Version Changes
Revised. Amendments from Version 3
There is an addition to the discussion regarding the effect of post-vaccination examination time on antibody levels. We also add a discussion on the relationship between antibodies in the blood and human milk.
Abstract
Background: Breast milk is a critical element in developing a baby’s immunity through immune transfer. Antibodies are an essential unit of immunity against infection with the SARS-CoV-2 virus. This paper explores antibodies in breast milk in postpartum women who have been vaccinated or exposed to coronavirus disease 2019 (COVID-19). Duration of antibody appearance was studied to determine the adequate time in transferring antibodies by breastfeeding.
Methods: Three databases, PubMed, Google Scholar, and ScienceDirect, were used as sources of articles. Inclusion criteria applied in selecting articles were prospective observational study or experimental design study in English, evaluating antibodies in breast milk, and conducted between 2019–2021. Article quality and risk of bias were assessed with Critical Appraisal Skills Programme (CASP). The data found were synthesized in a narrative manner.
Results: This systematic review included 20 articles. A total of 306 postpartum women who were infected with COVID-19, 20 postpartum women who had viral symptoms and 495 postpartum women who had been vaccinated were studied. Immunoglobulin A (IgA) and immunoglobulin G (IgG) antibodies were found in the breast milk of infected and vaccinated postpartum women. SARS CoV-2 infection is associated with the presence of IgA dominant, whereas vaccination is related to the presence of IgG dominant. Antibodies persisted from day 10 of onset to 10 months in infected postpartum women and started from three days to six weeks in vaccinated postpartum women. Meta-analysis could not be carried out due to the variety of articles.
Conclusions: Antibodies found in breast milk in infected and vaccinated postpartum women have different dominant types. Further research needs to be done regarding the mechanism of antibody transfer in breast milk, longer research duration and studies that directly examine the comparison of antibodies in breast milk in vaccinated and infected postpartum women.
Registration: PROSPERO ( CRD42022340859, 23 June 2022).
Keywords: COVID-19, maternal health, neonatal health, human milk, antibody, vaccine
Introduction
SARS CoV-2 became a worldwide pandemic and caused changes in all aspects of life including in mothers with postpartum and neonatal period. 1 Coronavirus disease 2019 (COVID-19) infection during pregnancy, birth and postpartum is associated with a significant increase in morbidity and mortality in mothers and babies. New mothers and their infants are more susceptible to infection compared to the general population. 2 COVID-19-related postpartum deaths have been reported in Brazil, with an estimated 106 deaths in 2020. 3 In addition, babies are also vulnerable to COVID-19 infection. The babies born to mothers infected with COVID-19 are more likely to be admitted to the neonatal unit. 4 Case overview of COVID-19 in children in previous studies were seen to be less frequent, less severe, and the mortality rate very low, but there is growing evidence that they are as susceptible as adults. 5 Infants with severe respiratory failure and the prolonged clinical course associated with SARS-CoV-2 exposure may be due to extreme prematurity, immature lungs, and immunocompromised status. 6
Breast milk is the only food needed in the first 6 months of life. 7 Breastmilk also the best source protection for babies. 8 Breast milk contains various kinds of antibodies that provides protection to the baby. One way for babies to get additional antibody protection is through vaccination. In the case of COVID-19, vaccination is one of the main protections but the provision of a COVID-19 vaccine for newborns and babies are not yet available. A recent study found that a COVID-19 vaccination regimen consisting of BNT162b2 was found to be safe, immunogenic, and efficacious in children aged five to 11 years. 9 When compared with infants, postpartum mothers have the opportunity to get antibodies through vaccination. The COVID-19 vaccine is an effective way to prevent COVID-19. 10 In influenza outbreaks in previous years, influenza vaccines have been shown to increase serum antibodies and reduce disease severity in both mother and baby.
Breastfeeding can protect for at least six months because breast milk contains consistently high levels of actively produced anti-influenza immunoglobulin A (IgA). The results of this research found that breastfeeding can provide protection for at least the first 6 months because breast milk contains consistently high levels of actively produced anti-influenza immunoglobulin A (IgA). Infants with fever have fewer episodes of respiratory illness, which implies that breastfeeding may provide local mucosal protection. 11 The risk of COVID-19 can be reduced through breastfeeding among children as has been documented for other infections compared to formula feeding. 12
The risk of being exposed to the virus and providing nutrition is a dilemma, especially regarding the content of substances contained in breast milk. Breastfeeding mothers do not understand the substances contained in breast milk, whether viruses or protective substances when giving it to their babies. This condition also exacerbated by the rise of misinformation on social media about the content of the SARS-CoV-2 virus in breast milk, causing breastfeeding issues during the pandemic. 13 Breastfeeding mothers will be distraught and ask themselves whether the coronavirus can be transmitted through breast milk and what they can do to protect themselves and their babies. 8 The World Health Organization (WHO) said breastfeeding does not need to be stopped during COVID-19 infection or after the mother’s vaccination. 14 Research conducted by United Nations Children’s Fund (UNICEF) in five countries in South Asia found that less than 25% of interviewees understood that it was safe to continue breastfeeding. They preferred to give formula milk, 15 whereas a meta-analysis conducted in 2020 showed that the SARS-CoV-2 genome is generally not found in the breast milk of breastfeeding mother infected with COVID-19. 16 After the virus content information has been clearly explained, the protection points become an essential part to know. Previous studies have found that there are antibodies in the breast milk of COVID-19-infected mothers, but the duration of the presence of these antibodies is unknown. 17 Infected and vaccinated postpartum women allow the formation of an antibody against COVID-19, but the long-term impact on antibody composition and functional activity is unclear. 18 Given this context, the purpose of this research is to determine the status of antibodies in breast milk following COVID-19 infection and vaccination. After finding records of the presence of antibodies, the duration of the appearance of antibodies will also be studied. Knowledge about the duration of antibodies in breast milk determines the adequate time in transferring antibodies by breastfeeding. The current results can be used as a guideline for recommendations to continue breastfeeding and health promotions that emphasize the presence of antibodies in breast milk in vaccinated postpartum mothers.
Methods
Study design and search strategy
The status of antibodies in breast milk after exposure to COVID-19 and after vaccination and the duration of antibodies appearing in breast milk were explored by compiling a systematic review. The preparation of this report follows the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines. 19 , 39 This systematic review is registered in PROSPERO ( CRD42022340859, 23 June 2022). Data were collected in July-September 2021. English-language research conducted from 2020 to 2021 according to topics was searched in PubMed (RRID:SCR_004846), Google Scholar (RRID:SCR_008878), and Science Direct. The keywords used were a combination of Medical Subject Heading (MeSH) terms and relevant keywords in a different order: “breast milk”, “COVID-19”, “antibody”, “immunoglobulin”, “vaccine”, “severe”, “acute respiratory syndrome coronavirus 2”, “coronavirus disease 2019”, “SARS-CoV-2”. The author also uses synonyms in the search. In the articles found, the researcher also examined the literature in the bibliography, including manuscripts that were not captured in the electronic literature search.
