Abstract
Hepatocellular carcinoma (HCC), the most prevalent malignancy of the digestive tract, is characterized by a high mortality rate and poor prognosis, primarily due to its initial diagnosis at an advanced stage that precludes any surgical intervention. Recent advancements in systemic therapies have significantly improved oncological outcomes for intermediate and advanced‐stage HCC, and the combination of locoregional and systemic therapies further facilitates tumor downstaging and increases the likelihood of surgical resectability for initially unresectable cases following conversion therapies. This shift toward high conversion rates with novel, multimodal treatment approaches has become a principal pathway for prolonged survival in patients with advanced HCC. However, the field of conversion therapy for HCC is marked by controversies, including the selection of potential surgical candidates, formulation of conversion therapy regimens, determination of optimal surgical timing, and application of adjuvant therapy post‐surgery. Addressing these challenges and refining clinical protocols and research in HCC conversion therapy is essential for setting the groundwork for future advancements in treatment strategies and clinical research. This narrative review comprehensively summarizes the current strategies and clinical experiences in conversion therapy for advanced‐stage HCC, emphasizing the unresolved issues and the path forward in the context of precision medicine. This work not only provides a comprehensive overview of the evolving landscape of treatment modalities for conversion therapy but also paves the way for future studies and innovations in this field.
Keywords: advanced hepatocellular carcinoma, conversion therapy, immune checkpoint inhibitors, locoregional treatment, molecular targeted therapy, prognosis, resection
Setting a foundation for future research and advancements in HCC treatment, aligning with the emerging paradigm of precision medicine; addressing the need for a holistic approach in managing advanced‐stage HCC, and advocating for a balance between aggressive treatment and quality of life considerations.
Abbreviations
- AEs
adverse events
- ALPPS
Associating Liver Partition and Portal vein ligation for Staged hepatectomy
- BCLC
Barcelona Clinic Liver Cancer
- CNLC
Chinese National Liver Cancer
- DCR
disease control rate
- DoR
duration of response
- HAIC
hepatic arterial infusion chemotherapy
- ICI
immune checkpoint inhibitor
- MDT
multidisciplinary team
- ORR
objective response rate
- OS
overall survival
- pCR
pathological complete response
- PD‐1/PD‐L1
programmed cell death protein‐1/programmed cell death protein‐ligand 1
- PR
partial response
- PVE
portal vein embolization
- PVTT
portal vein tumor thrombosis
- RFS
recurrence‐free survival
- RT
radiotherapy
- TACE
transarterial chemoembolization
- TKI
tyrosine kinase inhibitor
- uHCC
unresectable hepatocellular carcinoma
1. INTRODUCTION
Hepatocellular carcinoma (HCC), a highly malignant form of digestive tract cancer, presents significant challenges in oncological management due to its aggressive nature and poor prognosis, with persistently high incidence and mortality rates worldwide. 1 , 2 Although surgical resection remains the primary approach for achieving long‐term survival in HCC patients, fewer than 30% of patients are eligible for surgery due to the majority being diagnosed at advanced stages. 3 , 4 For those with advanced‐stage HCC who are not candidates for resection, the 5‐year survival rate is fewer than 20%, indicating an extremely poor overall prognosis. 5 Therefore, it is crucial to effectively improve the long‐term survival of these patients to enhance HCC prognosis and alleviate the socioeconomic burden.
Conversion therapy provides a second chance for patients with late‐stage HCC who are no longer candidates for surgery, by offering a means to downgrade initially unresectable HCC (uHCC) to a resectable condition through a variety of treatment modalities. 4 , 6 , 7 The reasons for HCC being initially deemed unresectable can be categorized into surgical and oncological unresectability, with the former encompassing factors such as the patient's inability to tolerate surgery, inadequate liver function, or insufficient future liver remnant volume, while the later referring to situations in which the tumor could technically be resected, but surgery would not offer a survival benefit over non‐surgical treatments. In this context, conversion therapy has emerged as a critical approach for enabling patients with uHCC to access surgical treatment options and secure long‐term survival. Studies show that patients with uHCC who become eligible for resection following conversion therapy exhibit a postoperative 5‐year overall survival (OS) rate of 24.9%–57.0%, comparable with the 30%–50% survival rate observed in those who undergo initial curative resection. 8 However, the heterogeneous nature of advanced HCC has led to a lack of consensus or guidelines for managing this specific population, introducing uncertainty in conversion therapy applications. In the era of precision medicine, selecting ideal treatment regimens, accurately assessing and selecting suitable surgical candidates, and implementing perioperative management have become focal points in the field of HCC conversion therapy. This review aims to comprehensively analyze and discuss the current state and challenges of conversion therapy for HCC, incorporating insights from preliminary international research and clinical practices. Our goal is to clarify the objectives and significance of conversion therapy and explore the new opportunities and challenges brought about by emerging treatment modalities.
2. CURRENT STATUS AND POTENTIAL CANDIDATE SELECTION FOR CONVERSION THERAPY IN HCC
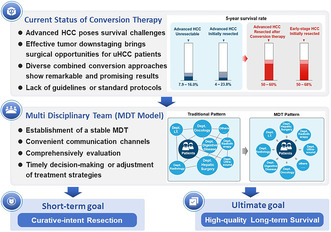
Currently, the successful liver resection for initially uHCC following conversion therapy has garnered significant attention, bringing great encouragement to both patients and physicians. Evidence suggests that advanced HCC patients who achieve R0 resection after conversion therapy have a comparable prognosis with that of patients with initially resectable HCC. Moreover, R0 resection after conversion therapy serves as an independent protective factor for long‐term survival in the uHCC population. 9 , 10 , 11 These findings underscore the critical role of conversion therapy in treating advanced HCC, yet the low surgical rate following successful conversion therapy remains a major limitation to its broader clinical application. Historically, patients who could not tolerate surgical resection due to insufficient residual liver volume could undergo surgical procedures such as PVE or ALPPS to increase the size of the remaining liver. However, PVE has shown limited effectiveness in increasing remnant liver volume and carries a high risk of complications, while the high mortality rate of ALPPS has also largely hampered its clinical application. 12 , 13
Currently, the rapid emergence and development of treatment modalities such as immunotherapy, molecular targeted therapy, and transcatheter arterial interventions have propelled HCC conversion therapy to a new level. Not only has it demonstrated notable efficacy in reducing tumor burden, but it has also exhibited significant improvements in long‐term survival, regardless of whether surgical resection is ultimately performed. 7 This means that patients with advanced‐stage HCC may benefit from high‐intensity and multidisciplinary conversion therapy regimens, enhancing their likelihood of subsequently receiving curative resection. Compared with America and European countries, China demonstrates a more proactive approach to conversion therapy for initially uHCC. 5 According to the BCLC staging system, patients in stages III B and C are inclined toward systemic treatment, while in the CNLC staging system, these patients corresponded to stages IIb and IIIa, for which surgical treatment remains a viable option with wide recommendation 6 , 7 , 14 (Figure 1). This variation in treatment approaches may be attributed to the high prevalence of patients with advanced‐stage HCC. 15 In addition, the criteria for resection after conversion therapy are also different and often depend on the tumor characteristics, such as whether there is shrinkage or necrosis of primary tumors, reduction in tumor number, regression of vascular thrombi, and disappearance of extrahepatic lesions based on modified RECIST (mRECIST) standards. 7 However, it should be noted that, although the scope and potential candidates of conversion therapy are continuously expanding, some patients may still have difficulty benefiting from it, especially those with Child–Pugh B/C liver function, who may not tolerate intensive treatment regimens and may even suffer severe organ damage. This issue should be taken seriously in clinical practice.
