Abstract
Purpose
Patients with locally advanced esophageal squamous cell carcinoma (ESCC) scheduled for neoadjuvant radiotherapy still have a poor prognosis. This study was to explore the prognostic value of the pretreatment systemic immune-inflammation index (SII) in patients with locally advanced ESCC after neoadjuvant radiotherapy (NRT).
Materials and Methods
Eighty-two consecutive patients with ESCC scheduled for neoadjuvant radiotherapy between 2011 and 2017 were enrolled in this study. SII values (SII = platelet × neutrophil/lymphocyte), prognostic nutritional index values (PNI = albumin concentration (g/L) + 5 × total lymphocyte count (109/L)), platelet–lymphocyte ratio (PLR), and neutrophil–lymphocyte ratio (NLR) were retrospectively collected and calculated before treatment. The Cut-off Finder application was applied to find out the cut-off points of the SII, NLR, PNI and PLR. A regression model was used to examine prognostic factors for overall survival (OS) rates.
Results
The median follow-up was 44 months (3 to 83). Sixty patients (73.17%) underwent surgery as scheduled. This study found that factors improving OS were a lower SII (≤916.6 × 109/L) (P=0.040) and neoadjuvant chemoradiotherapy (NCRT) (P=0.034). The patients with a lower SII and NCRT had a better OS (P< 0.001). Moreover, additionally, a higher SII was associated with a lower resectability rate (P=0.035).
Conclusion
The SII can predict resectability in ESCC patients following neoadjuvant radiotherapy. Both the SII and neoadjuvant chemoradiotherapy appear to influence OS.
Keywords: ESCC, NCRT, SII, resectable
Introduction
Esophageal cancer is the sixth leading cause of cancer fatality and the eighth most commonly diagnosed cancer in the world.1 The esophageal cancer is mainly divided into squamous cell carcinoma and adenocarcinoma, with esophageal squamous cell carcinoma (ESCC) accounting for about 85%.2 Studies have shown that NCRT, neoadjuvant chemotherapy combined with immunotherapy/chemoradiotherapy (CRT), and neoadjuvant chemotherapy (NCT) are the main treatments which improve the survival rate of patients with resectable locally advanced ESCC.3 The use of chemoradiotherapy as preoperative treatments has been validated by several recent clinical trials,4–6 and the prognosis of preoperative chemoradiotherapy is better than that of surgery alone. However, the prognosis is still poor.7 At present, there are few researches to compare the prognosis of patients with NCT and those with NCRT. Consequently, it is essential to investigate the effect of NCT/NCRT on the prognosis of patients with locally advanced ESCC and to determine personalized clinical care.
Over the years, there has been much evidence that patients’ neutrophils, lymphocytes, platelets and other inflammatory indicators, as well as nutritional indicators, are closely related to the prognosis of several solid tumours.8–11 These peripheral venous blood parameters, such as PLR, SII, NLR and PNI, have some effect on the prognosis prediction of ESCC.12–14 The SII, which depends on neutrophil counts, platelet counts and lymphocyte counts, has been one of the independent factors for prognosis prediction of ESCC in recent years.15 At present, the mechanism of how SII affects the prognosis of esophageal cancer is still unclear. Some studies suggest that neutrophils may activate endothelial cells and parenchymal cells, enhancing the adhesion of circulating tumor cells and promoting distant metastasis.16 Lymphocytes typically play a pivotal role in anti-tumor immunity,17 while tumor cell-activated platelets, in turn, induce the opening of the endothelial barrier, promoting the metastasis and dissemination of tumor cells.18 According to retrospective studies, NLR, PLR, and SII have certain predictive value for the efficacy of neoadjuvant chemoradiotherapy in patients with locally advanced colorectal cancer.19
However, few studies regarding the SII in locally advanced ESCC patients who underwent NCRT are available, and its role is still controversial. Consequently, the aim was to investigate what the predictive value of the SII, PNI, PLR and NLR in locally advanced ESCC patients who underwent NRT/NCRT.
Patients and Methods
Patients
Eighty-two consecutive patients with ESCC treated with NRT at the Fujian Provincial Cancer Hospital in China from April 2011 to November 2017 were assessed. The inclusion criteria were as follows: (1) diagnosed with ESCC by endoscopic biopsy; (2) underwent NRT and intended to undergo follow-up surgery; (3) no distant metastasis; and (4) No pathological confirmation of cancer other than esophageal cancer. Patients with unresectable T4 or a significant medical disease or those who did not complete NRT were excluded. All patients were diagnosed by clinical examination, endoscopic biopsy, oesophagus barium X, computerized tomography of the thorax and abdomen, and bone scintigraphy and restaged according to the 8th edition of the American Joint Committee on Cancer (AJCC) tumour-node-metastasis (TNM) classification system (15). All patients’ serological tests were obtained within 7 days of neoadjuvant therapy. The Ethics Committee of Fujian Cancer Hospital gave consent (Ethics number: K2023-165-01), and all participating patients signed informed consent forms. The study complies with the Declaration of Helsinki.