Study selection
The inclusion criteria applied in selecting articles were prospective observational study or experimental design study in English, evaluating antibodies in breast milk, and the study was conducted during the COVID-19 pandemic between 2019 and 2021. The exclusion criteria for this study were case reports, animal studies, letters to editors, study reviews, abstracts without full text. Manuscripts that only discussed breast milk in healthy postpartum or breastfeeding mothers were not included.
Data collection process
Two authors (EMK and NAR) performed title and/or abstract screening independently of the included articles using standard Microsoft Excel (RRID:SCR_016137) forms. The data obtained were combined in one folder and then an assessment was carried out. Each author analyzed all existing manuscripts, and then the results were compared with each other. A third external collaborator was consulted (Hari Paraton) to address disagreements in consensus.
Data items
The outcomes from this study were the presence of antibodies and the duration of the appearance of antibodies in breast milk. The antibodies studied were IgG and IgA against COVID-19. The tool used to detect the presence of antibodies is ELISA or similar tools. The authors realized that not all manuscripts had explanations for these two antibody levels, therefore the studies that only discussed one type of antibody were also included in the systematic review. The authors also realized that not all studies that discussed antibodies also explained the duration of the appearance. Based on the consideration of the limited number of articles, the authors discussed the articles that discusses the time when antibodies can be detected. Unclear information was included in the exclusion criteria.
Study risk of bias assessment
Authors conducted a risk of bias assessment study using critical appraisal tools. The Critical Appraisal Skills Programme (CASP) was used to assess the formal article by two independent research team members, EMK and NAR. The use of CASP is based on the 1994 JAMA ‘Users’ guides to the medical literature, which is used for both randomized controlled trials and systematic reviews. This checklist was adapted from Guyatt GH, Sackett DL, and Cook DJ and is used by health care practitioners. 20 CASP results are concluded into the category of moderate overall quality and low overall quality. 21 The results of the analysis are presented in Table 1.
Table 1. The results of the critical appraisal using CASP checklist.
| Author | Did the study address a clearly focused issue? | Was the cohort recruited in an acceptable way? | Was the exposure accurately measured to minimize bias? | Was the outcome accurately measured to minimize bias? | Have the authors identified all important confounding factors? | Have the authors taken account of the confounding factors in the design and/or analysis? | Was the follow up of subjects complete enough? | Was the follow up of subjects long enough? | How precise are the results? | Do you believe the results? | Can the results be applied to the local population? | Do the results of this study fit with other available evidence? | What are the implications of this study for practice? | Overall quality assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Juncker et al. 22 | Y | Y | Y | Y | Y | Y | C | C | IgA p < .001 and IgG p = .011 | Y | Y | Y | Y | M |
| Juncker et al. 40 | Y | Y | Y | Y | N | N | Y | Y | p = .070 | Y | Y | Y | Y | M |
| Narayanaswamy et al. 41 | Y | Y | Y | Y | Y | Y | C | C | p > 0.05 | Y | Y | Y | Y | M |
| Bäuerl et al. 42 | Y | Y | Y | Y | N | N | C | C | p < 0.0001 | Y | Y | Y | Y | M |
| Duncombe et al. 43 | Y | Y | Y | Y | N | N | C | C | NA | Y | Y | Y | Y | M |
| Pace et al. 44 | Y | Y | Y | Y | N | N | C | C | NA | Y | Y | N | Y | M |
| Fox et al. 45 | Y | Y | Y | Y | N | N | Y | Y | IgA p < 0.0001, and IgG p = 0.017 | Y | Y | N | Y | M |
| Demers-Mathieu et al. 46 | Y | Y | Y | Y | N | N | C | C | p = 0.002 | Y | Y | Y | Y | M |
| Lechosa-Muñiz et al. 23 | Y | Y | Y | Y | Y | Y | C | C | p < 0.001, p = 0.001 | Y | Y | Y | Y | M |
| Gray et al. 25 | Y | Y | Y | Y | Y | Y | Y | Y | p < .0001 | Y | Y | Y | Y | M |
| Low et al. 47 | Y | Y | Y | Y | N | N | Y | Y | NA | Y | Y | Y | Y | M |
| Jakuszko et al. 48 | Y | Y | Y | Y | N | N | C | C | p < 0.001 | Y | Y | Y | Y | M |
| Guida et al. 49 | Y | Y | Y | Y | N | N | C | C | p = 0.145 | Y | Y | Y | Y | M |
| Ramìrez et al. 50 | Y | Y | Y | Y | N | N | C | C | p < .001 | Y | Y | Y | Y | M |
| Perl et al. 51 | Y | Y | Y | Y | N | N | C | C | IgA p < .001 and IgG p = .004 | Y | Y | Y | Y | M |
| Golan et al. 30 | Y | Y | Y | Y | N | N | C | C | p < 0.0001 | Y | Y | Y | Y | M |
| Valcarce et al. 52 | Y | Y | Y | Y | Y | Y | C | C | p = 0.005 | Y | Y | Y | Y | M |
| Selma-Roy et al. 24 | Y | Y | Y | Y | Y | Y | C | C | p < 0.0001 | Y | Y | Y | Y | M |
| Juncker et al. 53 | Y | Y | Y | Y | Y | Y | C | C | p < .001 | Y | Y | Y | Y | M |
| Rosenberg-Friedman et al. 54 | Y | Y | Y | Y | Y | Y | C | C | p < 0.0125 | Y | Y | Y | Y | M |
Table 2. Characteristics of research on breastfeeding mothers infected with COVID-19 and antibody status in breast milk.
| Authors, year, country | Research time | Study type | COVID-19 confirmation | Control group | Sampling time | No. of infected mothers | Antibody test | No. of mothers showing antibodies, n (%) | Time when antibodies can be detected | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IgA | IgG | IgM | |||||||||
| Juncker et al., 22 2021, Netherlands | October 2020 and February 2021 | Prospective cohort | PCR | Healthy lactating mother | An average of eight weeks after getting a positive result | 165 | ELISA | 98 (59) | No data available | No data available | 10 months after COVID-19 confirmation |
| Juncker et al., 40 2021, Netherlands | May–August 2020 | Longitudinal follow-up | PCR and symptoms | No control used in this research | After the clinical symptoms in 14–143 days | 24 | ELISA | 24 (100) | No data available | No data available | Five months (143 days) after the onset of COVID-19 symptoms |
| Narayanaswamy et al., 41 2021, MMC Worchester, MA | March–September 2020 | Prospective cohort | PCR | Bilateral colostrum sample pre-pandemic as control (n = 8) | 48 hours of delivery | 15 | ELISA | 11 (73) | 11 (73) | 5 (33) | No data available |
| Bäuerl et al., 42 2021, Spain | April–December 2020 | Prospective multicenter longitudinal study | PCR | Whole milk pre-pandemic control (n = 13) | Before and after delivery <7 days and 15 days | 60 | ELISA | 51 (72.9) | 45 (64.3) | 51 (72.9) | 206 days after PCR confirmation |
| Duncombe et al., 43 2021, USA | No data available | Prospective longitudinal cohort study | PCR | PCR negative control (n = 3) | Three weeks to six months post-illness onset | 2 | ELISA | 2 (100) | 2 (100) | 1 (50) | 10 days to six months after symptom onset |
| Pace et al., 44 2021, USA | No data available | Prospective study | PCR | Milk sample pre-pandemic control (n = 10) | Average 12.0 ± 8.9 days after onset of symptoms | 18 | ELISA | 14 (76) | 15 (80) | No data available | No data available |
| Fox et al., 45 2020, USA | December 2019 | Prospective study | PCR and symptoms | Milk sample pre-pandemic control (n = 10) | 14–30 days after symptoms | 15 | ELISA | 12 (80) | 2 (13) | 1 (7) | No data available |
| Demers-Mathieu et al., 46 2021, USA | No data available | Unpaired experimental design | PCR | 20 with viral symptom control and 16 unexposed | 16–84 days after infection | 7 | ELISA | Y, no data available | Y, no data available | Y, no data available | No data available |
Note: COVID-19 = coronavirus disease 2019, IgA = immunoglobulin A, IgG = immunoglobulin G, IgM = immunoglobulin M.
