Abstract
Background
Penetrating thoracic injuries have a significant risk of morbi-mortality. Despite the advancements in damage control methods, a subset of patients with severe pulmonary vascular lesions and bronchial injuries persists. In some of these cases, post-traumatic pneumonectomy is required, and perioperative extracorporeal membrane oxygenation (ECMO) support may be required due to right ventricular failure and respiratory failure.
Case description
A male was brought to the emergency department (ED) with a penetrating thoracic injury, presenting with massive right hemothorax and active bleeding that required ligation of the right pulmonary hilum to control the bleeding. Subsequently, he developed right ventricular dysfunction and ARDS, necessitating a dynamic hybrid ECMO configuration to support his condition and facilitate recovery.
Conclusions
Penetrating thoracic injuries with severe pulmonary vascular lesions may need pneumonectomy to control bleeding. ECMO support reduces the associated mortality by decreasing the complications rate. A multidisciplinary team is essential to achieve good outcomes in severe compromised patients.
Keywords: ECMO, Traumatic pneumonectomy, Case report, Penetrating thoracic injury
Background
The reported incidence of lung resection in traumatic injuries is 0.08% [1]. Pneumonectomy is performed even less commonly, occurring at an incidence of 0.01% amongst trauma patients. Traumatic pneumonectomy (TP) is a rare but critical intervention in thoracic trauma, with a mortality rate ranging from 50 to 100% [1]. A complete pneumonectomy may be required to control hilar hemorrhage or if pulmonary and bronchial injuries are beyond repair. However, the mortality rates of TP are high. Wagner et al. suggested rapid pneumonectomy for unstable patients with central hilar vascular destruction [2]. Urgent TP is typically conducted following hemorrhage from major hilar or bronchial injuries where lung salvage is unfeasible [1, 3, 4]. Early post-TP fatalities can result from acute right heart failure due to a sudden increase in pulmonary artery pressure. This strain on the thin-walled right ventricle leads to subsequent left ventricular dysfunction [1]. Right heart failure and pulmonary edema in the remaining lung tissue can manifest within hours post-pneumonectomy [5]. However, in extremis patients a more aggressive and timely initiation of this procedure should be warranted [1].
The use of extracorporeal membrane oxygenation (ECMO) in trauma patients can serve as an adjunct therapy when conventional treatments are ineffective. The indications for ECMO in trauma cases, however, remain uncertain, and clinical outcomes vary due to the need for anticoagulation, hemorrhage risk, and coagulopathy. Despite these challenges, advancements in device technology have led to improved outcomes and reduced bleeding rates, establishing ECMO as a viable strategy in managing acute severe dysfunction [6, 7]. A recent meta-analysis describes the prognosis for adult trauma patients requiring ECMO, concluding that it is beneficial for severely traumatized patients by improving their prognosis and serving as a valuable tool in managing severe trauma-related cardiorespiratory failure, hemorrhagic shock, and cardiac arrest [8]. The use of ECMO to support the recovery of a patient after a pneumonectomy could be a tool for supporting the heart in right ventricular dysfunction and in cases of developed acute respiratory distress syndrome (ARDS). We present a case of penetrating thoracic trauma necessitating TP, supported by ECMO.
