Abstract
INTRODUCTION
Patients with subjective memory complaints (SMC) may include subgroups with different neuropsychological profiles and risks of cognitive impairment.
METHODS
Cluster analysis was performed on two datasets (n: 630 and 734) comprising demographic and neuropsychological data from SMC and healthy controls (HC). Survival analyses were conducted on clusters. Bayesian model averaging assessed the predictive utility of clusters and other biomarkers.
RESULTS
Two clusters with higher and lower than average cognitive performance were detected in SMC and HC. Assignment to the lower performance cluster increased the risk of cognitive impairment in both datasets (hazard ratios: 1.78 and 2.96; P log‐rank: 0.04 and <0.001) and was associated with lower hippocampal volumes and higher tau/amyloid beta 42 ratios in cerebrospinal fluid. The effect of SMC was small and confounded by mood.
DISCUSSION
This study provides evidence of the presence of cognitive clusters that hold biological significance and predictive value for cognitive decline in SMC and HC.
Highlights
Patients with subjective memory complaints include two cognitive clusters.
Assignment to the lower performance cluster increases risk of cognitive impairment.
This cluster shows a pattern of biomarkers consistent with incipient Alzheimer's disease pathology.
The same cognitive cluster structure is found in healthy controls.
The effect of memory complaints on risk of cognitive decline is small and confounded.
Keywords: Alzheimer's disease, Bayesian model averaging, biomarkers, cluster analysis, cognitive impairment, neuropsychological profiles, subjective memory complaints
1. BACKGROUND
Subjective memory complaints (SMC) refer to individuals' self‐perceived decline in memory function without objective evidence of cognitive impairment. 1 , 2 Methods used to detect memory complaints range from simple questions regarding one's perception of memory function 3 to more comprehensive questionnaires such as the Metamemory in Adulthood Questionnaire. 4 Similarly, the neuropsychological criteria used to define normal cognitive function have not been uniform, using varying degrees of stringency and comprehensiveness. 5
The prevalence of SMC, particularly among older adults, is remarkably high. Previous studies report a prevalence ranging from 25% to 50% in this population. 6 Additionally, SMC have been shown to have a negative impact on the quality of life for older individuals. 7
Despite high prevalence, the clinical relevance and nosological position of SMC remain controversial. When interpreting previous studies, it is important to differentiate between memory complaints as a symptom and SMC as a clinical entity. It is also essential to differentiate SMC from subjective cognitive decline (SCD), which refers to self‐perceived cognitive decline in domains beyond just memory, such as attention and executive functions. 8 Notably, the analysis of SMC patients focuses on the amnestic presentations of Alzheimer's disease (AD), in contrast to the more widely applicable concept of SCD.
Cross‐sectional studies have primarily found a link between memory complaints and mood and other psychological factors. 2 , 9 However, there are exceptions that demonstrate an association between memory complaints and objective cognitive performance 10 , 11 or have yielded mixed results. 12 , 13
On the other hand, longitudinal studies have mainly shown an increased risk of cognitive impairment among patients with SMC, as summarized in the meta‐analysis conducted by Mitchell et al. 14 Nevertheless, this finding is not universally observed across all studies. 15 , 16 Furthermore, even when the association is present, the magnitude of the risk may be small, 17 and its relevance at the population level may be limited. 18 Memory complaints by older adults have also been shown to be inconsistent over time. 19
Research in patients with SMC has revealed structural and functional changes in the brain, as well as patterns of biomarker levels that fall between those observed in healthy controls and patients with mild cognitive impairment (MCI) due to AD. 20 , 21 , 22 , 23 , 24 , 25 , 26 These findings have led to the hypothesis that SMC represent a stage within the continuum of the degenerative process associated with AD. 27 , 28
Discrepancies among studies may arise from variations in several factors, such as the definitions used for cognitive complaints, the selection of neuropsychological tests, the study designs, the duration of follow‐up periods, the source of the samples, and the statistical methods used. These conflicting observations also raise the possibility that SMC include different populations with distinct neuropsychological profiles and risks of cognitive impairment. Unsupervised machine learning methods may help unravel these kinds of questions. 29 Specifically, clustering algorithms can be applied to a battery of neuropsychological variables to identify clusters of cognitive performance. 30 In this line, Jessen et al. identified three clusters among 2389 unimpaired subjects, which corresponded to subjects without memory complaints, with general memory complaints, and with both general memory complaints and complaints regarding tasks of daily living. 31 However, the value of these clusters to predict cognitive decline or to identify people with early AD is unknown.
In this study, two observational datasets of subjects with normal cognitive function at baseline were analyzed with the main aim of identifying the presence (or absence) of cognitive clusters in patients with SMC. Additionally, if cognitive clusters were found, the study aimed to investigate the association between cluster assignments and the risk of cognitive impairment.
As secondary analyses, we assessed (in one of the datasets) the presence (or absence) of cognitive clusters in healthy controls, the association between SMC and risk of cognitive impairment, and the predictive value of cognitive clusters and SMC on risk of cognitive decline compared to that of other clinical and biomarker variables, including brain volumetric measures, brain [18F]Fluorodeoxyglucose (FDG) uptake, brain florbetapir uptake, apolipoprotein E (APOE) genotype, and levels of biomarkers in cerebrospinal fluid (CSF).
2. METHODS
2.1. Clínica Universidad de Navarra dataset
2.1.1. Description of the Clínica Universidad de Navarra sample
The Clínica Universidad de Navarra (CUN) database (n = 945) retrospectively collected data on patients with SMC who were treated at the Memory Unit of CUN (Pamplona, Spain) between 2001 and 2017. SMC was defined as self‐reported perception of memory difficulties without objective evidence of cognitive impairment, indicated by test scores > −1.5 standard deviation (SD) of the mean (see below). Note that these criteria allow the inclusion of cases that would be classified as subtle cognitive decline in more recent classifications. 32
All participants were evaluated by an experienced neurologist on cognitive disorders. The initial assessment included a medical and history review, an interview with a family member or friend, and a general and neurological examination. All participants underwent laboratory tests (full blood count, biochemistry, vitamin B12, folate, glucose, lipids, syphilis serology, and thyroid function), neuropsychological assessment, and brain magnetic resonance imaging (MRI). The neuropsychological evaluation included the following tests or scales: the Mini‐Mental State Examination (MMSE), 33 the memory evaluation included in the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) battery, 34 categorical fluency measured by the number of animals named in 1 minute, phonological fluency measured by the number of words beginning with the letter P named in 1 minute, 35 the Stroop Color and Word Test (SCWT), 36 Raven's matrices test, 37 the Boston Naming Test, 38 the Trail Making Test Parts A and B (TMT‐A and TMT‐B), 39 and the Geriatric Depression Scale with 30 items (GDS‐30). 40 All participants were reviewed in a multidisciplinary consensus meeting to determine a clinical diagnosis. We excluded patients with MCI or dementia at baseline. 41 , 42 Participants were also excluded if they had major neurological or systemic illnesses that could cause cognitive impairment, current or past major psychiatric disease (e.g., schizophrenia, major depression, or bipolar disorder), history of alcohol or substance abuse, significant MRI abnormalities (brain tumors, large cerebral infarct, or bleeding), or past head trauma with loss of consciousness. In follow‐up visits, patients were evaluated by a neurologist, and a neuropsychological assessment was performed to establish clinical progression to amnestic MCI or dementia due to AD.
RESEARCH IN CONTEXT
Systematic review: A PubMed review shows that the clinical relevance of subjective memory complaints (SMC) remains controversial. Cross‐sectional studies primarily associate SMC with mood, while longitudinal studies reveal an increased risk of cognitive impairment. These conflicting data suggest the presence of subgroups within SMC with varying risks of cognitive impairment.
Interpretation: Two clusters with higher and lower than average cognitive performance were identified in patients with SMC and healthy controls. Assignment to the lower performance cluster increased the risk of cognitive impairment and was associated with a pattern of biomarkers consistent with incipient Alzheimer's disease pathology. The effect of SMC on the risk of cognitive decline was small and confounded by mood.
Future directions: Current knowledge advocates for a proactive strategy to detect dementing disorders. Individuals performing below average on neuropsychological tests, even within normal ranges, have an increased risk of cognitive decline, justifying more stringent follow‐up.
