Abstract
Epithelial cells are known to be a major target for human cytomegalovirus (HCMV) infection; however, the analysis of virus-cell interactions has been difficult to approach due to the lack of in vitro models. In this study, we established a polarized epithelial cell model using a colon epithelial cell-derived cell line (Caco-2) that is susceptible to HCMV infection at early stages of cellular differentiation. Infection of polarized cells was restricted to the basolateral surface whereas virus was released apically, which was consistent with the apical and not basolateral surface localization of two essential viral glycoproteins, gB and gH. HCMV infection resulted in the development of a cytopathology characteristic of HCMV infection of colon epithelium in vivo, and infection did not spread from cell to cell. The inability of HCMV to infect Caco-2 cells at late stages of differentiation was due to a restriction at the level of viral entry and was consistent with the sequestration of a cellular receptor for HCMV. These observations provide the first evidence that restriction of HCMV replication in epithelial cells is due to a receptor-mediated phenomenon.
Human cytomegalovirus (HCMV) is an opportunistic agent that causes severe disease in immunosuppressed individuals. The clinical pathology associated with infection varies depending on the particular etiology of the immunosuppression. In iatrogenically immunosuppressed patients, HCMV disease of the gastrointestinal (GI) tract is generally limited to the cecum and ascending colon, whereas in AIDS patients, disease is multifocal and can involve any region of the GI tract, with the colon as the most frequently infected site, followed by the esophagus (1, 3, 5). HCMV infection of the lung is most severe in bone marrow transplant patients, where it causes pneumonia in approximately 15% of allograft recipients and is associated with 20% mortality (1). In AIDS patients, HCMV frequently infects the lung, but the infection progresses more slowly and rarely results in pneumonia (8). HCMV infection of the retina is unique to patients with AIDS and is responsible for retinitis in an estimated 20% of patients with advanced human immunodeficiency virus disease (8).
Despite the observed differences in clinical pathology, the cell types infected by HCMV and the histopathology associated with infection are comparable in all immunosuppressed individuals. In infected organs, HCMV can be identified in epithelial and endothelial cells, macrophages, and fibroblasts; however, the pathologic mechanism of HCMV-induced disease is not completely understood (26). Two major mechanisms have been proposed to account for HCMV pathology. In the GI tract and the retina, pathological findings support a role for ischemia in the disease process. Additionally, the identification of HCMV in epithelial cells in the GI tract, lung, and retina suggests that disease may also be a consequence of a direct cytopathic effect (CPE) of the virus (7, 12, 25).
Currently, most in vitro studies of the HCMV infection process are conducted in nonpolarized normal human fibroblast (HF) cells. This cell type has certain advantages for the study of HCMV, such as permissiveness to infection, extensive characterization, and ease of growth in culture. However, the cell type used to study HCMV can profoundly affect many characteristics of the infection process, including productivity of virus replication, viral gene expression, and protein trafficking. Consequently, HCMV replication in HF cells may not be representative of the replicative process within polarized epithelial cells. To date, studies of HCMV in epithelial cells in vitro have been hampered by the absence of a suitable polarized epithelial cell model system. A number of primary epithelial cells have been shown to support replication of HCMV, but poor characterization and difficulty of growth in culture have limited their utility. HCMC and ARPE-19 are two epithelial cell lines that have been shown to support productive viral replication (27, 30). However, HCMC cells are poorly characterized, and ARPE-19 cells are difficult to grow in culture and are not representative of epithelial cells from nonretinal tissues. In the present study, we have established a polarized epithelial model that is permissive for HCMV infection. Viral infection was dependent on the state of cellular differentiation (defined by transepithelial resistance [TER]), which appeared to be due to the availability of a polarized cell surface receptor. These observations provide the first evidence that restriction of HCMV infection in epithelia is due to a receptor-mediated phenomenon.
MATERIALS AND METHODS
Cell lines and virus.
HCMV Towne strain and HV5.111, a recombinant HCMV (Toledo strain) that expresses green fluorescent protein (GFP) under the control of the cellular elongation factor 1α (EF1α) promoter (31), were propagated in normal human dermal fibroblast cells (NHDF) by standard methods. HV5.111 was constructed using pQ91, which consists of the BamHI (position 197042)-to-SalI (position 200171) fragment of HCMV (AD169 strain) containing US9 and US10 cloned into pUC21. A cassette consisting of the GFP gene (EGFP; Clontech, Calif.) under the control of the EF1α promoter and the Escherichia coli guanosine-hypoxanthine phosphoribosyltransferase gene under the control of the mouse phosphoglycerate kinase promoter was inserted into pQ91 at the ApaI site between US9 and US10 to create pQ111. pQ111 was then digested with BamHI and SalI to release the vector prior to its transfection into NHDF to generate recombinant virus as described elsewhere (32). Caco-2 cells were obtained from the American Type Culture Collection (Rockville, Md.) and cultured at 37°C in an atmosphere of 5% CO2 in complete medium (Dulbecco’s modified essential medium [BioWhittaker, Walkersville, Md.] containing 20% fetal bovine serum and supplemented with 4 mM l-glutamine, 200 μg of penicillin G per ml, and 200 μg of streptomycin sulfate per ml). In all experiments, Caco-2 cell monolayers were grown on 12-mm-diameter, 3-μm-pore-size polycarbonate filters (Corning Costar Corp., Acton, Mass.) Cells were plated at a density of 5 × 105 cells/filter and fed at 4-day intervals. At times indicated, the TER of Caco-2 cell monolayers was measured with an EVOM epithelial voltohmeter with an ENDOHM-12 chamber (World Precision Instruments, Sarasota, Fla.) according to the manufacturer’s instructions.
HCMV infection of Caco-2 cells.
Caco-2 cell monolayers were infected with HCMV Towne either basolaterally or apically, by addition of virus to either the basolateral or apical media at a multiplicity of infection (MOI) of 25 (except for virus cellular spread assay). Prior to infection, monolayers were washed once with Dulbecco’s phosphate-buffered saline (DPBS). After addition of virus, infection was allowed to proceed for 1 h at 37°C in an atmosphere of 5% CO2. When volume was limiting, the infection procedure was repeated with fresh virus until the desired MOI was achieved. After infection, monolayers were washed three times with DPBS, fresh complete medium was added, and cells were cultured at 37°C in an atmosphere of 5% CO2. TER was measured immediately after addition of the fresh complete medium to ensure that monolayers had remained intact during the infection procedure.
Immunofluorescence microscopy.
