Abstract
Objective
Intestinal fibrosis is considered an inevitable consequence of chronic IBD, leading to stricture formation and need for surgery. During the process of fibrogenesis, extracellular matrix (ECM) components critically regulate the function of mesenchymal cells. We characterised the composition and function of ECM in fibrostenosing Crohn’s disease (CD) and control tissues.
Design
Decellularised full-thickness intestinal tissue platforms were tested using three different protocols, and ECM composition in different tissue phenotypes was explored by proteomics and validated by quantitative PCR (qPCR) and immunohistochemistry. Primary human intestinal myofibroblasts (HIMFs) treated with milk fat globule-epidermal growth factor 8 (MFGE8) were evaluated regarding the mechanism of their antifibrotic response, and the action of MFGE8 was tested in two experimental intestinal fibrosis models.
Results
We established and validated an optimal decellularisation protocol for intestinal IBD tissues. Matrisome analysis revealed elevated MFGE8 expression in CD strictured (CDs) tissue, which was confirmed at the mRNA and protein levels. Treatment with MFGE8 inhibited ECM production in normal control HIMF but not CDs HIMF. Next-generation sequencing uncovered functionally relevant integrin-mediated signalling pathways, and blockade of integrin αvβ5 and focal adhesion kinase rendered HIMF non-responsive to MFGE8. MFGE8 prevented and reversed experimental intestinal fibrosis in vitro and in vivo.
Conclusion
MFGE8 displays antifibrotic effects, and its administration may represent a future approach for prevention of IBD-induced intestinal strictures.
INTRODUCTION
Despite the widespread use of immunosuppressants and biologics, two-thirds of patients with Crohn’s disease (CD) require surgery due to stricture-related intestinal obstruction.1 No specific antistricture therapies are available. Traditionally, intestinal fibrosis has been considered an inevitable and irreversible complication of chronic gut inflammation,1 2 but recent evidence from multiple organs, including the intestine, challenges this paradigm,3 4 prompting a renewed interest in a better understanding of the pathogenesis of stricture formation and development of antifibrotic therapeutics. This is particularly important as mechanistic models suggest that fibrosis may progress independently of inflammation.1
A key component of intestinal fibrosis is the excessive deposition of the extracellular matrix (ECM).5 The major source of ECM in CD strictures (CDs) are mesenchymal cells, such as myofibroblasts, that arise from multiple sources, increase in numbers and get activated by distinct pathways including cytokines, growth factors such as transforming growth factor (TGF)-β1 or microbial components.1 However, other cell types, such as epithelial cells, are also well-known contributors to the ECM pool.6 7 The ECM is a complex protein network that provides not only physical support to all locally residing cells but also influences their function.5 It is now clear that the modulatory function of the ECM is mediated by its ability to signal to both immune and non-immune cells8 through multiple mechanisms including cell surface integrins.8 Data emerged that under pathological conditions, such as CD, the components and functional properties of ECM are altered, modulating the proliferation, activation and migration of local myofibroblasts and their ECM production.1 2 4 Despite its obvious importance, the composition or specific function of the ECM in stricturing CD has been only superficially explored.
To fill this knowledge gap and foster the search for antifibrotic drugs, we established functional three-dimensional (3D) ECM scaffolds from CDs and control resection tissues, by developing, validating and comparing three different ECM decellularisation methods. A comprehensive proteomic matrisome analysis of the decellularised gut by mass spectrometry revealed a previously unknown upregulation of the unique ECM protein milk fat globule-epidermal growth factor 8 (MFGE8) in CDs. MFGE8 messenger RNA (mRNA) and protein upregulation was confirmed in human tissues, and MFGE8 was found to exert antifibrotic properties in human intestinal myofibroblast (HIMF). RNA sequencing and functional experiments revealed signalling through integrin αvβ5 and focal adhesion kinase (FAK). Such antifibrotic MFGE8 function was demonstrable in HIMF from the normal intestine but not CDs, which was linked with downregulation of integrin β5. In CDs, MFGE8 prevented and reversed experimental intestinal fibrosis, pointing to MFGE8 as a vital player in the pathogenesis of intestinal fibrosis and a potential therapeutic target.
RESULTS
Development and validation of decellularised intestinal tissue models
A robust model is essential to perform a relevant functional analysis of intestinal ECM, and therefore, we developed, tested and compared three different protocols for human full-thickness gut tissue decellularisation.9–11 Each protocol differed based on the main detergent: sodium dodecyl sulfate, sodium deoxycholate (SDC) or peracetic acid (online supplemental figure S1; patient demographics can be found in online supplemental table S1). Prespecified criteria for selection of the optimal protocol included removal of all cellular components as indicated by multiple methods, preservation of structural integrity, retention of key ECM molecules and and ability to re-adhere to HIMF. The detailed results for establishing the tissue model can be found in online supplemental results. All three protocols retained the major ECM components collagen I (COLI), COLIII and fibronectin (FN) as well as other matrisome components (online supplemental results, online supplemental figures S2 and S3), showing a marked drop in DNA and RNA content (online supplemental figure S4A,B) and a dramatic reduction in gene expression levels of housekeeping genes (online supplemental figure S4C). Compared with native tissue only, the SDC protocol resulted in a complete absence of F-actin by immunofluorescence (IF) staining (online supplemental figure S4D); a lack of detectability of protein for E-cadherin, cytokeratin-19, vimentin, β-tubulin, phosphatase and tensin homolog, glyceraldehyde 3-phosphate dehydrogenase and α-smooth muscle actin (α-SMA; online supplemental figure S4E); and HIMF adherence to decellularised ECM (online supplemental figure S4F). The SDC protocol was therefore selected as the optimal model based on the above results and used for matrisome analysis.
Increased expression of MFGE8 in strictured CD matrisome
To comprehensively define the gut ECM composition, we performed proteomic analysis of decellularised ECM from normal control (NL, colon), UC (colon), non-strictured CD (CDns, ileum) and strictured CD (CDs, ileum) tissues using liquid chromatography-mass spectrometry (LC-MS) as previously described.12 Proteins that passed quality control were interrogated for the core matrisome,13 an omic inventory of ECM components and functions. We used a defined and similar protein amount of decellularised ECM across samples and groups to ascertain the investigation of their relative contribution.
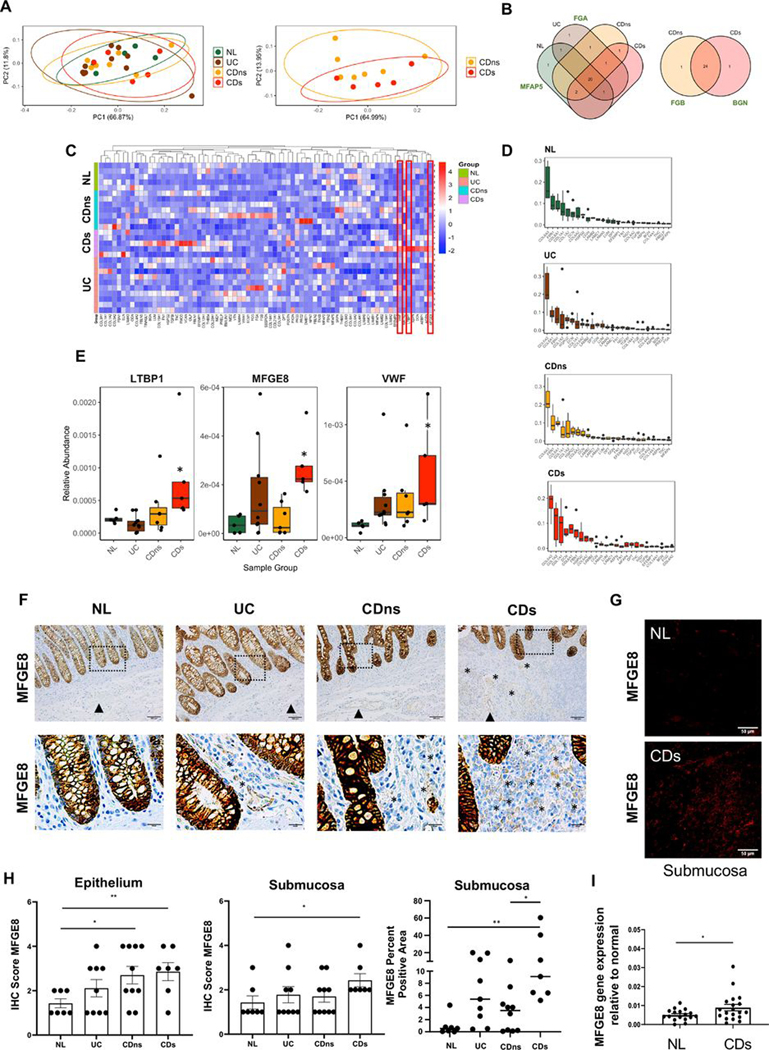
Principal component analysis and Venn diagram of the ECM matrisome revealed no distinct clustering in any tissue type (NL, UC, CDns and CDs) (figure 1A,B). 20 out of 29 components were present in all tissue types with only 2 uniquely expressed: fibrinogen-α in UC and microfibrillar-associated protein antigen 5 in NL. Overall, the quality of ECM components was largely comparable among CDs, CDns, UC and NL. When comparing CDs and CDns, 24 out of 26 components were present in both, except for fibrinogen-β, which was only expressed in CDns and biglycan only expressed in CDs (figure 1B,C).
Figure 1.
