Abstract
Purpose:
The effects of thoracic radiation therapy (RT) on physical functioning and quality of life (QoL) are incompletely defined. We determined the associations between thoracic RT dose volume metrics, physical activity, and QoL in patients with cancer.
Methods and Materials:
Participants with breast cancer, lung cancer, or mediastinal lymphoma treated with radiation with or without chemotherapy were enrolled in a prospective, longitudinal cohort study. Data were collected pre-RT, immediately post-RT, and 5 to 9 months post-RT. At each timepoint, self-reported physical activity was assessed via the Godin—Shephard Leisure-Time Physical Activity Questionnaire, and QoL metrics were assessed via Functional Assessment of Chronic Illness Therapy Fatigue and Dyspnea Scales. Multivariable adjusted linear regression models were stratified by breast cancer alone and lung cancer and lymphoma combined.
Results:
One hundred thirty participants were included in the study. In breast cancer (n = 80), each 1-Gy increase in mean heart dose was associated with worse Functional Assessment of Chronic Illness Therapy Fatigue scores (−1.0; 95% confidence interval [CI], −1.9 to −0.2; P = .021); similar associations were observed between V5 and fatigue (−2.5; 95% CI, −4.4 to −0.6; P = .010 for each 10% increase in V5). In lung cancer and lymphoma (n = 50), each 10% increase in V5 was associated with decreased physical activity (Godin−Shephard Leisure-Time Physical Activity Questionnaire score −2.3; 95% CI, −4.3 to −0.4; P = .017). Although the associations between baseline levels of physical activity and fatigue and dyspnea were of borderline significance in breast cancer alone (P <.10), increased physical activity over time was associated with improvements in fatigue and dyspnea across all cancer types (P < .05 for all).
Conclusions:
Higher cardiac RT dose was associated with worse fatigue and physical activity across breast cancer, lung cancer, and mediastinal lymphoma. Longitudinal increases in physical activity were associated with concurrent improvements in QoL measures. Strategies to increase physical activity and decrease cardiac RT dose may improve physical functioning and QoL for patients with cancer.
Introduction
Radiation therapy (RT) field site, size, and dose are known to have an important impact on quality of life (QoL).1,2 However, the effects of thoracic RT on QoL metrics of fatigue and dyspnea are incompletely defined.3 Importantly, evidence suggests that exercise during and after chemoradiation can improve fatigue and physical functioning in cancer patients.4–6 This relationship has been shown to be particularly notable among cancer survivors partaking in moderate-to-vigorous physical activity (MVPA).7 However, the relationships between specific RT dose volume metrics, exercise, and QOL metrics such as fatigue and dyspnea have not been comprehensively studied. The purpose of this study was to define the associations and quantify the potential interrelationships among thoracic RT dose volume metrics, physical activity levels, and QoL metrics during and after RT.
Methods and Materials
Participants with breast cancer, lung cancer, or mediastinal lymphoma treated with photon or proton thoracic RT with or without chemotherapy were enrolled in a longitudinal prospective cohort study from 2015 to 2018. All participants received RT with curative intent. Participants who had received prior thoracic RT and those with a life expectancy <12 months were excluded. The study was approved by the institutional review board at The University of Pennsylvania School of Medicine. All participants provided written, informed consent.
This was a secondary analysis of the Cardiotoxicity of Radiation Therapy cohort study (NCT02769299) and included data collected at 3 time points: pre-RT, immediately post-RT, and 5 to 9 months post-RT. Participants included in this analysis all had completed questionnaires at baseline and at least 1 additional follow-up time point. At each time point, self-reported physical activity was assessed via the Godin—Shephard Leisure-Time Physical Activity Questionnaire (GSLTPAQ) (Appendix EA). This 3-question scoring system quantifies the amount and type of physical activity reported (strenuous, moderate, mild) and the amount of MVPA, defined by the North American public health physical activity guidelines.8 QoL metrics of fatigue and dyspnea were assessed via the Functional Assessment of Chronic Illness Therapy (FACIT) Fatigue and Dyspnea Scales, which are internally and externally validated health-related QoL questionnaires.9 FACIT Fatigue is a 13-item questionnaire scored from 0 to 4, and FACIT Dyspnea is a 10-item survey scored from 0 to 3 (Appendix EB and EC). Higher GSLTPAQ scores indicate greater physical activity, higher FACIT Fatigue scores less fatigue, and higher FACIT Dyspnea scores worse dyspnea.
