Abstract
BACKGROUND
The reported actual risk of rupture for vertebral artery dissection (VAD) in patients presenting with headache is very low, ranging from 0.4% to 1.0%. The authors report a case in which the dissection site dilated rapidly within several hours after the dissection occurred resulting in subarachnoid hemorrhage (SAH).
OBSERVATIONS
A 49-year-old healthy man who had participated in a marathon noticed a headache while running. Magnetic resonance imaging (MRI) performed 2 days later revealed no findings suspicious for right VAD, but a string sign was observed in the left side, suggesting left VAD. Three hours following MRI, he developed severe headaches and became unconscious at home, prompting emergency services to rush him to the hospital. A computed tomography scan showed diffuse SAH and a rapidly enlarged aneurysmal dilatation in the right vertebral artery. He underwent endovascular internal trapping to prevent rebleeding. He was discharged without any neurological symptoms. No recurrence or new dissection occurred after 2 years of follow-up.
LESSONS
Even in the absence of typical imaging findings, strict management, such as blood pressure control, is required when clinical findings strongly suggest VAD, and differentiation of VAD from primary headache is important.
Keywords: aneurysm, headache, unruptured, subarachnoid hemorrhage, vertebral artery dissection
ABBREVIATIONS: CT = computed tomography, CTA = CT angiography, IAD = intracranial arterial dissection, MRI = magnetic resonance imaging, SAH = subarachnoid hemorrhage, VA = vertebral artery, VAD = VA dissection, VADA = VA dissecting aneurysm
Subarachnoid hemorrhage (SAH) caused by intracranial arterial dissection (IAD) can be fatal. In Japan, 3.2% of SAH cases are attributed to IAD.1 A nationwide survey covering 632 cases of IAD revealed that 193 cases (30.5%) resulted in SAH.2 Among these, 109 cases (56.5%) had a favorable outcome, 82 cases (42.5%) had poor outcomes, and there were 50 fatalities (25.9%). Many patients with SAH resulting from IAD have experienced headaches several days before the hemorrhage.3 This observation indicates that IADs causing SAH remain unruptured for a period between their onset and rupture. However, there are no established methods for the management of unruptured IAD and the prevention of SAH.
There are few reports regarding the dynamic morphological changes in imaging findings of impending rupture of an IAD.4, 5 We report the case of a vertebral artery dissection (VAD) that ruptured 2 days following the onset of headache. Initial imaging findings did not reveal typical imaging signs; however, the dissection site dilated rapidly within several hours after the dissection occurred, causing SAH. This case is valuable from the perspective of rupture prevention and underscores the importance of monitoring the progression toward rupture in VADs.
Illustrative Case
A 49-year-old healthy man visited a private doctor with the chief complaint of pulsatile headache in the right temporal and occipital regions. The patient had participated in a marathon 2 days before, noticed a headache while running, and had intermittent pulsatile headaches that worsened after the marathon. The next day, he took painkillers, but the headache did not improve. Therefore, he visited a local private clinic. The patient had no nausea or vomiting during the clinical course. His blood pressure was high at 155/97 mm Hg, and his baseline blood pressure was unknown. An imaging study was performed at 18:30 hours using a 0.3-T open magnetic resonance imaging (MRI) scanner (AIRIS Vento Plus, FUJIFILM Co.). These images revealed no findings suspicious for dissection in the right vertebral artery (VA), but a string sign was observed in the left VA, suggesting dissection of the left VA (Fig. 1). The practitioner considered the possibility of VAD, prescribed antihypertensive drugs to control the patient’s blood pressure, and recommended close follow-up observation. The patient remained calm after returning home but subsequently experienced a severe headache and self-initiated a call to the fire department at 22:07 hours to request an ambulance. The emergency personnel arrived at his home at 22:15 hours. Upon receiving no response when ringing his doorbell, they entered the residence and discovered the patient unconscious; they promptly transported him to the emergency department via ambulance.
