Abstract
Nitric oxide (NO), a signaling molecule, regulates biological functions in multiple organs/tissues, including the epidermis, where it impacts permeability barrier homeostasis, wound healing, and antimicrobial defense. In addition, NO participates in cutaneous inflammation, where it exhibits pro-inflammatory properties via the cyclooxygenase/prostaglandin pathway, migration of inflammatory cells, and cytokine production. Yet, NO can also inhibit cutaneous inflammation through inhibition of T cell proliferation and leukocyte migration/infiltration, enhancement of T cell apoptosis, as well as through down-regulation of cytokine production. Topical applications of NO-releasing products can alleviate atopic dermatitis in humans and in murine disease models. The underlying mechanisms of these discrepant effects of NO on cutaneous inflammation remain unknown. In this review, we briefly review the regulatory role of NO in cutaneous inflammation and its potential, underlying mechanisms.
Keywords: nitric oxide, dermatitis, inflammation
INTRODUCTION
NO takes on divergent roles in regulating a variety of cellular functions. Physiological levels of NO are required to maintain normal cellular function, while excessive NO can compromise cellular functions. For example, low levels of NO can increase cardiac output, while high levels decrease cardiac function [1]. Similarly, low concentrations of NO (5 to 50 μmol/l) increase, while high concentrations (10 mM/l) decrease the beating rate of the sinoatrial node in vitro.[2] Deficiency in endothelial nitric oxide synthase (eNOS) increases blood pressure in mice [3, 4]. While knockout of eNOS alone lowers survival rate by 50%, knockout of all three NOS isoforms—inducible NOS (iNOS), neural NOS(nNOS), and eNOS—reduces the 10-month survival rate by 80% in mice [5]. In contrast, other studies showed iNOS deficiency increases survival rates and improves cardiac function in coronary ligated mice [6]. In the liver, NO produced by eNOS is required to maintain functional homeostasis and prevent the development of liver diseases. Stimulation by various stimuli, resulting in excess NO synthesized by iNOS, can provoke and/or exacerbate hepatic pathology [7]. Conversely, inhibition of iNOS activity alleviates damage to both the liver and the kidneys in bile duct-ligated rats [8]. Moreover, inhalation of NO improves lung function in both humans and animals [9–11]. Likewise, supplemental NO can improve tolerance to aerobic and anaerobic exercise in untrained or moderately trained humans [12]. Furthermore, inhibition of NO synthesis decreases T regulatory cells, worsens renal damage, and increases blood pressure in rats [13, 14]. Collectively, this evidence indicates that NO can diversely regulate the biological function of multiple cells/tissues.
Studies have also demonstrated a regulatory role for NO in cutaneous function, including stimulation of keratinocyte migration, proliferation, and improvement of cutaneous wound healing [15–19]. Previous studies showed that deficiency in iNOS delays cutaneous wound healing [20]. A requirement for NO in keratinocyte differentiation, lipid production, and epidermal permeability barrier has also been demonstrated, though a negative impact of NO on the epidermal permeability barrier has been shown [21, 22]. The antimicrobial properties of NO also have been well illustrated. For example, topical applications of NO-releasing nanoparticles for 2 days significantly accelerated the healing of wounds infected with Acinetobacter baumannii along with a marked reduction in bacterial burden [23]. Similarly, topical applications of NO-releasing nanoparticles for 3 days accelerated wound closure and decreased both inflammation and minimal bacterial burden in cutaneous wounds infected by methicillin-resistant Staphylococcus aureus.[24] Likewise, exogenous NO gas, used 8 h daily for 3 days, also reduces the bacterial count in Staphylococcus aureus-infected cutaneous wound [25]. Finally, NO also regulates cutaneous and extracutaneous inflammation [26–28], which is the subject of this review.
NO PRODUCTION AND REGULATION
NO is synthesized by 3 NO synthases (NOS) (EC 1.14.13.39)—nNOS (NOS1), iNOS (NOS2), and eNOS (NOS3), which are expressed in almost all tissues and various cell types. NOS converts arginine to citrulline, producing NO (Fig. 1). [29, 30] Flavin mononucleotide, flavin adenine dinucleotide, nicotinamide adenine dinucleotide phosphate, and (6R-)5,6,7,8-tetrahydro-L-biopterin serve as cofactors for NO synthase. However, the distribution of these three isoforms varies in different tissues. For example, nNOS is mainly expressed in the neurons and the brain, while eNOS is primarily expressed in endothelial cells, but both eNOS and nNOS are constitutively expressed in all tissues. [31–33] Likewise, iNOS is normally expressed at low levels in almost all tissues. Keratinocytes express all three isoforms of NOS, and macrophages, neutrophils, T cells, and fibroblasts also express various NOS. [33–35]
Fig. 1.
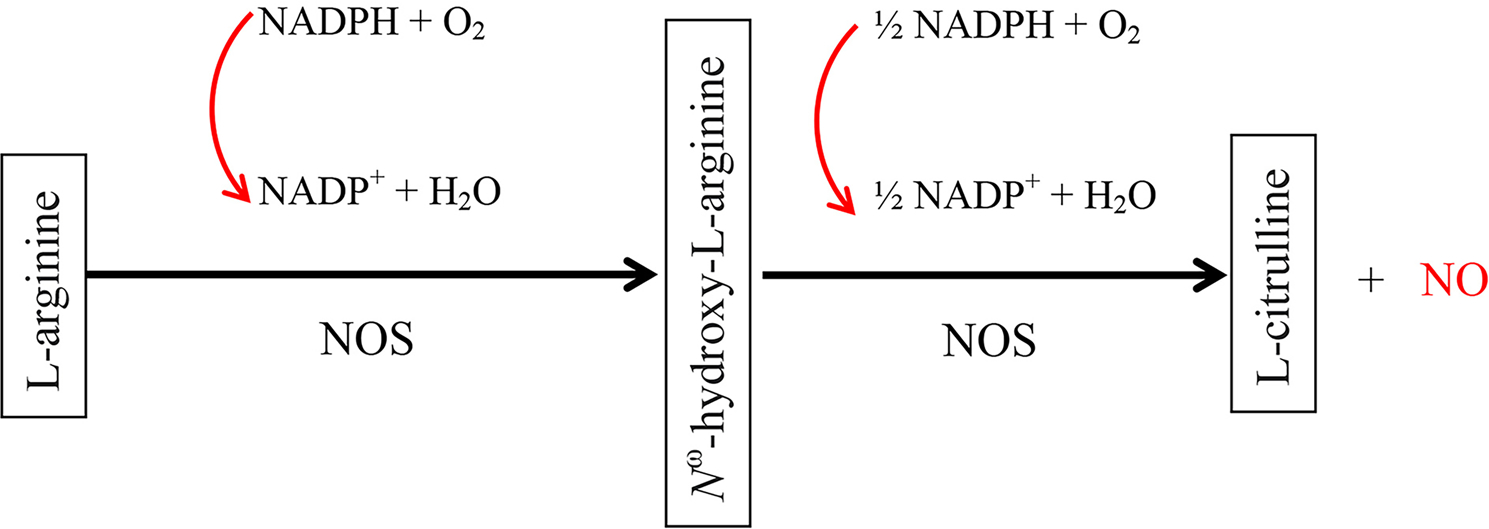
Schematic diagram of enzymatic production of nitric oxide from L-arginine: Nitric oxide synthases first catalyze 5-electron oxidation of a guanidino nitrogen of L-arginine (L-Arg) to generate Nω-hydroxy-L-arginine (NOHLA), via activating oxygen at heme and transferring electrons from 1 molecule of nicotinamide-adenine-dinucleotide phosphate (NADPH) to flavin-adenine-dinucleotide and flavin-mononucleotide, followed by oxidation of Nω-hydroxy-L-arginine, consuming 0.5 molecules of NADPH and one molecule of oxygen, to produce citrulline and nitric oxide. This diagram is modified from previous publications [29, 30].
The expression and activity of NOS are regulated by a number of factors. eNOS and nNOS activity can be regulated by intracellular calcium via calmodulin-calcium interactions. High calcium level increases the binding of calmodulin to NOS, resulting in an increase in NO production. However, vascular endothelial growth factor-induced activation of eNOS can occur via both calcium-dependent and -independent manners [36]. Expression levels of mRNA for nNOS can be up-regulated by physical, chemical, and biological factors, such as heat, electrical stimulation, light exposure, colchicine, and allergic substances, as well as hypoxia [37]. Injuries and certain pathological conditions also increase nNOS expression in neural tissue [38–40]. Likewise, cutaneous wounding increases nNOS expression in keratinocytes [41]. In contrast, hypoxia and TNF-α down-regulate eNOS expression [37]. Interferon α∕β (IFNα∕β) lipopolysaccharide (LPS) and estrogen up-regulate eNOS expression and activity [42–45]. Moreover, mechanical stimulation of the skin increases cutaneous NO production by both nNOS and eNOS [46]. iNOS activity is also regulated by calcium-dependent and -independent signaling pathways, at least in some cells [47]. Estrogen and pro-inflammatory cytokines increase, while glucocorticoids decrease iNOS expression [48]. It is worth noting that some stimuli-induced iNOS expression in keratinocytes varies with the origin of the keratinocyte. For example, induction of iNOS expression by IFNγ is more profound in differentiating epidermal keratinocytes than in HaCat cells or mucosal keratinocytes [49]. Inhibitory effects of IL-10 and IL-4, but not IL-13, on NO production and iNOS expression have also been demonstrated, possibly by down-regulation of IL-1 production [50]. The mechanisms whereby Th1 and Th2 cytokines differentially regulate cutaneous NO production remain unknown. But studies show upregulation of NO production by Th1 cytokines, such as IL-2 and IF is mediated by increased TNF-α production at least in macrophages because TNF-α antibody attenuates Th1 cytokine-induced increases in NO production [51]. Also, the p40 subunit of IL-12 is crucial for the induction of iNOS expression in mouse microglia [52]. Thus, the unique structure of some Th1 cytokines and the upregulation of TNF-α production can be ascribed to the upregulation of NO production by Th1 cytokines. Bacterial infection can increase iNOS expression and NO production, as well [53]. Additionally, either UVA or UVB irradiation increases NO production in the epidermis and the dermis. [54, 55] Our recent studies showed that disruption of the epidermal permeability barrier also increases iNOS mRNA expression in the epidermis [21].