Matrisome analysis of decellularised intestinal resection tissues from patients with IBD and controls. Mass spectrometry analysis of extracellular matrix (ECM) proteins of different tissue phenotypes was performed, and results were calculated relative to the total amount of ECM. (A) Principal component analysis of the relative abundance of matrisome proteins produced by normal (NL), UC, non-strictured Crohn’s disease (CDns) and strictured Crohn’s disease (CDs). Ellipses indicate 95% CIs. No separation of the matrisome components was noted between NL, UC, CDns and CDs (left graph) as well as between CDns and CDs (right graph; n=27). (B) Proteins detected in each tissue phenotype. Venn diagrams were created using present matrisome components among the four phenotypes. Most matrisome components were expressed by all phenotypes with the only uniquely expressed proteins being delineated next to the Venn diagram (n=27). (C) Unsupervised hierarchical clustering of matrisome proteins (normalised relative abundance) for each phenotype indicating a large qualitative and quantitative overlap of matrisome expression across all phenotypes. The three molecules with differential expression between the phenotypes are marked with red boxes (n=27). (D) Top 25 ECM proteins for each phenotype ranked by level of abundance. The top 5 matrisome proteins across the tissue phenotypes NL, UC, CDns and CDs were collagen VI, fibrillin 1, collagen 1, decorin and perlecan (n=27). (E) Relative abundance of matrisome molecules with significant difference between the phenotypes. The three molecules identified were latent latent transforming growth factor-β binding protein 1, milk fat globule-epidermal growth factor 8 (MFGE8) and von Willebrand Factor (n=27). * indicates a significant difference compared with NL. (F) Immunohistochemistry (IHC) staining for MFGE8 expression in colon tissues identified intestinal epithelial cells as the major source of MFGE8. Slides are representative of n=33. Dotted box inserts represent the magnified areas in the lower row. (G) Immunofluorescence for MFGE8 focusing on the submucosa as the area with the highest ECM expression reveals increased MFGE8 in CDs compared with NL. Slides are representative of n=12. (H) Quantification for MFGE8 expression using a blinded IHC score indicated upregulation of MFGE8 in the epithelium of CDns and CDs compared with NL and in the submucosa of CDs compared with NL (n=33). Right panel depicts automatic quantification of the MFGE8 surface area in the submucosa. (I) Real-time PCR analysis revealed that MFGE8 gene expression was elevated in CDs resection tissues compared with NL (n=36). Data are presented as mean±SEM. *, p<0.05; **, p<0.01. NL, normal; CDs, strictured Crohn’s disease; CDns, non-strictured Crohn’s disease; FGA, fibrinogen-α; FGB, fibrinogen-ß; MFAP5, microfibrillar-associated protein antigen 5; BGN, biglycan; LTBP1, latent transforming growth factor-β binding protein 1; MFGE8, milk fat globule-epidermal growth factor; VWF, von Willebrand Factor; IHC, immunohistochemistry.
COLVI, fibrillin 1, COLI, decorin and perlecan were the top 5 matrisome proteins in all tissue groups, a result consistent with the overall ECM composition in most organs14 (figure 1D). When we interrogated the matrisome dataset for quantitatively different proteins, we identified MFGE8, latent TGF-β binding protein 1 (LTBP1) and von Willebrand factor (VWF) as upregulated in CDs to NL (figure 1E). Since MFGE8 showed increased expression in the inflamed intestines associated with fibrosis (UC and CDs) compared with NL and CDns and preliminary evidence is available on MFGE8 in acute experimental IBD,15 16 MFGE8 was chosen for further functional exploration.
We validated the matrisome analysis in native surgical resection tissue sections, where MFGE8 was strongly expressed in epithelial cells across all tissue types with a much weaker expression in the lamina propria and submucosa. Immunohistochemistry (IHC) scores of MFGE8 intensity increased in the epithelium of CDns and CDs compared with NL, which was confirmed by automatic quantification of the MFGE8 per cent surface area (figure 1F,H). We determined the expression of MFGE8 in epithelial cell washes from primary human resection tissues from NL and CDs and found an increase in MFGE8 in the CDs-derived epithelial cells compared with NL (online supplemental figure S5A). To exert its function on fibroblasts, MFGE8 would need to be secreted by epithelial cells. We hence additionally measured MFGE8 secretion by the intestinal epithelial cell line HT29 in response to interleukin (IL)-1 β, tumour necrosis factor (TNF), TGF-β1 or lipopolysaccharide (LPS) for 24 hours. Epithelial cells spontaneously secreted MFGE8. IL-1β increased MFGE8 secretion in HT29 cells compared with untreated, while TNF, TGF-β1 or LPS decreased MFGE8 secretion (online supplemental figure S5B), suggesting that epithelial MFGE8 secretion is regulated by inflammatory stimuli present in IBD. Submucosal MFGE8 expression based on IHC scoring was increased only in CDs compared with NL but not in CDns or UC compared with NL (figure 1F,H). We additionally performed automatic quantification determining the area of the submucosa positive for MFGE8 staining and confirmed the upregulation of MFGE8 expression in CDs compared with CDns and NL (figure 1H). Additional IF analysis separately exposing epithelium and submucosa images confirmed higher MFGE8 expression in CDs compared with NL in the epithelium and submucosa (figure 1G, online supplemental figure S6). Confirmatory, MFGE8 gene expression increased in CDs full-thickness intestinal tissue compared with NL (figure 1I).
Antifibrotic effect of MFGE8 on experimental intestinal fibrosis in vivo
We first tested the potential modulatory effect of MFGE8 on dextran sodium sulfate (DSS) experimental colitis. We administered MFGE8 intrarectally since our goal was the functional exploration of the intestinal matrisome. In the acute model, mice receiving DSS lost weight, while the no DSS groups gained weight (online supplemental figure S7A). Mice receiving DSS and MFGE8 displayed lower clinical scores (composite of weight loss, rectal bleeding and stool consistency) compared with DSS alone (online supplemental figure S7B). MFGE8 administration modestly reversed the shortening of the colon length seen in the DSS group (online supplemental figure S7C) but had no effect on the histological inflammatory score (online supplemental figure S7D).
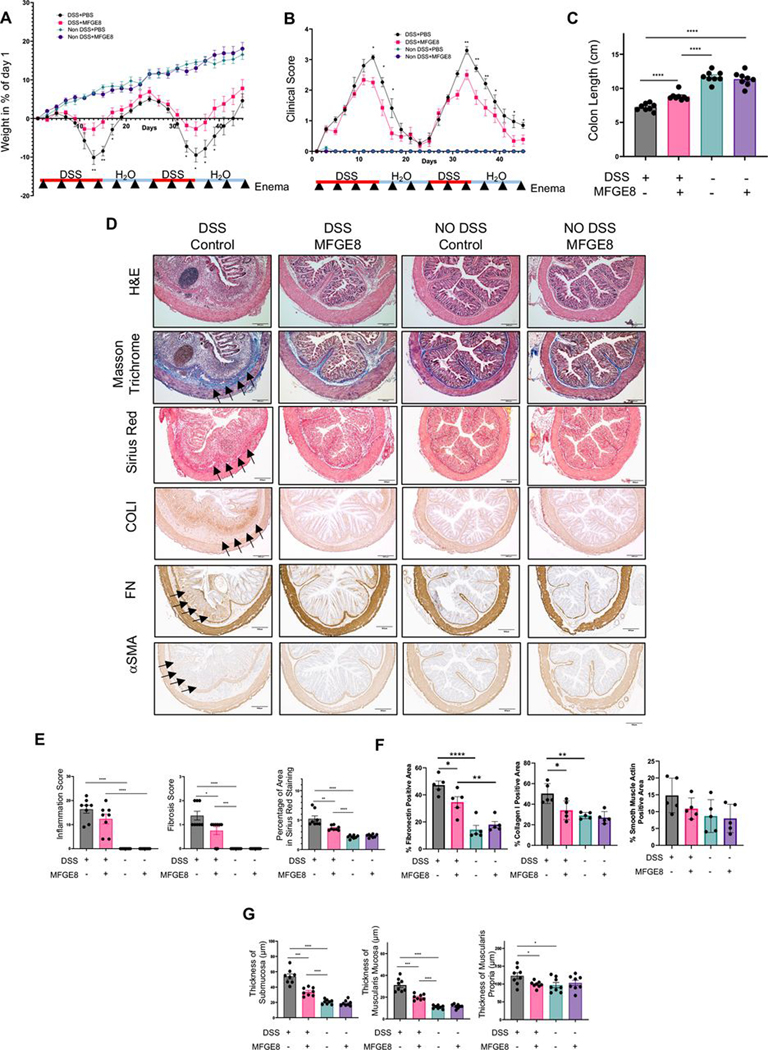
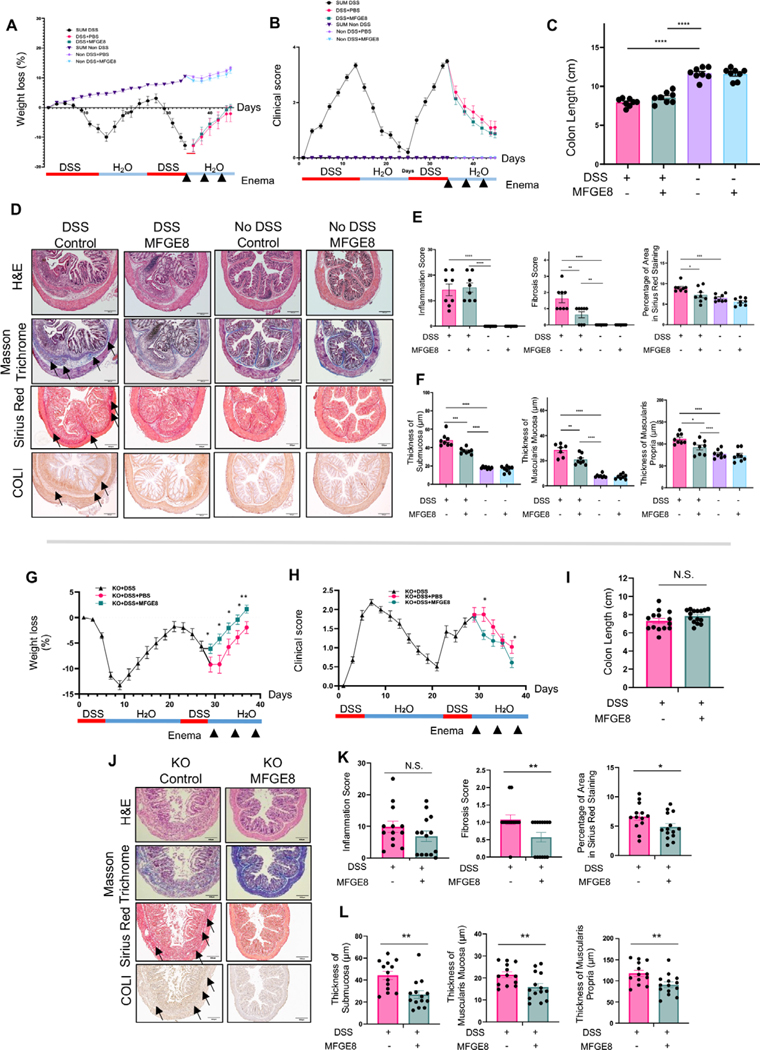
We next explored the potential action of MFGE8 on experimental intestinal fibrosis in the chronic DSS model (MFGE8 enemas every 4 days for 6 weeks). In the DSS group, MFGE8-treated animals lost less weight and had a reduced clinical score and reduced colon shortening compared with no MFGE8-treated animals (figure 2A–C). On a histopathologic assessment, prophylactic MFGE8 administration did not reduce the inflammatory score but reduced the fibrosis score as well as sirius red (collagen-positive area) staining in DSS-treated animals (figure 2D,E). This was accompanied by a reduction in the FN and COLI-positive surface area and a trend towards reduction in α-SMA in the DSS-treated animals receiving MFGE8 compared with DSS alone (figure 2D,F). Given that all ECM molecules tested were downregulated in response to MFGE8, we focused our readouts to sirius red for the remainder of the animal experiments. In DSS-treated animals, MFGE8 also reduced the increased thickness of the submucosa, muscularis mucosa and muscularis propria compared with no DSS-treated animals (figure 2G). No DSS-treated animals did not show any difference in weight, clinical scores, colon length, inflammation, fibrosis or intestinal layer thickness irrespective of MFGE8 administration (figure 2A–F).