Given concerns regarding differences in cardiac RT dose for various cancers, stratified analyses were performed for (1) breast cancer alone and (2) lung cancer and lymphoma combined. The latter groups were combined given similar cardiac dose and limited sample size within each individual cancer type. Standard parametric or nonparametric statistics were used to describe participant characteristics and changes in physical activity and QoL measures over time according to variable distribution. Pairwise comparisons of physical activity and QoL metrics at baseline and the post-RT time points were evaluated with the Wilcoxon signed-rank test. To understand the relationships among cardiac RT dose, physical activity, and QoL, generalized estimating equation linear regression models with an independence correlation structure and a robust variance estimator were used; each model was adjusted for age, sex (in lung cancer and lymphoma), chemotherapy (as part of current treatment; categorized as none, preceding, or concurrent), RT duration, and time point of assessment. Three series of analyses were performed to determine (1) the associations between RT dose—volume metrics and changes in physical activity or QoL, also adjusted for the baseline physical activity or QoL measure; (2) the longitudinal associations between baseline physical activity level and post-RT changes in QoL, also adjusted for the baseline QoL measure and mean heart dose (MHD); and (3) the longitudinal associations between concurrent physical activity and QoL, also adjusted for MHD. In sensitivity analyses for the lung and lymphoma subgroup, models were additionally adjusted for cancer type. Statistical analyses were performed using STATA 13.1 (StataCorp, College Station, TX), and figures were generated using R 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria). A P value <.05 was considered statistically significant.
Results
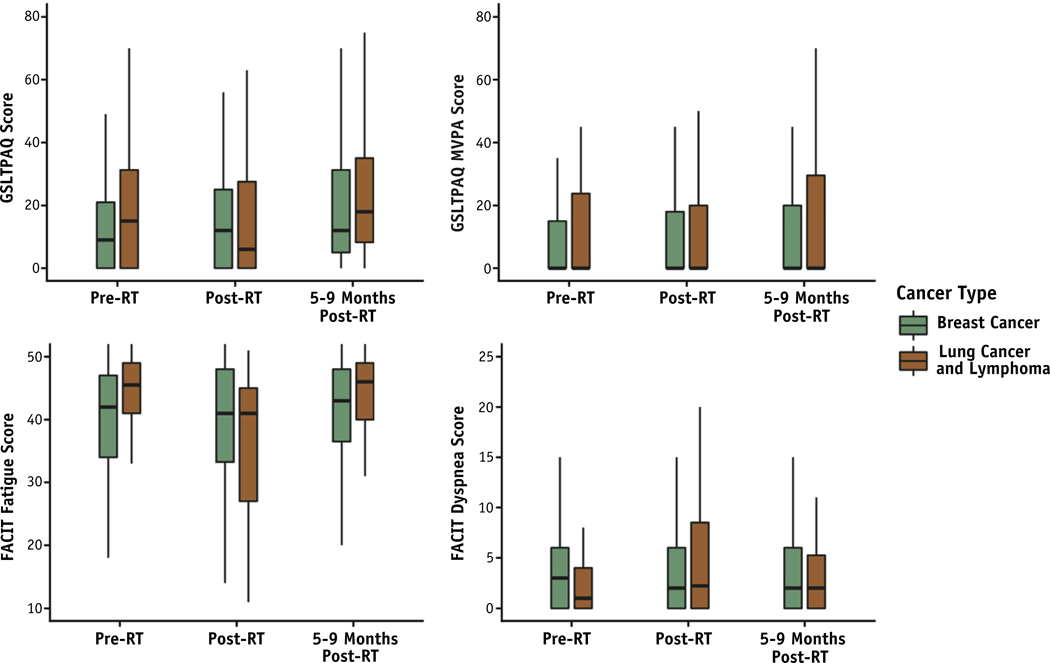
Among 130 study participants, the median age was 53.5 years and 78.5% were female (Table 1). The median MHD was 1.4 Gy among participants with breast cancer (n = 80) and 9.3 Gy among participants with lung cancer or lymphoma (n = 50). Questionnaires were completed by 120 participants immediately post-RT and by 114 participants 5 to 9 months post-RT. Immediately post-RT, participants with breast cancer reported a modest but significant reduction in dyspnea (P = .017); at 5 to 9 months post-RT, they reported a significant increase in physical activity (P = .012) and improved fatigue (P = .006) in comparison with pre-RT levels (Fig. 1, Table ED). In contrast, lung cancer and lymphoma participants reported worse fatigue (P = .001) and increased dyspnea (P = .013) immediately post-RT; fatigue subsequently improved by 5 to 9 months post-RT (Fig. 1, Table EE). Across both cohorts, baseline median GSLTPAQ MVPA levels were low.
