Abstract
Introduction
Venous thromboembolism (VTE) is associated with significant morbidity and mortality in cancer patients. Our study compares the mortality in hospitalized VTE patients among the four most common gastrointestinal (GI) malignancies which include esophageal, gastric, pancreatic, and colorectal cancer.
Methods
A retrospective study was conducted utilizing the Nationwide Inpatient Sample database (NIS) from 2016 to 2019. Patients with VTE were identified using ICD-10 codes from all primary discharge diagnoses. Only deep venous thrombosis (DVT) and pulmonary embolism (PE) were considered. Patients with VTE were further divided into groups: esophageal cancer, gastric cancer, pancreatic cancer, and colorectal cancer, and compared with patients who did not have these malignancies. The adjusted odds ratio (aOR) was calculated using multivariate regression analysis.
Results
Among 999,559 patients discharged with a VTE diagnosis, 25,775 (2.6%) had one of the four GI malignancies. Among patients with one of the four included GI malignancy diagnosis, 18,816 (73%) patients had PE and 6,959 (27%) patients had DVT. The study shows that adults admitted to the hospitals for VTE have higher mortality when compared to patients who did not have GI malignancies, with esophageal cancer having the highest inpatient mortality with an aOR of 2.701; 95% confidence interval (CI) 1.989–3.669, p value <0.000. For the remaining GI cancers, gastric cancer had an aOR 1.576; CI: 1.094–2.269, p value 0.015, and pancreatic cancer had an aOR of 1.736; 95% CI: 1.445–2.085, p value <0.000. Patients with colorectal cancer had no significant increase in the odds of mortality with aOR of 1.213; 95% CI: 0.988–1.489, p value 0.066.
Conclusions
This study demonstrates that VTE in hospitalized patients with esophageal cancer is associated with greater mortality compared to other GI malignancies.
Keywords: Gastrointestinal malignancies, Esophageal cancer, Deep vein thrombosis, Pulmonary embolism, Venous thromboembolism
Introduction
Venous thromboembolism (VTE) including pulmonary embolism (PE) and deep venous thrombosis (DVT) affects around 600,000 Americans each year with an estimated mortality of 10–30% within the first month of diagnosis [1]. The mortality rate dramatically increases in cancer patients and is considered the second leading cause of death after progression of the cancer itself [2, 3]. In addition to short-term morbidity of VTE in this population, long-term consequences dictated by higher VTE recurrence and major bleeding events have been reported in this population compared to non-cancer VTE patients [4]. Furthermore, malignancy is considered a strong risk factor for developing VTE with an incidence of 4–20% among cancer patients [5]. Emerging data are also suggesting that among all first diagnosed VTEs approximately 20–30% are associated with cancer [5].
Apart from patients’ related factors and transient events, multiple cancer-specific factors play a role in increasing the likelihood of developing VTE including cancer site, histology, stage, and treatment [6]. For example, gastrointestinal (GI), hematological and lung cancers carry higher risk of thrombosis compared to many others, and compared to localized tumors, metastatic cancers are associated with increased risk of VTE [7]. With regard to treatment, chemotherapeutic agents such as cisplatin-based agents and bevacizumab have been reported to increase the likelihood of thrombosis, not to mention the increased risk of VTE due to surgical interventions as part of cancer treatment [7].
Notably, GI cancers are among the top 10 cancers leading to VTE [7]. The incidence of VTE in GI cancer patients, according to Amis et al., is around 8.6% compared to 3.3% in non-GI cancers [8]. Developing VTE in patients with GI cancers can be catastrophic, leading to increased overall mortality. Majmudar et al. [9] and his team reported a mortality rate of 44.4% at 6 months in gastric cancer patients complicated with VTE. In this study, we aimed to investigate the impact of acute VTE in patients admitted to hospitals with GI malignancies in terms of mortality, total length of stay, and total hospital charges.