Treatments
These patients were retrospectively divided into the NRT group, with a total radiotherapy dose of 32–45 Gy (1.8–2.15 Gy/fraction) only (11 cases) and the NCRT group with a radiotherapy dose of 32–45 Gy (1.8–2.15 Gy/fraction) combined with chemotherapy (71 cases). Of the 82 patients, 36 were treated with intensity-modulated radiotherapy (IMRT), and 46 were treated with two-dimensional radiotherapy (2DRT). Most of the regimens in patients with chemotherapy were paclitaxel concomitant with nedaplatin (39 cases), cisplatin (9 cases), or carboplatin (19 cases). The rest of them received 5-fluorouracil combined with cisplatin (4 cases). Finally, four to six weeks after neoadjuvant therapy, sixty (73.17%) patients underwent surgical resection as scheduled, and twenty-two (26.83%) were not completed due to progression or physical intolerance, A total of 2 patients underwent esophageal cancer two-field radical surgery, while 58 patients underwent esophageal cancer three-field radical surgery. Of the patients who did not undergo surgery, 20 underwent a total radiotherapy dose of 20–30 Gy (2.0–2.1 Gy per fraction) combined with chemotherapy (paclitaxel concomitant with nedaplatin (16 cases) or cisplatin (4 cases)), 1 received a total radiotherapy dose of 20–30 Gy (2.0–2.1 Gy per fraction) only, and 1 did not receive any other treatment.
Follow-Up
Within 2 years of treatment, the patients were hospitalized in our hospital or the clinic and were followed up 1 time every 3 months and every 6 months thereafter. During follow-up, the main means of examination were endoscopy, oesophagus barium X, and computerized tomography of the thorax and abdomen. All patients completed the follow-up until November 2018 or death. OS was defined as the time from diagnosis to death due to any cause or last follow-up of the patient. The cut-off point of inflammatory indicators and nutritional indicators affecting survival was determined by the Cut-off Finder application.
Statistical Analyses
The primary endpoint was OS. SPSS software version 21.0 was used for this study analysis. The chi-square test or Fisher’s exact test was used to compare categorical variables. Spearman correlation analyses were used to analyse the links between PNI and NLR, SII, and PLR. The Kaplan–Meier method was curved to plot the OS, and the Log rank test was used to analyse the differences. P values below 0.05 were considered statistically significant. To analyze the predictive value of high and low SII levels on the OS of ESCC patients using receiver operating characteristic (ROC) curves. The Cox proportional hazards model was used to perform the univariate and multivariate analyses. Multivariate analysis was carried out when univariate analysis was significant (P < 0.05).
Results
Patient Characteristics and Treatment Outcomes
Eighty-two patients were evaluated in our study. The patient characteristics are shown in Table 1. The median age was 59 years (38 to 76 years). There were 71 (86.6%) male and 11 (13.4%) female patients. There were 9 (11.0%) cases with stage II disease, 48 (58.5%) cases with stage III disease, 24 (29.3%) cases with stage IV disease, and one (1.2%) case with unknown stage. Of these cases, 21 (25.6%) were in the upper thoracic segment, while the remaining 61 (64.4%) were in the middle thoracic or lower thoracic segments. Regarding the primary tumour length, 46 (56.1%) were ≤7 cm, and 35 (42.6%) were >7 cm. Thirty (36.6%) had a smoking history, and 11 (13.4%) had an alcohol consumption history.
Table 1.
Patient Characteristics
| Characteristic | N(%) |
|---|---|
| Age(years) | |
| ≤60 | 47(57.3) |
| >60 | 35(42.7) |
| Sex | |
| Male | 71(86.6) |
| Female | 11(13.4) |
| T stage | |
| T2+T3 | 53(64.6) |
| T4 | 28(34.1) |
| Unknown | 1(1.3) |
| Lymph node metastasis | |
| Negative | 12(14.6) |
| Positive | 69(84.1) |
| Unknown | 1(1.3) |
| Primary tumour location | |
| Upper thoracic | 21(25.6) |
| Middle thoracic, lower thoracic | 61(64.4) |
| Smoking | |
| Yes | 30(36.6) |
| No | 52(63.4) |
| Alcohol consumption | |
| Yes | 71(86.6) |
| No | 11(13.4) |
| Primary tumour length | |
| ≤7 cm | 46(56.1) |
| >7 cm | 35(42.6) |
| Unknown | 1(1.3) |
| Chemoradiotherapy | |
| Yes | 71(86.6) |
| No | 11(13.4) |
| Chemotherapeutic regimens | |
| Paclitaxel concomitant with nedaplatin | 39(47.6) |
| Paclitaxel concomitant with cisplatin | 9(10.9) |
| Paclitaxel concomitant with carboplatin | 19(23.2) |
| 5-fluorouracil combined with cisplatin | 4(4.9) |
| No | 11(13.4) |
| Radiotherapy mode | |
| IMRT | 36(43.9) |
| 2DRT | 46(56.1) |
| Completed the operation as scheduled | |
| Yes | 60(73.2) |
| No | 22(26.8) |
| Operative method | |
| Esophageal cancer three-field radical surgery | 58(70.7) |
| Esophageal cancer two-field radical surgery | 2(2.5) |
| No | 22(26.8) |
| NLR | |
| ≤3.525 | 65(79.3) |
| >3.525 | 17(20.7) |
| PLR | |
| ≤209.6 | 68(82.9) |
| >209.6 | 14(17.1) |
| SII | |
| ≤916.6 | 62(75.6) |
| >916.6 | 20(24.4) |
| PNI | |
| ≤45.25 | 21(25.6) |
| >45.25 | 61(74.4) |
Abbreviations: IMRT, intensity-modulated radiotherapy; 2DRT, two-dimensional radiotherapy; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index; PNI, prognostic nutritional index.