Table 3. Characteristics of research on vaccinated breastfeeding mothers and antibody status in breast milk.
| Authors | Year | Country | Research time | Study type and sample timing | Vaccine type | Number of vaccinated women and the dose given | Antibody test | Antibody type | Finding | Time when antibodies can be detected | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgA | IgG | IgM | ||||||||||
| Lechosa-Muñiz et al. 23 | 2021 | Spain | 1 April 2021 to 30 April 2021 | Observational study 30 days after the second dose of the vaccine and 30 days after the first dose. | BNT162b2 mRNA-1273 ChAdOx1-S | 20 women with a single dose of ChAdOx1-S. 70 women with two doses of BNT162b2, 20 women with two doses of mRNA-1273. | ELISA | Yes | Yes | NA | Breastfeeding mothers offer their infants IgA and IgG isotype antibodies directed against SARS-CoV-2 protein S in breast milk. | No data available |
| Gray et al. 25 | 2021 | No data available | 17 December 2020, and 23 February 2021 | Prospective cohort study two to six weeks after the second vaccine. | BNT162b2 Pfizer/BioNTech or mRNA-1273 Moderna/National Institutes of Health (NIH) | 31 women with two doses of RNA vaccine. | ELISA | Yes | Yes | Yes | The second vaccine dose increased IgG, but not IgA, in maternal blood and breast milk. | No data available |
| Low et al. 47 | 2021 | Singapore | No data available | Longitudinal study. Five time points: pre-vaccination (T1), 1–3 days after dose one (T2), 7–10 days after dose 1 (T3), 3–7 days after dose two of COVID-19 mRNA vaccine (T4), and 4–6 weeks after dose two of COVID-19 mRNA vaccine (T5). | BNT162b2 (Pfizer-BioNTech) | 14 lactating healthcare workers after the second dose | ELISA | Yes | Yes | NA | 86% (12/14) of the individuals produced SARS-CoV-2-specific IgA, and 100% (14/14) individuals produced SARS-CoV-2-specific IgG in human milk. | 86% of individuals produce SARS-CoV-2-specific IgA, and 100% of individuals produce SARS-CoV-2-specific IgG in breast milk within 3-7 days after administration second dose of BNT162b2 vaccine and decreased over time. Significant IgG decreased to 4-6 weeks after the second dose. |
| Jakuszko et al. 48 | 2021 | Poland | No data available | Prospective cohort Sampling after the first dose of vaccine, on day 8 ± 1, on day 22 ± 2 (immediately before the second dose), on day 29 ± 3 (which is also day 7 ± 3 after the second dose), and on day 43 ± 4 (which is also day 21 ± 4 after the second dose). | mRNA vaccine BNT162b2 (Pfizer-BioNTech) | 32 breastfeeding women. | ELISA | Yes | Yes | NA | There are IgG and IgA in breast milk. IgG is detectable in serum levels. | Serum and breast milk antibody concentrations were highest on day 29 ± 3 and decreased on day 43 ± 4. The immune response to vaccination was most vital 7 ± 3 days after the second dose of vaccine. Strong secretion of IgA and IgG antibodies in breast milk for six weeks after vaccination. |
| Guida et al. 49 | 2021 | Italy | No data available | Cohort prospective observational study, 17 breastfeeding women and 10 volunteers 20 days after the first dose and seven volunteers seven days after the second dose. | mRNA BNT162b2 vaccine | 10 women after the first dose, seven women after the second dose. | ELISA | Yes | Yes | No | Anti-SARS CoV-2 S-IgA, IgG, and IgM antibodies in the breast milk of vaccinated women. | 7 days after the second dose. |
| Ramìrez et al. 50 | 2021 | No data available | 22 February to 4 April 2021 | Prospective cohort, 14 days after their second dose of vaccine. | 94%, BNT162b2 mRNA COVID-19 vaccine and 6% COVID-19 mRNA-1273 vaccine | 98 vaccinated, and the 24 control participants. | The SARS-CoV-2 IgG Architect Abbott® | Yes | Yes | NA | All participants had IgG antibodies, and 89% of them had IgA antibodies against SARS-CoV-2 in breast milk. | No data available |
| Perl et al. 51 | 2021 | Israel | December 2020 and January 2021 | Prospective cohort study before administration of the vaccine and then once weekly for six weeks starting at week two after the first dose. | All participants received two doses of the Pfizer-BioNTech | 44 breastfeeding women after the first dose. | ELISA | Yes | Yes | NA | Strong neutralizing effects, protective effect against infection in the infant due to SARS-CoV-2. | SARS-CoV-2-specific IgA and IgG antibodies in breast milk six weeks after vaccination. IgA secretion was early, followed by a spike in IgG after four weeks (a week after the second vaccine). |
| Golan et al. 30 | 2021 | USA | December 2020 to June 2021 | Prospective cohort, 50 breastfeeding women collected from a subset of infants whose mothers received the vaccine during lactation or pregnancy (4–15 weeks after mothers’ second dose). | mRNA46-based vaccines for COVID-19 (mRNA-1273 and BNT162b2) | 50 breastfeeding mothers after second dose. | ELISA | Yes | Yes | Yes | Anti-SARS-CoV-2 IgG and IgM levels significantly increased in maternal plasma, and significant transfer of anti-SARS-CoV-2 IgA and IgG antibodies to milk. Anti-SARS-CoV-2 IgG antibodies were not detected in the plasma of infants whose mothers were vaccinated during lactation. | No data available |
| Valcarce et al. 52 | 2021 | USA | December 2020 to March 2021 | Prospective observational study, 22 lactating health care workers. There were three time points (pre-vaccination, post-first vaccine, and post-second vaccine doses). | SARS-CoV-2 mRNA vaccine (Pfizer-BioNTech or Moderna) | 20 lactating health care mothers. | ELISA | Yes | Yes | Yes | Significant secretion of SARS-CoV-2-specific IgA and IgG in human milk and plasma is discovered after SARS-CoV-2 vaccination. | No data available |
| Selma-Royo et al. 24 | 2021 | Spain | January to April 2021 | Prospective cohort observational study, 75 lactating women. Seven time points were collected from baseline up to 25 days after the 1st dose, and same points were collected for mRNA vaccines 30 days after second dose. | mRNA vaccines (BNT162b2 and mRNA-1273) and adenovirus-vectored vaccine (ChAdOx1 nCoV-19) | 75 lactating women. | ELISA | Yes | Yes | NA | High intervariability for IgA antibodies. IgG levels were significantly higher than those observed in milk from women who had COVID-19, while IgA levels were lower. | No data available |
| Juncker et al. 53 | 2021 | Netherlands | 2021 | Prospective longitudinal study, 16 samples of human milk were collected according to a schedule: one sample before the first vaccination and one sample on days 3, 5, 7, 9, 11, 13, and 15–17 days after the first vaccination. This schedule was the same for the second vaccination. | mRNA based BNT162b2 vaccine | 20 women after the second dose, six women after the first dose. | ELISA | Yes | Yes | NA | A SARS-CoV-2-specific antibody IgA response was observed in human milk. | IgA starting to increase between days 5–7 after the first dose and declining after day 15, on average. |
| Rosenberg-Friedman et al. 54 | 2021 | No data available | 2021 | Prospective cohort study approximately five months postpartum in the first dose (mean 154 days, range 68−382) and the second dose 21 days later. | BNT162b2 mRNA vaccination | 10 lactating health care providers in first dose and second dose. | ELISA | Yes | Yes | NA | IgG and IgA with neutralization capacity. | 14 days after the second dose. |
Note: COVID-19 = coronavirus disease 2019, IgA = immunoglobulin A, IgG = immunoglobulin G, IgM = immunoglobulin M.
