Abstract
BACKGROUND
Neurolymphomatosis (NL) is a rare disease defined as an invasion of lymphoma into peripheral nerves, nerve roots, or nerve plexuses, including the cranial nerves. No clear treatment protocols have yet been defined for this pathology.
OBSERVATIONS
A woman in her 40s had a primary central nervous system lymphoma diagnosed from an intracranial tumor biopsy and underwent chemotherapy and radiation therapy. After she complained of pain in the trunk and extremities, magnetic resonance imaging and [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET) performed 25 months after initial diagnosis revealed multiple lesions in the nerve ganglia, plexuses, and peripheral nerves from the cervical to the sacral spinal cord. Cerebrospinal fluid cytology revealed atypical lymphocytes and lymphoma dissemination in the spinal cavity. Based on these findings, NL was diagnosed. An intrathecal antineoplastic regimen temporarily reduced abnormal uptake of FDG, but the lesion recurred. After additional high-dose methotrexate therapy, FDG accumulation in the previously identified lesions disappeared. However, peripheral neuropathic pain and paraplegia remained. The patient died 9 months after the initial diagnosis of NL.
LESSONS
The authors reported a case of NL following primary central nervous system lymphoma. In this case, FDG-PET proved useful for diagnosis, and high-dose methotrexate therapy was temporarily effective.
Keywords: primary central nervous system lymphoma, neurolymphomatosis, [18F]fluorodeoxyglucose positron emission tomography, cytology, case report
ABBREVIATIONS: Ara-C = Adriamycin, CSF = cerebrospinal fluid, CT = computed tomography, DLBCL = diffuse large B-cell lymphoma, FDG = [18F]fluorodeoxyglucose, HD-MTX = high-dose methotrexate, IL-2R = interleukin 2 receptor, MRI = magnetic resonance imaging, NL = neurolymphomatosis, PCNSL = primary central nervous system malignant lymphoma, PET = positron emission tomography, PSL = prednisolone
Neurolymphomatosis (NL) is a rare disease defined as the invasion of lymphoma into the peripheral nerves, nerve roots, or nerve plexuses, including the cranial nerves. This pathological condition shows a poor prognosis, and while early diagnosis and treatment are desirable, no clear protocols for treatment have yet been devised.1–3
Herein, we report a case of NL secondary to primary central nervous system malignant lymphoma (PCNSL) in which [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET) proved useful for both diagnosis and the evaluation of therapeutic effects.
Illustrative Case
History and Examination
A woman in her 40s presented to a local physician with left upper-extremity clumsiness and gait disturbance. Gadolinium-enhanced magnetic resonance imaging (MRI) of the head showed scattered intracranial lesions in the supra- and infratentorial areas. The patient was referred to the Department of Neurosurgery for further investigation and treatment. At her first visit to our hospital, she was conscious with mild left hemiplegia. Within a month, she was admitted to our hospital with worsening gait disturbance and somnolence. MRI was performed at the first visit to our hospital and on admission 1 month later (Fig. 1). FDG-PET after admission showed localized high uptake (maximum standardized uptake value: 19.0) in the left cerebellar hemisphere, but no lesions were apparent in the trunk. Tissue was biopsied from contrast-enhanced lesions in the left cerebellar hemisphere. Histological examination showed diffuse proliferation of large, atypical lymphocytes with large oval nuclei. Immunostaining revealed that these cells were positive for CD20, CD5, CD10, and bcl-6 (Fig. 2). Based on these findings, the pathological diagnosis was diffuse large B-cell lymphoma (DLBCL).
FIG. 1.
