Gastroenterology, like many other disciplines, is expanding rapidly. In the past four years there have been exciting advances in screening, diagnosis, and therapy. This article describes some of the most clinically relevant developments.
Methods
We selected the topics after discussion with colleagues and attending the British Society of Gastroenterology meeting, Birmingham 2002. We subjectively assessed the most important recent innovations and evaluated these in more depth by searching Medline and the Cochrane Controlled Trials Register. We also hand searched recent issues of Gastroenterology, Gut, and Gastrointestinal Endoscopy.
Screening
Gastric cancer
Gastric cancer is the second commonest cause of cancer mortality worldwide, causing around 660 000 deaths annually. In England and Wales it is the fifth commonest cause of cancer death, with an annual mortality of about 7000. A meta-analysis of nested case-control studies reported that patients infected with Helicobacter pylori were nearly six times more likely to develop distal gastric adenocarcinoma than uninfected controls.1 A recent randomised trial suggests that eradication of H pylori will improve gastric atrophy and intestinal metaplasia, which are thought to be premalignant changes.2 Studies have also identified subgroups of people infected with H pylori who may be at particular risk of developing gastric cancer.3 A randomised trial showed that H pylori screening and treatment might save money because of the reduced costs of treating dyspepsia.4
Oesophageal and proximal gastric adenocarcinoma have been increasing in recent years, and this parallels the fall in prevalence of H pylori infection. Some investigators have therefore suggested that H pylori infection protects against the development of cancers of the proximal stomach and oesophagus. This hypothesis is not supported by a meta-analysis of nested case-control trials,1 but the benefits and harms of population screening and treatment for H pylori can properly be evaluated only in a randomised controlled trial. Unfortunately, trials to evaluate the efficacy of this primary prevention approach require large numbers of people and long term follow up. Funding bodies may prefer trials that detect disease early as these require fewer participants and shorter follow up. It is therefore uncertain whether population H pylori screening and treatment will ever be rigorously evaluated.
Recent developments
Wireless capsule endoscopy images the small bowel better than other endoscopic techniques and may replace upper gastrointestinal endoscopy and colonoscopy
The most cost effective method of colorectal cancer screening is being evaluated, and a national screening programme is likely to be introduced in the next few years
Helicobacter pylori “test and treat” is more cost effective than endoscopy for managing dyspepsia
New endoscopic treatments are being developed for gastro-oesophageal reflux disease
Infusions with tumour necrosis factor α antibodies are useful in severe Crohn's disease
Colorectal cancer
Colorectal cancer is less important than gastric cancer in global terms, but it is a notable cause of death in many developed countries. In the United Kingdom, colorectal cancer is responsible for almost 16 000 deaths each year. Many cancers develop from adenomatous polyps, so detection and removal of polyps should reduce mortality. Three methods of screening have been proposed: faecal occult blood testing, flexible sigmoidoscopy, and colonoscopy (table 1). Faecal occult blood testing reduced mortality from colorectal cancer by up to 23% in four randomised controlled trials.5 The sensitivity is increased with faecal DNA analysis for the adenomatous polyposis coli (APC) gene, which was reported to detect 57% of colorectal cancers in one case-control study.6 DNA analysis will remain a research tool for some years, but it could lead to a cheap, accurate, non-invasive test for colorectal cancer.
Table 1.
Comparison of three approaches to screening for colorectal cancer
| Faceal occult blood
|
Flexible sigmoidoscopy
|
Colonoscopy
|
|
|---|---|---|---|
| Evidence for benefit from randomised controlled trials | Systematic review of four trials showed 16% reduction* (95% CI 7% to 23%) in colorectal cancer mortality | No evidence for reduction in mortality. Evidence that colorectal cancer detected at an earlier stage | No evidence for reduction in mortality or earlier detection. |
| Surveillance interval | 1-2 years | Uncertain | Uncertain |
| Age range evaluated | 45-75 years | 55-64 years | Uncertain |
| Colonoscopy rate in compliant subjects | 2% | 5% | 100% |
| Facilities required | Testing unit plus increased provision for colonoscopy | Flexible sigmoidoscopy facilities in secondary care, primary care, or mobile units plus increased provision for colonoscopy |
Facilities in secondary care for extra colonoscopy |
| Compliance | 60-90% | 40% | Uncertain |
| Cost | Relatively inexpensive | Moderately expensive† | Very expensive† |
| Potential reduction in mortality assuming 100% compliance | 23% (95% CI 11% to 43%) | 70% | 100% |
| Perforation rate | 0.006%‡ | 0.01% | 0.5% |
Number relates to all patients randomised to faecal occult blood screening and therefore incorporates the effect of non-compliance.
