Sprague–Dawley rat pups exposed by gavage from PND21–40 to 50 mg TBBPA/kg bw per day (Yu et al., 2018).
In a two‐generation study in CD‐1 mice exposed via drinking water to 200 μg TBBPA/L (approximately 1 μg/mouse per day, equivalent to 35 μg/kg bw per day according to the authors), F2 animals from treated parents exhibited significant increases in the absolute weight of prostate, seminal vesicles and epididymis and a decrease in testicular weight. There was no effect on sperm or spermatogenesis. An increased incidence of apoptosis (number of TUNEL positive cells, without cytological evidence of which cell types are affected) in the testes and changes in the morphometry of seminiferous tubules (decreased epithelial thickness, but without changes in the tubule diameters) was observed in F1‐TBBPA treated animals and F2 animals from F1‐treated females. There was no effect on spermatozoa or spermatogenesis (Zatecka et al., 2013).
CD‐1 mice were exposed from PND0‐56 (via lactation PND0–21 and direct exposure of pups from PND21–56) to 0, 15, 150 or 1500 ng/mL TBBPA in drinking water (according to the authors equivalent to 0, 8, 86 or 880 μg/kg bw per day for dams, and to 0, 2, 19 or 221 μg/kg bw per day for weaned pups) (Li et al., 2022). The authors did not take into account the consumption of water by pups from PND14–21, while for weaned pups, calculations were done between PND21 and PND42. On PND7, TBBPA‐treated male pups presented significantly lower body weight at all doses. Reduced seminiferous tubule area coupled with decreased Sertoli cell and germ cell numbers and marker gene expression occurred in the testes of the two highest dose groups, along with reduced cell proliferation and disordered arrangement of Sertoli cell nuclei. On PND15, most of these testicular alterations were still observed in TBBPA‐treated males, and cytoskeleton damage in Sertoli cells was also observed. On PND56, pups had no severe reproductive impairment; no effect was observed on body weight nor on absolute testis weight, anogenital distance, sperm count and motility, and the serum testosterone level was unaffected. However, TBBPA exposure at the two highest doses resulted in dose‐dependent reductions in the seminiferous tubule area coupled with decreased number of Sertoli cells and spermatogonia and the number of stage VII–VIII seminiferous tubules (at 1500 ng/mL), and cytoskeleton damage in Sertoli cells, along with downregulated expression of marker genes for Sertoli cells, spermatogonia and spermatocyte. The authors concluded that these observations demonstrate that postnatal exposure to TBBPA retarded and disturbed testis development in early life (within the first 2 weeks after birth) and ultimately caused adverse outcomes in adult testes, probably due to the decrease of number of Sertoli cells and dysfunctions in interactions with the other cells of the seminiferous epithelium (Li et al., 2022). The CONTAM Panel noted that this drinking water study was well conducted; however, the concentrations in the drinking water were not confirmed by analysis of TBBPA, which may be important, e.g. because of the low solubility of TBBPA in water (see Section 1.3.1). The CONTAM Panel considered that there is a high level of uncertainty regarding the doses calculated from the concentrations in drinking water.
In another study from the same laboratory, CD1‐mice were exposed to TBBPA postnatally (PND0–56) via drinking water to 0, 150 or 1500 ng/mL (according to the authors equivalent to about 0, 50 or 500 μg/kg bw per day) (Xiong et al., 2023). On PND56, exposure resulted in slight reductions in the anogenital distance, damages in the seminiferous tubules (reduction in seminiferous tubule area and seminiferous epithelium height), reduction of the number of germ cells. The aim of the study was mainly to assess the effect of TBBPA exposure on the testicular susceptibility to follow‐up stress in adulthood. For that, on PND56 a single injection (ip) of busulfan was administered. Within 4 weeks after injection, this leads to spermatogenic impairment (decreased sperm count) in TBBPA‐treated mice but not in non‐treated ones. Busulfan injection caused a significant decrease in testis weight in the mice treated with 1500 ng TBBPA/mL. Three of eight mice exposed to TBBPA at 1500 ng/mL and busulfan developed testicular atrophy, and one individual with testicular atrophy was also observed in the group exposed to 150 ng/mL and busulfan. From PND56–84, animals received sterile water only. On PND84, TBBPA treatment also led to an exacerbated alteration, over the already detected in the previous study with TBBPA only exposure, of microtubule and microfilament damage in seminiferous tubule cells (Sertoli cells) at 150 and 1500 ng/mL. According to the authors, exposure in prepubertal stages of mice to TBBPA would lead to a lower resistance to stressors (busulfan in this case) in the testis, with potential consequences on fertility in males (Xiong et al., 2023). The CONTAM Panel noted that this drinking water study was well conducted; however, the concentrations in the drinking water were not confirmed by analysis of TBBPA, which may be important, e.g. because of the low solubility of TBBPA in water (see Section 1.3.1). The CONTAM Panel considered that there is a high level of uncertainty regarding the doses calculated from the concentrations in drinking water.
Female mice were dietary exposed to 500 μg TBBPA/kg bw per day for 2 weeks prior to mating and time‐mated in trios with unexposed males (i.e. 2:1 female to male ratio) (Reed et al., 2022). In allogeneic pregnancies in mice but not syngeneic pregnancies TBBPA exposure was associated with higher rates of conceptus haemorrhaging that were positively linked to fetal resorption, suggesting mechanisms that involve altered maternal–fetal immune tolerance (Reed et al., 2022).
TBBPA (up to 1000 mg/kg bw per day) exposure for 7 days by gavage was negative for agonistic and antagonistic response with binding affinities relative to the natural 17‐β‐oestradiol in an uterotrophic assay using C57BL/6J ovariectomised adult female mice (Ohta et al., 2012).
TBBPA derivatives
In studies conducted by the NTP (2017), TBBPA‐bDiBPrE was orally administered by gavage to F344/NTac rats at doses of 0, 62.5, 125, 250, 500 or 1000 mg/kg bw, and to B6C3F1/N mice at doses of 0, 125, 250, 500, 1000 or 2000 mg/kg bw for 14 weeks (5 days per week). There were no changes in the number of spermatozoa or spermatids, sperm motility or testis or epididymis weights of dosed males and there were no oestrous cycle changes in dosed females.
The effects of TBBPA‐bDiBPrE on postnatal testis development were investigated in CD‐1 mice by Li, Xiong, Zhang, et al. (2023). Dams and their progeny (10 pups per dam) were administered TBBPA‐bDiBPrE at 0, 150 or 3000 ng/mL (expected doses by the authors of 0, 50 or 1000 μg/kg bw per day) in drinking water from PND0–21. Offspring were further exposed directly from PND22–56. TBBPA‐bDiBPrE concentrations in drinking water were measured on days 0, 1 and 2. According to the authors, based on the daily water consumption and body weight, the estimated daily intakes by dams were 0, 59 ± 9 or 1195 ± 150 μg/kg bw per day, and by weaned pups were 0, 50 ± 9 or 1117 ± 172 μg/kg bw per day. On PND 7, three male pups were sampled from each litter. On PND21, pups were weaned, and one male pup from each of five litters in each group was assigned for extended treatment to the same doses until PND56. TBBPA‐bDiBPrE was detectable in serum from suckling pups on PND14 demonstrating that pups are exposed to TBBPA‐bDiBPrE through milk from the treated dams. On PND7, a decrease in body weight was found in the high dose pups, with no changes in the anogenital distance. On PND7, neonatal males from this group presented reduced seminiferous tubule area, reduced germ cell population as well as reduced number of Sertoli cells per seminiferous tubule. Examination of Sertoli cell microtubule cytoskeleton showed that the microtubule amount per seminiferous tubule was also reduced at 150 ng/mL. When exposure to TBBPA‐bDiBPrE continued to adulthood (PND56), no significant alterations in body weight, testis weight and serum testosterone level were observed, but the sperm count in cauda epididymis was significantly decreased in the high‐dose group, with no significant changes in sperm morphology. On PND56, testes in both dosed groups displayed a smaller seminiferous tubule area. In the two dose groups, the percentage of stage VIII seminiferous tubules was also significantly reduced in a dose‐dependent manner. According to the authors, a few immature germ cells improperly appeared in the lumen. Immature germ cells were also found in the cauda epididymis in the high‐dose group. There were significant decreases in the number of spermatocytes and spermatids per seminiferous tubule in the high‐dose group. Microtubule damage was still remarkable in Sertoli cells in adult testes on PND56; microtubule tracks in stages VI − VII tubules became shorter and thinner in a dose‐dependent manner (Li, Xiong, Zhang, et al., 2023). The CONTAM Panel considered that there is a high level of uncertainty regarding the doses calculated from the concentrations in drinking water, noting that this compound is around a thousand times less soluble than TBBPA. 32
In a follow‐up study (Li, Xiong, Chen, et al., 2023), exposure of five male CD‐1 mice/group (exposed from PND0‐21 via the dams and then directly from PND22) to 0, 150 or 3000 ng/mL continued until 8 months of age. Based on the daily water consumption and body weight, the average daily intakes of TBBPA‐bDiBPrE for dams and weaned males were estimated by the authors to be approximately 50 and 1000 μg/kg bw per day. In addition, male mice exposed from PND0 up to 8‐month age were allowed to mate with non‐treated females for the evaluation of fertility. Following the 8‐month exposure to both doses, body weight, anogenital distance and serum testosterone levels were not significantly altered. In pregnant females, no difference in the gestation period with respect to the control group was detected, but the male‐to‐female sex ratio of the offspring of the TBBPA‐bDiBPrE‐treated males was significantly reduced in the high‐dose group, potentially due to a decreasing trend of the Y‐bearing sperm population in TBBPA‐bDiBPrE treated mice. Testis weight showed a non‐statistically significant decreasing trend. However, reduced epididymal sperm count was observed in the high‐dose group. Morphologically abnormal spermatozoa (amorphous head, no hook, folded neck, and curly or bent tails) increased with increased doses. The percentage of stages VII–VIII seminiferous tubules was significantly reduced even in the low‐dose group. The testes of TBBPA‐bDiBPrE‐treated mice showed reduced seminiferous epithelium height and seminiferous area in stages VII–VIII seminiferous tubules. According to the authors, immature germ cells improperly appeared in the lumen in the treated groups and cell debris was also observed in the epididymis, particularly in the caput epididymidis. There were decreases in the numbers of spermatogenic cells at different developmental stages. Fewer Sertoli cells were observed per seminiferous tubule in treated testes. Moreover, microtubule of Sertoli cells became shorter and thinner following long‐term exposure with a dose‐dependent decrease. High‐dose TBBPA‐bDiBPrE‐treated mice had fewer offspring with a lower number of males. The CONTAM Panel considered that there is a high level of uncertainty regarding the doses calculated from the concentrations in drinking water.
Summary of developmental and reproductive effects
No effect on reproduction were observed in 2‐generation reproductive toxicity studies by gavage in Sprague–Dawley rats up to 1000 mg TBBPA/kg bw per day. No maternal toxicity, embryotoxicity, fetotoxicity, variations or malformations were observed in a developmental toxicity study in Sprague–Dawley rats exposed by gavage from GD0–19 up to 1000 mg TBBPA/kg bw per day. A delay in the time to preputial separation was found in F1 Wistar Han rats exposed by gavage to 250 mg/kg bw per day.
Although studies by gavage in rats TBBPA did not show effects on conventional reproductive endpoints, in studies in mice dosed with TBBPA (150 and 1500 ng/mL) in drinking water, it was reported that postnatal exposure of pups (during lactation and directly) to TBBPA retarded and disturbed testis development in early life. The CONTAM Panel considered that there is a high level of uncertainty regarding the doses calculated from the concentrations in drinking water. Cytological and molecular analysis in the studies administering TBBPA via drinking water suggest that reproduction can be more affected in spermatogenesis than in oogenesis. The studies indicate that Sertoli cells are affected and consequently also the spermatogenic differentiation in the seminiferous epithelium.
The CONTAM Panel noted that the sensitivity to TBBPA might be different in rats and mice but noted the different route of exposure in the studies identified.
Three months exposure by gavage of adult male and female rats and mice to TBBPA‐bDiBPrE was not toxic for the reproduction up to 1000 or 2000 mg/kg bw, respectively. Postnatal exposure of neonatal male mice to TBBPA‐bDiBPrE (at 150 and 1500 ng/mL) through drinking water impaired testis development, reduced germ cell population and the number of Sertoli cells per seminiferous tubule, disrupted spermatogenesis and caused histopathological changes in adult testes (reduced seminiferous tubule area, microtubule damage in Sertoli cells). The CONTAM Panel considered that there is a high level of uncertainty regarding the doses calculated from the concentrations in drinking water.
3.1.2.4. Immunotoxicity studies
Studies considered in the previous EFSA assessment
The previous Opinion (EFSA CONTAM Panel, 2011c) reviewed a few studies that investigated immune parameters. Studies conducted in rats showed no effects of TBBPA on the immune system at oral (diet) doses up to 3000 mg/kg bw per day for 28 days (van der Ven et al., 2008) or 1000 mg/kg bw per day via gavage for 13 weeks (MPI Research, 2002b, as cited in ECB, 2006).
A 28‐day dietary study in mice indicated that TBBPA exposure at 1700 mg/kg bw per day increased the viral titre of host immunity to respiratory syncytial virus (RSV) on day 5 post infection and there were slight histological changes in lung tissue (Watanabe et al., 2010). Cytokine analysis of bronchoalveolar lavage fluid (BALF) from RSV‐infected mice treated with TBBPA, revealed altered concentrations of several cytokines (tumour necrosis factor (TNF)‐α, interleukin (IL)‐6 and interferon (IFN)‐γ, IL‐4, IL‐10, Th2) in comparison to infected mice not treated with TBBPA. An increase of the fraction of immature B‐lymphocytes in the bronchoalveolar lavage fluid was also observed.
Studies published since the previous EFSA assessment
TBBPA
Takeshita et al. (2013) examined the effect of Brazilian propolis (AF‐08) on the exacerbation of RSV infection by TBBPA exposure in mice. Four‐week‐old female BALB/c were given feed mixed with 1% TBBPA alone, 0.02% AF‐08 alone or 1% TBBPA and 0.02% AF‐08 for 4 weeks and then intranasally infected with RSV. Although the corresponding TBBPA dose was not given in the article, it was estimated to be approximately 1700 mg/kg bw per day in a previous study from the same laboratory using the same inclusion rate and age of mice (Watanabe et al., 2010). The pulmonary viral titre was approximately doubled in mice fed the diet containing 1% TBBPA, compared with the control. Animals given 1% TBBPA combined with 0.02% propolis showed significantly lower pulmonary viral titres compared with those fed 1% TBBPA alone, suggesting a protective effect. AF‐08 alone had no effect on pulmonary viral titre in RSV‐infected mice. Neither TBBPA nor propolis treatment affected the titres of RSV antibodies in sera of mice. Serum INF‐γ concentrations were elevated in TBBPA exposed mice but reduced below that of unexposed controls following co‐treatment with propolis.
The effects of TBBPA on perinatal immunity of mice was evaluated using an RSV infection model (Watanabe et al., 2017). TBBPA was mixed at 0, 0.01, 0.1 or 1.0% into the powder diet given to 8‐week‐old pregnant female mice from GD10 to weaning of the pups on PND21 (equivalent to doses of 0, 20, 200 or 2000 mg/kg bw per day). 33 On PND28, two to three female offspring per group were infected with RSV (strain A2) intranasally. Body weight, food consumption and general condition of TBBPA‐treated dams were unaffected in comparison to controls. There was no effect on litter size, survival rate, food consumption, behaviour or other signs of toxicity in offspring of the TBBPA‐treatment group up to weaning in comparison to controls. On Day 5 post‐infection, the pulmonary viral titre was 20‐fold higher in offspring from females given feed containing 0.1% TBBPA, compared to controls. There was a trend for an increase in viral titre also in pups from dams fed 0.01% TBBPA, but this was not statistically significant. However, pups from dams fed a diet containing 1.0% TBBPA showed > 100‐fold reduction in pulmonary viral titres compared with pups from unexposed dams. Qualitative analysis indicated histological changes in lung tissues (interstitial pneumonia) of RSV‐infected offspring, which appeared to be exacerbated in offspring from females fed diets with 0.1 and 1% TBBPA in comparison to the control. Gene expression of interleukin (IL)‐24 in lung tissue in offspring from TBBPA‐treated mice was elevated by 3.59‐fold in a microarray assay, but without statistics shown.
TBBPA derivatives
No studies were identified.
Summary of immunotoxic effects
The limited studies on immunotoxic effects suggest that exposure to TBBPA directly from the diet or in utero can have effects on immune system in mice (200 mg/kg bw per day), increasing the titre of RSV virus following infection. The increase in viral titre was associated with changes in serum cytokine concentrations. Rats exposed to much higher doses of TBBPA (up to 3000 mg/kg bw per day) showed no effects on the immune system.
3.1.2.5. Neurotoxicity/neurobehavioural studies
Studies considered in the previous EFSA assessment
Few in vivo neurotoxicity studies were reported in the previous EFSA Opinion (EFSA CONTAM Panel, 2011c) and have provided contradictory results, especially about to the developmental neurotoxic potential of TBBPA.
No effects were observed in adult mice exposed to a single TBBPA administration (0.75 or 11.5 mg/kg bw by gavage) at PND10 (Eriksson et al., 2001).
No behavioural effects were reported in adult Sprague–Dawley rats daily exposed to TBBPA for 13 weeks at doses 0, 100, 300 or 1000 mg/kg bw per day by gavage (MPI Research, 2002b, as cited by ECB, 2006).
Two two‐generation studies conducted in rats, one using Crl:CD(SD) IGS BR rats (Schroeder, 2002, as cited by Williams and deSesso, 2010), and the other using Wistar rats (MPI Research, 2002a, 2003, as cited by ECB, 2006), did not show any behavioural effects in F2 pups tested for locomotor activity, spatial learning; passive avoidance learning and auditory perception. Doses used for exposure ranged from 0 to 1000 mg TBBPA/kg bw per day by gavage.
In addition, several studies did not show any histological modifications nor synaptic‐related protein expression in brains of rats exposed during development to TBBPA (Saegusa et al., 2009; Viberg & Eriksson, 2011).
A one‐generation reproductive toxicity study (Lilienthal et al., 2008) was considered in the previous Opinion. This study was conducted in Wistar rats with exposure to TBBPA (0, 3, 10, 30, 100, 300, 1000 or 3000 mg/kg bw per day) starting prior to mating (10 weeks in females and 2 weeks in males), during mating, gestation and lactation. After weaning (PND21) pups were directly exposed to the same doses as those used in the parents. Impairments in brainstem auditory evoked potentials were reported in F1 animals of 50–110 days of age, more related to cochlear disturbances in females and effects on transmission of the signal in the cochlear nerve in males. The authors calculated BMDL5 values ranging from 1 to 40 mg/kg bw for hearing threshold disturbances (depending on the signal frequency tested) in females, whereas a BMDL5 of about 8 mg/kg bw based on auditory dysfunction was calculated in both sexes. These BMDL5s were not considered further for the risk characterisation in the previous Opinion due to the high level of uncertainty in the BMD modelling, and because the increased thresholds in the brainstem auditory evoked potentials were difficult to interpret.
Behavioural alterations in the open field, contextual fear conditioning and Y maze tests 3 h after dosing were reported in 3‐week‐old mice exposed to a single dose of TBBPA at three different dose levels (0.1, 5 or 250 mg/kg bw, Nakajima et al., 2009). These results were not considered for risk characterisation in the previous Opinion due to unclear dose–response relationships regarding the results obtained in the different behavioural paradigms used and the limited information given about the experimental protocol.
Studies published since the previous EFSA assessment
TBBPA
CD‐1 mice (5 animals per group) were orally treated (from the tip of a pipette) with 0.2 mg TBBPA/kg bw per day or oil (vehicle) from GD8 through PND21 (Kim et al., 2015). The litters were not culled, and dams and pups were kept together until weaning on PND21. At PND21, one male pup per litter was randomly selected and tested in the open field. In parallel, three other male pups from each litter were randomly selected and housed together until postnatal week (PNW) 15. This formed a group described as a triad by the authors of the study. Twenty triads of cohabited littermates per group were considered for the behavioural testing. The animals of each triad were tested for their social behaviour and interest for exploration of an unfamiliar male mouse of the same age and strain in a 3‐chambers test at PNW10, and the social hierarchy assessed using a tube test (PNW11) and a urine marking test (PNW12–14). Social dominance was assessed a second time using the tube test at PNW15. Results showed a higher variation in litter sizes reported in the TBBPA group (5, 7, 11, 14, 15 pups/litter) compared to control litters (11–15 pups/litter). There were no significant changes in average pup weight adjusted for litter size at birth. No significant differences between TBBPA‐exposed animals and controls in the open‐field were noted at PNW10. In the sociability 3‐chamber test at PNW10, TBBPA‐exposed animals displayed reduced interest in social novelty as they moved significantly shorter distances and spent less time in the zone of wire cage housing the unfamiliar male mouse. No differences in the organisation of the social hierarchy were observed at PNW11 and PNW15 (tube test) and PNW12–14 (urine marking test). A LOAEL of 0.2 mg/kg bw per day was identified based on the time spent to explore the unfamiliar mouse in the 3‐chamber test. The CONTAM Panel noted that this effect is indicative of disturbances of social behaviour and therefore considered as adverse. A possible link between the oestrogenicity of TBBPA and its effects on social behaviour is suspected due to the known relationship between sociability and gonadal hormones disturbances as reported in gonadectomised male and female mice (Karlsson et al., 2015).
Male C57Bl6/J mice (5‐week‐old) were administered TBBPA by gavage at 0, 20, 100 or 500 mg/kg bw per day for 2 weeks (PND42–56), and behavioural tests were performed from PND56–65. Mice were assessed on day 2 and 8 of test trial for learning and memory in a passive avoidance test (Kim et al., 2017). TBBPA significantly reduced the latency to entry the aversive compartment on day 2 at doses 100 and 500 mg/kg bw per day, and on day 8 only at the highest dose 500 mg/kg bw per day. The memory retention impairment was associated with a reduction in hippocampal neurogenesis possibly mediated by neuroinflammation and BDNF‐CREB (Brain‐Derived Neurotrophic Factor‐cAMP Response Element‐Binding protein) signalling. The NOAEL was 20 mg/kg bw per day based on memory retention impairment at day 2.
In a first experiment (one dose level, pilot study) by Rock et al. (2019), Wistar rat dams were orally exposed using cookie treats to 0 or 0.1 mg TBBPA/kg bw daily from GD9 to PND21 to assess offspring (both sexes) activity and anxiety‐related behaviours. At PND1, litters were standardised to 10 pups with a 5M:5F sex ratio whenever possible. Pups were tested for anxiety in a light/dark box (PND110–120) and an elevated plus maze (PND185–192), and activity in running wheel apparatus for 63 h (PND160–170). No significant differences between the two experimental groups were found in both mazes except for an increasing activity in TBBPA‐exposed females compared to controls (p < 0.05) during the 3 days of testing in the running wheel apparatus.
The second experiment by Rock et al. (2019) consisted of Wistar female rat dams orally administered TBBPA at 0, 0.1, 25 or 250 mg/kg bw per day by gavage from GD6 to PND21. Pups were then directly dosed from PND22 to PND90. Litters were standardised to 8 (4M:4F sex ratio whenever possible) on PND4. Rats were assessed for locomotor activity and anxiety in the open field (two times, at PND90 and PND145–150), the light/dark box (PND150–155) and the elevated plus maze (PND155–160). No effects of TBBPA exposure were found in female offspring in both mazes. In males, time in the centre and entries into the centre measured in the open‐field at PND90 were significantly reduced while latency to enter the centre was significantly increased only in the TBBPA 25 mg/kg bw per day exposure group. At PND145, only the latency to enter the centre was significantly reduced in males of all three TBBPA exposure groups, but the CONTAM Panel noted high variability of this behaviour in the control group and the lack of dose‐dependency. In the light/dark box, a significant linear trend with the dose was observed for latency to enter the light part of the maze. The same trend was reported for number of entries in the light box, but it was not significant. In the elevated plus maze, a significant linear trend with the dose was observed for open arm entries with a significant variation compared to controls found in both the TBBPA 25 and 250 mg/kg bw per day dose groups. No significant effect of exposure was observed for time spent in either the open arms or the closed arms. A NOAEL of 0.1 mg/kg bw per day and a LOAEL of 25 mg/kg bw per day were identified based on the reduced number of entries in the light box in males. The doses used in the study, especially the large gap between the lowest and the middle one, were selected based on TBBPA effective doses used in previous studies conducted by the NTP and in the first experiment reported in the present paper.
A study from Park et al. (2016) investigated the effect of TBBPA on hearing function by recording the auditory brainstem response after 30 days of treatment with 0 or 250 mg/kg bw per day of TBBPA by gavage in ICR mice aged 8 weeks. The results showed higher average auditory brainstem response threshold in TBBPA‐exposed animals compared to controls in all the frequency ranges tested.
In a two‐generation toxicity study, Sprague–Dawley rats were exposed by gavage to 0, 10, 100 or 1000 mg TBBPA/kg bw per day (Cope et al., 2015). The study was performed according to OECD TG 416. Parental males and females were treated with TBBPA prior to and during mating, and females through gestation and lactation until weaning of the pups, followed by treatment of the pups. One male and one female from each litter in each dose group were randomly selected to become F1 parents, and the same procedure was applied for F2 generation and TBBPA exposure. The neurological examination of the F1 parental animals at GD9 and GD18 and PND4 and PND14 consisted in the assessment of a set of behavioural and physiological markers of well‐being and good development. F2 generation animals underwent neurological examinations including an FOB (Functional Observation Battery), the assessment of motor activity and coordination, emotionality, conditioned learning and spatial memory. No behavioural effects of TBBPA were observed in the F1 and F2 generations. Postmortem brain morphometric analyses of samples from all major brain regions were performed in F2 generation 11‐ and 60‐day old animals. The sole histological lesion was a significant reduction of thickness of the parietal cortex at the level of infundibulum in 11‐day‐old animals at the maternal dose of 1000 mg/kg bw per day. This reduction has been observed in both males and females and was not associated with histological changes in other parts of the parietal cortex and behavioural modifications related to motor activity, emotionality or learning performances. A NOAEL of 100 mg/kg bw per day for this effect was identified.
Song et al. (2024) conducted a study in CD‐1 mice by exposing lactating females to 0, 15, 150 or 1500 ng TBBPA/mL in drinking water equivalent to 0, 5, 50 and 500 μg TBBPA/kg per day according to the authors, 5 females per group of treatment (see also Section 3.1.2.2.2). At PND21, pups were weaned and maintained for continuous exposure to TBBPA according to the same protocol as for the mother. On PND35 and PND56, one male per litter was selected and tested for neurobehavioural performances in the open‐field and the elevated plus maze. On PND35, the distance covered by the animals in the open field (including both border and centre) was significantly increased in the group exposed to TBBPA at 15 ng/mL whereas no significant effects have been observed with the two highest doses. In the elevated plus maze, the time spent in the closed arms was reduced in a significant way in the lowest dose group and increased in the highest dose group. Such results were in line with concomitant changes in the exploration of open arms as reflected by a significant reduction in the number of open arm entries observed in mice exposed to TBBPA at 1500 ng/mL. All these results suggest some contrasting effects of TBBPA on the level of anxiety of juvenile mice according to the dose (reduction of anxiety level at TBBPA at 15 ng/mL and increase at TBBPA at 1500 ng/mL). In adulthood (PND56), no significant variations in the open field have been measured whereas a significant increase in the number of open arm entries in the elevated plus maze has been observed in mice exposed to TBBPA at 15 ng/mL. No other significant changes in this maze have been reported. Concomitant reductions in the serum level of serotonin were observed in mice exposed to the two highest doses of TBBPA at both stages (PND35 and PND56). The unclear dose–response relationship regarding the behavioural results reported in this study does not allow to identify a NOAEL or LOAEL. The CONTAM Panel considered that there is a high level of uncertainty regarding the doses calculated from the concentrations in drinking water. The CONTAM Panel noted that this drinking water study was generally well conducted, however, the concentrations in the drinking water were not confirmed by analysis of TBBPA, which may be important, e.g. because of the low solubility of TBBPA in water (see Section 1.3.1).
TBBPA derivatives
No studies were identified.
Summary of neurotoxicity/neurobehavioural effects
Whereas few in vivo studies regarding the neurotoxicity of TBBPA were available in the previous EFSA Opinion published in 2011, seven new in vivo studies have been added to the present Opinion. Some of them suggest the potential neurotoxicity of TBBPA. The study from Lilienthal et al. (2008), detailed in the previous Opinion, reported the ototoxicity of TBBPA in rats early exposed. The CONTAM Panel could not identify a NOAEL/LOAEL, and the increased thresholds in the brainstem auditory evoked potentials were difficult to interpret.
Several new studies demonstrate the ability of TBBPA to induce long‐term behavioural disturbances after an early exposure. The study by Kim et al. (2015) reported a reduction in the interest for social novelty of adult mice exposed to TBBPA at 0.2 mg/kg bw per day (GD8–PND21). Kim et al. (2017) reported a memory retention impairment in a passive‐avoidance test performed in adult mice (PND56–65) previously exposed for 2 weeks (PND42–56) to TBBPA by gavage leading to the identification of a NOAEL of 20 mg/kg bw per day. Rock et al. (2019) reported two experiments: a 1st experiment that reported the spontaneous locomotor activity in a running wheel apparatus for 72 h of adult rats orally exposed to 0.1 mg/kg bw per day of TBBPA (GD9–PND21), and a second experiment that assessed the level of activity and anxiety of adult animals orally exposed to 0.1, 25 or 250 mg/kg bw per day of TBBPA (GD6–PND21). A NOAEL of 0.1 mg/kg bw per day and a LOAEL of 25 mg/kg bw per day were identified based on the increase of the level of anxiety reported in the second experiment. The CONTAM Panel noted the large gap between the lowest and middle dose. The CONTAM Panel also noted that the 1st experiment in this study was a one dose level study, and that the measurement of the level of activity in the running wheel was not investigated in the 2nd experiment. However, such results are supportive of those reported by Kim et al. (2015).
3.1.2.6. Genotoxicity studies
Studies considered in the previous EFSA assessment
No studies were found in the open literature, but in the ECB risk assessment report (ECB, 2006), in the literature review by NIEHS (2002) and in the IPCS report (WHO/IPCS, 1995) a number of in vitro genotoxicity tests were reported.
Negative results were obtained in reverse mutation assays with TBBPA on Salmonella Typhimurium with strains TA92, TA98, TA100, TA1535, TA1537 and TA1538 and in yeast Saccharomyces cerevisiae strains D3 or D4 in the presence and in the absence of metabolic activation (Ethyl Corp., 1981; Litton Bionetics, Inc., 1976, 1977a, 1977b; Mortelmans et al., 1986; SRI Int., 1978, as cited by NIEHS, 2002; Velsicol Chemical Corporation, 1978, as cited in ECB, 2006). TBBPA gave also a negative response in an unconventional in vitro Sp5/V79 and SPD recombination assay (Helleday et al., 1999, as cited in ECB, 2006).
In the presence and absence of metabolic activation, TBBPA was negative in an in vitro chromosomal aberration test in human peripheral lymphocytes (BioReliance, 2001, as cited in ECB, 2006) and it did not induce sister chromatid exchanges in CHO cells (Cavagnaro and Cortina, 1984, as cited by WHO/IPCS, 1995). It was also negative in vitro in a rat hepatocyte unscheduled DNA synthesis assay (Cavagnaro and Sernau, 1984, as cited by WHO/IPCS, 1995).
It was concluded that the in vitro data indicate that TBBPA was not genotoxic. In vivo genotoxicity data were not available.
Studies published since the previous EFSA assessment
Since the publication of the previous Opinion, genotoxicity studies have been identified for TBBPA and TBBPA‐bDiBPrE. Details of the in vitro studies and in vivo assays are reported in Tables 10 and 11, respectively.
TABLE 10.
In vitro genotoxicity studies on TBBPA and TBBPA‐bDiBPrE published since the previous Opinion.
| Type of test experimental test system | Test substance | Exposure conditions | Result | Reference |
|---|---|---|---|---|
|
Reverse gene mutation assay (Ames test) S. Typhimurium TA98, TA100, TA1535, TA1537 and E. coli WP2 uvrA |
TBBPA Purity: NR Vehicle: NR |
± S9 mix −S9: tested up to 156 μg/plate, with TA1537, up to 625 μg/plate with TA1535, and over 2500 μg/plate with other strains +S9: tested up to 313 μg/plate with TA1537 and up to 5000 μg/plate with other strains Metabolic activation from liver S9 fraction from phenobarbital and 5,6‐benzoflavone‐induced rats Positive and negative controls: used |
Negative | Shibuya (2001 as described in NICNAS, 2020) |
|
Reverse gene mutation assay (Ames test) S. Typhimurium strains TA98, TA100, TA1535, TA1537 and TA1538 |
TBBPA Purity: NR Vehicle: DMSO |
± S9 mix Tested up to 500 μg/plate Metabolic activation with liver S9 fraction from Aroclor 1254‐induced rats Positive and negative controls: used |
Negative | Curren et al. (1981 as described in NICNAS, 2020) |
|
Reverse gene mutation assay (Ames test) S. Typhimurium TA98, TA100, TA1535 or TA1537 |
TBBPA Purity: 99% Vehicle: DMSO |
Preincubation method ± S9 mix 0, 100, 333, 1000, 3333 or 10,000 μg/plate Metabolic activation with S9 fraction from Aroclor 1254‐induced rat and hamster liver Positive and negative controls: used |
Negative | NTP (2014) |
|
Reverse gene mutation assay (Ames test) S. Typhimurium strains TA98 or TA100, or in E. coli strain WP2 uvrA/pKM101 |
TBBPA Purity: 99% Vehicle: DMSO |
Preincubation method ± S9 mix 0, 50, 100, 250, 500, 1000, 3000 or 6000 μg/plate Metabolic activation with S9 fraction from Aroclor 1254‐induced rat liver Positive and negative controls: used |
Negative | NTP (2014) |
|
Chromosomal aberration test Chinese hamster lung cells |
TBBPA Purity: NR Vehicle: DMSO |
± S9 mix Exposure: −S9: 6h expo. up to 6.5 μg/mL, 24h expo; up to 60 μg/mL +S9: 6h expo. up to 30 μg/mL Metabolic activation with liver S9 fraction from phenobarbital and 5,6‐benzoflavone‐induced rats Positive controls: MMC and CP |
Negative | Yamakage (2001, as described in NICNAS, 2020) |
|
Chromosomal aberration test Human peripheral lymphocytes |
TBBPA Purity: NR Vehicle: DMSO |
± S9 mix Exposure: −S9: 4‐h expo up to 100 μ/mL, 20‐h expo: up to 75 μg/mL +S9: 4‐h expo up to 50 μg/mL Harvesting: 20 h after initiation of treatment Metabolic activation with liver S9 fraction from Aroclor 1254‐induced rats Positive controls: MMC and CP |
Negative | Gudi (2001, as described in NICNAS, 2020) |
|
γH2AX Metabolically competent HepaRG cells |
TBBPA Purity: NR Vehicle: DMSO |
0, 2.5, 10 or 20 μM Measures using automated in situ detection of γH2AX positive cells after 1, 7 and 14 days Cytotoxicity: MTT assay |
Negative No cytotoxicity |
Quesnot et al. (2016) |
|
Comet assay (alkaline) Human acute monocytic leukaemia (THP‐1) |
TBBPA Purity: > 98% Vehicle: DMSO |
−S9 mix 0, 5, 20, 40, 60, 80 or 100 μg/mL Exposure: 1 h Positive control: no |
Positive at all concentrations Concentration related increase of % tail DNA |
Wang et al. (2020) |
|
Comet assay (alkaline) Human peripheral blood mononuclear cells |
TBBPA Purity: 99% Vehicle: DMSO |
−S9 mix 0, 0.01, 0.1, 1, 10 μg/mL Exposure: 24 h Positive control: H2O2 |
Positive Concentration‐related increases in SSB or DSB (as measured by the % DNA in the Comet tail) from 0.1 μg/mL Oxidative damage to DNA pyrimidines (at 0.1 and 1 μg/mL) or purines (from 0.01 μg/mL) using the enzymes endo III or hOGG1, respectively |
Barańska, Woźniak, et al. (2022) |
|
Comet assay (neutral) Human peripheral blood mononuclear cells |
TBBPA Purity: 99% Vehicle: DMSO |
−S9 mix 0, 0.01, 0.1, 1, 10 μg/mL Exposure: 24 h |
Positive Increases in DSB at 1 and 10 μg/mL |
Barańska, Woźniak, et al. (2022) |
|
Reverse gene mutation assay (Ames test) S. Typhimurium strains TA98, TA100 or TA102 |
TBBPA‐bDiBPrE Purity: 94% Vehicle: DMSO |
Preincubation method ± S9 mix 0, 100, 333, 1000, 3333, 10,000 μg/plate Metabolic activation: Aroclor 1254‐induced male Sprague–Dawley rat liver Positive and negative controls used |
Negative | NTP (2017) |
Abbreviations: CP, cyclophosphamide monohydrate; DMSO, dimethyl sulfoxide; DSB, double strand breaks; H2O2, hydrogen peroxide; MMC, cross‐linking agent mitomycin C; MTT, tetrazolium salt (3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide); NR, not reported; SSB, single strand break.
TABLE 11.
In vivo genotoxicity studies on TBBPA and TBBPA‐bDiBPrE.
| Type of test experimental test system | Test substance | Exposure conditions | Result | Reference |
|---|---|---|---|---|
|
Micronucleus test Peripheral blood samples B6C3F1/N mice 5M, 5F |
TBBPA Purity: 99% Vehicle: corn oil |
0, 10, 50, 100, 500, 1000 mg/kg bw Exposure: gavage for 3 months (5 days/week), harvesting at end of treatment Analyses of 2000 normochromatic erythrocyte No positive control |
Inconclusive (low relevance) No increases in the frequencies of micronucleated erythrocytes No significant changes in the percentage of circulating PCE indicating no bone marrow toxicity Systemic toxicity: Increases in absolute and relative liver weights in 500 mg/kg bw males (114% of control for both) and 1000 mg/kg bw males (111% and 119% of control, respectively) and females (113% and 112% of control, respectively). Absolute and relative kidney weights were significantly decreased (86% and 91% of control, respectively) and spleen (108% and 116% of control, respectively) weights were significantly increased in 1000 mg/kg bw males Increased incidences of renal tubule cytoplasmic alterations at 500 and 1000 mg/kg bw per day in males No toxicokinetic study in mice. Low bioavailability of TBBPA in rats |
NTP (2014) |
|
Micronucleus test Peripheral blood samples B6C3F1/N mice 5M, 5F |
TBBPA‐bDiBPrE Purity: 94% Vehicle: corn oil |
0, 125, 250, 500, 1000, 2000 mg/kg bw Exposure: gavage for 3 months (5 days/week), harvesting at end of treatment Analyses of 2000 normochromatic erythrocyte No positive control |
Inconclusive (low relevance). No increases in the frequencies of micronucleated erythrocytes No significant changes in the percentage of circulating PCE indicating no bone marrow toxicity No signs of systemic toxicity |
NTP (2017) |
Abbreviations: F, female; M, male; PCE, polychromatic erythrocytes.
TBBPA
TBBPA did not induce reverse gene mutation in S. Typhimurium strains TA98, TA100, TA1535, TA1537, TA1538 and Escherichia coli WP2 uvrA and WP2 uvrA/pKM101 with and without metabolic activation (Curren et al., 1981, as described in NICNAS, 2020; NTP, 2014; Shibuya, 2001).
TBBPA did not cause structural chromosome aberrations or polyploidy in an in vitro chromosomal aberration test in Chinese hamster lung cells in the absence or presence of metabolic activation (Yamakage, 2001, as described in NICNAS, 2020). No induction of chromosomal aberrations was observed in human peripheral lymphocytes exposed to TBBPA in presence or absence of rat liver S9 (Gudi and Brown, 2001, as described in NICNAS, 2020).
Metabolically competent HepaRG cells were exposed daily to 0, 2.5, 10 or 20 μM TBBPA and genotoxicity was measured using automated in situ detection of γH2AX positive cells after 1, 7 and 14 days. The toxicity was evaluated by MTT assay. TBBPA did not induce γH2AX positive cells and was not cytotoxic at the concentrations tested (Quesnot et al., 2016).
TBBPA was tested in an alkaline Comet assay in human peripheral blood mononuclear cells (PBMCs) exposed for 24 h to concentrations of 0, 0.01, 0.1, 1 or 10 μg/mL. Concentration‐related increases in single strand breaks (SSB) or double strand breaks (DSB) (as measured by the % DNA in the Comet tail) were observed from 0.1 μg/mL. Increases in DSB were also observed at 1 and 10 μg/mL in the neutral version of the Comet assay. TBBPA induced oxidative damage to DNA pyrimidines (at 0.1 and 1 μg/mL) or purines (from 0.01 μg/mL) as detected by a modified comet assay using the enzymes endo III or hOGG1, respectively, in the alkaline Comet assay (Barańska, Woźniak, et al., 2022).
Concentration related increases of % tail DNA were also observed in an alkaline Comet assay in human acute monocytic leukaemia (THP‐1) cells exposed for 1 h to 5, 20, 40, 60, 80 or 100 μg TBBPA/mL (Wang et al., 2020).
In vivo, no increases in the frequencies of micronucleated erythrocytes were observed in peripheral blood samples from male or female B6C3F1/N mice after 3 months exposure (5 days/week) to TBBPA (10–1000 mg/kg bw) by gavage. The results of this study have low relevance as the conditions of testing were not in compliance with OECD TG 474 (see Table 11). No significant changes in the percentage of circulating polychromatic erythrocytes (PCE) were observed in dosed mice, indicating that bone marrow exposure was not demonstrated, and only mild systemic toxicity was observed (NTP, 2014).
TBBPA derivatives
TBBPA‐bDiBPrE was not mutagenic in S. Typhimurium strains TA98, TA100 or TA102, with or without rat liver S9 (NTP, 2017).
No significant increases in the frequencies of micronucleated erythrocytes were observed in peripheral blood samples from male or female B6C3F1/N mice exposed to TBBPA‐bDiBPrE (125–2000 mg/kg bw) after 3 months exposure (5 days/week) by gavage. No significant changes in the percentage of polychromatic erythrocytes (PCE) were seen in these mice, indicating that bone marrow exposure was not demonstrated and no signs of systemic toxicity were observed (NTP, 2017).
Summary of genotoxicity
In summary, TBBPA was not mutagenic in vitro in bacterial, yeast and mammalian mutagenicity assays in the presence or absence of metabolic activation; it did not cause structural chromosome aberrations in Chinese hamster lung and human peripheral lymphocytes. In in vitro Comet assays, TBBPA induced SSB and DSB as well as oxidised DNA bases in human peripheral blood mononuclear cells. It also induced SSB in THP‐1 cells. Due to the lack of validation and appropriate OECD guidelines for the in vitro Comet assay, these results were regarded of limited relevance. Moreover, in the absence of other positive results, these in vitro Comet assay results were not considered a sign of mutagenic potential.
In vivo, no increase in micronuclei was observed in a peripheral blood micronucleus test in mice. The results of this test were of low relevance with no convincing evidence for target organ exposure. In view of the lack of effect in vitro, according to EFSA Scientific Committee (2011) it is not necessary to test in vivo.
Based on the available evidence TBBPA is not genotoxic.
TBBPA‐bDiBPrE was not mutagenic in vitro in S. Typhimurium. In vivo, no increase in micronuclei was observed in a peripheral blood micronucleus test in mice exposed at very high dose. However, there was no convincing evidence for target organ exposure. Based on these very limited data no conclusion can be taken on the genotoxic potential of TBBPA‐bDiBPrE.
3.1.2.7. Carcinogenicity
Studies considered in the previous EFSA assessment
The previous Opinion reported that there were no long‐term toxicity or carcinogenicity studies of TBBPA in animals. The CONTAM Panel noted however that TBBPA was not genotoxic in vitro (EFSA CONTAM Panel, 2011c).
Administration of TBBPA to neonatal rats via lactation and then for 2 weeks after weaning followed by administration of 7,12‐dimethylbenz(a)anthracene (DMBA) and N‐bis(2‐hydroxypropyl)nitrosamine (DHPN) later in life, increased the incidence of thyroid follicular adenomas and transitional cell papillomas in the urinary bladder, but no increase in the incidence of malignant tumours (Imai et al., 2009).
Studies published since the previous EFSA assessment
TBBPA
Since the previous Opinion, the NTP has performed carcinogenicity studies on TBBPA in rats and mice at doses of 0, 250, 500 or 1000 mg/kg bw administered by gavage 5 days per week for 2 years (corresponding to 0, 179, 357 or 714 mg/kg bw per day) (NTP, 2014; also reported in Dunnick et al., 2015) (see details in Appendix D, Table D1).
In rats, there was no effect on survival. There was a dose‐related decrease in body weight in males by 12% but not in females. There were no non‐neoplastic effects in males but abnormalities in the uterus and ovary were reported in females. In the males, the only neoplastic lesions were interstitial cell adenoma of the testis, with a low incidence. For the females, increased incidences of uterine tumours were reported from review of transverse sections, and the tissues were subjected to additional longitudinal review. When the two reviews were combined, there were significant positive trends in the incidences of adenocarcinoma and of combined adenoma, adenocarcinoma and malignant mixed Müllerian tumour (MMMT), the incidences being statistically significant in the 500 and 1000 mg/kg bw groups. Uterine atypical hyperplasia, which is considered to be a preneoplastic lesion, was also observed. Table 12 summarises the tumour incidences in rats. The NTP report concluded that there was ‘equivocal evidence of carcinogenic activity’ in male rats based on the occurrence of testicular adenoma. There was ‘clear evidence of carcinogenic activity’ in female rats based on increased incidences of uterine epithelial tumours (predominantly uterine adenocarcinoma).
TABLE 12.
Summary of tumour incidences in rats in the NTP (2014) study.
| 0 mg/kg bw a | 250 mg/kg bw | 500 mg/kg bw | 1000 mg/kg bw | |
|---|---|---|---|---|
| Females – Uterus b | ||||
| Atypical endometrial hyperplasia | 2 c | 13 d | 11 d | 13 d |
| Adenoma | 3 | 2 | 4 | 6 |
| Adenocarcinoma | 4I | 10 | 15 d | 16 d |
| MMMT | 0 | 4 | 0 | 2 |
| Adenoma, adenocarcinoma or MMMT (combined) e | 6 f | 11 | 16 d | 19 d |
| Males – Testis | ||||
| Interstitial cell adenoma | 0 | 0 | 1 | 3 |
Abbreviation: MMMT, Malignant mixed Müllerian tumour.
Groups of 50 rats were dosed by gavage 5 days per week for 2 years (averaged doses = 0, 179, 357, 714 mg/kg bw per day).
Based on original transverse review and additional longitudinal review, combined.
Statistically significant trend at p ≤ 0.05.
Statistically significant at p ≤ 0.01 in pairwise comparisons with the control group.
One of 5 peer reviewers did not agree that MMMT should be combined with uterine epithelial tumours.
Statistically significant trend at p ≤ 0.01.
In mice, there was early mortality at the top dose of 1000 mg/kg bw, which was considered to have been in part due to gastrointestinal toxicity arising from the gavage method of dosing. Therefore, the top dose mice were not examined for occurrence of tumours. In male mice dose‐related non‐neoplastic effects were observed in the liver, kidney and forestomach, whereas in female mice effects were only reported in the forestomach. There was a statistically significant increase in liver hepatoblastoma in males, with no statistically significant dose‐related trend (Table 13). The number of survivors at termination of the study in the 0, 250, 500 and 1000 mg/kg bw groups were 33, 26, 39 and 12, respectively, suggesting that the lower number of tumours at 500 mg/kg bw than at 250 mg/kg bw was not related to survival. Also in male mice, there was a significant positive trend for the incidence of adenoma or carcinoma (combined) of the cecum or colon, but these were within the historical control range and not statistically significant compared to controls. There was also a significant positive trend for the incidence of haemangiosarcoma (all organs), and the incidence in the 500 mg/kg bw group was significantly increased, but these were also within the historical control range.
TABLE 13.
Summary of tumour incidences in male mice in the NTP (2014) study.
| 0 mg/kg bw a | 250 mg/kg bw | 500 mg/kg bw | |
|---|---|---|---|
| Hepatocellular adenoma (including multiple) | 32 | 33 | 38 |
| Hepatocellular carcinoma (including multiple) | 11 | 15 | 17 |
| Hepatocellular adenoma or Hepatocellular carcinoma | 39 | 39 | 43 |
| Hepatoblastoma | 2 | 11 b | 8 |
| Hepatocellular carcinoma or hepatoblastoma | 12 | 24 b | 20 |
| Hepatocellular adenoma, hepatocellular carcinoma or hepatoblastoma | 39 | 42 | 43 |
| Caecum or colon: adenoma or carcinoma | 0 c | 0 | 3 |
| Haemangiosarcoma (all organs) | 1 c | 5 | 8 b |
| Haemangioma or haemangiosarcoma | 3 c | 5 | 9 |
Groups of 50 mice were dosed by gavage 5 days per week for 2 years (averaged doses = 0, 179, 357 mg/kg bw per day). A higher dose group of 1000 mg/kg bw was not assessed for tumours due to early mortality.
Statistically significant at p ≤ 0.05 in pairwise comparisons with the control group.
Statistically significant trend at p ≤ 0.05.
No neoplastic lesions were reported in female mice. The number of survivors at termination of the study in the 0, 250, 500 and 1000 mg/kg bw groups were 40, 31, 36 and 4, respectively.
The NTP report noted that hepatocellular adenoma, hepatocellular carcinoma and hepatoblastoma are considered to represent a biological and morphological continuum. The incidences of hepatoblastoma in this study were considered by the NTP to be ‘some evidence of carcinogenic activity, but not clear evidence, because the combined incidences of hepatocellular carcinomas and hepatoblastomas were significant only in the 250 mg/kg group and the trend test was not significant’. The NTP further concluded that there was ‘no evidence of carcinogenic activity’ in female mice administered 250 or 500 mg/kg bw. The CONTAM Panel concluded that the carcinogenicity study in mice was limited by the mortality in the top dose group of 1000 mg/kg bw, which prevented assessment of tumours in that dose group, and the study did not provide a robust basis for risk assessment.
TBBPA derivatives
Following the findings of carcinogenicity of TBBPA, the NTP included TBBPA‐bDiBPrE in its testing programme. However, while a 3‐month study and genotoxicity studies were conducted (see Section 3.1.2.6 above) carcinogenicity studies have not been conducted (NTP, 2017, see details in Appendix D, Table D2).
Summary on carcinogenicity
TBBPA has been found to induce testicular adenoma and uterine epithelial tumours (predominantly uterine adenocarcinoma) in rats. In mice there was some evidence of an increased incidence of hepatoblastoma in males, with no carcinogenic effects in females at the assessed doses. The CONTAM Panel concluded that the carcinogenicity study in mice was limited by the mortality in the top dose group, such that tumours could only be assessed in the control, low and mid‐dose groups with no significant dose response relationship, and it did not provide a robust basis for risk assessment.
No carcinogenicity studies were available for the TBBPA derivatives.
3.1.3. Observations in humans
In the previous EFSA Opinion on TBBPA and its derivatives, no epidemiological studies were identified (EFSA CONTAM Panel, 2011c).
Since then, nine epidemiological studies (15 publications) have been identified assessing thyroid function (n = 4 publications), neurotoxicity (n = 3 publications), cancer (n = 1 publication), lipid and sugar metabolism (n = 3 publications), birth outcomes (n = 2 publications) and other outcomes (n = 3 publications). The populations under study were from Belgium (n = 1), China (n = 5), Republic of Korea (n = 1), USA (n = 2). Most of these studies were cross‐sectional in design and of a small sample size. Exposure assessment for other contaminants was rarely reported and in the few studies in which it was performed, adjustment for these contaminants when assessing associations with TBBPA was rarely incorporated into the analysis.
Among the assessed studies, the Guangxi Zhuang Birth Cohort (GZBC) in China (n = 2023 mother–child pairs) assessed multiple contaminants and endpoints and represented the main body of evidence for many of the health outcome categories discussed below (7 publications, Huang et al., 2022; Liang et al., 2020, 2021, 2022, 2023a, 2023b; Tang et al., 2023). To avoid unnecessary repetition, the CONTAM Panel provided an overview of the study characteristics here and highlighted the individual endpoint results under the relevant section. The study estimated serum TBBPA levels in pregnancy (UPLC–MS/MS, 1st or 2nd trimester), as well as other bisphenols (BPA, BPB, BPF and BPS). TBBPA was detected in 66% of the samples in the full study population of 2023 (ng/mL; geometric mean: 0.520; range: < LOD–187; IQR: < LOD–0.620) (Liang et al., 2020). The study is a large general population cohort with a prospective design and information on many possible confounding variables was taken into consideration. Limitations include the relatively small study sub‐populations analysed for some endpoints, the determination of TBBPA in a single spot serum sample given its short half‐life, and the relatively large proportion of samples in which TBBPA concentrations were below the LOD. The use of serum samples for measurement of the bisphenols may constitute another limitation as it has been proposed that urine is the optimal medium for biomonitoring of these compounds since higher urinary concentrations (compared to blood) reduce the likelihood that external contamination of the sample obscures true exposures.
3.1.3.1. Thyroid function and disease
Kiciński et al. (2012) in a cross‐sectional study in Belgium assessed the association between blood TBBPA levels (among other BFRs), thyroid hormone profiles (FT4, FT3, TSH) and neurobehaviour in adolescents (n = 515). A low‐exposure setting was observed (ng/L; LOQ, 15; IQR, < LOQ; P95, 22; max, 186) and the statistical analysis pertained to a comparison between the concentrations > LOQ compared to the concentrations < LOQ. No statistically significant associations were observed.
Kim and Oh (2014) assessed the correlation between serum TBBPA levels and thyroid hormone profiles in 26 infants with congenital hypothyroidism, 12 healthy infants and their respective mothers in Republic of Korea. A cross‐sectional study exposure assessment was implemented with TBBPA levels measured after diagnosis and correlation analyses run separately within the four groups. TBBPA concentrations ranged from < LOD to 713 ng/g lipid (mean: 45.6 ng/g lipid) in the 76 serum samples. No statistically significant correlations were observed for TBBPA.
Huang et al. (2022) reporting on the GZBC in China (n = 446) assessed the association between serum TBBPA levels and thyroid hormone profiles in pregnancy (1st or 2nd trimester; TT3, TT4, FT3, FT4, TSH). In the multiple adjusted analyses in this cross‐sectional assessment, the TBBPA middle tertile (tertile boundaries not reported) was statistically significantly associated with a decrease in TT3 (% change; 95% CI: −8.26; −15.82, −0.01). No statistically significant results were observed for any other thyroid function biomarker at the TBBPA middle or high tertile (1st or 2nd trimester) and the direction of the observed effect showed varying consistency [e.g. 1st trimester, % change, 95% CI; TT3, −8.26 (−15.82, −0.01); TT4, −3.51 (−11.05, 4.68); FT3, 0.91 (−4.88, 7.05); FT4, 1.49 (−5.31, 8.78); TSH, −26.96 (−57.92, 26.76)].
Finally, Xi et al. (2023) in the Shanghai‐Minhang Birth Cohort study attempted to evaluate the association between various maternal bisphenol analogue levels including TBBPA and thyroid hormone profiles. Urine samples were used and the LOD for TBBPA was 0.001 ng/mL (LC–MS/MS). Due to the small number of samples with TBBPA above the LOQ (1.7%), TBBPA was not considered further in the statistical analyses.
In summary, a small number of small cross‐sectional studies assessed the association between TBBPA levels and thyroid hormone profiles or congenital hypothyroidism. One study including a population from China reported a single statistically significant association for a decrease in TT3 levels. Among other limitations, the lack of prospective epidemiological evidence, the small number of studies, the small study sample sizes, the lack of consistency and replication of the associations under study render this body of evidence insufficient for further hazard identification and risk characterisation.
3.1.3.2. Neurotoxicity/neurobehavioural effects
As discussed above, Kiciński et al. (2012) in a cross‐sectional study in Belgium assessed the association between blood TBBPA levels (among other BFRs), neurobehaviour and thyroid hormones' profile in adolescents (n = 515). Neurobehaviour was evaluated by four tests included in the Neurobehavioural Evaluation System (Continuous Performance test, Digital‐Symbol, Digital Span, Finger Tapping). A low‐exposure setting was observed (ng/L; LOQ, 15; IQR, < LOQ; P95, 22; max, 186) and the statistical analysis pertained to a comparison between the concentrations above the LOQ compared to the concentrations below the LOQ. No statistically significant associations were observed.
Zhu et al. (2023) in a case–control study (n cases = 122, n controls = 164) in China investigated the associations between concentrations of TBBPA (among other contaminants) in the placenta and risk for neural tube defects assessed in a cross‐sectional fashion. No statistically significant associations were observed for TBBPA.
Manivannan et al. (2019) in a postmortem toxicological study in the USA used liver and adipose tissues of Alzheimer's disease patients (n = 12) and age‐matched controls (n = 12) to assess the occurrence and concentrations of 11 environmental contaminants including TBBPA. The comparison between TBBPA concentrations in Alzheimer's disease and control subjects was not reported.
The cumulative evidence on potential TBBPA neurotoxicity in humans stems from a small number of small non‐prospective epidemiological studies that have assessed neurobehaviour in adolescents, neural tube defects and Alzheimer's disease in various populations and with no endpoint assessed in more than one study. Adjustment for exposure assessment for other contaminants was not reported and no statistically significant signals were observed. The cumulative evidence is not sufficient to support or refute a TBBPA neurotoxicological effect in humans.
3.1.3.3. Lipid and sugar metabolism
In the GZBC a prospective nested case–control design was used to study associations of TBBPA and other bisphenols measured in maternal serum (collected in the first trimester of pregnancy) with risk of gestational diabetes mellitus (Tang et al., 2023). An oral glucose tolerance test (75 g) was used to screen for gestational diabetes mellitus at 24–28 weeks of gestation. This study selected 100 mothers with gestational diabetes mellitus and 400 controls. TBBPA was associated with a reduced risk of gestational diabetes mellitus in single chemical models adj OR for high versus low TBBPA exposure category: 0.42; 95% CI: 0.22, 0.79; adj OR for log increase in TBBPA: 0.67 (0.54, 0.85). Mixture analyses were performed to evaluate the combined effect of all five compounds (BPA, BPB, BPF, BPS and TBBPA) together, using BKMR and G‐computational models. In these mixture models, the bisphenol mixture was associated with a decreased risk of gestational diabetes mellitus. The relatively small size of the case group (n = 100) is a limitation of the study, in addition to limitations mentioned above.
A study in the USA (Ilozumba et al., 2022) measured Triclosan, BPA, TBBPA and tetrabromobenzoic acid concentrations in urine samples from 302 women with newly diagnosed breast cancer, with the aim to test their associations with demographic characteristics and body fatness. TBBPA was not detectable in the urine samples so no further analyses were performed.
A cross‐sectional study in China (Li et al., 2024) measured TBBPA and debromination products (TriBBPA, DBBPA and 2‐MBBPA) in hair samples of 598 adult participants from across China. Logistic regression models showed that TBBPA hair concentration was associated with a small increase in risk of metabolic syndrome (adj odds ratio (OR): 1.02, 95% CI: 1.01, 1.05).
3.1.3.4. Birth outcomes
Liang et al. (2020) examined the associations between pregnancy serum bisphenol levels and birth size in 2023 mother‐infant pairs from the same GZBC study in China. Concentrations of TBBPA and other bisphenols were determined in first trimester serum samples. Because a large proportion (34%) of TBBPA concentrations were < LOD, analyses used the following categories: low (< LOD), middle (between LOD–median) and high (> median). Higher TBBPA concentrations were associated with a decrease in birth weight (adj β middle vs. low: −75.35, 95% CI: −114.37, −36.33, adj β high vs. low: −44.94, 95% CI: −84.01, −5.88) with a significant p‐value for trend over the categories (p = 0.023). Associations were stronger and statistically significant in males (p for trend 0.007) compared to females (p for trend 0.699). An association with decreasing birth length and birth weight z‐score were also seen in males (p for trend 0.026 and 0.039, respectively) and with decreasing ponderal index in females (p for trend 0.037). Strengths of this study are the large sample size; limitations are described above for this cohort.
In the same GZBC study population (n = 2023), Liang et al. (2021) examined the effects of bisphenol mixtures (measured in first trimester serum) on the risk of preterm birth. There were 113 cases of preterm birth in the cohort. Single exposure models showed an increased risk of preterm birth with higher TBBPA concentration in males only (OR middle vs. low = 3.57 (1.65, 7.71); OR high vs. low = 2.44 (1.10, 5.45)). Cubic spline models showed that this association had an inverse U‐shape. In mixture analyses, bisphenol mixtures were related to an increased risk of PTB, with BPF, BPS and BPA having the greatest positive contribution and with little contribution from TBBPA. Strengths and limitations are those pointed out above for the same cohort. Additionally, in this study, the number of cases of preterm birth was relatively small (n = 113).
3.1.3.5. Cancer
Zhang et al. (2023) implemented a case–control study framework but with a cross‐sectional exposure assessment (after the cancer diagnosis) and assessed the association between TBBPA levels in breast adipose tissue (UPLC‐MS/MS) and breast cancer diagnosis in China (n cases = 180, n controls = 80). Control definition included women with benign breast disease classified as BIRADS category 3 or 4A. TBBPA was detected in 89% of the samples (controls: median, IQR, ng/g; 3.69, 1.78–6.02). TBBPA levels showed a statistically significant association with breast cancer diagnosis (adjusted OR, 95% CI; 1.15, 1.02–1.30, continuous TBBPA increase; 3.07, 1.12–8.40, 1st vs. 2nd; 4.59, 1.47–14.32, 1st vs. 3rd; estimates adjusted for age, BMI, menarche age, menopause status and parity).
The evidence supporting the proposed association between TBBPA and breast cancer is limited mainly by the lack of longitudinal data, the small sample size and the absence of replication.
3.1.3.6. Other endpoints
The GZBC cohort was used by Liang et al. (2023a) to examine associations between bisphenols measured in first trimester serum samples and leucocyte telomere length in cord blood, in 801 mother–child pairs. Increased TBBPA levels were associated with increased relative telomere length in cord blood by 3.31% (95% CI: 0.67%, 6.01%; per continuous ln‐TBBPA increase) among mothers < 28 years old, but not in the total study population. Limitations of the study are described above.
In 2035 pregnant women of the same GZBC study, Liang et al. (2022) examined associations between bisphenols (measured in first trimester serum samples) and haemoglobin levels and the risk of anaemia measured in the first, second and third trimester. TBBPA levels were analysed continuously and categorised into low, middle and high groups. TBBPA levels in the high‐exposure group were associated with a higher risk of anaemia in the third trimester (OR high vs. low = 1.46; 95% CI: 1.07, 1.99), but showed no association with haemoglobin levels. Mixture analysis showed that elevated levels of the bisphenol mixture (all five compounds combined) were associated with a decrease in haemoglobin levels in the second trimester. Limitations of the study are described above.
Lastly, 230 children from the GZBC study were followed up for bone mineral density measurements at a mean age of 4.6 years, and associations between the bisphenols measured in maternal first trimester serum samples and bone mineral density parameters were evaluated (Liang et al., 2023b). In single exposure models, each natural log (Ln) unit increase in TBBPA was associated with a 0.007 m/s (95% CI: −0.015, 0.000) decrease in Ln‐transformed speed of sound, one of the bone mineral density parameters measured, among girls. There were no associations in the total population or in boys, and no associations between TBBPA and the other bone mineral density parameters studied (bone mineral density z‐scores and risk of low bone mineral density).
3.1.4. Mode of action
In its previous Opinion, the CONTAM Panel reported that experimental animal models have shown that TBBPA might affect thyroid hormone homeostasis and the immune system, and that it might exert weak oestrogenic activity (EFSA CONTAM Panel, 2011c). The liver was not a critical target for TBBPA. From the limited available studies, exposure to TBBPA during development indicated a possibility of neurodevelopmental behavioural or reproductive or teratogenic effects of TBBPA. The in vitro data indicated that TBBPA was not genotoxic. TBBPA had not been tested for carcinogenicity. However, based on the weight of evidence (absence of genotoxicity in vitro, no indications of changes in cell proliferation in studies with up to 90 days repeated administration, no immunosuppression) there were no indications that TBBPA might be carcinogenic.
In the previous Opinion, in vitro mechanistic studies, conducted with TBBPA at low μM concentrations, indicated that effects on the thyroid hormone system could be mediated via binding to the transport protein transthyretin, and antagonistic action towards TRα‐ and TRβ‐dependent gene expression. Inhibition of oestradiol sulfotransferase activity was suggested as the key event responsible for the weak oestrogenic activity of TBBPA. Possible modes of action for neurotoxicity and neurodevelopmental effects included inhibition of uptake of neurotransmitters dopamine, glutamate and gamma‐amino‐n‐butyric acid (GABa) into synaptosomes.
At low micromolar concentrations, TBBPA increased reactive oxygen species (ROS) formation, intracellular Ca2+ and extracellular glutamate in primary culture of granule cells from rat cerebellum, leading to apoptosis‐like nuclear shrinkage, chromatin condensation, DNA fragmentation and cell death (Reistad et al., 2007). Cellular signalling systems were implicated in the possible mode of action in immune effects.
Since then, numerous new studies have been published on toxicity and on possible modes of action of TBBPA. There is now evidence of carcinogenicity for TBBPA by a non‐genotoxic mode of action. Therefore, the mechanistic data in relation to absence of genotoxicity are of particular importance. The new data available confirm that liver is not a critical target organ. The only notable thyroid effect in TBBPA‐treated rats by gavage is significant reduction of serum TT4 levels occurring at doses from 100 mg/kg bw per day in rats. This change was not accompanied by changes in serum TT3 or TSH levels. Therefore, the thyroid is not considered anymore as a critical target organ of TBBPA. Kidney lesions have been observed in neonatal mice or rats exposed to high doses of TBBPA in utero or during lactation as well as in adult mice. Postnatal exposure of mice to TBBPA via drinking water retarded and disturbed testis development in early life and ultimately caused adverse outcomes in adult testes. No effect on reproduction or development were observed in 2‐generation reproductive toxicity studies in Sprague–Dawley rats up to 1000 mg TBBPA/kg bw per day. The ability of TBBPA exposure to impair later in life behavioural functions (memory retention impairment in mice or increase in the level of anxiety of rats) after an early exposure to TBBPA was demonstrated. The limited studies on immunotoxic effects suggest that exposure to TBBPA directly from the diet or in utero can have effects on immune system in mice.
