Abstract
Background
A global increase in cannabis use has led to questions about its effects on fertility. The rise in consumption amongst women of reproductive age is a growing concern, as this group is vulnerable in terms of reproductive health. Ample evidence suggests that the psychoactive component of cannabis, Δ9-Tetrahydrocannabinol (THC), interacts with the endocannabinoid system (ECS), that helps regulate mammalian reproduction. This study aimed to research the epigenetic effects of THC in bovine granulosa cells (GCs) by (1) investigating global DNA methylation via measuring 5-mC and 5-hmC levels; (2) measuring key methylation regulators, including the methylating enzymes DNMT1, DNMT3a, DNMT3b and the demethylases TDG and TET1/2/3; and (3) assessing fertility-associated miRNAs key in developmental competency, including miR-21, -155, -33b, -324 and -346.
Methods
Bovine GCs were used as a translational model for reproductive toxicity in humans. To determine THC effects, GCs were isolated from Cumulus-Oocyte-Complexes (COCs) from bovine ovaries, cultured in vitro for 7 days, or until confluent, and cryopreserved at passage 1 (P1). For experimentation, cells were thawed, cultured until passage 2 (P2), serum restricted for 24-h and treated for 24-h in one of five groups: control, vehicle (1:1:18 ethanol: tween: saline) and three clinically relevant THC doses (0.032, 0.32 and 3.2 μM). Global methylation was assessed by measuring 5-mC and 5-hmC levels with flow cytometry. To assess mRNA and protein expression of methylation regulators and miRNA profiles, qPCR and Western Blotting were utilized. Shapiro-Wilk test was used to determine normality within datasets. One-way ANOVA was applied to determine statistical significance using GraphPad Prism 6.0.0.
Results
Results indicate a significant decrease (p = 0.0435) in 5-mC levels following low THC exposure, while no changes were observed in 5-hmC levels. A significant increase in DNMT1 following high THC exposure at the RNA level (p < 0.05) and a significant increase following low THC exposure at the protein level (p = 0.0048) were also observed. No significant differences were observed in DNMT3a/3b, TDG, TET1/2/3 mRNAs or in any of the miRNAs analyzed.
Conclusions
This research suggests that THC mainly affects DNA methylation, but not miRNA profiles, ultimately altering gene expression and likely impairing oocyte competence, maturation, and fertilization potential.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40360-024-00763-5.
Keywords: Cannabis, THC, Granulosa cells, Fertility, DNA methylation, MicroRNAs
Background
Infertility affects approximately 1 in 6 adults, or about 17.5% of the population worldwide [1] and it is clinically defined as the inability to achieve pregnancy after 12 months of unprotected sexual intercourse. Approximately 37% of infertility cases have been linked to female, 29% to male, and 18% to both reproductive systems [2], while over 25% of cases have no known cause [3, 4]. Causes for female infertility include ovulatory, tubal, uterine or hormone disorders [4, 5]. Factors contributing to ovulatory disorders include polycystic ovary syndrome (PCOS), endocrine disorders, maternal age, lifestyle and environmental factors [6, 7]. Couples’ experiencing infertility often resort to Assisted Reproductive Technology (ART) leading to 3.2 million ART cycles performed annually at a 10% annual growth rate, and more than 9 million babies born using this technology [1, 8]. Still, improvements are needed, as the success rate for each cycle is only approximately 25% [9]. With the decline in global fertility, and the overall low ART success rates, there is a growing interest in identifying potential environmental and social factors contributing to fertility disorders [2].
The effects of recreational drugs and alcohol use on health and reproduction has been linked to several human disorders [2, 10]. Cannabis is now one of the most accepted and widely used drugs in the Western world [11–15]. The perceived risk of cannabis has declined with its increased acceptance [16], leading to aclimb in cannabis consumption. Furthermore, a study in Colorado found 70% of dispensaries recommended cannabis use to pregnant women for treating nausea, while 36% stated using cannabis was safe during pregnancy [17]. With a large support from the public and media attention, it is not surprising that The National Institute on Drug Abuse found that cannabis use among young adults (ages 19–30) has risen by 13.2% from 2011 to 2021 [18]. In Canada, the percentage of young adults reporting cannabis use in 2021 was 37% and 49% between the ages of 16–19 and 20–24, respectively [16]. The majority of Canadian users (>90%) also reported cannabis having no effect or being beneficial in their social lives, personal relationships, mental and physical health, home life, school and work performance [16] showing that cannabis users mainly have positive attitudes when evaluating the effects of cannabis on their health and wellness. Likewise, Canadian youth have documented multiple reasons for using cannabis, including social anxiety, identity formation, social acceptability, perceived acceptability, and the lower perceived risk when compared to other substances [19]. In addition to the higher frequency of cannabis consumption, Δ9-Tetrahydrocannabinol (THC) potency in cannabis has also drastically increased from 4% in 1995 [11] to 20.5% in 2022 [20, 21]. Likewise, the ratio of THC to cannabidiol (CBD) has drastically risen over the last 20 years [22, 23]. The rise in THC potency in recreational cannabis products can be attributed to increased customer demand of products containing high THC. It can also be attributed to changes in cultivation practices and technological advances, such as indoor hydroponic cultivation, cross breeding, genetic manipulations and in general, improved access to potent seeds [24].
Recreational cannabis is derived from the plant cannabis sativa, consisting of rich metabolites including phytocannabinoids THC and CBD. Different intake methods and the type of cannabis consumed causes various psychological effects [25]. THC is the main psychoactive component in cannabis and elicits its effects via the endocannabinoid system (ECS), an important modulatory system responsible for maintain homeostasis, regulating energy balance, lipid metabolism, cell growth, immune functions, and reproductive physiology [15, 26–28]. The ECS comprises endocannabinoids (eCBs), such as anandamide (AEA) and 2-arachidonyl glycerol (2-AG), cannabinoid receptors CB1 and CB2, and the enzymes involved in their synthesis, breakdown, and transportation. The CB1 and CB2 receptors are grouped under the transmembrane-spanning G-protein coupled family of receptors [25]. Their activation results in downstream cellular physiological changes [25, 27, 29–33], such as lowered cAMP and AC levels, initiation of mitogen-activated protein (MAP) kinases, and inhibition of calcium channels [27, 32, 34–36].
THC can directly modulate the ECS, which has been detected throughout the female reproductive system including the placenta, uterus, endometrium, ovary, embryo, oocyte, follicular fluid and granulosa cells (GCs) [15, 37–40]. The ECS controls various aspects of reproduction, including the release of gonadotropins, steroid hormone synthesis, the production and release of male and female gametes, and pregnancy [15, 41]. The primary receptor involved in the cellular response to THC, CB1, has been detected in the ovary, oviduct, uterus and placenta [41]. The regulation of eCBs is important to reproductive success, thus, enhanced ECS signalling by exogenous cannabinoids may impair fertility [27]. This is especially concerning with the rise in young adults and expecting mothers using cannabis [42]. It is therefore critical to further study the potential effects of cannabis on oocyte competency, maturation, and pre-implantation embryonic development [43, 44].
More recently, experts have questioned how THC exposure to gametes could impair fertility. Although some research has identified no conclusive link between cannabis use and female reproductive health [45, 46], some studies have found effects to placental formation [47–49], altered LH and FHS levels [50, 51], fewer oocytes and with lower quality, lower in vitro fertilization (IVF) success rates [52], reduced fecundability [53] and disruption to early embryonic development and maturation [54]. A study by Ryan et al. [51] found increased menstrual cycle length and basal FSH concentrations in response to increased THC concentrations in the rhesus macaques, suggesting ovulatory disruption. Similarly, THC-exposed GCs had increased proliferation, decreased apoptosis and increased VEGF and PGE2 secretion, which have been associated with ovarian dysfunction [55]. Furthermore, THC can affect placental development [49]. 24-h THC exposure increased NAPE-specific phospholipase D (NAPE-PLD) and decreased Fatty acid amine hydrolase (FAAH) levels in human placenta. Interestingly, 72 h treatment showed an opposite trend of decreased NAPE-PLD, increased FAAH levels, and increased AEA levels [56]. Chang et al. [47] showed that THC inhibited trophoblast cell migration and invasion by activating the STAT3 signaling pathway, ultimately affecting placental development. They also found, using cannabinoid receptor inhibitors, that THC dysregulated trophoblast function partly through CB1 and CB2 receptors [47].
Recent studies have focused on prenatal cannabis exposure and heritable alterations in the genome [11, 12, 57]. Epigenetic mechanisms plays a role in regulating gene expression and can be a source of heritable changes from cannabis exposure [11]. Additionally, cross-generational impacts of environmental insults are assumed to be mediated through epigenetic mechanisms [13, 33]. DNA methylation is an epigenetic mechanism that is highly involved in regulating gene expression and is vulnerable to environmental stressors [13, 58]. DNA methyltransferases (DNMTs), such as DNMT1, DNMT3a and DNMT3b, mediate DNA methylation by maintaining methyl marks and establishing de novo DNA methylation patterns. Demethylation is regulated by a second group of enzymes: Ten-eleven translocation (TETs) (TET1/2/3) and Thymine DNA glycosylase (TDG). Several studies have reported cannabis causing epigenetic dysregulation [33, 59], with 6640 differentially methylated CpG sites in human sperm between cannabis users versus non-users [11]. Enriched CpG sites were associated with Hippo signaling pathways and pathways in cancer, which they further replicated in THC exposed rodents, indicating THC may be causing these epigenetic modifications [11]. A study by Fuchs Weizman et al. [57] found a decrease in DNMT3b (de novo methylator) both in vivo and in vitro and decreased global methylation patterns in vitro, in GCs following THC exposure. miRNAs are other epigenetic factors important in development and are affected by cannabis [60, 61]. Martίnez-Peña et al. [55] found prenatal THC exposure resulted in differentially expressed miRNAs, including miR-122-5p in the rat ovary. Moreover, a transcriptomic analysis in our lab showed altered miRNA expression in THC exposed sperm [62]. The epigenome thus offers insight into the effects of environmental stressors on changes at the cellular level, based on altered signalling pathways and gene expression.
As the goal for our research is to ultimately analyze epigenetic patterns in the oocyte and embryo, which cannot be performed in human samples, we used bovine cells as a translational model. The bovine species is an excellent translational model for human reproduction as bovine and humans share similarities in ovarian function, oocyte characteristics, metabolic requirements, genome activation and embryo development [3], and humans and cows are both single ovulators. Additionally, Rodriguez-Osorio et al. [63] showed that DNMTs have a higher degree of conservation in their protein sequence between bovine and humans compared to other species.
In this study, fertility-associated miRNAs, including miR-21, miR-155, miR-346, miR-33b and miR-324 were assessed. miR-21 is highly expressed in murine GCs [64], ovine follicles [65], and is upregulated during human ovulation [66]. miR-21 regulates transcripts involved in cell cycle and apoptosis [66], therefore promoting follicular cell survival during ovulation [67], and its suppression leading to apoptosis in GCs [64]. miR-155 is another key miRNA as its dysregulation leads to ovulatory pathology such as PCOS [67, 68]. By regulating PDCD4, miR-155 activates the PI3K/AKT and JNK pathways, promoting cell proliferation, migration and invasion [67]. This is particularly interesting as PI3K/AKT pathways are also modulated by the ECS. Another miRNA linked to developmental competency is miR-33b which was found to be upregulated in GCs of PCOS patients [69], suggesting its role in ovarian function. In fact, increased miR-33b expression inhibited cell growth and enhanced apoptosis by reducing the Wnt-β-catenin signalling pathway [70] and targeting TGDBRI and SMAD7 [69]. Evidence indicates that miR-324 may be linked with PCOS due to its downregulation in PCOS patients compared to controls [71, 72]. Jiang and Ma [72] also found decreased miR-324 expression in their PCOS rat model and discovered that miR-324 may affect apoptosis and GCs proliferation by directly targeting WNT2B. Lastly, miR-346 has a poorly understood role in folliculogenesis, although is important in embryogenesis by regulating EG-VEGF, crucial for embryo implantation, and by repressing MMP-2 and MMP-9 [73]. Previous research in our laboratory indicated that mir-33b, miR-324 and miR-346 were significantly downregulated following THC exposure in sperm [62].