Figure 2.
Milk fat globule-epidermal growth factor 8 (MFGE8) exerts antifibrotic properties in chronic dextran sodium sulfate (DSS)-induced colitis. (A–G) Chronic DSS colitis was induced in Balb/C mice by two cycles of 3.5% DSS administration and recovery. 3600 ng or recombinant mouse MFGE8 or vehicle control was applied as enema every 4 days starting from the first day of DSS administration. The severity of DSS-induced colitis was evaluated by measuring (A) body weight loss and (B) calculating the clinical score consisting of blood in stool, weight loss and stool consistency. MFGE8 and DSS-treated animals have reduced weight loss and lower clinical score. (C) Colon length was less reduced in MFGE8-treated and DSS-exposed mice compared with DSS alone. (D) Representative images from mouse colon sections stained withH&E, Masson’s trichrome (MT), sirius red (SR), collagen I (COLI), fibronectin (FN) or α-smooth muscle actin (α-SMA). Slides are representative of n=5–8 per group. Arrows point towards the area of fibrosis. (E) Inflammation score was determined by an IBD pathologist in a blinded fashion using H&E sections. There was no difference in DSS-treated animal irrespective of exposure to MFGE8 or not. Fibrosis score as determined by an IBD pathologist in a blinded fashion using MT sections and automatic quantification using SR sections was analysed. MFGE8 reduced the fibrosis score per cent sirius red area in DSS-exposed animals. (F) MFGE8 reduced the per cent positive area for FN and COLI and showed a trend of reduction of α-SMA. (G) MFGE8 reduced the thickness of the submucosa, muscularis mucosa and muscularis propria in DSS-exposed animals. Data are presented as mean±SEM (n=8 per group from two independent experiments). *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001. DSS, dextran sodium sulfate; MFGE8, milk fat globule-epidermal growth factor 8; PBS, phosphate-buffered saline; α-SMA, α-smooth muscle actin; FN, fibronectin; COLI, collagen I.
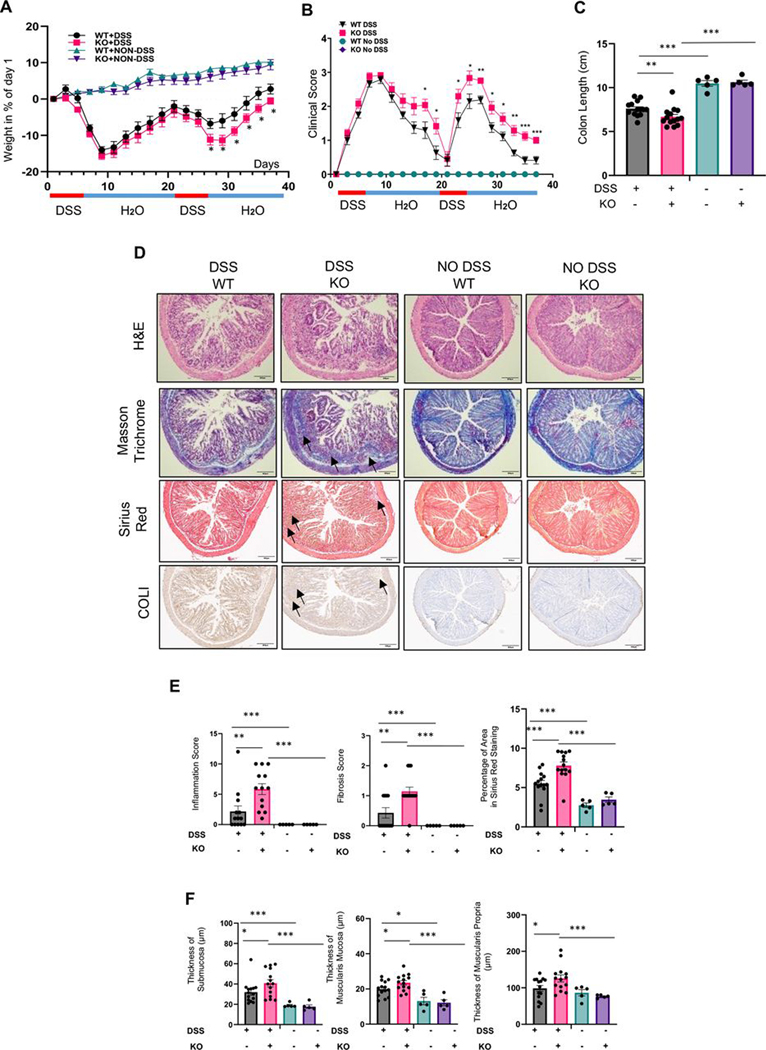
To confirm the antifibrotic action of MFGE8, we took an in vivo loss-of-function approach in chronic DSS colitis by using MFGE8 knockout (KO) mice (MFGE8 systemically deleted). In the DSS group, MFGE8 KO animals lost more weight, had a higher clinical score and reduced colon length compared with wild-type (WT) animals at the end of the second DSS cycle (figure 3A–F). On histopathologic assessment, DSS-treated MFGE8 KO mice had more colonic inflammation, increased fibrosis scores, collagen-positive area and increased thickness of the submucosa, muscularis mucosa and muscularis propria compared with DSS-treated WT mice (figure 3D–F). Animals not treated with DSS showed no difference in any of the endpoints irrespective of being KO or WT (figure 3A–F).
Figure 3.
Milk fat globule-epidermal growth factor 8 (MFGE8) knockout (KO) mice show increased fibrosis in chronic dextran sodium sulfate (DSS)-induced colitis. (A–F) Chronic DSS colitis was induced in C57/BL6 wild-type (WT) or MFGE8 KO mice by two cycles of 3% DSS administration and recovery (6-day DSS and 13-day recovery). The severity of DSS-induced colitis was evaluated by measuring (A) body weight loss and (B) calculating the clinical score consisting of blood in stool, weight loss and stool consistency. MFGE8 KO mice have higher weight loss and increased clinical score. (C) Colon length was shorter in DSS-exposed KO mice compared with DSS-exposed WT mice. (D) Representative images from mouse colon sections stained with H&E, Masson’s trichrome (MT), sirius red (SR) and collagen I (COLI). Slides are representative of n=14 per DSS groups and n=5 per no DSS groups. Arrows point towards the area of fibrosis. (E) Inflammation score was determined by an IBD pathologist in a blinded fashion using H&E sections. When exposed to DSS, MFGE8 KO mice had a higher inflammation score compared with WT mice. Fibrosis score as determined by an IBD pathologist in a blinded fashion using MT sections and automatic quantification using SR sections was analysed. MFGE8 KO mice had increased fibrosis score and per cent sirius red area in DSS-exposed animals compared with WT mice. (F) MFGE8 KO mouse colons had an increased thickness of the submucosa, muscularis mucosa and muscularis propria in DSS-exposed animals compared with WT mice. Data are presented as mean±SEM (n=15 per DSS group and n=5 per no DSS groups from two independent experiments). *, p<0.05; **, p<0.01; ***, p<0.001. WT, wild type; DSS, dextran sodium sulfate; KO, knockout; COLI, collagen I.
We confirmed the antifibrotic properties of MFGE8 on experimental intestinal fibrosis in an additional intestinal fibrosis model, the chronic trinitrobenzene sulfonic acid (TNBS) model (MFGE8 enemas every 4 days for 4 weeks, online supplemental figure S8). In the TNBS group, MFGE8-treated animals had a numerically, but not significantly, reduced clinical score. No difference in weight loss was noted (online supplemental figure S8A,B). MFGE8-treated animals had a reduced colon shortening compared with no MFGE8-treated animals receiving TNBS (online supplemental figure S8C). On a histopathologic assessment, prophylactic MFGE8 administration did not reduce the inflammatory score but reduced the fibrosis score (online supplemental figure S8D,E). Sirius red (collagen-positive area) staining in TNBS-treated animals (online supplemental figure S8D,E) was lower in MFGE8-treated animals compared with controls. In TNBS-treated animals, MFGE8 did not reduce the increased thickness of the submucosa, muscularis mucosa or muscularis propria compared with untreated animals (online supplemental figure S8D,F).
Taken together, these results suggest that MFGE8 has antifibrotic properties in vivo.
MFGE8 has an antifibrotic effect on primary human intestinal myofibroblasts from normal controls but not strictured CD
We investigated the antifibrotic mechanisms of MFGE8 using primary HIMFs as they are the chief profibrotic cell type and producer of ECM,2 and they can be found in close physical proximity of MFGE8-positive areas, particularly in CDs (online supplemental figure S5).