Table 1.
Baseline characteristics of patients receiving thoracic RT for breast cancer, lung cancer, and lymphoma*
| Overall, n = 130 | Breast cancer, n = 80 | Lung cancer and lymphoma, n = 50 | |
|---|---|---|---|
|
| |||
| Age, y | 53.5 [42.0–62.0] | 53.5 [44.0–62.0] | 54.5 [33.0–66.0] |
| Sex, n (%) | |||
| Female | 102 (78.5) | 80 (100.0) | 22 (44.0) |
| Race, n (%) | |||
| Caucasian | 101 (77.7) | 57 (71.2) | 44 (88.0) |
| Black or African American | 25 (19.2) | 20 (25.0) | 5 (10.0) |
| Asian/Pacific Islander/Other | 4 (3.1) | 3 (3.8) | 1 (2.0) |
| BMI (kg/m2) | 28.9 ± 6.6 | 29.4 ± 6.9 | 28.2 ± 6.1 |
| Medical history | |||
| Hypertension, n (%) | 42 (32.3) | 28 (35.0) | 14 (28.0) |
| Hypercholesterolemia, n (%) | 40 (30.8) | 22 (27.5) | 18 (36.0) |
| Diabetes, n (%) | 13 (10.0) | 9 (11.2) | 4 (8.0) |
| Current or prior smoking, n (%) | 58 (44.6) | 32 (40.0) | 26 (52.0) |
| Treatment characteristics Chemotherapy as part of treatment regimen, n (%) | |||
| None | 16 (12.3) | 16 (20.0) | 0 (0.0) |
| Preceding | 88 (67.7) | 64 (80.0) | 24 (48.0) |
| Concurrent | 26 (20.0) | 0 (0.0) | 26 (52.0) |
| Primary radiation technique, n (%) | |||
| Protons (passive scattering) | 10 (7.7) | 3 (3.8) | 7 (14.0) |
| Protons (scanning) | 28 (21.5) | 8 (10.0) | 20 (40.0) |
| 3D conformal | 59 (45.4) | 57 (71.2) | 2 (4.0) |
| IMRT | 33 (25.4) | 12 (15.0) | 21 (42.0) |
| Total radiation dose, Gy | 52.7 [50.4–60.0] | 52.6 [50.4–60.0] | 50.4 [36.0–66.6] |
| MHD, Gy | 2.7 [1.2–6.9] | 1.4 [1.0–2.6] | 9.3 [6.2–16.1] |
| V5, % | 10.3 [2.2–26.8] | 2.9 [1.4–8.8] | 38.1 [20.9–59.3] |
| V30, % | 0.9 [0–7.3] | 0.1 [0–0.9] | 12.5 [5.0–23.4] |
| Outcome measures | |||
| GSLTPAQ score | 11.3 [0–25.0] | 9.0 [0–21.0] | 15.0 [0–32.0] |
| GSLTPAQ MVPA score | 0 [0–15.0] | 0 [0–15.0] | 0 [0–24.0] |
| FACIT Fatigue score | 43.3 [36.0–48.0] | 42.0 [34.0–47.0] | 45.5 [41.0–49.0] |
| FACIT Dyspnea score | 2.0 [0–5.0] | 3.0 [0–6.0] | 1.0 [0–4.0] |
Abbreviations: 3D = 3-dimensional; BMI = body mass index; FACIT = Functional Assessment of Chronic Illness Therapy; GSLTPAQ = Godin—Shephard Leisure-Time Physical Activity Questionnaire; IMRT = intensity modulated radiation therapy; MHD = mean heart dose; V5 and V30 indicate the percent volume of heart receiving 5 Gy and 30 Gy, respectively; MVPA = moderate-to-vigorous physical activity; RT = radiation therapy.