Effect measures
Due to the limited number of manuscripts, a meta-analysis could not be completed. The effect that was measured in this study was the number of respondents’ breast milk presentations where antibodies were found in breast milk. The obtained durations were also combined descriptively.
Data abstraction and synthesis
The study selection process was through a review of the inclusion and exclusion criteria. As suggested in a systematic review of the literature, the analysis in this study was based on the findings of each study. The steps started from extracting the relevant results, sorting, and examining them to identify sub-themes and themes. The type of synthesis used is in the form of narrative synthesis. Narrative synthesis was chosen because it allowed authors to gather insight into the antibody content in breast milk and we did not perform this meta-analysis. The data are arranged in Table 2 contains the characteristics of research on mothers infected with COVID-19 and antibody status in breast milk and is written in a systematic table including (1) authors, year, country, (2) research time, (3) study type, (4) COVID-19 confirmation, (5) control group, (6) sampling time, (7) number of infected mothers, (8) antibody test, (9) number of mothers showing antibodies, and (10) duration time. Table 3 contains characteristics of research on mothers with COVID-19 vaccination and antibody status in breast milk and is written in a systematic table including (1) authors, year, country, (2) research time, (3) study type and sample timing, (4) vaccine type, (5) number of vaccinated women and the dose given, (6) antibody test, (7) type of antibody, (8) finding, and (9) duration time. Heterogeneity in the data was explored including duration of baseline antibody measurement and sample characteristics.
Reporting bias assessment
The researcher was unable to form the funnel plot due to the limited number of the manuscript. Funnel plots have a similar function to forest plots. This plot serves to assess the intervention effect of individual studies on some measure or precision of each study. The risk assessment of bias in this review was carried out using a risk approach that emerged and was reported as a research limitation.
Certainty assessment
The main domain used to assess the certainty of evidence was the risk of bias through critical appraisal and the inconsistency of the results of the research included in the review.
Results
Results of article screening
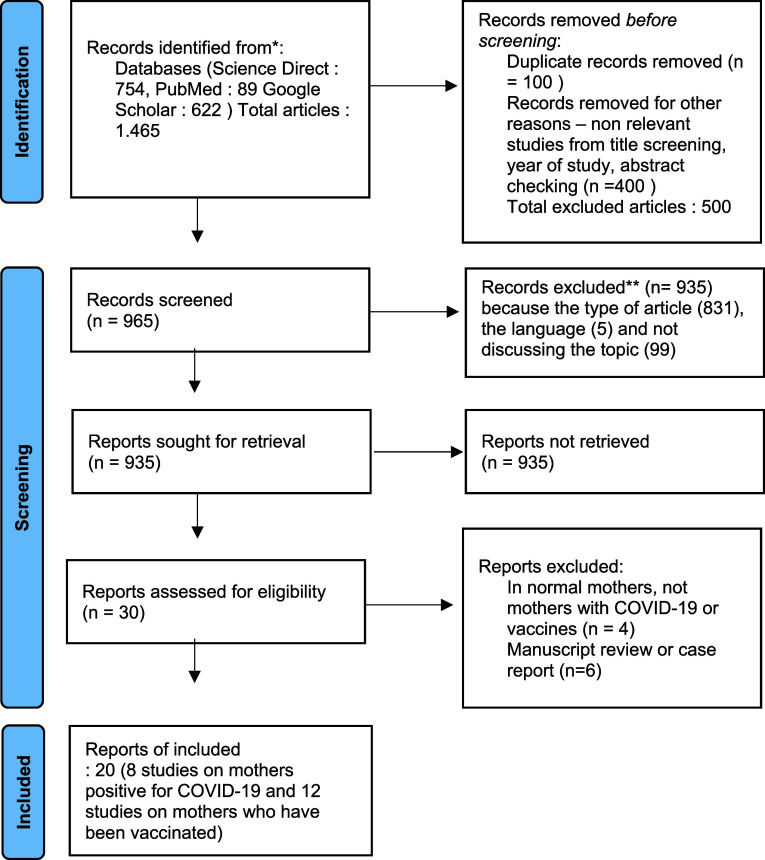
In searching the database, 1,465 abstracts were found from searches with relevant keywords. The authors then screened results for possible inclusion. The findings of 935 articles were excluded from the eligibility list. The articles did not meet the criteria, including the type of manuscript, the language used, and the topics discussed under the proposed title. After this stage, the researcher tried to re-examine the assessment results, but the manuscript finally entered the exclusion criteria. After screening, 30 full-text articles were selected and examined in detail to determine eligibility. Furthermore, 20 articles were determined that met the requirements. There were eight studies on the antibody status of infected breastfeeding mothers and 12 studies on antibody status of vaccinated breastfeeding mothers included. Figure 1 shows the study selection flowchart. The quality of the research is in the moderate category and is arranged in Table 1.
Figure 1. Flowchart study selection.
COVID-19, coronavirus disease 2019.