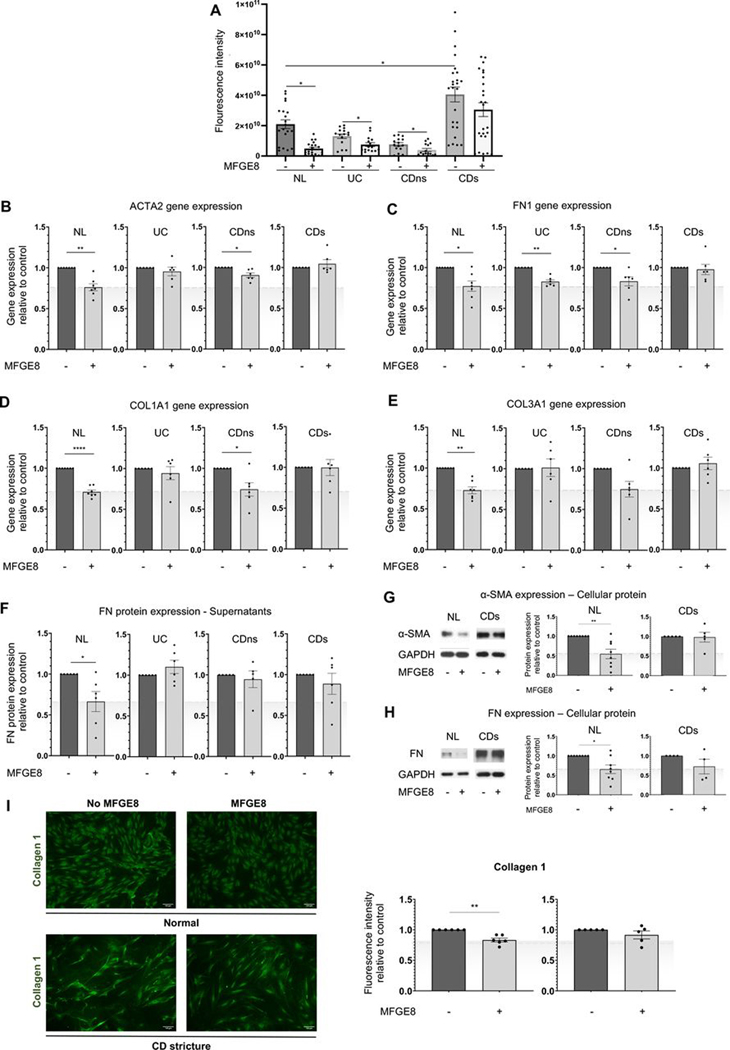
We first assessed the actual deposition of ECM components in a novel assay. After exposure to MFGE8 for 5 days, HIMFs were removed, and the deposited ECM was quantified in an automated unbiased manner (figure 4A). Using FN, a major ECM component as a test deposited product, CDs at baseline deposited a significantly higher amount of FN compared with NL, UC and CDns. When exposed to MFGE8, HIMF in the NL, UC and CDns groups significantly reduced FN deposition, but this did not occur when CDs cells were used (figure 4A).
Figure 4.
Milk fat globule-epidermal growth factor 8 (MFGE8) shows antifibrotic effects on primary human intestinal myofibroblasts (HIMFs) derived from normal (NL) but not patients with stricturing Crohn’s disease (CD). Primary HIMFs were isolated from freshly resected intestinal tissues from NL, UC, non-strictured CD (CDns) and strictured CD (CDs). (A) Deposition of fibronectin (FN) was measured using an extracellular matrix deposition assay. An increase in FN deposition was noted at baseline between the CDs HIMF and all other phenotypes. On exposure to MFGE8, NL, UC and CDns HIMF reduced FN, but CDs did not (n=3–5 cell lines per group with 5 independent experiments per line). (B–F) NL HIMF exposed to MFGE8 reduced gene expression of α-smooth muscle actin (α-SMA, ACTA2), FN (FN1), collagen I (COLI, COL1A1), COLIII (COL3A1) and FN protein. CD HIMF did not show any response to ACTA2, FN1, COLA1A1 and COL3A1 gene expression by quantitative PCR (qPCR) and FN protein by ELISA. HIMF UC only reduced FN1 gene expression, and CDns reduced ACTA2, FN1 and COL1A1 gene expression in response to MFGE8 (n=5–7 per group). (G–I) HIMF NL reduced cellular protein expression of α-SMA and FN (immunoblot) and COLI (immunocytochemistry), which was not observed in HIMF CDs (n=4–8). *, p<0.05; **, p<0.01; ****, p<0.0001. α-SMA, α-smooth muscle actin; CDs, strictured CD; CDns, non-strictured CDns; COL, collagen; FN, fibronectin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase;MFGE8, milk fat globule-epidermal growth factor 8; NL, normal.
To corroborate these findings, MFGE8 inhibited gene expression of representative ECM molecules in NL HIMF, such as ACTA2 (gene for α-SMA), FN1 (gene for FN), COL1A1 (gene for COLI) and COL3A1 (gene for COLIII) compared with untreated HIMF. MFGE8 reduced secreted FN as well as cellular FN and COLI and the expression of α-SMA in NL HIMF compared with untreated cells (figure 4B–I). In contrast, MFGE8 had no effect on HIMF derived from CDs compared with untreated, which was seen for gene expression for ACTA2, FN1, COL1A1 and COL1A3 and protein expression for FN, COLI and α-SMA (figure 4B–I). In UC HIMF, MFGE8 reduced gene expression for FN1 and in CDns HIMF gene expression for ACTA2, FN1 and COL1A1 compared with untreated.
Hence, MFGE8 exerts an antifibrotic effect on NL HIMF but not CDs-derived HIMF. The effect of MFGE8 on HIMF cytokine secretion can be found in online supplemental figure S9 and online supplemental results.
Next-generation sequencing of HIMF points to integrin signalling for MFGE8
To understand the lack of antifibrotic response of CDs HIMF to MFGE8, we globally assessed differentially expressed genes in HIMF exposed or not to MFGE8 using next-generation RNA sequencing.17–19 The detailed analysis can be found in online supplemental results.
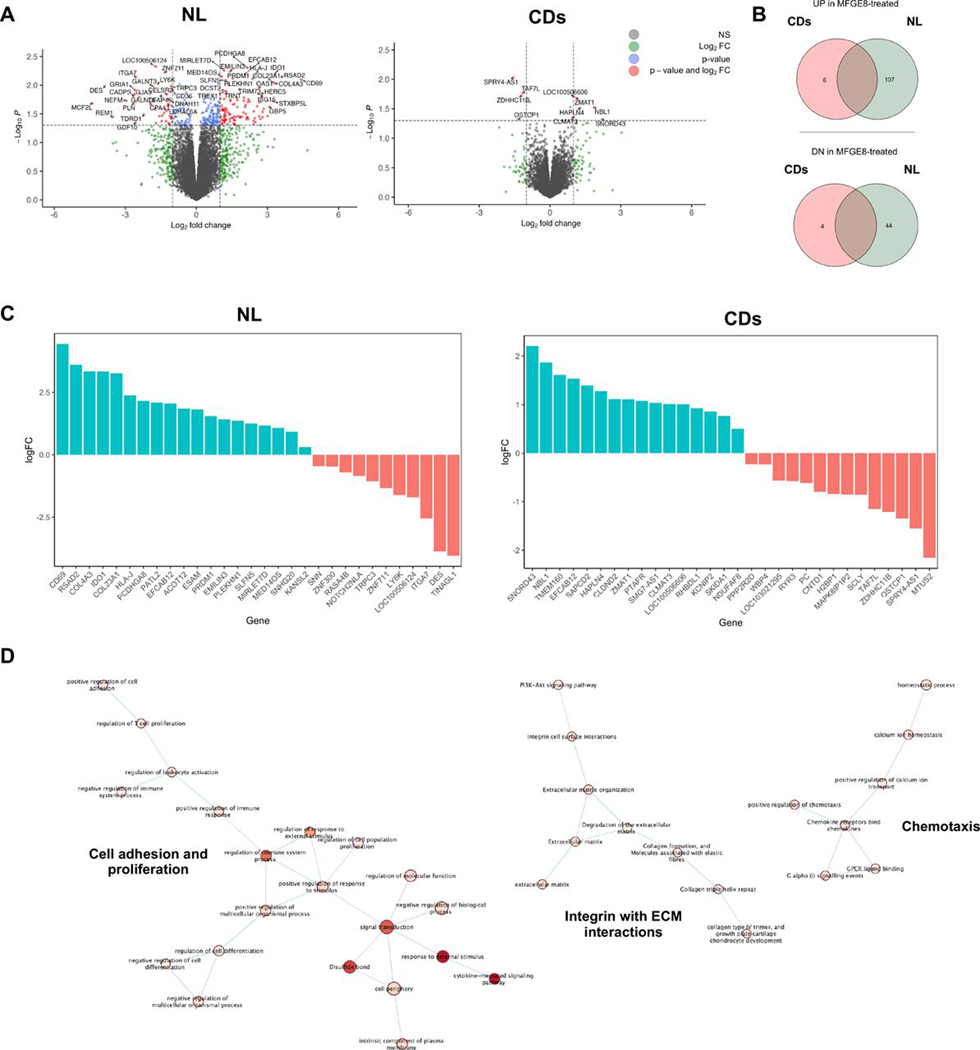
Briefly, volcano plot, Venn diagram and top upregulated or downregulated genes (figure 5A–C) revealed a robust separation of the gene profiles in the absence and presence of MFGE8 in NL HIMF with 107 genes upregulated and 44 genes downregulated (twofold change, p<0.01). Global gene expression analysis indicates that the MFGE8 was a leading regulator of ECM organisation and ECM-cell interactions. In contrast, the response of CDs HIMF to MFGE8 revealed only six upregulated and four downregulated (twofold change, p<0.01) genes, as displayed in heatmap, volcano plot, Venn diagram and top upregulated or downregulated genes (figure 5A–C).
Figure 5.