Case presentation
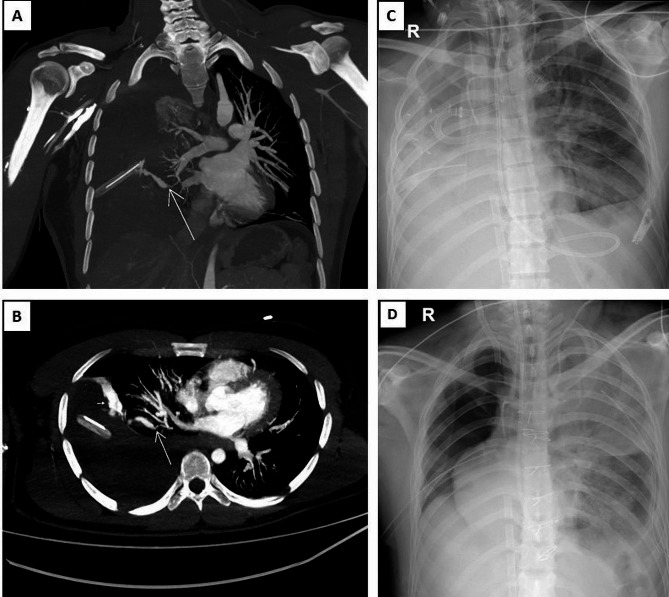
A 34-year-old male, previously healthy, was admitted to the emergency department (ED) following a penetrating thoracic trauma caused by a stab wound. Upon arrival, his temperature was 36.5 °C, pulse 77/min and blood pressure were 60/40 mmHg. Pulse oximetry on room air showed an oxygen saturation of 91%. Examination revealed three stab wounds: the first in the posterior cervical region, the second in the right posterior hemithorax at T4 with a 2 cm scapular line, and the third in the right lumbar region at L1 with a 3 cm wound. Auscultation of the chest showed decreased breath sounds on the right. The eFAST showed right hemothorax. The emergent thoracostomy drained 450 mL of blood. Thoraco-abdominal Angio-CT was performed, ruling out vascular or spinal cord injuries, but finding a massive right hemothorax from an artery of the lower right lobe with active bleeding to the pleural cavity (Fig. 1A, B). At this point the chest tube output reached 1250 ml.
Fig. 1.
Thoracic Trauma and ECMO support. A y B. Multiplanar reconstruction with maximum intensity projection. Coronal and axial lung views showing bleeding from a posterior basal subsegmental artery of the lower right lobe (arrow) with extravasation of the contrast medium into the pleural cavity resulting in a massive coagulated hemothorax. C. Chest X-Ray shows central ECMO cannulation as a support after lung pneumonectomy. D. Chest X-Ray shows peripheral ECMO cannulation as pulmonary support due to respiratory failure
Due to the severity of the situation, the patient was taken to the operative room (OR) for damage control surgery by the trauma surgery team through a midline sternotomy. Intraoperatively, a wound was discovered on the posterior face of the pulmonary hilum that compromised the central portion of the three pulmonary lobes, predominantly affecting the lower lobe, with active bleeding and air leak from the wounds. The pulmonary hilum was clamped to control the bleeding, but due to persistent bleeding and poor oxygenation, a right pulmonary hilum ligation was performed. During the surgery, the patient experienced a cardiac arrest, necessitating direct cardiac compressions in cycles of less than 2 min and a return to spontaneous circulation. Chest tubes, mediastinal packing and delayed sternal closure using the VAC® therapy system was left in place.
The patient was transferred to the Intensive Care Unit (ICU), where his condition deteriorated, presenting with refractory multiple organ dysfunction unresponsive to vasopressor support, respiratory failure characterized by severe hypoxemia and restrictive disorder, and severe hypercapnia. A transesophageal echocardiogram (TEE) was performed, revealing significant right ventricular dilatation and septal flattening, with underfilled left cardiac chambers due to impaired filling of the left heart—indicative of severe right ventricular dysfunction that progressed to cardiogenic shock (Table 1; Hour 0). Consequently, the intensivist team and the ECMO support group decided to initiate peripheral veno-arterial (VA-ECMO) using a femoro-femoral configuration.
Table 1.