Expression of viral and cellular proteins in Caco-2 cell monolayers was determined by indirect immunofluorescence using a modification of the method of Molloy et al. (21). All procedures were performed at room temperature unless stated otherwise. Cells grown on filters were rinsed in DPBS and fixed for 15 min in DPBS containing 4% paraformaldehyde, 0.1 mM CaCl2, and 0.1 mM MgCl2. After three washes with DPBS, filter autofluorescence was quenched by two 15-min incubations in DPBS (pH 8.0) containing NaBH4 (1 μg/ml). Filters were washed three times in DPBS, and cells were permeabilized and blocked by incubation for 15 min in DPBS containing 2% normal goat serum and 0.4% Triton X-100. Filters were then washed three times in wash buffer (DPBS containing 0.2% Triton X-100 and 0.2% [wt/vol] bovine serum albumin) and excised from inserts. Filters were incubated cell side down in primary antibody diluted in DPBS containing 0.1% Triton X-100. The primary antibodies used were a rabbit anti-IE86 antibody (R638) (9) (used at a dilution of 1/150), a mouse monoclonal anti-gB antibody (27-156) (33) (used at a dilution of 1/150), and a mouse monoclonal anti-gH antibody (14-4B) (2) (used at a dilution of 1/50). Cells were incubated with primary antibody either overnight at 4°C or for 2 h at room temperature. No difference in level of staining was observed between the two different incubation conditions. After incubation, filters were washed three times with wash buffer and incubated in DPBS containing the appropriate fluorescein isothiocyanate-conjugated secondary antispecies antibody. Epifluorescence was visualized with a Nikon Optiphot fluorescence microscope.
Virus entry assay.
Caco-2 cell monolayers were infected at an MOI of 25 with HV5.111. At 4 days postinfection (p.i.), cells were rinsed in DPBS and fixed for 15 min in DPBS containing 4% paraformaldehyde, 0.1 mM CaCl2, and 0.1 mM MgCl2. Cells were permeabilized and blocked by incubation for 15 min in DPBS containing 2% normal goat serum and 0.4% Triton X-100. Cells were then counterstained with propidium iodide (PI) at 1 μg/ml in DPBS for 5 min; after washing, GFP- and PI-positive cells were visualized with a Nikon Optiphot fluorescence microscope.
MIEP activity assay.
Caco-2 cell monolayers were infected with a recombinant adenovirus vector, AdgD1(E1−), expressing a reporter gene (encoding herpes simplex virus type 1 [HSV-1] glycoprotein D [gD]) under the control of the HCMV major immediate-early (IE) promoter (MIEP) (22). After addition of virus to both basolateral and apical media, infection was allowed to proceed for 1 h at 37°C in an atmosphere of 5% CO2. After infection, monolayers were washed three times with DPBS, fresh complete medium was added, and cells were cultured at 37°C in an atmosphere of 5% CO2. At 3 days p.i., cells were rinsed in DPBS and fixed for 15 min in DPBS containing 4% paraformaldehyde, 0.1 mM CaCl2, and 0.1 mM MgCl2. Cells were then stained for the presence of HSV-1 gD with mouse anti-HSV-1 gD monoclonal antibodies LP2 (22) and DL6 (14) at a dilution of 1/200 and counterstained with PI as described above. HSV-1 gD- and PI-positive cells were visualized with a Nikon Optiphot fluorescence microscope.
Virus cellular spread assay.
Caco-2 cell monolayers at a TER of 250 Ω · cm2 were infected at an MOI of 1 by addition of HCMV to the basolateral media. Cells were then cultured in complete medium containing 0.1% human anti-HCMV immunoglobulin (IgG) and fed at 4-day intervals. At days 8, 16, 24, and 32 p.i., cell monolayers were fixed and stained for an HCMV IE protein IE86 as described previously. The degree of cell-to-cell spread over the 32-day period was quantitated by the change in number of IE86-positive cells within each plaque of infection. IE86-positive cells were visualized by indirect immunofluorescence microscopy after staining with IE86-specific antibody R638 as outlined above.
Virus growth assay.
Caco-2 cell monolayers at a TER of 250 Ω · cm2 were infected at an MOI of 25 by addition of HCMV to the basolateral media. Monolayers were fed by complete replacement of media at 4-day intervals. At various times p.i., separate supernatant fractions were harvested from basolateral and apical wells, and cell fractions were harvested by trypsinization of filters with trypsin-EDTA (BioWhittaker). Supernatant fractions were clarified by centrifugation, and cell fractions were washed three times with DPBS before storage at −70°C. The level of virus in each fraction was determined by titration on NHDF. Briefly, cell fractions were sonicated to release cell-associated virus prior to titration. Serial dilutions of the various fractions were then added to 80% confluent NHDF in 96-well microtiter plates. After 24 h, NHDF were fixed and stained for IE86, and IE86-positive cells were counted to determine virus titer.
Immunohistochemistry.
Caco-2 cell monolayers were fixed as described above at intervals p.i. as indicated in Results. Filters were then dehydrated through graded alcohols and xylene, embedded on edge in paraffin, and sectioned. After predigestion with 0.1% protease, cut sections were stained with a mouse monoclonal antibody directed against the HCMV p52 early gene product (CCH2; DAKO Corp., Carpinteria, Calif.) for 1 h at room temperature. Following three DPBS washes, sections were incubated for an additional hour with biotinylated goat anti-mouse IgG followed by avidin-biotin-horseradish peroxidase complex (Vectastain kit; Vector Laboratories). Sections were washed as before, and p52 was visualized by addition of diaminobenzidine (Sigma, St. Louis, Mo.) in 0.05% H2O2. After a final wash in H2O, sections were counterstained with hematoxylin, dehydrated, and coverslipped with Permocent (Fisher Scientific).
Electron microscopy.
Localization of virus progeny within Caco-2 cells was determined by electron microscopy. Cells were fixed and prepared for microscopy essentially as described by Jourdan et al. (17). At day 20 p.i., Caco-2 cell monolayers were rinsed three times with DPBS and fixed with 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.4) for 30 min at room temperature. Filters were then washed with DPBS and postfixed with 1.5% osmium tetroxide in sodium phosphate buffer for 30 min at room temperature. Filters were dehydrated in a graded ethanol series, cut into strips, and embedded in epoxy resin. Ultrathin sections were double stained with uranyl acetate and lead citrate and viewed with a Philips EM 300 electron microscope.
RESULTS
HCMV infection of Caco-2 cells is differentiation state dependent and occurs preferentially at the basolateral membrane.