The CONTAM Panel decided to focus its current evaluation of modes of action on the endpoints of most relevance for the hazard characterisation, i.e. carcinogenicity, neurotoxicity, thyroid hormone signalling, reproductive and other endocrine related effects and immunotoxicity. Oxidative stress, apoptosis and mitochondrial dysfunction are also addressed as these can be involved in a number of apical effects.
Since the previous EFSA Opinion further studies on the possible mode of action of TBBPA have been published (see Appendix E, E1, E2, E3, E4, E5) and the most relevant aspects for the risk assessment are described below.
There were insufficient data on the mode of action of the TBBPA derivatives included in the TORs to allow a comparison with TBBPA.
3.1.4.1. Oxidative stress, apoptosis and mitochondrial dysfunction
Expression levels of SOD were significantly increased in rats exposed by gavage from PND18–48 at 500 mg/kg bw. The production of 8‐OHdG was strongly induced in the testis at 500 mg/kg bw and in the kidney at 250 and 500 mg/kg bw but not in the liver (Choi et al., 2011).
At high concentrations, TBBPA (10, 20 or 40 mM) significantly increased intracellular ROS, malondialdehyde (MDA) and the ratio of oxidised/reduced glutathione (GSSG/GSH) in a human liver cell line (L02 cells). It also decreased the cell mitochondrial membrane potential, caused the release of cytochrome c to cytoplasm and promoted the expression of caspase‐9 and caspase‐3, and finally increased the level of apoptosis. Moreover, TBBPA promoted the expression of antioxidant genes related to Nrf2 (Zhang, Wang, et al., 2019).
Exposure of human peripheral blood mononuclear cells (PBMCs) for 1 or 24 h to TBBPA (0.0001 to 1 μg/mL) increased ROS (including hydroxyl radical) formation. In addition, TBBPA induced lipid peroxidation and caused oxidative damage to proteins in the cells (Włuka et al., 2020).
In a human stem cell‐based model of spermatogenesis (mixed population of spermatogonia, primary and secondary spermatocytes and spermatids), TBBPA affected spermatogonia and primary spermatocytes after acute treatment (1 day) at concentrations of 25–200 μM. TBBPA affects spermatogenesis through decreases of the mitochondrial membrane potential and ROS generation, ultimately causing apoptosis (Steves et al., 2018).
At concentrations of 20–80 μM, TBBPA exposure of cultured bovine cumulus–oocyte complexes impaired oocyte maturation and subsequent embryonic development by inducing oocyte meiotic failure (defective spindle assembly and chromosome alignment). TBBPA induced also oxidative stress and early apoptosis. Moreover, TBBPA exposure caused mitochondrial dysfunction, displaying a decrease in mitochondrial membrane potential, mitochondrial content, mtDNA copy number and ATP levels, which are regulated by the expression of pyruvate dehydrogenase kinase 3 (Guo et al., 2022).
TBBPA (≥ 20 μM for 48 h) significantly decreased cell viability and increased apoptosis in RIN‐m5F cells derived from rat pancreatic b‐cells, thus, TBBPA‐induced apoptotic cell death. In addition, TBBPA‐induced mitochondrial dysfunction, ROS generation, the formation of certain nitrogen species and elevation of inflammatory cytokine levels in vitro (Suh et al., 2017).
In mouse neural stem‐like C17.2 cells, TBBPA‐induced apoptosis accompanied by increased reactive oxygen species generation and mitochondrial dysfunction (Cho et al., 2020).
Generation of ROS, mitochondrial membrane potential dysfunction, cell apoptosis and overload of intracellular free Ca2+ demonstrated that the cytotoxicity induced by TBBPA in A549 cells (non‐small cell lung cancer) was mediated by oxidative stress (Yu et al., 2019). In human peripheral blood mononuclear cells (PBMCs) TBBPA triggered apoptosis, increasing cytosolic Ca2+ concentration, decreasing transmembrane mitochondrial potential, activating caspase‐8, −9 and −3, as well as increasing DNA fragmentation and chromatin condensation. TBBPA induced apoptosis mainly by the involvement of the mitochondrial pathway. The accelerated apoptosis of lymphocytes (main population of PBMCs) caused by TBBPA may lead to disorders in the immune system function and possibly be involved in cancer development (Barańska, Bukowska, et al., 2022). Zieminska, Lenart, et al. (2017) examined the role of calcium transients induced by TBBPA in triggering oxidative stress and cytotoxicity in primary cultures of rat cerebellar granule cells (CGC). In CGC calcium imbalance and oxidative stress both mediate acute cytotoxicity of TBBPA. A causal relationship between these mechanisms depends on the concentration of TBBPA. At a concentration of 10 μM TBBPA, an increase in cytosolic Ca2+ concentration is a primary event triggering oxidative stress, depolarisation of mitochondria and reduction of the neuronal viability. At a concentration of 25 μM TBBPA additional calcium‐independent mechanisms of induction of oxidative stress and cytotoxicity emerge.
In summary, based on in vitro studies in several cell types it was shown that ROS induction, and the resulting oxidative damage, apoptosis and mitochondrial dysfunction may be mechanisms whereby TBBPA exerts toxic effects: carcinogenicity, kidney and testis toxicity, impaired oocyte maturation, neurotoxicity and immunotoxicity. Moreover, increasing cytosolic Ca2+ concentration may be a primary event triggering oxidative damage and neurotoxicity (see Section 3.1.4.4).
3.1.4.2. Carcinogenicity
TBBPA has been found to induce uterine epithelial tumours (predominantly uterine adenocarcinoma) and atypical endometrial hyperplasia in female rats, testicular adenoma in male rats and hepatoblastoma in male mice. It was not tumourigenic in female mice (NTP, 2014). Atypical endometrial hyperplasia is considered to be a preneoplastic lesion in women, resulting from high levels of oestrogens and insufficient levels of progesterone‐like hormones (Dunnick et al., 2015). The primary tumour sites in the rat have led to suggestions that sex hormone imbalance is involved in the mechanism. Interaction of TBBPA with sex hormones is discussed further below (see Section 3.1.4.3).
3.1.4.2.1. Evidence for a genotoxic mode of action
TBBPA was not mutagenic in vitro in bacterial, yeast and mammalian mutagenicity assays in the presence or absence of metabolic activation; it did not cause structural chromosome aberrations in Chinese hamster lung and human peripheral lymphocytes. In in vitro Comet assays, TBBPA induced SSB and DSB as well as oxidised DNA bases in human peripheral blood mononuclear cells. It also induced SSBs in THP‐1 cells. Due to the lack of validation and appropriate OECD guidelines for the in vitro Comet assay, these results were regarded of limited relevance. Moreover, in the absence of other positive results, these in vitro Comet assay results were not considered a sign of mutagenic potential. In vivo, no increase in micronuclei were observed in a peripheral blood micronucleus test in mice. The results of this test were of low relevance with no convincing evidence for target organ exposure. In view of the lack of effect in vitro, this uncertainty in the result of the in vivo study does not raise a concern for genotoxicity (see Section 3.1.2.6).
Concentration‐related increases in SSB or DSB were observed in an alkaline Comet assay in PBMCs exposed to TBBPA (0.01–10 μg/mL) (Barańska, Woźniak, et al., 2022). In addition, it was shown that TBBPA induced oxidative damage to DNA pyrimidines (at 0.1 and 1 μg/mL) or purines (from 0.01 μg/mL) using the enzymes endo III or hOGG1, respectively. It was also observed that PBMCs efficiently repaired DNA lesions induced by 10 μg/mL TBBPA as shown by a decrease in the extent of DNA damage, but were unable to remove completely damaged DNA after 120 min post‐incubation period. No changes in the supercoiling of plasmid molecules were observed in an in vitro plasmid relaxation assay (Barańska, Woźniak, et al., 2022). The CONTAM Panel noted that this assay does not provide unequivocal conclusions on the ability of a compound to bind to DNA.
Analysis of Tp53 mutations was performed in Wistar Han rat uterine carcinomas induced by chronic exposure to TBBPA to evaluate if the incidences of these mutations in treated rats differed from spontaneous tumours (NTP, 2014). Exons 5 to 8 of the rat Tp53 gene were examined for mutations in spontaneous uterine adenocarcinomas and adenocarcinomas from TBBPA‐dosed rats. A statistically significant increase in the incidence of Tp53 mutations was observed in uterine adenocarcinomas from treated rats compared to spontaneous tumours from control rats, with no relationship with the dose of TBBPA. TBBPA‐dosed rats also had multiple mutations per tumour. The authors concluded that uterine carcinogenesis in dosed animals may be at least partly driven by alterations in the Tp53 signalling pathway. There was no difference between the mutation spectra of spontaneous tumours and those from TBBPA‐dosed animals. The CONTAM Panel noted that the lack of difference in the mutational spectra favours the hypothesis of an increase in the level of endogenous damage, i.e. secondary non‐direct genotoxic events.
Other studies (see Section 3.1.4.1) contribute to the ample evidence for mitochondrial involvement in the process of cell death by apoptosis and the role of reactive oxygen formation, e.g. studies of stress gene induction (e.g. bcl‐2, bax) or activation of caspase‐3 and caspase‐9, and studies of oxidative stress.
3.1.4.2.2. Evidence for a non‐genotoxic mode of action
With respect to uterine cancer, a major hypothesis is that TBBPA results in decreased oestrogen metabolism, by competitive inhibition of oestradiol sulfation. TBBPA and oestrogen have similar affinities for sulfotransferases, particularly SULT1E1, therefore TBBPA exposure could decrease sulfoconjugation and hence inactivation and excretion of oestradiol, leading to increased levels of oestradiol in the uterus. This mode of action is considered to be relevant to humans (Dunnick et al., 2015; Lai et al., 2015). Dunnick et al. (2015) further hypothesised that differences in the capacity and/or capability of conjugating enzymes between rats and mice could explain why uterine tumours were observed in rats but not in mice. Borghoff et al. (2016) reported that sulfation of TBBPA is saturated at dose levels of 250 mg/kg bw per day and above (see Section 3.1.1.1.3), i.e. the doses at which tumours have been reported.
Effects on oestrogen metabolism and homeostasis were implicated as a major mode of action for the histological changes detected in the uterus of the chronically exposed rats to TBBPA (Sanders et al., 2016). Target genes as Thra, Ttr, Thrb, Ers1, Esr2 and Ppara were detected as deregulated in the uterus in TBBPA‐treated rats. In addition, some genes such as Ccnd2 and Cdk4 involved with the Cyclin D complex were up‐regulated in the distal uterus, which could be related to cell‐cycle regulation and potentially implicated in tumour formation (Zhao et al., 2006). In summary, changes in expression of genes associated with both cell cycle regulation and growth as detected in the uterus of TBBPA‐exposed rats may potentially induce tumorigenesis in the uterus of rats chronically exposed to TBBPA by an increase of the oestrogenic activity.
A further possibility for an effect of TBBPA on oestrogen levels, is by inhibition of hydroxysteroid‐dehydrogenase‐17β (HSD17β), which has been observed in vitro. HSD17β metabolises oestradiol into oestrone, which is less active, thus inhibition of HSD17β could increase oestrogen activity (Dunnick et al., 2015).
Harvey et al. (2015) investigated mutations in 22 of the uterine carcinomas induced by TBBPA in the NTP (2014) study, comparing them to mutations in 10 spontaneous uterine carcinomas from control animals in other NTP studies. The number of mutations in tumour protein 53 (Tp53) was 15/22 in the tumours from TBBPA‐treated animals and 2/10 in tumours from control animals. In addition, there was overexpression of human epidermal growth factor receptor 2 in the TBBPA‐induced tumours compared to spontaneous uterine carcinomas in the control animals. Similar to spontaneous carcinomas, tumours in TBBPA‐exposed rats were oestrogen receptor‐alpha positive and progesterone receptor negative by immunohistochemistry. The authors concluded that the morphologic and molecular features of uterine carcinomas in TBBPA‐exposed rats resemble those of high‐grade type I tumours in women.
Lai et al. (2015) proposed a sequence of events for TBBPA induction of uterine tumours, involving:
Competitive inhibition of oestrogen sulfo‐conjugation, leading to decrease oestrogen elimination and increased serum oestrogen levels;
Increased expression of hormone‐responsive genes promoting DNA synthesis and cell proliferation;
Promotion (proliferative or survival advantage) of cells containing Tp53 mutations;
Formation of uterine tumours.
Similarly, Wikoff et al. (2016) argued that the evidence did not support a mode of action for the uterine carcinogenicity of TBBPA involving direct interaction with ERs or dopamine receptors and proposed an adverse outcome pathway (AOP) commencing with inhibition of oestrogen sulfotransferase, leading to increased oestrogen bioavailability in target tissues, increased oestrogen‐related gene expression and cell proliferation, generation of reactive quinones and subsequent DNA damage, leading to endometrial hyperplasia and ultimately uterine tumours. The authors argued that these events only occur at high dose, repeated exposures to TBBPA that are associated with inhibition of sulfotransferases and are not relevant for human exposure.
Other effects have also been proposed as involved in TBBPA induction of uterine tumours. Guan et al. (2021) found that TBBPA at pM‐nM concentrations induced proliferation of Ishikawa cells, a human endometrial carcinoma cell line. The increased proliferation was associated with elevation on NF‐κB expression and ubiquitin‐mediated degradation of IκB.
The induction by TBBPA of testicular tumours in the rat could be related to altered homeostasis of androgens, for example by its androgenic activity (Dunnick et al., 2015). Generation of ROS has also been proposed as a possible mode of action in carcinogenicity of TBBPA (see discussion above).
Hoffman and colleagues have performed a series of studies on mode of action of TBBPA in induction of ovarian cancer (Hoffmann, Fiedor, et al., 2017; Hoffmann, Gogola, et al., 2017; Hoffmann et al., 2018). TBBPA has not been shown to induce ovarian cancer, and since the results of these studies were dependent on the ovarian cancer cell line used, the relevance to TBBPA induced cancer is unclear.
The EFSA Panel on Plant Protection Products and their Residues (PPR Panel) has developed a putative AOP network leading to uterine adenocarcinoma (EFSA PPR Panel, 2023). This involves a number of molecular initiating events (MIEs, induction of aromatase, inhibition of SULT1E1 and inhibition of HSD17β), leading to increased oestradiol availability as a common key event. The AOP continues with activation of ERα, epigenetic modulation, altered expression of factors ruling proliferation, increased proliferation and genetic instability, leading to development of uterine adenocarcinoma.
As noted above, IARC classified TBBPA as probably carcinogenic to humans based on the sufficient evidence in animals and strong mechanistic evidence. Guyton et al. (2018) published an assessment of the 10 key characteristics (KC) of carcinogens applied by IARC. They noted that TBBPA was classified on the basis of strong mechanistic evidence related to KC5 (induces oxidative stress), KC7 (is immunosuppressive) and KC8 (modulates receptor‐mediated effects, particularly with respect to thyroid‐hormone receptor and PPAR‐γ), and that these are also relevant for humans. Notably, the KCs related to genotoxicity (KC1 (Is electrophilic or can be metabolically activated), KC2 (Is genotoxic), KC3 (Alters DNA repair or causes genomic instability)) were not found to be associated with TBBPA (Guyton et al., 2018; IARC, 2018; key characteristics of human carcinogens from Smith et al., 2016, see Appendix F, F1). Further discussion of TBBPA induction of oxidative stress, immunosuppression and receptor‐mediated effects is included in other sub‐sections of this chapter.
In summary, there is evidence that the carcinogenicity of TBBPA occurs via non‐genotoxic mechanisms, which are likely to have thresholds for effects due to the multiple biochemical events involved.
3.1.4.3. Reproductive effects, including changes in sex hormones
No effect on reproduction or development were observed in Sprague–Dawley rats exposed by gavage up to 1000 mg TBBPA/kg bw per day (Cope et al., 2015). A delay in the time to preputial separation was found in F1 Wistar Han rats exposed by gavage to 250 mg/kg bw per day (Brown et al., 2020).
3.1.4.3.1. Endocrine effects
In its previous Opinion, the CONTAM Panel reported that competitive inhibition of oestradiol sulfotransferase activity (and not a direct modulation of ER‐mediated gene expression) might be the key event responsible for the weak oestrogenic activity of TBBPA (EFSA CONTAM Panel, 2011c).
TBBPA showed both oestrogenic and antioestrogenic effects, indicating both receptor‐dependent and –independent mechanisms. The oestrogenic activity of TBBPA using ERE‐luciferase reporter assay in MCF‐7 cells may only be observed at high concentrations (1 × 10−6–1 × 10−4 M) and these responses are largely mediated by receptor independent mechanisms. Anti‐oestrogenic activity of TBBPA (1 × 10−5 M) was also shown by addition to the 17‐β‐oestradiol assay system in MCF‐7 cells (Kitamura et al., 2005). In vivo, TBBPA (> 20 mg/kg bw, 3 days) exhibited oestrogenic activity by increasing uterus weight in ovarectomised mice (Kitamura et al., 2005) TBBPA did not show androgenic or anti‐androgenic activity in the ARE‐luciferase reporter assay using NIH3T3 cells without expressing AR (Kitamura et al., 2005).
Gosavi et al. (2013) showed that TBBPA can bind to and inhibit oestrogen sulfotransferase. TBBPA bound to the steroid‐metabolising enzyme SULT1E1; it can mimic oestradiol binding to the active site of the enzyme. TBBPA had a weak androgen receptor activation in vitro with recombinant yeast systems carrying human androgen receptor (hAR).
Using a set of yeast strains expressing the oestrogen receptor (ERα) gene, oestrogen‐related receptor (ERRc) gene, androgen receptor (AR) gene or progesterone receptor (PR) gene, TBBPA showed ERα and PR antagonistic activities but was not an AR agonist. The authors concluded that TBBPA exhibited oestrogenic activity (Li et al., 2010). TBBPA showed clear GR (glucocorticoid receptor) and AR (androgen receptor) antagonism in specific yeast receptor bioassays. In addition, it was shown that testosterone secretion was increased by TBBPA in murine MA‐10 Leydig cells. Steroid levels of MA‐10 cells were evaluated showing that TBBPA exposure caused an induction of P4, 17‐OH‐P4, deoxycorticosterone (DOC), androstenedione, α‐T as well as β‐T levels compared to DMSO (Roelofs et al., 2015).
To evaluate the oestrogenic effects of TBBPA in vitro on MCF‐7 human oestradiol‐dependent breast adenocarcinoma cancer cells, the proliferation test (E‐screen assay) was used. TBBPA (up to 20 μM) has not shown any oestrogenic effect mediated by the oestrogen receptor α. It did not induce the endogenous oestrogen‐dependent TFF1 (trefoil factor 1) gene expression (Dorosh et al., 2011). TBBPA showed statistically significant oestrogenic activity in MCF‐7 cells and decreased cell proliferation when co‐treated with 17β‐oestradiol, thereby indicating potential antioestrogenic effects (Krivoshiev et al., 2016). Cao et al. (2017) also showed that TBBPA induced a low level of cell proliferation in MCF‐7 cells (MCF‐7BUS breast adenocarcinoma cells). In the MVLN assay (an MCE‐7‐derived cell line stably transfected with the luciferase reporter gene and an oestrogen‐responsive element) TBBPA exhibited no oestrogenic activity. An H‐bonding interaction with Thr347 was identified as an important structural feature for the recognition of TBBPA at the E2 binding site of ERα.
TBBPA in the nanomolar range significantly induced oestradiol secretion by human choriocarcinoma‐derived placental JEG‐3 cells According to the authors, these data may indicate that TBBPA alters placental oestradiol production in ways that may reflect its ability to interfere with normal placental development during early pregnancy (Honkisz and Wójtowicz, 2015).
TBBPA exposure (up to 1000 mg/kg bw per day) for 7 days by gavage was negative for agonistic and antagonistic response in an uterotrophic assay using C57BL/6J ovariectomised adult female mice (Ohta et al., 2012).
To investigate the effects of TBBPA on the activity of the ABC transporters, an uptake assay was performed using membrane vesicles overexpressing the specific transporters and incubated with known substrates at concentrations ranging from 1 to 100 μM. The effect of TBBPA on breast cancer resistance protein (BCRP), multidrug resistance proteins 1 and 4 (MRP1 and MRP4), and P‐glycoprotein (P‐gp) was investigated using membrane vesicles overexpressing these transporters. TBBPA inhibited all transporters tested. Using transporter‐overexpressing Madin‐Darby canine kidney cells it was shown that TBBPA was not transported by BCRP or by P‐gp. To investigate the toxicological implications of these findings, testosterone secretion and expression of steroidogenic genes were determined in murine Leydig (MA‐10) cells upon exposure to TBBPA. TBBPA concentration dependently increased testosterone secretion by MA‐10 cells. Inhibition of the Mrps by MK‐571 completely blocked testosterone secretion elicited by TBBPA, which could not be explained by coinciding changes in expression of steroidogenic genes. Therefore, the authors hypothesise that transporter‐mediated efflux of testosterone precursors out of MA‐10 cells is inhibited by TBBPA resulting in higher availability for testosterone production (Dankers et al., 2013).
3.1.4.3.2. Changes in gene expression and epigenetic mechanisms
TBBPA was able to increase apoptosis of testicular cells and other changes in the first and second generations of CD‐1 mice exposed via drinking water to 35 μg/kg bw per day (Zatecka et al., 2013; see Section 3.1.2.3). Certain effects of TBBPA can be transmitted to the next generation not via changes in the DNA sequence but via altered patterns of gene expression concerning genes encoding heat shock protein (Hsp) (Hsp70‐2, Hsc70t, Hsp60) and genes involved in apoptosis (Bax and Bcl‐2). A significant decreased expression of Sox9 (in Sertoli cells) was also detected, potentially causing the reduction of the thickness of the seminiferous epithelium (Zatecka et al., 2013).
C57Bl/6J mouse pups were exposed via drinking water to 35 μg/kg bw per day TBBPA during the gestation, lactation, pre‐pubertal and pubertal periods up to the age of 70 days. TBBPA treatment did not result in statistically significant changes in any of the conventional reproductive endpoints (anogenital distance, weight of the reproductive organs, sperm morphology and concentration), but TBBPA‐treated animals had lower testosterone levels. TBBPA exposure resulted in significantly higher number of TUNEL positive cells in spermatozoa, in altered protamine ratios and increased sperm DNA fragmentation. It had the potential to alter the epigenetic marking of sperm chromatin through generation of an anomalous content and distribution of protamines (decreased protamine 1/protamine 2 ratio and increased total protamine/DNA ratio) (Zatecka et al., 2014).
Using the mouse C18‐4 spermatogonial cell line (spermatogonia immortalised by SV40) as a model to characterise the testicular toxicity of TBBPA it was shown that TBBPA (25 μM) exposure (24–72 h) decreased the number of spermatogonia in vitro. TBBPA induced dose‐ and time‐dependent alterations in nuclear morphology, cell cycle, DNA damage responses (detected as y‐H2AX positive expression) and perturbation of the cytoskeleton (Liang et al., 2017).
In an in vitro three‐dimensional (3D) mouse testicular cell co‐culture model (incorporation of Sertoli and Leydig cells with C18 spermatogonia cells) and by employing a high‐content image (HCA)‐based single‐cell analysis it was shown that TBBPA induced dose‐ and time‐dependent changes in a wide spectrum of adverse endpoints, including nuclear morphology, DNA synthesis, DNA damage (early DNA damage response marker, γ‐H2AX) and cytoskeletal structure (F‐actin cytoskeleton). The co‐cultured testicular cells were more sensitive than the C18‐4 spermatogonial cells. Single‐cell‐based assays not only showed the levels of the averaged population, but also revealed changes in the sub‐population (cells with stretching F‐actin and cells without stretching F‐actin, multinucleated and single nucleated cells, mitotic phenotypic features, … see also below) (Yin et al., 2020).
Pregnant Wistar Han rat dams (GD6–PND21) and their progeny (PND22–90) were exposed by gavage to 0, 0.1, 25 or 250 mg TBBPA/kg bw per day (Brown et al., 2020). mRNA expression of some steroidogenic enzymes and receptors in the testes were different from that in controls on PND90 and 1‐year‐old time points. Cyp11a1 and Hsd3b1 showed a decrease of expression level in the 250 mg/kg bw group.
Postnatal exposure (PND0–56) of CD‐1 mice via drinking water to 500 μg/kg bw per day resulted on PND56 in reduction of the number of germ cells along with down‐regulation of the expression of the genes encoding proteins of the meiotic synaptonemal complex (Scyp3 (spermatocyte marker)) (Xiong et al., 2023). Historical developmental exposure to TBBPA had little effect on the expression of Ki67 (spermatogonia marker) and Acrv1 (spermatid marker). The microtubule and microfilament cytoskeleton are a sensitive target to TBBPA relative to spermatogenic parameters (Xiong et al., 2023).
Yin et al. (2023) examined the selective toxicity of TBBPA (0, 1, 5 and 10 μM for 24 and 48 h) on mouse testicular cells, including Sertoli cells, Leydig cells and spermatogonia. TBBPA treatment showed a dose‐dependent decrease of cell number in both spermatogonia cell and Sertoli cells but not in Leydig cells. TBBPA treatment induced changes in nuclear size and caused differential cell cycle arrest among the three different types of testicular cells. TBBPA treatment significantly decreased nuclear roundness in spermatogonia, nuclear smoothness and nuclear roundness in Leydig cells and nuclear smoothness in Sertoli cells. No changes in nuclear smoothness were observed in spermatogonia. TBBPA reduced the percentage of BrdU positive cells (marker of DNA synthesis) in spermatogonia and also in Sertoli cells. Alterations in the cytoskeleton were also observed. TBBPA treatment caused F‐actin total intensity induction in the different cells. TBBPA significantly increased the number of γ‐H2AX positive cells in spermatogonia and Sertoli cells. It increased Methyl‐CpG binding domain 1 (MBD1, marker of methylation) total intensity in spermatogonia and Sertoli cells. TBBPA significantly increased the autophagosome membrane protein LC3B (to monitor autophagy activity) level in spermatogonia and Sertoli cells. TBBPA targeted spermatogonia and Sertoli cells and less Leydig cells (Yin et al., 2023).
Potential molecular mechanisms underlying TBBPA‐caused changes in testis development were investigated in CD‐1 mice exposed from PND0–56 (via lactation PND0–21 and direct exposure of pups from PND21–56) to 0, 15, 150, 1500 ng/mL TBBPA in drinking water (Li et al., 2022) RT‐PCR was conducted to analyse the expression of marker genes for Sertoli, Leydig and germ cells, blood‐testis barrier‐associated genes as well as genes involved in thyroid hormone and Notch signalling pathways. Notch signalling in Sertoli cells is involved in regulation of the balance between maintenance and differentiation of gonocytes in the perinatal testis (Garcia and Hofmann, 2013). TBBPA exerted a limited effect on thyroid hormone signalling pathway involved in testis development. However, TBBPA‐treated testes displayed higher expression of Notch marker genes, implying Notch signalling activation. The authors concluded that in TBBPA‐treated testes, the reduced Sertoli cell population size and abnormal cytoskeleton could be at least partly responsible for the decrease in the tubular area. As each Sertoli cell supports a specific number of germ cells, TBBPA‐caused alterations in Sertoli cells are responsible for the reductions in the spermatogonia number per tubule, the percentage of stage VII–VIII seminiferous tubules and the expression levels of spermatogonia and spermatocyte marker genes. Moreover, the down‐regulated expression of blood‐testis barrier‐associated genes might imply the structural and functional abnormalities of the blood‐testis barrier.
In a study on TBBPA‐bDiBPrE (Li, Xiong, Zhang, et al., 2023, see details in Table D2, Appendix D), using the mouse Sertoli cell line (TM4) it was shown that microtubule cytoskeleton in Sertoli cells is a sensitive target of TBBPA‐bDiBPrE implying the impairment of the blood‐testis barrier integrity as well as potential dysfunctions inside the intercellular interactions of the seminiferous epithelium. Assays of tubulin polymerisation and molecular docking analysis suggested an interaction of tubulin with TBBPA‐bDiBPrE, able to interfere with tubulin dynamics in testis exposed to TBBPA‐bDiBPrE.
Exposure via drinking water of 5 male CD‐1 mice/group during lactation and directly from PND0 until 8 months of age to TBBPA‐bDiBPrE average daily intakes for dams and weaned males estimated to be approximately 50 and 1000 μg/kg bw per day. Decreases in the numbers of spermatogenic cells at different developmental stages were observed. Gene expression of Ki67, Ssxb1, Acrv1 and Prm2, markers for spermatogonia, spermatocytes, spermatids and spermatozoa, respectively, show down‐regulation in the high dose group. Exposure to low‐dose also resulted in significant down‐regulation of Acrv1 and Prm2 expression. Both doses led to higher expression of apoptosis marker genes (Caspase 3, Caspase 9, Bax and Bcl2). All data demonstrate that long‐term exposure to low‐dose TBBPA‐bDiBPrE disrupted spermatogenesis in mice (Li, Xiong, Chen, et al., 2023).
In summary, although TBBPA did not cause effects on some conventional reproductive endpoints (anogenital distance, weight of the reproductive organs, sperm morphology and concentration) in rats and mice exposed by gavage, the histological, cellular and molecular alterations observed in mice exposed postnatally through drinking water demonstrate that TBBPA disturbed testis development and caused adverse testicular outcomes in adulthood. TBBPA targeted spermatogonia and Sertoli cells and to a lesser extent Leydig cells. TBBPA exerted a limited effect on thyroid hormone signalling pathway involved in testis development. However, Notch signalling activation in Sertoli cells was involved, which can lead to abnormalities in spermatogenesis, affecting fertility (Garcia and Hofmann, 2013). The authors concluded that in TBBPA‐treated testes, the reduced Sertoli cell population size and abnormal cytoskeleton could at least partly be responsible for the decrease in the tubular area. TBBPA‐caused alterations in Sertoli cells are responsible for the reductions in the spermatogonia number per tubule. The down‐regulated expression of Sertoli cell marker genes associated with the blood‐testis barrier, might imply the structural and functional abnormalities of the blood‐testis barrier. In addition, it was shown that effects on spermatogenesis may result from decreases of the mitochondrial membrane potential and ROS generation, ultimately causing apoptosis. Some effects of TBBPA can be transmitted to the next generation not via changes in the DNA sequence but via changes in gene expression of selected genes encoding proteins and regulatory genes, such as those encoding microRNAs that play an important role during spermatogenesis. It was also shown in some studies that TBBPA has a weak oestrogenic activity and TBBPA could also exert testicular toxicity by interaction with ATP‐binding cassette (ABC) transporters (BCRP, MRP1, MRP4 and P‐gp) that are expressed in the blood‐testis barrier.
3.1.4.4. Neurotoxicity
Many new publications related to brain mode of action of TBBPA have been published since the previous Opinion (40 studies), mainly related to impacts on cell viability, cytotoxicity and apoptosis, intracellular Ca2+ concentration, mitochondrial metabolism and oxidative stress, cell electrophysiology, synaptic plasticity and brain connectivity and receptor interactions and neurotransmitter contents. Details are described in Appendix E and an overview of the most relevant aspects is described below.
3.1.4.4.1. Cytotoxicity and cell viability
A large set of studies showed the ability of TBBPA to reduce cell viability in various cell culture models as rat PC12 cell line (Abe et al., 2023; Gu et al., 2019; Hendriks et al., 2014; Liu, Ren, et al., 2016), human neuroblastoma SH‐SY5Y neuronal cell line (Al‐Mousa and Michelangeli, 2012), human neuroblastoma SK‐N‐MC cell line (Choi et al., 2023), rat primary cerebellar granule cell cultures (Lenart et al., 2017; Zieminska et al., 2012a, 2012b; Zieminska, Lenart, et al., 2017; Zieminska, Salinska, et al., 2015), mouse primary hippocampal or cortical cell cultures (Szychowski and Wojtowicz, 2016; Wojtowicz et al., 2014) or various neural stem cell lines including iPSC, neural stem cells, neurons, astrocytes, oligodendrocytes and fibroblasts (Cho et al., 2020; Klose et al., 2021; Pei et al., 2016). TBBPA concentrations used over the different studies were within the range 1–100 μM. A LC50 value of 15 μM has been reported for cytotoxicity of TBBPA in SH‐SY5Y neuronal cell line (Al‐Mousa and Michelangeli, 2012).
3.1.4.4.2. Apoptosis
The reduction in cell viability is related to apoptosis as indicated by the induction of caspase activity (Al‐Mousa and Michelangeli, 2012; Cho et al., 2020; Wojtowicz et al., 2014), decrease in PPARγ protein expression (Wojtowicz et al., 2014), increase in apoptotic cell death (Choi et al., 2023; Szychowski and Wojtowicz, 2016), changes in cell morphology (Choi et al., 2023; Gu et al., 2019) and delayed overexpression of pro‐apoptotic genes (Lenart et al., 2017). Exposure of HEI‐OC1 auditory cell cultures to TBBPA (125 mg/mL) increased the expression of the pro‐apoptotic protein Bax and the caspase‐3 activity while it reduced the expression of the anti‐apoptotic Bcl‐2 protein (Park et al., 2016). In vivo exposure to TBBPA to 3‐week‐old mice (50 and 100 mg/kg bw per day, 28 days) induced concomitant increase in the expression of the pro‐apoptotic gene Bax and reduction in the expression of the anti‐apoptotic one Bcl‐2 in the hippocampus (Wang & Dai, 2022). A perinatal exposure to TBBPA (GD10‐PND20, 100–10,000 ppm in soy‐free diet) in rats increased the number of apoptotic bodies in the hippocampal sub‐granular zone at doses of 1000 or 10,000 ppm (Saegusa et al., 2012). In addition, TBBPA 20 μM was shown to promote autophagy as another cell death mechanism in human neuroblastoma SK‐N‐MC cell line (Choi et al., 2023).
3.1.4.4.3. Oxidative stress
Apoptosis is mediated by active intrinsic mechanisms like the production of ROS and the induction of oxidative stress (Redza‐Dutordoir and Averill‐Bates, 2016) and the alterations in cellular Ca2+ homeostasis (Ermak and Davies, 2002). TBBPA has been shown to induce the production of ROS measured through different fluorimetric methodologies (Al‐Mousa and Michelangeli, 2012; Cho et al., 2020; Choi et al., 2023; Hendriks et al., 2014; Szychowski and Wojtowicz, 2016; Zieminska et al., 2012a, 2012b; Zieminska, Lenart, et al., 2017, Zieminska, Ruszczynska, et al., 2017) and its modulation by adding ROS scavengers like ascorbic acid or GSH (Zieminska et al., 2012b). TBBPA (≥ 7.5 μM) was also shown to induce dose‐dependent reductions in GSH content and catalase activity (Zieminska, Lenart, et al., 2017) and in antioxidant nuclear Nrf2 levels of expression (Choi et al., 2023). The NOX system which is one of the major producers of ROS within the cell was induced by TBBPA in SK‐N‐MC cells as reflected by the increasing production of NOX4 during the incubation of cells with TBBPA 20 μM (Choi et al., 2023). In addition, results obtained in C17.2 cells, a mouse neural progenitor cell line, indicated the ability of TBBPA to create mitochondrial stress and dysfunction and to disrupt mitochondrial biogenesis linked to the excessive ROS production (Cho et al., 2020). The exposure of human neuroblastoma SK‐N‐MC cells with TBBPA 20 μM markedly induced mitochondrial ROS generation and significantly increased mitochondrial superoxide production relative to untreated cells (Choi et al., 2023). Exposure of HEI‐OC1 auditory cell line to TBBPA (125 mg/mL) also increased the accumulation of ROS and the expression and secretion of the pro‐inflammatory cytokine IL‐6 that were prevented by pretreating the cell cultures with the antioxidant compound N‐acetylcysteine (Park et al., 2016). TBBPA was then hypothesised to trigger auditory hair cell death and to lead to hearing loss as observed in mice exposed to TBBPA (250 mg/kg bw per day) for 30 days (Park et al., 2016). A significant increase in ROS production was reported in the hippocampus of 3‐week‐old mice exposed to TBBPA for 28 days (50 and 100 mg/kg bw per day) which is correlated with the significant reduction in the GSH level measured in the same brain region (Wang & Dai, 2022). TBBPA 20 μM also promoted ROS‐mediated activation of various protein kinases in human neuroblastoma SK‐N‐MC cells, including the PI3K/Akt survival signalling pathway, the nuclear Nrf2 translocation and the cell death promoting MAPK/ERK signalling pathways (Choi et al., 2023).
A recent RNA sequencing analysis of PC12 cells has been performed to assess the neurotoxicity of TBBPA (Abe et al., 2023). Differential expression analysis showed that TBBPA affected the expression of 636 genes with 242 genes shared with the ones dysregulated by another flame retardant, namely HBCDDs. Genes that were dysregulated by TBBPA were mostly related to endoplasmic reticulum stress response and necroptosis in addition to ROS production and apoptosis‐related signalling genes.
3.1.4.4.4. Intracellular calcium homeostasis
The disruption of Ca2+ cell homeostasis can then be toxic and cause cell death. In this context, TBBPA has been shown to increase intracellular Ca2+ concentration in various cell types including rat neuronal neuroblastic PC12 and B35 cell lines (≥ 1 μM TBBPA) (Hendriks, van Kleef, van den Berg, et al., 2012; Hendriks et al., 2014), SH‐SY5Y neuronal cell line (20 μM TBBPA) (Al‐Mousa and Michelangeli, 2012), human neuroblastoma SK‐N‐MC cell line (20 μM TBBPA) (Choi et al., 2023) or primary rat cerebellar granule cells (5–25 μM TBBPA) (Zieminska et al., 2014; Zieminska, Lenart, et al., 2017; Zieminska, Ruszczynska, et al., 2017). Several studies suggest the ability of TBBPA to impair the Ca2+ intracellular concentration as the result of an imbalance resulting from the inhibition of the Ca2+ influx into neurons (Hendriks, van Kleef, van den Berg, et al., 2012; Hendriks et al., 2014; Meijer et al., 2014) and its release from intracellular stores (Al‐Mousa and Michelangeli, 2012; Zieminska et al., 2012b). Effects of TBBPA on intracellular Ca2+ content, ROS production and mitochondrial membrane potential using different fluorescent probes, have been reported in PC12 cells (Zieminska, Lenart, et al., 2017). The authors suggested the Ca2+ imbalance induced by TBBPA at 10 μM to be a primary event leading to oxidative stress, depolarisation of mitochondria and cytotoxicity, with additional portion of oxidative stress and cytotoxicity independent of Ca2+ observed at a concentration of 25 μM TBBPA. In the same cell system, TBBPA (10–50 μM) inhibited the O‐GlcNAcase expression and activity, a glycosylation enzyme of importance in the brain, which in turn led to increasing intracellular Ca2+, oxidative stress, inflammation, repressed proliferation, interference with cell cycle and apoptosis (Gu et al., 2019).
Pharmacological modulation with specific agonists or antagonists of receptors involved in the Ca2+ mobilisation like ryanodine receptors and glutamate NMDA or AMPA receptor in TBBPA‐exposed cells confirm the ability of TBBPA to imbalance the cell Ca2+ homeostasis. Co‐application of specific antagonist NMDA receptor MK‐801 with ryanodine in primary rat cerebellar granular cells prevented the concentration‐dependently increase or intracellular Ca2+ cell content whereas exposure to the specific antagonist ryanodin receptor bastadin 12 remained ineffective (Zieminska et al., 2015). TBBPA (25 μM) strongly depolarised granular cells in cerebellar brain slices and primary cell cultures which was partly reduced by the application of specific glutamate NMDA receptor MK‐801, glutamate AMPA receptor CQNX or voltage‐gated sodium channel blocker TTX (Diamandakis et al., 2015, 2019). Combined application of MK‐801, CQNX and TTX completely prevented the TBBPA‐induced cell depolarisation (Diamandakis et al., 2015, 2019). In parallel, TBBPA (10–40 μM) had no effect on the specific binding of MNDA receptor glutamate, suggesting the implication of the glutamatergic neurotransmission pathway without any effect of TBBPA on the endogenous binding of glutamate to such receptors (Diamandakis et al., 2019).
3.1.4.4.5. Neurotransmission and synaptic plasticity
TBBPA exhibited moderate overall activity in a set of cell‐free neurochemical assays including four receptor assays (dopamine D2 receptor, GABAA receptor, glutamate NMDA, muscarinic acetylcholine receptor) and three enzyme activity assays (glutamine synthase, acetylcholine esterase, monoamine oxidase) all working across 20 vertebrate species (Arini et al., 2017). All the results obtained in this system placed TBBPA in the last third of the 80 substances tested with the interaction of TBBPA with the glutamate NMDA receptor being the most affected endpoint (Arini et al., 2017).
TBBPA was shown to exert several adverse effects on functional neurotransmission endpoints including receptors (Hendriks, van Kleef, van den Berg, et al., 2012; Hendriks, van Kleef & Westerink, 2012; Renner et al., 2021), enzyme activities (Wang & Dai, 2022), the presynaptic SNPA‐25 protein expression (Zieminska et al., 2016) and neurotransmitter concentrations (Bagley et al., 2018).
TBBPA acts as full (≥ 10 μM) and partial (≥ 0.1 μM) agonist on human GABAA receptors (Hendriks, van Kleef, van den Berg, et al., 2012) and nicotinic acetylcholine receptors (Hendriks, van Kleef, van den Berg, et al., 2012; Hendriks, van Kleef & Westerink, 2012) in Xenopus oocytes injected with human complementary DNA coding for both receptors. The neurotoxicity of TBBPA (9 levels of concentration ranging 0.01 to 1000 μM) has been studied using human automated midbrain organoids derived from smNPCs (small molecule neural precursor cell cultures) that is established to be efficient to identify the nigrostriatal dopaminergic toxicity of chemicals (Renner et al., 2021). TBBPA was then identified as a selective toxicant for dopaminergic neurons in nigrostriatal automated midbrain organoids generated from two independent cell lines (Renner et al., 2021).
In vivo exposure to TBBPA to 3‐week‐old mice (50 and 100 mg/kg bw per day, 28 days) induced concomitant increase in the hippocampal activity of acetylcholine esterase and reduction in the activity of choline acetyltransferase (Wang & Dai, 2022).
In vitro 24 h exposure of rat primary cerebellar granule cell cultures to TBBPA 1–100 μM increased the SNAP‐25 presynaptic protein expression (mRNA and protein) (Zieminska et al., 2016). As a member of the well‐conserved SNARE protein complex present in presynaptic and vesicular membranes, SNAP‐25 is a synaptosomal‐associated protein highly involved in intracellular vesicular trafficking, regulation of exocytosis and neurotransmitter release and appeared to be disrupted by TBBPA.
The effects of perinatal exposure to TBBPA on brain neurotransmitter contents (dopamine, GABA, serotonin) measured in three brain regions of interest (caudate putamen, substantia nigra, dorsal raphe) were assessed in rat pups aged PND115 issued from dams dosed with TBBPA 1 or 10 mg/kg bw twice a day from GD6 to PND21. The results suggest disturbances in dopamine, and GABA brain contents, but not serotonin, with variations related to the sex, brain regions and TBBPA exposure (Bagley et al., 2018).
Numerous studies demonstrated a large set of neurotoxic effects in vitro following acute exposure to TBBPA, including changes in cell viability, production of ROS, calcium homeostasis, receptor function and neurotransmitter activity, and raised the question of the effects on synaptic plasticity, especially when the exposure occurs during the early brain development period.
A single oral administration of 48.9 mg/kg bw TBBPA to mice on PND10 failed to impair the hippocampal synaptic plasticity assessed at PND17–19 by measuring ex vivo extracellular field recordings and levels of expression of several postsynaptic proteins involved in long‐term potentiation (CaMK‐II, GAP‐43, Glu‐R1, PSD‐95 and synaptophysin) (Hendriks et al., 2015). The exposure of 3‐week‐old mice to TBBPA (50 and 100 mg/kg bw per day) for 28 days decreased the hippocampal expression of the synaptic memory‐related proteins BDNF and PSD‐95 (Wang and Dai, 2022).
Overall, it can be concluded that a single oral exposure to TBBPA on PND10 may not be sufficient to affect normal brain development, function or plasticity to an adverse degree while a repeated exposure (which can be considered as more realistic for the human situation) was shown to affect the hippocampal synaptic plasticity. Gestational/lactational studies indicate that developmental exposure to TBBPA (GD10–PND20, 100–10,000 ppm in soy‐free diet) in rats resulted in alterations of neuronal migration in the hippocampus (Saegusa et al., 2012) and increased vimentin‐positive immature astrocytes and Ret‐positive oligodendrocytes populations in the same brain area (Fujimoto et al., 2013) at both doses of 1000 and 10,000 ppm.
3.1.4.4.6. Developmental neurotoxicity
Few recent studies aimed to assess the developmental neurotoxicity of TBBPA exposure using human or rodent neural stem cell‐derived systems (Behl et al., 2015; Klose et al., 2021, 2022; Liang, Liang, Yin, et al., 2019; Liang, Liang, Zhou, et al., 2019; Yin et al., 2018), invertebrate animal models (Zhang, Ireland, et al., 2019) or zebrafish (Park et al., 2016).
Developmental toxicity and neurotoxicity of TBBPA was tested in a battery of cell‐based in vitro assays to evaluate its effects on the differentiation of mouse embryonic stem cells, proliferation of progenitor cells, outgrowth of neurites from various differentiated neurons and firing activity in neural networks. In addition, developmental toxicity of TBBPA has been assessed using two alternative animal models including nematode (Caenorhabditis elegans) and zebrafish (Danio renio). TBBPA impaired the mouse stem cell differentiation, the C. elegans larva development and the zebrafish embryonic development with respective points of departure of 41.0, 10.1 and 4.6 μM. It also impaired the human stem cell neurite outgrowth with a concentration of 12.2 μM as a point of departure. TBBPA showed a significant activity in several assays and suggested this compound as a developmental toxicant and neurotoxicant (Behl et al., 2015). Using a human cell‐based developmental neurotoxicity in vitro battery covering a large variety of neurodevelopmental endpoints, TBBPA affected various endpoints including neural progenitor cell proliferation, neural crest cell migration, neurite morphology, oligodendrocyte differentiation and cell viability, that confirms TBBPA is able to cause developmental neurotoxicity (Klose et al., 2022).
The developmental neurotoxicity of TBBPA was also observed in a mouse stem‐cell based system (1–250 μM, Yin et al., 2018) or a human‐based one (0.001–250 μM, Liang, Zhou, et al., 2019). In both systems, TBBPA impaired the neural differentiation and concomitantly disturbed the NOTCH signalling pathway. The Notch system is a highly conserved signalling pathway that is crucial to regulate cell proliferation, cell fate, differentiation and cell death in all metazoans. In addition, the results from Liang, Zhou, et al. (2019) suggest that the effects of TBBPA on early stages of neural development could be modulated by T3 cell signalling.
Differentiation of human stem cell progenitors into neural ectoderm was studied in the presence of TBBPA 1 or 5 μM and the whole concomitant transcriptional changes analysed (Liang, Liang, Yin, et al., 2019). A set of transcriptional factors crucial for neural development was then identified to be dysregulated by TBBPA, including ‘regulation of glucose metabolism process’ (2 genes), ‘positive regulation of transcription from RNA polymerase II promoter’ (5 genes), ‘midbrain development’ (2 genes) and ‘nervous system development’ (3 genes). TBBPA also induced in a dose‐dependent way the WNT and AHR signalling pathways, whereas earlier studies had shown no effect of TBBPA on AHR‐related enzyme activities (see EFSA CONTAM Panel, 2011c). Such results suggest that TBBPA is able to disrupt not only the development of the nervous system at early stages, but also neuron generation at later stages.
TBBPA was shown to interfere with oligodendrocyte development and maturation in vitro by dysregulation of oligodendrogenesis‐associated genes like MBP, KLF9 and EGR1 (Klose et al., 2021). Two mechanisms of action were then identified: the first one through the disruption of the thyroid‐regulated pathway and the maturation of oligodendrocytes, and the second one through the thyroid‐independent dysregulation of cholesterol metabolism and the reduction of the number of oligodendrocytes as a consequence. Comparative analyses of human and rat neural progenitor cells (NPCs) indicated that human oligodendrogenesis is more sensitive to endocrine disruption by TBBPA (Klose et al., 2021).
TBBPA also affected the blood–brain barrier integrity. The study from Cannon et al. (2019) assessed the effect of TBBPA on the expression and activity of three blood–brain barrier transporters in Sprague–Dawley rats (15–20 weeks) orally administered once with 250 mg/kg bw of TBPPA. Three ATP‐binding cassette efflux transporters were considered, the P‐glycoprotein (P‐gp), the breast cancer resistant protein (BCRP) and the multidrug resistance‐associated protein 2 (MRP2) transport proteins. Significant changes in the luminal accumulation of P‐gp according to the gender and reduction in BCRP in both sexes have been observed in vivo and ex vivo. The results also showed a significant dose‐dependent variation of the blood–brain barrier permeability according to the transporter and the gender considered with a LOAEL of 1 nM.
Two studies performed in alternative animal models are also indicative of the neurotoxicity and ototoxicity of TBBPA. The study from Park et al. (2016) indicated the loss of neuromasts in zebrafish exposed to TBBPA 2.5–20 μg/mL as observed in ex vivo rat cochlear explants. As a correlate, auditory brainstem response threshold was increased in mice orally exposed to TBBPA for 30 days at a dose of 250 mg/kg bw per day, indicating that TBBPA exposure causes hearing loss possibly related to hair cell death. In freshwater planarians Dugesia japonica (Zhang, Ireland, et al., 2019), TBBPA 10 μM (and higher doses) impaired unstimulated behaviour on day 12 in regenerating but not adult animals, suggesting a developmental selective defect. In addition, TBBPA induced lethality in 24% of adult planarians at day 12 and caused eye regeneration defects suggesting any over toxic effects. TBBPA 100 μM induced specific sublethal effects on scrunching in adult animals without any correlation with the inhibition of cholinesterase activity as observed with the other BFRs studied. Results suggest that TBBPA caused adverse effects in both adult and developing planarians and may be considered as a developmental neurotoxicant.
In summary, all the mechanistic studies reviewed here support the observations that TBBPA can have adverse effects on the nervous system in mammals. At low micromolar concentrations, TBBPA impaired cell viability in various primary and cell line systems with the cell death related to the induction of apoptosis while autophagy has been recently reported as a second cell death mechanism possibly related to TBBPA. TBBPA increased ROS formation and cytosolic Ca2+ concentration that contributed to dysregulation of cell cycle and generation of apoptosis. Experimental evidence suggests the implication of the glutamatergic neurotransmission pathway on the cell calcium homeostasis in the toxicity of TBBPA but without any effect on the specific binding of glutamate NMDA receptor. Calcium mobilisation from its internal stores was also concerned. Recent ex vivo studies showed the ability of TBBPA to impair the synaptic plasticity in the hippocampus, rather following repeated exposure than acute administration. GABAergic, glutamatergic, dopaminergic and cholinergic neurotransmitter systems were affected by TBBPA at different levels including receptors, enzyme activities and brain neurotransmitter concentrations. Recent in vitro studies using in vitro stem cell‐based systems indicated the potentiality of TBBPA to be a developmental neurotoxicant that affects not only the neuronal proliferation, differentiation and connectivity but also the maturation of other cell types as oligodendrocytes, astrocytes and brain–blood barrier integrity.
3.1.4.5. Thyroid effects
Reports on TBBPA‐induced changes in levels of thyroid hormones in experimental animals were contradictory (see Section 3.1.2.2.2). In the previous Opinion in vitro mechanistic studies indicated significant effects of TBBPA on the thyroid hormone system (binding to transport protein transthyretin, antagonistic action towards TRα‐ and TRβ‐dependent gene expression). Since the previous Opinion several additional studies have become available on the mode of action of thyroid effects.
The thyroid hormone agonist/antagonist activities of TBBPA were evaluated using a yeast (Saccharomyces cerevisiae Y190) two‐hybrid assay incorporating the human thyroid hormone α (TRα), both with and without possible metabolic activation by rat liver S9. In the absence of S9, TBBPA exhibited agonistic activity which increased markedly in the presence of metabolic activation. TBBPA (10−6–10−4 M) inhibited the binding of T3 to TRα demonstrating its T3 antagonist activity both in the presence and absence of S9 (Terasaki et al., 2011).
Thyroid hormone receptors recruit corepressors or coactivators to the promoters of target genes to regulate their transcription. Corepressors such as nuclear hormone receptor corepressor (NCoR) are recruited by unliganded thyroid hormone receptors, whereas coactivators such as steroid receptor coactivator‐2 (SRC2) are recruited when T3 is bound to thyroid hormone receptors. TBBPA was found to interfere with the ability of the hTRα1 ligand binding domain (LBD) to bind both NCoR and SRC2. TBBPA behaved similarly to T3 in promoting the release of NCoR from LBD, whereas it failed to promote LBD interactions with SRC2. However, it did reduce the T3‐induced interactions between LBD and the coactivator peptide. TBBPA in the micromolar range can affect the regulation of transcription by both the apo‐ and the holo‐TRα1, with potential disruption of the expression of genes that are either up‐ or down‐regulated by T3 (Lévy‐Bimbot et al., 2012).
TBBPA modified T3‐mediated up‐regulation of gene expression in neural cells. TBBPA at micromolar concentrations has an antagonist effect on T3 response in HEK293–Gal4TRa1luc cells (derived from human embryonic kidney 293 cells). The effects of TBBPA are mediated by the ligand binding domain of TRα1 and reflect the ability of TBBPA to act as a low‐affinity antagonist ligand of the receptor. TBBPA (10−8 M) displayed little if any activity on a neural reporter cell line C17.2α–HrLuc (cells derived from mouse cerebellum). A slight but significant antagonist effect of TBBPA was observed when T3 was at a low concentration (10−9 M). At a higher concentration of 10−5 M, TBBPA has a broader influence, outside of the thyroid hormone signalling pathway. Transcriptomics analysis (RNAseq) in mouse neuronal precursor C17.2α cells showed enrichment in genes encoding secreted proteins (IGF1 and PDGFB for example), some with roles in neurodevelopment. Gene Set enrichment analysis also indicated effects involving epidermal growth factors and TGFβ response pathways (Guyot et al., 2014).
T4 and reverse triiodothyronine (rT3) deiodination kinetics were measured by incubating pooled human liver microsomes with T4 or rT3. TBBPA was a potent deiodinase (DI) activity inhibitor with an IC50 of 2.1 μM. It may disrupt thyroid hormone homeostasis through the inhibition of DI activity in vivo (Butt et al., 2011).
TBBPA significantly decreased thyroid hormone‐dependent rat pituitary tumour GH3 cell proliferation, indicating its antagonistic activity. TBBPA can reduce GH3 cell proliferation when co‐incubated with T3. Genes involved in regulation of thyroid hormones, thyrotropin releasing hormone receptor (Trhr) and Tsh were upregulated, but downregulation of thyroid receptor β (Trb) mRNA was observed. The study indicates that downregulation of TSHR, TPO, TG and NIS expression is associated with TBBPA induced disruption of thyroid hormone synthesis and its effects on thyroid function (Hu et al., 2023).
The effect of TBBPA on histone and RNA polymerase II (RNAPII) in thyroid hormone‐response genes encoding thyroid hormone receptor β (Thrβ) and thyroid hormone‐induced basic leucine zipper protein (Thibz) from Xenopus laevis XL58‐TRE‐Luc cells was assessed. TBBPA (1 μM) affects thyroid hormone‐induced histone and RNAPII modifications. TBBPA may influence epigenetically a cascade of T3‐mediated gene regulation (Otsuka et al., 2014).
Exposure of C57BL/6 mice to 0.02 mg TBBPA/kg bw per day for 5 weeks downregulated the protein expression levels of the thyrotropin receptor (TSHR), the sodium/iodide symporter (NIS) and thyroperoxidase (TPO) in the thyroid. At a dose of 20 mg TBBPA/kg bw per day, protein abundance of thyroglobulin (TG) was also decreased (Hu et al., 2023). The Thyroid Hormone Action Indicator (THAI) transgenic mouse model was used to assess tissue‐specific changes in thyroid hormone activity. Ninety‐day‐old THAI mice were exposed by gavage to 0 or 150 mg TBBPA/kg bw per day for 6 days. The expression of the thyroid hormone‐responsive luciferase reporter in these animals was measured in peripheral tissue samples and by in vivo imaging (14‐day‐long treatment accompanied with imaging on day 7, 14 and 21 from the first day of treatment). Based on the mRNA level of the thyroid hormone‐responsive luciferase reporter system, thyroid hormone action remained unchanged in the heart, interscapular brown adipose tissue, skeletal muscle, skin, small intestines and liver. However, TBBPA disrupted thyroid hormone signalling in the bone. It was shown that in the small intestine, thyroid hormone action was decreased after 2 weeks of treatment and then recovered after a recovery week. TBBPA also impaired the global thyroid hormone economy by decreasing the circulating free T4 levels. In the promoter assays, TBBPA showed a direct stimulatory effect on the hdio3 promoter, indicating a potential mechanism for silencing thyroid hormone action (Sinkó et al., 2022).
The effects of TBBPA on T3‐induced and spontaneous Xenopus laevis (which share many similarities with thyroid hormone dependent development in higher vertebrates) metamorphosis were investigated. TBBPA, at micromolar concentrations, disrupts thyroid hormone‐dependent development in a developmental stage dependent manner. TBBPA exhibits an antagonistic activity at the developmental stages when animals have high endogenous thyroid hormone levels, whereas it acts as an agonist at the developmental stages when animals have low endogenous thyroid hormone levels (Zhang et al., 2014).
In the review by Lai et al. (2015), it was reported that the potential modes of action for thyroid changes induced by TBBPA administration were expected to exhibit a threshold for adverse effects due to the ability of the mammalian organism to compensate small changes in thyroid hormone levels.
In summary, while evidence of effects of TBBPA on the thyroid and thyroid hormone system were ambiguous, there was mechanistic information on how this BFR could cause thyroid toxicity. These include antagonistic and agonistic effects on the thyroid hormone receptors through direct competition or interference with recruitment of corepressors and coactivators, downregulation of genes necessary for thyroid gland function and deiodination of thyroid hormone. Evidence from a reporter mouse model indicated that bone might be more susceptible to TBBPA induced thyroid hormone disruption than other tissues.
3.1.4.6. Immunotoxicity
In the previous EFSA Opinion on TBBPA the CONTAM Panel noted, based on limited mechanistic data available at the time, that TBBPA has effects on immune system signalling processes in vitro, at low μM concentrations or greater, but that the significance of these effects to people exposed to low levels of TBBPA in the diet was unknown (EFSA CONTAM Panel, 2011c). New studies have expanded the understanding of how TBBPA might affect the immune system. TBBPA has been found to impede the ability of Natural Killer (NK) cells to bind to and lyse target cells (Hurd & Whalen, 2011). This may involve a reduced expression of cell surface proteins needed for NK cells to bind to target cells. NK lytic function depends on activation of Mitogen Activated Protein Kinases (MAPKs) and MAPK kinases (MAPKKs). Activating phosphorylation of MAPK p44/42 was stimulated by TBBPA at concentrations of 0.5 μM and higher, and MAPK p38 at 2.5 μM and higher concentrations (Cato et al., 2014; Whalen & Kibakaya, 2011). The authors proposed that this might affect the activation of NK cells in response to the presence of tumorous target cells. However, activation of PKC and PKD, which are upstream of the MAPKK and MAPK in NK cells were unaffected by TBBPA exposure (Rana & Whalen, 2015).
Secretion of the inflammatory cytokine IFN‐γ by NK cells, monocyte depleted peripheral mononuclear cells (MD‐PBMCs) and peripheral blood mononuclear cells (PBMCs) was generally decreased at TBBPA concentrations ranging from 0.05 to 5 μM, but the response varied greatly among cells from different donors (Almughamsi & Whalen, 2016; Reid & Whalen, 2011). Similarly, all three immune cell preparations showed large variability in changes in IL‐1β secretion induced by exposure to TBBPA (Anisuzzaman & Whalen, 2016). Treatment of the immune cell preparations with 0.05–5 μM TBBPA caused either increased or decreased secretion of IL‐1β depending on concentration and exposure time (1–6 days).
Evidence that TBBPA may stimulate immune cells comes from in vitro experiments on splenocytes and bone marrow dendritic cells (BMDCs) from atopic prone NC/Nga mice (Koike et al., 2013). Exposure to 1 or 10 μg/mL of TBBPA increased the percentage of CD86+MHC class II+ cells in splenocytes. The percentage of TCR+ cells was increased in splenocytes by exposure to 0.1, 1.0 or 10 μg/L of TBBPA but there was no dose–response. TBBPA exposure in the same concentration range increased IL‐4 production in splenocytes with the highest response observed at the lowest exposure concentration of 0.1 μg/mL. There were however no effects on BMDC differentiation from TBBPA exposure at concentrations up to 10 μg/mL.
Exposures of NK cells, monocyte‐depleted (MD) peripheral blood mononuclear cells (PBMCs) (individually or in combination) to TBBPA (0.05–5 μM) decreased the secretion of TNFα from all immune cell preparations regardless of their composition, with significant reduction being observed at the lowest concentration tested (Yasmin & Whalen, 2018). The murine RAW 264.7 macrophage cell line was exposed to TBBPA at non‐cytotoxic concentrations ranging from 1 to 100 nM (Wang et al., 2019). TBBPA upregulated the expression of pro‐inflammatory cytokines, including IL‐1β, IL‐6 and TNF‐α, but attenuated LPS‐stimulated expression of the same pro‐inflammatory cytokines. TBBPA also attenuated LPS‐stimulated expression of anti‐inflammatory cytokines, including IL‐4, IL‐10 and IL‐13. TBBPA reduced the abundance of mRNA for several antigen‐presenting‐related genes, including H2–K2, H2‐Aa, CD80 and CD86. This is in contrast to results by Koike et al. (2013), who found that the percentage CD86 expressing cells in splenocytes was increased by TBBPA exposure. TBBPA impaired the phagocytic activity of RAW 264.7 macrophages (neutral red phagocytosis assays; Wang et al., 2019). TBBPA exposure caused activating NF‐κB p65 phosphorylation (p‐p65) activation, while it reduced LPS‐stimulated p‐p65 protein levels. DCFH‐DA staining assays indicated that generation of reactive oxygen species only occurred at the highest exposure concentration of 100 μM while most other significant changes were observed at 10 and 100 nM with few changes already at 1 nM (H2‐K1 expression; p65 phosphorylation with or without LPS stimulation).
A transcriptomics study on female Wistar Han rats orally exposed to TBBPA at 250 mg/kg bw per day for five consecutive days found that differentially expressed genes in uterine tissue were statistically enriched in gene sets belonging to at least 10 immune‐system related canonical pathways (Hall et al., 2017). Many of the immune response pathways showed negative regulation in response to TBBPA exposure and the authors suggested that TBBPA exposure at this dose‐level may trigger immunosuppression.
Exposure to TBBPA increased expression of IL‐6 and ICAM‐1 proteins in BEAS‐2B cells at a non‐cytotoxic concentration of 1 μg/mL (Koike et al., 2013). Expression of IL‐8 was dose‐dependently decreased with significant effect at TBBPA concentrations of 0.1 μg/mL. There was also a dose‐dependent increase in EGF production and EGFR phosphorylation at higher exposure concentrations. TBBPA showed weak ligand activity for ERα and for thyroid hormone receptors (TRα and TRβ) at least at higher concentrations, and TR antagonist partially attenuated the TBBPA‐induced increase of the expression of ICAM‐1 and IL‐6. The results indicate that TBBPA has the potential of affecting the expression of proinflammatory proteins in bronchial epithelial cells. This might involve stimulation of EGFR and/or TR and potentially ER activity.
The effects of TBBPA on the production of sex steroids, cytokines and oxidative stress was investigated in the placental explant cultures (Arita et al., 2018). Explants were exposed to 0.005–50 μM TBBPA in the presence and absence of heat‐killed E. coli to assess effects on the immune response. None of the treatments caused cytotoxicity. TBBPA exposure increased production of testosterone, interleukin‐6 (IL‐6) and 8‐isoprostane (8‐IsoP) with effects observed at 0.005 μM TBBPA. There was no increase in testosterone production in response to TBBPA in E. coli challenged placental explants as the testosterone concentration in the untreated controls was already high. Oestradiol concentrations tended to decrease in E. coli challenged TBBPA exposed explants with a lowest effective concentration of 0.5 μM. In the presence of E. coli stimulation, TBBPA increased production of tumour necrosis factor α (TNFα), IL‐6 and IL‐10 at exposures of 5 μM and higher. Placental production of interleukin‐1β in response to E. coli challenge was supressed by TBBPA concentrations of 0.5 μM and above. An increase in the concentration of 8‐IsoP at the lower exposure levels (0.005–0.5 μM; without E. coli) indicates a possible involvement of oxidative stress, but another oxidative stress biomarker, haem oxygenase‐1 (HO‐1), showed only increased concentrations at higher levels of exposure (5–50 μM).
Production of cytokines was also affected by exposure of the placental cell line HTR‐8/SVneo to TBBPA (Park et al., 2014). Concentrations of IL‐6 and prostaglandin E2 (PGE2) in the culture medium were increased following exposure to a non‐cytotoxic concentration of 10 μM TBBPA and also at a concentration of 20 μM which caused significant cell death. The increase in PGE2 production was likely caused by TBBPA stimulation of cyclic oxygenases (COX) as the effect was abrogated by COX inhibitors. Further analyses revealed increased expression at the mRNA level of a large number of immune related genes with a lowest effective concentration of 10 μM.
Yao et al. (2021) dosed male mice by oral gavage with 30 μg/kg bw per day TBBPA or 50 μg/kg bw per day TBBPA‐bDiBPrE either once or for 7 days. There were alterations in the lipid profile of the liver, and transcriptomics analysis revealed that sets of genes regulated by the treatment were enriched in Gene Ontology (GO) terms relating metabolic regulation, lipid metabolism and immune response. These responses generally suggested an immunosuppressive effect although this was not followed up on in the study by additional experiments.
In summary, there is mounting evidence for effects of TBBPA on cytokine production and responses to inflammatory stimuli, supporting observations in animal studies (see Section 3.1.2.4). It is possible that some of these effects may occur in vitro at nanomolar and low micromolar concentrations, but the molecular initiating event(s) for these effects remain(s) elusive.
3.1.5. Consideration of critical effects and dose–response modelling
3.1.5.1. Consideration of critical effects
In the previous Opinion (EFSA CONTAM Panel, 2011c), thyroid hormone homeostasis was considered the main target for TBBPA toxicity. The CONTAM Panel identified a Reference Point (BMDL10 calculated by the authors of the study) of 16 mg/kg bw per day for a 10% decrease in circulating T4 (van der Ven et al., 2008).