For the current analysis, we restricted the sample to subjects > 50 years old who had valid results in all neuropsychological tests used for clustering (see Section 2.1.2). This resulted in a final sample of 630 cases. Table 1 presents the main clinical and demographic characteristics of these patients at baseline. The sample included 243 subjects without follow‐up, 211 subjects with follow‐up < 1 year (mean = 33.3 days, SD = 84.2; median = 8 days, interquartile range [IQR] = 4 to 12, range = 1 to 364), and 176 subjects with follow‐up > 1 year (mean = 1670.5 days, SD = 1137.8; median = 1374.5, IQR = 800.2 to 2382.2, range = 367 to 5163). Note that the limited sample size of the patients with long follow‐up does not affect cluster analysis, which is a cross‐sectional analysis performed on baseline characteristics, but it reduces the sample available to evaluate the risk of cognitive decline (see Section 2.1.3).
TABLE 1.
Demographic and neuropsychological characteristics of the study samples at baseline.
| Characteristics | CUN dataset (n = 630) | ADNI dataset (n = 734) |
|---|---|---|
| Age (years) | 66 (60, 72) | 71 (67, 76) |
| Sex | ||
| Male | 326 (52%) | 309 (42%) |
| Female | 304 (48%) | 425 (58%) |
| Education | ||
| Levels 0–1: 233 (37%) | 16 (15, 18) | |
| Levels 2–4: 397 (63%) | – | |
| Memory complaints | ||
| Yes | 630 (100%) | 387 (53%) |
| No | 0 (0%) | 347 (47%) |
| GDS | ||
| 15 items | – | 0 (0, 1) |
| 30 items | 8 (5, 12) | – |
| MMSE | 29 (28, 30) | 29 (29, 30) |
| Delayed recall | 5 (4, 6) | 7 (6, 8) |
| Categorical fluency | 17 (13, 20) | 21 (17, 25) |
| Phonological fluency | 13 (10, 17) | – |
| Boston Naming Test | 52 (49, 55) | – |
| SCWT | 45 (41, 51) | – |
| Raven's matrices | 29 (26, 32) | – |
| TMT‐A (seconds) | 41 (31, 55) | 31 (25, 38) |
| TMT‐B (seconds) | 95 (72, 135) | 72 (55, 93) |
Note: Descriptive statistics are given as median (interquartile range) or frequency (percentage). Educational attainment is encoded as an ordered variable in the CUN dataset (0 = no formal education; 1 = pre‐college (primary education); 2 = college or vocational training; 3 = bachelor degree/diploma; 4 = doctorate/master's degree), while in the ADNI dataset, it is represented in years. Delayed recall corresponds to the CERAD items in the CUN dataset and the ADAS‐Cog items in the ADNI dataset. There were three missing values for GDS‐30 in the CUN dataset.
Abbreviations: ADAS‐Cog, Alzheimer´s Disease Assessment Scale‐Cognitive Subscale; ADNI, Alzheimer's Disease Neuroimaging Initiative; CERAD, Consortium to Establish a Registry for Alzheimer´s Disease; CUN, Clínica Universidad de Navarra; GDS, Geriatric Depression Scale; MMSE, Mini‐Mental State Examination; SCWT, Stroop Color and Word Test; TMT‐A, Trail Making Test Part A; TMT‐B, Trail Making Test Part B.
The study obtained approval from the ethics committee of the institution, and it was performed in accordance with the ethical standards laid down in the Declaration of Helsinki and its revisions. All participants gave written consent. There was no discrimination in the selection of patients based on sex or ethnicity.
2.1.2. Cluster analysis
The data underwent preprocessing before conducting the cluster analysis. This process included: (1) standardization of age and education variables; (2) standardization of the neuropsychological variables after stratifying them by age (categorized into four groups according to quartiles), sex, and education (categorized into two groups: without studies or with primary studies, or with secondary studies or higher). This second step had two main aims: (1) to obtain in‐sample standardized values of the neuropsychological variables and (2) to design a procedure that could be applied under the same conditions to samples of different origins, increasing its reproducibility. The number of strata was selected as a compromise between using too many, which would reduce the precision of the estimates, and using too few, which would increase the risk of residual confounding. Sex was not included in the cluster analysis itself, as binary variables tend to exert a disproportionate influence on clustering results.
The neuropsychological variables used for clustering included scores from the following tests or scales: MMSE, items recalled in CERAD delayed recall task, categorical fluency (animals), phonological fluency (letter P), SCWT, Raven's matrices test, items correctly named in Boston Naming Test, and time to complete TMT‐A and TMT‐B. We selected these tests with the aim to cover the main neuropsychological domains involved in AD.
Cluster tendency was assessed using the Hopkins statistics and a graphical representation of Pearson correlation‐based distances between observations.
The number of clusters and the clustering algorithm were chosen according to three measures of the internal quality of the clusters: silhouette width, Dunn index, and connectivity. The tested algorithms were: k‐means, agglomerative hierarchical, divisive hierarchical (DIANA), partition around medoids (PAM), and fuzzy analysis (FANNY) clustering.
The interpretation of the clusters was evaluated by comparing across clusters the values of the neuropsychological tests used for clustering and those of other clinical and demographic variables. Bivariate statistical tests such as the Wilcoxon rank sum test and Pearson chi‐squared test were applied to the variables not used for clustering.
The relative importance of the variables in determining the clusters was assessed using variable selection using random forests (VSURF).
2.1.3. Association between cognitive cluster assignment at baseline and risk of cognitive impairment
To perform these longitudinal analyses, the CUN database was further filtered to include only patients with a minimum follow‐up period of 1 year. This resulted in a subsample of 176 subjects, among whom there were 58 events defined as progression to amnestic MCI (57 cases) or dementia (1 case). The patients with follow‐up periods > 1 year were older at baseline (median = 68.0 years, IQR = 62.8–73.3) than those with shorter follow‐up periods (median = 65.0 years; IQR = 59.0–72.0) or no follow‐up (median = 66.0 years; IQR = 60.0–71.5; Kruskal–Wallis test P = 0.002), but there were not significant differences in sex (P = 0.327), education level (P = 0.881), or MMSE scores (P = 0.625).
The association of cognitive clusters with risk of cognitive impairment was assessed using various methods. These included the Kaplan–Meier method with the log‐rank test, the comparison of restricted mean survival times (RMST), and a Cox proportional hazards (PH) regression model adjusted for age, sex, education, and mood (measured with the GDS‐30). Additionally, the incidence rate ratio was estimated using g‐computation with a negative binomial model adjusted for the same covariates. Survival curves adjusted for age, sex, education, and mood using the direct method were also calculated.
2.2. Alzheimer's Disease Neuroimaging dataset
2.2.1. Description of the Alzheimer's Disease Neuroimaging Initiative sample
The second dataset included participants from the Alzheimer's Disease Neuroimaging Initiative (ADNI) 2 and 3 cohorts who had normal cognitive function at baseline. The dataset consisted of a total of 739 participants (adni.loni.usc.edu, accessed on April 15, 2023). It comprised two diagnostic groups: one with SMC with a total of 388 participants, and another group without memory concerns (CN) with a total of 351 participants. This basal diagnostic classification was not available in ADNI 1.
The ADNI was launched in 2003 as a public–private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial MRI, positron emission tomography, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. For up‐to‐date information, see www.adni‐info.org.
The definitions of terms can be found in the ADNI procedures manuals. It is important to note that in the ADNI 2 and 3 cohorts, the definition of normal cognitive function is stringent, requiring scores no less than approximately 0.5 SD below the mean of the Weschler Memory Scale Logical Memory II. Individuals with scores between approximately 0.5 and 1.5 SD below the mean are classified as early (amnestic) MCI, which may overlap with subtle cognitive impairment. 32 This has to be distinguished from late (amnestic) MCI, which requires scores < 1.5 SD below the mean, similar to the traditional definition. The patients of the SMC group had to score ≥ 16 on the first 12 questions of the Cognitive Change Index.
By restricting the analysis to individuals with valid results in all the neuropsychological tests used for clustering, we obtained a sample of 734 cases, with 347 in the CN group and 387 in the SMC group (Table 1). The median time of follow‐up was 1451 days (IQR = 377 to 2212) for the CN group and 756 days (IQR = 0 to 1481) for the SMC group.