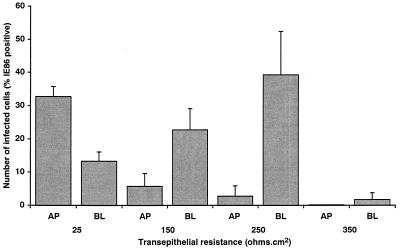
The first series of experiments were designed to determine whether HCMV could infect Caco-2 cells. In other studies using a number of different cell types, HCMV has been shown to require an appropriate state of cellular differentiation for infection (11, 13, 24, 28, 29). Caco-2 cells are unique in their ability to differentiate after reaching confluency. Therefore, HCMV was added to Caco-2 cell monolayers at increasing states of cellular differentiation as measured by TER. Increase in TER has been shown to parallel the appearance of other markers of cellular differentiation in this cell type, including alkaline phosphatase activity, carcinoembryonic antigen expression, and dome formation (19), and consequently TER serves as an ideal marker of the state of cellular differentiation in Caco-2 cells. In addition, as many viruses infect polarized cell monolayers by preferentially entering at either the apical or basolateral surface, HCMV was added either to the apical or the basolateral media of the monolayers. Monolayers were infected at an MOI of 25, which was shown to result in maximal infection of Caco-2 cells (data not shown). At day 8 p.i., the level of infection was determined by staining monolayers for the presence of IE86 protein. The results presented in Fig. 1 show that HCMV can infect Caco-2 cells, but the efficiency of infection is dependent on the state of cellular differentiation. Maximal infection (≈40% IE86 positive) occurred when cells were infected at early stages of differentiation (≤250 Ω · cm2). In contrast, at later stages of differentiation when the cells reached a TER of 350 Ω · cm2, a significant drop in the ability of HCMV to infect Caco-2 cells was observed (1.8% IE86 positive), suggesting a loss in the HCMV-susceptible cellular phenotype as the cells continued differentiation. The results presented in Fig. 1 also demonstrate that HCMV infection of Caco-2 cell monolayers is polarized and occurs preferentially at the basolateral membrane. The preference HCMV displayed for the basolateral membrane was maximal (14-fold; P < 0.000001) when monolayers were infected at a TER of 250 Ω · cm2. At a TER of 150 Ω · cm2, the preference for the basolateral membrane, although only fourfold, was still significant (P < 0.003). At a TER of 25 Ω · cm2, HCMV infection appeared to occur preferentially at the apical surface, presumably due to a reduced ability of HCMV to pass through the 3-μm-pore-size filter. The low level of infection of monolayers infected at a TER of 350 Ω · cm2 precluded the determination of any preference of polarity of infection with any level of confidence. In summary, HCMV preferentially infects Caco-2 cells at the basolateral surface in a differentiation-state dependent manner, with maximal infection occurring at early stages of cellular differentiation.
FIG. 1.
HCMV infection of Caco-2 cells is differentiation state dependent and occurs preferentially at the basolateral membrane. HCMV was added to Caco-2 cell monolayers at increasing states of cellular differentiation as measured by TER. HCMV was added to the either apical (AP) or the basolateral (BL) media of Caco-2 cell monolayers at an MOI of 25. At day 8 p.i., monolayers were fixed, and the level of infection was determined by staining monolayers for the presence of HCMV IE86, followed by visualization of epifluorescence with a Nikon Optiphot fluorescence microscope.
gB and gH are distributed to the same subcellular sites in HCMV-infected Caco-2.
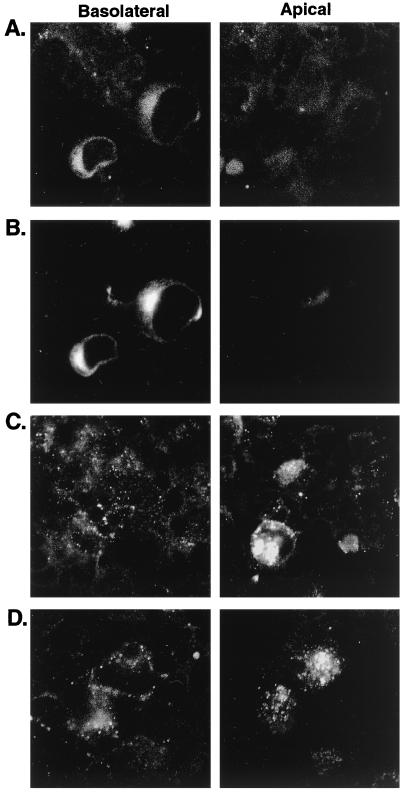
In a number of cell lines, HCMV can enter the cell and express IE genes but is unable to express late genes and produce progeny virus (15). To determine whether HCMV-infected Caco-2 cells can express early and late genes, Caco-2 cells were infected basolaterally at a TER of 250 Ω · cm2 and stained for the presence of gB (early) and gH (late) glycoproteins. Five percent of HCMV-infected Caco-2 cells expressed both gB and gH. Expression was first observed at day 12 and persisted for the duration of the experiment (28 days). By confocal microscopy on permeabilized cells, the majority of gB and gH were colocalized intracellularly in an endoplasmic reticulum- or Golgi-like staining pattern (Fig. 2A and B). Staining of nonpermeabilized cells demonstrated that gB (Fig. 2C) and gH (Fig. 2D) were localized to the apical surface of infected cells, although they were present in much lower concentrations on the cell surface than was observed intracellularly. In summary, HCMV-infected Caco-2 cells express early (gB) and late (gH) genes, which colocalize and are distributed intracellularly and to the apical cell surface.
FIG. 2.
gB and gH are distributed to the same subcellular sites in HCMV-infected Caco-2. Caco-2 cell monolayers at a TER of 250 Ω · cm2 were infected basolaterally at an MOI of 25. At day 16 p.i., monolayers were fixed and stained for the presence of gB (early) and gH (late) glycoproteins. Confocal microscopy demonstrated that gB and gH were colocalized. In permeabilized cells (A and B), the majority of gB (A) and gH (B) were observed to be colocalized intracellularly in an endoplasmic reticulum- or Golgi-like staining pattern. In nonpermeabilized cells (C and D), both gB (C) and gH (D) were observed to be localized to the apical surface of infected cells, although they were present in much lower concentrations on the cell surface than was observed intracellularly.
HCMV infection in Caco-2 cells is productive, and progeny virus is both localized intracellularly and released into the apical supernatant.