Since then, new toxicological studies with TBBPA have been carried out using different experimental designs with single or repeated dosing during gestation, postnatally or in adulthood. Except for a limited number of studies on TBBPA‐bDiBPrE, no data are available on TBBPA derivatives.
Four studies involved dosing TBBPA to mice via drinking water (Li et al., 2022; Song et al., 2024; Xiong et al., 2023; Zatecka et al., 2013). These studies investigated effects in the thyroid, neurotoxicity/neurobehaviour or reproductive toxicity and reported effects at exceptionally low levels (150 and 1500 ng/mL, reported to be about 0.05 and 0.5 mg/kg bw per day) (see Sections 3.1.2.2.2, 3.1.2.3 and 3.1.2.5).
The CONTAM Panel noted that thyroid effects and reproductive toxicity appeared at much higher doses when TBBPA was administered by gavage. In a study by gavage in mice, an increased height of thyroid follicular epithelial cells was observed in males after exposure by gavage to 20 mg/kg bw per day (Hu et al., 2023). Some studies in rats using gavage have reported decreased TT4 levels at doses ≥ 100 mg/kg bw per day for thyroid toxicity (Choi et al., 2011; Cope et al., 2015; NTP, 2014). In another study in rats no effects on thyroid hormone levels or thyroid histopathology were observed up to 1000 mg/kg bw per day (Osimitz et al., 2016).
Neither exposure of adults nor exposure of offspring during development to TBBPA had effects on conventional reproductive endpoints in male rats, including reproductive organ weight, sperm count, serum testosterone level and testicular histology. In the gavage studies, no adverse effects were reported at doses up to 1000 mg/kg bw per day on reproduction (2‐generation study) or developmental toxicity in Sprague–Dawley rats (Cope et al., 2015). A delay in the time to preputial separation was found in F1 Wistar Han rats exposed by gavage (GD6‐PND21) to 250 mg/kg bw per day (Brown et al., 2020).
The CONTAM Panel noted that the drinking water studies were generally well conducted, however, the concentrations in the drinking water were not confirmed by analysis of TBBPA, which may be important, e.g. because of the low solubility of TBBPA in water (see Section 1.3.1). The authors reported that the daily intake of TBBPA in μg/kg bw per day was estimated based on the daily water consumption and body weight. This may be the case for dams but not for the pups, as no drinking water consumption was measured. The CONTAM Panel considered that there is a high level of uncertainty regarding the doses received by the animals, and therefore, no NOAELs/LOAELs were identified from these studies, and no dose–response assessment was performed. The potential relevance of these studies is considered further in the uncertainty analysis (see Section 3.5).
The data available on TBBPA showed that liver is not a critical target organ, because adverse effects were reported only at doses higher than other effects, i.e. 500 mg/kg bw per day or higher (Choi et al., 2011; Dunnick et al., 2017; Tada et al., 2006, 2007).
The only notable thyroid effect in rats exposed by gavage was a significant reduction of serum TT4 levels occurring at doses from 100 mg/kg bw per day (Cope et al., 2015; NTP, 2014). In mice exposed by gavage there was no effect on serum TT3, TT4 or TSH levels. The only effect observed was an increased height of thyroid follicular epithelial cells in male mice after exposure to 20 mg/kg bw per day (Hu et al., 2023).
Kidney lesions have been observed in neonatal mice or rats exposed by gavage to high doses (> 100 mg/kg bw per day) of TBBPA in utero or during lactation as well as in adult mice (Fukuda et al., 2004; NTP, 2014; Tada et al., 2006).
No effect on reproduction or development were observed in 2‐generation reproductive toxicity studies in Sprague–Dawley rats exposed by gavage up to 1000 mg TBBPA/kg bw per day (Cope et al., 2015). A delay in the time to preputial separation was found in F1 Wistar Han rats exposed by gavage (GD6–PND21) to 250 mg/kg bw per day (Brown et al., 2020).
TBBPA has been demonstrated to impair behavioural performances related to memory in adult mice (Kim et al., 2017) and to increase anxiety in adult rats perinatally exposed to the contaminant (Rock et al., 2019). A two‐generation study reported a reduction in the parietal cortex thickness in F2 rats early exposed to TBBPA through the parental and the F1 generation (Cope et al., 2015). The lowest dose of TBBPA reported to increase the level of anxiety was 25 mg/kg bw per day in adult rats perinatally exposed from GD6 to PND90 (Rock et al., 2019, 2nd experiment), while a dose of 100 mg/kg bw per day was demonstrated to impair the memory retention in adult mice postnatally exposed for 2 weeks (Kim et al., 2017). The CONTAM Panel noted the large gap between the lowest and the middle doses in the Rock et al. (2019, 2nd experiment) such that a reliable NOAEL could not be identified. Two studies with one dose level showed the ability of TBBPA to alter social behaviour in adulthood in mice (Kim et al., 2015) and locomotor activity in adulthood in rats (Rock et al., 2019, 1st experiment) at similar low dose levels (respectively, 0.2 and 0.1 mg/kg bw per day, exposure during gestation and lactation). A large set of new data related to the TBBPA modes of action in the brain support the view that TBBPA is potentially neurotoxic with multiple molecular and cellular changes observed in vitro and ex vivo, including cell viability and cytotoxicity, oxidative stress, apoptosis, intracellular calcium changes, signalling pathways modifications, synaptic plasticity and neurotransmitter concentration and receptor expression. Some in vitro studies performed using human or rodent stem cell‐based systems suggest TBBPA to be a developmental neurotoxicant.
The limited studies on immunotoxic effects suggest that high doses (200 mg/kg bw per day, Watanabe et al., 2017) of TBBPA directly from the diet or in utero can have effects on immune system in mice.
TBBPA has been found to induce uterine epithelial tumours (predominantly uterine adenocarcinoma) and atypical endometrial hyperplasia in female rats, testicular adenoma in male rats and hepatoblastoma in male mice. It was not tumourigenic in female mice (NTP, 2014). The available evidence indicates that TBBPA is not genotoxic (see Section 3.1.2.6) and there are a number of plausible non‐genotoxic modes of action for carcinogenicity of TBBPA. The carcinogenicity of TBBPA is considered to be relevant for humans, with modes of action that are likely to have thresholds for effects due to the multiple biochemical events involved (see Section 3.1.4.2). The lowest dose of TBBPA reported to cause a significant increase in tumours was 500 mg/kg bw (357 mg/kg bw per day), for adenocarcinomas of the uterus in female rats. A significant increase in atypical endometrial hyperplasia of the uterus (which is considered to be a preneoplastic lesion) was observed at the dose of 250 mg/kg bw (179 mg/kg bw per day), the lowest dose tested.
The cumulative evidence on TBBPA effects in humans stems from a small number of small epidemiological studies of usually cross‐sectional design that have assessed mostly thyroid function and neurotoxicity in mostly Chinese populations. Exposure assessment for other contaminants was rarely reported and in the few studies in which it was performed, adjustment for these contaminants was rarely incorporated into the TBBPA analysis. For neither neurotoxicity nor thyroid function, was the cumulative evidence sufficient to support or refute a TBBPA effect in humans.
Based on all data available and the potential relevance to humans of the endpoints considered for the risk assessment, the CONTAM Panel considered that the critical effects of TBBPA are neurobehavioural changes, carcinogenicity and the preneoplastic lesion atypical endometrial hyperplasia of the uterus.
There were insufficient data on the toxicity of TBBPA‐bDiBPrE to identify a reference point, and no data on the other derivatives.
3.1.5.2. Dose–response analysis
The Panel performed benchmark dose (BMD) modelling according to the 2022 EFSA Guidance on the use of the BMD approach in risk assessment (EFSA Scientific Committee, 2022, see Section 2.2). The results of the BMD modelling for the critical studies on neurotoxicity/neurobehaviour in mice (Kim et al., 2017) and rats (Cope et al., 2015; Rock et al., 2019, 2nd experiment) and carcinogenicity in rats (NTP, 2014) are summarised in Table 14. Details of the BMD analyses including the individual reports of the modelling are shown in Annex C.
TABLE 14.
Benchmark dose (BMD) modelling for the critical carcinogenicity and neurotoxicity studies of TBBPA (for details of the BMD analyses, see Annex C).
| Reference | Observed effect | BMD; BMDU (mg/kg bw per day) | BMDL10 (mg/kg bw per day) |
|---|---|---|---|
| Rock et al. (2019, 2nd experiment) | Latency(s) to enter light box in mice | 330; 707 | 61.6 a |
| Light box entries in mice | 353; 707 | 77.6 | |
| Open arms entries in mice | 141; 656 | 3.5 a | |
| Kim et al. (2017) | Decrease learning and memory in a passive avoidance test (day 2) in mice | 79.3; 1008 | 10.3 a |
| Cope et al. (2015) | Reduction in brain parietal thickness in rats | 476; 845 | 154 |
| NTP (2014) | Uterus adenocarcinoma in rats | 297; 1175 | 88.1 |
| Uterus adenoma, adenocarcinoma, MMMT combined in rats b | 327; 1236 | 115 | |
| Testis interstitial cell adenoma in rats | 1226; 2045 | 734 | |
| Uterine atypical endometrial hyperplasia in rats | 223; 1090 | 41.6 |
The Panel selected a BMR of 10% for the neurodevelopmental effects. This value is considered to reflect the natural variability of neurobehavioural endpoints, in the absence of any biological consideration of severity to justify a different BMR. This is also in line with the approach taken for the update of the risk assessment on PBDEs (EFSA CONTAM Panel, 2024). For the carcinogenicity the Panel selected the default BMR of 10% for quantal data.
For the one dose level neurobehavioural studies by Kim et al. (2015) and Rock et al. (2019) no BMD modelling could be performed. The LOAELs were 0.2 mg/kg bw per day, based on decreased interest in social interaction in mice, and 0.1 mg/kg bw per day for increased running wheel activity in female mice, respectively.
3.1.6. Approach for risk characterisation
There is evidence that the carcinogenicity of TBBPA occurs via non‐genotoxic mechanisms (see Sections 3.1.2.6 and 3.1.4.2).
Considering the new data available, i.e. chronic/carcinogenicity toxicity study, and additional neurobehavioural and reproductive toxicity studies, the CONTAM Panel considered the toxicological data were sufficient to establish a HBGV.
The CONTAM Panel decided to set the HBGV on a daily basis, i.e. a tolerable daily intake (TDI), because of the low half‐life of TBBPA and lower accumulation compared to, e.g. PBDEs (see Section 3.1.1).
The lowest BMDL10 from the carcinogenicity study was 42 mg/kg bw per day, based on the incidence of the preneoplastic lesion, uterine atypical endometrial hyperplasia, in rats. Effects on neurodevelopment were reported at lower doses than this BMDL10. Therefore, it was decided to base the TDI on the LOAEL of 0.2 mg/kg bw per day for decreased interest in social interaction (Kim et al., 2015) in mice. This effect was considered of higher relevance to humans than the increase in the level of activity at 0.1 mg/kg bw per day as measured by Rock et al. (2019).
The CONTAM Panel considered that the default uncertainty factor of 100 was sufficient to cover uncertainties and variability with respect to kinetic and dynamic differences between animal species and humans and within the human population. An additional factor was applied to account for the absence of a NOAEL in the critical study. The EFSA Guidance on default factors indicates that the size of this factor should be determined on a case‐by‐case basis (EFSA Scientific Committee, 2012), while ECHA (2012) suggests using a factor between 3 (as minimum/majority of cases) and 10 (as maximum/exceptional cases). The Panel decided to apply a factor of 3, considered to be sufficiently conservative.
Based on the above, a TDI for TBBPA of 0.7 μg/kg bw per day was established.
There were insufficient or lack of data on the toxicity of the five TBBPA derivatives included in the TORs to derive a Reference Point for any of the derivatives. Furthermore, there were insufficient data on the mode of action of any of the TBBPA derivatives included in the TORs to allow a comparison with TBBPA that would support assignment to an assessment group for the purpose of combined risk assessment.
3.2. Occurrence data
3.2.1. Occurrence data on food submitted to EFSA
An initial number of 2958 analytical results on TBBPA, TBBPA‐bME and TBBPA‐bDiBPrE in food were available in the EFSA database. Data were reported by six Member States plus Norway and United Kingdom between year 2011 and 2021. The raw occurrence data set on TBBPA and its derivatives in food as extracted from the EFSA data warehouse is available at the EFSA Knowledge Junction community. 34
The occurrence data submitted to EFSA were not systematically checked for possible duplications with the data reported in the literature (see Section 3.2.3). This might have resulted in a partial overlap between the data reported in the scientific literature and the data reported to EFSA and used in the current exposure assessment.
The occurrence data were carefully evaluated, and a list of validation steps was applied before being used to estimate dietary exposure. Data providers were contacted to clarify inconsistencies identified during the data check. The following paragraphs describe modifications that were made to the initial data set based on the feedback received and/or expert judgement.
Analytical results reported as referring to fat weight (n = 858) were converted to whole weight using the provided fat percentage. Some food classified under ‘Animal fat’ that were reported with low fat content were reclassified to fresh meat and vice versa, based on data provider clarifications.
Where analytical results were reported as not corrected for recovery, the reported result was multiplied by the reported recovery factor. When no indication was provided on the application of a correction factor it was assumed that the result was reported as corrected. A recovery factor equal to one was assumed to indicate 100% recovery. A number of analytical results were reported as not corrected for recovery and no recovery factor was provided (n = 599). In this case the recovery was assumed to be 100%.
No analytical results were reported to be related to ‘suspect sampling’, thus none was excluded based on sampling strategy.
Analytical results reported for pooled samples were excluded as information on sample size was missing (n = 12) and it was not possible to ensure a proportionate representation of the individual samples by weighting the reported analytical results for the number of samples pooled.
Food categories that had less than six samples at the Level 1 of the FoodEx2 classification were excluded. This included: 15 analytical results, 100% left‐censored, for ‘Grains and grain‐based products,’ ‘Legumes, nuts, oilseeds and spices’, ‘Composite dishes’, ‘Seasoning, sauces and condiments’, ‘Fruit and vegetable juices and nectars (including concentrates)’ and ‘Products for non‐standard diets, food imitates and food supplements’, and one quantified result for ‘Sugar and similar, confectionery and water‐based sweet desserts’, for TBBPA.
In addition, the CONTAM Panel decided to also exclude analytical results for food categories that had 100% left‐censored results at the Level 1 of the Foodex2 classification. This included for TBBPA, 22 analytical results for ‘Vegetables and vegetable products’, 8 analytical results for ‘Fruit and fruit products’ and 28 analytical results for ‘Animal and vegetable fats and oils and primary derivatives thereof’. For TBBPA‐bME and TBBPA‐bDiBPrE it included 49 analytical results each, for ‘Milk and dairy products’.
A total of 37 analytical results for TBBPA on processed fish and preserved meat were excluded for having a very high LOD (4 μg/kg) and LOQ (12 μg/kg).
After these exclusions, TBBPA concentrations for foods belonging to the food categories ‘Grains and grain‐based products, ‘Composite dishes’ and ‘Animal and vegetable fats and oils and primary derivatives thereof’ and some dairy and egg products were derived from TBBPA concentration in the relevant raw primary commodity, e.g. concentrations in butter calculated from concentrations in milk (see Section 2.3.2).
After the described cleaning procedure, 163 analytical results were excluded while 2795 analytical results were made available to be included in the dietary exposure assessment to TBBPA (n = 2090), TBBPA‐bME (n = 359) and TBBPA‐bDiBPrE (n = 346).
The number of analytical results per year and country in this final data set is presented in Table 15. Most results for TBBPA were provided by France (34%) and Norway (24%) while only France reported data on the two TBBPA derivatives TBBPA‐bME and TBBPA‐bDiBPrE. Overall France reported 60% of all analytical results. Nine samples were reported as of non‐EU origin while 1863 samples were of unknown origin (64%). The majority of the data (60%) were reported between 2014 and 2016.
TABLE 15.
Number of analytical results reported for TBBPA and TBBPA derivatives (TBBPA‐bME and TBBPA‐bDiBPrE) across different European countries and years (country of sampling; final cleaned data set).
| Compound | Country | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Total | % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TBBPA | Denmark | 10 | 20 | 20 | 50 | 2% | ||||||||
| France | 172 | 209 | 191 | 183 | 185 | 1 | 941 | 34% | ||||||
| Germany | 47 | 47 | 2% | |||||||||||
| Ireland | 45 | 45 | 2% | |||||||||||
| Netherlands | 37 | 57 | 53 | 40 | 1 | 188 | 7% | |||||||
| Norway | 59 | 100 | 151 | 144 | 96 | 71 | 59 | 680 | 24% | |||||
| United Kingdom | 139 | 139 | 5% | |||||||||||
| TBBPA Total | Total | 37 | 219 | 348 | 258 | 360 | 345 | 152 | 144 | 96 | 71 | 60 | 2090 | 75% |
| TBBPA‐bDiBPrE | France | 168 | 174 | 4 | 346 | 12% | ||||||||
| TBBPA‐bME | France | 180 | 175 | 4 | 359 | 13% | ||||||||
| Total | 37 | 219 | 348 | 606 | 709 | 353 | 152 | 144 | 96 | 71 | 60 | 2795 | 100% | |
| % | 1% | 8% | 12% | 22% | 25% | 13% | 5% | 5% | 3% | 3% | 2% | 100% | – |
Special attention was given to the verification of analytical methods. The majority of the results referred to LC–MS methods, followed by GC–MS.
Left‐censored data in the final data set accounted between 64% and 82% for TBBPA, 85%–97% for TBBPA‐bDiBPrE and 60%–98% for TBBPA‐bME of the analytical results across FoodEx2 Level 1 food categories (Table 16).
TABLE 16.
Number of samples, % of left‐censored results and LB and UB mean concentration and maximum quantified concentration for each Level 1 of the FoodEx2 classification from occurrence data submitted to EFSA for TBBPA and two derivatives TBBPA‐bME and TBBPA‐bDiBPrE (ng/kg ww) in the final data set.
| Abbreviation | FoodEx2 Level 1 | N | %LC | Mean LB | Mean MB | Mean UB a | Max quant. |
|---|---|---|---|---|---|---|---|
| TBBPA | Meat and meat products | 418 | 64% | 1.42 | 16.81 | 32.21 | 100 |
| TBBPA | Fish and seafood | 1376 | 78% | 17.52 | 51.41 | 85.29 | 13,000 |
| TBBPA | Milk and dairy products | 157 | 82% | 0.17 | 4.02 | 7.86 | 4.71 |
| TBBPA | Eggs and egg products | 139 | 74% | 0.46 | 3.90 | 7.34 | 5.66 |
| TBBPA‐bDiBPrE | Meat and meat products | 90 | 97% | 0.31 | 6.62 | 12.94 | 11.51 |
| TBBPA‐bDiBPrE | Fish and seafood | 188 | 85% | 17.29 | 22.70 | 28.11 | 1700 |
| TBBPA‐bDiBPrE | Eggs and egg products | 39 | 97% | 0.24 | 6.32 | 12.40 | 9.49 |
| TBBPA‐bME | Meat and meat products | 103 | 98% | 0.001 | 0.25 | 0.49 | 0.08 |
| TBBPA‐bME | Fish and seafood | 188 | 60% | 0.43 | 0.48 | 0.53 | 10.00 |
| TBBPA‐bME | Eggs and egg products | 39 | 97% | 0.002 | 1.07 | 2.15 | 0.07 |
The mean UB concentration is in certain cases higher than the maximum quantified value because it is calculated using the substitution method described in Section 2.3.2 thus, it is affected by analytical methods LOQs, that can be higher than maximum quantified values.
Proportions of non‐detected, non‐quantified and quantified analytical results across FoodEx2 Level 1 food categories are presented in Figure 3.
FIGURE 3.
Proportions of non‐detected, non‐quantified and quantified analytical results across FoodEx2 Level 1 food categories.
The highest quantified results for TBBPA were found in ‘Fish and seafood’. The highest quantified result was found in ‘Fish liver’ (13 μg/kg ww) followed by results for ‘Ocean perch’ and ‘Pollack’ (2.87, 1.73 μg/kg ww, respectively). The highest quantified results not belonging to the category ‘Fish and seafood’ was found in ‘Animal fresh fat tissue’ (up to 0.1 μg/kg ww) of the ‘Meat and meat products’ food category.
For TBBPA‐bDiBPrE the highest quantified results were found in ‘Fish and seafood’ (highest value in ‘Clams’ 0.7 μg/kg ww, followed by ‘Sea bass’ at 0.3 μg/kg ww). For TBBPA‐bME the highest quantified results were found in ‘Mussels’ with highest value of 0.01 μg/kg ww.
TBBPA LB and UB mean concentrations for the different Foodex2 food categories were calculated as described in Section 2.3.2. Table 16 shows the LB and UB mean concentrations and maximum quantified concentration for each Level 1 of the Foodex2 classification from occurrence data submitted to EFSA on TBBPA and the two derivatives TBBPA‐bME and TBBPA‐bDiBPrE.
Occurrence data for TBBPA‐bME and TBBPA‐bDiBPrE were not further used as the CONTAM Panel deemed not possible to identify a Reference Point or perform a risk assessment for them due to lack of, or limited, toxicological studies on these TBBPA derivatives (see Section 3.1.5.1).
No occurrence data were submitted to EFSA for food for infants. Thus, the CONTAM Panel decided to use for the dietary exposure assessment to TBBPA, LB and UB mean concentrations of TBBPA in infant and follow‐on formula identified in two studies from the literature (see Section 2.3.2).
Table 17 shows the ranges across various food categories for each Level 2 of the FoodEx2 classification of TBBPA mean concentrations, used for the exposure assessment, obtained from occurrence data submitted to EFSA, extracted from the literature (in the case of infant and follow‐on formula) and calculated as described in Section 2.3.2.
TABLE 17.
TBBPA mean concentration ranges (ng/kg ww) across various food categories (N = number of food subcategories) for each Level 2 (Level 4 for infant and follow on formula) of the FoodEx2 classification from occurrence data submitted to EFSA, extracted from the literature and calculated as described in Section 2.3.2.
| FoodEx2 Level 1 | FoodEx2 Level 2 | N of food subcategories | min LB | median LB | max LB |
|---|---|---|---|---|---|
| Alcoholic beverages | Unsweetened spirits and liqueurs | 1 | 0.1 | 0.1 | 0.1 |
| Animal and vegetable fats and oils and primary derivatives thereof | Animal and vegetable fats/oils | 15 | 1.1 | 4.7 | 394.4 |
| Animal and vegetable fats and oils and primary derivatives thereof | Fat emulsions and blended fats | 1 | 4.0 | 4.0 | 4.0 |
| Composite dishes | Dishes, incl. Ready to eat meals (excluding soups and salads) | 29 | 0.5 | 1.8 | 9.9 |
| Composite dishes | Soups and salads | 6 | 0.2 | 0.5 | 11.8 |
| Composite dishes | Spoonable desserts and ice creams (generic) | 2 | 0.1 | 0.1 | 0.1 |
| Eggs and egg products | Processed eggs | 8 | 0.5 | 2.4 | 4.3 |
| Eggs and egg products | Unprocessed eggs | 11 | 0.4 | 0.5 | 0.5 |
| Fish and seafood | Crustaceans | 18 | 0.3 | 0.9 | 1.6 |
| Fish and seafood | Fish (meat) | 129 | 0.1 | 1.9 | 109.3 |
| Fish and seafood | Fish and seafood processed | 30 | 19.7 | 19.7 | 19.7 |
| Fish and seafood | Fish offal | 9 | 118.1 | 118.1 | 119.9 |
| Fish and seafood | Molluscs | 21 | 1.1 | 1.2 | 4.0 |
| Food products for young population | Follow‐on formulae, liquid | 4 | 116.0 | 116.0 | 116.0 |
| Food products for young population | Follow‐on formulae, powder | 4 | 928.0 | 928.0 | 928.0 |
| Food products for young population | Infant formulae, liquid | 4 | 47.1 | 47.1 | 47.1 |
| Food products for young population | Infant formulae, powder | 3 | 376.8 | 376.8 | 376.8 |
| Grains and grain‐based products | Breakfast cereals | 1 | 0.2 | 0.2 | 0.2 |
| Grains and grain‐based products | Fine bakery wares | 64 | 0.1 | 0.8 | 1.4 |
| Grains and grain‐based products | Pasta, doughs and similar products | 10 | 0.1 | 0.9 | 0.9 |
| Meat and meat products | Animal blood | 1 | 1.1 | 1.1 | 1.1 |
| Meat and meat products | Animal edible offal, non‐muscle, other than liver and kidney | 10 | 1.1 | 1.1 | 1.1 |
| Meat and meat products | Animal fresh fat tissues | 5 | 1.1 | 1.6 | 2.9 |
| Meat and meat products | Animal kidney | 7 | 1.1 | 1.1 | 1.1 |
| Meat and meat products | Animal liver | 16 | 2.7 | 2.8 | 3.4 |
| Meat and meat products | Animal meat dried | 4 | 1.1 | 1.1 | 1.1 |
| Meat and meat products | Animal other slaughtering products | 23 | 1.1 | 1.1 | 1.1 |
| Meat and meat products | Canned‐tinned meat | 4 | 1.1 | 1.1 | 1.1 |
| Meat and meat products | Mammals and birds meat | 51 | 0.5 | 0.8 | 1.8 |
| Meat and meat products | Marinated meat | 1 | 0.6 | 0.6 | 0.6 |
| Meat and meat products | Meat and meat products | 1 | 1.1 | 1.1 |
1.1 |
| Meat and meat products | Meat specialties | 8 | 1.1 | 1.1 |
1.1 |
| Meat and meat products | Preserved/processed fat tissues | 3 | 1.1 | 1.1 |
1.1 |
| Meat and meat products | Processed whole meat products | 29 | 1.1 | 1.1 |
1.1 |
| Meat and meat products | Sausages | 48 | 1.1 | 1.1 | 1.1 |
| Milk and dairy products | Cheese | 128 | 1.7 | 1.7 | 1.7 |
| Milk and dairy products | Dairy dessert and similar | 10 | 0.0 | 0.1 | 0.2 |
| Milk and dairy products | Fermented milk or cream | 24 | 0.2 | 0.2 | 1.3 |
| Milk and dairy products | Milk and dairy powders and concentrates | 8 | 0.5 | 1.9 | 5.1 |
| Milk and dairy products | Milk, whey and cream | 22 | 0.2 | 0.2 | 1.3 |
Abbreviations: LB, lower bound; N, number of food subcategories.
In the previous EFSA Opinion on TBBPA and its derivatives (EFSA CONTAM Panel, 2011c), occurrence data on TBBPA were submitted to EFSA by four European countries (Ireland, Norway, Spain and the UK) for a total of 652 food samples covering the period from 2003 to 2010. The food category with most analytical results was ‘Fish and other seafood (including amphibians, reptiles, snails and insects)’ (n = 465), followed by ‘Meat and meat products (including edible offal)’ (n = 49), ‘Milk and dairy products’ (n = 40), ‘Animal and vegetable fats and oils’ (n = 41) and ‘Eggs and egg products’ (n = 27). Less than 10 samples were reported for the remaining food categories. All analytical results on TBBPA were reported as less than the LOQs of the different methods used (in general ≤ 1 μg/kg ww).
3.2.2. Food processing
No suitable data were identified in the scientific literature with respect to the effects of cooking and processing on levels of TBBPA and the derivatives considered in the TORs in food.
3.2.3. Previously reported occurrence data in the open literature
Occurrence data on TBBPA and its derivatives reported in the open literature until 2011 were summarised in the previous EFSA Opinion (EFSA CONTAM Panel, 2011c).
Since the previous Opinion, some studies have been published in peer‐reviewed journals reporting the occurrence of TBBPA and two derivatives (TBBPA‐bMeE and TBBPA‐bDiBPrE) in food samples. Data from selected studies since 2010 are summarised in Appendix G (Tables G1 and G2), giving a short overview on the occurrence of TBBPA and its derivatives in food collected in European countries but this information is not claimed to be exhaustive.
As shown in Tables G1 and G2, a low detection frequency of TBBPA was observed in food samples across Europe, which is in accordance with the detection frequency of TBBPA in the data submitted to EFSA (see Section 3.2.1). Some of the authors indicated that the studies were performed in response to Commission Recommendation 2014/118/EU on the monitoring of traces of TBBPA and other BFRs in food, and the resulting data might have been submitted to EFSA.
Rivière et al. (2019) determined the concentration of TBBPA in food for infants of 0–3 years (169 samples) and few composite foods (36 samples) collected in France and reported a detection frequency of TBBPA of around 30%. Considering upper bound levels, the mean concentration of TBBPA in growing‐up milk 35 was 21.7 ng/kg ww, while infant formula and follow‐on formula contained TBBPA at 48.6 and 60.1 ng/kg ww, respectively. Martínez et al. (2019) also analysed infant formula samples. A total number of 50 samples from Spain were examined and TBBPA was detected in five samples at levels of 0.57 μg/L (< 1–1.9 μg/L) (J Rovira Solano, 2024, personal communication). Lankova et al. (2013) analysed six infant formula samples, but none of the samples was found to contain TBBPA (LOQ = 50–150 ng/kg). No information on food for infants has been submitted to EFSA.
Venisseau et al. (2018) reported measurements of TBBPA, TBBPA‐bMeE and TBBPA‐bDiBPrE in 15 fish oils, 15 fish meals, 114 fishes, 154 crustaceous and molluscs, 72 milk samples, 57 eggs, 28 sheep livers and 152 meat samples collected in France. TBBPA was detected in almost half of the samples in sheep liver, fish and seafood, in ranges between < LOD and 22.76 ng/kg ww, between < LOD and 68 ng/kg ww and between < LOD and 26 ng/kg ww, respectively. TBBPA was also detected in meat (ranging between < LOD and 22.76 ng/kg ww), eggs (ranging between < LOD and 5.66 ng/kg ww) and milk (ranging between < LOD and 2.70 ng/kg ww) in lower frequencies. TBBPA‐bMeE was mainly detected in seafood (54% detection frequency) and in fish (19% detection frequency), with reported ranges between < LOD and 3 ng/kg ww and between < LOD and 90 ng/kg ww, respectively. TBBPA‐bDiBPrE was detected in 19% of the fish samples analysed, ranging between < LOD and 300 ng/kg ww, whereas in the seafood samples, the detection frequency was 5% with values reaching 1700 ng/kg ww. As confirmed by the authors, most of the occurrence data reported in this paper have been submitted to EFSA, including data for the two TBBPA derivatives.
Poma et al. (2018) reported occurrence of TBBPA in Belgian food samples from animal and plant origin (n = 183 samples). TBBPA was detected in 2% of the composite food samples analysed. TBBPA was detected only in three samples up to 96 ng/kg ww, all belonging to the category ‘meat and meat products’. In the Netherlands, de Winter‐Sorkina et al. (2003) reported TBBPA concentrations in different food products including dairy products, animal products, fish and vegetable oils. TBBPA was quantified only in two cheese products out of the 13 samples analysed (ranges < 0.1–0.09 μg/kg) and six out of the 17 fish samples analysed (range 0.001–3.4 μg/kg). Later, Gebbink et al. (2019) determined levels of TBBPA in 107 animal‐derived food samples. TBBPA was detected in three samples above the LOQ: in one bovine meat sample (0.10 μg/kg lipid), one broiler meat sample (0.054 μg/kg lipid) and one haddock fillet (0.063 μg/kg ww). Garcia Lopez et al. (2018) reported occurrence of TBBPA in Irish foods (n = 53 samples), including milk, eggs, fish and meat samples. TBBPA was only detected in one farmed salmon sample at 0.01 μg/kg ww.
Since 2010 several studies have been published reporting occurrence data on TBBPA and its derivatives in fish and seafood collected both from marine and freshwater regions. Some of these studies identified in the literature are presented in Tables G1 and G2 in Appendix G.
Aznar‐Alemany et al. (2017) analysed TBBPA in 42 samples from 10 species of fish and seafood consumed in Europe. Samples were collected from the Mediterranean Sea, the North Sea and the north‐east Atlantic Ocean, while three samples were imported from the Pacific Ocean and one from India. TBBPA was detected only in 40% of the mackerel samples and in 50% of the monkfish samples (mean values of 2.76 and 24.5 μg/kg lipid, respectively). Considering the average percentage of fat in mackerel (12.7%) and monkfish (0.23%), TBBPA levels are expected to be on average at 0.35 μg/kg ww (range: 0.095–1.0 μg/kg ww) in mackerel and 0.056 μg/kg (range: 0.042–0.064 μg/kg ww) in monkfish. Within the data submitted to EFSA, the levels of TBBPA in monkfish (n = 58, range < LOQ–0.07 μg/kg ww) are similar to those reported in the literature, whereas TBBPA in mackerel (n = 35, range < LOQ–0.02 μg/kg ww) is two orders of magnitude lower than those reported in the literature.
Chessa et al. (2019) analysed TBBPA in 24 samples of wild fish and seafood species and 16 samples of farmed bivalve molluscs from six different locations collected from Sardinia (Italy). All the data were left‐censored (< 0.05 μg/kg ww).
With regards to freshwater and estuaries sites, Kotthoff et al. (2017) reported levels of TBBPA and TBBPA‐bMeE in breams collected from six different sites in Europe and in soles from the Netherlands. TBBPA was detected in all samples ranging between 0.5 and 1.2 μg/kg ww in breams, and 0.5–0.7 μg/kg ww in soles. TBBPA‐bMeE was found < LOD (0.4 μg/kg ww).
In the Czech Republic, Hlouskova et al. (2013) and Svihlikova et al. (2015) investigated the levels of TBBPA in fish from different freshwater sites. Hlouskova et al. (2013) detected TBBPA in 11 out of the 48 samples of different fish species analysed, and reported a mean value of 1.29 μg/kg ww. Svihlikova et al. (2015) reported a detection frequency of 9 out of the 59 samples with a mean value of 1.39 μg/kg ww.
Several publications were also identified reporting the occurrence of TBBPA in food outside Europe, such as from China, the Republic of Korea and Tunisia (see Table H1, Appendix H). The most recent study from China by Zhao et al. (2023) reported mean values (for values lower than LOD the ½ of the LOD was considered for the calculations) of TBBPA in aquatic food, meat, eggs and dairy products at 19.0, 97.7, 13.2 and 3.73 ng/kg ww, respectively. The detection frequency in this study was very high, 100% or close to 100%. TBBPA in meat and eggs was found at higher levels in China when compared to mean concentrations (UB values) in Europe. For aquatic food and dairy products the levels of TBBPA reported in China are close to the levels detected in Europe. Interestingly, the only values reported for TBBPA concentration in vegetables and fruits was from China (Wang et al., 2019). TBBPA was detected in 5 out of the 13 vegetable samples that were analysed with a mean concentration of 0.161 μg/kg ww (median < LOD, range 0.5–5 μg/kg ww). In the same study 8 fruit samples were examined and TBBPA was found in one sample, banana (0.277 μg/kg ww). It is worth noting that the mean levels reported in this study for the other food categories (fish/seafood, meat, eggs and dairy products) were an order of magnitude higher as compared to the levels of TBBPA in the same food categories reported in the most recent study from China by Zhao et al. (2023). In the Republic of Korea, Lee et al. (2020) reported TBBPA levels in different food samples, mainly aquatic and meat‐based food. The levels of TBBPA reported in foods in the Republic of Korea were in the same range as those reported in China by Zhao et al. (2023).
In summary, TBBPA levels in food reported in the open literature were very low, i.e. the majority of data reported were left‐censored, which is in accordance with the data submitted to EFSA. Comparison of the concentrations of TBBPA and derivatives reported in literature with the data submitted to EFSA is impracticable and possible only in few instances due to the high number of left‐censored data and differences in the reporting of the data.
3.3. Exposure assessment for humans
3.3.1. Current dietary exposure assessment
The CONTAM Panel assessed the dietary exposure to TBBPA following the methodology described in Section 2.6. A summary of the TBBPA occurrence data including the number of results and concentrations across the FoodEx2 level food categories as used for exposure assessment is presented in Section 3.2.1.
3.3.1.1. Mean and high dietary exposure
Table 18 shows the summary statistics of the estimated chronic dietary exposure to TBBPA for each age group. Detailed mean and 95th percentile dietary exposure estimates for all age group and population groups and dietary surveys are presented in Annex B (Table B3). The special population groups ‘Pregnant women’, ‘Lactating women’ and ‘Vegetarians’ resulted in mean and P95 exposure estimates within the range of the adult population group and thus will not be further discussed.
TABLE 18.
Mean and P95 dietary exposure (LB and UB) to TBBPA (range across surveys).
| Age group | Mean dietary exposure (ng/kg bw day) | P95 dietary exposure (ng/kg bw day) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | |||||||
| N | Min | Max | Min | Max | N | Min | Max | Min | Max | |
| Infants a | 12 | 1 | 5.4 | 8.4 | 30 | 11 | 4.7 | 11 | 28 | 85 |
| Toddlers | 15 | 0.13 | 3.2 | 1.2 | 11 | 14 | 0.88 | 7.8 | 6.1 | 37 |
| Other children | 19 | 0.015 | 0.19 | 0.28 | 0.75 | 19 | 0.028 | 1.3 | 0.50 | 3.7 |
| Adolescents | 21 | < 0.01 | 0.028 | 0.11 | 0.26 | 20 | 0.013 | 0.16 | 0.25 | 0.5 |
| Adults | 22 | < 0.01 | 0.016 | 0.078 | 0.16 | 22 | 0.011 | 0.057 | 0.16 | 0.35 |
| Elderly | 19 | < 0.01 | 0.014 | 0.075 | 0.16 | 19 | 0.011 | 0.073 | 0.16 | 0.32 |
| Very elderly | 14 | < 0.01 | 0.014 | 0.091 | 0.17 | 10 | 0.01 | 0.046 | 0.19 | 0.32 |
Mean dietary exposure to TBBPA ranged across surveys and LB and UB estimates, from < 0.01 ng/kg bw per day in Adolescents, Adults, Elderly and Very elderly to 30 ng/kg bw day in Infants.
P95 dietary exposure to TBBPA ranged across surveys and LB and UB estimates, from 0.01 ng/kg bw per day in Very elderly to 85 ng/kg bw day in Infants.
In the previous EFSA Opinion (EFSA CONTAM Panel, 2011c) all data submitted to EFSA were reported as left‐censored and, therefore, a meaningful exposure assessment for TBBPA was not possible for any population group. Nevertheless, the EFSA's CONTAM Panel provided a worst‐case exposure assessment of TBBPA for adults with a high fish consumption and for toddlers with a high cow's milk consumption. The CONTAM Panel notes that a comparison of the current data with results from the previous Opinion therefore is not possible due to a number of facts, such as improvements in instrumental analysis, different percentage of left‐censored data, consideration of further food commodities, more occurrence and consumption data submitted to EFSA, and a higher level of stratification of the food categories and age groups.
3.3.1.2. Contribution of different food groups to the exposure estimates
The percentage contribution of each individual food category at the Level 1 and 3 of the Foodex2 classification to the total mean LB chronic dietary exposure of TBBPA was estimated across dietary surveys and is presented in Annex B (Tables B4 and B5).
Contribution of the respective food groups was calculated over LB exposure estimates to avoid that the high contribution of certain food groups could be artificially driven by the treatment of the left‐censored data.
Table 19 describes the contribution of each food category to the overall mean LB exposure to TBBPA as number of surveys in which contribution was higher than 10% and the percentage contribution range across dietary surveys (minimum and maximum) for all age classes.
TABLE 19.
Contribution of each food category to the overall mean LB exposure to TBBPA as number of surveys in which contribution was higher than 10% and the percentage contribution range across dietary surveys (minimum and maximum) for all age classes. No % range shown for categories that did not contribute more than 10% in the age class (–).
| Food | Infants (12) | Toddlers (15) | Other children (19) | Adolescents (21) | Adults (22) | Elderly (19) | Very elderly (14) | N survey >10% |
|---|---|---|---|---|---|---|---|---|
| Fish and seafood | – | – | 16 (2.6–59.3) | 21 (17.8–65.5) | 22 (21–76.4) | 19 (27.9–80.3) | 13 (3.6–79.9) | 91 |
| Meat and meat products | – | – | 13 (2.5–29) | 21 (11.3–36.2) | 22 (10.7–48.3) | 18 (8.5–35.3) | 14 (10.1–43.5) | 88 |
| Milk and dairy products | – | – | 15 (2.4–42.5) | 20 (8.2–40.7) | 18 (7.1–31.1) | 16 (6.6–27.3) | 12 (6.7–28.6) | 81 |
| Food products for young population | 12 (97.2–99.8) | 15 (82.2–99.5) | 10 (0–91.4) | 2 (0–10.7) | – | – | – | 39 |
| Grains and grain‐based products | – | – | 3 (0.01–16.3) | 6 (0.005–18.1) | 1 (1.8–12.5) | 1 (1.6–13.1) | 1 (1.1–15.2) | 12 |
| Animal and vegetable fats and oils | – | – | 2 (0.1–11) | 1 (0.4–12.5) | 1 (0.2–10.6) | 2 (0.3–12.6) | 3 (0.6–13.4) | 9 |
| Composite dishes | – | – | 2 (0–42.3) | – | 1 (0–21.8) | 1 (0–21) | 1 (0–14.1) | 5 |
The food categories with the highest number of surveys in which the contribution was higher than 10% are ‘Fish and seafood’, ‘Meat and meat products’ and ‘Milk and dairy products’ for Other children, Adolescents, Adults, Elderly and Very elderly.
For the age groups Infants and Toddlers, ‘Foods for young populations’ had the highest number of surveys in which the contribution was greater than 10%. Highest percentage contribution was found in Infants and Toddlers for ‘Foods for the young population’ (> 99%) and for ‘Fish and seafood’ in the Elderly (80.3%). ‘Meat and meat products’ have contributed up to 48.3% in Adults and ‘Milk and milk products’ up to 42.5% in Other children, across surveys and age groups.
Within this FoodEx2 Level 1 food categories, the main contributors were:
– ‘Marine Fish’ and ‘Fish and seafood processed’ within the ‘Fish and seafood’ category,
– ‘Mammals and birds meat’ within the ‘Meat and meat products’ category,
– ‘Cheese’ and ‘Milk’ within the ‘Milk and Dairy products’ category, and
– ‘Infant and follow‐on formulae’ within the ‘Foods for the young population’ category.
Figure 4 shows the percentage contribution and the contribution in ng/kg bw per day of food categories to the total exposure to TBBPA for each survey for adults, toddlers and infants.
FIGURE 4.
Percentage contribution (top) and the contribution in ng/kg bw per day (bottom) of food categories to the total exposure to TBBPA for each survey for adults, toddlers and infants.
3.3.1.3. Breastfed infants
For the exposure assessment of breastfed infants, an age of 3 months was selected, equivalent to a weight of about 6.1 kg, with an estimated average daily consumption of 800 mL and a high consumption of 1200 mL of human milk, with a mean fat content of 3.5%. The occurrence data were taken from the studies reported in the literature from European countries since the publication of the previous Opinion. These data were extracted from Table 8 (see Section 3.1.1.3).
The TBBPA mean concentrations ranged from 0.05 to 3.5 ng/g lipid. A higher mean value of 18.7 ng/g lipid was reported in a study in which a hydrolysis step was introduced, thus the levels corresponded to ‘Total TBBPA’ (Martínez et al., 2019, see Section 3.1.1.3).
The exposure scenario based on average human milk consumption and the range of mean concentrations of TBBPA (0.05 to 3.5 ng/g lipid) resulted in an exposure between 0.230 and 16.07 ng/kg bw per day. For infants with high human milk consumption this would result in an exposure between 0.344 and 24.1 ng/kg bw per day.
Considering the highest mean TBBPA concentration reported in the study in which a hydrolysis step was applied to cleave conjugates (MB mean: 18.7 ng/g lipid, Martínez et al., 2019), exposure estimates would be 85.8 and 128.8 ng/kg bw per day, for mean and high consumption, respectively.
3.3.1.4. Formula‐fed infants
Consumption data on infant formula for infants less than 16 weeks of age across European countries are limited. Therefore, the CONTAM Panel performed a dedicated scenario for infants below 16 weeks of age using the consumption data published in the Guidance of the EFSA Scientific Committee (SC) on the risk assessment of substances present in food intended for infants below 16 weeks of age (EFSA Scientific Committee, 2017). For assessing the exposure to substances which do not accumulate in the body, the EFSA Guidance recommended values of 200 and 260 mL/kg bw per day as conservative mean and high level consumption values. These values are derived from data for infants aged 2–4 weeks, when formula consumption is highest, expressed on a body weight basis. Considering infants around 2 months of age (with a body weight of 5 kg), this would lead to daily consumption values of 1000 mL for mean consumption, and of 1300 mL for high consumption. For the exposure estimation, the occurrence data on the infant formula samples expressed in μg/kg ww were converted to μg/L assuming a density of 1, which is a small error which gives rise to negligible additional uncertainty in the exposure estimates.
The same occurrence data extracted from the literature on levels in infant formula that were used for the exposure assessment of infants above 16 weeks of age were used for this scenario (see Section 2.3.2).
Two possible scenarios were considered: one for average consumers (scenario 1) using the mean occurrence values that are considered as the most representative for chronic exposure estimates, and a second scenario (scenario 2) where infants were expected to be loyal consumers of the same brand highly contaminated. In scenario 2, the P95 occurrence levels for TBBPA in infant formula were used. The different exposure estimates are shown in Table 20.
TABLE 20.
Dietary exposure estimates for TBBPA via the consumption data of ‘Infant formula’ using default consumption data as recommended by the EFSA Scientific Committee and the occurrence data from the literature (Section 2.3.2).
| Exposure (ng/kg bw per day) | Mean consumption of infant formula | P95 consumption of infant formula |
|---|---|---|
| Scenario 1 (mean TBBPA occurrence) | ||
| LB | 9.40 | 12.2 |
| UB | 116 | 151 |
| Scenario 2 (P95 TBBPA occurrence) | ||
| LB | 36.2 | 47.1 |
| UB | 200 | 260 |
For scenario 1, mean exposure (LB–UB) was 9.40–116 ng/kg bw per day, while the P95 exposure (LB–UB) was 12.2–151 ng/kg bw per day. For scenario 2, mean exposure (LB–UB) was 36.2–200 ng/kg bw per day, while the P95 exposure (LB–UB) was 47.1–260 ng/kg bw per day.
3.3.2. Previously reported dietary exposure
Since the previous Opinion, a limited number of studies have been published in peer‐reviewed journals reporting estimates of human dietary exposure to TBBPA in Europe. Those studies are summarised in Appendix G along with the underlying occurrence data. No studies have been retrieved reporting estimates of human dietary exposure to any of the TBBPA derivatives considered in this Opinion.
Rivière et al. (2019) investigated dietary exposure to BFRs, including TBBPA, in young children. Concentrations of TBBPA were obtained from the infant total diet study (TDS) performed in France (Hulin et al., 2014) and consumption data for 705 children under 3 years of age were collected as individual, consecutive 3‐day weight food records, excluding totally or partially breastfed infants. The dietary exposure to TBBPA was calculated using data on individual consumption and levels of TBBPA (UB) for four age groups, i.e. 1–4 months, 5–6 months, 7–12 months and 13–36 months. Mean dietary exposure to TBBPA was found to range between 0.968 and 9.94 ng/kg bw per day for the 1–3 years of age group and the 1–4 months of age group, respectively. Moreover, the 90th percentile dietary exposure to TBBPA was calculated and reported to range between 1.8 and 31.3 ng/kg bw per day for the 1–3 years of age group and the 1–4 months of age group, respectively.
Martínez et al. (2019) reported exposure calculations to TBBPA for infants of different age groups, i.e. < 1‐month old, between 1‐ and 6‐month‐old and between 6 and 12‐month‐old, fed exclusively with infant formula. In total 50 infant formula samples were analysed and milk intake figures were obtained from the US‐EPA exposure handbook (USEPA, 2011). Mean exposure estimates were calculated between 0.05 and 0.08 μg/kg bw per day for the > 6–12 months of age group and the < 1 month of age group, respectively.
Aznar‐Alemany et al. (2017) estimated the dietary exposure to TBBPA via intake of commercial seafood species available in European markets. In the course of this study, TBBPA levels were measured in 42 samples from 10 fish and seafood species and detected only in half of the mackerel and monkfish samples. Consumption data were obtained from a consumer survey of 2824 individuals in total, in five European countries, i.e. Belgium, Ireland, Italy, Portugal and Spain (Jacobs et al., 2015) representing western, northern and southern Europe and covering a heterogeneous population in terms of seafood consumption habits. Exposure estimates were calculated for each country separately, using both UB and LB scenarios. Overall, adults from southern European countries (Spain and Portugal) showed the highest mean exposure to TBBPA via seafood of 1.3 ng/kg bw per day, due to the highest total consumption of seafood in those countries.
Several studies were retrieved reporting TBBPA exposure estimates outside Europe. In the most recent study performed in China from Zhao et al. (2023), consumption data from the 6th Chinese TDS were considered, where daily consumption values of each food group were calculated according to the 3‐day dietary survey. The mean estimated daily intake was found to be 156 ± 695 pg/kg bw per day. This was an order of magnitude lower as compared to the previous 5th Chinese TDS (1.51 ng/kg bw per day, Shi et al., 2017b).
3.3.3. Non‐dietary sources of exposure
After food, the next largest source of exposure to BFRs for non‐occupational exposed individuals is from dust. Table 2 in Section 1.3.3.2 summarises studies that report occurrence of TBBPA in dust and includes those where an estimate of exposure has been made. Several of these studies originate from Europe, although there are differences in study design.
Fromme et al. (2014) estimated a ‘High’ dust intake for adults of 0.045 ng/kg bw per day and a ‘High’ dust intake for toddlers of 0.525 ng/kg bw per day on the basis of a study conducted in Germany (considering in both cases an average daily intake of house dust of 30 mg for adults and 60 mg for toddlers).
Abdallah et al. (2016) estimated a dust intake based on median concentrations in indoor dust in France and an average dust intake (20 and 50 mg per day for adults and toddlers, respectively) of 2.2 and 5.6 ng per day, for adults and toddlers, respectively. The authors also estimated the dust intake based on P95 concentrations in dust and a high dust ingestion (50 and 200 mg per day for adults and toddlers, respectively) of 16.8 and 67.3 ng per day, for adults and toddlers, respectively.
Besis et al. (2017) made estimates based on a study conducted in Greece in car dust and reported values for adults of 0.00063 ng/kg bw per day, and for toddlers, the estimate was 0.004 ng/kg bw per day.
Tay et al. (2017) estimated the dust intake based on median concentrations in settled dust in Norway and on an average dust intake (30 mg per day for adults) to be 28 pg/kg bw per day.
Harrad and Abdallah (2011) estimated the exposure of adults and toddlers to be 4.4 and 3.8 ng per day, respectively based on an average dust intake (20 mg per day for adults and 50 mg for toddlers) and median concentrations in car dust.
One study was identified on a TBBPA derivative; Tao et al. (2016) determined TBBPA‐bDiBPrE in dust from homes and offices and estimate an intake of 15 and 23 ng per day for adults and toddlers, respectively, based on median concentrations in dust and average dust intake values of 20 and 50 mg for adults and toddlers, respectively.
Other studies made estimates of exposure from dust in car vehicles, or made estimates of combined flame retardants, or combined exposure from the analysis of dermal hand wipes and exposure to settled dust. Details can be found in Table 2.
Direct comparisons with studies conducted in other global regions is difficult due to the differences in study design (e.g. proximity to industrial sites, way in which samples were taken etc.) but these studies are summarised in Table 2.
Most estimates of intake of TBBPA as a result of exposure from dust are in the order of a few tens of pg/kg bw per day or less, although there are estimates that high level exposure for some individuals can be several hundred pg/kg bw per day. These estimates are based on a small number of ad hoc studies reported in the literature rather than as a result of a formal robust process such as that made for dietary exposure in this assessment. While the extreme values suggest that for some individuals exposure from dust can approach the normal range of exposures found as a result of intake from the diet, current information suggests that for most of the population, diet represents the largest source of exposure to TBBPA.
A comparison was made between using direct measurements from hand wipes with an indirect estimation from settled dust concentrations in order to assess dermal exposure to halogenated flame retardants (Tay et al., 2018). TBBPA accounted for 77% of the total mass of the flame retardants that were measured, and direct dermal exposure of participants to TBBPA via hand wipes was estimated to be 150–18,000 pg/kg bw per day. The mean TBBPA masses (1300 ng, equivalent to 1200 pg/cm2) found in this study were much higher than those reported in children's hand wipes in the USA (0.4 ng) as reported by Stapleton et al. (2014), but lower than those detected in patch samples attached to worker's clothing at an electronic dismantling facility in Finland of 6700 pg/cm2 (Mäkinen et al., 2009). This finding was unexpected by the authors because around 90% of TBBPA is used as a reactive intermediate in the manufacture of epoxy and polycarbonate resins whereas only 10% of TBBPA is used as an additive flame retardant.
TBBPA has been reported in a variety of other studies including dishcloths (Gallisti et al., 2017), plastic toys (Brandsma et al., 2022; Fatunsin et al., 2020), cosmetics (Pawar et al., 2017), camping tents (Keller et al., 2014), thermo cups and kitchen utensils (Samsonek and Puype, 2013), e‐Cigarette Liquids (Wei et al., 2019), the surface of phones and personal computers (Zheng et al., 2017). These studies suggest that these sources of exposure are less important in terms of overall exposure for most of the population when compared to diet or dust.
3.4. Risk characterisation
The CONTAM Panel evaluated the current chronic dietary exposure using mean LB and UB levels of TBBPA in various food groups and using the consumption surveys from European countries available in the Comprehensive Food Consumption database (see Section 3.3.1.1).
The mean LB and UB exposure estimates are presented in Table 18. Mean dietary exposure to TBBPA ranged across surveys from < 0.01 ng/kg bw per day in Adolescents, Adults, Elderly and Very elderly to 30 ng/kg bw day in Infants, while P95 dietary exposure ranged from 0.01 ng/kg bw per day in Very elderly to 85 ng/kg bw day in Infants.
These exposure estimates are all below the TDI of 0.7 μg/kg bw per day. Therefore, the CONTAM Panel concluded that the chronic dietary exposure to TBBPA in the European population does not raise a health concern.
For the breastfed infants scenario, the exposure estimates obtained using the range of mean concentrations reported in the literature from European samples ranged between 0.230 and 16.07 ng/kg bw per day and between 0.344 and 24.1 ng/kg bw per day, for average and high human milk consumption, respectively. A higher exposure estimate of 128.8 ng/kg bw per day was obtained using the highest mean TBBPA concentration reported in the study in which a hydrolysis step was applied to cleave conjugates (see Section 3.3.1.3). These estimates were below the TDI.
For formula‐fed infants, the highest exposure estimates were (LB–UB) 47.1–260 ng/kg bw day obtained considering P95 occurrence values and high consumption of infant formula (see Section 3.3.1.4). These exposure estimates were below the TDI.
No risk characterisation could be performed for any of the five TBBPA derivatives included in the TORs, due to insufficient or lack of data both on the toxicity and occurrence (see Sections 3.1.5.1 and 3.2.1).
3.5. Uncertainty analysis
The aim of the uncertainty analysis is to identify and quantify uncertainties affecting the risk assessment for TBBPA in food and assess the overall certainty of the main conclusions, as recommended in the EFSA Guidance on uncertainty analysis (EFSA Scientific Committee, 2018).
As the risk assessment for TBBPA followed the normal approach of the CONTAM Panel, including standardised elements to address some sources of uncertainty, e.g. default uncertainty factors and use of EFSA's Comprehensive Database on consumption, the uncertainty analysis followed the approach for a standardised assessment (Section 3 of the Guidance).
The combined impact of the identified uncertainties was quantified in a tiered approach. Considering that all the exposure estimates were below the TDI for TBBPA (Section 3.3.1), it was considered appropriate to quantify the combined impact of all the uncertainties affecting the risk characterisation in a single judgement, as described in Section 3.2 of the Guidance (EFSA Scientific Committee, 2018). This proved sufficient to reach a clear conclusion for the present assessment. The certainty of the conclusion on the genotoxic potential of TBBPA was quantified separately.
The following sections report the methods and results for each step in the uncertainty analysis. Further details of the expert knowledge elicitation (EKE) that was performed for the risk characterisation are documented in Annex D.
3.5.1. Identification of sources of uncertainty
Sources of uncertainty related to the exposure assessment, hazard assessment and risk characterisation for the current TBBPA assessment were listed and discussed (see Appendix I). It was then considered which of these were non‐standard 36 sources of uncertainty and which would have most impact on the outcome of the risk assessment. Standard sources of uncertainty 37 were not considered further in the uncertainty analysis, as explained in Section 3 of the EFSA Guidance on uncertainty analysis (EFSA Scientific Committee, 2018).
Uncertainties affecting the exposure assessment are described and prioritised in Table I1 (Appendix I). All of the non‐standard sources of uncertainty were judged to have negligible or low impact on the assessment, including the high proportion of left‐censored occurrence data.
Most of the uncertainties affecting the hazard assessment were judged to have negligible or low impact on the assessment (Table I2, Appendix I). The exceptions to this were as follows:
Moderate impact: No NOAEL could be identified in the critical study (Kim et al., 2015), as this was a one dose level study.
Moderate impact: Four studies in which mice were dosed with TBBPA via drinking water (Li et al., 2022; Song et al., 2024; Xiong et al., 2023; Zatecka et al., 2013) reported effects on the thyroid, neurotoxicity/neurodevelopment or reproduction toxicity at exceptionally low levels whereas other studies where TBBPA was administered by gavage have reported effects only at three orders of magnitude higher dose levels. These studies were generally well conducted, however, the concentrations in the drinking water were not confirmed by analysis of TBBPA, which may be important, e.g. because of the low solubility of TBBPA in water. The authors reported that the daily intake of TBBPA in μg/kg bw per day was estimated based on the daily water consumption and body weight. This may be the case for dams but not for the pups, as no drinking water consumption was measured. The CONTAM Panel considered that there is a high level of uncertainty regarding the doses received by the animals, and therefore, no NOAELs/LOAELs were identified from these studies, and no dose–response assessment was performed.
Uncertainties affecting risk characterisation are described and prioritised in Table I3 (Appendix I). All of the non‐standard sources of uncertainty were judged to have negligible or low impact on the assessment.
3.5.2. Genotoxicity
Uncertainties affecting the evidence on the genotoxic potential of TBBPA (limited relevance of the in vitro Comet assay and lack of convincing evidence for target organ exposure in a blood micronucleus test) were assessed to have negligible impact on the assessment (Table I2, Appendix I). In view of the weight of evidence that carcinogenicity of TBBPA is via non‐genotoxic mechanisms (see Sections 3.1.2.7, 3.1.3.1, 3.1.5.2 and 3.1.6), it was considered sufficient to quantify the certainty of this conclusion by the simpler process of expert group discussion rather than a formal or semi‐formal EKE (see section 12.6 in EFSA Scientific Committee, 2018). Having discussed the evidence and uncertainties involved, the experts agreed that TBBPA is carcinogenic but almost certainly (≥ 99% probability) not via non‐genotoxic mechanisms.
3.5.3. Risk characterisation
As stated above, considering that all the exposure estimates were below the TDI for TBBPA (Section 3.4), it was considered appropriate to quantify the combined impact of all the uncertainties affecting the risk characterisation in a single judgement. This was done by a semi‐formal process of expert knowledge elicitation (semi‐formal EKE, annex B.8 of EFSA Scientific Committee, 2018).
The question to be addressed in the EKE was formulated as follows:
What is your % probability that, if all of the identified non‐standard uncertainties affecting the assessment were resolved (e.g. by obtaining more or better data), current dietary exposure to TBBPA would not raise a health concern for any type of health effect for any of the population groups and surveys considered, at either the mean or P95 of chronic exposure?’
Eight experts (five toxicology experts, one epidemiology expert and two exposure assessment experts) provided judgements and reasoning on this question. Their individual judgements of the required probability ranged from 80% to 99%; the detailed judgements are documented in Annex D together with a detailed description of the EKE procedure.
After discussing and refining their individual judgements and reasoning, the experts worked towards a consensus conclusion based on the following considerations:
The highest TBBPA exposure estimate (for formula‐fed infants at the P95 of both consumption and occurrence) was a factor of 2.6 below the TDI based on the LOAEL of 0.2 mg/kg bw per day for decreased interest in social interaction in mice. This is slightly less than the additional factor of 3 recommended by the EFSA Scientific Committee (2017) to allow for increased inter‐individual toxicokinetic variability when considering infants below 16 weeks of age. However, this was an UB estimate of exposure and the true exposure is likely to be much closer to the LB, which was a factor of 14 below the TDI (see Section 2.3.2). Exposure estimates for all other population groups were at least a factor of 5 below the TDI, making it almost certain that current dietary exposure to TBBPA does not raise a health concern.
TBBPA was concluded to be carcinogenic but almost certainly (≥ 99% probability) via non‐genotoxic mechanisms (see Section 3.5.2). The lowest acceptable BMDL10 for a cancer endpoint (41.6 mg/kg bw per day for uterine atypical endometrial hyperplasia from NTP, 2014) was two orders of magnitude above the LOAEL of 0.2 mg/kg bw per day for decreased interest in social interaction.
There was a LOAEL of 0.1 mg/kg bw per day identified for increased level of activity in the running wheel apparatus (1st experiment in Rock et al., 2019), which is a factor of 2 closer to the dietary exposure estimates than the LOAEL of 0.2 mg/kg bw per day for decreased interest in social interaction (Kim et al., 2015) which is the Reference Point for the establishment of the TDI. However, the relevance for humans and adversity of an increased activity level in the running wheel apparatus was uncertain. This, together with the other uncertainties affecting this endpoint and the exposure estimates, made it extremely unlikely that this effect would raise a health concern.
Effects on thyroid, reproduction and neurotoxicity endpoints were reported at exceptionally low levels in a series of studies where mice were exposed to TBBPA via drinking water (Li et al., 2022; Song et al., 2024; Xiong et al., 2023; Zatecka et al., 2013). These studies were generally well‐conducted but the concentrations in the drinking water were not confirmed by analysis of TBPPA, which may be important, e.g. because of the low solubility of TBBPA in water. Thus there is a high level of uncertainty regarding the doses received by the animals. If resolving this uncertainty would confirm the occurrence of effects at lowest level reported in these studies (5 μg/kg bw per day), it could result in a lower tolerable intake, which would be exceeded by the UB estimates of exposure for Infants in the general population and formula‐fed infants, and also by the highest estimates of exposure for breastfed infants (which were MB but based on ‘total TBBPA‘ rather than ‘free TBBPA’). Taking account of the high uncertainty regarding the calculation of the dose levels in these toxicity studies and the uncertainties affecting the different exposure estimates, the experts considered it possible but very unlikely that these effects raise a health concern.
Based on these considerations, the experts agreed on a consensus judgement of 90%–95% probability that current dietary exposure to TBBPA would not raise a health concern for any of the surveys and population groups considered, including breastfed and formula‐fed infants.
A lower probability of 80% was given by one expert noting the increasing evidence of the sensitivity of the developing brain to chemical exposure, including MOA studies with TBBPA reported in the Opinion. These indicate some probability that relevant effects of TBBPA may be found at lower dose levels in future, though these might be intermediate rather than apical (see Annex D).
3.5.4. Summary of the uncertainty analysis
Uncertainties affecting each part of the assessment were systematically identified and prioritised, and their combined impact on the main conclusions was quantified by expert judgement.
Uncertainties affecting assessment of genotoxicity were assessed to have negligible impact on the conclusion. Considering the weight of evidence involved, the CONTAM Panel concluded that TBBPA is carcinogenic but almost certainly (≥ 99% probability) via non‐genotoxic mechanisms.
Considering that all the exposure estimates were below the TDI for TBBPA, and taking account of all associated uncertainties, the CONTAM Panel concluded with 90%–95% certainty 38 that current dietary exposure to TBBPA would not raise a health concern for any of the surveys and population groups considered.
4. CONCLUSIONS
TBBPA and its derivatives have been used commercially as both additive BFR and reactive BFR. They can enter the environment as a result of releases from production sites but probably more importantly via migration from products, especially where it has been used as an additive flame retardant. The environmental exposure could be either during normal use of the product or at end of life, e.g. at landfill or e‐waste sites. When these compounds enter the environment, they may undergo degradation under both aerobic and anaerobic conditions to form lower brominated analogues, and eventually completely dehalogenated to bisphenol A (BPA). TBBPA is less bioaccumulative than other BFRs or POPs, but is still found to accumulate in foods at higher trophic levels.
As required by the Terms of Reference, the present Opinion focusses on TBBPA and five TBBPA derivatives, i.e. TBBPA bismethyl ether (TBBPA‐bMeE), TBBPA bis(2‐hydroxyethyl) ether (TBBPA‐bOHEtE), TBBPA bisallyl ether (TBBPA‐bAE), TBBPA bis(glycidyl ether) (TBBPA‐bGE) and TBBPA bis(2,3‐dibromopropyl)ether (TBBPA‐bDiBPrE).
The assessment is an update of the EFSA CONTAM Panel Opinion on TBBPA and its derivatives in Food published by EFSA in 2011. It takes into account the occurrence data in food and biological samples submitted to EFSA after the publication of the previous Opinion, as well as the newly available scientific information of relevance to hazard identification and characterisation.
No risk characterisation could be performed for any of the five TBBPA derivatives included in the TORs, due to insufficient or lack of data both on the toxicity and occurrence (see Sections 3.1.5.1 and 3.2.1).
4.1. Hazard identification and characterisation
4.1.1. Toxicokinetics
TBBPA is well absorbed through the gastrointestinal tract in rodents. Due to extensive metabolism, the oral bioavailability in rats is less than 5%. No data were identified in humans.
TBBPA is mainly metabolised to glucuronide and sulfate conjugates in rodents and humans.
Rodent data with repeated exposure to TBBPA showed no accumulation in tissues due to rapid elimination.
Several studies have measured concentrations of TBBPA in human samples, as a conjugate mostly in serum and partly in human milk.
In rodents, most of the TBBPA and metabolites are eliminated in the faeces.
In humans, only urine samples have been analysed where TBBPA‐glucuronide and TBBPA‐sulfate have been detected.
In rodents, there is evidence of transplacental transfer and transfer via lactation of TBBPA.
Elimination half‐lives of TBBPA in rodents have been reported to be about half a day, and in humans about 2–3 days.
4.1.2. Toxicity in experimental animals
TBBPA has shown some evidence of effects on the liver in mice and rats. Increases in liver weight were generally small (< 12%) and occurred at doses of 500 mg/kg bw per day or higher. Histopathological changes were reported in some studies, without a clear dose–response relationship.