FIGURE 1.
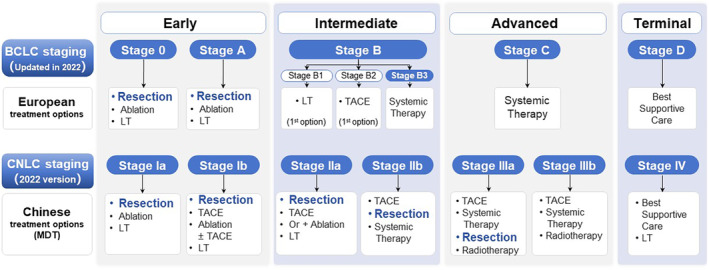
Comparison of stage‐dependent recommended treatment approaches based on CNLC staging versus BCLC staging. BCLC, Barcelona Clinic Liver Cancer; CNLC, Chinese National Liver Cancer; HCC, hepatocellular carcinoma; LT, liver transplantation; MDT, Multidisciplinary Team; TACE, transarterial chemoembolization.
The emergence of various TKIs and ICIs has shown tremendous potential and promising prospects in treating HCC, with an increasing number of combined therapies based on these agents being developed and used in clinical practice. 16 The ORR serves as a crucial indicator for evaluating the efficacy of conversion therapy, which also encompasses factors such as DCR, DoR, and depth of pathological response. 7 , 17 , 18 To achieve the best conversion outcomes, current strategies predominantly involve combining TKIs or/and ICIs with local therapies such as TACE and HAIC, while prioritizing options with high ORR values and low rates of AEs to maximize the success of following conversion resection. In general, the selection of conversion treatment regimens should adhere to the principles of safety, efficacy, and accessibility. Below are the current major advancements in locoregional and systemic therapies for HCC conversion treatment.
3. LOCALIZED VASCULAR INTERVENTIONS
Locoregional therapies play a crucial role in the management of advanced HCC. 3 As the primary vascular interventional approaches, TACE and HAIC have gained great acceptance and are widely recommended as important therapeutic options for HCC conversion due to their high tumor response rates, repeatability, and minimally invasive and safety characteristics. 19 , 20 , 21 Currently, TACE remains the mainstream treatment regimen in treating uHCC. 3 , 7 A retrospective study involving 82 patients with advanced HCC who achieved successful downstaging after TACE showed that those who underwent additional salvage resection had a longer survival time (49 months vs. 31 months) and higher 5‐year OS rate (26% vs. 10%) compared with those who continued conservative treatment. 22 However, for patients with tumor diameter greater than 10 cm, the efficacy of TACE is not satisfactory, with a DCR of fewer than 50%, conversion rate of fewer than 10%, and a median OS of only 6.5–9.1 months. 23 , 24 This may be attributed to the abundant blood supply and collateral circulation in large tumors, which makes it difficult to achieve complete embolization when the embolic agent dosage is limited.
In fact, TACE primarily achieves the goal of limiting HCC growth using embolic agents to block the tumor blood supply. However, it inevitably impedes the further cytotoxic effect of other drugs on the tumor. Additionally, TACE causes significant damage to normal liver tissue, which suggests that the anti‐tumor effect based on embolizing tumor blood supply may not outweigh the adverse reactions. 19 With the continuous refinement and optimization of chemotherapy regimens, HAIC, developed in parallel with TACE, abandoned the use of embolic agents and extended the delivery duration of chemotherapy drugs. 20 , 25 This approach effectively diminishes embolism‐related complications and yields substantial anti‐tumor effects, leading clinical physicians to progressively shift their focus from TACE to HAIC in the era of conversion therapy for HCC.
Modern HAIC therapy primarily employs the FOLFOX chemotherapy regimen, demonstrating superior tumor regression and higher ORR compared with TACE. 26 , 27 Findings from prospective studies consistently indicate that the HAIC treatment group exhibits significantly improved OS and higher surgical rate after downstaging therapies, with the former reaching an impressive ORR of up to 54.1%, while experiencing significantly lower rates of severe adverse events compared with the TACE group. 28 , 29 A Phase III clinical trial comparing HAIC with sorafenib in uHCC (FOHAIC‐1) revealed significantly higher ORR (31.5% vs. 1.5%) and conversion resection rates (12.3% vs. 0.8%) in the HAIC group for all lesions compared with the sorafenib group. 30 Another retrospective study similarly found a significantly superior ORR in the HAIC treatment group compared with the sorafenib group (47.8% vs. 9.1%), with 26.1% of patients achieving salvage resection after successful downstaging. 31 Consistently, in a cohort of 247 HCC patients with PVTT, a combination of HAIC and sorafenib demonstrated higher ORR compared with sorafenib monotherapy, with approximately 12.8% of patients successfully receiving salvage resection, which was a 10% increase compared with the control group. 32
The combination of HAIC and RT has also proven to be an effective strategy for advanced‐HCC patients with PVTT, and increasing radiation is often accompanied by a higher conversion resection rate without increased incidence of severe AEs. 33 A retrospective study in Japan revealed a conversion resection rate of 13.5% among HCC patients with PVTT who underwent HAIC combined with RT, with a marked 3‐year survival rate of 71% versus 18% for the non‐surgical group. 34 Another real‐world study showed that the surgical conversion rate for HAIC combined with RT was 26.5%, and the median postoperative disease‐specific survival in the combination group was 62 months, which was significantly longer than the 15 months in the resection‐first group. 35 The above results indicated that the HAIC‐based combined therapies can exert a synergistic anti‐tumor effect, while ensuring safety for advanced HCC with PVTT. In clinical practice, the selection of HAIC and TACE as locoregional therapy for uHCC is a widely discussed topic that requires consideration of their technical features and application scenarios. Generally, HAIC is considered to have a higher response rate in treating advanced HCCs, making it the preferred option for patients with heavy tumor burden and PVTT, 36 but subsequent treatments are required to ensure sustained anti‐tumor effect due to its short‐term efficacy. Conversely, TACE mainly causes extensive intra‐tumoral necrosis, thus having limited effect on reducing tumor volume. In addition, literature has reported that early TACE–HAIC combination therapy can maximize the likelihood of successful conversion in chemotherapy‐resistant HCC, while careful monitoring of the patient's liver function is necessary. 37 However, the question whether combining HAIC or TACE with systemic treatment could lead to a higher conversion rate for advanced HCC still requires further in‐depth exploration.