Daily Record of Hemodynamics, Vasopressor Support, Ventilatory Parameters, Drainage, and ECMO Status Over the First Eight Days in ICU
| *Day 0: Hour 6 | Day 0: Hour 12 | Day 1: Hour 24 | Day 1: Hour 36 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Hemodynamic Parameters | ||||||||||
| Heart Rate (bpm) | 96 | 131 | 132 | 126 | 121 | 71 | 74 | 76 | 91 | 85 |
| Arterial Pressure (mmHg) | 95/80 | 73/59 | 100/52 | 83/63 | 92/56 | 60/56 | 80/64 | 81/65 | 101/72 | 107/82 |
| Hemoglobin (mg/dL) | 9,8 | 12,4 | 11,4 | 10 | 8,6 | 10,1 | 8,9 | 9,5 | 9,9 | 10,8 |
| Platelets (per µL) | 151,000 | 80,000 | 66,000 | 26,000 | 31,000 | 51,000 | 67,000 | 59,000 | 69,000 | 80,000 |
| pH | 7.2 | 7.21 | 7.07 | 7.43 | 7.41 | 7.47 | 7.37 | 7.34 | 7.42 | 7.47 |
| pCO2 (mmHg) | 35 | 42 | 57 | 37 | 42 | 37 | 48 | 48 | 37 | 36 |
| pO2 (mmHg) | 110 | 87 | 109 | 91 | 66 | 137 | 66 | 140 | 130 | 112 |
| HCO3 (mEq/L) | 17.5 | 16.8 | 16.5 | 25 | 26.3 | 26.9 | 27 | 25 | 24 | 25 |
| BE (mEq/L) | -11 | -10.6 | -13.6 | 0.3 | 12 | 3.2 | 2.4 | 0.1 | 0 | 1 |
| Lactate (mmol/L) | 7.41 | 6.4 | 5 | 3.3 | 3.7 | 2.3 | 0.9 | 0.6 | 1 | 0.9 |
| Creatinine (mg/dL) | 1.18 | 0.97 | 1.8 | 2.2 | 2.3 | 2.29 | 2.6 | 2.5 | 1.72 | 1.4 |
| Bilirubin (mg/dL) | - | - | 1.15 | 1.8 | 3.16 | 3.25 | 2.2 | 2.9 | 4.4 | 4.2 |
| Vasopressor Support | ||||||||||
| Norepinephrine (mcg/kg/min) | 0,5 | 0,343 | 0,343 | 0,343 | 0,343 | 0,0067 | 0,026 | 0,026 | - | - |
| Vasopressin (UI/min) | - | 0,047 | 0,047 | 0,0633 | 0,047 | 0,04 | 0,04 | 0,06 | - | - |
| Dobutamine (cc/hr) | - | - | 6,4 | - | 10 | - | - | - | ||
| Milrinone (cc/hr) | - | - | - | 13,5 | 13,5 | 13,5 | 13,5 | 11,8 | 16,6 | 16,6 |
| Ventilatory Parameters | ||||||||||
| Respiratory Rate | 22 | 22 | 35 | 10 | 10 | 10 | 10 | 10 | 11 | 12 |
| Oxygen Saturation | 100 | 94 | 82 | 99 | 92 | 99 | 98 | 99 | 99 | 98 |
| Ventilation Mode | VC | VC | PC | PC | APC | PC | PC | PC | APC | PC |
| FiO2 (%) | 0.40 | 50 | 100 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| IMV (times/min) | 16 | 22 | 35 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| PEEP (cm H2O) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 8 |
| Tidal Volume (mL) | 480 | 430 | 260 | 221 | 110 | 110 | 111 | 200 | 471 | 360 |
| Drainages | ||||||||||
| Left Thoracic Tube (cc) | - | 750 | 1372 | 1850 | 2250 | 700 | 400 | 253 | 120 | 420 |
| Right Thoracic Tube (cc) | - | 430 | 805 | 850 | 900 | 250 | 300 | 470 | 420 | 418 |
| VAC (cc) | - | 150 | 400 | 500 | 700 | 300 | 350 | 350 | 0 | 500 |
| ECMO Record | ||||||||||
| Sweep Gas Flow (SGF) L/min | - | - | 5,6 | 6,1 | 5,5 | 4 | 6,96 | 8 | 8,25 | 8 |
| ECMO Flow (L/min) | - | - | 4,5 | 4,0 | 4,0 | 3,6 | 4,8 | 4,8 | 3,6 | 4,0 |
*Day 0: Refers to the day of the trauma, 6 h post-admission to the ICU following damage control surgery
Three hours after VA-ECMO cannulation, the patient exhibited signs of ischemia in the lower right limb, indicated by asymmetric Near-Infrared Spectroscopy (NIRS) readings. He was promptly taken back to the OR. Initially, switch to central VA-ECMO support using a femoral and ascending aorta configuration. Subsequently, a femoral cutdown was performed, involving the removal of arterial cannula, followed by an arterial embolectomy, which restored perfusion to the right lower limb. Finally, the thoracic surgery team performed a right pneumonectomy (Fig. 1C).
