Abstract
The in vivo passage of a neutralization-sensitive, laboratory-adapted simian-human immunodeficiency virus (SHIV-HXBc2) generated a pathogenic, neutralization-resistant virus, SHIV-HXBc2P 3.2. SHIV-HXBc2P 3.2 differs from SHIV-HXBc2 only in 13 amino acid residues of the viral envelope glycoproteins. Here we used antibody competition analysis to examine the structural changes that occurred in the SHIV-HXBc2P 3.2 gp120 exterior envelope glycoprotein. The relationships among the antibody epitopes on the conserved gp120 core of SHIV-HXBc2 and SHIV-HXBc2P 3.2 were similar. The third variable (V3) loop was more closely associated with the fourth conserved (C4) region and CD4-induced epitopes on the gp120 core in the HXBc2P 3.2 gp120 glycoprotein compared with the HXBc2 gp120 glycoprotein. Rearrangements of the second variable (V2) loop with respect to the CD4 binding site and associated epitopes were evident in comparisons of the two gp120 glycoproteins. Thus, the in vivo evolution of a neutralization-resistant virus involves conformational adjustments of the V2 and V3 variable loops with respect to the conserved receptor-binding regions of the gp120 core.
Human immunodeficiency virus type 1 (HIV-1) has evolved mechanisms to evade the humoral immune response to its envelope glycoproteins, gp120 and gp41 (20). This is a property that HIV-1 shares with other lentiviruses (1, 5, 14, 15, 20), suggesting that it might be necessary for the development or maintenance of a persistent, transmissible infection in vivo. The evasion mechanism(s) is manifested by the restricted ability of potentially neutralizing antibodies to bind to the native, trimeric form of the fusogenic envelope glycoprotein complex, as it exists on the surface of virions or virus-infected cells (20). In most well-studied examples, antibody binding to this complex results in virus neutralization. In most cases, neutralization occurs by inhibition of the binding of virions to the CD4 antigen and the coreceptor molecules, associations that trigger conformational changes in the viral envelope glycoproteins and hence virus-cell fusion (20, 27, 28, 29).
Upon in vitro passage of HIV-1, structural changes occur in the envelope glycoproteins that are associated with the acquisition of a more neutralization-sensitive phenotype, perhaps as an indirect consequence of selection for viral variants that more efficiently interact with cell surface receptors (20). How this occurs is not yet well understood. However, studying the antibody response to the HIV-1 envelope glycoproteins during natural infections has been facilitated by the development of the simian-human immunodeficiency virus (SHIV)-monkey model (12, 13, 21, 23). SHIVs are recombinant viruses in which segments of the HIV-1 genome (typically the tat, rev, vpu, and env genes) are inserted into a simian immunodeficiency virus backbone. SHIVs therefore express HIV-1 envelope glycoproteins yet, unlike HIV-1, efficiently infect monkeys (12, 13, 21, 23). One of the prototypic SHIVs was SHIV-HXBc2, which contains the envelope glycoproteins derived from a T-cell line-adapted X4 HIV-1 strain (12). SHIV-HXBc2, like HIV-1 HXBc2, is sensitive to most of the neutralizing monoclonal antibodies (MAbs) that are able to recognize epitopes present on the HXBc2 envelope glycoproteins (10, 11). In rhesus macaques, SHIV-HXBc2 replicated only to low levels and did not cause pathology within a 3-year period (11). Repeated in vivo passage of SHIV-HXBc2 resulted in the generation of a virus called KU-1 that could cause rapid CD4+ T-lymphocyte depletion and AIDS in infected monkeys (8). The KU-1 env gene was cloned and inserted into the parental SHIV-HXBc2 to create SHIV-HXBc2P 3.2, a virus that caused precipitous CD4+ T-lymphocyte loss and induced AIDS-like disease in rhesus macaques (4). Thus, mutations within the KU-1 env gene that resulted in only a few amino acid differences from the HXBc2 envelope glycoproteins were sufficient to account for the acquired immunopathogenicity of SHIV-HXBc2P 3.2. SHIV-HXBc2P 3.2 was also significantly more resistant to neutralization by soluble CD4 and neutralizing antibodies than the parental SHIV-HXBc2 (4). However, the epitopes for the neutralizing MAbs were retained on the monomeric SHIV-HXBc2P 3.2 gp120 envelope glycoprotein (4). Thus, in vivo passage of a virus expressing neutralization-sensitive HIV-1 envelope glycoproteins generated a closely related virus with a high degree of neutralization resistance, a property typical of primary HIV-1 isolates.
