Abstract
The ability of rabbit hemorrhagic disease virus to agglutinate human erythrocytes and to attach to rabbit epithelial cells of the upper respiratory and digestive tracts was shown to depend on the presence of ABH blood group antigens. Indeed, agglutination was inhibited by saliva from secretor individuals but not from nonsecretors, the latter being devoid of H antigen. In addition, erythrocytes of the rare Bombay phenotype, which completely lack ABH antigens, were not agglutinated. Native viral particles from extracts of infected rabbit liver as well as virus-like particles from the recombinant virus capsid protein specifically bound to synthetic A and H type 2 blood group oligosaccharides. Both types of particles could attach to adult rabbit epithelial cells of the upper respiratory and digestive tracts. This binding paralleled that of anti-H type 2 blood group reagents and was inhibited by the H type 2-specific lectin UEA-I and polyacrylamide-conjugated H type 2 trisaccharide. Young rabbit tissues were almost devoid of A and H type 2 antigens, and only very weak binding of virus particles could be obtained on these tissues.
Rabbit hemorragic disease virus (RHDV) is a noncultivable calicivirus that infects rabbits and causes epidemics of an acute fatal hepatitis. The disease is characterized by high morbidity and mortality rates for adult animals. Death is the result of a widespread circulation dysfunction associated with disseminated intravascular coagulation and necrotizing hepatitis lesions (14, 24). Large quantities of virus particles are found in several organs, especially the liver, which is considered the major site of virus replication (6, 14, 19, 27). The viral genome consists of a single-stranded RNA of nearly 7.5 kb, packaged in a small icosahedral capsid (3, 15). The capsid protein has an estimated molecular mass of 60 kDa (VP60) (16), and expression of the corresponding cDNA in insect cells infected with a recombinant baculovirus yields a protein that spontaneously assembles into virus-like particles (VLPs). These VLPs are both antigenically and morphologically similar to native RHDV particles (11, 23). Yet very little is known about the pathogenesis of naturally occurring RHDV infections, and identification of the cellular receptor(s) used by the virus to establish infection would lead to a better understanding of the pathogenesis of RHDV.
RHDV is known to agglutinate human erythrocytes (2, 25), and previous studies demonstrated that its hemagglutinin receptor on human red blood cells corresponds to a developmental antigen which is not expressed on fetal cells and is mainly carried by polyglycosylceramides (26). The glycolipid nature of the receptor on human red blood cells suggests that the carbohydrate moiety could be recognized by the virus capsid protein. Carbohydrate antigens of the histo-blood group family are developmental antigens that can be shared among various mammal species, and the presence of some of these antigens has been detected on epithelial cells of the rabbit digestive tract (1, 17, 21). In the present study, we first tested the ability of the virus to use carbohydrate blood group antigens for hemagglutination of human erythrocytes. The presence of such antigens on epithelial cells of the higher respiratory and digestive tracts, likely entry doors for the virus, was then correlated with the ability of RHDV particles or VLPs to attach to these cells.
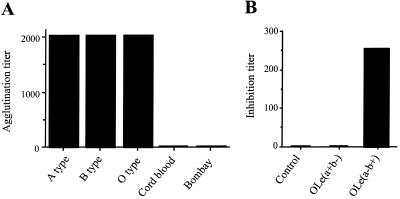
RHDV hemagglutinating activity depends on the presence of ABH blood group antigens. RHDV agglutinates human red blood cells but not erythrocytes from rabbits or other mammals (2, 7). A distinctive characteristic of human erythrocytes is the presence of ABH antigens. Those from other mammals are devoid of such antigens (21). This prompted us to test the hemagglutinating activity of RHDV on human red blood cells, which have either low or no expression of ABH antigens. To this end, the liver of one adult New Zealand rabbit dead after an experimental infection with RHDV strain VHD L4/90-10 (kindly supplied by IFFA Laboratory, Lyon, France) was used as a source of the virus and prepared as previously described (26). A liver extract from a noninfected rabbit was used as a negative control. Human red blood cells, phenotyped for ABH and Lewis antigens, and saliva were obtained from the Blood Transfusion Center (Nantes, France). The hemagglutination assay was carried out in microtitration plates with V-bottomed wells with serial dilutions from 12.5% (wt/vol) liver suspensions as previously described (26). As shown in Fig. 1A, the virus-containing liver preparation strongly agglutinated human adult red blood cells irrespective of their ABO phenotype. However, cord blood cells, which present only small amounts of ABH epitopes compared to adults cells (4), as well as erythrocytes of the rare Bombay phenotype, which are completely devoid of such epitopes, were not agglutinated at all. Bombay individuals lack ABH epitopes because of inactivating mutations in the gene (FUT1) encoding the α1,2-fucosyltransferase responsible for the synthesis of the H antigen on erythrocytes (8, 12). To confirm that the presence of the H antigen was required for agglutination to occur, a hemagglutination inhibition test was performed as previously described (26), using saliva from O blood group individuals of either the secretor [O,Le(a−, b+)] or the nonsecretor [O,Le(a+, b−)] phenotype. The former possess large amounts of H antigen in their saliva, of which the latter are devoid. This absence of antigen in the saliva of about 20% of Europeans is due to inactivating mutations in the FUT2 gene, which encodes an α1,2-fucosyltransferase responsible for the synthesis of the H antigen in saliva (9, 12). As depicted in Fig. 1B, the H antigen-containing saliva from a secretor strongly abolished agglutination, whereas saliva from a nonsecretor did not.
