Abstract
The human T-cell leukemia virus type 1 (HTLV-1) Tax oncoprotein is a 40-kDa nuclear phosphoprotein which functions in the viral replication cycle as a transcriptional trans-activator of the viral long terminal repeat. Tax interacts with a variety of different transcription factors, including the CREB binding protein (CBP)/p300 family of transcriptional accessory proteins. We demonstrate that a Tax mutant defective for the CBP/p300 interaction retains the capacity to immortalize primary human T lymphocytes when it is expressed from a functional molecular clone of HTLV-1. Thus, immortalization of HTLV-1-infected cells appears to be independent of Tax-induced alterations in CBP/p300 function.
Human T-cell leukemia virus type 1 (HTLV-1) infects and immortalizes CD4+ T cells in vitro and is associated with the development of adult T-cell leukemia in vivo (31, 48). The Tax protein of HTLV-1 has been demonstrated to have transforming activity in rodent fibroblast transformation models (49, 54) and is leukemogenic when it is expressed in transgenic mice (26). Tax functions in the viral replication cycle as a transcriptional trans-activator of the viral long terminal repeat (LTR) (15, 22, 33, 51) through interactions with the cyclic AMP response element binding protein / activating transcription factor (CREB/ATF) family of transcriptional activators (2, 10, 13, 14, 55, 61, 62). In addition to activating the LTR through CREB/ATF, Tax is also capable of increasing the activity of the NF-κB (7, 32, 42, 53), p67SRF (23, 53), and Sp1/Egr-1 (56) pathways of transcriptional activation. Unregulated expression of cellular genes through these pathways likely contributes to the transforming activity of Tax.
Activation of the LTR by Tax occurs through interactions of Tax with CREB and the CREB response elements (CREs) found in three 21-nucleotide repeats in the LTR (2, 11, 55, 61, 62). The interaction of Tax with C-G-rich motifs that flank the CREs is important for CRE-CREB-Tax ternary complex formation, making the interaction specific to the viral CREs (41). Tax also interacts with the transcriptional coactivators CREB binding protein (CBP) and p300 (35, 38, 59). CBP and p300 are related multifunctional proteins that display histone acetyltransferase activities (8, 46) and interact with a variety of inducible transcription factors, including CREB (4, 16, 39), NF-κB/Rel (24, 47), p53 (5, 27, 43), c-Myb (18, 45), and c-Jun (9). In addition, CBP and p300 have also been shown to interact with a number of general transcription factors, including RNA polymerase II, transcription factor IIB, and TATA binding protein (1, 19, 36, 44, 60). Tax interacts with CBP in a region of CBP known as the KIX domain (amino acids 450 to 780), which has also been shown to bind to a number of other cellular proteins, including c-Jun, c-Myb, and p53 (9, 18, 45, 57). By binding to Tax, CBP can be recruited to the Tax response element-Tax-CREB ternary complex in a manner that is independent of the phosphorylation of CREB at serine 133 (25, 38), which is otherwise required for CREB-CBP binding (16).
The interaction of Tax with CBP has been shown to alter the binding of other proteins to CBP, including c-Myb, p53, and c-Jun, apparently by competition for binding to the KIX domain (17, 57, 58). This competition for CBP binding can lead to altered activities of other transcriptional activation pathways, which may contribute to the transforming activity of Tax (17, 57, 58). In addition to Tax, other viral proteins with transforming activities have been shown to interact with CBP/p300, including adenovirus E1A (3, 20) and simian virus 40 large T antigen (6, 21). However, a role for the interaction of Tax with CBP or p300 in the process of cellular immortalization by HTLV-1 has not been established.