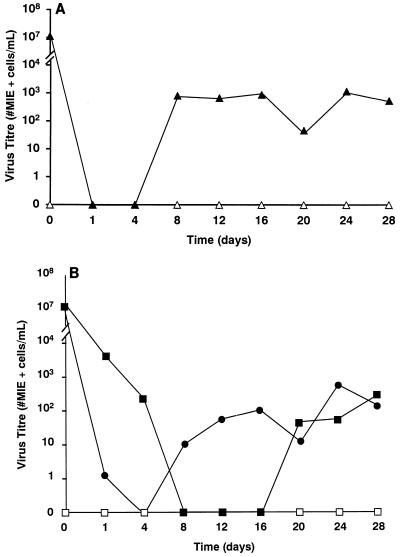
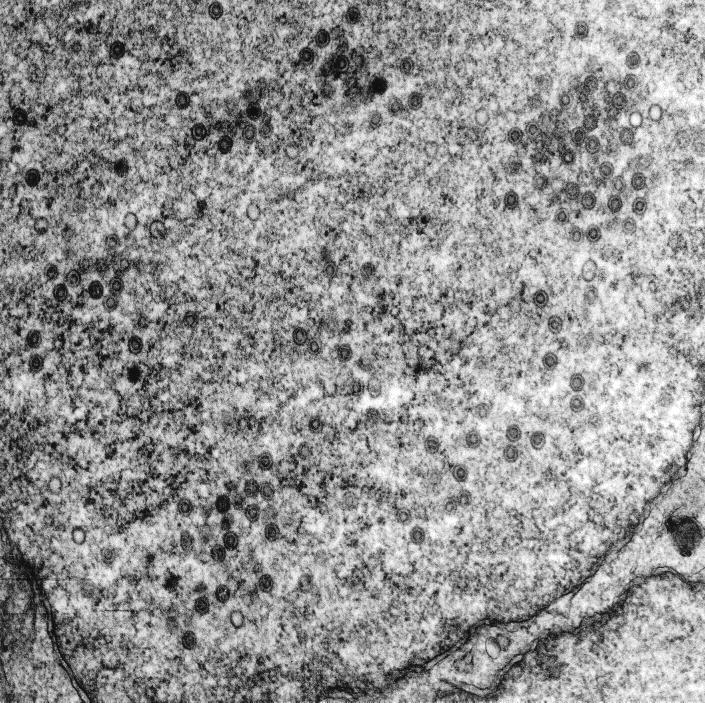
The expression of gB and gH in HCMV-infected Caco-2 cells suggested that infection was productive. To determine directly whether HCMV infection of Caco-2 cells was productive, Caco-2 cells were infected basolaterally at a TER of 250 Ω · cm2, and at various times p.i., cells and the apical and basolateral supernatants were collected separately for virus titration. HCMV infection was shown to be productive in Caco-2 cells (Fig. 3). The pattern of progeny virus distribution paralleled the distribution of gB and gH observed by confocal microscopy, with virus being distributed both intracellularly and released apically (Fig. 3). Virus was observed only at late times (>day 20) p.i. in the basolateral media, which corresponded to the time at which the cellular monolayer was observed to be perturbed (see below), and presumably resulted from the leakage of apically released virus into the basolateral well. Finally, the observation of HCMV capsids in the nuclei of HCMV-infected Caco-2 cells by electron microscopy confirmed that the infection was productive (Fig. 4).
FIG. 3.
HCMV infection in Caco-2 cells is productive, with progeny virus localized intracellularly and released into the apical supernatant. A single-step virus growth curve was performed to determine whether HCMV infection of Caco-2 cells was productive. Caco-2 cell monolayers at a TER of 250 Ω · cm2 were infected basolaterally at an MOI of 25 (filled symbols), or 0 (open symbols). At various times p.i., cells (▴) (A), and apical (●) and basolateral (■) supernatants (B) were collected separately and titered for virus.
FIG. 4.
Electron microscopy showing HCMV capsids in the nuclei of HCMV-infected Caco-2 cells. Caco-2 cell monolayers at a TER of 250 Ω · cm2 were infected basolaterally at an MOI of 25. At day 20 p.i., monolayers were fixed, cut into ultrathin sections, and embedded before being viewed with a Philips EM 300 electron microscope.
HCMV infection in Caco-2 cells is cytopathic and causes cytomegaly with basophilic nuclear inclusions.
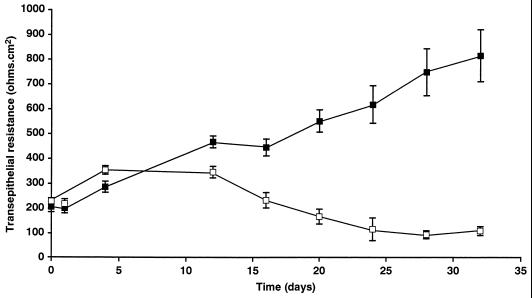
In vivo, HCMV infection of epithelial cells is cytopathic and causes cytomegaly with cytoplasmic and intranuclear inclusions. Experiments were designed to determine whether Caco-2 cells infected with HCMV in vitro exhibited a cytopathology comparable to that observed for epithelial cells in vivo. Caco-2 monolayers were infected basolaterally at 250 Ω · cm2, and TER was measured at various times p.i. TER was monitored to determine the effect of HCMV infection on the integrity of the monolayer, with a decrease in TER being suggestive of a CPE on the Caco-2 cells. As the results in Fig. 5 demonstrate, HCMV infection of Caco-2 cells resulted in a pronounced disruption of the monolayer, observed as a decrease in TER beginning at day 12 p.i. The initial decline in TER coincided with the onset of expression of gB and gH, and with the appearance of large holes in the monolayer. To determine whether in vitro-infected Caco-2 cells exhibited HCMV cytopathology characteristic of epithelial infection in vivo, cells were immunostained with an antibody for the HCMV early protein p52 and then counterstained with hematoxylin. Approximately 2% of total Caco-2 cells exhibited classic HCMV cytopathology consisting of cytomegaly with basophilic nuclear inclusions (Fig. 6), which correlated positively with the level of gB- and gH-positive cells seen by immunofluorescence.
FIG. 5.
HCMV infection of Caco-2 cells results in disruption of the monolayer, which is observed as a decrease in TER. Caco-2 cell monolayers at a TER of 250 Ω · cm2 were infected basolaterally at an MOI of 25 (□) or 0 (■). TER was then measured at various times p.i.
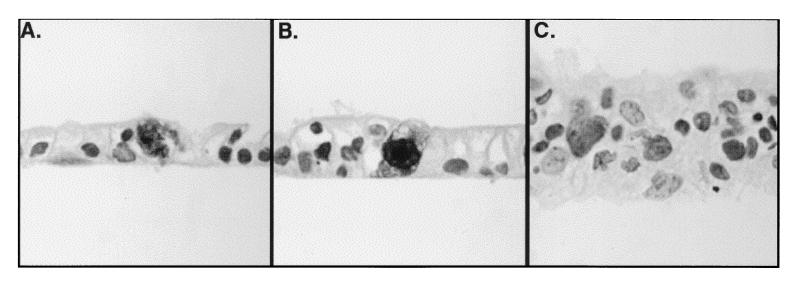
FIG. 6.