Therefore, this study seeks to investigate the relationship between pharmacologically relevant concentrations of THC and the female reproductive system by assessing DNA methylation and miRNA profiles in GCs following THC exposure. In this investigation, GCs are used as they are an excellent indicator of oocyte health and crucial to oocyte development by providing a suitable microenvironment during oogenesis [74–81]. THC concentrations were chosen based on THC plasma concentrations detected in recreational users and after therapeutic use [82] and are in line with previous research in our laboratory that assessed THC’s effects in gametes and blastocysts [54], and GCs [83]. In addition, THC was detected in follicular fluid of ART patients at a concentration of 0.03243 μM, which is the range of the therapeutic dose (0.032 μM, [THC]) utilized in our study [57].
Herein, we analyzed the effects of THC on epigenetic mechanisms, such as DNA methylation, by measuring 5-methylcytosine (5-mC) and 5-hydroxymethyl cytosine (5-hmC) levels and DNMT1/3a/3b, TET1/2/3 and TDG mRNA and protein expression in GCs. We further looked at the effects of THC on miRNA expression of fertility-associated miRNAs: miR-21, miR-155, miR-346, miR-33b and miR-324. We hypothesized that THC alters epigenetic mechanisms, such as DNA methylation patterns and miRNA profiles, in bovine GCs which might ultimately impact oocyte developmental competency.
Methods
Granulosa cells retrieval
Bovine (Bos Taurus) ovaries were collected from local abattoirs (Cargill Meat Solutions, Guelph, Ontario, Canada and Highland Packers, Stoney Creek, Ontario, Canada) and transported to the laboratory in sterile warmed saline solution supplemented with penicillin/streptomycin (1%) (University of Guelph, Ontario, Canada) under controlled temperatures of 34–36 °C. GCs used in this study were retrieved and cultured as previously described by Sabry et al. [84]. Briefly, using an aspiration pump set-up, follicles ranging from 2 to 22 mm were aspirated using a sharp 18-gauge needle. GCs were mechanically stripped from aspirated cumulus-oocyte-complexes (COCs) and washed in phosphate-buffered saline (PBS) (Wisent, Saint-Jeane Baptist, QC, Canada) and in 1× Dulbecco’s Modified Eagle Medium (DMEM) (Gibco) containing glutamine (2 mM) (Sigma Aldrich) and penicillin/streptomycin (1%). Cells were resuspended in DMEM supplemented with 20% fetal bovine serum (FBS) (10% total serum-Gibco, 12,483,020) and cultured at 38.5 °C in 5% CO2 for 7 days, or until 100% confluent, with media replacement every 48 h. Once confluent, GCs were cryopreserved at passage 1 (P1) in 70% DMEM, 20% FBS and 10% DMSO (Sigma D5879) in liquid nitrogen for further experimentation.
In vitro granulosa cell culture
Frozen GCs were thawed and resuspended in DMEM supplemented with 20% FBS and incubated at 38.5 °C in 5% CO2 for 72 h, or until >80% confluency was reached. Cells were then trypsinized, resuspended in DMEM supplemented with 10% FBS, split into 6-well plates at a seeding density of 2 × 105 cells, incubated for 24 h, serum starved using OptiMEM™ Reduced Serum Media (Thermo Fisher) for 24 h, and incubated at 38.5 °C for 24 h in one of five treatment groups: a control containing only OptiMEM media, vehicle (1:1:18 ethanol: tween: saline), or GC cell treatment with three clinically relevant concentrations of THC (0.032, 0.32 and 3.2 µM). Cells were snap-frozen in liquid nitrogen and stored at −80 °C for RNA or protein extraction. For all biological replicates, cells were treated at passage 2 (P2), following 120 h of cell culture post-thaw.
5-mC and 5-hmC detection by flow cytometry
Following 24-h treatment, GCs were washed, added to DMEM supplemented with 10% FBS and centrifuged at 5000 × g for 3 min at 4 °C. Cells were then fixed in 4% PFA (AAA1131336 Fisher Scientific) at 37 °C for 30 min, chilled on ice and centrifuged at 5000 × g for 3 min at 4 °C. Cells were resuspended in PBS supplemented with 10% FBS and re-centrifuged under the same conditions. To allow cell permeabilization, cells were resuspended in PBS + 0.1% Triton™ X-100 (Sigma Aldrich) + 5% BSA and gently rocked for one hour at room temperature.
Following cell fixation and permeabilization, 5-mC and 5-hmC levels were detected. A negative isotype control and no stain control were included for both 5-mC and 5-hmC detection. The negative isotype control was included for each primary antibody (5-mC and 5-hmC) to measure the level of fluorescence for non-specific antibody binding. A no stain control was used to control for any background autofluorescence and was used to determine the negative population of cells. Cells were centrifuged at 5000 × g for 3 min at 4 °C and resuspended in 50 µl of primary antibody, 5-methylcytocine (5-mC) (Abcam ab10805), (1:100 in PBS + 0.1% Triton™ X-100 + 5% BSA) and were gently rocked for an hour at room temperature. Negative isotype control for 5-mC was added, Mouse igG1, kappa monoclonal isotype control [15-6E10A7] (Abcam ab170190) (1:1000 in PBS + 0.1% Triton™ X-100 + 5% BSA). The no-stain control was incubated in PBS + 0.1% Triton™ X-100 + 5% BSA only. At the end of the 1-h, cells were added to 450 µL of PBS + 0.1% Triton™ X-100 and were centrifuged at 5000 × g for 3 min at 4 °C. Cells were then resuspended in PBS + 0.1% Triton™ X-100 and were centrifuged under the same conditions. At this point, cells were resuspended in the secondary antibody, Goat pAb to Ms. IgG (Abcam ab1501133) (1:2000 in PBS + 0.1% Triton™ X-100 + 5% BSA) and placed in the dark at room temperature for 45 min. Cells were then added of PBS + 0.1% Triton™ X-100 and were centrifuged at 5000 × g for 3 min at 4 °C. Cells were then resuspended in PBS + 0.1% Triton™ X-100 and were centrifuged under the same conditions. Finally, cells were resuspended in PBS + 5% BSA and were read using Flow Cytometry.
The same protocol was repeated to detect 5-hmC levels using a primary antibody, 5-hydroxymethylcytocine (Abcam AB214728) and a negative isotype control, Rabbit IgG monoclonal [EPR25A] isotype control (ab 172730), both diluted to 1:100 in PBS + 0.1% Triton™ X-100 + 5% BSA. The 5-hmC secondary antibody, Goat pAb to Rb IgG (Abcam ab 150077) was diluted to 1:2000 in PBS + 0.1% Triton™ X-100 + 5% BSA.
To assess 5-mC and 5-hmC levels, flow cytometry was used. Once cells were strained through a 40uM filter (Avantar—VWR) into fluorescence-activated cell sorting (FACS) tubes (Fisher Brand), cells were read using BD Accuri C6 Flow Cytometer and 50,000 events were recorded with a slow fluidics rate. An 8 and 6-peak bead validation was performed before machine use. To visualize 5-mC flow cytometry results, confocal images were taken with Olympus FV1200 Confocal Microscope using DAPI as a counter-stain. Results were analyzed using the Flowjo software on four biological replicates.
RNA extraction and cDNA synthesis
RNA was extracted from frozen GCs using the RNeasy Plus Micro Kit (Qiagen, Toronto, Canada; 74,034) following manufacturer’s protocol. Briefly, Buffer RLT Plus was added to samples, transferred to gDNA Eliminator Spin Columns and centrifuged. 70% ethanol was added, transferred to RNeasy MinElute Spin columns, and centrifuged. Columns were then washed using RW1 and RPE buffers, and 80% ethanol. Membranes were dried before adding 17 µL of RNAse-free water to elute RNA. RNA concentrations were measured using a Nanodrop 2000c (Thermo Fisher Scientific, Waltham, MA, USA).
mRNA was reverse transcribed (RT) into cDNA using the QuantaBio qScript cDNA Supermix (VWR, Mississauga, Canada; 95,048). Briefly, 4 µL of qScript cDNA Supermix was added to RNA samples. 1000 ng of RNA was then reverse transcribed (RT) in a T100 Thermal Cycler (Bio-Rad, Mississauga, Canada) under the following conditions: 5 min at 25 °C, 30 min at 42 °C, and 5 min at 85 °C. A no template control (NTC) excluding RNA, and no reverse transcription control (NRT) excluding the reverse transcriptase enzyme, were included. cDNA samples were stored at −20 °C until qPCR analysis.
Quantitative polymerase chain reaction (qPCR)
The CFX96 Touch Real-Time PCR Detection System (Biorad, 1,725,201) and SsoFast EvaGreen Supermix (Biorad, 1,725,201) were used to determine the mRNA expression of 3 genes involved in DNA methylation (DNMT1, DNMT3a, DNMT3b) and 4 genes involved in demethylation (TET1, TET2, TET3 and TDG), following the protocol: 5 min at 95 °C, followed by 44 cycles at 95 °C for 10 s, 60 °C for 10 s and 72 °C for 10 s, as previously described by Saleh et al. [85]. Primer sequences and primer efficiencies are summarized in Table 1. All primers were purchased from Qiagen, except for TDG, which was designed and sequenced [86]. Relative mRNA expression was determined using the efficiency-corrected method (∆∆Ct) with tyrosine 3-monooxygensae/tryptophan 5-monooxygensae activation protein zeta (YWHAZ) and peptidylprolyl isomerase (PPIA) as reference genes. A calibrator consisting of cDNA from GCs was used to account for inter-run variability. A minimum of five biological replicates were quantified in technical triplicates for each primer set. NTC, NRT and water were measured as RT and qPCR negative controls. mRNA expression profiles were then analyzed using Bio-Rad CFX Maestro software.
Table 1.
mRNA primer sequences
| Gene symbol | Gene full name | GenBank accession # | Product size (bp) | Primer sequence (5’- 3’) | Efficiency (%) | Source |
|---|---|---|---|---|---|---|
| DNMT1 | DNA (cytosine-5)-methyltransferase 1 | NM_182651 | 136 |
F: TTAGCACCTCATTTGCCGAGTA R: TAGGTGGAGTCAGGGTTGCTCT |
104.2 | Sabry et al. [86] |
| DNMT3a | DNA (cytosine-5)-methyltransferase 3a | NM_001206502.1 | 110 |
F: GCGTTAGTGACAAGAGGGACA R: AAGGTTCCCCCAGAAGTAGC |
100.1 | Sabry et al. [86] |
| DNMT3b | DNA (cytosine-5)-methyltransferase 3b | NM_181813.2 | 103 |
F: GAAACCAGGACTCGGTCTGA R: GGCCTCGGGTAGAACGTAG |
100.4 | |
| TET1 | Ten-eleven translocation methylcytosine dioxygenase 1 | XM_003587999.2 | 214 |
F: TTCCCACGGCTCGGTTCT R: RTTTCTGTTCGGAGGCTTTAGTTT |
100.9 | Sabry et al. [86] |
| TET2 | Ten-eleven translocation methylcytosine dioxygenase 2 | XM_005207682.1 | 285 |
F:AAGGCTGAGGGACGAGAACGA R:GAGACGGAGATGGTATCAAGAATGG |
102.0 | |
| TET3 | Ten-eleven translocation methylcytosine dioxygenase 3 | XM_005212473.1 | 118 |
F: TCCTTCGGTTGTTCCTGGAG R: TCTTCCGGAGCACTTCTTCC |
100.1 | |
| TDG | Thymine DNA-glycosylase | NM_001083696.2 | 159 |
F: GAACGCGGGCAGCTATTCTC R: GTCTCTCGTGTGGGTTCCTG |
99.0 | Sabry et al. [86] |
Western blotting
Proteins were extracted from GCs and were lysed in radioimmunoprecipitation assay (RIPA) buffer with protease inhibitors (Bio tool B14001 and B15001) with 4 freeze/thaw cycles using liquid nitrogen, sonication in ice water bath and centrifugation at 12 000 × g at 4 °C for 10 min. Protein concentration was determined using Bio-Rad DC Protein Assay Kit (BioRad, Mississauga, ON) following manufacturers’ instructions.
