Abstract
Introduction of DNA into normal and immunodeficient mice, alone or in complex with VP1 pseudocapsids, has been compared to DNA transfer by viral infection. Similar to natural infection and in contrast to plasmid alone, VP1 pseudocapsids efficiently introduced DNA, which remained for months in normal mice and possibly longer in B- and T-cell-deficient mice.
Several gene transfer systems using adeno- and retroviruses as vectors have the undesirable drawback of retaining viral genes that can potentially interact with host genes (17). To avoid such complications, in vivo approaches have been made using self-assembled murine polyomavirus major capsid protein, VP1, as a vector (7, 11, 15). In the work reported here, the tissue distribution and influence of the immune response on gene transfer in vivo by VP1 pseudocapsids was studied in detail, and the data obtained were compared to those observed with natural virions. A polyomavirus specific PCR (4) was used to follow the spread and the longevity of DNA introduced by this route up to 6 months postinoculation (p.i.). Polyomavirus mutant dl1023—with a deletion at position 5278 to 5286, which abolishes viral replication, and cloned into pBR322 (total, 9 kb)—was used as a substrate to compare delivery either as “naked” DNA or by pseudocapsids. Both routes were compared with inoculation by natural virions into normal mice, where infection has been shown to be limited in time (5), and in B-cell-deficient (μMT) (10) and T-cell-deficient (CD4−/− CD8−/−) mice (14), where infection persists (2, 3). To quantify DNA copy numbers in selected tissues, real-time PCR was used with heart and bone of normal, CD4−/− CD8−/−, and μMT mice on C57BL/6 background (10, 14). VP1 pseudocapsids were prepared and purified from Sf9 insect cells (6, 15). The 9-kb dl1023-pBR322 recombinant plasmid (12) was purified using the maxi kit (QIAGEN GmbH). For intraperitoneal inoculation of each mouse, 5 μg of plasmid was mixed with 150 μg of sonicated (3 × 30 s) polyomavirus VP1 pseudocapsids in 150 μl of phosphate-buffered saline and was incubated at room temperature for 30 min. Mice were inoculated intraperitoneally either with 5 μg of dl1023-pBR322 plasmid alone or in complex with 150 μg of VP1 pseudocapsids or with approximately 5 × 107 PFU of large-plaque A2 strain of polyomavirus (8). Sera from the inoculated mice were tested for the presence of polyomavirus antibodies by hemagglutination inhibition tests (2). To monitor the persistence and tissue distribution of the introduced viral information, the mice were sacrificed up to 6 to 8 months p.i., and a polyomavirus PCR was performed as described previously except that 0.06 mM myogenin (PCR internal control) primers were used (4). For real-time quantification (9, 13) polyomavirus VP1 DNA forward primer 4867 to 4889 (5′-CTC CGA ACT CAT TAC ACC CTC C-3′), reverse primer 4967 to 4987 (5′-TTA CGG GAC TCT CGG CAG A-3′), and internal fluorogenic oligonucleotide probe 4904 to 4926 (5′-FAM-ATT TCG CCA TCA AGG GCA GCG-TAMRA-3′) were used. As internal control, myogenin forward primer 3257 to 3277 (5′-TCC CTT ACG TCC ATC GTG GA-3′), reverse primer 3316 to 3336 (5′-CAG TTG GGC ATG GTT TCG TC-3′), and internal probe 3282 to 3310 (5′-FAM-TCA CGG TGG AGG ATA TGT CTG TTG CCT T-TAMRA-3′) were used. Polyomavirus VP1 and myogenin PCRs were performed separately, in triplicate. Each reaction mixture contained 100 ng of template DNA, primers (0.3 μM), and a fluorogenic Taqman probe (0.1 μM) in TaqMan PCR universal master mix (PE Applied Biosystems) in a final volume of 50 μl incubated at 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min in an ABI 7700 sequence detection system.
Pseudocapsid–dl1023-pBR322 complexes were inoculated into 11 adult normal C57BL/6 mice, and mice were analyzed up to 3 months p.i. by a polyomavirus specific PCR. During this period 7 of 11 mice had polyomavirus DNA in several tissues (Table 1). For comparison, dl1023-pBR322 plasmid was inoculated into nine adult, normal C57BL/6 mice and tested up to 2 months p.i. Polyomavirus DNA could be observed in 2 of 3 mice within 1 week p.i. but in only 1 of 6 mice thereafter. As a positive control, polyomavirus was inoculated into 23 adult, normal C57BL/6 mice. In 10 of 23 mice, monitored for 8 months p.i., polyomavirus DNA could be observed in many tissues. However, the presence of polyomavirus DNA became more limited with time and, by 6 to 8 months p.i., was no longer detected by PCR (Table 1).