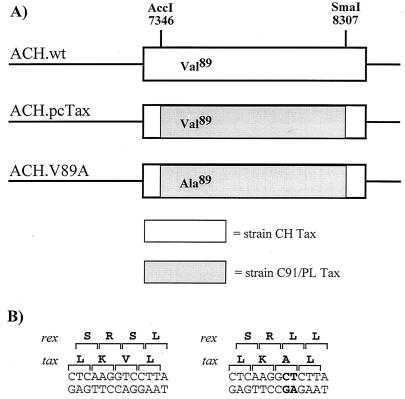
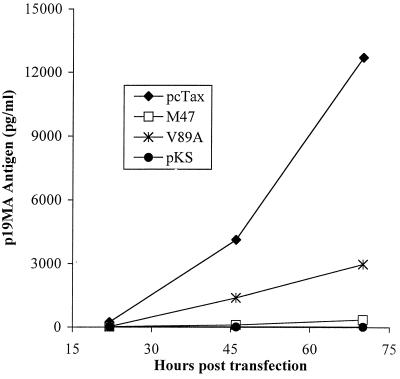
To examine the potential role of the Tax-CBP/p300 interaction in cellular transformation by HTLV-1, a molecular clone (37) containing a valine 89-to-alanine mutation in the tax gene was constructed (Fig. 1A). This mutation is in a region of Tax (amino acids 81 to 95) that displays some similarity to the kinase-inducible domain of CREB (amino acids 129 to 140), which binds the KIX domain of CBP (29). Harrod et al. have previously described three Tax mutations in this region (K85A, K88A, and V89A) that produce mutants that are defective for the Tax-CBP interaction but that allow the mutant to retain the ability to interact with CREB (29). The K88A and V89A mutants were demonstrated in this study to be completely defective for the Tax-CBP interaction by a glutathione S-transferase binding experiment and were also defective for CRE-CREB-Tax-CBP quaternary complex formation in gel shift assays. The V89A Tax mutation was chosen for this study because the net charge of Tax is not changed by this substitution, thus making it the least likely to have additional structural effects among the three possible mutations. In the context of the molecular clone, this mutation also creates an S108L substitution in the Rex protein, whose open reading frame (ORF) overlaps the Tax ORF (Fig. 1B). To confirm that this recombinant clone was capable of producing virus particles, the pACH.V89A plasmid was transfected into 293T cells and virus particle production was measured to 72 h posttransfection by a p19 matrix antigen enzyme-linked immunosorbent assay (Cellular Products, Buffalo, N.Y.). The ACH.V89A clone produced approximately 30% of the levels of virus produced by the wild-type ACH. pcTax clone (Fig. 2). This differed from the levels observed with the ACH.M47 mutant, another CREB activation mutant containing a mutation in the carboxy-terminal region of Tax (52), which produced <5% of the levels of wild-type virus. Consistent with the observed levels of virus particle production, transient cotransfections with an HTLV-LTR-luciferase reporter construct together with the ACH.V89A mutation demonstrated approximately 30% of wild-type activity (data not shown). As the relative levels of virus particle production correlated with the relative level of LTR activation, it is unlikely that this mutation adversely affects the Rex protein. This level of LTR activation is consistent with that observed in two previous studies that utilized this mutant (29, 58). Thus, as expected, V89A mutant Tax is partially defective for HTLV-LTR activation, presumably due to the defect in the Tax-CBP/p300 interaction.
FIG. 1.
Construction of the ACH.V89A Tax mutant. (A) The ACH.pcTax and ACH.V89A constructs used in this study contain the tax gene of the HTLV-1 strain C91/PL between the AccI site at position 7346 and the SmaI site at position 8307 in the ACH molecular clone. (B) The TC-to-CT nucleotide substitution encoding the V89A mutation was introduced by overlap extension PCR. This mutation results in a Val89-to-Ala89 substitution in the Tax ORF and a Ser108-to-Leu108 substitution in the overlapping Rex ORF.
FIG. 2.
Viral particle production by ACH.V89A. Human 293T kidney fibroblast cells seeded to 30% confluence in six-well plates were transfected with 3 μg of ACH.pcTax, ACH.V89A, ACH.M47, or an empty vector plasmid with LipofectAMINE (Gibco, Bethesda, Md.). Virus particle production was measured by p19 matrix antigen enzyme-linked immunosorbent assay on conditioned supernatants at the indicated time points posttransfection. The ACH.V89A clone produced approximately 30% of the amount of virus produced by the wild-type clone, while the ACH.M47 mutant virus produced <5% of the wild-type amount. Shown are the means of results of duplicate transfections in one trial; the data are representative of multiple experiments.