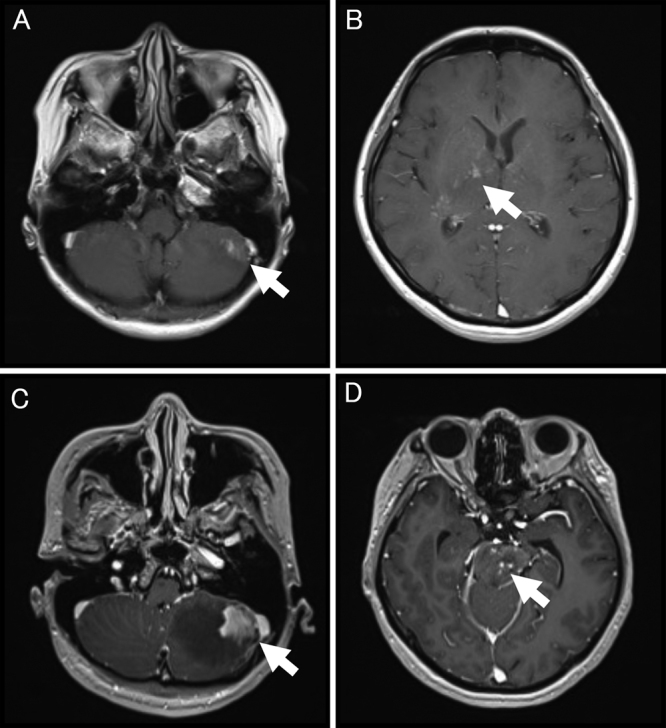
Axial gadolinium-enhanced T1-weighted MRI of the brain on admission (A and B) and 1 week before biopsy (C and D). MRI on admission shows abnormal enhancements scattered in the right basal ganglia (A, white arrow) and below the tentorium cerebelli (B, left cerebellum, white arrow). Images before biopsy show a lesion extending to the left cerebellum (C, white arrow) and new abnormal enhancement of the brainstem (D, white arrow).
FIG. 2.
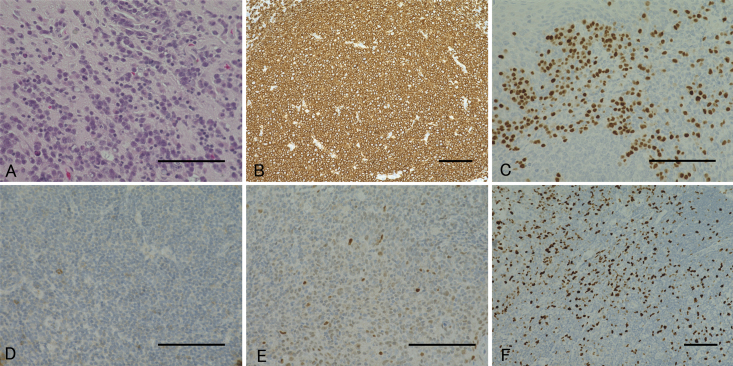
Pathological findings of the biopsied lesion. Hematoxylin and eosin staining shows marked diffuse infiltration of large lymphomatous cells (A), and immunostaining reveals these cells as CD20-positive (B), MUM-1–positive (C), CD10-negative (D), weakly positive for bcl-6 (E), and MIB-1–positive (F). Bar = 100 μm (A–F).
Initial Treatment
Initial treatment comprised high-dose methotrexate (HD-MTX) administered at 100 mg/kg every 2 weeks for a total of 6 courses. Symptoms improved after starting treatment, and lesions reduced in size over time, resulting in complete remission. Additional radiotherapy was considered, but the patient and her family declined this option, so she was discharged home after chemotherapy.
First Recurrence
MRI at 19 months after the completion of initial chemotherapy revealed a new, asymptomatic, contrast-enhanced lesion in the right frontal lobe subcortex. Recurrent PCNSL was diagnosed, and 2 additional courses of HD-MTX were administered. Contrast-enhanced lesions disappeared after treatment.
Second Recurrence
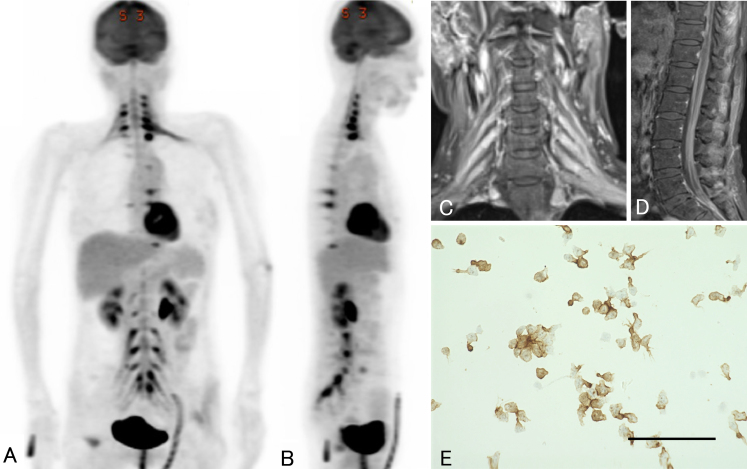
Three months after starting the second course of treatment, she was readmitted with decreased spontaneity. MRI revealed recurrent lesions in the left basal ganglia. An additional course of HD-MTX was administered. After chemotherapy, the elevated blood MTX concentration was prolonged. Contrast-enhanced computed tomography (CT) of the abdomen revealed acute tubular necrosis. In consideration of the risk of renal failure, the patient underwent radiotherapy with no additional HD-MTX therapy. Whole-brain radiation was started, but she complained of pain in the extremities and trunk and gradually became somnolent. Brain MRI performed at the end of radiotherapy revealed mild ventricular enlargement, although the intracranial neoplastic lesion had shrunk. At the same time, FDG-PET of the head and trunk was performed. Multiple FDG-rich lesions were observed in the ganglia, plexuses, and peripheral nerves from the cervical to sacral spinal cord (Fig. 3). Gadolinium-enhanced MRI of the spinal cord also showed multiple contrast-enhanced lesions at the same site, and the spinal cord cavity and cauda equina showed diffuse enhancement (Fig. 3). Examination of cerebrospinal fluid (CSF) findings from lumbar puncture showed increased cell counts, increased protein, and elevated levels of the tumor marker soluble interleukin-2 receptor(IL-2R) (Table 1). Clusters of atypical lymphocytes were identified in CSF. Immunostaining revealed that the atypical lymphocytes were CD20-positive, suggesting intraspinal dissemination of lymphoma (Fig. 3). Based on these findings, NL secondary to PCNSL was diagnosed.