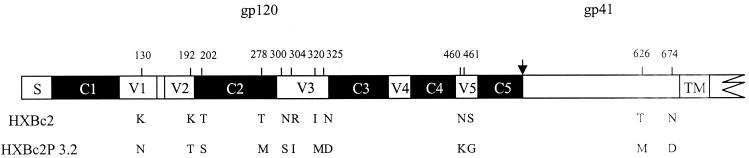
The envelope glycoprotein changes that occurred in the SHIV-HXBc2P 3.2 envelope glycoproteins upon in vivo passage are shown in Fig. 1. Compared with the parental HXBc2 envelope glycoproteins, the HXBc2P 3.2 envelope glycoproteins exhibit changes in five regions: the first variable/second variable (V1/V2) stem-loops, the second conserved (C2) region, the V3 region, the V5 region, and the gp41 ectodomain. The locations and nature of these changes are of interest. Three of the changes occur within the V1/V2 stem-loop structure, which has been implicated in modulation of neutralization sensitivity in other contexts (3, 32, 33). One of the observed changes, within the V1 loop, involves the acquisition of an N-linked glycosylation site in the HXBc2P 3.2 envelope glycoprotein. Sugar moieties hypothetically could contribute to steric masking of neutralization epitopes on the HIV-1 envelope glycoproteins. Conversely, loss of N-linked glycosylation sites at particular locations could assist in the tighter packing of envelope glycoprotein regions involved in the masking of neutralization epitopes. Three of the HXBc2P 3.2-associated changes, one in the C2 region and two in the gp41 ectodomain, involve the loss of potential N-linked glycosylation sites. The C2 change occurs in the gp120ℒD loop, which is located within a heavily glycosylated, outer domain rim that flanks the recessed CD4-binding region of gp120 (9, 31). Four amino acid changes occur in the base of the V3 loop, another region that can modulate HIV-1 neutralization sensitivity (7, 25, 34). None of these changes involve V3 residues previously implicated in coreceptor interactions (24), consistent with our observations that both HXBc2 and HXBc2P 3.2 envelope glycoproteins utilize the CXCR4 receptor almost exclusively (4). Finally, two residues within the HXBc2P 3.2 gp120 V5 region are altered compared with the gp120 glycoprotein of the parental HXBc2 virus.
FIG. 1.
Comparison of primary amino acid sequences of the HXBc2 and HXBc2P 3.2 envelope glycoproteins. The HIV-1 gp120 glycoprotein and part of the gp41 glycoprotein are depicted. S, signal peptide; TM, transmembrane region; V1 to V5, gp120 variable regions; C1 to C5, gp120 conserved regions. The arrow denotes the gp120-gp41 cleavage site. Residues that differ between the HXBc2 and HXBc2P 3.2 envelope glycoproteins are denoted by numbers, with the amino acid in single-letter code noted beneath the sequence.
To examine the structural basis of neutralizing antibody resistance, we performed a comparative topological analysis of the antibody epitopes on the monomeric forms of the HXBc2 and HXBc2P 3.2 gp120 exterior envelope glycoproteins, using procedures described in detail elsewhere (2, 18). The sources of monomeric gp120s from HXBc2 and HXBc2P 3.2 were culture supernatants from 293T cells transfected with the respective env genes. The gp120 proteins were captured directly from the supernatants onto a solid phase by adsorbed sheep polyclonal antibody D7324 (2, 18, 19). This antibody was raised to a peptide spanning the C-terminal 15 amino acids of HIV-1 LAI, a well-conserved region that is proximal to a segment of the gp41-binding site on gp120 (6, 16, 30). Via D7324, the gp120 molecule is captured with a geometry that roughly mimics its orientation on virions, with the position of the solid phase corresponding to that of the viral membrane. Antibodies are able to react with exposed epitopes on the captured gp120 proteins (18).
In initial experiments, antibody titration curves were performed with biotin-labeled MAbs (bio-MAbs) to determine the relative affinities of each MAb for the HXBc2 and HXBc2P 3.2 gp120 glycoproteins. The concentration of each bio-MAb required to achieve 50% of maximal binding to the two gp120 glycoproteins is shown in Table 1. All antibodies used in the study exhibited roughly similar affinities for the HXBc2 and HXBc2P 3.2 gp120 glycoproteins, with the exception of two V3 loop-directed antibodies. The G3-1472 and 110.I antibodies both bound the HXBc2P 3.2 gp120 glycoprotein with lower affinity than the HXBc2 envelope glycoprotein. This difference in affinity probably results from a subset of the V3 loop sequence differences between HXBc2 and HXBc2P 3.2 gp120 glycoproteins. The binding curves of the bio-MAbs were used to select the optimal concentration of each bio-MAb to use in the cross-competition experiments. Typically, we used a bio-MAb concentration that led to the generation of a signal at an optical density at 492 nm of approximately 1.20, which usually represented approximately 70% of the saturating binding concentration.
TABLE 1.