A total of 40 µg of protein was prepared by combining equal volumes of 3× Reducing Buffer plus 5% ß-mercaptoethanol (Sigma Aldrich, M6250). Samples were denatured at 90 °C and loaded onto a 15% polyacrylamide gel in the XCell SureLock Mini-Cell Electrophoresis System (Invitrogen; Burlington, ON, Canada) containing 5X Tris-Glycine Buffer. Gels were run for 2 h at 125 V and transferred onto nitrocellulose membranes (Bio-Rad, 1,620,115) in an Invitrogen wet transfer western blot apparatus (Invitrogen; Burlington, ON, Canada) containing 1X Towbins Buffer, for 2 h at 40 V. Membranes were blocked for 1 h in 5% skim milk in TBST. DNMT1 primary antibody (1:5000 in 5% skim milk in TBST) was incubated overnight at 4 °C and the anti-rabbit IgG HRP-linked secondary antibody (Cell Signalling Technology, Whitby, ON, Canada; 70,735) (1:2000 in 5% skim milk in TBST) was incubated for 1 h at room temperature. Membranes were then washed, placed in Clarity Western ECL Blotting Substrate (Bio-Rad 170-5060) and imaged using Bio-Rad ChemiDoc XRS + Imaging System. Beta Actin (Cell Signalling Technology, 4967) was used as a loading control (1:2000 in 5% BSA in TBST) and incubated for 1 h at room temperature, followed by incubation with the secondary antibody anti-mouse IgG HRP-Linked antibody (Cell Signalling Technology, 7076) (1:5000 in 5% skim milk in TBST) for 1 h at room temperature. Protein bands were quantified by densitometric analysis using Image Lab software from Bio-Rad and values were expressed as a ratio between DNMT1 expression and loading control (Beta Actin) for each sample. Four biological replicates were used for this set of experiments.
MicroRNA analysis
Total RNA was extracted from frozen GCs as previously described. RNA was reverse transcribed using the miRCURY LNA RT Kit (Qiagen 339,340) by first diluting RNA samples to 5 ng/µL in nuclease-free water. Each sample was added to 2 µL of 5× miRCURY SYBR Green RT Reaction Buffer (Qiagen) and 1 µL of 10× miRCURY RT Enzyme Mix (Qiagen). NTC and NRT controls were included. RNA samples were then reverse transcribed under the following conditions: 60 min at 42 °C and 5 min at 4 °C. The resulting cDNA was then stored at −20 °C for qPCR analysis.
miR-21, miR-155, miR-324, miR-346 and miR-33b expressions were quantified by qPCR as described above. Primer sequences are summarized in Table 2. Master Mix composition included 1 µL primer mix, 1 µL RNase-free water and 5 µL 2× miRCURY SYBR Green Master Mix. 3 µL of 1:15 diluted cDNA and 7 µL of Master Mix was added to each well and placed in the CFX96 Touch Real-Time PCR Detection System (Biorad, 1,725,201) under the following conditions: 95 °C for 2 min followed by 40 cycles of 95 °C for 10 s and 56 °C for 60 s, ending with a melt curve analysis from 60–95 °C. Relative microRNA expression was determined using the efficiency-corrected method (∆∆Ct) with miR-93 and miR-132 used as reference genes according to the GeNorm algorithm [87]. A calibrator was used to account for inter-run variability. A minimum of three biological replicates were quantified in technical triplicates for each primer sequence. NTC, NRT and water were measured as RT and qPCR negative controls. microRNA expression profiles were then analyzed using the Bio-Rad CFX Maestro software system.
Table 2.
microRNA primer sequences
| miRNA | Primer ID | Accession # | Primer sequence (5’- 3’) | Efficiency (%) |
|---|---|---|---|---|
| miR-21 | hsa-miR-21-5p | MIMATI0000076 | UAGCUUAUCAGACUGAUGUUGA | 101.3 |
| miR-155 | hsa-miR-155-5p | MIMAT0000646 | UUAAUGCUAAUCGUGAUAGGGGUU | 99.5 |
| miR-324 | hsa-miR-324-5p | MIMAT0000761 | CGCAUCCCCUAGGGCAUUGGUG | 101.1 |
| miR-346 | hsa-miR-346 | MIMAT0000826 | UGUCUGCCCGCAUGCCUGCCUCU | 100.3 |
| miR-93 | hsa-miR-93-5p | MIMAT0000093 | CAAAGUGCUGUUCGUGCAGGUAG | 100.3 |
| miR-132 | hsa-miR-132-3p | MIMAT0000426 | UAACAGUCUACAGCCAUGGUCG | 100.7 |
| miR-33b | hsa-miR-33b-5p | MIMAT0003301 | GUGCAUUGCUGUUGCAUUGC | 100.3 |
*miRNA primers were predesigned and validated by Qiagen. Primer efficiencies were tested in this study
Statistical analysis
GraphPad Prism 6 statistical software was used for all statistical tests. The Shapiro-Wilk Test was used to determine normality within datasets. A One-Way Analysis of Variance (ANOVA) was used on normally distributed datasets and the Kruskal-Wallis test was used on non-normally distributed data. Data were considered statistically significant using a two-tailed p-value < 0.05. A Tukey’s post-hoc test was used on datasets shown to be statistically significant to compare differences among treatment groups. Data were presented as mean ± the standard error of the mean (SEM).
Results
5-mC and 5-hmC Levels in granulosa cells following 24-hour THC exposure
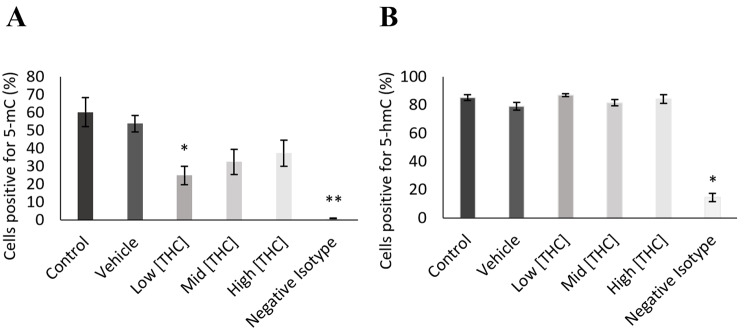
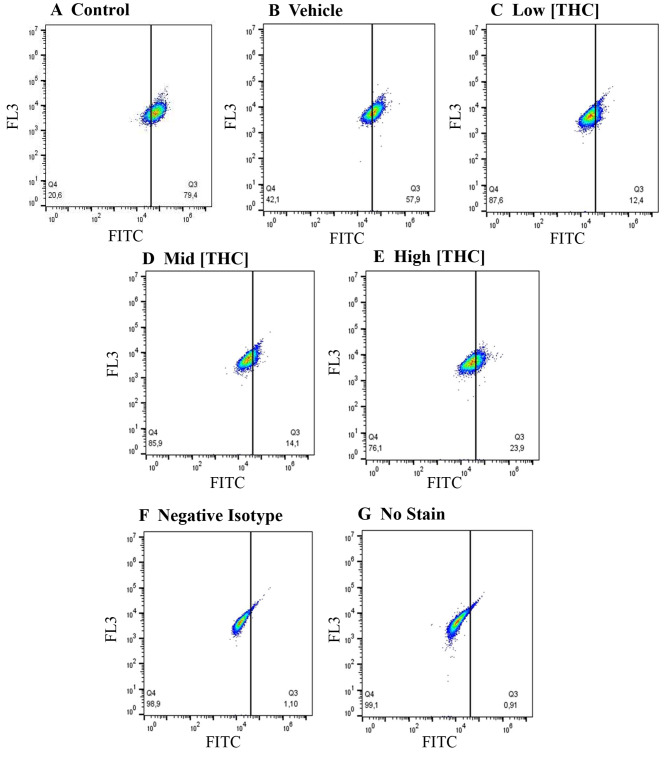
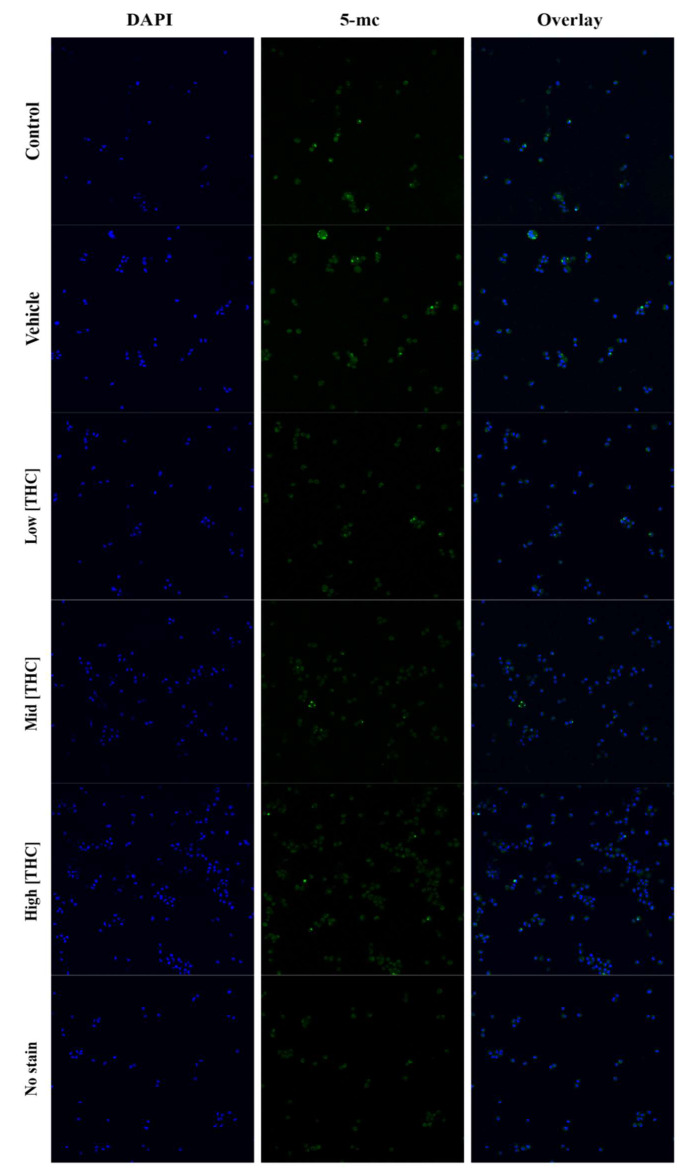
5-mC and 5-hmC levels were detected in 24 h treated GCs using flow cytometry and gated against no stain control (Figs. 1 and 2). As seen in Fig. 1(A), the 5-mC negative isotype control was significantly reduced compared to control (p = 0.0001284, n = 4) and 5-mC levels were significantly decreased in the low THC group (p = 0.0435, n = 4). As depicted in Fig. 1(B), the 5-hmC negative isotype was significantly reduced compared to control (p = 0.0154, n = 4), however, no differences were observed for 5-hmC levels across treatment groups (Fig. 1B). An overall reduction in 5-mC staining in THC-treated cells can be further visualized in flow cytometry plots (Fig. 2) and confocal images (Fig. 3) taken using Olympus FV1200 Confocal Microscope. As seen in Fig. 2, representative flow cytometry plots were gated into two quadrants- left quadrant representing the negative cell population, and right quadrant showing cells positive for 5-mC. In Fig. 3, blue fluorescence represents nuclear DNA stain (DAPI), while green fluorescence denotes 5-mC staining.
