Abstract
INTRODUCTION
Leveraging the nonmonolithic structure of Latin America, which represents a large variability in social determinants of health (SDoH) and high levels of genetic admixture, we aim to evaluate the relative contributions of SDoH and genetic ancestry in predicting dementia prevalence in Latin American populations.
METHODS
Community‐dwelling participants aged 65 and older (N = 3808) from Cuba, Dominican Republic, Mexico, and Peru completed the 10/66 protocol assessments. Dementia was diagnosed using the cross‐culturally validated 10/66 algorithm. Multivariate linear regression models adjusted for SDoH were used in the main analysis. This study used cross‐sectional data from the 1066 population‐based study.
RESULTS
Individuals with higher proportions of Native American (>70%) and African American (>70%) ancestry were more likely to exhibit factors contributing to worse SDoH, such as lower educational levels (p < 0.001), lower socioeconomic status (p < 0.001), and higher frequency of vascular risk factors (p < 0.001). After adjusting for measures of SDoH, there was no association between ancestry proportion and dementia probability, and ancestry proportions no longer significantly accounted for the variance in cognitive performance (African predominant p = 0.31 [‐0.19, 0.59] and Native predominant p = 0.74 [‐0.24, 0.33]).
DISCUSSION
The findings suggest that social and environmental factors play a more crucial role than genetic ancestry in predicting dementia prevalence in Latin American populations. This underscores the need for public health strategies and policies that address these social determinants to effectively reduce dementia risk in these communities.
Highlights
Countries in Latin America express a large variability in social determinants of health and levels of admixture.
After adjustment for downstream societal factors linked to SDoH, genetic ancestry shows no link to dementia.
Population ancestry profiles alone do not influence cognitive performance.
SDoH are key drivers of racial disparities in dementia and cognitive performance.
Keywords: ancestry, dementia, Latinos, prevalence, risk factors, social determinants of health
1. BACKGROUND
Alzheimer's disease and related dementias (ADRD) disproportionately affect racial and ethnic minorities. 1 , 2 , 3 Recent studies show a rapid increase in dementia rates among Latinos compared to non‐Latino white. 4 Approximately 12% of older Latino adults are diagnosed with Alzheimer's dementia (AD), representing the fastest‐growing proportion of AD cases among different ethnic groups in the United States. 2 , 5 , 6 , 7 , 8 Furthermore, existing differences in the prevalence of AD, age at onset, clinical presentation, and disease progression among Latino subgroups (e.g., Caribbean Hispanics vs. Non‐Caribbean Hispanics) suggest greater disease heterogeneity within the Latino community. 9 , 10 Despite these differences, research studies exploring the main drivers for AD disparities in Latinos relative to non‐Latino whites and within Latino subgroups are still limited.
“Latino” serves as an umbrella term that encompasses a diverse range of geographic and ethnic backgrounds, largely reflective of the rich cultural, linguistic, and historical diversity found in Latin America. This region, unlike areas with more homogeneous structures, showcases a complex tapestry of cultures resulting from centuries of interactions among Indigenous, European, and African populations. Latin American populations, formed through genetic admixture of ancestral populations from Africa, the Americas, and Europe 11 , 12 , 13 represent the world's largest admixed groups, showcasing extensive genetic and phenotypic diversity. 14 , 15
Latin American countries also share experiences related to colonialism, social stratification, and evolving national identities. The impact of colonialism and the legacy of caste systems have contributed to persistent social inequalities and discrimination, influencing the region's social determinants of health (SDoH). These disparities can manifest in various forms, including unequal access to education, employment, and healthcare, as well as systemic racism. This extensive diversity is key to understanding the significant heterogeneity in disease patterns observed among Latino populations. Moreover, this context provides a framework for a more nuanced analysis of how social, cultural, and economic factors contribute to health disparities, including conditions like dementia and cognitive decline.
Given the vast diversity and complex historical legacies within Latin American communities, it is crucial to dissect specific factors contributing to differences in ADRD in Latinos, including genetic admixture, cultural diversity, and various SDoH. However, race/ethnicity correlates with both genetic ancestry and socioeconomic factors, which complicates efforts to better explore the influence of biological (e.g., genetic admixture) versus environmental interactions and SDoH. 16 , 17 Quantitative admixture proportions are a more accurate and reliable measure of individual genetic ancestry and allow to separate effects of genetic admixture and SDoH attributable to ethnic differences. 18 Previous studies of genetic ancestry and dementia have not systematically controlled for SDoH, which are significant confounders and a major threat to study validity and findings. 19 Understanding the relative importance of biological and social causes of disparities is critical for proposing effective interventions to reduce these disparities.
Overall, modern Latin American populations offer an optimal setting to examine the interplay between genetic ancestry and SDoH on dementia prevalence. Their unique combination of high admixture and existing social disparities creates a unique opportunity to test the relative contribution of SDoH and genetic ancestry to ADRD within Latinos. By focusing on Latin America, researchers can better understand the unique challenges and opportunities within this diverse region, providing a more accurate framework for studies and interventions.
In this study, we take advantage of the remarkable diversity in genetic ancestry that exists among Latino populations to assess if this variation may, in part, explain racial/ethnic differences in disease risk after controlling for SDoH. The present study uses genetic ancestry to describe the population(s) from which an individual's recent biological ancestors originated, as reflected in the DNA inherited from those ancestors. It is not our intent to define groups according to race, as race is self‐described and reflects a social construct primarily defined by physical characteristics and shaped by geographic, cultural, and sociopolitical forces. 20 , 21 , 22 Also, although our participants have a Latino origin (ethnicity), 23 , 24 we recognize the diversity in cultural origin and background traditions of Latino populations.
We used data collected through the 10/66 population‐based study 25 , 26 to estimate the prevalence of dementia and cognitive impairment according to genetic ancestry in a population‐based study. In addition, we sought to examine the impact of genetic ancestry on cognitive performance after controlling for sociodemographic, health and social disparities. The present research features a regional, multicenter study using the same protocols and diagnostic assessments from four Latin American (LatAm) countries.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources to explore how social determinants of health (SDoH) compare to genetic ancestry in predicting dementia risk among Latin American populations. There are no epidemiological studies exploring the relative contributions of SDoH and genetic ancestry in predicting dementia risk in Latin American populations.
Interpretation: This cohort study found that social determinants of health, rather than genetic ancestry, significantly predict dementia risk in Latin American populations. Adjusting for social determinants of health significantly reduces the apparent influence of genetic ancestry on dementia risk.
Future directions: The findings emphasize the greater importance of social and environmental factors over genetic predispositions in addressing dementia risk, suggesting a need for public health strategies and policies that focus on these determinants in Latin American communities.
