Instigating effective treatment regimens in a way that improves patient adherence is vital to tackling the global resurgence of tuberculosis
About one third of the world's population has latent tuberculosis, caused by Mycobacterium tuberculosis infection.1 From this pool, roughly 9 million cases of active tuberculosis emerge annually, resulting in 2-3 million deaths. Most new cases occur in the most populated nations—India and China—but the highest rates of disease are seen in sub-Saharan Africa, the Indonesian and Philippine archipelagos, Afghanistan, Bolivia, and Peru. In these regions case rates typically exceed 300 cases per 100 000 per year.1,2 Although the incidence of tuberculosis declined in North America and western Europe throughout most of the latter half of the 20th century, case rates have increased over the past 10 years mainly because of immigration, HIV/AIDS, and the neglect of tuberculosis control programmes.3,4 One vital factor in curbing the increase of tuberculosis is the instigation of proper treatment that not only encompasses an effective regimen but also ensures compliance with and response to treatment. This review highlights current treatment recommendations for tuberculosis.
Summary points
Many people worldwide have latent or active tuberculosis, and the number of active cases is expected to increase in the future
The most common cause of treatment failure and acquired drug resistance is non-adherence; predicting non-adherence is highly problematic
Directly observed therapy is the most effective means of combatting non-adherence; intermittent (less than daily) regimens facilitate the therapy
Testing the susceptibility of Mycobacterium tuberculosis to drugs is essential for identifying resistance and tailoring treatment
Managing multidrug resistant tuberculosis is complex and should, when possible, be done in specialised programmes
Sources and selection criteria
We performed a Medline search of the past 10 years using the key words “tuberculosis and treatment or drug therapy” to find pertinent literature. We also searched bibliographies from review articles on the treatment of tuberculosis for relevant references.
Principles of chemotherapy and rationale for multidrug regimen
Treatment using more than one drug is based on two principles: preventing acquired drug resistance and enhancing efficacy. Tubercle bacilli undergo random chromosomal mutations that have made them resistant to every drug used to treat tuberculosis. Fortunately, these mutations are infrequent.5 Because they are unlinked (in terms of chromosomal location or function) and specific to a drug or drug class, spontaneous generation of an organism with multiresistance is extremely improbable. Acquired drug resistance for tuberculosis is almost always caused by inadequate treatment. This can include failure of the patient to take the prescribed drugs, failure of the physician to prescribe appropriately, failure of the healthcare system to ensure that drugs are available, or—rarely—malabsorption of the drug(s) due to dysfunction of the digestive system or substandard bioavailability of the preparation.
Treatment that uses a combination of drugs (table 1) has been shown to accelerate the response of the disease to treatment and to shorten the length of treatment required to cure.6 Rifampicin and isoniazid are the main drugs used today, rifampicin being the more important agent in terms of reducing the duration of treatment and assuring favourable outcomes.6 Nine month regimens using rifampicin and isoniazid, together with an introductory phase of streptomycin or ethambutol, or both, have been predicted to cure 95% or more patients.7 Studies from the UK's Medical Research Council showed that, if pyrazinamide is included in the treatment for the first two months, the length of treatment could be reduced to six months and still retain cure rates of 95% or more.8
Table 1.
Current regimens for treatment of drug susceptible tuberculosis
| Regimen
|
Initial phase
|
Continuation phase
|
|---|---|---|
| Daily* | 2 months of isoniazid, rifampicin, and pyrazinamide, with or without ethambutol | 4 months of isoniazid and rifampicin |
| Intermittent† | 2 weeks of daily isoniazid, rifampicin, pyrazinamide and streptomycin or ethambutol‡ | 24 weeks of twice weekly isoniazid and rifampicin‡ |
| 8 weeks of thrice weekly isoniazid, rifampicin, pyrazinamide and streptomycin or ethambutol§ | 18 weeks of thrice weekly isoniazid and rifampicin§ |
The daily regimen is used when patients self administer their drugs. There is enough redundancy that, if patients miss some of their doses, the outcome will remain acceptable.