Antibody status in breast milk from infected breastfeeding mother
A total of 306 breastfeeding mothers who were positive for COVID-19 and 20 of them who had viral symptoms and had a very high likelihood that they had been exposed to COVID-19 were studied to determine antibody status in breast milk. Samples were taken within 48 hours after delivery to six months after infection. Table 2 describes characteristics of research on breastfeeding mothers infected with COVID-19 and antibody status in breast milk. The samples included in this review are breastfeeding mothers with positive PCR confirmation and mothers who had a very high likelihood that they had been exposed to COVID-19 when viewed from the symptoms they had. Almost all studies used controls, namely comparisons with healthy breastfeeding mothers and breastmilk samples taken during the pre-pandemic period. One study did not include controls, namely a longitudinal study. Detection of all antibodies was performed using ELISA. Most studies found that breast milk contains immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM). IgA was the dominant antibody found, while IgG and IgM were not always found in individuals. However, infected breastfeeding mothers did not always have IgA antibodies against COVID-19, which was found in a study by Juncker et al. (2021), who found that 59% of mothers had these antibodies. 22
Antibody status in breast milk from vaccinated breastfeeding mother
The characteristics of research on vaccinated breastfeeding mothers and antibody status in breast milk can be seen in Table 3. A total of 495 breastfeeding mothers who had been vaccinated, received the first and second doses of various types of vaccines and had never had COVID-19 were analyzed. IgG and IgA antibodies were found in the breast milk of vaccinated mothers. No studies showed IgM data. Administration of the second dose of the vaccine was associated with an increase in IgG in breast milk. Despite evidence of increased antibodies, anti-SARS-CoV-2 IgG antibodies were not detected in the plasma of infants whose mothers were vaccinated during breastfeeding. 30 This study is one of 20 studies examining infant plasma. In the study, infant blood was collected with a heel stick at 5-15 weeks after breastfeeding mothers were vaccinated the second dose. Eight blood samples were collected from infants aged 68 days to 1 year. IgG levels were higher than IgA levels in the breast milk of vaccinated breastfeeding mothers.
Time when antibodies can be detected
In mothers infected with COVID-19, the appearance of antibodies was detected on day 10 and could persist for up to 10 months. IgA levels are known to decrease over time, while IgG is relatively stable. In vaccinated mothers, antibody status differed between mothers vaccinated with first dose and second dose Most studies found the presence of antibodies after the first dose, although in small amounts. Early appearance could be detected three days after vaccination up to six weeks in both first dose and second dose of vaccination. The highest levels and immune response were found about four weeks after vaccination, but at the second dose, the antibody appeared between days four and 10. After that, there may be a chance to experience a decline before the sixth week, especially on day 15 or day 43 ± 4. These studies stopped examining antibodies at the sixth week. Therefore, the data provided are limited to detection at the sixth week.
Comparison of antibody levels by type of vaccine
Not all studies address differences in antibody levels based on the type of vaccine received. There are four studies discussing antibodies in blood and breast milk samples. Research that discusses the comparison of antibodies in breast milk conducted by Lechosa-Muñiz et al. (2021), 23 Selma-Royo et al. (2021), 24 Gray et al. (2021) 25 and Valcarce et al. (2021). 52 The mean antibody titers found in breast milk were found to be different for each vaccine type, 0.41, 0.45, and 0.09 (AU) for mothers who received BNT162b2, mRNA-1273, and ChAdOx1-S (one dose), respectively. No differences could be found between those vaccinated with BNT162b2 vs. mRNA-1273. 23 Research conducted by Selma-Royo et al. (2021) found that mothers vaccinated with Moderna and BioNTech/Pfizer after the first dose had a higher increase in breast milk anti-SARS-CoV-2 IgG than Oxford/AstraZeneca ( p < 0.0001) and BioNTech/Pfizer ( p = 0.002 ). In addition, the number of doses received also determines the amount of antibodies present in the breastmilk. COVID-19 vaccination induced anti-SARS-CoV-2 IgA and IgG in breast milk with higher levels after the 2nd dose. 24 This is consistent with the study of Gray et al. (2021) who found that booster doses of the vaccine increased SARS CoV-2 IgG, but not IgA in breast milk. 25 Another study conducted by Valcarce et al. (2021) found that both mRNA vaccines produced statistically significant SARS-CoV-2-specific IgA and IgG in breast milk and plasma. 52
In serum, when analyzing IgG, the mean antibody titres observed in the serum of lactating mothers were different according to the type of vaccine they received, being 0.32, 0.30, and 0.16 (AU) for mothers who received BNT162b2, mRNA-1273, and ChAdOx1-S (one dose), respectively. When analyzing IgA, the mean antibody titres observed in the serum of lactating mothers showed differences according to the type of vaccine administered: 0.12, 0.16, and 0.02 (AU) for mothers who received BNT162b2, mRNA-1273, and ChAdOx1-S (one dose) vaccines, respectively. 23 In the comparison of antibodies from samples in the maternal sera and umbilical cord in a study conducted by Gray et al. (2021) found that neutralizing antibody titers were lower in the umbilical cord than maternal sera, although this proved not to be statistically significant ( p = 0.05). Vaccine booster doses increase SARS CoV-2 IgG, but not IgA, in blood. 25 The mean of SARS-CoV-2 IgG in plasma was a significantly higher at post second vaccine dose in mothers vaccinated with Pfizer vs. mothers vaccinated with Moderna ( p = 0.005). 52 The type of vaccine, the number of doses, and the location of sampling have a role in the number of antibodies found.
Discussion
The antibody status in the breast milk of breastfeeding mothers who have been exposed to COVID-19 or are highly suspected and those who have been vaccinated against COVID-19 differs. The dominant antibody in mothers infected with COVID-19 is IgA, while the predominant antibody in vaccinated mothers is IgG, according to Young et al. (2021). 18 Vaccination is associated with an increase in dominant IgG and IgA antibodies after direct viral infection. These results differentiate antibodies in breast milk between vaccinated and infected mothers with COVID-19. Breast milk from both groups, namely mothers who are infected with COVID-19 and mothers who are vaccinated showed neutralizing activity against live SARS-CoV-2 virus, which could be attributed to SARS-CoV-2 IgA and IgG antibodies. 18 The dominant IgA response in breast milk can be found according to previous studies, namely the result of natural infection. 26
Antibody transfer through breast milk is an evolutionary strategy for enhancing immunity early in life. 27 Research conducted by Pullen et al. (2021) in which they applied a serologic system to characterize SARS-CoV-2 specific antibodies in maternal serum and breast milk found a preferential transfer of antibodies capable of causing neutrophil phagocytosis and neutralization. Distinct SARS-CoV-2-specific antibody response was observed in serum and breast milk from individuals previously infected with SARS-CoV-2, with a predominant transfer of IgA and IgM into breast milk. 27 IgA is the most essential class of Ig provided by breast milk to infants because it acts in the intestines while the function of secretory IgA (SIgA) in infants is still in development. 28 The presence of IgM antibodies was found in this study although not all studies examined the presence of IgM even if only in a small sample. The presence of IgM in some samples suggests the possibility that breast milk may have a protective effect on the newborn. 29
Although IgG are present in breast milk, they are functionally attenuated. 27 Emerging data from vaccinated pregnant and lactating women suggest that vaccine-induced transfer may be altered due to unusually high levels of IgG antibody induced by mRNA vaccines approved by the current Emergency Use Authorization. 25 Vaccine gives the baby strong IgA and IgG antibodies and may increase immunity compared to natural infection. 27 Nevertheless, the IgG transfer scheme still needs to be studied further because this study has not been able to find out about the transfer mechanism. A study conducted by Golan et al. (2021) found that no IgG was found in the plasma of infants whose mothers were vaccinated during lactation. 30 Although high levels of IgG were found in breast milk, these antibodies may not be transferred effectively to the baby. IgA antibodies produced after vaccination with the Pfizer/BioNTech vaccine resist the gastric phase but are degraded during the intestinal phase of the infant’s digestion. By contrast, IgG is more susceptible to degradation in both digestive phases. 31 The results of another study found that maternal SARS-CoV-2 IgG was efficiently transferred across the placenta when infection occurs more than two months before delivery. Passive immunity inherited from the mother can last in infants for up to six months. Neonates can mount a strong antibody response to perinatal SARS-CoV-2 infection. 32 Antibodies shown from breastfeeding may have a protective effect on the recipient infant, provided that the infant has not increased its immune response to infection. 33
Studies showing the duration of antibody persistence in breast milk are limited. Antibodies can be detected in mothers who are breastfeeding and infected with COVID-19 as early as day 10 and can last for up to 10 months. IgA levels are known to decrease over time, while IgG is relatively stable. In lactating mothers who were vaccinated, the trial was only up to six weeks in duration. Antibodies have been detected after the first dose of vaccination for three days and can last up to six weeks. Before six weeks, it can decrease. It is not known whether the antibody status will still decrease or persist at a certain point. Individual research by Young et al. (2021) showed that IgG began to decline by 90 days after the second vaccine dose. 18 This suggests that further research is needed to investigate the exact duration of antibodies in breast milk after vaccination.