Global gene expression in primary human intestinal myofibroblasts (HIMFs) derived from normal but not patients with stricturing Crohn’s disease (CD) in response to milk fat globule-epidermal growth factor 8 (MFGE8). Primary HIMFs from normal (NL; n=4) or stricture CD (CDs; n=3) were exposed to 500 ng/mL MFGE8 or left untreated for 12 hours prior to undergoing unbiased global gene expression by next-generation sequencing. (A) Annotated volcano plot with upregulated (left) and downregulated (right) genes on exposure to MFGE8. Significant genes are depicted in red. (B) Significantly upregulated (top) and downregulated (bottom) gene exposure of HIMF to MFGE8 for NL and CDs. (C) Top 30 regulated genes—upregulated (blue) and downregulated (red) genes—on exposure of HIMF to MFGE8. (D) Pathway enrichment analysis comparing differential gene expression in HIMF NL and CDs exposed to MFGE8 reveals induction of pathways associated with cell adhesion and proliferation, chemotaxis and extracellular matrix-integrin interactions. NL, normal; CDs, strictured Chron’s disease; MFGE8, milk fat globule-epidermal growth factor 8; ECM, extracellular matrix.
To elucidate pathways involved in the differential response of NL and CDs, we used uniquely upregulated genes in HIMF NL in response to MFGE8 that were not regulated in HIMF CDs as input for GO genesSet enrichment analysis, which revealed interferon (IFN)-α/β signalling, cytokine-mediated signalling and cell surface receptor signalling as the major pathways (online supplemental tables S2–S4). ECM-related pathways indicated induction of ECM organisation (HSA-1474244), ECM-integrin interaction (HSA-216083), cell adhesion (HAS-04514), proliferation (GO:0042127) and chemotaxis (GO:0050921) (figure 5D). However, MFGE8 neither affected NL HIMF proliferation nor migration (online supplemental figure S10). This suggests that MFGE8 exerts antifibrotic effects on NL HIMF, through reduction in ECM production and potentially integrin pathways.
Focal adhesion kinase and integrin αvβ5 mediate the antifibrotic effects of MFGE8 on HIMF
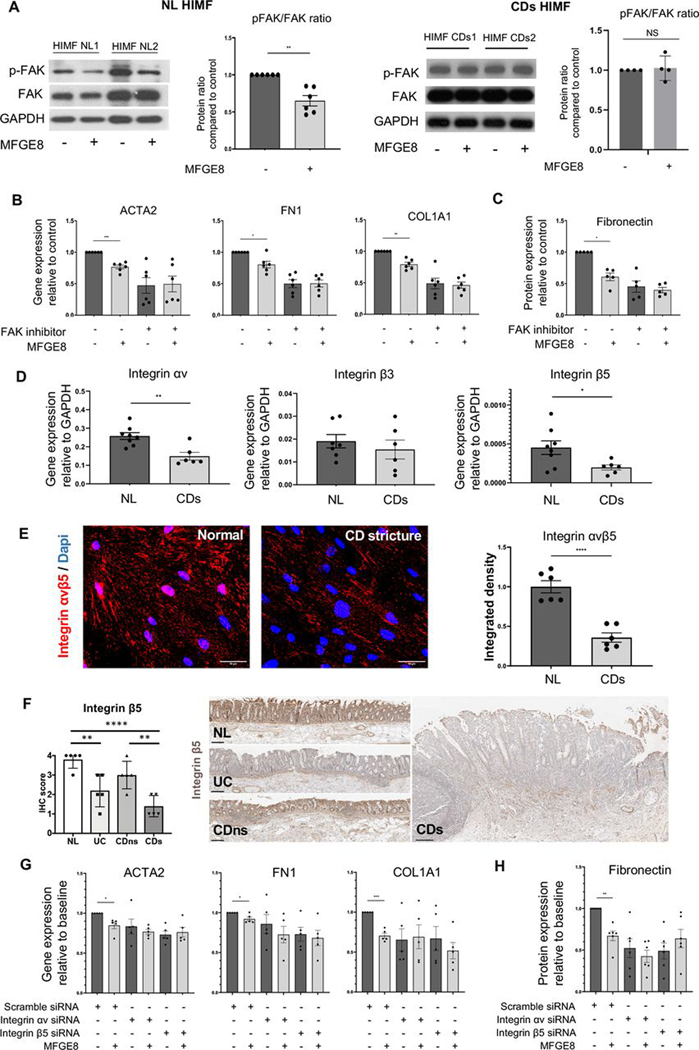
We next investigated a possible role of FAK (critical for ECM-cell interactions15 20) and found that exposure of NL HIMF to MFGE8, but not CDs HIMF to MFGE8, decreased FAK phosphorylation (lower phospho-FAK (p-FAK)/FAK ratio; figure 6A, left panel and right panel), suggesting that a lack of response of CDs HIMF might be due to blunted FAK signalling. NL HIMF exposure to a small-molecule F AK phosphorylation inhibitor (FAK inhibitor 14) showed a reduced expression of ACTA2, FN1 and COL1A1 genes and FN protein compared with no FAK inhibitor. FAK phosphorylation inhibitor-exposed NL HIMF lost the response to MFGE8 (figure 6B,C).
Figure 6.
Milk fat globule-epidermal growth factor 8 (MFGE8) exerted antifibrotic effects on primary human intestinal myofibroblasts (HIMFs) through focal adhesion kinase (FAK) pathway and integrin αvβ5. (A) Immunoblot analysis of FAK activation in normal (NL) and Chron’s disease stricture (CDs) primary HIMF exposed to 500 ng/mL MFGE8 for 1 hour. NL HIMF but not CDs HIMF reduced phosphorylation of FAK (n=4–6 per group). The experiments were not designed to quantitatively compare baseline FAK/pFAK between NL and CDs. (B) Gene expression of HIMF NL for α-smooth muscle actin (α-SMA, ACTA2), fibronectin (FN1) and collagen I, (COLI, COL1A1) untreated or after exposure to MFGE8 for 12 hours in the presence or absence of a FAK phosphorylation inhibitor. Inhibition of FAK phosphorylation reduced the baseline expression of ACTA2, FN1 and COL1A1 and rendered HIMF NL non-responsive to MFGE8 (n=6 per group). (C) Protein expression of HIMF NL for FN untreated or after exposure to MFGE8 for 48 hours in the presence or absence of a FAK phosphorylation inhibitor. Inhibition of FAK phosphorylation reduced the baseline expression of FN and rendered HIMF NL non-responsive to MFGE8 (n=5 per group). (D) Gene expression of integrins αv, β3 and β5 in HIMF NL and HIMF CDs. HIMF CDs showed a lower expression of integrins αv and β5 but not β3 (n=6 per group). (E) Immunofluorescence of integrin αvβ5 in HIMF. NL and HIMF CDs. HIMF CDs showed a lower expression of integrin αvβ5 (n=6). (F) Immunohistochemistry of integrin β5 in freshly resected intestinal tissues. Integrin β5 expression was reduced in CDs compared with NL and non-strictured CD (CDns) (n=5 per group) (G) Gene expression of HIMF NL for ACTA2, FN1 and COL1A1 untreated or after exposure to MFGE8 for 12 hours in the presence or absence of a small interfering RNA (siRNA) targeting integrin αv or β5. Inhibition of integrin αv or β5 rendered HIMF NL non-responsive to MFGE8 (n=5 per group). (G) Protein expression of HIMF NL for FN untreated or after exposure to MFGE8 for 48 hours in the presence or absence of siRNA targeting integrin αv or β5. Inhibition of integrin αv or β5 rendered HIMF NL non-responsive to MFGE8 (n=6 per group). *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001. NS: not significant. HIMF, human intestinal myofibroblast; NL, normal; CDs, strictured Chron’s disease; FAK, focal adhesion kinase; FN, fibronectin; COL, collagen; siRNA, small interfering RNA; MFGE8, milk fat globule-epidermal growth factor 8; siRNA, small interfering RNA.
As functional exploration of all integrins is not practically feasible, we focused on integrins known to bind MFGE8, namely, αvβ3 and αvβ5.21 22 Gene and/or protein expression of αv and β5, but not β3, was significantly lower in HIMF CDs compared with NL (figure 6D,E). The only binding partner of integrin β5 is integrin αv.23 Concordant with the in vitro results, integrin β5 expression in freshly resected intestinal tissues was downregulated in mesenchymal cells in the mucosa and submucosa of freshly resected CDs and UC compared with NL as well as in CDs compared with CDns (figure 6F).
Knockdown of αv or β5 (online supplemental figure S11) with small interfering RNA (siRNA) decreased the baseline gene expression of α-SMA, FN and COL1A1 as well as protein expression of FN. After knockdown of αv or β5, MFGE8 did not further reduce the profibrotic gene expression compared with untreated in NL HIMF (figure 6G,H). These results validate integrin αvβ5 as an important mediator of the antifibrotic effects of MFGE8 on HIMF.
Reversal of intestinal fibrosis in vivo by administration of MFGE8
Finally, we investigated whether MFGE8 could reverse fibrosis in the chronic DSS model (MFGE8 enemas every 4 days after fibrosis was established; figure 7A). In DSS-treated animals, MFGE8 did not alter weight loss, clinical score, colon length or histological inflammation (figure 7A–E). Of relevance, MFGE8 reduced fibrosis score, collagen-positive area in DSS-treated animals and thickness of the submucosa, muscularis mucosa and muscularis propria (figure 7E,F). Endpoints were not different in the no DSS-treated animals irrespective of MFGE8 administration (figure 7A–E).
Figure 7.