Selection of the Optimal Cut-Off Point
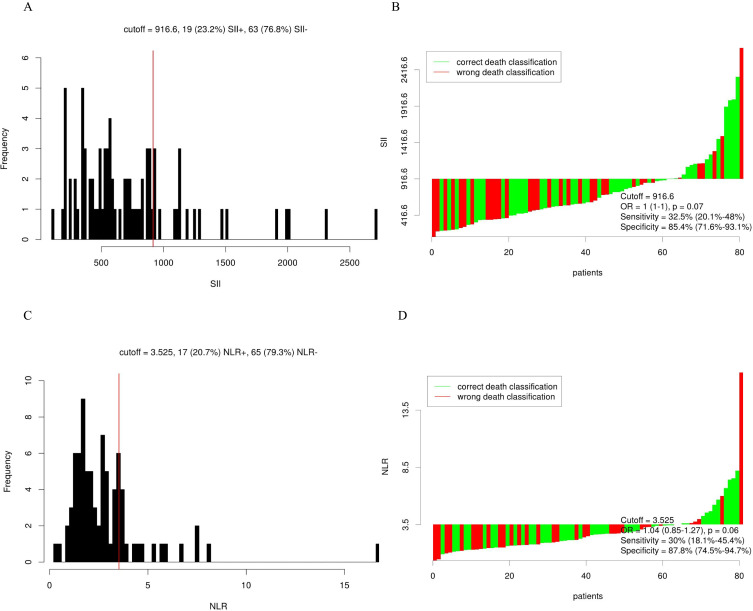
According to Cut-off Finder software, the optimal cut-off values of inflammatory biomarkers and nutritional indicators were as follows (Table 1 and Figure 1): SII = platelet count × neutrophil/lymphocyte (P × N/L) and the patients were divided into a low SII group (≤916.6 × 109/L) and a high SII group (>916.6 × 109/L). PNI = albumin concentration (g/L)+ five times the total lymphocyte absolute count (109/L), and the patients were divided into a low PNI (≤45.25) group and a high PNI (>45.25) group. Homologous, NLR = neutrophil/lymphocyte ratio (N/L), and the patients were divided into a low NLR group (≤3.525) and a high NLR group (>3.525). Lastly, PLR = platelet–lymphocyte ratio (P/L), and the patients were divided into a low PLR group (≤209.6) and a high PLR group (>209.6).
Figure 1.
Distribution-based cut-off optimization of SII and NLR in ESCC patients. (A) Histograms of SII expression values. (B) Waterfall plot of optimal dichotomization for SII expression values. (C) Histograms of NLR expression values. (D) Waterfall plot of optimal dichotomization for NLR expression values.
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; SII, systemic immune-inflammation index; ESCC, esophageal squamous cell carcinoma.
Correlation Between Baseline SII, NLR and Clinicopathological Characteristics and Resectability Rate
Table 2 shows the correlation between clinicopathological characteristics and SII and NLR within 7 days prior to treatment. The SII was significantly associated with T stage (p=0.006), alcohol consumption history (p=0.021), and primary tumour length (p=0.023). Patients with a lower SII were more likely to have more early T stage, an alcohol consumption history, and a larger primary tumour length. Furthermore, patients with a low SII were more likely to have a high resectability rate (p=0.035). Nevertheless, the NLR seemed to have no significant correlation with clinicopathologic features and resectability rate in our study (P=0.217, Table 2).
Table 2.