TABLE 1.
Detection of polyomavirus DNA by PCR in organs and tissue of normal, CD4−/− CD8−/−, and μMT C57BL/6 mice inoculated with VP1–dl1023-pBR322 complexes or polyomavirusa
| No. of polyomavirus DNA-positive organ and tissue samples
|
||||||
|---|---|---|---|---|---|---|
| Normal mice
|
CD4−/− CD8−/− mice
|
μMT mice
|
||||
| Organ or tissue | VP1–dl1023-pBR322 (0–3 m.p.i.) | Polyomavirus (0–8 m.p.i.) | VP1–dl1023-pBR322 (0–6 m.p.i.) | Polyomavirus (0–2 m.p.i.) | VP1–dl1023-pBR322 (0–6 m.p.i.) | Polyomavirus (0–6 m.p.i.) |
| Brain | 5 | 9 | 6 | 5 | 4 (1 ND) | 13 |
| Parotid | 4 | 8 | 6 | 7 (1 ND) | 3 | 13 |
| Thymus | 2 (2 ND) | 6 | (6 ND) | 7 (1 ND) | 1 (6 ND) | 13 |
| Lungs | 6 | 7 | 6 | 6 (2 ND) | 7 | 13 |
| Bone | 5 | 10 | 5 | 7 (1 ND) | 7 | 13 |
| Heart | 5 | 9 | 6 | 8 | 7 | 13 |
| Stomach | 5 | 7 | 0 (5 ND) | 6 | 5 (1 ND) | 13 |
| Liver | 5 | 8 | 5 | 8 | 7 | 13 |
| Spleen | 6 | 10 | 5 | 7 | 6 | 13 |
| Kidney | 7 | 8 | 5 | 8 | 7 | 13 |
| L.N. | 6 | 6 | 5 | 8 | 4 | 13 |
| Gonads | 5 | 7 (1 ND) | 6 | 7 | 5 | 13 |
| Skin | 1 | 7 (1 ND) | 5 | 4 (4 ND) | 5 | 13 |
| Blood | 5 | 5 | 0 (5 ND) | 7 (1 ND) | 1 (3 ND) | 13 |
| No. of polyomavirus-positive mice/total no. of mice (%) | 7/11 (64) | 10/23 (43) | 6/6 (100) | 8/8 (100) | 8/9 (89) | 13/13 (100) |
m.p.i., months p.i.; ND, not defined; L.N., lymph nodes.
Pseudocapsid–dl1023-pBR322 complexes were also inoculated into six adult CD4−/− CD8−/− mice and nine adult μMT mice, and analyses were performed at regular intervals up to 6 months p.i. In all CD4−/− CD8−/− mice and eight of nine μMT mice, polyomavirus DNA was detected in most tissues (Table 1). Plasmid alone was inoculated into six adult CD4−/− CD8−/− and three adult μMT mice and was tested at different time points up to 2 months p.i. Polyomavirus DNA was not detected in any of the μMT mice but was detectable in several tissues of the three CD4−/− CD8−/− mice 2 weeks p.i., whereas by 1 month only traces of DNA could be detected in one of three CD4−/− CD8−/− mice. As a positive control, polyomavirus was inoculated into 8 adult CD4−/− CD8−/− and 13 μMT mice. All mice analyzed up to 2 to 6 months p.i. contained polyomavirus DNA in almost all tissues (Table 1).
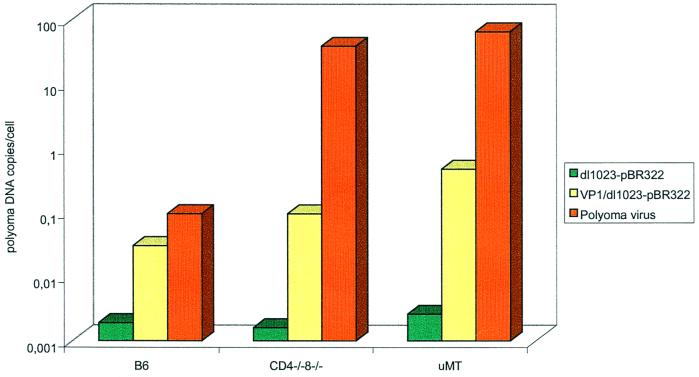
To quantify and compare the levels of DNA in tissues from the different delivery methods, real-time PCR was carried out. DNA obtained from bone and heart under the following conditions was analyzed: (i) 1 to 6 months p.i. from normal, CD4−/− CD8−/−, and μMT mice inoculated with VP1–dl1023-pBR322 complexes; (ii) 1 to 2 months p.i. from the same groups of mice inoculated with dl1023-pBR322 only; and (iii) 1 to 6 months p.i. from mice infected with polyomavirus. Variations in individual mice within the different groups were observed. Comparing median values, normal mice inoculated with pseudocapsid–dl1023-pBR322 complexes carried 10- to 50-fold higher copy numbers than mice inoculated with plasmid alone (Fig. 1). In immunodeficient mice, a 50- to 100-fold difference could be observed between the two delivery systems (Fig. 1). In general, the number of DNA copies found in normal mice inoculated with pseudocapsid–dl1023-pBR322 complexes was similar to that found in normal mice infected with polyomavirus. However, due to less-inhibited viral replication in immunodeficient mice inoculated with polyomavirus, their viral copy number was considerably higher (100- to 1,000-fold) than that found in normal mice (Fig. 1).