The only notable thyroid effect in rats exposed to TBBPA by gavage was a significant reduction of serum TT4 levels occurring at doses from 100 mg/kg bw per day. The only effect observed in mice was an increased height of thyroid follicular epithelial cells in males exposed by gavage at 20 mg/kg bw per day.
Kidney lesions were observed in neonatal mice or rats following exposure of dams (during gestation and lactation) by gavage to high doses (> 100 mg/kg bw per day) of TBBPA as well as in adult mice.
No effect on reproduction or development were observed in 2‐generation reproductive toxicity studies in rats exposed by gavage up to 1000 mg TBBPA/kg bw per day. In another study, a delay in the time to preputial separation was observed in F1 male pups of rat dams exposed by gavage (GD6–PND21) to 250 mg/kg bw per day.
TBBPA can affect the immune system. Exposure to TBBPA (200 mg/kg bw per day) directly from the diet or in utero can have effects on immune system in mice with associated changes in serum cytokine concentrations in offspring from TBBPA exposed dams.
TBBPA was shown to induce behavioural disturbances later in life after an early exposure by gavage in utero, during lactation or postnatally in rats and mice. Effects reported included memory deficit, anxiety and/or locomotor activity perturbations with effects appearing at a wide range of doses. Histological alterations were noted in brain areas implicated in such behaviours (parietal cortex, hippocampus).
Among the various behavioural disorders, impairments in sociability and social recognition were reported in adult male mice perinatally exposed to TBBPA at a dose of 0.2 mg/kg bw per day.
Based on the evidence available, TBBPA is not genotoxic.
TBBPA has been found to induce testicular adenoma and uterine epithelial tumours (predominantly uterine adenocarcinoma) in rats. In mice there was some evidence of increased incidence of hepatoblastoma in males, with no carcinogenic effects in females at the assessed doses, which were not considered to provide a robust basis for risk assessment.
4.1.3. Observations in humans
The epidemiolocal evidence on TBBPA pertains to various endpoints, including thyroid function and neurotoxicity.
The cumulative evidence is non‐longitudinal consisting of a small number of small studies. Exposure assessment for other contaminants was rarely reported and in the few studies in which it was performed, adjustment for these contaminants was rarely incorporated into the TBBPA analysis.
The lack of prospective epidemiological evidence, the small number of studies, the small study sample sizes, the lack of consistency and replication of the associations under study render this body of evidence insufficient.
4.1.4. Mode of action
Based on in vitro studies in several cell types it was shown that generation of reactive oxygen species (ROS), and the resulting oxidative damage, apoptosis and mitochondrial dysfunction may be mechanisms whereby TBBPA exerts toxic effects: carcinogenicity, kidney and testis toxicity, impaired oocyte maturation, neurotoxicity and immunotoxicity. Moreover, increasing cytosolic Ca2+ concentration may be a primary event triggering oxidative damage and neurotoxicity.
As a correlate with the increasing cytosolic Ca2+ concentration, TBBPA induced some electrophysiology and glutamate neurotransmission disturbances in various neural cell types. Recent studies using new alternative in vitro neurotoxicity models indicated the potentiality of TBBPA to be a developmental neurotoxicant that affects both neuronal and glial cell types.
There is evidence that the carcinogenicity of TBBPA occurs via non‐genotoxic mechanisms which are likely to have thresholds for effects due to the multiple biochemical events involved.
4.1.5. Critical effects and dose–response analysis
The evidence from the available human data did not provide a sufficient basis for the risk assessment. Thus, the CONTAM Panel considered the data from studies in experimental animals to identify Reference Points for the human risk characterisation.
The CONTAM Panel concluded that neurobehavioural changes and carcinogenicity were the critical effects for the hazard characterisation.
Since TBBPA is carcinogenic, but via non‐genotoxic mechanisms, and considering the new data that had become available since the previous Opinion, the CONTAM Panel considered it appropriate to set a Tolerable Daily Intake (TDI).
Dose–response modelling of the data from the carcinogenicity study in rats resulted in a BMDL10 of 42 mg/kg bw per day, based on the incidence of the preneoplastic lesion, uterine atypical endometrial hyperplasia.
Effects on neurodevelopment were reported at lower doses than this BMDL10 in studies with one dose level meaning that the data could not be modelled. A LOAEL of 0.2 mg/kg bw per day for decreased interest in social interaction in adult male mice pups exposed from GD8–PND21 via the dams was identified as the most appropriate Reference Point for TBBPA risk characterisation.
Applying the default uncertainty factor of 100 for inter‐ and intraspecies variability, and a factor of 3 to extrapolate from LOAEL to NOAEL, a TDI for TBBPA of 0.7 μg/kg bw per day was established.
There were insufficient or lack of data on the toxicity of the five TBBPA derivatives included in the TORs to derive Reference Points for any of the derivatives.
There were insufficient data on the mode of action of any of the TBBPA derivatives included in the TORs to allow a comparison with TBBPA that would support assignment to an assessment group for the purposes of combined risk assessment.
4.2. Occurrence and exposure for the European population
4.2.1. Occurrence in food
Following data cleaning, 2090 analytical results were made available to be included in the assessment of dietary exposure to TBBPA.
Left‐censored results in the final data set for TBBPA accounted between 64% and 82% of all analytical results.
The highest quantified results for TBBPA were found in ‘Fish and seafood’. The highest quantified result was found in fish liver (13 μg/kg ww) followed by results for ‘Ocean perch’ and ‘Pollack’ (2.9 and 1.7 μg/kg ww, respectively). The highest quantified results not belonging to the category ‘Fish and seafood’ was found in ‘Animal fresh fat tissue’ (0.1 μg/kg ww) of the ‘Meat and meat products’ food category.
No occurrence data were submitted to EFSA for food for infants. Thus, the CONTAM Panel decided to use for the dietary exposure assessment to TBBPA, LB and UB mean concentrations of TBBPA in infant and follow‐on formula identified in two studies from the literature.
In the studies on human milk from European countries identified in the literature, TBBPA mean concentrations ranged from 0.05 to 3.5 ng/g lipid. In a study in which a hydrolysis step was introduced to cleave conjugates, a higher mean value of 18.7 ng/g lipid was reported.
Following data cleaning a total of 359 analytical results were available for TBBPA‐bME and 346 analytical results for TBBPA‐bDiBPrE, submitted to EFSA by one European country. The highest concentrations were reported in ‘Clams’ for TBBPA‐bDiBPrE (0.7 μg/kg ww) and in ‘Mussels’ for TBBPA‐bME (0.01 μg/kg ww).
The occurrence data on TBBPA‐bME and TBBPA‐bDiBPrE were not further used as the CONTAM Panel deemed not possible to identify a Reference Point or perform a risk assessment for them due to lack of, or limited, toxicological studies.
4.2.2. Exposure assessment
Mean dietary exposure to TBBPA ranged across surveys and LB and UB estimates, from < 0.01 ng/kg bw per day in Adolescents, Adults, Elderly and Very Elderly to 30 ng/kg bw day in Infants.
P95 dietary exposure to TBBPA ranged across surveys and LB and UB estimates, from 0.01 ng/kg bw per day in Very elderly to 85 ng/kg bw day in Infants.
The food categories with the highest number of surveys in which the contribution was higher than 10% are ‘Fish and seafood’, ‘Meat and meat products’ and ‘Milk and dairy products’ for Other children, Adolescents, Adults, Elderly and Very elderly. For the age groups Infants and Toddlers, ‘Foods for young populations’ had the highest number of surveys in which the contribution was greater than 10%.
The highest percentage contribution was found in Infants and Toddlers for ‘Foods for the young population’ (> 99%) and in the Elderly for ‘Fish and seafood’ (80.3%). ‘Meat and meat products’ have contributed up to 48.3% in Adults and ‘Milk and milk products’ up to 42.5% in other children, across surveys and age groups.
An exposure scenario for breastfed infants using the range of TBBPA mean concentrations in human milk samples from European countries reported in the literature, resulted in daily exposure estimates for average human milk consumption between 0.23 and 16.1 ng/kg bw per day. For infants with high human milk consumption this resulted in an exposure between 0.34 and 24.1 ng/kg bw per day.
Considering the highest mean TBBPA concentration in human milk reported in a study in which a hydrolysis step was applied to cleave conjugates, exposure estimates were 85.8 and 129 ng/kg bw per day, for mean and high consumption, respectively.
An exposure scenario for formula‐fed infants below 16 weeks of age considering mean TBBPA occurrence data at the LB, resulted in daily exposure estimates of 9.4 and 12.2 ng/kg bw per day, respectively for mean and P95 infant formula consumption. At the UB, it resulted in estimates of 116 and 151 ng/kg bw per day, respectively.
Considering P95 TBBPA occurrence data at the LB, it resulted in daily exposure estimates of 36.2 and 47.1 ng/kg bw per day, for mean and P95 infant formula consumption. At the UB, it resulted in estimates of 200 and 260 ng/kg bw per day, respectively.
The available data suggest that for most of the population, diet represents the largest source of exposure to TBBPA.
No suitable data were identified in the scientific literature with respect to the effects of cooking and processing on levels of TBBPA and the TBBPA derivatives considered.
4.3. Risk characterisation
The exposure estimates to TBBPA for the European population, including breastfed and formula‐fed infants, are all below the TDI of 0.7 μg/kg bw per day.
The CONTAM Panel concluded that the chronic dietary exposure to TBBPA in the European population does not raise a health concern.
An uncertainty analysis was performed. Based on the weight of evidence, the CONTAM Panel concluded that TBBPA is carcinogenic but almost certainly (≥ 99% probability) via non‐genotoxic mechanisms. Considering that all the exposure estimates were far below the TDI for TBBPA, and taking account of all associated uncertainties, the CONTAM Panel concluded with 90%–95% certainty 39 that current dietary exposure to TBBPA would not raise a health concern for any of the surveys and population groups considered.
5. RECOMMENDATIONS
The CONTAM Panel made the following recommendations:
More data on occurrence of TBBPA in human milk and food for infants, with more sensitive analytical methods, to enable a more robust exposure assessment for infants.
Data on the occurrence of TBBPA in food of plant origin.
Studies to understand the contribution of TBBPA conjugates to the overall exposure.
More toxicokinetic data on TBBPA in humans and rodents.
More reproductive studies, allowing a comparison between rats and mice, both in male and females.
Developmental neurotoxicity studies on TBBPA to better characterise the dose–response relationship, and explore sensitive endpoints and species differences.
More information on the mode of action of developmental neurotoxicity.
Information that would allow understanding of the large differences between the doses inducing effects in studies performed by gavage or drinking water administration.
In order to conduct a risk assessment for the TBBPA derivatives, the CONTAM Panel made the following recommendations:
Data on the occurrence of the TBBPA derivatives in food, with sensitive analytical methods.
Occurrence of TBBPA derivatives in human milk and food for infants, with more sensitive analytical methods, to enable an exposure assessment for infants.
Information that would allow hazard identification and characterisation for the TBBPA derivatives.
6. DOCUMENTATION AS PROVIDED TO EFSA
Data on the study design and levels reported in Martínez et al. (2019) were provided by Joaquim Rovira Solano following a personal communication and used in Section 2.3.2 Data validation and analysis.
Abbreviations
- 8‐IsoP
8‐Isoprostane
- 8‐OHdG
8‐hydroxy‐2′‐deoxyguanosine
- ABC transporters
ATP‐binding cassette transporters
- Adj OR
adjusted odds ratio
- AHR
aryl hydrocarbon receptor
- Akt
protein kinase B
- ALT
alanine transaminase
- AMPA
α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid
- AMU
Assessment and Methodological Support Unit
- ANSES
French Agency for Food, Environmental and Occupational Health and Safety
- AOP
adverse outcome pathway
- AP‐1
activator protein‐1
- APCI
atmospheric pressure chemical ionisation
- APPI
atmospheric pressure photoionization
- AR
androgen receptor
- AST
aspartate aminotransferase
- ATP
adenosine triphosphate
- AUC
area under the curve
- BALF
bronchoalveolar lavage fluid
- BCRP
breast cancer resistance protein
- BDNF
brain‐derived neurotrophic factor
- BFRs
brominated flame retardants
- BIRADS
breast imaging reporting and data system
- BKMR
Bayesian Kernel machine regression
- BMD
benchmark dose
- BMDCs
bone marrow dendritic cells
- BMDL
benchmark dose lower confidence limit
- BMDL5
benchmark dose lower confidence limit for a benchmark response of 5%
- BMDL10
benchmark dose lower confidence limit for a benchmark response of 10%
- BMI
body mass index
- BMR
benchmark response
- BPA
bisphenol A
- bw
body weight
- CAR
constitutive androstane receptor
- carboxy‐H2DCFDA
6‐carboxy‐2′,7′‐dichlorodihydrofluorescein diacetate
- CAS
chemical abstract service
- CEP Panel
Panel on Food Contact Materials, Enzymes and Processing Aids
- CGC
cerebellar granule cells
- CI
confidence Interval
- CLH
harmonised classification and labelling
- CNQX
6‐cyano‐7‐nitroquinoxaline‐2,3‐dione
- COCs
cumulus–oocyte complexes
- CONTAM Panel
Panel on Contaminants in the Food Chain
- CoRAP
Community Rolling Action Plan
- COT
Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment
- COX
cyclic oxygenases
- CP
cyclophosphamide monohydrate
- CREB
cAMP response element‐binding
- CYP
cytochrome
- DBBPA
dibromobisphenol A
- DBDPE
decabromodiphyenyl ethane
- DCF
dichlorofluorescein
- DCFH‐DA
dichloro‐dihydro‐fluorescein diacetate
- DHPN
N‐bis(2‐hydroxypropyl)nitrosamine
- DI
deiodinase
- DMBA
7,12‐dimethylbenz(a)anthracene
- DMSO
dimethyl sulfoxide
- DOC
deoxycorticosterone
- DPI
diphenyleneiodonium chloride
- DSB
double strand breaks
- DTU
technical University of Denmark
- ECB
European Chemicals Bureau
- ECHA
European Chemicals Agency
- ED
endocrine disruptors
- EEC
European Economic Community
- EGF
epidermal growth factor
- EGFR
EGF receptor
- EKE
expert knowledge elicitation
- ER
oestrogen receptor
- ERK
extracellular signal‐regulated kinases
- EROD
ethoxyresorufin‐O‐deethylase
- ERRc
oestrogen‐related receptor
- ES
ectoplasmic specialisation
- EU
European Union
- EURL
European Reference Laboratory
- F
female
- FOB
functional observation battery
- FT3
free triiodothyronine
- FT4
free thyroxine
- GABA
gamma‐amino‐n‐butyric acid
- GAPS‐Megacities
global atmospheric passive sampling network
- GC
gas chromatography
- GD
gestational day
- GHS
globally harmonised system of classification and labelling of chemicals
- GO
gene ontology
- GPR30
G protein‐coupled receptor 30
- GR
glucocorticoid receptor
- GSH
glutathione
- GSSG
oxidised glutathione
- GZBC
Guangxi Zhuang Birth Cohort
- H2O2
hydrogen peroxide
- hAR
human androgen receptor
- HBCDDs
hexabromocyclododecanes
- HBGV
health‐based guidance value
- HCA
high‐content image
- HO‐1
haem oxygenase‐1
- HPLC
high performance liquid chromatography
- HSD17β
hydroxysteroid‐dehydrogenase‐17β
- IARC
International Agency for Research on Cancer
- IC
half‐maximal inhibitory concentration
- ICAM‐1
intercellular adhesion molecule‐1
- ICNA
Industrial Chemicals (Notification and Assessment) Act
- IFN
interferon
- IL
interleukin
- ip
intraperitoneal
- IPCS
International Programme on Chemical Safety
- IQR
inter quartile range
- iv
intravenous
- IκB
inhibitor of κB
- KC
key characteristics
- LA
Louisiana
- LB
lower bound
- LBD
ligand binding domain
- LC
liquid chromatography
- LC50
lethal concentration 50
- LD50
lethal dose, 50%
- LOAEL
lowest observed adverse effect level
- LOD
limit of detection
- LOQ
limit of quantification
- LPS
lipopolysaccharide
- M
male
- MAPK
mitogen‐activated protein kinase
- MB
medium bound
- MBBPA
monobromobisphenol A
- MBD1
methyl‐CpG binding domain 1
- MD
monocyte depleted
- MDA
malondialdehyde
- MIE
molecular initiating event
- MK‐801
(+)‐5‐methyl‐10,11‐dihydro‐5H‐dibenzo[a,d]·cyclohepten‐5,10‐imine hydrogen maleate
- MLs
maximum levels
- MMC
cross‐linking agent mitomycin C
- MMMTs
Malignant mixed Müllerian tumours
- MMP‐9
matrix metalloproteinase‐9
- MOA
mode of action
- MOE
margin of exposure
- MRP
multidrug resistance proteins
- MS
mass spectrometry
- MTT
tetrazolium salt (3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide)
- NAC
N‐acetyl‐L‐cysteine
- NADPH
nicotinamide adenine dinucleotide phosphate
- NCoR
nuclear hormone receptor corepressor
- ND
not detected
- NF‐κB
nuclear factor‐kappaB
- NICNAS
National Industrial Chemicals Notification and Assessment Scheme
- NIEHS
National Institute of Environmental Health Sciences
- NIS
sodium/iodide symporter
- NK
natural killer
- NMDA
N‐methyl‐d‐aspartate
- NOAEL
no‐observed‐adverse‐effect level
- NOX
NADPH oxidase
- NPCs
neural progenitor cells
- NR
not reported
- NTP
National Toxicology Program
- OCSPP
Office of Chemical Safety and Pollution Prevention
- OECD
Organisation for Economic Co operation and Development
- OPFRs
organophosphate flame retardants
- P95
95th percentile
- PBDEs
polybrominated diphenyl ethers
- PBK
physiologically based kinetic
- PBMCs
peripheral blood mononuclear cells
- PBPK
physiologically based pharmacokinetic
- PBT
persistent, bioaccumulative and toxic
- PCE
polychromatic erythrocytes
- PCR
polymerase chain reaction
- PDK3
pyruvate dehydrogenase kinase 3
- PEC
priority existing chemical
- PGE2
prostaglandin E2
- P‐gp
P‐glycoprotein
- PI3K
phosphatidylinositol 3‐kinase
- PK
protein kinase
- PLE
pressurised liquid extraction
- PND
postnatal day
- PNW
postnatal week
- POPs
persistent organic pollutants
- PPAR
peroxisome proliferator‐activated receptor
- PPR
plant protection products and their residues
- PPS
preputial separation
- PR
progesterone receptor
- PS
phosphatidylserine
- QA
quality assurance
- QC
quality control
- QuEChERS
Quick, Easy, Cheap, Effective, Rugged and Safe
- RAC
ECHA's Committee for Risk Assessment
- RAR
retinoic acid receptor
- REACH
Registration, Evaluation, Authorisation and Restriction of Chemicals
- RNAPII
RNA polymerase II
- RoHS
restriction of hazardous substances
- ROS
reactive oxygen species
- RPC
raw primary commodity
- RSV
respiratory syncytial virus
- RT‐PCR
reverse transcription polymerase chain reaction
- SC
Scientific Committee
- SD
standard deviation
- smNPCs
small molecule neural precursor cell cultures
- SOD
superoxide dismutase
- SOP
standard operational procedures
- SPD
single‐parent ditype
- SPE
solid‐phase extraction
- SRC2
steroid receptor coactivator‐2
- SSB
single strand breaks
- SULT1E1
steroid sulfotransferase 1E1
- SVHC
substance of very high concern
- Τ4
thyroxine
- TBARS
thiobarbituric acid reactive substance
- TBBPA
tetrabromobisphenol A
- TBBPA‐bAE (TBBPA‐BAE)
TBBPA bisallyl ether
- TBBPA‐bDiBPrE (TBBPA‐BDBPE)
TBBPA bis(2,3‐dibromopropyl) ether
- TBBPA‐bGE (TBBPA‐BGE)
TBBPA bis(glycidyl ether)
- TBBPA‐bMeE (TBBPA‐BME)
TBBPA bismethyl ether
- TBBPA‐bOHEtE (TBBPA‐BHEE)
TBBPA bis(2‐hydroxyethyl) ether
- TBBPS
tetrabromobisphenol S
- TCBPA
trichlorobisphenol A
- TDI
tolerable daily intake
- TDS
total diet study
- TFF1
trefoil factor 1
- TG
thyroglobulin
- TGFβ
transforming growth factor beta
- Th2
T helper type 2 cells
- THAI
thyroid hormone action indicator
- Thibz
thyroid hormone‐induced basic leucine zipper protein
- Thrβ
thyroid hormone receptor β
- TNF
tumour necrosis factor
- TORs
terms of reference
- TPO
thyroperoxidase
- TR
thyroid hormone receptor
- Trhr
thyrotropin releasing hormone receptor
- TriBBPA
tribromobisphenol A
- TSCA
Toxic Substances Control Act
- TSH
thyroid‐stimulating Hormone
- TSHR
thyrotropin receptor
- TT3
total triiodothyronine
- TT4
total thyroxine
- TTX
tetrodotoxin
- TUNEL
terminal deoxynucleotidyl transferase dUTP Nick End Labeling
- UB
upper bound
- UGT
UDP glucuronosyltransferase
- UK
United Kingdom
- UPLC
ultra‐high performance liquid chromatography
- USA
United States of America
- US‐EPA
United States Environmental Protection Agency
- vPvB
very persistent and very bioaccumulative
- w/w
weight/weight
- WHO
World Health Organisation
- ww
wet weight
CONFLICT OF INTEREST
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
REQUESTOR
European Commission
QUESTION NUMBER
EFSA‐Q‐2018‐00434
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
PANEL MEMBERS
Margherita Bignami, Laurent Bodin, James Kevin Chipman, Jesús del Mazo, Bettina Grasl‐Kraupp, Christer Hogstrand, Laurentius (Ron) Hoogenboom, Jean‐Charles Leblanc, Carlo Stefano Nebbia, Elsa Nielsen, Evangelia Ntzani, Annette Petersen, Salomon Sand, Dieter Schrenk, Tanja Schwerdtle, Christiane Vleminckx, and Heather Wallace.
Supporting information
Protocol for the risk assessments for human health related to the presence of brominated flame retardants (BFRs) in food
Benchmark dose analysis
Uncertainty analysis – protocol and results of the EKE
Outcome of the public consultation
ACKNOWLEDGEMENTS
The Panel wishes to thank the following for the support provided to this scientific output: Federico Cruciani and Eleni Gkimprixi. The Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
APPENDIX A. Levels in dust from non‐european countries
A.1.
The Table below, which does not claim to be a comprehensive review of the literature, provides an overview of the occurrence of TBBPA and TBBPA derivatives in dust samples collected in non‐European countries.
TABLE A1.
Occurrence of TBBPA and TBBPA derivatives in dust samples from non‐European countries.
| Country sampling year | Number of samples | Concentration (ng/g) | Exposure via dust ingestion (ng/kg bw per day) | Reference |
|---|---|---|---|---|
| TBBPA | ||||
|
South Africa 2012–2013 |
N = 14 Cars N = 7 Houses N = 7 Offices N = 8 Laboratories |
Median: Cars: 1156 Houses: 120 Offices: 492 Laboratories: 269 |
Adults: 0.08 Teenager: 0.08 Toddlers: 0.60 |
Abafe and Martincigh (2016) |
|
USA NR |
N = 29 Surfaces of 100 cm2 (fridge, microwave, printer) |
Range (ng/100 cm2): LOQ–4.18 |
NR | Di Napoli‐Davis and Owens (2013) |
|
USA 2007–2013 |
N = 4 gyms N = 4 houses |
Mean: Gyms: 50 Houses: 680 |
NR | La Guardia and Hale (2015) |
|
USA 2015–2018 |
N = 84 samples from classrooms and other non‐residential spaces |
Median, mean: 95, 1400 |
NR | Rodgers et al. (2020) |
|
USA 2011–2012 |
N = 19 houses | Median: 209 | NR | Schreder and La Guardia (2014) |
|
Republic of Korea 2009–2016 |
N = 46 houses N = 18 offices N = 6 kindergartens N = 19 cars N = 10 public indoor environments N = 25 schools |
Mean Cars: 126.8 Offices: 1386 Homes: 146.8 Public indoor: 515.9 Schools: 388.3 Kindergartens: 246.2 |
Average estimated daily intakes for general population (1–60 years old): 3.03 |
Barghi et al. (2017) |
|
Republic of Korea 1990–2001 |
N = 42 houses | Mean: 213 | NR | Kweon et al. (2018) |
|
China NR |
N = 15 samples in total 2 RA: residential area 2 OA: office building area 5 EW: e‐waste dismantling workshop 3 RW: raw material‐crushing workshop 3 BW: secondary copper blast furnace workshop |
Mean RA: 9350 OA: 5500 EW: 23,800 RW: 19,200 BW: 7400 |
NR | Liu et al. (2020) |
|
China 2016 |
N = 43 road dust |
Range: < LOQ–74.1 |
Estimation of daily intake via dust ingestion (range) for Adults: 0.0007–0.0021 Toddlers: 0.0014–0.0043 |
Lu, He, et al. (2018) |
|
China 2012 |
Residential houses N = 52 indoor N = 52 outdoor |
Mean Indoor: 3435 Outdoor: 1998 |
Estimation of daily intake via dust ingestion (range) adults: 0.04–7.50 children: 0.31–58.54 |
Wu et al. (2016) |
|
China 2014 |
N = 30 Houses N = 27 Offices |
Range, Median Houses: 7.37–113, 20.1 Offices: 8.18–171, 39.5 |
Estimation of daily intake via dust ingestion (Median) Adults: 0.009 Toddlers: 0.084 |
Wang et al. (2018) |
|
Thailand 2019–2021 |
Indoor Houses N = 13 e‐waste site N = 23 urban area N = 12 rural area |
Range, Median e‐waste site: 44–2300, 720 Urban area: 3.5–300, 68.6 Rural area: 2.0–201, 17 |
Estimation of daily intake via dust ingestion (range) Adults: < 0.01–0.15 Children: 0.02–0.90 Toddlers: 0.06–2.58 |
Waiyarat et al. (2022) |
|
Vietnam 2015 |
Indoor dust N = 3 workplaces N = 3 floor N = 3 settled on shelves |
Range Workplaces: 4900‐9100 Floor: 780–13,000 Settled: 9700‐19,000 |
Only estimated daily exposure for inhalation (median) shown Workers: 1.6 |
Wannomai et al. (2021) |
| TBBPA‐derivatives | ||||
|
USA 2006–2011 |
N = 16 houses In different years |
Median Houses in 2006: 260 Houses in 2011: 200 |
NR | Dodson et al. (2012) |
APPENDIX B. Literature search
B.1.
Information on physicochemical properties, production and industrial use, environmental fate and levels, analytical methods, previous assessments and legislation was gathered from the previous EFSA Opinion on TBBPA and its derivatives (EFSA CONTAM Panel, 2011c), assessments by international bodies (by checking the original websites of the relevant organisations) and from current EU legislation. Literature searches were also conducted to identify new information in reviews and other peer‐reviewed publications, also in the fields of previously reported occurrence data and exposure assessments, food processing and non‐dietary exposure. The information was summarised in a narrative way based on expert knowledge and judgement.
Details of the literature search performed are shown in Table B1 Search strings were run in the databases indicated. Since October 2019 (see Section 2.2), literature searches were performed in August 2022 and June 2023. The outcome of the searches was saved in separate EndNote files and an automatic duplicate detection run. The references were then transferred to DistillerSR (a web‐based systematic review software) where another automatic duplicate detection was again made. The selection for relevance based on title and abstract and full text was done by EFSA staff and WG members.
Until the time of endorsement for public consultation and adoption, the literature was monitored via WoS‐Web of Science to identify studies relevant for the risk assessment.
TABLE B1.
Details of the literature search.
|
((((((ALL=(TBBPA)) OR ALL=(tetrabromobisphenol A)) OR ALL=(TBBPA bismethyl ether)) OR ALL=(TBBPA bis(2‐hydroxyethyl) ether)) OR ALL=(TBBPA bisallyl ether)) OR ALL=(Tetrabromobisphenol A bis(glycidyl ether))) OR ALL=(TBBPA bis(2,3‐dibromopropyl)ether) Sources: Web of science (all databases) and PUBMED Period: 01.01.2011–25.08.2022 |
25.08.2022 |
1766 results Removal of duplicates: 1732 results |
|
((((((ALL=(TBBPA)) OR ALL=(tetrabromobisphenol A)) OR ALL=(TBBPA bismethyl ether)) OR ALL=(TBBPA bis(2‐hydroxyethyl) ether)) OR ALL=(TBBPA bisallyl ether)) OR ALL=(Tetrabromobisphenol A bis(glycidyl ether))) OR ALL=(TBBPA bis(2,3‐dibromopropyl)ether) Sources: Web of Science (all databases) and PUBMED Period: 25.08.2022–20.06.2023 |
20.06.2023 |
Total without duplicates: 214 results |
APPENDIX C. Occurrence of TBBPA in human samples from non‐European countries reported in the literature
C.1.
The Table below, which does not claim to be a comprehensive review of the literature, provides an overview of the occurrence of TBBPA in human samples collected in non‐European countries.
TABLE C1.
Concentration of TBBPA in human samples from non‐European countries.
| Country year | Number of samples | Concentration (ng/g lipid) | Comments | Reference |
|---|---|---|---|---|
| Human milk | ||||
|
Japan 2005–2006 |
n = 9 |
Mean, median (range) (MB): 1.04, 0.72 (0.39–2.22) |
– | Fujii, Nishimura, et al. (2014) |
|
Japan 2008–2010 |
n = 64 |
Mean, median (range): 48, 3.2 (< 0.0–2200) |
Detected in 97% of the samples analysed | Fujii et al. (2018) |
|
Japan 2011–2013 |
n = 12 |
Mean (range): 0.0644 (0.00674–1.28) ng/mL |
– | Akiyama et al. (2015) |
|
Japan 2012–2013 |
n = 19 |
Mean (Range): 1.9 (< 0.018–8.7) |
– | Nakao et al. (2015) |
|
China 2011 |
n = 103 |
Mean, median (range): 0.41, 0.10 (< LOD‐12.46) |
Detected in 53% of the samples | Shi et al. (2013) |
|
China 2011 |
n = 29 pooled samples |
Mean, median (range): 7.58, 1.21 (0.14–66.8) |
Detected in all pools analysed | Shi et al. (2017a) |
|
China 2014 |
n = 111 (from 37 mothers) |
Mean, median (range): 5.59, 1.57 (< LOD‐42) |
Detected in 64% of the samples analysed | Huang et al. (2020) |
|
China 2018 |
n = 105 |
Mean, median (range): 0.761, 0.271 (< LOD–34.8) |
Detected in 99% of the samples analysed | Zhao and Shi (2021) |
|
USA 2004–2005 |
n = 43 |
Range: < 0.03–0.550 |
Detected in 35% of the samples | Carignan et al. (2012) |
| Serum | ||||
|
Republic of Korea NR |
n = 26 Congenital hypothyroidism mother‐infant pairs n = 12 mother‐infant pairs w/o congenital hypothyroidism |
Mean (range): With congenital hypothyroidism: Infants: 83 (< LOD–713) Mothers: 8.61 (< LOD–48.25) w/o congenital hypothyroidism: Infants: 72.5 (< LOD–457.4) Mothers: 10.7 (< LOD–74.0) |
– | Kim and Oh (2014) |
|
Japan 1989–1999 2010 |
n = 60 Adult males |
Range: Years 1989–1999: < 50–950 pg/g ww Year 2010: < 50–420 pg/g ww |
Detected in 28% of the samples analysed | Fujii, Harada, et al. (2014) |
|
Japan 2006 |
n = 10 Adult females |
Mean, median (range): 40.5, 1.0 (< LOQ–238) pg/g ww |
– | Fujii, Nishimura, et al. (2014) |
|
China 2016 |
n = 181 Pregnant women |
Median (range): < LOD (< LOD–5.83) ng/mL |
Detected in 30% of the samples analysed | Li et al. (2020) |
|
USA 2009 |
n = 12 Male firefighters after exposure to fire event |
ND | – | Shaw et al. (2013) |
| Adipose tissue | ||||
|
USA NR |
n = 24 Adults with and w/o Alzheimer disease (post‐mortem) |
Median (P25–P75): 0.67 (0.51–0.91) ng/g ww |
– | Manivannan et al. (2019) |
| Liver | ||||
|
USA NR |
n = 24 Adults with and w/o Alzheimer disease (post‐mortem) |
Median (P25–P75): 1.85 (1.13–4.32) ng/g ww |
– | Manivannan et al. (2019) |
| Urine | ||||
|
USA 2006–2010 |
n = 302 Women with breast cancer |
ND | – | Ilozumba et al. (2022) |
|
China NR |
n = 10 adults from e‐waste area n = 10 adults from non e‐waste area |
ND | – | Lin et al. (2020) |
|
China 2020 |
n = 120 Wuxi population n = 121 Taishun population Males and females (3–86 years old) |
ND Geometric mean: 0.122 ng/mL |
– Detected in 4.2% of the samples analysed |
Wei et al. (2022) |
|
China 2020 |
n = 86 Chengdu population n = 104 Nantong population n = Shehong population |
Mean (range): 4.41 (< 0.193–33.7) 0.140 (< 0.193–0.500) 0.135 (< 0.193–0.136) |
Detected in 44.2% of the samples analysed Detected in 2.88% of the samples analysed Detected in 0.909% of the samples analysed |
Xu, Hu, et al. (2022) |
|
China 2013 |
n = 94 Individuals residing near a bisphenol AF manufacturing plant |
ND | – | Yang et al. (2014) |
|
China NR |
n = 20 parent–child pairs |
Average: Children: 0.171 μg/L Parents: 0.181 μg/L |
Detected in 80% of the samples analysed in both groups | Zhang et al. (2016) |
|
China NR |
n = 1157 male and females general population (< 18–> 59 years old) |
Mean (range): 0.04 (< 0.061–2.25) μg/L |
Detected in 7.3% of the samples analysed | Huang et al. (2023) |
| Hair | ||||
|
Republic of Korea, Iran 2017 |
n = 24 Korea n = 15 Iran |
Mean (range): Korea: 5.00 (ND–16.04) ng/g Iran: 0.23 (ND–1.08) ng/g |
HBCDDs | Barghi et al. (2018) |
|
China 2019–2020 |
n = 598 (362 females and 236 males, average age of 62.6 ± 11.9 years old) |
Range: < LOD ~ 1299 ng/g Median: 1.86 ng/g Significant higher concentrations of TBBPA in female hair than in male hair |
Detected in 75% of the samples analysed | Li et al. (2024) |
APPENDIX D. Toxicological studies on TBBPA and TBBPA derivatives identified since the previous Opinion
TABLE D1.
Summary of the outcomes of toxicological studies of TBBPA in experimental animals sorted by exposure route.
| Reference |
Purity Supplier (lot number) |
Species tested (strain) Number of animals per dose group Age Gender |
Route of administration Exposure doses Vehicle Study duration | Parameter(s) studied and effects reported | NOAEL/LOAEL |
|---|---|---|---|---|---|
| Diet | |||||
| Watanabe et al. (2010). Effects of tetrabromobisphenol A, a brominated flame retardant, on the immune response to respiratory syncytial virus infection in mice |
Purity: NR Tokyo Kasei (NR) |
Mice (BALB/c) 6–7 per group 5 weeks at study start F |
Oral (diet) 0%, 1% in diet (RSV and mock infected) Vehicle: none Duration: 4 weeks |
Effects in TBBPA‐treated mice:
Increased % of double‐positive CD4 + CD8+ cells in BALF, in infected mice when also treated with TBBPA |
LOAEL = 1700 mg/kg bw (estimated) |
| Watanabe et al. (2017). Perinatal exposure to tetrabromobisphenol A (TBBPA), a brominated flame retardant, exacerbated the pneumonia in respiratory syncytial virus (RSV)‐infected offspring mice |
Purity: ≥ 98.0% Tokyo Kasei (T556F) |
Mice (BALB/c) 10 (control) or 8 (TBBPA) animals per group 7 weeks at pairing F |
Oral (diet) 0%, 0.01%, 0.1%, 1% in diet, corresponding to 0, 20, 200 and 2000 mg/kg bw (RSV infected animals) Vehicle: none Duration: GD10–PND21 |
Effects in TBBPA‐treated mice:
Elevated gene expression of IL‐24 in RSV‐infected offspring. |
NOAEL = 20 mg/kg bw (exacerbation of interstitial pneumonia) |
| Gavage | |||||
| Choi et al. (2011). Molecular mechanism of Tetrabromobisphenol A (TBBPA)‐induced target organ toxicity in Sprague–Dawley male rats |
97% purity Sigma‐Aldrich USA (NR) |
Rats (Sprague–Dawley Crl:CD) 9 M per group PND18 (peripubertal) M |
Oral (gavage) 0, 125, 250, 500 mg/kg per day Vehicle: Corn oil 30 days (PND18–48) |
No effect on body weight gain or weights of kidney, spleen, testes, epididymides and adrenals. No histological findings in the liver, kidney, testis and thyroid glands No effect on total CYP content, and levels of CYP1A1, CYP1A2, CYP3A1, cytb5, total GSH and MDA Effects from 250 mg/kg bw per day:
Effects at 500 mg/kg bw per day:
|
NOAEL = 125 mg/kg bw per day LOAEL = 250 mg/kg bw per day NOAEL thyroid = 125 mg/kg bw per day |
| Ohta et al. (2012). Ovariectomised mouse uterotrophic assay of 36 chemicals |
Purity: > 95% Wako Pure Chemical (NR) |
Mice (C57BL/6J) 6 animals per group 8 weeks at study start F |
Oral (gavage) 0, 1000 mg/kg bw per day Vehicle: corn oil Duration: 7 days |
No effect in uterine weight up to 1000 mg/kg bw per day | NOAEL = 1000 mg/kg bw per day |
| Takeshita et al. (2013). Effect of Brazilian propolis on exacerbation of Respiratory Syncytial Virus infection in mice exposed to Tetrabromobisphenol A, a brominated flame retardant |
Purity: > 93% Tokyo Kasei (NR) |
Mice (BALB/c) 5–7 animals per group 5 weeks old F |
Oral (gavage) Soy‐free diet mixed with 0, 1% (w/w) TBBPA alone or mixed with 0.02% (w/w) of Brazilian propolis Vehicle: powder formula for rodents Duration: 4 weeks Intranasal infection with respiratory syncytial virus (RSV) |
Effects at an estimated dose of 1700 mg/kg bw:
|
LOAEL = 1700 mg/kg bw (estimated) |
|
NTP (2014). NTP Technical Report on the toxicology studies of Tetrabromobisphenol A (CASRN 79‐94‐7) in F344/NTac rats and B6C3F1/N mice and toxicology and carcinogenesis studies of Tetrabromobisphenol A in Wistar Han [Crl:WI(Han)] rats and B6C3F1/N mice (Gavage Studies) 90‐day study in rats |
Purity: 99% Albemarle Corporation (25317K‐1) |
Rats (F344/NTac) 10 animals per sex and dose group 5–6 weeks M/F |
Oral (gavage) 0, 10, 50, 100, 500, 1000 mg/kg bw (corresponding to 0, 7.1, 35.7, 71.4, 357, 714 mg/kg bw per day) Vehicle: corn oil Duration: 14 weeks, 5 days per week |
Dose‐related decreases in TT4 concentrations on day 4 and at week 14 in 500 and 1000 mg/kg M and F. This effect was observed with less consistency in the 100 mg/kg groups | NOAEL = 35.7 mg/kg bw per day |
|
NTP (2014). NTP Technical Report on the toxicology studies of Tetrabromobisphenol A (CASRN 79‐94‐7) in F344/NTac rats and B6C3F1/N mice and toxicology and carcinogenesis studies of Tetrabromobisphenol A in Wistar Han [Crl:WI(Han)] rats and B6C3F1/N mice (Gavage Studies) 90‐day study in mice |
Purity: 99% Albemarle Corporation (25317K‐1) |
Mouse (C6B3F1) 10 animals per sex and dose group 6–7 weeks M/F |
Oral (gavage) 0, 10, 50, 100, 500, 1000 mg/kg bw (corresponding to 0, 7.1, 35.7, 71.4, 357, 714 mg/kg bw per day) Vehicle: corn oil Duration: 14 weeks, 5 days per week |
Increases in absolute and relative liver weights in 500 mg/kg bw M (114% of control for both) and 1000 mg/kg bw M (111% and 119% of control, respectively) and F (113% and 112% of control, respectively) Absolute and relative kidney weights significantly decreased (86% and 91% of control, respectively) and spleen (108% and 116% of control, respectively) weights were significantly increased in 1000 mg/kg bw M Increased incidences of renal tubule cytoplasmic alterations in the 500 and 1000 mg/kg M mice. The severity of the lesion in the 1000 mg/kg group was greater than that in the 500 mg/kg bw group |
NOAEL = 71.4 mg/kg bw per day |
| NTP (2014). NTP Technical Report on the toxicology studies of Tetrabromobisphenol A (CASRN 79‐94‐7) in F344/NTac rats and B6C3F1/N mice and toxicology and carcinogenesis studies of Tetrabromobisphenol A in Wistar Han [Crl:WI(Han)] rats and B6C3F1/N mice (Gavage Studies) |
Purity: 99% Supplier: Albemarle Corporation (M032607KA) |
Rats (Wistar Han, [Crl:WI(Han)]) 50 or 60 animals per group 5–6 weeks at study start M/F |
Oral (gavage) 0, 250, 500, 1000 mg/kg bw, 5 days per week, corresponding to 0, 179, 357, 714 mg/kg bw per day Vehicle: corn oil Duration: 2‐years |
Decreased body weight by 12% at 1000 mg/kg bw (M) No effect on mortality or clinical signs Germinal (testicular) epithelium atrophy (≥ 250 mg/kg bw) and testicular interstitial cell adenomas (≥ 500 mg/kg bw) Neoplastic and non‐neoplastic uterine lesions:
6%, 4%, 8%, 12% at 0, 250, 500, 1000 mg/kg bw
8%, 20%, 30%, 32% at 0, 250, 500, 1000 mg/kg bw.
4%, 26%, 22%, 26% at 0, 250, 500, 1000 mg/kg bw.
0%, 8%, 0, 4% at 0, 250, 500, 1000 mg/kg bw |
LOAEL = 179 mg/kg bw per day averaged over the week |
| NTP (2014). NTP Technical Report on the toxicology studies of Tetrabromobisphenol A (CASRN 79‐94‐7) in F344/NTac rats and B6C3F1/N mice and toxicology and carcinogenesis studies of Tetrabromobisphenol A in Wistar Han [Crl:WI(Han)] rats and B6C3F1/N mice (Gavage Studies) |
Purity: 99% Albemarle Corporation (M032607KA) |
Mice (B6C3F1/N) 50 animals per group Age: 5–6 weeks at study start M/F |
Oral (gavage) 0, 250, 500, 1000 mg/kg bw, 5 days per week, corresponding to 0, 179, 357, 714 mg/kg bw per day Vehicle: corn oil Duration: 2‐years |
Neoplastic lesions:
Adenoma or carcinoma of the cecum or colon. Combined incidence: 0%, 0%, 6% at 0, 250, 500 mg/kg bw (Significant trend, historical control (HC): 0%–2%)
(4%, 22%, 16% at 0, 250, 500 mg/kg bw. HC: 0%–6%)
(all organs, significant trend) No neoplastic effects in F |
No clear dose response relationship |
| Cope et al. (2015). A reproductive, developmental and neurobehavioural study following oral exposure of tetrabromobisphenol A on Sprague–Dawley rats |
Purity: NR (HPLC determined, but not stated) Albermale Corporation (25948M‐1NR) |
Rats (Crl: CD1 (Sprague–Dawley) IGS BR) Multigenerational study including 2 generations:
Age:
M, F (F1 and F2 generations) 2‐generation reproductive |
Oral (gavage) 0, 10, 100, 1000 mg/kg bw per day Vehicle: Corn Oil Duration: Parental or F0 generation: 10 weeks prior to mating +3 weeks of gestation and lactation F1 generation: 10 weeks prior to mating +3 weeks of gestation and lactation In accordance to OECD guideline. |
Neurotoxicity/neurobehavioural effects:
Other effects:
|
NOAEL = 100 mg/kg bw per day (based on parietal cortex thickness of F2 animals aged of 11 days) NOAEL = 10 mg/kg bw per day (thyroid toxicity) |
|
Rats (Crl: CD1 (Sprague–Dawley) IGS BR) 25 F/group 6–8 week‐old at study start F Developmental |
Oral (gavage) 0, 100, 300, 1000 mg/kg bw per day Vehicle: corn oil Duration: organogenesis period (F) in accordance with OECD guideline |
No evidence of maternal toxicity (mortality, clinical signs) up to 1000 mg/kg bw per day. No evidence of embryo/fetotoxicity (pre‐/post‐implantation losses, fetal weight, sex ratio, external anomalies) up to 1000 mg/kg bw per day |
NOAEL = 1000 mg/kg bw per day | ||
| Kim B et al. (2015). Endocrine disruptors alter social behaviours and indirectly influence social hierarchies via changes in body weight |
Purity: 97% Sigma‐Aldrich (NR) |
Mice (CD1) 5 dams/group 8 weeks at study start F (dams), M (offspring) |
Oral (tip of pipette) 0, 0.2 mg/kg bw per day Vehicle: corn oil Duration: GD8–PND21 |
No effect of TBBPA on dam on body weight and pup weight adjusted for litter weight No significant differences between TBBPA‐exposed animals and controls in the open‐field Reduced interest in social novelty of TBBPA‐exposed animals in the sociability 3‐chamber test No difference in social hierarchies in a tube test at PNW11 and 15 whereas the social dominance of pups was inversely related to body weight irrespective of treatment Developmental exposure to TBBPA shift behaviour towards increased anxiety and decreased interest in social interactions, and reproduces negative associations between social hierarchy status |
LOAEL = 0.2 mg/kg bw per day (the only dose tested) |
| Yang et al. (2016). Toxic Effects of Tetrabromobisphenol A on Thyroid Hormones in SD Rats and the Derived‐reference Dose |
Purity: not stated Supplier NR |
Rats (Sprague–Dawley) At least 15 M and 15 F per group (from US‐EPA guidelines) Juvenile animals (from US‐EPA guidelines) M, F |
Oral (gavage) (from US‐EPA guidelines) 0, 5, 50, 250, 1000 mg/kg bw per day Vehicle: NR M rats were exposed from PND23 through PND53. F rats were exposed from PND22 through PND42 (from US‐EPA guidelines) [Study design in line with male (Guideline 890.1450) and female (Guideline 890.1450) Pubertal assay]. |
TT4 (M, F), TT3 (M, F) and TSH (F) serum levels of TBBPA‐treated animals changed following a non‐monotonic dose‐effect relationship at the dose range of 0–1000 mg/kg bw per day TSH (M) serum levels decreased with increasing dose up to 1000 mg/kg bw per day BMDL calculations performed by the authors (mg/kg bw per day): TT3 (BMDL, F): 220.01 TT3 (BMDL, M): 5.62 TT4 (BMDL, F): 948.32 TT4 (BMDL, M): 42.99 TSH (BMDL, F): 618.78 TSH (BMDL, M): 28.35 |
No statistical analysis performed |
| Osimitz et al. (2016). Subchronic toxicology of Tetrabromobis phenol A in rats |
Purity: 99% Albemarle Corporation, Chemtura Corporation, Israeli Chemical (composite of three commercial lots) |
Rats (CD® (Crl: CD (SD) IGS BR)) 10 animals/group (plus 5 animals/recovery group) 6 weeks on arrival M/F |
Oral (gavage) 0, 100, 300, 1000 mg/kg bw per day Vehicle: corn oil Duration: 13 weeks, 7 days/week + 6‐week post‐treatment recovery period for additional control and high‐dose animals |
No effects on: mortality, clinical signs, body weight, absolute and relative‐to‐body organ weights, feed consumption, histopathology, urinalysis, ophthalmology, FOB, motor activity, serum TSH and TT3, or other serum clinical chemistry measurements up to 1000 mg/kg bw per day Serum TT4 levels: Significantly decreased in TBBPA‐treated animals (on day 33 in M and F and on day 90 in M) These effects were not considered adverse |
NOAEL = 1000 mg/kg bw per day |
| Sanders et al. (2016). Disruption of oestrogen homeostasis as a mechanism for uterine toxicity in Wistar Han rats treated with tetrabromobisphenol A |
Purity: 97% Sigma‐Aldrich (NR) |
Rats (Wistar Han) 10 animals per group 12 weeks at study start F |
Oral (gavage) 0, 250 mg/kg bw per day Vehicle: 1:1:3 ratio of ethanol, Cremophor EL® and water Duration: 5‐days |
|
LOAEL = 250 mg/kg bw per day (thyroid) NOAEL = 250 mg/kg bw per day (reproduction) |
| Park et al. (2016). Tetrabromobisphenol‐A induces apoptotic death of auditory cells and hearing loss |
Purity: > 95% Sigma‐ Aldrich (NR) |
Mice (ICR) 10 animals/group 4 weeks (18–20 g) at study start Gender NR |
Oral (gavage) 0, 250 mg/kg bw per day Vehicle NR Duration: 30‐days |
Hearing loss at 250 mg/kg bw per day (only one dose used) | Hearing loss at 250 mg/kg bw per day (only one dose used) |
| Kim et al. (2017). High dose tetrabromobisphenol A impairs hippocampal neurogenesis and memory retention |
Purity: 97% Sigma‐Aldrich (NR) |
Mice (C57BL/6) 4–8 animals/group 5‐weeks at study start M |
Oral (gavage) 0, 20, 100, 500 mg/kg bw per day Vehicle: corn oil Duration: 14 days |
No effect on proliferation of newly generated cells in the hippocampus up to 500 mg/kg bw per day Memory retention affected by TBBPA at 100 and 500 mg/kg bw per day at day 2 of testing and at 500 mg/kg bw per day at day 8 of testing Reduced survival of newly generated cells in the hippocampus (dentate gyrus) at 100 and 500 mg/kg bw per day Increasing number of glial cells in the same part of hippocampus (astrocytes and microglial cells) at 100 and 500 mg/kg bw per day |
NOAEL = 20 mg/kg bw per day (based on memory retention impairment at day 2 of testing, and based on immunohistochemical findings in hippocampus) |
| Dunnick et al. (2017). Tetrabromobisphenol A activates the hepatic interferon pathway in rats |
Purity: NR Albemarle Corporation (M032607 K) |
Rats (Wistar Han, [Crl:WI(Han)]) 25 animals per group 5–6 weeks at study start F |
Oral (gavage) 0, 25, 250, 1000 mg/kg bw, 5‐days/week, corresponding to 0, 18, 179, 714 mg/kg bw per day Vehicle: corn oil Duration: 13‐weeks |
|
NOAEL = 179 mg/kg bw per day LOAEL = 714 mg/kg bw per day |
| Yu et al. (2018). Combined effects of cadmium and tetrabromobisphenol a (TBBPA) on development, antioxidant enzymes activity and thyroid hormones in female rats |
Purity: ≥ 99% Sigma‐Aldrich (NR) |
Rats (Sprague–Dawley) 6–7 pups per group PND21 F |
Oral (gavage) 0 (blank), 0 (vehicle), 50 mg/kg bw per day (with or without co‐exposure to CdCl2) Vehicle: 0.5% (w/v) CMC solution Duration: 20 days |
Findings in TBBPA‐treated rats in comparison to vehicle control:
No effect on body weight gain, uterus weight or ovary weight |
NOAEL = 50 mg/kg bw per day (reproduction) NOAEL = 50 mg/kg bw per day (thyroid) LOAEL = 50 mg/kg bw per day (kidney) |
| Rock et al. (2019). Sex‐specific behavioural effects following developmental exposure to tetrabromobisphenol A (TBBPA) in Wistar rats |
Purity: 97% Fluka Analytical (NR) 1st experiment (pilot study) |
Rats (Wistar) 8 litters (control) and 5 litters (TBBPA) GD9–PND21 M, F (offspring) |
Oral (food treat: 20 μL of the vehicle, 100% ethanol, or 20 μL containing 30 μg TBBPA in solution was pipetted onto ¼ of a soy‐free food treat pellet; consumption was monitored to ensure the dam ate the entire treat) 0, 0.1 mg/kg bw per day Vehicle: ethanol Duration: GD9–PND21 (dam) |
No effect on anxiety assessed in the light (dark box and in the elevated plus maze) in both M and F Increasing motor activity in F during the 3 days of testing in the running wheel apparatus No effects in M |
LOAEL = 0.1 mg/kg bw per day |
| Rock et al. (2019). Sex‐specific behavioural effects following developmental exposure to tetrabromobisphenol A (TBBPA) in Wistar rats |
Purity: 97% Sigma‐Aldrich (MKBG4059 V) 2nd experiment (main study) |
Rats (Wistar) 20–24 litters/group GD6–PND90 M, F (offspring) |
Oral (gavage) 0, 0.1, 25, 250 mg/kg bw per day Vehicle: Sesame Oil Duration: Dams: GD6–PND21 Pups: PND22–90 |
AT PND90:
At PND145:
|
NOAEL = 0.1 mg/kg bw per day (based on the number of entries in the light box in M) |
| Brown et al. (2020). Developmental exposure to Tetrabromobisphenol A Has minimal impact on male rat reproductive health |
97% purity Sigma‐Aldrich (#MKBG4059 V) |
Rats (Wistar Han) 20–24 pregnant dams/group and their offspring, equalised to 8 pups (4 M and 4 F per dam if possible) on PND1 |
Oral (gavage) 0, 0.1, 25, 250 mg/kg bw per day Vehicle: Sesame oil Dams treated from GD6 to weaning at PND21 Pups gavaged from PND22–90 M pups allowed to age to 1‐year without treatment |
Anogenital distance on PND4 (M pups): delay at 250 mg/kg bw per day Preputial separation: delay at 250 mg/kg bw per day No effect on: Gross morphology of testes, sperm, prostate, or secondary sex organs at PND21 (prepubertal), PND42 (pubertal), 90 (adult) and 1‐year (senescence) |
NOAEL = 25 mg/kg bw per day |
| Yao et al. (2021). Toxicity of Tetrabromobisphenol A and its derivative in the mouse liver following oral exposure at environmentally relevant levels |
Purity: NR NR (NR) |
Mice (C57BL/6) 5–7 animals/group 8 weeks old M |
Oral (gavage) 0, 30 μg/kg bw Vehicle: olive oil Duration: assessment at 6h after single gavage, 24 h after single gavage, and after daily exposure for 7 consecutive days |
No histopathological changes in the liver, and no alterations in hepatic enzymes (ALT and AST) in the serum | NOAEL = 30 μg/kg bw per day (only dose tested) |
| Hu et al. (2023). Bisphenol analogues induce thyroid dysfunction via the disruption of the thyroid hormone synthesis pathway |
Purity: NR TCI (NR) |
Mice (C57BL/6) 8 animals/sex/group 6–8 weeks old M, F |
Oral (gavage) 0, 0.002, 0.02, 2, 20 mg/kg bw per day Vehicle: corn oil Exposure: 5 weeks |
No effect on serum TT3, TT4 or TSH levels Increased height of follicular epithelial cells in M at 20 mg/kg bw per day (not evaluated at this dose in F) |
NOAEL = 2 mg/kg bw per day |
| Drinking water | |||||
| Zatecka et al. (2013). Effect of tetrabromobisphenol A on induction of apoptosis in the testes and changes in expression of selected testicular genes in CD1 mice |
Purity: NR Sigma (NR) |
Mice (CD1) 20 animals/group Age NR M (F1, F2) F (P, F1, F2) |
Oral (drinking water) 0, 200 μg/L (estimated by the authors as equal to 0, 35 μg/kg bw per day) Vehicle: none Duration: 2‐generations |
No effect on body weight and anogenital distance in F1 and F2 TBBPA‐treated animals No effect on the number of progeny and sex ratio in both generations F2 animals from TBBPA‐treated parents exhibited a statistically significant increase in the absolute weight of prostate by 25% and seminal vesicles by 19% and a decrease in testicular weight by 6%. The weight of epididymis in F2 animals from F1 treated males was also significantly increased Increased incidence of apoptosis in the testes (F1, F2). Changes in the morphometry of seminiferous tubules (decreased epithelial thickness) (F1, F2) No effect on sperm quality and spermatogenesis |
LOAEL = 200 μg/mL (35 μg/kg bw per day) |
| Li et al. (2022). Effects of postnatal exposure to tetrabromobisphenol A on testis development in mice and early key events |
Purity: NR Tokyo Chemical Industry (NR) |
Mice (CD‐1) 5 pups/group Newborn M |
Oral (drinking water) 0, 15, 150, 1500 ng/mL + 0.001% DMSO in water (estimated by the authors as equal to 8, 86, 880 μg/kg bw per day for dams, and to 2, 19, 221 μg/kg bw per day for pups post‐weaning) (body weight calculated at PND21) Vehicle: water Duration: PND0‐PND56 |
On PND7: significant lower body weight, reduced tubule area, decreased Sertoli cell and germ cell numbers and marker gene expression occurred in the testes of the two highest dose groups, along with reduced cell proliferation and disordered arrangement of Sertoli cell nuclei On PND15: most of these testicular alterations still observed in TBBPA‐treated males, and cytoskeleton damage in Sertoli cells also observed On PND56: no effect on body weight nor on testis weight, anogenital distance, sperm count, motility in male pups and serum testosterone level At two highest doses: dose‐dependent reductions in the seminiferous tubule area coupled with decreased Sertoli cells and spermatogonia and the number of stage VII–VIII seminiferous tubules (at 1500 ng/mL), and cytoskeleton damage in Sertoli cells, along with down‐regulated expression of marker genes for Sertoli cells, spermatogonia and spermatocyte |
No NOAEL/LOAEL identified a |
| Xiong et al. (2023). Postnatal exposure to low‐dose tetrabromobisphenol A increases the susceptibility of mammal testes to chemical‐induced spermatogenic stress in adulthood |
Purity: NR NR (NR) |
Mice (ICR (CD‐1)) 5 pups per dose group Newborn M Dams: PND0–21 Offspring: PND22–84 |
Oral (drinking water) 0, 150, 1500 ng/mL (estimated by the authors to about 0, 50, 500 μg/kg bw per day) Vehicle: water Duration: PND0–PND56. testing after PND56 and at PND84 Busulfan injection (i.p.) in adulthood (PND56) |
Slight reductions in the anogenital distance, the seminiferous tubule area and seminiferous epithelium height in the high‐dose group Reduction of the number of germ cells, along with down‐regulation of the expression of the corresponding genes Frailer microtubule tracks in Sertoli cells in TBBPA‐treated testes compared to controls, implying microtubule damage From PND56–84, animals received sterile water only Consistent with microtubule damage, on PND84, TBBPA treatment also led to microfilament damage in seminiferous tubules, characterised by reduced apical ectoplasmic specialisation (ES) and basal ES Shorter and thinner microtubule tracks appeared to show severer microtubule damage with increased dose Busulfan injection in adulthood led to spermatogenic impairment in postnatally TBBPA‐treated mice but not in non‐treated ones |
No NOAEL/LOAEL identified a |
| Song et al. (2024). Tetrabromobisphenol A exerts thyroid disrupting effects but has little overt impact on postnatal brain development and neurobehaviors in mice |
Purity: > 97% Tokyo Chemical Industry (NR) |
Mice (CD‐1) Number: 5 litters/group Age: Dams: PND1–21 Offspring: PND22–56 M offspring tested |
Oral (drinking water) 0, 15, 150, 1500 ng/mL + 0.01% DMSO in water (estimated by the authors to about 0, 5, 50, 500 μg/kg bw per day for the dams) Vehicle: DMSO+water Duration: Dams: PND1–21 Pups: PND22–56 |
On PND15, at 1500 ng/mL: thyroid histological alteration in male pups (evidence of shrinkage in the follicle size and colloid) On PND35 the serum levels of TT3 and TT4 were lower in the two highest dose groups On PND56, the thyroid injury was more severe in male offspring than during lactation In addition to the same effect as those observed on PND15, compensatory follicular hyperplasia and colloids loss were particularly pronounced in the two highest dose groups and blood sinuses between the thyroid follicles appeared to increase in these two groups Significant decreases in the serum TT3 and TT4 levels at the two highest doses During puberty and adulthood, the thyroid morphological alterations became more pronounced in the TBBPA‐treated animals Contradictory effects of TBBPA on the level of anxiety at PND35 according to the dose (reduction of anxiety at 15 ng/mL and higher anxiety level at 1500 ng/mL) Reduction of the serum level of serotonin at the two highest concentrations |
No NOAEL/LOAEL identified a |
Abbreviations: BMDL, Benchmark dose lower bound; CYP, Cytochrome; ED, embryonic day; EROD, Ethoxyresorufin‐O‐deethylase; ES, ectoplasmic specialisation; F, females; GD, gestational day; GSH, Glutathione; LOAEL, lowest‐observed‐adverse‐effects level; M, males; NOAEL, no‐observed‐adverse‐effect level; NR, not reported; OECD, Organisation for Economic Co‐operation and Development; PND, postnatal day; PNW, postnatal week; PPS, Preputial separation; ROS, reactive oxygen species; T3, triiodothyronine; T4, thyroxine; TSH, thyroid‐stimulating hormone; TT3, total triiodothyronine; TT4, total thyroxine; UDP‐GT, UDP‐glucuronosyltransferase.
The CONTAM Panel considered that there is a high level of uncertainty regarding the concentrations of TBBPA in the drinking water and the doses calculated from these concentrations, and thus, no NOAEL/LOAEL was identified from this study.
TABLE D2.
Summary of the outcomes of toxicological studies of TBBPA‐bDiBPrE, the only TBBPA derivative considered in the TORs for which toxicity data have been identified, in experimental animals.
| Reference | Purity Supplier (lot number) | Species tested (strain) Number of animals per dose group Age Gender | Route of administration Exposure doses Vehicle Study duration | Parameter(s) studied and effects reported | NOAEL/LOAEL |
|---|---|---|---|---|---|
| NTP et al. (2017). Tetrabromobisphenol A‐biS(2,3‐dibromopropyl ether) (CASRN 21850–44‐2) administered by gavage to F344/NTac rats and B6C3F1/N mice |
Purity: 94% Great Lakes Chemical Corporation (534106) |
Rats (F344/NTac) 2 × 10 animals per sex and group 5–6 weeks at study start M, F |
Oral (gavage) 0, 62.5, 125, 250, 500, 1000 mg/kg bw (0, 45, 89, 179, 357, 714 mg/kg bw per day) Vehicle: corn oil Duration: 23‐days, 5‐days/week 14‐weeks, 5‐days/week |
Effects at ≥ 125 mg/kg bw:
No effects up to 1000 mg/kg bw on: clinical findings, changes in clinical chemistry parameters, haematology or effects on body weights, absolute and relative‐to‐body organ weights (see methods). There were no gross or histologic lesions, changes in the number of sperm or spermatids, sperm motility or oestrus cycle |
NOAEL = 714 mg/kg bw per day [on the basis that microsomal protein and enzyme changes are not adverse] |
|
Purity: 94% Great Lakes Chemical Corporation (534106) |
Mice (B6C3F1/N) 10 animals per sex and group 6–7 weeks at study start M, F |
Oral (gavage) 0, 125, 250, 500, 1000, 2000 mg/kg bw (0, 89, 179, 357, 714, 1429 mg/kg bw per day) Vehicle: corn oil Duration: 14‐weeks, 5‐days/week |
Effects at ≥ 250 mg/kg bw:
Effects at ≥ 125 mg/kg bw:
No effects up to 2000 mg/kg bw per day on: clinical findings, changes in clinical chemistry parameters, haematology or effects on body weights, absolute and relative‐to‐body organ weights (see methods). There were no gross or histologic lesions, changes in the number of sperm or spermatids, sperm motility or oestrus cycle |
NOAEL = 1429 mg/kg bw per day [on the basis that microsomal protein and enzyme changes are not adverse] | |
| Shockley et al. (2020a). Comparative toxicity and liver transcriptomics of legacy and emerging brominated flame retardants following 5‐day exposure in the rat |
Purity: 94.5% Great Lakes Chemical Corporation (534106) |
Rats (Harlan SD) 6 animals per dose group 7 weeks old M |
Oral (gavage) 0, 0.1, 1, 10, 100, 1000 μmol/kg bw per day (corresponding to 0, 0.09, 0.94, 9.4, 94, 944 mg/kg bw per day) Vehicle: corn oil Duration: 5 days Necroscopy 1 day after the last dose |
No statistically significant effects on mortality, body weight, liver weight or histopathological changes in the liver Decrease in T4 at the top dose (74% of control) No significant liver disease related transcript changes |
NOAEL = 94 mg/kg bw per day LOAEL = 944 mg/kg bw per day |
| Yao et al. (2021). Toxicity of Tetrabromobisphenol A and its derivative in the mouse liver following oral exposure at environmentally relevant levels |
Purity: NR NR (NR) |
Mice (C57BL/6) 5–7 animals per group 8 weeks old M |
Oral (gavage) 0, 50 μg/kg bw Vehicle: olive oil Duration: assessment at 6h after single gavage, 24 h after single gavage, and after daily exposure for 7 consecutive days |
No histopathological changes in the liver, and no alterations in hepatic enzymes (ALT and AST) in the serum | NOAEL = 50 μg/kg bw |
| Li, Xiong, Zhang, et al. (2023). Tetrabromobisphenol A‐bis (2, 3‐dibromopropyl ether) impairs postnatal testis development in mice: the microtubule cytoskeleton as a sensitive target |
Purity: > 98% MREDA (NR) |
Mice (CD‐1) 15 dams (10 M pups/dam) Age: 0–56 days PND7: 3 pups/group PND21: weaning PND21‐56: 1 M pup/5 litter/group |
Oral (drinking water) 0, 150, 3000 ng/mL (expected doses: 0, 50, 1000 μg/kg bw per day) Solvent: DMSO Estimated by the authors as equal to: Dams: 0, 59 ± 9, 1195 ± 150 μg/kg bw per day Weaned pups: 0, 50 ± 9, 1117 ± 172 μg/kg bw per day Duration: PND0–56 |
TBBPA‐bDiBPrE was detectable in serum from suckling pups on PND14 demonstrating that pups are exposed to TBBPA‐bDiBPrE through milk from the treated dams PND7: decrease body weight in high dose group, with no changes in the anogenital distance. Neonatal M from this group presented reduced seminiferous tubule area, reduced germ cell population as well as reduced Sertoli cells per seminiferous tubule. Examination of Sertoli cell microtubule cytoskeleton showed that the microtubule amount per seminiferous tubule was also reduced at 150 ng/mL PND56: no significant alterations in body weight, testis weight and serum testosterone level, but sperm count in cauda epididymis was significantly decreased in the high‐dose group, with no significant changes in sperm morphology. Testes in both dosed groups displayed a smaller seminiferous tubule area. In the two dose groups, the percentage of stage VIII seminiferous tubules was also significantly reduced in a dose‐dependent manner. A few immature germ cells improperly appeared in the lumen. Immature germ cells were also found in the cauda epididymis in the high dose group. There were significant decreases in the number of spermatocytes and spermatids per seminiferous tubule in the high‐dose group. Microtubule damage was still remarkable in adult testes; microtubule tracks in stages VI − VII tubules became shorter and thinner in a dose‐dependent manner. Microtubule cytoskeleton in Sertoli cells is a sensitive target of TBBPA‐bDiBPrE implying the impairment of the blood‐testis barrier integrity |
No NOAEL/LOAEL identified a |
| Li, Xiong, Chen, et al. (2023). Extended exposure to tetrabromobisphenol A‐bis(2,3‐dibromopropyl ether) leads to subfertility in male mice at the late reproductive age |
Purity: > 98% MREDA (NR) |
CD‐1 mice 15 dams (10 M pups/dam) Age: 0–8 months PND7: 3 pups/group PND21: weaning PND21‐8 months: 1 M pup/5 litters/group |
Oral (drinking water) 0, 150, 3000 ng/mL (expected doses: 0, 50, 1000 μg/kg bw per day) Solvent: DMSO Duration: PND0‐8 months In addition, male mice exposed from PND0 up to 8‐month age were allowed to mate with non‐treated females for the evaluation of fertility |
At 8 months: body weight, anogenital distance and serum testosterone levels were not significantly altered Testis weight showed a non‐statistically significant decreasing trend. Reduced epididymal sperm count was observed in the high‐dose group Morphologically abnormal spermatozoa (amorphous head, no hook, folded neck and curly or bent tails) increased with increased doses. The percentage of stages VII‐VIII seminiferous tubules was significantly reduced even in the low‐dose group. The testes of treated mice showed reduced seminiferous epithelium height and seminiferous area in stages VII–VIII seminiferous tubules. Immature germ cells improperly appeared in the lumen in the treated groups and cell debris was also observed in the epididymis, particularly in the caput epididymidis There were decreases in the numbers of spermatogenic cells at different developmental stages. Fewer Sertoli cells were observed per seminiferous tubule in treated testes. Moreover, microtubule of Sertoli cells became shorter and thinner following long‐term exposure with a dose‐dependent decrease. High‐dose treated mice had fewer offspring with a female‐biased sex ratio |
No NOAEL/LOAEL identified a |
Abbreviations: F, females; GD, gestational day; LOAEL, lowest‐observed‐adverse‐effects level; M, males; NOAEL, no‐observed‐adverse‐effect level; NR, not reported; PND, postnatal day.