4. TARGETED AND IMMUNOTHERAPY‐DOMINATED SYSTEMIC THERAPY
Molecular targeted therapy serves as the cornerstone for systemic treatment of advanced‐stage HCC. Since 2007, sorafenib, a classical multi‐targeted TKI, has played a crucial role as the first‐line treatment for HCC for over a decade until lenvatinib demonstrated non‐inferiority to sorafenib and gained approval as a first‐line option for HCC. 38 In a retrospective study involving uHCC with tumor burdens exceeding the up‐to‐seven criteria, lenvatinib exhibited significantly superior ORR compared with TACE, making it a promising option for targeted therapy in HCC conversion treatment. 39 In addition, a novel TKI, donafenib, has been approved for use in uHCC patients who have not received systemic therapy, and demonstrated significant extension in median survival time and a 17% reduction in risk of death when compared with sorafenib in advanced HCC. However, donafenib may have limited efficacy in the conversion treatment of uHCC due to its relatively low ORR of 4.6%. 40
With the emergence of MDT pattern and precision treatment for HCC, the TKI monotherapy‐based conversion approach is no longer sufficient to meet the expectation of long‐term survival benefits for uHCC patients, raising an urgent issue of exploring safe and effective combination regimens (Figure 2). The unveiling of data from the IMbrave 150 trial has ushered in a new era of immunotherapy combined with anti‐angiogenic antibodies for treating HCC in the advanced stage. 41 In this study, the “T+A” (atezolizumab plus bevacizumab) regimen achieved an ORR of 30%, with 8% complete responses and 22% partial responses. The median DoR was up to 18.1 months, far exceeding the results showed in similar Phase III trials, and laid the foundation for maintaining disease in a state of remission after discontinuing conversion therapy. Furthermore, Kudo et al. demonstrated that treatment with “T+A” regimen for TACE‐unsuitable uHCC patients achieved a complete response (CR) rate of 35%, with approximately two‐thirds of these patients achieving drug‐free status without any observed recurrence. 42 As ORR is a crucial indicator in conversion therapy and lenvatinib has shown a relatively high ORR in clinical studies, current conversion therapy regimens are often based on lenvatinib in combination with other approaches. In particular, the combination with ICI has been proven to be a feasible treatment strategy for initially converting uHCC to resectable HCC, with a conversion rate of 15.9% and a pCR rate of 60%. 43 Multiple retrospective studies have shown that the ORR ranged from 33.3% to 55.6% and the conversion rate was between 18.8% and 42.4% in patients with advanced uHCC or HCC with macrovascular invasion who received combination therapy with lenvatinib and anti‐PD‐1 antibodies. 44 , 45 , 46 In addition, the long‐term survival of patients who exhibited a positive response to combined TKI/anti‐PD‐1 antibodies and fulfilled the eligibility criteria for conversion resection in initially uHCC has been reported, with 1‐year RFS and OS of 75% and 95.8%, respectively. Furthermore, achieving pCR after systemic treatment was strongly correlated with favorable RFS post‐resection. 47
FIGURE 2.
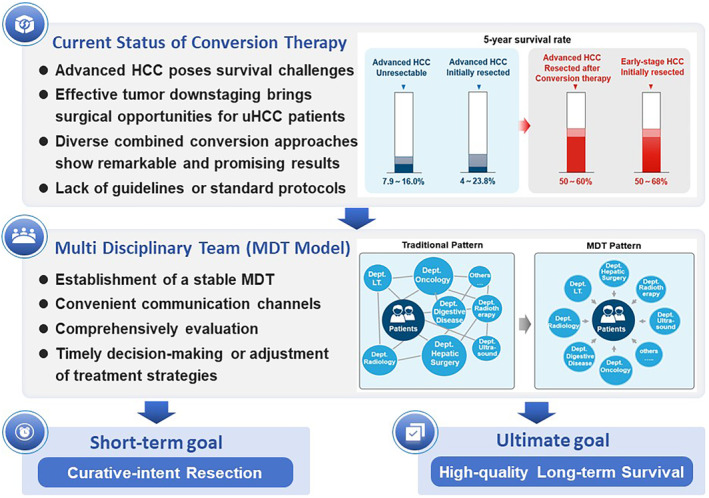
Multidisciplinary treatment of HCC promotes the emergence of novel treatment paradigm. HCC, hepatocellular carcinoma; LT, liver transplantation; MDT, Multidisciplinary Team; uHCC, unresectable hepatocellular carcinoma.
5. LOCAL COMBINED SYSTEMIC TREATMENT REGIMENS
While systemic treatment has been demonstrated to provide survival benefits for uHCC patients, ensuring effective local tumor control remains equally imperative. This scenario has given rise to the strategy of integrating locoregional and systemic approaches, which has gained wide acceptance among clinicians. Recently, various regimens have presented encouraging synergistic effects, including the combination of TACE and sorafenib, 48 TACE and lenvatinib 49 (including newly published TACTICS‐L trial 50 ), HAIC and sorafenib, 32 and HAIC plus lenvatinib and toripalimab, 51 with reported ORR ranging from 40.8% to 71.3%. The recently published phase II prospective trial (START‐FIT study) reported for the first time the potential application prospects of sequential TACE and radiotherapy followed by using avelumab (an anti‐PD‐L1 agent) for advanced HCC. 52 The results showed that 55% of patients met the criteria for successful conversion resection, with 12% of patients undergoing curative resection or radiofrequency ablation, while the remaining patients maintained close monitoring after radiological remission assessment. Another single‐center, single‐arm phase II clinical trial including 36 patients with advanced HCC showed that the ORR based on mRECIST criteria reached 41.7% after sequential nivolumab following yttrium‐90 resin microsphere radioembolization, with a CR rate of 11% and a PR rate of 31%. 53 This suggests that selective internal RT is expected to play a more dominant role in HCC conversion therapy in the future.
Despite the rapid advancements in research on the combined therapies for advanced HCC, the exploration and optimization of conversion strategies by physicians seem to be endless. Considering that the existing approaches are far from enough to meet the rising demands of conversion treatment for uHCC, there is an imperative for more aggressive and potent treatment strategies. In this context, a triple combination therapy consisting of TACE/HAIC along with molecular targeted therapy and immunotherapy has emerged. In 2021, Liu et al. 54 first reported an impressive ORR of 68.2% among 22 advanced‐stage HCC patients who were treated with TACE in combination with lenvatinib and camrelizumab. In addition, a single‐arm study by Zhang et al. 55 demonstrated a surprising conversion resection rate of 60% and a median response time of 50.5 days among initially uHCC patients following a triple combination regimen involving HAIC, TKIs, and ICIs. Similarly, an ongoing prospective, double‐blinded, randomized phase 3 LEAP‐012 study (NCT04246177) is designed to investigate whether adding lenvatinib plus pembrolizumab to TACE could offer enhanced clinical benefit in patients with inoperable intermediate‐stage HCC. 56 The outcomes of this trial are anticipated to address the unmet need for an effective treatment paradigm for uHCC patients who currently have limited options. Interestingly, in another similar randomized, double‐blinded, phase 3 trial (LEAP‐002), the outcomes were somewhat disappointing, as the combination of lenvatinib and pembrolizumab did not achieve the prespecified significance for enhanced OS and progression‐free survival when compared with lenvatinib with a placebo, 57 suggesting that this may not necessitate a modification in clinical guidelines. Efforts to compare the efficacy of triple and dual combination regimens have been made, but a consensus on their effectiveness has not yet been reached. In a real‐world study, the triple combination modality of TACE, lenvatinib, plus toripalimab exhibited a higher conversion rate, improved disease‐free survival, superior treatment responses, and a comparable safety profile compared with dual combination therapy using lenvatinib and TACE. 58 Another systematic review, encompassing 741 patients from 15 studies receiving the triple combination of TACE/HAIC, TKIs, and ICIs, revealed an overall ORR and DCR of 60.6% and 88.5%, respectively, with 35.9% of patients achieving conversion resection. Subgroup analyses further provided additional evidence for the superiority of triple therapy over other regimens in terms of CR, ORR, DCR, and conversion rate, thereby suggesting a potential clinical advantage for uHCC patients in terms of both short‐ and long‐term outcomes, with no significant rise in severe AEs. 59 In addition, there are ongoing clinical trials exploring even more than triple combination therapies, such as TACE in conjunction with deflazacort, tucatinib, and lenvatinib (EMERALD‐3 trial; NCT05301842), which may hold promise for offering more effective and potential strategies for uHCC patients.