Concentrations of bio-MAbs yielding 50% maximal binding
| Epitope | bio-MAb | 50% binding concna
|
||
|---|---|---|---|---|
| HXBc2 | HXBc2P 3.2 | Ratio, HXBc2P 3.2/HXBc2 | ||
| C1-C4 (D) | A32 | 0.1 | 0.06 | 0.6 |
| C1-C5 (D) | 212A | 0.2 | 0.15 | 0.75 |
| C1 (L) | 135/9 | 0.003 | 0.002 | 0.66 |
| CD4i (D) | 48d | 0.2 | 0.3 | 1.5 |
| 17b | 0.7 | 0.3 | 0.43 | |
| CD4BS (D) | IgGb12 | 0.67 | 0.2 | 0.3 |
| F91 | 0.025 | 0.025 | 1.0 | |
| CD4 (D) | sCD4 | 0.007 | 0.015 | 2.5 |
| C4 (L) | G3-508 | 0.001 | 0.001 | 1.0 |
| G3-519 | 0.067 | 0.067 | 1.0 | |
| C4-V3 (D) | G3-299 | 0.025 | 0.025 | 1.0 |
| V2 (D) | SC258 | 0.24 | 0.2 | 0.83 |
| G3-4 | 0.025 | 0.025 | 1.0 | |
| G3-136 | 0.067 | 0.67 | 1.0 | |
| CRA-3 | 0.8 | 0.45 | 0.56 | |
| V2 (L) | BAT085 | 0.15 | 0.075 | 0.5 |
| 11/4C | 1/600b | 1/600b | 1.0 | |
| V3 (L) | G3-1472 | 0.001 | 0.025 | 25 |
| 110.5 | 0.015 | 0.02 | 1.3 | |
| 110.I | 0.024 | 0.25 | 10.4 | |
| 110.J | 0.22 | 0.8 | 3.6 | |
| C3-V4 (D) | 2G12 | 0.25 | 0.15 | 0.6 |
The HXBc2 and HXBc2P 3.2 gp120 glycoproteins were captured on an enzyme-linked immunosorbent assay plate using the polyclonal antiserum D7324. The bio-MAbs were incubated with the captured gp120 glycoproteins at concentrations of 3 μg/ml and threefold serial dilutions thereof. The bound antibody was detected as described elsewhere (18), titration curves were generated, and half-maximum binding concentrations were calculated. D, discontinuous epitope; L, linear epitope.
Hybridoma supernatants were used.
The antibody cross-competition analysis was performed by measuring the extent of the binding of each bio-MAb, at its predetermined, optimal concentration, to gp120 in the presence or absence of competitor MAbs. The competitors were added at a fixed concentration of 5 or 10 μg/ml, this usually representing a saturating concentration. Each competitor MAb was tested in triplicate, and each experiment was usually performed three or more times, ensuring that at least nine individual enzyme-linked immunosorbent assay wells contribute to the datum point for each competitor MAb. The extent of bio-MAb binding in the absence of the competitor MAb was defined as 100%. The ratio between the binding of the bio-MAb in the presence and absence of each competitor was then calculated as a percentage. A value of <50% is indicative of significant inhibition of the binding of the bio-MAb by the competitor; one of >125% means that the competitor MAb has enhanced the binding of the bio-MAb, most probably by inducing a conformational change in the gp120 molecules that improves the accessibility or integrity of the epitope for the bio-MAb (2, 18).
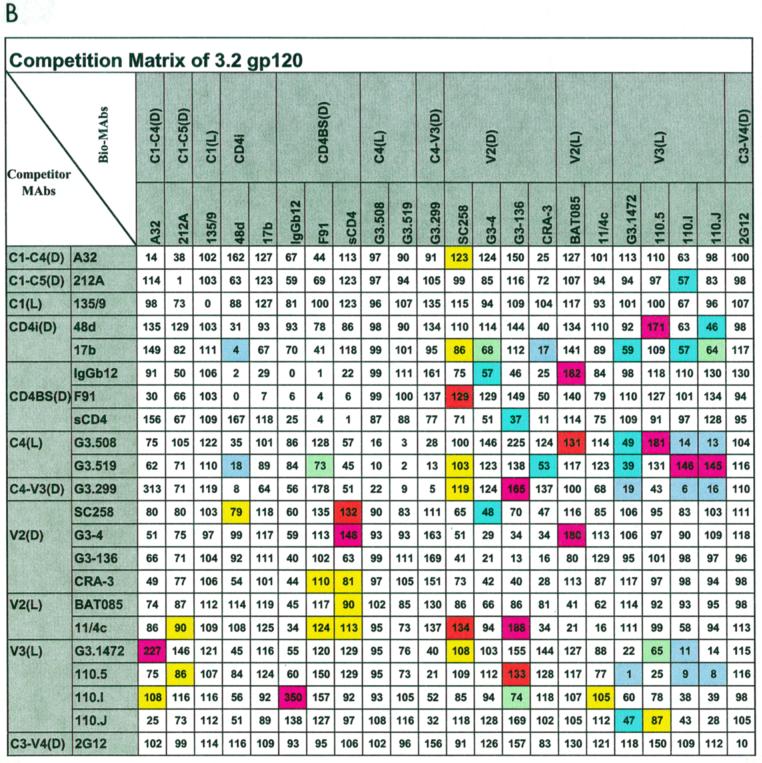
The antibody competition matrices are shown in Fig. 2. We have highlighted in color on the matrices those MAb combinations that lead to significant and highly reproducible differences in the extent of competition observed when the HXBc2 and HXBc2 3.2P gp120s are compared. Competition values that differ by ≥40% between the HXBc2 and HXBc2P 3.2 gp120 glycoproteins are thus colored according to the observed degree of inhibition or enhancement.
FIG. 2.