2. METHODS
2.1. Setting and study participants
The primary analyses in this study included data from the 10/66 study, a population‐based study focused on community‐dwelling participants aged 65 years and older. 25 , 26 The 10/66 study is a multinational research initiative with the goal of providing comprehensive evidence on dementia prevalence and incidence in low‐ and middle‐income countries (LMIC). The 10/66 cohort comprises adults aged 65 years and over living in 9 LMICs (India, China, Nigeria, Cuba, Dominican Republic, Brazil, Venezuela, Mexico and Peru). 27 These sites were selected purposively to maximize their accessibility and relationship with local research groups and stakeholders. 26 While four countries (China, India, Peru, and Mexico) included separate urban and rural catchment sites, the remaining five countries included data from urban areas only. 27 The rural sites were remote areas with low population density and an agricultural lifestyle, whereas urban sites were areas with low or mixed socioeconomic status households (areas that were predominantly middle‐class or high‐income earners were excluded). 27 The sample size calculations for each country have been reported in the 10/66 study protocol. 27 Eligible participants were identified by door‐knocking all households in the catchment area. 26
The 10/66 study's broader aim is to guide the development and implementation of policies for enhancing the health and social well‐being of older individuals in these regions. It takes an innovative approach to dementia research in LMIC by introducing the 10/66 Dementia Diagnosis to address challenges in diagnosing dementia among older individuals with limited education. 27 , 28 The studies also employ standardized protocols across all sites to ensure consistency and reliability in the research process. In summary, participants received an assessment lasting three hours, including a participant interview, physical examination, cognitive assessment, blood draw, and informant interview. 27 Genetic ancestry data were specifically collected in four Latin American countries: Cuba, the Dominican Republic, Mexico, and Peru.
For this study, we used cross‐sectional data from the baseline survey (Wave 1) of the 10/66 project and included participants from Dominican Republic, Mexico, and Peru, for whom genetic ancestry information was available. Genetic data sampling in Cuba was selectively gathered due to budgetary limitations. Consistent with the Cuban cohort's established dementia prevalence of 11.4%, 10 we retained a random sample of participants without dementia and employed a two‐stage stratified random sampling method to select 45 (11.4% of samples) individuals with dementia in the 10/66 study. Stratification criteria encompassed gender, educational level, and multimorbidity. This method aimed to attenuate selection bias, ensuring that the sample closely mirrored the demographic structure of the Cuban population (refer to Supplemental Material Table 1). In primary analyses, we pooled respondents across the four 10/66 sites to increase statistical power.
Written informed consent was obtained from all participants and their study partners. Local institutional review boards and the King's College London Research Ethics Committee approved this project. The full protocol for the 10/66 population‐based surveys is available in open‐access publications. 26 , 27
2.2. Measures
The 10/66 protocols included, but were not limited to: a cognitive assessment, a structured interview of geriatric mental status, sociodemographic data and risk factors for dementia, a full neurological disease assessment, and a physical and neurological exam. All interviewers and field examiners received uniform and standardized training in Spanish language by qualified clinicians. Full details are available elsewhere. 25 The measures directly related to the present analyses are described below.
2.2.1. Cognitive performance and dementia status
Dementia was diagnosed using the cross‐culturally validated 10/66 dementia diagnosis algorithm, for which strong concurrent and predictive validity has been demonstrated. 28 , 29 Dementia diagnosis was established following: (i) a structured clinical interview; (ii) a cognitive test battery, including (a) the Community Screening Instrument for Dementia (CSI‐D), 30 (b) a verbal fluency task, and (c) the modified Consortium to Establish a Registry for Alzheimer's Disease (CERAD) 10‐word list learning task with delayed recall 31 ; and (iii) an informant interview (CSI‐D) 30 for evidence of cognitive and functional decline. The Clinical Dementia Rating® (CDR®) 32 scale was used to define dementia severity.
2.2.2. Covariates
Age was ascertained using documented age, or an event calendar provided by participant or informant report. To evaluate the impact of social disparities on dementia and cognitive function, we included educational attainment, gender, socioeconomic status (SES), place of residence (urban vs. rural), and multimorbidity as covariates in our analysis. Gender was assessed according to the participant's self‐report. We categorized educational attainment as: (1) no education, (2) some education but not completed primary school, (3) completed primary school, (4) completed secondary school, (5) completed tertiary education (college) or above. Socioeconomic status was assessed according to the number of reported household assets (motor vehicles; television; refrigerator and/or freezer; water and electrical utilities; telephone; plumbed toilet; plumbed bathroom). Participants were also asked to disclose whether they received income from an occupational or government pension. Country of residence and urban versus rural region were also included as covariates. Multimorbidity was defined as the presence of two or more of the following diseases: depression, heart disease, arthritis, diabetes, respiratory disease, stroke, and/or hypertension. These covariates reflect different aspects of SDoH, providing a comprehensive framework for understanding their influence on health outcomes. To avoid possible collinearity in the analysis, dementia was not included in the multimorbidity variable.
2.2.3. Assessment of genetic ancestry
Global genetic ancestry data was available only in a subsample of the 10/66 study (3808/11613). Individual admixture proportions (African/European/Native American) were determined using sixty highly informative ancestry‐informative markers. These markers have been shown to be sufficient to estimate three‐way individual admixture proportions with a standard error of less than 0.1. 33 , 34 , 35 The ADMIXMAP program 36 , 37 (http://homepages.ed.ac.uk/pmckeigu/admixmap/) was run with K = 3 corresponding to the three ancestral continental population groups: African, European, and Native American. The ADMIXMAP results were used to generate individual ancestry proportion contribution from each of the three continental ancestry groups. Genotyping was performed by KBiosciences (http://kbioscience.co.uk), using the KASPar chemistry allele‐specific polymerase chain reaction SNP genotyping system (http://www.kbioscience.co.uk/genotyping/genotyping_chemistry.htm).
Using genetic ancestry proportions, we further classified the study sample into three predominant genetic ancestry categories: predominant African ancestry (when the proportion of African genetic ancestry exceeds 70%), predominant European ancestry (when the proportion of European ancestry exceeds 70%), and predominant Native American ancestry (when the proportion of Native American ancestry surpasses 70%). The 70% cutoff is a commonly used threshold in studies examining genetic ancestry and related health outcomes. This approach is particularly useful in regions with high levels of genetic admixture, as it balances the need for specificity with inclusivity. In our study, the 70% cut‐off is designed to capture individuals who predominantly identify with a specific ancestry while allowing for some level of admixture and reducing ambiguity in defining predominant genetic ancestry. Participants for whom no single genetic ancestry exceeded 70%, were consider the more variable admixed ancestry group.
2.3. Statistical analysis
2.3.1. Descriptive statistics
Descriptive statistics were calculated for all variables. Sociodemographic characteristics, crude dementia prevalence, and genetic ancestry categories were described according to the country of origin. Similarly, sociodemographic characteristics, dementia prevalence and cognitive performance were described by genetic ancestry categories. For continuous variables, we used means and standard deviations (SD), or median and interquartile range (IQR). For categorical variables, these included counts and proportions in each category. Kruskal–Wallis test and chi‐squared test were used to calculate p‐values.
2.3.2. Multivariate logistic regression
To understand the relationship between predominant genetic ancestry and other covariates with dementia, we fit sequentially logistic regression models with (1) basic adjustment for age; (2) adjustment for age and gender; (3) multivariable adjustment for age, gender, CDR global score, education level, and SES (assets); (4) multivariable adjustment for age, gender, CDR global score, education level, SES (assets), country of origin, rural area, and multimorbidity.
2.3.3. Multivariate linear regression
To evaluate the effect of predominant genetic ancestry on dementia and subsequent cognitive performance domains (i.e., memory, words, logical memory, language, orientation, and animal naming), we used multivariate linear regression models adjusted for age, gender, CDR global score, education level, assets, country, rural area, and multimorbidity.
All analyses were performed in Stata 17 MP (College Station, TX) and graphs were created using MATLAB and Stata. All p‐values were from two‐sided tests, and results were deemed statistically significant at p < 0.05.