Although current findings indicate that a more proactive and robust triple therapy seems to offer better treatment outcomes for patients, as the variety of treatment regimens increases, ensuring safety becomes an unavoidable concern that deserves utmost emphasis. Elevated levels of alanine aminotransferase, aspartate aminotransferase, and hypertension were identified as the most common AEs, with no reported occurrence of fatal AEs. 59 Moreover, the combination of TKIs and ICIs has been linked to an elevated risk of liver function impairment, fatigue, rash, and hypothyroidism. 51 , 54 , 59 , 60 , 61 These data have proposed the likelihood of using liver function as a criterion for assessing the suitability of triple therapy, and it should only be considered in patients with relatively preserved liver function. Of note, while the triple therapy of TACE/HAIC, TKIs, and ICIs has shown preliminary encouraging results, it should be approached with great caution and critical assessment as the reports on systemic treatment combined with or without locoregional therapy for converting uHCC are predominantly from small‐sample retrospective studies, making it difficult to avoid type II statistic errors and impacting the reliability of the results. Additionally, the significant heterogeneity of advanced HCCs and the great diversity of triple therapy regimens, including different patterns of vascular intervention, and selection of TKI and ICI agents, are important factors contributing to the divergences between the included studies. Thus, future randomized controlled trials with larger sample sizes and multi‐regional centers are warranted to optimize the selection and sequencing of these treatment modalities, as well as to identify candidate populations that would benefit the most from these approaches.
6. APPROACHES TO CONVERSION RESECTION FOR HCC: OPPORTUNISTIC VS. CONSERVATIVE PATTERNS
The optimal timing for surgical intervention after successful downstaging therapy in initial uHCC remains under debate. The prevailing viewpoint suggests that surgery should be performed after achieving significant tumor regression and maintaining stability for a specific duration, although the length of the observation period varies widely, ranging from 42 to 298 days. 62 In addition, factors such as the time it takes for the medication to become effective, toxic side effects and preoperative cessation of medication need to be taken into account. Indeed, the determination of successful conversion for uHCC lacks objective criteria and is primarily reliant on subjective assessment by clinicians, which can be largely influenced by the surgeon's experience, surgical expertise, and comprehensive capabilities of the hospital where they practice. Many clinicians advocate for timely curative resection following successful conversion therapy, emphasizing the need to seize the valuable opportunity for resectability. The rationale behind this urgency lies in the potential risk of losing surgical options in the event of tumor re‐progression. However, in the real‐world scenario, many patients who have achieved effective tumor control through conversion therapy, including notable tumor shrinkage or complete normalization of tumor serum biomarkers, frequently exhibit hesitancy toward undergoing surgery and instead opt to maintain their current status. This reluctance primarily arises from concerns related to surgery and the potential disturbance of the immune microenvironment postoperatively, which may contribute to the risk of tumor recurrence. Existing research has reported a postoperative recurrence rate of up to 36.6% within almost 12–16.8 months following conversion therapy. 47 , 62 , 63 Such a rapid recurrence often signifies accelerated tumor progression and the failure of previous treatment regimens, thus necessitating the development of alternative therapeutic approaches and undoubtedly imposing a significant psychological and economic burden on patients.
These challenges have given rise to an alternative model for conversion resection, in which surgery is postponed after successful conversion therapy; close monitoring is continuingly maintained while adhering to the existing regimens. In this case, surgery is only considered when there is an increase in serum biomarkers or the appearance of any newly emerged lesions, which indicates the ineffectiveness of the current treatment regimens and the more aggressive tumor biological behavior. 64 , 65 The two approaches represent the primary models for HCC conversion therapy and can be viewed as a comparison between opportunism and observationalism; however, no concrete evidence ascertains which approach can yield greater survival benefits for patients. As such, further prospective research comparing the OS and especially postoperative OS of patients treated with these two approaches is highly warranted to better guide clinical decision‐making when patients are experiencing successful conversion therapy for initial uHCC. Of course, the choice of treatment also depends on the type of physician in charge of patients. For example, from the perspective of surgeons who are skilled in surgical treatment, prompt surgical resection is highly recommended to achieve a curative effect once conversion therapy is successful with the aim, while internists may lean more toward a conservation strategy to maintain the current treatment plan. This discrepancy also highlights the importance of multidisciplinary management of conversion therapy for HCC.
Importantly, many studies have reported complete pathological responses in 19.5%–39.1% of patients undergoing conversion resection, 62 , 66 , 67 in which the resected samples have completely necrotized, eliminating the necessity for additional curative resection in these patients. Therefore, a critical issue has gradually been raised regarding how to preoperatively assess the extent of necrosis in the lesions after conversion therapy, which is expected to provide first‐line evidence for surgical decision‐making. Although numerous studies are currently underway to establish different models for predicting the degree of lesion necrosis through methods including novel serum biomarkers, liquid biopsy techniques, radiomics, and AI training, 68 , 69 , 70 , 71 , 72 , 73 the predictive efficacy remains unsatisfactory. Thus, there is an urgent need for further exploration of advanced imaging techniques and novel, promising serum biomarkers to achieve more accurate and precise prediction of tumor necrosis before conversion resection.