Compared to the CUN dataset, several neuropsychological variables were not available in the ADNI data. Specifically, the Boston Naming Test was not included (not available in ADNI 3), and SCWT, Raven's matrices test, and phonological fluency task with the letter P were not available in both ADNI 2 and ADNI 3. The number of recalled words for the Alzheimer´s Disease Assessment Scale‐Cognitive Subscale (ADAS‐Cog) delayed recall task was calculated as 10 minus task 4 of ADAS v11 (ADASQ4) score recorded in the adnimerge table. The GDS scores in ADNI corresponded to the version with 15 items (GDS‐15).
The inclusion of the baseline diagnosis variable, labeling the SMC and CN groups, enabled us to analyze the association between memory complaints and the risk of cognitive impairment.
2.2.2. Cluster analysis and association between cognitive clusters or SMC assignment and risk of cognitive impairment
The cluster analysis and the assessment of the association between cognitive clusters and the risk of cognitive impairment were conducted as described for the CUN dataset.
The effect of SMC assignment on the risk of cognitive decline was evaluated using similar procedures. The association between SMC and other demographic, clinical, and neuropsychological variables was analyzed using standard bivariate tests.
Similar to the CUN database, the longitudinal analyses in the ADNI dataset were restricted to patients with a minimum follow‐up period of 1 year. The resulting subsample consisted of 514 patients, with 269 in the CN group and 245 in the SMC group. The number of events observed was 65 (amnestic MCI 64, dementia 1). The patients with follow‐up periods > 1 year (n = 514) had a median age at baseline of 70.9 years (IQR = 67.2–75.8), while those with no follow‐up (n = 177) had a median age of 68.5 years (IQR = 64.6–73.9), and those with follow‐up < 1 year (n = 43) had a median age of 74.9 years (IQR = 68.2–81.4; Kruskal–Wallis test P < 0.001), but there were no significant differences in sex (P = 0.100), years of education (P = 0.858), or MMSE scores (P = 0.416).
2.2.3. Association between cognitive clusters or SMC assignment and biomarker measurements
Taking advantage of the availability of neuroimaging and other biomarker data in the ADNI project, we also analyzed the association between cluster assignment or basal diagnosis (SMC vs. CN groups) and volumetric brain measurements; APOE genotype; FDG uptake at the angular, temporal, and posterior cingulate regions; 43 florbetapir cortical uptake normalized by the whole cerebellum; 44 and levels of CSF biomarkers (amyloid beta 1‐42 [Aβ42], total tau, phosphorylated P‐181 tau [p‐tau], tau/Aβ42 ratio, and p‐tau/Aβ42 ratio).
2.2.4. Bayesian model averaging of Cox PH regression models of time to cognitive impairment
A Bayesian model averaging (BMA) analysis of Cox PH models was used to investigate the relative importance of cognitive clusters, along with other clinical, demographic, and biomarker variables, in determining the risk of cognitive impairment. This analysis required a dataset with no missing values, resulting in a sample size of 144 cases (89 CN and 55 SMC). To prevent redundancy, we selected the following variables as representatives of neurodegenerative changes or Aβ42 amyloidosis: hippocampal volumes, posterior limbic FDG uptake, tau/Aβ42 ratio in CSF, and number of APOE ε4 alleles. Note that our objective was not to develop a model for predicting cognitive impairment. Instead, we used BMA to gain a better understanding of the relative predictive value of the variables. Specifically, we focused on evaluating the posterior probabilities of the coefficients that represent the variables, where a posterior probability of 0 for a coefficient indicates that the variable was excluded from the models, making it an ineffective predictor.
2.3. Statistical software
Statistical analyses were done with the R software (version 4.2.2) 45 and the R packages ADNIMERGE (0.0.1, accessed on April 15, 2023), 46 hopkins (1.0), 47 clValid (0.7), 48 factoextra (1.0.7), 49 pheatmap (1.0.12), 50 survival (3.5‐0), 51 survminer (0.4.9), 52 survRM2 (1.0‐4), 53 riskCommunicator (1.0.1), 54 FHtest (1.5), 55 adjustedCurves (0.10.1), 56 VSURF (1.2.0), 57 and BMA (3.18.17). 58
3. RESULTS
3.1. CUN dataset
3.1.1. Cluster analysis
The heatmap of the correlation‐based distances matrix provided graphical evidence suggesting the existence of cognitive clusters in the CUN sample, which consisted solely of patients with SMC (Figure 1). The Hopkins statistics yielded a value very close to one (0.999974), further supporting the presence of distinct clusters.
FIGURE 1.
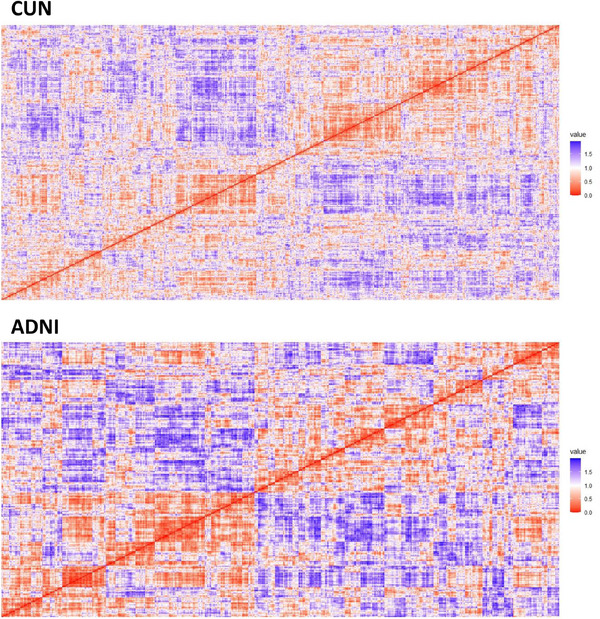
Heatmap displaying the correlation‐based distance matrices of the clinical and neuropsychological variables used for clustering in the CUN (Clínica Universidad de Navarra) and ADNI (Alzheimer's Disease Neuroimaging Initiative) datasets.
Taking into consideration the internal quality of the clusters, the analysis conducted using the clValid package indicated the existence of two clusters and suggested the use of either agglomerative (hclust) or divisive (DIANA) hierarchical clustering algorithms. After reviewing the results of both methods, we ultimately selected the DIANA algorithm. A cluster plot illustrating the two first principal components, the silhouette width plot, and the dendrograms of the clusters may be found in supporting information (Figures S1–S, 3).
The two clusters identified in the analysis were characterized by subjects with higher‐than‐average (cluster 1) and lower‐than‐average (cluster 2) standardized (Z) scores in the neuropsychological tests (Figure 2). In Figure 2, the sign of the TMT‐A and TMT‐B variables was reversed to align with the direction of the other variables, where higher values indicate better performance. A comparison of other clinical, demographic, and biomarker variables between clusters is presented in Table 2.
FIGURE 2.
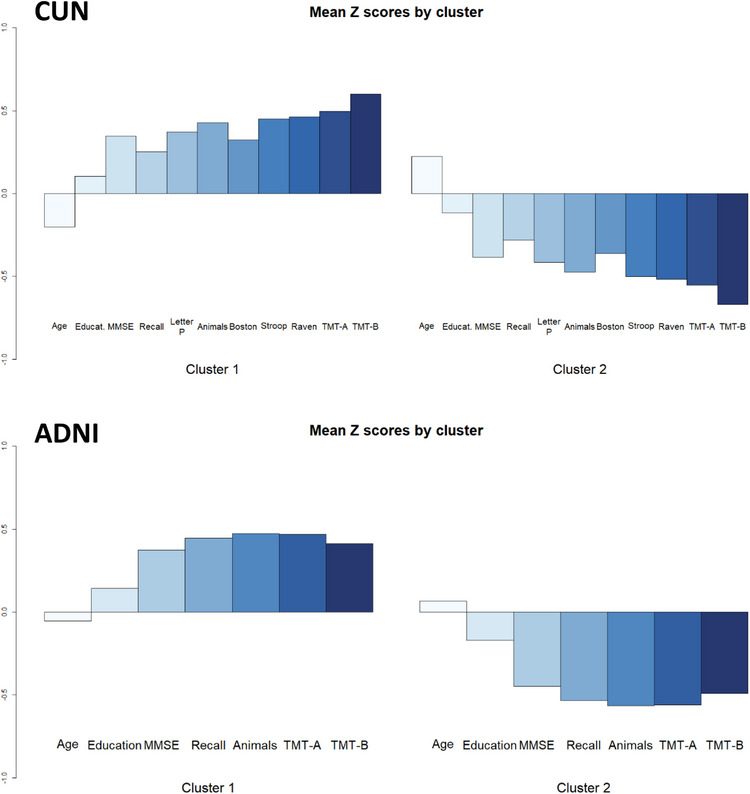
Mean standardized (Z) scores of the variables used for clustering in the CUN and ADNI datasets. The sign of TMT‐A and TMT‐B was reversed to align with the direction of the other variables. ADNI, Alzheimer's Disease Neuroimaging Initiative; CUN, Clínica Universidad de Navarra; MMSE, Mini‐Mental State Examination; TMT‐A, Trail Making Test, Part A; TMT‐B, Trail Making Test, Part B.