REGULATION OF CUTANEOUS INFLAMMATION BY NO
The link between NO and inflammation in extracutaneous tissues has been well established. In inflammatory bowel diseases, such as Crohn’s disease (CD) and ulcerative colitis, circulating levels of NO correlate positively with levels of Th17 cytokines (IL-17A, IL-23, IL-6, IFN-γ, and IL-12) [56, 57]. Pertinently, inhibition of iNOS attenuated edema and neutrophil infiltration in 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced acute colitis in rats [58]. The pathogenic role of NO in inflammation was further evidenced by the observation that inhibition of iNOS decreased, while an NO donor increased the levels of TNF-α and IL-6 in the culture medium of inflamed mucosa and renal epithelial cells [59, 60]. Similarly, inhibition of iNOS decreases IL-6 production in lung tissue infected by group B streptococcus [61]. Interestingly, inhibition of iNOS increases expression levels of IL-4 and IL-10 while decreasing IL-2 and IFNγ.[62] Other studies showed that inhibition of NOS with L-NG-nitro arginine methyl ester (L-NAME) increased expression levels of TNF-α, IL-1β, IL-6, MMP-2, and MMP-9 in a mouse model of osteoarthritis [63]. Studies have also demonstrated a regulatory role for NO in cutaneous inflammation in vitro and in vivo (Table 1).[64–93].
Table 1.
Regulatory Role of NO in Cutaneous Inflammation
| Proinflammation | |||
|---|---|---|---|
|
| |||
| Models | Methods | Results | References |
| Keratinocytes stimulated with IgE | Pretreatment of keratinocytes with 1 mM L-NMMA, a NOS inhibitor, for 1 h, followed by stimulation with IgE for 48 h; or keratinocytes were cultured with 100 μM SNP for 48 h | SNP increased IL-6 and TNFα L-NMMA inhibited IgE-induced increases in IL-6 and TNFα: |
[64] |
| Topical TPA-indued inflammation in mice | Topical application of aminoguanidine or LNMMA 30 min prior to TPA application, skin samples were taken 4 h after TPA application. Or skin was treated with SNP, SNAP, or carboxy-PTIO for 2 h | NOS inhibitor decreased COX2 in TPA-treated skin; NO donors increased COX2 and PGE2 expression in normal mouse skin; NO scavenger (carboxy-PTIO) decreased COX2 expression |
[65] |
| Inflammation induced by subcutaneous injection of carrageenin in male Wistar rats | Mouse hind paws were injected subcutaneously with 300 μg carrageenin. In some experiments, animals were injected intraperitoneally with 5 mg L-NMMA 2 h before, and 24 and 48 h after the administration of carrageenin; the draining lymph node (DLN) was collected 48 h after the administration of carrageenin | L-NMMA decreased the carrageenin-induced increase in skin swelling, without marked reduction in inflammatory infiltrates; L-NMMA did not affect the proliferation of T cells from DLN but inhibited cytokine production upon stimulation with concanavalin A |
[66] |
| Paw edema was induced by subplantar injection of 0.1 ml of 1% A-carrageenin or 3% dextran. Carrageenin and dextran were given alone or in combination with L-arginine, D-arginine, L-NAME, or L-NMMA. The volume of the paw was measured with plethysmometry immediately after the injection. Subsequent readings of the volume of the same paw were carried out at 30- or 60-min intervals. In some experiments paw was treated with dexamethasone sodium phosphate (0.1 mg/kg) given s.c. 2 h prior to the induction of inflammation | Both L-NAME and L-NMMA dose-dependently inhibited the increase in vascular permeability and edema; L-arginine increased the inflammatory responses and reversed the inhibitory effects of L-NAME and L-NMMA; In dexamethasone-treated rats, L-arginine enhanced the dextran-induced edema and the early phase of carrageenin-induced edema but did not affect the inhibition by dexamethasone in the late phase of carrageenin-induced edema |
[67] | |
| Paw edema was induced by subplantar injection of 0.1 ml of 1% carrageenin. Either L-NMMA or L-NAME was given intravenously (30–300 mg kg− 1) 1 h before or after carrageenan administration | Both L-NMMA or L-NAME inhibited paw edema at all-time points; The selective iNOS inhibitors, L-NIL, or AG, did not inhibit paw edema during the first 4 h after carrageenan treatment, but inhibited paw edema at subsequent time points (from 5 to 10 h); All NOS inhibitors inhibited PGE2 production and neutrophil infiltration |
[68] | |
| Orally given L-NAME in the drinking water at a dose of approximately 75 μM/rat/day for 2 and 4 weeks. Paw edema was induced by subplantar injection of carrageenin | L-NAME reduced carrageenin-induced paw edema; No changes in vascular permeability, as assessed by Evans blue extravasation, were observed in L-NAME- treated animals |
[69] | |
| 60 min prior to induction of inflammation with subcutaneous injection of carrageenin, L-NMMA was given by microdialysis | L-NMMA inhibited the increases in cutaneous PGE2, 6-keto-PGFlα, and COX2 induced by carrageenin | [70] | |
| Substance p-induced edema in rats | Intradermal injection of substance P (0.03–1 nmol) with or without concurrent injection of L-NAME (0.1 μM) or L-NMMA (0.1 μM). Edema was assessed 15 min after | L-NAME and L-NMMA inhibited substance P-induced edema and blood flow; L-arginine (1 pmol) prevented the inhibitory effects of L-NAME |
[71] |
| Mustard oil-induced inflammation in rat hind paws | L-NAME (43 μM /kg i.v.) 15 min prior to application of mustard oil. Edema was measured up to 2 h after mustard oil application | L-NAME decreased blood flow without changes in edema | [72, 73] |
| UVB-irradiated rat ears | Rat ears were irradiated with UVB at an intensity of 5.4 J/cm2 (each side of the ear) for 20 min. L-NAME (25 nM) was intradermally injected 24 h after irradiation | L-NAME had no effect on ear edema | [74] |
| UVB-irradiated mouse skin | Mice were irradiated with UVB at an intensity of 20 mW/cm2 for 9 min. Either L-NAME (100 nM/ site) or L-arginine (10 μM/site) or L-NAME 100 nmol + L-arginine 10 μM was intradermally injected 17.5 h after irradiation | L-NAME abolished UVB-induced increase in blood flow. The effect of L-NAME was reversed partially by the co-injection of L-arginine | [75] |
| Complete Freund’s adjuvant-induced paw inflammation in mice | 30 min prior to induction of paw inflammation by intraplantar injection of complete Freund’s adjuvant (CFA), 7-NINA (selective nNOS inhibitor), AG (selective iNOS inhibitor), L-NAME (a non-selective NOS inhibitor), but not L-NIO (selective eNOS inhibitor), at a dose of 50 mg/kg body weight was given intraperitoneally | Pretreatment with 7-NINA, AG, L-NAME, but not L-NIO, significantly attenuated thermal hyperalgesia induced by CFA; Pretreatment with the NOS inhibitors prevented the CFA-induced increases in TNF-α and IL-1β mRNA expression; 7-NINA and L-NAME increased while either AG or L-NIO decreased IL-10 mRNA expression |
[76] |
| Complete Freund’s adjuvant-induced paw inflammation in NOS KO (nNOS, iNOS, and eNOS) knockout mice | Paw inflammation was induced by intraplantar injection of 10 μl of complete Freud’s adjuvant | Expression levels of TNF-α, IL-1β and IL-10 mRNA in nNOS-, iNOS-, or eNOS-KO mice were lower than that in WT mice at 6 to 24 h, except for TNF-α in eNOS-KO and IL-10 in iNOS-KO mice at 24 h, where they were higher or equal to WT controls | [76] |
| Inflammation induced by intradermal injection of histamine or bradykinin in rabbits | Histamine (3 nM/site), bradykinin (0.1 nM/site), or PGE1 (0.1 nM/site) was co-injected with either SNP (0.4–400 nM/site), SIN-1 (0.4–400 nM/site) or L-NAME (0–400 nM/site) | NO donors did not affect vascular permeability induced by either histamine or bradykinin or PGE1; L-NAME inhibited plasma leakage induced by a combination of PGE1 and bradykinin or histamine; L-arginine or SNP reversed the inhibitory effect of L-NAME on plasma leakage |
[77] |
| Inflammation induced by intradermal injection of histamine, bradykinin, or zymosan-activated plasma in guinea pigs | Histamine, bradykinin, or zymosan-activated plasma was intradermally injected alone or with L-NAME. Inflammation was assessed 2 h after | Given L-NAME with bradykinin ( 10−10 M/site) caused a dose-dependent inhibition of bradykinin-induced edema formation; At a dose of 10− 6 M/ site, L-NAME inhibited bradykinin-induced edema formation by 89%; SNP reversed the inhibitory effect of L-NAME on bradykinin-induced edema; L-NAME inhibited eosinophil infiltrate induced by zymosan-activated plasma, passive cutaneous anaphylaxis reaction, platelet-activating factor, and bradykinin |
[78] |
| Reverse passive Arthus reaction in guinea pigs | Inflammation was induced by intradermal injection of antigen with or without L-NAME. Skin edema was assessed 4 h after induction of inflammation | L-NAME suppressed edema formation by 68%, whereas a cyclo-oxygenase inhibitor (ibuprofen) suppressed edema by 27% | [79] |
| Inflammation in cutaneous wound of nNOS, iNOS, and e NOS KO mice | A 6-mm full-thickness wound was created on the dorsal surface and covered with a semi-occlusive dressing. Wounds were treated daily for 7 days (d 0–6) with topical application of 50 μl of either SP ( 10−7 M) or normal saline by infusion with a 26-gauge needle through the dressing onto the wound bed | NOS KO delayed wound healing. Densities of macrophage, leukocyte, and dendritic cells were lower in all NOS KO mice than in the controls at d 3 and d 7 post wound | [80] |
| Inflammation in cutaneous wound of iNOS KO mice | A 2.5 cm longitudinal incision was created on the back of the mice | TGF-β1 expression was increased by 50 to 100% in iNOS KO wounds on postoperative days 5 and 7; VEGF and IL-4 expression were elevated by 25 to 100% in wild type compared with iNOS-KO mice |
[81] |
| Allergic contact dermatitis in Balb/C mice | Dermatitis was induced by topical application of 0.4% DNFB to mouse ears. Intradermal injection of 10 μl aminoguanidine (50 mM) or L-NMA (100 mM) into the ears | NOS inhibitors decreased ear thickness and inflammatory infiltrates | [82] |