7. POSTOPERATIVE ADJUVANT THERAPY FOLLOWING CONVERSION RESECTION FOR HCC
Upon undergoing conversion therapy, patients frequently present with advanced‐stage tumors characterized by larger diameters, multiple tumor lesions, and the presence of major vascular invasion, all of which are independent risk factors for postoperative recurrence. 74 , 75 Therefore, it is imperative to administer postoperative adjuvant therapy to reduce the risk of recurrence in these high‐risk populations. Currently, there is no consensus or standard regarding the selection of adjuvant therapy regimens and treatment duration following conversion resection for HCC. However, insights can be drawn from the postoperative adjuvant strategies for liver metastases from colorectal cancer, which typically involve maintaining the pre‐existing chemotherapy regimen for 6 months or adjusting drug dosages and shortening the course of chemotherapy based on patient tolerance. 76 A real‐world study by Pan et al. 77 found that among advanced HCC patients who underwent FOLFOX‐HAIC‐based conversion therapy, receiving postoperative adjuvant therapy significantly improved long‐term survival outcomes, especially in patients with macrovascular invasion and positive hepatitis B surface antigen. Evidence has also indicated that the use of TKI or ICI alone as adjuvant therapy can improve the long‐term prognosis of patients with uHCC after conversion resection. 78 However, the failure of the STORM trial has demonstrated that the effectiveness of sorafenib, a representative TKI drug, is unsatisfactory in the adjuvant treatment of HCC after surgery, as it does not provide definite survival benefits to patients. 79 , 80 In terms of immunotherapy, ICIs can restore the anti‐tumor immune response by blocking the PD‐1/PD‐L1 axis and maintaining memory T cells both in the tumor microenvironment and peripheral blood circulation, which have the potential to eliminate residual tumor cells and play a crucial role in immune surveillance against tumor recurrence after curative resection. 81 , 82 , 83 The recently published results of the IMbrave 050 study showed that adjuvant therapy with atezolizumab in combination with bevacizumab significantly improved the RFS of patients who underwent curative resection or radiofrequency ablation for HCC, implying strong evidence for the development of immunotherapy‐based adjuvant strategies for HCC. 84 Indeed, from the perspective of reducing side effects or avoiding drug resistance, it is generally preferred to continue the original regimen(s) or certain drugs in the following period of adjuvant treatment. 85 In addition, for patients who have undergone local treatments such as radiotherapy and embolization, systemic therapy may be considered as postoperative treatment as the lesions have been surgically removed. 7 , 86
Currently, there is no consensus on the optimal duration of adjuvant therapy following conversion surgery for HCC. Considering adjuvant therapy aims to reduce recurrence and extend survival, strategies for patients at high risk of recurrence can similarly inform the development of postoperative adjuvant treatment approaches for those undergoing conversion surgery. 85 , 87 , 88 , 89 A meta‐analysis revealed that adjuvant sorafenib therapy post‐resection enhanced OS and RFS, reducing recurrence with variable treatment durations from 4 to nearly 71 months. 90 Similarly, the encouraging positive results of the IMbrave 050 trial have gradually positioned immunotherapy as a critical choice for postoperative adjuvant treatment, with 12 months of atezolizumab combining bevacizumab significantly improving RFS in high‐risk patients. 84 Align with these findings, a recently published retrospective study also showed that adjuvant immunotherapy led to better RFS and OS compared with active surveillance following curative‐intent surgical resection for intermediate/advanced‐stage HCC. 91 The immunotherapy regimen included pembrolizumab, sintilimab, camrelizumab, toripalimab, and tislelizumab, with a treatment period of 12 months unless there was a recurrence or severe intolerable complications. Additionally, it is noteworthy that whether the duration of postoperative adjuvant therapy can be adjusted based on achieving a pCR in resected specimens remains an unanswered question. In summary, the development of individualized and precise adjuvant therapy regimens and protocols should consider multiple factors, including the patient's surgical treatment, tumor biology, patient compliance, drug response, and financial implications. In addition, it is also expected that more multicenter prospective studies with a larger sample size will be conducted to furnish real‐world evidence and to better guide clinical practice.
8. SUMMARY AND FUTURE PERSPECTIVES
Conversion therapy is primarily aimed at patients with HCC who cannot achieve complete resection through oncological or surgical means. Due to great advancements in surgical techniques and drug development, the conversion therapy for HCC has also yield impressive outcomes, with the following states recently: (1) currently, the majority of data on conversion therapy for HCC is derived from retrospective studies, resulting in significant discrepancies in reported conversion rates across different studies; (2) due to significant discrepancy in tumor staging among patients undergoing treatment, different approaches are implemented, thus leading to varying extent of survival benefits; (3) therapies based on lenvatinib, combining additional systemic and local treatments, have shown higher ORR and conversion rates, yet still require further support from large‐scale, high‐quality prospective clinical studies; (4) although there is no strict limit on the duration of conversion therapy, and it is usually aimed at achieving R0 resection, the investigation of novel biomarkers for early prediction of disease progression is particularly crucial; and (5) notably, multi‐drug combination regimens, while offering stronger conversion effects, have a significantly higher incidence of severe AEs (Grade ≥ 3) compared with dual‐drug combinations or monotherapy with TKIs/ICIs alone. Therefore, finding the optimal balance between drug efficacy and safety is of paramount importance. Last, postoperative adjuvant treatment should be personalized based on the patient's individual conditions, such as preoperative and perioperative risk factors, to maximize survival benefits after curative resection.
The current state of HCC conversion therapy has sparked extensive discussion and raised numerous hot issues, particularly regarding the necessity and timing of surgical intervention post‐conversion therapy, and addressing these concerns requires more high‐quality evidence from prospective trials or translational research (Figure 3). Despite the winding and thorny path ahead, it is firmly believed that with the increasing disclosure of positive results from a growing number of clinical trials and the fast updating of drug regimens, the conversion therapy for HCC will undoubtedly become more standardized, safe, and effective under the unwavering pursuit of survival benefits by physicians, thereby bringing new dawn and hope to more advanced HCC patients.
FIGURE 3.
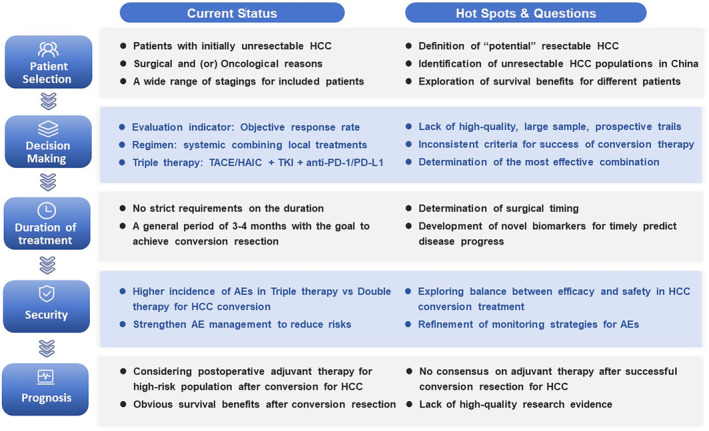
Current status, hotspots, and challenges in conversion therapy for HCC. AE, adverse event; HAIC, hepatic arterial infusion chemotherapy; HCC, hepatocellular carcinoma; PD‐1/PD‐L1, programmed cell death protein‐1/programmed cell death protein‐ligand 1; TACE, transarterial chemoembolization; TKIs, tyrosine kinase inhibitors.
AUTHOR CONTRIBUTIONS
Ming‐Da Wang: Conceptualization; data curation; formal analysis; resources; writing – original draft. Xue‐Jun Xu: Conceptualization; data curation; formal analysis; writing – original draft. Ke‐Chun Wang: Conceptualization; data curation; formal analysis; investigation; writing – original draft. Yong‐Kang Diao: Conceptualization; formal analysis; methodology; software. Jia‐Hao Xu: Investigation; methodology; project administration; supervision; visualization. Li‐Hui Gu: Formal analysis; investigation; resources; software; supervision. Lan‐Qing Yao: Conceptualization; formal analysis; investigation; methodology; software; validation. Chao Li: Conceptualization; resources; software; supervision; validation; visualization. Guo‐Yue Lv: Funding acquisition; resources; supervision; visualization; writing – review and editing. Tian Yang: Conceptualization; funding acquisition; resources; supervision; validation; visualization; writing – review and editing.
ACKNOWLEDGEMENTS
None.