TABLE 2.
Demographic and neuropsychological characteristics of the study samples at baseline according to cognitive cluster assignment.
| CUN dataset (n = 630) | ADNI dataset (n = 734) | |||||
|---|---|---|---|---|---|---|
| Characteristics | Cluster 1 (n = 332) | Cluster 2 (n = 298) | P | Cluster 1 (n = 400) | Cluster 2 (n = 334) | P |
| Age (years) | 65 (59, 70) | 69 (62, 75) | – | 70 (67, 76) | 71 (67, 76) | – |
| Sex | ||||||
| Male | 167 (50%) | 159 (53%) | 0.473 | 168 (42%) | 141 (42%) | 0.999 |
| Female | 165 (50%) | 139 (47%) | 232 (58%) | 193 (58%) | ||
| Education | ||||||
| 0–1 | 116 (35%) | 117 (39%) | – | 17 (16, 18) | 16 (14, 18) | – |
| 2–4 | 216 (65%) | 181 (61%) | ||||
| Memory complaints | ||||||
| Yes | 332 (100%) | 298 (100%) | – | 200 (50%) | 187 (56%) | 0.119 |
| No | 0 (0%) | 0 (0%) | 200 (50%) | 147 (44%) | ||
| GDS | ||||||
| 15 items | – | – | – | 0 (0, 1) | 1 (0, 1) | 0.004 |
| 30 items | 7 (4, 11) | 9 (5, 13) | <0.001 | – | – | – |
| MMSE | 29 (28, 30) | 28 (27, 29) | – | 30 (29, 30) | 29 (28, 30) | – |
| Delayed recall | 5 (4, 7) | 4 (3, 5) | – | 8 (7, 9) | 7 (5, 8) | – |
| Categorical fluency | 19 (16, 21) | 14 (11, 17) | – | 23 (20, 26) | 18 (15, 21) | – |
| Phonological fluency | 15 (12, 18) | 11 (9, 14) | – | – | – | – |
| Boston Naming Test | 54 (51, 56) | 50 (47, 53) | – | – | – | – |
| SCWT | 48 (44, 53) | 42 (38, 47) | – | – | – | – |
| Raven's matrices | 32 (28, 34) | 27 (24, 29) | – | – | – | – |
| TMT‐A (seconds) | 32 (28, 34) | 52 (40, 69) | – | 27 (23, 32) | 36 (30, 44) | – |
| TMT‐B (seconds) | 76 (59, 93) | 131 (99, 170) | – | 59 (49, 76) | 86 (71, 112) | – |
Note: Descriptive statistics are given as median (interquartile range) or frequency (percentage). Educational attainment is encoded as an ordered variable in the CUN dataset (0 = no formal education; 1 = pre‐college [primary education]; 2 = college or vocational training; 3 = bachelor degree/diploma; 4 = doctorate/master's degree), while in the ADNI dataset, it is represented in years. Delayed recall corresponds to the CERAD items in the CUN dataset and the ADAS‐Cog items in the ADNI dataset. P values correspond to Wilcoxon rank sum test or Pearson chi‐squared test. Note that it is not appropriate to apply hypothesis tests to compare the variables used for clustering, which explains the absence of their P values.
Abbreviations: ADAS‐Cog, Alzheimer's Disease Assessment Scale‐Cognitive Subscale; ADNI, Alzheimer's Disease Neuroimaging Initiative; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; CUN, Clínica Universidad de Navarra; GDS, Geriatric Depression Scale; MMSE, Mini‐Mental State Examination; SCWT, Stroop Color and Word Test; TMT‐A, Trail Making Test Part A; TMT‐B, Trail Making Test Part B.
According to the VSURF analysis, the most important variable for determining the cognitive clusters was the time taken to complete the TMT‐B task (mean variable importance = 0.124; see Figure S4 in supporting information).
3.1.2. Association of cognitive clusters with risk of cognitive impairment
The participants in the lower‐than‐average cluster (cluster 2) exhibited a higher risk of developing cognitive impairment compared to the members of the higher‐than‐average cluster (cluster 1; Figure 3, log‐rank test P = 0.04). The RMST values in days were 2537 for cluster 1 and 2143 for cluster 2, resulting in a difference of 394 days (95% confidence interval [CI] = 94 to 694, P = 0.010). A Cox PH model adjusting for age, sex, education, and mood showed a hazard ratio (HR) of cognitive impairment for cluster 2 compared to cluster 1 of 1.742 (CI = 0.994 to 3.053, P = 0.052), and it did not violate the PH assumption. Age was significantly associated with cognitive impairment (HR = 1.093, CI = 1.043 to 1.144, P < 0.001), and there was an almost significant association between education and cognitive decline (HR = 0.740, CI = 0.547–1.001, P = 0.051). The incidence rate ratio estimated using a negative binomial model indicated an increased rate of cognitive impairment in cluster 2 compared to cluster 1: 1.802 (CI = 1.158–3.177). The adjusted survival curves for cognitive clusters were similar to the unadjusted curves (see Figure S5 in supporting information).
FIGURE 3.
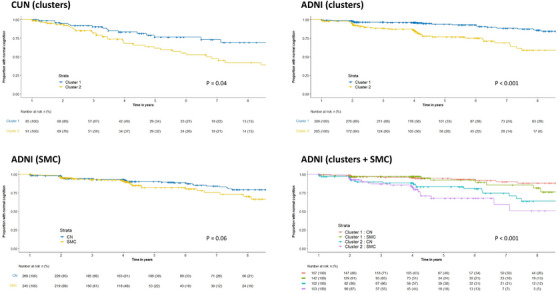
Kaplan–Meier survival curves stratified by cognitive cluster in the CUN dataset, and stratified by cluster, initial diagnostic classification (SMC vs. CN) or both in the ADNI dataset. The P values correspond to the log‐rank test. ADNI, Alzheimer's Disease Neuroimaging Initiative; CN, cognitively normal; CUN, Clínica Universidad de Navarra; SMC, subjective memory complaints.
3.2. ADNI dataset
3.2.1. Cluster analysis
The application of the same clustering methods to the ADNI sample again revealed the presence of two clusters representing higher‐than‐average (cluster 1) and lower‐than‐average (cluster 2) neuropsychological performance in both the CN and SMC groups (Figures 1 and 2, Table 2, and Figures S1–S, 3). The Hopkins statistics yielded a value of 0.9985181, confirming a strong tendency toward clustering. The findings were consistent when analyzing all cases together or examining the CN and SMC groups separately (not shown).
In this dataset, the most important variable to determine the clusters was the categorical fluency (mean variable importance = 0.092), followed by TMT‐A (0.082), ADAS‐Cog delayed recall (0.081), MMSE (0.059), and TMT‐B (0.058; see Figure S4).
3.2.2. Association of cognitive clusters with biomarkers
The comparison of biomarker values between the two cognitive clusters showed significant differences in whole brain, hippocampal and entorhinal volumes, posterior limbic FDG activity, and tau/Aβ42 and p‐tau/Aβ42 CSF ratios (Table 3). In particular, the lower‐than‐average performance cluster (cluster 2) showed reduced values of brain volumes and posterior limbic FDG activity, and higher values of tau/Aβ42 and p‐tau/Aβ42 CSF ratios compared to the higher‐than‐average performance cluster. Interestingly, the differences were more significant for biomarkers of brain injury or dysfunction than for those of Aβ42 amyloidosis.
TABLE 3.