Normally distributed continuous variables are summarized with the mean ± standard deviation; nonnormally distributed continuous variables are summarized with the median [interquartile range].
Fig. 1.
GSLTPAQ and QoL over time in breast cancer and lung cancer and lymphoma participants treated with radiation therapy. Boxplots depict the median and range before, immediately after, and 5 to 9 months after radiation therapy. Higher GSLTPAQ scores indicate greater physical activity, higher FACIT fatigue scores indicate less fatigue, and higher FACIT dyspnea scores indicate worse dyspnea. Abbreviations: FACIT = Functional Assessment of Chronic Illness Therapy; GSLTPAQ = Godin−Shephard Leisure-Time Physical Activity Questionnaire; MVPA = moderate-to-vigorous physical activity; QoL = quality of life.
Among participants with breast cancer, each 1-Gy increase in MHD was associated with worse fatigue (−1.0; 95% confidence interval [CI], −1.9 to −0.2; P = .021), and each 10% increase in V5 (the percent volume of the heart receiving 5 Gy) was associated with worse fatigue (−2.5; 95% CI, −4.4 to 0.6; P = .010) (Table 2). Among participants with lung cancer and lymphoma, each 1-Gy increase in MHD tended toward reduced physical activity as measured by the overall GSLTPAQ score (−0.5; 95% CI, −1.1 to 0.1; P = .083), although this was not statistically significant. Each 10% increase in heart V5 was associated with a significant reduction in overall physical activity (−2.3; 95% CI, −4.3 to −0.4; P = .017). These changes were not seen with GSLTPAQ MVPA.
Table 2.
Longitudinal associations between radiation dose (MHD and V5) and subsequent changes in GSLTPAQ scores and QoL metrics as measured by FACIT Fatigue or FACIT Dyspnea scales over time*
| Breast cancer, n = 80 | Lung cancer and lymphoma, n = 50 | ||||
|---|---|---|---|---|---|
|
|
|
||||
| RT dose volume exposure | Physical activity and QoL outcome measures | β-coefficient (95% CI)† | P value | β-coefficient (95% CI)† | P value |
|
| |||||
| MHD | GSLTPAQ score | 1.2 (−1.5 to 3.8) | .403 | −0.5 (−1.1 to 0.1) | .083 |
| GSLTPAQ MVPA score | 1.4 (−1.1 to 3.8) | .266 | −0.2 (−0.8 to 0.3) | .379 | |
| FACIT Fatigue score | −1.0 (−1.9 to −0.2) | .021 | −0.1 (−0.4 to 0.2) | .577 | |
| FACIT Dyspnea score | 0.1 (−0.6 to 0.7) | .888 | 0 (−0.1 to 0.1) | .954 | |
| V5 | GSLTPAQ score | 0.4 (−2.1 to 3.0) | .758 | −2.3 (−4.3 to −0.4) | .017 |
| GSLTPAQ MVPA score | 0.8 (−1.6 to 3.1) | .519 | −1.3 (−3.0 to 0.4) | .144 | |
| FACIT Fatigue score | −2.5 (−4.4 to −0.6) | .010 | −0.3 (−1.2 to 0.6) | .476 | |
| FACIT Dyspnea score | −0.5 (−2.1 to 1.0) | .507 | 0.1 (−0.3 to 0.5) | .725 | |
Abbreviations: CI = confidence interval; FACIT = Functional Assessment of Chronic Illness Therapy; GSLTPAQ = Godin—Shephard Leisure-Time Physical Activity Questionnaire; MHD = mean heart dose; MVPA = moderate-to-vigorous physical activity; QoL = quality of life; RT = radiation therapy; V5 = the percent volume of heart receiving 5 Gy.