| Allergic contact dermatitis in iNOS KO and wild type mice | Dermatitis was induced by topical application of ovalbumin; the mice were intraperitoneally injected with 20 mg/kg L-NAME after sensitization | Ear swelling and the frequency of scratching for the OVA-sensitized mice decreased significantly after the administration of l-NAME; Ear swelling and the frequency of scratching in the sensitized animals were significantly suppressed in iNOS KO mice; Skin histamine content and number of mast cells were also lower in iNOS KO mice than in the controls |
[83] |
| Allergic contact dermatitis induced by picryl chloride (PC1) in mice | Ear swelling was observed at 2 h (early phase) and 24 h (late phase) after application of the antigen to PC1-sensitized CBA/J mice. L-NAME or L-arginine was intravenously injected at the time of the PC1 challenge | Intravenous injection of L-NAME dose-dependently inhibited the antigen-induced contact hypersensitivity reaction; Low-dose (1 mg/kg) L-NAME inhibited the early-phase reaction, but not the late-phase reaction. High-dose (250 mg/kg) L-NAME inhibited both early- and late- phase reactions. High-dose (250 mg/kg) L-arginine increased both early and late phase reactions Injection of L-NAME (250 mg/kg) with PCI significantly decreased IL-2 and IFN-γcompared with PCI alone; Administration of L-arginine (250 mg/kg) significantly increased the production of IL-2 and IFN-γ by the lymph node cells |
[84] |
| Humans with atopic dermatitis | 15 patients with atopic dermatitis were topically treated with 1% NLA twice daily for 4 weeks | Improvements in pruritus and erythema were observed in 80 and 66% of patients, respectively | [85] |
| Normal human skin | 0.02 ml zeolite zinc (33% wt/wt) or 0.02 ml zeolite manganese NO (33% wt/wt) or 0.04 ml acidified NO2−, was applied topically to the volar aspect of the forearm once every 8 h for 2 consecutive days | Topical Ze-NO induced a dermal CD4+ T cell to infiltrate and IFN-γ secretion. The acidified nitrite caused dermal infiltrate of CD3+, CD4+, CD8+, and CD68+ cells and neutrophils. Suction blisters were created in Ze-NO- treated and control skin. IFN-γ, but not IL-4, was detected in Ze-NO-treated skin | [86] |
| Application of 0.5% sodium nitrite with 2% ascorbic acid three times daily over 48 h produced mild skin inflammation, whereas 5% nitrite with 2% ascorbic acid produced marked inflammation | NO increased staining intensity of CD3, CD4, CD8, CD68, VCAM-1 and ICAM-1, and neutrophil infiltration | [87] | |
| Anti-inflammation | |||
| Allergic contact dermatitis in mice | Ear inflammation was induced by topical application of 20 μl of 0.3% (v/v) DNFB. iNOS was inhibited with an intraperitoneal injection of L-NIL (2.5 mg in 0.5 ml PBS twice daily) for 6 consecutive days starting 1 day before sensitization | iNOS inhibitor increased ear thickness and augmented FITC-induced migration of cutaneous DCs, including Langerin( +) LCs and Langerin( —) dermal DCs, to draining lymph nodes; iNOS inhibitor enhanced LC survival in vitro |
[88] |
| Irritant contact dermatitis in mice | Irritant contact dermatitis was induced by topical application of 5% benzalkonium chloride to mouse ears. Test products were applied either 15 min before or 5 min after benzalkonium chloride application | Topical NO-hydrocortisone (NCX 1022) (either pre or posttreatment) was more effective than hydrocortisone in reduction of ear thickness during the initial stages of inflammation (from 1 to 5 h); NCX 1022, but not hydrocortisone, significantly inhibited granulocyte recruitment and reduced the number of infiltrated cells and disruption of the tissue architecture compared to hydrocortisone-treated tissues; NCX 1022 was more effective than hydrocortisone in inhibiting benzalkonium chloride-induced leukocyte adhesion to the endothelium |
[89] |
| Mouse model of atopic dermatitis | Atopic dermatitis was induced by sensitization with Ovalbumin. Berdazimer sodium (6%), a NO donor, was applied topically for 24 h | NO donor decreased Staphylococcus aureus colonization and expression of IL-4 and IL-13 | [90] |
| Patients with atopic dermatitis | A NO donor, berdazimer sodium (2 or 6%), was applied topically twice daily for 2 weeks | NO donor decreased eczema area and severity index scores; NO donor decreased expression levels of inflammation-related genes |
[90] |
| Nickel-induced inflammation in the mouse dermis | Allergic inflammation was induced by the intradermal injection of nickel chloride. An iNOS inhibitor, L-NIL (100 μM), was co-administrated with nickel | NOS inhibitor increased ear swelling | [91] |
| iNOS KO mice | Inflammation caused by subcutaneous implantation | iNOS KO mice exhibited increased neutrophil and macrophage infiltration and collagen deposition; No changes in CXCL1 and CCL2 expression were observed |
[92] |
| Nickel-induced inflammation in humans | Skin was topically treated with NOS inhibitor, L-NMMA, for 4 consecutive days, followed by nickel patch testing, then irradiated with a solar simulator | NOS inhibitor increased epidermal S-100+ dendritic cells and decreased dermal S-100+ dendritic cells; Erythema index and sunburn cells were decreased in NOS inhibitor-treated area |
[93] |
L-NMMA, NG- monomethyl L-arginine; L-NAME, NG- nitro-L-arginine methyl ester; NLA, Nω-nitro-L-arginine; L-NIL, N-iminoethyl-L-lysine; AG: aminoguanidine; L-NIO, L-N(5)-(l-iminoethyl)-ornithine; 7-NINA, 7-nitroindazole sodium salt; SNP, Sodium nitroprusside; SIN-1, 3-Morpholinosydnonimine; TPA, 12-O-tetradecanoylphorbol-13-acetate; DNFB, 2,4-dinitrofluorobenzene
Pro-inflammatory Effects
NO has long been considered a key mediator in the development of cutaneous inflammation under various conditions. In both atopic dermatitis and allergic contact dermatitis, expression levels of eNOS and iNOS are increased in the dermis [94], although the absence of iNOS mRNA in the atopic epidermis was reported [95]. In psoriasis, another common inflammatory dermatosis, nNOS is expressed in all layers of the involved and uninvolved epidermis of psoriatic patients but only expressed in keratinocytes in the granular layer and eccrine sweat glands of normal skin. Expression of iNOS was also found in the epidermis of normal skin, while strong staining of iNOS was observed in both keratinocytes and in the papillary dermis of psoriatic skin. [95–97] Topical applications of an NO donor increased the number of CD3-, CD4-, and CD-68 positive cells in the skin [87]. Likewise, circulating levels of NO correlate positively with the severity of psoriasis [98]. All of these studies suggest a link between NO and inflammatory dermatoses in humans.
The link between NO and cutaneous inflammation is further supported by a number of in vivo and in vitro studies using NOS inhibitors and NOS knockout mice. In keratinocyte cultures, pre-treatment of keratinocytes with a NOS inhibitor (NG-monomethyl-l-arginine, l-NMMA) decreased IgE-induced secretion of TNF-α and IL-6, while an NO donor increased cytokine production [64]. In vivo, pretreatment of mice with an iNOS inhibitor prevented TPA-induced increases in COX2 expression [65]. Similarly, intraperitoneal injection of either nNOS or iNOS inhibitor (but not an eNOS inhibitor) lowered expression levels of TNF-α and IL-1β in a mouse model of plantar inflammation induced by complete Freund’s adjuvant [76]. In addition, intravenous injection of NOS inhibitor (L-NAME) decreased ear swelling as well as the contents of IL-2 and IFNγ in lymph nodes of mice with cutaneous inflammation induced by topical applications of picryl chloride [84]. In humans, topical application of 5% NO−2 solution increased NO content in the superficial dermis, accompanied by an increase in infiltrates of macrophages and neutrophils in both the epidermis and the dermis, along with increased dermal infiltration of T cells [86]. Likewise, topical applications of 5% nitrite cream markedly increased inflammatory infiltrates in both the dermis and the epidermis in humans.[87] Correspondingly, topical applications of an NOS inhibitor (Nω-nitro-L-arginine) twice daily for 4 weeks reduced pruritus and erythema in patients with atopic dermatitis in subjects who responded poorly to topical steroids [85]. Direct evidence of NO in regulating cutaneous inflammation has been demonstrated in NOS knockout mice. In a mouse model of ovalbumin-specific immunoglobulin E-induced dermatitis, iNOS knockout mice displayed no increase in either cutaneous mast cell infiltration or circulating levels of pro-inflammatory cytokines, such as TNF-α, in comparison to wild-type controls [83]. Moreover, deficiency in either iNOS, nNOS, or eNOS decreased the densities of inflammatory cells, including dendritic cells, macrophages, and leukocytes in cutaneous wounds in mice [80]. Notably, the effects of NOS inhibitors on inflammation depend on their route of administration, at least in a rat model of carrageenin-induced pleurisy. For example, a single intrapleural injection of the iNOS inhibitors [S-(2-aminoethyl) isothiourea or N-(3-(aminomethyl)-benzyl) acetamidine] or eNOS inhibitor [l-N5(1-iminoethyl)-ornithine] exacerbated inflammation at early stages (1–6 h), while systemically administering NOS inhibitors ameliorated the severity of inflammation throughout the induction of carrageenin-induced pleurisy in rats [99]. Studies also demonstrated that inhibition of NOS increased vasodilation without changes in edema provoked by topical mustard oil [72, 73], suggesting regulation of NO in cutaneous inflammation varies in different inflammatory models. Nonetheless, these studies demonstrate a regulatory role for NO in cutaneous inflammation.