Fig. 1.
Global methylation levels detected by 5-mC and 5-hmC staining using flow cytometry. A) 5-mC (n = 4) and B) 5-hmC (n = 4) levels in GCs following 24-hour treatment with low (0.032 µM), mid (0.32 µM), and high (3.2 µM) concentrations of THC. Bars represent ± SEM. *p < 0.05, **p < 0.0005
Fig. 2.
5-mC representative flow cytometry plots. The proportion of no stain (left quadrant) to 5-mC stained (right quadrant) in GCs can be visualized. Plots represent A) control, B) vehicle, C) low THC (0.032 µM), D) mid THC (0.32 µM) E) high THC (3.2 µM), F) negative isotype control and G) no stain control
Fig. 3.
Confocal images of GCs stained with nuclear stain DAPI (blue) and 5-mC antibody (green) following 24-hour treatment with low (0.032 µM), mid (0.32 µM), and high (3.2 µM) concentrations of THC. Images captured using an Olympus FV1200 Confocal Microscope
mRNA and protein expression of DNA methylation genes
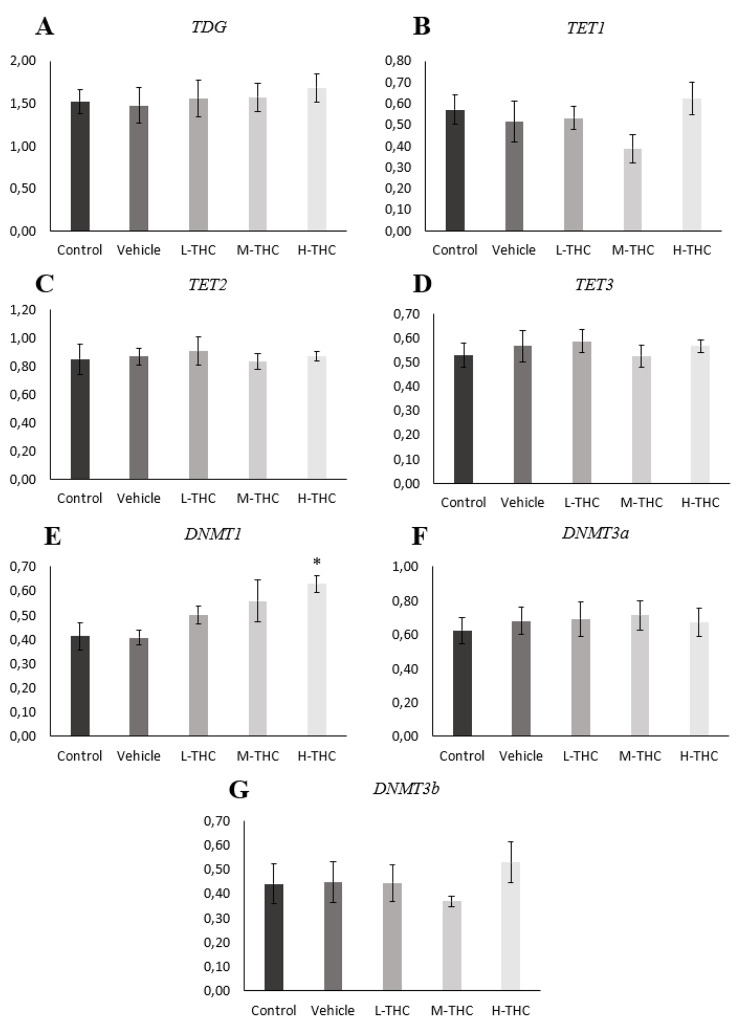
To further evaluate altered 5-mC levels, mRNA expression of key enzymes involved in DNA methylation, including DNMTs (DNMT1, DNMT3a, DNMT3b), TET enzymes (TET1, TET2, TET3) and TDG, was quantified. A minimum of four biological replicates were assessed in GCs following exposure to low (0.032 µM), mid (0.32 µM), and high (3.2 µM) THC. mRNA expression was quantified by qPCR and normalized to reference genes YWHAZ and PPIA. As seen in Fig. 4(E), DNMT1 mRNA expression appeared to increase with higher concentrations of THC, however, the increase was statistically significant only following high THC exposure (p < 0.05, n = 4). In contrast, no significant changes were observed in DNTM3a, DNMT3b, TET1, TET2, TET3 or TDG expression (Fig. 4).
Fig. 4.
Relative mRNA expression in GCs normalized to housekeeping genes YWHAZ and PPIA. A) TDG (n = 6), B) TET1 (n = 6), C) TET2 (n = 6), D) TET3 (n = 6), E) DNMT1 (n = 4), F) DNMT3a (n = 6) and G) DNMT3b (n = 5) expression following 24-hour treatment in low (0.032 µM), mid (0.32 µM), and high (3.2 µM) concentrations of THC. Bars represent ± SEM. *p < 0.05
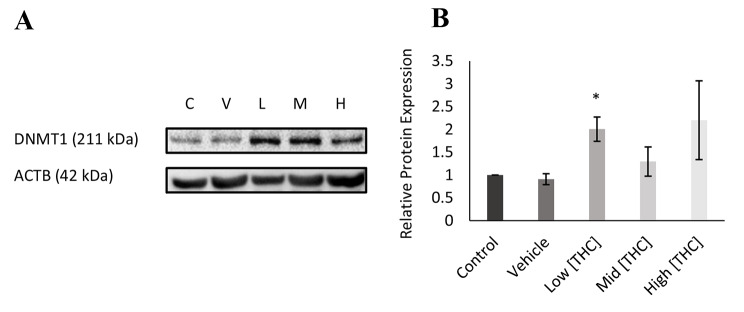
To further analyze the effects of THC on DNMT1 expression, DNMT1 proteins were quantified by western blotting with Beta-actin as a loading control. As seen in the densitometry analysis (Fig. 5) and visual representation (Fig. 5), DNMT1 expression appeared to increase overall following THC exposure, although was only statistically significant following low THC exposure (p = 0.00498, n = 4).
Fig. 5.
Western blot displaying DNMT1 protein expression relative to loading control ACTB. Expression was qualified in GCs following 24-hour treatment with low (0.032 µM), mid (0.32 µM), and high (3.2 µM) concentrations of THC A) DNMT1 protein expression (n = 4) and B) densitometry analysis. Bars represent ± SEM. *p = 0.00498. Blots were cropped for representation. Full original uncropped blots can be found in the supplementary material Figure S1
MicroRNA expression in THC-treated granulosa cells
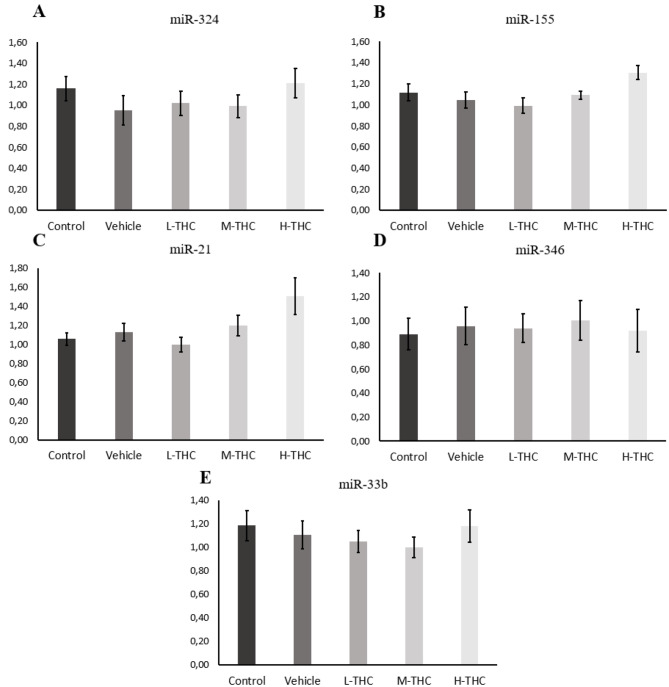
The relative expression of selected fertility associated miRNAs was assessed in GCs following THC exposure. A minimum of three biological replicates was measured by qPCR on the five miRNAs selected (miR-324, miR-155, miR-21, miR-346 and miR-33b) relative to housekeeping miRNAs: miR-93 and miR-132. As seen in Fig. 6(C), miR-21 expression appears to increase with increasing concentrations of THC, although not statistically significant. Overall, no significant changes were seen in miR-324, miR-155, miR-346 and miR-33b expression following THC exposure (Fig. 6).
Fig. 6.
Relative miRNA expression normalized to housekeeping genes miR-93 and miR-132. A) miR-324 (n = 6), B) miR-155 (n = 3), C) miR-21 (n = 6) D) miR-346 (n = 6) and E) miR-33b (n = 7) expression in GCs following 24-hour treatment with low (0.032 µM), mid (0.32 µM), and high (3.2 µM) concentrations of THC. Bars represent ± SEM
Discussion
The goal of this study was to explore the potential effects of THC on epigenetic mechanisms that are known to mediate development, such as DNA methylation and miRNA profiles, in bovine GCs. With the rise in cannabis consumption among reproductive age-women, and the increase in THC potency within cannabis preparation, it is important to further investigate how THC may interact with the ECS within the female reproductive system. The ECS has a prominent role in mammalian reproduction and is present in all female reproductive tissues [15, 33, 40].
To investigate the effects of THC on GCs, three physiologically relevant THC doses were used: a therapeutic low dose (0.032 µM THC), and a recreational mid and high dose, (0.32 µM; 3.2 µM THC, respectively). Therapeutic cannabis is often administered by prescribed drugs such as Marinol ©, which contains a synthetic THC: dronabinol. The low therapeutic level of THC employed in our investigation is reflective of peak plasma concentrations detected 1.5 h after consuming the recommended dose of 10 mg of Marinol © [88]. Likewise, Fuchs Weizman et al. [57] measured THC in follicular fluid of patients undergoing ART in the order of 0.03243 μM, which is comparable with our therapeutic dose of 0.032 μM. Recreational cannabis use is more difficult to predict due to the variability amongst users. As a result, a 10× concentration range of 0.32 and 3.2 µM was employed in this investigation to reflect heavy recreational use. These values were additionally reported by Whan et al. [82] as being representative of blood plasma concentrations found in therapeutic and recreational cannabis users. THC concentrations chosen for this study are also reflective of previously published research from our laboratory investigating THC on sperm parameters [62], GC viability [83], and oocyte and embryo quality [54].
Previously in our laboratory, GCs exposed to therapeutic and recreational doses of THC did not display compromised cell viability or apoptosis [83]. Misner et al. [54] performed a transcriptome analysis which revealed that THC was dysregulating transcript expression in COCs. While it has been documented that THC can cause epigenetic modifications in sperm [11, 58, 62, 89], less is known regarding its epigenetic effects on the female reproductive tract.
Epigenetic mechanisms have an important biological function as they regulate gene expression. The present study focused specifically on DNA methylation and miRNAs. As previously mentioned in more details, DNA methylation is the process of adding methyl groups to cytosine, it is typically mediated by DNMTs and is mainly associated with transcriptional silencing [90, 91]. In addition, DNA also undergoes demethylation, with 5-hmC an intermediate in the demethylation process.