FIG. 1.
(A) Agglutination of human erythrocytes from cord blood or from adults of A, B, and O and Oh (Bombay) phenotypes by an RHDV liver extract. Cord blood and Bombay erythrocytes have small amounts of ABH antigens and no ABH antigens, respectively. Hemagglutination assay titers were defined as the reciprocal of the last serial two-fold dilutions that gave detectable agglutination. (B) Inhibition of agglutination of adult blood group O erythrocytes by saliva from a nonsecretor [OLe(a+b−)] or a secretor [OLe(a−b+)]. As a control, PBS was used in place of saliva. Inhibition titers correspond to the reciprocal of the last dilution that completely inhibited agglutination.
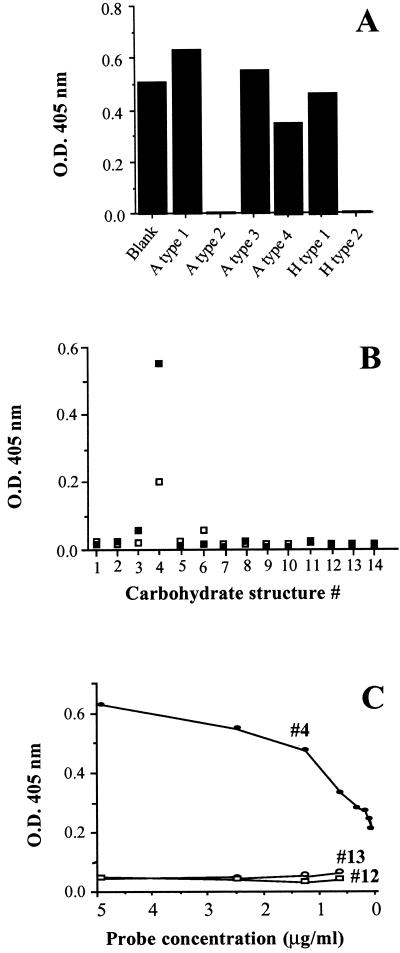
RHDV binds to A and H type 2 antigens. In order to define more precisely the specificity of the virus for antigens of the ABH family, liver extracts containing virus particles were incubated on a set of immobilized synthetic oligosaccharides. These oligosaccharides, coupled to a silica solid support (SYNSORB), were obtained from Chembiomed Ltd. and from R. U. Lemieux (Edmonton, Alberta, Canada). A total of 100 μl of a 1/50 dilution (in phosphate-buffered saline [PBS]) of the viral suspension (25% [wt/vol]) were incubated for 1 h at 37°C under gentle agitation on 10 mg of wet SYNSORB. After adsorption, supernatants were recovered and tested for the presence of viral particles by capture enzyme-linked immunosorbent assay (ELISA). For this experiment, Nunc immunoplates were coated with purified chicken anti-RHDV (AFSSA, Ploufragan, France) by an overnight incubation at 37°C in a wet atmosphere. After a blocking step, absorbed RHDV liver extracts were incubated for 1 h at 37°C. RHDV binding was detected using an anti-VP60 monoclonal antibody (MAb)(10C5; data not shown), followed by alkaline phosphatase-conjugated anti-mouse antibody (Sigma, St. Louis, Mo.). Reactions were revealed using p-nitrophenyl phosphate (Sigma) as a substrate (structures of the carbohydrates used are given in Table 1). Of the six oligosaccharides tested, two completely adsorbed the viral reactivity (Fig. 2A). They correspond to the A and H type 2 antigens. Closely related structures did not absorb any reactivity. The