Antibody competition matrices for the HXBc2 (A) and HXBc2P 3.2 (B) gp120 glycoproteins. Each matrix displays the results of cross-competition experiments in which the binding MAb (listed in the top row) is biotin labeled and the competitor MAb (listed in the left-hand column) is unlabeled. Numbers in the individual boxes refer to the extent of binding of the test bio-MAb to gp120 in the presence of each competitor MAb, expressed as a percentage of control (no competitor = 100%). A number greater than 100 indicates that the competitor MAb has enhanced the binding of the bio-MAb; one less than 100 indicates that inhibition has occurred. In practice, only values of >125 or <75 are considered experimentally significant. On the two matrices, we have highlighted competition values that differ by ≥40% between the HXBc2 and HXBc2P 3.2 gp120s, to indicate significant differences between the two proteins with respect to MAb binding. The highlighted boxes are colored according to the degree of observed competition or enhancement (see scale beneath the matrix).
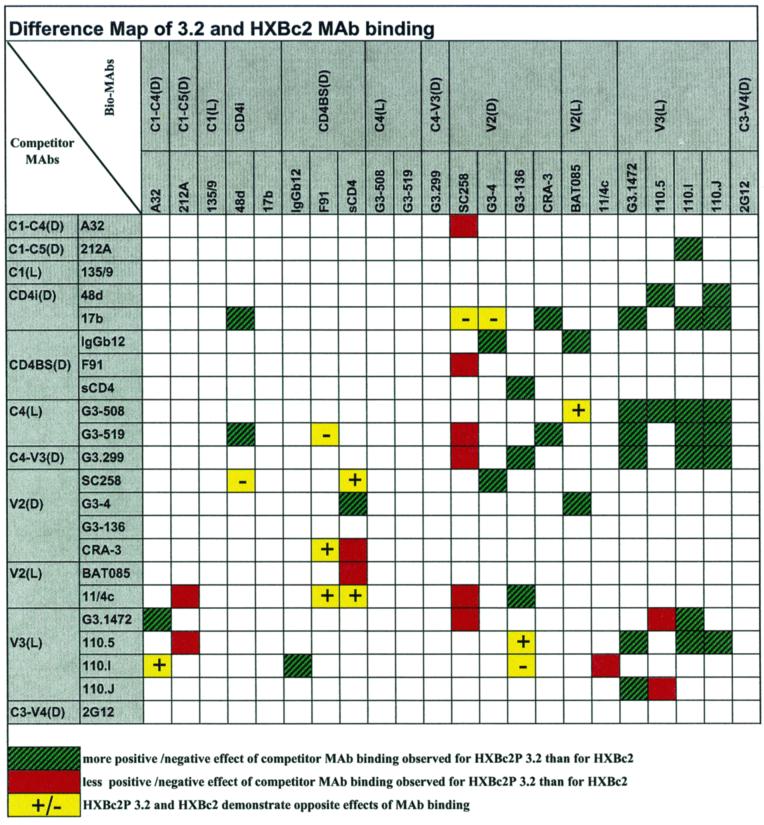
Figure 3 shows a difference map, which highlights competition values that exhibit ≥40% difference between the two gp120 glycoproteins. Green squares indicate instances where either a more positive or more negative effect of competitor MAb binding was observed for the HXBc2P 3.2 gp120 than for the HXBc2 gp120. In those instances where differences in MAb affinity for the two glycoproteins do not explain the results, the more pronounced effects of competitor MAb binding indicate a closer relationship of the two epitopes on the HXBc2P 3.2 gp120 glycoprotein. A less positive or negative effect of competitor MAb binding for the HXBc2P 3.2 gp120 than for the HXBc2 gp120 probably indicates a more distant relationship between the antibody epitopes on the HXBc2P 3.2 gp120 glycoprotein, in those instances where the results cannot be explained by differences in MAb affinity for the HXBc2 and HXBc2P 3.2 gp120 glycoproteins.
FIG. 3.
Difference map of the HXBc2 and HXBc2P 3.2 gp120 envelope glycoproteins. Boxes where the difference between the values for the HXBc2 and HXBc2P 3.2 gp120 glycoproteins differ by ≥40% are highlighted. Green squares indicate instances where either a more positive or more negative effect of competitor MAb binding was observed for the HXBc2P 3.2 gp120 than for the HXBc2 gp120; red squares indicate instances where a less positive or less negative effect of competitor MAb binding was observed for the HXBc2P 3.2 gp120 than for the HXBc2 gp120; yellow squares indicate instances where opposite effects of the competitor MAb were seen for the two gp120 glycoproteins. A plus sign indicates that the competitor MAb exerted a positive effect on the binding of the labeled antibody to the HXBc2P 3.2 envelope glycoprotein; a minus sign indicates that the competitor MAb exerted a negative effect on the binding of the labeled antibody to the HXBc2P 3.2 envelope glycoproteins.
Several general features of the difference map are noteworthy. There are 35 green squares but only 13 red and 12 yellow squares. The predominance of green squares suggests that there may be a greater overall proximity of antibody epitopes on the HXBc2P 3.2 gp120 glycoprotein than on the HXBc2 gp120 glycoprotein. The general positions of the highlighted squares on the matrix are also revealing. The paucity of highlighted squares in the upper left quadrant, which details the relationship between conserved epitopes in the gp120 core, indicates little overall difference between the antigenic structures of the HXBc2 and HXBc2P 3.2 gp120 cores. This is consistent with the locations of most of the amino acid differences between the two proteins in the gp120 variable regions (Fig. 1). It is also consistent with the expectation that the intra- and interdomain relationships in the gp120 core will be conserved among virus variants. This expectation is based on the high degree of conservation of the gp120 residues within the core domains and in the domain interfaces in primate immunodeficiency viruses (9, 31).