FIG. 3.
Images from FDG-PET, spinal MRI, and CSF cytology performed 25 months after initial diagnosis. Coronal (A) and sagittal (B) FDG-PET shows abnormal FDG accumulation in the ganglia, plexuses, and peripheral nerves from the cervical cord to the sacral cord. Coronal gadolinium-enhanced T1-weighted MRI of the cervical spinal cord (C) also shows multiple areas of contrast enhancement in the ganglia and plexuses. Sagittal gadolinium-enhanced T1-weighted MRI of the lumbar level (D) shows diffuse contrast effects within the spinal cavity. Immunostaining (E) shows aggregates of large, atypical lymphocytes positive for CD20 antibody. Bar = 100 μm.
TABLE 1.
Findings from the CSF examination during intrathecal injection treatment
| CSF Study | Before IT | After 5 Cycles of IT | After 9 Cycles of IT |
|---|---|---|---|
| Color | Colorless | Colorless | Colorless |
| Cell count (/μl) | 22 | 2 | <1 |
| Protein (mg/dl) | 1182 | 149 | 180 |
| Sugar (mg/dl) | 10 | 61 | 60 |
| Chloride (mEq/l) | 101 | 125 | 129 |
| β2-microglobulin (mg/l) | 13.5 | 2.3 | 1.6 |
| IL-2R (U/ml) | 9820 | 130 | 245 |
IT = intrathecal therapy.
Treatment for NL
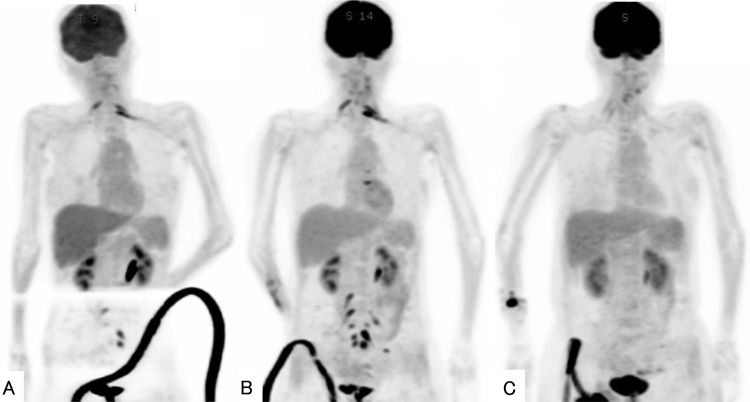
Additional HD-MTX was not administered because of the deteriorating renal function. An intrathecal antineoplastic regimen was administered using MTX at 15 mg/day, Adriamycin (Ara-C) at 4 mg/day, and prednisolone at 10 mg. At the time of injection, CSF was collected and tested. The number of cells and protein levels in CSF both decreased with an antineoplastic regimen compared to the start of treatment (Table 1). Cytological examination of the CSF after 5 intrathecal injections showed no atypical lymphocytes. The concentration of soluble IL-2R in CSF decreased after the 5 injections then increased again after the ninth injection. FDG-PET after 5 intrathecal injections (Fig. 4) showed reduced FDG-PET uptakes in the cervical, thoracic, lumbar, and sacral spinal cords. After 9 intrathecal injections, lesions showing enhanced FDG-PET uptake were again observed (Fig. 4). As a result, the intrathecal antineoplastic regimen did not lead to complete remission. After confirming a recovery of renal function, additional HD-MTX was administered. After 2 additional courses of treatment, the FDG-accumulating lesions in the trunk disappeared (Fig. 4). In addition, MRI confirmed the disappearance of intracranial lesions and shrinkage of the enlarged ventricles. No increase in cerebrospinal pressure was observed during an antineoplastic regimen.