ability of the virus or of VLPs to bind to oligosaccharides of the blood group antigen family was then tested by direct ELISA. Neoglycoconjugate probes containing a synthetic oligosaccharide linked to polyacrylamide (PAA) via a spacer arm were obtained from Syntesome (Munich, Germany). ELISA plates were coated with PAA neoglycoconjugates at 10 μg/ml in PBS overnight at 37°C in a humid chamber. Then the RHDV liver suspension (a 1/10 dilution of suspension at 25% [wt/vol]) or VLPs at 1 μg/ml were added and incubated for 1 h at 37°C. VLPs which had spontaneously assembled from recombinant capsid protein VP60 were kindly supplied by D. Rasschaert (Institut National de la Recherche Agronomique, Nouzilly, France) and prepared as previously described (11). Attachment of either virus particles from the liver extract or purified VLPs was detected using the anti-VP60 MAb 10C5 as described above. Figure 2B and C show that a significant dose-dependent binding of both the native virus and the VLPs was detected on the H type 2 trisaccharide exclusively.
TABLE 1.
Structures of carbohydrates used in this studya
| Name | Identification no. | Structure |
|---|---|---|
| N-Acetyllactosamine | 1 | Galβ1-4GlcNAcβ1-R1 |
| H-disaccharide | 2 | Fucα1-2Galβ1-R1 |
| H type 1 | 3 | Fucα1-2Galβ1-3GlcNAcβ1-R1,2 |
| H type 2 | 4 | Fucα1-2Galβ1-4GlcNAcβ1-R1,2 |
| A-disaccharide | 5 | GalNAcα1-3Galβ1-R1 |
| A-trisaccharide | 6 | GalNAcα1-3(Fucα1-2)Galβ1-R1 |
| Sialyl-Lea | 7 | NeuAcα2-3Galβ(Fucα1-4)1-3GlcNAcβ1-R1 |
| Sialyl-Lex | 8 | NeuAcα2-3Galβ(Fucα1-3)1-4GlcNAcβ1-R1 |
| 3 sialyllactose | 9 | NeuAcα2-3Galβ1-4Glcβ1-R1 |
| 6 sialyllactose | 10 | NeuAcα2-6Galβ1-4Glcβ1-R1 |
| Le-disaccharide | 11 | Fucα1-4GlcNAcβ1-R1 |
| Leb | 12 | Fucα1-2Galβ1-3(Fucα1-4)GlcNAcβ1-R1 |
| Ley | 13 | Fucα1-2Galβ1-4(Fucα1-3)GlcNAcβ1-R1 |
| Lea | 14 | Galβ1-3(Fucα1-4)GlcNAcβ1-R1 |
| Lex | 15 | Galβ1-4(Fucα1-3)GlcNAcβ1-R1 |
| A type 1 | 16 | GalNAcα1-3(Fucα1-2)Galβ1-3GlcNAcβ1-R2 |
| A type 2 | 17 | GalNAcα1-3(Fucα1-2)Galβ1-4GlcNAcβ1-R2 |
| A type 3 | 18 | GalNAcα1-3(Fucα1-2)Galβ1-3GalNAcα1-R2 |
| A type 4 | 19 | GalNAcα1-3(Fucα1-2)Galβ1-3GalNAcβ1-R2 |
R1,2, linkage through an aliphatic chain to polyacrylamide (R1) or silica beads (R2).
FIG. 2.