The type of vaccine is related to the number of antibodies detected in breast milk even though IgG status is more dominant than IgA and IgM. Post-vaccination IgG levels reached levels similar to those of terminally ill COVID-19 patients and demonstrated a decreased breadth of antibody responses targeting the endemic coronavirus. 34 Another study conducted by Dashdorj et al. (2021) found that IgG levels from high to low were Pfizer/BioNTech, AstraZeneca, Sputnik V and Sinopharm, respectively. 35 These different responses also allow different amounts of antibodies to be transferred into breast milk.
Based on the results of the opportunity for antibody transfer, breastfeeding is highly recommended for mothers infected with COVID-19 and mothers who have been vaccinated. Research conducted by Verd et al. (2021) found that in a sample of children visiting emergency services with potential symptoms of COVID-19, a higher prevalence of positive SARS-CoV-2 reverse transcriptase (RT)-PCR test results among those who were formula-fed exclusively compared to those who have been breastfed. Breastfeeding may reduce the risk of exposure to COVID-19 and other infections in children, compared to formula feeding. 12 It is vital to continue to breastfeed according to the available time duration. Women with confirmed COVID-19 should be advised to adhere standard precautions for contact with breastfeeding. 36
There are clear and up-to-date recommendations on the duration of breastfeeding from the American Academy of Pediatrics, UNICEF and WHO. WHO and UNICEF recommend: early initiation of breastfeeding within 1 hour of birth; exclusive breastfeeding for the first 6 months of life; and the introduction of complementary (solid) foods that are nutritionally adequate and safe at the age of 6 months along with continuous breastfeeding until the age of 2 years or older. 55 , 56 In the first 6 months this is important because the baby’s production of sIgA is low during that period. 37 Apart from direct breastfeeding, every mother has the right to choose to breastfeed her baby and health workers need to help ensure good handwashing practices before and after expressing the milk. 38
Due to varying collection times, certain patients’ milk and serum samples may contain no Ig A in vaccinated women. The interval between sample and vaccination has a direct bearing on variations in antibody concentrations. Neutralizing anti-SARS-CoV-2 antibodies (IgA and IgG) were examined in breast milk and blood samples from women who had received vaccinations at least 20 days after the immunization cycle ended. This research was conducted by Scrimin et al. in 2022. IgA was absent from all forty-two milk samples, while anti-SARS-CoV-2 IgG was present in all of them. IgA’s inherent kinetics could be connected to the quicker fall in IgA. The absence of IgA may suggest a sharp fall following immunization, even if regular breastfeeding would help it last. 57 In a study conducted by Esteve-Palau et al. (2021), lactating mothers who were vaccinated against SARS-CoV2 with the Pfizer-BioNTech COVID-19 vaccine and who were 18 years of age or older were included. The study examined the levels of SARS-CoV-2-specific antibodies in the breast milk of mRNA-vaccinated women over time and their correlation with serum antibody levels. Each participant had serum and breast milk samples taken simultaneously at three different time points: two weeks following the first vaccination dose (time point 1), two weeks following the second vaccination dose (time point 2), and four weeks following the second vaccination dose (time point 3). Following the second dosage, breast milk’s IgG(S1) levels rose and showed a positive correlation with matching serum levels. 58
Notable is the link between antibody concentrations in blood and milk. A study conducted on animals revealed that during lactation, there were alterations in blood and milk metabolism. Milk has the potential to predict blood metabolites and metabolic condition, as confirmed by the association between blood and milk. 59 The antibodies, or immunoglobulins, present in milk and colostrum are identical to those present in blood or mucosal secretions. According to Hurley (2011), they are a class of proteins with a variety of defensive bioactivities. 60
Limitations and recommendations
Most of these studies only involve a small number of samples, and they did not find a mechanism for antibody transfer during breastfeeding. We recommend that future research explores antibody testing with a more extended research duration (more than six weeks) in vaccinated postpartum women. In order to ensure that the reported time is more accurate, multiple individual studies that directly discuss the comparison of antibodies in vaccinated mothers with mothers exposed to COVID-19 and the assessment of antibody transfer mechanism by breastfeeding may be useful. In addition, the researcher was unable to compile a meta-analysis because it included various types of academic papers resulting in a lack of homogeneity.
The strength of this study is that there have not been many studies that have reviewed the comparison of antibody types and duration of antibodies in infected and vaccinated postpartum women. The results obtained can support evidence-based health promotion to support continued breastfeeding during the pandemic and allow for the right time to support antibody transfer.
Conclusions
IgA and IgG antibodies were found in the breast milk of infected and vaccinated postpartum women. Infection with SARS CoV-2 is related with the presence of IgA, but vaccination is associated with increased IgG. Antibody levels persisted from day 10 of onset to 10 months in infected breastfeeding mothers and start from three days to six weeks in vaccinated breastfeeding mothers. Antibodies produced in breast milk in infected and vaccinated postpartum women have different dominant types. Further research needs to be done regarding the mechanism of antibody transfer in breast milk, longer research duration and studies that directly examine the comparison of antibodies in breast milk in vaccinated and infected postpartum women.