FIG. 4.
After 5 cycles of an antineoplastic regimen, FDG-PET shows a reduced number of lesions with increased FDG uptake in the cervical, thoracic, lumbar, and sacral spinal cord (A). After 9 cycles of intrathecal therapy, an increased number of lesions with elevated uptake of FDG-PET is observed again (B). After 2 additional courses of systemic HD-MTX, FDG-accumulating lesions have disappeared (C).
Radiation therapy was not added for NL. After treatment, pain in the upper and lower extremities decreased, but paraplegia remained. The patient was transferred to another hospital for care and died 2 months later, 9 months after the initial diagnosis of NL. The cause of death was respiratory failure.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
We encountered a case of NL secondary to PCNSL. In this case, FDG-PET was useful for the diagnosis and evaluation of therapeutic effects. NL is an extremely rare disease that can occur as a primary disease or as a recurrence or progression of previously treated disease,2 but the incidence of these forms has not been clearly defined. According to the International PCNSL Collaborative Group report, NL has been identified in 50 patients in 16 years. Most cases of NL are associated with non-Hodgkin’s lymphoma (90%), but some are associated with acute leukemia (10%).3 To the best of our knowledge, very few reports have described PCNSL followed by NL.4
Time is required to confirm the diagnosis of NL. Antemortem diagnosis is achieved for about 20%–55% of NLs.2, 5 Cytomorphological analysis of CSF reportedly yields evidence of dissemination in approximately 20% of cases.2, 6 The diagnostic sensitivity of MRI for NL has been reported as 70%–80%, compared to 80%–90% for FDG-PET.3 The diagnosis of NL in the present case required evidence from nerve biopsy and autopsy. FDG-PET has already been reported as useful for diagnosing NL.7, 8 In our case, 1 month passed from the onset of symptoms to the diagnosis of NL. FDG-PET was very helpful in diagnosing NL.
NL has been treated with various combinations of systemic chemotherapy, intrathecal chemotherapy, and radiation therapy. Rituximab is a monoclonal antibody directed against the protein CD20 and is used as a regimen for non-Hodgkin’s lymphoma ( rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone). However, because of the large molecule, rituximab cannot cross the blood-brain or blood-nerve barriers. Rituximab did not improve NL outcomes in some reported cases.9 HD-MTX therapy is particularly effective, due at least in part to its ability to cross the blood-brain barrier. However, clear protocols for treatment remain lacking. In this case, we administered HD-MTX and an intrathecal antineoplastic regimen based on previous reports.10, 11 An analysis of 50 patients with NL showed that 46% responded to treatment, with a median overall survival of 10 months.3
In our case, the number of atypical cells was reduced by multiple intrathecal antineoplastic regimens. On the other hand, tumor marker levels in CSF re-elevated during the intrathecal antineoplastic regimen. It is known that IL-2 levels are elevated in blood in NL.12 There are no reports that have investigated IL-2 in CSF over time, as in our case. There was a correlation between FDG-PET findings and IL-2 levels in CSF. It may be used as a biomarker of response to treatment in NL. FDG-PET revealed a recurrence of NL at the end of an intrathecal antineoplastic regimen. It proved temporarily effective, but NL did not have a complete response. After confirming the recovery of renal function, HD-MTX therapy was again administered and a reduction of the lesion was confirmed on FDG-PET. PET-CT has also been reported as useful for evaluating therapeutic efficacy in NL.13 In our case, serial FDG-PET was also useful for judging therapeutic efficacy. This is a rare case in which remission and recurrence of NL were observed using FDG-PET. Periodic FDG-PET may be useful for follow-up of NL.