Although the precise underlying mechanisms of the pro-inflammatory effect of NO have not been fully clarified, several mechanisms could contribute to these effects. First, during the development of inflammation, vascular dilation and leukocyte migration are crucial events leading to inflammatory infiltration and edema. Studies showed that fewer migrated leukocytes and smaller vessel diameters were observed in iNOS-deficient mice than in the normal wild-type mice upon induction of inflammation with LPS [100]. NOS inhibitors such as L-NAME and L-NMMA inhibited the carrageenin-induced increase in vascular permeability and paw edema in rat skin [67]. The inhibitory effects of NO on inflammatory infiltration and edema are likely via prostaglandin E (PGE) because NOS inhibitors reduced, while NO donors increased PGE production and potentiated arachidonic acid-induced paw edema in rats [101, 102]. Moreover, inhibition of NOS attenuated cutaneous edema induced by either bradykinin or histamine [103] or zymosan-activated plasma-induced infiltration of eosinophil and neutrophils [78]. Thus, NO-induced increases in vascular permeability and leukocyte migration account in part for the pro-inflammatory effect of NO in cutaneous inflammation.
Secondly, NO can activate the promoter of the IL-8 gene and increase IL-8 mRNA expression in human melanoma cells and lung epithelial cells in vitro.[104–106] Correspondingly, NO donors enhance LPS-induced IL-8 production in neutrophils [107]. Likewise, NO donors and L-arginine increase expression levels of IL-6, IL-1β, and TNF-α, as well as macrophage inflammatory proteins [59–61, 108]. Inhibition of iNOS decreases TNF-α, and IL-6 production by 66 and 27%, respectively, in vitro.[59] Similarly, nNOS deficiency decreases TNF-α, IL-1β, and IL-6 production in macrophages stimulated with LPS [109]. Notably, nNOS, but neither iNOS nor eNOS, is involved in cytokine production in response to stimulation with LPS. Hence, stimulation of cytokine production can be an additional mechanism by which NO triggers/exacerbates inflammation.
Finally, NO can enhance proliferation and differentiation of Th1 cells, accompanied by an elevation in INFγ production [110], events that could also contribute to the pro-inflammatory effect of NO in cutaneous inflammation. In summary, the pro-inflammatory effects of NO can be collectively attributed to increased vascular permeability and cytokine production, as well as stimulation of inflammatory cell migration and T cell proliferation and differentiation.
Anti-inflammatory Effects
NO also exerts anti-inflammatory properties in cutaneous and extracutaneous tissues. For example, inhibition of NOS enhances the recruitment and extravasation of leukocytes in postcapillary venules [111]. Conversely, administration of an NO donor (3-morpholinosydnonimine, SIN-1) inhibits both platelet-activating factor- and integrin-induced leukocyte adhesion in venules [112, 113]. NO-releasing derivatives of mesalamine are more effective than mesalamine alone in the inhibition of colon inflammation and reduction of IL-1β production by splenocytes [114]. Similarly, co-culture of peripheral blood monocytes with NO donor reduced secretion of IL-1α, IL-6, IL-8, and TNF-α in comparison to the vehicle treatment [115]. Evidence indicates certain anti-inflammatory properties of NO are also demonstrated in the skin. For instance, acne is a common skin disorder accompanied by cutaneous inflammation. Preincubation of keratinocytes with NO donor for 1 h dose-dependently decreased Propionibacterium (P.) acnes–induced secretion of IL-6 and IL-8 as compared to those without NO donor treatment. Allergic contact dermatitis is another common cutaneous inflammatory disorder. Previous studies showed that intraperitoneal injections of an iNOS inhibitor for 6 days dramatically augmented ear thickness induced by topical dinitrofluorobenzene (DNFB) in mice [88]. Likewise, inhibition of iNOS enhanced nickel-induced ear thickness by over 70% as compared to vehicle-treated ears [91]. Consistent with these findings in murine models, topical applications of an NO donor twice-daily for two weeks lowered eczema area and severity scores by 23% from the baseline, along with suppression of gene expression related to innate immunity (Th2, Th17/Th22, Th9, and Th1) pathways in humans with atopic dermatitis [90]. Taken together, these studies demonstrate the anti-inflammatory benefits of NO in cutaneous inflammation.
The underlying mechanisms whereby NO attenuates cutaneous inflammation can be attributable to reductions in cytokine production and inflammatory infiltration, and stimulation of CD4 + T cells to differentiate into Treg cells. Previous studies showed that P. acnes increased expression levels of IL-1β, IL-6, IL-8, and TNF in vivo and in vitro via activation of the NLRP3 inflammasome [115–119] and that an NO donor can inhibit NLRP3 inflammasome activity, leading to reductions in IL-8 and IL-1β [120]. Likewise, an NO donor inhibited the secretion of IL-6 and other inflammatory proteins by monocytes by over 50% [121]. In contrast, inhibition of NO production increases expression levels of pro-inflammatory cytokines, such as IL-1β, IL-6, and TNFβ, while an NO donor inhibited the expression of these cytokines in LPS-treated macrophages [122–125]. An NO donor (NOC-18) also inhibited proliferation and differentiation of Th17 cells, as well as the secretion of IL-17a and IL-22 [126], while increasing CLA+CD25+Foxp3+ regulatory T cells through the canonical soluble guanylyl cyclase–cyclic guanosine monophosphate (sGC-cGMP) signaling pathway [127], which negatively regulates inflammation. Moreover, either sodium nitroprusside or NOC12 (NO donors) can dose-dependently enhance T lymphocyte apoptosis and down-regulate expression levels of Bcl-2 protein, leading to reductions in T cell survivability [128]. The anti-inflammatory properties of NO can be ascribed to the enhancement of T cell apoptosis as well as inhibition of cytokine expression.
NO can also inhibit the migration and adhesion of inflammatory cells. Prior to sensitization with 2,4-dinitrofluorobenzene, daily intraperitoneal injection of iNOS inhibitor for 6 days dramatically increases lymphocytic infiltration into the dermis and dendritic cell migration into the lymph nodes in mice [88]. In a rat model of pleurisy, low doses of an NO donor (NOC-18 at a dose of 10 mg/kg) inhibited leukocyte infiltration by 20% 4 h after induction of pleurisy, while high doses of the same NO donor (30 mg/kg) increased leucocytes by 33% [129]. In a mouse model of irritant contact dermatitis induced by topical benzalkonium chloride, topical NO-hydrocortisone (NCX 1022) is more effective than hydrocortisone alone in inhibition of granulocyte recruitment and leukocyte adhesion to endothelia in addition to the reduction in the number of infiltrated cells [89]. Evidence indicates that NO-induced inhibition of neutrophil migration is mediated by cyclic GMP-intercellular adhesion molecule I [130]. Inhibition of migration and adhesion of inflammatory cells could therefore contribute to the anti-inflammatory properties of NO.
The exact underlying mechanisms for the divergent effects of NO on inflammation are unknown. Evidence indicates that NO is required in the induction of inflammation. Low levels of NO can increase endothelial permeability, facilitating the migration of inflammatory cells to the inflamed site and cytokine production. [131–134] Moreover, low levels of NO increase cyclic GMP and β 2 integrin, resulting in enhanced chemotaxis and neutrophil aggravation. Thus, either NOS deficiency or administration of NOS inhibitor attenuates inflammatory response to stimuli. In contrast, high levels of NO inhibit inflammation. Previous studies showed that high levels of NO increase peroxynitrite production and ADP ribosylation of actin, leading to reductions in neutrophil infiltration [132]. High levels of NO also can induce neutrophil and T cell apoptosis. [132, 134]. In addition, NO donor inhibited histamine release and mast cell degranulation, while NOS inhibitor enhanced LPS–induced histamine release [135]. Hence, NO also exhibits anti-inflammatory properties, particularly in the case of inflamed skin. However, further studies are required to define which levels of NO are considered low or high.
CONCLUSIONS
NO exhibits both pro-inflammatory and anti-inflammatory properties via positive or negative regulation of cytokine expression, inflammatory cell proliferation, and adhesion, as well as T cell apoptosis. Although it appears that high levels of NO inhibit inflammation, further studies are needed to determine the optimal level of NO that can inhibit cutaneous inflammation and whether NO donors could improve cutaneous inflammation in clinical settings.
FUNDING
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Disease of the National Institutes of Health under award number R01 AR061106, administered by the Northern California Institute for Research and Education, with resources from the Research and Development Service, Department of Veterans Affairs Medical Center, San Francisco. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DECLARATIONS
Competing Interests The authors declare no competing interests.