Cost depends on surveillance interval recommended.
Calculated from the number of extra colonoscopies that testing will generate.
Flexible sigmoidoscopy could have a greater effect on colorectal cancer mortality than faecal occult blood screening and is being evaluated in three randomised controlled trials. Preliminary results from a UK study in which 40 674 people had flexible sigmoidoscopy, suggest that the procedure is acceptable; high risk polyps were detected in 1.2%, and 0.3% had colorectal cancer.7 Sixty two per cent of the colorectal cancers were detected early and potentially curable, with only 26% being inoperable.7 This compares favourably with the spectrum of disease seen in patients presenting with symptoms.
Although flexible sigmoidoscopy looks promising, data on death rates from colorectal cancer are needed before such screening can be advocated. There is also uncertainty about the optimum age for screening and whether this should be done “once in a lifetime” or at regular intervals. The introduction of flexible sigmoidoscopy screening will inevitably place extra burden on endoscopy services. Nurses could shoulder some of this burden, as studies have shown their diagnostic accuracy is similar to that of a doctor.8
Flexible sigmoidoscopy may miss up to 30% of lesions because they occur beyond the reach of the endoscope.9 Colonoscopy will detect these lesions, and this approach is being investigated in the United States.10 However, colonoscopy is more expensive, less acceptable to patients, and is associated with a 0.5% perforation rate, which could offset any benefits.
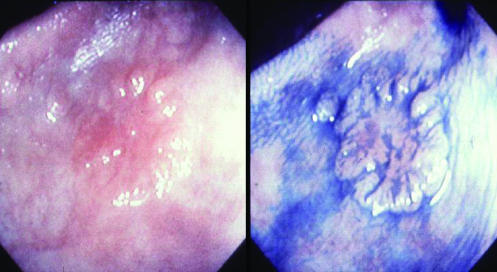
The detection of colorectal cancer could be enhanced by wider recognition that some neoplastic lesions are flat and therefore difficult to detect. In one UK series of 1000 unselected patients evaluated by a single endoscopist trained in Japanese techniques of careful evaluation of the mucosa, 117/321 (36%) of adenomas identified were flat or depressed as were four out of six Dukes' A adenocarcinomas detected.11 Training in identifying these subtle lesions (fig 1) could improve the detection of early colorectal cancer.
Figure 1.
Early flat colonic neoplasm before and after spraying with indigo carmine dye to aid visualisation
New diagnostic imaging techniques
Wireless capsule endoscopy
A videotelemetry capsule has been developed that is small enough (11×27 mm) to be swallowed.12 This is now commercially available but is being used in only a few hospitals. The images obtained are transmitted to aerials taped to the body as the capsule passes through the gastrointestinal tract by peristalsis. These images are then stored in a recorder carried by the patient. Potentially, the whole of the gastrointestinal tract can be seen without the need for uncomfortable and invasive endoscopy. Images of the upper gastrointestinal tract and colon are inferior to those obtained by upper gastrointestinal endoscopy and colonoscopy. The capsule is also unable to take biopsy samples or conduct any therapeutic procedure.
At present, the capsule is most likely to be used for imaging the small bowel. This part of the gastrointestinal tract is difficult to access with an endoscope, and radiology misses important lesions in this area such as angiodysplasia (fig 2). Around 40% of patients with obscure gastrointestinal bleeding and normal appearances on endoscopy and colonoscopy have been estimated to have a small bowel lesion. Capsule endoscopy has been shown to be better than push enteroscopy (endoscopy of the small bowel) for detecting small bowel lesions, mainly because the more distal small bowel can be visualised.13 This promises to be a real advance for patients with recurrent iron deficiency anaemia and normal results on other investigations.
Figure 2.