Biomarker values in the ADNI sample according to cognitive cluster assignment.
| Characteristics | Cluster 1 | Cluster 2 | P |
|---|---|---|---|
| Whole brain (mL) | 1070 (994, 1136) | 1023 (962, 1102) | <0.001 |
| n | 362 | 304 | |
| Hippocampus (µL) | 7641 (7150, 8226) | 7343 (6809, 7893) | <0.001 |
| n | 346 | 296 | |
| Entorhinal (µL) | 4044 (361, 4463) | 3884 (3534, 4312) | 0.019 |
| n | 352 | 285 | |
| PET FDG (SUVR) | 1.31 (1.23, 1.37) | 1.26 (1.17, 1.34) | <0.001 |
| n | 185 | 170 | |
| PET Florbetapir (SUVR) | 1.05 (1.00, 1.19) | 1.07 (1.00, 1.20) | 0.802 |
| n | 258 | 223 | |
| Aβ42 (pg/mL) | 1012 (731, 1384) | 952 (701, 1263) | 0.088 |
| n | 116 | 103 | |
| Tau (pg/mL) | 207 (172, 283) | 219 (176, 313) | 0.172 |
| n | 163 | 141 | |
| P‐tau (pg/mL) | 19.2 (15.1, 26.1) | 19.9 (15.5, 28.8) | 0.246 |
| n | 162 | 141 | |
| Tau/Aβ42 | 0.19 (0.13, 0.35) | 0.28 (0.15, 0.37) | 0.034 |
| n | 116 | 103 | |
| P‐tau/Aβ42 | 0.02 (0.01, 0.03) | 0.03 (0.01, 0.04) | 0.048 |
| n | 115 | 103 | |
| APOE ε4 (no. alleles) | |||
| 0 | 244 (67%) | 187 (68%) | 0.645 |
| 1 | 110 (30%) | 79 (28%) | |
| 2 | 10 (3%) | 11 (4%) | |
| n | 364 | 277 |
Note: Descriptive statistics are given as median (interquartile range) or frequency (percentage). PET FDG is a measurement of FDG uptake at the angular, temporal, and posterior cingulate regions. PET florbetapir is a composite measurement of cortical florbetapir uptake normalized by the cerebellum. Aβ42, tau, and p‐tau indicate the levels of biomarkers in cerebrospinal fluid. The symbol “n” indicates the number of cases available for analysis. P values correspond to Wilcoxon rank sum test or Pearson chi‐squared test.
Abbreviations: Aβ, amyloid beta; APOE, apolipoprotein E; FDG, [18F]Fluorodeoxyglucose; PET, positron emission tomography; p‐tau, phosphorylated P‐181 tau; SUVR, standardized uptake value ratio.
3.2.3. Association of cognitive clusters with risk of cognitive impairment
The assignment to the lower‐than‐average performance cluster (cluster 2) significantly increased the risk of cognitive impairment according to the log‐rank test (Figure 3, P < 0.001). Additionally, a comparison of the RMST in days of the two clusters (cluster 1 = 2847; cluster 2 = 2495; difference = 352, CI = 192 to 511, P < 0.001) underscores this association. Furthermore, a Cox PH regression model, controlling for age, sex, education, mood (GDS‐15), and SMC, revealed that the HR for cluster 2 versus cluster 1 was 2.957 (CI = 1.682–5.199, P < 0.001). The Cox PH model also showed an increased risk of cognitive impairment for age (HR = 1.079, CI = 1.034–1.126, P < 0.001), GDS‐15 (HR = 1.234, CI = 1.004–1.518, P = 0.046), and SMC (HR = 1.743, CI = 1.003–3.030, P = 0.049), and it did not violate the PH assumption. The incidence rate ratio of cognitive impairment for the cluster 2 versus cluster 1 groups was 2.479 (CI = 1.534–4.072). The adjusted survival curves for cognitive clusters and SMC were similar to the unadjusted curves (see Figure S5).
3.2.4. Association of SMC with risk of cognitive impairment
SMC assignment was not a statistically significant predictor of cognitive decline according to the log‐rank test (P = 0.06) and the incidence rate ratio (1.391, CI = 0.844–2.454), and it was marginally significant in the Cox PH model described above (P = 0.049). However, visual inspection of the survival curves suggested an increased risk of late progression (after 4 years), especially for cluster 2 (lower‐than‐average performance) participants (Figure 3). In this line, the Fleming–Harrington test showed a significant effect of SMC after adjusting the lambda parameter to 1 to detect delayed effects: P = 0.028. The comparison of the RMST in days of the SMC versus CN groups, adjusted for age, sex, and education also showed a significant difference between groups (CN − SMC = 166, CI = 15–316, P = 0.031), but it was reduced after adjusting for GDS‐15 (CN − SMC = 141, CI = −5 to 288, P = 0.059). Note that the number of subjects at risk at late time points was limited, which reduced the power of the standard tests at those stages (see risk tables in Figure 3).
Comparative analyses between participants with and without memory concerns (SMC vs. CN groups) showed a significant association at baseline with GDS‐15 (higher in SMC) and categorical fluency (lower in SMC), but not with the remaining variables, including brain volumes, biomarkers in CSF, brain florbetapir uptake, and the FDG posterior limbic measurement (Table 4).
TABLE 4.
Demographic, neuropsychological, and biomarker values of the ADNI sample at baseline according to the initial diagnostic classification (SMC vs. CN groups).
| Characteristics | SMC (n = 387) | CN (n = 347) | P |
|---|---|---|---|
| Age (years) | 70 (66, 75) | 71 (67, 76) | 0.015 |
| Sex | |||
| Male | 152 (39%) | 157 (45%) | 0.116 |
| Female | 235 (61%) | 190 (55%) | |
| Education (years) | 16 (15, 18) | 16 (15, 18) | 0.944 |
| Cluster | |||
| 1 | 200 (52%) | 200 (58%) | 0.119 |
| 2 | 187 (48%) | 147 (42%) | |
| GDS‐15 | 1 (0, 2) | 0 (0, 1) | <0.001 |
| MMSE | 29 (29, 30) | 29 (28, 30) | 0.251 |
| Delayed recall | 7 (6, 8) | 7 (6, 9) | 0.852 |
| Categorical fluency | 20 (17, 24) | 22 (18, 25) | 0.006 |
| TMT‐A (seconds) | 30 (25, 38) | 31 (25, 39) | 0.254 |
| TMT‐B (seconds) | 73 (56, 95) | 70 (55, 91) | 0.296 |
| Whole brain (mL) | 1055 (985, 1129) | 1044 (976, 1121) | 0.584 |
| Hippocampus (µL) | 7540 (7056, 8090) | 7573 (6946, 8058) | 0.526 |
| Entorhinal (µL) | 4023 (3591, 4464) | 3915 (3551, 4374) | 0.162 |
| PET FDG (SUVR) | 1.29 (1.20, 1.37) | 1.28 (1.19, 1.35) | 0.446 |
| PET florbetapir (SUVR) | 1.06 (1.00, 1.20) | 1.07 (1.01, 1.19) | 0.260 |
| Aβ42 (pg/mL) | 1046 (774, 1351) | 957 (714, 1279) | 0.219 |
| Tau (pg/mL) | 216 (168, 300) | 211 (178, 300) | 0.945 |
| P‐tau (pg/mL) | 18.9 (14.6, 26.3) | 19.5 (15.5, 27.1) | 0.646 |
| Tau/Aβ42 | 0.19 (0.14, 0.37) | 0.23 (0.13, 0.38) | 0.384 |
| P‐tau/Aβ42 | 0.02 (0.01, 0.03) | 0.02 (0.01, 0.04) | 0.311 |
| APOE ε4 (no. alleles) | |||
| 0 | 205 (64%) | 226 (71%) | 0.048 |
| 1 | 108 (34%) | 81 (25%) | |
| 2 | 8 (2%) | 13 (4%) |
Note: Descriptive statistics are given as median (interquartile range) or frequency (percentage). Delayed recall corresponds to the ADAS‐Cog items. PET FDG is a measurement of FDG uptake at the angular, temporal, and posterior cingulate regions. PET florbetapir is a composite measurement of cortical florbetapir deposition normalized by the cerebellum. Aβ42, tau, and P‐tau indicate the levels of biomarkers in cerebrospinal fluid. p values correspond to the Wilcoxon's rank sum test or the Pearson's Chi‐squared test.