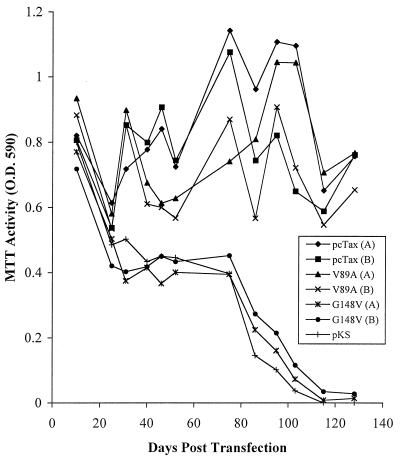
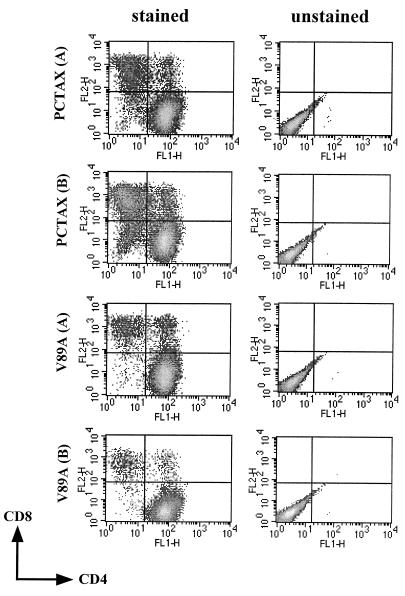
To examine the effect of the V89A mutation on the ability of the ACH molecular clone to immortalize transfected primary peripheral blood mononuclear cells (PBMC), 107 PBMC were isolated from uninfected donors by Ficoll-Paque (Amersham Pharmacia, Piscataway, N.J.) purification, activated for 72 h with a solution containing 10 μg of phytohemagglutinin-P (Sigma, St. Louis, Mo.) per ml and 50 U of interleukin-2 (IL-2) per ml, and transfected by electroporation with the pACH.V89A mutant molecular clone. Cellular viability was monitored to 90 to 120 days posttransfection by MTT conversion assays (28). As positive and negative controls, PBMC were also transfected with the wild-type ACH.pcTax plasmid and ACH.G148V, which contains a point mutation in the tax gene that results in a Tax protein which is incapable of activating the NF-κB pathway. We have previously shown that the NF-κB pathway is important for the immortalization of infected cells using a different Tax mutant, designated M22 (50, 52). Results of one experiment in which each clone was transfected in duplicate and an additional empty vector was transfected as a control are shown in Fig. 3. Like the cells transfected with the ACH.pcTax molecular clones, the cells transfected with the ACH.V89A mutant continued to proliferate indefinitely. Two additional experiments performed with PBMC from a different donor also resulted in immortalization of transfected cells with the ACH.V89A plasmid. Conversely, the cells transfected with an empty vector or the ACH.G148V clone proliferated only transiently and were not immortalized in a total of three or eight attempts, respectively. The cells immortalized with the wild-type clone as well as the V89A mutant were both of a T-helper cell phenotype, in that the majority of cells in the immortalized cell cultures expressed CD4 and lacked expression of CD8 (Fig. 4). Thus, it appears that the interaction of Tax with CBP/p300 is not required for the IL-2-dependent immortalization of HTLV-1-infected cells. Furthermore, the results with the G148V NF-κB activation mutant confirm our previous results with the M22 Tax mutant (50).
FIG. 3.
Immortalization of transfected PBMC by the ACH.V89A clone. Uninfected PBMC were activated for 72 h with a solution containing 10 μg of phytohemagglutinin-P and 50 U of IL-2 per ml. Ten million cells were transfected by electroporation as previously described (50) with 25 μg of ACH.pcTax, ACH.V89A, ACH.G148V, or the pBluescript KS plasmid. Cellular viability was monitored by MTT conversion assays. The ACH.pcTax and ACH.V89A plasmid-transfected cells were immortalized, while the ACH.G148V plasmid- and empty vector-transfected cells were not immortalized. O.D. 590, optical density at 590 nm.
FIG. 4.