Data availability
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
Reporting guidelines
Zenodo: PRISMA checklist for ‘Records of antibodies in breast milk in postpartum women who have been vaccinated or exposed to COVID-19: A systematic review. https://doi.org/10.5281/zenodo.6785317. 39
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Acknowledgement
We would like to thank Hari Paraton as an external contributor to this paper who was consulted to address disagreements in consensus during the data collection process.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 4; peer review: 2 approved]
References
- 1. Kotlar B, Gerson E, Petrillo S, et al. : The impact of the COVID-19 pandemic on maternal and perinatal health: a scoping review. Reprod. Health. 2021;18(1):10. 10.1186/s12978-021-01070-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Villar J, Ariff S, Gunier RB, et al. : Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021 Aug 1;175(8):817–826. 10.1001/jamapediatrics.2021.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gurzenda S, Castro MC: COVID-19 poses alarming pregnancy and postpartum mortality risk in Brazil. EClinicalMedicine. 2021;36:100917. 10.1016/j.eclinm.2021.100917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allotey J, Stallings E, Bonet M, et al. : Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ. 2020;41(September):81–82. 10.1097/01.aoa.0000744128.44930.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liguoro I, Pilotto C, Bonanni M, et al. : SARS-COV-2 infection in children and newborns: a systematic review. Eur. J. Pediatr. 2020/05/18. 2020 Jul;179(7):1029–1046. 10.1007/s00431-020-03684-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kumar VHS, Prasath A, Blanco C, et al. : Respiratory Failure in an Extremely Premature Neonate with COVID-19. Child (Basel, Switzerland). 2021 Jun 4;8(6):477. 10.3390/children8060477 Reference Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schindler-Ruwisch J, Phillips KE: Breastfeeding During a Pandemic: The Influence of COVID-19 on Lactation Services in the Northeastern United States. J. Hum. Lact. 2021;37(2):260–268. 10.1177/08903344211003898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. UNICEF: Breastfeeding during the COVID-19 pandemic Tips on keeping your baby healthy and safe. 2021. Reference Source
- 9. Walter EB, Talaat KR, Sabharwal C, et al. : Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022;386(1):35–46. 10.1056/NEJMoa2116298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. CDC: COVID-19 Vaccines While Pregnant or Breastfeeding. 2021. Reference Source
- 11. Schlaudecker EP, Steinhoff MC, Omer SB, et al. : IgA and neutralizing antibodies to influenza a virus in human milk: a randomized trial of antenatal influenza immunization. PLoS One. 2013 Aug 14;8(8):e70867–e70867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verd S, Ramakers J, Vinuela I, et al. : Does breastfeeding protect children from COVID-19? An observational study from pediatric services in Majorca, Spain. Int. Breastfeed. J. 2021;16(1):83. 10.1186/s13006-021-00430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bavel JJV, Baicker K, Boggio PS, et al. : Using social and behavioural science to support COVID-19 pandemic response. Nat. Hum. Behav. 2020;4(5):460–471. 10.1038/s41562-020-0884-z [DOI] [PubMed] [Google Scholar]
- 14. WHO: WHO recommends continuing breastfeeding during COVID-19 infection and after vaccination. 2021. Reference Source
- 15. UNICEF: Misinformation and formula milk donations are threatening breastfeeding during COVID-19 Breast milk is a baby’s ‘first vaccine”.’ 2020. Reference Source
- 16. Low JM, Low YW, Zhong Y, et al. : Titres and neutralising capacity of SARS-CoV-2-specific antibodies in human milk: a systematic review. Arch. Dis. Child - Fetal Neonatal Ed. 2021 Jul 12. fetalneonatal-2021-322156. Reference Source [DOI] [PubMed] [Google Scholar]
- 17. UNICEF: Breastfeeding Safely During The COVID-19 Pandemic. 2021.
- 18. Young BE, Seppo AE, Diaz N, et al. : Association of Human Milk Antibody Induction, Persistence, and Neutralizing Capacity With SARS-CoV-2 Infection vs mRNA. Vaccination. 2021;176:159–159. 10.1001/jamapediatrics.2021.4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, et al. : Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009 Jul 21;6(7):e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Critical Appraisal Skills Programme (CASP): CASP Checklist: Cohort Study. Casp UK. 2018; (2018):7. Reference Source [Google Scholar]
- 21. Long HA, French DP, Brooks JM: Optimising the value of the critical appraisal skills programme (CASP) tool for quality appraisal in qualitative evidence synthesis. Res. Methods Med. Heal. Sci. 2020 Aug 6;1(1):31–42. 10.1177/2632084320947559 [DOI] [Google Scholar]
- 22. Juncker HG, Romijn M, Loth VN, et al. : Antibodies Against SARS-CoV-2 in Human Milk: Milk Conversion Rates in the Netherlands. J. Hum. Lact. 2021;37:469–476. 10.1177/08903344211018185 [DOI] [PubMed] [Google Scholar]
- 23. Lechosa-Muñiz C, Paz-Zulueta M, Mendez-Legaza JM, et al. : Induction of sars-cov-2-specific igg and iga in serum and milk with different sars-cov-2 vaccines in breastfeeding women: A cross-sectional study in northern spain. Int. J. Environ. Res. Public Health. 2021;18(16):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Selma-Royo M, Bauerl C, Mena-Tudela D, et al. : Anti-Sars-Cov-2 IgA And IgG In Human Milk After Vaccination Is Dependent On Vaccine Type And Previous Sars-Cov-2 Exposure A Longitudinal Study. medRxiv. 2021. 2021.05.20.21257512. 10.1101/2021.05.20.21257512v1 [DOI] [PMC free article] [PubMed]
- 25. Gray KJ, Bordt EA, Atyeo C, et al. : Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am. J. Obstet. Gynecol. 2021;225(3):303.e1–303.e17. 10.1016/j.ajog.2021.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keulen BJ, Romijn M, Bondt A, et al. : Human milk from previously covid-19-infected mothers: The effect of pasteurization on specific antibodies and neutralization capacity. Nutrients. 2021;13(5). 10.3390/nu13051645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pullen KM, Atyeo C, Collier ARY, et al. : Selective functional antibody transfer into the breastmilk after SARS-CoV-2 infection. Cell Rep. 2021;37(6):109959. 10.1016/j.celrep.2021.109959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rio-Aige K, Azagra-Boronat I, Castell M, et al. : The Breast Milk Immunoglobulinome. Nutrients. 2021;13. 10.3390/nu13061810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peng S, Zhu H, Yang L, et al. : A study of breastfeeding practices, SARS-CoV-2 and its antibodies in the breast milk of mothers confirmed with COVID-19. Lancet Reg. Heal. - West Pacific. 2020;4:100045. 10.1016/j.lanwpc.2020.100045 Reference Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Golan Y, Prahl M, Cassidy AG, et al. : COVID-19 mRNA Vaccination in Lactation: Assessment of adverse effects and transfer of anti-SARS-CoV2 antibodies from mother to child. medRxiv Prepr. Serv. Heal. Sci. 2021. Reference Source [Google Scholar]