The intermittent regimens are intended for directly observed therapy.
This regimen (initial and continuation phase) entails a total of 62 doses and has yielded over 95% success rates for the past 22 years in Denver, Colorado.14
This regimen (initial and continuation phase) uses 78 doses and has also resulted in success rates of approximately 95% in Hong Kong, where it is the standard regimen.8
A regimen of rifampicin, isoniazid, and pyrazinamide given to patients who have strains of the bacilli resistant to isoniazid—the most common type of resistance—is thought to result in treatment failure and acquired resistance to rifampicin. Therefore, the American Thoracic Society and US Centers for Disease Control and Prevention recommended in 1994 that a fourth drug, ethambutol, should initially be included in the treatment for patients in whom the bacilli might be susceptible to resistance.9 Such individuals may be immigrants from regions known to have a high prevalence of resistance, people from urban areas, or individuals with a medical history that might predispose them to resistance; arbitrarily, the fourth drug was to be included in areas in which the level of resistance was known to be 4% or more. In 1998, the British Thoracic Society embraced regimens that use four drugs for the initial phase of treatment as standard practice.
It is recommended that all patients with tuberculosis undergo a test for HIV. Supplements of pyridoxine (vitamin B6)—not to exceed a daily dose of 50 mg—are suggested for patients taking isoniazid to prevent peripheral neuritis. Particular attention should be given to patients at risk of neuropathy, including patients who are malnourished or pregnant. Baseline liver function tests and periodic and regular monitoring are advocated in view of the potential hepatotoxicity of isoniazid, rifampicin, and pyrazinamide. The risk of major liver damage is less than 1%, but mild asymptomatic increases in transaminase blood concentrations are seen in up to 20% of patients. Doses of ethambutol should be carefully adjusted in patients with renal impairment. In addition, patients taking ethambutol should have their visual acuity checked initially and monitored monthly (Snellen acuity and Ishihara colour). They should be instructed to report promptly any perceived disturbances in their vision.
Hospital admission is not routinely indicated for patients with tuberculosis unless the clinical illness merits such care, extenuating psychosocial circumstances exist, or patients have prognostic factors associated with poor short term outcome (respiratory failure or death) such as lymphopenia, advanced age, or alcoholism.10 To prevent nosocomial transmission, patients with tuberculosis (and suspected cases) should be placed in rooms of negative pressure and frequent air changes and, ideally, the means to filter out or lethally irradiate the tubercle bacilli with ultraviolet γ radiation.
The first line agents and their common drug toxicities are listed in table 2. A low and unavoidable risk of relapse is present after treatment. For the regimens described in table 1, the probability of relapse is less than 5%.11 Most recurrences occur within six months and the disease usually has the same drug susceptibility profile as before treatment.11 Current guidelines do not state the need for surveillance after treatment, especially when drugs have been given under supervision. Rather, patients should be instructed to return to their clinic or physician after treatment when their clinical status changes; in these instances, suitable tests, including examination of sputum samples and chest radiographs, should be carried out.
Table 2.