Generalized estimating equation linear regression modeled the association between the change in each activity and QoL measure from baseline and heart radiation therapy dose exposure metrics. Models were adjusted for age, sex (for lung cancer and lymphoma only), chemotherapy exposure (none, preceding, or concurrent), RT duration, time point of assessment, and baseline GSLTPAQ or FACIT score. Note that higher overall GSLTPAQ scores indicate greater physical activity, higher FACIT fatigue scores indicate less fatigue, and higher FACIT dyspnea scores indicate worse dyspnea.
β-coefficients represent the difference in the change from baseline at each post-RT time point for a given physical activity or QoL outcome measure for every 1-Gy increase in MHD or for each 10% increase in V5.
Among participants with breast cancer, every 10-point increase in baseline GSLPTAQ MVPA scores tended toward modest improvements in fatigue scores over time (0.5; 95% CI, 0–1.0; P = .060) (Table 3). Additionally, increased baseline overall GSLTPAQ scores tended to be associated with modest improvements in fatigue (P = .070) and dyspnea (P = .065) over time, although these were not statistically significant. In lung cancer and lymphoma, there was no significant association between baseline physical activity levels and subsequent changes in fatigue or dyspnea. In multivariable models, increases in physical activity over time, as measured by the overall GSLTPAQ and GSLTPAQ MVPA scores, were concurrently associated with less fatigue and dyspnea in both breast cancer and lung cancer and lymphoma (Table 4). In sensitivity analyses including adjustment for cancer type in the lung cancer and lymphoma subgroups, results were similar (Tables EF–EI).
Table 3.
Association between baseline GSLTPAQ scores and changes in QoL metrics as measured by FACIT Fatigue or Dyspnea over time*
| Change in QoL measure in breast cancer, according to physical activity (n = 80) | Change in QoL measure in lung cancer and lymphoma, according to physical activity (n = 50) | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Baseline physical activity measure | QoL measure | β-coefficient (95% CI)† | P value | β-coefficient (95% CI)† | P value |
|
| |||||
| Baseline overall GSLTPAQ score | |||||
| FACIT Fatigue score | 0.5 (0–1.0) | .070 | 0.6 (−0.4 to 1.5) | .229 | |
| FACIT Dyspnea score | −0.3 (−0.7 to 0) | .065 | −0.1 (−0.6 to 0.4) | .706 | |
| Baseline GSLTPAQ MVPA score | |||||
| FACIT Fatigue score | 0.5 (0–1.0) | .060 | 0.3 (−0.6 to 1.2) | .498 | |
| FACIT Dyspnea score | −0.2 (−0.5 to 0.0) | .087 | −0.1 (−0.6 to 0.4) | .683 | |
Abbreviations: CI = confidence interval; FACIT = Functional Assessment of Chronic Illness Therapy; GSLTPAQ = Godin—Shephard Leisure-Time Physical Activity Questionnaire; MVPA = moderate-to-vigorous physical activity; QoL = quality of life.
Generalized estimating equation linear regression modeled the association between the change in each QoL measure from baseline and baseline physical activity scores. Models were adjusted for age, sex (for lung cancer and lymphoma only), mean heart dose, radiation therapy duration, chemotherapy exposure (none or preceding/concurrent), time point of assessment, and baseline FACIT score. Note that higher overall GSLTPAQ and GSLTPAQ MVPA scores indicate greater physical activity, higher FACIT fatigue scores indicate less fatigue, and higher FACIT dyspnea scores indicate worse dyspnea.
β-coefficients represent the difference in the change from baseline at each post—radiation therapy time point for a given QoL outcome measure for each 10-point increase in overall GSLTPAQ or GSLTPAQ MVPA score at baseline.