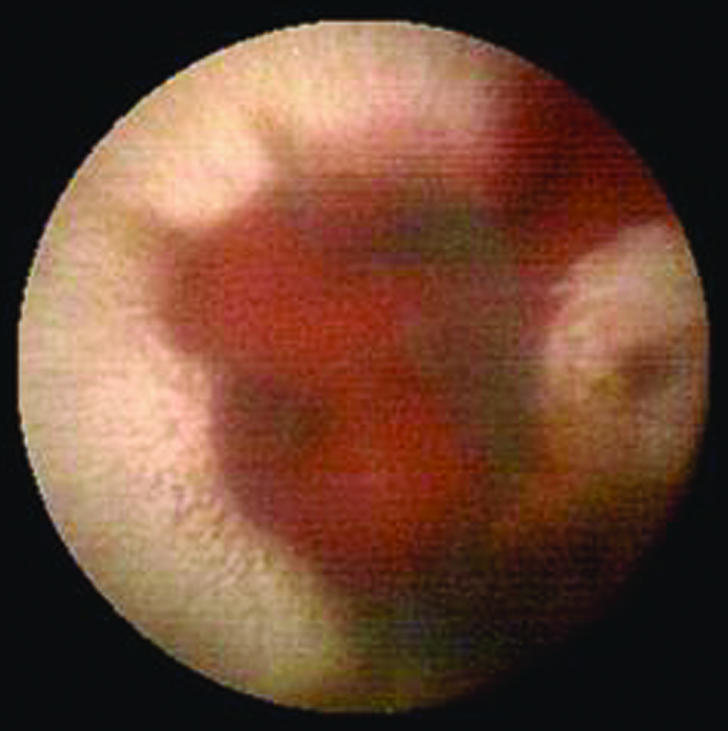
Small bowel angiodysplasia with active bleeding seen with wireless capsule endoscopy
Virtual colonoscopy
Colonoscopy is the most accurate method of imaging the lower gastrointestinal tract. The demand for this procedure is likely to increase if colorectal screening is introduced. The problems with colonoscopy are that it is uncomfortable for the patient, requires intravenous sedation, has a perforation rate of 0.5%, and has a mortality of about 0.1%. Virtual colonoscopy (or computed tomographic colonoscopy) was first described in 1994 and may overcome many of the limitations of endoscopic colonoscopy. It combines conventional spiral computed axial tomography with recent developments in virtual reality computer technology.14 The two-dimensional images generated by helical computed tomography are reconstructed into three-dimensional images by software that simulates the interior of the colon as it would be viewed through an endoscope.
The limitations of the technique include expense, poor image quality due to stool and fluid, and difficulty interpreting images.15 Finally, if abnormalities are found conventional colonoscopy is needed to obtain a tissue diagnosis. Software developments and computer interpretation of images should reduce costs and shorten reporting time.14
Treatment
Management of undiagnosed dyspepsia
Patients with dyspepsia have traditionally been referred for endoscopy to exclude underlying upper gastrointestinal malignancy and make a definite diagnosis to rationalise treatment. H pylori causes most peptic ulcer disease, and a systematic review shows that eradication of H pylori will also benefit a few patients with non-ulcer dyspepsia.16
Young dyspeptic patients can be managed with a non-invasive test for H pylori infection without the need for endoscopy. Patients who test positive should have eradication therapy, which will cure the peptic ulcer disease. H pylori negative patients can be reassured and treated empirically. Gastrointestinal malignancy is unlikely in patients without alarm symptoms such as weight loss or anaemia. Four randomised controlled trials have confirmed that H pylori “test and treat” is more cost effective than endoscopy,17,18 and many guidelines now recommend this approach for young dyspeptic patients (box). The age cut-off for this strategy varies depending on the local incidence of upper gastrointestinal cancer, but recent British and Scottish guidelines suggest the threshold can be raised to include patients under 55 years old (box).