FUNDING INFORMATION
This study was supported by the National Natural Science Foundation of China (No. 81972726 and 82273074 for Yang T; 82372813 for Wang M; U20A20360 for Lv G), Dawn Project Foundation of Shanghai (No. 21SG36 for Yang T), Shanghai Health and Hygiene Discipline Leader Project (No. 2022XD001 for Yang T), Shanghai Outstanding Academic Leader Program (No. 23XD1424900 for Yang T), the Natural Science Foundation of Shanghai (No. 22ZR1477900 for Wang M), and Shanghai Science and Technology Committee Rising‐Star Program (No. 22QA1411600 for Wang M). The funding bodies had no role in the study design, data collection, analysis, interpretation of data, or writing of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENTS
Approval of the research protocol by an Institutional Reviewer Board: N/A.
Informed Consent: N/A.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.
Wang M‐D, Xu X‐J, Wang K‐C, et al. Conversion therapy for advanced hepatocellular carcinoma in the era of precision medicine: Current status, challenges and opportunities. Cancer Sci. 2024;115:2159‐2169. doi: 10.1111/cas.16194
Ming‐Da Wang, Xue‐Jun Xu, Ke‐Chun Wang contributed equally to this work.
Contributor Information
Guo‐Yue Lv, Email: lvgy@jlu.edu.cn.
Tian Yang, Email: yangtianehbh@smmu.edu.cn.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. [DOI] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 3. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345‐1362. [DOI] [PubMed] [Google Scholar]
- 4. Singal AG, Llovet JM, Yarchoan M, et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78(6):1922‐1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Q, Cao M, Lei L, et al. Burden of liver cancer: from epidemiology to prevention. Chin J Cancer Res. 2022;34(6):554‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xie DY, Zhu K, Ren ZG, Zhou J, Fan J, Gao Q. A review of 2022 Chinese clinical guidelines on the management of hepatocellular carcinoma: updates and insights. Hepatobil Surg Nutr. 2023;12(2):216‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun HC, Zhou J, Wang Z, et al. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobil Surg Nutr. 2022;11(2):227‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lau WY, Lai EC. Salvage surgery following downstaging of unresectable hepatocellular carcinoma—a strategy to increase resectability. Ann Surg Oncol. 2007;14(12):3301‐3309. [DOI] [PubMed] [Google Scholar]
- 9. Zhao HT, Cai JQ. Chinese expert consensus on neoadjuvant and conversion therapies for hepatocellular carcinoma. World J Gastroenterol. 2021;27(47):8069‐8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arita J, Ichida A, Nagata R, et al. Conversion surgery after preoperative therapy for advanced hepatocellular carcinoma in the era of molecular targeted therapy and immune checkpoint inhibitors. J Hepatobiliary Pancreat Sci. 2022;29(7):732‐740. [DOI] [PubMed] [Google Scholar]
- 11. Shindoh J, Kawamura Y, Kobayashi Y, et al. Prognostic impact of surgical intervention after lenvatinib treatment for advanced hepatocellular carcinoma. Ann Surg Oncol. 2021;28(12):7663‐7672. [DOI] [PubMed] [Google Scholar]
- 12. Zhang J, Huang H, Bian J, et al. Safety, feasibility, and efficacy of associating liver partition and portal vein ligation for staged hepatectomy in treating hepatocellular carcinoma: a systematic review. Ann Transl Med. 2020;8(19):1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan A, Zhang WY, Chok K, et al. ALPPS versus portal vein embolization for hepatitis‐related hepatocellular carcinoma: a changing paradigm in modulation of future liver remnant before major hepatectomy. Ann Surg. 2021;273(5):957‐965. [DOI] [PubMed] [Google Scholar]
- 14. Xu X, Lau WY, Yang T. The updated BCLC staging system needs further refinement: a surgeon's perspective. J Hepatol. 2022;76(5):1239‐1240. [DOI] [PubMed] [Google Scholar]
- 15. Xie D, Shi J, Zhou J, Fan J, Gao Q. Clinical practice guidelines and real‐life practice in hepatocellular carcinoma: a Chinese perspective. Clin Mol Hepatol. 2023;29(2):206‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cappuyns S, Corbett V, Yarchoan M, Finn RS, Llovet JM. Critical appraisal of guideline recommendations on systemic therapies for advanced hepatocellular carcinoma: a review. JAMA Oncol. 2023;3:404. doi: 10.1001/jamaoncol.2023.2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin ZP, Hu XL, Chen D, et al. Clinical efficacy of targeted therapy, immunotherapy combined with hepatic artery infusion chemotherapy (FOLFOX), and lipiodol embolization in the treatment of unresectable hepatocarcinoma. J Physiol Pharmacol. 2022;73(6):755‐762. doi: 10.26402/jpp.2022.6.08 [DOI] [PubMed] [Google Scholar]
- 18. Xu S, Lai R, Zhao Q, Zhao P, Zhao R, Guo Z. Correlation between immune‐related adverse events and prognosis in hepatocellular carcinoma patients treated with immune checkpoint inhibitors. Front Immunol. 2021;12:794099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28‐36. [DOI] [PubMed] [Google Scholar]
- 20. Iwamoto H, Shimose S, Shirono T, Niizeki T, Kawaguchi T. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma in the era of chemo‐diversity. Clin Mol Hepatol. 2023;29(3):593‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Si T, Huang Z, Khorsandi SE, Ma Y, Heaton N. Hepatic arterial infusion chemotherapy versus transarterial chemoembolization for unresectable hepatocellular carcinoma: a systematic review with meta‐analysis. Front Bioeng Biotechnol. 2022;10:1010824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Y, Huang G, Wang Y, et al. Is salvage liver resection necessary for initially unresectable hepatocellular carcinoma patients downstaged by transarterial chemoembolization? Ten years of experience. Oncologist. 2016;21(12):1442‐1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang YH, Wu JC, Chen SC, et al. Survival benefit of transcatheter arterial chemoembolization in patients with hepatocellular carcinoma larger than 10 cm in diameter. Aliment Pharmacol Ther. 2006;23(1):129‐135. [DOI] [PubMed] [Google Scholar]
- 24. Xue T, Le F, Chen R, et al. Transarterial chemoembolization for huge hepatocellular carcinoma with diameter over ten centimeters: a large cohort study. Med Oncol. 2015;32(3):64. [DOI] [PubMed] [Google Scholar]
- 25. Song MJ. Hepatic artery infusion chemotherapy for advanced hepatocellular carcinoma. World J Gastroenterol. 2015;21(13):3843‐3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mei J, Yu H, Qin L, Jia Z. FOLFOX‐HAIC for unresectable large hepatocellular carcinoma: the effectiveness has yet to be determined. J Clin Oncol. 2022;40(16):1841. [DOI] [PubMed] [Google Scholar]
- 27. Zhao M, Guo Z, Zou YH, et al. Arterial chemotherapy for hepatocellular carcinoma in China: consensus recommendations. Hepatol Int. 2023;18(1):4‐31. [DOI] [PubMed] [Google Scholar]
- 28. Li QJ, He MK, Chen HW, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized phase III trial. J Clin Oncol. 2022;40(2):150‐160. [DOI] [PubMed] [Google Scholar]
- 29. He MK, Le Y, Li QJ, et al. Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non‐randomized study. Chin J Cancer. 2017;36(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lyu N, Wang X, Li JB, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: a biomolecular exploratory, randomized, phase III trial (FOHAIC‐1). J Clin Oncol. 2022;40(5):468‐480. [DOI] [PubMed] [Google Scholar]