Materials and Methods
Data Source
This retrospective study was conducted utilizing the National (Nationwide) Inpatient Sample (NIS) database for the years 2016–2019. NIS is the largest publicly available all-payer inpatient care database in the USA, containing data for more than seven million hospital stays. Its large sample size is ideal for developing national and regional estimates and enables analyses of rare conditions, uncommon treatments, and special populations. Data are collected from all the States participating in the Health Cost and Utilization Project (HCUP) and represent more than 97% of the US population [10]. The NIS includes clinical and non-clinical variables for each hospital stay, including up to 40 discharge diagnoses, and 25 procedures using the International Classification of Diseases, Tenth Revision, Clinical Modification/Procedure Coding System (ICD-10). Since NIS is de-identified data that are publicly available, it is exempt from Institutional Review Board (IRB) review and approval.
Study Population
Patients with VTE were identified using ICD-10 diagnoses I26XX and I82XXX from all listed primary discharge diagnoses. Patients younger than 18 years of age, and those with missing information for age, gender, or race were excluded from the analysis. Patients with GI malignancies were identified using ICD 10 codes: C25X for pancreatic cancer, C15X for esophageal cancer, C16X for gastric cancer, and C18x and C20x for colorectal cancer. We further divided the patient population into five groups: no malignancy, pancreatic cancer, esophageal cancer, gastric cancer, and colorectal cancer.
Definitions of Variables
The NIS pre-defined variables were used to identify each patient’s age (in years); gender (male or female); race (White, African American, Hispanic, Asian/Pacific Islander, Native American, or others); insurance (Medicare, Medicaid or private). The Charlson comorbidity index (CCI) was used to assess the comorbidity burden since comorbid conditions are known to influence outcomes of hospitalization negatively. It has been a widely used index to measure the severity of comorbidity burden from administrative databases [11]. The higher score indicates a more substantial burden of comorbidity. Mortality was the primary endpoint, while total hospital charge and hospital length of stay were secondary endpoints.
Statistical Analysis
Analyses were performed using STATA, version 17.0 (StataCorp LLC, College Station, TX). NIS is based on a complex sampling design that includes stratification, clustering, and weighting. This software facilitates analysis to produce nationally representative unbiased results, variance estimates, and p values.
Univariable logistic regression analysis was used to calculate unadjusted odds ratios (ORs) for the primary and secondary outcomes. A second logistic regression model was built by using only variables that were associated with the outcome of interest on univariable regression analysis at p < 0.05 and was also used to adjust for the potential patient- and hospital-level confounders. The Fisher’s exact test was then utilized to compare proportions, and the Student’s t test was also applied to compare continuous variables. All p values were two-sided, with 0.05 as the threshold for statistical significance.
Results
There were 142 million discharges included in the NIS database between the years 2016 and 2019, of which 999,559 patients were admitted to the hospital with VTE, 25,775 (2.6%) had one of the four GI malignancies included. Compared with patients who didn’t have GI cancers, esophageal cancer was more seen in males with a prevalence of 78.6%. CCI with a score of 3 or higher was more reported in patients with pancreatic cancer (90.3%). The white race was the most reported race in all cancers and was most predominant in esophageal cancer. More than 50% of all included patients were covered by Medicare which was more reported in patients with pancreatic cancer. Demographics and patients’ characteristics are shown in Table 1.
Table 1.