Dyspepsia guidelines recommending H pylori “test and treat”
Age cut-off (years)
British Society of Gastroenterology(www.bsg.org.uk/clinical_prac/guidelines/dyspepsia.htm) <55
European Society for Primary Care Gastroenterology(www.espcg.org/guidelines/hpguide.html) <45
European Helicobacter pylori Study Group19 <45
American Gastroenterology Association20 <45
Scottish Intercollegiate Guidelines Network(www.sign.ac.uk/guidelines/published/index.html) <55
Gastro-oesophageal reflux disease
Eradication of H pylori has no role in treating gastro-oesophageal reflux disease.21 Proton pump inhibitors are effective in gastro-oesophageal reflux disease, but as it is a chronic disorder patients often need to take these expensive drugs long term. Other approaches have therefore been developed for long term treatment of reflux disease. These include an endoscopic suturing device to tighten the lower oesophageal sphincter,22 endoscopic submucosal implantation of gelatinous microspheres in the lower oesophagus,23 and radiofrequency energy delivery to the lower oesophageal sphincter.24 Uncontrolled studies in small numbers of patients have suggested all these approaches reduce acid reflux for up to six months, but larger randomised controlled trials with long term follow up are required.
Other developments
Variations in the NOD2 gene on chromosome 16 are strongly associated with susceptibility to Crohn's disease, with an odds ratio of 3.0 for heterozygous and 22 for the homozygous genotypew1
Mutations in the HFE gene are present in most patients with haemachromatosis. This has simplified diagnosis,w2 but the appropriateness of population screening is debatable as many people with the mutation may never develop the diseasew3 w4
Barrett's oesophagus predisposes to oesophageal adenocarcinoma, and endoscopic surveillance is often recommended, although most people do not develop neoplasia. Cyclin D1 overexpression in biopsy specimens from patients with Barrett's oesophagus entering a surveillance programme was associated with an odds ratio of 7 for the development of adenocarcinomaw5
Irritable bowel syndrome
Irritable bowel syndrome affects 10-20% of the population and is a chronic disorder that often does not respond to treatment. The serotonin receptors 5-HT3 and 5-HT4 are involved in the sensory and motor functions of the gut and are potential targets for new drugs. A selective 5-HT3 antagonist, alosetron, was shown to improve symptoms of diarrhoea predominant irritable bowel syndrome in women; 41% of women responded to active treatment compared with 29% of placebo controls.25 The drug was released in the United States but was associated with 49 cases of ischaemic colitis26 and five deaths. The company therefore withdrew the drug.27 Further 5-HT3 and 5-HT4 antagonists, 5-HT4 agonists, and other serotonergic drugs are being evaluated.28 It is hoped that these will benefit some patients with irritable bowel syndrome without causing severe adverse events.
Inflammatory bowel disease
Ulcerative colitis and Crohn's disease are idiopathic inflammatory disorders of unknown aetiology. About one third of patients with Crohn's disease and ulcerative colitis do not respond to conventional medical treatments. This subgroup presents a challenge to gastroenterologists, but there have been important advances in treatment.
Infliximab is a human-mouse chimeric monoclonal IgG1 antibody directed against tumour necrosis factor α. The National Institute for Clinical Excellence recommends infliximab in patients with severe Crohn's disease who do not respond to immunomodulating drugs and corticosteroids and for whom surgery is inappropriate.29
Thalidomide has recently been shown to reduce production of tumour necrosis factor α in vitro, and initial case series suggest treatment may achieve almost 50% remission in patients with refractory Crohn's disease.30 Women were given strict contraceptive advice before agreeing to start treatment because of the drug's teratogenic effects. The thalidomide molecule is being modified to try to increase therapeutic effects and decrease toxicity.
Intravenous cyclosporin has been shown to be effective in inducing remission in severe ulcerative colitis,31 but the disease almost inevitably relapses. Nevertheless, the drug is a useful addition to the armoury against inflammatory bowel disease as it allows the disease to be brought under control so that the patient can be better prepared for surgery.
Additional educational resources
Sandborn WJ, Targan SR. Biologic therapy of inflammatory bowel disease. Gastroenterology 2002;122:1592-608
Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002;34:417-29
Rossini FP, Pennazio M. Small bowel endoscopy. Endoscopy 2002;31:13-20
Harris A, Misiewicz JJ. ABC of the upper gastrointestinal tract: management of Helicobacter pylori. BMJ 2001;323:1047-50
Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med 2002;137:132-41.