Table 4.
Longitudinal associations between GSLTPAQ scores and concurrent changes in QoL metrics as measured by FACIT Fatigue or Dyspnea over time*
| Change in QoL measure in breast cancer, according to physical activity (n = 80) | Change in QoL measure in lung cancer and lymphoma, according to physical activity (n = 50) | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Physical activity measure | QoL measure | β-coefficient (95% CI)† | P value | β-coefficient (95% CI)† | P value |
|
| |||||
| Overall GSLTPAQ score | |||||
| FACIT Fatigue score | 1.6 (1.0–2.3) | <.001 | 1.3 (0.5–2.1) | .001 | |
| FACIT Dyspnea score | −0.9 (−1.3 to −0.4) | <.001 | −0.6 (−1.0 to −0.2) | .003 | |
| GSLTPAQ MVPA score | |||||
| FACIT Fatigue score | 1.8 (1.1–2.4) | <.001 | 1.3 (0.4–2.1) | .003 | |
| FACIT Dyspnea score | −0.8 (−1.3 to −0.4) | <.001 | −0.6 (−1.1 to −0.2) | .004 | |
Abbreviations: CI = confidence interval; FACIT = Functional Assessment of Chronic Illness Therapy; GSLTPAQ = Godin—Shephard Leisure-Time Physical Activity Questionnaire; MVPA = moderate-to-vigorous physical activity; QoL = quality of life.
Generalized estimating equation linear regression modeled the concurrent association between QoL and physical activity scores over time, both before and after radiation therapy. Models were adjusted for age, sex (in lung cancer and lymphoma only), mean heart dose, radiation therapy duration, chemotherapy exposure (none, preceding, or concurrent), and the time point of assessment. Note that higher overall GSLTPAQ and GSLTPAQ MVPA scores indicate greater physical activity, higher FACIT fatigue scores indicate less fatigue, and higher FACIT dyspnea scores indicate worse dyspnea.
β-coefficients represent the concurrent difference in each QoL outcome measure for every 10-point increase in overall GSLTPAQ and GSLTPAQ MVPA scores at each time point.
Discussion
In participants with breast cancer undergoing RT with an overall low MHD, physical activity and fatigue improved over time. Among participants with lung cancer or lymphoma, fatigue and dyspnea worsened immediately post-RT, but fatigue improved over time. There was a significant association between higher radiation dose to the heart (MHD and V5) and greater fatigue in breast cancer, and higher V5 and decreased physical activity in lung cancer and lymphoma. In breast cancer, high baseline levels of overall physical activity, and particularly MVPA, tended toward improved fatigue post-RT. Among all participants, when accounting for RT dose and duration and chemotherapy exposure, increased physical activity over time was concurrently associated with less fatigue and dyspnea.
This study adds to previous evidence suggesting that higher thoracic RT doses are associated with worse QoL,3 and it adds to growing evidence that physical activity levels and QoL metrics in patients with cancer are closely related.4–7 Previous studies have shown that in patients with lung cancer, higher RT heart dose is associated with worse cardiovascular outcomes and survival.10 However, how cardiac effects of RT mediate QoL parameters of fatigue is unknown. Recommended levels of physical activity positively affect cardiac conditioning in patients with cancer,4 and any increase in physical activity is considered clinically meaningful for cardiovascular health.11 Although it is known that patients tend to decrease physical activity levels after receiving a cancer diagnosis,12 our findings suggest that increased levels of physical activity are associated with improved QoL. Additionally, increased MVPA levels before the initiation of chemoradiation for treatment of certain cancers may improve QoL during and after treatment. These associations between exercise and QoL for patients undergoing thoracic RT provide incremental value to our current understanding of the effects of RT.