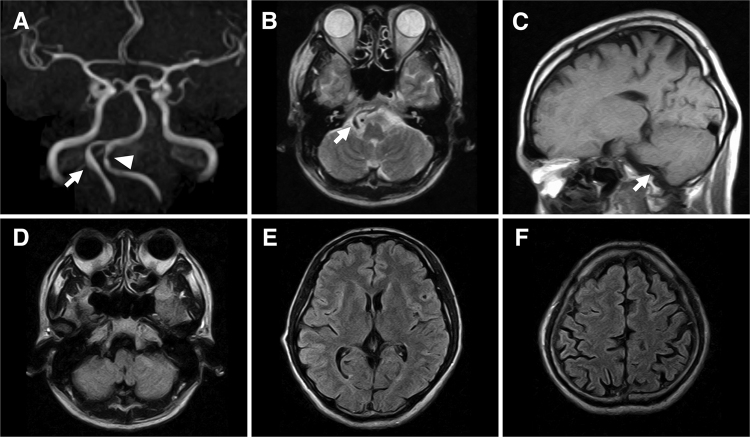
FIG. 1.
MRI was performed at 18:30 hours, after the patient had visited a private clinic on the 2nd day after the onset of headache. A: Magnetic resonance angiography (MRA) image showing a normal right VA (arrow) and mild stenosis (arrowhead) of the left VA. B: An axial T2-weighted image at the level of the VA shows a normal flow void sign. C: A sagittal T1-weighted image showing a flow void sign in the right VA without a T1-hyperintensity sign, indicating an intramural hematoma. D–F: Serial axial fluid-attenuated inversion recovery images showing no SAH at either the supra- or infratentorial cisterns.
Upon admission, the patient was in a coma, and emergency head computed tomography (CT) was performed at 23:00 hours. CT revealed diffuse SAH, and CT angiography (CTA) revealed aneurysmal dilatation of the right VA with an intimal flap (Fig. 2). The patient was diagnosed with SAH due to the rupture of a right VA dissecting aneurysm (VADA), classified as Hunt and Kosnik grade IV or Fisher grade 3. Endovascular internal trapping of the VADA was immediately performed to prevent rebleeding (Fig. 3A–C). MRI obtained the following day revealed a right lateral medullary infarction (Fig. 3D). Postoperatively, the patient started to regain consciousness, but the patient still had sensory disturbance of the right face, dysphagia, and hiccups. There were no complications of cerebral vasospasm, and a ventriculoperitoneal shunt was placed after 6 weeks for normal pressure hydrocephalus. The postshunting course was uneventful, and the initial neurological symptoms had resolved by the time of discharge (discharge modified Rankin Scale score 0). No recurrence or new dissection occurred after 2 years of follow-up (Fig. 3E and F).
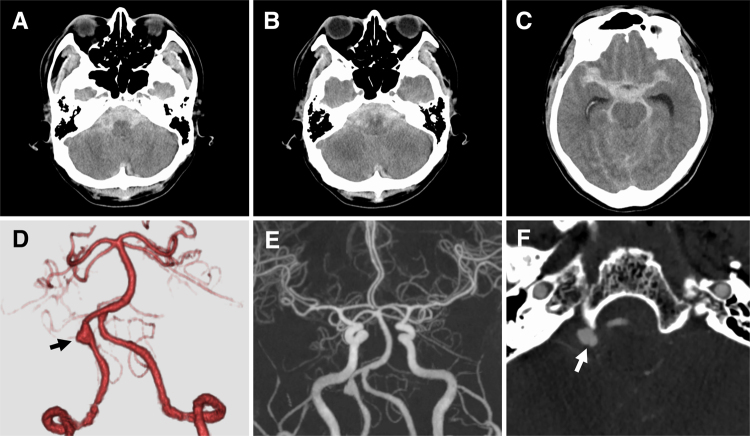
FIG. 2.
Imaging was performed at 23:00 hours, after the patient had been transported to our hospital via an ambulance. A–C: Emergency CT scans showing Fisher grade 3 SAH associated with acute hydrocephalus. A clot of SAH is predominant in the posterior fossa. D and E: CTA images showing aneurysmal dilatation of the right VA (arrow) and no other lesion suspected of dissection. F: The source image of the CTA image reveals an intimal flap (arrow).
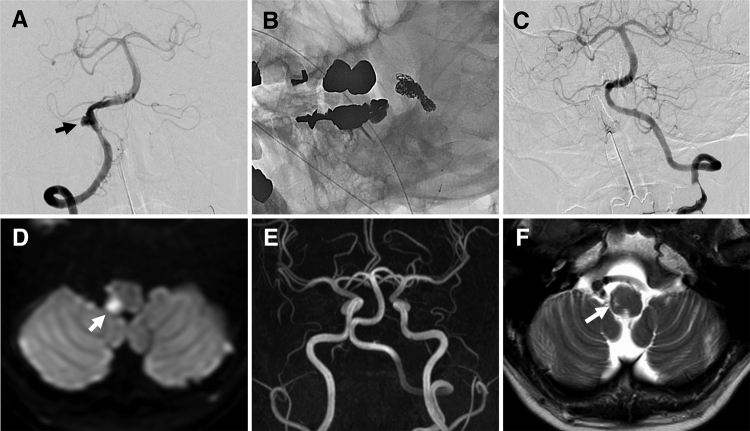
FIG. 3.