Study population characteristics
| Variable | No malignancy | GI malignancy | |||
|---|---|---|---|---|---|
| esophageal | gastric | pancreatic | colorectal | ||
| Male gender, n (%) | 481,588 (48) | 785,753 (78.6) | 583,243 (58.4) | 509,575 (51) | 495,481 (49.6) |
| Age, mean, years | 63.0 | 66.3 | 66.6 | 67.7 | 65.9 |
| Race, n (%) | |||||
| White | 707,588 (70.8) | 861,020 (86.1) | 571,748 (57.2) | 717,683 (71.8) | 693,294 (69.4) |
| African American | 190,616 (19.1) | 55,376 (5.5) | 218,704 (21.9) | 171,924 (17.2) | 194,214 (19.4) |
| Hispanic | 65,271 (6.5) | 53,476 (5.4) | 120,847 (12.1) | 63,972 (6.4) | 74,267 (7.4) |
| Asian/Pacific Islander | 10,096 (1) | 7,897 (0.8) | 38,383 (3.8) | 14,494 (1.5) | 13,694 (1.4) |
| Native American | 3,898 (0.4) | 1,999 (0.2) | 17,292 (1.7) | 2,599 (0.3) | 3,598 (0.4) |
| Other | 21,990 (2.2) | 19,791 (2) | 32,586 (3.3) | 28,887 (2.9) | 20,491 (2.1) |
| Insurance n (%) | |||||
| Medicare | 528,767 (52.9) | 554,255 (55.5) | 558,254 (55.9) | 589,140 (58.9) | 537,463 (53.8) |
| Medicaid | 124,145 (12.4) | 71,269 (7.1) | 126,644 (12.7) | 77,466 (7.8) | 116,549 (11.7) |
| Private | 276,078 (27.6) | 336,451 (33.7) | 281,976 (28.2) | 293,770 (29.4) | 298,468 (29.9) |
| Self-pay | 40,882 (4.1) | 11,895 (1.2) | 19,192 (1.9) | 15,993 (1.6) | 22,390 (2.2) |
| No change | 3,598 (0.4) | 0 (0) | 0 (0) | 2,099 (0.2) | 1,399 (0.14) |
| Others | 26,088 (2.6) | 25,689 (2.6) | 13,394 (1.3) | 21,191 (2.1) | 23,289 (2.3) |
| CCI, n (%) | |||||
| Score of 0 | 315,061 (31.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Score of 1 | 232,298 (23.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Score of 2 | 166,227 (16.6) | 219,703 (22) | 165,027 (16.5) | 96,557 (9.7) | 161,329 (16.1) |
| Score of 3 or more | 285,974 (28.6) | 779,856 (78) | 834,531 (83.5) | 903,001 (90.3) | 838,230 (83.9) |
Among patients with GI malignancies included in our study, 18,816 (73%) patients had PE and 6,959 (27%) patients had DVT. Colorectal cancer was the most common reported GI malignancy, followed by pancreatic, gastric, and esophageal. Patients with esophageal cancer had the highest percentage of PE (81.38%) among all GI cancers we studied. Patients with colorectal cancer had the highest percentage of DVT (30.92%) among all GI malignancies we studied. Table 2 shows the number and percentage of patients with VTE divided by the type of GI malignancy out of the total number of cancer patients included in the study.
Table 2.
Percentage and number of patients with VTE divided by type of GI malignancy
| Thromboembolism | GI malignancy | |||
|---|---|---|---|---|
| esophageal | gastric | pancreatic | colorectal | |
| Pulmonary embolism | 2,099 (8.1%) | 1,999 (7.76%) | 7,197 (27.92%) | 7,597 (29.47%) |
| Deep vein thrombosis | 480 (1.8%) | 640 (2.48%) | 2,499 (9.7%) | 3,399 (13.19%) |
VTE, venous thromboembolism; GI, gastrointestinal.
Mortality
For adult patients admitted to the hospital with VTE and compared to patients without GI malignancies, patients with esophageal cancer had the highest odds of mortality with almost 3 times higher odds of dying (adjusted odds ratio (aOR) 2.701; 95% confidence interval (95% CI) 1.989–3.669, p value <0.000). Patients with Pancreatic cancer had the second-highest odds of mortality out of the 4 chosen GI malignancies with 73.6% increased odds of dying (aOR 1.736; 95% CI: 1.445–2.085, p value <0.000). Patients with Gastric cancer as well had 57.6% increased odds of mortality (aOR 1.576; 95% CI: 1.094–2.269, p value 0.015). Patients with colorectal cancer had no significant increase in the odds of mortality (aOR 1.213; 95% CI: 0.988–1.489, p value 0.066). Compared to male patients, female patients had 8.6% higher odds of mortality (aOR 1.086; 95% CI: 1.024–1.152, p value 0.006). Also, for each 1-year increase in age, there was a 1.8% increase in odds of mortality (aOR 1.018; CI: 1.016–1.021, p value <0.000).