REFERENCES
- 1.Massion PB, Feron O, Dessy C, and Balligand JL. 2003. Nitric oxide and cardiac function: Ten years after, and continuing. Circulation Research 93 (5): 388–398. [DOI] [PubMed] [Google Scholar]
- 2.Musialek P, Lei M, Brown HF, Paterson DJ, and Casadei B. 1997. Nitric oxide can increase heart rate by stimulating the hyperpolarization-activated inward current, I(f). Circulation Research 81 (1): 60–68. [DOI] [PubMed] [Google Scholar]
- 3.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, and Smithies O. 1996. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences of the USA 93 (23): 13176–13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyamoto Y, Saito Y, Kajiyama N, Yoshimura M, Shimasaki Y, Nakayama M, Kamitani S, Harada M, Ishikawa M, Kuwahara K, Ogawa E, Hamanaka I, Takahashi N, Kaneshige T, Teraoka H, Akamizu T, Azuma N, Yoshimasa Y, Yoshimasa T, Itoh H, Masuda I, Yasue H, and Nakao K. 1998. Endothelial nitric oxide synthase gene is positively associated with essential hypertension. Hypertension 32 (1): 3–8. [DOI] [PubMed] [Google Scholar]
- 5.Cau SB, Carneiro FS, and Tostes RC. 2012. Differential modulation of nitric oxide synthases in aging: Therapeutic opportunities. Frontiers in Physiology 3: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu YH, Carretero OA, Cingolani OH, Liao TD, Sun Y, Xu J, Li LY, Pagano PJ, Yang JJ, and Yang XP. 2005. Role of inducible nitric oxide synthase in cardiac function and remodeling in mice with heart failure due to myocardial infarction. American Journal of Physiology. Heart and Circulatory Physiology 289: H2616–H2623. [DOI] [PubMed] [Google Scholar]
- 7.Iwakiri Y, and Kim MY. 2015. Nitric oxide in liver diseases. Trends in Pharmacological Sciences 36 (8): 524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahmoud MF, Zakaria S, and Fahmy A. 2015. Can chronic nitric oxide inhibition improve liver and renal dysfunction in bile duct ligated rats? Advances in Pharmacological Sciences 2015:298792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinberger B, Heck DE, Laskin DL, and Laskin JD. 1999. Nitric oxide in the lung: Therapeutic and cellular mechanisms of action. Pharmacology & Therapeutics 84 (3): 401–411. [DOI] [PubMed] [Google Scholar]
- 10.Kinsella JP, Parker TA, Galan H, Sheridan BC, Halbower AC, and Abman SH. 1997. Effects of inhaled nitric oxide on pulmonary edema and lung neutrophil accumulation in severe experimental hyaline membrane disease. Pediatric Research 41 (4 Pt 1): 457–463. [DOI] [PubMed] [Google Scholar]
- 11.Kozłowska Z, Owsiańska Z, Wroblewska JP, et al. 2020. Genotype-phenotype correlation in two Polish neonates with alveolar capillary dysplasia. BMC Pediatrics 20 (1): 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bescós R, Sureda A, Tur JA, and Pons A. 2012. The effect of nitric-oxide-related supplements on human performance. Sports Medicine (Auckland, N. Z.) 42 (2): 99–117. [DOI] [PubMed] [Google Scholar]
- 13.Ramirez LA, Gillis EE, Musall JB, Mohamed R, Snyder E, El-Marakby A, and Sullivan JC. 2020. Hypertensive female Sprague-Dawley rats require an intact nitric oxide synthase system for compensatory increases in renal regulatory T cells. American Journal of Physiology Renal Physiology 319 (2): F192–F201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brinson KN, Elmarakby AA, Tipton AJ, Crislip GR, Yamamoto T, Baban B, and Sullivan JC. 2013. Female SHR have greater blood pressure sensitivity and renal T cell infiltration following chronic NOS inhibition than males. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 305 (7): R701–R710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhan R, Yang S, He W, Wang F, Tan J, Zhou J, Yang S, Yao Z, Wu J, and Luo G. 2015. Nitric oxide enhances keratinocyte cell migration by regulating Rho GTPase via cGMP-PKG signalling. PLoS One 10(3):e0121551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malone-Povolny MJ, Maloney SE, and Schoenfisch MH. 2019. Nitric oxide therapy for diabetic wound healing. Advanced Healthcare Materials 8(12):e1801210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witte MB, Kiyama T, and Barbul A. 2002. Nitric oxide enhances experimental wound healing in diabetes. British Journal of Surgery 89 (12): 1594–1601. [DOI] [PubMed] [Google Scholar]
- 18.Shi HP, Wang SM, Zhang GX, Zhang YJ, and Barbul A. 2007. Supplemental L-arginine enhances wound healing following trauma/hemorrhagic shock. Wound Repair Regen. 15 (1): 66–70. [DOI] [PubMed] [Google Scholar]
- 19.Krischel V, Bruch-Gerharz D, Suschek C, Kröncke KD, Ruzicka T, and Kolb-Bachofen V. 1998. Biphasic effect of exogenous nitric oxide on proliferation and differentiation in skin derived keratinocytes but not fibroblasts. The Journal of Investigative Dermatology 111 (2): 286–291. [DOI] [PubMed] [Google Scholar]
- 20.Kitano T, Yamada H, Kida M, Okada Y, Saika S, and Yoshida M. 2017. Impaired healing of a cutaneous wound in an inducible nitric oxide synthase-knockout mouse. Dermatology Research and Practice 2017: 2184040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dang E, Man G, Zhang J, Lee D, Mauro TM, Elias PM, and Man MQ. 2020. Inducible nitric oxide synthase is required for epidermal permeability barrier homeostasis in mice. Experimental Dermatology 29 (10): 1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Man MQ, Wakefield JS, Mauro TM, and Elias PM. 2021. Role of nitric oxide in regulating epidermal permeability barrier function. Experimental Dermatology 10.1111/exd.14470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mihu MR, Sandkovsky U, Han G, Friedman JM, Nosanchuk JD, and Martinez LR. 2010. The use of nitric oxide releasing nanoparticles as a treatment against Acinetobacter baumannii in wound infections. Virulence. 1 (2): 62–67. [DOI] [PubMed] [Google Scholar]
- 24.Martinez LR, Han G, Chacko M, Mihu MR, Jacobson M, Gialanella P, Friedman AJ, Nosanchuk JD, and Friedman JM. 2009. Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection. The Journal of Investigative Dermatology 129: 2463–2469. [DOI] [PubMed] [Google Scholar]
- 25.Ghaffari A, Jalili R, Ghaffari M, Miller C, and Ghahary A. 2007. Efficacy of gaseous nitric oxide in the treatment of skin and soft tissue infections. Wound Repair Regen. 15: 368–377. [DOI] [PubMed] [Google Scholar]
- 26.Wallace JL 2005. Nitric oxide as a regulator of inflammatory processes. Memorias do Instituto Oswaldo Cruz 100: S5–9. [DOI] [PubMed] [Google Scholar]
- 27.Moilanen E, and Vapaatalo H. 1995. Nitric oxide in inflammation and immune response. Annals of Medicine 27 (3): 359–367. [DOI] [PubMed] [Google Scholar]
- 28.Medeiros R, Figueiredo CP, Passos GF, and Calixto JB. 2009. Reduced skin inflammatory response in mice lacking inducible nitric oxide synthase. Biochemical Pharmacology 78 (4): 390–395. [DOI] [PubMed] [Google Scholar]
- 29.Modified from Knowles RG, and Moncada S. 1994. Nitric oxide synthases in mammals. Biochemical Journal 298( Pt 2) (Pt 2):249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stuehr D, Pou S, and Rosen GM. 2001. Oxygen reduction by nitric-oxide synthases. Journal of Biological Chemistry 276 (18): 14533–14536. [DOI] [PubMed] [Google Scholar]
- 31.Förstermann U, and Sessa WC. 2012. Nitric oxide synthases: regulation and function. European Heart Journal 33(7):829–37, 837a-837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Förstermann U, and Kleinert H. 1995. Nitric oxide synthase: Expression and expressional control of the three isoforms. Naunyn-Schmiedeberg’s Archives of Pharmacology 352 (4): 351–364. [DOI] [PubMed] [Google Scholar]
- 33.Mattila JT, and Thomas AC. 2014. Nitric oxide synthase: Non-canonical expression patterns. Frontiers in Immunology 5: 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jianjun Yang J, Zhang R, Lu G, Shen Y, Peng L, Zhu C, Cui M, Wang W, Arnaboldi P, Tang M, Gupta M, Qi CF, Jayaraman P, Zhu H, Jiang B, Chen SH, He JC, Ting AT, Zhou MM, Kuchroo VK, Morse HC 3rd., Ozato K, Sikora AG, and Xiong H. 2013. T cell–derived inducible nitric oxide synthase switches off Th17 cell differentiation. Journal of Experimental Medicine 210 (7): 1447–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilchrist M, McCauley SD, and Befus AD. 2004. Expression, localization, and regulation of NOS in human mast cell lines: Effects on leukotriene production. Blood 104 (2): 462–469. [DOI] [PubMed] [Google Scholar]
- 36.Devika NT, and Jaffar Ali BM. 2013. Analysing calcium dependent and independent regulation of eNOS in endothelium triggered by extracellular signaling events. Molecular BioSystems 9: 2653–2664. [DOI] [PubMed] [Google Scholar]
- 37.Förstermann U, Boissel JP, and Kleinert H. 1998. Expressional control of the “constitutive” isoforms of nitric oxide synthase (NOS I and NOS III). The FASEB Journal 12 (10): 773–790. [PubMed] [Google Scholar]