3. RESULTS
3.1. Sample descriptions
Summary descriptive statistics for the study sample by country are presented in Table 1. Average ancestry proportions are 32.8% ± 29.2% European, 19.0% ± 27.3% African, and 48.2% ± 36.9% Native American ancestry. We observed extensive variation in ancestry between countries. Each Latin American country has a unique pattern of three‐way continental genetic admixture characterized by specific proportions of African, European, and Native American ancestry (Figure 1). The Mexican and Peruvian samples (Figure 1B) showed a predominantly Native American ancestry (mean/[± standard deviation] 78.7% [± 21.2%] and 64.2% [± 22.1%], respectively), followed by European contributions (18.7% [± 20.5%] and 29.4% [± 21.3%], respectively) and African ancestry (2.6% [± 3.7%] and 6.4% [± 10.8%], respectively). The Cuban sample (Figure 1B) showed the least regional variation with low levels of Native ancestry (2.7% [± 3.6%]) and high levels of European ancestry (80.6% [± 22.3%]) and African ancestry (16.8% [± 21.9%]). The Dominican Republic sample showed the highest proportion of African ancestry across the whole sample and, similar to the Cuban sample, showed little contribution from Native American ancestry. Details about ancestry contributions per country and in the pooled cohort are shown in Figure 1.
TABLE 1.
Sociodemographic and health characteristics of participants by country
| Parameter | Cuba | DR a | Peru | Mexico |
|---|---|---|---|---|
| N (%) | 393 (10.3) | 1 107 (29.1) | 730 (19.2) | 1 578 (41.4) |
| Female gender b , no. (%) | 259 (65.9) | 756 (68.3) | 477 (65.3) | 1 008 (63.9) |
| Age, mean (SD) | 74.7 (8.0) | 75.0 (7.3) | 74.5 (7.0) | 74.1 (6.5) |
| Education level c , no. (%) | ||||
| None | 9 (2.3) | 216 (19.5) | 12 (1.6) | 402 (25.5) |
| Some, did not complete primary school | 72 (18.3) | 554 (50.1) | 42 (5.8) | 693 (43.9) |
| Completed primary school | 116 (29.5) | 209 (18.9) | 241 (33.0) | 283 (17.9) |
| Completed secondary school | 111 (28.2) | 83 (7.5) | 246 (33.7) | 108 (6.8) |
| Tertiary school (college) | 85 (21.6) | 35 (3.2) | 183 (25.1) | 90 (5.7) |
| Socio‐economic status (assets) Median (IQR) | 6 (5‐6) | 5 (4‐6) | 6 (6‐7) | 6 (4‐6) |
| Multimorbidity, no. (%) | 212 (53.9) | 730 (65.9) | 308 (42.2) | 719 (45.6) |
| Dementia, no. (%) | 45 (11.5) | 133 (12.0) | 71 (9.7) | 127 (8.0) |
| Ancestry percentage, mean (SD) | ||||
| African | 16.8 (21.9) | 51.5 (27.3) | 6.4 (10.8) | 2.6 (3.7) |
| European | 80.6 (22.3) | 38.1 (26.9) | 29.4 (21.3) | 18.7 (20.5) |
| Native American | 2.7 (3.6) | 10.4 (7.2) | 64.2 (22.1) | 78.7 (21.2) |
| Predominant genetic ancestry categories (>70%), no. (%) | ||||
| Predominant African ancestry | 17 (4.3) | 337 (30.4) | 1 (0.1) | 0 |
| Predominant European ancestry | 296 (75.3) | 159 (14.4) | 35 (4.8) | 31 (2.0) |
| Predominant Native American ancestry | 0 | 1 (0.1) | 327 (44.8) | 1 083 (68.6) |
| Admixed Ancestry d | 80 (20.4) | 610 (55.1) | 367 (50.3) | 464 (29.4) |
Abbreviations: IQR, interquartile range; SD, standard deviation.
DR: Dominican Republic.
Female gender: 1 missing value in Dominican Republic.
Education level: 10 missing value in Dominican Republic, 6 in Peru, and 2 in Mexico.
Includes participants for whom no single genetic ancestry (African, European, or Native American) exceeds 70%.
FIGURE 1.
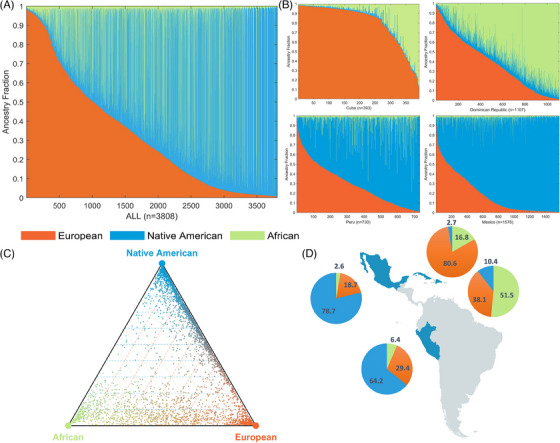
Genetic ancestry in Latin American populations. Legend: The figure depicts ancestry contributions of putative ancestral source populations in four admixed Latin American populations. (A) Admixture plots showing the fractions of African, Native American and European ancestry for all countries combined. (B) Admixture plots showing the fractions of African, Native American, and European ancestry among admixed individuals for each country. Each individual is represented as a column with the admixture fractions color‐coded as shown in the legend. (C) Triangle plots showing the relative ancestry contributions – African, European, Native American – to admixed individuals from four Latin American populations. (D) Pie charts showing the average ancestry values for each population next to their geographic location
3.2. Estimates of genetic ancestry and social disparities of health
The sociodemographic characteristics of the sample by predominant genetic ancestry categories are presented in Table 2. Individuals with predominant Native American ancestry and African ancestry were more likely to exhibit factors contributing to worse SDoH, such as lower educational levels (p < 0.001) and lower SES (p < 0.001). Notably, higher vascular risk factors and comorbidities were observed in populations with predominant African ancestry (p < 0.001).
TABLE 2.