Compared to White race as a reference, there were 9.8% higher odds of mortality in African American patients (aOR 1.098; 95% CI: 1.019–1.184, p value 0.014) and 49.4% higher odds of mortality in Asian/Pacifier Islander patients (aOR 1.494; 95% CI: 1.180–1.891, p value 0.001). Hispanic and Native American patients had no statistically significant increase in mortality when compared to the white race. Compared to Medicare as a reference, patients with private insurance and no insurance (self-pay) were more likely to die with it being 68.1% increased odds of dying in self-paying patients and 12.2% in private insurance patients (aOR 1.681; 95% CI: 1.424–1.985, p value <0.000) and (aOR 1.122; 95% CI: 1.026–1.227, p value 0.012), respectively. Charlson Comorbidity score was also associated with a statistically significant increase in the odds of mortality; when compared to a score of zero as a reference, patients with scores of one, two, and three or more had higher odds of mortality (OR 1.835; CI: 1.648–2.044, p value <0.000), (OR 2.12; CI: 1.903–2.381, p value <0.000), and (OR 3.742; CI: 3.391–4.129, p value <0.000), respectively (as exhibited in Table 3).
Table 3.
The multivariate logistic regression analysis of the association between different GI malignancies and mortality in patients admitted with VTE
| Variable | adjusted OR | p value | 95% CI | |
|---|---|---|---|---|
| GI malignancy | ||||
| Esophageal cancer | 2.701 | 0.000 | 1.989 | 3.669 |
| Gastric cancer | 1.576 | 0.015 | 1.094 | 2.269 |
| Pancreatic cancer | 1.736 | 0.000 | 1.445 | 2.085 |
| Colorectal cancer | 1.213 | 0.066 | 0.988 | 1.489 |
| Age | 1.018 | 0.000 | 1.016 | 1.021 |
| Gender | 1.086 | 0.006 | 1.024 | 1.152 |
| Race | ||||
| African American | 1.098 | 0.014 | 1.019 | 1.184 |
| Hispanic | 1.122 | 0.064 | 0.993 | 1.267 |
| Asian/Pacific Islander | 1.494 | 0.001 | 1.180 | 1.891 |
| Native American | 1.312 | 0.245 | 0.830 | 2.073 |
| Other | 1.542 | 0.000 | 1.296 | 1.835 |
| CCI | ||||
| Score of 1 | 1.835 | 0.000 | 1.648 | 2.044 |
| Score of 2 | 2.129 | 0.000 | 1.903 | 2.381 |
| Score of 3 or more | 3.742 | 0.000 | 3.391 | 4.129 |
| Insurance | ||||
| Medicaid | 1.086 | 0.189 | 0.960 | 1.228 |
| Private | 1.122 | 0.012 | 1.026 | 1.227 |
| Self-pay | 1.681 | 0.000 | 1.424 | 1.985 |
| No charge | 0.528 | 0.150 | 0.221 | 1.261 |
| Others | 1.611 | 0.000 | 1.344 | 1.932 |
VTE, venous thromboembolism; GI, gastrointestinal.