Correlations Between the SII, NLR, and Clinicopathological Variables in Patients with ESCC
| Parameter | SII | NLR | ||||
|---|---|---|---|---|---|---|
| Low (n) | High (n) | P | Low (n) | High (n) | P | |
| Age | 0.072 | 0.337 | ||||
| ≤60 | 39 | 8 | 36 | 9 | ||
| >60 | 23 | 12 | 26 | 8 | ||
| Sex | 0.279 | 0.109 | ||||
| Male | 52 | 19 | 54 | 17 | ||
| Female | 10 | 1 | 19 | 0 | ||
| T stage | 0.006 | 0.073 | ||||
| T2+T3 | 45 | 8 | 45 | 8 | ||
| T4 | 16 | 12 | 19 | 9 | ||
| Lymph node metastasis | 0.72 | 0.295 | ||||
| Negative | 10 | 2 | 11 | 1 | ||
| Positive | 51 | 18 | 53 | 16 | ||
| Primary tumour location | 0.211 | 1.000 | ||||
| Upper thoracic | 18 | 3 | 17 | 4 | ||
| Middle thoracic, lower thoracic | 44 | 17 | 48 | 13 | ||
| Smoking | 0.715 | 0.901 | ||||
| Yes | 22 | 8 | 24 | 6 | ||
| No | 40 | 12 | 41 | 11 | ||
| Alcohol consumption | 0.021 | 0.690 | ||||
| Yes | 5 | 6 | 8 | 3 | ||
| No | 57 | 14 | 57 | 14 | ||
| Primary tumour length | 0.023 | 0.719 | ||||
| ≤7 | 39 | 7 | 37 | 9 | ||
| >7 | 22 | 13 | 27 | 8 | ||
| Chemoradiotherapy | 0.126 | 0.227 | ||||
| Yes | 56 | 15 | 7 | 4 | ||
| No | 6 | 5 | 58 | 13 | ||
| Completed the operation as scheduled | 0.035 | 0.217 | ||||
| Yes | 13 | 9 | 15 | 7 | ||
| No | 49 | 11 | 50 | 10 | ||
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; SII, systemic immune-inflammation index; ESCC, esophageal squamous cell carcinoma.
Predictors of OS in the Univariate and Multivariate Analyses
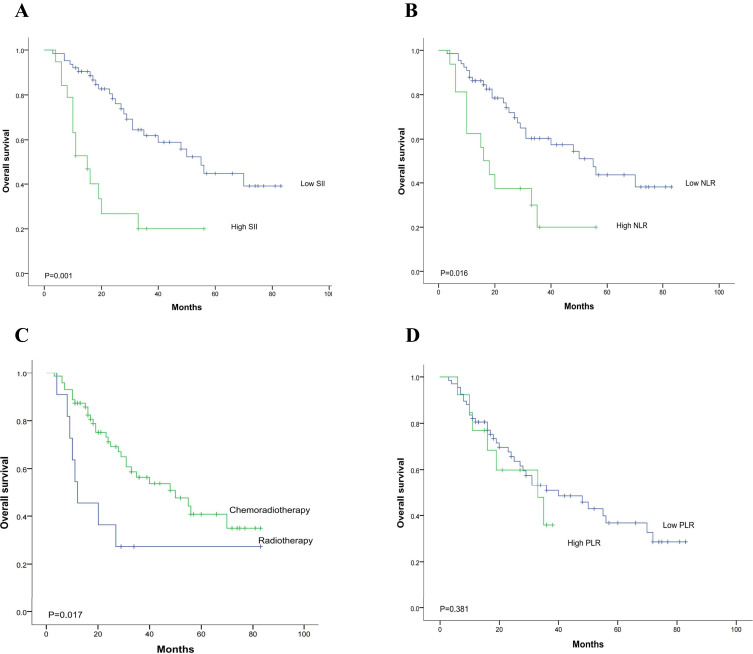
Among the 82 patients, the 1-year OS rates were 81.7%, 3-years OS rates were 52.6%, and the 5-years OS rates were 38.8%, and the median survival time was 40 months (range, 3 to 83 months). Kaplan–Meier survival analysis Results shown in Figure 2, and the univariate analysis results shown in Table 3. The results indicated that high SII, high NLR, and NRT with radiotherapy only had a significant correlation with poor prognosis, with unadjusted hazard ratios (HRs) of 2.923 (95% CI, 1.468–5.819), 2.338 (95% CI, 1.144–4.776), and 0.398 (95% CI, 0.181–0.873), respectively. In the matched 82-patient survival analysis, the median OS was 55 months for ESCC patients who has an SII ≤916.6 and 16 months for ESCC patients with an SII >916.6. The 3-year OS rate of patients was 60.5% in the low SII group versus 27.5% in the high SII group (p = 0.001). Analogously, the median OS was 55 months for ESCC patients with an NLR ≤3.525 and 20 months for ESCC patients with an NLR >3.525. The 3-year OS rate of patients was 59.6% in the low NLR group versus 23.3% in the high NLR group (p = 0.016). In addition, ESCC patients in the NCRT group had a markedly higher 3-year OS rate than those in the NRT group (57.0% vs 27.3%; p = 0.017). Although not reaching statistical significance, the low PLR group also showed a trend towards improved OS rates compared with that of the high PLR group.
Figure 2.
Kaplan–Meier survival curves for the 82 ESCC patients stratified based on (A) SII, (B) NLR, (C) chemoradiotherapy, and (D) PLR. (A) The 3-year OS rate of patients in the low SII group was significantly better than that of patients in the high SII group (60.5% vs 27.5%; p =0.001). (B) The 3-year OS rate of patients in the low NLR group was significantly better than that of patients in the high NLR group (59.6% vs 23.3%; p =0.016). (C) The 3-year OS rate of patients in the NCRT group was significantly better than that of patients in the NRT group (57.0% vs 27.3%; p =0.017). (D) The 3-year OS rate of patients in the low PLR group versus the high PLR group (55.9% vs 36.7%, p =0.381).
Abbreviation: PLR, platelet-to-lymphocyte ratio.
Table 3.