HCMV infection of Caco-2 cells causes cytopathology characterized by cytomegaly with basophilic cytoplasmic nuclear inclusions. Caco-2 cell monolayers at a TER of 250 Ω · cm2 were infected basolaterally at an MOI of 25 (A and B) or 0 (C). At day 20 p.i., monolayers were fixed and stained with an antibody for the HCMV early protein p52 and then counterstained with hematoxylin.
Virus does not spread from cell-to-cell through the Caco-2 cell monolayer.
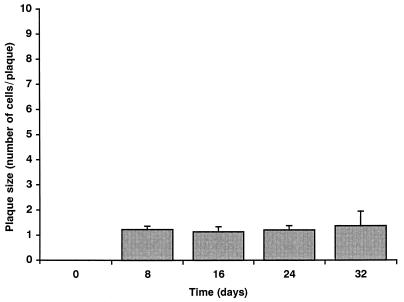
In NHDF, HCMV is unable to spread from cell to cell in the presence of neutralizing antibody (23). However, recent studies have shown that HCMV infection can spread in monolayers of ARPE-19 cells (30). To address the question of lateral cell-to-cell spread in Caco-2 cell monolayers, monolayers at a TER of 250 Ω · cm2 were infected at an MOI of 1 with HCMV. Cells were then cultured in complete medium containing 0.1% human anti-HCMV IgG to neutralize extracellular virus progeny. At days 8, 16, 24 and 32 p.i., cell monolayers were fixed and stained for the HCMV IE protein IE86. The degree of cell-to-cell spread over the 32-day period was quantitated by the change in number of IE86-positive cells within each plaque of infection. The number of IE86-positive cells per plaque did not increase over the 32-day period (Fig. 7), demonstrating that HCMV infection in Caco-2 cells does not spread from cell to cell, which is consistent with studies of NHDF in vitro and the pathology observed in vivo.
FIG. 7.
HCMV does not spread from cell to cell through the Caco-2 cell monolayer. Caco-2 cell monolayers at a TER of 250 Ω · cm2 were infected basolaterally at an MOI of 1. Cells were then cultured in complete medium containing 0.1% human anti-HCMV IgG. At days 8, 16, 24, and 32, monolayers were fixed and stained for HCMV IE86, and epifluorescence was visualized with a Nikon Optiphot fluorescence microscope. The degree of cell-to-cell spread over the 32-day period was quantitated by the change in number of IE86-positive cells per plaque of infection.
The dependence of HCMV infection on state of cellular differentiation is due to a restriction at the level of viral entry.
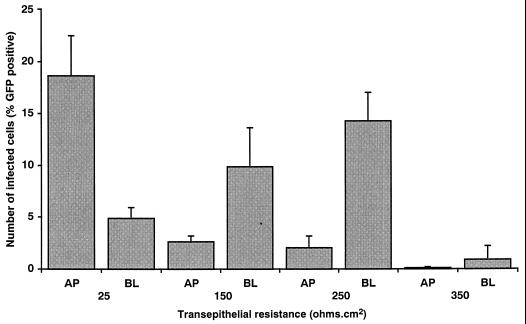
The decreased expression of IE86 in cells infected at a TER of 350 Ω · cm2 suggested that the block to infection was early in the replicative cycle of the virus, even perhaps at the level of viral entry. Activity of the MIEP in the majority of Caco-2 cells regardless of cellular differentiation state [determined by using a recombinant adenovirus vector, AdgD1(E1−), expressing a reporter gene under the control of the HCMV MIEP (data not shown)] suggested that the block to infection was at the level of viral entry. Therefore, virus entry assays using HV5.111, a recombinant HCMV that constitutively expresses a reporter gene, encoding GFP, were conducted to determine directly whether the restriction of HCMV infection in Caco-2 cells at a TER of 350 Ω · cm2 was at the level of virus entry; HV5.111 is constructed in a Toledo background and places the GFP gene under the control of the constitutively active promoter of EF1α, an essential component of the protein translational machinery. HV5.111 was added to either the basolateral or the apical media of Caco-2 monolayers at increasing TER, and GFP-positive cells were counted at 4 days p.i. (time of maximal GFP expression). The results presented in Fig. 8 show that the relative levels of GFP-positive Caco-2 cells infected with HV5.111 were comparable to the levels of IE86-positive cells seen after infection with Towne (Fig. 1) with respect to both the dependency of infection on TER and the preferential infection at the basolateral membrane. Although the genomes of Toledo and Towne have significant differences, including the presence of 13-kbp deletion in the former (4), these differences clearly had no effect on the polarity and differentiation state dependency of infection in Caco-2 cells.
FIG. 8.
The dependence of HCMV infection on state of cellular differentiation is due to a restriction at the level of viral entry. Infection studies using a recombinant HCMV (HV5.111) that constitutively expresses GFP were conducted to determine whether the restriction of HCMV infection in Caco-2 cells at late times of differentiation was at the level of virus entry; HV5.111 places GFP under the control of the constitutively active promoter of EF1α, an essential component of the protein translational machinery. HV5.111 was added at an MOI of 25 to either the apical (AP) or the basolateral (BL) media of Caco-2 cell monolayers at increasing states of cellular differentiation as measured by TER. At day 4 p.i. (time of maximal GFP expression), monolayers were fixed, and the level of infection was determined by visualization of GFP-positive cells with a Nikon Optiphot fluorescence microscope.
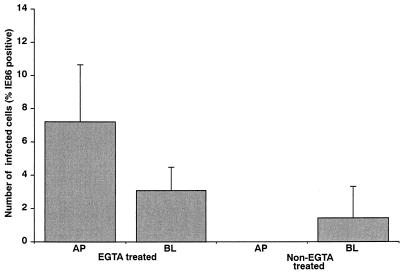
The differentiation state-dependent restriction of viral entry is due to an inaccessibility of the viral receptor.
The inability of virus to enter the cells could be due either to a decrease in the concentration of a viral receptor(s) at the cell surface or to a decrease in its accessibility at the cell surface. To distinguish between these two alternatives, the effect of EGTA on the ability to infect Caco-2 cell monolayers at late (350 Ω · cm2) stages of differentiation was determined. EGTA treatment of Caco-2 cells disrupts cell-to-cell junctions and permits access to molecules that may have been sequestered to sites of cell-to-cell contact between polarized epithelial cells and thereby inaccessible to virus. As shown in Fig. 9, EGTA treatment of Caco-2 cells at a late, noninfectable stage of differentiation returned the cells to an infectable phenotype. This ability of EGTA to reverse the differentiation state-dependent inhibition of HCMV infection is consistent with the inability of virus to enter these cells being due, in large part, to the sequestration of a viral receptor to a region that is inaccessible to the virus with increased state of differentiation.