Adsorption of native RHDV on immobilized blood group-active oligosaccharides. (A) After incubation of an RHDV liver extract on oligosaccharide-conjugated beads (structures 3, 4, 16, 17, 18, and 19 as given in Table 1), the presence of virus particles was detected using a capture ELISA. (B) Direct binding of native RHDV particles from a liver extract (open symbols) or of VLPs (closed symbols) on a series of blood group-related oligosaccharides conjugated to polyacrylamide (structures 1 through 14). Binding was revealed by an ELISA using the anti-VP60 MAb 10C5. (C) Binding of VLPs to decreasing amounts of the H type 2 (#4), the Leb (#12), and the Ley (#13) polyacrylamide probes, measured by an ELISA using MAb 10C5. O. D. 405 nm, optical density at 405 nm.
ABH antigens are built up by sequential addition of monosaccharide units on precursor structures carried by either glycolipids or glycoproteins. Four main precursor types are known which have in common a terminal galactose in β linkage to the subterminal sugar. They differ by this subterminal sugar, either an N-acetylglucosamine or an N-acetylgalactosamine, and by its linkage, either α or β, to core glycans or peptidic chains. Addition of a fucose in α1,2 to the galactose residue of the precursors yields the H antigens. The A and B antigens can then be formed by addition of either an N-acetylgalactosamine or a galactose in α1,3 linkage to the galactose of the H antigens (20). The results presented above clearly show that native RHDV and VLPs recognize the A and H type 2 blood group antigens. Thus, the capsid protein VP60 behaves like a lectin. Its carbohydrate specificity is quite similar to that of the plant lectin UEA-I in that it is restricted to antigens based on type 2 precursor and requires the presence of an α1,2-linked fucose. However, at variance with this plant lectin, its binding site is not masked after the H type 2 trisaccharide is replaced by the N-acetylgalactosamine that yields the A type 2 structure. This would explain why human A-type red blood cells are agglutinated as efficiently as O-type cells. Although it could not be directly tested, recognition of the B type 2 antigen is likely, since the virus also strongly agglutinates human erythrocytes with a B phenotype.
RHDV binds to histo-blood group antigens of rabbit epithelial cells. ABH histo-blood group antigen expression is not restricted to erythrocytes. Instead, they are widely distributed among tissue types. They have been found on epithelial cells of the digestive tracts of all terrestrial vertebrates tested. However, their appearance on red blood cells seems to be a recent event in phylogenetic terms, since expression on this cell type is restricted to anthropoid apes. We therefore tested whether RHDV could bind to tissues from two young (6 weeks old) and two adult rabbits, fixed in 95% ethanol and paraffin embedded. Sections were incubated with 200 μl of either RHDV or control liver extracts diluted 1/10 or with VLPs at 5 μg/ml. After washings in PBS, RHDV or VLP binding was detected using MAb 10C5 followed by biotinylated secondary antibody (Vector Labs, Burlingame, Calif.) and peroxidase-conjugated avidin (Vector). Reactions were revealed with 3-amino-9-ethylcarbazole. Counterstaining was performed with Harris hematoxylin. The presence of histo-blood group antigens on these tissues was determined using the anti-H MAb 7 E11, specific for H type 2 determinants (data not shown), and the anti-A blood group antigen 2A-1#8, specific for all types of A structures: A types 1, 2, 3, 4, ALeb, and ALey (13). Their binding was detected as described above for antibody 10C5. The presence of H type 2 antigen was also detected using peroxidase-labeled UEA-I lectin (Sigma) at 1 μg/ml. No binding of either native RHDV or VLPs was observed on liver, kidney, heart, or spleen. In contrast, a clear labeling of epithelial cells was detected in the trachea, large bronchi of the lung, concha nasalis, tonsils, or small intestine. Strikingly, a parallel labeling was observed with the anti-H MAb 7 E11 and with peroxidase-labeled UEA-I lectin in all these tissues. A parallel labeling was also observed using the anti-A MAb, with the exception of biliary ducts, which were weakly stained (Table 2). Since both MAb 7 E11 and UEA-I specifically recognize the H type 2 antigen, the parallel labeling suggested that RHDV and VLPs could bind to epithelial cells via this antigen. To test this possibility, competitions of RHDV and of VLPs binding on the epithelial cells of the trachea were carried out using unlabeled UEA-I or PAA neoglycoconjugates. To this end, RHDV liver extracts or VLPs were coincubated with either unconjugated UEA-I lectin at 40 μg/ml or PAA neoglycoconjugates at 50 μg/ml. Binding was revealed as described above using MAb 10C5. A near-complete inhibition of the virus binding was obtained by coincubation with UEA-I. Similarly, attachment of VLPs was almost completely inhibited by the H type 2 neoglycoconjugate but not by the Lex neoglycoconjugate used as a control (Table 3 and Fig. 3A, B, C and D). Taken together, these results indicate that binding of native RHDV or VLPs to rabbit epithelial cells depends on the recognition of A or H type 2 antigens.