Sociodemographic characteristics and cognitive performance by predominant genetic ancestry categories
| Predominant genetic ancestry categories | |||||
|---|---|---|---|---|---|
| Characteristics | Predominant African ancestry | Predominant European ancestry | Predominant Native American ancestry | Admixed Ancestry d | p‐Value |
| N (%) | 355 (9.3) | 521 (13.7) | 1 411 (37.1) | 1 521 (39.9) | |
| Age, mean (SD) | 75.6 (7.4) | 74.8 (7.3) | 74.2 (6.5) | 74.5 (7.0) | 0.040 |
| Gender female a , no. (%) | 240 (67.6) | 331 (63.5) | 882 (62.5) | 1 047 (68.8) | 0.002 |
| Socio‐economic status (assets) Median (IQR) | 5 (4‐6) | 6 (5‐6) | 6 (4‐6) | 6 (5‐6) | <0.001 |
| Educational level b , no. (%) <0.001 | |||||
| None | 83 (23.4) | 31 (6.0) | 327 (23.2) | 198 (13.0) | |
| Some, did not complete primary school | 186 (52.4) | 119 (22.8) | 563 (39.9) | 493 (32.4) | |
| Completed primary school | 53 (14.9) | 144 (27.6) | 275 (19.5) | 377 (24.8) | |
| Completed secondary school | 21 (5.9) | 129 (24.8) | 126 (8.9) | 272 (17.9) | |
| Tertiary school (college) | 10 (2.8) | 93 (17.9) | 119 (8.4) | 171 (11.2) | |
| Countries <0.001 | |||||
| Cuba | 17 (4.8) | 296 (56.8) | 1 (0.1) | 80 (5.3) | |
| Dominican Republic | 337 (94.9) | 159 (30.5) | 327 (23.2) | 610 (40.1) | |
| Peru | 1 (0.3) | 35 (6.7) | 0 (0) | 367 (24.1) | |
| Mexico | 0 (0) | 31 (6.0) | 1 083 (76.8) | 464 (30.5) | |
| Urban or rural sites <0.001 | |||||
| Urban | 355 (100) | 521 (100) | 708 (50.2) | 1 458 (95.9) | |
| Rural | 0 | 0 | 703 (49.8) | 63 (4.1) | |
| Mutimorbidities | 243 (65.9) | 277 (53.2) | 642 (45.5) | 816 (53.6) | < 0.001 |
| Dementia, no. (%) | 49 (13.8) | 56 (10.7) | 124 (8.8) | 147 (9.7) | 0.036 |
| CDR c , no. (%) <0.002 | |||||
| 0 | 160 (45.1) | 272 (52.2) | 667 (47.3) | 724 (47.6) | |
| 0.5 | 152 (42.8) | 198 (38.0) | 646 (45.8) | 634 (41.7) | |
| 1 | 31 (8.7) | 35 (6.7) | 82 (5.8) | 116 (7.6) | |
| 2 | 9 (2.5) | 11 (2.1) | 12 (0.9) | 29 (1.9) | |
| 3 | 3 (0.8) | 5 (1.0) | 4 (0.3) | 28 (1.2) | |
| CDR sum of boxes, median (IQR) | 0.5 (0‐1.5) | 0.5 (0‐1) | 0.5 (0‐1) | 0.5 (0‐1) | <0.001 |
| Cognitive performance in non‐dementia cases | |||||
| Cognitive score d , median (IQR) | 29.8 (27.2‐31.4) | 30.9 (29.2‐32.0) | 30.0 (27.9‐31.4) | 30.6 (28.7‐31.8) | <0.001 |
| Word delayed recall, mean (SD) | 3.9 (2.1) | 4.6 (2.2) | 4.1 (2.1) | 4.3 (2.1) | <0.001 |
| Logical memory, median (IQR) | 4 (4‐5) | 5 (4‐5) | 4 (3‐5) | 4 (3‐5) | <0.001 |
| Language, median (IQR) | 6 (6‐6) | 6 (6‐6) | 6 (6‐6) | 6 (6‐6) | <0.001 |
| Orientation, median (IQR) | 9 (7‐9) | 9 (8‐9) | 9 (8‐9) | 9 (8‐9) | <0.001 |
| Animal naming, mean (SD) | 12.8 (4.6) | 16.2 (6.7) | 14.5 (5.5) | 15.0 (5.9) | <0.001 |
Abbreviations: IQR, interquartile range; SD, standard deviation.
Female gender: 1 missing value in the “Predominant European ancestry” group.
Education level: 2 missing values in the “Predominant African ancestry” group, 5 in the “Predominant European ancestry” group, 1 in the “Predominant Native American ancestry” group, and 10 in “Admixed Ancestry” group.
CDR: Clinical Dementia Rating.
Includes participants for whom no single genetic ancestry (African, European, or Native American) exceeds 70%.
3.3. Genetic admixture, dementia, and cognitive performance
In the base model logistic regression where only age was accounted for, participants in the predominant African ancestry group showed a higher probability of dementia (p = 0.04) (see Table 2). Figure 2 illustrates the probability of dementia across ages, categorized by predominant genetic ancestry. However, in fully adjusted models for downstream societal risk factors linked to SDoH (e.g., education, SES, multimorbidity), predominant genetic ancestry categories were not associated with dementia probability. A detailed table of results for the stepwise logistic regression can be found in Supplemental Material Tables 2‐5.
FIGURE 2.
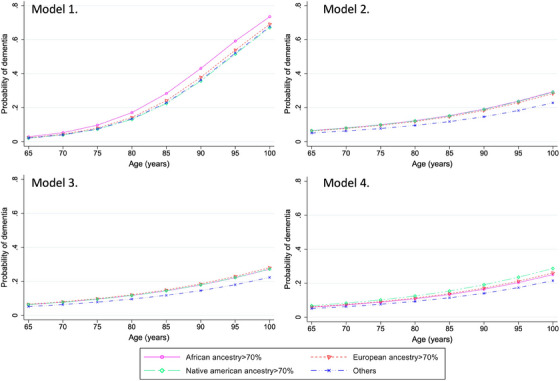
Dementia probability by age according to genetic ancestry. Legend: Model 1 (nonadjusted base model). Model 2 (adjusted by gender and CDR). Model 3 (adjusted by gender, CDR, education level, social‐economic status (assets)). Model 4 (adjusted by gender, CDR, education level, socioeconomic status (assets), country, rural area, and multimorbidity). CDR, Clinical Dementia Rating® scale. Others: refer to the Admixed Ancestry group, which includes participants for whom no single genetic ancestry (African, European, or Native American) exceeds 70%.
Consistent patterns of results were observed in adjusted models for cognitive performance. In unadjusted analysis, participants with predominant Native American ancestry and predominant African ancestry showed lower cognitive performance than those with predominant European ancestry and more variable admixed ancestry populations (Table 2). However, after adjusting for all the covariates (age, gender, education level, assets, country, rural area, and multimorbidity), predominant genetic ancestry categories did not significantly account for variance in overall cognitive performance. In cognitive domain‐specific models, we found no evidence for associations between predominant genetic ancestry categories and episodic memory, verbal fluency, language, or orientation. The only exception is that the African predominant category showed lower performance in logical memory (β = 0.21, CI: 0.04‐0.38, p = 0.01). Table 3 presents the findings from multivariate linear regression models examining the associations between predominant genetic ancestry categories and cognitive performance. Detailed results tables can be found in the Supplemental Materials (Table 2‐11).
TABLE 3.
Association between the predominant genetic ancestry categories and cognitive outcomes adjusted by age, sex, CDR, education, assets, country, rural area, and multimorbidity
| Cognitive outcomes | Predominant genetic ancestry categories | Coefficient | p‐Value | 95% CI |
|---|---|---|---|---|
| Cognitive composite score | Predominant African ancestry | 0.20 | 0.31 | [−0.19, 0.59] |
| Predominant European ancestry | 0.36 | 0.06 | [−0.02, 0.73] | |
| Predominant Native American ancestry | 0.05 | 0.74 | [−0.24, 0.33] | |
| Admixed Ancestry a | Base model | |||
| Word delayed recall | Predominant African ancestry | −0.06 | 0.59 | [−0.28, 0.16] |
| Predominant European ancestry | −0.11 | 0.30 | [−0.33, 0.10] | |
| Predominant Native American ancestry | −0.02 | 0.84 | [−0.18, 0.15] | |
| Admixed Ancestry | Base model | |||
| Logical memory | Predominant African ancestry | 0.21 | 0.01 | [0.04, 0.38] |
| Predominant European ancestry | 0.02 | 0.80 | [−0.14, 0.19] | |
| Predominant Native American ancestry | −0.08 | 0.19 | [−0.21, 0.04] | |
| Admixed Ancestry | Base model | |||
| Language | Predominant African ancestry | 0.10 | 0.20 | [−0.05, 0.24] |
| Predominant European ancestry | 0.11 | 0.14 | [−0.03, 0.25] | |
| Predominant Native American ancestry | 0.05 | 0.38 | [−0.06, 0.16] | |
| Admixed Ancestry | Base model | |||
| Orientation | Predominant African ancestry | 0.05 | 0.54 | [−0.11, 0.21] |
| Predominant European ancestry | 0.09 | 0.26 | [−0.07, 0.24] | |
| Predominant Native American ancestry | −0.00 | 0.96 | [−0.12, 0.12] | |
| Admixed Ancestry | Base model | |||
| Animal naming | Predominant African ancestry | −0.54 | 0.08 | [−1.15, 0.07] |
| Predominant European ancestry | 0.44 | 0.14 | [−0.15, 1.03] | |
| Predominant Native American ancestry | −0.04 | 0.87 | [−0.49, 0.42] | |
| Admixed Ancestry | Base model | |||
Abbreviations: CDR, Clinical Dementia Rating®; CI, confidence interval.