The CONTAM Panel considered that there is a high level of uncertainty regarding the concentrations of TBBPA in the drinking water and the doses calculated from these concentrations, and thus, no NOAEL/LOAEL was identified from this study.
APPENDIX E. Mode of action studies identified since the previous Opinion
E.1.
The Tables below include studies on the possible mode of action of TBBPA published since the previous Opinion and described in Section 3.1.4. The studies for immunotoxicity are summarised in Section 3.1.4.6.
Table E1.
Studies on oxidative stress, apoptosis and mitochondrial dysfunction.
| Reference | Comments |
|---|---|
| Tetz et al. (2013). Troubleshooting the dichlorofluorescein assay to avoid artefacts in measurement of toxicant‐stimulated cellular production of reactive oxidant species | TBBPA facilitated conversion of 6‐carboxy‐2′,7′‐dichlorodihydrofluorescein diacetate (carboxy‐H2DCFDA) to the fluorescent dichlorofluorescein (DCF) moiety in the absence of serum. The authors concluded that because TBBPA increased fluorescence in the absence of cells, the increased DCF fluorescence observed with TBBPA in the presence of cells cannot be attributed to cellular ROS and may, instead, be the result of chemical activation of carboxy‐H2DCFDA to the fluorescent DCF moiety. This paper is not informative about TBBPA mode of action, but casts doubt on the reliability of any ROS studies using the DCF assay |
| Wang et al. (2014). Quest for the binding mode of tetrabromobisphenol A with Calf thymus DNA | Studies of the binding interaction of TBBPA with calf thymus DNA using multispectroscopic and molecular modelling methods. It was concluded that TBBPA might enter the groove of calf thymus DNA, and that hydrogen bonds and hydrophobic forces play a major role in the binding. Study is of little relevance since we do not consider DNA binding in the genotoxicity section and it is not about covalent binding |
| Krivoshiev et al. (2015). Elucidating toxicological mechanisms of current flame retardants using a bacterial gene profiling assay | Gene expression profiles were monitored using 12 E. coli reporter strains. The bacterial stress‐gene assay demonstrates the induction of a number of stress genes by TBBPA. Clustering suggested that TBBPA results in membrane and protein damage as mode of action with oxidative damage as underlying mechanism |
| Szychowski et al. (2016). Tetrabromobisphenol A (TBBPA)‐stimulated reactive oxygen species (ROS) production in cell‐free model using the 2′,7′‐dichlorodihydrofluorescein diacetate (H(2)DCFDA) assay‐limitations of method | Study casts doubt on the reliability of ROS studies using the DCF assay, similarly to Tetz et al. (2013) |
| Choi et al. (2011). Molecular mechanism of tetrabromobisphenol A (TBBPA)‐induced target organ toxicity in Sprague–Dawley male rats | 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG), a biomarker of DNA oxidative damage was measured in the kidney, liver and testes of Sprague–Dawley rats following oral exposure to TBBPA for 30 days (PND18‐48). Expression levels of SOD were significantly increased at 500 mg/kg bw. 500 mg TBBPA/kg bw strongly induced the production of 8‐OhdG in the testis. It was also increased in the kidney at 250 and 500 mg/kg bw |
| Shao et al. (2017). Mechanism of synergistic DNA damage induced by the hydroquinone metabolite of brominated phenolic environmental pollutants and Cu(II): Formation of DNA‐Cu complex and site‐specific production of hydroxyl Radicals | This paper claims that 2,6‐dibromohydroquinone (2,6‐DBrHQ) is a major metabolite of TBBPA. 2,6‐DBrHQ was reacted with plasmid DNA, or with calf‐thymus DNA, in cell‐free model systems. In the presence, but not absence, of Cu(II), 2,6‐DBrHQ induced double strand breaks and 8‐oxodG formation. It is not clear if this metabolite is formed in mammalian systems and the relevance of these reactions in sub‐cellular systems is unclear |
| Yu et al. (2019). OPFRs and BFRs induced A549 cell apoptosis by caspase‐dependent mitochondrial pathway | Generation of ROS, mitochondrial membrane potential (MMP) dysfunction, cell apoptosis and overload of intracellular free Ca2+ demonstrated that the cytotoxicity induced by TBBPA in A549 cells (non‐small cell lung cancer) was mediated by oxidative stress. The cell survival rate significantly increased when pretreated with Ac‐DEVD‐CHO, suggesting that the caspase‐3 dependent mitochondrial pathway may have played a primary role in the process of A549 cell apoptosis |
| Zhang, Wang, et al. (2019). Regulation of TBBPA‐induced oxidative stress on mitochondrial apoptosis in L02 cells through the Nrf2 signalling pathway | At very high concentrations, TBBPA (10, 20 and 40 mM) significantly increased intracellular ROS, malondialdehyde (MDA) and the ratio of oxidised/reduced glutathione (GSSG/GSH) in human hepatocytes (L02 cells). TBBPA also decreased the cell MMP, caused the release of cytochrome c to cytoplasm and promoted the expression of caspase‐9 and caspase‐3, and finally increased the level of apoptosis. The ROS inhibitor N‐acetyl‐L‐cysteine (NAC) relieved the oxidative stress responses, and prevented the decrease of MMP and increase of apoptosis. Moreover, TBBPA promoted the expression of antioxidant genes related to Nrf2 |
| Włuka et al. (2020). Tetrabromobisphenol A, terabromobisphenol S and other bromophenolic flame retardants cause cytotoxic effects and induce oxidative stress in human peripheral blood mononuclear cells (in vitro study) | Exposure of human peripheral blood mononuclear cells (PBMCs) for 1 or 24 h to TBBPA reduced PBMCs viability and ATP level as well as increased reactive oxygen species (including hydroxyl radical) formation. In addition, TBBPA induced lipid peroxidation and caused oxidative damage to proteins in the incubated cells |
| Barańska, Bukowska, et al. (2022). Determination of Apoptotic Mechanism of Action of Tetrabromobisphenol A and Tetrabromobisphenol S in Human Peripheral Blood Mononuclear Cells: A Comparative Study | The apoptotic potential and mechanism of action of TBBPA in human peripheral blood mononuclear cells (PBMCs) was assessed. TBBPA triggered apoptosis by phosphatidylserine (PS) externalisation on cellular membrane, increasing cytosolic Ca2+ level, decreasing transmembrane mitochondrial potential, activating caspase‐8, −9 and −3, as well as increasing PARP‐1 cleavage, DNA fragmentation and chromatin condensation. TBBPA induced apoptosis mainly by the involvement of the mitochondrial pathway. The accelerated apoptosis of lymphocytes (main population of PBMCs) caused by TBBPA may lead to disorders in the immune system function and possibly be involved in cancer development |
| Steves et al. (2018). Ubiquitous Flame‐Retardant Toxicants Impair Spermatogenesis in a Human Stem Cell Model | Using a human stem cell‐based model of spermatogenesis, it was shown that TBBPA affected spermatogonia and primary spermatocytes after acute treatment (1 day) at concentrations that are physiologically relevant (25–200 μM). TBBPA exposure impairs cell cycle progression in in vitro cultures but did not affect haploid sperm viability. TBBPA affect spermatogenesis through decreases of the mitochondrial membrane potential and ROS generation, ultimately causing apoptosis |
| Guo et al. (2022). Tetrabromobisphenol Exposure Impairs Bovine Oocyte Maturation by Inducing Mitochondrial Dysfunction | TBBPA (20, 40 and 80 μM) exposure of bovine cumulus–oocyte complexes (COCs) impaired oocyte maturation because of the abnormality of nuclear and cytoplasmic maturation. TBBPA exposure induced mitochondrial dysfunction, which resulted in oxidative stress and early apoptosis by mediating the expression of superoxide dismutase 2 (SOD2). In addition, mitochondrial dysfunction led to insufficient energy supply to disrupt spindle assembly and chromosome alignment via regulating pyruvate metabolism mediated by pyruvate dehydrogenase kinase 3 (PDK3). The authors suggested that these abnormalities can induce fertilisation failure and subsequent embryonic development arrest |
| Suh et al. (2017). Tetrabromobisphenol A induces cellular damages in pancreatic β‐cells in vitro | RIN‐m5F cells derived from rat pancreatic b‐cells were exposed to different concentrations of TBBPA for 48 h. TBBPA (≥ 20 μM) significantly decreased cell viability and increased apoptosis, thus, TBBPA‐induced apoptotic cell death. In addition, TBBPA‐induced mitochondrial dysfunction, ROS generation, the formation of certain nitrogen species and elevation of inflammatory cytokine levels in vitro |
| Zieminska, Lenart, et al. (2017). The Role of Ca2+ Imbalance in the Induction of Acute Oxidative Stress and Cytotoxicity in Cultured Rat Cerebellar Granule Cells Challenged with Tetrabromobisphenol A | The role of calcium transients induced by TBBPA in triggering oxidative stress and cytotoxicity was examined in primary cultures of rat cerebellar granule cells (CGC). In CGC calcium imbalance and oxidative stress both mediate acute cytotoxicity of TBBPA. A causal relationship between these mechanisms depends on the concentration of TBBPA. At a concentration of 10 μM TBBPA, an increase in [Ca2+]i is a primary event triggering oxidative stress, depolarisation of mitochondria and reduction of the neuronal viability. At a concentration of 25 μM TBBPA additional calcium‐independent mechanisms of induction of oxidative stress and cytotoxicity emerge |
| Cho et al. (2020). Tetrabromobisphenol A‐induced apoptosis in neural stem cells through oxidative stress and mitochondrial dysfunction | The potential involvement of oxidative stress and mitochondrial dysfunction in TBBPA‐mediated neurotoxicity was investigated in neural stem‐like C17.2 cells isolated from neonatal mouse cerebellum and immortalised. TBBPA was more cytotoxic to neural stem cells than to neurons, astrocytes, or fibroblasts isolated from Sprague–Dawley rat cortices. TBBPA‐induced neural stem cell apoptosis was accompanied by increased reactive oxygen species generation and mitochondrial dysfunction. At a molecular level, TBBPA‐induced apoptosis was determined to be mediated by c‐Jun N‐terminal kinase‐p53 pathway activation. According to the authors, these findings suggest that the adverse effects of TBBPA on hippocampal neurogenesis are due to the inhibition of neural stem cell expansion |
| Zhao et al. (2019). Metabolic perturbation, proliferation and reactive oxygen species jointly contribute to cytotoxicity of human breast cancer cell induced by tetrabromo and tetrachloro bisphenol A | Low micromolar levels (0–10 μM) of TBBPA exposure of MCF‐7 cells (human breast cancer cells) induced cell proliferation and activated the energy metabolism of both glycolysis and amino acid. Higher concentrations of TBBPA (10–50 μM) induce inhibition of cell proliferation in a concentration dependent manner. TBBPA induce the generation of ROS and MDA in accordance with the depletion of GSH, GSH‐Px and SOD activities in MCF‐7 cells at micromolar levels (10–50 μM) |
| Su et al. (2020). TBBPA stimulated cell migration of endometrial cancer via the contribution of NOX‐generated ROS in lieu of energy metabolism | Low doses of TBBPA (10−12–10−9 mol/L) could promote the migration of endometrial cancer cells. NADPH oxidase (NOX)‐driven ROS instead of energy metabolism was sensitive to TBBPA stimulation. In addition, molecular docking supported a link between TBBPA ligand and NOX receptor |
| Lee et al. (2019). Tetrabromobisphenol A Induces MMP‐9 Expression via NADPH Oxidase and the activation of ROS, MAPK and Akt Pathways in Human Breast Cancer MCF‐7 Cells | Treatment with TBBPA of human breast cancer MCF‐7 cells significantly induced the expression and promoter activity of matrix metalloproteinase‐9 (MMP‐9). TBBPA‐induced MMP‐9 expression was mediated by the nuclear factor‐kappaB (NF‐κB) and activator protein‐1 (AP‐1) transcription activation as a result of the phosphorylation of the Akt and the mitogen‐activated protein kinase (MAPK) signalling pathways. TBBPA‐induced activation of Akt/MAPK pathways and MMP‐9 expression were attenuated by a specific NADPH oxidase inhibitor (Diphenyleneiodonium chloride (DPI)), and the ROS scavenger (N‐acetyl cysteine (NAC)). These results suggest that TBBPA can induce cancer cell metastasis by releasing MMP‐9 via ROS‐dependent MAPK, and Akt pathways in MCF‐7 cells |
Table E2.
Studies on mode of action of carcinogenicity/genotoxicity.
| Reference | Comments |
|---|---|
| Song et al. (2013). Investigation of tetrabromobisphenol A interacting with DNA nitrogen bases using experimental and density functional theory methods | As studied by UV–Vis spectroscopy, TBBPA placed into a quartz cuvette interacted as an electrophile with guanine, adenine and cytosine; and as a nucleophile with thymine. The interactions of TBBPA with the nitrogen bases followed the order: guanine > adenine > thymine > cytosine. The TBBPA‐guanine complex exhibited the highest charge transfer, the largest bonding constant, and the most negative interaction energy. The authors concluded that TBBPA interacts directly with DNA nitrogen bases. The CONTAM Panel considered these data, based on chemical interaction rather than in a biological model, to be of limited relevance |
| Harvey et al. (2015). Uterine Carcinomas in Tetrabromobisphenol A‐exposed Wistar Han Rats Harbour Increased Tp53 Mutations and Mimic High‐grade Type I Endometrial Carcinomas in Women | Uterine carcinomas induced by TBBPA in the NTP (2014) study were found to have an increase in tumour protein 53 (Tp53 mutations), and overexpression of human epidermal growth factor receptor 2 compared to spontaneous uterine carcinomas. Tumours in TBBPA‐exposed rats were oestrogen receptor‐alpha positive and progesterone receptor negative by immunohistochemistry. The authors concluded that the morphologic and molecular features of uterine carcinomas in TBBPA‐exposed rats resemble those of high‐grade type I tumours in women, suggesting that exposure to TBBPA may pose an increased cancer risk |
| Lai et al. (2015). Tetrabromobisphenol A (TBBPA): Possible modes of action of toxicity and carcinogenicity in rodents | Review discussing possible MOAs for T4 changes and uterine tumours induced by TBBPA. MOA may increase circulating oestrogens by a competitive inhibition of oestrogen conjugation and produce uterine tumours by promoting pre‐existing Tp53‐mutations due to increased oestrogen levels resulting in increased cell proliferation |
| Hoffmann, Fiedor, et al. (2017). Bisphenol A and its derivatives tetrabromobisphenol A and tetrachlorobisphenol A induce apelin expression and secretion in ovarian cancer cells through a peroxisome proliferator‐activated receptor gamma‐dependent mechanism | TBBPA at low nM concentrations increased apelin (an adipokine) expression and secretion in the epithelial ovarian cancer cell line OVCAR‐3, which involved the peroxisome proliferator‐activated receptor gamma but not oestrogen receptors. Apelin stimulated proliferation of OVCAR‐3 cells. The authors suggested that TBBPA induce ovarian cancer cell progression by up‐regulating apelin, which acts as a mitogenic factor in these cells. The results depended on the specific ovarian cancer cell line used and relevance to uterine cancer is unclear |
| Hoffmann, Gogola, et al. (2017). Stimulation of ovarian cell proliferation by tetrabromobisphenol A but not tetrachlorobisphenol A through G protein‐coupled receptor 30 | OVCAR‐3 and KGN cells were used as in vitro models to represent epithelial ovarian cancers and graulosa cell tumours, respectively. TBBPA, stimulated OVCAR‐3 and KGN cell proliferation. The stimulatory effects were reversed by pre‐treatment with a G protein‐coupled receptor 30 (GPR30) antagonist in both cell lines. GPR30 is a novel membrane receptor that promotes specific binding of endogenous and exogenous oestrogens The authors suggested that TBBPA stimulates ovarian cancer cell proliferation through the GPR30 pathway |
| Hoffmann et al. (2018). Bisphenol A and its derivatives decrease expression of chemerin, which reverses its stimulatory action in ovarian cancer cells | At low nM concentrations, TBBPA decreased chemerin expression and secretion in granulosa cell tumour COV434 cells by both peroxisome proliferator‐activated receptor γ and oestrogen receptor signalling pathways. Since chemerin inhibited TBBPA‐induced cell proliferation, the authors suggested that TBBPA down‐regulation of chemerin expression, may act as a mitogenic factor in ovarian cancer cells |
| Guyton et al. (2018). Application of the key characteristics of carcinogens in cancer hazard identification | Assessment of the key characteristics used by IARC in determining whether animal carcinogenicity is relevant for humans |
| Gorowska‐Wojtowicz et al. (2019). Leydig cell tumorigenesis – implication of G‐protein coupled membrane oestrogen receptor, peroxisome proliferator‐activated receptor and xenoestrogen exposure. In vivo and in vitro appraisal | Study primarily about possible aetiology of Leydig cell tumours, particularly with respect to involvement of PPAR. TBBPA affected PPARα expression |
| Lyu et al. (2020). TBBPA regulates calcium‐mediated lysosomal exocytosis and thereby promotes invasion and migration in hepatocellular carcinoma | TBBPA and TBBPA‐BDBPE at pM‐uM concentrations promoted migration and invasion of HepG2 cells in a transwell model system. Associated with changes in number and distribution of lysosomes and calcium ino‐mediated lysosomal exocytosis |
| Shockley et al. (2020b). Transcriptomic data from the rat liver after 5 days of exposure to legacy or emerging brominated flame retardants | This is accompanying data providing toxicogenomic data for others to use |
| Guan et al. (2021). The promotion of tetrabromobisphenol A exposure on Ishikawa cells proliferation and pivotal role of ubiquiti‘n‐mediated I kappa B ‘degradation | TBBPA at pM‐nM concentrations induced proliferation of Ishikawa cells, a human endometrial carcinoma cell line, associated with elevation on NF‐κB expression and ubiquitin‐mediated degradation of IκB. This was assumed to be analogous to progression of endometrial carcinoma |
| Xu, Su, et al. (2022). Constructing an MCF‐7 breast cancer cell‐based transient transfection assay for screening RAR? (Ant)agonistic activities of emerging phenolic compounds | TBBPA was found to have antagonistic activity on the retinoic acid receptor α (RARα) in MCF‐7 breast carcinoma cells were transiently transfected with a RARα expression vector (pEF1α‐RARα‐RFP) and a reporter vector containing a retinoic acid reaction element (pRARE‐TA‐Luc). Relevance for TBBPA carcinogenicity is unclear |
| Liu et al. (2022). Short‐term tetrabromobisphenol A exposure promotes fibrosis of human uterine fibroid cells in a 3D culture system through TGF‐beta signalling | TBBPA increased cell proliferation and promoted fibrosis, and upregulated expression of profibrotic genes in a 3D human uterine leiomyoma (ht‐UtLM) spheroid culture model. Relevance to carcinogenicity unclear |
| Cheng and Volz (2022). Halogenated bisphenol a analogues induce PPARγ‐independent toxicity within human hepatocellular carcinoma cells | TBBPA induces toxicity within HepG2 cells in a PPARγ‐independent manner |
Table E3.
Studies on mode of action of reproductive effects.
| Reference | Comments |
|---|---|
| Honkisz and Wojtowicz (2015). Modulation of estradiol synthesis and aromatase activity in human choriocarcinoma JEG‐3 cells exposed to tetrabromobisphenol A | TBBPA in the nanomolar range significantly induced oestradiol secretion by human choriocarcinoma‐derived placental JEG‐3 cells compared to that of controls after 24, 48 or 72 h of exposure. In addition, TBBPA induced changes in aromatase protein expression associated with upregulation of aromatase enzymatic activity and cAMP levels. According to the authors, these data may indicate that TBBPA alters placental oestradiol production in ways that may reflect its ability to interfere with normal placental development during early pregnancy |
| Ohta et al. (2012). Ovariectomized mouse uterotrophic assay of 36 chemicals | TBBPA (up to 1000 mg/kg bw per day) exposure for 7 days by gavage was negative for agonistic and antagonistic response in an uterotrophic assay using C57BL/6J ovariectomised adult female mice |
| Li et al. (2010). In vitro profiling of endocrine disrupting effects of phenols | The ability of TBBPA to activate oestrogen receptor (ER), androgen receptor (AR), progesterone receptor (PR) and oestrogen‐related receptor (ERR) were determined using a set of recombined yeast strains transformed with the ERα gene, ERRc gene, AR gene or pR gene. TBBPA showed ERα and PR antagonistic activities but was not an AR agonist. It exhibited also the ability to reverse the ERR inhibition induced by 4‐hydroxytamoxifen. The authors concluded that TBBPA exhibited oestrogenic activity |
| Dorosh et al. (2011). Assessing oestrogenic effects of brominated flame retardants Hexabromocyclododecane and Tetrabromobisphenol A on MCF‐7 Cells | TBBPA has not shown any oestrogenic effect mediated by the oestrogen receptor α in vitro in MCF‐7 human oestradiol‐dependent breast cancer cells. It did not induce the endogenous oestrogen‐dependent TFF1 (trefoil factor 1) gene expression |
| Riu et al. (2011). Characterization of novel ligands of ER alpha, Er beta, and PPAR gamma: the case of halogenated bisphenol A and their conjugated metabolites |
TBBPA and its sulfonated metabolite did not bind to, or activate ERα ‘As observed in stably transfected cell line assays with which we showed that TBBPA was not able to activate ERα (Riu et al., 2011), TBBPA did not bind to the immobilised ERα‐LBD, with most of the loaded radioactivity recovered in washing fractions.’ TBBPA was completely metabolised within 24 h into glucuronic acid (13.5 ± 2.9%) and sulfate (86.5 ± 2.9%) conjugates of TBBPC. Like TBBPA, its sulfonated conjugate did not bind to ERα, but it did bind to PPARα immobilised to beads in an affinity chromatography column |
| Lee et al. (2012). Evaluation of in vitro screening system for estrogenicity: comparison of stably transfected human estrogen receptor‐alpha transcriptional activation (OECD TG455) assay and estrogen receptor (ER) binding assay | TBBPA does not bind to or transactivate ERα in ERα binding and stably transfected transcriptional activation (STTA) and assays |
| Dankers et al. (2013). Endocrine Disruptors Differentially Target ATP‐Binding Cassette Transporters in the Blood‐Testis Barrier and Affect Leydig Cell Testosterone Secretion In Vitro | To investigate the effects of TBBPA on activity of the ABC transporters, an uptake assay was performed using membrane vesicles overexpressing the specific transporters incubated with known substrates at concentrations ranging from 1 to 100 μM. The effect of TBBPA on breast cancer resistance protein (BCRP), multidrug resistance proteins 1 and 4 (MRP1 and MRP4) and P‐glycoprotein (P‐gp) was investigated using membrane vesicles overexpressing these transporters. TBBPA inhibited all transporters tested. Using transporter‐overexpressing Madin‐Darby canine kidney cells it was shown that TBBPA was not transported by BCRP or by P‐gp. To investigate the toxicological implications of these findings, testosterone secretion and expression of steroidogenic genes were determined in murine Leydig (MA‐10) cells upon exposure to TBBPA. TBBPA concentration dependently increased testosterone secretion by MA‐10 cells. Inhibition of the Mrps by MK‐571 completely blocked testosterone secretion elicited by TBBPA, which could not be explained by coinciding changes in expression of steroidogenic genes. Therefore, the authors hypothesise that transporter‐mediated efflux of testosterone precursors out of MA‐10 cells is inhibited by TBBPA resulting in higher availability for testosterone production |
| Gosavi et al. (2013). Mimicking of Estradiol Binding by Flame Retardants and Their Metabolites: A Crystallographic Analysis | The authors showed that TBBPA can bind to and inhibit oestrogen sulfotransferase. TBBPA bound to the steroid‐metabolising enzyme SULT1E1; it can mimic estradiol binding to the active site of the enzyme. TBBPA had a weak androgenic activity in vitro with recombinant yeast systems carrying human androgen receptor (hAR) |
| Huang et al. (2013). Hormonal effects of tetrabromobisphenol A using a combination of in vitro and in vivo assays | In an in vivo bioassay on mosquitofish (Gambusia affinis), the gene expression patterns of vitellogenin (Vtg), oestrogen receptors (ERα and ERβ) and androgen receptors (ARa and ARβ) were evaluated in adult males and juveniles after exposure to TBBPA for 60 days. Significant up‐regulation of Vtg, ERa and Erβ mRNAs was observed in the liver after exposure to 500 nM of TBBPA. In the testis, the lowest concentration of TBBPA (50 nM) markedly induced Vtg, ERβ and ARβ mRNA expression, but the same concentration significantly inhibited ARa mRNA expression. In addition, in juveniles, 100 nM of TBBPA significantly up‐regulated the expression of Vtg, ERβ and ARa mRNAs. However, TBBPA did not cause histological alterations in the liver and testis of adult male mosquitofish |
| Zatecka et al. (2013). Effect of tetrabrombisphenol A on induction of apoptosis in the testes and changes in expression of selected testicular genes in CD1 mice | TBBPA was able to increase apoptosis of testicular cells and changes in the morphometry of seminiferous tubule in the first and second generations of CD‐1 mice exposed via drinking water to 35 μg/kg bw per day (Zatecka et al., 2013, see Section 3.1.2.4). This study suggests that permanent exposure to TBBPA slightly enhances its effect in the next generation depending on whether the parents were affected or not. According to the authors, this hypothesis is supported by the finding that changes in gene expression were observed even in F2 generation mice, where only the father was exposed to TBBPA. Some effects of TBBPA can be transmitted to the next generation not via changes in the DNA sequence but via epigenetic changes: via changes in gene expression of selected genes encoding proteins that play an important role during spermatogenesis (genes of acrosomal proteins, androgen‐responsive genes, heat shock protein (Hsp) genes, genes encoding proteins responsible for the regulation of apoptosis and a gene expressed in Sertoli cells: Sox 9 |
| Zatecka et al. (2014). The effect of tetrabromobisphenol A on protamine content and DNA integrity in mouse spermatozoa | C57Bl/6J mouse pups were exposed to 35 μg/kg bw TBBPA during the gestation, lactation, pre‐pubertal and pubertal periods up to the age of 70 days. TBBPA treatment did not result in statistically significant changes in any of the general reproductive system parameters measured, but TBBPA‐treated animals had lower testosterone levels. According to the authors, in addition to the resulting increased sperm DNA damage (significantly higher number of TUNEL positive cells in spermatozoa from TBBPA‐treated animals as compared to the controls), TBBPA exposure also has the potential to alter the epigenetic marking of sperm chromatin through generation of an anomalous content and distribution of protamines |
| Roelofs et al. (2015). Structural bisphenol analogues differentially target steroidogenesis in murine MA‐10 Leydig cells as well as the glucocorticoid receptor | Effects of TBBPA on steroidogenesis were investigated in vitro. TBBPA showed clear GR (glucocorticoid receptor) and AR (androgen receptor) antagonism in specific yeast receptor bioassays. In addition, it was shown that testosterone secretion was increased by TBBPA in murine MA‐10 Leydig cells. Steroid levels of MA‐10 cells were evaluated showing that TBBPA exposure caused an induction of P4, 17‐OH‐P4, deoxycorticosterone (DOC), androstenedione, a‐T as well as β‐T levels compared to DMSO |
| Krivoshiev et al. (2016). Assessing in‐vitro estrogenic effects of currently‐used flame retardants | The human breast adenocarcinoma cell line (MCF‐7) flow‐cytometric proliferation assay was used in order to establish potential oestrogen‐ disrupting effects of 12 currently‐used flame retardants. MCF‐7 cell line is oestrogen receptor α positive, and therefore responsive to oestrogens, TBBPA showed statistically significant estrogenic activity in this assay. TBBPA decreased cell proliferation when co‐treated with E2, thereby indicating potential antiestrogenic effects. Studies on the estrogenic activity of TBBPA in vitro are sometimes inconsistent. The estrogenic activity of TBBPA may only be observed at high concentrations and that these responses are largely mediated by receptor independent mechanisms. TBBPA exhibited estrogenic activity by increasing uterus weight in overiectomised mice (Kitamura et al., 2005), while also showing to increase aromatase activity in Wistar rats (van der Ven et al., 2008) |
| Liang et al. (2017). High‐Content Analysis Provides Mechanistic Insights into the Testicular Toxicity of Bisphenol A and Selected Analogues in Mouse Spermatogonial Cells | Using the mouse C18‐4 spermatogonial cell line (spermatogonia immortalised by SV40) as a model to characterise the testicular toxicity of TBBPA it was shown that TBBPA (25 μM) exposure (24–72 h) decreased the number of mouse spermatogonia in vitro. TBBPA induce dose‐ and time‐dependent alterations in nuclear morphology, cell cycle, DNA damage responses (detected as y‐H2AX positive expression) and perturbation of the cytoskeleton |
| Cao et al. (2017). Experimental and computational insights on the recognition mechanism between the oestrogen receptor alpha with bisphenol compounds | The oestrogenic potency of TBBPA was investigated by the E‐Screen bioassay in MCF‐7BUS breast adenocarcinoma cells and by luciferase activity in MVLN cells. TBBPA exhibited no oestrogenic activity in the MVLN assay, while it induced a low proliferation of MCF‐7 cells. An H‐bonding interaction with Thr347 was identified as an important structural feature for the recognition of TBBPA at the E2 binding site of Era |
| Liang, Zhou, et al. (2019). Embryoid body‐based RNA‐seq analyses reveal a potential TBBPA multifaceted developmental toxicity | Human embryonic stem cells were induced to differentiate into neural ectoderm in the presence of TBBPA. Induced neural ectoderm cells were positive for several neural markers, including NESTIN, TFAP2A and SOX2, indicating high induction efficiency. It was shown that transcription factors crucial for neural development, such as ZIC1, ZIC3, HES3, IGFBP3 and DLX5, were dysregulated. TBBPA might also influence axon growth/guidance and neuron transmission‐related processes, by dysregulating genes including CNTN2, SLIT1, LRRC4C, RELN, CBLN1, CHRNB4 and GDF7. TBBPA may not only disrupt the development of the nervous system at early stages, but also dysregulate neuron generation at later stages, by interfering with processes related to axon growth/guidance and neuron transmission. Furthermore, TBBPA exposure seemed to interfere with the WNT and AHR signalling pathways |
| Pawlicki et al. (2019). Do G‐protein coupled oestrogen receptor and bisphenol A analogs influence on Leydig cell epigenetic regulation in immature boar testis ex vivo? | The membrane bound G‐protein coupled oestrogen receptor (GPER) is expressed in boar testes and is thought to mediate non‐genomic oestrogen signalling required for male reproductive function. Testicular fragments from 7‐day‐old pigs were treated with a GEPR antagonist (G‐15) and/or TBBPA (10 nM). TBBPA reduced expression of GPER protein in testis fragments to the same extent as an equimolar concentration of G‐15. Both TBBPA and G‐15 (separate treatments) reduced oestrogen secretion from the testis fragments. While G‐15 reduced expression of collagen fibres, exposure to TBBPA increased Col6a1 mRNA and COL6A1 protein abundance. TBBPA exposure also caused increased expression of PLIN1 and PC3 protein and altered abundance of mRNA for Drosha (upregulation) and Dicer (downregulation) indicating possible effects on miRNA processing |
| Yin et al. (2020). High‐Content Image‐Based Single‐Cell Phenotypic Analysis for the Testicular Toxicity Prediction Induced by Bisphenol A and Its Analogs Bisphenol S, Bisphenol AF, and Tetrabromobisphenol A in a Three‐Dimensional Testicular Cell Co‐culture Model | In an in vitro three‐dimensional (3D) mouse testicular cell co‐culture model and by employing a high‐content image (HCA)‐based single‐cell analysis it was shown that TBBPA induce dose‐ and time‐dependent changes in a wide spectrum of adverse endpoints, including nuclear morphology, DNA synthesis, DNA damage and cytoskeletal structure. The co‐cultured testicular cells were more sensitive than the C18 spermatogonial cells. Single‐cell‐based assays not only showed the levels of the averaged population, but also revealed changes in the sub‐population |
| Sheikh and Beg (2020). Structural binding interactions of tetrabromobisphenol A with sex steroid nuclear receptors and sex hormone‐binding globulin | Structural binding characterisation of TBBPA against the nuclear receptor oestrogen receptor alpha (ERa), oestrogen receptor β (ERβ), androgen receptor (AR), progesterone receptor (PR) and the steroid transport protein, sex hormone‐binding globulin (SHBG) revealed tight binding of TBBPA in the binding pocket of these proteins |
| Shi et al. (2023). Halogen atoms determine the inhibitory potency of halogenated bisphenol A derivatives on human and rat placental 11β‐hydroxysteroid dehydrogenase 2 | The effects of TBBPA on human and rat placental 11β‐hydroxysteroid dehydrogenase 2 (11β‐HSD2), a critical enzyme for placental function by inactivating maternal cortisol were investigated. Species‐dependent inhibition was observed; TBBPA was a potent inhibitor of human 11β‐HSD2 and less potent of rat 11β‐HSD2. Evaluation of several BPA derivatives showed that they bind steroid active sites and that inhibition was structure‐dependent; the halogen atoms and hydrophobicity determine the inhibitory potency of halogenated BPA derivatives |
| Yin et al. (2023). High‐content analysis of testicular toxicity of BPA and its selected analogs in mouse spermatogonial, Sertoli cells, and Leydig cells revealed BPAF induced unique multinucleation phenotype associated with the increased DNA synthesis | The selective toxicity of TBBPA (0, 1, 5 and 10 μM for 24 and 48 h) was examined on mouse testicular cells, including Sertoli cells, Leydig cells and spermatogonia using a high‐content image (HCA)‐based single‐cell analysis. TBBPA treatment showed a dose‐dependent decrease of cell number in both spermatogonia cell and Sertoli cells but not in Leydig cells. TBBPA treatment induced changes in nuclear size and caused differential cell cycle arrest among the three different types of testicular cells. Nuclear shape measurements showed that TBBPA treatment significantly decreased nuclear roundness in spermatogonia, nuclear smoothness and nuclear roundness in Leydig cells and nuclear smoothness in Sertoli cells. No changes in nuclear smoothness were observed in spermatogonia. TBBPA reduced the percentage of BrdU positive cells (marker of DNA synthesis) in spermatogonia and also in Sertoli cells. Alterations in the cytoskeleton were also observed; TBBPA treatment caused F‐Actin total intensity induction in the different cells. TBBPA significantly increased the number of γ‐H2AX positive cells in spermatogonia and Sertoli cells. It increased Methyl‐CpG binding domain 1 (MBD1, marker of methylation) total intensity in spermatogonia and Sertoli cells. TBBPA significantly increased the autophagosome membrane protein LC3B (to monitor autophagy activity) level in spermatogonia and Sertoli cells. TBBPA targeted spermatogonia and Sertoli cells and less Leydig cells |
| Brown et al. (2020). Developmental exposure to tetrabromobisphenol A has minimal impact on male rat reproductive health | Pregnant Wistar rat dams (GD6‐PND21) and their progeny (PND22‐PND90) were exposed by gavage to 0, 0.1, 25, or 250 mg TBBPA/kg bw per day. A delay in the time to preputial separation was found in the 250 mg/kg bw per day group. Spermatogenesis was not disrupted. The mRNA expression levels of some steroidogenic enzymes and receptors in the testes were different from that in controls in PND90 and 1‐year‐old time points. At PND90 cholesterol side‐chain cleavage (Cyp11a1) showed a significant dose response effect and a decrease of expression level at the highest dose. Three‐beta‐hydroxysteroid dehydrogenase (Hsd3b1) had a significant downward trend and difference of expression in the 250 mg/kg bw treatment at 1 year, but not at PND90. There was no change of expression at either age in hydroxysteroid (17beta) dehydrogenase 3 (Hsd17b3), 5a‐reductase (Srd5a1), or oestrogen receptors alpha (Esr1) or beta (Esr2) |
| Li et al. (2022). Effects of postnatal exposure to tetrabromobisphenol A on testis development in mice and early key events | Potential molecular mechanisms underlying TBBPA‐caused changes in testis development were investigated in CD‐1 mice exposed from PND0‐56 (via lactation PND0‐21 and direct exposure of pups from PND21‐56) to 0, 15, 150, 1500 ng/mL TBBPA in drinking water. The total RNA of testes was extracted. RT‐PCR was conducted to analyse the expression of marker genes for Sertoli cell (Sox9 and Cst12), Leydig cells (Cyp17a1 and Star), germ cells (Vasa, Stra8, Ssxb1, Sycp3, Plzf, Lhx, Etv5 and Pou5f2), and blood‐testis barrier‐associated genes (Nectin1 and Nectin2) as well as genes involved in TH (Thrb, Klf9, Shh and Dio3) and Notch signalling (Notch1, Dll1, Dll4 and Hes1) pathways. On PND7, no robust or dose‐associated changes in the expression of TH‐response genes were observed, except for Shh upregulation in the 1500 ng/mL TBBPA group. This suggest that TBBPA exerted a limited effect on TH signalling pathway involved in testis development. However, TBBPA‐treated testes displayed higher expression of Notch signalling receptor (Notch1), ligand (Dll1 and Dll4), and target (Hes1), implying Notch signalling activation. The authors concluded that in TBBPA‐treated testes, the reduced Sertoli cell population size and abnormal cytoskeleton could be at least partly responsible for the decrease in the tubular area. As each Sertoli cell supports a specific number of germ cells, TBBPA‐caused alterations in Sertoli cells are responsible for the reductions in the spermatogonia number per tubule, the percentage of stage VII–VIII seminiferous tubules and the expression levels of spermatogonia and spermatocyte marker genes. Moreover, the down‐regulated expression of Nectin 1 and Nectin 2, Sertoli cell marker genes associated with the blood–testis barrier, might imply the structural and functional abnormalities of the blood–testis barrier |
Table E4.
Studies on mode of action of neurotoxicity.
| Reference | Comments |
|---|---|
| Saegusa et al. (2012). Transient aberration of neuronal development in the hippocampal dentate gyrus after developmental exposure to brominated flame retardants in rats | Exposure of rats GD10‐PND20 to TBBPA 100, 1000, 10,000 ppm → aberration in neuronal migration at PND20 in DG of hippocampus + increase in apoptotic bodies in subgranular zone. At PND77, increase in the number of the mature neurons. Effects > 1000 ppm for TBBPA. Not effect on thyroid |
| Hendriks, van Kleef, van den Berg, et al. (2012). Multiple Novel Modes of Action Involved in the In Vitro Neurotoxic Effects of Tetrabromobisphenol‐A |
TBBPA acts as full or partial agonist of human GABAA receptors in Xenopus oocytes + electrophysiology Antagonism of AchR in neuronal B35 cells → reduction in Ach‐induced increase in [Ca2+]i + increase in basal [Ca2+]i in B35 and dopaminergic PC12 cells → TBBPA‐induced inhibition of voltage‐gated Ca2+ channels → Imbalance resulting from the influx of Ca2+ into neurons and its release from intracellular stores |
| Hendriks, van Kleef and Westerink (2012). Modulation of human alpha(4)beta(2) nicotinic acetylcholine receptors by brominated and halogen‐free flame retardants as a measure for in vitro neurotoxicity |
Effects of TBBPA on human Ach receptors expressed in Xenopus oocytes through electrophysiological approaches → TBBPA acts as an AcHR antagonist (3–100 μM) |
| Zieminska et al. (2012a). Acute Cytotoxicity Evoked by Tetrabromobisphenol A in Primary Cultures of Rat Cerebellar Granule Cells Outweighs the Effects of Polychlorinated Biphenyls |
Acute toxicity of TBBPA (1–50 μM) on rat cerebellar granule cells Reduced viability, alteration of Ca2+ signalling (increases in 45Ca uptake and intracellular Ca2+ concentration), increase in ROS production |
| Zieminska et al. (2012b). Synergistic neurotoxicity of oxygen–glucose deprivation and tetrabromobisphenol A in vitro: role of oxidative stress |
Effects of TBBPA (2.5–7.5 μM) on oxidative metabolism of rat cerebellar granule cells (normoxic vs. oxygen–glucose deprivation) → Involvement of oxidative stress in the neurotoxicity of TBBPA and synergistic effects of TBBPA + OGD |
| Al‐Mousa and Michelangeli (2012). Some Commonly Used Brominated Flame Retardants Cause Ca2+‐ATPase Inhibition, Beta‐Amyloid Peptide Release and Apoptosis in SH‐SY5Y Neuronal Cells |
Effects of TBBPA (1–30 μM) on cytotoxicity of SH‐SY5Y neuronal cells → TBBPA cytotoxic at low concentration (LC50 = 15 ± 4 μM) → through apoptosis and induction of caspase activity + increases in [Ca2+]i (through microsomal Ca2+‐ATPse inhibition) and ROS → depolarisation of the mitochondria + release of cytochrome c → significant increase in Ab‐42 secretion |
| Fujimoto et al. (2013). Increased Cellular Distribution of Vimentin and Ret in the Cingulum of Rat Offspring After Developmental Exposure to Decabromodiphenyl Ether or 1,2,5,6,9,10‐Hexabromocyclododecane |
Effects of TBBPA on white matter development in juvenile rats early exposed (10, 100, 1000 ppm in soy‐free diet, GD10‐PND20, 8 pups/litter) 1M and 1f/litter used at PND20 for histology = IHC of vimentin+ and Ret+ cells → no effect of TBBPA on V+ and Ret+ cells in cerebral white matter (overexpression indicative of developmental hypothyroidism) |
| Meijer et al. (2014). Comparison of plate reader‐based methods with fluorescence microscopy for measurements of intracellular calcium levels for the assessment of in vitro neurotoxicity |
Effects of TBBPA (10–100 μM) on [Ca2+]i metabolism in PC12 cells by two methods: single cell fluorescent microscopy (Fura‐2) and plate‐based reader method From the microscopy methodology: → Reduction of the depolarisation‐evoked increase in [Ca2+]i (TBBPA 10 μM) → increase in basal [Ca2+]i (TBBPA 10 or 100 μM) |
| Wojtowicz et al. (2014). PPAR‐gamma Agonist GW1929 But Not Antagonist GW9662 Reduces TBBPA‐Induced Neurotoxicity in Primary Neocortical Cells |
Effects of TBBPA (1–100 nM; 1–100 μM) on apoptosis and cytotoxicity of mouse primary cortical neuronal cell cultures → Increases in LDH (10–100 μM) and caspase‐3 (100 nM–100 μM) activities → TBBPA 10 μM decreases the expression of PPAR‐gamma protein (induced caspase activity and apoptosis) |
| Zieminska et al. (2014). Bastadin 12 and ryanodine reveal similarities between thapsigargin‐ and tetrabromobisphenol A‐induced intracellular Ca2+ release in cultured cerebellar granule cells |
Effects of TBBPA (5–25 μM) on Ca2+ signalling in rat primary cerebellar granule cell cultures → TBBPA dose‐related increases in [Ca2+]i concentration (same release with pharmacological agents bastadin 12 and ryanodine) → co‐administration of bastadin + ryanodine prior TBBPA inhibits the TBBPA‐induced release of [Ca2+]i |
| Hendriks et al. (2014). A comparison of the in vitro cyto‐ and neurotoxicity of brominated and halogen‐free flame retardants: prioritization in search for safe(r) alternatives |
Effects of TBBPA (1–100 μM) on [Ca2+]i metabolism in rat PC12 or B35 cells → reduction of cell viability with TBBPA 100 μM in both culture cells → increases in ROS production with 10 μM TBBPA → no modification of basal [Ca2+]i → reduction of the depolarisation‐increase of [Ca2+]i induced by K+ through inhibition of voltage‐gated Ca2+ channels |
| Hendriks et al. (2015). Effects of neonatal exposure to the flame retardant tetrabromobisphenol‐A, aluminum diethylphosphinate or zinc stannate on long‐term potentiation and synaptic protein levels in mice |
Effects of TBBPA (single oral administration at PND10‐aged mice, 211 μM/kg bw = 48.9 mg/kg bw, gavage) on hippocampal synaptic plasticity at PND17‐19 → Hippocampal slices & electrophysiological signals long‐term potentiation between cells of the Schaffer collateral‐commissural pathway → TBBPA has no effects on basal excitability of neurons but induced a minor decrease in post‐tetanic LTP (not significant) → no significant modifications of expression of synaptic proteins involved in LTP (CamKII, GAP‐43, GluR1, PSD95, synaptophysin) → no significant increase in the brain and muscle tissue level of TBBPA compared to controls |
| Zieminska, Salinska, et al. (2015). Tetrabromobisphenol‐A‐evoked oxidative stress and cytotoxicity in cultured neurons: Involvement of zinc |
Effects of TBBPA (5–25 μM) on cytotoxicity and oxidative stress in primary cultures of cerebellar granule cells → increases in cellular DCFH and rhodamine fluorescence # enhanced production of ROS and mitochondrial depolarisation → contribution of the enhancement of intracellular Zinc concentration in mediating the TBBPA‐induced oxidative stress and cytotoxicity |
| Zieminska, Stafiej, et al. (2015). Role of Ryanodine and NMDA Receptors in Tetrabromobisphenol A‐Induced Calcium Imbalance and Cytotoxicity in Primary Cultures of Rat Cerebellar Granule Cells |
Effects of TBBPA (7.5–25 μM) on Ca2+ signalling in rat primary cerebellar granule cell cultures and role of ryanodine and NMDA receptors in mediating the cytotoxicity induced by TBBPA → increasing uptake of Ca2+ with the dose of TBBPA (significant at the 3 doses) → abolition of this increase by the glutamate antagonist MK‐801 but not by the RyR antagonist bastadin 12 → complete abolition when exposed to a combined application of both antagonists → TBBPA ≥ 10 μM reduced neuronal viability, abolished by the use of NMDA and/or RyR antagonists alone or in combination |
| Diamandakis et al. (2015). Tetrabromobisphenol‐A modulates activity of NMDA receptors in isolated fractions of rat brain cortical membranes and depolarizes cultured neurons |
Effects of TBBPA (0.156–80 μM) on NMDA‐glutamate binding using membranes isolated from rat brain cortex → Bell‐like concentration effect on the binding of [3H]‐MK‐801, a glutamate NMDAR antagonist, with a maximum value at 20 μM of TBBPA → TBBPA ≥ 10 μM decreased [3H]glutamate binding → low modulation of [3H]‐glycine binding by TBBPA TBBPA interacts in a complex way with glutamate‐NMDAR signalling pathway, possibly related to the TBBPA‐evoked membrane depolarisation of neurons |
| Behl et al. (2015). Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity |
Effects of TBBPA (range of μM concentrations) on various in vitro and in vivo endpoints
→ TBBPA (≥ 10–30 μM) was active in multiple assays suggesting potent developmental toxicity and neurotoxicity of this BFR |
| Zieminska et al. (2016). Select putative neurodevelopmental toxins modify SNAP‐25 expression in primary cultures of rat cerebellar granule cells |
Effects of TBBPA (1–100 μM) on SNPA‐25 expression (mRNA and protein) in rat primary cerebellar granule cell cultures → TBBPA 25 μM increased SNAP‐25 mRNA and protein expression by 49% and 79%, respectively TBBPA altered in vitro the SNPA‐25 protein expression known to be strongly implicated in neurotransmission and neurotransmitter secretion |
| Liu, Ren, et al. (2016). The potential neurotoxicity of emerging tetrabromobisphenol A derivatives based on rat pheochromocytoma cells |
Effects of TBBPA and 4 derivatives on cell viability and apoptosis, dopamine secretion and acetylcholine esterase activity and oxidative stress in PC12 cells → Significant reduction of cell viability (≥ 10 μM) → Just cell viability results presented for TBBPA (referred to other references for other results related to TBBPA) |
| Pei et al. (2016). Comparative neurotoxicity screening in human iPSC‐derived neural stem cells, neurons and astrocytes |
Effects of TBBPA (10 or 100 μM) on cytotoxicity assessed in various cell culture models (iPSC, neural SC, neurons, astrocytes). Cytotoxicity assessed using a MTT assay and a luciferase‐based assay → TBBPA 100 μM highly cytotoxic for both cell models (MTT assay) → TBBPA 100 μM cytotoxic for neurons and astrocytes (luciferase‐based assay) |
| Szychowski and Wojtowicz (2016). TBBPA causes neurotoxic and the apoptotic responses in cultured mouse hippocampal neurons in vitro |
Effects of TBBPA (1 nM–100 μM) on cell viability and apoptosis in mouse primary hippocampal neuronal cell cultures → increase in apoptotic cells with TBBPA ≥ 1 μM + caspase‐3 activity ≥ 100 nM → increase in LDH activity with TBBPA ≥ 50 nM → increase in the ROS generation with TBBPA ≥ 1–10 μM (transient period of incubation, 30 min, 3 or 24 h) according to the concentration of the fluorescent probe |
| Park et al. (2016). Tetrabromobisphenol‐A induces apoptotic death of auditory cells and hearing loss in zebrafish | The present study investigated the effects of TBBPA on hearing function in vivo and in vitro. Auditory Brainstem Response (ABR) threshold was markedly increased in mice after oral administration of TBBPA (250 mg/kg bw per day, 30 days), indicating that TBBPA causes hearing loss. TBBPA induced ex vivo the loss of both zebrafish neuromasts and hair cells in the rat cochlea in a dose‐dependent manner with a concomitant increase in the percentage of apoptotic cells. Exposure of HEI‐OC1 cells to TBBPA increased the expression of the pro‐apoptotic Bax protein and the activity of caspase‐3 while the expression of the anti‐apoptotic protein Bcl‐2 was significantly reduced. TBBPA also increased the accumulation of ROS and the expression and secretion of the pro‐inflammatory cytokine IL‐6. Pretreatment of HEI‐OC1 cells with the antioxidant compound N‐acetylcysteine abolished the ROS accumulation and increased the cell viability. Taken together, these data strongly suggest that TBBPA causes the accumulation of ROS and inflammatory cytokines, which trigger hair cell death and eventually lead to hearing loss. |
| Lenart et al. (2017). Altered expression of genes involved in programmed cell death in primary cultured rat cerebellar granule cells acutely challenged with tetrabromobisphenol A |
Effects of TBBPA (10 and 25 μM) on gene expression related to cell death and apoptosis in primary rat cerebellar granule cell cultures → significant reduction of cell viability after 30 min of exposure to TBBPA 25 μM → changes in the expression of a limited number of genes over the 84 studied: no induction of programmed necrosis, early suppression of the autophagy and anti‐apoptotic genes followed by a delayed activation of pro‐apoptotic genes |
| Zieminska, Lenart, et al. (2017). The role of Ca2+ Imbalance in the Induction of Acute Oxidative Stress and Cytotoxicity in Cultured Rat Cerebellar Granule Cells Challenged with Tetrabromobisphenol A |
Effects of TBBPA (10 and 25 μM) on Ca2+ transients in primary rat cerebellar granule cell cultures, and on oxidative stress and cytotoxicity → TBBPA increased [Ca2+]i and ROS production using the measurement of fluorescence of fluo‐3 (Ca2+) and DCFH (ROS). Modulation of the TBBPA‐related radical production by free radical scavengers → TBBPA reduced the membrane potential of mitochondria using the fluorescent probe rhodamine. Such increases were maintained also when free radical scavengers were added → Simultaneous reduction of GSH and catalase activity with the concentration of TBBPA → dose‐related reduction of cell viability with the concentration of TBBPA. Prevented by the addition of free radical scavengers and ryanodine and NMDA receptors At 10 μM TBBPA Ca2+ imbalance is a primary event, inducing oxidative stress, depolarisation of mitochondria and cytotoxicity At a concentration of 25 μM TBBPA induced an additional Ca2+−independent portion of oxidative stress and level of cytotoxicity. |
| Zieminska, Ruszczynska, et al. (2017). Tetrabromobisphenol A disturbs zinc homeostasis in cultured cerebellar granule cells: A dual role in neurotoxicity |
Effects of TBBPA (10 and 25 μM) on Zn2+ transients in primary rat cerebellar granule cell cultures through the application of TPEN, a Zn2+ chelator → potentiation of the Ca2+ uptake induced by TBBPA or glutamate → enhancement of the ROS production induced by TBBPA + reduction in the membrane potential (only with TBBPA 25 μM) A role for intracellular Zn2+ homeostasis in cytoprotection Complex interaction between the Zn2+ homeostasis and intracellular Ca2+ signalling involved in the TBBPA‐related cytotoxicity |
| Arini et al. (2017). A cell‐free testing platform to screen chemicals of potential neurotoxic concern across 20 vertebrate species |
Testing the neurotoxicity of 80 chemicals including TBBPA in a set of cell‐free neurochemical assays (4 receptor assays: dopamine D2, GABAR, glutamate NMDAR, muscarinic AchR and 3 enzyme activity assays: AchE, glutamine synthase, MAO) all working across 20 vertebrate species. TBBPA exhibited moderate overall activity in this system, placing it in the last third of the 80 substances tested. The interaction of TBBPA with the glutamate NMDA receptor appeared as the most affected endpoint. |
| Bagley et al. (2018). IR‐MALDESI mass spectrometry imaging of underivatized neurotransmitters in brain tissue of rats exposed to tetrabromobisphenol A |
Effects of perinatal exposure to TBBPA on brain neurotransmitter contents (dopamine, GABA, serotonin) measured in 3 brain regions of interest (caudate putamen, substantia nigra, dorsal raphe) in rat pups → Dams dosed with TBBPA 1 or 10 mg/kg bw twice a day from GD6 to PND21 (gavage, sesame oil as vehicle) → Brain neurotransmitter contents measured in pups aged PND115 using IR‐MALDESI mass spectrometry imaging Results suggest disturbances in dopamine, and GABA brain contents, but not serotonin, with variations related to the sex, brain regions and TBBPA exposure |
| Yin et al. (2018). TBBPA and Its Alternatives Disturb the Early Stages of Neural Development by Interfering with the NOTCH and WNT Pathways |
Developmental neurotoxicity of TBBPA assessed using a mouse neural stem cell‐based system → TBBPA caused cytotoxicity in proliferating mESCs only at concentrations much higher than 1 μM (IC50 = 102 μM) → TBBPA induced an increase of intracellular Ca2+ concentration and stimulated an oxidative stress at levels of concentrations much higher than 1 μM → TBBPA stimulated in an abnormal way neurogenesis during embryonic development using two different approaches (embryoid body‐based NPC induction and ESC monolayer neural differentiation) and measuring the expression of neural progenitor markers (Pax6, Sox1 and 3) and neurogenesis genes (Map2, NeuroD, Dcx) → TBBPA upregulated the expression of NOTCH effectors (Hes1 and 5) but not the WNT target genes TBBPA impaired the early embryonic neural development, mostly through the dysregulation of the NOTCH signalling pathway |
| Cannon et al. (2019). Tetrabromobisphenol A (TBBPA) Alters ABC Transport at the Blood–Brain Barrier | Effects of TBBPA on the expression and activity of 3 blood–brain barrier transporters in Sprague–Dawley rats (15–20 weeks) orally administered once with 250 mg/kg bw of TBPPA. Three ATP binding cassette efflux transporters were considered for this study, namely the P‐glycoprotein (P‐gp), the brain cancer resistant protein (BRCP) and the multidrug resistance‐associated protein 2 (MRP2) transport proteins. In vivo measurement of the luminal accumulation of specific fluorescent substrates of each transporter showed significant changes of P‐gp permeability according to the gender (2‐fold increase in males and 45% decrease in females) and significant reductions of BCRP transport in both sexes. No significant variations were observed regarding the MRP2 permeability. Ex vivo measurement of the luminal accumulation of substrates in freshly isolated brain capillaries exposed to 100 nM of TBBPA showed the same significant changes confirming the results from the in vivo experiments. A dose–response relationship study (TBBPA doses ranging 1–1000 nM) on the P‐gp and BRCP permeability was performed using the ex vivo methodology. The results showed significant dose‐related variations of the BBB permeability according to the transporter (P‐gp and BRCP) and the gender considered that allow to consider with a LOAEL of 1 nM from all the dose‐effect relationship studied |
| Liang, Liang, Zhou, et al. (2019). Typical halogenated flame retardants affect human neural stem cell gene expression during proliferation and differentiation via glycogen synthase kinase 3 beta and T3 signalling |
Developmental neurotoxicity of TBBPA assessed using a human neural stem cell‐based system → TBBPA did not alter cell viability and proliferation at concentration levels as high as 1 μM. → TBBPA rather altered the neural differentiation (reduced the expression of hNSC identity markers as SOX2, NES and TUBB3) → TBBPA disturbed the NOTCH signalling pathway rather than GSK3β signalling → Effects of TBBPA on early stages of neural development possibly modulated by T3 cell signalling (impairment of SOX3 expression when cells were cultured with a free‐T3 medium) TBBPA affect the differentiation of hNSCs, possibly by perturbating T3‐cell signalling |
| Liang, Liang, Yin, et al. (2019). Toxicogenomic analyses of the effects of BDE‐47/209, TBBPA/S and TCBPA on early neural development with a human embryonic stem cell in vitro differentiation system |
Toxicogenomic effects of TBBPA (10 or 100 nM, 1 or 5 μM) on differentiation of human embryonic stem cells into neural ectoderm. → TBBPA did not impair the cell viability during the differentiation → TBBPA impaired the expression of 26 genes among the 47 dysregulated by the 5 BFRs studied TBBPA mostly interfered with embryonic brain development at early stages whereas TBBPA‐like chemicals also dysregulate processes related to axon growth/guidance, neuron transmission and Wnt and AhR signalling pathways |
| Zhang, Ireland, et al. (2019). Screening for neurotoxic potential of 15 flame retardants using freshwater planarians |
Testing the general toxicity and developmental neurotoxicity of TBPPA (0.01 to 100 μM) in freshwater planarians → TBBPA caused adverse effects relative to morphology and behaviour in both adult and regenerating planarians → TBBPA induced sublethal effects in regenerating animals and toxicity concomitant with lethality in adult planarians → TBBPA impaired unstimulated behaviour in regenerating animals, not in adults, suggesting a developmental selective defect Results suggest the potent developmental neurotoxicity of TBBPA considering both morphological and behavioural endpoints in adult and regenerating planarians |
| Diamandakis et al. (2019). Tetrabromobisphenol A‐induced depolarization of rat cerebellar granule cells: ex vivo and in vitro studies |
Effects of TBBPA on plasma membrane potential and depolarization in primary rat cerebellar granule cells using whole‐cell current clam recording and the measurement of the fluorescent probe oxonol VI as an indicator of membrane potential. The contribution of NMDA and AMPA receptors, voltage‐gated sodium channels and intracellular calcium mobilisation was tested using their selective antagonists or inhibitors. Direct interactions of TBBPA with NMDARs were tested by measuring the specific binding of radiolabelled NMDAR ligands to isolated rat cortical membrane fraction. → TBBPA depolarised CGC at concentration # 25 μM in cerebellar slices (whole‐cell current clamp electrophysiology) and ≥ 7.5 μM in primary cell cultures (fluorescent probe oxonol VI) → depolarisation partly or completely reduced with TTX, ligands of ryanodine receptors (ryanodine and/or bastadin 12) and of glutamate ionotropic receptors (CNQX and/or MK801) → no effect of TBBPA on the specific binding of NMDA receptors ligands (NMDA, Glycine, ketamine) Cell depolarisation induced by TBBPA mediated by ionotropic glutamate receptors and VOC‐Na + and modulation of endogenous glutamate rather than direct action on the binding of glutamate to its own receptors |
| Gu et al. (2019). New insights into mechanism of bisphenol analogue neurotoxicity: implications of inhibition of O‐GlcNAcase activity in PC12 cells |
Studying the effects of TBBPA (25, 50 or 100 μM) on the O‐GlcNAcase expression and activity, a glycosylation enzyme of importance in the brain, in PC12 cell cultures → All the 7 bisphenol analogues tested significantly inhibited OGA activity and up‐regulated protein O‐GlcNAcylation level with TBBPA being the strongest → TBBPA altered cell proliferation and cell cycle, and induced apoptosis in exposed cells → TBBPA increased the intracellular Ca2+ concentration and the ROS production over time in a dose‐dependent way TBBPA impairs the neural proliferation and differentiation of neural‐related cells in vitro (PC12 cells) with the impact of the OGA enzyme as a new molecular target for bisphenol analogues. |
| Cho et al. (2020). Tetrabromobisphenol A‐Induced Apoptosis in Neural Stem Cells Through Oxidative Stress and Mitochondrial Dysfunction |
Study assessing the potential involvement of oxidative stress and mitochondrial dysfunction in (12.5–200 μM) TBBPA‐mediated neurotoxicity in neural stem cells → TBBPA was more cytotoxic to neural stem cells than to neurons, astrocytes, or fibroblasts → TBBPA induced neural stem cell apoptosis was accompanied by increased reactive oxygen species generation and mitochondrial dysfunction → TBBPA‐induced apoptosis was mediated by c‐Jun N‐terminal kinase‐p53 pathway activation Adverse effects of TBBPA on hippocampal neurogenesis are due to the inhibition of neural stem cell expansion |
| Renner et al. (2021). Cell‐Type‐Specific High Throughput Toxicity Testing in Human Midbrain Organoids |
The neurotoxicity of TBBPA (9 levels of dose ranging 0.01 to 1000 μM) has been studied using human automated midbrain organoids derived from smNPCs (small molecule neural precursor cell cultures). This in vitro AMO system is established to be efficient to identify the nigrostriatal dopaminergic toxicity of chemicals TBBPA was then identified as a selective toxicant for dopaminergic neurons in nigrostriatal AMOs generated from two independent cell lines |
| Klose et al. (2021). TBBPA Targets Converging Key Events of Human Oligodendrocyte Development Resulting in Two Novel AOPs |
Effects of TBBPA on oligodendrocyte development using neural progenitor cell cultures (human and rat) → TBBPA (0.01, 0.1 or 1 μM) significantly reduced in a dose‐dependent way the thyroid‐dependent oligodendrocyte maturation in both human and rats NPCs → TBBPA dysregulated T3‐regulated transcripts in human NPCs, mostly related to glia and oligodendrocyte development → TBBPA deregulated a gene cluster involved in cholesterol metabolism independently of thyroid‐signalling, some of the genes being involved in differentiation or myelination of oligodendrocytes TBBPA interferes with oligodendrocyte development and maturation through two MoA: (1) impairment of oligodendrocyte maturation by dysregulation of thyroid‐dependent oligodendrogenesis‐associated genes, and (2) disruption of cholesterol homeostasis with reduction of the number of oligodendrocytes independently of thyroid‐signalling |
| Klose et al. (2022). Neurodevelopmental toxicity assessment of flame retardants using a human DNT in vitro testing battery |
Assessment of neurotoxicity of 15 organophosphorous FRs including TBBPA using a human cell‐based developmental neurotoxicity battery covering a large variety of neurodevelopmental endpoints → Most endpoints were affected at concentrations also inducing cytotoxicity → Endpoints affected by TBBPA: oligodendrocyte differentiation, neural crest cell migration, neural progenitor cell proliferation, neurite morphology, cell toxicity and viability |
| Wang and Dai (2022). Comparative Effects of Brominated Flame Retardants BDE‐209, TBBPA, and HBCD on Neurotoxicity in Mice |
The study aimed to assess the neurotoxicity of TBBPA in mice orally exposed at 50 or 100 mg/kg bw per day for 28 days. Animals were assessed for their cognitive performances (Morris water maze and object recognition test). Thus, oxidative stress, apoptosis‐related proteins and learning and memory‐related proteins were measured in hippocampus → TBBPA increased the ROS level of production only at the highest dose used for exposure while the GSH level of expression was significantly reduced at both doses → TBBPA disrupted the Bax/Bcl‐2 balance at a dose of 100 mg/kg/day and favoured the expression of the pro‐apoptotic factor Bax with a concomitant reduction in the expression of the anti‐apoptotic Bcl‐2 protein → TBBPA significantly reduced BDNF and PSD‐95 expression levels, suggesting the impact of such chemical on synaptic plasticity. Concomitant increase in AchE activity has been reported in hippocampus of the same animals, that was significantly correlated with the reduction of ChAT in the same part of the brain Such results indicated the ability of TBBPA to induce some neurotic effects in a highly sensitive part of the brain, the hippocampus, but with moderate consequences on behavioural learning and memory abilities. Results of this study suggest that TBBPA may be considered as a less potent neurotoxicant compared to two other BFRs tested, BDE‐209 and HBCDD |
| Abe et al. (2023). Tetrabromobisphenol A and hexabromocyclododecane, brominated flame retardants, trigger endoplasmic reticulum stress and activate necroptosis signalling in PC12 cells |
Transcriptomic study of the effects of TBBPA (5, 10 or 12,5 μM) on differential gene expression in PC12 cells → TBBPA impair the cell viability at doses of 10 or 12.5 μM → TBBPA impaired the expression of 636 genes with 242 genes shared with the ones dysregulated by HBCDD → most of the genes disrupted by TBBPA are related to the endoplasmic reticulum stress response → implication of necroptosis in the cell lethality induced by TBBPA TBBPA activate specific cell signalling pathways, most related to endoplasmic reticulum stress response and necroptosis, in addition to the activation to the apoptosis‐related signalling |
| Choi et al. (2023). Apigenin attenuates tetrabromobisphenol A‐induced cytotoxicity in neuronal SK‐N‐MC cells |
The study investigated the cytoprotective effects of apigenin, a promising dietary flavonoid to treat or prevent inflammatory‐ and cytotoxic‐related disorders, in human neuroblastoma SK‐N‐MC cell line exposed to TBBPA → TBBPA (5 to 100 μM) reduced the cell viability at concentrations higher than 20 μM that is prevented by apigenin 1 μM → TBBPA 20 μM induced apoptosis, necrosis and autophagy that is prevented by apigenin 1 μM → TBBPA 20 μM induced pro‐inflammatory signalling, ROS production and increased the intracellular Ca2+ concentration, all of them being abolished by apigenin 0.01, 0.1 or 1.0 μM → TBBPA‐induced mitochondrial dysfunction was attenuated by apigenin 0.01–1.0 μM → TBBPA‐dysregulation of ERK and Akt signalling pathways was restored by apigenin 0.01–1.0 μM TBBPA caused reduction in cell viability, increased intracellular Ca2+ concentrations, induced oxidative damages and triggered mitochondrial dysfunction in various cells, all prevented by apigenin |
Table E5.