Univariate and Multivariate Cox Proportional Hazards Regression Models for Overall Survival in Patients with ESCC
| Characteristic | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | P | HR | (95% CI) | P | |
| Age | ||||||
| ≤60/>60 | 1.070 | 0.554–2.068 | 0.839 | |||
| Gender | ||||||
| Male/Female | 0.711 | 0.218–2.322 | 0.568 | |||
| T stage | ||||||
| T2-3/T4 | 0.937 | 0.478–1.836 | 0.848 | |||
| Lymph node metastasis | ||||||
| Negative/Positive | 1.222 | 0.476–3.136 | 0.675 | |||
| Primary tumour location | ||||||
| Upper thoracic/middle +lower thoracic | 1.612 | 0.737–3.527 | 0.225 | |||
| Smoking | ||||||
| Yes/No | 0.966 | 0.485–1.924 | 0.922 | |||
| Alcohol consumption | ||||||
| Yes/No | 1.855 | 0.815–4.220 | 0.133 | |||
| Primary tumour length | ||||||
| ≤7 cm/>7 cm | 1.515 | 0.801–2.866 | 0.195 | |||
| NLR | ||||||
| ≤3.525/>3.525 | 2.338 | 1.144–4.776 | 0.016 | 1.107 | 0.421–2.908 | 0.837 |
| PLR | ||||||
| ≤209.6/>209.6 | 1.448 | 0.626–3.350 | 0.381 | |||
| SII | ||||||
| ≤916.6/>916.6 | 2.923 | 1.468–5.819 | 0.001 | 2.665 | 1.045–6.794 | 0.040 |
| PNI | ||||||
| ≤45.25/>45.25 | 1.605 | 0.733–3.511 | 0.381 | |||
| Chemoradiotherapy | ||||||
| Yes/No | 0.398 | 0.181–0.873 | 0.017 | 0.423 | 0.190–0.937 | 0.034 |
| Radiotherapy mode | ||||||
| IMRT/2DRT | 1.152 | 0.585–2.266 | 0.681 | |||
Abbreviations: IMRT, intensity-modulated radiotherapy; 2DRT, two-dimensional radiotherapy; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index; PNI, prognostic nutritional index; ESCC, esophageal squamous cell carcinoma.
Meanwhile, the multivariate analyses showed that the SII (HR; 2.665, 95% CI, 1.045–6.794; p = 0.040) and NCRT (HR; 2.665, 95% CI, 0.190–0.937; p = 0.034) were independent prognostic factors for OS, as seen in Table 3.
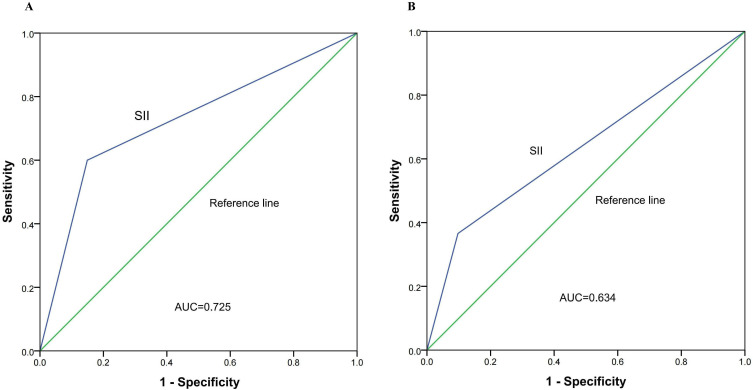
The ROC Curve of SII Predicts Overall Survival
ROC curves (AUC) were created to assess the predictive efficiency of the two groups of pre-treatment SII values for 1-year OS and 3-year OS in ESCC patients. Figure 3A and B represent the ROC curves for 1-year OS and 3-year OS, respectively. The AUC for SII was 0.725 (p = 0.007; 95% CI 0.568–0.883) and 0.634 (p = 0.037, 95% CI 0.513–0.755), respectively.
Figure 3.
Predictive ability of the SII by ROC curves in 1 year (A) and 3 years (B).
Abbreviation: ROC, receiver operating characteristic.