A: A digital subtraction angiography image showing aneurysmal dilatation (arrow) associated with a slight string sign at the proximal portion of the right VA. B: A nonsubtracted left oblique image obtained at the end of embolization, showing the coils that were placed during aneurysmal dilatation and in the right VA. C: A final left VA angiogram showing no opacification of the right VA or VADA. D: An axial diffusion-weighted image obtained on the day after the procedure, showing a right lateral medullary infarction (arrow). E: Two-year follow-up MRA image showing that there was no recurrence of the aneurysm and that the left VA had no abnormal findings. F: Axial T2-weighted image showing an old lateral medullary infarction (arrow).
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
In this report, we present a case in which the dissection site of the VA exhibited rapid expansion within 3 hours following MRI, leading to hemorrhage. To the best of our knowledge, this is the first case report in which there were acute morphological changes due to an impending rupture of VAD on MRI.
IAD is more common in Asian populations than in Western populations. IAD is a significant vascular lesion among younger individuals, and the mean age of onset is 50.4 years.6 The predominant symptom of unruptured intracranial VAD is severe occipital headache, either in isolation or accompanied by localized neurological deficits due to ischemia or mass effects. In cases of rupture, patients exhibit symptoms of SAH, necessitating immediate surgery to prevent fatal rebleeding.6 On the other hand, unruptured VAD does not typically lead to a poor prognosis; thus, conservative treatment of these lesions is recommended, unless the dissecting aneurysm enlarges or shows signs of compression.7, 8
It has been reported that 50%–60% of all IAD patients present with SAH;6 however, recent advancements in imaging technology have increasingly led to the detection of unruptured VAD patients who present only with headache or posterior neck pain.7, 9 The reported actual risk of rupture for VAD patients presenting with headache is very low, ranging from 0.4% to 1.0%.6 According to Mizutani,3 96% of patients experience SAH within 3 days following the onset of headache; it is considered that aneurysmal dilatation leading to hemorrhage often occurs within this short period. In this case, imaging evaluation was conducted 2 days after onset and just prior to rupture, revealing that aneurysmal dilatation does not progress gradually; instead, there are cases in which it undergoes an instantaneous completion of its morphological transformation. Pathologically, hemorrhagic VADs show dissections between the media and adventitia layers, indicating that aneurysmal dilatation is more likely to cause SAH.10 Clinicians should consider the possibility that devastating rupture may occur due to rapid dilatation of the aneurysm within a few days, as suggested by the clinical course of our case.
Regarding the medical management of non–hemorrhagic-onset VAD, a study involving 41 patients who were managed solely with rest and antihypertensive therapy reported imaging improvements in approximately half of the subjects within a week.11 Anticoagulation therapy is generally contraindicated and should be avoided in the acute phase;12 however, favorable outcomes and safety were reported when anticoagulation therapy was given to 81 patients with IAD without aneurysmal expansion.13 However, as suggested by our case in which aneurysmal dilatation occurred rapidly, blood pressure management is crucial in the acute treatment of non–hemorrhagic-onset VAD, and anticoagulation therapy should be avoided.
It is important to diagnose VAD early and during the onset of headaches. However, the diagnosis of VAD is often challenging due to the nonspecific nature of clinical symptoms. Acute unilateral headache and neck pain, particularly when the pain persists or intensifies, are symptoms suggestive of VAD.14 Initial imaging findings in VAD often reveal fusiform dilatation or the "pearl-and-string" sign, yet the absence of an intramural hematoma, intimal flap, or double lumen can complicate the identification of dissection.4 Detection of an intramural hematoma can take 48–72 hours after its onset, and these hematomas manifest as a high signal on T1-weighted MRI scans.6 Many patients with VAD are typically diagnosed 5.8–9.8 days after symptom onset; therefore, the critical 3-day window of high bleeding risk is missed.3, 7 For early detection and appropriate intervention, it is crucial to remember that there may be individuals suffering from undetected IAD among patients thought to have primary headaches.