Length of Stay and Total Hospital Charges
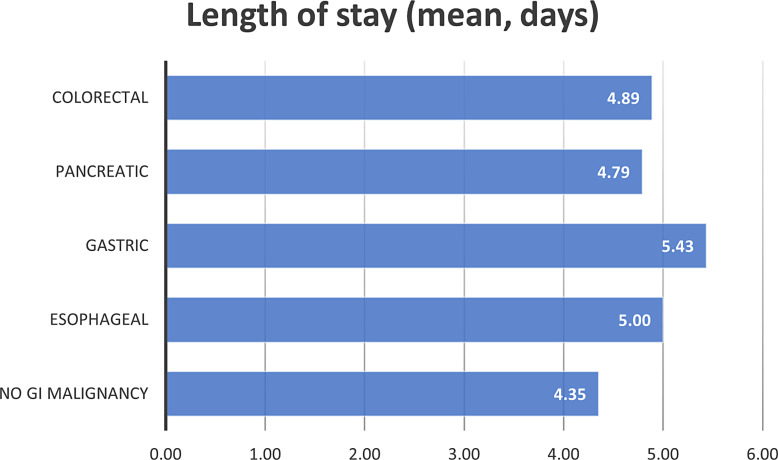
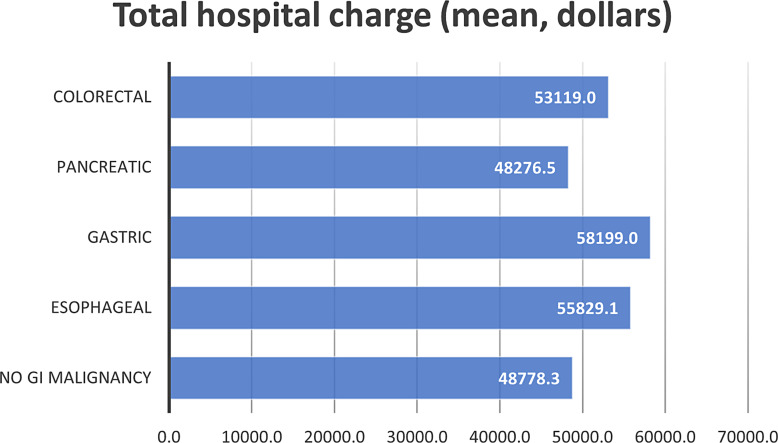
The mean length of stay for patients who were admitted due to VTE and without GI malignancies was 4.35 days. On the other hand, patients with gastric, esophageal, colorectal, and pancreatic who were admitted due to VTE were found to have the following mean length of stay: 5.43, 5.0, 4.89, and 4.79 days, respectively. In addition, patients admitted due to VTE with no GI malignancy had a mean of 48,778.3 dollars in total hospital costs. Patients admitted with VTE with GI malignancy including gastric, esophageal, colorectal, and pancreatic cancers had a mean total hospital cost of 58,199.0, 55,829.1, 53,119.0, and 48,276.5 dollars, respectively. Figures 1 and 2 shows the mean length of stay and mean total hospital charge in patients with VTE, respectively.
Fig. 1.
The mean length of stay for gastrointestinal malignancies included in the analysis.
Fig. 2.
The mean total hospital charge for gastrointestinal malignancies included in the analysis.
Discussion
In our study, we found that the prevalence of GI malignancies among VTE patients is low. However, the latter carried a higher inpatient mortality rate particularly in esophageal, pancreatic, and gastric cancer patients, compared to those with no known GI malignancy. In this population, the hypercoagulable state is linked to the tumor biology dictated by the expression of tissue factor which in turn binds to factor VII, leading to activation of the coagulation cascade [12, 13]. The other mechanism implicated is tumor lysis caused by active chemotherapy treatment which incites releasing of intracellular procoagulants such as the Willebrand factor [14]. Therefore, early recognition of such complications is pivotal to prevent mortality, especially in those with a good response to chemotherapy. Of GI malignancies we studied, esophageal cancer was associated with the highest inpatient mortality rate when it is complicated by VTE followed by pancreatic and gastric cancer, respectively. Despite a report suggesting an increase of 1 year-mortality following colorectal cancer diagnosis in patients with VTE [15], our study failed to show an increase of inpatient mortality among those patients.