Cell surface phenotype of ACH.V89A-immortalized cells. Immortalized cells were stained with anti-CD4 antibody–fluorescein isothiocyanate and anti-CD8 antibody–phycoerythrin and analyzed on a Becton Dickinson FACSCAN. Cells immortalized with the ACH.pcTax and ACH.V89A plasmid are predominately CD4+ and CD8−. The cell lines examined were derived from the experiment whose results are shown in Fig. 3. FL1-H and FL2-H, CD4 and CD8 levels, respectively.
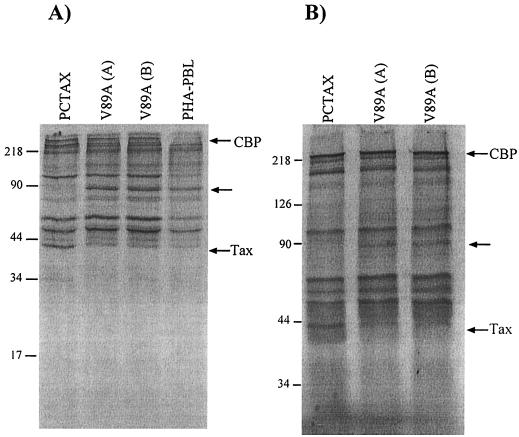
To further confirm that the V89A mutant was defective for CBP binding in the context of the immortalized cells, whole-cell lysates were produced from immortalized cells by lysing 3 × 106 cells labeled for 18 h with [35S]Trans-label (ICN, Costa Mesa, Calif.) in 1 ml of radioimmunoprecipitation assay buffer, followed by immunoprecipitation with an anti-CBP antibody (Santa Cruz Biotech, Santa Cruz, Calif.). Immunoprecipitated proteins were resolved on either sodium dodecyl sulfate (SDS)–10% or SDS–7.5% polyacrylamide assay gels. A 40-kDa protein that was absent from cells immortalized with V89A mutant virus or from uninfected cells was coprecipitated in cells immortalized with wild-type HTLV-1 (Fig. 5). This protein is similar in size to the Tax proteins detected at equivalent levels in both ACH.pcTax- and ACH.V89-immortalized cells as determined by immunoblot analysis with anti-Tax antibodies (not shown). Thus, it appears that V89A mutant Tax fails to interact with CBP in immortalized cells, confirming that this interaction is dispensable for immortalization. Interestingly, a 90-kDa protein coprecipitated with CBP in cells that expressed the V89A mutant Tax but not wild-type Tax, suggesting that Tax competes with this protein for CBP binding. None of the proteins that have been demonstrated to bind to the KIX domain of CBP have a molecular mass of 90 kDa, so we are unable to speculate as to the identity of this protein.
FIG. 5.
Coimmunoprecipitation of Tax and CBP in wild-type- but not V89A mutant-immortalized cells. Immortalized cells were labeled with [35S]methionine, and CBP was immunoprecipitated from whole-cell lysates by anti-CBP polyclonal antibody. A 40-kDa band that was absent from V89A mutant-immortalized or uninfected cells was coimmunoprecipitated from wild-type-immortalized cells. Shown are results of two independent experiments in which cells were separated on an SDS–10% (A) or an SDS–7.5% (B) polyacrylamide gel. Note that binding of Tax to CBP appears to displace an unidentified 90-kDa CBP-interacting protein. Molecular mass markers (in kilodaltons) are noted at the left sides of the gels.
Although the interaction of Tax with members of the CBP/p300 family is well established, the role that this interaction plays in cellular immortalization is not known. The results of this study indicate that the Tax-CBP/p300 interaction is not required for cellular immortalization. This result is consistent with those of our previous studies utilizing the CREB activation-deficient M47 mutant Tax (50, 52), which was reported in one study to be capable of binding p300 but not CBP (12). The ACH.M47 mutant clone also retains the ability to immortalize infected cells despite substantially reduced LTR activation (50). However, unlike cultures immortalized with the M47 virus, which were demonstrated to have a high proportion of CD8+ cells, the cells immortalized with the V89A virus were, like wild-type immortalized cells, mostly CD4+ cells. This difference may be due to a higher level of Tax activation of the LTR by the V89A mutant in CD4+ cells or differences in interactions with cellular proteins between the two mutants that are responsible for the observed phenotypes. It is of interest that the M47 mutant Tax, but not the V89 mutant Tax, is also defective in its interaction with p300/CBP-associated factor (30, 34). The difference in cell surface phenotype aside, these results also confirm that, while it is important for activation of the LTR, CREB/ATF activation appears to be dispensable for cellular immortalization.