During the ICU stay, veno-venous hemofiltration and dual vasopressor support were necessary, along with multiple blood transfusions. The hemodynamic, ventilatory parameters, vasopressor support, drainages and ECMO record during the first eight days of VA-ECMO cannulation are detailed in Table 1. The characteristics of the timeline, surgical procedures and ECMO cannulation configurations are presented in Table 2.
Table 2.
Key Events and Interventions of the patient
| Day | Hour | Event | Procedure Details |
|---|---|---|---|
| 0 | 0 | Damage Control Surgery | |
| 0 | 6 | Admission to ICU after damage control surgery | |
| 1 | 29 | Initiation of peripheral VA-ECMO | Femoro-femoral cannulation (15–25 French) |
| 1 | 33 | Initiation of central VA-ECMO | Aorto-femoral cannulation (17–25 French) |
| Femoral Arterial Embolectomy | |||
| Pneumonectomy | |||
| 7 | Switch to VV-ECMO | Jugulo-femoral cannulation (18–25 French) | |
| Sternal Closure | |||
| 11 | Removal of Right Thoracostomy | ||
| 12 | Percutaneous Tracheostomy | ||
| 61 | Removal of VV-ECMO Support | ||
| 112 | Transfer to Intermediate Care Unit (UCIN) | ||
| 124 | Transfer to General Ward | ||
| 133 | Discharge to Home with Home Care Services |
Sternal closure and successful switch from central VA-ECMO to peripheral veno-venous (VV-ECMO) were achieved on the 7th day, due to cardiac function recovery and to prevent hypoxemia and acute lung or respiratory failure (Fig. 1D). On the 11th day, the right thoracostomy was removed, and on the 12th day, he underwent a percutaneous tracheostomy.
The patient experienced several episodes of bronchial obstruction due to thrombus formation, which were treated with bronchoalveolar lavage. He also developed pneumonia caused by multidrug-resistant Pseudomonas aeruginosa, necessitating antimicrobial therapy. On the 61st day, the patient was successfully weaned off VV-ECMO but required oxygen support with high-flow nasal cannula for three additional days.
Subsequently, he required oxygen support with venturi mask and was transferred to the intermediate care unit on day 112. Then, he was transferred to a general hospitalization ward for complete recovery, resulting in a total of 133 days of in-hospital care. Table 4 describes the timeline of hospitalizations. On day 133, the patient was discharged with home care arrangements.
Discussion and conclusions
This case involves a young male with penetrating trauma who was admitted to the ED hemodynamically unstable. A CT scan revealed the source of bleeding in the right hemithorax. He was taken to the OR for a median sternotomy approach for damage control surgery. There was a major lesion from the right pulmonary hiluim profusely bleeding, necessitating clamping and ligation of the right pulmonary hilum, packing of the thorax, and transfer to the ICU for stabilization before completing pneumonectomy. Subsequent complications from the hilum ligation were managed with dynamic hybrid ECMO support, leading to the patient’s recovery and survival from a fatal injury.
The ligation of the hilium and posterior TP carries a high mortality rate. The postoperative course is also associated with a rate of 72% of serious pulmonary, pleural space and cardiovascular complications [1, 7]. The patient exhibits right ventricular failure and pulmonary edema with severe acute hypoxemia. This pulmonary edema is known as post-pneumonectomy pulmonary edema, described very rarely and associated with mortality rates such as 80 to 100% [9]. VA-ECMO supports lung rest while keeping low mechanical ventilator parameters [10]. Once the right heart adapted, VA-ECMO was no longer needed.