TABLE 2.
Binding of RHDV and the UEA-I lectin to rabbit tissuesa
| Antibody or virus | Labeling intensity for:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Trachea | Lungb | Concha nasalis | Tonsils | Small intestine | Liverc | Spleen | Kidney | Muscle | |
| Control | − | − | − | − | − | − | − | − | − |
| RHDV | ++++ | ++ | ++++ | +++ | +++ | − | − | − | − |
| Anti-A | ++++ | ++ | ++++ | ++++ | ++++ | + | − | − | − |
| Anti-H | +++ | + | +++ | ++ | +++/− | − | − | − | − |
| UEA-I | ++++ | ++ | ++++ | +++ | +++/− | − | − | − | − |
Tissue sections were incubated with either an RHDV liver preparation, MAb 2A#8 (anti-A) or 7 E11 (anti-H), or peroxidase-labeled UEA-I. Binding of the MAbs was detected using a biotin–avidin-peroxidase method. Binding of the virus was detected using the anti-VP60 MAb 10C5. Controls for specificity were performed by incubating a liver extract from an uninfected rabbit followed by 10C5 (control). Intensity of labeling was scored from very strong (++++) to completely negative (−). +++/−, areas of heterogeneous labeling. Only extremely weak binding of RHDV could be observed on human stomach and small intestine. Labeling was always restricted to epithelial cells.
Labeling was restricted to large bronchi.
Labeling was restricted to biliary ducts.
TABLE 3.
Histochemical labeling of young (6-week-old) and adult rabbit tracheaea
| Antibody or virus | Labeling intensity for:
|
|
|---|---|---|
| Adult trachea | Young trachea | |
| Anti-A | ++++ | − |
| Anti-H | +++ | + |
| UEA-I | ++++ | ++ |
| RHDV | ++++ | + |
| VLPs | +++ | ± |
| RHDV + UEA-I | ± | ND |
| VLP + H type 2 | ± | ND |
| VLP + Lex | ++++ | ND |
Intensity of labeling was scored from very strong (++++) to completely negative (−). ±, weak labeling. Binding of the anti-A and anti-H MAbs was revealed using a biotin–avidin-peroxidase method. Binding of UEA-I was revealed using a peroxidase-labeled lectin. Binding of RHDV and VLPs was revealed using the anti-VP60 MAb (10C5) and the biotin–avidin-peroxidase method. Inhibition of RHDV binding was tested by coincubation with unlabeled UEA-I lectin at 40 μg/ml. Inhibition of VLPs binding was tested by coincubation with polyacrylamide-conjugated H type 2 and Lex trisaccharides at 50 μg/ml. ND, not determined.
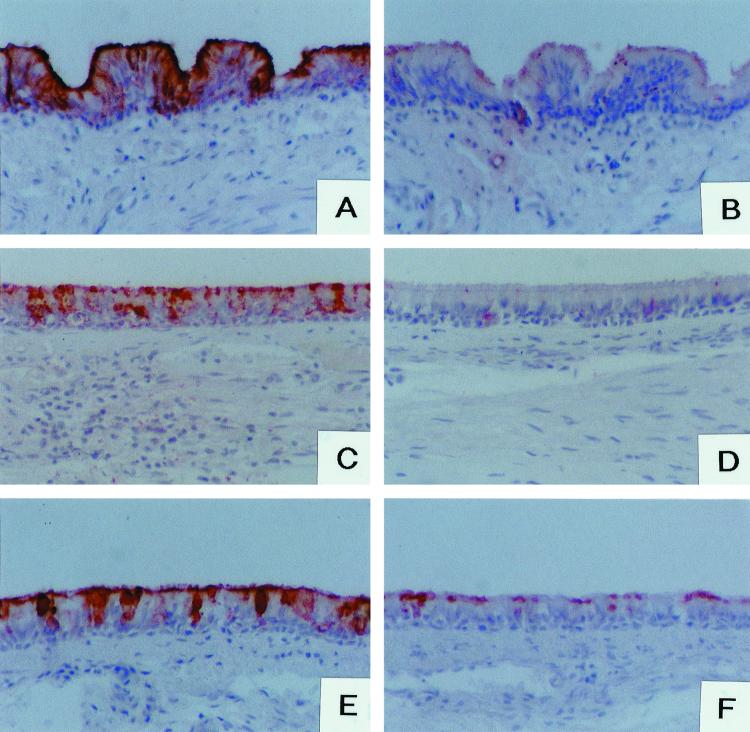
FIG. 3.