FIG. 9.
The differentiation state-dependent restriction of viral entry is due to an inaccessibility of the viral receptor. HCMV Towne was added at an MOI of 25 to either the apical (AP) or the basolateral (BL) media of Caco-2 cell monolayers (TER of 350 Ω · cm2) that had been pretreated with 10 mM EGTA to disrupt cell-to-cell junctions. At day 8 p.i., monolayers were fixed, and the level of infection was determined by visualization of IE86-positive cells with a Nikon Optiphot fluorescence microscope.
DISCUSSION
In the present study, we have established that HCMV infection of polarized epithelial cells is dependent on the state of cellular differentiation. In these cells, HCMV infection was restricted to the basolateral surface, suggesting polarized expression of a viral cognate cellular receptor(s) at the plasma membrane. At later periods postdifferentiation, when cells were resistant to infection, receptors appear to have become sequestered in a region of the membrane surface that is inaccessible to HCMV. In addition to the polarized entry of the virus at the basolateral surface, HCMV also exhibited polarized release of virus into the apical supernatant. The inability of virus to infect the apical cell surface as well as the limited lateral spread of progeny virus from cell to cell also restricted virus infection in culture. This in vitro system accurately reproduces the pathology of HCMV infection in the bowel in vivo in which virus initially spreads hematogenously but is thereafter restricted to focal sites of ulceration.
Prior to this study study, ARPE-19, a nonimmortalized limited-passage retinal pigment epithelial line, was the only characterized epithelial line known to be permissive for HCMV infection (30). However, although retinal pigment epithelial cells are an epithelial cell type, they differ in many aspects from epithelial cells of nonretinal tissues, including in their distribution of cell surface proteins between basolateral and apical membranes. As the life cycle of HCMV is influenced greatly by cell type, our concern was that HCMV replication in ARPE-19 cells may not be representative of replication in epithelial cells derived from nonretinal tissues. In addition, the utility of ARPE-19 cells for in vitro studies is potentially limited due to their slow growth in culture, requiring 6 to 8 weeks to form an intact monolayer. On the basis of IE protein expression, Caco-2 and ARPE-19 cells were infected to similar levels at comparable MOIs of HCMV. In Caco-2 cells, 5% of infected (IE-positive) cells expressed E (gB) and L (gH) proteins, revealing that only a fraction of infected cells progressed to a productive infection. In both cell types, cell surface gB (and gH in Caco-2 cells) accumulated at the apical surface of the cells, which was consistent with the release of virus into the apical supernatant. A comparison with the number of ARPE-19 cells productively infected was not possible, as the percentage of these cells expressing E and L proteins was not shown in the study of Tugizov et al. (30). The incongruity observed for Caco-2 cells between the number of infected (IE-positive) cells compared to the number of productively infected (E- and L-positive) cells has also been observed for epithelial cells in the GI tract in vivo (26). The mechanism(s) responsible for this restriction of viral replication in Caco-2 cells in vitro and in GI epithelial cells in vivo is not known. Surprisingly, preliminary studies showed chemical agents such as sodium butyrate, retinoic acid, and dexamethasone that are capable of inducing HCMV replication in a number of different cell types (11, 24, 28, 34) were unable to increase the number of Caco-2 cells that progressed to a productive infection (data not shown).
In Caco-2 cells, similar to ARPE-19 cells (30), HCMV was cytopathic and resulted in a disruption of the monolayer at a comparable rate. In Caco-2 cells, CPE was observed by a decrease in TER and the presence of holes in the monolayer by immunofluorescence. Histologically, HCMV infection of Caco-2 cells resulted in cytomegaly with basophilic cytoplasmic and nuclear inclusions similar to those in HCMV-infected cells in vivo (3). The presence of an E protein (p52) in all Caco-2 cells exhibiting CPE, combined with the observation that a similar number of cells expressed gB and gH, suggests that CPE was a characteristic of productively infected cells as has been proposed for HCMV-infected cells in vivo (26). By electron microscopy, the apical brush borders of all infected cells containing nuclear capsids were deficient of villi, suggesting a more subtle effect of the virus on these cells prior to complete cytolysis (data not shown). In ARPE-19 cells, HCMV similarly caused a decrease in the TER of the cell monolayer; however, immunohistochemistry and electron microscopy of infected cells were not conducted in these studies (30). Maidji et al. (18) have suggested that the increased paracellular permeability in ARPE-19 cells was mediated by HCMV protein US9. However, in Caco-2 cells the drop in TER was observed only at late times coincident with the appearance of progeny virus. As US9 is expressed only at early times of infection (16), the drop in TER in Caco-2 cells was likely due to a direct consequence of the CPE of the production of progeny virus in these cells, not an effect of US9. Regardless of the precise mechanism, the ability of HCMV to induce cytopathology in Caco-2 and ARPE-19 cells in vitro demonstrates that HCMV can cause direct damage to these different epithelial cell types. However, the degree to which a direct cytopathic, compared to an indirect ischemic, effect of the virus contributes to pathology observed in vivo remains to be established.
Although HCMV infection of Caco-2 and ARPE-19 cells exhibited many similarities, the two cell types also displayed a number of significant differences. First, the membrane surface at which infection occurred differed between the two cell types. In Caco-2 cells, virus entered preferentially at the basolateral membrane, whereas in ARPE-19 cells, virus was shown to enter preferentially at the apical membrane surface. The difference in polarity of infection between Caco-2 and ARPE-19 cells may represent a difference in the behavior of HCMV in retinal compared to nonretinal epithelial cells, as it is consistent with the proposed hematogenous spread of HCMV in these tissues in vivo. Hematogenous spread requires that virus is able to enter the membrane surfaces of epithelial cells oriented toward the vessels carrying the HCMV-infected blood. In the retina, retinal pigment epithelial cells are oriented with their apical surfaces toward the overlying retinal arterioles and veins that appear to be the source of HCMV infection within the retina; the basolateral surface of these cells faces the underlying choroid capillary layer, which does not appear to be involved in the spread of infection to the overlying retinal layers. Likewise, in the GI tract, epithelial cells are oriented with their basolateral surfaces toward the underlying capillaries and venules of the lamina propria and submucosal regions which appear to be source of HCMV infection. Consequently, the apical surface of retinal pigment epithelial cells and the basolateral surface of the GI epithelial cells would be expected to be susceptible to viral entry, which is exactly what we observe for the polarity of HCMV entry in Caco-2 compared to ARPE-19 cells. However, this does not exclude the possibility that other factors may also contribute to the polarity of infection observed in vivo. For example, in the retina the proposed inability of HCMV to pass through the relatively impermeable Bruch’s membrane that separates the basolateral surface of the retinal pigment epithelial cells from the choroid capillaries has been suggested to account for the lack of viral spread from these vessels into overlying retinal layers (12). At very early times postconfluence (TER of 25 Ω · cm2), HCMV infection did appear to occur preferentially at the apical surface. At this time, medium is able to flow freely between the apical and basolateral compartments. Consequently, the preferential infection at the apical membrane presumably reflects a reduced (approximately threefold) ability of HCMV to gain access to the basolateral surface of the cells through the 3-μm-pore-size filters.