We first assessed global DNA methylation, showing 5-mC levels significantly reduced following low THC exposure. Similarly, a study by Fuchs Weizman et al. [57] found 100 and 500 ng/mL THC-exposed human GCs had decreased DNA methylation by 76.2 and 83.8%, respectively. They further analyzed the effects of a combined treatment of THC, 11-OH-THC and 11-COOH-THC based on follicular fluid concentrations measured in vivo (0.03243, 0.007 and 0.05495 μM, respectively). The combined treatment led to a 37.2% decrease in 5-mC levels following acute (24 h) exposure [57]. In a follow up study, they found THC-exposed GCs had different methylation profiles, with 3679 differentially methylated sites compared to controls [28]. Similar results were also observed by Murphy et al. [11], where 3979 CpG sites were found to be differentially methylated in human sperm exposed to THC, 78% of which had reduced methylation. In contrast, 5-hmC acts as a demethylation intermediate controlled by TET enzymes [92, 93]. No changes in 5-hmC paired with altered 5-mC levels might indicate that THC is affecting DNMTs, but not TET enzymes. To better understand the functional effects of THC-altered 5-mC levels, identifying differentially methylated regions (DMRs) in THC exposed GCs would be valuable and would add significance and strength to this research. We could then isolate which genes are being impacted and how that would affect developmental competency. For instance, by evaluating DMRs in THC exposed rat and human sperm, Murphy et al. [11] identified several KEGG pathways enriched by THC, including Hippo signalling, MAPK signaling, and pathways related to cancer. Hippo signalling is particularly important in follicular activation, growth, maturation and steroidogenesis [94]. In fact, Hippo signalling knockout models led to decreased bovine and mouse GC proliferation and function [95, 96]. Whereas Hippo signaling over-expression resulted in decreased GC proliferation in hens [97, 98], and supported human GC proliferation and growth, but disrupted steroidogenesis [99]. Similarly, MAPK activity in cumulus GCs plays an important role in meiotic resumption, ovulation and luteinization [100, 101]. DMRs were also identified by Fuchs Weizman et al. [28] in THC-exposed GCs. These regions were associated with epigenetic modifications, transcription factors, cell proliferation, apoptosis, post-translational modifications, and extra cellular matrix remodeling [28]. Of the DMRs discovered, they found 47% were hypermethylated, while 53% were hypomethylated [28]. The reported total 5-mC levels in this investigation rather than DMRs, do not account for the percentage of DNA being hypermethylated versus hypomethylated.
Previous work in our laboratory by Misner et al. [54] observed decreased connexin 37 and 43 levels in COCs exposed to low THC, while no changes were detected following mid or high THC exposure. In addition, they found 62 differentially expressed genes in the low THC group compared to controls, whereas only a handful of genes were differentially expressed in the mid and high groups [54]. Gene expression is related to the accessibility of transcription machinery to DNA, partially controlled by methylation. Thus, paired with the results in the present study, upregulated gene expression may be a result of decreased 5-mC levels. If transcript levels were increased in COCs exposed to THC, perhaps there is an increase in transcripts being sent from the surrounding GCs due to decreased DNA methylation. The bidirectional communication between the oocyte and its surrounding GCs is essential for folliculogenesis, oocyte maturation and competence acquisition [79, 81, 102]. Throughout folliculogenesis, the oocyte and surrounding GCs transfer molecules, including ions, cAMP, metabolites, amino acids and RNA transcripts [81].
Global DNA methylation is governed by establishing new methylation patterns, maintaining methylation patterns during cell replication and active demethylation. Together, DNMTs and TETs/TDG establish, maintain and erase CpG methyl marks, contributing to overall gene expression [90]. The detected increase in DNMT1 mRNA and protein expression may be indicative of increased cell replication, but previous research in our lab found no significant changes in cell counts following THC exposure [83]. Cells might be increasing DNMT1 transcripts to compensate for reduced 5-mC marks following THC exposure. Previous research by Vassall et al. [101] found a significant increase in DNMT1 in the medaka fish ovary following THC treatment. Although our results showed no changes in DNMT3a or DNMT3b expression, Vassall et al. [103] found decreased DNMT3a, while Fuchs Weizman et al. [57] found reduced DNMT3b expression, both in vivo and confirmed in vitro, in THC exposed human GCs. Smith et al. [104] also found TET3 upregulated in blood lymphocytes paired with detected THC levels, following acute cannabis smoking. These varied results are likely from different study designs, such as exposure route (in vitro vs inhalation), time of exposure, different models and the effects of other cannabis components, such as CBD. Therefore, future studies looking at CBD effects and a combination of THC and CBD effects would be needed to overcome the limitation of assessing only the role of THC.
In addition to DNA methylation, DNMT1 is directly involved in DNA damage repair to maintain chromosome integrity [105–107]. Following the recruitment of DNMT1 to DNA repair sites, DNMT1 regulates the rate of ATR signaling [107]. ATR is a major regulator of the DNA damage response in cells during replication as it controls replication origin firing, replication fork stability, cell cycle checkpoints, and DNA repair [108]. Therefore, it is plausible that THC might be affecting DNA integrity, resulting in increased DNMT1 expression.
THC acts on the ECS, which in turn activates multiple pathways including P13K/AKT, MAPK and cAMP [27, 32]. Notably, the AKT1 pathway stabilises DNMT1 expression, and a rise in DNMT1 expression depends on AKT1 [109, 110]. Likewise, P1K3/AKT signaling can increase DNMT1 expression [111], which can then lead to decreased E-cadherins [110]. E-cadherins promote cell-to-cell contact with the surrounding GCs [112], which maintains follicular integrity [113]. It can be speculated that THC may be altering the P1K3/AKT pathway, subsequently resulting in increased DNMT1 expression.
To further elucidate the epigenetic effects of THC on GCs, we assessed fertility-associated miRNAs, important in cell survival, apoptosis, cell growth and proliferation during folliculogenesis. THC may be increasing miR-21 expression in a dose dependent manner, although these changes were not statistically significant. No significant changes were observed in the other miRNAs analyzed, contradicting previous work in our laboratory on THC-treated sperm showing a decrease in miR-33b, miR-324, and miR-346 expression [62], suggesting a different impact of THC on the female and male gametes. To our knowledge, no other studies have documented the effects of THC on these miRNAs in gametes, making this research novel and even more valuable.
Similar to our observations, Jackson et al. [114] and Sido et al. [115] showed that both AEA and THC significantly upregulated miR-21 in mouse lymphocytes, suggesting that miR-21 might likely be a target of the ECS. Our results indicated no significant changes in miR-155 expression, but other studies have shown a decrease in miR-155 expression from combined exposure to THC and CBD [116], and to CBD alone [117]. Interestingly, Juknat et al. [117] found that CBD had a greater effect on miRNA profiles compared to THC. Paired with our findings, this suggests THC has a smaller effect on miRNA expression compared to other epigenetic mechanisms, such as DNA methylation. It would be worth investigating additional miRNAs important in folliculogenesis, including miR-212, miR-214, miR-99a, miR-100 and miR-218. miR-212 and miR-214 are synthesized by GCs and sent to the oocyte for meiotic resumption, while miR-99a, miR-100 and miR-218 are involved in follicular maturation [81, 118]. This would allow to overcome one of the limitations of this study, that is the restricted number of target miRs investigated.
Epigenetic mechanisms often work together to regulate gene expression, therefore THC’s effects should be assessed on other epigenetic modulators, such as histone modifications. The interaction of histones and DNA affects chromatin integrity, which in turn regulates gene expression [119]. Histone modifications are important in oocyte development [120] and can be altered from THC exposure [119, 121–123]. In fact, Fuchs Weizman et al. [28] found DMRs from THC exposure were associated with histone methyltransferase SMYD3 and ZFP37, which alter histone acetylation and methylation. Another possible avenue for this research might consist in investigating THC effects on other types of ncRNAs, such as long-noncoding RNAs (lncRNAs), short interfering RNAs (siRNAs) and circular RNAs (circRNAs), as these ncRNAs have been proven to be involved in primary ovarian insufficiencies [124], in post transcriptional regulation during oocyte maturation [125], in meiotic resumption, spindle formation and chromosome alignment [126] and play a role in epigenetic regulation, being correlated to oocyte and embryo competency [127, 128].
As the oocyte and its surrounding GCs communicate in a bidirectional manner, it is important to address how THC’s effects on DNA methylation in GCs impact the oocyte. The epigenome, including DNA methylation, is passed down to future progeny. Hofmeister et al. [125] discovered that 99.998% of the methylated genome was effectively inherited across multiple generations, concluding that DNA methylation is extremely stable across generations, thus, THC’s ability to alter DNA methylation can produce transgenerational impacts.
The potential consequences of perturbations in DNA methylation could be detrimental to oocyte growth and development. Changes in DNA methylation patterns could lead to enriched pathways such as hippo signalling and MAPK signalling [91]. These pathways are important in follicular activation, growth [92, 93], meiotic resumption, ovulation and luteinization [97, 98]. Any disruptions to these critical processes may lead to cellular apoptosis, interrupt steroidogenesis [96] and disrupt follicular integrity [108]. Therefore, oocyte growth and maturation may be negatively compromised by such epigenetic changes.
The strength of this research lays on the clinical relevance of the doses used and the novelty in the investigation of specific microRNAs and epigenetics changes that can have an effect not only on the individual, but also on generations to come. As previously mentioned the oocyte and the surrounding granulosa cells are constantly in a bidirectional communication, therefore further studies looking at these epigenetic changes directly on the oocytes will add significance to this research that has the limitation of being conducted only in granulosa cells. However, this study is still extremely important because of the lack of knowledge and studies in the field.
Conclusions
In summary, the results presented in this paper support the hypothesis that THC alters epigenetic mechanisms, such as DNA methylation patterns, but does not affect miRNAs in bovine GCs. Altered DNA methylation will subsequently lead to changes in gene expression, which may affect developmental processes that require precisely regulated gene activity, ultimately impairing oocyte competence. Overall, the importance of this research lays in advancing our understanding of how cannabis use may affect fertility, ultimately providing scientific evidence to better advise patients undergoing ARTs about the effect(s) of cannabis consumption. Furthermore, this information will be valuable in developing guidelines similar to the ones established for other recreationally-used substances, such as alcohol and tobacco.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge all members of the Reproductive Health and Biotechnology Laboratory (University of Guelph), especially the Lab manager Monica Antenos and research technicians Elizabeth St. John and Allison MacKay and previous master student Vivien Truong for providing technical training and assistance. Lastly, thank you to Cargill Meat Solutions (Guelph, ON) and Highland Packers (Stoney Creek, ON) for supplying the ovaries necessary for this project.
Abbreviations
- AEA
Anandamide
- ANOVA
Analysis of variance
- ART
Assisted reproductive technology
- BSA
Bovine serum albumin
- CB1
Endocannabinoid receptor 1
- CB2
Endocannabinoid receptor 2
- CBD
Cannabidiol
- cDNA
Complementary DNA
- circRNA
circular RNAs
- COC
Cumulus-oocyte-complex
- DMEM
Dubecco’s modified Eagle’s medium
- DMRs
Differentially methylated regions
- DNMT
DNA methyltransferase
- eCB
Endocannabinoids
- ECS
Endocannabinoid system
- ECL
Enhanced chemiluminescence
- FAAH
Fatty acid amine hydrolase
- FBS
Fetal bovine serum
- GC
Granulosa cell
- IVF
In vitro fertilization
- lncRNA
Long non-coding RNAs
- miRNA
Micro-ribonucleic acid
- mRNA
Messenger ribonucleic acid
- PBS
Phosphate-buffered saline
- PCOS
Polycystic ovary syndrome
- PFA
Paraformaldehyde
- qPCR
Quantitative polymerase chain reaction
- RIPA
Radioimmunoprecipitation assay
- RT
Reverse transcription
- SEM
Standard error of the mean
- siRNA
Small interfering RNAs
- sncRNA
Small non-coding RNAs
- TBST
Tris buffered saline with tween
- TDG
Thymine DNA glycosylase
- TET
Ten-eleven translocation
- THC
Delta-9-tetrahydrocannabinol
- 2-AG
2-arachidonyl glycerol
- 5-mC
5-methyl cytosine
- 5-hmC
5-hydroxymethyl cytosine
Author contributions
S.F., R.S., L.A.F. contributed to study design and conceptualized experiments. S.F. performed the majority of experiments, data analysis and writing of manuscript. L.C. performed some protein experiments. R.S. contributed to protocol design and optimizations. M.S.N. contributed to editing of the manuscript. J.Y.K. provided supervision and supplied T.H.C. for this study. L.A.F. provided supervision, funding and contributed to writing and editing of the manuscript.