Histochemical staining of rabbit tracheae. Adult rabbit trachea sections (A through D) were incubated with either native RHDV particles from liver extract (A and B) or VLPs (C and D). RHDV liver extract or VLPs were coincubated with either the UEA-I lectin (B), Lex-polyacrylamide conjugate structure 15 (C), or the H type 2-polyacrylamide conjugate (D). Binding of peroxidase-labeled UEA-I lectin to epithelial cells from adult and 6-week-old rabbits is shown in panels E and F, respectively.
Absence of RHDV binding to young rabbit epithelial cells correlates with low expression of A and H antigens. Under natural conditions, adult rabbits are highly susceptible to infection. However, young animals are not, and susceptibility progressively increases from 1 to 3 months of age (18, 27). We therefore tested whether native RHDV or VLPs would bind to young rabbit epithelial cells as strongly as they did to adult epithelial cells. As shown in Table 3, it was observed that almost no binding was detectable on the tracheae of 6-week-old rabbits. Likewise, epithelial cells from these young animals did not express detectable amounts of A histo-blood group antigen and expressed much smaller amounts of H type 2 antigen as deduced from the weak labeling given by MAb 7 E11 and UEA-I (Table 3 and Fig. 3E and F).
In order to determine when the A and H antigens appear on rabbit epithelial cells, their expression was tested weekly by immunofluorescence on buccal epithelial cells. These cells from four rabbits aged from 3 to 12 weeks and from four adults were collected using cotton swabs. After recovery in PBS, cells were labeled with the anti-A MAb diluted 1/2 followed by fluorescein isothiocyanate (FITC)-labeled goat anti-mouse immunoglobulins (Sigma) or with FITC-conjugated UEA-I at 20 μg/ml. No histo-blood group A antigen expression was detected until the eighth week, when it appeared on a subset of cells. Expression on young rabbit cells increased until 12 weeks, when it was almost as high as on cells from adults. In contrast, the H antigen was detected from the third week on. However, the staining of cells from the younger animals was clearly weaker than that of cells from adults, with full expression being reached at 10 weeks.
The mechanism by which RHDV infects rabbits is unknown at present. We were unable to detect attachment of either native or recombinant virus particles to liver sections. Yet large numbers of viral particles can be isolated from the livers of infected animals, and their presence within hepatocytes has been detected by immunostaining and in situ hybridization (5, 22). Moreover, a recent report describes in vitro infection of rabbit hepatocytes by RHDV (10). Yet ABH antigens were not detected on rabbit hepatocytes, a situation similar to that found in humans. It is thus unlikely that RHDV uses histo-blood group antigens as receptors on hepatocytes. Nevertheless, upper respiratory and digestive tract epithelial cells are likely the first to encounter virus particles at the time of infection. These cells could be a primary site of viral replication. This is in agreement with the fact that tracheitis is a frequent early sign of the disease (6, 24, 27). We were able to observe that both native and recombinant virus particles can attach to them through recognition of A or H type 2 antigens. In addition, very little binding was observed on tracheae from young animals, which turned out to express only small amounts of the antigens compared to adults. This correlates with the very low infectivity of RHDV in young rabbits. These observations suggest, yet do not prove, that the histo-blood group-specific lectin activity of RHDV could participate in the infectious process, and these observations warrant further study.
Acknowledgments
We are grateful to D. Rasschaert for his generous gift of purified VLPs and to J. Rocher for excellent technical assistance. We are also grateful to Y. Petit-Le Roux for skillful assistance in the preparation of MAbs.
This work was supported by INSERM and ENVN.