Supplementary Material
Footnotes
Competing interests: PM has received fees for speaking and research funds from AstraZeneca, Wyeth Laboratories, and Abbott Laboratories.
Extra references are available on bmj.com
References
- 1.Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–353. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881–1888. doi: 10.1093/jnci/92.23.1881. [DOI] [PubMed] [Google Scholar]
- 3.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 4.Mason J, Feltbower R, Crocombe W, Duffett S, Axon ATR, Drummond, et al. The effect of population H pylori screening and treatment on dyspepsia in the community: an economic analysis of a randomised controlled trial. Aliment Pharmacol Ther. 2002;16:559–568. doi: 10.1046/j.1365-2036.2002.01204.x. [DOI] [PubMed] [Google Scholar]
- 5.Towler B, Irwig L, Glasziou P, Kewenter J, Weller D, Silagy C. A systematic review of the effects of screening for colorectal cancer using the faecal occult blood test, Hemoccult. BMJ. 1998;317:559–565. doi: 10.1136/bmj.317.7158.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traverso G, Shuber A, Levin B, Johnson C, Olsson L, Schoetz DJ, et al. Detection of APC mutations in fecal DNA from patients with colorectal tumors. N Engl J Med. 2002;346:311–320. doi: 10.1056/NEJMoa012294. [DOI] [PubMed] [Google Scholar]
- 7.UK Flexible Sigmoidoscopy Screening Trial Investigators. Single flexible sigmoidoscopy screening to prevent colorectal cancer: baseline findings of a UK multicentre randomised trial. Lancet. 2002;359:1291–2000. doi: 10.1016/S0140-6736(02)08268-5. [DOI] [PubMed] [Google Scholar]
- 8.Schoenfeld P, Lipscomb S, Crook J, Dominguez J, Butler J, Holmes L, et al. Accuracy of polyp detection by gastroenterologists and nurse endoscopists during flexible sigmoidoscopy: a randomized trial. Gastroenterology. 1999;117:312–318. doi: 10.1053/gast.1999.0029900312. [DOI] [PubMed] [Google Scholar]
- 9.Imperiale T, Wagner D, Lin C, Rogge J, Ransohoff D. Risk of advanced neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343:169–174. doi: 10.1056/NEJM200007203430302. [DOI] [PubMed] [Google Scholar]
- 10.Lieberman D, Weiss D Veterans Affairs Cooperative Study Group 380. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med. 2001;345:555–560. doi: 10.1056/NEJMoa010328. [DOI] [PubMed] [Google Scholar]
- 11.Rembacken BJ, Fujii T, Cairns A, Dixon MF, Yoshida S, Chalmers DM, et al. Flat and depressed colonic neoplasms: a prospective study of 1000 colonoscopies in the UK. Lancet. 2000;355:1211–1214. doi: 10.1016/s0140-6736(00)02086-9. [DOI] [PubMed] [Google Scholar]
- 12.Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. doi: 10.1038/35013140. [DOI] [PubMed] [Google Scholar]
- 13.Appleyard M, Fireman Z, Glukhovsky A, Jacob H, Shreiver R, Kadirkamanathan S, et al. A randomised trial comparing wireless capsule endoscopy with push enteroscopy for the detection of small-bowel lesions. Gastroenterology. 2000;119:1431–1438. doi: 10.1053/gast.2000.20844. [DOI] [PubMed] [Google Scholar]
- 14.Halligan S, Fenlon HM. Science, medicine, and the future: virtual colonoscopy. BMJ. 1999;319:1249–1252. doi: 10.1136/bmj.319.7219.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenlon HM, Nunes DP, Schroy PC, 3rd, Barish MA, Clarke PD, Ferrucci JT. A comparison of virtual and conventional colonoscopy for the detection of colorectal polyps. N Engl J Med. 1999;341:1496–1503. doi: 10.1056/NEJM199911113412003. [DOI] [PubMed] [Google Scholar]