Dosages of first line antituberculosis drugs and major adverse effects
| Drug
|
Dosage
|
Adverse effects
|
|
|---|---|---|---|
| Daily
|
Twice or thrice weekly
|
||
| Isoniazid | 5 mg/kg oral (maximum 300 mg) | 900 mg twice weekly 600 mg thrice weekly |
Hepatitis, peripheral neuritis, drug induced lupus, seizures, and hypersensitivity with rash and fever. Drug interactions with dilantin and disulfiram |
| Rifampicin | 10 mg/kg oral (maximum 600 mg) | 10 mg/kg 600 mg twice weekly 600 mg thrice weekly |
Orange body secretions, flu-like syndrome, hepatitis, thrombocytopenia, nausea, anorexia, diarrhoea, renal failure, and multiple drug interactions |
| Pyrazinamide | 25-30 mg/kg oral | 30-35 mg/kg | Hyperuricemia, hepatitis, rash, nausea, and anorexia |
| Ethambutol | 25 mg/kg initial 2 months, then 15 mg/kg oral | 50 mg/kg twice weekly 30 mg/kg thrice weekly |
Optic neuritis and gastrointestinal discomfort |
| Streptomycin | 15 mg/kg intravenously or intramuscularly (maximum 1.0 g) 5 days a week | 15 mg/kg (maximum 1.5 g) twice weekly or thrice weekly | Ototoxicity, vestibular dysfunction, nephrotoxicity, rash, and hypersensitivity reactions |
Non-adherence to treatment and directly observed therapy (DOT)
Some patients with tuberculosis, as in virtually all chronic disorders, will fail to take their drugs.12 Unique public health philosophies and practices have evolved, however, which have helped to tackle this problem. For example, the public in industrialised nations has come to expect the air to be free of tuberculosis, in the same way that the water is free of such potentially lethal pathogens as typhoid and cholera. This has led to mandated treatment, quarantine, or even short term incarceration of patients in the United States or other countries.13
DOT programmes use a nurse or surrogate to deliver and observe patients taking all the doses of their drugs, rather than relying on patients to take them on their own. To facilitate DOT, intermittent (less frequently than daily) regimens have been used. Two popular intermittent regimens are represented in table 1; these six month regimens have been shown to be comparable in efficacy to daily treatment of equal length. The patients may either come to a health facility (clinic based DOT)14 (fig 1) or be seen elsewhere—for example, at home, work, or shelter (community based DOT).15 Preparations using drug combinations—for example, isoniazid and rifampicin (Rifamate; Hoechst Marion Roussel) and isoniazid, rifampicin, and pyrazinamide (Rifater; Hoechst Marion Roussel)—are also available, and may improve adherence. Whether such drug combinations prove to be more beneficial, given their increased cost and the reduced ability to discriminate which drug is responsible for toxicity or intolerance, has yet to be shown.
Figure 1.
Directly observed therapy in Rangoon, Burma. The two Buddhist nuns are receiving antituberculosis drugs at a public health facility that is also a poultry market
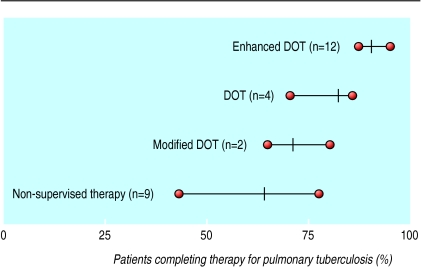
DOT is highly effective at promoting successful treatment. A comparison of self treatment versus various forms of DOT has shown that completion of treatment is significantly higher when the treatment is supervised (fig 2).16 Some observers have argued that DOT is an infringement of individual liberty,17 but well designed DOT programmes can be regarded as enhancing the health service and as a manifestation of community care.15 Successful DOT programmes provide a variety of incentives and enablers (practices that facilitate the treatment programme) to make them “consumer friendly.” Incentives may include rewards for making oneself available for treatment—for example, provision of social services, food stamps, assistance with housing or, in some cases, cash payments for the inconvenience. Some enablers facilitate treatment by being open during convenient hours, being in accessible locations, and providing help with transport, child care in clinics, or comprehensive services at a single site—for example, radiology, blood drawing, and sputum induction services.
Figure 2.