Our study is limited by a small sample size, which did not allow comparisons by chemotherapy exposure in breast cancer and resulted in the combination of lung cancer and lymphoma subgroups in our analyses. Additionally, we only studied a select group of cancers. Changes were observed over a relatively short period of time (ie, less than 1 year post-RT), and the third time point for follow-up was broad (5–9 months post-RT). Finally, other studies using the FACIT Fatigue scales have defined a minimum clinically important difference to assess clinically relevant changes in FACIT fatigue scores.13 However, because the minimum clinically important difference has been shown to vary by population and context and has not been specifically studied in this particular population,14 we are limited in our understanding of the greater clinical significance of the changes in fatigue observed in this study. Additional work is needed to assess types, duration, and frequency of exercises that could be included in exercise prescriptions to affect the greatest benefit in patients with cancer. Future studies should not only include longer-term follow-up to better inform clinical practice but also assess the interaction between chemotherapy and radiation on physical activity and QoL.
Conclusions
Among participants with breast cancer, higher cardiac RT dose—volume metrics were associated with worse fatigue, but higher baseline levels of physical activity, particularly MVPA levels, tended toward improved fatigue post-RT. Among patients with lung cancer or lymphoma, increased cardiac volume receiving 5 Gy was also associated with reduced physical activity post-RT. Across breast cancer, lung cancer, and lymphoma, increased levels of physical activity were associated with improvements in QoL over time. Strategies to decrease cardiac RT dose and encourage high levels of physical activity before, during, and after RT may improve QoL in patients with cancer receiving thoracic RT.
Supplementary Material
Acknowledgments
This work was supported by Abramson Cancer Center and Radiation Oncology Pilot Grant Award (B.K.), by R01 HL 118018 (B.K.), and by an Investigator Initiated Award from Roche Diagnostics (B.K.).
Footnotes
Disclosures: none.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2020.10.018.
References
- 1.Takahashi T, Hondo M, Nishimura K, et al. Evaluation of quality of life and psychological response in cancer patients treated with radiotherapy. Radiat Med 2008;26:396–401. [DOI] [PubMed] [Google Scholar]
- 2.Yucel B, Akkas EA, Okur Y, et al. The impact of radiotherapy on quality of life for cancer patients: A longitudinal study. Support Care Cancer 2014;22:2479–2487. [DOI] [PubMed] [Google Scholar]
- 3.Movsas B, Hu C, Sloan J, et al. Quality of life analysis of a radiation dose—escalation study of patients with non—small-cell lung cancer: A secondary analysis of the Radiation Therapy Oncology Group 0617 randomized clinical trial. JAMA Oncol 2016;2:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Med Sci Sport Exercise 2019;51: 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galvão DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol 2005;23:899–909. [DOI] [PubMed] [Google Scholar]
- 6.Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: A meta-analysis. JAMA Oncol 2017;3:961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: A meta-analysis. Cancer Epidemiol Biomarkers Prev 2011;20:123–133. [DOI] [PubMed] [Google Scholar]
- 8.Godin G. The Godin-Shephard leisure-time physical activity questionnaire. Health Fitness J Can 2011;4:18–22. [Google Scholar]
- 9.Acaster S, Dickerhoof R, DeBusk K, Bernard K, Strauss W, Allen LF. Qualitative and quantitative validation of the FACIT-fatigue scale in iron deficiency anemia. Health Qual Life Outcomes 2015;13:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speirs CK, DeWees TA, Rehman S, et al. Heart dose is an independent dosimetric predictor of overall survival in locally advanced non—small cell lung cancer. J Thorac Oncol 2017;12:293–301. [DOI] [PubMed] [Google Scholar]
- 11.Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA 2018;320:2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luctkar-Flude MF, Groll DL, Tranmer JE, Woodend K. Fatigue and physical activity in older adults with cancer: A systematic review of the literature. Cancer Nursing 2007;30:E35. [DOI] [PubMed] [Google Scholar]
- 13.Cella D, Eton DT, Lai J-S, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) Anemia and Fatigue Scales. J Pain Symptom Manage 2002;24:547–561. [DOI] [PubMed] [Google Scholar]
- 14.Nordin Å, Taft C, Lundgren-Nilsson Å, Dencker A. Minimal important differences for fatigue patient reported outcome measures—a systematic review. BMC Med Res Methodol 2016;16:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.