Abbreviations: Aβ, amyloid beta; ADAS‐Cog, Alzheimer's Disease Assessment Scale‐Cognitive Subscale; ADNI, Alzheimer's Disease Neuroimaging Initiative; APOE, apolipoprotein E; CN, normal controls without memory concerns; CUN, Clínica Universidad de Navarra; FDG, [18F]Fluorodeoxyglucose; GDS, Geriatric Depression Scale; MMSE, Mini‐Mental State Examination; PET, positron emission tomography; p‐tau, phosphorylated P‐181 tau; SMC, subjective memory concerns; SUVR, standardized uptake value ratio; TMT‐A, Trail Making Test part A; TMT‐B, Trail Making Test part B.
3.2.5. Bayesian model averaging of Cox PH regression models of time to cognitive impairment
BMA of the Cox PH regression models applied to the ADNI sample showed that the variables most useful for estimating the risk of cognitive impairment were the cognitive cluster assignment (posterior probability to be included in the models = 90%), the tau/Aβ42 ratio in CSF (85%), the posterior limbic FDG uptake (51%), the hippocampal volume (51%), and the GDS‐15 score (48%; Figure 4, and Figure S6 in supporting information). The remaining variables exhibited lower results: education (28%), SMC (22%), age (22%), number of APOE ε4 alleles (18%), and sex (11%).
FIGURE 4.
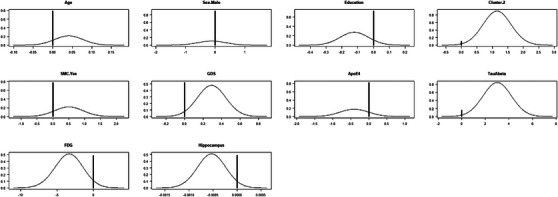
Posterior probabilities of the coefficients that represent the variables analyzed by Bayesian model averaging. High values at 0 indicate a low probability to be included in the models. APOE, apolipoprotein E; FDG, fluorodeoxyglucose; GDS, Geriatric Depression Scale; SMC, subjective memory complaints; TauAbeta, tau/Aβ42 ratio in cerebrospinal fluid.
4. DISCUSSION
This study shows that patients with SMC exhibit a heterogeneous cognitive performance, characterized by two distinct clusters with higher‐than‐average and lower‐than‐average results. This cognitive cluster structure is also observed in healthy individuals without memory concerns, and it consistently predicts cognitive impairment in both groups. Specifically, being assigned to the lower‐than‐average cognitive cluster at baseline significantly increases the risk of subsequent cognitive decline, even after accounting for factors such as age, sex, education, and mood. Furthermore, the lower performance group exhibited a pattern of biomarker changes consistent with incipient neurodegeneration and Aβ42 amyloidosis. In comparison, the effect of SMC on the risk of cognitive decline is smaller and delayed (over 4 years), mainly limited to the group of individuals with lower initial cognitive performance, and at least partially confounded by mood. Among various clinical and demographic variables, as well as neuroimaging and other biomarker measurements, the cognitive cluster assignment proved to be the most valuable variable for predicting cognitive impairment, featuring in 90% of all models selected by BMA.
The identification of two distinct cognitive clusters among patients with SMC aligns with previous research investigating cognitive profiles in various populations. 59 , 60 , 61 Several studies have reported heterogeneity in cognitive performance among individuals with SMC, subtle cognitive decline, MCI, and dementia due to AD. 31 , 62 , 63 , 64 , 65 Our study extends these findings by demonstrating that a cognitive cluster structure like that found in patients with SMC is also present in healthy individuals without memory concerns. This suggests that cognitive heterogeneity may be a fundamental aspect of cognitive functioning across populations.
The biological relevance of the observed cognitive clusters is shown by differences between clusters in terms of neuroimaging and other biomarkers. In particular, the reduced hippocampal, entorhinal, and whole brain volumes; the posterior limbic hypometabolism; and the increased tau/Aβ42 and p‐tau/Aβ42 CSF ratios observed in the participants with lower cognitive performance align with previous studies linking structural and functional brain changes to cognitive decline and neurodegenerative processes. 66 , 67 Interestingly, differences between clusters appear to be more pronounced for biomarkers of neurodegeneration rather than those related to amyloidosis. This observation is consistent with the findings of Edmonds et al. in patients with subtle cognitive decline, suggesting that neurodegeneration often precedes amyloidosis. 32 It is also noteworthy that among the tested measurements of amyloidosis, only differences in the tau/Aβ42 and p‐tau/Aβ42 ratios in CSF reached statistical significance, highlighting their greater sensitivity compared to other determinations. 26 These findings do not rule out the effect of differences in cognitive reserve among clusters. Cognitive reserve refers to the brain's ability to withstand age‐related changes or disease‐related pathology while maintaining cognitive functioning. 68 In fact, both neurodegeneration and cognitive reserve could interact to determine differences in future cognitive decline. It is worth noting that no significant associations were found between the biomarkers and the baseline diagnostic classification used in the ADNI database (SMC vs. CN groups). This observation raises questions about the relevance of SMC as a distinct biological entity.
The consistent predictive value of cognitive cluster assignment for cognitive decline highlights its potential clinical utility. Previous research has demonstrated that objective cognitive performance is a robust indicator of cognitive decline and dementia risk. 69 Cognitive cluster assignment outperformed SMC and other variables in predicting cognitive impairment, appearing in the majority of models selected by BMA. This suggests that assessing an individual's cognitive cluster assignment at baseline could offer valuable insights into their future risk of cognitive decline. However, this specific aim was not pursued in the present study, as we primarily used cluster analysis as an exploratory tool to help us understand the structure of the data.
The relationships among objective cognitive function, SMC, and mood is a complex issue that requires further investigation. Comparing the bivariate and multivariable models incorporating these variables in our study provides interesting insights. At baseline, GDS scores, interpreted as surrogate markers of mood, are significantly associated with SMC. Cross‐sectional comparisons at baseline also reveal a significant association between cognitive clusters and GDS, but not between cognitive clusters and SMC. Longitudinal analyses show a significant association between cognitive clusters and cognitive decline, as well as between GDS and cognitive decline. In contrast, the effect of SMC on the risk of cognitive impairment is small, it is at least partially confounded by mood, and it seems to be modified by baseline cognitive performance. These results agree with previous studies showing that the predictive value of SMC for future cognitive impairment is modest and may be influenced by other factors such as age, mood, and initial cognitive performance. 15 , 16 Mood disturbances, such as depression and anxiety, are known to be associated with subjective cognitive complaints. 2 , 9 These mood‐related factors can influence an individual's perception of their cognitive abilities, leading to an overestimation of memory problems. Additionally, mood disturbances may contribute to cognitive impairment or exacerbate cognitive decline. 70 The confounding effect of mood on the relationship between SMC and cognitive decline emphasizes the importance of considering both objective cognitive performance measures and mood factors in the assessment of cognitive impairment risk.
The results of this study also suggest that the association between SMC and cognitive impairment may be influenced by the criteria used to define normal cognition. Previous studies which demonstrated a stronger association between SMC and both the risk of cognitive impairment and biomarker profiles indicative of early AD may have included individuals with subtle cognitive impairment due to less strict criteria for defining normal cognition. 14 The inclusion of individuals with subtle cognitive impairment in the normal cognition group could inflate the observed association between SMC and cognitive decline, suggesting a stronger link than what may truly exist. In contrast, the ADNI cases included in this study used a more stringent criterion for normal cognition, considering scores > ≈ −0.5 SD from the mean as normal.
Taken together, our data challenge the notion of including SMC in the continuum of cognitive impairment associated with AD. 28 Moreover, from a conceptual standpoint, it does not seem appropriate to merge two distinct concepts, namely objective cognitive function and subjective perception of memory, on a one‐dimensional spectrum. In contrast, our results support the concept of a continuum of objective cognitive changes that would start with subjects at the low range of normality (i.e., our lower performance cluster) and then eventually progress to subtle cognitive decline, MCI, and overt dementia.