Rate of completion of treatment for various directly observed therapy (DOT) regimens versus non-supervised therapy.16 Enhanced DOT standard=DOT plus incentives and enablers; modified DOT= supervision for only part of the treatment, typically during initial hospitalisation, followed by self supervision; non-supervised therapy= self administered therapy. Figure adapted from Chaulk and Kazandjian16
Concern exists that governments cannot afford to provide DOT, but recent analyses show that, by assuring prompt cure, preventing relapses, and lessening acquired drug resistance, DOT programmes result in net savings to the community.18,19 The impact of DOT programmes may be seen in the reduction in the number of cases of tuberculosis in the 1990s in the United States, together with an increase in the proportion of patients receiving DOT in 1990 from 4% to over 70% by 2000.20 From 1995 to 2000, the rate of tuberculosis in the United States fell by an average of 7.8% per year. Although the broad implementation of DOT was not the only intervention during this period (improved measures to limit nosocomial transmission were also introduced), we believe it was the major factor driving these improved rates.
Treatment of different groups
Treatment in developing countries
In theory, the diagnosis and treatment of tuberculosis is the same in developing countries and industrialised countries, but economic limitations mean that significant differences exist in practice. As advocated by the World Health Organization's DOT short course policy, microscopy of sputum is the primary and often sole means of diagnosis in nations that have limited resources. It has notable limitations: firstly, diagnosis by microscopy of unconcentrated sputum is far less sensitive than that of concentrated sputum smears (better) or sputum culture (best); secondly, culture of the tubercle bacilli is required for the early detection of drug resistance, which may compromise the response of the bacteria to standard treatment.
Historically, many of the poorer nations have used a highly economical drug regimen consisting of isoniazid and thiacetazone given for 15 to 18 months, typically costing a total of only US$10-15 per person. Although the regimen is attractive in terms of cost, it is undesirable because the treatment takes longer, has marginal efficacy, and is ineffective in the presence of resistance to isoniazid. Regimens containing thiacetazone also have a greater risk of causing potentially lethal cutaneous drug reactions in people with AIDS.21 Most nations have now developed the standard WHO six month regimen, which includes isoniazid, rifampicin, pyrazinamide, and ethambutol. Because of the profoundly deleterious effects of resistance to rifampicin, strong emphasis is placed on DOT when rifampicin is used.
Treatment of HIV and tuberculosis
Treatment of people who have tuberculosis and AIDS raises four key issues.22 Firstly, patients may fail to properly absorb the antituberculosis drugs, which may increase the risk of treatment failure, relapses, and acquired drug resistance.23 Secondly, drug-drug interactions may compromise antiretroviral and anti-tuberculosis treatment, as well as increase the risk of acquired drug resistance and toxicity.24 We recommend that people who have both disorders are managed by clinicians who have special experience and interest in this patient population. Because the anti-retroviral drugs are less readily available in most developing countries than in the developed world, treatment of tuberculosis in people with AIDS as recommended by the US Centers for Disease Control and Prevention is not possible in many countries.25,26 Thirdly, because antiretroviral therapy reconstitutes CD4 lymphocyte numbers and immune function, patients may experience a paradoxical worsening of symptoms or other manifestations—for example, worsening of infiltrates on chest radiographs, enlarging pleural or pericardial effusions, swelling on lymph nodes—from pre-existing infections including tuberculosis.27 Delaying the initiation of antiretroviral therapy until the patient has completed several months of tuberculosis treatment reduces the risk and severity of such reactions but does not totally obviate the hazards. Fourthly, patients seem to have a modestly increased risk of relapse.28 Despite this, the 1994 guidelines of the US Centers for Disease Control and Prevention and the American Thoracic Society recommended the standard six month regimen, with the caveat that treatment should be prolonged in “slow responders.”9
Treatment of multidrug resistant tuberculosis
Multidrug resistant tuberculosis—which occurs when tuberculosis strains are resistant to at least isoniazid and rifampicin—is important clinically because it substantially increases the risks of treatment failure, further acquired resistance, and death. Its prevalence varies widely and generally reflects poorly organised treatment practices.29 People who are particularly at risk include those with histories of treatment for tuberculosis, those from high risk areas, and patients or healthcare workers from institutions (hospitals, clinics, prisons, or nursing homes) in which there has been epidemic transmission of resistant strains.