In our experience, the decision to defer pneumonectomy is based on data from hospitals in Cali, which show a significantly reduced mortality rate of less than 40% when pneumonectomy is postponed in unstable patients [11]. This delay allows time to correct hypothermia, acidosis, and coagulopathy, which are crucial for patient survival. At that time, our institution did not have ECMO support available; notably, the only patient who died following pneumonectomy suffered from severe pulmonary hypertension, a condition that could potentially have been managed with ECMO. During the damage control surgery, the patient was exsanguinated and very unstable. Immediate ligation and transfer to the ICU were critical for stabilizing the patient over the next 24 h, allowing for a second operation to complete the pneumonectomy [4]. This approach provided time to improve metabolic homeostasis. Recent meta-analyses indicate that ECMO is beneficial for severely traumatized patients by improving their prognosis and serving as a valuable tool in managing severe trauma-related cardiorespiratory failure, hemorrhagic shock, and cardiac arrest [8].
ECMO support is also associated with numerous complications [9], such as thrombosis or embolia that require blood products to correct coagulopathy. In this patient, lower limb ischemia was present, however it was related to femoral/arterial cannula ratio. Stroke is a major concern; however, we achieved a balance between thrombosis and coagulopathy and had no neurological events. In this instance, an infectious complication arises from ventilator-associated pneumonia (VAP) caused by multidrug resistant Pseudomonas aeruginosa. A dynamic hybrid ECMO configuration was considered, and the patient was converted to VV-ECMO support until the pneumonia resolved. Dynamic configurations in severe cases have been associated with a lower incidence of mortality in high-risk patients [12].
In conclusion, penetrating thoracic injuries with severe pulmonary vascular lesions sometimes necessitate a pneumonectomy. ECMO support presents as an alternative that can reduce the associated mortality by offering support during the adaptation to pathophysiological cardiopulmonary changes. A multidisciplinary team is essential to minimize complications associated with pneumonectomy, ECMO use, and prolonged hospital stays.
Acknowledgements
We extend our sincere gratitude to all co-authors for their dedication and collaborative effort throughout the study. Special thanks are owed to the medical personnel of the Intensive Care Unit and Operating Room at Fundacion Valle del Lili in Cali, Colombia for their invaluable support and expertise, which were instrumental in the management and documentation of this case. We are also deeply appreciative of the support from the Clinical Research Center of Fundación Valle del Lili, whose assistance was pivotal in conducting this study. Our acknowledgment extends to the University Hospital Fundación Valle del Lili for providing the essential resources and environment that facilitated our research. Lastly, our deepest appreciation goes to the patient involved in this case report; their cooperation and consent have been crucial in allowing us to share these significant findings with the wider medical community.
Abbreviations
- TP
Traumatic pneumonectomy
- ECMO
Extracorporeal Membrane Oxygenation
- CT
Computed Tomography
- ICU
Intensive Care Unit
- ARDS
Acute Respiratory Distress Syndrome
- VA
ECMO-Venoarterial Extracorporeal Membrane Oxygenation
- OR
Operating room
- CPB
Cardiopulmonary bypass
- VV
ECMO-Venovenous extracorporeal membrane oxygenation
- VAP
Ventilator-associated pneumonia
Author contributions
This case report was developed through the significant contributions of each author, ensuring the work’s integrity and accuracy from the beginning to the end. AISO and DPG were chiefly responsible for the conception and design of the study, guiding its direction and framework. AMA and ACAO led the data acquisition efforts, gathering and documenting all relevant clinical information comprehensively. The initial draft of the manuscript was prepared by AMA and ACAO, focusing on the case’s significance and findings. The manuscript underwent substantial refinement in clarity, accuracy, and content through the critical reviews provided by AFG, DFBR, NFTE, and MVG. CAGG was responsible for capturing and analyzing the images, adding valuable visual insigh.ts into the case. All authors have reviewed and approved the final manuscript, committing to be accountable for all aspects of the work, particularly in addressing any questions related to its accuracy or integrity.