Funding
This research was funded by the Department of Biomedical Sciences, University of Guelph, OVC and Ontario Graduate Scholarship.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.1 in 6 people globally affected by infertility: WHO. Available from: https://www.who.int/news/item/04-04-2023-1-in-6-people-globally-affected-by-infertility. Accessed 8 June 2023. [PMC free article] [PubMed]
- 2.de Angelis C, Nardone A, Garifalos F, Pivonello C, Sansone A, Conforti A, et al. Smoke, alcohol and drug addiction and female fertility. Reprod Biol Endocrinol. 2020;18(1):21. doi: 10.1186/s12958-020-0567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santos RR, Schoevers EJ, Roelen BA. Usefulness of bovine and porcine IVM/IVF models for reproductive toxicology. Reprod Biol Endocrinol. 2014;12:117. doi: 10.1186/1477-7827-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koysombat K, Abbara A, Dhillo WS. Current pharmacotherapy and future directions for neuroendocrine causes of female infertility. Exp Opin Pharmacother. 2023;24(1):37–47. doi: 10.1080/14656566.2022.2064217. [DOI] [PubMed] [Google Scholar]
- 5.Fertility problems: assessment and treatment. NICE; 2017. Available from: https://www.nice.org.uk/guidance/cg156/evidence. Accessed 8 June 2023.
- 6.What are some possible causes of female infertility? NICHD - Eunice Kennedy Shriver National Institute of Child Health and Human Development. 2017. Available from: https://www.nichd.nih.gov/health/topics/infertility/conditioninfo/causes/causes-female. Accessed 8 June 2023.
- 7.Ojo OA, Nwafor-Ezeh PI, Rotimi DE, Iyobhebhe M, Ogunlakin AD, Ojo AB. Apoptosis, inflammation, and oxidative stress in infertility: a mini review. Toxicol Rep. 2023;10:448–62. doi: 10.1016/j.toxrep.2023.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhnt AK, Passet-Wittig J. Families formed through assisted reproductive technology: causes, experiences, and consequences in an international context. Reprod Biomed Soc Online. 2022;14:289–96. doi: 10.1016/j.rbms.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgaud M, Bretin B, Reignier A, Vos JD, David L. Du nouveau dans les modèles d’étude de l’embryon humain. Med Sci (Paris) 2023;39(2):129–36. doi: 10.1051/medsci/2023018. [DOI] [PubMed] [Google Scholar]
- 10.Hajizadeh M. Legalizing and regulating Marijuana in Canada: review of potential economic, social, and health impacts. Int J Health Policy Manag. 2016;5(8):453–56. doi: 10.15171/ijhpm.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy SK, Itchon-Ramos N, Visco Z, Huang Z, Grenier C, Schrott R, et al. Cannabinoid exposure and altered DNA methylation in rat and human sperm. Epigenetics. 2018;13(12):1208–21. doi: 10.1080/15592294.2018.1554521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibn Lahmar Andaloussi Z, Taghzouti K, Abboussi O. Behavioural and epigenetic effects of paternal exposure to cannabinoids during adolescence on offspring vulnerability to stress. Int J Dev Neurosci. 2019;72:48–54. doi: 10.1016/j.ijdevneu.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Osborne AJ, Pearson JF, Noble AJ, Gemmell NJ, Horwood LJ, Boden JM, et al. Genome-wide DNA methylation analysis of heavy cannabis exposure in a New Zealand longitudinal cohort. Transl Psychiatry. 2020;10(1):1–10. doi: 10.1038/s41398-020-0800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrott R, Murphy SK. Cannabis use and the sperm epigenome: a budding concern? Environ Epigenet. 2020;6(1):dvaa002. doi: 10.1093/eep/dvaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popescu-Spineni D, Guja L, Cristache C, Pop-Tudose M, Munteanu A. The influence of endocannabinoid system on women reproduction. Acta Endo (Buc). 2022. 10.4183/aeb.2022.209. [DOI] [PMC free article] [PubMed]
- 16.Health Canada. Canadian Cannabis survey 2021: summary. 2021. Available from: https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/research-data/canadian-cannabis-survey-2021-summary.html. 11 May 2023.
- 17.Dickson B, Mansfield C, Guiahi M, Allshouse AA, Borgelt LM, Sheeder J, et al. Recommendations from Cannabis dispensaries about first-trimester Cannabis use. Obstetrics Gynecol. 2018;131(6):1031. doi: 10.1097/AOG.0000000000002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institute on Drug Abuse. Marijuana and hallucinogen use among young adults reached all time-high in 2021. 2022. Available from: https://nida.nih.gov/news-events/news-releases/2022/08/marijuana-and-hallucinogen-use-among-young-adults-reached-all-time-high-in-2021. 10 May 2023.
- 19.Leos-Toro C, Fong GT, Meyer SB, Hammond D. Cannabis health knowledge and risk perceptions among Canadian youth and young adults. Harm Reduct J. 2020;17(1):54. doi: 10.1186/s12954-020-00397-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ElSohly MA, Chandra S, Radwan M, Majumdar CG, Church JC. A comprehensive review of Cannabis potency in the United States in the last decade. Biol Psych. 2021;6(6):603–06. doi: 10.1016/j.bpsc.2020.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Shim M, Nguyen H, Grootendorst P. Lessons from 20 years of medical Cannabis use in Canada. PLoS ONE. 2023;18(3):e0271079. doi: 10.1371/journal.pone.0271079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in Cannabis potency over the last two decades (1995-2014) - analysis of current data in the United States. Biol Psychiatry. 2016;79(7):613–19. doi: 10.1016/j.biopsych.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madras BK. Tinkering with THC-to-CBD ratios in Marijuana. Neuropsychopharmacology. 2019;44(1):215–16. doi: 10.1038/s41386-018-0217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lafaye G, Karila L, Blecha L, Benyamina A. Cannabis, cannabinoids, and health. Dialog Clin Neurosci. 2017;19(3):309–16. doi: 10.31887/DCNS.2017.19.3/glafaye. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.du Plessis SS, Agarwal A, Syriac A. Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J Assist Reprod Genet. 2015;32(11):1575–88. doi: 10.1007/s10815-015-0553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hehemann MC, Raheem OA, Rajanahally S, Holt S, Chen T, Fustok JN, et al. Evaluation of the impact of marijuana use on semen quality: a prospective analysis. Ther Adv Urol. 2021;13:17562872211032484. doi: 10.1177/17562872211032484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fonseca BM, Rebelo I. Cannabis and Cannabinoids in reproduction and fertility: where we stand. Reprod Sci. 2022;29(9):2429–39. doi: 10.1007/s43032-021-00588-1. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs Weizman N, Wyse BA, Montbriand J, Jahangiri S, Librach CL. Cannabis significantly alters DNA methylation of the human ovarian follicle in a concentration-dependent manner. Mol Hum Reprod. 2022;gaac022. 10.1093/molehr/gaac022. [DOI] [PMC free article] [PubMed]
- 29.Battista N, Pasquariello N, Di Tommaso M, Maccarrone M. Interplay between endocannabinoids, steroids and cytokines in the control of human reproduction. J Neuroendocrinol. 2008;20 Suppl 1:82–89. doi: 10.1111/j.1365-2826.2008.01684.x. [DOI] [PubMed] [Google Scholar]
- 30.Cabral GA, Rogers TJ, Lichtman AH. Turning over a new leaf: cannabinoid and endocannabinoid modulation of immune function. J Neuroimmune Pharmacol. 2015;10(2):193–203. doi: 10.1007/s11481-015-9615-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu HC, Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiatry. 2016;79(7):516–25. doi: 10.1016/j.biopsych.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 2018;19(3):833. doi: 10.3390/ijms19030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cecconi S, Rapino C, Di Nisio V, Rossi G, Maccarrone M. The (endo)cannabinoid signaling in female reproduction: what are the latest advances? Prog Lipid Res. 2020;77:101019. doi: 10.1016/j.plipres.2019.101019. [DOI] [PubMed] [Google Scholar]
- 34.Gomez Del Pulgar T, Velasco G, Guzmán M. The CB1 cannabinoid receptor is coupled to the activation of protein kinase B/Akt. Biochem J. 2000;347(2):369–73. doi: 10.1042/bj3470369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54(2):161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 36.Velasco G, Galve-Roperh I, Sánchez C, Blázquez C, Haro A, Guzmán M. Cannabinoids and ceramide: two lipids acting hand-by-hand. Life Sci. 2005;77(14):1723–31. doi: 10.1016/j.lfs.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Peralta L, Agirregoitia E, Mendoza R, Expósito A, Casis L, Matorras R, et al. Expression and localization of cannabinoid receptors in human immature oocytes and unfertilized metaphase-II oocytes. Reprod BioMed Online. 2011;23(3):372–79. doi: 10.1016/j.rbmo.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 38.López-Cardona AP, Sánchez-Calabuig MJ, Beltran-Breña P, Agirregoitia N, Rizos D, Agirregoitia E, et al. Exocannabinoids effect on in vitro bovine oocyte maturation via activation of AKT and ERK1/2. Reproduction. 2016;152(6):603–12. doi: 10.1530/REP-16-0199. [DOI] [PubMed] [Google Scholar]
- 39.Kuzma-Hunt AG, Truong VB, Favetta LA. Glucocorticoids, stress and delta-9 tetrahydrocannabinol (THC) during early embryonic development. Int J Mol Sci. 2021;22(14):7289. doi: 10.3390/ijms22147289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rava A, Trezza V. Emerging roles of endocannabinoids as key lipid mediators for a successful pregnancy. Int J Mol Sci. 2023;24(6):5220. doi: 10.3390/ijms24065220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alharthi NS. Endocannabinoid system components: a crucial role in regulation of disease. J Adv Pharm Educ Res. 2022;12(3):72–81. doi: 10.51847/FIVP7AOddG. [DOI] [Google Scholar]
- 42.Koto P, Allen VM, Fahey J, Kuhle S. Maternal cannabis use during pregnancy and maternal and neonatal outcomes: a retrospective cohort study. BJOG. 2022;129(10):1687–94. doi: 10.1111/1471-0528.17114. [DOI] [PubMed] [Google Scholar]
- 43.Chavarro JE. Marijuana and reproduction: time to raise the evidence bar to a new high. Fertil Sterility. 2018;109(5):793–94. doi: 10.1016/j.fertnstert.2018.01.045. [DOI] [PubMed] [Google Scholar]
- 44.Corsi DJ, Hsu H, Weiss D, Fell DB, Walker M. Trends and correlates of cannabis use in pregnancy: a population-based study in Ontario, Canada from 2012 to 2017. Can J Public Health. 2019;110(1):76–84. doi: 10.17269/s41997-018-0148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kasman AM, Thoma ME, McLain AC, Eisenberg ML. Association between use of marijuana and time to pregnancy in men and women: findings from the National Survey of Family Growth. Fertil Steril. 2018;109(5):866–71. doi: 10.1016/j.fertnstert.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 46.Wise LA, Wesselink AK, Hatch EE, Rothman KJ, Mikkelsen EM, Sørensen HT, et al. Marijuana use and fecundability in a North American preconception cohort study. J Epidemiol Community Health. 2018;72(3):208–15. doi: 10.1136/jech-2017-209755. [DOI] [PubMed] [Google Scholar]
