Abstract
Background
Uveal melanoma (UM) has a poor prognosis once liver metastases occur. The melphalan/Hepatic Delivery System (melphalan/HDS) is a drug/device combination used for liver-directed treatment of metastatic UM (mUM) patients. The purpose of the FOCUS study was to assess the efficacy and safety of melphalan/HDS in patients with unresectable mUM.
Methods
Eligible patients with mUM received treatment with melphalan (3.0 mg/kg ideal body weight) once every 6 to 8 weeks for a maximum of six cycles. The primary end point was the objective response rate (ORR). The secondary end points included duration of response (DOR), overall survival (OS), and progression-free survival (PFS).
Results
The study enrolled 102 patients with mUM. Treatment was attempted in 95 patients, and 91 patients received treatment. In the treated population (n = 91), the ORR was 36.3 % (95 % confidence interval [CI], 26.44–47.01), including 7.7 % of patients with a complete response. Thus, the study met its primary end point because the lower bound of the 95 % CI for ORR exceeded the upper bound (8.3 %) from the benchmark meta-analysis. The median DOR was 14 months, and the median OS was 20.5 months, with an OS of 80 % at 1 year. The median PFS was 9 months, with a PFS of 65 % at 6 months. The most common serious treatment-emergent adverse events were thrombocytopenia (15.8 %) and neutropenia (10.5 %), treated mostly on an outpatient basis with observation. No treatment-related deaths were observed.
Conclusion
Treatment with melphalan/HDS provides a clinically meaningful response rate and demonstrates a favorable benefit-risk profile in patients with unresectable mUM (study funded by Delcath; ClinicalTrials.gov identifier: NCT02678572; EudraCT no. 2015-000417-44).
Supplementary Information
The online version contains supplementary material available at 10.1245/s10434-024-15293-x.
Uveal melanoma (UM) accounts for approximately 3 % to 5 % of all melanoma cases in the United States and represents the most common intraocular malignancy in adults.1 During its natural history, up to 50 % of patients with UM will eventually experience the development of metastatic disease, most often to the liver (~90 % of cases).1 The prognosis of metastatic UM (mUM) patients with hepatic metastases is dismal, with a median overall survival (mOS) of approximately 1 year.2,3
Treatment of mUM is challenging because, aside from tebentafusp (limited to HLA-A*02:01-positive patients), commonly used systemic therapies rarely produce durable responses or significant survival benefit.1,4,5 Immune checkpoint inhibitors (ICIs), including pembrolizumab, nivolumab and ipilimumab, have shown limited efficacy in mUM patients, with objective response rates (ORRs) for single-agent or dual immune checkpoint blockade (ICB) ranging from 0 to 16.7 % in retrospective chart analyses.6–9 The combination of nivolumab and ipilimumab showed an ORR of 18 % in a small, prospective single-center study10 and 11.5 % in a prospective multicenter study11 with mUM patients. A recent review12 reported overall ORR, median progression-free survival (mPFS), and mOS of 9.2 %, 3.0 months, and 11.5 months, respectively, for ICI in mUM.
Because the vast majority of patients with mUM will have liver metastases, often leading to liver failure,13 the National Comprehensive Cancer Network guidelines recommend for those patients, among other options, liver-directed therapies, including transarterial chemoembolization (TACE), radioembolization or immunoembolization, and thermal ablation, as well as locoregional perfusion procedures delivering high-dose chemotherapeutic agents, namely, intrahepatic perfusion (IHP), a surgical procedure, and percutaneous hepatic perfusion (PHP), a minimally invasive procedure.14
The PHP procedure requires the use of a hepatic delivery system (HDS), commercially available in Europe as CHEMOSAT (Delcath Systems, Inc., Queensbury, NY), and in the United States as the HEPZATO KIT (melphalan/HDS; Delcath Systems, Inc., Queensbury, NY), recently approved by the U.S. Food and Drug Administration (FDA) as the first approved liver-directed treatment for unresectable mUM patients.15
Ideally, a liver-directed treatment should treat the whole liver and target all radiographically evident and occult metastases, allow for retreatment for optimized tumor control, and achieve an acceptable benefit/risk profile. Radioembolization and TACE fulfill some of these requirements and generally achieve better control of hepatic metastases than systemic therapy. However, these procedures have limitations with respect to repeatability of treatment and ability to treat the whole liver.13 Although IHP treats the whole liver and has shown promising tumor response rates, its use is restricted by a high risk of morbidity and mortality due to its invasive nature. Most patients undergo only one treatment, which significantly limits its use and patient outcomes.16,17 The PHP procedure is a minimally invasive technique optimized to chemosaturate the whole liver without major surgery, and most patients are able to receive multiple treatments.