This case highlights the rapid formation of aneurysmal dilatation within the dissecting lesion resulting in VAD rupture. It underscores the necessity for strict blood pressure management and close observation for patients with symptomatic but unruptured VAD, while this approach may not change ultimately outcomes of patients. It is unclear from this single case, but the accumulation of future cases could lead to further studies that predict which dissections may cause pseudoaneurysms.
Lessons
The present case emphasizes that even if the initial examination does not reveal the typical imaging findings of VAD, drastic morphological changes secondary to a VAD can occur in patients with nonruptured VAD who present with headache. The reported actual risk of hemorrhagic change for VAD patients presenting with headache is very low; however, strict management, such as blood pressure control, is required when clinical findings strongly suggest VAD.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Mori, Fujita, Iwakura, Kohta. Acquisition of data: Mori, Fujita, Iwakura, Imura, Onobuchi. Analysis and interpretation of data: Fujita, Iwakura, Kimura. Drafting the article: Mori, Fujita, Iwakura, Kohta. Critically revising the article: Fujita, Iwakura. Reviewed submitted version of manuscript: Fujita, Iwakura, Kohta, Kimura. Approved the final version of the manuscript on behalf of all authors: Mori. Administrative/technical/material support: Fujita, Iwakura. Study supervision: Fujita, Iwakura, Sasayama.
Correspondence
Tatsuya Mori: Kobe University Graduate School of Medicine, Kobe, Japan. tatsuya.mori.1987@gmail.com.
References
- 1.Mizutani T. A fatal, chronically growing basilar artery: a new type of dissecting aneurysm. J Neurosurg. 1996;84(6):962-971. [DOI] [PubMed] [Google Scholar]
- 2.Tajima Y, Ono JI, Higuchi Y, Machida T, Saeki N, Yamaura A. A nationwide study of intracranial arterial dissection in the vertebrobasilar system (part 2): outcomes in hemorrhagic-onset cases. Surg Cereb Stroke (Jpn). 2015;43(4):252-256. [Google Scholar]
- 3.Mizutani T. Natural course of intracranial arterial dissections: clinical article. J Neurosurg. 2011;114(4):1037-1044. [DOI] [PubMed] [Google Scholar]
- 4.Inoue S, Fujita A, Shinoda K, et al. A case of intracranial vertebral artery dissection undetected by CT, MRI, and MRA at the onset of headache that caused subarachnoid hemorrhage seven days later. J Neuroendovascular Ther. 2022;16(5):265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naito I, Iwai T, Sasaki T. Management of intracranial vertebral artery dissections initially presenting without subarachnoid hemorrhage. Neurosurgery. 2002;51(4):930-937. [DOI] [PubMed] [Google Scholar]
- 6.Debette S, Compter A, Labeyrie MA, et al. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol. 2015;14(6):640-654. [DOI] [PubMed] [Google Scholar]
- 7.Kai Y, Nishi T, Watanabe M, et al. Strategy for treating unruptured vertebral artery dissecting aneurysms. Neurosurgery. 2011;69(5):1085-1091. [DOI] [PubMed] [Google Scholar]
- 8.Santos-Franco JA, Zenteno M, Lee A. Dissecting aneurysms of the vertebrobasilar system. A comprehensive review on natural history and treatment options. Neurosurg Rev. 2008;31(2):131-140. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi N, Murayama Y, Yuki I, et al. Natural course of dissecting vertebrobasilar artery aneurysms without stroke. AJNR Am J Neuroradiol. 2014;35(7):1371-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasaki O, Ogawa H, Koike T, Koizumi T, Tanaka R. A clinicopathological study of dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1991;75(6):874-882. [DOI] [PubMed] [Google Scholar]
- 11.Echigo T, Matsui H, Oka H, et al. Clinical features of unruptured vertebral artery dissection presenting as isolated occipital headache and/or neck pain. Article in Japanese. No Shinkei Geka. 2013;41(4):305-310. [PubMed] [Google Scholar]
- 12.Engelter ST, Brandt T, Debette S, et al. Antiplatelets versus anticoagulation in cervical artery dissection. Stroke. 2007;38(9):2605-2611. [DOI] [PubMed] [Google Scholar]
- 13.Metso TM, Metso AJ, Helenius J, et al. Prognosis and safety of anticoagulation in intracranial artery dissections in adults. Stroke. 2007;38(6):1837-1842. [DOI] [PubMed] [Google Scholar]
- 14.Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522. [DOI] [PubMed] [Google Scholar]