Includes particpants for whom no single genetic ancestry (African, European, or Native American) exceeds 70%.
4. DISCUSSION
In the present study, we report the first comprehensive characterization of the role of genetic ancestry in cognitive performance and dementia in Latin America while controlling for SDoH. In our approach, we leverage the non‐monolithic structure of Latin American countries, which represents a large variability in SDoH and high levels of admixture from African, European, and Native American ancestral source populations. First, we describe substantial SDoH disparities among different ancestry groups in Latin America, stemming from enduring disadvantages and structural racism rooted in the colonial period. Second, after adjustment for downstream societal risk factors linked to SDoH, no association is observed between genetic ancestry and dementia. Third, our study provides evidence that population ancestry profiles alone do not influence cognitive performance. These findings highlight that social and environmental factors likely play more critical roles in determining racial disparities in dementia and cognitive performance.
The observed ancestry proportions are consistent with previous genetic studies. 11 , 12 , 15 , 38 , 39 The extensive genetic ancestry structure that exists within and between Latin American countries is the result of well‐documented historical factors. 13 , 40 Native American populations in the Caribbean faced larger enslavement and extermination, while African slaves were introduced as substitutes, transforming the Caribbean into the main recipient of African slaves in the region. 13 This historical context helps to explain the observed ancestry proportions in the Caribbean relative to Mexico and South America. Similarly, in Latin America, both colonization and postcolonization history have played significant roles in shaping the historical racial discrimination or structural racism that contributes to the observed SDoH. 41 , 42
Disentangling the contributions of genetic, societal, and environmental factors toward disparities in dementia has important clinical and public health implications for improving care and reducing stigma around dementia disparities. Prior studies reported racial differences in dementia prevalence in Latino and African‐American groups. 43 , 44 , 45 , 46 Most studies in this area have been focused on US settings, 47 , 48 , 49 , 50 , 51 suggesting that racial and ethnic differences in cognitive health are often attributed to historical oppression and discrimination and observed disparities are influenced by evolving systems of racism, systemic inequalities, and other SDoH.
Similar to our findings, in a community study, African‐American participants were more likely than white participants to develop dementia. However, after controlling for demographics, apolipoprotein E4 (ApoE4) status, comorbidities, lifestyle factors, and socioeconomic status, observed associations between race and dementia prevalence largely disappeared. 52 Our findings add to the existing body of literature implicating societal and environmental risk—including socioeconomic factors, educational attainment, access to health care, and experiences of racial segregation and structural racism—in the existing disparities in dementia risk. ,
We also observed no significant association between predominant genetic ancestry categories and cognitive performance after adjusting for potential confounders, including education, vascular comorbidities, and others. Prior cohort studies have identified differences in cognitive performance across ethnic groups 53 , 54 , 55 ; however, consistent with our observation, differences in education play a major role in these differences. Our study findings suggest that cognitive performance is largely influenced by upstream societal risk factors linked to SDoH. Future cognitive studies among ethnically diverse cohorts should comprehensively assess societal confounders to clarify mechanisms that might underlie the observed lower performance among groups. In clinical practice, consideration of societal confounders rather than the so‐called “race/ethnic norms” will likely be better, nonbiased approaches to personalized medicine. These findings may also have implications for explaining observed differences in dementia prevalence for Latino populations in the United States, where SDoH also plays a significant role in observed disparities. Although the history of racialization in Latin America has distinct characteristics compared to that in the United States (establishment of complex caste systems in Latin America versus One Drop Rule in the United States), the social constructs of race and ethnicity in both regions have significantly influenced health outcomes and cognitive health, often through discrimination and unequal access to resources.
It is important to highlight that monogenic variants have also been associated with differential effects on dementia risk across ancestry groups. 56 , 57 , 58 ApoE4, an established genetic risk factor for AD, is enriched in individuals with higher African ancestry. 18 , 59 , 60 , 61 However, its effect seems attenuated in individuals with predominant African ancestry compared to those with predominant Native American and European ancestry. 18 , 61 , 62 , 63 Although we could not assess the effect of specific variants in our population, known nongenetic risk factors cumulatively explain more than 56% of all dementia cases in Latin America, 64 and genetic variants with lower frequency in the population may not be predominant drivers of population‐level disparities. Similarly, recent genome‐wide association meta‐analyses with higher representation of African descent cohort have shown that while the major pathways involved in Alzheimer's disease etiology in African American individuals are similar to those in non‐Hispanic White individuals, the disease‐associated loci within these pathways differ. 65 , 66 Overall, the existing evidence and the one provided in our study do not imply that possible biological variability within ancestry groups is irrelevant to health but do suggest that social and environmental factors may explain a greater proportion of dementia cases. Therefore, while genetic predispositions may play a role in dementia risk, these factors alone cannot explain the observed disparities.
The present study is not without limitations. First, while we believe that our method for identifying dementia cases was sensitive to capturing people with dementia, and our diagnosis was based on a formal clinical assessment, we were not able to include biomarker and/or neuroimaging data to confirm these diagnoses. In addition, our study focus is limited to global ancestry, and no additional genome‐wide genotyping or local ancestry was available in the cohort, so we cannot rule out the possibility of a higher frequency of protective or susceptibility rarer variants in specific ancestry groups or the potential differential effect of known genes in dementia risk. Although we cannot exclude the role of all genetic variants, according to our findings, this is not the main driver of observed differences across ancestry groups. Second, the cross‐sectional design prevents us from inferring causality, and future longitudinal studies are required to explore causal relationships over time and assess the progression of dementia and cognitive changes. Third, our study did not include specific measures of systemic racism, a critical factor influencing health disparities, including those related to dementia and cognitive function, which may lead to an incomplete understanding of the underlying causes of observed differences among various groups. Finally, our results need to be interpreted with caution; Latin American countries are very diverse, and we expect to see distinct ancestry proportions across the region. This observation is also true within the same country, where different regions show large variability in ancestry proportions. Similarly, the extent to which our findings are generalizable to Latinos in the United States requires careful consideration. While there are similarities in the broad concept of “Latino,” significant differences in social, cultural, and historical contexts between US‐based Latinos and Latin American populations remain. In the United States, Latinos often face unique forms of discrimination and systemic racism compared to their counterparts in Latin America, impacting their health and social outcomes. In addition, the diversity in ancestry proportions and cultural backgrounds may not directly align between Latin America and US‐based Latino populations. In summary, given the diversity within Latino populations, varying cultural influences, and distinct socio‐economic factors, future studies will benefit from a more comprehensive and detailed genetic assessment of individuals from a broader range of geographic areas.