- 16.Moayyedi P, Soo S, Deeks J, Forman D, Mason J, Innes M, et al. Systematic review and economic evaluation of Helicobacter pylori eradication treatment for non-ulcer dyspepsia. BMJ. 2000;321:659–664. doi: 10.1136/bmj.321.7262.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Delaney BC, Innes MA, Deeks J, Wilson S, Oakes R, Moayyedi P, et al. Initial management strategies for dyspepsia. Cochrane Database Syst Rev 2001;(3):CD001961. [DOI] [PubMed]
- 18.McColl KEL, Murray LS, Gillen D, Walker A, Wirz A, Fletcher J, et al. Randomised trial of endoscopy with testing for Helicobacter pylori compared with non-invasive H pylori testing alone in the management of dyspepsia. BMJ. 2002;324:999–1002. doi: 10.1136/bmj.324.7344.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malfertheiner P, Megraud F, O'Morain C, Hungin AP, Jones R, Axon A, et al. Current concepts in the management of Helicobacter pylori infection—the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167–180. doi: 10.1046/j.1365-2036.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 20.American Gastroenterological Association medical position statement: evaluation of dyspepsia. Gastroenterology. 1998;114:579–581. doi: 10.1016/s0016-5085(98)70541-4. [DOI] [PubMed] [Google Scholar]
- 21.Moayyedi P, Bardhan KD, Young L, Dixon MF, Brown L, Axon ATR. The effect of Helicobacter pylori eradication on reflux symptoms in gastro-esophageal reflux disease in patients: a randomised controlled trial. Gastroenterology. 2001;121:1120–1126. doi: 10.1053/gast.2001.29332. [DOI] [PubMed] [Google Scholar]
- 22.Filipi CJ, Lehman GA, Rothstein RI, Raijman I, Stiegmann GV, Waring P, et al. Transoral, flexible endoscopic suturing for treatment of GERD: a multicentre trial. Gastrointestinal Endoscopy. 2001;53:416–422. doi: 10.1067/mge.2001.113502. [DOI] [PubMed] [Google Scholar]
- 23.Feretis C, Benakis P, Dimopoulos C, Dailianas A, Filalithis P, Stamou KM, et al. Endoscopic implantation of plexiglas (PMMA) microspheres for the treatment of GERD. Gastrointest Endosc. 2001;55:423–426. doi: 10.1067/mge.2001.113912. [DOI] [PubMed] [Google Scholar]
- 24.Triadafilopoulos G, DiBaise JK, Nostrant TT, Stollman NH, Anderson PK, Wolfe M, et al. The Stretta procedure for the treatment of GERD: 6 and 12 month follow-up of the US open label trial. Gastrointestinal Endosc. 2002;55:145–156. doi: 10.1067/mge.2002.121227. [DOI] [PubMed] [Google Scholar]
- 25.Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-based trial. Lancet. 2000;355:1035–1040. doi: 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- 26.Freidel D, Thomas R, Fisher RS. Ischemic colitis during treatment with alosetron. Gastroenterology. 2001;120:557–560. doi: 10.1053/gast.2001.21177. [DOI] [PubMed] [Google Scholar]
- 27.Horton R. Lotonex and the FDA: a fatal erosion of integrity. Lancet. 2001;357:1544–1545. doi: 10.1016/S0140-6736(00)04776-0. [DOI] [PubMed] [Google Scholar]
- 28.Camilleri M. Management of the irritable bowel syndrome. Gastroenterology. 2001;120:652–658. doi: 10.1053/gast.2001.21908. [DOI] [PubMed] [Google Scholar]
- 29.National Institute of Clinical Excellence. Guidance on the use of infliximab for Crohn's disease. London: NICE; 2002. . (Technology appraisal guidance No 40.) [Google Scholar]
- 30.Ehrenpreis ED, Kane SV, Cohen LB, Cohen RD, Hanaver SB, et al. Thalidomide therapy for patients with refractory Crohn's disease: an open-label trial. Gastroenterology. 1999;117:1271–1277. doi: 10.1016/s0016-5085(99)70276-3. [DOI] [PubMed] [Google Scholar]
- 31.D'Haens G, Lemmens L, Geboes K, Vandeputte L, Van Acker F, Mortelmans L, et al. Intravenous cyclosporin versus intravenous corticosteroids as single therapy for severe attacks of ulcerative colitis. Gastroenterology. 2001;120:1323–1329. doi: 10.1053/gast.2001.23983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.