Funding
Funded by Fundación Valle del Lili.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Informed consent was obtained from the patient. The study has received approval from the Institutional Ethical Review Board of Fundación Valle del Lili (N°2023.193).
Consent for publication
The consent for publication was obtained from the patient.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Phillips B, Turco L, Mirzaie M, Fernandez C. Trauma pneumonectomy: a narrative review. Int J Surg Lond Engl. 2017;46:71–4. doi: 10.1016/j.ijsu.2017.08.570. [DOI] [PubMed] [Google Scholar]
- 2.Wang FY, Fang B, Yu ZH, Shao JS, Wen WB, Zhou LX. Severe thoracic trauma caused left pneumonectomy complicated by right traumatic wet lung, reversed by extracorporeal membrane oxygenation support—a case report. BMC Pulm Med. 2019;19(1):30. doi: 10.1186/s12890-019-0790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin MJ, McDonald JM, Mullenix PS, Steele SR, Demetriades D. Operative management and outcomes of traumatic lung resection. J Am Coll Surg. 2006;203(3):336–44. doi: 10.1016/j.jamcollsurg.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 4.García A, Millán M, Ordoñez CA, Burbano D, Parra MW, Caicedo Y et al. Cirugía de control de daños del trauma pulmonar. Colomb Médica [Internet]. 2021 Jun [cited 2023 Apr 13];52(2). http://www.scielo.org.co/scielo.php?script=sci_abstract&pid=S1657-95342021000200404&lng=en&nrm=iso&tlng=es
- 5.Baumgartner F, Omari B, Lee J, Bleiweis M, Snyder R, Robertson J, et al. Survival after trauma pneumonectomy: the pathophysiologic balance of shock resuscitation with right heart failure. Am Surg. 1996;62(11):967–72. [PubMed] [Google Scholar]
- 6.Veno-venous. ECMO in ARDS after post-traumatic pneumonectomy | SpringerLink [Internet]. [cited 2023 Oct 24]. https://link.springer.com/article/10.1007/s00134-013-3116-4.
- 7.Fuentes PA. Pneumonectomy: historical perspective and prospective insight. Eur J Cardiothorac Surg. 2003;23(4):439–45. doi: 10.1016/S1010-7940(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Zhang L, Huang X, Ma N, Wang P, Li L, et al. ECMO in adult patients with severe trauma: a systematic review and meta-analysis. Eur J Med Res. 2023;28(1):412. doi: 10.1186/s40001-023-01390-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kazior MR, Streams JR, Dennis BM, King AB, Henson CP, Slinger P, et al. Pulmonary complications after Trauma Pneumonectomy. J Cardiothorac Vasc Anesth. 2020;34(7):1952–61. doi: 10.1053/j.jvca.2020.01.057. [DOI] [PubMed] [Google Scholar]
- 10.Dotiwala A, Kalakoti P, Grier LR, Quispe M, Scott LK, Conrad SA, et al. Penetrating thoracic injury requiring emergency pneumonectomy supported with two ECMO runs: a testament to multidisciplinary critical care medicine. Trauma Case Rep. 2023;44:100779. doi: 10.1016/j.tcr.2023.100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia A, Martinez J, Rodriguez J, Millan M, Valderrama G, Ordoñez C, et al. Damage-control techniques in the management of severe lung trauma. J Trauma Acute Care Surg. 2015;78(1):45–50. doi: 10.1097/TA.0000000000000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C, Xie H, Li J, Qin B, Lu J, Zhang J, et al. Dynamic and hybrid configurations for extracorporeal membrane oxygenation: an analysis of the Chinese extracorporeal Life Support Registry. ASAIO J Am Soc Artif Intern Organs 1992. 2022;68(4):547–52. doi: 10.1097/MAT.0000000000001535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.