Our study had several strengths, including a relatively large sample and diversity included in the study by leveraging data from multiple LatAm countries generated using the same methodology for participant assessment. The inclusion of comprehensive measures of SDoH allowed us to accurately account for social‐mediated potential confounders. Our results suggest that previously reported disparities in dementia prevalence and cognitive performance are largely attributable to SDoH and provide new evidence against a genetic admixture hypothesis for increased disease susceptibility. Our findings underscore the importance of addressing SDoH and societal factors to mitigate increased risks in more vulnerable populations. While this work is an important start to disentangling the role of ancestry from SDoH in race/ethnic‐based dementia disparities, several opportunities for future research remain. First, additional studies could focus on the incidence of dementia and the patterns of cognitive change over time. Longitudinal studies could shed light on the progression of dementia and the risk factors contributing to cognitive decline. Second, another important avenue for research involves distinguishing between different types of dementia, such as Alzheimer's disease and vascular dementia, to determine how SDoH and genetic ancestry influence these conditions. Finally, future inclusion of neighborhood‐level factors and systemic discrimination will be critical to understanding the broader context of dementia and how socio‐economic conditions, access to healthcare, and experiences of discrimination affect cognitive health and dementia risk. A significant avenue to include in future analysis is the use of a social deprivation index a robust framework to better identify patterns of social inequality across different groups and understand how deprivation impacts cognitive health over time.
5. CONCLUSIONS
In summary, covariate adjustment of SDoH sharply attenuated associations between genetic ancestry, dementia probability, and cognitive performance, while statistically significant associations between SDoH and dementia persisted. These findings highlight that social and environmental factors, as opposed to genetic ancestry, likely play more critical roles in determining racial disparities in cognitive performance and subsequent dementia prevalence.
AUTHOR CONTRIBUTIONS
All authors worked collectively to develop the protocols and methods described in this paper. Jorge J Llibre‐Guerra and Miao Jiang had full access to all the data in the study and takes responsibility for the integrity and accuracy of the data analysis. (1) Acquisition of data: Jorge J Llibre‐Guerra, Ana Luisa Sosa, Daisy Acosta, Ivonne Z. Jimenez‐Velasquez, Mariella Guerra, Aquiles Salas, Juan C Llibre‐Guerra, Isaac Acosta, and Juan J. Llibre Rodriguez. (2) Research project: A. Conception: Jorge J Llibre‐Guerra and Juan J Llibre Rodriguez. B. Organization and project administration: Jorge J Llibre‐Guerra and Juan J Llibre Rodriguez. C. Execution: Jorge J Llibre‐Guerra and Juan J Llibre Rodriguez. (3) Statistical Analysis: A. Design: Jorge J Llibre‐Guerra and Miao Jiang. B. Execution: Miao Jiang. C. Review and Critique: All authors. (4) Manuscript: A. Writing of the first draft: Jorge J Llibre‐Guerra and Miao Jiang. B. Review and Critique: All authors.
CONFLICT OF INTEREST STATEMENT
Llibre‐Guerra JJ, Miao J, Rodriguez‐Salgado AM, Acosta I, Sosa AL, Acosta D, Jiménez‐Velázquez IZ, Guerra M, Salas A, Llibre‐Guerra JC, Díaz Sánchez N, Prina M, Renton A, Albanese E, Yokoyama J, Llibre‐Rodríguez J, report no conflict of interest or relevant financial disclosure related to this manuscript. Author disclosures are available in the supporting information.
CONSENT STATEMENT
Written informed consent was obtained from all participants and their study partners. This project was approved by local institutional review boards and the King's College London Research Ethics Committee. The full protocol for the 10/66 population‐based surveys is available in an open‐access publication.
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
This is a secondary analysis of data collected by the 10/66 Dementia Research Group (www.alz.co.uk/1066). The principal investigators, data custodians and responsible parties for research governance in each site are Juan Llibre Rodriguez (Cuba), Daisy Acosta (Dominican Republic), Mariella Guerra (Peru), Aquiles Salas (Venezuela), Ana Luisa Sosa (Mexico), KS Jacob (Vellore, India), Joseph D Williams (Chennai, India), Ivonne Jimenez (Puerto Rico) and Yueqin Huang (China). The 10/66 Dementia Research Group's research has been funded by the Wellcome Trust Health Consequences of Population Change Program (GR066133 – Prevalence phase in Cuba and Brazil; GR080002‐ Incidence phase in Peru, Mexico, Argentina, Cuba, Dominican Republic, Venezuela, and China), the World Health Organization (India, Dominican Republic, and China), the US Alzheimer's Association (IIRG – 04 – 1286 ‐ Peru, Mexico, and Argentina), the Puerto Rico State Legislature (Puerto Rico), and FONACIT/ CDCH/ UCV (Venezuela). Secondary data analysis on genetic ancestry and dementia in the 10/66 Latin American countries is supported by NIH‐NIA (K01AG073526) and Alzheimer Association (AARFD‐21‐851415). Miao Jiang has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska‐Curie grant agreement No 801076, through the SSPH + Global PhD Fellowship Programme in Public Health Sciences (GlobalP3HS) of the Swiss School of Public Health. The content is solely the responsibility of the authors and does not represent the official views of WT, AA or NIH‐NIA. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Llibre‐Guerra JJ, Jiang M, Acosta I, et al. Social determinants of health but not global genetic ancestry predict dementia prevalence in Latin America. Alzheimer's Dement. 2024;20:4828–4840. 10.1002/alz.14041
Jorge J. Llibre‐Guerra and Miao Jiang Joint first author.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer Dement. 2016;12:216‐224. doi: 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. 2020 Alzheimer's disease facts and figures. Alzheimer Dement. 2020;16:391‐460. doi: 10.1002/alz.12068 [DOI] [PubMed] [Google Scholar]