Studies on mode of action for thyroid toxicity.
| Reference | Comments |
|---|---|
| Terasaki et al. (2011). Assessment of thyroid hormone activity of halogenated bisphenol A using a yeast two‐hybrid assay | The thyroid hormone agonist/antagonist activities of TBBPA were evaluated using a yeast two‐hybrid assay incorporating the human thyroid hormone a (TRα), both with and without possible metabolic activation by rat liver S9. In the absence of S9 TBBPA exhibited agonist activity. The activities of TBBPA increased markedly after metabolic activation S9. TBBPA inhibited the binding of T3 to TRα demonstrating its T3 antagonist activity both in presence and absence of S9. Metabolic activation by S9 significantly increased his agonist and antagonist potential |
| Butt et al. (2011). Halogenated Phenolic Contaminants Inhibit the In Vitro Activity of the Thyroid‐Regulating Deiodinases in Human Liver |
T4 and reverse triiodothyronine (rT3) deiodination kinetics were measured by incubating pooled human liver microsomes with T4 or rT3 and monitoring the production of T3, rT3, 3,3′‐diiodothyronine and 3‐monoiodothyronine by liquid chromatography tandem mass spectrometry TBBPA was a potent deiodinase (DI) activity inhibitor |
| Freitas et al. (2011). Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay | A stable luciferase reporter gene assay was developed based on the thyroid hormone responsive rat pituitary tumour GH3 cell line that constitutively expresses both thyroid hormone receptor isoforms. Stable transfection of the pGL4CP‐SV40‐2xtaDR4 construct into the GH3 cells resulted in a highly sensitive cell line (GH3.TRE‐Luc), which was further optimised into an assay that allowed the detection of T3 and T4 concentrations in the picomolar range after only 24 h of exposure |
| Matsubara et al. (2012). An improved thyroid hormone reporter assay to determine the thyroid hormone‐like activity of amiodarone, bithionol, closantel and rafoxanide | Reporter gene assays using the thyroid hormone responsive element (TRE) connected to the luciferase reporter gene have been developed with the ability to detect T3 activity at 10−11 M. The assay successfully detected thyroid hormone‐like activities of several model chemicals as well as a flame retardant, TBBPA |
| Levy‐Bimbot et al. (2012). Tetrabromobisphenol‐A disrupts thyroid hormone receptor alpha function in vitro: Use of fluorescence polarisation to assay corepressor and coactivator peptide binding |
TBBPA interfere with the ability of the hTRα1 ligand binding domain (LBD) to bind both nuclear hormone receptor corepressor (NCoR) and steroid receptor coactivator‐2 (SRC2). TBBPA behaved similarly to T3 in promoting the release of NCoR from LBD, whereas it failed to promote LBD interactions with SRC2. However, it did reduce the T3‐induced interactions between LBD and the coactivator peptide. This study suggests that TBBPA in the micromolar range can affect the regulation of transcription by both the apo‐ and the holo‐TRα1, with potential disruption of the expression of genes that are either up‐ or down‐regulated by T3 TBBPA, can induce an inactive conformation of hTRα1 in which the ability of the receptor to recruit either corepressors or coactivators is hampered. TBBPA could therefore disrupt the function of both unliganded hTRα1 (apo‐TRα1) and T3‐bound hTRα1 (holo‐TRα1) |
| McIver et al. (2013). Can thyroid hormone mimics affect thyroid hormone measurement by immunoassay | Hormone‐relevant concentrations of TBBPA were added to thyroid hormone‐stripped human serum and subjected to 6 different thyroid hormone immunoassays. TBBPA was negative in all of the thyroid hormone immunoassays tested (Total T3 in‐house RIA, Total T4 and T3 Beckman–Coulter Access, Free T4 and T3 Abbott Architect) except at very high concentration where it gave a marginally positive result in one immunoassay (in house T4 radioimmunoassay (RIA). Serum TBBPA is very unlikely to give false positive results in thyroid hormone immunoassays |
| Oka et al. (2013). Establishment of transactivation assay systems using fish, amphibian, reptilian and human thyroid hormone receptors | For the screening of chemicals with a potential thyroid hormone and anti‐thyroid hormone activities, a transient transactivation assay systems using thyroid hormone receptors (TRα and TRβ) from three frog species, a fish, an alligator and a human was developed |
| Zhang et al. (2014). Tetrabromobisphenol A Disrupts Vertebrate Development via Thyroid Hormone Signalling Pathway in a Developmental Stage‐Dependent Manner | TBBPA has been demonstrated in vitro to disrupt the TH signalling pathway at the transcriptional level. The effects of TBBPA on T3‐induced and spontaneous Xenopus laevis (which share many similarities with TH dependent development in higher vertebrates) metamorphosis were investigated. In a 6‐day T3‐induced metamorphosis assay using premetamorphic tadpoles, 10–1000 nM TBBPA exhibited inhibitory effects on T3‐ induced expression of TH‐response genes and morphological changes in a concentration‐dependent manner, with a weak stimulatory action on tadpole development and TH‐response gene expression in the absence of T3 induction. In a spontaneous metamorphosis assay, it was found that TBBPA promoted tadpole development from stage 51 to 56 (pre and prometamorphic stages) but inhibited metamorphic development from stage 57 to 66 (metamorphic climax). TBBPA, even at low concentrations, disrupts TH‐dependent development in a developmental stage dependent manner, i.e. TBBPA exhibits an antagonistic activity at the developmental stages when animals have high endogenous TH levels, whereas it acts as an agonist at the developmental stages when animals have low endogenous TH levels |
| Guyot et al. (2014). Toxicogenomic analysis of the ability of brominated flame retardants TBBPA and BDE‐209 to disrupt thyroid hormone signalling in neural cells | In vitro analysis of the ability of TBBPA, to alter thyroid hormone response based on a model neural cell line and genome‐wide analysis of gene expression. The HEK293–Gal4TRa1luc cells were derived from human embryonic kidney 293 cells 293 cells. TBBPA (_ > 10−6 M) has a visible antagonist effect on T3 response. The experimental setting strongly suggests that the effects of TBBPA is mediated by the ligand binding domain of TRα1, and thus reflects the ability of TBBPA to act as low‐affinity antagonist ligand of the receptor. The ability of TBBPA to interfere with T3 response in a neural reporter cell line C17.2α (cells derive from mouse cerebellum) with Hr‐Luc gene was also investigated. TBBPA at 10_8M display little if any activity on C17.2a–HrLuc cells. A slight but significant antagonist effect of TBBPA was observed when T3 was at low concentration. TBBPA is able to modify T3‐mediated up‐regulation of gene expression in neural cells. At high concentration, TBBPA has a broader influence, outside of the TH signalling pathway, which may also be relevant to neurotoxicity |
| Lai et al. (2015). Tetrabromobisphenol A (TBBPA): Possible modes of action of toxicity and carcinogenicity in rodents | This review analyses several modes of action that may account for the observed thyroxine hormone changes and the uterine tumours. It concludes that the potential modes of action for thyroid changes induced by TBBPA are expected to exhibit a threshold for adverse effects due to the ability of the mammalian organism to compensate small changes in thyroid hormone levels. Regarding MoAs for the uterine tumours, TBBPA does not exert genotoxic or estrogenic effects. Available evidence suggests that TBBPA may increase levels of circulating oestrogens by a competitive inhibition of oestrogen conjugation and produce uterine tumours by promoting pre‐existing Tp53‐mutations due to increased oestrogen levels resulting in increased cell proliferation |
| Skledar and Masic (2016). Bisphenol A and its analogs: Do their metabolites have endocrine activity? Environmental Toxicology and Pharmacology 47: 182–199 | Review – Knowledge about the metabolic pathways and enzymes involved in metabolic biotransformations is essential for understanding and predicting mechanisms of toxicity. Bisphenols are metabolised predominantly by the glucuronidation reaction, which is considered their most important detoxification pathway, as based on current knowledge, glucuronides do not have activity on endocrine receptors. In contrast, several oxidative metabolites of bisphenols with enhanced endocrine activities are presented, and these findings indicate that oxidative metabolites of bisphenols can still have endocrine activities in humans |
| Stavreva et al. (2016). ‘Novel cell‐based assay for detection of thyroid receptor beta‐interacting environmental contaminants’ |
A novel cell assay, based on the translocation of a green fluorescent protein (GFP) – tagged chimeric molecule of glucocorticoid receptor (GR) and the thyroid receptor beta (TRβ) from the cytoplasm to the nucleus in the presence of TR ligands. Unlike the constitutively nuclear TRβ, this GFP‐GR‐TRβ chimera is cytoplasmic in the absence of hormone while translocating to the nucleus in a time‐ and concentration‐dependent manner upon stimulation with T3 and thyroid hormone analogue, triiodothyroacetic acid (TRIAC), while the reverse triiodothyronine (rT3) was inactive. GFP‐GR‐TRβ chimera does not show any cross‐reactivity with the GR‐activating hormones, providing a clean system for the screening of TR beta ‐interacting EDCs. TBBPA induced GFP‐GR‐TRβ translocation at micro molar concentrations Screening of over 100 concentrated water samples from different geographic locations in the United States |
| Matsui et al. (2016). Development of yeast reporter assays for the enhanced detection of environmental ligands of thyroid hormone receptors alpha and beta from Xenopus tropicalis |
In order to detect TH disruptors for amphibians, a reporter assay was developed using yeast strains expressing the thyroid hormone receptors (TRs) α and β together with the transcriptional coactivator SRC‐1 These yeast strains responded to endogenous THs (T2 (3,5‐Diiodo‐L‐thyronine), T3 and T4) in a dose‐dependent manner. They detected the TR ligand activities of some artificial chemicals suspected to exhibit TH disrupting activities, as well as TR ligand activity in river water collected downstream of sewage plant discharges |
| Iakovleva et al. (2016). Tetrabromobisphenol A Is an Efficient Stabilizer of the Transthyretin Tetramer |
In this study TBBPA efficiently stabilises the tetrameric structure of plasma protein transthyretin (TTR). The X‐ray crystal structure shows TBBPA binding in the thyroxine binding pocket with bromines occupying two of the three halogen binding sites. TBBPA binds TTR with an extremely high selectivity in human plasma Preservation of tetrameric integrity impairs the ability of TTR to exert a cytotoxic effect. TBBPA significantly decreases the TTR dissociation rate under acidic denaturation conditions. TBBPA prevents TTR toxicity in human SH‐SY5Y neuroblastoma cells |
| Mengeling et al. (2017). A multi‐tiered, in vivo, quantitative assay suite for environmental disruptors of thyroid hormone signaling | Examination of potential TH disruption in the tractable, in vivo model system of amphibian metamorphosis. A quantitative, precocious‐metamorphosis assay suite was developed using 1‐week post‐fertilisation (PF) Xenopus laevis tadpoles in order to assess disruption of TH signalling. TBBPA although not effective in antagonising cell proliferation, significantly inhibited the TRE‐luciferase reporter |
| Lu, Zhan, et al. (2018). Thyroid Disruption by Bisphenol S Analogues via Thyroid Hormone Receptor beta: in Vitro, in Vivo, and Molecular Dynamics Simulation Study | The potential endocrine‐disrupting effects of Bisphenol S and its analogues towards thyroid hormone receptor (TR) β was investigated. The recombinant two‐hybrid yeast assay showed that TBBPA has potent antagonistic activity towards TRβ |
| Kollitz et al. (2018). The Affinity of Brominated Phenolic Compounds for Human and Zebrafish Thyroid Receptor beta: Influence of Chemical Structure | The goal of this study was to determine how structural differences of 3 Brominated Phenolic Compounds (BPC) classes impact binding affinities for the thyroid receptor beta (TRβ) in humans and zebrafish. In general, structural variations impacted binding affinities of both receptors in a similar manner. TRβ affinity increased with increasing halogen size and decreasing electronegativity, as well as increasing the degree of halogenation. The bridge group of the diphenyl compounds have a role in determining TRβ affinities. Ring orientations that diverge from that observed for T3 may negatively impact binding affinities |
| Ren et al. (2020). Binding and Activity of Tetrabromobisphenol A Mono‐Ether Structural Analogs to Thyroid Hormone Transport Proteins and Receptors | The aim of the study was to explore the potential thyroid hormone (TH) system–disrupting effect of TBBPA mono‐ether structural analogs |
| Zhang et al. (2021). Tetrabromobisphenol A induces THR beta‐mediated inflammation and uterine injury in mice at environmentally relevant exposure concentrations | To investigate whether TBBPA induces adverse outcomes at environmentally relevant exposure doses, BALB/c mice were exposed by gavage to TBBPA (5 and 50 mg/kg bw) for 14 and 28 days. The internal doses of TBBPA in mice serum were nearly the internal doses in volunteers. TBBPA significantly increased the secretion of some pro‐inflammatory cytokines and suppressed immune responses in mice under such serum concentrations after 14‐ and 28‐days exposure. Uterine oedema was observed in TBBPA‐treated mice. In a primary uterine cells model (primary cells from mice uterus tissues from BALB/c mice), it was shown that TBBPA exposure suppressed THRβ expression, leading to the activation of the inflammatory PI3K/NF‐κB signalling pathway |
| An et al. (2023). Daily exposure to low concentrations Tetrabromobisphenol A interferes with the thyroid hormone pathway in HepG2 cells | HepG2 cells were exposed to low concentrations of TBBPA daily to investigate the changes in gene regulation, mainly related to pathways associated with the endocrine system. qPCR confirmed that prolonged exposure gradually activated the thyroid hormone and parathyroid hormone signalling pathways. The expression levels of genes related to the thyroid hormone signalling pathway (six major signal pathways, including the oxytocin signalling pathway, parathyroid hormone synthesis, secretion and action, thyroid hormone signalling pathway, insulin signalling path‐ way, progesterone‐mediated oocyte maturation and oestrogen signalling pathway) were upregulated after five generations of exposure to 1 and 81 nM TBBPA. Co‐exposure to 81 nM TBBPA and 0.5 nM thyroid hormone receptor antagonist for five generations significantly reduced the expression of thyroid hormone and parathyroid hormone receptors. 81 nM TBBPA inhibited the activation of the Ras pathway and downregulated Ras gene expression level, indicating the association between the toxic effect and thyroid hormone receptors. The experiments revealed that the thyroid hormone pathway regulated the induction of the Ras signalling pathway by TBBPA |
| Sinkó et al. (2022). Tetrabromobisphenol A and Diclazuril Evoke Tissue‐Specific Changes of Thyroid Hormone Signaling in Male Thyroid Hormone Action Indicator Mice | Tissue‐specific changes of TH action were assessed in 90‐day‐old Thyroid Hormone Action Indicator (THAI) transgenic mouse model exposed by gavage to 0 or 150 mg TBBPA/kg bw per day for 6 days by measuring the expression of a TH‐responsive luciferase reporter in peripheral tissue samples and by in vivo imaging (14‐day‐long treatment accompanied with imaging on day 7, 14 and 21 from the first day of treatment). By measuring the mRNA level of the TH‐responsive luciferase reporter system, TH action remained unchanged in the heart, interscapular brown adipose tissue, skeletal muscle, skin, small intestines and liver. However, TBBPA disrupted TH signalling in the bone. By in vivo imaging, it was shown that in the small intestine, TH action was decreased after 2 weeks of treatment and then recovered after the recovery week. TBBPA impaired also the global TH economy by decreasing the circulating free T4 levels. In the promoter assays, TBBPA showed a direct stimulatory effect on the hdio3 promoter, indicating a potential mechanism for silencing TH action |
| Hu et al. (2023). Bisphenol analogues induce thyroid dysfunction via the disruption of the thyroid hormone synthesis pathway | Exposure to TBBPA of 0.02 mg/kg bw per day downregulated the protein expression levels of thyrotropin receptor, the sodium/iodide symporter, thyroperoxidase. The TH‐dependent effects were further determined using the T‐Screen assay at 10−11 M to 10−5 M concentrations. TBBPA significantly decreased TH‐dependent rat pituitary tumour GH3 cell proliferation, indicating its antagonistic activity. TBBPA can reduce GH3 cell proliferation when co‐incubated with T3. The gene responsible for THs synthesis of thyrotropin releasing hormone receptor and TSH were upregulated, but downregulation of thyroid receptor β was observed. TBBPA disrupts THs synthesis and thyroid function, which are associated with the down regulation of TSHR, TPO, TG and NIS expression. Down regulation of TRβ and up regulation of TRHR and TSHβ was demonstrated, further inhibiting GH3 cells proliferation and inducing potential THs‐disrupting effects |
APPENDIX F. The key characteristics of human carcinogens
TABLE F1.
Key characteristics of human carcinogens (from Smith et al., 2016, and cited in Guyton et al., 2018).
| Key characteristic (KC) | Example relevant evidence |
|---|---|
| 1. Is electrophilic or can be metabolically activated | Parent compound or metabolite with an electrophilic structure (e.g. epoxide, quinone, etc.), formation of DNA and protein adducts |
| 2. Is genotoxic | DNA damage (DNA strand breaks, DNA‐protein cross‐links, unscheduled DNA synthesis), intercalation, gene mutations, cytogenetic changes (e.g. chromosome aberrations, micronuclei) |
| 3. Alters DNA repair or causes genomic instability | Alterations of DNA replication or repair (e.g. topoisomerase II, base‐excision or double‐strand break repair) |
| 4. Induces epigenetic alterations | DNA methylation, histone modification, microRNAs |
| 5. Induces oxidative stress | Oxygen radicals, oxidative stress, oxidative damage to macromolecules (e.g. DNA, lipids) |
| 6. Induces chronic inflammation | Elevated white blood cells, myeloperoxidase activity, altered cytokine and/or chemokine production |
| 7. Is immunosuppressive | Decreased immunosurveillance, immune system dysfunction |
| 8. Modulates receptor‐mediated effects | Receptor in/activation (e.g. ER, PPAR, AHR) or modulation of endogenous ligands (including hormones) |
| 9. Causes immortalization | Inhibition of senescence, cell transformation |
| 10. Alters cell proliferation, cell death or nutrient supply | Increased proliferation, decreased apoptosis, changes in growth factors, energetics and signalling pathways related to cellular replication or cell‐cycle control, angiogenesis |
Abbreviations: ER, oestrogen receptor; PPAR, peroxisome proliferator‐activated receptor.
APPENDIX G. Occurrence of TBBPA and TBBPA derivatives in food and dietary exposure assessment from European countries reported in the literature since the previous Opinion
TABLE G1.
Literature data on occurrence and dietary exposure of TBBPA in food samples from European countries.
| Country | Year | Food matrices analysed | Food matrices TBBPA detected (detection frequency) | Mean (range) | Exposure estimates | Reference |
|---|---|---|---|---|---|---|
| General food categories | ||||||
| France | 2011–2012 |
Food for infants (0–3 years old) and composite foods Food for infants: Milk‐based beverage Cereals Milk‐based desserts Growing‐up milk Soups and puree Fruit puree Vegetable‐based ready‐to‐eat meal Meat/fish‐based ready‐to‐eat meal Infant formula Follow‐on formula Composite foods: Dairy products, meat, pastries, chocolate, fish N = 205 |
Growing‐up milk (9/9) Vegetable‐based ready‐to‐eat meal (2/20) Meat/fish‐based ready‐to‐eat meal (4/45) Infant formula (17/28) Follow‐on formula (24/33) Chocolate (1/2) Mixed dishes (1/4) Fish (1/3) Meat (1/2) Pastries (1/1) Poultry (1/2) |
21.7 (6.79–75.0) ng/kg ww 10.4 (6.45–14.7) ng/kg ww (UB) 12.7 (7.08–141) ng/kg ww (UB) 48.6 (7.34–321) ng/kg ww (UB) 60.1 (9.12–581) ng/kg ww (UB) 61.9 (60.6–63.2) ng/kg ww (UB) 26.9 (18.3–31.7) ng/kg ww (UB) 18.4 (16.6–21.4) ng/kg ww (UB) 25.4 (21.4–29.3) ng/kg ww (UB) 914 ng/kg ww (UB) 53.6 (23.3–83.8) ng/kg ww (UB) |
Consumption data: Consumption survey for 705 children aged 0–3 years, excluding totally or partially breastfed infants Exposure estimates based on UB scenario: Mean: 0.968 to 9.94 ng/kg bw per day for the 1–3 years of age group and the 1–4 months of age group, respectively P90: 1.8–31.3 ng/kg bw per day for the 1–3 years of age group and the 1–4 months of age group, respectively |
Rivière et al. (2019) |
| France | 2014–2016 |
Food samples Fish Crustaceous/molluscs Milk Egg Sheep liver Meat N = 577 (feed excluded) |
Fish (51/114) Crustaceous/molluscs (92/154) Milk (12/72) Egg (15/57) Sheep liver (14/28) Meat (56/152) |
< LOD (< LOD–68) ng/kg ww 0.6 (< LOD‐26) ng/kg ww < LOD (< LOD–2.70) ng/kg ww < LOD (< LOD–5.66) ng/kg ww 0.079 (< LOD–22.76) ng/kg ww < LOD (< LOD–17.73) ng/kg ww Median instead of mean LOD 0.001–0.002 μg/kg from Bichon et al 2018 |
No exposure assessment |
Venisseau et al. (2018) |
| Belgium | 2015–2016 |
Food samples ‘Fish and fish products’ (n = 61 including fish, crustaceans and molluscs) ‘Meat and meat products’ (n = 35) ‘Milk and dairy products’ (n = 38, including liquid milk, desserts and cheese) ‘Food for infants and young children’ (n = 18), ‘Animal and vegetable fat’ (n = 9) ‘Grains and grain‐based products’ (n = 7), ‘Eggs and egg products’ (n = 4) ‘Potatoes and derived products’ (n = 4), ‘Other food’ (stock: n = 4, food supplements: n = 1; vegetables: n = 2) N = 183 |
All samples (2%) Fish sticks Canned King crabs Turkey delicatessen |
0.8 ng/kg ww (LB) 39 ng/kg ww 8 ng/kg ww 96 ng/kg ww |
No exposure assessment | Poma et al. (2018) |
| The Netherlands | NR |
Dairy and diary products (22 pooled samples), Eggs (9 pooled samples), Meat and poultry (21 pooled samples), Animal fats (7 pooled samples), Fish (17 pooled samples), vegetable oil (6 pooled samples) N = 82 |
Cheese (2/13) Fish (6/17) |
< 0.1–0.09 μg/kg 0.001–3.4 μg/kg |
Consumption data: Data obtained from the third Dutch National Food Consumption Survey (DNFCS) Exposure estimates based on MB scenario: Mean: 0.04 ng/kg bw per day |
de Winter‐Sorkina et al., 2003 |
| The Netherlands | 2009–2014 |
Food samples (various foods from terrestrial and aquatic animal origin) N = 107 |
Bovine meat (1/13) Broiler meat (1/15) Haddock fillet (1/5) |
0.10 μg/kg lipid 0.054 μg/kg lipid 0.063 μg/kg ww |
No exposure assessment | Gebbink et al. (2019) |
| Ireland | 2015 |
Food samples Milk (12 samples), eggs (yolk only, 12 samples), fish (10 samples), carcass fat taken from cattle, pigs, lambs and chickens (12 samples in total), and liver (bovine, porcine, ovine, equine and avian, 7 samples in total) N = 53 |
1 farmed salmon sample | 0.01 μg/kg ww | No exposure assessment | Garcia Lopez et al. (2018) |
| Spain | NR |
Infant formula N = 50 |
Infant formula (5/50) |
0.57 (< LOD–1.9) μg/L (MB) (14.6 μg/kg lipid) LOD (1 μg/L) |
Consumption data: Data on milk intake were obtained from the US‐EPA exposure handbook (USEPA, 2011) with monthly temporal resolution Mean exposure estimates: 0.05–0.08 μg/kg bw per day for the > 6–12 months of age group and the < 1 month of age group, respectively |
Martínez et al. (2019) |
| The Czech Republic | NR |
Infant formula N = 6 |
Infant formula (0/6) | LOQ (50–150 ng/kg) | No exposure assessment | Lankova et al. (2013) |
| Fish and seafood | ||||||
|
Europe Italy, Spain, Portugal, Greece, Denmark, the Netherlands, France, Ireland, India 10 species from the Mediterranean Sea, the North Sea and the north‐east Atlantic Ocean, including 3 samples imported from the Pacific Ocean (cod and tuna) and one from India (shrimp) |
2014–2015 |
Fish and seafood (mainly marine) Cod (3) Mackerel (11) Monkfish (4) Mussel (10) Nile perch (1) Plaice (1) Salmon (3) Seabream (2) Shrimp (1) Tuna (6) N = 42 |
Mackerel (40% in 11 samples) Monkfish (2/4) |
2.76 (< 0.84 (LOD)–6.56) μg/kg lipid; fat 3.44%–36.5% 24.5 (< 0.84 (LOD)–48.6) μg/kg lipid; fat 0.17%–0.26% |
Consumption data: Data for adults from Belgium, Ireland, Italy, Portugal and Spain Exposure estimates for each country separately (UB scenario): Mean: 1.3 × 10−3 μg/kg bw per day (Spain and Portugal) P95: 1.3 × 10−3 μg/kg bw per day (Belgium) |
Aznar‐Alemany et al. (2017) |
| Italy | 2017–2018 |
Fish and seafood (marine) Wild fish and seafood species (N = 24) Seafood farmed bivalve molluscs, from six different locations (N = 16) |
< 0.05 (LOD) μg/kg ww in all samples | No exposure assessment | Chessa et al. (2019) | |
|
EU area Six sites: The Netherlands, Sweden, UK (2 sites), France, Germany |
2007–2013 |
Fish (freshwaters and estuaries) Bream (Abramis brama) from all sites and sole (Solea solea) only from the Netherlands N = 43 |
Bream (35/36) Sole (7/7) |
0.5–1.2 μg/kg ww 0.5–0.7 μg/kg ww |
No exposure assessment | Kotthoff et al. (2017) |
| Czech Republic | 2010 |
Fish (freshwater) Fish muscle tissue (27 common breams, 1 common carp, 1 crucian carp, 8 European chubs, 1 European perch, 1 grayling, 1 gudgeon, 1 rainbow trout, 6 roaches, 1 rudd) N = 48 |
Fish (11/48) Species not reported |
1.29 (0.14–4.43) μg/kg ww Median: 0.46 μg/kg ww |
No exposure assessment | Hlouskova et al. (2013) |
| The Czech Republic | 2010 |
Fish (freshwater) 26 European chubs, 22 common breams, 4 asps, 3 roaches, 2 crucian carps, 1 common carp and 1 nase carp N = 59 |
Fish (9/59) Species not reported |
1.39 (0.160–6.06) μg/kg ww 60.8 (4.99–203) μg/kg lipid Median: 0.318 μg/kg ww (19.2 μg/kg lipid) |
No exposure assessment | Svihlikova et al. (2015) |
Abbreviations: dw, dry weight; LB, lower bound; LOD, limit of detection; LOQ, limit of quantification; MB, medium bound; UB, upper bound; ww, wet weight.
TABLE G2.
Literature data on occurrence of TBBPA derivatives (TBBPA‐bMeE and TBBPA‐bDiBPrE) in food samples from European countries.
| Derivative | Country | Year | Food matrices analysed | Food matrices TBBPA derivative detected (detection frequency) | Mean (range) | Reference |
|---|---|---|---|---|---|---|
| General food categories | ||||||
| TBBPA‐bMeE | France | 2014–2016 |
Food samples Fish, Crustaceous/molluscs, Milk, Egg, Sheep liver, Meat N = 577 (feed excluded) |
Fish (22/114) Crustaceous/molluscs (83/154) Milk (1/72) Egg (1/57) Sheep liver (0/28) Meat (1/152) |
< LOD (< LOD–3) ng/kg ww < LOD (< LOD–90) ng/kg ww < LOD (< LOD–0.04) ng/kg ww < LOD (< LOD–0.069) ng/kg ww – < LOD (< LOD–0.077) ng/kg ww LODs not reported. LOQ = 0.4 ng/kg (as reported by Bichon et al., 2018) |
Venisseau et al. (2018) |
| TBBPA‐bDiBPrE | France | 2014–2016 |
Food samples Fish, Crustaceous/molluscs, Milk, Egg, Sheep liver, Meat N = 577 (feed excluded) |
Fish (22/114) Crustaceous/molluscs (8/154) Milk (1/72) Egg (1/57) Sheep liver (0/28) Meat (3/152) |
< LOD (< LOD–300) ng/kg ww < LOD (< LOD–1700) ng/kg ww < LOD (< LOD–4.41) ng/kg ww < LOD (< LOD–9.49) ng/kg ww – < LOD (< LOD–11.51) ng/kg ww LODs not reported. LOQ = 21 ng/kg (as reported by Bichon et al., 2018) |
Venisseau et al. (2018) |
| Fish and seafood | ||||||
| TBBPA‐bMeE |
EU area Six sites: The Netherlands, Sweden, UK (2 sites), France, Germany |
2007–2013 |
Fish (freshwaters and estuaries) Bream (Abramis brama) from all sites and sole (Solea solea) only from the Netherlands N = 43 |
All concentrations < 0.4 μg/kg (LOD) | Kotthoff et al. (2017) | |
| TBBPA‐bDiBPrE | Italy | 2010 |
Liver of fish (freshwater) 4 common carps (Cyprinus carpio), 2 breams (Abramis brama), 3 sanders (Sander lucioperca) and 3 sheatfishes (Silurus glanis) |
All concentrations < LOD: 0.66 μg/kg dw | Viganò et al. (2015). Emerging and priority contaminants with endocrine active potentials in sediments and fish from the River Po (Italy). Environmental Science and Pollution Research, 22, 14050–14066 | |
| TBBPA‐bDiBPrE | Italy | 2010 |
Clams N = 327 |
All concentrations < LOD: 0.66 μg/kg dw | Casatta et al. (2015). Tracing endocrine disrupting chemicals in a coastal lagoon (Sacca di Goro, Italy): Sediment contamination and bioaccumulation in Manila clams. Science of the Total Environment, 511, 214–222 | |
Abbreviations: dw, dry weight; LOD, limit of detection; LOQ, limit of quantification; MB, medium bound; UB, upper bound; ww, wet weight.
APPENDIX H. Occurrence of TBBPA in food from non‐European countries reported in the literature
H.1.
The Table below, which does not claim to be a comprehensive review of the literature, provides an overview of the occurrence of TBBPA in food samples collected in non‐European countries.
TABLE H1.
Literature data on occurrence of TBBPA in food samples from non‐European countries.
| Country | Year | Food matrices analysed | Food matrices TBBPA detected (detection frequency) | Mean (range) of TBBPA levels | Exposure estimates | Reference |
|---|---|---|---|---|---|---|
| China | 2016–2019 |
Animal‐derived foods N = 96 |
Aquatic food (100%) Meat (100%) Egg (91.7%) Dairy products (95.8%) |
19.0 (2.56–97.4) ng/kg ww Median: 11.1 ng/kg ww 97.7 (13.7–1.5 × 103) ng/kg ww Median: 24.6 ng/kg ww 13.2 (< LOD‐35.5) ng/kg ww Median: 8.52 ng/kg ww 3.73 (0.427–32.1) ng/kg ww Median: 1.6 ng/kg ww |
Consumption data: 6th Chinese Total Diet Study (TDS) Exposure estimates (not clear whether LB or UB scenario was used): Mean estimated daily intake: 156 ± 695 pg/kg bw per day P95: 411 pg/kg bw per day |
Zhao et al. (2023). ‘Legacy and novel brominated flame retardants in animal‐derived foods from China Total Diet Study (CTDS): Temporal trends, evidence of substitution and dietary exposure assessment.’ J Hazard Mater 443(Pt A): 130223 |
| China | 2013 |
Food stuff samples meat/meat product, Egg, Fish/seafood, Dairy products, Vegetables, Fruit and berry, Oil, Soybean and nut, Cereal product N = 90 |
Meat/meat product (12/17) Egg (6/16) Fish/seafood (5/18) Dairy products (6/11) Vegetables (5/13) Fruit and berry (banana) (1/8) |
1.26 μg/kg ww 0.22 μg/kg ww 0.162 μg/kg ww 0.02 μg/kg ww 0.161 μg/kg ww 0.035 μg/kg ww |
Consumption data: Data from ‘The report of hygiene and human health status of Beijing, 2011″ Exposure estimates (LB scenario): 164 ng/day for adult via food |
Wang et al. (2019). Tetrabromobisphenol A, hexabromocyclododecane isomers and polybrominated diphenyl ethers in foodstuffs from Beijing, China: Contamination levels, dietary exposure and risk assessment. Science of the Total Environment, 666, 812–820 |
| China | 2013 |
Eggs samples (chicken, goose) N = 40 |
27–420 (1.6–890) μg/kg lipid Median: 6–27 μg/kg lipid |
No exposure assessment | Zeng et al. (2016). Habitat‐ and species‐dependent accumulation of organohalogen pollutants in home‐produced eggs from an electronic waste recycling site in South China: Levels, profiles and human dietary exposure. Environmental Pollution, 216, 64–70 | |
| China | 2011 |
Composite samples (aquatic foods, meat and meat products, eggs and egg products, milk and milk products) N = 80 |
Aquatic foods (18/20) Meat and meat products (19/20) Eggs and egg products (10/20) Milk and milk products (19/20) |
3.05a ± 4.26 (ND‐13.5) μg/kg lipid 1.78 ± 2.67 (ND‐10.9) μg/kg lipid 3.12 ± 7.54 (ND‐33.1) μg/kg lipid 5.76 ± 11.8 (ND‐52) μg/kg lipid |
Consumption data: 5th Chinese Total Diet Study (TDS) Exposure estimates (UB scenario): Mean intake: 1.51 ng/kg bw per day |
Shi et al. (2017b). Dietary exposure assessment of Chinese population to tetrabromobisphenol‐A, hexabromocyclododecane and decabrominated diphenyl ether: Results of the 5th Chinese Total Diet Study. Environmental Pollution, 229, 539–547 |
| Republic of Korea | 2016 |
Eight food categories Vegetables (rice, kimchi, brown rice) Fish (11 species) Fish products (fish cake and canned tuna) Shellfish (5 species) Meat (duck) Meat products (sausage and ham) Cephalopod (4 species) Crustacea (shrimp and crab) N = 301 |
Rice Fish Fish products Shellfish Meat Meat products Cephalopod Crustacea |
0.015 ± 0.025 (< 0.010 (LOD)–0.069) μg/kg ww < 0.010 (LOD)–0.933 μg/kg ww < LOD–0.191 μg/kg ww < 0.004 (LOD)–0.240 μg/kg ww < 0.006 (LOD)–0.026 μg/kg ww < 0.006 (LOD)‐0.238 μg/kg ww < 0.017 (LOD)–0.315 μg/kg ww < 0.004 (LOD)–0.045 μg/kg ww |
Consumption data: 19,386 samples of the Korean population Exposure assessment (UB scenario): Mean: 0.157 ng/kg bw per day; P95: 0.412 ng/kg bw per day |
Lee et al. (2020). Assessment of Tetrabromobisphenol and Hexabromocyclododecanes exposure and risk characterisation using occurrence data in foods. Food and Chemical Toxicology, 137 111121 |
| Tunisia (Bizerte lagoon) | 2018 |
Fish and seafood [8 species (3 fish, 5 shellfish (1 crustacean and 4 molluscs))] N = 24 [each species, three pools of 3 to 60 individual samples] |
European eel Clams |
0.01 (< 0.01 (LOQ)–0.04) μg/kg ww 0.11 (0.07–0.14) μg/kg ww |
Consumption data: FAO 2016 Exposure assessment (UB scenario): Mean estimated intake: 0.0004 ng/kg bw per day |
Mahfoudhi et al. (2023). First study of bromophenols and hexabromocyclododecanes in seafood from North Africa (case of Bizerte Lagoon, Tunisia): occurrence and human health risk. Environ Sci Pollut Res Int 30, 64499–64516 |
APPENDIX I. Identification of sources of uncertainty
I.1.
A systematic approach was used to identify sources of uncertainty affecting the assessment of TBBPA. A previously‐prepared list of sources of uncertainty commonly encountered in risk assessments of the CONTAM Panel was reviewed to identify and describe those sources that applied to the present assessment. In addition, the Panel considered each part of the present assessment in turn to identify any additional sources of uncertainty, beyond those in the existing list. Subsequently, the Panel considered which of those sources of uncertainty would have most impact on the outcome of the exposure assessment, and of the hazard identification and characterisation.
A complete list of the sources of uncertainty identified is presented in Tables I1, I2, I3. The most important uncertainties are listed together with a qualitative evaluation of their potential impact on the assessment in four categories: negligible, low, medium and high. These qualitative ratings were used later in the analysis to prioritise consideration of the main sources of uncertainty and to facilitate the assessment of overall uncertainty.
TABLE I1.
Uncertainties identified and their impact on the outcome of the exposure assessment for TBBPA in food.
| Description of the uncertainty | Impact on the exposure assessment a | |
|---|---|---|
| Occurrence data | ||
| Analytical measurements | Performance (e.g. specificity for the target compounds) of the analytical method (GC‐ECD, GC–MS, etc.) | 1 – Low impact. Methods for TBBPA are well established. Most of the data were analysed by LC–MS and some by GC–MS using isotope labelled standards |
| Proportion of left‐censored data and magnitude of difference between risk estimates for LB and UB exposures | 1 – Low impact. Although the proportion of left‐censored data is high, the uncertainty is addressed by using the substitution method, and the use of the UB which is an overestimation of the exposure estimates. The impact of this uncertainty was considered to be medium for the exposure assessment, but low for the risk characterisation | |
| Consideration of recovery (e.g. correction carried out or not) | 1 – Low impact. Methods for TBBPA use isotope labelled standards that do not need to be corrected for recovery | |
| Data reporting | Potential errors in reporting the occurrence data (e.g. in the classification of the food category, unit of measurement, parameter, fat vs. whole weight, etc.) – unidentified errors (not apparent from the data provided) | 1 – Low impact. Data cleaning procedures that identifies outliers and clarifications received by data providers aim to solve most of the possible reporting errors. Although some errors might remain unidentified their overall impact on the results should be low |
| Missing information in reporting the occurrence data (e.g. analytical method) | 1 – Low impact. Clarifications received by data providers | |
| Missing or unclear information about the treatment/processing applied prior to the analysis of the sample that is submitted to EFSA | 0 – Negligible impact. Limited information is available on processing, but in general the food is analysed raw. Since sample treatment/processing impact on TBBPA concentration is considered negligible, the impact of this uncertainty is negligible | |
| Use of food categories at high (often not enough specified) FoodEx/FoodEx2 level | 1 – Low impact. A total of 90% of analytical results were reported at Foodex2 Level 3 or lower | |
| Other uncertainties linked to occurrence data, factors and assumptions | Possible effect of food preparation on the chemical concentrations | 1 – Low impact. No information available on the impact of food processing on the concentration of TBBPA. Assuming that TBBPA will behave in a similar way to lipophilic POPs, i.e. that TBBPA associates with lipids, and amounts in food may be reduced if fats that come from the food during cooking or processing are discarded; on the other hand if moisture is lost during the heating of foods but no fat is discarded, then the total amount will remain unchanged but the concentrations may increase. Overall, the impact of these changes would be low |
| Uncertainty in the reporting of fat content by data providers and need for conversion to whole weight | 0 – Negligible impact. When results were expressed on fat content, fat percentage was provided and this allowed the conversion of concentration to whole weight. Some values were clarified with data providers, and data were corrected accordingly | |
| Representativeness of the data | Limited number of analytical results per food categories expected to contribute to the exposure | 1 – Low impact. No data for infant formula and/or follow‐on formula were submitted to EFSA. Data from the literature was used: two studies were identified, reporting on 120 samples on infant formula and follow‐on formula collected in two Member States (Spain and France). Considering a common market, these data were considered sufficiently representative and used in the exposure assessment. The impact of this uncertainty was considered to be medium for the exposure assessment, but low for the risk characterisation |
|
1 – Low impact. Analytical results were only available for a limited number of food categories. Where possible, concentrations were inferred to obtain occurrence data in composite food and derivatives, for food for which occurrence data were available in the raw primary commodity In addition, although very limited number of samples in food categories of non‐animal origin all left‐censored, and these categories were not included in the exposure assessment, TBBPA is not expected to be present at high concentrations in these categories, thus the impact on both the exposure assessment and overall risk assessment is considered low | ||
| Extrapolation of data from one food category to others, and other assumptions |
1 – Low impact.
Overall, the impact of this uncertainty was considered to be medium for the exposure assessment, but low for the risk characterisation |
|
| Sampling strategy not fully random | 0 – Negligible impact. There were no samples reported as ‘suspect sampling’. Thus it is assumed that sampling was sufficiently randomised | |
| Uneven distribution of the data per year (e.g. recent years not sufficiently represented) | 1 – Low impact. Only the most recent data (from 2011 onwards) were considered in the assessment | |
| Uneven distribution of the data per country (e.g. large number of MSs not sufficiently represented) | 1 – Low impact. Data coming from six Member States plus UK and NO. Most results were provided by France (58%) and Norway (23%). Considering a common market, these data were considered sufficiently representative for the exposure assessment | |
| Limited number of analytical results per variables that could explain higher/lower levels, such as production method (e.g. wild vs. farmed), processing (e.g. peeled vs. raw), etc | 1 – Low impact. The additional information provided is limited but no specific aspects have been identified to have a major impact | |
| Human milk data | Proportion of left‐censored data and inclusion of hydrolysis step to cleave conjugates | 1 – Low impact. The study reporting the highest mean concentration in human milk (Martínez et al., 2019) used the MB approach and included a hydrolysis step in the sample preparation to cleave conjugates. Thus the results refer to ‘total TBBPA’ (conjugated + unconjugated). Therefore, the concentration is likely to be an overestimate of the bioavailable fraction of the TBBPA |
| Consumption data | ||
| Data reporting |
Unidentified errors in reporting consumption data, e.g. in the classification of the food, portion size, body weight estimation, memory errors, capacity to report details in dietary surveys Different dietary survey methodologies (e.g. dietary record vs. 24‐h re‐call), dietary software, interview options, use of portion‐size measurement Use of national standard recipes and ingredients factors for composite dishes (potentially leading to, e.g. underestimation of minor ingredients, overestimation of standard ingredients) Different sample size and response rate of the dietary surveys Long‐term (chronic) exposure assessed based on few days of consumption per individual information about processing/cooking method not consistently reported in consumption records |
1 – Low impact. Uncertainties and limitations related to the use of the EFSA Comprehensive Food Consumption Database have been described by EFSA (EFSA, 2011a). These uncertainties are common to dietary exposure assessments performed using the Comprehensive Database, and have the potential to cause either an over‐ or under‐estimation of the exposure No specific uncertainties affecting the food consumption data used for the TBBPA exposure assessment were identified |
| Representativeness of the data | Availability of food consumption data for special population groups, including consumers only of specific foods of special interest, or following special diets | 1 – Low impact. ‘Consumers only’ of mostly contaminated foods are covered by 95th percentile exposure estimates. Pregnant, lactating women and vegetarian's surveys provided similar exposure results as adults from the general population. No other specific population groups that might have a particular exposure to TBBPA were identified |
| Exposure estimates | ||
| Non‐dietary exposure | Sources of exposure other than dietary – how much important is dietary exposure to the total | 1 – Low impact. Exposure from dust can vary greatly between individuals and age classes, and can be significant for some individuals, although not as great as exposure from diet. It can be particularly relevant for young children. Limited data on dermal and inhalation and other potential non‐dietary routes of exposure |
| Exposure assessment scenario | Consumers loyalty to specific brands or from specific local areas not considered | 0 – Negligible impact. Exposure assessment was made for the general population. Brand loyalty and consumer's only scenarios were considered not relevant for the TBBPA exposure assessment. High exposures due to variability across individuals are covered by the 95th percentile of the exposure estimates |
Abbreviations: ECD, electron capture detector; FR, France; GC, gas chromatography; LB, lower bound; LC, liquid chromatography; MS, mass spectrometry; MSs, Member States; NO, Norway; POPs, persistent organic pollutants; UB, upper bound; UK, United Kingdom.
0 – Uncertainty with negligible impact; 1 – Uncertainty with low impact; 2 – Uncertainty with medium impact; 3 – Uncertainty with high impact.
TABLE I2.
Uncertainties identified and their impact on the outcome of the hazard identification and characterisation.
| Description of the uncertainty | Impact on the hazard identification and characterisation a | |
|---|---|---|
| Chemical composition and analytical methods | ||
| Dosing and chemical composition | Uncertainty associated with the dose in the critical studies used in the risk assessment | 1 – Low impact. Many of the studies are conducted using gavage dosing |
| Hazard identification and characterisation | ||
| ADME | ADME in relation to the critical studies | 1 – Low impact. TK data on rat are available by gavage or i.v. route. Only one in vivo study was identified in mice (oral exposure). One In vitro study showed that the TBBPA glucuronidation abilities in mice was 1.1‐fold those observed in human |
| Relevance in humans, genetic background/susceptibility/sensitive populations | 0 – Negligible impact. Immature metabolism for young children could lead to underestimation of the risk. This is covered in the default UF for intraindividual variability | |
| Accumulation potential | 0 – Negligible impact. Studies in rats with single and repeated exposure show rapid clearance. Data on bioaccumulation indicates that TBBPA is weakly bioaccumulative compared to HBCDDs or PBDEs | |
| Little information on transfer rate to animal products | 0 – Negligible impact. No studies are available but no impact on the risk assessment | |
| Transfer via mother's milk | 0 – Negligible impact. Based on studies in rats, low levels of TBBPA in the pups have been detected | |
| Toxicity studies in experimental animals: critical endpoints and critical study design | Limitations in the study design of the studies that can result in uncertainties |
Carcinogenicity: 0 – Negligible impact. The critical rat study was conducted by NTP (2014) and considered to be of high quality. The mouse study was compromised by high mortality in the top dose group and is not considered to be a critical study Neurotoxicity: 1 – Low impact. The critical study (Kim et al., 2015) is a one dose level study |
| Relevance for humans of the adverse effect/biomarkers of adversity |
Carcinogenicity: 0 – Negligible impact. The uterine tumours reported in rats are relevant for humans Neurotoxicity: 0 – Negligible impact. The effect in the critical study (increased anxiety and decreased interest in social interaction) is indicative of disturbances of social behaviour and is considered adverse. Moreover, mechanistic data, indicating epigenetic modifications at lower doses of TBBPA, provide supporting evidence that exposure to TBBPA has adverse effects on the nervous system in mammals |
|
| Effects tested only in one species, one sex or certain age groups or at one time point |
Carcinogenicity: 0 – Negligible impact. Both male and female rats and mice were tested Neurotoxicity: 1 – Low impact. Only M pups tested in the critical study (Kim et al., 2015) |
|
| Consistency between studies on same/similar endpoints: high dose versus low dose |
Carcinogenicity: 0 – Negligible impact. Not relevant Neurotoxicity: 0 – Negligible impact. Different effects were seen at similar levels in different studies |
|
| Genotoxicity | Uncertainty in the assessment of genotoxicity | 0 – Negligible impact. Based on the evidence available, TBBPA is not genotoxic (see Section 3.1.2.6 ) |
| Selection of reference point | NOAEL/LOAEL approach | 2 – Moderate impact. No NOAEL could be identified in the critical study (Kim et al., 2015). One dose level study |
| Weaknesses in non‐critical studies and uncertainty about whether the endpoints they tested might have been critical if the weaknesses were not present |
2 – Moderate impact. Four studies involved dosing TBBPA to mice via drinking water (Zatecka et al., 2013; Li et al., 2022; Xiong et al., 2023; Song et al., 2024). These studies reported effects on the thyroid, neurotoxicity/neurodevelopment or reproduction toxicity at exceptionally low effect levels whereas other studies where TBBPA was administered by gavage have reported effects only at three orders of magnitude higher dose levels. The CONTAM Panel noted that the studies were generally well conducted. However, the concentrations in drinking water were not confirmed by analysis of TBBPA, which may be important, e.g. because of the low solubility of TBBPA in water. The authors reported that the daily intake in μg/kg bw per day was estimated based on the daily water consumption and body weight. This may be the case for dams but not for the pups, as no drinking water consumption was measured. The CONTAM Panel considered therefore that there is a high level of uncertainty regarding the doses received by the animals, and no NOAELs/LOAELs were identified, or dose–response assessment performed. If effects occurred at the levels reported in these studies, the impact on the hazard assessment and TDI would be high, but the impact on the risk characterisation would be moderate due to the low exposure estimates 1 – Low impact. The lowest LOAEL was identified from a one dose level study (Rock et al., 2019, 1st experiment) for an increased level of activity in the running wheel apparatus. The relevance for humans and adversity of this effect was considered uncertain 1 – Low impact. Increasing evidence of the sensitivity of the developing brain to chemical exposure, including MOA studies with TBBPA reported in the Opinion. These indicate some probability that relevant effects of TBBPA may be found at lower dose levels in future, though these might be intermediate rather than apical |
|
Abbreviations: ADME, absorption, distribution, metabolism, excretion; BMD, benchmark dose; BMDL, Benchmark Dose Lower Confidence Limit; BMR, Benchmark Response; DSB, double strand breaks; MOA, mode of action; NTP, National Toxicology Program; OECD, Organisation for Economic Co‐operation and Development; SSB, single strand breaks; TDI, Tolerable Daily Intake; TK, Toxicokinetic; UF, Uncertainty factor.
0 – Uncertainty with negligible impact; 1 – Uncertainty with low impact; 2 – Uncertainty with medium impact; 3 – Uncertainty with high impact.
TABLE I3.
Uncertainties identified and their impact on the outcome of the risk characterisation.
| Description of the uncertainty | Impact on the hazard identification and characterisation a | |
|---|---|---|
| Establishment of the HBGV (TDI) | Inter‐species differences not correctly covered by standard UFs | The default UFs for inter‐species differences in kinetics and dynamics are applied and are considered sufficient. This is therefore considered a standard uncertainty |
| Intra‐species differences not correctly covered by standard UFs | The default UFs for intra‐species differences in kinetics and dynamics are applied and are considered sufficient. This is therefore considered a standard uncertainty | |
| Extrapolation from LOAEL to NOAEL not correctly covered by the factor of 3 | 1 – Low impact. A factor of 3 to extrapolate from LOAEL to NOAEL was applied and is considered sufficiently conservative | |
| Risk characterisation for infants under 16 weeks of age | Additional factor for increased inter‐individual toxicokinetic variability compared to adult population | In the absence of data to support a case‐specific factor, the default factor of 3 is considered (EFSA Scientific Committee, 2017). This is therefore considered a standard uncertainty |
| Case‐by‐case consideration of additional toxicodynamic variability in this age group (EFSA Scientific Committee, 2017) | 0 – Negligible impact. The critical study included exposure of offspring via gestation and lactation | |
Abbreviations: HBGV, Health Based Guidance Value; TDI, Tolerable Daily Intake; UF, Uncertainty factor.
0 – Uncertainty with negligible impact; 1 – Uncertainty with low impact; 2 – Uncertainty with medium impact; 3 – Uncertainty with high impact.
1.
ANNEX A. Protocol for the risk assessments for human health related to the presence of brominated flame retardants (BFRs) in food
A.1.
The Annex is provided as a separate pdf file containing the risk assessment protocol applied by the CONTAM Panel to up‐date the previous risk assessments of brominated flame retardants (BFRs) in food, and is available under the Supporting Information section on the online version of the Scientific output.
ANNEX B. Occurrence data on TBBPA and TBBPA derivatives in food submitted to EFSA, dietary surveys per country and age group available in the EFSA Comprehensive Database considered in the exposure assessment, and chronic dietary exposure to TBBPA and the contribution of different food groups to the dietary exposure
B.1.
The Annex is provided as a separate excel file, containing the occurrence data submitted to EFSA, the dietary surveys per country and age group, and the chronic dietary exposure to TBBPA, and is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.11551015.
ANNEX C. Benchmark dose analysis
C.1.
The Annex is provided as a separate pdf file containing the results of the benchmark dose (BMD) analysis, and is available under the Supporting Information section on the online version of the Scientific output.
ANNEX D. Uncertainty analysis – protocol and results of the EKE
D.1.
The Annex is provided as a separate pdf file containing the details and outcome of the EKE performed for the uncertainty analysis, and is available under the Supporting Information section on the online version of the Scientific output.
ANNEX E. Outcome of the public consultation
E.1.
The Annex is provided as a separate pdf file containing the comments received during the public consultation and the replies by the CONTAM Panel, and is available under the Supporting Information section on the online version of the Scientific output.
EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , Schrenk, D. , Bignami, M. , Bodin, L. , Chipman, J. K. , del Mazo, J. , Grasl‐Kraupp, B. , Hogstrand, C. , Hoogenboom, L. R. , Leblanc, J.‐C. , Nebbia, C. S. , Nielsen, E. , Ntzani, E. , Petersen, A. , Sand, S. , Schwerdtle, T. , Wallace, H. , Benford, D. , Hart, A. , … Vleminckx, C. (2024). Update of the scientific opinion on tetrabromobisphenol A (TBBPA) and its derivatives in food. EFSA Journal, 22(7), e8859. 10.2903/j.efsa.2024.8859
Adopted: 4 June 2024
Annexes A, C, D and E are available under the Supporting Information section
Notes
Probabilities assessed by expert judgement in the uncertainty analysis are reported in conclusions as % certainty for the more probable outcome, following EFSA's guidance on communication of uncertainty (EFSA, 2019).
Referred as TBBPA‐DHEE by Okeke et al. (2022).
Referred as TBBPA‐DBPE by Okeke et al. (2022).
Referred as TBBPA‐DHEE and TBBPA‐MHEE by Zhang, Dong, et al. (2018).
Results below limit of detection (LOD) or below the limit of quantification (LOQ).
Council Regulation (EEC) No 315/93 of 8 February 1993 laying down Community procedures for contaminants in food. OJ L 37, 13.2.1993, p. 1.
Commission Regulation (EU) No 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food. OJ L 119/103, 5.5.2023, pp. 103–157.
Regulation (Ec) No 1907/2006 Of The European Parliament And Of The Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. OJ L 396, 30.12.2006, pp. 1–849.
EC/ List numbers: 201‐236‐9 (TBBPA); 221‐346‐0 (TBBPA‐bGE); 244‐617‐5 (TBBPA‐bDiBPrE); 246‐850‐8 (TBBPA‐bAE); 253‐693‐9 (TBBPA‐bMeE); 306‐832‐3; 400‐440‐7; 420‐850‐1; 436‐220‐2; 468‐980‐6; 500‐107‐7; 500‐399‐6; 600‐581‐6; 926‐564‐6; 944‐461‐4.
Current call: https://www.efsa.europa.eu/en/call/call‐continuous‐collection‐chemical‐contaminants‐occurrence‐data‐food‐and‐feed‐2023.
This study was not cited in the previous EFSA assessment on TBBPA and its derivatives (EFSA CONTAM Panel, 2011c) and it is included in the current assessment for completeness.
This study was not cited in the previous EFSA assessment on TBBPA and its derivatives (EFSA CONTAM Panel, 2011c) and it is included in the current assessment for completeness.
This study was not cited in the previous EFSA assessment on TBBPA and its derivatives (EFSA CONTAM Panel, 2011c) and it is included in the current assessment for completeness.
The study was not captured in the previous Opinion, but it is reported in the current update for completeness.
Referred as TBBPA‐DBPE by Shockley et al. (2020a).
This study was cited in the previous EFSA assessment on TBBPA (EFSA CONTAM Panel, 2011c) and it is included in the current assessment to provide insight into additional observations by the study authors not reported in the previous Opinion.
Equivalent doses were calculated based on default factors for converting chemical substance concentrations in drinking water into daily doses in experimental animal studies according to EFSA Scientific Committee (2012).
‘Growing‐up milk’ is interpreted as growing‐up formula, milk‐based, for young children (1–3 years).
Non‐standard uncertainties are defined by EFSA Scientific Committee (2018) as “Any deviations from a standardised procedure or standardised assessment element that lead to uncertainty regarding the result of the procedure. For example, studies that deviate from the standard guidelines or are poorly reported, cases where there is doubt about the applicability of default values, or the use of non‐standard or ‘higher tier’ studies that are not part of the standard procedure.”
Standard uncertainties are defined by EFSA Scientific Committee (2018) as ‘Sources of uncertainty that are considered (implicitly or explicitly) to be addressed by the provisions of a standardised procedure or standardised assessment element. For example, uncertainties due to within and between species differences in toxicity are often addressed by a default factor of 100 in chemical risk assessment.”
Probabilities assessed by the uncertainty analysis are reported in conclusions as % certainty for the more probable outcome, following EFSA's guidance on communication of uncertainty (EFSA, 2019).
The impact of uncertainties on each part of the risk assessment was quantified using % probabilities assessed by expert judgement, following EFSA's guidance on uncertainty analysis (EFSA Scientific Committee, 2018). When reporting conclusions, the resulting probabilities are expressed as % certainty for the more probable outcome, following EFSA's guidance on communication of uncertainty (EFSA, 2019).
REFERENCES
- Abafe, O. A. , & Martincigh, B. S. (2016). Determination and human exposure assessment of polybrominated diphenyl ethers and tetrabromobisphenol A in indoor dust in South Africa. Environmental Science and Pollution Research, 23, 7038–7049. [DOI] [PubMed] [Google Scholar]
- Abb, M. , Stahl, B. , & Lorenz, W. (2011). Analysis of brominated flame retardants in house dust. Chemosphere, 85, 1657–1663. [DOI] [PubMed] [Google Scholar]
- Abdallah, M. A. (2016). Environmental occurrence, analysis and human exposure to the flame retardant tetrabromobisphenol‐A (TBBP‐A) – A review. Environment International, 94, 235–250. [DOI] [PubMed] [Google Scholar]
- Abdallah, M. A. , & Harrad, S. (2011). Tetrabromobisphenol‐A, hexabromocyclododecane and its degradation products in UK human milk: Relationship to external exposure. Environment International, 37, 443–448. [DOI] [PubMed] [Google Scholar]
- Abdallah, M. A. E. , Bressi, M. , Oluseyi, T. , & Harrad, S. (2016). Hexabromocyclododecane and tetrabromobisphenol‐A in indoor dust from France, Kazakhstan and Nigeria: Implications for human exposure. Emerging Contaminants, 2, 73–79. [Google Scholar]
- Abe, N. , Sasaki, M. , & Nakajima, A. (2023). Tetrabromobisphenol A and hexabromocyclododecane, brominated flame retardants, trigger endoplasmic reticulum stress and activate necroptosis signaling in PC12 cells. Environmental Toxicology and Pharmacology, 98, 104056. [DOI] [PubMed] [Google Scholar]
- Akiyama, E. , Kakutani, H. , Nakao, T. , Motomura, Y. , Takano, Y. , Sorakubo, R. , Mizuno, A. , Aozasa, O. , Tachibana, K. , Doi, T. , & Ohta, S. (2015). Facilitation of adipocyte differentiation of 3T3‐L1 cells by debrominated tetrabromobisphenol A compounds detected in Japanese breast milk. Environmental Research, 140, 157–164. [DOI] [PubMed] [Google Scholar]
- Al‐Mousa, F. , & Michelangeli, F. (2012). Some commonly used brominated flame retardants cause Ca2+‐ATPase inhibition, beta‐amyloid peptide release and apoptosis in SH‐SY5Y neuronal cells. PLoS One, 7, 33059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almughamsi, H. , & Whalen, M. M. (2016). Hexabromocyclododecane and tetrabromobisphenol A alter secretion of interferon gamma (IFN‐γ) from human immune cells. Archives of Toxicology, 90(7), 1695–1707. 10.1007/s00204-015-1586-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, T. C. , Lu, L. R. , & Li, G. Y. (2023). Daily exposure to low concentrations tetrabromobisphenol A interferes with the thyroid hormone pathway in HepG2 cells. Fundamental Research, 3, 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisuzzaman, S. , & Whalen, M. M. (2016). Tetrabromobisphenol A and hexabromocyclododecane alter secretion of IL‐1β from human immune cells. Journal of Immunotoxicology, 13(3), 403–416. 10.3109/1547691X.2015.1111960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANSES (French Agency for food, environmental and occupation health safety) . (2016). Opinion of the French Agency for Food, Environmental and Occupational Health & Safety on the dietary exposure of children under three years of age to certain substances . https://www.anses.fr/en
- ANSES (French Agency for food, environmental and occupation health safety) . (2017a). Connaissances relatives à la réglementation, à l'identification, aux propriétés chimiques, à la production et aux usages des substances de la famille des polybromés et particulièrement du 2,2′,4,4′‐tétrabromodiphényl éther (n° CAS: 5436‐43‐1) et du décabromodiphényléther (décaBDE) (n°1163‐19‐5). Tome 1. Saisine n° 2009‐SA‐0331 . https://www.anses.fr/en
- ANSES (French Agency for food, environmental and occupation health safety) . (2017b). Connaissances relatives aux données de contamination et aux expositions par des composés de la famille des polybromés. Tome 2. Saisine n° 2009‐SA‐0331 . https://www.anses.fr/en
- ANSES (French Agency for food, environmental and occupation health safety) . (2017c). Connaissances relatives aux données de toxicité sur les composés de la famille des polybromés. Tome 3. Saisine n° 2009‐SA‐0331 . https://www.anses.fr/en
- Antignac, J.‐P. , Cariou, R. , Maume, D. , Marchand, P. , Monteau, F. , Zalko, D. , Berrebi, A. , Cravedi, J.‐P. , Andre, F. , & Le Bizec, B. (2008). Exposure assessment of fetus and newborn to brominated flame retardants in France: Preliminary data. Molecular Nutrition & Food Research, 52, 258–265. [DOI] [PubMed] [Google Scholar]
- Arini, A. , Mittal, K. , Dornbos, P. , Head, J. , Rutkiewicz, J. , & Basu, N. (2017). A cell‐free testing platform to screen chemicals of potential neurotoxic concern across twenty vertebrate species. Environmental Toxicology and Chemistry, 36, 3081–3090. [DOI] [PubMed] [Google Scholar]
- Arita, Y. , Pressman, M. , Getahun, D. , Menon, R. , & Peltier, M. R. (2018). Effect of Tetrabromobisphenol A on expression of biomarkers for inflammation and neurodevelopment by the placenta. Placenta, 68, 33–39. 10.1016/j.placenta.2018.06.306 [DOI] [PubMed] [Google Scholar]
- Aznar‐Alemany, O. , Trabalon, L. , Jacobs, S. , Barbosa, V. L. , Tejedor, M. F. , Granby, K. , Kwadijk, C. , Cunha, S. C. , Ferrari, F. , Vandermeersch, G. , Sioen, I. , Verbeke, W. , Vilavert, L. , Domingo, J. L. , Eljarrat, E. , & Barcelo, D. (2017). Occurrence of halogenated flame retardants in commercial seafood species available in European markets. Food and Chemical Toxicology, 104, 35–47. [DOI] [PubMed] [Google Scholar]
- Badée, J. , Qiu, N. , Collier, A. C. , Takahashi, R. H. , Forrest, W. F. , Parrott, N. , Schmidt, S. , & Fowler, S. (2019). Characterization of the ontogeny of hepatic UDP‐glucuronosyltransferase enzymes based on glucuronidation activity measured in human liver microsomes. The Journal of Clinical Pharmacology, 59, S42–S55. [DOI] [PubMed] [Google Scholar]
- Bagley, M. C. , Ekelöf, M. , Rock, K. , Patisaul, H. , & Muddiman, D. C. (2018). IR‐MALDESI mass spectrometry imaging of underivatized neurotransmitters in brain tissue of rats exposed to tetrabromobisphenol A. Analytical and Bioanalytical Chemistry, 410, 7979–7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barańska, A. , Bukowska, B. , & Michałowicz, J. (2022). Determination of apoptotic mechanism of action of tetrabromobisphenol A and tetrabromobisphenol S in human peripheral blood mononuclear cells: A comparative study. Molecules, 27, 6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barańska, A. , Woźniak, A. , Mokra, K. , & Michałowicz, J. (2022). Genotoxic mechanism of action of TBBPA, TBBPS and selected bromophenols in human peripheral blood mononuclear cells. Frontiers in Immunology, 13, 869741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barghi, M. , Shin, E. S. , Choi, S. D. , Dahmardeh Behrooz, R. , & Chang, Y. S. (2018). HBCD and TBBPA in human scalp hair: Evidence of internal exposure. Chemosphere, 207, 70–77. [DOI] [PubMed] [Google Scholar]
- Barghi, M. , Shin, E. S. , Kim, J. C. , Choi, S. D. , & Chang, Y. S. (2017). Human exposure to HBCD and TBBPA via indoor dust in Korea: Estimation of external exposure and body burden. Science of the Total Environment, 593, 779–786. [DOI] [PubMed] [Google Scholar]
- Behl, M. , Hsieh, J. H. , Shafer, T. J. , Mundy, W. R. , Rice, J. R. , Boyd, W. A. , Freedman, J. H. , Hunter, E. S., 3rd , Jarema, K. A. , Padilla, S. , & Tice, R. R. (2015). Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicology and Teratology, 52, 181–193. [DOI] [PubMed] [Google Scholar]
- Bergman, A. , Ryden, A. , Law, R. J. , de Boer, J. , Covaci, A. , Alaee, M. , Birnbaum, L. , Petreas, M. , Rose, M. , Sakai, S. , Van den Eede, N. , & van der Veen, I. (2012). A novel abbreviation standard for organobromine, organochlorine and organophosphorus flame retardants and some characteristics of the chemicals. Environment International, 49, 57–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besis, A. , Christia, C. , Poma, G. , Covaci, A. , & Samara, C. (2017). Legacy and novel brominated flame retardants in interior car dust – implications for human exposure. Environmental Pollution, 230, 871–881. [DOI] [PubMed] [Google Scholar]
- Bhattacharya, A. , & Khare, S. K. (2016). Sustainable options for mitigation of major toxicants originating from electronic waste. Current Science, 111, 1946–1954. [Google Scholar]
- BioReliance . (2001). An in vitro mammalian chromosome aberration test with tetrabromobisphenol‐A [Unpublished]. As cited in ECB (2006).