Caco-2 and ARPE-19 cells also differed in the ability to support the spread of infection from cell to cell in the presence of neutralizing antibodies. In our studies with Caco-2 cells, HCMV did not spread from cell to cell, which was consistent with previous studies of HF cells in vitro, but in variance with studies of HCMV replication in ARPE-19 cells (23, 30). Because HCMV spreads inefficiently in individuals with competent immune systems, the importance of lateral cell-to-cell spread for HCMV in vivo is questionable. Indeed, even in heavily immunosuppressed individuals with significantly impaired immune responses, lesions within the retina and GI tract are focal.
The ability of HCMV to productively infect Caco-2 cells was differentiation state dependent, with maximal infection occurring at early stages of differentiation (≤250 Ω · cm2). The dependency of HCMV permissiveness on cellular differentiation state has been observed for many other cell types, including Tera-2 cells (11, 24), macrophages (13), and TPC-1 cells (28). However, in all of these cells the restriction of productive HCMV infection was due to the inactivity of the MIEP. HCMV replication has also been shown to be reduced in confluent monolayers of nonpolarized fibroblasts, presumably as a consequence of decreased metabolic activity. In contrast, in Caco-2 cells, our experiments using the AdgD1(E1−) expressing a reporter gene (encoding gD) under the control of the HCMV MIEP demonstrate that Caco-2 cells can support expression of the MIEP at all stages of differentiation. Consequently, the observed decrease in number of IE86-positive cells at late stages of differentiation in Caco-2 cells must be due to a block prior to IE expression. HCMV entry involves an initial heparan-dependent attachment that is followed by a receptor-dependent fusion between the viral envelope and the cellular membrane (6). The ability of EGTA pretreatment of cells at a TER of 350 Ω · cm2 to increase their level of infection was consistent with the inability of virus to enter these cells being due to the inaccessibility of a viral receptor in the more differentiated cell type. Interestingly, a similar behavior was observed for Listeria monocytogenes infection of Caco-2 cells (10). Similar to HCMV, Listeria infection of Caco-2 cells occurred preferentially at the basolateral surface, was impaired in cells at later times of differentiation, and could be returned to maximal levels in these cells by pretreatment of the cells with EGTA, showing that the restriction was due to an inaccessibility of a cellular receptor. Recently, the cellular receptor for L. monocytogenes was shown to be the adhesion molecule E-cadherin, which suggests that its inaccessibility with increased differentiation may be due its sequestration into lateral junctional complexes (20). This would suggest that the receptor for HCMV may similarly be a junctional protein localized at the lateral membrane in differentiated Caco-2 cells. However, alternative explanations for the observed effect of EGTA on Caco-2 cell monolayers are possible. For example, the loss of tight junctions may result in the reorganization of intracellular components such as actin and microtubles which could be required for the targeted transport of the incoming virion capsid to the nucleus.
In summary, the Caco-2 cell line represents an HCMV-permissive, well-characterized line that grows quickly in culture and is representative of epithelial cells from all nonretinal tissues of the body. We propose that HCMV infection of Caco-2 cells is an ideal system in which to study HCMV pathogenesis in a polarized epithelial cell type that is relevant to HCMV infection of epithelial in vivo.
ACKNOWLEDGMENTS
We thank David C. Johnson and Ashlee V. Moses for invaluable advice throughout the project, and we thank David C. Johnson for supplying the AdgD1(E1−) and anti-HSV-1 gD antibodies DL6 and LP2. We are also grateful to Aurelie Snyder at the OHSU Core Laboratory Facility, Michael Webb at the Oregon Regional Primate Research Centre, and Alex J. Merz from the laboratory of Maggie So for technical advice throughout this study, and we thank Andrew Townsend at Extreme Images for assistance with graphic illustrations.
REFERENCES
- 1.Britt W J, Alford C A. Cytomegalovirus. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. New York, N.Y: Lippincott-Raven Press; 1996. pp. 2493–2523. [Google Scholar]
- 2.Britt W J, Vugler L, Butfiloski E J, Stephens E B. Cell surface expression of human cytomegalovirus (HCMV) gp55-116 (gB): use of HCMV-recombinant vaccinia virus-infected cells in analysis of the human neutralizing antibody response. J Virol. 1990;64:1079–1085. doi: 10.1128/jvi.64.3.1079-1085.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burakoff R, Eglow R. Infections of the esophagus. In: Haubrich W S, Schaffner F, Berk J E, editors. Bockus gastroenterology. 5th ed. Philadelphia, Pa: The W. B. Saunders Company; 1995. pp. 503–517. [Google Scholar]
- 4.Cha T-A, Tom E, Kemble G W, Duke M, Mocarski E S, Spaete R R. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J Virol. 1996;70:78–83. doi: 10.1128/jvi.70.1.78-83.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chejfec G. Diseases of the digestive system: esophagus. In: Damjanov I, Linder J, editors. Anderson’s pathology. 10th ed. St. Louis, Mo: Mosby; 1996. pp. 1647–1660. . 1660. [Google Scholar]
- 6.Compton T, Nowlin D M, Cooper N R. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology. 1993;193:834–841. doi: 10.1006/viro.1993.1192. [DOI] [PubMed] [Google Scholar]
- 7.Crawford S W. Respiratory disease in bone marrow and hematopoietic stem cell transplantation. In: Fishman A P, editor. Fishman’s pulmonary diseases and disorders. 3rd ed. New York, N.Y: McGraw-Hill; 1998. pp. 2137–2152. [Google Scholar]
- 8.Drew W L, Jacobson M A. Cytomegalovirus. In: Cohen P T, Sande M A, Volberding P A, editors. The AIDS knowledge base: a textbook on HIV disease from the University of California, San Francisco, and the San Francisco General Hospital. 2nd ed. New York, N.Y: Lippincott-Raven Press; 1994. pp. 6.13-1–6.13-12. [Google Scholar]