- 3. Gaugler J, James B, Johnson T, Scholz K, Weuve J. 2016 Alzheimer's disease facts and figures. Alzheimer Dement. 2016;12:459‐509. doi: 10.1016/j.jalz.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 4. Wortmann M. Dementia: a global health priority—Highlights from an ADI and World Health Organization report. Alzheimers Res Ther. 2012;4:1‐3. doi: 10.1186/alzrt143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vega IE, Cabrera LY, Wygant CM, Velez‐Ortiz D, Counts SE, Abisambra J. Alzheimer's disease in the latino community: intersection of genetics and social determinants of health. J Alzheimer's Disease. 2017;58:979‐992. doi: 10.3233/JAD-161261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chin AL, Negash S, Hamilton R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis Assoc Disord. 2011;25:187‐195. doi: 10.1097/WAD.0b013e318211c6c9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer's disease and related dementias in the United States (2015‐2060) in adults aged ≥65 years. Alzheimer Dement. 2019;15:17‐24. doi: 10.1016/j.jalz.2018.06.3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen C, Zissimopoulos JM. Racial and ethnic differences in trends in dementia prevalence and risk factors in the United States. Alzheimer Dement. 2018;4:510‐520. doi: 10.1016/j.trci.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nitrini R, Bottino CMC, Albala C, et al. Prevalence of dementia in Latin America: a collaborative study of population‐based cohorts. Int Psychogeriatr. 2009;21:622‐630. doi: 10.1017/S1041610209009430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodriguez JJL, Ferri CP, Acosta D, et al. Prevalence of dementia in Latin America, India, and China: a population‐based cross‐sectional survey. Lancet North Am Ed. 2008;372:464‐474. doi: 10.1016/S0140-6736(08)61002-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bryc K, Velez C, Karafet T, et al. Genome‐wide patterns of population structure and admixture among Hispanic/Latino populations. Proc Natl Acad Sci USA. 2010;107:8954‐8961. doi: 10.1073/PNAS.0914618107/SUPPL_FILE/PNAS.200914618SI.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Homburger JR, Moreno‐Estrada A, Gignoux CR, et al. Genomic insights into the ancestry and demographic history of South America. PLoS Genet. 2015;11:e1005602. doi: 10.1371/journal.pgen.1005602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moreno‐Estrada A, Gravel S, Zakharia F, et al. Reconstructing the population genetic history of the caribbean. PLoS Genet. 2013;9:e1003925. doi: 10.1371/journal.pgen.1003925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ruiz‐Linares A, Adhikari K, Acuña‐Alonzo V, et al. Admixture in Latin America: geographic structure, phenotypic diversity and self‐perception of ancestry based on 7,342 individuals. PLoS Genet. 2014;10:e1004572. doi: 10.1371/journal.pgen.1004572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chacón‐Duque JC, Adhikari K, Fuentes‐Guajardo M, et al. Latin Americans show wide‐spread Converso ancestry and imprint of local Native ancestry on physical appearance. Nat Commun. 2018;9:5388. doi: 10.1038/s41467-018-07748-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laveist T, Pollack K, Thorpe R, Fesahazion R, Gaskin D. Place, not race: disparities dissipate in southwest Baltimore when blacks and whites live under similar conditions. Health Aff (Millwood). 2011;30(10):1880‐1887. doi: 10.1377/hlthaff.2011.0640. n.d.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Navarro V. Race or class versus race and class: mortality differentials in the United States. Lancet North Am Ed. 1990;336(8725):1238‐1240. doi: 10.1016/0140-6736(90)92846-A [DOI] [PubMed] [Google Scholar]
- 18. Llibre‐Guerra JJ, Li Y, Allen IE, et al. Race, genetic admixture, and cognitive performance in the Cuban population. J Gerontol A. 2021;77(2):331‐338. doi: 10.1093/gerona/glab063 [DOI] [PubMed] [Google Scholar]
- 19. Adkins‐Jackson PB, George KM, Besser LM, et al. The structural and social determinants of Alzheimer's disease related dementias. Alzheimer Dement. 2023;19:3171‐3185. doi: 10.1002/ALZ.13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fontanarosa PB, Bauchner H. Race, ancestry, and medical research. JAMA. 2018;320:1539. doi: 10.1001/jama.2018.14438 [DOI] [PubMed] [Google Scholar]
- 21. Ioannidis JPA, Powe NR, Yancy C. Recalibrating the use of race in medical research. JAMA. 2021;325(7):623‐624. doi: 10.1001/jama.2021.0003 [DOI] [PubMed] [Google Scholar]
- 22. Borrell LN, Elhawary JR, Fuentes‐Afflick E, et al. Race and genetic ancestry in medicine — a time for reckoning with racism. New England J Med. 2021;384:474‐480. doi: 10.1056/NEJMMS2029562/SUPPL_FILE/NEJMMS2029562_DISCLOSURES.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manly JJ. Deconstructing race and ethnicity. Med Care. 2006;44:S10‐S16. doi: 10.1097/01.mlr.0000245427.22788.be [DOI] [PubMed] [Google Scholar]
- 24. Ali‐Khan SE, Krakowski T, Tahir R, Daar AS. The use of race, ethnicity and ancestry in human genetic research. Hugo J. 2011;5:47‐63. doi: 10.1007/s11568-011-9154-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prina AM, Mayston R, Wu Y‐T, Prince M. A review of the 10/66 dementia research group. Soc Psychiatry Psychiatr Epidemiol. 2018;54:1‐10. doi: 10.1007/s00127-018-1626-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prina AM, Acosta D, Acostas I, et al. Cohort profile: the 10/66 study. Int J Epidemiol. 2016;46:dyw056. doi: 10.1093/ije/dyw056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prince M, Ferri CP, Acosta D, et al. The protocols for the 10/66 dementia research group population‐based research programme. BMC Public Health. 2007;7:165. doi: 10.1186/1471-2458-7-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prince MJ, De Rodriguez JL, Noriega L, et al. The 10/66 Dementia Research Group's fully operationalised DSM‐IV dementia computerized diagnostic algorithm, compared with the 10/66 dementia algorithm and a clinician diagnosis: a population validation study. BMC Public Health. 2008;8:219. doi: 10.1186/1471-2458-8-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prince M, Acosta D, Chiu H, Scazufca M, Varghese M. Dementia diagnosis in developing countries: a cross‐cultural validation study. Lancet. 2003;361:909‐917. doi: 10.1016/S0140-6736(03)12772-9 [DOI] [PubMed] [Google Scholar]
- 30. Hall KS, Hendrie HC, Rodgers DD, et al. The development of a dementia screening interview in two distinct languages. Int J Methods Psychiatr Res. 1993;3:1‐28. [Google Scholar]
- 31. Sosa AL, Albanese E, Prince M, et al. Population normative data for the 10/66 Dementia Research Group cognitive test battery from Latin America, India and China: a cross‐sectional survey. BMC Neurol. 2009;9:48. doi: 10.1186/1471-2377-9-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412‐2414. doi: 10.1212/WNL.43.11.2412-A [DOI] [PubMed] [Google Scholar]
- 33. Smith MW, Patterson N, Lautenberger JA, et al. A high‐density admixture map for disease gene discovery in African Americans. Am Hum Genet. 2004. doi: 10.1086/420856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith MW, Lautenberger JA, Shin HD, et al. Markers for mapping by admixture linkage disequilibrium in African American and Hispanic Populations. Am Hum Genet. 2001;74(5):1001‐1013. doi: 10.1086/323922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Teruel BM, Rodríguez JJL, McKeigue P, et al. Interactions between genetic admixture, ethnic identity, APOE genotype and dementia prevalence in an admixed Cuban sample; a cross‐sectional population survey and nested case‐control study. BMC Med Genet. 2011;12:43. doi: 10.1186/1471-2350-12-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoggart CJ, Shriver MD, Kittles RA, Clayton DG, McKeigue PM. Design and analysis of admixture mapping studies. Am Hum Genet. 2004;74(5):965‐978. doi: 10.1086/420855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shriner D, Overview of Admixture Mapping n.d. doi: 10.1002/0471142905.hg0123s76 [DOI] [PubMed]