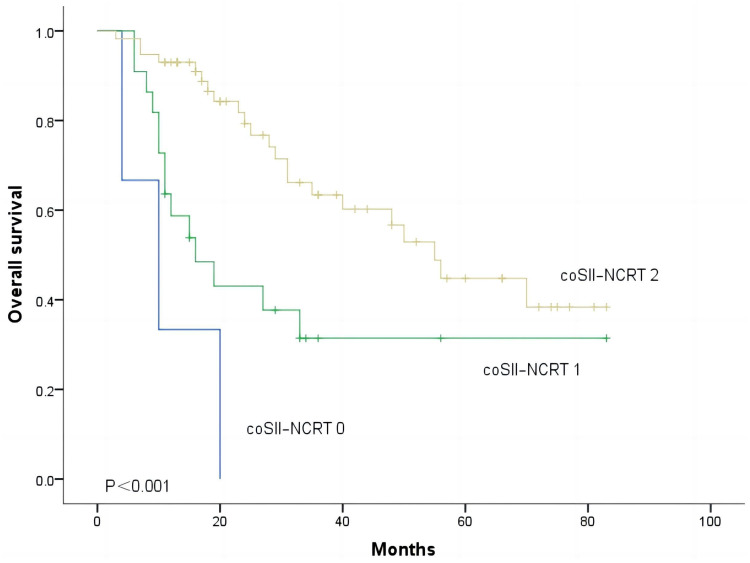
Correlation Between SII and NCRT on Survival
In the end, we sought to predict OS in coSII–NCRT ESCC patients. The coSII–NCRT 0 group was defined as the combination of patients in the high SII and the NRT group, the coSII–NCRT 1 group was defined as the combination of patients in the high SII and the NCRT group or the low SII and the NRT group, and the coSII–NCRT 2 group was defined as the combination of patients in the low SII and the NCRT group. The 1-year OS rate of patients in the coSII–NCRT 2 group was significantly better than that in the coSII–NCRT 0 group and the coSII–NCRT 1 group (92.9% vs 66.3% vs 20%), and the 3-year OS rates of patients in these three groups were 61.9%, 41.3%, and 0.0%, respectively. As Figure 4 shows, according to coSII–NCRT, patients who underwent NRT can be divided into three groups with different prognoses (p<0.001).
Figure 4.
Kaplan–Meier survival curves for ESCC patients according to the combination of SII and NCRT.
Abbreviation: coSII–NCRT, combination of SII and NCRT.
Discussion
In our study, we analysed the prognostic values of different peripheral venous blood parameters, NLR, PNI, ALI and SII, in newly diagnosed locally advanced ESCC after NRT/NCRT. We found that the SII was associated with alcohol consumption history, T stage, and primary tumour length. Only the SII and NCRT were found to be independent risk factors for OS in locally advanced ESCC. Moreover, as far as we know, this is the first time to demonstrate that a preoperative low SII (≤916.6) and treatment with NCRT were independent prognostic predictors for OS in patients with locally advanced ESCC who were ready for surgery.
An increasing number of pretreatment peripheral blood indicators have been used as relevant biomarkers to predict the prognosis of solid tumour patients. Previous studies have shown that a lower SII can lead to a better prognosis in tumour patients and is an independent influencing factor for better prognosis in solid tumours for instance classical Hodgkin lymphoma, non-small cell lung cancer, and cervical cancer.10,20,21 In addition, a meta-analysis specifically reviewed 10 studies, including 7087 patients with bladder cancer, and found that the SII was related to poor prognosis. It was also found that a high SII before treatment was related to poor tumour differentiation, high tumour stage, lymph node involvement, tumour size ≥3 cm and other factors.22 Recently, Chang et al23 also studied esophageal cancer patients’ treatment with immune checkpoint inhibitors and revealed that high pretreatment SII values can affect patients’ PFS and OS. These results are consistent with the results in this study. However, what sets this study apart from previous studies is the inclusion of locally advanced ESCC patients who received NRT for the first time and were initially operable. Moreover, through univariate analysis. It also found that patients with a low SII were more seem to have a high reserve rate (p=0.035). Perhaps, patients with a high SII value before treatment could have a lower surgical rate after subsequent neoadjuvant radiation therapy. When the initial treatment plan is formulated, radical radiotherapy and chemotherapy should be directly performed, which will improve the survival of patients to a certain extent. Further large-scale research is needed exploring multiple factor correlation analysis.
Currently, the mechanism by which SII affects the prognosis of solid tumour patients is still being explored. To our knowledge, the SII is an inflammatory indicator included of three types of inflammatory cells: platelets, neutrophils, and lymphocytes. Among them, platelets act as protective “clones”, which can protect circulating tumour cells from immune damage. In addition, they can lead to epithelial mesenchymal transformation and promote tumour cell metastasis to distant areas.24,25 Platelets can release vascular endothelial growth factor, which can promote the formation of tumour neovascularization, thereby promoting the occurrence and development of tumours.26 Neutrophils can also increase tumour activity by secreting various inflammatory mediators, coving interleukin (IL)-22, IL-10, and IL-6.27 This result was also verified in an in vitro study of breast cancer.28 Lymphocytes have an important position in immune monitoring and defence.29,30 Based on the above, a high SII value may promote the occurrence and development of tumours, evade immune surveillance, and ultimately lead to poor prognosis in tumour patients.
At present, there is still insufficient evidence to suggest that surgically treating locally advanced esophageal cancer with NCRT is more effective than NRT. In this study, it was shown that receiving synchronous radiotherapy and chemotherapy is an independent influencing factor for surgically resectable patients, and Kaplan‒Meier survival curve validation showed that patients with higher SII values combined with only receiving NRT have a significantly worse prognosis than those with lower SII values and those who receive NCRT. This may be related to the fact that effective chemotherapy drugs can inhibit the accelerated reproliferation of residual tumour cells during radiotherapy.31 The results are similar to previous studies, showing that patients with locally advanced esophageal cancer who received neoadjuvant chemoradiotherapy and had a high SII value before treatment had poor prognosis.32 In addition, another study showed that a higher neutrophil-to-lymphocyte ratio during radiotherapy may be an important adverse prognostic factor for cervical cancer,33 and it was also found that a low lymphocyte value before radiotherapy is associated with a high distant metastasis rate. Wang et al34 found that patients with rectal cancer who received neoadjuvant chemoradiotherapy had worse prognosis if they had higher platelet levels before treatment. Sun et al35 also found that in rectal cancer patients receiving neoadjuvant chemoradiotherapy, a low SII value before treatment is associated with good treatment response to chemoradiotherapy.