Initial therapy for patients with suspected multidrug resistant tuberculosis might reasonably use extended empirical regimens, especially if patients have extensive lung disease or perilous extrapulmonary forms of tuberculosis such as miliary or meningeal disease. For patients with proved disease it is important to give at least four drugs to which the mycobacteria are susceptible—usually three oral drugs and one injectable drug. Generally, an injectable drug such as an aminoglycoside is given for three to six months after the initial date of conversion of sputum cultures from testing positive for M tuberculosis to testing negative, and the patient continues to take oral antimycobacterial drugs for 15-18 months after the last positive sputum culture.
Additional educational resources
Cohn DL, Bustreo F, Raviglione MC. Drug-resistant tuberculosis: review of the worldwide situation and the WHO/IUATLD Global Surveillance Project. International Union Against Tuberculosis and Lung Disease. Clin Infect Dis 1997;24(suppl):121-30S.
Schwoebel V, Lambregts-van Weezenbeek CS, Moro ML, Drobniewski F, Hoffner SE, Raviglione MC, et al. Standardization of antituberculosis drug resistance surveillance in Europe. Recommendations of a World Health Organization (WHO) and International Union Against Tuberculosis and Lung Disease (IUATLD) Working Group. Eur Respir J 2000;16:364-71.
Bass JB Jr, Farer LS, Hopewell PC, O'Brien R, Jacobs RF, Ruben F, et al. Treatment of tuberculosis and tuberculosis infection in adults and children. American Thoracic Society and the Centers for Disease Control and Prevention. Am J Respir Crit Care Med 1994;149:1359-74.
Centers for Disease Control, Division of Tuberculosis Elimination. Core curriculum on tuberculosis. 1994. Atlanta, GA: Centers for Disease Control, 1994.
American Thoracic Society, Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med 2000;161(suppl):221-47S.
Joint Tuberculosis Committee of the British Thoracic Society. Control and prevention of tuberculosis in the United Kingdom: code of practice 2000. Thorax 2000;55:887-901.
Iseman MD, Huitt GA. Treatment of multidrug-resistant tuberculosis. In: Bastian I, Portaels F, eds. Multidrug-resistant tuberculosis: resurgent and emerging infectious diseases. Dordrecht: Kluwer, 2000:175-90.
Raviglione MC, Pio A. Evolution of WHO policies for tuberculosis control, 1948-2001. Lancet 2002;359:775-80.
Wagner KR, Bishai WR. Issues in the treatment of Mycobacterium tuberculosis in patients with human immunodeficiency virus infection. AIDS 2001;15(suppl):203-12S.
National Center for HIV, STD and TB Prevention. Division of tuberculosis elimination. www.cdc.gov/nchstp/tb (accessed 13 September 2002).
American Lung Association fact sheet: tuberculosis and HIV. www.lungusa.org/diseases/tbhivfac.html (accessed 13 September 2002).