- 47.Chang X, Bian Y, He Q, Yao J, Zhu J, Wu J, et al. Suppression of STAT3 signaling by Δ9-tetrahydrocannabinol (THC) induces trophoblast dysfunction. Cell Physiol Biochem. 2017;42(2):537–50. doi: 10.1159/00047760. [DOI] [PubMed] [Google Scholar]
- 48.Chang X, Li H, Li Y, He Q, Yao J, Duan T, et al. RhoA/MLC signaling pathway is involved in Δ9-tetrahydrocannabinol-impaired placental angiogenesis. Toxicol Lett. 2018;285:148–55. doi: 10.1016/j.toxlet.2017.12.031. [DOI] [PubMed] [Google Scholar]
- 49.Lo JO, Hedges JC, Girardi G. Impact of cannabinoids on pregnancy, reproductive health, and offspring outcomes. Am J Clin Exp Obstet Gynecol. 2022;227(4):571–81. doi: 10.1016/j.ajog.2022.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mendelson H, Mello K, Skupny ST, Lex W. Marihuana smoking suppresses luteinizing hormone in women.J Pharmacol Exp Ther. 1986 Jun;237(3):862–6. [PubMed]
- 51.Ryan KS, Mahalingaiah S, Campbell LR, Roberts VHJ, Terrobias JJD, Naito CS, et al. The effects of delta-9-tetrahydrocannabinol exposure on female menstrual cyclicity and reproductive health in rhesus macaques. F&S Sci. 2021;2(3):287–94. doi: 10.1016/jxfss.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klonoff-Cohen HS, Natarajan L, Victoria Chen R. A prospective study of the effects of female and male marijuana use on in vitro fertilization (IVF) and gamete intrafallopian transfer (GIFT) outcomes. Am J Clin Exp Obstet Gynecol. 2006;194(2):369–76. doi: 10.1016/j.ajog.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 53.Mumford SL, Flannagan KS, Radoc JG, Sjaarda LA, Zolton JR, Metz TD, et al. Cannabis use while trying to conceive: a prospective cohort study evaluating associations with fecundability, live birth and pregnancy loss. Hum Reprod. 2021;36(5):1405–15. doi: 10.1093/humrep/deaa355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Misner MJ, Taborek A, Dufour J, Sharifi L, Khokhar JY, Favetta LA. Effects of Delta-9 tetrahydrocannabinol (THC) on oocyte competence and early embryonic development. Front Toxicol. 2021;3:14. doi: 10.3389/ftox.2021.647918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martínez-Peña AA, Petrik JJ, Hardy DB, Holloway AC. Delta-9-tetrahydrocannabinol increases vascular endothelial growth factor (VEGF) secretion through a cyclooxygenase-dependent mechanism in rat granulosa cells. Reprod Toxicol. 2022;111:59–67. doi: 10.1016/j.reprotox.2022.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Maia J, Midão L, Cunha SC, Almada M, Fonseca BM, Braga J, et al. Effects of cannabis tetrahydrocannabinol on endocannabinoid homeostasis in human placenta. Arch Toxicol. 2019;93(3):649–58. doi: 10.1007/s00204-019-02389-7. [DOI] [PubMed] [Google Scholar]
- 57.Fuchs Weizman N, Wyse BA, Szaraz P, Defer M, Jahangiri S, Librach CL. Cannabis alters epigenetic integrity and endocannabinoid signalling in the human follicular niche. Hum Reprod. 2021;36(7):1922–31. doi: 10.1093/humrep/deab104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schrott R, Rajavel M, Acharya K, Huang Z, Acharya C, Hawkey A, et al. Sperm DNA methylation altered by THC and nicotine: vulnerability of neurodevelopmental genes with bivalent chromatin. Sci Rep. 2020;10(1):16022. doi: 10.1038/s41598-020-72783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Resendiz M, Watkins DS, Öztürk NC, Zhou FC. Chapter 32 - environmental influence on epigenetics. In: Tollefsbol TO, editor. Handbook of epigenetics. 3rd. Academic Press; 2023. pp. 639–68. [Google Scholar]
- 60.Szutorisz H, Hurd YL. High times for cannabis: epigenetic imprint and its legacy on brain and behavior. Neurosci Biobehav Rev. 2018;85:93–101. doi: 10.1016/j.neubiorev.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dinu AR, Rogobete AF, Bratu T, Popovici SE, Bedreag OH, Papurica M, et al. Cannabis Sativa revisited—crosstalk between microRNA expression, inflammation, oxidative stress, and endocannabinoid response system in critically ill patients with sepsis. Cells. 2020;9(2):307. doi: 10.3390/cells9020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Truong VB, Davis OS, Gracey J, Neal MS, Khokhar JY, Favetta LA. Sperm capacitation and transcripts levels are altered by in vitro THC exposure. BMC Mol Cell Biol. 2023;24:6. doi: 10.1186/s12860-023-00468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodriguez-Osorio N, Wang H, Rupinski J, Bridges SM, Memili E. Comparative functional genomics of mammalian DNA methyltransferases. Reprod BioMed Online. 2010;20(2):243–55. doi: 10.1016/j.rbmo.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 64.Carletti MZ, Fiedler SD, Christenson LK. MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells1. Biol Reprod. 2010;83(2):286–95. doi: 10.1095/biolreprod.109.081448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McBride D, Carré W, Sontakke SD, Hogg CO, Law A, Donadeu FX, et al. Identification of miRNAs associated with the follicular–luteal transition in the ruminant ovary. Reproduction. 2012;144(2):221–33. doi: 10.1530/REP-12-0025. [DOI] [PubMed] [Google Scholar]
- 66.Yerushalmi GM, Salmon-Divon M, Ophir L, Yung Y, Baum M, Coticchio G, et al. Characterization of the miRNA regulators of the human ovulatory cascade. Sci Rep. 2018;8(1):15605. doi: 10.1038/s41598-018-33807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kamalidehghan B, Habibi M, Afjeh SS, Shoai M, Alidoost S, Almasi Ghale R, et al. The importance of small non-coding RNAs in human reproduction: a review article. Appl Clin Genet. 2020;13:1–11. doi: 10.2147/TACG.S207491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bahmyari S, Jamali Z, Khatami SH, Vakili O, Roozitalab M, Savardashtaki A, et al. microRNAs in female infertility: an overview. Cell Biochem Funct. 2021;39(8):955–69. doi: 10.1002/cbf.3671. [DOI] [PubMed] [Google Scholar]
- 69.Li Y, Xiang Y, Song Y, Wan L, Yu G, Tan L. Dysregulated miR-142, -33b and -423 in granulosa cells target TGFBR1 and SMAD7: a possible role in polycystic ovary syndrome. Mol Hum Reprod. 2019;25(10):638–46. doi: 10.1093/molehr/gaz014. [DOI] [PubMed] [Google Scholar]
- 70.Zhang J, Xu Y, Liu H, Pan Z. MicroRNAs in ovarian follicular atresia and granulosa cell apoptosis. Reprod Biol Endocrinol. 2019;17:9. doi: 10.1186/s12958-018-0450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song DK, Sung YA, Lee H. The role of serum MicroRNA-6767-5p as a biomarker for the diagnosis of polycystic ovary syndrome. PLoS ONE. 2016;11(9):e0163756. doi: 10.1371/journal.pone.0163756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang YC, Ma JX. The role of MiR-324-3p in polycystic ovary syndrome (PCOS) via targeting WNT2B. Eur Rev Med Pharmacol Sci. 2018;22(11):3286–93. doi: 10.26355/eurrev_201806_15147. [DOI] [PubMed] [Google Scholar]
- 73.Su MT, Tsai PY, Tsai HL, Chen YC, Kuo PL. miR-346 and miR-582-3p-regulated EG-VEGF expression and trophoblast invasion via matrix metalloproteinases 2 and 9. BioFactors. 2017;43(2):210–19. doi: 10.1002/biof.1325. [DOI] [PubMed] [Google Scholar]
- 74.Hashimoto S, Saeki K, Nagao Y, Minami N, Yamada M, Utsumi K. Effects of cumulus cell density during in vitro maturation on the developmental competence of bovine oocytes. Theriogenology. 1998;49(8):1451–63. doi: 10.1016/S0093-691X(98)00091-0. [DOI] [PubMed] [Google Scholar]
- 75.Atef A, François P, Christian V, Marc-André S. The potential role of gap junction communication between cumulus cells and bovine oocytes during in vitro maturation. Mol Reprod Dev. 2005;71(3):358–67. doi: 10.1002/mrd.20281. [DOI] [PubMed] [Google Scholar]
- 76.Coticchio G, Albertini DF, De Santis L. Oogenesis. London: Springer; 2013. [Google Scholar]
- 77.Uyar A, Torrealday S, Seli E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil Steril. 2013;99(4):979–97. doi: 10.1016/j.fertnstert.2013.01.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bunel A, Nivet AL, Blondin P, Vigneault C, Richard FJ, Sirard MA. Cumulus cell gene expression associated with pre-ovulatory acquisition of developmental competence in bovine oocytes. Reprod Fertil Dev. 2014;26(6):855–65. doi: 10.1071/RD13061. [DOI] [PubMed] [Google Scholar]
- 79.Melo EO, Cordeiro DM, Pellegrino R, Wei Z, Daye ZJ, Nishimura RC, et al. Identification of molecular markers for oocyte competence in bovine cumulus cells. Anim Genet. 2017;48(1):19–29. doi: 10.1111/age.12496. [DOI] [PubMed] [Google Scholar]
- 80.Richani D, Dunning KR, Thompson JG, Gilchrist RB. Metabolic co-dependence of the oocyte and cumulus cells: essential role in determining oocyte developmental competence. Human Reprod Update. 2021;27(1):27–47. doi: 10.1093/humupd/dmaa043. [DOI] [PubMed] [Google Scholar]
- 81.Martinez CA, Rizos D, Rodriguez-Martinez H, Funahashi H. Oocyte-cumulus cells crosstalk: new comparative insights. Theriogenology. 2023;205:87–93. doi: 10.1016/j.theriogenology.2023.04.009. [DOI] [PubMed] [Google Scholar]
- 82.Whan LB, West MCL, McClure N, Lewis SEM. Effects of delta-9-tetrahydrocannabinol, the primary psychoactive cannabinoid in marijuana, on human sperm function in vitro. Fertil Sterility. 2006;85(3):653–60. doi: 10.1016/j.fertnstert.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 83.Dufour J, Sabry R, Khokhar JY, Favetta LA. Delta-9 tetrahydrocannabinol (THC) effects on the cortisol stress response in bovine granulosa cells. Toxicol In Vitro. 2023;88:105549. doi: 10.1016/j.tiv.2022.105549. [DOI] [PubMed] [Google Scholar]
- 84.Sabry R, Saleh AC, Stalker L, LaMarre J, Favetta LA. Effects of bisphenol A and bisphenol S on microRNA expression during bovine (Bos taurus) oocyte maturation and early embryo development. Reprod Toxicol. 2021;99:96–108. doi: 10.1016/j.reprotox.2020.12.001. [DOI] [PubMed] [Google Scholar]
- 85.Saleh AC, Sabry R, Mastromonaco GF, Favetta LA. BPA and BPS affect the expression of anti-Mullerian hormone (AMH) and its receptor during bovine oocyte maturation and early embryo development. Reprod Biol Endocrinol. 2021;19(1):119. doi: 10.1186/s12958-021-00773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sabry R, May DR, Favetta LA. The relationship between miR-21, DNA methylation, and bisphenol a in bovine COCs and granulosa cells. Front Cell Dev Biol. 2023;11:1294541. doi: 10.3389/fcell.2023.1294541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuzma-Hunt AG, Sabry R, Davis OS, Truong VB, Khokhar JY, Favetta LA. THC and sperm: impact on fertilization capability, pre-implantation in vitro development and epigenetic modifications. PLoS ONE. 2024;19(3):e0298697. doi: 10.1371/journal.pone.0298697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.FDA and Cannabis: research and drug approval process. FDA. 2023 Feb 27; Available from: https://www.fda.gov/news-events/public-health-focus/fda-and-cannabis-research-and-drug-approval-process. 15 June 2023.