REFERENCES
- 1.Breimer M E, Hansson G C, Karlsson K A, Leffler H, Pimlot W, Samuelsson B E. Selected ion monitoring of glycosphingolipid mixtures. Identification of several blood group type glycolipids in the small intestine of an individual rabbit. Biomed Mass Spectrom. 1979;6:231–241. doi: 10.1002/bms.1200060603. [DOI] [PubMed] [Google Scholar]
- 2.Capucci L, Scicluna M T, Lavazza A. Diagnosis of viral haemorrhagic disease of rabbits and the European brown hare syndrome. Rev Sci Tech Off Int Epizoot. 1991;10:347–370. doi: 10.20506/rst.10.2.561. [DOI] [PubMed] [Google Scholar]
- 3.Clarke I N, Lambden P R. The molecular biology of caliciviruses. J Gen Virol. 1997;78:291–301. doi: 10.1099/0022-1317-78-2-291. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda M N, Levery S B. Glycolipids of fetal, newborn and adult erythrocytes: glycolipid pattern and structural study of H3-glycolipid from newborn erythrocytes. Biochemistry. 1983;22:5034–5040. doi: 10.1021/bi00290a024. [DOI] [PubMed] [Google Scholar]
- 5.Gelmetti D, Griecon V, Rossi C, Capucci L, Lavazza A. Detection of rabbit haemorrhagic disease virus (RHDV) by in situ hybridization with a digoxigenin labelled RNA probe. J Virol Methods. 1998;72:219–226. doi: 10.1016/S0166-0934(98)00030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guittré C, Ruvoën-Clouet N, Barraud L, Cherel Y, Baginski I, Prave M, Ganière J P, Trépo C, Cova L. Early stages of rabbit haemorrhagic disease virus infection monitored by polymerase chain infection. Zentbl Vetmed Reihe B. 1996;43:109–118. doi: 10.1111/j.1439-0450.1996.tb00294.x. [DOI] [PubMed] [Google Scholar]
- 7.Hanchun Y, Xu W Y, Du N. Hemagglutination characteristics of rabbit haemorrhagic disease virus. 1991. p. 67. . Proceedings of the International Symposium on Rabbit Haemorrhagic Disease. Beijing, People's Republic of China. Chinese Association of Animal and Veterinary Sciences, Beijing, China. [Google Scholar]
- 8.Kelly R J, Ernst L K, Larsen R D, Bryant J G, Robinson J S, Lowe J B. Molecular basis for H blood group deficiency in Bombay (Oh) and para-Bombay individuals. Proc Natl Acad Sci USA. 1994;91:5843–5847. doi: 10.1073/pnas.91.13.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly R J, Rouquier S, Giorgi D, Lennon G G, Lowe J B. Sequence and expression of a candidate for the human secretor blood group α(1,2) fucosyltransferase gene (FUT2) J Biol Chem. 1995;270:4640–4649. doi: 10.1074/jbc.270.9.4640. [DOI] [PubMed] [Google Scholar]
- 10.König M, Thiel H J, Meyers G. Detection of viral proteins after infection of cultured hepatocytes with rabbit hemorrhagic disease virus. J Virol. 1998;72:4492–4497. doi: 10.1128/jvi.72.5.4492-4497.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurent S, Vautherot J-F, Madelaine M-F, Le Gall G, Rasschaert D. Recombinant rabbit hemorrhagic disease virus capsid protein expressed in baculovirus self-assembles into viruslike particles and induces protection. J Virol. 1994;68:6794–6798. doi: 10.1128/jvi.68.10.6794-6798.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Pendu J, Cartron J P, Lemieux R U, Oriol R. The presence of at least two different H blood group-related β-d-gal α-2-l-fucosyltransferases in human serum and genetics of blood group H substances. Am J Hum Genet. 1985;37:749–760. [PMC free article] [PubMed] [Google Scholar]
- 13.Le Pendu J, Le Cabellec M, Bara J. Immunohistochemical analysis of antibodies against ABH and other glycoconjugates in normal human pyloric and duodenal mucosae. Trans Clin Biol. 1997;1:41–46. doi: 10.1016/s1246-7820(97)80009-2. [DOI] [PubMed] [Google Scholar]