Treatment of latent infection for people exposed to multidrug resistant bacilli is problematic because the only drugs widely deemed appropriate are isoniazid and rifampicin. A Delphi survey of a panel of experts on tuberculosis failed to reach a defined consensus on the most appropriate regimen for people exposed to multidrug resistant tuberculosis, although a combination of pyrazinamide and ciprofloxacin was considered somewhat appropriate.30 Experimentally, the combination of pyrazinamide and ofloxacin has been shown to have a favourable intramacrophage antimycobacterial effect.31 In light of the recent cases of severe hepatotoxicity associated with preventive treatment comprising either pyrazinamide and rifampicin32 or pyrazinamide and fluoroquinolone,33,34 however, fluoroquinolone monotherapy without pyrazinamide may be considered for people whose tuberculin skin test recently converted who have been exposed to multidrug resistant tuberculosis, with the caveat that long term efficacy data on these treatments are lacking.35
Potential chemotherapeutics
The Darwinian principle of natural selection predicts that drug resistant strains of tuberculosis will continue to develop. Research into new forms of treatment is therefore important. Fluoroquinolones are the most promising new agents for treatment of tuberculosis.36 Additional potential therapeutics include other classes of pharmaceuticals36 such as oxaolidinones (eg linezolid), treatments that affect the immune system such as improvement of BCG or Mycobacterium vaccae vaccines with or without cytokine augmentation treatment,37 and sterilisation of the semidormant population by targeting the citrate lyase pathway.38
Future directions
The immediate challenges for the control of tuberculosis include developing curative regimens that are shorter or that require patients to take drugs less frequently. Ideally, future regimens would have both features—that is, a once weekly regimen requiring that patients be treated for only four months. Such regimens would greatly facilitate monitoring compliance.36 The more compelling long term issue is the development of an improved vaccine that would have an epidemiological impact. BCG does reduce morbidity and mortality in infants but has little effect on adult pulmonary disease, which is the primary cause of death and virtually the only source of transmission. Unfortunately, because the reservoir of currently infected people is so huge, the benefits of an improved vaccine would not have substantive impact for decades. Finally, it is crucial that new, affordable and non-toxic drugs be developed to replace those lost to drug resistance.
Supplementary Material
Acknowledgments
EDC gratefully acknowledges the support by RO1-HL66112-01A1, Parke-Davis Atorvastatin Research Award, and the American Lung Association Career Investigator Award. The authors are also grateful for the kind support from Bettina Garthwaite Lowerre Foundation for mycobacteriology research.
Footnotes
Competing interests: None declared.
A box containing information for patients can be found on bmj.com
References
- 1.Raviglione MC, Snider DEJ, Kochi A. Global epidemiology of tuberculosis: morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 2.WHO report 2002: Global tuberculosis control: surveillance, planning, financing. Geneva: World Health Organization; 2002. [Google Scholar]
- 3.Burwen DR, Bloch AB, Griffin LD, Ciesielski CA, Stern HA, Onorato IM. National trends in the concurrence of tuberculosis and acquired immunodeficiency syndrome. Arch Intern Med. 1995;155:1281–1286. [PubMed] [Google Scholar]
- 4.Cantwell MF, Snider DEJ, Cauthen GM, Onorato IM. Epidemiology of tuberculosis in the United States, 1985 through 1992. JAMA. 1992;272:535–539. [PubMed] [Google Scholar]