- 31. Pieri M, Nicolaidou V, Paphiti I, et al. : Survival of vaccine-induced human milk SARS-CoV-2 IgG and IgA immunoglobulins across simulated human infant gastrointestinal digestion. medRxiv. 2021. 2021.06.17.21259021. 10.1101/2021.06.17.21259021v1 [DOI] [PMC free article] [PubMed]
- 32. Song D, Prahl M, Gaw SL, et al. : Passive and active immunity in infants born to mothers with SARS-CoV-2 infection during pregnancy: prospective cohort study. BMJ Open. 2021 Jul 1;11(7):e053036. 10.1136/bmjopen-2021-053036 Reference Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Favara DM, Ceron-Gutierrez ML, Carnell GW, et al. : Detection of breastmilk antibodies targeting SARS-CoV-2 nucleocapsid, spike and receptor-binding-domain antigens. Emerg. Microbes Infect. 2020 Dec;9(1):2728–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Röltgen K, Nielsen SCA, Arunachalam PS, et al. : mRNA vaccination compared to infection elicits an IgG-predominant response with greater SARS-CoV-2 specificity and similar decrease in variant spike recognition. medRxiv Prepr. Serv. Heal. Sci. 2021 Apr 7. 2021.04.05.21254952. Reference Source [Google Scholar]
- 35. Dashdorj NJ, Wirz OF, Röltgen K, et al. : Direct comparison of antibody responses to four SARS-CoV-2 vaccines in Mongolia. Cell Host Microbe. 2021;29(12):1738–1743.e4. 10.1016/j.chom.2021.11.004 Reference Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pramana C, Suwantoro J, Sumarni N, et al. : Breastfeeding in postpartum women infected with COVID-19. Int. J. Pharm. Res. 2020;12(14):1857–1862. [Google Scholar]
- 37. Palmeira P, Carneiro-Sampaio M: Immunology of breast milk. Rev. Assoc. Med. Bras. 2016 Sep 1;62:584–593. 10.1590/1806-9282.62.06.584 [DOI] [PubMed] [Google Scholar]
- 38. Marinelli KA, Lawrence RM: Safe Handling of Containers of Expressed Human Milk in all Settings During the SARS-CoV-2 (COVID-19) Pandemic. J. Hum. Lact. 2020;36:498–501. 10.1177/0890334420919083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kurniawati EM: Records of antibodies in breast milk in postpartum women who have been vaccinated or exposed to COVID-19: A systematic review. [Dataset]. Zenodo. 2022. 10.5281/zenodo.6785318 [DOI] [PMC free article] [PubMed]
- 40. Juncker HG, Romijn M, Loth VN, et al. : Human Milk Antibodies Against SARS-CoV-2: A Longitudinal Follow-Up Study. J. Hum. Lact. 2021;37:485–491. 10.1177/08903344211030171 [DOI] [PubMed] [Google Scholar]
- 41. Narayanaswamy V, Pentecost B, Alfandari D, et al. : Humoral and Cell-Mediated Immune Response in Colostrum from Women Diagnosed Positive for SARS-CoV-2. Breastfeed. Med. 2021;16:987–994. 10.1089/bfm.2021.0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bäuerl C, Randazzo W, Sánchez G, et al. : SARS-CoV-2 RNA and antibody detection in breast milk from a prospective multicentre study in Spain. Arch. Dis. Child - Fetal Neonatal Ed. 2021. fetalneonatal-2021-322463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duncombe CJ, Mcculloch DJ, Shuey KD, et al. : Dynamics of breast milk antibody titer in the six months following SARS-CoV-2 infection. J. Clin. Virol. 2021;142(January):104916. 10.1016/j.jcv.2021.104916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pace RM, Williams JE, Järvinen KM, et al. : Characterization of sars-cov-2 rna, antibodies, and neutralizing capacity in milk produced by women with covid-19. MBio. 2021;12(1):1–11. 10.1128/mBio.03192-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fox A, Marino J, Amanat F, et al. : Evidence of a significant secretory-IgA-dominant SARS-CoV-2 immune response in human milk following recovery from COVID-19. medRxiv. 2020.
- 46. Demers-Mathieu V, Dapra C, Mathijssen G, et al. : Human milk antibodies against s1 and s2 subunits from sars-cov-2, hcov-oc43, and hcov-229e in mothers with a confirmed covid-19 pcr, viral symptoms, and unexposed mothers. Int. J. Mol. Sci. 2021;22(4):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Low JM, Gu Y, Ng MSF, et al. : Codominant IgG and IgA expression with minimal vaccine mRNA in milk of BNT162b2 vaccinees. npj Vaccines. 2021;6(1):105. 10.1038/s41541-021-00370-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jakuszko K, Kościelska-Kasprzak K, Żabińska M, et al. : Immune response to vaccination against covid-19 in breastfeeding health workers. Vaccines. 2021;9(6):1–10. 10.3390/vaccines9060663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guida M, Terracciano D, Cennamo M, et al. : COVID-19 vaccine mRNABNT162b2 elicits human antibody response in milk of breastfeeding women. Vaccines. 2021;9(7):3–9. 10.3390/vaccines9070785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ramìreza DSR, Pérez MML, Pérez MC, et al. : SARS-CoV-2 Antibodies in Breast Milk After Vaccination. Pediatrics. 2021;148:e2021052286. 10.1542/peds.2021-052286 [DOI] [PubMed] [Google Scholar]
- 51. Perl SH, Uzan-Yulzari A, Klainer H, et al. : SARS-CoV-2–Specific Antibodies in Breast Milk After COVID-19 Vaccination of Breastfeeding Women. JAMA. 2021 May 18;325(19):2013–2014. 10.1001/jama.2021.5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Valcarce V, Stafford LS, Neu J, et al. : Detection of SARS-CoV-2-Specific IgA in the Human Milk of COVID-19 Vaccinated Lactating Health Care Workers. Breastfeed. Med. 2021; XX(Xx):1–6. [DOI] [PubMed] [Google Scholar]
- 53. Juncker HG, Mulleners SJ, Gils MJ, et al. : The Levels of SARS-CoV-2 Specific Antibodies in Human Milk Following Vaccination. J. Hum. Lact. 2021;37(3):477–484. 10.1177/08903344211027112 [DOI] [PubMed] [Google Scholar]
- 54. Rosenberg-Friedman M, Kigel A, Bahar Y, et al. : BNT162b2 mRNA vaccine elicited antibody response in blood and milk of breastfeeding women. Nat. Commun. 2021;12(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. WHO : Infant and Young Child Feeding. 2021. https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding 34176363
- 56. Meek JY, Noble L: Section on Breastfeeding; Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics. 2022;150(1): e2022057988. 10.1542/peds.2022-057988 [DOI] [PubMed] [Google Scholar]
- 57. Scrimin F, Campisciano G, Comar M, et al. : IgG and IgA Antibodies Post SARS-CoV-2 Vaccine in the Breast Milk and Sera of Breastfeeding Women. Vaccines (Basel). 2022;10(1):125. 10.3390/vaccines10010125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Esteve-Palau E, Gonzalez-Cuevas A, Guerrero ME, et al. : Quantification of Specific Antibodies Against SARS-CoV-2 in Breast Milk of Lactating Women Vaccinated With an mRNA Vaccine. JAMA Netw Open. 2021 Aug 2;4(8): e2120575. 10.1001/jamanetworkopen.2021.20575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Andjelić B, Djoković R, Cincović M, et al. : Relationships between Milk and Blood Biochemical Parameters and Metabolic Status in Dairy Cows during Lactation. Metabolites. 2022;12(8):733. 10.3390/metabo12080733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hurley WL, Theil PK: Perspectives on immunoglobulins in colostrum and milk. Nutrients. 2011 Apr;3(4):442–474. 10.3390/nu3040442 [DOI] [PMC free article] [PubMed] [Google Scholar]