Compared to White race, the African American race was significantly associated with higher mortality in patients with GI malignancy complicated with VTE. The driving factor behind this result is unclear to us but might be due to genetic predisposition versus social determinants of health. Interestingly, self-pay patients as well as Medicaid insurance carriers had higher inpatient mortality rate in our population. However, there was no difference in mortality between cancer and non-cancer patients presenting with VTE who had private health insurance. The explanations for these results specifically in Medicaid patients are probably due to the quality of care provided, the socioeconomic status, and the associated comorbidities. On the other hand, the ability to continue to pay for treatment might be a major challenge in self-pay patients, leading to an increase in mortality.
While the treatment of VTE in cancer patients is similar to non-cancer patients which is basically anticoagulation, the treatment of the former remains complicated. The challenge stems from the higher than usual bleeding risk, recurrence of VTE and comorbidities in cancer patients. According to a study that was done at Mayo Clinic, the risk of bleeding in patients with GI cancer was similar to those with non-GI cancer treated with apixaban, rivaroxaban, and enoxaparin. Apixaban, however, carried a higher rate of bleeding in luminal GI cancer compared to non-GI cancer [16]. This might partially explain our finding that patients with luminal cancers had higher total hospital charges compared to pancreatic cancer patients. Also, complications of VTE and its treatment in this population might explain the increased total hospital stay in patients with GI cancers who presented with VTE.
The strength of this study stems from the size of the sample, which makes the results more representative of the general population; the use of NIS which is the largest publicly available inpatient database gives the option to adjust all outcomes to the most common baseline characteristics of both patients and hospitals to minimize confounding factors as much as possible, and it analyzes multiple demographics for patients with VTE and GI malignancy. However, there are a few limitations such as being a retrospective study, which makes it susceptible to nonrandomization. Also, the NIS database includes an administrative database, which means that administrative codes were used to identify VTE, GI malignancy, and other diagnoses, leading to possible misclassifications, undercodings, or overcodings. The misclassification will, however, be seen as an error rather than a bias since it is likely to occur equally across all arms. Errors do not change the nature of the relationship between two variables; rather, statistical significance between them becomes more difficult to establish.
Conclusion
This study investigated the impact of VTE on GI cancer patients. It showed increased odds of mortality in esophageal, gastric, and pancreatic cancer patients compared to those without GI cancer who presented to the hospital with VTE, whereas VTE in colorectal cancer patients did not increase the odds of mortality. Early recognition of VTE is of high importance to initiate treatment and prevent further progression of thrombosis, especially in those with a good response to treatment. However, treatment can be challenging due to the higher risk of bleeding and recurrence of VTE as well as cancer patients’ associated comorbidities.
Statement of Ethics
Since NIS is de-identified data that are publicly available, it is exempt from the Institutional Review Board (IRB) review and approval. Patient consent was not required as this study was based on publicly available data.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding has been received in the preparation of data or the manuscript.
Author Contributions
Mohammad Darweesh: conceptualizing the article, review of literature, drafting the methodology and results, critical revision of language and accuracy of literature and citation of all drafts, review of article, and editing. Metri Haddaden: conceptualizing the article, review of literature, review the analysis, drafting the discussion, final review of the article, and editing. Zainab Fatima: conceptualizing the article, review of literature, review the analysis, drafting the discussion, final review of article, and editing. Mahmoud Mansour: conceptualizing the article, review of literature, drafting the methodology and result, styling and figures editing, review of article, and editing. Rami Dalbah and Suhib Fahmawi: conceptualizing the article, review of literature, drafting the introduction, styling and figures editing, review of article, and editing. Rateb Mahfouz: conceptualizing the article, review of literature, drafting the methodology and result on, styling and figures editing, review of article, and editing. Adham E. Obeidat: conceptualizing the article, review of literature, drafting the discussion, styling, and figures editing, review of article, and editing. Bhavesh Gajjar: conceptualizing the article, review of literature, reviewed the final draft with figures and tables, critical appraisal and editing of every draft after it is made, critical appraisal of the final draft.