- 9.Fish K N, Depto A S, Moses A V, Britt W, Nelson J A. Growth kinetics of human cytomegalovirus are altered in monocyte-derived macrophages. J Virol. 1995;69:3737–3743. doi: 10.1128/jvi.69.6.3737-3743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaillard J-L, Finlay B B. Effect of polarization and differentiation on entry of Listeria monocytogenes into the enterocyte-like Caco-2 cell line. Infect Immun. 1996;64:1299–1308. doi: 10.1128/iai.64.4.1299-1308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonczol L, Andrews P W, Plotkin S A. Cytomegalovirus replicates in differentiated but not in undifferentiated human embryonal carcinoma cells. Science. 1984;224:159–161. doi: 10.1126/science.6322309. [DOI] [PubMed] [Google Scholar]
- 12.Ho M. Cytomegalovirus. In: Mandell G L, Gordon R, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1992. pp. 1351–1364. [Google Scholar]
- 13.Ibanez C E, Schrier R, Ghazal P, Wiley C, Nelson J A. Human cytomegalovirus productively infects primary differentiated macrophages. J Virol. 1991;65:6581–6588. doi: 10.1128/jvi.65.12.6581-6588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isola V J, Eisenberg R J, Siebert G R, Heilman C J, Wilcox W C, Cohen G H. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J Virol. 1989;63:2325–2334. doi: 10.1128/jvi.63.5.2325-2334.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeang K T, Chin G, Hayward G S. Characterization of cytomegalovirus immediate-early genes. I. Nonpermissive rodent cells overproduce the IE94K protein from CMV (Colburn) Virology. 1982;121:393–403. doi: 10.1016/0042-6822(82)90177-5. [DOI] [PubMed] [Google Scholar]
- 16.Jones T R, Muzithras V P. Fine mapping of transcripts expressed from the US6 gene family of human cytomegalovirus strain AD169. J Virol. 1991;65:2024–2036. doi: 10.1128/jvi.65.4.2024-2036.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jourdan N, Maurice M, Delautier D, Quero A M, Servin A L, Trugnan G. Rotavirus is released from the apical surface of cultured human intestinal cells through nonconventional vesicular transport that bypasses the Golgi apparatus. J Virol. 1997;71:8268–8278. doi: 10.1128/jvi.71.11.8268-8278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maidji E, Tugizov S, Jones T, Zheng Z, Pereira L. Accessory human cytomegalovirus glycoprotein US9 in the unique short component of the viral genome promotes cell-to-cell transmission of virus in polarized epithelial cells. J Virol. 1996;70:8402–8410. doi: 10.1128/jvi.70.12.8402-8410.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mariadason J M, Barkla D H, Gibson P R. Effect of short-chain fatty acids on paracellular permeability in Caco-2 intestinal epithelium model. Am J Physiol. 1997;272:G705–G712. doi: 10.1152/ajpgi.1997.272.4.G705. [DOI] [PubMed] [Google Scholar]
- 20.Mengaud J, Ohayon H, Gounon P, Mege R-M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 21.Molloy S S, Thomas L, VanSlyke J K, Stenberg P E, Thomas G. Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J. 1994;13:18–33. doi: 10.1002/j.1460-2075.1994.tb06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muggeridge M I, Wu T T, Johnson D C, Glorioso J C, Eisenberg R J, Cohen G H. Antigenic and functional analysis of a neutralization site of HSV-1 glycoprotein D. Virology. 1990;174:375–387. doi: 10.1016/0042-6822(90)90091-5. [DOI] [PubMed] [Google Scholar]
- 23.Navarro D, Paz P, Tugizov S, Topp K, La Vail J, Pereira L. Glycoprotein B of human cytomegalovirus promotes penetration into cells, transmission of infection from cell to cell, and fusion of infected cells. Virology. 1993;197:143–158. doi: 10.1006/viro.1993.1575. [DOI] [PubMed] [Google Scholar]
- 24.Nelson J A, Groudine M. Transcriptional regulation of the human cytomegalovirus major immediate-early gene is associated with induction of DNase I-hypersensitivity sites. Mol Cell Biol. 1986;6:452–461. doi: 10.1128/mcb.6.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodgers V D. Enteric manifestations of immunodeficiency states. In: Haubrich W S, Schaffner F, Berk J E, editors. Bockus gastroenterology. 5th ed. Philadelphia, Pa: The W. B. Saunders Company; 1995. pp. 1146–1173. [Google Scholar]
- 26.Sinzger C, Grefte A, Plachter B, Gouw A S, The T H, Jahn G. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J Gen Virol. 1995;76:741–750. doi: 10.1099/0022-1317-76-4-741. [DOI] [PubMed] [Google Scholar]
- 27.Smith J D. Human cytomegalovirus: demonstration of permissive epithelial cells and nonpermissive fibroblastic cells in a survey of human cell lines. J Virol. 1986;60:583–588. doi: 10.1128/jvi.60.2.583-588.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka J, Sadanari H, Sato H, Fukuda S. Sodium butyrate-inducible replication of human cytomegalovirus in a human epithelial cell line. Virology. 1991;185:271–280. doi: 10.1016/0042-6822(91)90774-6. [DOI] [PubMed] [Google Scholar]
- 29.Topp K, Rothman A L, La Vail J L. Herpes virus infection of RPE and MDCK cells: polarity of infection. Exp Eye Res. 1997;64:343–354. doi: 10.1006/exer.1996.0209. [DOI] [PubMed] [Google Scholar]
- 30.Tugizov S, Maidji E, Pereira L. Role of apical and basolateral membranes in replication of human cytomegalovirus in polarized retinal pigment epithelial cells. J Gen Virol. 1996;77:61–74. doi: 10.1099/0022-1317-77-1-61. [DOI] [PubMed] [Google Scholar]
- 31.Uetsuki T, Naito A, Nagata S, Kaziro Y. Isolation and characterization of the human chromosomal gene for polypeptide chain elongation factor 1-alpha. J Biol Chem. 1989;264:5791–5798. [PubMed] [Google Scholar]
- 32.Vieira J, Schall T J, Corey L, Geballe A P. Functional analysis of the human cytomegalovirus US28 gene by insertion mutagenesis with the green fluorescent protein gene. J Virol. 1998;72:8158–8165. doi: 10.1128/jvi.72.10.8158-8165.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner B, Kropff B, Kalbacher H, Britt W, Sundqvist V A, Ostberg L, Mach M. A continuous sequence of more than 70 amino acids is essential for antibody binding to the dominant antigenic site of glycoprotein gp58 of human cytomegalovirus. J Virol. 1992;66:5290–5297. doi: 10.1128/jvi.66.9.5290-5297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinshenker B G, Wilton S, Rice G P A. Phorbol ester-induced differentiation permits productive human cytomegalovirus infection in a monocytic cell line. J Immunol. 1988;140:1625–1631. [PubMed] [Google Scholar]