Milk fat globule-epidermal growth factor 8 (MFGE8) reverses fibrosis but does not affect inflammation in chronic dextran sodium sulfate (DSS)-induced colitis. (A–F) Chronic DSS colitis was induced in Balb/C mice by two cycles of 3.5% DSS administration and recovery. 3600 ng or recombinant mouse MFGE8 or vehicle control was applied as enema every 4 days starting from the end of the second cycle of DSS administration (therapeutic administration in already established fibrosis). The severity of DSS-induced colitis was evaluated by measuring (A) body weight loss and (B) the clinical score consisting of blood in stool, weight loss and stool consistency. The therapeutic administration of MFGE8 did not affect weight loss and clinical score. (C) Colon length was not changed in MFGE8-treated and DSS-exposed mice compared with DSS alone. (D) Representative images from mouse colon sections stained with H&E, Masson’s trichrome (MT), sirius red (SR) and collagen I (COLI). Slides are representative of n=8 per group. Arrows point towards the area of fibrosis. (E) Inflammation score was determined by an IBD pathologist in a blinded fashion using H&E sections. There was no difference in DSS-treated animal irrespective of exposure to MFGE8 or not. Fibrosis score as determined by an IBD pathologist in a blinded fashion using MT sections and automatic quantification using SR sections was analysed. MFGE8 reduced the fibrosis score and per cent Sirius red area in DSS-exposed animals. (F) MFGE8 reduced the thickness of the submucosa, muscularis mucosa and muscularis propria in DSS-exposed animals. Data are presented as mean±SEM (n=8 per group from two independent experiments). *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001. Next, chronic DSS colitis was induced in MFGE8 knockout (KO) mice by two cycles of 3% DSS administration and recovery. 3600 ng mg of recombinant MFGE8 or vehicle control was applied as enema every 4 days starting from the end of the second cycle of DSS administration (MFGE8 rescue in already established fibrosis). The severity of DSS-induced colitis was evaluated by measuring (G) body weight loss and (H) calculating the clinical score consisting of blood in stool, weight loss and stool consistency. MFGE8 KO mouse receiving MFGE8 has a lower weight loss and improved clinical score. (I) Colon length in MFGE8 KO mice was not different when MFGE8 was administered. (J) Representative images from mouse colon sections stained with H&E, MT, SR and COLI. Slides are representative of n=14 per group. (K) Inflammation score was determined by an IBD pathologist in a blinded fashion using H&E sections. When exposed to MFGE8, MFGE8 KO mice showed no difference in inflammation score compared with untreated mice. Fibrosis score as determined by an IBD pathologist in a blinded fashion using MT sections and automatic quantification using SR sections was analysed. MFGE8 administration to MFGE8 KO mice reduced the fibrosis score and per cent sirius red area compared with untreated MFGE8 KO mice. (L) MFGE8 KO mice receiving MFGE8 had a reduced thickness of the submucosa, muscularis mucosa and muscularis propria compared with untreated MFGE8 KO mice. Data are presented as mean±SEM (n=14 per group from two independent experiments). (M) Integrin β5 on immunohistochemistry is increased in chronic DSS fibrosis compared with no DSS (n=5 per group). *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001. DSS, dextran sodium sulfate; MFGE8, milk fat globule-epidermal growth factor 8; PBS, phosphate-buffered saline; COLI, collagen I; NS, not significant.
To confirm these findings, we used MFGE8 KO mice as a loss-of-function approach in the chronic DSS colitis model and tested whether MFGE8 can rescue an established fibrotic phenotype (MFGE8 emenas every 4 days after fibrosis was established; figure 7G). MFGE8 led to a reduction in weight loss and clinical score, while the colon length and the inflammatory score remained unchanged (figure 7I–K). Importantly, administration of MFGE8 led to a reduction of the fibrosis score, collagen-positive area and thickness of the submucosa, muscularis mucosa and muscularis propria compared with controls in the DSS-treated animals (figure 7J–L).
We confirmed the lack of anti-inflammatory properties of MFGE8 in freshly isolated human lamina propria mononuclear cells (LPMCs), which were exposed to three different concentrations of MFGE8, including the concentration eliciting an effect on HIMF. Cytokines in the cell supernatant were determined using an array consisting of IL-1β, IL-6, IL-8, IL-10, IL-12, TNF, monocyte chemoattractant protein-1 (MCP-1), TGF-β1, IFNγ and Regulated on Activation, Normal T Cell Expressed and Secreted (RANTES). The cytokines being detectable were IL-1β, IL-6, IL-8, IL12, RANTES and MCP-1. MFGE8 in all three tested concentrations did not affect the amount of any of the detectable cytokines in the LPMC supernatants (online supplemental figure S12).
Finally, to investigate whether FAK signalling is relevant for the action of topically administered MFGE8, we induced TNBS fibrosis and prophylactically administered MFGE8 (MFGE8 enemas every 4 days for 4 weeks) and/or a FAK inhibitor small molecule (Y15, 10 mg/kg, administered every 4 days from the onset of the first TNBS cycle) (online supplemental figure S8). There was no significant difference in weight loss or clinical score in the TNBS-exposed animals, irrespective of administration of vehicle, Y15 or Y15 and MFGE8. Mice exposed to TNBS showed a reduced colon shortening when treated with Y15 or Y15 and MFGE8 (online supplemental figure S8A–C). On a histopathologic assessment, prophylactic Y15 or Y15 and MFGE8 did not reduce the inflammatory score, and Y15 alone did not reduce the fibrosis score, but the combination of Y15 and MFGE8 reduced the fibrosis score. Y15 but not the combination of Y15 and MFGE8 reduced the area of sirius red (collagen-positive area) staining in TNBS-treated animals, but there was no additional benefit noted in when administering Y15 alone versus Y15 combined with MFGE8 (online supplemental figure S8D,E). Neither Y15 nor Y15 and MFGE8 reduced the increased thickness of the submucosa or muscularis mucosa in TNBS-treated animals (online supplemental figure S8D,F). Of note and overall, when MFGE8 was coadministered with the FAK inihibtor Y15, no additional difference was noted in any of the endpoints (online supplemental figure S8A–F). This suggests that once intestinal FAK signalling is blocked, MFGE8 does not exert an additional effect on intestinal fibrosis.
DISCUSSION
Strikingly limited knowledge is available in fibrostenotic CD regarding the critical interaction of intestinal ECM with mesenchymal cells.4 24 ECM composition in intestinal fibrosis is still unclear, and no reliable experimental platform exists for its examination.25 26 Only one study used a 3D decellularised human intestinal scaffold, but these were derived from normal duodenum and not inflamed or fibrostenotic ileal or colonic tissues. Thus, to develop a reliable system to study ECM composition and function in CD, we tested three decellularisation protocols9–11 and selected one based on SDC because of complete cell removal, preserved structure and capacity to bind HIMF.
Native ECM is a complex mix of proteins (the matrisome) whose individual components can exert both profibrotic and antifibrotic properties.4 5 27 We initially explored the ECM composition of NL, UC, CDns and CDs using decellularised tissues, and three matrisome components were found to be elevated in CDs, namely, MFGE8, VWF and LTBP1. MFGE8 was mainly expressed in the intestinal epithelium and upregulated in CDs. MFGE8 is secreted in increased amounts by intestinal epithelial cells after exposure to IL-1β. This is relevant as most ECM is produced by mesenchymal cells, but in this case, an epithelial secreted ECM component exterted function on fibroblasts. MFGE8 belongs to the family of ECM glycoproteins with multiple functions,28–31 but recent studies also indicate an antifibrotic effect in the heart,32 33 liver34 and kidney35 and amelioration of inflammation in acute DSS-induced and TNBS-induced colitis on systemic administration.16 Based on these preliminary reports, we performed an exhaustive series of experiments to explore its potential role in CDs.
To provide a proof of principle for the action of MFGE8, we tested the effects of MFGE8 on inflammation and fibrosis in vivo using a gain-of-function and loss-of-function approach using two different animal models. We chose to administer MFGE8 intrarectally to maximise intestinal exposure and minimise systemic effects. MFGE8 did not prevent histological inflammation but reduced the degree of fibrosis in the DSS and TNBS models when administered prophylactically. Hence, MFGE8 could be an effective preventive approach in the human clinical setting. To corroborate this finding, we next induced DSS fibrosis in MFGE8 KO mice that exhibited a higher degree of inflammation and fibrosis, confirming the antifibrotic effect of MFGE8. While we cannot provide experimental evidence to support this hypothesis, we believe that the difference in inflammation between the topical MFGE8 administration (no change) and the systemic MFGE8 KO animals (increase in inflammation) could be explained by either the topical, non-systemic nature of the effect with the enemas or by the absence of MFGE8 since birth in the KO animals and hence influence on immune system development. Interestingly, prior work by Aziz and colleagues15 suggested that intravenous and hence systemic administration of MFGE8 exerted anti-inflammatory properties in acute DSS colitis, but when we administered MFGE8 topically in DSS colitis, then no anti-inflammatory effect was observed. To support this finding, human LPMCs isolated from a patient with CD did not show any difference in the production of proinflammatory cytokines regardless of the exposure to different concentrations of MFGE8.
We hence next focused on MFGE8 and three major cellular functions relevant to fibrosis in vitro: ECM production, migration and proliferation. It is believed that those in vitro functions are relevant to the in vivo process of fibrogenesis. NL HIMF exposed to MFGE8 reduced the expression of FN and COLI1 as well as α-SMA. Surprisingly, MFGE8 had no such effect on CDs HIMF, while UC and CDns HIMF exhibited a blunted response compared with NL HIMF. This is in our opinion relevant as it is not frequently observed that different functions of mesenchymal cells found in vivo are preserved after cell isolation and culture. Given that isolation protocols and passage numbers of the HIMF were the same in all cell groups, the noted differences could suggest preserved cell properties intrinsic to each group or priming by a certain mediator or the inflammatory milieu the cells were exposed to in situ. The lack of response in CDs HIMF indicates that even when levels of MFGE8 are elevated and in proximity to HIMF in situ, MFGE8 may not be able to exert its antifibrotic effect. Interestingly, MFGE8 showed no effect on HIMF proliferation or migration. This is in contrast to other systems and cell types, like enhancement of hepatocyte proliferation34 or inhibition of neutrophil migration.36 These differential results are most likely due to the tissue-specific and cell-specific actions of MFGE8, with our findings suggesting a selective intestinal antifibrotic effect mediated by a reduction in ECM production. The functional findings add to our knowledge for the few ECM or matrisome molecules that have shown a functional effect on mesenchymal cells in IBD, such as hyaluronan, FN or lysyl oxidase.12 37 38 Of note, those ECM molecules have exerted profibrotic or proinflammatory properties and are derived from mesenchymal cells, whereas MFGE8 is an antifibrotic epithelial-derived ECM molecule. One may argue that the relatively lower amounts of MFGE8 in the submucosa compared with the epithelium in IBD may make this molecule less likely to be pathogenetically relevant. We however believe that the experimental mouse fibrosis data and the profound and consistent effect of MFGE8 in vitro point towards its importance in the development of strictures in CD.