- 38.Herdegen T, Brecht S, Mayer B, Leah J, Kummer W, Bravo R, and Zimmermann M. 1993. Long-lasting expression of JUN and KROX transcription factors and nitric oxide synthase in intrinsic neurons of the rat brain following axotomy. Journal of Neuroscience 13 (10): 4130–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villar MJ, Ceccatelli S, Bedecs K, Bartfai T, Bredt D, Synder SH, Hökfelt T. 1994. Upregulation of nitric oxide synthase and galanin message-associated peptide in hypothalamic magnocellular neurons after hypophysectomy. Immunohistochemical and in situ hybridization studies. Brain Research 650(2):219–28. [DOI] [PubMed] [Google Scholar]
- 40.Zhang ZG, Chopp M, Gautam S, Zaloga C, Zhang RL, Schmidt HH, Pollock JS, and Förstermann U. 1994. Upregulation of neuronal nitric oxide synthase and mRNA, and selective sparing of nitric oxide synthase-containing neurons after focal cerebral ischemia in rat. Brain Research 654 (1): 85–95. [DOI] [PubMed] [Google Scholar]
- 41.Boissel JP, Ohly D, Bros M, Gödtel-Armbrust U, Förstermann U, and Frank S. 2004. The neuronal nitric oxide synthase is upregulated in mouse skin repair and in response to epidermal growth factor in human HaCaT keratinocytes. The Journal of Investigative Dermatology 123 (1): 132–139. [DOI] [PubMed] [Google Scholar]
- 42.Kaku Y, Nanri H, Sakimura T, Ejima K, Kuroiwa A, and Ikeda M. 1997. Differential induction of constitutive and inducible nitric oxide synthases by distinct inflammatory stimuli in bovine aortic endothelial cells. Biochimica et Biophysica Acta 1356 (1): 43–52. [DOI] [PubMed] [Google Scholar]
- 43.Bucher M, Ittner KP, Zimmermann M, Wolf K, Hobbhahn J, and Kurtz A. 1997. Nitric oxide synthase isoform III gene expression in rat liver is up-regulated by lipopolysaccharide and lipoteichoic acid. FEBS Letters 412 (3): 511–514. [DOI] [PubMed] [Google Scholar]
- 44.Neugarten J, Ding Q, Friedman A, Lei J, and Silbiger S. 1997. Sex hormones and renal nitric oxide synthases. Journal of the American Society of Nephrology 8 (8): 1240–1246. [DOI] [PubMed] [Google Scholar]
- 45.Lantin-Hermoso RL, Rosenfeld CR, Yuhanna IS, German Z, Chen Z, and Shaul PW. 1997. Estrogen acutely stimulates nitric oxide synthase activity in fetal pulmonary artery endothelium. American Journal of Physiology 273 (1 Pt 1): L119–L126. [DOI] [PubMed] [Google Scholar]
- 46.Ikeyama K, Denda S, Tsutsumi M, and Denda M. 2010. Neuronal nitric oxide synthase in epidermis is involved in cutaneous circulatory response to mechanical stimulation. The Journal of Investigative Dermatology 130 (4): 1158–1166. [DOI] [PubMed] [Google Scholar]
- 47.Zhang B, Crankshaw W, Nesemeier R, Patel J, Nweze I, Lakshmanan J, and Harbrecht BG. 2015. Calcium-mediated signaling and calmodulin-dependent kinase regulate hepatocyteinducible nitric oxide synthase expression. Journal of Surgical Research 193 (2): 795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pautz A, Art J, Hahn S, Nowag S, Voss C, and Kleinert H. 2010. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide 23 (2): 75–93. [DOI] [PubMed] [Google Scholar]
- 49.Arany I, Brysk MM, Brysk H, and Tyring SK. 1996. Induction of iNOS mRNA by interferon-gamma in epithelial cells is associated with growth arrest and differentiation. Cancer Letters 110 (1–2): 93–96. [DOI] [PubMed] [Google Scholar]
- 50.Linehan JD, Kolios G, Valatas V, Robertson DA, and Westwick J. 2005. Immunomodulatory cytokines suppress epithelial nitric oxide production in inflammatory bowel disease by acting on mononuclear cells. Free Radical Biology & Medicine 39 (12): 1560–1569. [DOI] [PubMed] [Google Scholar]
- 51.Taub DD, and Cox GW. 1995. Murine Th1 and Th2 cell clones differentially regulate macrophage nitric oxide production. Journal of Leukocyte Biology 58 (1): 80–89. [DOI] [PubMed] [Google Scholar]
- 52.Saha RN, and Pahan K. 2006. Regulation of inducible nitric oxide synthase gene in glial cells. Antioxidants & Redox Signaling 8 (5–6): 929–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wienerroither S, Rauch I, Rosebrock F, Jamieson AM, Bradner J, Muhar M, Zuber J, Müller M, and Decker T. 2014. Regulation of NO synthesis, local inflammation, and innate immunity to pathogens by BET family proteins. Molecular and Cellular Biology 34 (3): 415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holliman G, Lowe D, Cohen H, Felton S, and Raj K. 2017. Ultraviolet radiation-induced production of nitric oxide: A multi-cell and multi-donor analysis. Science and Reports 7 (1): 11105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang HR, Tsao DA, Wang SR, and Yu HS. 2003. Expression of nitric oxide synthases in keratinocytes after UVB irradiation. Archives of Dermatological Research 295 (7): 293–296. [DOI] [PubMed] [Google Scholar]
- 56.Rafa H, Saoula H, Belkhelfa M, Medjeber O, Soufli I, Toumi R, de Launoit Y, Moralès O, Nakmouche M, Delhem N, and Touil-Boukoffa C. 2013. IL-23/IL-17A axis correlates with the nitric oxide pathway in inflammatory bowel disease: Immunomodulatory effect of retinoic acid. Journal of Interferon and Cytokine Research 33 (7): 355–368. [DOI] [PubMed] [Google Scholar]
- 57.Rafa H, Amri M, Saoula H, Belkhelfa M, Medjeber O, Boutaleb A, Aftis S, Nakmouche M, and Touil-Boukoffa C. 2010. Involvement of interferon-γ in bowel disease pathogenesis by nitric oxide pathway: A study in Algerian patients. Journal of Interferon and Cytokine Research 30 (9): 691–697. [DOI] [PubMed] [Google Scholar]
- 58.Kankuri E, Vaali K, Knowles RG, Lähde M, Korpela R, Vapaatalo H, and Moilanen E. 2001. Suppression of acute experimental colitis by a highly selective inducible nitric-oxide synthase inhibitor, N-[3-(aminomethyl)benzyl]acetamidine. Journal of Pharmacology and Experimental Therapeutics 298 (3): 1128–1132. [PubMed] [Google Scholar]
- 59.Kankuri E, Hämäläinen M, Hukkanen M, Salmenperä P, Kivilaakso E, Vapaatalo H, and Moilanen E. 2003. Suppression of pro-inflammatory cytokine release by selective inhibition of inducible nitric oxide synthase in mucosal explants from patients with ulcerative colitis. Scandinavian Journal of Gastroenterology 38 (2): 186–192. [DOI] [PubMed] [Google Scholar]
- 60.Demirel I, Vumma R, Mohlin C, Svensson L, Säve S, and Persson K. 2012. Nitric oxide activates IL-6 production and expression in human renal epithelial cells. American Journal of Nephrology 36 (6): 524–530. [DOI] [PubMed] [Google Scholar]
- 61.Raykova VD, Glibetic M, Ofenstein JP, and Aranda JV. 2003. Nitric oxide-dependent regulation of pro-inflammatory cytokines in group B streptococcal inflammation of rat lung. Annals of Clinical and Laboratory Science 33 (1): 62–67. [PubMed] [Google Scholar]
- 62.Holán V, Krulová M, Zajícová A, and Pindjáková J. 2002. Nitric oxide as a regulatory and effector molecule in the immune system. Molecular Immunology 38 (12–13): 989–995. [DOI] [PubMed] [Google Scholar]
- 63.Hsu CC, Lin CL, Jou IM, Wang PH, and Lee JS. 2017. The protective role of nitric oxide-dependent innate immunosuppression in the early stage of cartilage damage in rats: Role of nitric oxide in cartilage damage. Bone & Joint Research 6 (4): 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bécherel PA, Mossalayi MD, Ouaaz F, Le Goff L, Dugas B, Paul-Eugène N, Frances C, Chosidow O, Kilchherr E, Guillosson JJ, et al. 1994. Involvement of cyclic AMP and nitric oxide in immunoglobulin e-dependent activation of Fc epsilon RII/CD23+ normal human keratinocytes. The Journal of Clinical Investigation 93 (5): 2275–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chun KS, Cha HH, Shin JW, Na HK, Park KK, Chung WY, and Surh YJ. 2004. Nitric oxide induces expression of cyclooxygenase-2 in mouse skin through activation of NF-kappaB. Carcinogenesis 25 (3): 445–454. [DOI] [PubMed] [Google Scholar]
- 66.Ianaro A, O’Donnell CA, Di Rosa M, and Liew FY. 1994. A nitric oxide synthase inhibitor reduces inflammation, down-regulates inflammatory cytokines and enhances interleukin-10 production in carrageenin-induced oedema in mice. Immunology 82 (3): 370–375. [PMC free article] [PubMed] [Google Scholar]
- 67.Ialenti A, Ianaro A, Moncada S, and Di Rosa M. 1992. Modulation of acute inflammation by endogenous nitric oxide. European Journal of Pharmacology 211 (2): 177–182. [DOI] [PubMed] [Google Scholar]
- 68.Salvemini D, Wang ZQ, Wyatt PS, Bourdon DM, Marino MH, Manning PT, and Currie MG. 1996. Nitric oxide: A key mediator in the early and late phase of carrageenan-induced rat paw inflammation. British Journal of Pharmacology 118 (4): 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Medeiros MV, Binhara IM, Moreno Júnior H, Zatz R, De Nucci G, and Antunes E. 1995. Effect of chronic nitric oxide synthesis inhibition on the inflammatory responses induced by carrageenin in rats. European Journal of Pharmacology 285 (2): 109–114. [DOI] [PubMed] [Google Scholar]