Conclusion
The SII is a significant predictor of the prognosis of patients with resectable LA-ESCC who receiving NRT. Patients with low SII values receiving NCRT may improve their prognosis. In summary, research on prognostic factors for LA-ESCC remains a key focus in the field of esophageal cancer, and continuous exploration and discovery are needed. In clinical work, better personalized diagnosis and treatment plans should be developed for patients based on their individual situations to obtain a better prognosis.
Acknowledgments
No assistance in the preparation of this article is to be declared.
Funding Statement
This study was supported by the Startup Fund for scientific research, Fujian Medical University (Grant number:2023QH1171); Joint Funds for the Innovation of Science and Technology, Fujian Province (Grant number:2021Y9227and 2023Y9412); Fujian ProvincialClinical Research Center for Cancer Radiotherapy and Immunotherapy (Grant number:2020Y2012); Natural Science Foundation of Fujian Province (Grant number:2023J011254); the Science Foundation for The Excellent Youth Scholars of Fujian Provincial Health Commission (Grant number:2022ZQNZD009); the Special Research Funds for Local Science and Technology Development Guided by Central Government(Grant number:2023L3020).
Data Sharing Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Disclosure
The authors declare that there is no conflict of interest in this work.
References
- 1.Erratum. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca a Cancer J Clinicians. 2020;70:313. [DOI] [PubMed] [Google Scholar]
- 2.Morgan E, Soerjomataram I, Rumgay H, et al. the global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 2022;163:649–658.e2. doi: 10.1053/j.gastro.2022.05.054 [DOI] [PubMed] [Google Scholar]
- 3.Leng X, Daiko H, Han Y, Mao Y. Optimal preoperative neoadjuvant therapy for resectable locally advanced esophageal squamous cell carcinoma. Ann NY Acad Sci. 2020;1482:213–224. doi: 10.1111/nyas.14508 [DOI] [PubMed] [Google Scholar]
- 4.Klevebro F, Johnsen G, Johnson E, et al. Morbidity and mortality after surgery for cancer of the oesophagus and gastro-oesophageal junction: a randomized clinical trial of neoadjuvant chemotherapy vs. neoadjuvant chemoradiation. European j Surg Oncol. 2015;41:920–926. doi: 10.1016/j.ejso.2015.03.226 [DOI] [PubMed] [Google Scholar]
- 5.Stahl M, Walz M, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851–856. doi: 10.1200/JCO.2008.17.0506 [DOI] [PubMed] [Google Scholar]
- 6.Burmeister B, Thomas J, Burmeister E, et al. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised Phase II trial. Eur J Canc. 2011;47:354–360. doi: 10.1016/j.ejca.2010.09.009 [DOI] [PubMed] [Google Scholar]
- 7.Tang H, Wang H, Fang Y, et al. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy followed by minimally invasive esophagectomy for locally advanced esophageal squamous cell carcinoma: a prospective multicenter randomized clinical trial. Annals of Oncology. 2023;34:163–172. doi: 10.1016/j.annonc.2022.10.508 [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, Du J, Zhong X, et al. The prognostic value of the systemic immune-inflammation index for patients with bladder cancer after radical cystectomy. Front Immunol. 2022;13:1072433. doi: 10.3389/fimmu.2022.1072433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka S, Uchino J, Yokoi T, et al. Prognostic nutritional index and lung immune prognostic index as prognostic predictors for combination therapies of immune checkpoint inhibitors and cytotoxic anticancer chemotherapy for patients with advanced non-small cell lung cancer. Diagnostics. 2022; 12. doi: 10.3390/diagnostics13010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang H, Liu Q, Zhu L, et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep. 2019;9:3284. doi: 10.1038/s41598-019-39150-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polk N, Budai B, Hitre E, Patócs A, Mersich T. Corrigendum: High Neutrophil-to-Lymphocyte Ratio (NLR) and systemic immune-inflammation index (SII) are markers of longer survival after metastasectomy of patients with liver-only metastasis of rectal cancer. Pathol Oncol Res. 2022;28:1610658. doi: 10.3389/pore.2022.1610658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Y, Chen J, Yao X, et al. Patterns and prognostic predictive value of perineural invasion in esophageal squamous cell carcinoma. BMC Cancer. 2022;22:1287. doi: 10.1186/s12885-022-10386-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu X, Han R, Zhong Y, Weng N, Zhang A. Correction to: post treatment NLR is a predictor of response to immune checkpoint inhibitor therapy in patients with esophageal squamous cell carcinoma. Can Cell Inter. 2021;21:693. doi: 10.1186/s12935-021-02401-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao F, Wang L, Zhang W, Wang L, Zhao L. Preoperative Prognostic Nutritional Index is a Significant Predictor of Survival in Esophageal Squamous Cell Carcinoma Patients. Nutr Canc. 2021;73:215–220. doi: 10.1080/01635581.2020.1757129 [DOI] [PubMed] [Google Scholar]