A potential limitation of our work is the origin of the resection samples that formed the basis for this manuscript. The NL and UC samples were derived from the colon and the CD samples from the ileum. We however found a striking similarity in the matrisome between all groups and controlled the CDs samples with CDns, both of which were derived from the ileum. The same approach was used for the isolated HIMF.
Our unbiased next-generation sequencing analysis confirmed the antifibrotic signalling of MFGE8 in NL HIMF with 151 genes being significantly upregulated or downregulated compared with only 10 differentially expressed genes in CDs HIMF. Interestingly, when comparing differentially regulated genes in CDs with NL HIMF in response to MFGE8, pathway analysis revealed cell with ECM interactions through integrins as a potential candidate explaining the distinct response. Integrins are heterodimeric cell surface receptors that mediate cell-ECM and cell-cell interactions, and their modulation is now feasible in human clinical trials.39 We next turned our attention to FAK, a non-receptor tyrosine kinase to integrins, mediating ECM-integrin signal transduction.40 In fibrotic conditions, activation of FAK is closely related to the increased expression of α-SMA and collagen in fibrotic liver tissue in vivo and promotes the activation of hepatic stellate cells in vitro, implying a possible profibrotic role of FAK in fibrogenesis.41 42 NL HIMF exposed to MFGE8 reduced FAK signalling (less phosphorylation), whereas CDs HIMF did not. FAK inhibition made NL HIMF unresponsive to MFGE8, suggesting the activation of FAK is the downstream pathway of MFGE8-integrin interaction. This was confirmed in vivo in TNBS-induced fibrosis where topical coadministration of a FAK inihibitor and MFGE8 did not have stronger antifibrotic properties compared with administration of either alone.
Since at least 24 integrin heterodimers with a combination of 18 α-chains and 8 β-chains exist,43 we focused our attention on the subunits αv, β3 and β5, which are known to bind MFGE8.21 22 The expression of integrins αv and β5, but not β3, was lower in CDs HIMF compared with NL HIMF potentially explaining its role in MFGE8 signalling. Knocking down either of the two integrin subunits rendered NL HIMF unresponsive to the antifibrotic action of MFGE8, indicating that the defective antifibrotic response of CDs HIMF to MFGE8 may be due to their lower αvβ5 expression. Of relevance, integrin β5 expression in mesenchymal cells in the CDs intestine was lower compared with the NL intestine, suggesting that HIMF in vitro retains the integrin β5 expression found in situ. MFGE8 has also been reported to signal via TGF-β1/Smad2/332 and Akt/protein kinase B-glycogen synthase kinase-3β/mammalian target of rapamycin pathway44 in experimental cardiac systems, but we saw no such effect (data not shown).
We finally tested if MFGE8 is able to reverse fibrosis. These results are of particular importance to the pathogenesis of fibrosis in CD because in most patients that are candidates for antifibrotic therapies, a marked degree of fibrosis is already established at the time of treatment. Strikingly, the MFGE8 antifibrotic effects were evident also in the therapeutic setting after fibrosis was clearly established. To corroborate this finding, we were able to rescue the excessive ECM accumulation in the MFGE8 KO mice by local administration of MFGE8 after fibrosis was already established. Given the mechanistic findings of MFGE8 signalling through FAK, we finally performed an experiment in TNBS fibrosis topically coadministering a FAK inhibitor with MFGE8. FAK inhibitor alone reduced intestinal fibrosis. This is expected given that FAK is mediating signalling for essentially all integrins and the known effect of integrin activation on fibrogenesis. However, once FAK signalling was blocked, the administration of MFGE8 did not exert an additional antifibrotic effect. This indicates that the signalling pathway identified in vitro may also be relevant in vivo.
With improved understanding of the mechanisms of fibrogenesis and discovery of novel molecules with apparent antifibrotic effect, as demonstrated by our results, the therapeutic spectrum of agents to manage fibrosis in CD is widening.4 A large international effort is underway building a pathway to testing antifibrotic medications in stricturing CD.4 The first randomised controlled clinical trial testing an antifibrotic therapy approach has begun (NCT 05013385). Despite its ability to reverse experimental murine fibrosis, the in vitro effect of MFGE8 was only found on NL, UC and CDns but not CDs HIMF. MFGE8 may hence be best positioned as a preventative rather than a therapeutic agent for stricturing CD. Alternative future and more complex approaches may include sensitisation of HIMF to the action of MFGE8 by, for instance, anti-inflammatory or other comedications that may upregulate integrin β5 or the selective upregulation of integrin β5 in HIMF.
MATERIALS AND METHODS
Procurement of intestinal tissues
Briefly, full-thickness freshly resected intestinal specimens from subjects with CD (ileum) and controls, comprising UC (colon), diverticular disease (colon) and apparently healthy tissue (colon; constipation, healthy margin of resections from patients with colorectal cancer; termed NL for normal), were procured as previously described.19 45–47 CD specimens were classified based on gross anatomy into CDs and CDns. CDns represented purely inflammatory disease without the presence of internal penetrating disease. CDs resections included strictures with and without the copresence of internal penetrating disease. We did not find any functional differences within the CDs group when analysing the presence or absence of internal penetrating disease separately, and hence, all strictures were combined. This procurement system was validated by histopathologic evaluation performed by a trained IBD pathologist (IOG). Tissue blocks were procured for further processing including cell isolation; formalin-fixed, paraffin-embedded sectioning; and decellularisation. This work was approved by Cleveland Clinic Institutional Review Board (IRB) 17–1167.
Isolation and culture of primary human intestinal myofibroblasts
HIMFs were isolated and cultured as previously described19 45 47 and used between passages 4 and 8. Briefly, HIMFs were obtained as explants of surgically resected intestinal mucosa, grown to subconfluence in Dulbecco’s minimal essential medium supplemented with 10% fetal bovine serum (GIBCO, Life Technologies Corporation, Grand Island, New York, USA) and antibiotics and established as long-term cultures fed twice weekly and subcultured at confluence.
Decellularisation of colonic tissue sections, matrisome analysis, DNA and RNA extraction from colon tissue, HT29 stimulation experiments, cytokine cytometric bead array, DSS-induced colitis and isolation and culture of primary HIMFs can be found in online supplemental materials and methods.
Stimulation of primary human intestinal myofibroblasts with milk fat globule-epidermal growth factor 8 in vitro
HIMFs were isolated and cultured from NL, UC, CDns and CDs tissue. They were subsequently plated into 6-well tissue culture plates (Corning, New York, USA) for RNA extraction and qPCR, 24-well tissue culture plates (Corning, New York, USA) for protein extraction and 3-well glass chamber slides (IBIDI GmbH, Martinsried, Germany) for staining. Cells were seeded at a concentration of 20 000 cells/cm2 for all experiments. In all instances, cells were seeded, cultured overnight at 37°C prior to serum deprivation and followed by treatment with MFGE8 (R&D, Minneapolis, 2767-MF - 050) or vehicle in serum-free medium at 500 ng/mL. This concentration was chosen based on the optimal effects on suppression of ECM production in HIMFs. Cells were harvested for RNA analysis at 12 hours and protein analysis at 48 hours. Cells in three-well chamber slides were fixed at 48 hours for IF analysis.
Next-generation RNA sequencing analysis, FAK pathway inhibition, RNA interference, migration assay, cell proliferation assay, quantitative reverse transcriptase PCR procedure, IF, immunoblotting, IHC, ELISA and ECM deposition assay for HIMFs can be found in online supplemental materials and methods. The primers used for quantitative and qualitative PCR can be found in online supplemental table S5. The negative controls for the staining experiments can be found in online supplemental figure S13.
Statistical analysis
Data were analysed using analysis of Student’s t-test or variance for independent groups. In case of non-parametric distribution of data, comparisons were performed using Mann-Whitney/Wilcoxon or Kruskal-Wallis test, followed by Dunn’s test for multiple comparisons. Values were expressed as mean±SE, and statistical significance was set at p<0.05. All analyses were performed using GraphPad Prism (V.9; GraphPad Software Inc., San Diego, California, USA).
Supplementary Material
WHAT YOU NEED TO KNOW
The extracellular matrix (ECM) regulates mesenchymal cell function.
ECM composition in IBD in general and Crohn’s disease (CD)-associated strictures in particular remain unknown.
Milk fat globule-epidermal growth factor 8 (MFGE8) regulates multiple cellular functions in chronic inflammation, but its role in CD intestinal fibrosis has not been explored.
WHAT THIS STUDY ADDS
The expression of MFGE8 is increased in the CD stricture CDs matrisome.
In vivo experiment and in vitro experiment indicate an antifibrotic effect of MFGE8 mediated by the integrin αvβ5 and focal adhesion kinase.
CDs tissue-derived myofibroblasts do not respond to the antifibrotic effect of MFGE8.
HOW MIGHT IT IMPACT ON CLINICAL PRACTICE IN THE FORESEEABLE FUTURE
This study comprehensively investigated the antifibrotic effects of MFGE8 on CD and its underlying mechanisms.
MFGE8 may serve as a preventive agent for the treatment of CD intestinal fibrosis in the future.
Acknowledgements
We thank Dr Judith Drazba, Dr John Peterson, Andrelie Branicky and Apryl Helmick from the Lerner Research Institute Microscopy and Image Core for their help in microscopy and image analysis. We also thank Dr. Belinda Willard (Director, Proteomics Core, Cleveland Clinic) for proteomics analysis. We thank Dr Pieter W Faber (Director, University of Chicago Genomics Facility) for facilitating RNA sequencing. We acknowledge the support of the Departments of Colorectal Surgery and Pathology of the Cleveland Clinic. Tissue samples were provided by the Human Tissue Procurement Facility of the Cleveland Clinic through the services of the Biorepository Core funded by 2 P30 DK097948–06. Proteomics analysis was supported by National Institutes of Health-funded shared instrument grant 1S10OD023436–01.
Funding
This work was supported by the Helmsley Charitable Trust through the Stenosis Therapy and Anti-Fibrotic Research (STAR) Consortium (No. 3081 and No. 2210–05567 to FR), the Crohn’s and Colitis Foundation (No. 569125 to FR), the National Institute of Health (NIDDK R01DK123233 & R01DK132038 to FR), the National Institutes of Health (NIDDK 2 P30 DK097948 to CF and FR), the National Science Foundation of China (81970483, 82170537 and 82222010 to RM and 82200573 to SL) and the Bureau of Science and Technology of Guangzhou (No. 2023A04J2171).