FIG. 1.
Quantitative comparison of polyomavirus DNA levels by real-time PCR in heart and bone of normal C57BL/6, CD4−/− CD8−/−, and μMT mice with dl1023-pBR322 plasmid alone 1 to 2 months p.i. or with pseudocapsid–dl1023-pBR322 complexes or the polyomavirus A2 strain up to 6 months p.i., represented as the median of data for each group. (Ten-fold serial dilutions of myogenin DNA from uninfected mice were used as standards by estimating that 500 ng of DNA corresponded to 105 cells.)
This study monitored and compared the persistence of polyomavirus DNA introduced either as “naked” DNA, by VP1 pseudocapsids, or in virions in normal and immunodeficient mice. DNA introduced by the pseudocapsid route could be observed in almost all (14 of 15) immunodeficient mice and was widely distributed to almost all tissues up to 6 months p.i. (Table 1). In normal C57BL/6 mice, DNA was introduced into a more limited number of mice (7 of 11). Although the total number of polyomavirus DNA-positive tissues and -positive mice was similar in different groups of mice, the median value of polyomavirus DNA copies per cell in heart and bone in normal C57BL/6 mice was somewhat less than that observed in immunodeficient mice (0.05 versus 0.1 to 0.5) (Fig. 1). From these data, we conclude that the immune system may influence the persistence of viral DNA introduced by pseudocapsids but does not totally eliminate it.
The difference obtained between normal and immunodeficient mice may reflect the fact that dl1023 DNA is competent to express immunoreactive polyomavirus early region proteins (1), and these may be recognized more efficiently in mice with a normal immune system. The small difference observed between different animal groups when using plasmid alone may reflect its shorter-term survival and the 10 to 100 lesser amounts of viral DNA copies introduced per cell (Fig. 1).
Our results also suggest that at least some of the difference seen in DNA transfer between normal and T-cell-deficient mice may be due to antibody responses (measured as a hemagglutination inhibition response) against capsids. Interestingly, this response was clearly lower against pseudocapsids (1:1,600) than against virus (1:6,000) despite the fact that 103 to 105 more pseudocapsid particles than virions were introduced into each mouse (data not shown). Titers (1:80) were obtained in uninfected mice or mice inoculated with plasmid alone (data not shown). μMT mice were not tested since these mice do not generate antibody responses (2).
Overall, our data confirm the results of Soeda et al. (15), who showed the feasibility of using polyomavirus VP1 pseudocapsids to introduce DNA larger than that actually protected by the carrier system (7, 16) into different types of cells in vivo. Furthermore, we show that pseudocapsids introduce heterologous DNA to a wide range of tissue more efficiently than heterologous DNA alone and that the DNA can persist for months in normal mice and in greater quantity and for a longer time in immunodeficient mice. The fact that polyomavirus VP1 pseudocapsids target tissues far beyond the point of inoculation can be advantageous for some gene therapy applications, such as overcoming an enzymatic defect. Nevertheless, because a wide tissue distribution can also be a disadvantage, and because an antibody response is induced after VP1 inoculation, modifying pseudocapsids to alter targeting specificities and avoid immune recognition would be desirable. Studies to achieve this aim are in progress.
Acknowledgments
This work was supported by a European Community grant (BIO4-CT97-147), the Swedish Cancer Foundation (1753-B99-15XCC), and the Stockholm County Council. T.D. was supported by the Swedish Cancer Foundation (4081-B99-02PBG).