- 15.Feng J, Chen S, Yang X. Systemic immune-inflammation index (SII) is a useful prognostic indicator for patients with squamous cell carcinoma of the esophagus. Medicine. 2017;96:e5886. doi: 10.1097/MD.0000000000005886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph E DL, Beverly RKW, Leo TF. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res. 2004;10:10. [DOI] [PubMed] [Google Scholar]
- 17.Joseph CYC, David LC, Connie I D, et al. The lymphocyte-to-monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resectable colorectal cancer. Ann Surg. 2016;265:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben ZS, Mark LK. Platelets and tumor cells: a new form of border control. Cancer Cell. 2013;24:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Liu X, Xu M, Chen K, Li S, Guan G. Prognostic value of pretreatment systemic inflammatory markers in patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy. Sci Rep. 2020;10:8017. doi: 10.1038/s41598-020-64684-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirili C, Paydas S, Kapukaya T, Yılmaz A. Systemic immune-inflammation index predicting survival outcome in patients with classical Hodgkin lymphoma. Biomarkers Med. 2019;13:1565–1575. doi: 10.2217/bmm-2019-0303 [DOI] [PubMed] [Google Scholar]
- 21.Coutu B, Johnson K, Bhirud A, et al. Systemic Immune-Inflammatory Index Association with Survival in Patients Undergoing Trimodality Therapy for Lung Cancer. Oncology. 2022;100:247–256. doi: 10.1159/000520989 [DOI] [PubMed] [Google Scholar]
- 22.Li J, Cao D, Huang Y, et al. The Prognostic and Clinicopathological Significance of Systemic Immune-Inflammation Index in Bladder Cancer. Front Immunol. 2022;13:865643. doi: 10.3389/fimmu.2022.865643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang L, Cheng Q, Ma Y, et al. Prognostic Effect of the Controlling Nutritional Status Score in Patients With Esophageal Cancer Treated With Immune Checkpoint Inhibitor. J Immunother. 2022;45:415–422. doi: 10.1097/CJI.0000000000000438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanger BZ, Kahn ML. Platelets and tumor cells: a new form of border control. Cancer Cell. 2013;24:9–11. doi: 10.1016/j.ccr.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma C, Fu Q, Diggs L, et al. Platelets control liver tumor growth through P2Y12-dependent CD40L release in NAFLD. Canc Cell. 2022;40:986–998.e5. doi: 10.1016/j.ccell.2022.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franco A, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126:582–588. doi: 10.1182/blood-2014-08-531582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan K, Chong S, Wong F, et al. Neutrophils contribute to inflammatory lymphangiogenesis by increasing VEGF-A bioavailability and secreting VEGF-D. Blood. 2013;122:3666–3677. doi: 10.1182/blood-2012-11-466532 [DOI] [PubMed] [Google Scholar]
- 28.Park J, Wysocki R, Amoozgar Z, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci trans med. 2016;8:361ra138. doi: 10.1126/scitranslmed.aag1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 30.Chen L, Zhang F, Sheng X, Zhang S. Decreased pretreatment lymphocyte/monocyte ratio is associated with poor prognosis in stage Ib1-IIa cervical cancer patients who undergo radical surgery. Onco Targets Ther. 2015;8:1355–1362. doi: 10.2147/OTT.S82174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maquilan G, Grover S, Xanthopoulos E, et al. Analysis of the relationship between response to chemotherapy and response to radiation therapy in patients with non-small cell lung cancer receiving sequential treatment. American j Clin Oncol. 2018;41:391–395. doi: 10.1097/COC.0000000000000288 [DOI] [PubMed] [Google Scholar]
- 32.Wei-Xiang Q, Xiaoyan W, Chengqiang L, et al. Pretreatment absolute lymphocyte count is an independent predictor for survival outcomes for esophageal squamous cell carcinoma patients treated with neoadjuvant chemoradiotherapy and pembrolizumab: an analysis from a prospective cohort. Thorac Canc. 2023;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao M, Gao Z, Gu X, Yang X, Wang S, Fu J. Predictive significance of lymphocyte level and neutrophil-to-lymphocyte ratio values during radiotherapy in cervical cancer treatment. Canc Med. 2023;12:15820–15830. doi: 10.1002/cam4.6221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang P, Wang Z, Liu Y, Xie J, Ren Y. Prognostic value of platelet-associated biomarkers in rectal cancer patients received neoadjuvant chemoradiation: a retrospective study. Canc Radiotherap. 2021;25:147–154. doi: 10.1016/j.canrad.2020.06.030 [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Huang Z, Chi P. An inflammation index-based prediction of treatment response to neoadjuvant chemoradiotherapy for rectal mucinous adenocarcinoma. Int J Clin Oncol. 2020;25:1299–1307. doi: 10.1007/s10147-020-01670-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.