Although this patient was diagnosed with NL secondary to DLBCL, no peripheral nerve biopsy of the affected area was performed. In previous reports, biopsy has remained the gold standard of diagnosis (sensitivity 88%), but half of patients with NL did not undergo biopsy because of the difficulty in obtaining a biopsy specimen from the relevant area and the potential for permanent nerve damage and false-negative diagnoses.3, 14 Since biopsy is an invasive examination, FDG-PET has been reported as useful for diagnosing NL. In our case, CD20-positive atypical cells were observed in the CSF, confirming the diagnosis of NL.
Lessons
We encountered an extremely rare case of NL secondary to PCNSL. When peripheral neuropathy develops after PCNSL, differentiating NL from other pathologies is important. MRI of the spinal cord and FDG-PET/CT of the trunk appear useful for diagnosis.
Disclosures
The authors report no conflict of interest concerning the materials ormethods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Asano, Aishima. Acquisition of data: Horiguchi. Analysis and interpretation of data: Asano. Drafting the article: Asano, Horiguchi. Critically revising the article: Kakinuma, Nagaki, Aishima. Reviewed submitted version of manuscript: Tosaka. Approved the final version of the manuscript on behalf of all authors: Asano. Administrative/technical/material support: Yoshimoto. Study supervision: Yamada, Aishima, Tosaka, Yoshimoto.
Correspondence
Hirofumi Asano: Gunma University Graduate School of Medicine, Maebashi, Gunma, Japan. asano.h29@gmail.com.
References
- 1.Kelly JJ, Karcher DS. Lymphoma and peripheral neuropathy: a clinical review. Muscle Nerve. 2005;31(3):301-313. [DOI] [PubMed] [Google Scholar]
- 2.Baehring JM, Damek D, Martin EC, Betensky RA, Hochberg FH. Neurolymphomatosis. Neuro Oncol. 2003;5(2):104-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grisariu S, Avni B, Batchelor TT, et al. Neurolymphomatosis: an International Primary CNS Lymphoma Collaborative Group report. Blood. 2010;115(24):5005-5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi YJ, Shin JA, Kim YH, et al. Neurolymphomatosis of brachial plexus in patients with non-Hodgkin’s lymphoma. Case Rep Oncol Med. 2013;2013:492329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosso SM, Bruin HG, Wu KL, Bent MJ. Diagnosis of neurolymphomatosis with FDG PET. Neurology. 2006;67(4):722-723. [DOI] [PubMed] [Google Scholar]
- 6.Fischer L, Martus P, Weller M, et al. Meningeal dissemination in primary CNS lymphoma: prospective evaluation of 282 patients. Neurology. 2008;71(14):1102-1108. [DOI] [PubMed] [Google Scholar]
- 7.Tsang HHC, Lee EYP, Anthony MP, Khong PL. 18F-FDG PET/CT diagnosis of vagus nerve neurolymphomatosis. Clin Nucl Med. 2012;37(9):897-898. [DOI] [PubMed] [Google Scholar]
- 8.Salm LP, Hiel BV, Stokkel MPM. Neurolymphomatosis diagnosed by (18)F-FDG PET-CT. Clin Nucl Med. 2013;38(6):e261-e262. [DOI] [PubMed] [Google Scholar]
- 9.Gan HK, Azad A, Cher L, Mitchell PLR. Neurolymphomatosis: diagnosis, management, and outcomes in patients treated with rituximab. Neuro Oncol. 2010;12(2):212-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jisun J, Sun WK, Duk HS. Neurolymphomatosis: a single-center experience of neuromuscular manifestations, treatments, and outcomes. J Neurol. 2021;268:851-859. [DOI] [PubMed] [Google Scholar]
- 11.Yuichiro O, Yasuhiro K, Yotaro O, et al. Two cases of neurolymphomatosis with fatal bilateral vocal cord paralysis that were diagnosed with 18F-fluorodeoxyglucose positron emission tomography (FDG PET)/CT. Intern Med. 2017;56:1193-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosei M, Yoshiaki A, Kentaro N, et al. Diagnosis of intravascular large B cell lymphoma: novel insights into clinicopathological features from 42 patients at a single institution over 20 years. Br J Haematol. 2019;187(3):328-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin M, Kilanowska J, Taper J, Chu J. Neurolymphomatosis–diagnosis and assessment of treatment response by FDG PET-CT. Hematol Oncol. 2008;26(1):43-45. [DOI] [PubMed] [Google Scholar]
- 14.Bent MJ, Bruin HG, Bos GM, Rivière GB, Smitt PAS. Negative sural nerve biopsy in neurolymphomatosis. J Neurol. 1999;246(12):1159-1163. [DOI] [PubMed] [Google Scholar]