- Borghoff, S. J. , Wikoff, D. , Harvey, S. , & Haws, L. (2016). Dose‐and time‐dependent changes in tissue levels of tetrabromobisphenol A (TBBPA) and its sulfate and glucuronide conjugates following repeated administration to female Wistar Han Rats. Toxicology Reports, 3, 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady, U. E. (1979). Pharmacokinetic study of tetrabromobisphenol A (BP‐4A) in rats. Report from Athens, Georgie, University of Georgia (Report to Velsicol Chemical Corporation, Chicago, submitted to WHO by the Brominated Flame Retardant Industry Panel) as cited in WHO/IPCS (1995).
- Brandsma, S. H. , Leonards, P. E. G. , Koekkoek, J. C. , Samsonek, J. , & Puype, F. (2022). Migration of hazardous contaminants from WEEE contaminated polymeric toy material by mouthing. Chemosphere, 294, 133774. [DOI] [PubMed] [Google Scholar]
- Bredsdorff, L. , Olesen, P. T. , Beltoft, V. M. , Sharma, A. K. , Hansen, M. , Nørby, K. , Ravn‐Haren, G. , & Ekstrøm, J. (2023). Identifying and collecting relevant literature related to the toxicity of polybrominated diphenyl ethers (PBDEs), tetrabromobisphenol A (TBBPA) and brominated phenols. EFSA Supporting Publication 2023:EN‐8014, 201 pp.
- Brown, P. R. , Gillera, S. E. , Fenton, S. E. , & Yao, H. H. (2020). Developmental exposure to tetrabromobisphenol A has minimal impact on male rat reproductive health. Reproductive Toxicology, 95, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu, D. , Zhuang, H. , Yanga, G. , & Ping, X. (2015). A real‐time immuno‐PCR assay for the detection of tetrabromobisphenol A. Analytical Methods, 7, 99–106. [Google Scholar]
- Butt, C. M. , Wang, D. , & Stapleton, H. M. (2011). Halogenated phenolic contaminants inhibit the in vitro activity of the thyroid‐regulating deiodinases in human liver. Toxicological Sciences, 124, 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon, R. E. , Trexler, A. W. , Knudsen, G. A. , Evans, R. A. , & Birnbaum, L. S. (2019). Tetrabromobisphenol A (TBBPA) alters ABC transport at the blood‐brain barrier. Toxicological Sciences, 169, 475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, H. , Wang, F. , Liang, Y. , Wang, H. , Zhang, A. , & Song, M. (2017). Experimental and computational insights on the recognition mechanism between the estrogen receptor α with bisphenol compounds. Archives of Toxicology, 91, 3897–3912. [DOI] [PubMed] [Google Scholar]
- Carignan, C. C. , Abdallah, M. A. , Wu, N. , Heiger‐Bernays, W. , McClean, M. D. , Harrad, S. , & Webster, T. F. (2012). Predictors of tetrabromobisphenol‐A (TBBP‐A) and hexabromocyclododecanes (HBCD) in Milk from Boston mothers. Environmental Science and Technology, 46, 12146–12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou, R. , Antignac, J. P. , Zalko, D. , Berrebi, A. , Cravedi, J. P. , Maume, D. , Marchand, P. , Monteau, F. , Riu, A. , Andre, F. , & Le Bizec, B. (2008). Exposure assessment of French women and their newborns to tetrabromobisphenol‐A: Occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere, 73, 1036–1041. [DOI] [PubMed] [Google Scholar]
- Casatta, N. , Mascolo, G. , Roscioli, C. , & Viganò, L. (2015). Tracing endocrine disrupting chemicals in a coastal lagoon (Sacca di Goro, Italy): Sediment contamination and bioaccumulation in Manila clams. Science of the Total Environment, 511, 214–222. [DOI] [PubMed] [Google Scholar]
- Cato, A. , Celada, L. , Kibakaya, E. C. , Simmons, N. , & Whalen, M. M. (2014). Brominated flame retardants, tetrabromobisphenol A and hexabromocyclododecane, activate mitogen‐activated protein kinases (MAPKs) in human natural killer cells. Cell Biology and Toxicology, 30(6), 345–360. 10.1007/s10565-014-9289-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavagnaro, J. , & Cortina, T. A. (1984). In vitro sister chromatid exchange in Chinese hamster ovary cells with GLCC 785‐104C (Final report. Report to Great Lakes Chemical Corporation, West Lafayette, IN, submitted to WHO by the Brominated Flame Retardant Industry Panel). As cited in WHO/IPCS (1995). Hazleton Biotechnologies Corporation. [Google Scholar]
- Cavagnaro, J. , & Sernau, R. C. (1984). Unscheduled DNA synthesis rat hepatocytes assay, GLCC 785‐104C (Final report. Report to Great Lakes Chemical Corporation, West Lafayette, IN, submitted to WHO by the Brominated Flame Retardant Industry Panel). As cited in WHO/IPCS (1995). Hazleton Biotechnologies Corporation. [Google Scholar]
- Chao, J. , Zeng, L. , Li, R. , & Zhou, Y. (2021). Molecularly imprinted polymer‐capped wrinkled silica‐quantum dot hybrid particles for fluorescent determination of tetra bromo bisphenol A. Microchimica Acta, 188, 126. [DOI] [PubMed] [Google Scholar]
- Cheng, V. , & Volz, D. C. (2022). Halogenated bisphenol A analogues induce PPARγ‐independent toxicity within human hepatocellular carcinoma cells. Current Research in Toxicology, 3, 100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessa, G. , Cossu, M. , Fiori, G. , Ledda, G. , Piras, P. , Sanna, A. , & Brambilla, G. (2019). Occurrence of hexabromocyclododecanes and tetrabromobisphenol A in fish and seafood from the sea of Sardinia – FAO 37.1.3 area: Their impact on human health within the European Union marine framework strategy directive. Chemosphere, 228, 249–257. [DOI] [PubMed] [Google Scholar]
- Cho, J. H. , Lee, S. , Jeon, H. , Kim, A. H. , Lee, W. , Lee, Y. , Yang, S. , Yun, J. , Jung, Y. S. , & Lee, J. (2020). Tetrabromobisphenol A‐induced apoptosis in neural stem cells through oxidative stress and mitochondrial dysfunction. Neurotoxicity Research, 38, 74–85. [DOI] [PubMed] [Google Scholar]
- Choi, E. M. , Park, S. Y. , Suh, K. S. , & Chon, S. (2023). Apigenin attenuates tetrabromobisphenol A‐induced cytotoxicity in neuronal SK‐N‐MC cells. Journal of environmental science and health. Part A, Toxic/Hazardous Substances & Environmental Engineering, 58, 152–162. [DOI] [PubMed] [Google Scholar]
- Choi, J. S. , Lee, Y. J. , Kim, T. H. , Lim, H. J. , Ahn, M. Y. , Kwack, S. J. , Kang, T. S. , Park, K. L. , Lee, J. , Kim, N. D. , & Jeong, T. C. (2011). Molecular mechanism of tetrabromobisphenol A (TBBPA)‐induced target organ toxicity in Sprague‐Dawley male rats. Toxicological Research, 27, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo, G. , Lee, I. S. , & Oh, J. E. (2019). Species and habitat‐dependent accumulation and biomagnification of brominated flame retardants and PBDE metabolites. Journal of Hazardous Materials, 371, 175–182. [DOI] [PubMed] [Google Scholar]
- CLH Report . (2020). Proposal for harmonised classification and labelling. Based on Regulation (EC) No 1272/2008 (CLP Regulation), Annex VI, Part 2. International Chemical Identification: 2,2′,6,6′‐tetrabromo‐4,4′‐isopropylidenediphenol; tetrabromobisphenol‐A (TBBPA). Norwegian Environment Agency P.O. Box 5672 Torgarden, 7485 Trondheim, Norway. Co‐submitter: The Danish Environmental Protection Agency.
- Colnot, T. , Kacew, S. , & Dekant, W. (2014). Mammalian toxicology and human exposures to the flame retardant 2,2′,6,6′‐tetrabromo‐4,4′‐isopropylidenediphenol (TBBPA): Implications for risk assessment. Archives of Toxicology, 88, 553–573. [DOI] [PubMed] [Google Scholar]
- Cope, R. B. , Kacew, S. , & Dourson, M. (2015). A reproductive, developmental and neurobehavioral study following oral exposure of tetrabromobisphenol A on Sprague‐Dawley rats. Toxicology, 329, 49–59. [DOI] [PubMed] [Google Scholar]
- Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment . (2004). COT statement on tetrabromobisphenol A‐ Review of toxicological data. COT statement 2004/02 July 2004.
- COT (Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment) . (2020). Addendum to the Overarching Statement on the potential risks from contaminants in the diet of infants aged 0 to 12 months and children aged 1 to 5 years . https://cot.food.gov.uk/2020‐StatementsandPositionpapers#addendum‐to‐the‐overarching‐statement‐on‐contaminants‐in‐the‐diet‐of‐children‐2020
- Covaci, A. , Voorspoels, S. , Abdallah, M. A. E. , Geens, T. , Harrad, S. , & Law, R. J. (2009). Analytical and environmental aspects of the flame retardant tetrabromobisphenol‐A and its derivatives. Journal of Chromatography A, 1216, 346–363. [DOI] [PubMed] [Google Scholar]
- Cunha, S. C. , Oliveira, C. , & Fernandes, J. O. (2017). Development of QuEChERS‐based extraction and liquid chromatography‐tandem mass spectrometry method for simultaneous quantification of bisphenol A and tetrabromobisphenol A in seafood: Fish, bivalves, and seaweeds. Analytical and Bioanalytical Chemistry, 409, 151–160. [DOI] [PubMed] [Google Scholar]
- Dankers, A. C. , Roelofs, M. J. , Piersma, A. H. , Sweep, F. C. , Russel, F. G. , van den Berg, M. , van Duursen, M. B. , & Masereeuw, R. (2013). Endocrine disruptors differentially target ATP‐binding cassette transporters in the blood‐testis barrier and affect Leydig cell testosterone secretion in vitro. Toxicological Sciences, 136, 382–391. [DOI] [PubMed] [Google Scholar]
- de Winter‐Sorkina, R. , Bakker, M. I. , van Donkersgoed, G. , & van Klaveren, J. D. (2003). Dietary intake of brominated flame retardants by the Dutch population . RIVM report 310305001/2003. RIKILT report number 2003.019. https://www.rivm.nl/bibliotheek/rapporten/310305001.pdf
- de Wit, C. A. , Kierkegaard, A. , Ricklund, N. , & Sellström, U. (2010). Emerging brominated flame retardants in the environment. Brominated Flame Retardants, 241–286. In: Eljarrat, E., Barceló, D. (eds) Brominated Flame Retardants. The Handbook of Environmental Chemistry, vol 16. Springer, Berlin, Heidelberg. 10.1007/698_2010_73 [DOI] [Google Scholar]
- Di Napoli‐Davis, G. , & Owens, J. E. (2013). Quantitation of tetrabromobisphenol‐A from dust sampled on consumer electronics by dispersed liquid‐liquid microextraction. Environmental Pollution, 180, 274–280. [DOI] [PubMed] [Google Scholar]
- Diamandakis, D. , Salińska, E. , Zieminska, E. , & Lazarewicz, J. (2015). Tetrabromobisphenol‐A modulates activity of NMDA receptors in isolated fractions of rat brain cortical membranes and depolarizes cultured neurons. Toxicology Letters, 238, S281. [Google Scholar]
- Diamandakis, D. , Zieminska, E. , Siwiec, M. , Tokarski, K. , Salinska, E. , Lenart, J. , Hess, G. , & Lazarewicz, J. W. (2019). Tetrabromobisphenol A‐induced depolarization of rat cerebellar granule cells: Ex vivo and in vitro studies. Chemosphere, 223, 64–73. [DOI] [PubMed] [Google Scholar]
- Dirtu, A. C. , Jaspers, V. L. B. , Cernat, R. , Neels, H. , & Covaci, A. (2010). Distribution of PCBs, their hydroxylated metabolites and other phenolic contaminants in human serum from two European countries. Environmental Science and Technology, 44, 2876–2883. [DOI] [PubMed] [Google Scholar]
- Dirtu, A. C. , Roosens, L. , Geens, T. , Gheorghe, A. , Neels, H. , & Covaci, A. (2008). Simultaneous determination of bisphenol A, triclosa, and tetrabromobisphenol A in human serum using solidphase extraction and gas chromatography‐electron capture negative‐ionization mass spectrometry. Analytical and Bioanalytical Chemistry, 391, 1175–1181. [DOI] [PubMed] [Google Scholar]
- Dodson, R. E. , Perovich, L. J. , Covaci, A. , Van den Eede, N. , Ionas, A. C. , Dirtu, A. C. , Brody, J. G. , & Rudel, R. A. (2012). After the PBDE phase‐out: A broad suite of flame retardants in repeat house dust samples from California. Environmental Science and Technology, 46, 13056–13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorosh, A. , Děd, L. , Elzeinová, F. , & Pěknicová, J. (2011). Assessing oestrogenic effects of brominated flame retardants hexabromocyclododecane and tetrabromobisphenol A on MCF‐7 cells. Folia Biologica, 57, 35–39. [PubMed] [Google Scholar]
- Dubaisi, S. , Caruso, J. A. , Gaedigk, R. , Vyhlidal, C. A. , Smith, P. C. , Hines, R. N. , Kocarek, T. A. , & Runge‐Morris, M. (2019). Developmental expression of the cytosolic sulfotransferases in human liver. Drug Metabolism and Disposition, 47(6), 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour, P. , Pirard, C. , & Charlier, C. (2017). Determination of phenolic organohalogens in human serum from a Belgian population and assessment of parameters affecting the human contamination. Science of the Total Environment, 599, 1856–1866. [DOI] [PubMed] [Google Scholar]
- Dunnick, J. K. , Morgan, D. L. , Elmore, S. A. , Gerrish, K. , Pandiri, A. , Ton, T. V. , Shockley, K. R. , & Merrick, B. A. (2017). Tetrabromobisphenol A activates the hepatic interferon pathway in rats. Toxicology Letters, 266, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnick, J. K. , Sanders, J. M. , Kissling, G. E. , Johnson, C. L. , Boyle, M. H. , & Elmore, S. A. (2015). Environmental chemical exposure may contribute to uterine cancer development: Studies with tetrabromobisphenol A. Toxicologic Pathology, 43, 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorakova, D. , Pulkrabova, J. , Gramblicka, T. , Polachova, A. , Buresova, M. , López, M. E. , Castaño, A. , Nübler, S. , Haji‐Abbas‐Zarrabi, K. , Klausner, N. , Göen, T. , Mol, H. , Koch, H. M. , Vaccher, V. , Antignac, J. P. , Haug, L. S. , Vorkamp, K. , & Hajslova, J. (2021). Interlaboratory comparison investigations (ICIs) and external quality assurance schemes (EQUASs) for flame retardant analysis in biological matrices: Results from the HBM4EU project. Environmental Research, 202, 111705. [DOI] [PubMed] [Google Scholar]
- ECB (European Chemicals Bureau) . (2006). European Commission – Joint Research Centre Institute for Health and Consumer Protection . European Union risk assessment report. 2,2′,6,6′‐tetrabromo‐4,4′‐isopropylidenediphenol, (tetrabromobisphenol‐A or TBBP‐A). Part II – human health. CAS No: 79‐94‐7 EINECS No: 201‐236‐9. Series: 4th Priority List Volume: 63.
- ECHA (European Chemicals Agency) . (2012). Chapter R.8: characterisation of dose [concentration]‐response for human health. In Guidance on information requirements and chemical safety assessment, 186 pp. [Google Scholar]
- ECHA (European Chemicals Agency) . (2013). Registration dossier for 1,1'‐(isopropylidene)bis[3,5‐dibromo‐4‐(2,3‐dibromopropoxy)benzene], EC number: 244‐617‐5, CAS number: 21850‐44‐2. Last modified: 30 March 2022. Available at: https://echa.europa.eu/sv/registration‐dossier/‐/registereddossier/13876/1/1
- EFSA (European Food Safety Authority) . (2010a). Standard sample description for food and feed. EFSA Journal, 8(1), 1457. 10.2903/j.efsa.2010.1457 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2010b). Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal, 8(3), 1557. 10.2903/j.efsa.2010.1557 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2011a). Report on the development of a food classification and description system for exposure assessment and guidance on its implementation and use. EFSA Journal, 9(12), 2489. 10.2903/j.efsa.2011.2489 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2011b). The food classification and description system FoodEx 2 (draft‐revision 1). EFSA Supporting Publications, 8(12), EN‐215. 10.2903/sp.efsa.2011.EN-215 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2013). Standard sample description ver. 2.0. EFSA Journal, 11(10), 3424. 10.2903/j.efsa.2013.3424 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2015). The food classification and description system FoodEx2 (revision 2). EFSA Supporting Publication, 12(5), EN‐804. 10.2903/sp.efsa.2015.EN-804 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Arcella, D. , Ioannidou, S. , & Sousa, R. (2018). Internal report on the harmonisation of dilution factors to be used in the assessment of dietary exposure . 10.5281/zenodo.1256085 [DOI]
- EFSA (European Food Safety Authority) , Dujardin, B. , & Kirwan, L. (2019). Annex A to the technical report on the raw primary commodity (RPC) model – Input data [data set]. Zenodo. 10.5281/zenodo.2537955 [DOI]
- EFSA (European Food Safety Authority) , Arcella, D. , Cascio, C. , Dujardin, B. , Gergelová, P. , Gómez Ruiz, J. A. , Zs, H. , Ioannidou, S. , Medina Pastor, P. , & Riolo, F. (2021). Technical report on handling occurrence data for dietary exposure assessments. EFSA Supporting Publication, 18(12), EN‐7082. 10.2903/sp.efsa.2021.EN-7082 [DOI] [Google Scholar]
- EFSA CEP Panel (EFSA Panel on Food Contact Materials, Enzymes and Processing Aids) , Lambré, C. , Barat Baviera, J. M. , Bolognesi, C. , Chesson, A. , Cocconcelli, P. S. , Crebelli, R. , Gott, D. M. , Grob, K. , Lampi, E. , Mengelers, M. , Mortensen, A. , Rivière, G. , Silano, V. , Steffensen, I.‐L. , Tlustos, C. , Vernis, L. , Zorn, H. , Batke, M. , … Van Loveren, H. (2023). Scientific opinion on the re‐evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA Journal, 21(4), 6857. 10.2903/j.efsa.2023.6857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) . (2011a). Scientific Opinion on hexabromocyclododecanes (HBCDDs) in food. EFSA Journal, 9(7), 2296. 10.2903/j.efsa.2011.2296 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) . (2011b). Scientific Opinion on polybrominated diphenyl ethers (PBDEs) in food. EFSA Journal, 9(5), 2156. 10.2903/j.efsa.2011.2156 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) . (2011c). Scientific Opinion on tetrabromobisphenol A (TBBPA) in food. EFSA Journal, 9(12), 2477. 10.2903/j.efsa.2011.2477 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) . (2012a). Scientific opinion on brominated flame retardants (BFRs) in food: Brominated phenols and their derivatives. EFSA Journal, 10(4), 2634. 10.2903/j.efsa.2012.2634 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) . (2012b). Scientific opinion on emerging and novel brominated flame retardants (BFRs) in food. EFSA Journal, 10(10), 2908. 133 pp. 10.2903/j.efsa.2012.2908 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , Schrenk, D. , Bignami, M. , Bodin, L. , Chipman, J. K. , del Mazo, J. , Grasl‐Kraupp, B. , Hogstrand, C. , Hoogenboom, L. , Leblanc, J.‐C. , Nebbia, C. S. , Nielsen, E. , Ntzani, E. , Petersen, A. , Sand, S. , Schwerdtle, T. , Wallace, H. , Benford, D. , Fuerst, P. , … Vleminckx, C. (2021). Scientific Opinion on theupdate of the risk assessment of hexabromocyclododecanes (HBCDDs) in food. EFSA Journal, 19(3), 6421. 10.2903/j.efsa.2021.6421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) . (2024). Update of the risk assessment of polybrominated diphenyl ethers (PBDEs) in food. EFSA Journal, 22(1), e8497. 10.2903/j.efsa.2024.8497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , Hernandez‐Jerez, A. F. , Adriaanse, P. , Aldrich, A. , Berny, P. , Coja, T. , Duquesne, S. , Focks, A. , Millet, M. , Pelkonen, O. , Pieper, S. , Tiktak, A. , Topping, C. J. , Widenfalk, A. , Wilks, M. , Wolterink, G. , Angeli, K. , Recordati, C. , Van Duursen, M. , … Marinovich, M. (2023). Scientific Opinion on the development of adverse outcome pathways relevant for the identification of substances having endocrine disruption properties. Uterine adenocarcinoma as adverse outcome. EFSA Journal, 21(2), 7744. 47 pp. 10.2903/j.efsa.2023.7744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee . (2011). Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal, 9(9), 2379. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee . (2012). Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal, 10(3), 2579. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee . (2017). Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age. EFSA Journal, 15(5), 4849. 10.2903/j.efsa.2017.4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee . (2018). Guidance on uncertainty analysis in scientific assessments. EFSA Journal, 16(1), 5123. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , More, S. J. , Bampidis, V. , Benford, D. , Bennekou, S. H. , Bragard, C. , Halldorsson, T. I. , Hernandez‐Jerez, A. F. , Koutsoumanis, K. , Naegeli, H. , Schlatter, J. R. , Silano, V. , Nielsen, S. S. , Schrenk, D. , Turck, D. , Younes, M. , Benfenati, E. , Castle, L. , Cedergreen, N. , … Hogstrand, C. (2019). Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA Journal, 17(3), 5634. 10.2903/j.efsa.2019.5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee . (2022). Guidance on the use of the benchmark dose approach in risk assessment. EFSA Journal, 20(10), 7584. 10.2903/j.efsa.2022.7584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehtyl Corporation . (1981). Salmonella/microsomal assay‐tetrabromobisphenol A EPA/OTS doc number 878216192. As cited in ECB (2006).
- Eriksson, J. , Rahm, S. , Green, N. , Bergman, Å. , & Jakobsson, E. (2004). Photochemical transformations of tetrabromobisphenol A and related phenols in water. Chemosphere, 54, 117–126. [DOI] [PubMed] [Google Scholar]
- Eriksson, P. , Jakobsson, E. , & Fredriksson, A. (2001). Brominated flame retardants: A novel class of developmental neurotoxicants in our environment? Environmental Health Perspectives, 109, 903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermak, G. , & Davies, K. J. (2002). Calcium and oxidative stress: From cell signaling to cell death. Molecular Immunology, 38, 713–721. [DOI] [PubMed] [Google Scholar]
- Eulaers, I. , Jaspers, V. L. , Pinxten, R. , Covaci, A. , & Eens, M. (2014). Legacy and current‐use brominated flame retardants in the Barn Owl. Science of the Total Environment, 472, 454–462. [DOI] [PubMed] [Google Scholar]
- Fatunsin, O. T. , Oluseyi, T. O. , Drage, D. , Abdallah, M. A. , Turner, A. , & Harrad, S. (2020). Children's exposure to hazardous brominated flame retardants in plastic toys. Science of the Total Environment, 720, 137623. [DOI] [PubMed] [Google Scholar]
- Feng, A. H. , Chen, S. J. , Chen, M. Y. , He, M. J. , Luo, X. J. , & Mai, B. X. (2012). Hexabromocyclododecane (HBCD) and tetrabromobisphenol A (TBBPA) in riverine and estuarine sediments of the Pearl River Delta in southern China, with emphasis on spatial variability in diastereoisomer‐ and enantiomer‐specific distribution of HBCD. Marine Pollution Bulletin, 64, 919–925. [DOI] [PubMed] [Google Scholar]
- Freitas, J. , Cano, P. , Craig‐Veit, C. , Goodson, M. L. , Furlow, J. D. , & Murk, A. J. (2011). Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay. Toxicology in Vitro, 25, 257–266. [DOI] [PubMed] [Google Scholar]
- Fromme, H. , Hilger, B. , Kopp, E. , Miserok, M. , & Völkel, W. (2014). Polybrominated diphenyl ethers (PBDEs), hexabromocyclododecane (HBCD) and "novel" brominated flame retardants in house dust in Germany. Environment International, 64, 61–68. [DOI] [PubMed] [Google Scholar]
- Fujii, Y. , Harada, K. H. , Hitomi, T. , Kobayashi, H. , Koizumi, A. , & Haraguchi, K. (2014). Temporal trend and age‐dependent serum concentration of phenolic organohalogen contaminants in Japanese men during 1989–2010. Environmental Pollution, 185, 228–233. [DOI] [PubMed] [Google Scholar]
- Fujii, Y. , Kato, Y. , Masuda, N. , Harada, K. H. , Koizumi, A. , & Haraguchi, K. (2018). Contamination trends and factors affecting the transfer of hexabromocyclododecane diastereomers, tetrabromobisphenol A, and 2,4,6‐tribromophenol to breast milk in Japan. Environmental Pollution, 237, 936–943. [DOI] [PubMed] [Google Scholar]
- Fujii, Y. , Nishimura, E. , Kato, Y. , Harada, K. H. , Koizumi, A. , & Haraguchi, K. (2014). Dietary exposure to phenolic and methoxylated organohalogen contaminants in relation to their concentrations in breast milk and serum in Japan. Environment International, 63, 19–25. [DOI] [PubMed] [Google Scholar]
- Fujimoto, H. , Woo, G. H. , Morita, R. , Itahashi, M. , Akane, H. , Nishikawa, A. , & Shibutani, M. (2013). Increased cellular distribution of vimentin and ret in the cingulum of rat offspring after developmental exposure to decabromodiphenyl ether or 1,2,5,6,9,10‐hexabromocyclododecane. Journal of Toxicologic Pathology, 26, 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, N. , Ito, Y. , Yamaguchi, M. , Mitumori, K. , Koizumi, M. , Hasegawa, R. , Kamata, E. , & Ema, M. (2004). Unexpected nephrotoxicity induced by tetrabromobisphenol A in newborn rats. Toxicology Letters, 150, 145–155. [DOI] [PubMed] [Google Scholar]
- Gallistl, C. , Lok, B. , Schlienz, A. , & Vetter, W. (2017). Polyhalogenated compounds (chlorinated paraffins, novel and classic flame retardants, POPs) in dishcloths after their regular use in households. Science of the Total Environment, 595, 303–314. [DOI] [PubMed] [Google Scholar]
- Ganci, A. P. , Vane, C. H. , Abdallah, M. A. , Moehring, T. , & Harrad, S. (2019). Legacy PBDEs and NBFRs in sediments of the tidal river Thames using liquid chromatography coupled to a high resolution accurate mass Orbitrap mass spectrometer. Science of the Total Environment, 658, 1355–1366. [DOI] [PubMed] [Google Scholar]
- Garcia Lopez, M. , Driffield, M. , Fernandes, A. R. , Smith, F. , Tarbin, J. , Lloyd, A. S. , Christy, J. , Holland, M. , Steel, Z. , & Tlustos, C. (2018). Occurrence of polybrominated diphenylethers, hexabromocyclododecanes, bromophenols and tetrabromobisphenols A and S in Irish foods. Chemosphere, 197, 709–715. [DOI] [PubMed] [Google Scholar]
- Garcia, T. X. , & Hofmann, M. C. (2013). NOTCH signaling in Sertoli cells regulates gonocyte fate. Cell Cycle, 12, 2538–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier, L. T. , Laurich, B. , Hebert, C. E. , Drake, C. , & Letcher, R. J. (2019). Tetrabromobisphenol‐A‐Bis(dibromopropyl ether) flame retardant in eggs, regurgitates, and feces of herring gulls from multiple North American Great Lakes locations. Environmental Science & Technology, 53, 9564–9571. [DOI] [PubMed] [Google Scholar]
- Gebbink, W. A. , van der Lee, M. K. , Peters, R. J. B. , Traag, W. A. , Dam, G. T. , Hoogenboom, R. , & van Leeuwen, S. P. J. (2019). Brominated flame retardants in animal derived foods in The Netherlands between 2009 and 2014. Chemosphere, 234, 171–178. [DOI] [PubMed] [Google Scholar]
- Gerecke, A. C. , Giger, W. , Hartmann, P. C. , Heeb, N. V. , Kohler, H.‐P. E. , Schmid, P. , Zennegg, M. , & Kohler, M. (2006). Anaerobic degradation of brominated flame retardants in sewage sludge. Chemosphere, 64, 311–317. [DOI] [PubMed] [Google Scholar]
- Geyer, H. J. , Schramm, K. W. , Feicht, E. A. , Fried, K. W. , Henkelmann, B. , Lenoir, D. , Darnerud, P. O. , Aune, M. , Schmid, P. , & McDonald, T. A. (2004). Terminal elimination half‐lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Extended abstract. Conference: Dioxin.
- Gil‐Solsona, R. , Castaño‐Ortiz, J. M. , Muñoz‐Mas, R. , Insa, S. , Farré, M. , Ospina‐Alvarez, N. , Santos, L. H. M. L. M. , García‐Pimentel, M. , Barceló, D. , & Rodriguez‐Mozaz, S. (2022). A holistic assessment of the sources, prevalence, and distribution of bisphenol A and analogues in water, sediments, biota and plastic litter of the Ebro Delta (Spain). Environmental Pollution, 314, 120310. [DOI] [PubMed] [Google Scholar]
- Gorowska‐Wojtowicz, E. , Duliban, M. , Kudrycka, M. , Dutka, P. , Pawlicki, P. , Milon, A. , Zarzycka, M. , Placha, W. , Kotula‐Balak, M. , Ptak, A. , Wolski, J. K. , & Bilinska, B. (2019). Leydig cell tumorigenesis – implication of G‐protein coupled membrane estrogen receptor, peroxisome proliferator‐activated receptor and xenoestrogen exposure. In vivo and in vitro appraisal. Tissue & Cell, 61, 51–60. [DOI] [PubMed] [Google Scholar]
- Gosavi, R. A. , Knudsen, G. A. , Birnbaum, L. S. , & Pedersen, L. C. (2013). Mimicking of estradiol binding by flame retardants and their metabolites: A crystallographic analysis. Environmental Health Perspectives, 121, 1194–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, S. Y. , Ekpeghere, K. I. , Ki, H. Y. , Lee, I. S. , Kim, D. H. , Choo, G. , & Oh, J. E. (2017). Brominated flame retardants in marine environment focused on aquaculture area: Occurrence, source and bioaccumulation. Science of the Total Environment, 601, 1182–1191. [DOI] [PubMed] [Google Scholar]
- Gu, Y. X. , Liang, X. X. , Yin, N. Y. , Yang, Y. , Wan, B. , Guo, L. H. , & Faiola, F. (2019). New insights into mechanism of bisphenol analogue neurotoxicity: Implications of inhibition of O‐GlcNAcase activity in PC12 cells. Archives of Toxicology, 93, 2661–2671. [DOI] [PubMed] [Google Scholar]
- Guan, G. , Su, H. , Wei, X. , Zheng, Y. , & Jin, X. (2021). The promotion of tetrabromobisphenol A exposure on Ishikawa cells proliferation and pivotal role of ubiquitin‐mediated I kappa B′ degradation. Ecotoxicology and Environmental Safety, 207, 111254. [DOI] [PubMed] [Google Scholar]
- Guerra, P. , Eljarrat, E. , & Barceló, D. (2011). Determination of halogenated flame retardants by liquid chromatography coupled to mass spectrometry. Trac‐Trends in Analytical Chemistry, 30, 842–855. [Google Scholar]
- Guo, J. , Min, C. G. , Zhang, K. Y. , Zhan, C. L. , Wang, Y. C. , Hou, S. K. , Ma, X. , & Lu, W. F. (2022). Tetrabromobisphenol exposure impairs bovine oocyte maturation by inducing mitochondrial dysfunction. Molecules, 27, 8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot, R. , Chatonnet, F. , Gillet, B. , Hughes, S. , & Flamant, F. (2014). Toxicogenomic analysis of the ability of brominated flame retardants TBBPA and BDE‐209 to disrupt thyroid hormone signaling in neural cells. Toxicology, 325, 125–132. [DOI] [PubMed] [Google Scholar]
- Guyton, K. Z. , Rusyn, I. , Chiu, W. A. , Corpet, D. E. , van den Berg, M. , Ross, M. K. , Christiani, D. C. , Beland, F. A. , & Smith, M. T. (2018). Application of the key characteristics of carcinogens in cancer hazard identification. Carcinogenesis, 39, 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmar, L. , Sjödin, A. , Höglund, P. , Thuresson, K. , Rylander, L. , & Bergman, Å. (2000). Biological halflives of polybrominated diphenyl ethers and tetrabromobisphenol A in exposed workers. Organohalogen Compounds, 47, 198–201. [Google Scholar]
- Hajeb, P. , Castano, A. , Cequier, E. , Covaci, A. , López, M. E. , Antuna, A. G. , Haug, L. S. , Henríquez‐Hernández, L. A. , Melymuk, L. , Luzardo, O. P. , & Thomsen, C. (2022). Critical review of analytical methods for the determination of flame retardants in human matrices. Analytica Chimica Acta, 1193, 338828. [DOI] [PubMed] [Google Scholar]
- Hakk, H. , Larsen, G. , Bergman, Å. , & Örn, U. (2000). Metabolism, excretion and distribution of the flame retardant tetrabromobisphenol‐A in conventional and bile‐duct cannulated rats. Xenobiotica, 30, 881–890. [DOI] [PubMed] [Google Scholar]
- Hall, S. M. , Coulter, S. J. , Knudsen, G. A. , Sanders, J. M. , & Birnbaum, L. S. (2017). Gene expression changes in immune response pathways following oral administration of tetrabromobisphenol A (TBBPA) in female Wistar Han rats. Toxicology Letters, 272, 68–74. 10.1016/j.toxlet.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, W. , Lei, L. , & Zhang, S. (2013). Adsorption of tetrabromobisphenol A on soils: Contribution of soil components and influence of soil properties. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 428, 60–64. [Google Scholar]
- Hanioka, N. , Isobe, T. , Saito, K. , Nagaoka, K. , Mori, Y. , Jinno, H. , Ohkawara, S. , & Tanaka‐Kagawa, T. (2024). Hepatic glucuronidation of tetrabromobisphenol A and tetrachlorobisphenol A: Interspecies differences in humans and laboratory animals and responsible UDP‐glucuronosyltransferase isoforms in humans. Archives of Toxicology, 5, 1–2. [DOI] [PubMed] [Google Scholar]
- Harrad, S. , & Abdallah, M. A. (2015). Concentrations of polybrominated diphenyl ethers, hexabromocyclododecanes and tetrabromobisphenol‐A in breast milk from United Kingdom women do not decrease over twelve months of lactation. Environmental Science and Technology, 49, 13899–13903. [DOI] [PubMed] [Google Scholar]
- Harrad, S. , & Abdallah, M. A. E. (2011). Brominated flame retardants in dust from UK cars – within‐vehicle spatial variability, evidence for degradation and exposure implications. Chemosphere, 82, 1240–1245. [DOI] [PubMed] [Google Scholar]
- Harvey, J. B. , Osborne, T. S. , Hong, H. H. , Bhusari, S. , Ton, T. V. , Pandiri, A. R. , Masinde, T. , Dunnick, J. , Peddada, S. , Elmore, S. , & Hoenerhoff, M. J. (2015). Uterine carcinomas in tetrabromobisphenol A‐exposed Wistar Han Rats Harbor increased Tp53 mutations and mimic high‐grade type I endometrial carcinomas in women. Toxicologic Pathology, 43, 1103–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, M. J. , Luo, X. J. , Yu, L. H. , Wu, J. P. , Chen, S. J. , & Mai, B. X. (2013). Diasteroisomer and enantiomer‐specific profiles of hexabromocyclododecane and tetrabromobisphenol A in an aquatic environment in a highly industrialized area, South China: Vertical profile, phase partition, and bioaccumulation. Environmental Pollution, 179, 105–110. [DOI] [PubMed] [Google Scholar]
- Health Canada . (2013). Screening assessment report of phenol, 4,4′‐(1‐methylethylidene) bis[2,6‐dibromo‐, (CAS RN. 79–94‐7); ethanol, 2,2′‐[(1‐ methylethylidene)bis[(2,6‐dibromo‐4,1‐phenylene)oxy]]bis, (CAS RN. 4162‐45‐2) and benzene, 1,1′‐(1‐methylethylidene)bis[3,5‐dibromo‐4‐(2‐propenyloxy)‐, (CAS RN. 25327‐89‐3) . https://www.ec.gc.ca/ese‐ees/BEE093E4‐8387‐4790‐A9CD‐C753B3E5BFAD/FSAR_TBBPA_EN.pdf
- Helleday, T. , Tuominen, K. , Bergman, A. , & Jenseen, D. (1999). Brominated flame retardants induce intragenic recombination in mammalian cells. Mutation Research, 439, 137–147. As cited in ECB (2006). [DOI] [PubMed] [Google Scholar]
- Hendriks, H. S. , Koolen, L. A. , Dingemans, M. M. , Viberg, H. , Lee, I. , Leonards, P. E. , Ramakers, G. M. , & Westerink, R. H. (2015). Effects of neonatal exposure to the flame retardant tetrabromobisphenol‐A, aluminum diethylphosphinate or zinc stannate on long‐term potentiation and synaptic protein levels in mice. Archives of Toxicology, 89, 2345–2354. [DOI] [PubMed] [Google Scholar]
- Hendriks, H. S. , Meijer, M. , Muilwijk, M. , van den Berg, M. , & Westerink, R. H. (2014). A comparison of the in vitro cyto‐ and neurotoxicity of brominated and halogen‐free flame retardants: Prioritization in search for safe(r) alternatives. Archives of Toxicology, 88, 857–869. [DOI] [PubMed] [Google Scholar]
- Hendriks, H. S. , van Kleef, R. G. , van den Berg, M. , & Westerink, R. H. (2012). Multiple novel modes of action involved in the in vitro neurotoxic effects of tetrabromobisphenol‐A. Toxicological Sciences, 128, 235–246. [DOI] [PubMed] [Google Scholar]
- Hendriks, H. S. , van Kleef, R. G. , & Westerink, R. H. (2012). Modulation of human α4β2 nicotinic acetylcholine receptors by brominated and halogen‐free flame retardants as a measure for in vitro neurotoxicity. Toxicology Letters, 213, 266–274. [DOI] [PubMed] [Google Scholar]
- Hloušková, V. , Lanková, D. , Kalachová, K. , Hrádková, P. , Poustka, J. , Hajšlová, J. , & Pulkrabová, J. (2013). Occurrence of brominated flame retardants and perfluoroalkyl substances in fish from the Czech aquatic ecosystem. The Science of the Total Environment, 461‐462, 88–98. [DOI] [PubMed] [Google Scholar]
- Hloušková, V. , Lanková, D. , Kalachová, K. , Hrádková, P. , Poustka, J. , Hajšlová, J. , & Pulkrabová, J. (2014). Brominated flame retardants and perfluoroalkyl substances in sediments from the Czech aquatic ecosystem. Science of the Total Environment, 470–471, 407–416. [DOI] [PubMed] [Google Scholar]
- Ho, K. L. , Yuen, K. K. , Yau, M. S. , Murphy, M. B. , Wan, Y. , Fong, B. M. , Tam, S. , Giesy, J. P. , Leung, K. S. , & Lam, M. H. (2017). Glucuronide and sulfate conjugates of tetrabromobisphenol A (TBBPA): Chemical synthesis and correlation between their urinary levels and plasma TBBPA content in voluntary human donors. Environment International, 98, 46–53. [DOI] [PubMed] [Google Scholar]
- Hoeting, J. A. , Madigan, D. , Raftery, A. E. , & Volinsky, C. T. (1999). Bayesian model averaging: a tutorial. Statistical Science, 14, 382–417. [Google Scholar]
- Hoffmann, M. , Fiedor, E. , & Ptak, A. (2017). Bisphenol A and its derivatives tetrabromobisphenol A and tetrachlorobisphenol A induce apelin expression and secretion in ovarian cancer cells through a peroxisome proliferator‐activated receptor gamma‐dependent mechanism. Toxicology Letters, 269, 15–22. [DOI] [PubMed] [Google Scholar]
- Hoffmann, M. , Gogola, J. , Kotula‐Balak, M. , & Ptak, A. (2017). Stimulation of ovarian cell proliferation by tetrabromobisphenol A but not tetrachlorobisphenol A through G protein‐coupled receptor 30. Toxicology in Vitro, 45, 54–59. [DOI] [PubMed] [Google Scholar]
- Hoffmann, M. , Rak, A. , & Ptak, A. (2018). Bisphenol A and its derivatives decrease expression of chemerin, which reverses its stimulatory action in ovarian cancer cells. Toxicology Letters, 291, 61–69. [DOI] [PubMed] [Google Scholar]
- Honkisz, E. , & Wojtowicz, A. K. (2015). Modulation of estradiol synthesis and aromatase activity in human choriocarcinoma JEG‐3 cells exposed to tetrabromobisphenol A. Toxicology in Vitro, 29, 44–50. [DOI] [PubMed] [Google Scholar]
- Hu, C. , Xu, Y. , Wang, M. , Cui, S. , Zhang, H. , & Lu, L. (2023). Bisphenol analogues induce thyroid dysfunction via the disruption of the thyroid hormone synthesis pathway. Science of the Total Environment, 900, 165711. [DOI] [PubMed] [Google Scholar]
- Hu, Y. , Pei, N. , Sun, Y. , Xu, X. , Zhang, Z. , Li, H. , & Mai, B. (2019). Halogenated flame retardants in mangrove sediments from the Pearl River Estuary, South China: Comparison with historical data and correlation with microbial community. Chemosphere, 227, 315–322. [DOI] [PubMed] [Google Scholar]
- Huang, D. Y. , Zhao, H. Q. , Liu, C. P. , & Sun, C. X. (2014). Characteristics, sources, and transport of tetrabromobisphenol A and bisphenol A in soils from a typical e‐waste recycling area in South China. Environmental Science and Pollution Research International, 21, 5818–5826. [DOI] [PubMed] [Google Scholar]
- Huang, G. Y. , Ying, G. G. , Liang, Y. Q. , Zhao, J. L. , Yang, B. , Liu, S. , & Liu, Y. S. (2013). Hormonal effects of tetrabromobisphenol A using a combination of in vitro and in vivo assays. Comparative Biochemistry and Physiology, Part C: Toxicology & Pharmacology, 157, 344–351. [DOI] [PubMed] [Google Scholar]
- Huang, H. , Liang, J. , Tang, P. , Yu, C. , Fan, H. , Liao, Q. , Long, J. , Pan, D. , Zeng, X. , Liu, S. , & Huang, D. (2022). Associations of bisphenol exposure with thyroid hormones in pregnant women: A prospective birth cohort study in China. Environmental Science and Pollution Research, 29, 87170–87183. [DOI] [PubMed] [Google Scholar]
- Huang, M. , Li, J. , Xiao, Z. , & Shi, Z. (2020). Tetrabromobisphenol A and hexabromocyclododecane isomers in breast milk from the general population in Beijing, China: Contamination levels, temporal trends, nursing infant's daily intake, and risk assessment. Chemosphere, 244, 125524. [DOI] [PubMed] [Google Scholar]
- Huang, S. , Wang, D. , Qi, Z. , Long, C. , Li, G. , & Yu, Y. (2023). A large‐scale nationwide study of urinary phenols in the Chinese population. Science of the Total Environment, 894, 164850. [DOI] [PubMed] [Google Scholar]
- Hulin, M. , Bemrah, N. , Nougadère, A. , Volatier, J. L. , Sirot, V. , & Leblanc, J. C. (2014). Assessment of infant exposure to food chemicals: The French Total Diet Study design. Food Additives and Contaminants, 31, 1226–1239. [DOI] [PubMed] [Google Scholar]
- Hurd, T. , & Whalen, M. M. (2011). Tetrabromobisphenol A decreases cell‐surface proteins involved in human natural killer (NK) cell‐dependent target cell lysis. Journal of Immunotoxicology, 8(3), 219–227. 10.3109/1547691X.2011.580437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iakovleva, I. , Begum, A. , Brännström, K. , Wijsekera, A. , Nilsson, L. , Zhang, J. , Andersson, P. L. , Sauer‐Eriksson, A. E. , & Olofsson, A. (2016). Tetrabromobisphenol A is an efficient stabilizer of the transthyretin tetramer. PLoS One, 11, 0153529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) . (2018). Some industrial chemicals. In IARC monographs on the evaluation of carcinogenic risks to humans, 115, 247–289. World Health Organization. https://publications.iarc.fr/Book‐And‐Report‐Series/Iarc‐Monographs‐On‐The‐Identification‐Of‐Carcinogenic‐Hazards‐To‐Humans/Some‐Industrial‐Chemicals‐2018 [Google Scholar]
- Ilozumba, M. N. , Shelver, W. L. , Hong, C. C. , Ambrosone, C. B. , & Cheng, T. Y. (2022). Urinary concentrations of Triclosan, bisphenol A, and brominated flame retardants and the Association of Triclosan with demographic characteristics and body fatness among women with newly diagnosed breast cancer. International Journal of Environmental Research and Public Health, 19, 4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai, T. , Takami, S. , Cho, Y.‐M. , Hirose, M. , & Nishikawa, A. (2009). Modifying effects of prepubertal exposure to potassium perchlorate and tetrabromobisphenol A on susceptibility to N‐bis(2‐hydroxypropyl)nitrosamine‐ and 7,12‐dimethylbenz(a)anthracene‐induced carcinogenesis in rats. Toxicology Letters, 185, 160–167. [DOI] [PubMed] [Google Scholar]
- Inthavong, C. , Hommet, F. , Bordet, F. , Rigourd, V. , Guérin, T. , & Dragacci, S. (2017). Simultaneous liquid chromatography etandem mass spectrometry analysis of brominated flame retardants (tetrabromobisphenol A and hexabromocyclododecane diastereoisomers) in French breast milk. Chemosphere, 186, 762–769. [DOI] [PubMed] [Google Scholar]
- IPCS/WHO (International Programme on Chemical Safety/World Health Organisation) . (1995). 2,2’,6,6’‐tetrabromo‐4,4’‐isopropylidenediphenol, (tetrabromobisphenol‐A or TBBP‐A). Part II –human health. https://www.inchem.org/documents/ehc/ehc/ehc172.htm
- Jacobs, S. , Sioen, I. , Pieniak, Z. , De Henauw, S. , Maulvault, A. L. , Reuver, M. , Fait, G. , Cano‐Sancho, G. , & Verbeke, W. (2015). Consumers' health risk‐benefit perception of seafood and attitude toward the marine environment: Insights from five European countries. Environmental Research Part B, 143, 11–19. [DOI] [PubMed] [Google Scholar]
- Jakobsson, C. , Thuresson, K. , Rylander, L. , Sjödin, A. , Hagmar, L. , & Bergman, Å. (2002). Exposure to polybrominated diphenyl ethers and tetrabromobisphenol A among computer technicians. Chemosphere, 46, 709–716. [DOI] [PubMed] [Google Scholar]
- Jeon, J. W. , Kim, C. S. , Kim, H. J. , Lee, C. H. , Hwang, S. M. , & Choi, S. D. (2021). Spatial distribution, source identification, and anthropogenic effects of brominated flame retardants in nationwide soil collected from South Korea. Environmental Pollution, 272, 116026. [DOI] [PubMed] [Google Scholar]
- Jin, J. , Peng, H. , Wang, Y. , Yang, R. , & Cui, J. (2006). An enhanced LC/MS/MS method for the determination of tetrabromobisphenol‐A (TBBP‐A) in soil using the quattro premier mass spectrometer. Organohalogen Compounds, 68, 85–88. [Google Scholar]
- Kamiya, Y. , Otsuka, S. , Miura, T. , Takaku, H. , Yamada, R. , Nakazato, M. , Nakamura, H. , Mizuno, S. , Shono, F. , Funatsu, K. , & Yamazaki, H. (2019). Plasma and hepatic concentrations of chemicals after virtual oral administrations extrapolated using rat plasma data and simple physiologically based pharmacokinetic models. Chemical Research in Toxicology, 32, 211–218. [DOI] [PubMed] [Google Scholar]
- Kang, M. J. , Kim, J. H. , Shin, S. , Choi, J. H. , Lee, S. K. , Kim, H. S. , Kim, N. D. , Kang, G. W. , Jeong, H. G. , Kang, W. , & Chun, Y. J. (2009). Nephrotoxic potential and toxicokinetics of tetrabromobisphenol A in rat for risk assessment. Journal of Toxicology and Environmental Health, Part A, 72(21–22), 1439–1445. [DOI] [PubMed] [Google Scholar]
- Karlsson, S. A. , Haziri, K. , Hansson, E. , Kettunen, P. , & Westberg, L. (2015). Effects of sex and gonadectomy on social investigation and social recognition in mice. BMC Neuroscience, 16, 83. 10.1186/s12868-015-0221-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefeni, K. K. , Okonkwo, J. O. , Olukunle, O. I. , & Botha, B. M. (2011). Brominated flame retardants: Sources, distribution, exposure pathways, and toxicity. Environmental Reviews, 19, 238–253. [Google Scholar]
- Keller, A. S. , Raju, N. P. , Webster, T. F. , & Stapleton, H. M. (2014). Flame retardant applications in camping tents and potential exposure. Environmental Science & Technology Letters, 1, 152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiciński, M. , Viaene, M. K. , Den Hond, E. , Schoeters, G. , Covaci, A. , Dirtu, A. C. , Nelen, V. , Bruckers, L. , Croes, K. , Sioen, I. , Baeyens, W. , Van Larebeke, N. , & Nawrot, T. S. (2012). Neurobehavioral function and low‐level exposure to brominated flame retardants in adolescents: A cross‐sectional study. Environmental Health, 11, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, A. H. , Chun, H. J. , Lee, S. , Kim, H. S. , & Lee, J. (2017). High dose tetrabromobisphenol A impairs hippocampal neurogenesis and memory retention. Food and Chemical Toxicology, 106, 223–231. [DOI] [PubMed] [Google Scholar]
- Kim, B. , Colon, E. , Chawla, S. , Vandenberg, L. N. , & Suvorov, A. (2015). Endocrine disruptors alter social behaviours and indirectly influence social hierarchies via changes in body weight. Environmental Health, 14, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, U. J. , & Oh, J. E. (2014). Tetrabromobisphenol A and hexabromocyclododecane flame retardants in infant‐mother paired serum samples, and their relationships with thyroid hormones and environmental factors. Environmental Pollution, 184, 193–200. [DOI] [PubMed] [Google Scholar]
- Kitamura, S. , Suzuki, T. , Sanoh, S. , Kohta, R. , Jinno, N. , Sugihara, K. , Yoshihara, S. , Fujimoto, N. , Watanabe, H. , & Ohta, S. (2005). Comparative study of the endocrine‐disrupting activity of bisphenol A and 19 related compounds. Toxicological Sciences, 84, 249–259. [DOI] [PubMed] [Google Scholar]
- Klose, J. , Pahl, M. , Bartmann, K. , Bendt, F. , Blum, J. , Dolde, X. , Förster, N. , Holzer, A. K. , Hübenthal, U. , Keßel, H. E. , Koch, K. , Masjosthusmann, S. , Schneider, S. , Stürzl, L. C. , Woeste, S. , Rossi, A. , Covaci, A. , Behl, M. , Leist, M. , … Fritsche, E. (2022). Neurodevelopmental toxicity assessment of flame retardants using a human DNT in vitro testing battery. Cell Biology and Toxicology, 8, 781–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose, J. , Tigges, J. , Masjosthusmann, S. , Schmuck, K. , Bendt, F. , Hübenthal, U. , Petzsch, P. , Köhrer, K. , Koch, K. , & Fritsche, E. (2021). TBBPA targets converging key events of human oligodendrocyte development resulting in two novel AOPs. Altex‐Alternatives to Animal Experimentation, 38, 215–234. [DOI] [PubMed] [Google Scholar]
- Knudsen, G. A. , Hall, S. M. , Richards, A. C. , & Birnbaum, L. S. (2018). TBBPA disposition and kinetics in pregnant and nursing Wistar Han IGS rats. Chemosphere, 192, 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen, G. A. , Jacobs, L. M. , Kuester, R. K. , & Sipes, I. G. (2007). Absorption, distribution, metabolism and excretion of intravenously and orally administered tetrabromobisphenol A [2, 3‐dibromopropyl ether] in male Fischer‐344 rats. Toxicology, 237(1–3), 158–167. [DOI] [PubMed] [Google Scholar]
- Knudsen, G. A. , Sanders, J. M. , Sadik, A. M. , & Birnbaum, L. S. (2014). Disposition and kinetics of tetrabromobisphenol A in female Wistar Han rats. Toxicology Reports, 1, 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike, E. , Yanagisawa, R. , & Takano, H. (2013). Brominated flame retardants, hexabromocyclododecane and tetrabromobisphenol A, affect proinflammatory protein expression in human bronchial epithelial cells via disruption of intracellular signaling. Toxicology in Vitro, 32, 212–219. 10.1016/j.tiv.2015.12.013 [DOI] [PubMed] [Google Scholar]
- Kollitz, E. M. , De Carbonnel, L. , Stapleton, H. M. , & Lee Ferguson, P. (2018). The affinity of brominated phenolic compounds for human and zebrafish thyroid receptor β: Influence of chemical structure. Toxicological Sciences, 163, 226–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp, E. K. , Fromme, H. , & Völkel, W. (2012). Analysis of common and emerging brominated flame retardants in house dust using ultrasonic assisted solvent extraction and on‐line sample preparation via column switching with liquid chromatography‐mass spectrometry. Journal of Chromatography A, 1241, 28–36. [DOI] [PubMed] [Google Scholar]
- Kotthoff, M. , Rudel, H. , & Jurling, H. (2017). Detection of tetrabromobisphenol A and its mono‐ and dimethyl derivatives in fish, sediment and suspended particulate matter from European freshwaters and estuaries. Analytical and Bioanalytical Chemistry, 409, 3685–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivoshiev, B. V. , Dardenne, F. , Blust, R. , Covaci, A. , & Husson, S. J. (2015). Elucidating toxicological mechanisms of current flame retardants using a bacterial gene profiling assay. Toxicology in Vitro, 29, 2124–2132. [DOI] [PubMed] [Google Scholar]
- Krivoshiev, B. V. , Dardenne, F. , Covaci, A. , Blust, R. , & Husson, S. J. (2016). Assessing in‐vitro estrogenic effects of currently‐used flame retardants. Toxicology in Vitro, 33, 153–162. [DOI] [PubMed] [Google Scholar]
- Kuester, R. K. , Sólyom, A. M. , Rodriguez, V. P. , & Sipes, I. G. (2007). The effects of dose, route, and repeated dosing on the disposition and kinetics of tetrabromobisphenol A in male F‐344 rats. Toxicological Sciences, 96(2), 237–245. [DOI] [PubMed] [Google Scholar]
- Kuramochi, H. , Kawamoto, K. , Miyazaki, K. , Nagahama, K. , Maeda, K. , Li, X. W. , Shibata, E. , Nakamura, T. , & Sakai, S. I. (2008). Determination of physicochemical properties of tetrabromobisphenol A. Environmental Toxicology and Chemistry: An International Journal, 27, 2413–2418. [DOI] [PubMed] [Google Scholar]
- Kweon, D. J. , Kim, M. K. , & Zoh, K. D. (2018). Distribution of brominated flame retardants and phthalate esters in house dust in Korea. Environmental Engineering Research, 23, 354–363. [Google Scholar]
- La Guardia, M. J. , & Hale, R. C. (2015). Halogenated flame‐retardant concentrations in settled dust, respirable and inhalable particulates and polyurethane foam at gymnastic training facilities and residences. Environment International, 79, 106–114. [DOI] [PubMed] [Google Scholar]
- Ladumor, M. K. , Bhatt, D. K. , Gaedigk, A. , Sharma, S. , Thakur, A. , Pearce, R. E. , Leeder, J. S. , Bolger, M. B. , Singh, S. , & Prasad, B. (2019). Ontogeny of hepatic sulfotransferases and prediction of age‐dependent fractional contribution of sulfation in acetaminophen metabolism. Drug Metabolism and Disposition, 47, 818–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, D. Y. , Kacew, S. , & Dekant, W. (2015). Tetrabromobisphenol A (TBBPA): Possible modes of action of toxicity and carcinogenicity in rodents. Food and Chemical Toxicology, 80, 206–214. [DOI] [PubMed] [Google Scholar]
- Lankova, D. , Lacina, O. , Pulkrabova, J. , & Hajslova, J. (2013). The determination of perfluoroalkyl substances, brominated flame retardants and their metabolites in human breast milk and infant formula. Talanta, 117, 318–325. [DOI] [PubMed] [Google Scholar]
- Lankova, D. , Svarcova, A. , Kalachova, K. , Lacina, O. , Pulkrabova, J. , & Hajslova, J. (2015). Multi‐analyte method for the analysis of various organohalogen compounds in house dust. Analytica Chimica Acta, 854, 61–69. [DOI] [PubMed] [Google Scholar]
- Lee, G. H. , Jin, S. W. , Kim, S. J. , Pham, T. H. , Choi, J. H. , & Jeong, H. G. (2019). Tetrabromobisphenol A induces MMP‐9 expression via NADPH oxidase and the activation of ROS, MAPK, and Akt pathways in human breast cancer MCF‐7 cells. Toxicological Research, 35, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. K. , Kim, T. S. , Kim, C. Y. , Kang, I. H. , Kim, M. G. , Jung, K. K. , Kim, H. S. , Han, S. Y. , Yoon, H. J. , & Rhee, G. S. (2012). Evaluation of in vitro screening system for estrogenicity: Comparison of stably transfected human estrogen receptor‐α transcriptional activation (OECD TG455) assay and estrogen receptor (ER) binding assay. Toxicological Sciences, 37, 431–437. [DOI] [PubMed] [Google Scholar]
- Lee, J. G. , Jeong, Y. , Kim, D. , Kang, G. J. , & Kang, Y. (2020). Assessment of tetrabromobisphenol and Hexabromocyclododecanes exposure and risk characterization using occurrence data in foods. Food and Chemical Toxicology, 137, 111121. [DOI] [PubMed] [Google Scholar]
- Lenart, J. , Zieminska, E. , Diamandakis, D. , & Lazarewicz, J. W. (2017). Altered expression of genes involved in programmed cell death in primary cultured rat cerebellar granule cells acutely challenged with tetrabromobisphenol A. Neurotoxicology, 63, 126–136. [DOI] [PubMed] [Google Scholar]
- Letcher, R. J. , & Chu, S. (2010). High‐sensitivity method for determination of tetrabromobisphenol‐S and tetrabromobisphenol‐A derivative flame retardants in great lakes herring gull eggs by liquid chromatography‐atmospheric pressure photoionization‐tandem mass spectrometry. Environmental Science and Technology, 44, 8615–8621. [DOI] [PubMed] [Google Scholar]
- Lévy‐Bimbot, M. , Major, G. , Courilleau, D. , Blondeau, J. P. , & Lévi, Y. (2012). Tetrabromobisphenol‐A disrupts thyroid hormone receptor alpha function in vitro: Use of fluorescence polarization to assay corepressor and coactivator peptide binding. Chemosphere, 87, 782–788. [DOI] [PubMed] [Google Scholar]
- Li, A. , Zhuang, T. , Shi, W. , Liang, Y. , Liao, C. , Song, M. , & Jiang, G. (2020). Serum concentration of bisphenol analogues in pregnant women in China. Science of the Total Environment, 707, 136100. [DOI] [PubMed] [Google Scholar]
- Li, F. , Jin, J. , Tan, D. , Wang, L. , Geng, N. , Cao, R. , Gao, Y. , & Chen, J. (2016). Hexabromocyclododecane and tetrabromobisphenol A in sediments and paddy soils from Liaohe River Basin, China: Levels, distribution and mass inventory. Journal of Environmental Sciences, 48, 209–217. [DOI] [PubMed] [Google Scholar]
- Li, J. , Chen, T. , Wang, Y. , Shi, Z. , Zhou, X. , Sun, Z. , Wang, D. , & Wu, Y. (2017). Simple and fast analysis of tetrabromobisphenol A, hexabromocyclododecane isomers, and polybrominated diphenyl ethers in serum using solid‐phase extraction or QuEChERS extraction followed by tandem mass spectrometry coupled to HPLC and GC. Journal of Separation Science, 40, 709–716. [DOI] [PubMed] [Google Scholar]
- Li, J. , Ma, M. , & Wang, Z. (2010). In vitro profiling of endocrine disrupting effects of phenols. Toxicology in Vitro, 24, 201–207. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Dong, M. , Xiong, Y. , Chang, Q. , Chen, X. , Fu, X. , Li, X. , & Qin, Z. (2022). Effects of postnatal exposure to tetrabromobisphenol A on testis development in mice and early key events. Archives of Toxicology, 96, 1881–1892. [DOI] [PubMed] [Google Scholar]
- Li, Y. Y. , Xiong, Y. M. , Chen, X. Y. , Sheng, J. Y. , Lv, L. , Li, X. H. , & Qin, Z. F. (2023). Extended exposure to tetrabromobisphenol A‐bis(2,3‐dibromopropyl ether) leads to subfertility in male mice at the late reproductive age. Archives of Toxicology, 97, 2983–2995. [DOI] [PubMed] [Google Scholar]
- Li, Y. Y. , Xiong, Y. M. , Zhang, S. Y. , Deng, J. L. , Xue, Q. , Hou, X. W. , Liu, W. B. , Li, X. H. , & Qin, Z. F. (2023). Tetrabromobisphenol A‐bis (2, 3‐dibromopropyl ether) impairs postnatal testis development in mice: The microtubule cytoskeleton as a sensitive target. Environment & Health, 1(3), 168–179. [Google Scholar]
- Li, Z. , Li, Z. , Zhou, Y. , Meng, W. , Li, J. , Zhou, Y. , He, C. , Dong, G. , & Yu, Y. (2024). Co‐occurrence of tetrabromobisphenol A and debromination products in human hair across China: Implications for exposure sources and health effects on metabolic syndrome. The Science of the Total Environment, 909, 168514. [DOI] [PubMed] [Google Scholar]
- Liang, S. , Liang, S. , Yin, N. , Hu, B. , & Faiola, F. (2019). Toxicogenomic analyses of the effects of BDE‐47/209, TBBPA/S and TCBPA on early neural development with a human embryonic stem cell in vitro differentiation system. Toxicology and Applied Pharmacology, 379, 114685. [DOI] [PubMed] [Google Scholar]
- Liang, S. , Liang, S. , Zhou, H. , Yin, N. , & Faiola, F. (2019). Typical halogenated flame retardants affect human neural stem cell gene expression during proliferation and differentiation via glycogen synthase kinase 3 beta and T3 signaling. Ecotoxicology and Environmental Safety, 183, 109498. [DOI] [PubMed] [Google Scholar]
- Liang, S. , Yin, L. , Shengyang, Y. K. , Hofmann, M. C. , & Yu, X. (2017). High‐content analysis provides mechanistic insights into the testicular toxicity of Bisphenol A and selected analogues in mouse Spermatogonial cells. Toxicological Sciences, 155, 43–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, S. , Zhou, H. , Yin, N. , Lu, Y. , & Faiola, F. (2019). Embryoid body‐based RNA‐seq analyses reveal a potential TBBPA multifaceted developmental toxicity. Journal of Hazardous Materials, 376, 223–232. [DOI] [PubMed] [Google Scholar]
- Liang, J. , Liu, S. , Liu, T. , Yang, C. , Wu, Y. , Tan, H. J. , Wei, B. , Ma, X. , Feng, B. , Jiang, Q. , & Huang, D. (2020). Association of prenatal exposure to bisphenols and birth size in Zhuang ethnic newborns. Chemosphere, 252, 126422. [DOI] [PubMed] [Google Scholar]
- Liang, J. , Yang, C. , Liu, T. , Tan, H. J. , Sheng, Y. , Wei, L. , Tang, P. , Huang, H. , Zeng, X. , Liu, S. , & Huang, D. (2021). Prenatal exposure to bisphenols and risk of preterm birth: Findings from Guangxi Zhuang birth cohort in China. Ecotoxicology and Environmental Safety, 228, 112960. [DOI] [PubMed] [Google Scholar]
- Liang, J. , Yang, C. , Liu, T. , Tang, P. , Huang, H. , Wei, H. , Liao, Q. , Long, J. , Zeng, X. , Liu, S. , & Huang, D. (2022). Single and mixed effects of prenatal exposure to multiple bisphenols on hemoglobin levels and the risk of anemia in pregnant women. Environmental Research, 207, 112625. [DOI] [PubMed] [Google Scholar]
- Liang, J. , Shao, Y. , Huang, D. , Yang, C. , Liu, T. , Zeng, X. , Li, C. , Tang, Z. , Juan, J. T. , Song, Y. , & Liu, S. (2023a). Effects of prenatal exposure to bisphenols on newborn leucocyte telomere length: a prospective birth cohort study in China. Environmental Science and Pollution Research, 30(10), 25013–25023. [DOI] [PubMed] [Google Scholar]
- Liang, J. , Pang, L. , Yang, C. , Long, J. , Liao, Q. , Tang, P. , Huang, H. , Wei, H. , Chen, Q. , Yang, K. , & Liu, T. (2023b). Effects of prenatal single and mixed bisphenol exposure on bone mineral density in preschool children: A population‐based prospective cohort study. Ecotoxicology and Environmental Safety, 267, 115665. [DOI] [PubMed] [Google Scholar]
- Lilienthal, H. , Verwer, C. M. , van der Ven, L. T. M. , Piersma, A. H. , & Vos, J. G. (2008). Exposure to tetrabromobisphenol A (TBBPA) in Wistar rats: Neurobehavioral effects in offspring from a one‐generation reproduction study. Toxicology, 246, 45–54. [DOI] [PubMed] [Google Scholar]
- Lin, M. , Ma, S. , Yu, Y. , Li, G. , Mai, B. , & An, T. (2020). Simultaneous determination of multiple classes of phenolic compounds in human urine: Insight into metabolic biomarkers of occupational exposure to E‐waste. Environmental Science & Technology Letters, 7, 323–329. [Google Scholar]
- Litton Bionetics, Inc . (1976). Mutagenicity evaluation of compound 279‐117‐2, final report, tetrabromobisphenol A [Unpublished]. As cited in ECB (2006).