The upper right quadrant of the difference map is heavily populated with highlighted squares, indicating substantial differences between HXBc2 and HXBc2P 3.2 gp120 glycoproteins in the relationship of the major variable loops, V2 and V3, to the conserved core (Fig. 3). That most of these squares are green suggests that the V2 and V3 loops interact more intimately with the HXBc2P 3.2 gp120 core than with the HXBc2 core. It is noteworthy that the lower left quadrant, which details the reciprocal effects of variable loop-directed MAbs on the binding of MAbs against the gp120 core, is less populated than the upper right quadrant. This indicates that MAb binding to a gp120 core epitope is more likely to register an effect on the binding of a variable loop-directed MAb than vice versa. Some insight into this observation derives from recent studies indicating an unusually high degree of flexibility among the gp120 core domains (19a). Ligands that bind the conserved, discontinuous structures in the gp120 core decrease this flexibility and limit the accessibility of gp120 to other ligands quite effectively (P. Kwong, R. Wyatt, W. Hendrickson, and J. Sodroski, unpublished observations). In contrast, MAbs against the gp120 variable loops do not alter the flexibility of the core; thus, the subsequent binding of ligands to the core involves a relatively flexible structure, diminishing the steric impact of the already bound antibody.
Specific highlighted data points within the difference map arise from two sources: (i) differences in the relative affinities of MAbs for the HXBc2 and HXBc2P 3.2 gp120 glycoproteins and (ii) conformational changes between the two gp120 molecules that alter the relationship of epitopes on the gp120 surface. The observed differences between the gp120 glycoproteins in the competition of the G3-1472 V3 MAb by several MAbs probably arises from the significantly lower affinity of the G3-1472 MAb for the HXBc2P 3.2 glycoprotein. Likewise, the differences observed for the HXBc2 and HXBc2P 3.2 glycoproteins in the ability of the 17b and 48d antibodies to compete for binding of the 110.I and possibly the 110.J anti-V3 MAbs could be a consequence of the somewhat lower affinity of these V3 MAbs for the HXBc2P 3.2 gp120. The altered pattern of V3 MAbs competing for the binding of other V3 MAbs to the two gp120 glycoproteins reflects the relative affinities of these MAbs for the HXBc2P 3.2 gp120 glycoprotein.
Most of the observed differences in the competition maps between the HXBc2 and HXBc2P 3.2 gp120 glycoproteins cannot, however, be explained by variation in the relative affinities of the involved MAbs for the two gp120 glycoproteins. The effects of C4-directed MAbs on the 110.I and 110.J V3 MAbs, as well as on the 110.5 MAb, likely reflect a decreased distance between the C4 and V3 regions on the HXBc2P 3.2 gp120 glycoprotein. The 48d antibody against a CD4-induced (CD4i) epitope also exhibits a greater effect on the binding of the 110.5 V3 MAb to the HXBc2P 3.2 gp120 glycoprotein. This is consistent with a V3 loop position on the HXBc2P 3.2 gp120 glycoprotein that is closer to the CD4i and C4 epitopes, which overlap considerably (9, 26, 31). The increased proximity of the V3 loop to the C4 and CD4i epitopes also explains the effects of G3-519 and 17b, C4 and CD4i MAbs, respectively, on the binding of the 48d antibody. The latter antibody recognizes a CD4i epitope but is strongly influenced by the V3 loop conformation and may even recognize elements of V3 as part of its epitope (26, 33). Thus, the few altered relationships among core epitopes of the HXBc2P 3.2 gp120 that are evident in the difference map involve core structures exhibiting intimate relationships with a variable loop.
The conformation of the V2 variable loop must also change in the HXBc2P 3.2 gp120 glycoprotein relative to that in the HXBc2 glycoprotein. The behavior of the V2 MAbs indicates the existence of two subsets of these epitopes. Most V2 epitopes appear to be closer to the CD4-binding site (CD4BS) and C4 epitopes in the HXBc2P 3.2 gp120 glycoprotein. By contrast, the SC258 epitope appears to be more distant from many gp120 epitopes in the HXBc2P 3.2 gp120 glycoprotein. Although this potentially could result from a lower affinity of the SC258 MAb for the HXBc2P 3.2 gp120 glycoprotein than for the HXBc2 gp120, direct assessment of the SC258 binding affinities for the two gp120 glycoproteins did not reveal any significant difference (Table 1). Together these data suggest conformational rearrangements within the V2 loop as well as movement of the V2 loop in relationship to the HXBc2P 3.2 gp120 core.
The 2G12 epitope, which is a carbohydrate-dependent structure (26a), does not appear to change its relationship to other gp120 epitopes in the HXBc2P 3.2 glycoprotein compared with the HXBc2 glycoprotein. This is consistent with the location of the 2G12 epitope on the gp120 outer domain (31). The 2G12 epitope is thus removed from the other gp120 neutralization epitopes, including the variable loops (18).