- 5.David HL. Probability distribution of drug-resistant mutants in unselected populations of Mycobacterium tuberculosis. Appl Microbiol. 1970;20:810–814. doi: 10.1128/am.20.5.810-814.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchison DA. Basic concepts in the chemotherapy of tuberculosis. In: Gangadharam PRJ, Jenkins PA, editors. Mycobacteria. II. Chemotherapy. New York: Chapman & Hall; 1998. pp. 15–50. [Google Scholar]
- 7.Hong Kong Chest Service/British Medical Research Council. Controlled trial of 6-month and 8-month regimens in the treatment of pulmonary tuberculosis: the results up to 24 months. Tubercle. 1979;60:201–210. doi: 10.1016/0041-3879(79)90001-1. [DOI] [PubMed] [Google Scholar]
- 8.Hong Kong Chest Service/British Medical Research Council. Controlled trial of 2, 4, and 6 months of pyrazinamide in 6-month, three-times weekly regimens for smear-positive pulmonary tuberculosis, including an assessment of a combined preparation of isoniazid, rifampin, and pyrazinamide: results at 30 months. Am Rev Respir Dis. 1991;143:700–706. doi: 10.1164/ajrccm/143.4_Pt_1.700. [DOI] [PubMed] [Google Scholar]
- 9.Ass JB, Jr, Farer LS, Hopewell PC, O'Brien R, Jacobs RF, Ruben F, et al. Treatment of tuberculosis and tuberculosis infection in adults and children. American Thoracic Society and the Centers for Disease Control and Prevention. Am J Respir Crit Care Med. 1994;149:1359–1374. doi: 10.1164/ajrccm.149.5.8173779. [DOI] [PubMed] [Google Scholar]
- 10.Barnes PF, Leedom JM, Chan LS, Wong SF, Shah J, Vachon LA, et al. Predictors of short-term prognosis in patients with pulmonary tuberculosis. J Infect Dis. 1988;158:366–371. doi: 10.1093/infdis/158.2.366. [DOI] [PubMed] [Google Scholar]
- 11.Iseman MD. A clinician's guide to tuberculosis. Baltimore: Lippincott, Williams & Wilkins; 1999. [Google Scholar]
- 12.Sbarbaro JA, Sbarbaro JB. Compliance and supervision of chemotherapy of tuberculosis. Sem Respir Infect. 1994;9:120–127. [PubMed] [Google Scholar]
- 13.Burman WJ, Cohn DL, Rietmeijer CA, Judson FN, Sbarbaro JA, Reves RR. Short-term incarceration for the management of noncompliance with tuberculosis treatment. Chest. 1997;112:57–62. doi: 10.1378/chest.112.1.57. [DOI] [PubMed] [Google Scholar]
- 14.Cohn DL, Catlin BJ, Peterson KL, Judson FN, Sbarbaro JA. A 62-dose, 6-month therapy for pulmonary and extrapulmonary tuberculosis: A twice-weekly, directly observed, and cost-effective regimen. Ann Intern Med. 1990;112:407–415. doi: 10.7326/0003-4819-76-3-112-6-407. [DOI] [PubMed] [Google Scholar]
- 15.Chaulk CP, Friedman M, Dunning R. Modeling the epidemiology and economics of directly observed therapy in Baltimore. Int J Tuberc Lung Dis. 2000;4:201–207. [PubMed] [Google Scholar]
- 16.Chaulk CP, Kazandjian VA. Directly observed therapy for treatment completion of pulmonary tuberculosis: consensus statement of the Public Health Tuberculosis Guidelines Panel. JAMA. 1998;279:943–948. doi: 10.1001/jama.279.12.943. [DOI] [PubMed] [Google Scholar]
- 17.Nardell EA. Beyond four drugs: public health policy and the treatment of the individual patient with tuberculosis [editorial] Am Rev Respir Dis. 1993;148:2–5. doi: 10.1164/ajrccm/148.1.2. [DOI] [PubMed] [Google Scholar]
- 18.Burman WJ, Dalton CB, Cohn DL, Butler JRG, Reves RR. A cost-effectiveness analysis of directly observed therapy vs self-administered therapy for treatment of tuberculosis. Chest. 1997;112:63–70. doi: 10.1378/chest.112.1.63. [DOI] [PubMed] [Google Scholar]
- 19.Moore RD, Chaulk CP, Griffiths R, Cavalcante S, Chaisson RE. Cost-effectiveness of directly observed versus self-administered therapy for tuberculosis. Am J Respir Crit Care Med. 1996;154:1013–1019. doi: 10.1164/ajrccm.154.4.8887600. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Reported tuberculosis in the United States. 2000. Atlanta, GA: Centers for Disease Control and Prevention; 2000. [Google Scholar]