Footnotes
Competing interests The Cleveland Clinic receives funds on her behalf from Celgene, Morphic, Pfizer, UCB, GB004 and Helmsley. SDH was consultant to Shionogi, Takeda and Guidepoint and receives research funding from the Crohn’s and Colitis Foundation and the American Society of Colon and Rectal Surgeons. CF receives speaker fees from UCB, Genentech, Sandoz and Janssen, and he is the consultant of Athos Therapeutics, Inc. FR is the consultant of Agomab, Allergan, AbbVie, Boehringer-Ingelheim, Celgene, Cowen, Genentech, Gilead, Gossamer, Guidepoint, Helmsley, Index Pharma, Jansen, Koutif, Metacrine, Morphic, Pfizer, Pliant, Prometheus Biosciences, Receptos, RedX, Roche, Samsung, Takeda, Techlab, Thetis, UCB and 89Bio.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Correction notice This article has been corrected since it published Online First. The author, Dr Atabai, name has been corrected.
Patient consent for publication Not applicable.
Ethics approval This study involves human participants and was approved by Cleveland Clinic IRB 17–1167. This is considered a minimal risk study.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
REFERENCES
- 1.Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology 2017;152:340–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Mao R, Kurada S, et al. Pathogenesis of fibrostenosing Crohn’s disease. Transl Res 2019;209:39–54. [DOI] [PubMed] [Google Scholar]
- 3.Latella G, Rieder F. Intestinal fibrosis: ready to be reversed. Curr Opin Gastroenterol2017;33:239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin S-N, Mao R, Qian C, et al. Development of antifibrotic therapy for stricturing Crohn’s disease: lessons from randomized trials in other fibrotic diseases. Physiol Rev 2022;102:605–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karsdal MA, Nielsen SH, Leeming DJ, et al. The good and the bad collagens of fibrosis - their role in signaling and organ function. Adv Drug Deliv Rev 2017;121:43–56. [DOI] [PubMed] [Google Scholar]
- 6.Bürger A, Wagner C, Viedt C, et al. Fibronectin synthesis by human tubular epithelial cells in culture: effects of PDGF and TGF-beta on synthesis and splicing. Kidney Int 1998;54:407–15. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi M, Ninomiya Y, Hayashi K, et al. Secretion of collagen types I and II by epithelial and endothelial cells in the developing chick cornea demonstrated by in situ hybridization and immunohistochemistry. Development 1988;103:27–36. [DOI] [PubMed] [Google Scholar]
- 8.Herrera J, Henke CA, Bitterman PB. Extracellular matrix as a driver of progressive fibrosis. J Clin Invest 2018;128:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen HJ, Wei Z, Sun J, et al. A recellularized human colon model identifies cancer driver genes. Nat Biotechnol 2016;34:845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giuffrida P, Curti M, Al-Akkad W, et al. Decellularized human gut as a natural 3D platform for research in intestinal fibrosis. Inflamm Bowel Dis 2019;25:1740–50. [DOI] [PubMed] [Google Scholar]
- 11.Keane TJ, Londono R, Turner NJ, et al. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 2012;33:1771–81. [DOI] [PubMed] [Google Scholar]
- 12.Mao R, Doyon G, Gordon IO, et al. Activated intestinal muscle cells promote preadipocyte migration: a novel mechanism for creeping fat formation in Crohn’s disease. Gut 2022;71:55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naba A, Clauser KR, Hoersch S, et al. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics 2012;11:M111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 2014;15:786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aziz MM, Ishihara S, Mishima Y, et al. MFG-E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin-dependent alphavbeta3 integrin signaling. J Immunol 2009;182:7222–32. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Brenner M, Yang WL, et al. Recombinant human MFG-E8 ameliorates colon damage in DSS-and TNBS-induced colitis in mice. Lab Invest 2015;95:480–90. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res 2012;40:4288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015;43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao S, Dejanovic D, Yao P, et al. Selective deletion of MyD88 signaling in α-SMA positive cells ameliorates experimental intestinal fibrosis via post-transcriptional regulation. Mucosal Immunol 2020;13:665–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao Y-Y, Zhang Z-H, Zhuang Z, et al. Recombinant milk fat globule-EGF factor-8 reduces apoptosis via integrin β3/FAK/PI3K/AKT signaling pathway in rats after traumatic brain injury. Cell Death Dis 2018;9:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu F, Chen Y, Hu Q, et al. MFGE8/Integrin β3 pathway alleviates apoptosis and inflammation in early brain injury after subarachnoid hemorrhage in rats. Exp Neurol 2015;272:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Datta R, Lizama CO, Soltani AK, et al. Autoregulation of insulin receptor signaling through MFGE8 and the αvβ5 integrin. Proc Natl Acad Sci U S A 2021;118:e2102171118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwal SK. Integrins and cadherins as therapeutic targets in fibrosis. Front Pharmacol 2014;5:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Haens G, Rieder F, Feagan BG, et al. Challenges in the pathophysiology, diagnosis, and management of intestinal fibrosis in inflammatory bowel disease. Gastroenterology 2022;162:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoshiba T. Decellularized extracellular matrix for cancer research. Materials (Basel) 2019;12:1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Southern BD, Grove LM, Rahaman SO, et al. Matrix-driven myosin II mediates the pro-fibrotic fibroblast phenotype. J Biol Chem 2016;291:6083–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Castro Brás LE, Frangogiannis NG. Extracellular matrix-derived peptides in tissue remodeling and fibrosis. Matrix Biol 2020;91–92:176–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni YQ, Zhan JK, Liu YS. Roles and mechanisms of MFG-E8 in vascular aging-related diseases. Ageing Res Rev 2020;64:101176. [DOI] [PubMed] [Google Scholar]
- 29.Neutzner M, Lopez T, Feng X, et al. MFG-E8/lactadherin promotes tumor growth in an angiogenesis-dependent transgenic mouse model of multistage carcinogenesis. Cancer Res 2007;67:6777–85. [DOI] [PubMed] [Google Scholar]
- 30.Gao C, Xie R, Yu C, et al. Thrombotic role of blood and endothelial cells in uremia through phosphatidylserine exposure and microparticle release. PLoS One 2015;10:e0142835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang W, Jiao J, Liu J, et al. MFG-E8 accelerates wound healing in diabetes by regulating “NLRP3 inflammasome-neutrophil extracellular traps” axis. Cell Death Discov 2020;6:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ge Z, Chen Y, Wang B, et al. MFGE8 attenuates Ang-II-induced atrial fibrosis and vulnerability to atrial fibrillation through inhibition of TGF-β1/Smad2/3 pathway. J Mol Cell Cardiol 2020;139:164–75. [DOI] [PubMed] [Google Scholar]
- 33.Wang B, Ge Z, Wu Y, et al. MFGE8 is down-regulated in cardiac fibrosis and attenuates endothelial-mesenchymal transition through Smad2/3-Snail signalling pathway. J Cell Mol Med 2020;24:12799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Zhang T, Wang K, et al. MFGE8 protects against CCl4 -induced liver injury by reducing apoptosis and promoting proliferation of hepatocytes. J Cell Physiol 2019;234:16463–74. [DOI] [PubMed] [Google Scholar]
- 35.Shi Z, Wang Q, Zhang Y, et al. Extracellular vesicles produced by bone marrow mesenchymal stem cells attenuate renal fibrosis, in part by inhibiting the RhoA/ROCK pathway, in a UUO rat model. Stem Cell Res Ther 2020;11:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aziz M, Yang WL, Corbo LM, et al. MFG-E8 inhibits neutrophil migration through αvβ₃-integrin-dependent MAP kinase activation. Int J Mol Med 2015;36:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soroosh A, Albeiroti S, West GA, et al. Crohn’s disease fibroblasts overproduce the novel protein KIAA1199 to create proinflammatory hyaluronan fragments. Cell Mol Gastroenterol Hepatol 2016;2:358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Bruyn JR, van den Brink GR, Steenkamer J, et al. Fibrostenotic phenotype of myofibroblasts in crohn’s disease is dependent on tissue stiffness and reversed by LOX inhibition. J Crohns Colitis 2018;12:849–59. [DOI] [PubMed] [Google Scholar]
- 39.Takada Y, Ye X, Simon S. The integrins. Genome Biol 2007;8:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee BY, Timpson P, Horvath LG, et al. FAK signaling in human cancer as a target for therapeutics. Pharmacol Ther 2015;146:132–49. [DOI] [PubMed] [Google Scholar]
- 41.Zhao X-K, Yu L, Cheng M-L, et al. Focal adhesion kinase regulates hepatic stellate cell activation and liver fibrosis. Sci Rep 2017;7:4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reif S, Lang A, Lindquist JN, et al. The role of focal adhesion kinase-phosphatidylinositol 3-kinase-akt signaling in hepatic stellate cell proliferation and type I collagen expression. J Biol Chem 2003;278:8083–90. [DOI] [PubMed] [Google Scholar]
- 43.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res 2010;339:269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng K-Q, Li J, She Z-G, et al. Restoration of circulating MFGE8 (Milk Fat Globule-EGF Factor 8) attenuates cardiac hypertrophy through inhibition of Akt pathway. Hypertension 2017;70:770–9. [DOI] [PubMed] [Google Scholar]
- 45.Rieder F, Georgieva M, Schirbel A, et al. Prostaglandin E2 inhibits migration of colonic lamina propria fibroblasts. Inflamm Bowel Dis 2010;16:1505–13. [DOI] [PubMed] [Google Scholar]
- 46.Strong SA, Pizarro TT, Klein JS, et al. Proinflammatory cytokines differentially modulate their own expression in human intestinal mucosal mesenchymal cells. Gastroenterology 1998;114:1244–56. [DOI] [PubMed] [Google Scholar]
- 47.Musso A, Condon TP, West GA, et al. Regulation of ICAM-1-mediated fibroblast-T cell reciprocal interaction: implications for modulation of gut inflammation. Gastroenterology 1999;117:546–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.