The current study has several limitations, mainly inherent to observational research. The external validity of the CUN dataset may be compromised due to retrospective data collection, along with a limited number of patients with long follow‐up periods. Specifically, of the 630 patients of the CUN sample, only 176 were followed up for > 1 year and included in the evaluation of the risk of cognitive decline. However, a comparison of patients with different follow‐up times did not reveal significant differences in their main clinical and demographic characteristics. Additionally, the ADNI cohorts are not population based but rely on the participation of motivated subjects. Another limitation is the absence of a scale to quantify the severity and persistence of SMC. 71 However, some studies suggest that the intensity of memory complaints does not significantly affect the risk of cognitive impairment. 72 Also, note that this study only analyzed memory complaints (SMC), making it particularly relevant to the amnestic presentations of AD. The broader concept of SCD likely encompasses the initial stages of other degenerative disorders as well, and future works in the field should better explore the differences between patients with amnestic and non‐amnestic cognitive complaints. In any case, the consistency of the observed cluster structure in two datasets of different origins, which use different criteria to define normal cognition (a classic cutoff of −1.5 SD for the CUN dataset and a strict cutoff of −0.5 SD for the ADNI dataset), supports the overall validity of our main results.
Looking ahead, these results and current knowledge discourage a passive approach to detecting dementing disorders solely based on evaluating patients with memory complaints, as already pointed out by Riedel‐Heller et al. 73 Instead, they advocate for a proactive strategy involving a comprehensive evaluation of at‐risk populations using clinical, analytical, and neuroimaging methods. Particularly, individuals performing below average on neuropsychological tests, even within normal ranges, have an increased risk of cognitive decline, justifying more stringent follow‐up. From a practical standpoint, this would likely necessitate a shift in the scope of clinical neurology practice toward healthier subjects and a greater emphasis on prevention‐focused procedures. However, widespread implementation of such a proactive strategy should await the development of non‐invasive, accurate, and affordable diagnostic methods, as well as safe and effective disease‐modifying treatments. Recent advances in the field suggest that both goals may be accomplished in the near future.
In conclusion, this study provides evidence of the presence of cognitive cluster structures that hold biological significance in both elderly patients with SMC and healthy controls. Furthermore, it highlights the predictive potential of cognitive cluster assignment in identifying individuals at risk of cognitive impairment. Our findings also challenge the conventional understanding of including SMC within the continuum of cognitive impairment associated with AD.
CONFLICT OF INTEREST STATEMENT
The authors do not have conflicts of interest to declare. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All participants provided informed consent.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors have nothing to report. Data collection and sharing for part of this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI; National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH‐12‐2‐0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol‐Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann‐La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Jiménez‐Huete A, Villino‐Rodríguez R, Ríos‐Rivera MM, et al. Clusters of cognitive performance predict long‐term cognitive impairment in elderly patients with subjective memory complaints and healthy controls. Alzheimer's Dement. 2024;20:4702–4716. 10.1002/alz.13903
Data used in preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in the analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp‐content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
REFERENCES
- 1. Jessen F, Wiese B, Bachmann C, et al. Prediction of dementia by subjective memory impairment effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67(4):414‐422. [DOI] [PubMed] [Google Scholar]
- 2. Balash Y, Mordechovich M, Shabtai H, Giladi N, Gurevich T, Korczyn AD. Subjective memory complaints in elders: depression, anxiety, or cognitive decline? Acta Neurol Scand. 2013;127(5):344‐350. [DOI] [PubMed] [Google Scholar]
- 3. Cook S, Marsiske M. Subjective memory beliefs and cognitive performance in normal and mildly impaired older adults. Aging Ment Health. 2006;10(4):413‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dixon RA, Hultsch DF, Hertzog C. The Metamemory in Adulthood (MIA) questionnaire. Psychopharmacol Bull. 1988;24:671‐688. [PubMed] [Google Scholar]
- 5. Abdulrab K, Heun R. Subjective Memory Impairment. A review of its definitions indicates the need for a comprehensive set of standardised and validated criteria. Eur Psychiatry. 2008;23(5):321‐330. [DOI] [PubMed] [Google Scholar]
- 6. Montejo P, Montenegro M, Fernandez MAM. Subjective memory complaints in the elderly: prevalence and influence of temporal orientation, depression and quality of life in a population‐based study in the city of Madrid. Aging Ment Heal. 2011;15(1):85‐96. [DOI] [PubMed] [Google Scholar]
- 7. Mol M, Carpay M, Ramakers I, Rozendaal N, Verhey F, Jolles J. The effect of perceived forgetfulness on quality of life in older adults; a qualitative review. Int J Geriatr Psychiatry. 2007;22(5):393‐400. [DOI] [PubMed] [Google Scholar]
- 8. Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19(3):271‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bartley M, Bokde AL, Ewers M, et al. Subjective memory complaints in community dwelling healthy older people: the influence of brain and psychopathology. Int J Geriatr Psychiatry. 2012;27(8):836‐843. [DOI] [PubMed] [Google Scholar]
- 10. Crumley JJ, Stetler CA, Horhota M. Examining the relationship between subjective and objective memory performance in older adults: a meta‐analysis. Psychol Aging. 2014;29(2):250‐263. [DOI] [PubMed] [Google Scholar]
- 11. Horn MM, Kennedy KM, Rodrigue KM. Association between subjective memory assessment and associative memory performance: role of ad risk factors. Psychol Aging. 2018;33(1):109‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lehrner J, Moser D, Klug S, et al. Subjective memory complaints, depressive symptoms and cognition in patients attending a memory outpatient clinic. Int Psychogeriatr. 2014;26(3):463‐473. [DOI] [PubMed] [Google Scholar]
- 13. Steinberg SI, Negash S, Sammel MD, et al. Subjective memory complaints, cognitive performance, and psychological factors in healthy older adults. Am J Alzheimers Dis Other Demen. 2013;28(8):776‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta‐analysis. Acta Psychiatr Scand. 2014;130(6):439‐451. [DOI] [PubMed] [Google Scholar]
- 15. Purser JL, Fillenbaum GG, Wallace RB. Memory complaint is not necessary for diagnosis of mild cognitive impairment and does not predict 10‐year trajectories of functional disability, word recall, or short portable mental status questionnaire limitations. J Am Geriatr Soc. 2006;54(2):335‐338. [DOI] [PubMed] [Google Scholar]
- 16. Yates JA, Clare L, Woods RT. Subjective memory complaints, mood and MCI: a follow‐up study. Aging Ment Heal. 2017;21(3):313‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. St John P, Montgomery P. Are cognitively intact seniors with subjective memory loss more likely to develop dementia? Int J Geriatr Psychiatry. 2002;17(9):814‐820. [DOI] [PubMed] [Google Scholar]
- 18. Tobiansky R, Livingston G, Mann A. The Gospel Oak Study stage IV: the clinical relevance of subjective memory impairment in older people. Psychol Med. 1995;25(4):779‐786. [DOI] [PubMed] [Google Scholar]
- 19. Howieson DB, Mattek N, Dodge HH, Erten‐Lyons D, Zitzelberger T, Kaye JA. Memory complaints in older adults: prognostic value and stability in reporting over time. SAGE Open Med. 2015;3. doi: 10.1177/2050312115574796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amariglio RE, Becker JA, Carmasin J, et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50(12):2880‐2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mosconi L, De Santi S, Brys M, et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry. 2008;63(6):609‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rami L, Fortea J, Bosch B, et al. Cerebrospinal fluid biomarkers and memory present distinct associations along the continuum from healthy subjects to AD patients. J Alzheimers Dis. 2011;23(2):319‐326. [DOI] [PubMed] [Google Scholar]