- 14.Marcato P S, Benazzi C, Vecchi G, Galeotti M, Salda L D, Sarli G, Lucidi P. Clinical and pathological features of viral haemorrhagic disease of rabbits and the European brown hare syndrome. Rev Sci Tech Off Int Epizoot. 1991;10:371–392. doi: 10.20506/rst.10.2.560. [DOI] [PubMed] [Google Scholar]
- 15.Meyers G, Wirblich C, Thiel H J. Rabbit hemorrhagic disease virus. Molecular cloning and nucleotide sequencing of a calicivirus genome. Virology. 1991;184:664–676. doi: 10.1016/0042-6822(91)90436-f. [DOI] [PubMed] [Google Scholar]
- 16.Meyers G, Wirblich C, Thiel H J. Genomic and subgenomic RNAs of rabbit hemorrhagic disease virus are both protein-linked and packaged into particles. Virology. 1991;184:677–686. doi: 10.1016/0042-6822(91)90437-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller-Podraza H, Stenhagen G, Larsson T, Andersson C, Karlsson K A. Screening for the presence of polyglycosylceramides in various tissues: partial characterization of blood group-active complex glycosphingolipids of rabbit and dog small intestines. Glycoconj J. 1997;14:231–239. doi: 10.1023/a:1018545922728. [DOI] [PubMed] [Google Scholar]
- 18.Morisse J P, Le Gall G, Boilletot E. Hepatitis of viral origin in leporidae. Introduction and aetiological hypotheses. Rev Sci Tech Off Int Epizoot. 1991;10:283–297. [PubMed] [Google Scholar]
- 19.Moussa A, Chasey D, Lavazza A, Capucci L, Smid B, Meyer G, Rossi C, Thiel H G, Vlasak R, Ronsholt L, Novotny N, McCullough K, Gavier-Widen D. Haemorrhagic disease of lagomorphs. Evidence for a calicivirus. Vet Microbiol. 1992;33:375–381. doi: 10.1016/0378-1135(92)90065-2. [DOI] [PubMed] [Google Scholar]
- 20.Oriol R, Le Pendu J, Mollicone R. Genetics of ABO, H, Lewis, X and related antigens. Vox Sang. 1986;51:161–171. doi: 10.1111/j.1423-0410.1986.tb01946.x. [DOI] [PubMed] [Google Scholar]
- 21.Oriol R, Mollicone R, Coullin P, Dalix A M, Candelier J J. Genetic regulation of the expression of ABH and Lewis antigens in tissues. APMIS. 1992;100:28–38. [PubMed] [Google Scholar]
- 22.Park J H, Ochiai K, Itakura C. Detection of rabbit haemorrhagic disease virus particles in the rabbit liver tissues. J Comp Pathol. 1992;107:329–340. doi: 10.1016/0021-9975(92)90008-i. [DOI] [PubMed] [Google Scholar]
- 23.Plana-Duran J, Bastons M, Rodriguez M J, Climent I, Cortés E, Vela C, Casal I. Oral immunization of rabbits with VP60 particles confers protection against rabbit haemorrhagic disease. Arch Virol. 1996;141:1423–1436. doi: 10.1007/BF01718245. [DOI] [PubMed] [Google Scholar]
- 24.Plassiart G, Guelfi J F, Ganière J P, Wang B, André-Fontaine G, Wyers M. Hematological parameters and visceral lesions relationships in rabbit viral haemorrhagic disease. J Vet Med Ser B. 1992;39:443–453. doi: 10.1111/j.1439-0450.1992.tb01192.x. [DOI] [PubMed] [Google Scholar]
- 25.Pu B Q, Qian N J, Cui S J. HA and HI tests for the detection of antibody titers to so called “haemorrhagic pneumonia” in rabbits. Chin J Vet Med. 1985;11:16–17. [Google Scholar]
- 26.Ruvoën-Clouet N, Blanchard D, André-Fontaine G, Ganière J P. Partial characterization of the human erythrocyte receptor for rabbit haemorrhagic disease virus. Res Virol. 1995;146:33–41. doi: 10.1016/0923-2516(96)80587-5. [DOI] [PubMed] [Google Scholar]
- 27.Xu Z J, Chen W X. Viral haemorrhagic disease in rabbits: a review. Vet Res Commun. 1989;13:205–212. doi: 10.1007/BF00142046. [DOI] [PubMed] [Google Scholar]