REFERENCES
- 1.Berke Z, Palmer S, Bergman T, Wester D, Svedmyr J, Linder S, Jörnvall H, Dalianis T. A short peptide eluted from the H-2Kb molecule of a polyomavirus-positive tumor corresponds to polyomavirus large T antigen peptide at amino acid 578 to 585 and induces polyomavirus-specific immunity. J Virol. 1996;70:3093–3097. doi: 10.1128/jvi.70.5.3093-3097.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berke Z, Mellin H, Heidari S, Wen T, Berglöf A, Klein G, Dalianis T. Adult X-linked immunodeficiency (XID) mice, IgM−/− single knockout and IgM−/−CD8−/− double knockout mice do not clear polyomavirus infection. In Vivo. 1998;12:143–148. [PubMed] [Google Scholar]
- 3.Berke Z, Wen T, Jin S, Klein G, Dalianis T. Polyomavirus persists in CD4−/−CD8−/− double knockout, but not in CD4−/− or CD8−/− single knockout mice. Virology. 1995;212:268–271. doi: 10.1006/viro.1995.1482. [DOI] [PubMed] [Google Scholar]
- 4.Berke Z, Dalianis T. Persistence of polyomavirus in mice infected as adults differs from that observed in mice infected as newborns. J Virol. 1993;67:4369–4371. doi: 10.1128/jvi.67.7.4369-4371.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berke Z, Dalianis T. Studies on polyomavirus persistence and polyomavirus-induced tumor development in relation to the immune system. Adv Cancer Res. 2000;79:249–276. doi: 10.1016/s0065-230x(00)79008-7. [DOI] [PubMed] [Google Scholar]
- 6.Forstová J, Krauzewicz N, Wallace S, Street A J, Dilworth S M, Beard S, Griffin B E. Cooperation of structural proteins during late events in the life cycle of polyomavirus. J Virol. 1993;67:1405–1413. doi: 10.1128/jvi.67.3.1405-1413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forstová J, Krauzewicz N, Sandig V, Elliott J, Palková Z, Strauss M, Griffin B E. Polyomavirus pseudocapsids as efficient carriers of heterologous DNA into mammalian cells. Hum Gene Ther. 1995;6:297–306. doi: 10.1089/hum.1995.6.3-297. [DOI] [PubMed] [Google Scholar]
- 8.Fried M, Griffin B E, Lund E, Robberson D L. Polyomavirus— study of wild-type, mutant and defective DNAs. Cold Spring Harbor Symp Quant Biol. 1974;39:45–52. doi: 10.1101/sqb.1974.039.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Heid C A, Stevens J, Livak K J, Williams P M. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 10.Kitamura D, Roes J, Kuhn R, Rajewski K. A B-cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 11.Krauzewicz N, Cox E, Soeda B, Clark B, Rayner S, Griffin B E. Sustained ex vivo and in vivo transfer of a reporter gene using polyomavirus pseudocapsids. Gene Ther. 2000;7:1094–1102. doi: 10.1038/sj.gt.3301219. [DOI] [PubMed] [Google Scholar]
- 12.Luthman H, Nilson M-G, Magnusson G. Non-contiguous segments of the polyoma genome required in cis for DNA replication. J Mol Biol. 1982;161:533–550. doi: 10.1016/0022-2836(82)90406-5. [DOI] [PubMed] [Google Scholar]
- 13.Orlando C, Pinzani P, Pazzagli M. Developments in quantitative PCR. Clin Chem Lab Med. 1998;36:255–269. doi: 10.1515/CCLM.1998.045. [DOI] [PubMed] [Google Scholar]
- 14.Schilham M W, Fung-Leung W R, Rahemtulla A, Kündig T, Zhang L, Potter J, Miller R G, Hengartner H, Mak T W. Alloreactive cytotoxic T cells can develop and function in mice lacking both CD4 and CD8. Eur J Immunol. 1993;23:1299–1304. doi: 10.1002/eji.1830230617. [DOI] [PubMed] [Google Scholar]
- 15.Soeda E, Krauzewicz N, Cox C, Stokrová J, Forstová J, Griffin B E. Enhancement by polylysine of transient, but not stable expression of genes carried into cells by polyoma VP1 pseudocapsids. Gene Ther. 1998;5:1410–1419. doi: 10.1038/sj.gt.3300748. [DOI] [PubMed] [Google Scholar]
- 16.Stokrová J, Palková Z, Fischer L, Richterová Z, Korb J, Griffin B E, Forstová J. Interactions of heterologous DNA with polyomavirus major structural protein, VP1. FEBS Lett. 1999;445:119–125. doi: 10.1016/s0014-5793(99)00003-4. [DOI] [PubMed] [Google Scholar]
- 17.Verma I M, Somia N. Gene therapy—promises, problems and prospects. Nature. 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]