- 21.Nunn P, Kibuga D, Gathna S, Brindle R, Imalingat A, Wasunna K, et al. Cutaneous hypersensitivity reactions due to thiacetazone in HIV-1 seropositive patients treated for tuberculosis. Lancet. 1991;337:627–630. doi: 10.1016/0140-6736(91)92447-a. [DOI] [PubMed] [Google Scholar]
- 22.Corbett EL, Steketee RW, ter Kuile FO, Latif AS, Kamali A, Hayes RJ. HIV-1/AIDS and the control of other infectious diseases in Africa. Lancet. 2002;359:2177–2187. doi: 10.1016/S0140-6736(02)09095-5. [DOI] [PubMed] [Google Scholar]
- 23.Peloquin CA, Nitta AT, Burman WJ, Brudney KF, Miranda-Massari JR, McGuinness ME, et al. Low antituberculosis drug concentrations in patients with AIDS. Ann Pharmacother. 1996;30:919–925. doi: 10.1177/106002809603000901. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control. Clinical update: impact of HIV protease inhibitors on the treatment of HIV-infected tuberculosis patients with rifampin. MMWR. 1996;45:921–925. [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Updated guidelines for the use of rifabutin or rifampin for the treatment and prevention of tuberculosis among HIV-infected patients taking protease inhibitors or nonnucleoside reverse transcriptase inhibitors. MMWR. 2000;49:185–189. [PubMed] [Google Scholar]
- 26.Wagner KR, Bishai WR. Issues in the treatment of Mycobacterium tuberculosis in patients with human immunodeficiency virus infection. AIDS. 2001;15(suppl):203–12S. doi: 10.1097/00002030-200100005-00024. [DOI] [PubMed] [Google Scholar]
- 27.Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med. 1998;158:157–161. doi: 10.1164/ajrccm.158.1.9712001. [DOI] [PubMed] [Google Scholar]
- 28.Pulido F, Pena J-M, Rubio R, Moreno S, Gonzalez J, Guijarro C, et al. Relapse of tuberculosis after treatment in human immunodeficiency virus-infected patients. Arch Intern Med. 1997;157:227–232. [PubMed] [Google Scholar]
- 29.Mahmoudi A, Iseman MD. Pitfalls in the care of patients with tuberculosis: common errors and their association with the acquisition of drug resistance. JAMA. 1993;270:65–68. [PubMed] [Google Scholar]
- 30.Passannante MR, Gallagher CT, Reichman LB. Preventive therapy for contacts of multidrug-resistant tuberculosis: a Delphi survey. Chest. 1994;106:431–434. doi: 10.1378/chest.106.2.431. [DOI] [PubMed] [Google Scholar]
- 31.Sbarbaro JA, Iseman MD, Crowle AJ. Combined effect of pyrazinamide and ofloxacin within the human macrophage. Tuberc Lung Dis. 1996;77:491–495. doi: 10.1016/s0962-8479(96)90045-3. [DOI] [PubMed] [Google Scholar]
- 32.American Thoracic Society update: fatal and severe liver injuries associated with rifampin and pyrazinamide for latent tuberculosis infection, and revisions in American Thoracic Society/CDC recommendations—United States, 2001. Am J Respir Crit Care Med. 2001;164:1319–1320. [Google Scholar]
- 33.Horn DL, Hewlett D, Alfalla C, Peterson S, Opal SM. Limited tolerance of ofloxacin and pyrazinamide prophylaxis against tuberculosis. N Engl J Med. 1994;330:1241. doi: 10.1056/nejm199404283301718. [DOI] [PubMed] [Google Scholar]
- 34.Ridzon R, Meador J, Maxwell R, Higgins K, Weismuller P, Onorato IM. Asymptomatic hepatitis in persons who received alternative preventive therapy with pyrazinamide and ofloxacin. Clin Infect Dis. 1997;24:1264–1265. doi: 10.1093/clinids/24.6.1264. [DOI] [PubMed] [Google Scholar]
- 35.American Thoracic Society, Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161(suppl):221–47S. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 36. Iseman MD. Tuberculosis therapy: (past), present and future. Eur Respir J 2002 (in press). [DOI] [PubMed]
- 37.Durban Immunotherapy Trial Group. Immunotherapy with Mycobacterium vaccae in patients with newly diagnosed pulmonary tuberculosis: a randomised controlled trial. Lancet. 1999;354:116–119. [PubMed] [Google Scholar]
- 38.McKinney JD, Honer zu Bentrup K, Munoz-Elias EJ, Miczak A, Chen B, Chan WT, et al. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature. 2000;406:735–738. doi: 10.1038/35021074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.