- 70.Toriyabe M, Omote K, Kawamata T, and Namiki A. 2004. Contribution of interaction between nitric oxide and cyclooxygenases to the production of prostaglandins in carrageenan-induced inflammation. Anesthesiology 101 (4): 983–990. [DOI] [PubMed] [Google Scholar]
- 71.Hughes SR, Williams TJ, and Brain SD. 1990. Evidence that endogenous nitric oxide modulates oedema formation induced by substance P. European Journal of Pharmacology 191 (3): 481–484. [DOI] [PubMed] [Google Scholar]
- 72.Lippe IT, Stabentheiner A, and Holzer P. 1993. Participation of nitric oxide in the mustard oil-induced neurogenic inflammation of the rat paw skin. European Journal of Pharmacology 232 (1): 113–120. [DOI] [PubMed] [Google Scholar]
- 73.Lippe IT, Stabentheiner A, and Holzer P. 1993. Role of nitric oxide in the vasodilator but not exudative component of mustard oil-induced inflammation in rat skin. Agents Actions 38 Spec No:C22–4. [DOI] [PubMed] [Google Scholar]
- 74.Benrath J, Eschenfelder C, Zimmerman M, and Gillardon F. 1995. Calcitonin gene-related peptide, substance P and nitric oxide are involved in cutaneous inflammation following ultraviolet irradiation. European Journal of Pharmacology 293 (1): 87–96. [DOI] [PubMed] [Google Scholar]
- 75.Warren JB, Loi RK, and Coughlan ML. 1993. Involvement of nitric oxide synthase in the delayed vasodilator response to ultraviolet light irradiation of rat skin in vivo. British Journal of Pharmacology 109 (3): 802–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Y, Boettger MK, Reif A, Schmitt A, Uçeyler N, and Sommer C. 2010. Nitric oxide synthase modulates CFA-induced thermal hyperalgesia through cytokine regulation in mice. Molecular Pain 6: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mariani-Pedroso SR, Bizeto L, Antunes E, Zatz R, and de Nucci G. 1995. Dissimilarity between prostaglandin E1 and nitric oxide donors as potentiators of plasma exudation in the rabbit skin in vivo. Prostaglandins Leukotrienes and Essential Fatty Acids 52 (6): 399–402. [DOI] [PubMed] [Google Scholar]
- 78.Teixeira MM, Williams TJ, and Hellewell PG. 1993. Role of prostaglandins and nitric oxide in acute inflammatory reactions in guinea-pig skin. British Journal of Pharmacology 110 (4): 1515–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Teixeira MM, Fairbairn SM, Norman KE, Williams TJ, Rossi AG, and Hellewell PG. 1994. Studies on the mechanisms involved in the inflammatory response in a reversed passive Arthus reaction in guinea-pig skin: Contribution of neutrophils and endogenous mediators. British Journal of Pharmacology 113 (4): 1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muangman P, Tamura RN, Muffley LA, Isik FF, Scott JR, Xie C, Kegel G, Sullivan SR, Liang Z, and Gibran NS. 2009. Substance P enhances wound closure in nitric oxide synthase knockout mice. Journal of Surgical Research 153 (2): 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Most D, Efron DT, Shi HP, Tantry US, and Barbul A. 2002. Characterization of incisional wound healing in inducible nitric oxide synthase knockout mice. Surgery. 132 (5): 866–876. [DOI] [PubMed] [Google Scholar]
- 82.Ross R, Gillitzer C, Kleinz R, Schwing J, Kleinert H, Förstermann U, and Reske-Kunz AB. 1998. Involvement of NO in contact hypersensitivity. International Immunology 10 (1): 61–69. [DOI] [PubMed] [Google Scholar]
- 83.Orita K, Hiramoto K, Kobayashi H, Ishii M, Sekiyama A, and Inoue M. 2011. Inducible nitric oxide synthase (iNOS) and α-melanocyte-stimulating hormones of iNOS origin play important roles in the allergic reactions of atopic dermatitis in mice. Experimental Dermatology 20 (11): 911–914. [DOI] [PubMed] [Google Scholar]
- 84.Morita H, Hori M, and Kitano Y. 1996. Modulation of picryl chloride-induced contact hypersensitivity reaction in mice by nitric oxide. The Journal of Investigative Dermatology 107 (4): 549–552. [DOI] [PubMed] [Google Scholar]
- 85.Morita H, Semma M, Hori M, and Kitano Y. 1995. Clinical application of nitric oxide synthase inhibitor for atopic dermatitis. International Journal of Dermatology 34 (4): 294–295. [DOI] [PubMed] [Google Scholar]
- 86.Mowbray M, Tan X, Wheatley PS, Rossi AG, Morris RE, and Weller RB. 2008. Topically applied nitric oxide induces T-lymphocyte infiltration in human skin, but minimal inflammation. The Journal of Investigative Dermatology 128 (2): 352–360. [DOI] [PubMed] [Google Scholar]
- 87.Ormerod AD, Copeland P, Hay I, Husain A, and Ewen SW. 1999. The inflammatory and cytotoxic effects of a nitric oxide releasing cream on normal skin. The Journal of Investigative Dermatology 113 (3): 392–397. [DOI] [PubMed] [Google Scholar]
- 88.Sugita K, Kabashima K, Yoshiki R, Ikenouchi-Sugita A, Tsutsui M, Nakamura J, Yanagihara N, and Tokura Y. 2010. Inducible nitric oxide synthase downmodulates contact hypersensitivity by suppressing dendritic cell migration and survival. The Journal of Investigative Dermatology 130 (2): 464–471. [DOI] [PubMed] [Google Scholar]
- 89.Hyun E, Bolla M, Steinhoff M, Wallace JL, Soldato PD, and Vergnolle N. 2004. Anti-inflammatory effects of nitric oxide-releasing hydrocortisone NCX 1022, in a murine model of contact dermatitis. British Journal of Pharmacology 143 (5): 618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guttman-Yassky E, Gallo RL, Pavel AB, Nakatsuji T, Li R, Zhang N, Messersmith E, and Maeda-Chubachi T. 2020. A nitric oxide-releasing topical medication as a potential treatment option for atopic dermatitis through antimicrobial and anti-inflammatory activity. The Journal of Investigative Dermatology 140 (12): 2531–2535.e2. [DOI] [PubMed] [Google Scholar]
- 91.Kuroishi T, Bando K, Endo Y, and Sugawara S. 2013. Metal allergens induce nitric oxide production by mouse dermal fibroblasts via the hypoxia-inducible factor-2α-dependent pathway. Toxicological Sciences 135 (1): 119–128. [DOI] [PubMed] [Google Scholar]
- 92.Cassini-Vieira P, Araújo FA, da Costa Dias FL, Russo RC, Andrade SP, Teixeira MM, and Barcelos LS, 2015. iNOS activity modulates inflammation, angiogenesis, and tissue fibrosis in polyether-polyurethane synthetic implants. Mediators Inflammation 2015:138461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kuchel JM, Barnetson RS, and Halliday GM. 2003. Nitric oxide appears to be a mediator of solar-simulated ultraviolet radiation-induced immunosuppression in humans. The Journal of Investigative Dermatology 121 (3): 587–593. [DOI] [PubMed] [Google Scholar]
- 94.Rowe A, Farrell AM, and Bunker CB. 1997. Constitutive endothelial and inducible nitric oxide synthase in inflammatory dermatoses. British Journal of Dermatology 136 (1): 18–23. [PubMed] [Google Scholar]
- 95.Bruch-Gerharz D, Fehsel K, Suschek C, Michel G, Ruzicka T, and Kolb-Bachofen V. 1996. A proinflammatory activity of interleukin 8 in human skin: Expression of the inducible nitric oxide synthase in psoriatic lesions and cultured keratinocytes. Journal of Experimental Medicine 184 (5): 2007–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ormerod AD, Weller R, Copeland P, Benjamin N, Ralston SH, Grabowksi P, and Herriot R. 1998. Detection of nitric oxide and nitric oxide synthases in psoriasis. Archives of Dermatological Research 290 (1–2): 3–8. [DOI] [PubMed] [Google Scholar]
- 97.Sirsjö A, Karlsson M, Gidlöf A, Rollman O, and Törmä H. 1996. Increased expression of inducible nitric oxide synthase in psoriatic skin and cytokine-stimulated cultured keratinocytes. British Journal of Dermatology 134 (4): 643–648. [DOI] [PubMed] [Google Scholar]
- 98.Orem A, Aliyazicioglu R, Kiran E, Vanizor B, Cimnocodeit G, and Deger O. 1997. The relationship between nitric oxide production and activity of the disease in patients with psoriasis. Archives of Dermatology 133 (12): 1606–1607. [DOI] [PubMed] [Google Scholar]
- 99.Paul-Clark MJ, Gilroy DW, Willis D, Willoughby DA, and Tomlinson A. 2001. Nitric oxide synthase inhibitors have opposite effects on acute inflammation depending on their route of administration. The Journal of Immunology 166 (2): 1169–1177. [DOI] [PubMed] [Google Scholar]
- 100.Iwama D, Miyahara S, Tamura H, Miyamoto K, Hirose F, and Yoshimura N. 2008. Lack of inducible nitric oxide synthases attenuates leukocyte-endothelial cell interactions in retinal microcirculation. British Journal of Ophthalmology 92 (5): 694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sautebin L, Ialenti A, Ianaro A, and Di Rosa M. 1995. Endogenous nitric oxide increases prostaglandin biosynthesis in carrageenin rat paw oedema. European Journal of Pharmacology 286 (2): 219–222. [DOI] [PubMed] [Google Scholar]