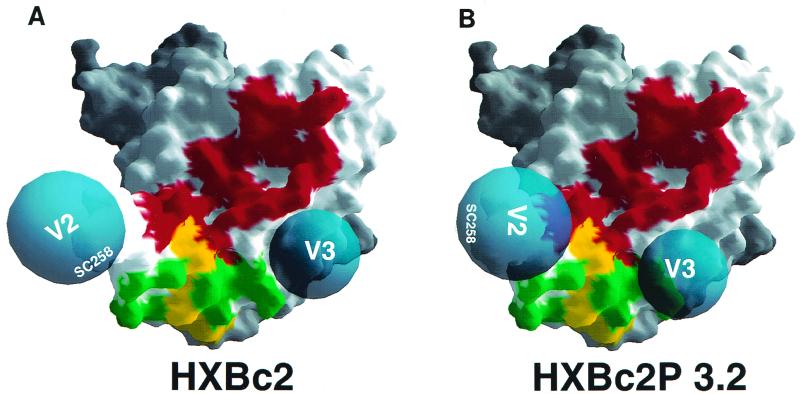
The antibody competition analysis was used to create models of the HXBc2 and HXBc2P 3.2 gp120 monomers (Fig. 4). In the models, the gp120 core corresponds to the HXBc2 structure crystallized in a complex with soluble CD4 and the Fab fragment of a CD4i antibody (9, 31). As discussed above, it is likely that free gp120 can assume many conformations (19a). However, as the only available detailed structure of the HIV-1 gp120 core is derived from this ternary complex, we have chosen to use it here. The assumptions underlying the models are that once the effects attributable to MAb affinity differences are eliminated, greater positive or negative effects in the antibody competition analysis indicate increased epitope proximity; thus, green squares in the difference map are modeled as greater proximity between the epitopes on the HXBc2P 3.2 gp120 glycoprotein compared with those on the HXBc2 gp120 glycoprotein. Additional constraints on the origins of the V2 and V3 strands imposed by the gp120 core structure (9) have been considered in the models.
FIG. 4.
Models of the HXBc2 and HXBc2P 3.2 gp120 envelope glycoproteins. The molecular surface of the HIV-1 gp120 core from the HXBc2 strain in a CD4-bound conformation is shown, with locations of the CD4-binding site (red) and the C4 (yellow) and CD4i (green) epitopes indicated. The red and green surfaces represent those regions that are ≤5 Å away (center-to-center distance) from CD4 and 17b in the ternary complex crystal (9). The deduced locations of the V2 and V3 loops in the HXBc2 and HXBc2P 3.2 gp120 glycoproteins are indicated. The sizes of the V2 and V3 loops, which contain sites for N-linked glycosylation, are only roughly approximated. The figure was drawn using the GRASP program.
Comparison of the models reveals a movement of the V2 and V3 loops in the HXBc2P 3.2 gp120 glycoprotein relative to the positions assumed in the HXBc2 gp120. A consequence of this movement is the approximation of the variable loops and the conserved neutralization epitopes near the receptor-binding regions. In particular, the conserved gp120 region near the CD4i epitopes that is implicated in chemokine receptor binding (22) is more effectively masked in the HXBc2P 3.2 gp120 model. This would essentially sequester the HXBc2P 3.2 chemokine receptor-binding surface from potentially neutralizing antibodies until binding to host cell CD4 occurs, at which time steric constraints limit the interaction of these antibodies with their epitope on gp120 (25). Masking of the receptor-binding regions of the HXBc2P 3.2 gp120 glycoprotein was not observed simply by measuring the affinity of MAbs directed against the CD4BS, CD4i, or C4 epitopes for the monomeric gp120 glycoproteins (Table 1). Such masking may be subtle in this context or difficult to detect due to the conformational flexibility of free gp120, alluded to above. Presumably, on the functional HIV-1 envelope glycoprotein trimer, the more limited flexibility results in an improved effectiveness of the overlying variable loops in diminishing recognition of the conserved epitopes by neutralizing antibodies.
The results are consistent with predictions made regarding the structural differences between primary and laboratory-adapted HIV-1 envelope glycoproteins (17a) and with the genetic mapping of neutralization resistance determinants within the gp120 glycoproteins of other primary HIV-1 isolates (5a, 25). Additional studies mapping the genetic determinants of neutralization resistance of SHIV-HXBc2P 3.2 should provide additional details of the structural basis of this resistance. It will be particularly important to understand the structural differences between the HXBc2 and HXBc2P 3.2 envelope glycoproteins in the context of the virion-associated oligomer. This understanding can guide attempts at intervention against HIV-1 infection by drugs and vaccines.
Acknowledgments
We thank the suppliers of MAbs and soluble CD4.
This work was supported by grants AI 39420, AI 36082, and AI 31783 from the National Institutes of Health. This work was also supported by the G. Harold and Leila Y. Mathers Foundation, the late William F. McCarty-Cooper, the Friends 10, and Douglas and Judith Krupp. J.P.M. is an Elizabeth Glaser Scientist of the Pediatric AIDS Foundation.