The Tax-CBP interaction is likely to be important for viral replication in the context of initial infection of quiescent cells in which CREB might not be phosphorylated and therefore be unable to bind CBP (16, 38). In vivo, the majority of cells encountered by HTLV-1 viral particles are likely to be quiescent; thus, the interaction between Tax and CBP/p300 may have evolved as a way to activate transcription from the LTR in newly infected cells. In addition, the experimental system utilized in this study does not address a potential role for a Tax-CBP/p300 interaction in the acquisition of IL-2-independent proliferation of immortalized cells. Nonetheless, understanding the complex interactions between Tax and cellular transcription factors in the context of virally infected immortalized cells will provide a better understanding of HTLV-1-mediated disease pathogenesis.
Acknowledgments
We thank Toni Portis for helpful discussions and critical review of the manuscript and John Harding for technical assistance.
This work was supported by PHS grant CA 64317 and training grant GM07076 (M.D.R.).
REFERENCES
- 1.Abraham S E, Lobo S, Yaciuk P, Wang H H, Moran E. p300 and p300-associated proteins, are components of TATA-binding protein (TBP) complexes. Oncogene. 1993;8:1639–1647. [PubMed] [Google Scholar]
- 2.Adya N, Giam C-Z. Distinct regions in human T-cell lymphotropic virus type I Tax mediate interactions with activator protein CREB and basal transcription factors. J Virol. 1995;69:1834–1841. doi: 10.1128/jvi.69.3.1834-1841.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arany Z, Sellers W R, Livingston D M, Eckner R. E1-A associated p300 and CREB-associated CBP belong to a conserved family of coactivators. Cell. 1994;77:799–800. doi: 10.1016/0092-8674(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 4.Arias J, Alberts A S, Brindle P, Claret F X, Smeal T, Karin M, Feramisco J, Montminy M. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature. 1994;370:226–229. doi: 10.1038/370226a0. [DOI] [PubMed] [Google Scholar]
- 5.Avantaggiati M L, Ogryzko V, Gardner K, Giordano A, Levine A S, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. doi: 10.1016/s0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- 6.Avavtaggiati M L, Carbone M, Graessmann A, Nakatani Y, Howard B, Levine A S. The SV40 large T antigen and adenovirus E1A oncoproteins interact with distinct isoforms of the transcriptional coactivator p300. EMBO J. 1996;15:2236–2248. [PMC free article] [PubMed] [Google Scholar]
- 7.Ballard D W, Bohnlein E, Lowenthal J W, Wano Y, Franza B R, Greene W C. HTLV-1 tax induces cellular proteins that activate the κB element in the IL-2 receptor alpha gene. Science. 1988;241:1652–1655. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- 8.Bannister A J, Kouzarides T. The CBP coactivator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 9.Bannister A J, Oehler T, Wilhelm D, Angel P, Kouzarides T. Stimulation of c-jun activity by CBP:c-jun residues Ser63/73 are required for CBP induced stimulation in vivo and CBP binding in vitro. Oncogene. 1995;11:2509–2514. [PubMed] [Google Scholar]
- 10.Bantignies F, Rousset R, Desbois C, Jalinot P. Genetic characterization of transactivation of the human T-cell leukemia virus type 1 promoter: binding of Tax to Tax-responsive element 1 is mediated by the cyclic AMP-responsive members of the CREB/ATF family of transcription factors. Mol Cell Biol. 1996;16:2174–2182. doi: 10.1128/mcb.16.5.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnhart M K, Connor L M, Marriott S J. Function of the human T-cell leukemia virus type 1 21-base-pair repeats in basal transcription. J Virol. 1997;71:337–344. doi: 10.1128/jvi.71.1.337-344.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bex F, Yin M J, Burney A, Gaynor R B. Differential transcriptional activation by human T-cell leukemia virus type 1 Tax mutants is mediated by distinct interactions with CREB binding protein and p300. Mol Cell Biol. 1998;18:2392–2405. doi: 10.1128/mcb.18.4.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodor J, Walker W, Flemington E, Spetz A-L, Habener J F. Modulation of Tax and PKA-mediated expression of HTLV-I promoter via cAMP response element binding and modulator proteins CREB and CREM. FEBS Lett. 1995;377:413–418. doi: 10.1016/0014-5793(95)01299-0. [DOI] [PubMed] [Google Scholar]