- 38. Silva‐Zolezzi I, Hidalgo‐Miranda A, Estrada‐Gil J, et al. Analysis of genomic diversity in Mexican Mestizo populations to develop genomic medicine in Mexico. Proc Natl Acad Sci USA. 2009;106:8611‐8616. doi: 10.1073/PNAS.0903045106/SUPPL_FILE/0903045106SI.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pena SDJ, di Pietro G, Fuchshuber‐Moraes M, et al. The genomic ancestry of individuals from different geographical regions of Brazil is more uniform than expected. PLoS One. 2011;6:e17063. doi: 10.1371/JOURNAL.PONE.0017063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Adhikari K, Mendoza‐Revilla J, Chacón‐Duque JC, Fuentes‐Guajardo M, Ruiz‐Linares A. Admixture in Latin America. Curr Opin Genet Dev. 2016;41:106‐114. doi: 10.1016/J.GDE.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 41. Norris ET, Wang L, Conley AB, et al. Genetic ancestry, admixture and health determinants in Latin America. Bmc Genomics [Electronic Resource]. 2018;19:861. doi: 10.1186/s12864-018-5195-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Glassman A, Gaziano TA, Buendia CPB, de Aguiar FCG. Confronting the chronic disease burden in Latin America and the Caribbean. Health Aff. 2010;29:2142‐2148. doi: 10.1377/hlthaff.2010.1038 [DOI] [PubMed] [Google Scholar]
- 43. Babulal GM, Quiroz YT, Albensi BC, et al. Perspectives on ethnic and racial disparities in Alzheimer's disease and related dementias: update and areas of immediate need. Alzheimer Dement. 2019;15:292‐312. doi: 10.1016/J.JALZ.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsoy E, Kiekhofer RE, Guterman EL, et al. Assessment of racial/ethnic disparities in timeliness and comprehensiveness of dementia diagnosis in California. JAMA Neurol. 2021;78(6):657‐665. doi: 10.1001/jamaneurol.2021.0399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kawas CH, Corrada MM, Whitmer RA. Diversity and disparities in dementia diagnosis and care: a challenge for all of us. JAMA Neurol. 2021;78(6):650‐652. doi: 10.1001/jamaneurol.2021.0285 [DOI] [PubMed] [Google Scholar]
- 46. Quiroz YT, Solis M, Aranda MP, et al. Addressing the disparities in dementia risk, early detection and care in Latino populations: highlights from the second Latinos & Alzheimer's Symposium. Alzheimer Dementia. 2022;18:1677. doi: 10.1002/ALZ.12589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Manly JJ, Jones RN, Langa KM, et al. Estimating the prevalence of dementia and mild cognitive impairment in the US: the 2016 Health and Retirement Study Harmonized Cognitive Assessment Protocol Project. JAMA Neurol. 2022;79:1242‐1249. doi: 10.1001/JAMANEUROL.2022.3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krieger N. Methods for the scientific study of discrimination and health: an ecosocial approach. Am J Public Health. 2012;102:936. doi: 10.2105/AJPH.2011.300544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Granot‐Hershkovitz E, Xia R, Yang Y, et al. Interaction analysis of ancestry‐enriched variants with APOE‐ɛ4 on MCI in the Study of Latinos‐Investigation of Neurocognitive Aging. Sci Rep. 2023;13:1‐9. doi: 10.1038/s41598-023-32028-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Granot‐Hershkovitz E, Tarraf W, Kurniansyah N, et al. APOE alleles’ association with cognitive function differs across Hispanic/Latino groups and genetic ancestry in the study of Latinos‐investigation of neurocognitive aging (HCHS/SOL). Alzheimer Dement. 2021;17:466‐474. doi: 10.1002/ALZ.12205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. González HM, Tarraf W, Schneiderman N, et al. Prevalence and correlates of mild cognitive impairment among diverse Hispanics/Latinos: study of Latinos‐Investigation of Neurocognitive Aging results. Alzheimer Dement. 2019;15:1507‐1515. doi: 10.1016/J.JALZ.2019.08.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yaffe K, Falvey C, Harris TB, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ. 2013;347:f7051‐f7051. doi: 10.1136/bmj.f7051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zahodne LB, Manly JJ, Azar M, Brickman AM, Glymour MM. Racial disparities in cognitive performance in mid‐ and late adulthood: analyses of two cohort studies. J Am Geriatr Soc. 2016;64:959‐964. doi: 10.1111/jgs.14113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Clark CM, DeCarli C, Mungas D, et al. Earlier onset of Alzheimer disease symptoms in Latino individuals compared with Anglo individuals. Arch Neurol. 2005;62:774‐778. doi: 10.1001/archneur.62.5.774 [DOI] [PubMed] [Google Scholar]
- 55. Gross AL, Mungas DM, Crane PK, et al. Effects of education and race on cognitive decline: an integrative study of generalizability versus study‐specific results. Psychol Aging. 2015;30:863‐880. doi: 10.1037/pag0000032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Reitz C, Jun G, Naj A, et al. Variants in the ATP‐binding cassette transporter (ABCA7), apolipoprotein E ϵ4, and the risk of late‐onset Alzheimer disease in African Americans. JAMA. 2013;309:1483‐1492. doi: 10.1001/jama.2013.2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mez J, Chung J, Jun G, et al. Two novel loci, COBL and SLC10A2, for Alzheimer's disease in African Americans. Alzheimer Dement. 2017;13:119‐129. doi: 10.1016/j.jalz.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Logue MW, Schu M, Vardarajan BN, et al. Two rare AKAP9 variants are associated with Alzheimer's disease in African Americans. Alzheimers Dement. 2014;10:609‐618. doi: 10.1016/j.jalz.2014.06.010. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Acosta D, Llibre‐Guerra JJ, Jiménez‐Velázquez IZ, Llibre‐Rodríguez JJ. Dementia research in the Caribbean Hispanic islands: present findings and future trends. Front Public Health. 2021;8:611998. doi: 10.3389/fpubh.2020.611998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nelson PT, Pious NM, Jicha GA, et al. APOE‐ε2 and APOE‐ε4 correlate with increased amyloid accumulation in cerebral vasculature. J Neuropathol Exp Neurol. 2013;72:708‐715. doi: 10.1097/NEN.0b013e31829a25b9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tang MX, Stern Y, Marder K, et al. The APOE‐ε4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. J Am Med Assoc. 1998;279:751‐755. doi: 10.1001/JAMA.279.10.751 [DOI] [PubMed] [Google Scholar]
- 62. Rajabli F, Feliciano BE, Celis K, et al. Ancestral origin of ApoE ε4 Alzheimer disease risk in Puerto Rican and African American populations. PLoS Genet. 2018;14:e1007791. doi: 10.1371/journal.pgen.1007791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hendrie HC, Murrell J, Baiyewu O, et al. APOE ε4 and the risk for Alzheimer disease and cognitive decline in African Americans and Yoruba. Int Psychogeriatr. 2014;26:977‐985. doi: 10.1017/S1041610214000167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mukadam N, Sommerlad A, Huntley J, Livingston G. Population attributable fractions for risk factors for dementia in low‐income and middle‐income countries: an analysis using cross‐sectional survey data. Lancet Glob Health. 2019;7:e596‐e603. doi: 10.1016/S2214-109X(19)30074-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sherva R, Zhang R, Sahelijo N, et al. African ancestry GWAS of dementia in a large military cohort identifies significant risk loci. Mol Psychiatry. 2023;28:1293‐1302. doi: 10.1038/s41380-022-01890-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kunkle BW, Schmidt M, Klein H‐U, et al. Novel Alzheimer disease risk loci and pathways in African American individuals using the African genome resources panel: a meta‐analysis. JAMA Neurol. 2021;78:102‐113. doi: 10.1001/JAMANEUROL.2020.3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