REFERENCES
- 1.Baldinotti F, Matteucci D, Mazzetti P, Gianelli C, Bandecchi P, Tozzini F, Bendinelli M. Serum neutralization of feline immunodeficiency virus is markedly dependent on passage history of the virus and host system. J Virol. 1994;68:4572–4579. doi: 10.1128/jvi.68.7.4572-4579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binley J, Wyatt R, Desjardins E, Kwong P, Hendrickson W, Moore J, Sodroski J. Analysis of the interaction of antibodies with a conserved, enzymatically deglycosylated core of the HIV-1 gp120 envelope glycoprotein. AIDS Res Hum Retroviruses. 1997;14:191–198. doi: 10.1089/aid.1998.14.191. [DOI] [PubMed] [Google Scholar]
- 3.Cao J, Sullivan N, Desjardins E, Parolin C, Robinson J, Wyatt R, Sodroski J. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1/V2 variable loops of the gp120 envelope glycoprotein. J Virol. 1997;71:9808–9812. doi: 10.1128/jvi.71.12.9808-9812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cayabyab M, Karlsson G, Etemad-Moghadam B, Hofmann W, Steenbeke T, Halloran M, Fanton J, Axthelm M, Letvin N, Sodroski J. Changes in the HIV-1 envelope glycoproteins responsible for the pathogenicity of a multiply passaged simian-human immunodeficiency virus (SHIV-HXBc2) J Virol. 1999;73:976–984. doi: 10.1128/jvi.73.2.976-984.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook R F, Berger S L, Rushlow K E, McManus J M, Cook S J, Harrold S, Raabe M L, Montelaro R C, Issel C J. Enhanced sensitivity to neutralizing antibodies in a variant of equine infectious anemia virus is linked to amino acid substitutions in the surface unit envelope glycoprotein. J Virol. 1995;69:1493–1499. doi: 10.1128/jvi.69.3.1493-1499.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Etemad-Moghadam B, Sun Y, Nicholson E, Karlsson G, Schenten D, Sodroski J. Determinants of neutralization resistance in the envelope glycoproteins of a simian-human immunodeficiency virus passaged in vivo. J Virol. 1999;73:8873–8879. doi: 10.1128/jvi.73.10.8873-8879.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helseth E, Olshevsky U, Furman C, Sodroski J. Human immunodeficiency virus type I gp120 envelope glycoprotein regions important for association with the gp41 transmembrane glycoprotein. J Virol. 1991;65:2119–2123. doi: 10.1128/jvi.65.4.2119-2123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogervorst E, de Jong J, van Wijk A, Bakker M, Valk M, Nara P, Goudsmit J. Insertion of primary syncytium-inducing (SI) and non-SI envelope V3 loops in human immunodeficiency virus type 1 (HIV-1) LAI reduces neutralization sensitivity to autologous, but not heterologous, HIV-1 antibodies. J Virol. 1995;69:6342–6351. doi: 10.1128/jvi.69.10.6342-6351.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joag S V, Li Z, Foresman L, Stephens E B, Zhao L-J, Adany I, Pinson D M, McClure H M, Narayan O. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J Virol. 1996;70:3189–3197. doi: 10.1128/jvi.70.5.3189-3197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwong P D, Wyatt R, Robinson J, Sweet R, Sodroski J, Hendrickson W. Structure of an HIV-1 gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li A, Baba T, Sodroski J, Zolla-Pazner S, Gorny M, Robinson J, Posner M, Katinger H, Barbas C, Burton D, Chou T-C, Ruprecht R. Synergistic neutralization of a chimeric SIV/HIV-1 virus with combinations of human anti-HIV-1 envelope monoclonal antibodies or hyperimmune globulins. AIDS Res Hum Retroviruses. 1997;13:647–655. doi: 10.1089/aid.1997.13.647. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Halloran M, Lord C, Watson A, Ranchalis J, Fung M, Letvin N, Sodroski J. Persistent infection of macaques with simian-human immunodeficiency viruses. J Virol. 1995;69:7061–7071. doi: 10.1128/jvi.69.11.7061-7067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Lord C I, Haseltine W, Letvin N L, Sodroski J. Infection of cynomolgus monkeys with a chimeric HIV-1/SIVmac virus that expresses the HIV-1 envelope glycoproteins. J Acquir Immune Defic Syndr. 1992;5:639–646. [PubMed] [Google Scholar]
- 13.Luciw P A, Pratt-Lowe E, Shaw K E S, Levy J A, Cheng-Mayer C. Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV) Proc Natl Acad Sci USA. 1995;92:7490–7494. doi: 10.1073/pnas.92.16.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Means R E, Greenough T, Desrosiers R C. Neutralization sensitivity of cell culture-passaged simian immunodeficiency virus. J Virol. 1997;71:7895–7902. doi: 10.1128/jvi.71.10.7895-7902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montefiori D C, Reimann K A, Wyand M S, Manson K, Lewis M G, Collman R G, Sodroski J G, Bolognesi D P, Letvin N L. Neutralizing antibodies in sera from macaques infected with chimeric simian-human immunodeficiency virus containing the envelope glycoproteins of either a laboratory-adapted variant or a primary isolate of human immunodeficiency virus type 1. J Virol. 1998;72:3427–3431. doi: 10.1128/jvi.72.4.3427-3431.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore J P, McCutchan F E, Poon S-W, Mascola J, Liu J, Cao Y, Ho D D. Exploration of antigenic variation in gp120 from clades A through F of human immunodeficiency virus type 1 by using monoclonal antibodies. J Virol. 1994;68:8350–8364. doi: 10.1128/jvi.68.12.8350-8364.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore J, Sattentau Q, Yoshiyama H, Thali M, Charles M, Sullivan N, Poon S-W, Fung M, Traincard F, Robinson J, Ho D D, Sodroski J. Probing the structure of the V2 domain of the human immunodeficiency virus type 1 surface glycoprotein gp120 with a panel of eight monoclonal antibodies: the human immune response to the V1 and V2 domains. J Virol. 1993;67:6136–6151. doi: 10.1128/jvi.67.10.6136-6151.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Moore J P, Ho D D. HIV-1 neutralization: the consequences of viral adaptation to growth on transformed T cells. AIDS. 1995;9:S117–S136. [PubMed] [Google Scholar]