- 14.Brauweiler A, Garl P, Franklin A A, Giebler H A, Nyborg J K. A molecular mechanism for human T-cell leukemia virus latency and Tax transactivation. J Biol Chem. 1995;270:12814–12822. doi: 10.1074/jbc.270.21.12814. [DOI] [PubMed] [Google Scholar]
- 15.Cann A J, Rosenblatt J D, Wachsman W, Shah N P, Chen I S Y. Identification of the gene responsible for human T-cell leukemia virus transcriptional regulation. Nature. 1985;318:571–574. doi: 10.1038/318571a0. [DOI] [PubMed] [Google Scholar]
- 16.Chrivia J C, Kwok R P S, Lamb N, Hagiwara M, Montminy M R, Goodman R H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 17.Colgin M A, Nyborg J K. The human T-cell leukemia virus type 1 oncoprotein Tax inhibits the transcriptional activity of c-Myb through competition for the CREB binding protein. J Virol. 1998;72:9396–9399. doi: 10.1128/jvi.72.11.9396-9399.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai P, Akimaru H, Tanaka Y, Hou D-X, Yasukawa T, Kanei-Ishii C, Takahishi T, Ishii S. CBP as a transcriptional coactivator of c-myb. Genes Dev. 1996;10:528–540. doi: 10.1101/gad.10.5.528. [DOI] [PubMed] [Google Scholar]
- 19.Dallas P B, Yaciuk P, Moran E. Characterization of monoclonal antibodies raised against p300: both p300 and CBP are present in intracellular TBP complexes. J Virol. 1997;71:1726–1731. doi: 10.1128/jvi.71.2.1726-1731.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckner R, Ewen M E, Newsome D, Gerdes M, DeCaprio J A, Lawrence J B, Livingston D M. Molecular cloning and functional analysis of the adenovirus E1A-associated 300 kD (p300) protein reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 21.Eckner, Ludlow J W, Nill N L, Oldread E, Arany Z, Modjtahedi N, DeCaprio J D, Livingstonm D M, Morgan J A. Association of p300 and CBP with simian virus 40 large T antigen. Mol Cell Biol. 1996;16:3454–3464. doi: 10.1128/mcb.16.7.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felber B K, Paskalis H, Kleinman-Ewing C, Wong-Staal F, Pavlakis G N. The pX protein of HTLV-I is a transcriptional transactivator of its long terminal repeats. Science. 1985;229:675–679. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- 23.Fujii M, Tsuchiya H, Chuhjo T, Akizawa T, Seiki M. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 1992;6:2066–2076. doi: 10.1101/gad.6.11.2066. [DOI] [PubMed] [Google Scholar]
- 24.Gerritsen M E, Williams A J, Neish A S, Moore S, Shi Y, Collins T. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci USA. 1997;94:2927–2932. doi: 10.1073/pnas.94.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giebler H A, Loring J E, Van Orden K, Colgin M A, Garrus J E, Escudero K W, Brauweiler A, Nyborg J K. Anchoring of CREB binding protein to the human T-cell leukemia virus type 1 promoter: a molecular mechanism of Tax transactivation. Mol Cell Biol. 1997;17:5156–5164. doi: 10.1128/mcb.17.9.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossman W J, Kimata J T, Wong F H, Zutter M, Ley T J, Ratner L. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1995;92:1057–1061. doi: 10.1073/pnas.92.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu W, Shi X L, Roeder R G. Synergistic activation of transcription by CBP and p53. Nature. 1997;387:819–823. doi: 10.1038/42972. [DOI] [PubMed] [Google Scholar]