- 23. Peter J, Scheef L, Abdulkadir A, et al. Gray matter atrophy pattern in elderly with subjective memory impairment. Alzheimers Dement. 2014;10(1):99‐108. [DOI] [PubMed] [Google Scholar]
- 24. Swinford CG, Risacher SL, Charil A, Schwarz AJ, Saykin AJ. Memory concerns in the early Alzheimer's disease prodrome: regional association with tau deposition. Alzheimers Dement (Amsterdam, Netherlands). 2018;10:322‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Harten AC, Visser PJ, Pijnenburg YAL, et al. Cerebrospinal fluid Aβ42 is the best predictor of clinical progression in patients with subjective complaints. Alzheimers Dement. 2013;9(5):481‐487. [DOI] [PubMed] [Google Scholar]
- 26. van Harten AC, Wiste HJ, Weigand SD, et al. Detection of Alzheimer's disease amyloid beta 1‐42, p‐tau, and t‐tau assays. Alzheimers Dement. 2022;18(4):635‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reisberg B, Prichep L, Mosconi L, et al. The pre‐mild cognitive impairment, subjective cognitive impairment stage of Alzheimer's disease. Alzheimers Dement. 2008;4(1):S98‐S108. [DOI] [PubMed] [Google Scholar]
- 28. Jessen F, Amariglio RE, Van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10(6):844‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mclachlan GJ. Cluster analysis and related techniques in medical research. Stat Methods Med Res. 1992;1(1):27‐48. [DOI] [PubMed] [Google Scholar]
- 30. Goldstein G. Application of cluster analysis to investigate neuropsychological heterogeneity in psychiatric and neurological patients. In: Allen DN, Goldstein G, eds. Cluster Analysis in Neuropsychological Research: Recent applications. Springer Science; 2013:37‐70. [Google Scholar]
- 31. Jessen F, Wiese B, Cvetanovska G, et al. Patterns of subjective memory impairment in the elderly: association with memory performance. Psychol Med. 2007;37(12):1753‐1762. [DOI] [PubMed] [Google Scholar]
- 32. Edmonds EC, Delano‐Wood L, Galasko DR, Salmon DP, Bondi MW. Subtle cognitive decline and biomarker staging in preclinical Alzheimer's disease. J Alzheimer's Dis. 2015;47(1):231‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Folstein MF, Folstein SE, McHugh PR. Mini‐mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 34. Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum GHA. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44(4):609‐614. [DOI] [PubMed] [Google Scholar]
- 35. Ruff RM, Light RH, Parker SB, Levin HS. Benton controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329‐338. [PubMed] [Google Scholar]
- 36. Stroop J. Studies of interference in serial verbal reaction. J Exp Psychol. 1935;18:643‐662. [Google Scholar]
- 37. Raven JC. The R.E.C.I. series of perceptual tests: an experimental survey. Br J Psychol. 1939;18:16‐34. [Google Scholar]
- 38. Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2nd ed. Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 39. Reitan RM. Validity of the trail making test as an indicator of organic brain damage. 1958;8(3):271‐276. [Google Scholar]
- 40. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37‐49. [DOI] [PubMed] [Google Scholar]
- 41. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McKhann G. The diagnosis of dementia due to Alzheimer's disease. Alzheimers Dement. 2011;7(3):263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Landau SM, Harvey D, Madison CM, et al. Associations between cognitive, functional, and FDG‐PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32(7):1207‐1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Landau SM, Lu M, Joshi AD, et al. Comparing PET imaging and CSF measurements of Aβ. Ann Neurol. 2013;74(6):826‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2021. https://www.R‐project.org/ [Google Scholar]
- 46. Weiner MW, Veitch DP, Aisen PS, et al. ADNIMERGE: R package for combining ADNI datasets. R package version 0.0.1; 2023. https://cran.r‐project.org/package=ADNIMERGE ‐
- 47. Hopkin A. hopkins: Tools for Assessing Cluster Tendency. R package version 1.0; 2022. https://cran.r‐project.org/package=hopkins
- 48. Brock G, Pihur V, Datta S, Datta S. clValid: An R package for cluster validation. J Stat Softw. 2008;25(4):1‐22. https://www.jstatsoft.org/v25/i04/ [Google Scholar]
- 49. Kassambara A, Mundt F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R package version 1.0.7; 2020. https://CRAN.R‐project.org/package=factoextra
- 50. Kolde R. pheatmap: Pretty. Heatmaps. R package version 1.0.12; 2019. https://CRAN.R-project.org/package=pheatmap
- 51. Therneau T. A Package for Survival Analysis in R. R package version 3.5-0; 2023. https://CRAN.R-project.org/package=survival
- 52. Kassambara A. survminer: Drawing Survival Curves using “ggplot2”. R package version 0.4.9; 2021. https://cran.r‐project.org/package=survminer
- 53. Lafitte F, Boulesteix A‐L. survRM2: Comparing Restricted Mean Survival Time. R package version 1.0‐4; 2021. https://cran.r‐project.org/package=survRM2
- 54. Böhning D, Filzmoser P, Maronna R, et al. riskCommunicator: Interface to the riskCommunicator API. R package version 1.0.1; 2018. https://cran.r‐project.org/package=riskCommunicator
- 55. Diks CGH. FHtest: Tests for right and interval‐censored survival data based on the Fleming‐Harrington Class. R package version 1.5; 2021. https://cran.r‐project.org/package=FHtest
- 56. Böhning D, Malley JD. Adjusted Curves: Adjusted Survival Curves. R package version 0.10.1; 2021. https://cran.r‐project.org/package=adjustedCurves
- 57. Genuer R, Poggi J‐M, Tuleau‐Malot C. VSURF: Variable Selection Using Random Forests. R package version 1.2.0; 2018.
- 58. Raftery A, Hoeting J, Volinsky C, Painter I, Yeung KY. BMA: Bayesian Model Averaging. R package version 3.18.17; 2022. https://CRAN.R‐project.org/package=BMA
- 59. Hendricks RM, Khasawneh MT. A systematic review of Parkinson's disease cluster analysis research. Aging Dis. 2021;12(7):1567‐1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Van Rooden SM, Heiser WJ, Kok JN, Verbaan D, Van Hilten JJ, Marinus J. The identification of Parkinson's disease subtypes using cluster analysis: a systematic review. Mov Disord. 2010;25(8):969‐978. [DOI] [PubMed] [Google Scholar]
- 61. Mirzaei G, Adeli H. Segmentation and clustering in brain MRI imaging. Rev Neurosci. 2019;30(1):31‐44. [DOI] [PubMed] [Google Scholar]
- 62. Cersonsky TEK, Mechery S, Thompson L, et al. Related amyloid burden and cortical atrophy in individuals with subtle cognitive decline. J Neuroimaging. 2022;32(6):1075‐1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Damian M, Hausner L, Jekel K, et al. Single‐domain amnestic mild cognitive impairment identified by cluster analysis predicts Alzheimer's disease in the European Prospective DESCRIPA Study. Dement Geriatr Cogn Disord. 2013;36(1‐2):1‐19. [DOI] [PubMed] [Google Scholar]
- 64. Hiu SKW, Bigirumurame T, Kunonga P, Bryant A, Pillai M. Neuropsychiatric inventory domains cluster into neuropsychiatric syndromes in Alzheimer's disease: A systematic review and meta‐analysis. Brain Behav. 2022;12(9):e2734. doi: 10.1002/brb3.2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ribaldi F, Rolandi E, Vaccaro R, Colombo M, Battista Frisoni G, Guaita A. The clinical heterogeneity of subjective cognitive decline: a data‐driven approach on a population‐based sample. Age Ageing. 2022;51(10):afac209. doi: 10.1093/ageing/afac209 [DOI] [PubMed] [Google Scholar]
- 66. Hansson O. Biomarkers for neurodegenerative diseases. Nat Med. 2021;27(6):954‐963. [DOI] [PubMed] [Google Scholar]
- 67. Mahaman YAR, Embaye KS, Huang F, et al. Biomarkers used in Alzheimer's disease diagnosis, treatment, and prevention. Ageing Res Rev. 2022;74:101544. doi: 10.1016/j.arr.2021.101544 [DOI] [PubMed] [Google Scholar]
- 68. Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11(11):1006‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Prado CE, Watt S, Treeby MS, Crowe SF. Performance on neuropsychological assessment and progression to dementia: a meta‐analysis. Psychol Aging. 2019;34(7):954‐977. doi: 10.1037/pag0000410 [DOI] [PubMed] [Google Scholar]
- 70. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Montejo Carrasco P, Montenegro‐Peña M, López‐Higes R, et al. Subjective memory complaints in healthy older adults: fewer complaints associated with depression and perceived health, more complaints also associated with lower memory performance. Arch Gerontol Geriatr. 2017;70:28‐37. [DOI] [PubMed] [Google Scholar]
- 72. Glodzik‐Sobanska L, Reisberg B, De Santi S, et al. Subjective memory complaints: presence, severity and future outcome in normal older subjects. Dement Geriatr Cogn Disord. 2007;24(3):177‐184. [DOI] [PubMed] [Google Scholar]
- 73. Riedel‐Heller SG, Matschinger H, Schork A, Angermeyer MC. Do memory complaints indicate the presence of cognitive impairment? Results of a field study. Eur Arch Psychiatry Clin Neurosci. 1999;249(4):197‐204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information