- 102.Sautebin L, Ialenti A, Ianaro A, and Di Rosa M. 1995. Modulation by nitric oxide of prostaglandin biosynthesis in the rat. British Journal of Pharmacology 114 (2): 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paul W, Douglas GJ, Lawrence L, Khawaja AM, Perez AC, Schachter M, and Page CP. 1994. Cutaneous permeability responses to bradykinin and histamine in the guineapig: Possible differences in their mechanism of action. British Journal of Pharmacology 111 (1): 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Andrew PJ, Harant H, and Lindley IJ. 1995. Nitric oxide regulates IL-8 expression in melanoma cells at the transcriptional level. Biochemical and Biophysical Research Communications 214 (3): 949–956. [DOI] [PubMed] [Google Scholar]
- 105.Sparkman L, and Boggaram V. 2004. Nitric oxide increases IL-8 gene transcription and mRNA stability to enhance IL-8 gene expression in lung epithelial cells. American Journal of Physiology. Lung Cellular and Molecular Physiology 287 (4): L764–L773. [DOI] [PubMed] [Google Scholar]
- 106.Kobayashi Y 2010. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. Journal of Leukocyte Biology 88 (6): 1157–1162. [DOI] [PubMed] [Google Scholar]
- 107.Corriveau CC, Madara PJ, Van Dervort AL, Tropea MM, Wesley RA, and Danner RL. 1998. Effects of nitric oxide on chemotaxis and endotoxin-induced interleukin-8 production in human neutrophils. Journal of Infectious Diseases 177 (1): 116–126. [DOI] [PubMed] [Google Scholar]
- 108.Van Dervort AL, Yan L, Madara PJ, Cobb JP, Wesley RA, Corriveau CC, Tropea MM, and Danner RL. 1994. Nitric oxide regulates endotoxin-induced TNF-alpha production by human neutrophils. The Journal of Immunology 152 (8): 4102–4109. [PubMed] [Google Scholar]
- 109.Baig MS, Zaichick SV, Mao M, de Abreu AL, Bakhshi FR, Hart PC, Saqib U, Deng J, Chatterjee S, Block ML, Vogel SM, Malik AB, Consolaro ME, Christman JW, Minshall RD, Gantner BN, and Bonini MG. 2015. NOS1-derived nitric oxide promotes NF-κB transcriptional activity through inhibition of suppressor of cytokine signaling-1. Journal of Experimental Medicine 212 (10): 1725–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Niedbala W B Cai, and F.Y. Liew. 2006. Role of nitric oxide in the regulation of T cell functions. Annals of the Rheumatic Diseases 65(Suppl 3):iii37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Granger DN, and Kubes P. 1996. Nitric oxide as antiinflammatory agent. Methods in Enzymology 269: 434–442. [DOI] [PubMed] [Google Scholar]
- 112.Gaboury J, Woodman RC, Granger DN, Reinhardt P, and Kubes P. 1993. Nitric oxide prevents leukocyte adherence: Role of superoxide. American Journal of Physiology 265 (3 Pt 2): H862–H867. [DOI] [PubMed] [Google Scholar]
- 113.Kubes P, Kurose I, and Granger DN. 1994. NO donors prevent integrin-induced leukocyte adhesion but not P-select-independent rolling in postischemic venules. American Journal of Physiology 267 (3 Pt 2): H931–H937. [DOI] [PubMed] [Google Scholar]
- 114.Wallace JL, Vergnolle N, Muscará MN, Asfaha S, Chapman K, McKnight W, Del Soldato P, Morelli A, and Fiorucci S. 1999. Enhanced anti-inflammatory effects of a nitric oxide-releasing derivative of mesalamine in rats. Gastroenterology 117 (3): 557–566. [DOI] [PubMed] [Google Scholar]
- 115.Qin M, Landriscina A, Rosen JM, Wei G, Kao S, Olcott W, Agak GW, Paz KB, Bonventre J, Clendaniel A, Harper S, Adler BL, Krausz AE, Friedman JM, Nosanchuk JD, Kim J, and Friedman AJ. 2015. Nitric oxide-releasing nanoparticles prevent Propionibacterium acnes-induced inflammation by both clearing the organism and inhibiting microbial stimulation of the innate immune response. The Journal of Investigative Dermatology 135 (11): 2723–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qin M, Pirouz A, Kim MH, Krutzik SR, Garbán HJ, and Kim J. 2014. Propionibacterium acnes induces IL-1β secretion via the NLRP3 inflammasome in human monocytes. The Journal of Investigative Dermatology 134 (2): 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, and Latz E. 2009. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. The Journal of Immunology 183 (2): 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kistowska M, Gehrke S, Jankovic D, Kerl K, Fettelschoss A, Feldmeyer L, Fenini G, Kolios A, Navarini A, Ganceviciene R, Schauber J, Contassot E, and French LE. 2014. IL-1β drives inflammatory responses to propionibacterium acnes in vitro and in vivo. The Journal of Investigative Dermatology 134 (3): 677–685. [DOI] [PubMed] [Google Scholar]
- 119.Li ZJ, Choi DK, Sohn KC, Seo MS, Lee HE, Lee Y, Seo YJ, Lee YH, Shi G, Zouboulis CC, Kim CD, Lee JH, and Im M. 2014. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. The Journal of Investigative Dermatology 134 (11): 2747–2756. [DOI] [PubMed] [Google Scholar]
- 120.Hernandez-Cuellar E, Tsuchiya K, Hara H, Fang R, Sakai S, Kawamura I, Akira S, and Mitsuyama M. 2012. Cutting edge: Nitric oxide inhibits the NLRP3 inflammasome. The Journal of Immunology 189 (11): 5113–5117. [DOI] [PubMed] [Google Scholar]
- 121.Wishah K, Malur A, Raychaudhuri B, Melton AL, Kavuru MS, and Thomassen MJ. 2002. Nitric oxide blocks inflammatory cytokine secretion triggered by CD23 in monocytes from allergic, asthmatic patients and healthy controls. Annals of Allergy, Asthma & Immunology 89 (1): 78–82. [DOI] [PubMed] [Google Scholar]
- 122.Dinakar C, Malur A, Raychaudhuri B, Buhrow LT, Melton AL, Kavuru MS, and Thomassen MJ. 1999. Differential regulation of human blood monocyte and alveolar macrophage inflammatory cytokine production by nitric oxide. Annals of Allergy, Asthma & Immunology 82 (2): 217–222. [DOI] [PubMed] [Google Scholar]
- 123.Persoons JH, Schornagel K, Tilders FF, De Vente J, Berkenbosch F, and Kraal G. 1996. Alveolar macrophages autoregulate IL-1 and IL-6 production by endogenous nitric oxide. American Journal of Respiratory Cell and Molecular Biology 14 (3): 272–278. [DOI] [PubMed] [Google Scholar]
- 124.Qiu HB, Chen DC, Pan JQ, Liu DW, and Ma S. 1999. Inhibitory effects of nitric oxide and interleukin-10 on production of tumor necrosis factor alpha, interleukin-1 beta, and interleukin-6 in mouse alveolar macrophages. Zhongguo Yao Li Xue Bao 20 (3): 271–275. [PubMed] [Google Scholar]
- 125.Deakin AM, Payne AN, Whittle BJ, and Moncada S. 1995. The modulation of IL-6 and TNF-alpha release by nitric oxide following stimulation of J774 cells with LPS and IFN-gamma. Cytokine 7 (5): 408–416. [DOI] [PubMed] [Google Scholar]
- 126.Niedbala W, Alves-Filho JC, Fukada SY, Vieira SM, Mitani A, Sonego F, Mirchandani A, Nascimento DC, Cunha FQ, and Liew FY. 2011. Regulation of type 17 helper T-cell function by nitric oxide during inflammation. Proceedings of the National Academy of Sciences of the USA 108 (22): 9220–9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yu C, Fitzpatrick A, Cong D, Yao C, Yoo J, Turnbull A, Schwarze J, Norval M, Howie SEM, Weller RB, and Astier AL. 2017. Nitric oxide induces human CLA+CD25+Foxp3+ regulatory T cells with skin-homing potential. The Journal of Allergy and Clinical Immunology 140 (5): 1441–1444.e6. [DOI] [PubMed] [Google Scholar]
- 128.Allione A, Bernabei P, Bosticardo M, Ariotti S, Forni G, and Novelli F. 1999. Nitric oxide suppresses human T lymphocyte proliferation through IFN-gamma-dependent and IFN-gamma-independent induction of apoptosis. The Journal of Immunology 163 (8): 4182–4191. [PubMed] [Google Scholar]
- 129.Ialenti A, Ianaro A, Maffia P, Sautebin L, and Di Rosa M. 2000. Nitric oxide inhibits leucocyte migration in carrageenin-induced rat pleurisy. Inflammation Research 49 (8): 411–417. [DOI] [PubMed] [Google Scholar]
- 130.Dal Secco D, Moreira AP, Freitas A, Silva JS, Rossi MA, Ferreira SH, and Cunha FQ. 2006. Nitric oxide inhibits neutrophil migration by a mechanism dependent on ICAM-1: Role of soluble guanylate cyclase. Nitric Oxide 15 (1): 77–86. [DOI] [PubMed] [Google Scholar]
- 131.Vallance P, and Hingorani A. 1999. Endothelial nitric oxide in humans in health and disease. International Journal of Experimental Pathology 80 (6): 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Armstrong R 2001. The physiological role and pharmacological potential of nitric oxide in neutrophil activation. International Immunopharmacology 1 (8): 1501–1512. [DOI] [PubMed] [Google Scholar]
- 133.Coleman JW 2001. Nitric oxide in immunity and inflammation. International Immunopharmacology 1 (8): 1397–1406. [DOI] [PubMed] [Google Scholar]
- 134.Tripathi P 2007. Nitric oxide and immune response. Indian Journal of Biochemistry & Biophysics 44 (5): 310–319. [PubMed] [Google Scholar]
- 135.Coleman JW 2002. Nitric oxide: A regulator of mast cell activation and mast cell-mediated inflammation. Clinical and Experimental Immunology 129 (1): 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