- 31. Lyu N, Kong Y, Mu L, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2018;69(1):60‐69. [DOI] [PubMed] [Google Scholar]
- 32. He M, Li Q, Zou R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7):953‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Byun HK, Kim HJ, Im YR, Kim DY, Han KH, Seong J. Dose escalation by intensity modulated radiotherapy in liver‐directed concurrent chemoradiotherapy for locally advanced BCLC stage C hepatocellular carcinoma. Radiother Oncol. 2019;133:1‐8. [DOI] [PubMed] [Google Scholar]
- 34. Hamaoka M, Kobayashi T, Kuroda S, et al. Hepatectomy after down‐staging of hepatocellular carcinoma with portal vein tumor thrombus using chemoradiotherapy: a retrospective cohort study. Int J Surg. 2017;44:223‐228. [DOI] [PubMed] [Google Scholar]
- 35. Chong JU, Choi GH, Han DH, et al. Downstaging with localized concurrent chemoradiotherapy can identify optimal surgical candidates in hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2018;25(11):3308‐3315. [DOI] [PubMed] [Google Scholar]
- 36. Gavriilidis P, Pawlik TM, Azoulay D. Comprehensive review of hepatocellular carcinoma with portal vein tumor thrombus: state of art and future perspectives. Hepatobiliary Pancreat Dis Int. 2023;23:221‐227. doi: 10.1016/j.hbpd.2023.10.009 [DOI] [PubMed] [Google Scholar]
- 37. Li B, Qiu J, Zheng Y, et al. Conversion to resectability using transarterial chemoembolization combined with hepatic arterial infusion chemotherapy for initially unresectable hepatocellular carcinoma. Ann Surg Open. 2021;2(2):e057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first‐line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non‐inferiority trial. Lancet. 2018;391(10126):1163‐1173. [DOI] [PubMed] [Google Scholar]
- 39. Kudo M, Ueshima K, Chan S, et al. Lenvatinib as an initial treatment in patients with intermediate‐stage hepatocellular carcinoma beyond up‐to‐seven criteria and child‐Pugh a liver function: a proof‐of‐concept study. Cancers (Basel). 2019;11(8):1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qin S, Bi F, Gu S, et al. Donafenib versus sorafenib in first‐line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open‐label, parallel‐controlled phase II–III trial. J Clin Oncol. 2021;39(27):3002‐3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894‐1905. [DOI] [PubMed] [Google Scholar]
- 42. Kudo M, Aoki T, Ueshima K, et al. Achievement of complete response and drug‐free status by atezolizumab plus bevacizumab combined with or without curative conversion in patients with Transarterial chemoembolization‐unsuitable, intermediate‐stage hepatocellular carcinoma: a multicenter proof‐of‐concept study. Liver Cancer. 2023;12(4):321‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu XD, Huang C, Shen YH, et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti‐PD‐1 antibody combinations. Liver Cancer. 2021;10(4):320‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gordan JD, Kennedy EB, Abou‐Alfa GK, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38(36):4317‐4345. [DOI] [PubMed] [Google Scholar]
- 45. Takeda H, Nishijima N, Nasu A, et al. Long‐term antitumor effect of lenvatinib on unresectable hepatocellular carcinoma with portal vein invasion. Hepatol Res. 2019;49(5):594‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hidaka M, Hara T, Soyama A, et al. The outcome of conversion liver resection surgery by lenvatinib treatment: a single center experience. Anticancer Res. 2022;42(6):3049‐3054. [DOI] [PubMed] [Google Scholar]
- 47. Zhu XD, Huang C, Shen YH, et al. Hepatectomy after conversion therapy using tyrosine kinase inhibitors plus anti‐PD‐1 antibody therapy for patients with unresectable hepatocellular carcinoma. Ann Surg Oncol. 2023;30(5):2782‐2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ding X, Sun W, Li W, et al. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first‐line treatment for hepatocellular carcinoma with portal vein tumor thrombus: a prospective randomized study. Cancer. 2021;127(20):3782‐3793. [DOI] [PubMed] [Google Scholar]
- 50. Kudo M, Ueshima K, Saeki I, et al. A phase 2, prospective, multicenter, single‐arm trial of transarterial chemoembolization therapy in combination strategy with lenvatinib in patients with unresectable intermediate‐stage hepatocellular carcinoma: TACTICS‐L trial. Liver Cancer. 2024;13(1):99‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. He MK, Liang RB, Zhao Y, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2021;13:17588359211002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chiang CL, Chiu K, Chan K, et al. Sequential transarterial chemoembolisation and stereotactic body radiotherapy followed by immunotherapy as conversion therapy for patients with locally advanced, unresectable hepatocellular carcinoma (START‐FIT): a single‐arm, phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8(2):169‐178. [DOI] [PubMed] [Google Scholar]
- 53. Tai D, Loke K, Gogna A, et al. Radioembolisation with Y90‐resin microspheres followed by nivolumab for advanced hepatocellular carcinoma (CA 209‐678): a single arm, single centre, phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6(12):1025‐1035. [DOI] [PubMed] [Google Scholar]
- 54. Liu J, Li Z, Zhang W, et al. Comprehensive treatment of trans‐arterial chemoembolization plus Lenvatinib followed by Camrelizumab for advanced hepatocellular carcinoma patients. Front Pharmacol. 2021;12:709060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang J, Zhang X, Mu H, et al. Surgical conversion for initially unresectable locally advanced hepatocellular carcinoma using a triple combination of angiogenesis inhibitors, anti‐PD‐1 antibodies, and hepatic arterial infusion chemotherapy: a retrospective study. Front Oncol. 2021;11:729764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Llovet JM, Vogel A, Madoff DC, et al. Randomized phase 3 LEAP‐012 study: transarterial chemoembolization with or without lenvatinib plus pembrolizumab for intermediate‐stage hepatocellular carcinoma not amenable to curative treatment. Cardiovasc Intervent Radiol. 2022;45(4):405‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Llovet JM, Kudo M, Merle P, et al. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP‐002): a randomised, double‐blind, phase 3 trial. Lancet Oncol. 2023;24(12):1399‐1410. [DOI] [PubMed] [Google Scholar]