- Litton Bionetics, Inc . (1977a). Mutagenicity evaluation of 859–82‐4. Final report. TSCATS [Unpublished Health and Safety Studies submitted to EPA]. EPA Document No. 88–7800185. Microfiche No. OTS0200512. Document No. 8EHQ‐0678‐0185D. From the RTK Net database. As cited in NIEHS (2002).
- Litton Bionetics, Inc . (1977b). Mutagenicity evaluation of tetrabromo bisphenol a (BP4‐a) . Final report. TSCATS [Unpublished Health and Safety Studies submitted to EPA]. Microfiche No. OS0206828. Document No. 878216106. Document No. 878216110. From the RTK Net database. As cited in NIEHS (2002).
- Liu, A. , Zhao, Z. , Qu, G. , Shen, Z. , Liang, X. , Shi, J. , & Jiang, G. (2019). Identification of transformation/degradation products of tetrabromobisphenol A and its derivatives. Trac‐Trends in Analytical Chemistry, 111, 85–99. [Google Scholar]
- Liu, A. , Zhao, Z. , Qu, G. , Shen, Z. , Shi, J. , & Jiang, G. (2018). Transformation/degradation of tetrabromobisphenol A and its derivatives: A review of the metabolism and metabolites. Environmental Pollution, 243, 1141–1153. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Ma, S. , Lin, M. , Tang, J. , Yue, C. , Zhang, Z. , Yu, Y. , & An, T. (2020). New mixed bromine/chlorine transformation products of tetrabromobisphenol A: Synthesis and identification in dust samples from an E‐waste dismantling site. Environmental Science & Technology, 54, 12235–12244. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Yu, L. , Castro, L. , Yan, Y. , Clayton, N. P. , Bushel, P. , Flagler, N. D. , Scappini, E. , & Dixon, D. (2022). Short‐term tetrabromobisphenol A exposure promotes fibrosis of human uterine fibroid cells in a 3D culture system through TGF‐beta signaling. FASEB Journal, 36, e22101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, K. , Li, J. , Yan, S. , Zhang, W. , Li, Y. , & Han, D. (2016). A review of status of tetrabromobisphenol A (TBBPA) in China. Chemosphere, 148, 8–20. [DOI] [PubMed] [Google Scholar]
- Liu, Q. , Ren, X. , Long, Y. , Hu, L. , Qu, G. , Zhou, Q. , & Jiang, G. (2016). The potential neurotoxicity of emerging tetrabromobisphenol A derivatives based on rat pheochromocytoma cells. Chemosphere, 154, 194–203. [DOI] [PubMed] [Google Scholar]
- Lu, J. F. , He, M. J. , Yang, Z. H. , & Wei, S. Q. (2018). Occurrence of tetrabromobisphenol A (TBBPA) and hexabromocyclododecane (HBCD) in soil and road dust in Chongqing, western China, with emphasis on diastereoisomer profiles, particle size distribution, and human exposure. Environmental Pollution, 242, 219–228. [DOI] [PubMed] [Google Scholar]
- Lu, L. , Zhan, T. , Ma, M. , Xu, C. , Wang, J. , Zhang, C. , Liu, W. , & Zhuang, S. (2018). Thyroid disruption by Bisphenol S analogues via thyroid hormone receptor β: In vitro, in vivo, and molecular dynamics simulation study. Environmental Science & Technology, 52, 6617–6625. [DOI] [PubMed] [Google Scholar]
- Lu, Z. , Letcher, R. J. , Chu, S. , Ciborowski, J. J. H. , Haffner, G. D. , Drouillard, K. G. , MacLeod, S. L. , & Marvin, C. H. (2015). Spatial distributions of polychlorinated biphenyls, polybrominated diphenyl ethers, tetrabromobisphenol A and bisphenol A in Lake Erie sediment. Journal of Great Lakes Research, 41, 808–817. [Google Scholar]
- Lyu, L. , Jin, X. , Li, Z. , Liu, S. , Li, Y. , Su, R. , & Su, H. (2020). TBBPA regulates calcium‐mediated lysosomal exocytosis and thereby promotes invasion and migration in hepatocellular carcinoma. Ecotoxicology and Environmental Safety, 192, 110255. [DOI] [PubMed] [Google Scholar]
- Mahfoudhi, G. , Ameur, W. B. , Malysheva, S. V. , Szternfeld, P. , Touil, S. , Driss, M. R. , & Joly, L. (2023). First study of bromophenols and hexabromocyclododecanes in seafood from North Africa (case of Bizerte Lagoon, Tunisia): Occurrence and human health risk. Environmental Science and Pollution Research International, 30, 64499–64516. [DOI] [PubMed] [Google Scholar]
- Mäkinen, M. S. , Mäkinen, M. R. , Koistinen, J. T. , Pasanen, A. L. , Pasanen, P. O. , Kalliokoski, P. J. , & Korpi, A. M. (2009). Respiratory and dermal exposure to organophosphorus flame retardants and tetrabromobisphenol A at five work environments. Environmental Science & Technology, 43, 941–947. [DOI] [PubMed] [Google Scholar]
- Manivannan, B. , Yegambaram, M. , Supowit, S. , Beach, T. G. , & Halden, R. U. (2019). Assessment of persistent, bioaccumulative and toxic organic environmental pollutants in liver and adipose tissue of Alzheimer's disease patients and age‐matched controls. Current Alzheimer Research, 16, 1039–1049. [DOI] [PubMed] [Google Scholar]
- Martínez, M. A. , Castro, I. , Rovira, J. , Ares, S. , Rodriguez, J. M. , Cunha, S. C. , Casal, S. , Fernandes, J. O. , Schuhmacher, M. , & Nadal, M. (2019). Early‐life intake of major trace elements, bisphenol A, tetrabromobisphenol A and fatty acids: Comparing human milk and commercial infant formulas. Environmental Research, 169, 246–255. [DOI] [PubMed] [Google Scholar]
- Matsubara, K. , Sanoh, S. , Ohta, S. , Kitamura, S. , Sugihara, K. , & Fujimoto, N. (2012). An improved thyroid hormone reporter assay to determine the thyroid hormone‐like activity of amiodarone, bithionol, closantel and rafoxanide. Toxicology Letters, 208, 30–35. [DOI] [PubMed] [Google Scholar]
- Matsui, S. , Ito‐Harashima, S. , Sugimoto, Y. , Takada, E. , Shiizaki, K. , Kawanishi, M. , & Yagi, T. (2016). Development of yeast reporter assays for the enhanced detection of environmental ligands of thyroid hormone receptors α and β from Xenopus tropicalis. Toxicology in Vitro, 37, 15–24. [DOI] [PubMed] [Google Scholar]
- McIver, C. R. , Shaw, I. C. , Gin, S. , & Ellis, M. J. (2013). Can thyroid hormone mimics affect thyroid hormone measurement by immunoassay? Clinical Biochemistry, 46, 1302–1304. [DOI] [PubMed] [Google Scholar]
- Meerts, I. A. , Assink, Y. , Cenijn, P. H. , van den Berg, J. H. , Bergman, C. , Koeman, J. H. , & Brouwer, A. (1999). Distribution of the flame retardant tetrabromobisphenol A in pregnant and fetal rats and effect on thyroid hormone homeostasis. Organohalogen Compounds, 40, 375–378. [Google Scholar]
- Meijer, M. , Hendriks, H. S. , Heusinkveld, H. J. , Langeveld, W. T. , & Westerink, R. H. (2014). Comparison of plate reader‐based methods with fluorescence microscopy for measurements of intracellular calcium levels for the assessment of in vitro neurotoxicity. Neurotoxicology, 45, 31–37. [DOI] [PubMed] [Google Scholar]
- Mengeling, B. J. , Wei, Y. , Dobrawa, L. N. , Streekstra, M. , Louisse, J. , Singh, V. , Singh, L. , Lein, P. J. , Wulff, H. , Murk, A. J. , & Furlow, J. D. (2017). A multi‐tiered, in vivo, quantitative assay suite for environmental disruptors of thyroid hormone signaling. Aquatic Toxicology, 190, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura, T. , Uehara, S. , Shigeta, K. , Yoshizawa, M. , Kamiya, Y. , Murayama, N. , Shimizu, M. , Suemizu, H. , & Yamazaki, H. (2021). Metabolic profiles of tetrabromobisphenol A in humans extrapolated from humanized‐liver mouse data using a simplified physiologically based pharmacokinetic model. Chemical Research in Toxicology, 34, 522–528. [DOI] [PubMed] [Google Scholar]
- Miyagi, S. J. , & Collier, A. C. (2011). The development of UDP‐glucuronosyltransferases 1A1 and 1A6 in the pediatric liver. Drug Metabolism and Disposition, 39(5), 912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales, K. H. , Ibrahim, J. G. , Chen, C.‐J. , & Ryan, L. M. (2006). Bayesian model averaging with applications to benchmark dose estimation for arsenic in drinking water. Journal of the American Statistical Association, 101(473), 9–17. 10.1198/016214505000000961 [DOI] [Google Scholar]
- Moreira Bastos, P. , Eriksson, J. , Green, N. , & Bergman, Å. (2008). A standardized method for assessment of oxidative transfrormations of brominated phenols in water. Chemosphere, 70, 1196–1202. [DOI] [PubMed] [Google Scholar]
- Mortelmans, K. , Haworth, S. , Lawlor, T. , Speck, W. , Tainer, B. , & Zeigler, E. (1986). Salmonella mutagenicity test: II. Results from the testing of 270 chemicals. Environmental Mutagenesis, 8, 1–119. As cited in ECB (2006). [PubMed] [Google Scholar]
- MPI Research . (2002a). An oral two generation reproductive, fertility and developmental neurobehavioural study of tetrabromobisphenol‐A in rats [Unpublished]. As cited in ECB (2006).
- MPI Research . (2002b). A 90‐day oral toxicity study of tetrabromobisphenol‐A in rats with a recovery group [Unpublished]. As cited in ECB (2006).
- MPI Research . (2003). Amendment to the final report. An oral two generation reproductive, fertility and developmental neurobehavioural study of tetrabromobisphenol‐A in rats (Unpublished report). As cited in ECB (2006).
- Nakajima, A. , Saigusa, D. , Tetsu, N. , Yamakuni, T. , Tomioka, Y. , & Hishinuma, T. (2009). Neurobehavioral effects of tetrabromobisphenol A, a brominated flame retardant, in mice. Toxicology Letters, 189, 78–83. [DOI] [PubMed] [Google Scholar]
- Nakao, T. , Akiyama, E. , Kakutani, H. , Mizuno, A. , Aozasa, O. , Akai, Y. , & Ohta, S. (2015). Levels of tetrabromobisphenol A, tribromobisphenol A, dibromobisphenol A, monobromobisphenol A, and bisphenol A in Japanese breast milk. Chemical Research in Toxicology, 28, 722–728. [DOI] [PubMed] [Google Scholar]
- Nakao, T. , Kakutani, H. , Yuzuriha, T. , & Ohta, S. (2022). Distribution, metabolism, excretion, and lactational transfer to pups of tetrabromobisphenol A and its metabolites in C57BL/6 mice. BPB Reports, 5(3), 50–58. [Google Scholar]
- NTP (National Toxicology Program) . (2014). Toxicology studies of tetrabromobisphenol A in F344/NTac rats and B6C3F1/N mice and toxicology and carcinogenesis studies of tetrabromobisphenol A in Wistar Han [Crl:WI(Han)] rats and B6C3F1/N mice (gavage studies). National Toxicology Program Technical Report Series, 587, NTP‐TR‐587. 10.22427/NTP-TR-587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP (National Toxicology Program) . (2017). Toxicity studies of tetrabromobisphenol A‐bis(2,3‐dibromopropyl ether) (CASRN 21850‐44‐2) administered by gavage to F344/NTac rats and B6C3F1/N mice. Toxicity Report Series, 85, 91 p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann, E. , Mehboob, H. , Ramírez, J. , Mirkov, S. , Zhang, M. , & Liu, W. (2016). Age‐dependent hepatic UDP‐glucuronosyltransferase gene expression and activity in children. Frontiers in Pharmacology, 7, 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICNAS (National Industrial Chemicals Notification and Assessment Scheme) . (2020). Priority existing chemical assessment report no. 42: Tetrabromobisphenol A. National Industrial Chemicals Notification and Assessment Scheme. https://www.industrialchemicals.gov.au/sites/default/files/2021‐06/PEC42‐Tetrabromobisphenol‐A‐TBBPA_0.pdf [Google Scholar]
- NIEHS (National Institute of Environmental Health Sciences) . (2002). Tetrabisphenol A: Review of toxicological literature. Contract No. N01‐ES‐65402. http://ntp.niehs.nih.gov/ntp/htdocs/Chem_Background/ExSumPdf/tetrabromobisphenola.pdf
- Ohta, R. , Takagi, A. , Ohmukai, H. , Marumo, H. , Ono, A. , Matsushima, Y. , Inoue, T. , Ono, H. , & Kanno, J. (2012). Ovariectomized mouse uterotrophic assay of 36 chemicals. The Journal of Toxicological Sciences, 37, 879–889. [DOI] [PubMed] [Google Scholar]
- Oka, T. , Mitsui‐Watanabe, N. , Tatarazako, N. , Onishi, Y. , Katsu, Y. , Miyagawa, S. , Ogino, Y. , Yatsu, R. , Kohno, S. , Takase, M. , Kawashima, Y. , Ohta, Y. , Aoki, Y. , Guillette, L. J., Jr. , & Iguchi, T. (2013). Establishment of transactivation assay systems using fish, amphibian, reptilian and human thyroid hormone receptors. Journal of Applied Toxicology, 33, 991–1000. [DOI] [PubMed] [Google Scholar]
- Okeke, E. S. , Huang, B. , Mao, G. , Chen, Y. , Zhengjia, Z. , Qian, X. , Wu, X. , & Feng, W. (2022). Review of the environmental occurrence, analytical techniques, degradation and toxicity of TBBPA and its derivatives. Environmental Research, 206, 112594. [DOI] [PubMed] [Google Scholar]
- Osimitz, T. G. , Droege, W. , & Hayes, A. W. (2016). Subchronic toxicology of tetrabromobisphenol A in rats. Human and Experimental Toxicology, 35, 1214–1226. [DOI] [PubMed] [Google Scholar]
- Otsuka, S. , Ishihara, A. , & Yamauchi, K. (2014). Ioxynil and tetrabromobisphenol A suppress thyroid‐hormone‐induced activation of transcriptional elongation mediated by histone modifications and RNA polymerase II phosphorylation. Toxicological Sciences, 138(2), 290–299. 10.1093/toxsci/kfu012 [DOI] [PubMed] [Google Scholar]
- Pan, Y. F. , Liu, S. , Tian, F. , Chen, H. G. , & Xu, X. R. (2022). Tetrabromobisphenol A and hexabromocyclododecanes in sediments from fishing ports along the coast of South China: Occurrence, distribution and ecological risk. Chemosphere, 302, 134872. [DOI] [PubMed] [Google Scholar]
- Parizek, O. , Gramblicka, T. , Parizkova, D. , Polachova, A. , Bechynska, K. , Dvorakova, D. , Stupak, M. , Dusek, J. , Pavlikova, J. , Topinka, J. , Sram, R. J. , & Pulkrabova, J. (2023). Assessment of organohalogenated pollutants in breast milk from The Czech Republic. Science of the Total Environment, 871, 161938. [DOI] [PubMed] [Google Scholar]
- Park, H. R. , Kamau, P. W. , Korte, C. , & Loch‐Caruso, R. (2014). Tetrabromobisphenol A activates inflammatory pathways in human first trimester extravillous trophoblasts in vitro. Reproductive Toxicology, 50, 154–162. 10.1016/j.reprotox.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, C. , Kim, S. J. , Lee, W. K. , Moon, S. K. , Kwak, S. , Choe, S. K. , & Park, R. (2016). Tetrabromobisphenol‐A induces apoptotic death of auditory cells and hearing loss. Biochemical and Biophysical Research Communications, 478, 1667–1673. [DOI] [PubMed] [Google Scholar]
- Pawar, G. , Abdallah, M. A. , de Sáa, E. V. , & Harrad, S. (2017). Dermal bioaccessibility of flame retardants from indoor dust and the influence of topically applied cosmetics. Journal of Exposure Science & Environmental Epidemiology, 27, 100–105. [DOI] [PubMed] [Google Scholar]
- Pawlicki, P. , Duliban, M. , Tuz, R. , Ptak, A. , Milon, A. , Gorowska‐Wojtowicz, E. , Tworzydlo, W. , Płachno, B. J. , Bilinska, B. , Knapczyk‐Stwora, K. , & Kotula‐Balak, M. (2019). Do G‐protein coupled estrogen receptor and bisphenol A analogs influence on Leydig cell epigenetic regulation in immature boar testis ex vivo? Animal Reproduction Science, 207, 21–35. [DOI] [PubMed] [Google Scholar]
- Pei, Y. , Peng, J. , Behl, M. , Sipes, N. S. , Shockley, K. R. , Rao, M. S. , Tice, R. R. , & Zeng, X. (2016). Comparative neurotoxicity screening in human iPSC‐derived neural stem cells, neurons and astrocytes. Brain Research, 1638, 57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, H. , Jin, J. , Wang, Y. , Liu, W. Z. , & Yang, R. M. (2007). Determination of tetrabromobisphenol‐A in soil by high performance liquid chromatography electrospray ion trap mass spectrometry. Chinese Journal of Analytical Chemistry, 35, 549–573. [Google Scholar]
- Pittinger, C. A. , & Pecquet, A. M. (2018). Review of historical aquatic toxicity and bioconcentration data for the brominated flame retardant tetrabromobisphenol A (TBBPA): Effects to fish, invertebrates, algae, and microbial communities. Environmental Science and Pollution Research, 25, 14361–14372. [DOI] [PubMed] [Google Scholar]
- Poma, G. , Malysheva, S. V. , Goscinny, S. , Malarvannan, G. , Voorspoels, S. , Covaci, A. , & Van Loco, J. (2018). Occurrence of selected halogenated flame retardants in Belgian foodstuff. Chemosphere, 194, 256–265. [DOI] [PubMed] [Google Scholar]
- Pratt, I. , Anderson, W. , Crowley, D. , Daly, S. , Evans, R. , Fernandes, A. , Fitzgerald, M. , Geary, M. , Keane, D. , Morrison, J. J. , & Reilly, A. (2013). Brominated and fluorinated organic pollutants in the breast milk of first‐time Irish mothers: Is there a relationship to levels in food? Food Additives and Contaminants Part a‐Chemistry Analysis Control Exposure and Risk Assessment, 30, 1788–1798. [DOI] [PubMed] [Google Scholar]
- Qu, G. , Liu, A. , Hu, L. , Liu, S. , Shi, J. , & Jiang, G. (2016). Recent advances in the analysis of TBBPA/TBBPS, TBBPA/TBBPS derivatives and their transformation products. Trac‐Trends in Analytical Chemistry, 83, 14–24. [Google Scholar]
- Quesnot, N. , Rondel, K. , Audebert, M. , Martinais, S. , Glaise, D. , Morel, F. , Loyer, P. , & Robin, M. A. (2016). Evaluation of genotoxicity using automated detection of γH2AX in metabolically competent HepaRG cells. Mutagenesis, 31(1), 43–50. 10.1093/mutage/gev059 [DOI] [PubMed] [Google Scholar]
- Rana, K. , & Whalen, M. (2015). Activation of protein kinase C and protein kinase D in human natural killer cells: effects of tributyltin, dibutyltin, and tetrabromobisphenol A. Toxicology Mechanisms and Methods, 25(9), 680–688. 10.3109/15376516.2015.1070226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redza‐Dutordoir, M. , & Averill‐Bates, D. A. (2016). Activation of apoptosis signalling pathways by reactive oxygen species. Biochimica et Biophysica Acta, 1863, 2977–2992. [DOI] [PubMed] [Google Scholar]
- Reed, J. M. , Spinelli, P. , Falcone, S. , He, M. , Goeke, C. M. , & Susiarjo, M. (2022). Evaluating the effects of BPA and TBBPA exposure on pregnancy loss and maternal–fetal immune cells in mice. Environmental Health Perspectives, 130, 037010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, J. , & Whalen, M. M. (2011). The brominated flame retardants hexabromcylododecane and tetrabromobisphenol A decrease secretion of interferon gamma from human natural killer cells. The FASEB Journal, 25, 1.21205780 [Google Scholar]
- Reindl, A. R. , & Falkowska, L. (2015). Flame retardants at the top of a simulated baltic marine food web – A case study concerning African penguins from the Gdansk Zoo. Archives of Environmental Contamination and Toxicology, 68, 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reistad, T. , Mariussen, E. , Ring, A. , & Fonnum, F. (2007). In vitro toxicity of tetrabromobisphenol‐A oncerebellar granule cells: cell death, free radical formation, calcium influx and extracellular glutamate. Toxicological Sciences, 96, 268–278. [DOI] [PubMed] [Google Scholar]
- Ren, X. M. , Yao, L. , Xue, Q. , Shi, J. , Zhang, Q. , Wang, P. , Fu, J. , Zhang, A. , Qu, G. , & Jiang, G. (2020). Binding and activity of tetrabromobisphenol A mono‐ether structural analogs to thyroid hormone transport proteins and receptors. Environmental Health Perspectives, 128, 107008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner, H. , Becker, K. J. , Kagermeier, T. E. , Grabos, M. , Eliat, F. , Günther, P. , Schöler, H. R. , & Bruder, J. M. (2021). Cell‐type‐specific high throughput toxicity testing in human midbrain organoids. Frontiers in Molecular Neuroscience, 14, 715054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riu, A. (2006). Devenir des retardateurs de flammes bromés chez le rat et l'homme: caractérisation des métabolites et évaluation de l'exposition foetale [PhD thesis, INP]. http://ethesis.inp‐toulouse.fr/archive/00000479/01/riu.pdf
- Riu, A. , le Maire, A. , Grimaldi, M. , Audebert, M. , Hillenweck, A. , Bourguet, W. , Balaguer, P. , & Zalko, D. (2011). Characterization of novel ligands of ERα, Erβ, and PPARγ: The case of halogenated bisphenol A and their conjugated metabolites. Toxicological Sciences, 122, 372–382. [DOI] [PubMed] [Google Scholar]
- Rivière, G. , Jean, J. , Gorecki, S. , Hulin, M. , Kolf‐Clauw, M. , Feidt, C. , Picard‐Hagen, N. , Vasseur, P. , Le Bizec, B. , & Sirot, V. (2019). Dietary exposure to perfluoroalkyl acids, brominated flame retardants and health risk assessment in the French infant total diet study. Food and Chemical Toxicology, 131, 110561. [DOI] [PubMed] [Google Scholar]
- Rock, K. D. , Gillera, S. E. A. , Devarasetty, P. , Horman, B. , Knudsen, G. , Birnbaumd, L. S. , Fenton, S. E. , & Patisaul, H. B. (2019). Sex‐specific behavioral effects following developmental exposure to tetrabromobisphenol A (TBBPA) in Wistar rats. Neurotoxicology, 75, 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers, K. M. , Covaci, A. , Poma, G. , Knox, K. , Allen, J. G. , Cedeno‐Laurent, J. , Rudel, R. A. , & Dodson, R. E. (2020). Flame retardant concentrations are lower in college spaces meeting the new furniture flammability standard TB117‐2013. Environmental Science & Technology Letters, 7, 833–839. [Google Scholar]
- Roelofs, M. J. , van den Berg, M. , Bovee, T. F. , Piersma, A. H. , & van Duursen, M. B. (2015). Structural bisphenol analogues differentially target steroidogenesis in murine MA‐10 Leydig cells as well as the glucocorticoid receptor. Toxicology, 329, 10–20. [DOI] [PubMed] [Google Scholar]
- Rovira, J. , Martínez, M. Á. , Mari, M. , Cunha, S. C. , Fernandes, J. O. , Marmelo, I. , Marques, A. , Haug, L. S. , Thomsen, C. , Nadal, M. , Domingo, J. L. , & Schuhmacher, M. (2022). Mixture of environmental pollutants in breast milk from a Spanish cohort of nursing mothers. Environment International, 166, 107375. [DOI] [PubMed] [Google Scholar]
- Saegusa, Y. , Fujimoto, H. , Woo, G.‐H. , Inoue, K. , Takahashi, M. , Mitsumori, K. , Hirose, M. , Nishikawa, A. , & Shibutani, M. (2009). Developmental toxicity of brominated flame retardants, tetrabromobisphenol Aand 1,2,5,6,9,10‐hexabromocyclododecane, in rat offspring after maternal exposure from mid‐gestation through lactation. Reproductive Toxicology, 28, 456–467. [DOI] [PubMed] [Google Scholar]
- Saegusa, Y. , Fujimoto, H. , Woo, G. H. , Ohishi, T. , Wang, L. , Mitsumori, K. , Nishikawa, A. , & Shibutani, M. (2012). Transient aberration of neuronal development in the hippocampal dentate gyrus after developmental exposure to brominated flame retardants in rats. Archives of Toxicology, 86, 1431–1442. [DOI] [PubMed] [Google Scholar]
- Saha, L. , Kumar, V. , Tiwari, J. , Rawat, S. , Singh, J. , & Bauddh, K. (2021). Electronic waste and their leachates impact on human health and environment: Global ecological threat and management. Environmental Technology & Innovation, 24, 102049. [Google Scholar]
- Saini, A. , Harner, T. , Chinnadhurai, S. , Schuster, J. K. , Yates, A. , Sweetman, A. , Aristizabal‐Zuluaga, B. H. , Jiménez, B. , Manzano, C. A. , Gaga, E. O. , & Stevenson, G. (2020). GAPS‐megacities: A new global platform for investigating persistent organic pollutants and chemicals of emerging concern in urban air. Environmental Pollution, 267, 115416. [DOI] [PubMed] [Google Scholar]
- Samsonek, J. , & Puype, F. (2013). Occurrence of brominated flame retardants in black thermo cups and selected kitchen utensils purchased on the European market. Food Additives and Contaminants Part a‐Chemistry Analysis Control Exposure & Risk Assessment, 30, 1976–1986. [DOI] [PubMed] [Google Scholar]
- Sanders, J. M. , Coulter, S. J. , Knudsen, G. A. , Dunnick, J. K. , Kissling, G. E. , & Birnbaum, L. S. (2016). Disruption of estrogen homeostasis as a mechanism for uterine toxicity in Wistar Han rats treated with tetrabromobisphenol A. Toxicology and Applied Pharmacology, 298, 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer, U. M. , Völkel, W. , & Dekant, W. (2006). Toxicokinetics of tetrabromobisphenol A in humans and rats after oral administration. Toxicological Sciences, 91(1), 49–58. [DOI] [PubMed] [Google Scholar]
- Schreder, E. D. , & La Guardia, M. J. (2014). Flame retardant transfers from U.S. households (dust and laundry wastewater) to the aquatic environment. Environmental Science & Technology, 48, 11575–11583. [DOI] [PubMed] [Google Scholar]
- Schroeder, R. (2002). An oral two generation reproductive, fertility, and developmental neurobehavioral study in tetrabromobisphenol A in rats. Study ID number: 474‐004. MPI Research, Inc. Study abstract available in HPV data summary and test plan for phenol, 4,4′‐isopropylidenbis[2,6‐dibromo‐ (tetrabromobisphenol A, TBBPA). Prepared by American Chemistry Council Brominated Flame Retardant Industry Panel (BFRIP). As cited in Williams and deSesso (2010). [Google Scholar]
- Shao, B. , Mao, L. , Qu, N. , Wang, Y. F. , Gao, H. Y. , Li, F. , Qin, L. , Shao, J. , Huang, C. H. , Xu, D. , & Xie, L. N. (2017). Mechanism of synergistic DNA damage induced by the hydroquinone metabolite of brominated phenolic environmental pollutants and Cu (II): Formation of DNA‐Cu complex and site‐specific production of hydroxyl radicals. Free Radical Biology and Medicine, 104, 54–63. [DOI] [PubMed] [Google Scholar]
- Shaw, S. D. , Berger, M. L. , Harris, J. H. , Yun, S. H. , Wu, Q. , Liao, C. , Blum, A. , Stefani, A. , & Kannan, K. (2013). Persistent organic pollutants including polychlorinated and polybrominated dibenzo‐p‐dioxins and dibenzofurans in firefighters from Northern California. Chemosphere, 91, 1386–1394. [DOI] [PubMed] [Google Scholar]
- Sheikh, I. A. , & Beg, M. A. (2020). Structural binding interactions of tetrabromobisphenol A with sex steroid nuclear receptors and sex hormone‐binding globulin. Journal of Applied Toxicology, 40, 832–842. [DOI] [PubMed] [Google Scholar]
- Shi, L. , Zhang, B. , Ying, Y. , Tang, Y. , Wang, S. , Zhu, Y. , Li, H. , Ge, R. S. , & Liu, Y. (2023). Halogen atoms determine the inhibitory potency of halogenated bisphenol A derivatives on human and rat placental 11β‐hydroxysteroid dehydrogenase 2. Food and Chemical Toxicology, 175, 113739. [DOI] [PubMed] [Google Scholar]
- Shi, Z. , Jiao, Y. , Hu, Y. , Sun, Z. , Zhou, X. , Feng, J. , Li, J. , & Wu, Y. (2013). Levels of tetrabromobisphenol A, hexabromocyclododecanes and polybrominated diphenyl ethers in human milk from the general population in Beijing, China. Science of the Total Environment, 452, 10–18. [DOI] [PubMed] [Google Scholar]
- Shi, Z. , Zhang, L. , Li, J. , & Wu, Y. (2018). Legacy and emerging brominated flame retardants in China: A review on food and human milk contamination, human dietary exposure and risk assessment. Chemosphere, 198, 522–536. [DOI] [PubMed] [Google Scholar]
- Shi, Z. , Zhang, L. , Zhao, Y. , Sun, Z. , Zhou, X. , Li, J. , & Wu, Y. (2017a). A national survey of tetrabromobisphenol‐A, hexabromocyclododecane and decabrominated diphenyl ether in human milk from China: Occurrence and exposure assessment. Science of the Total Environment, 599, 237–245. [DOI] [PubMed] [Google Scholar]
- Shi, Z. , Zhang, L. , Zhao, Y. , Sun, Z. , Zhou, X. , Li, J. , & Wu, Y. (2017b). Dietary exposure assessment of Chinese population to tetrabromobisphenol‐A, hexabromocyclododecane and decabrominated diphenyl ether: Results of the 5th Chinese Total Diet Study. Environmental Pollution, 229, 539–547. [DOI] [PubMed] [Google Scholar]
- Shin, E. S. , Jeong, Y. , Barghi, M. , Seo, S. H. , Kwon, S. Y. , & Chang, Y. S. (2020). Internal distribution and fate of persistent organic contaminants (PCDD/Fs, DL‐PCBs, HBCDs, TBBPA, and PFASs) in a Bos Taurus. Environmental Pollution, 267, 115306. [DOI] [PubMed] [Google Scholar]
- Shockley, K. R. , Cora, M. C. , Malarkey, D. E. , Jackson‐Humbles, D. , Vallant, M. , Collins, B. J. , Mutlu, E. , Robinson, V. G. , Waidyanatha, S. , Zmarowski, A. , Machesky, N. , Richey, J. , Harbo, S. , Cheng, E. , Patton, K. , Sparrow, B. , & Dunnick, J. K. (2020a). Comparative toxicity and liver transcriptomics of legacy and emerging brominated flame retardants following 5‐day exposure in the rat. Toxicology Letters, 332, 222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockley, K. R. , Cora, M. C. , Malarkey, D. E. , Jackson‐Humbles, D. , Vallant, M. , Collins, B. J. , Mutlu, E. , Robinson, V. G. , Waidyanatha, S. , Zmarowski, A. , Machesky, N. , Richey, J. , Harbo, S. , Cheng, E. , Patton, K. , Sparrow, B. , & Dunnick, J. K. (2020b). Transcriptomic data from the rat liver after five days of exposure to legacy or emerging brominated flame retardants. Data in Brief, 32, 106136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti, G. , Di Filippo, P. , Riccardi, C. , Pomata, D. , Sonego, E. , & Buiarelli, F. (2020). Occurrence of halogenated pollutants in domestic and occupational indoor dust. International Journal of Environmental Research and Public Health, 17, 3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkó, R. , Rada, K. , Kollár, A. , Mohácsik, P. , Tenk, M. , Fekete, C. , & Gereben, B. (2022). Tetrabromobisphenol A and diclazuril evoke tissue‐specific changes of thyroid hormone signaling in male thyroid hormone action indicator mice. International Journal of Molecular Sciences, 23, 14782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skledar, D. G. , & Masic, L. P. (2016). Bisphenol A and its analogs: Do their metabolites have endocrine activity? Environmental Toxicology and Pharmacology, 47, 182–199. [DOI] [PubMed] [Google Scholar]
- Smith, M. T. , Guyton, K. Z. , Gibbons, C. F. , Fritz, J. M. , Portier, C. J. , Rusyn, I. , DeMarini, D. M. , Caldwell, J. C. , Kavlock, R. J. , Lambert, P. F. , Hecht, S. S. , Bucher, J. R. , Stewart, B. W. , Baan, R. A. , Cogliano, V. J. , & Straif, K. (2016). Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environmental Health Perspectives, 124, 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, L. , Gao, J. , Han, K. , Wang, X. , & He, J. (2013). Investigation of tetrabromobisphenol A interacting with DNA nitrogen bases using experimental and density functional theory methods. Toxicological & Environmental Chemistry, 95, 970–983. [Google Scholar]
- Song, S. , Li, Y. , Lv, L. , Dong, M. , & Qin, Z. (2024). Tetrabromobisphenol A exerts thyroid disrupting effects but has little overt impact on postnatal brain development and neurobehaviors in mice. Journal of Environmental Sciences, 142, 1–10. [DOI] [PubMed] [Google Scholar]
- Sousa, S. , Pestana, D. , Faria, G. , Delerue‐Matos, C. , Calhau, C. , & Fernandes Domingues, V. (2023). Assessment of synthetic musks, polychlorinated biphenyls and brominated flame retardants in adipose tissue of obese northern Portuguese women – Metabolic implications. Science of the Total Environment, 894, 165015. [DOI] [PubMed] [Google Scholar]
- SRI Int . (1978). In vitro microbiological mutagenicity studies of Dow Chemical Company compounds (interim report). TSCATS [Unpublished Health and Safety Studies submitted to EPA]. Microfiche No. OTS0516107. Document No. 86‐870001204. From the RTK Net database. As cited by NIEHS (2002).
- Stapleton, H. M. , Misenheimer, J. , Hoffman, K. , & Webster, T. F. (2014). Flame retardant associations between children's handwipes and house dust. Chemosphere, 116, 54–60. 10.1016/j.chemosphere.2013.12.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavreva, D. A. , Varticovski, L. , Levkova, L. , George, A. A. , Davis, L. , Pegoraro, G. , Blazer, V. , Iwanowicz, L. , & Hager, G. L. (2016). Novel cell‐based assay for detection of thyroid receptor beta‐interacting environmental contaminants. Toxicology, 368‐369, 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steves, A. N. , Bradner, J. M. , Fowler, K. L. , Clarkson‐Townsend, D. , Gill, B. J. , Turry, A. C. , Caudle, W. M. , Miller, G. W. , Chan, A. W. , & Easley, C. A. (2018). Ubiquitous flame‐retardant toxicants impair spermatogenesis in a human stem cell model. Iscience, 3, 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, H. , Guan, G. , Ahmed, R. Z. , Lyu, L. , Li, Z. , & Jin, X. (2020). TBBPA stimulated cell migration of endometrial cancer via the contribution of NOX‐generated ROS in lieu of energy metabolism. Journal of Hazardous Materials, 400, 123204. [DOI] [PubMed] [Google Scholar]
- Suh, K. S. , Choi, E. M. , Rhee, S. Y. , Oh, S. , Kim, S. W. , Pak, Y. K. , Choe, W. , Ha, J. , & Chon, S. (2017). Tetrabromobisphenol A induces cellular damages in pancreatic β‐cells in vitro. Journal of Environmental Science and Health, Part A, 52, 624–631. [DOI] [PubMed] [Google Scholar]
- Sühring, R. , Barber, J. L. , Wolschke, H. , Kotke, D. , & Ebinghaus, R. (2015). Fingerprint analysis of brominated flame retardants and Dechloranes in North Sea sediments. Environmental Research, 140, 569–578. [DOI] [PubMed] [Google Scholar]
- Svihlikova, V. , Lankova, D. , Poustka, J. , Tomaniova, M. , Hajslova, J. , & Pulkrabova, J. (2015). Perfluoroalkyl substances (PFASs) and other halogenated compounds in fish from the upper Labe River basin. Chemosphere, 129, 170–178. [DOI] [PubMed] [Google Scholar]
- Szychowski, K. A. , Rybczyńska‐Tkaczyk, K. , Leja, M. L. , Wójtowicz, A. K. , & Gmiński, J. (2016). Tetrabromobisphenol A (TBBPA)‐stimulated reactive oxygen species (ROS) production in cell‐free model using the 2′,7′‐dichlorodihydrofluorescein diacetate (H(2)DCFDA) assay‐limitations of method. Environmental Science and Pollution Research, 23, 12246–12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szychowski, K. A. , & Wojtowicz, A. K. (2016). TBBPA causes neurotoxic and the apoptotic responses in cultured mouse hippocampal neurons in vitro. Pharmacological Reports, 68, 20–26. [DOI] [PubMed] [Google Scholar]
- Szymańska, J. A. , Sapota, A. , & Frydrych, B. (2001). The disposition and metabolism of tetrabromobisphenol‐A after a single ip dose in the rat. Chemosphere, 45, 693–700. [DOI] [PubMed] [Google Scholar]
- Tada, Y. , Fujitani, T. , Yano, N. , Takahashi, H. , Yuzawa, K. , Ando, H. , Kubo, Y. , Nagasawa, A. , Ogata, A. , & Kamimura, H. (2006). Effects of tetrabromobisphenol A, brominated flame retardant, in ICR mice after prenatal and postnatal exposure. Food Chemistry and Toxicology, 44, 1408–1413. [DOI] [PubMed] [Google Scholar]
- Tada, Y. , Fujitani, T. , Ogata, A. , & Kamimura, H. (2007). Flame retardant tetrabromobisphenol A induced hepatic changes in ICR male mice. Environmental Toxicology and Pharmacology, 23, 174–178. [DOI] [PubMed] [Google Scholar]
- Takeshita, T. , Watanabe, W. , Toyama, S. , Hayashi, Y. , Honda, S. , Sakamoto, S. , Matsuoka, S. , Yoshida, H. , Takeda, S. , Hidaka, M. , & Tsutsumi, S. (2013). Effect of Brazilian propolis on exacerbation of respiratory syncytial virus infection in mice exposed to tetrabromobisphenol A, a brominated flame retardant. Evidence‐Based Complementary and Alternative Medicine, 2013, 698206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, B. , Zeng, Y. H. , Luo, X. J. , Zheng, X. B. , & Mai, B. X. (2015). Bioaccumulative characteristics of tetrabromobisphenol A and hexabromocyclododecanes in multi‐tissues of prey and predator fish from an e‐waste site, South China. Environmental Science and Pollution Research, 22, 12011–12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, P. , Liang, J. , Liao, Q. , Huang, H. , Guo, X. , Lin, M. , Liu, B. , Wei, B. , Zeng, X. , Liu, S. , & Huang, D. (2023). Associations of bisphenol exposure with the risk of gestational diabetes mellitus: a nested case–control study in Guangxi, China. Environmental Science and Pollution Research, 30(10), 25170–25180. [DOI] [PubMed] [Google Scholar]
- Tao, F. , Abdallah, M. A. , & Harrad, S. (2016). Emerging and legacy flame retardants in UK indoor air and dust: Evidence for replacement of PBDEs by emerging flame retardants? Environmental Science and Technology, 50, 13052–13061. [DOI] [PubMed] [Google Scholar]
- Tay, J. H. , Sellström, U. , Papadopoulou, E. , Padilla‐Sánchez, J. A. , Haug, L. S. , & de Wit, C. A. (2017). Human exposure to legacy and emerging halogenated flame retardants via inhalation and dust ingestion in a Norwegian cohort. Environmental Science and Technology, 51, 8176–8184. [DOI] [PubMed] [Google Scholar]
- Tay, J. H. , Sellström, U. , Papadopoulou, E. , Padilla‐Sánchez, J. A. , Haug, L. S. , & de Wit, C. A. (2018). Assessment of dermal exposure to halogenated flame retardants: Comparison using direct measurements from hand wipes with an indirect estimation from settled dust concentrations. Environment International, 115, 285–294. [DOI] [PubMed] [Google Scholar]
- Tay, J. H. , Sellström, U. , Papadopoulou, E. , Padilla‐Sánchez, J. A. , Haug, L. S. , & de Wit, C. A. (2019). Serum concentrations of legacy and emerging halogenated flame retardants in a Norwegian cohort: Relationship to external exposure. Environmental Research, 178, 108731. [DOI] [PubMed] [Google Scholar]
- Terasaki, M. , Kosaka, K. , Kunikane, S. , Makino, M. , & Shiraishi, F. (2011). Assessment of thyroid hormone activity of halogenated bisphenol A using a yeast two‐hybrid assay. Chemosphere, 84, 1527–1530. [DOI] [PubMed] [Google Scholar]
- Tetz, L. M. , Kamau, P. W. , Cheng, A. A. , Meeker, J. D. , & Loch‐Caruso, R. (2013). Troubleshooting the dichlorofluorescein assay to avoid artifacts in measurement of toxicant‐stimulated cellular production of reactive oxidant species. Journal of Pharmacological and Toxicological Methods, 67, 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen, C. , Liane, V. H. , & Becher, G. (2007). Automated solid‐phase extraction for the determination of polybrominated diphenyl ethers and polychlorinated biphenyls in serum – Application on archived Norwegian samples from 1977 to 2003. Journal of Chromatography B, 846, 252–263. [DOI] [PubMed] [Google Scholar]
- Thomsen, C. , Lundanes, E. , & Becher, G. (2002). Brominated flame retardants in archived serum samples from Norway: A study on temporal a trends and role of age. Environmental Science and Technology, 36, 1414–1418. [DOI] [PubMed] [Google Scholar]
- USEPA (United States Environmental Protection Agency) . (2011). Exposure factors handbook (2011th ed.). U.S. Environmental Protection Agency. [Google Scholar]
- USEPA (United States Environmental Protection Agency) . (2020). Final scope of the risk evaluation for 4,4′‐(1‐methylethylidene)bis[2,6‐dibromophenol] (TBBPA). Supplemental file: Data extraction and evaluation tables for physical and chemical property studies CASRN: 79‐94‐7.
- van der Ven, L. T. M. , Van de Kuil, T. , Verhoef, A. , Verwer, C. M. , Lilienthal, H. , Leonards, P. E. G. , Schauer, U. M. D. , Cantón, R. F. , Litens, S. , De Jong, F. H. , Visser, T. J. , Dekant, W. , Stern, N. , Håkansson, H. , Slob, W. , Van den Berg, M. , Vos, J. G. , & Piersma, A. H. (2008). Endocrine effects of tetrabromobisphenol‐A (TBBPA) in Wistar rats as tested in a one‐generation reproduction study and a subacute toxicity study. Toxicology, 245, 76–89. [DOI] [PubMed] [Google Scholar]
- Vasiljevic, T. , & Harner, T. (2021). Bisphenol A and its analogues in outdoor and indoor air: Properties, sources and global levels. Science of the Total Environment, 789, 148013. [DOI] [PubMed] [Google Scholar]
- Velsicol Chemical Corporation . (1978). To determine the rates of absorption, distribution and excretion of tetrabromobisphenol A in rats [Unpublished]. As Cited by ECB (2006).
- Venisseau, A. , Bichon, E. , Brosseaud, A. , Vaccher, V. , Lesquin, E. , Larvor, F. , Durand, S. , Dervilly‐Pinel, G. , Marchand, P. , & Le Bizec, B. (2018). Occurrence of legacy and novel brominated flame retardants in food and feed in France for the period 2014 to 2016. Chemosphere, 207, 497–506. [DOI] [PubMed] [Google Scholar]
- Viberg, H. , & Eriksson, P. (2011). Differences in neonatal neurotoxicity of brominated flame retardants, PBDE 99 and TBBPA, in mice. Toxicology, 289, 59–65. [DOI] [PubMed] [Google Scholar]
- Viganò, L. , Guzzella, L. , Marziali, L. , Mascolo, G. , Bagnuolo, G. , Ciannarella, R. , & Roscioli, C. (2023). The last 50 years of organic contamination of a highly anthropized tributary of the Po River (Italy). Journal of Environmental Management, 326, 116665. [DOI] [PubMed] [Google Scholar]
- Viganò, L. , Mascolo, G. , & Roscioli, C. (2015). Emerging and priority contaminants with endocrine active potentials in sediments and fish from the River Po (Italy). Environmental Science and Pollution Research, 22, 14050–14066. [DOI] [PubMed] [Google Scholar]
- Waiyarat, S. , Boontanon, S. K. , Boontanon, N. , Fujii, S. , Harrad, S. , Drage, D. S. , & Abdallah, M. A. (2022). Exposure, risk and predictors of hexabromocyclododecane and tetrabromobisphenol‐A in house dust from urban, rural and E‐waste dismantling sites in Thailand. Chemosphere, 302, 134730. [DOI] [PubMed] [Google Scholar]
- Wang, B. , Wang, H. , Han, D. , Chen, J. , & Yin, Y. (2020). Studying the mixture effects of brominated flame retardants and metal ions by comet assay. Environmental Pollution, 267, 115677. [DOI] [PubMed] [Google Scholar]
- Wang, J. , & Dai, G. D. (2022). Comparative effects of brominated flame retardants BDE‐209, TBBPA, and HBCD on neurotoxicity in mice. Chemical Research in Toxicology, 35, 1512–1518. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Wang, Y. , Shi, Z. , Zhou, X. , & Sun, Z. (2018). Legacy and novel brominated flame retardants in indoor dust from Beijing, China: Occurrence, human exposure assessment and evidence for PBDEs replacement. Science of the Total Environment, 618, 48–59. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Zhao, X. , Wang, Y. , & Shi, Z. (2019). Tetrabromobisphenol A, hexabromocyclododecane isomers and polybrominated diphenyl ethers in foodstuffs from Beijing, China: Contamination levels, dietary exposure and risk assessment. The Science of the Total Environment, 666, 812–820. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Liu, J. , Liu, A. , Liu, Q. , Du, X. , & Jiang, G. (2012). Preparation and evaluation of mesoporous cellular foams coating of solid‐phase microextraction fibers by determination of tetrabromobisphenol A, tetrabromobisphenol S and related compounds. Analytica Chimica Acta, 753, 1–7. [DOI] [PubMed] [Google Scholar]
- Wang, Y. Q. , Zhang, H. M. , & Cao, J. (2014). Quest for the binding mode of tetrabromobisphenol A with calf thymus DNA. Spectrochimica Acta Part a‐Molecular and Biomolecular Spectroscopy, 131, 109–113. [DOI] [PubMed] [Google Scholar]
- Wannomai, T. , Matsukami, H. , Uchida, N. , Takahashi, F. , Viet, P. H. , Takahashi, S. , Kunisue, T. , & Suzuki, G. (2021). Inhalation bioaccessibility and health risk assessment of flame retardants in indoor dust from Vietnamese e‐waste‐dismantling workshops. Science of the Total Environment, 760, 143862. [DOI] [PubMed] [Google Scholar]
- Watanabe, W. , Hashiguchi, S. , Sakata, K. , Miyauchi, A. , Hirose, A. , Takeshita, T. , Konno, K. , Yoshida, H. , Sugita, C. , Kurokawa, M. , & Akashi, T. (2017). Perinatal exposure to tetrabromobisphenol A (TBBPA), a brominated flame retardant, exacerbated the pneumonia in respiratory syncytial virus (RSV)‐infected offspring mice. The Journal of Toxicological Sciences, 42, 789–795. [DOI] [PubMed] [Google Scholar]
- Watanabe, W. , Shimizu, T. , Sawamura, R. , Hino, A. , Konno, K. , Hirose, A. , & Kurokawa, M. (2010). Effects of tetrabromobisphenol A, a brominated flame retardant, on the immune response to respiratory syncytial virus infection in mice. International Immunopharmacology, 10, 393–397. [DOI] [PubMed] [Google Scholar]
- Wei, B. N. , Wei, B. , Goniewicz, M. , & O'Connor, R. J. (2019). Concurrent quantification of emerging chemicals of health concern in e‐cigarette liquids by high‐performance liquid chromatography‐tandem mass spectrometry. ACS Omega, 4, 15364–15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, X. , Hu, Y. , Zhu, Q. , Gao, J. , Liao, C. , & Jiang, G. (2022). Co‐exposure and health risks of several typical endocrine disrupting chemicals in general population in eastern China. Environmental Research, 204, 112366. [DOI] [PubMed] [Google Scholar]
- Whalen, M. M. , & Kibakaya, E. C. (2011). Tetrabromobisphenol A activates mitogen‐activated protein kinases in human natural killer cells. The FASEB Journal, 25, 1.21205780 [Google Scholar]
- WHO/ICPS (World Health Organization/International Programme on Chemical Safety) . (1995). Environmental health criteria 172: Tetrabromobisphenol A and derivatives. World Health Organization. http://www.inchem.org/documents/ehc/ehc/ehc172.htm [Google Scholar]
- WHO/ICPS (World Health Organization/International Programme on Chemical Safety) . (2009). Principles and methods for the risk assessment of chemicals in food, international programme on chemical safety, environmental health criteria 240. Chapter 6: Dietary exposure assessment of chemicals in food. http://www.who.int/ipcs/food/principles/en/index1.html
- Wikoff, D. S. , Rager, J. E. , Haws, L. C. , & Borghoff, S. J. (2016). A high dose mode of action for tetrabromobisphenol A‐induced uterine adenocarcinomas in Wistar Han rats: A critical evaluation of key events in an adverse outcome pathway framework. Regulatory Toxicology and Pharmacology, 77, 143–159. 10.1016/j.yrtph.2016.01.018 [DOI] [PubMed] [Google Scholar]
- Williams, A. L. , & de Sesso, J. M. (2010). The potential of selected brominated flame retardants to affect neurological development. Journal of Toxicology and Environmental Health, Part B, 13, 411–448. [DOI] [PubMed] [Google Scholar]
- Włuka, A. , Woźniak, A. , Woźniak, E. , & Michałowicz, J. (2020). Tetrabromobisphenol A, terabromobisphenol S and other bromophenolic flame retardants cause cytotoxic effects and induce oxidative stress in human peripheral blood mononuclear cells (in vitro study). Chemosphere, 261, 127705. [DOI] [PubMed] [Google Scholar]
- Wojtowicz, A. K. , Szychowski, K. A. , & Kajta, M. (2014). PPAR‐γ agonist GW1929 but not antagonist GW9662 reduces TBBPA‐induced neurotoxicity in primary neocortical cells. Neurotoxicity Research, 25, 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , Li, Y. , Kang, D. , Wang, J. , Zhang, Y. , Du, D. , Pan, B. , Lin, Z. , Huang, C. , & Dong, Q. (2016). Tetrabromobisphenol A and heavy metal exposure via dust ingestion in an e‐waste recycling region in Southeast China. Science of the Total Environment, 541, 356–364. [DOI] [PubMed] [Google Scholar]
- Xi, J. , Su, X. , Wang, Z. , Ji, H. , Chen, Y. , Liu, X. , Miao, M. , Liang, H. , & Yuan, W. (2023). The associations between concentrations of gestational bisphenol analogues and thyroid related hormones in cord blood: A prospective cohort study. Ecotoxicology and Environmental Safety, 256, 114838. 10.1016/j.ecoenv.2023.114838 [DOI] [PubMed] [Google Scholar]
- Xiong, Y. M. , Li, Y. Y. , Lv, L. , Chen, X. Y. , Li, X. H. , & Qin, Z. F. (2023). Postnatal exposure to low‐dose tetrabromobisphenol A increases the susceptibility of mammal testes to chemical‐induced spermatogenic stress in adulthood. Environment International, 171, 107683. [DOI] [PubMed] [Google Scholar]
- Xu, H. , Su, J. , Ku, T. , Liu, Q. S. , Liang, J. , Ren, Z. , Zhou, Q. , & Jiang, G. (2022). Constructing an MCF‐7 breast cancer cell‐based transient transfection assay for screening RARα (Ant) agonistic activities of emerging phenolic compounds. Journal of Hazardous Materials, 435, 129024. [DOI] [PubMed] [Google Scholar]
- Xu, L. , Hu, Y. , Zhu, Q. , Liao, C. , & Jiang, G. (2022). Several typical endocrine‐disrupting chemicals in human urine from general population in China: Regional and demographic‐related differences in exposure risk. Journal of Hazardous Materials, 424, 127489. [DOI] [PubMed] [Google Scholar]
- Yang, S. , Wang, S. , Liu, H. , & Yan, Z. (2012). Tetrabromobisphenol A: Tissue distribution in fish, and seasonal variation in water and sediment of Lake Chaohu, China. Environmental Science and Pollution Research, 19, 4090–4096. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Guan, J. , Yin, J. , Shao, B. , & Li, H. (2014). Urinary levels of bisphenol analogues in residents living near a manufacturing plant in south China. Chemosphere, 112, 481–486. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Ni, W. W. , Yu, L. , Cai, Z. , & Yu, Y. J. (2016). Toxic effects of tetrabromobisphenol A on thyroid hormones in SD rats and the derived‐reference dose. Biomedical and Environmental Sciences, 29, 295–299. [DOI] [PubMed] [Google Scholar]
- Yao, L. , Wang, Y. , Shi, J. , Liu, Y. , Guo, H. , Yang, X. , Liu, Y. , Ma, J. , Li, D. , Wang, Z. , Li, Z. , Luo, Q. , Fu, J. , Zhang, Q. , Qu, G. , Wang, Y. , & Jiang, G. (2021). Toxicity of tetrabromobisphenol A and its derivative in the mouse liver following oral exposure at environmentally relevant levels. Environmental Science and Technology, 55, 8191–8202. [DOI] [PubMed] [Google Scholar]
- Yin, L. , Hu, C. , & Yu, X. J. (2023). High‐content analysis of testicular toxicity of BPA and its selected analogs in mouse spermatogonial, Sertoli cells, and Leydig cells revealed BPAF induced unique multinucleation phenotype associated with the increased DNA synthesis. Toxicology in Vitro, 89, 105589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, L. , Siracusa, J. S. , Measel, E. , Guan, X. , Edenfield, C. , Liang, S. , & Yu, X. (2020). High‐content image‐based single‐cell phenotypic analysis for the testicular toxicity prediction induced by Bisphenol A and its analogs Bisphenol S, Bisphenol AF, and tetrabromobisphenol A in a three‐dimensional testicular cell co‐culture model. Toxicological Sciences, 173, 313–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, N. , Liang, S. , Liang, S. , Yang, R. , Hu, B. , Qin, Z. , Liu, A. , & Faiola, F. (2018). TBBPA and its alternatives disturb the early stages of neural development by interfering with the NOTCH and WNT pathways. Environmental Science & Technology, 52, 5459–5468. [DOI] [PubMed] [Google Scholar]
- Yu, P. , Yang, G. , Tan, H. , Cheng, Z. , Song, M. , Gu, Z. , & Li, X. (2011). Determination of pilocarpine in human plasma by LC‐APCI‐MS‐MS and application to a pharmacokinetic study. Chromatographia, 73, 921–927. [Google Scholar]
- Yu, X. , Yin, H. , Peng, H. , Lu, G. , Liu, Z. , & Dang, Z. (2019). OPFRs and BFRs induced A549 cell apoptosis by caspase‐dependent mitochondrial pathway. Chemosphere, 221, 693–702. [DOI] [PubMed] [Google Scholar]
- Yu, Y. , Ma, R. , Yu, L. , Cai, Z. , Li, H. , Zuo, Y. , Wang, Z. , & Li, H. (2018). Combined effects of cadmium and tetrabromobisphenol A (TBBPA) on development, antioxidant enzymes activity and thyroid hormones in female rats. Chemico‐Biological Interactions, 289, 23–31. [DOI] [PubMed] [Google Scholar]
- Zacs, D. , Rjabova, J. , Ikkere, L. E. , Bavrins, K. , & Bartkevics, V. (2018). Brominated flame retardants and toxic elements in the meat and liver of red deer (Cervus elaphus), wild boar (Sus scrofa), and moose (Alces alces) from Latvian wildlife. Science of the Total Environment, 621, 308–316. [DOI] [PubMed] [Google Scholar]
- Zatecka, E. , Castillo, J. , Elzeinova, F. , Kubatova, A. , Ded, L. , Peknicova, J. , & Oliva, R. (2014). The effect of tetrabromobisphenol A on protamine content and DNA integrity in mouse spermatozoa. Andrology, 2, 910–917. [DOI] [PubMed] [Google Scholar]
- Zatecka, E. , Ded, L. , Elzeinova, F. , Kubatova, A. , Dorosh, A. , Margaryan, H. , Dostalova, P. , & Peknicova, J. (2013). Effect of tetrabrombisphenol A on induction of apoptosis in the testes and changes in expression of selected testicular genes in CD1 mice. Reproductive Toxicology, 35, 32–39. [DOI] [PubMed] [Google Scholar]
- Zeng, Y. H. , Luo, X. J. , Tang, B. , & Mai, B. X. (2016). Habitat‐ and species‐dependent accumulation of organohalogen pollutants in home‐produced eggs from an electronic waste recycling site in South China: Levels, profiles, and human dietary exposure. Environmental Pollution, 216, 64–70. [DOI] [PubMed] [Google Scholar]
- Zhang, A. , Wang, R. , Liu, Q. , Yang, Z. , Lin, X. , Pang, J. , Li, X. , Wang, D. , He, J. , Li, J. , Zhang, M. , Yu, Y. , Cao, X. C. , Chen, X. , & Tang, N. J. (2023). Breast adipose metabolites mediates the association of tetrabromobisphenol A with breast cancer: A case‐control study in Chinese population. Environmental Pollution, 316, 120701. 10.1016/j.envpol.2022.120701 [DOI] [PubMed] [Google Scholar]
- Zhang, F. , Bartels, M. , Clark, A. , Erskine, T. , Auernhammer, T. , Bhhatarai, B. , Wilson, D. , & Marty, S. (2018). Performance evaluation of the GastroPlusTM software tool for prediction of the toxicokinetic parameters of chemicals. SAR and QSAR in Environmental Research, 29, 875–893. [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Ireland, D. , Sipes, N. S. , Behl, M. , & Collins, E. S. (2019). Screening for neurotoxic potential of 15 flame retardants using freshwater planarians. Neurotoxicology and Teratology, 73, 54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. H. , Zhang, Y. X. , Gui‐Xiang, J. I. , Huai‐Zhou, X. U. , Ji‐Ning, L. , & Li‐Li, S. (2016). Determination of bisphenol A, tetrabromobisphenol A and 4‐Tert‐Octylphenol in children and adults urine using high performance liquid chromatography‐tandem mass spectrometry. Chinese Journal of Analytical Chemistry, 44, 19–24. [Google Scholar]
- Zhang, W. , Li, A. , Pan, Y. , Wang, F. , Li, M. , Liang, Y. , Yao, X. , Song, J. , Song, M. , & Jiang, G. (2021). Tetrabromobisphenol A induces THR β‐mediated inflammation and uterine injury in mice at environmentally relevant exposure concentrations. Journal of Hazardous Materials, 407, 124859. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Wang, X. , Chen, C. , An, J. , Shang, Y. , Li, H. , Xia, H. , Yu, J. , Wang, C. , Liu, Y. , & Guo, S. (2019). Regulation of TBBPA‐induced oxidative stress on mitochondrial apoptosis in L02 cells through the Nrf2 signaling pathway. Chemosphere, 226, 463–471. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. F. , Xu, W. , Lou, Q. Q. , Li, Y. Y. , Zhao, Y. X. , Wei, W. J. , Qin, Z. F. , Wang, H. L. , & Li, J. Z. (2014). Tetrabromobisphenol A disrupts vertebrate development via thyroid hormone signaling pathway in a developmental stage‐dependent manner. Environmental Science & Technology, 48, 8227–8234. [DOI] [PubMed] [Google Scholar]
- Zhang, Z. , Dong, S. , Ge, D. , Zhu, N. , Wang, K. , Zhu, G. , Xu, W. , & Xu, H. (2018). An ultrasensitive competitive immunosensor using silica nanoparticles as an enzyme carrier for simultaneous impedimetric detection of tetrabromobisphenol A bis(2‐hydroxyethyl) ether and tetrabromobisphenol A mono(hydroxyethyl) ether. Biosensors & Bioelectronics, 105, 77–80. [DOI] [PubMed] [Google Scholar]
- Zhao, C. , Tang, Z. , Chung, A. C. , Wang, H. , & Cai, Z. (2019). Metabolic perturbation, proliferation and reactive oxygen species jointly contribute to cytotoxicity of human breast cancer cell induced by tetrabromo and tetrachloro bisphenol A. Ecotoxicology and Environmental Safety, 170, 495–501. [DOI] [PubMed] [Google Scholar]
- Zhao, M. , Kim, Y. T. , Yoon, B. S. , Kim, S. W. , Kang, M. H. , Kim, S. H. , Kim, J. H. , Kim, J. W. , & Park, Y. W. (2006). Expression profiling of cyclin B1 and D1 in cervical carcinoma. Experimental Oncology, 28(1), 44–48. [PubMed] [Google Scholar]
- Zhao, X. , Lyu, B. , Zhang, L. , Li, J. , Zhao, Y. , Wu, Y. , & Shi, Z. (2023). Legacy and novel brominated flame retardants in animal‐derived foods from China Total Diet Study (CTDS): Temporal trends, evidence of substitution, and dietary exposure assessment. Journal of Hazardous Materials, 443, 130223. [DOI] [PubMed] [Google Scholar]
- Zhao, X. , & Shi, Z. (2021). Legacy brominated flame retardants in human milk from the general population in Beijing, China: Biomonitoring, temporal trends from 2011 to 2018, and nursing infant's exposure assessment. Chemosphere, 285, 131533. [DOI] [PubMed] [Google Scholar]
- Zheng, X. , Sun, R. , Qiao, L. , Guo, H. , Zheng, J. , & Mai, B. (2017). Flame retardants on the surface of phones and personal computers. Science of The Total Environment, 609, 541–545. 10.1016/j.scitotenv.2017.07.202 [DOI] [PubMed] [Google Scholar]
- Zhu, H. , Cheng, Q. , Liu, J. , Jin, L. , Li, Z. , Ren, A. , & Wang, L. (2023). Associations among bisphenol A, its analogs, and chlorinated derivatives in placenta and risk for neural tube defects: A case‐control study. Science of the Total Environment, 900, 165586. [DOI] [PubMed] [Google Scholar]
- Zieminska, E. , Lenart, J. , Diamandakis, D. , & Lazarewicz, J. W. (2017). The role of Ca2+ imbalance in the induction of acute oxidative stress and cytotoxicity in cultured rat cerebellar granule cells challenged with tetrabromobisphenol A. Neurochemical Research, 42, 777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieminska, E. , Lenart, J. , & Lazarewicz, J. W. (2016). Select putative neurodevelopmental toxins modify SNAP‐25 expression in primary cultures of rat cerebellar granule cells. Toxicology, 370, 86–93. [DOI] [PubMed] [Google Scholar]
- Zieminska, E. , Ruszczynska, A. , & Lazarewicz, J. W. (2017). Tetrabromobisphenol A disturbs zinc homeostasis in cultured cerebellar granule cells: A dual role in neurotoxicity. Food and Chemical Toxicology, 109, 363–375. [DOI] [PubMed] [Google Scholar]
- Zieminska, E. , Salinska, E. , & Lazarewicz, J. W. (2015). Tetrabromobisphenol‐A‐evoked oxidative stress and cytotoxicity in cultured neurons: Involvement of zinc. Toxicology Letters, 238, S284–S285. [Google Scholar]
- Zieminska, E. , Stafiej, A. , Toczylowska, B. , Albrecht, J. , & Lazarewicz, J. W. (2015). Role of ryanodine and NMDA receptors in tetrabromobisphenol A‐induced calcium imbalance and cytotoxicity in primary cultures of rat cerebellar granule cells. Neurotoxicity Research, 28, 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieminska, E. , Stafiej, A. , Toczylowska, B. , & Lazarewicz, J. W. (2012a). Acute cytotoxicity evoked by tetrabromobisphenol A in primary cultures of rat cerebellar granule cells outweighs the effects of polychlorinated biphenyls. Polish Journal of Environmental Studies, 21, 1079–1087. [Google Scholar]
- Zieminska, E. , Stafiej, A. , Toczyłowska, B. , & Lazarewicz, J. W. (2012b). Synergistic neurotoxicity of oxygen‐glucose deprivation and tetrabromobisphenol A in vitro: Role of oxidative stress. Pharmacological Reports, 64, 1166–1178. [DOI] [PubMed] [Google Scholar]
- Zieminska, E. , Stafiej, A. , Toczylowska, B. , & Lazarewicz, J. W. (2014). Bastadin 12 and ryanodine reveal similarities between thapsigargin‐ and tetrabromobisphenol A‐induced intracellular Ca2+ release in cultured cerebellar granule cells. Journal of Physiology and Pharmacology, 65, 679–686. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol for the risk assessments for human health related to the presence of brominated flame retardants (BFRs) in food
Benchmark dose analysis
Uncertainty analysis – protocol and results of the EKE
Outcome of the public consultation