- 89.Innocenzi E, De Domenico E, Ciccarone F, Zampieri M, Rossi G, Cicconi R, et al. Paternal activation of CB2 cannabinoid receptor impairs placental and embryonic growth via an epigenetic mechanism. Sci Rep. 2019;9(1):17034. doi: 10.1038/s41598-019-53579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clark J, Rager JE. Chapter 1 - Epigenetics: an overview of CpG methylation, chromatin remodeling, and regulatory/noncoding RNAs. In: Fry RC, editor. Environmental Epigenetics in Toxicology and Public Health. Academic Press; 2020. pp. 3–32. [Google Scholar]
- 91.Huntriss J. Epigenetic reprogramming in the embryo. In: Epigenet reprod health (Vol. 21). Elsevier; 2021. pp. 97–116. 6 June 2023.
- 92.Shen L, Zhang Y. 5-Hydroxymethylcytosine: generation, fate, and genomic distribution. Curr Opin Cell Biol. 2013;25(3):289–96. doi: 10.1016/j.ceb.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stoyanova E, Riad M, Rao A, Heintz N. 5-Hydroxymethylcytosine-mediated active demethylation is required for mammalian neuronal differentiation and function. eLife. 2021;10:e66973. doi: 10.7554/eLife.66973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clark KL, George JW, Przygrodzka E, Plewes MR, Hua G, Wang C, et al. Hippo Signaling in the Ovary: emerging Roles in Development, Fertility, and Disease. Endocrine Rev. 2022;43(6):1074–96. doi: 10.1210/endrev/bnac013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Plewes MR, Hou X, Zhang P, Liang A, Hua G, Wood JR, et al. Yes-associated protein 1 is required for proliferation and function of bovine granulosa cells in vitro. Biol Reprod. 2019;101(5):1001–17. doi: 10.1093/biolre/ioz139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsoi M, Morin M, Rico C, Johnson RL, Paquet M, Gévry N, et al. Lats1 and Lats2 are required for ovarian granulosa cell fate maintenance. FASEB J. 2019;33(10):10819–32. doi: 10.1096/fj.201900609R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lyu Z, Qin N, Tyasi TL, Zhu H, Liu D, Yuan S, et al. The Hippo/MST pathway member SAV1 plays a suppressive role in development of the prehierarchical follicles in Hen Ovary. PLoS ONE. 2016;11(8):e0160896. doi: 10.1371/journal.pone.0160896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun X, Niu X, Qin N, Shan X, Zhao J, Ma C, et al. Novel insights into the regulation of LATS2 kinase in prehierarchical follicle development via the Hippo pathway in hen ovary. Poult Sci. 2021;100(12):101454. doi: 10.1016/j.psj.2021.101454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lv X, He C, Huang C, Hua G, Chen X, Timm BK, et al. Reprogramming of ovarian granulosa cells by YAP1 leads to development of high-grade cancer with mesenchymal lineage and serous features. Sci Bull (Beijing) 2020;65(15):1281–96. doi: 10.1016/j.scib.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liang CG, Su YQ, Fan HY, Schatten H, Sun QY. Mechanisms regulating oocyte meiotic resumption: roles of mitogen-activated protein kinase. Mol Endocrinol. 2007;21(9):2037–55. doi: 10.1210/me.2006-0408. [DOI] [PubMed] [Google Scholar]
- 101.Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, et al. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324(5929):938–41. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rimon-Dahari N, Yerushalmi-Heinemann L, Alyagor L, Dekel N. Ovarian folliculogenesis. In: Piprek RP, editor. Molecular mechanisms of cell differentiation in gonad development. Cham: Springer International Publishing; 2016. pp. 167–90. [DOI] [PubMed] [Google Scholar]
- 103.Vassall M, Chakraborty S, Feng Y, Faheem M, Wang X, Bhandari RK. Transcriptional alterations induced by Delta-9 tetrahydrocannabinol in the brain and gonads of adult medaka. J Xenobiotics. 2023;13(2):237–51. doi: 10.3390/jox13020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smith RC, Sershen H, Janowsky DS, Lajtha A, Grieco M, Gangoiti JA, et al. Changes in expression of DNA-methyltransferase and cannabinoid receptor mRNAs in blood lymphocytes after acute Cannabis smoking. Front Psychiatry. 2022;13:887700. doi: 10.3389/fpsyt.2022.887700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mortusewicz O, Schermelleh L, Walter J, Cardoso MC, Leonhardt H. Recruitment of DNA methyltransferase I to DNA repair sites. Proc Natl Acad Sci. 2005;102(25):8905–09. doi: 10.1073/pnas.0501034102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ha K, Lee GE, Palii SS, Brown KD, Takeda Y, Liu K, et al. Rapid and transient recruitment of DNMT1 to DNA double-strand breaks is mediated by its interaction with multiple components of the DNA damage response machinery. Human Molecular Genetics. 2011;20(1):126–40. doi: 10.1093/hmg/ddq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jin B, Robertson KD. DNA methyltransferases (DNMTs), DNA damage repair, and cancer. Adv Exp Med Biol. 2013;754:3–29. doi: 10.1007/978-1-4419-9967-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nam EA, Cortez D. ATR signaling: more than meeting at the fork. Biochem J. 2011;436(3):527–36. doi: 10.1042/BJ20102162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Estève PO, Chang Y, Samaranayake M, Upadhyay AK, Horton JR, Feehery GR, et al. A methylation and phosphorylation switch between an adjacent lysine and serine determines human DNMT1 stability. Nat Struct Mol Biol. 2011;18(1):42–48. doi: 10.1038/nsmb.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang RL, Peng LX, Yang JP, Zheng LS, Xie P, Wang MY, et al. IL-8 suppresses E-cadherin expression in nasopharyngeal carcinoma cells by enhancing E-cadherin promoter DNA methylation. Int J Oncol. 2015;48. 10.3892/ijo.2015.3226. [DOI] [PubMed]
- 111.Koh HB, Scruggs AM, Huang SK. Transforming growth factor-β1 increases DNA methyltransferase 1 and 3a expression through distinct post-transcriptional mechanisms in lung fibroblasts. J Biol Chem. 2016;291(37):19287–98. doi: 10.1074/jbc.M116.723080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yan H, Wen J, Zhang T, Zheng W, He M, Huang K, et al. Oocyte-derived E-cadherin acts as a multiple functional factor maintaining the primordial follicle pool in mice. Cell Death Dis. 2019;10(3):160. doi: 10.1038/s41419-018-1208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Piprek RP, Kloc M, Mizia P, Kubiak JZ. The central role of cadherins in gonad development, reproduction, and fertility. Int J Mol Sci. 2020;21(21):8264. doi: 10.3390/ijms21218264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jackson AR, Nagarkatti P, Nagarkatti M. Anandamide attenuates Th-17 cell-mediated delayed-type hypersensitivity response by triggering IL-10 production and consequent microRNA induction. PLoS ONE. 2014;9(4):e93954. doi: 10.1371/journal.pone.0093954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sido JM, Jackson AR, Nagarkatti PS, Nagarkatti M. Marijuana-derived Δ-9-tetrahydrocannabinol suppresses Th1/Th17 cell-mediated delayed-type hypersensitivity through microRNA regulation. J Mol Med. 2016;94(9):1039–51. doi: 10.1007/s00109-016-1404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Al-Ghezi ZZ, Miranda K, Nagarkatti M, Nagarkatti PS. Combination of cannabinoids, Δ9-tetrahydrocannabinol and cannabidiol, ameliorates experimental multiple sclerosis by suppressing neuroinflammation through regulation of miRNA-mediated signaling pathways. Front Immunol. 2019;10. Accessed 18 June 2023. [DOI] [PMC free article] [PubMed]
- 117.Juknat A, Gao F, Coppola G, Vogel Z, Kozela E. miRNA expression profiles and molecular networks in resting and LPS-activated BV-2 microglia—effect of cannabinoids. PLoS ONE. 2019;14(2):e0212039. doi: 10.1371/journal.pone.0212039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Di Pietro C. Exosome-mediated communication in the ovarian follicle. J Assist Reprod Genet. 2016;33(3):303–11. doi: 10.1007/s10815-016-0657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Meccariello R, Santoro A, D’Angelo S, Morrone R, Fasano S, Viggiano A, et al. The epigenetics of the endocannabinoid system. Int J Mol Sci. 2020;21(3):1113. doi: 10.3390/ijms21031113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Viveiros MM, De La Fuente R. Chapter 8 - epigenetic control of oocyte development. In: Leung PCK, Qiao J, editors. Human reproductive and prenatal genetics. Academic Press; 2019. pp. 173–92. [Google Scholar]
- 121.Yang X, Hegde VL, Rao R, Zhang J, Nagarkatti PS, Nagarkatti M. Histone modifications are associated with Δ9-tetrahydrocannabinol-mediated alterations in antigen-specific T cell responses. J Biol Chem. 2014;289(27):18707–18. doi: 10.1074/jbc.M113.545210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Prini P, Penna F, Sciuccati E, Alberio T, Rubino T. Chronic Δ9-THC exposure differently affects histone modifications in the adolescent and adult rat brain. Int J Mol Sci. 2017;18(10):2094. doi: 10.3390/ijms18102094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Prini P, Rusconi F, Zamberletti E, Gabaglio M, Penna F, Fasano M, et al. Adolescent THC exposure in female rats leads to cognitive deficits through a mechanism involving chromatin modifications in the prefrontal cortex. J Psychiatry Neurosci. 2018;43(2):87–101. doi: 10.1503/jpn.170082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang JH, Chen JH, Guo B, Fang Y, Xu ZY, Zhan L, et al. Recent insights into noncoding RNAs in primary ovarian insufficiency: focus on mechanisms and treatments. J Clin Endocrinol Metab. 2023;dgad070. 10.1210/clinem/dgad304. [DOI] [PubMed]
- 125.Cao Z, Gao D, Xu T, Zhang L, Tong X, Zhang D, et al. Circular RNA profiling in the oocyte and cumulus cells reveals that circARMC4 is essential for porcine oocyte maturation. Aging (Albany NY) 2019;11(18):8015–34. doi: 10.18632/aging.102315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stein P, Rozhkov NV, Li F, Cárdenas FL, Davydenk O, Vandivier LE, et al. Essential role for endogenous siRNAs during meiosis in mouse oocytes. PLoS Genet. 2015;11(2):e1005013. doi: 10.1371/journal.pgen.1005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wei L, Xia H, Liang Z, Yu H, Liang Z, Yang X, et al. Disrupted expression of long non-coding RNAs in the human oocyte: the possible epigenetic culprits leading to recurrent oocyte maturation arrest. J Assist Reprod Genet. 2022;39(10):2215–25. doi: 10.1007/s10815-022-02596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hofmeister BT, Lee K, Rohr NA, Hall DW, Schmitz RJ. Stable inheritance of DNA methylation allows creation of epigenotype maps and the study of epiallele inheritance patterns in the absence of genetic variation. Genome Biol. 2017;18:155. doi: 10.1186/s13059-017-1288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.