- 58. Qu WF, Ding ZB, Qu XD, et al. Conversion therapy for initially unresectable hepatocellular carcinoma using a combination of toripalimab, lenvatinib plus TACE: real‐world study. BJS Open. 2022;6(5):zrac114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ke Q, Xin F, Fang H, Zeng Y, Wang L, Liu J. The significance of transarterial chemo(embolization) combined with tyrosine kinase inhibitors and immune checkpoint inhibitors for unresectable hepatocellular carcinoma in the era of systemic therapy: a systematic review. Front Immunol. 2022;13:913464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yau T, Kang YK, Kim TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6(11):e204564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang F, Xu GL, Huang JT, et al. Transarterial chemoembolization combined with immune checkpoint inhibitors and tyrosine kinase inhibitors for unresectable hepatocellular carcinoma: efficacy and systemic immune response. Front Immunol. 2022;13:847601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Luo L, He Y, Zhu G, et al. Hepatectomy after conversion therapy for initially unresectable HCC: what is the difference. J Hepatocell Carcinoma. 2022;9:1353‐1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yi Y, Sun BY, Weng JL, et al. Lenvatinib plus anti‐PD‐1 therapy represents a feasible conversion resection strategy for patients with initially unresectable hepatocellular carcinoma: a retrospective study. Front Oncol. 2022;12:1046584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sun HC, Zhu XD. Downstaging conversion therapy in patients with initially unresectable advanced hepatocellular carcinoma: an overview. Front Oncol. 2021;11:772195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang ZF, Luo YJ, Lu Q, Dai SX, Sha WH. Conversion therapy and suitable timing for subsequent salvage surgery for initially unresectable hepatocellular carcinoma: what is new. World J Clin Cases. 2018;6(9):259‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang B, Shi X, Cui K, et al. Real‐world practice of conversion surgery for unresectable hepatocellular carcinoma – a single center data of 26 consecutive patients. BMC Cancer. 2023;23(1):465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang W, Hu B, Han J, et al. Surgery after conversion therapy with PD‐1 inhibitors plus tyrosine kinase inhibitors are effective and safe for advanced hepatocellular carcinoma: a pilot study of ten patients. Front Oncol. 2021;11:747950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Najmi Varzaneh F, Pandey A, Aliyari Ghasabeh M, et al. Prediction of post‐TACE necrosis of hepatocellular carcinoma usingvolumetric enhancement on MRI and volumetric oil deposition on CT, with pathological correlation. Eur Radiol. 2018;28(7):3032‐3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Casadei‐Gardini A, Orsi G, Caputo F, Ercolani G. Developments in predictive biomarkers for hepatocellular carcinoma therapy. Expert Rev Anticancer Ther. 2020;20(1):63‐74. [DOI] [PubMed] [Google Scholar]
- 70. Kaissis GA, Lohöfer FK, Hörl M, et al. Combined DCE‐MRI‐ and FDG‐PET enable histopathological grading prediction in a rat model of hepatocellular carcinoma. Eur J Radiol. 2020;124:108848. [DOI] [PubMed] [Google Scholar]
- 71. Ghidaglia J, Laurent V, Sebagh M, et al. Influence of key histological characteristics on 18F‐fluorodeoxyglucose /18F‐choline positron emission tomography positivity in hepatocellular carcinoma: a machine learning study. Front Med (Lausanne). 2023;10:1087957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gordic S, Corcuera‐Solano I, Stueck A, et al. Evaluation of HCC response to locoregional therapy: validation of MRI‐based response criteria versus explant pathology. J Hepatol. 2017;67(6):1213‐1221. [DOI] [PubMed] [Google Scholar]
- 73. Wang DD, Zhang JF, Zhang LH, et al. Clinical‐radiomics predictors to identify the suitability of transarterial chemoembolization treatment in intermediate‐stage hepatocellular carcinoma: a multicenter study. Hepatobiliary Pancreat Dis Int. 2023;22(6):594‐604. [DOI] [PubMed] [Google Scholar]
- 74. Tsilimigras DI, Bagante F, Moris D, et al. Recurrence patterns and outcomes after resection of hepatocellular carcinoma within and beyond the Barcelona clinic liver cancer criteria. Ann Surg Oncol. 2020;27(7):2321‐2331. [DOI] [PubMed] [Google Scholar]
- 75. Wang MD, Li C, Liang L, et al. Early and late recurrence of hepatitis B virus‐associated hepatocellular carcinoma. Oncologist. 2020;25(10):e1541‐e1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Georgilis E, Gavriatopoulou M, Tsilimigras DI, Malandrakis P, Theodosopoulos T, Ntanasis‐Stathopoulos I. Optimizing adjuvant therapy after surgery for colorectal cancer liver metastases: a systematic review. J Clin Med. 2023;12(6):2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pan Y, Yuan Z, Wang J, et al. Survival benefit and impact of adjuvant therapies following FOLFOX‐HAIC‐based conversion therapy with unresectable hepatocellular carcinoma: a retrospective cohort study. J Cancer Res Clin Oncol. 2023;149(16):14761‐14774. [DOI] [PubMed] [Google Scholar]
- 78. Zhou H, Song T. Conversion therapy and maintenance therapy for primary hepatocellular carcinoma. Biosci Trends. 2021;15(3):155‐160. [DOI] [PubMed] [Google Scholar]
- 79. Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double‐blind, placebo‐controlled trial. Lancet Oncol. 2015;16(13):1344‐1354. [DOI] [PubMed] [Google Scholar]
- 80. Zeng ZM, Mo N, Zeng J, et al. Advances in postoperative adjuvant therapy for primary liver cancer. World J Gastrointest Oncol. 2022;14(9):1604‐1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD‐L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15(3):971‐979. [DOI] [PubMed] [Google Scholar]
- 82. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fairfax BP, Taylor CA, Watson RA, et al. Peripheral CD8(+) T cell characteristics associated with durable responses to immune checkpoint blockade in patients with metastatic melanoma. Nat Med. 2020;26(2):193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Qin S, Chen M, Cheng AL, et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high‐risk hepatocellular carcinoma (IMbrave050): a randomised, open‐label, multicentre, phase 3 trial. Lancet. 2023;402(10415):1835‐1847. [DOI] [PubMed] [Google Scholar]
- 85. Chamseddine S, LaPelusa M, Kaseb AO. Systemic neoadjuvant and adjuvant therapies in the management of hepatocellular carcinoma‐a narrative review. Cancers (Basel). 2023;15(13):3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zeng JS, Zeng JX, Huang Y, Liu JF, Zeng JH. The effect of adjuvant transarterial chemoembolization for hepatocellular carcinoma after liver resection based on risk stratification. Hepatobiliary Pancreat Dis Int. 2023;22(5):482‐489. [DOI] [PubMed] [Google Scholar]
- 87. Zhang W, Zhang B, Chen XP. Adjuvant treatment strategy after curative resection for hepatocellular carcinoma. Front Med. 2021;15(2):155‐169. [DOI] [PubMed] [Google Scholar]
- 88. Zhu XD, Li KS, Sun HC. Adjuvant therapies after curative treatments for hepatocellular carcinoma: current status and prospects. Genes Dis. 2020;7(3):359‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Su YY, Li CC, Lin YJ, Hsu C. Adjuvant versus neoadjuvant immunotherapy for hepatocellular carcinoma: clinical and immunologic perspectives. Semin Liver Dis. 2021;41(3):263‐276. [DOI] [PubMed] [Google Scholar]
- 90. Huang S, Li D, Zhuang L, Sun L, Wu J. A meta‐analysis of the efficacy and safety of adjuvant sorafenib for hepatocellular carcinoma after resection. World J Surg Oncol. 2021;19(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Xu X, Wang MD, Xu JH, et al. Adjuvant immunotherapy improves recurrence‐free and overall survival following surgical resection for intermediate/advanced hepatocellular carcinoma a multicenter propensity matching analysis. Front Immunol. 2023;14:1322233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.