- 28.Hansen M B, Nielsen S E, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 29.Harrod R, Tang Y, Nicot C, Lu H S, Vassilev A, Nakatani Y, Giam C Z. An exposed KID-like domain in human T-cell lymphotropic virus type 1 Tax is responsible for the recruitment of coactivators CBP/p300. Mol Cell Biol. 1998;18:5052–5061. doi: 10.1128/mcb.18.9.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrod R, Kuo Y L, Tang Y, Yao Y, Vassilev A, Nakatani Y, Giam C Z. P300 and p300/cAMP-responsive element-binding protein associated factor interact with human T-cell lymphotropic virus type-1 Tax and a multi-histone acetyltransferase/activator-enhancer complex. J Biol Chem. 2000;21:11852–11857. doi: 10.1074/jbc.275.16.11852. [DOI] [PubMed] [Google Scholar]
- 31.Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita K, Shirakawa S, Miyoshi I. Adult T-cell leukemia antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue J, Seiki M, Taniguchi T, Tsuru S, Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 1986;5:2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue J, Yoshida M, Seiki M. Transcriptional (p40X) and post transcriptional (p27X-III) regulators are required for the expression and replication of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1987;84:3653–3657. doi: 10.1073/pnas.84.11.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang H, Lu H, Schiltz R L, Pise-Masison C A, Ogryzko V V, Nakatani Y, Brady J N. PCAF interacts with tax and stimulates tax transactivation in a histone-acetyltransferase-independent manner. Mol Cell Biol. 1999;19:8136–8145. doi: 10.1128/mcb.19.12.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kashanchi F, Duvall J F, Kwok R P S, Lundblad J R, Goodman R H, Brady J N. The coactivator CBP stimulates human T-cell lymphotropic virus type 1 Tax transactivation in vitro. J Biol Chem. 1998;273:34646–34652. doi: 10.1074/jbc.273.51.34646. [DOI] [PubMed] [Google Scholar]
- 36.Kee B L, Arias J, Montminy M R. Adaptor mediated recruitment of RNA polymerase II to a signal-dependent activator. J Biol Chem. 1996;271:2373–2375. doi: 10.1074/jbc.271.5.2373. [DOI] [PubMed] [Google Scholar]
- 37.Kimata J T, Wong F H, Wang J J, Ratner L. Construction and characterization of infectious human T-cell leukemia virus type 1 molecular clones. Virology. 1994;204:656–664. doi: 10.1006/viro.1994.1581. [DOI] [PubMed] [Google Scholar]
- 38.Kwok R P S, Laurance M E, Lundblad J R, Goldman P S, Shih H-M, Connor L M, Marriott S J, Goodman R H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature. 1996;380:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 39.Kwok R P S, Lundblod J R, Chrivia J C, Richards J P, Bachinger H P, Brennan R G, Roberts S G E, Green M R, Goodman R H. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 40.Langton B C, Sliwkowski M, Tran K V, Knapp S, Keitelman E, Smith C, Wallingford S, Liu H-L, Ralston J S, Brandis J, Coats S. Development and characterization of monoclonal antibodies to the HTLV-1 Tax (p40X) protein. Med Virol. 1988;8:295–302. [Google Scholar]
- 41.Lenzmeier B A, Giebler H A, Nyborg J K. Human T-cell leukemia virus type 1 Tax requires direct access to DNA for recruitment of CREB binding to the viral promoter. Mol Cell Biol. 1998;18:721–731. doi: 10.1128/mcb.18.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leung K, Nabel G J. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-κB-like factor. Nature. 1988;333:776–778. doi: 10.1038/333776a0. [DOI] [PubMed] [Google Scholar]
- 43.Lill N L, Grossman S R, Ginsberg D, DeCaprio J, Livingston D M. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 44.Nakajima T, Uchida C, Anderson S F, Lee C, Hurwitz J, Parvin J D, Montminy M. RNA helicase A mediates association of CBP with RNA polymerase II. Cell. 1997;90:1107–1113. doi: 10.1016/s0092-8674(00)80376-1. [DOI] [PubMed] [Google Scholar]
- 45.Oelgeschlager M, Janknecht R, Krieg J, Schreek S, Lusher B. Interaction of the co-activator CBP with Myb proteins: effects of Myb-specific transactivation and on the cooperativity with NF-M. EMBO J. 1996;15:2771–2780. [PMC free article] [PubMed] [Google Scholar]