Funding Statement
No funding has been received in the preparation of data or the manuscript.
Data Availability Statement
National Inpatient Sample (NIS) is a publicly available all-payer inpatient care database in the USA, containing data for more than seven million hospital stays. Further inquiries can be directed to the corresponding author.
References
- 1. Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 Suppl):S495–501. [DOI] [PubMed] [Google Scholar]
- 2. Sørensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343(25):1846–50. [DOI] [PubMed] [Google Scholar]
- 3. Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res. 2010;125(6):490–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li X, Partovi S, Gadani S, Martin C 3rd, Beck A, Vedantham S. Gastrointestinal malignancies and venous thromboembolic disease: clinical significance and endovascular interventions. Dig Dis Interv. 2020;4(3):260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sud R, Khorana AA. Cancer-associated thrombosis: risk factors, candidate biomarkers and a risk model. Thromb Res. 2009;123(Suppl 4):S18–21. [DOI] [PubMed] [Google Scholar]
- 6. Abdol Razak NB, Jones G, Bhandari M, Berndt MC, Metharom P. Cancer-associated thrombosis: an overview of mechanisms, risk factors, and treatment. Cancers. 2018;10(10):380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eichinger S. Cancer associated thrombosis: risk factors and outcomes. Thromb Res. 2016;140(Suppl 1):S12–S17. [DOI] [PubMed] [Google Scholar]
- 8. Tetzlaff ED, Cheng JD, Ajani JA. Thromboembolism in gastrointestinal cancers. Gastrointest Cancer Res. 2008;2(6):267–72. [PMC free article] [PubMed] [Google Scholar]
- 9. Majmudar K, Golemi I, Tafur AJ, Toro JD, Visonà A, Falgá C, et al. Outcomes after venous thromboembolism in patients with gastric cancer: analysis of the RIETE Registry. Vasc Med. 2020;25(3):210–7. [DOI] [PubMed] [Google Scholar]
- 10. HCUP national inpatient sample (NIS): healthcare cost and utilization Project (HCUP). Agency for healthcare research and quality. 2012. [cited 2022 Feb 15]: Available from: https://www.hcup-us.ahrq.gov/nisoverview.jsp [Google Scholar]
- 11. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. [DOI] [PubMed] [Google Scholar]
- 12. Wada H, Matsumoto K, Shiku H, Nakamura S, Suzuki H, Shigemori C. Tissue factor expression and metastatic potential of colorectal cancer. Thromb Haemost. 1998;80(12):894–8. [PubMed] [Google Scholar]
- 13. Khorana AA, Ahrendt SA, Ryan CK, Francis CW, Hruban RH, Hu YC, et al. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res. 2007;13(10):2870–5. [DOI] [PubMed] [Google Scholar]
- 14. Licciardello JT, Moake JL, Rudy CK, Karp DD, Hong WK. Elevated plasma von Willebrand factor levels and arterial occlusive complications associated with cisplatin-based chemotherapy. Oncology. 1985;42(5):296–300. [DOI] [PubMed] [Google Scholar]
- 15. Alcalay A, Wun T, Khatri V, Chew HK, Harvey D, Zhou H, et al. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol. 2006;24(7):1112–8. [DOI] [PubMed] [Google Scholar]
- 16. Houghton DE, Vlazny DT, Casanegra AI, Brunton N, Froehling DA, Meverden RA, et al. Bleeding in patients with gastrointestinal cancer compared with nongastrointestinal cancer treated with apixaban, rivaroxaban, or enoxaparin for acute venous thromboembolism. Mayo Clin Proc. 2021;96(11):2793–805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
National Inpatient Sample (NIS) is a publicly available all-payer inpatient care database in the USA, containing data for more than seven million hospital stays. Further inquiries can be directed to the corresponding author.