- 18.Moore J P, Sodroski J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70:1863–1872. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore J P, Wallace L A, Follett E A C, McKeating J A. An ELISA for antibodies to the envelope glycoprotein of divergent strains of HIV-1. AIDS. 1989;3:155–163. doi: 10.1097/00002030-198903000-00006. [DOI] [PubMed] [Google Scholar]
- 19a.Myszka D, Sweet R W, Hensley P, Brigham-Burke M, Kwong P D, Hendrickson W A, Wyatt R, Sodroski J, Doyle M. Energetics of the HIV gp120-CD4 binding reaction. Proc Natl Acad Sci USA. 2000;97:9026–9031. doi: 10.1073/pnas.97.16.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parren P H I, Moore J P, Burton D R, Sattentau Q J. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS. 1999;13:S137–S162. [PubMed] [Google Scholar]
- 21.Reimann K, Li J, Voss G, Lekutis C, Tenner-Racz K, Racz P, Lin W, Montefiori D, Lee-Paritz D, Collman R, Sodroski J, Letvin N. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J Virol. 1996;70:3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizzuto C, Wyatt R, Hernández-Ramos N, Sun Y, Kwong P D, Hendrickson W A, Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 23.Shibata R, Adachi A. SIV/HIV recombinants and their use in studying biological properties. AIDS Res Hum Retroviruses. 1992;8:403–409. doi: 10.1089/aid.1992.8.403. [DOI] [PubMed] [Google Scholar]
- 24.Speck R, Wehryl K, Platt E, Atchison R, Charo I, Kabat D, Chesebro B, Goldsmith M. Selective employment of chemokine receptors as human immunodeficiency virus type 1 coreceptors determined by individual amino acids within the envelope V3 loop. J Virol. 1997;71:7136–7139. doi: 10.1128/jvi.71.9.7136-7139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan N, Sun Y, Binley J, Lee J, Barbas C, Parren P, Burton D, Sodroski J. Determinants of human immunodeficiency virus type 1 envelope glycoprotein activation by soluble CD4 and monoclonal antibodies. J Virol. 1998;72:6332–6338. doi: 10.1128/jvi.72.8.6332-6338.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thali M, Moore J P, Furman C, Charles M, Ho D D, Robinson J, Sodroski J. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol. 1993;67:3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Trkola A, Purtscher M, Muster T, Ballaum C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore J, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody sensitive interactions between HIV-1 and its co-receptor CCR5. Nature. 1996;384:184–186. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 28.Ugolini S, Mondor I, Parren P W H I, Burton D R, Tilley S, Klasse P J, Sattentau Q J. Inhibition of virus attachment to CD4+ target cells is a major mechanism of T cell line-adapted HIV-1 neutralization. J Exp Med. 1997;186:1287–1298. doi: 10.1084/jem.186.8.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu L, Gerard N, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A, Desjardins E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 30.Wyatt R, Desjardins E, Olshevsky V, Nixon C, Binley J, Olshevsky U, Sodroski J. Analysis of the interaction of the human immunodeficiency virus type 1 gp120 envelope glycoprotein with the gp41 transmembrane glycoprotein. J Virol. 1997;71:9722–9731. doi: 10.1128/jvi.71.12.9722-9731.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyatt R, Kwong P D, Desjardins E, Sweet R, Robinson J, Hendrickson W, Sodroski J. The antigenic structure of the human immunodeficiency virus gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 32.Wyatt R, Moore J, Accola M, Desjardins E, Robinson J, Sodroski J. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J Virol. 1995;69:5723–5733. doi: 10.1128/jvi.69.9.5723-5733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wyatt R, Sullivan N, Thali M, Repke H, Ho D, Robinson J, Posner M, Sodroski J. Functional and immunologic characterization of human immunodeficiency virus type 1 envelope glycoproteins containing deletions of the major variable regions. J Virol. 1993;67:4557–4565. doi: 10.1128/jvi.67.8.4557-4565.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wyatt R, Thali M, Tilley S, Pinter A, Posner M, Ho D, Robinson J, Sodroski J. Relationship of the human immunodeficiency virus type 1 gp120 third variable loop to a component of the CD4 binding site in the fourth conserved region. J Virol. 1992;66:6997–7004. doi: 10.1128/jvi.66.12.6997-7004.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]