- 46.Ogryzko V V, Schlitz R L, Russanova V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 47.Perkins N D, Felzien L K, Betts J C, Leung K, Beach D H, Nabel G J. Regulation of NF-κB by cyclin-dependent kinases associated with the p300 coactivator. Science. 1997;275:523–527. doi: 10.1126/science.275.5299.523. [DOI] [PubMed] [Google Scholar]
- 48.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pozzati R, Vogel J, Jay G. The human T-lymphotropic virus type 1 tax gene can cooperate with the ras oncogene to induce neoplastic transformation of cells. Mol Cell Biol. 1990;10:413–417. doi: 10.1128/mcb.10.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robek M R, Ratner L. Immortalization of CD4+ and CD8+ T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J Virol. 1999;73:4856–4865. doi: 10.1128/jvi.73.6.4856-4865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seiki M, Inoue J, Takeda T, Yoshida M. Direct evidence that the p40x of human T-cell leukemia virus type-1 is a trans-activating transcriptional transactivator. EMBO J. 1986;5:561–565. doi: 10.1002/j.1460-2075.1986.tb04247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith M R, Greene W C. Identification of HTLV-1 tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki T, Hirai H, Fujisawa J, Fujita T, Yoshida M. A transactivator Tax of human T-cell leukemia virus type 1 binds to NF-κB p50 and serum response factor (SRF) and associates with enhancer DNAs of the NF-κB site and CArG box. Oncogene. 1993;8:2391–2397. [PubMed] [Google Scholar]
- 54.Tanaka A C, Takahashi C, Yamaoka S, Nosaka T, Maki M, Hatanaka M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type 1 in vitro. Proc Natl Acad Sci USA. 1990;87:1071–1075. doi: 10.1073/pnas.87.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tie F, Adya N, Greene W C, Giam C-Z. Interaction of the human T-lymphotropic virus type 1 Tax dimer with CREB and the viral 21-base-pair repeat. J Virol. 1996;70:8368–8374. doi: 10.1128/jvi.70.12.8368-8374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trejo S R, Fahl W E, Ratner L. The Tax protein of human T-cell leukemia virus type 1 mediates the transactivation of the c-sis/platelet-derived growth factor-B promoter through interactions with the zinc finger transcription factors Sp1 and NGFI-A/Egr-1. J Biol Chem. 1997;272:27411–27421. doi: 10.1074/jbc.272.43.27411. [DOI] [PubMed] [Google Scholar]
- 57.Van Orden K, Giebler H A, Lemasson I, Gonzales M, Nyborg J K. Binding of p53 to the KIX domain of CREB binding protein: a potential link to human T-cell leukemia virus type 1-associated leukemogenesis. J Biol Chem. 1999;274:26321–26328. doi: 10.1074/jbc.274.37.26321. [DOI] [PubMed] [Google Scholar]
- 58.Van Orden K, Yan J P, Ulloa A, Nyborg J K. Binding of the human T-cell leukemia virus Tax protein to the coactivator CBP interferes with CBP-mediated transcriptional control. Oncogene. 1999;18:3766–3772. doi: 10.1038/sj.onc.1202703. [DOI] [PubMed] [Google Scholar]
- 59.Yan J-P, Garrus J E, Giebler H A, Stargell L A, Nyborg J K. Molecular interactions between the coactivator CBP and the human T-cell leukemia virus Tax protein. J Mol Biol. 1998;281:395–400. doi: 10.1006/jmbi.1998.1951. [DOI] [PubMed] [Google Scholar]
- 60.Yang X, Ogryzko V V, Nishikawa J, Howard B H, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 61.Yin M-J, Gaynor R B. Complex formation between CREB and Tax enhances the binding affinity of CREB for the human T-cell leukemia virus type 1 21-base-pair repeats. Mol Cell Biol. 1996;16:3156–3168. doi: 10.1128/mcb.16.6.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yin M J, Paulssen E J, Seeler J-S, Gaynor R B. Protein domains involved in both in vivo and in vitro interactions between human T-cell leukemia virus type 1 Tax and CREB. J Virol. 1995;69:3420–3432. doi: 10.1128/jvi.69.6.3420-3432.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]