Results from early-phase clinical studies investigating the safety and efficacy of PHP using melphalan/HDS in mUM patients show encouraging signals of efficacy, including promising ORRs and OS relative to historic controls.18–22 The multicenter, open-label, phase 3 FOCUS study was designed to evaluate the efficacy and safety of melphalan/HDS in patients with unresectable mUM.
Methods
Patients
The study population included male and female patients 18 years of age or older with histologically verified mUM to the liver; liver tumor involvement of up to 50 %; at least one measurable liver lesion as assessed by contrast-enhanced computed tomography (CT) scans of the chest, abdomen, and pelvis as well as contrast-enhanced MRI of the liver (and brain and bone scans when indicated); and an Eastern Cooperative Oncology Group (ECOG)23 performance status of 0 to 1 at screening. Patients could be previously treated or treatment naïve, and could have limited extrahepatic disease amenable to resection or radiation.
The PHP procedure requires general anesthesia and active coagulation/anti-coagulation control. The eligibility criteria were designed to minimize the risks associated with the procedure (e.g., exclusion of patients with moderate or severe liver cirrhosis, portal hypertension, or New York Heart Association [NYHA] II–IV status). The eligibility criteria remained unchanged throughout the study and are provided in the study protocol. Detailed inclusion and exclusion criteria are mentioned in the Supplementary Appendix.
Study Design and Treatment
The FOCUS study, conducted at 23 centers across the United States and Europe, was initiated as a two-arm, controlled, randomized study. Eligible patients were randomized 1:1 to receive melphalan/HDS or best alternative care (BAC) including investigator’s choice of TACE, pembrolizumab, ipilimumab, or dacarbazine. Due to slow enrollment, with patient reluctance to receive BAC treatment, the study design was amended to a single-arm study, after which all eligible patients received treatment with melphalan/HDS.
The patients received melphalan treatment (3.0 mg/kg ideal body weight; maximum dose of 220 mg for a single treatment) once every 6 to 8 weeks for a maximum of six cycles. Before each treatment, liver venous outflow was isolated by a double-balloon catheter placed into the inferior vena cava. Melphalan was administered for 30 min via an infusion catheter placed in the hepatic artery. The infusion was followed by 30 min of washout with extracorporeal filtration to further reduce systemic exposure to melphalan. All the patients received granulocyte colony-stimulating factor within 72 h after each PHP procedure. Treatment procedures were administered by a team of medical or surgical oncology, interventional radiology, and anesthesiology personnel, and a perfusionist.
All lesions were assessed with follow-up imaging performed every 12 weeks until disease progression using the same techniques as at baseline. Further details are included in the Supplementary Appendix.
End Points and Assessments
The primary end point of the study was the objective response rate (ORR), as determined by the Independent Review Committee (IRC) based on Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.24 The secondary end points were duration of response (DOR), progression-free survival (PFS), overall survival (OS), and disease control rate (DCR).
Adverse events (AEs) were assessed by investigators and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.25 Post hoc analyses were performed to evaluate the relationship between tumor response and survival and to assess outcomes for the patients with hepatic-only disease versus hepatic and extrahepatic disease.
Study Oversight
The sponsor and all the authors contributed to various elements of the study design, protocol development, and data analysis. The protocol was approved by the institutional review board or independent ethics committee at each study center, as well as by all relevant competent authorities. The FOCUS study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines as outlined by the International Council for Harmonization. An IRC and an independent data safety monitoring board provided determination of efficacy and oversight of safety, respectively. The IRC was composed of board-certified radiologists with extensive experience in oncology. Imaging was assessed by two independent readers. Any disagreement about a patient’s response to treatment was adjudicated by a third reader. All the participants provided written informed consent. All the authors vouch for the accuracy and completeness of the data and for the fidelity of the study to the protocol.
Statistical Analysis
Once the study design was changed to a single-arm investigation, the primary end point was changed from OS to ORR in agreement with the FDA, and sample size re-estimation was implemented. A meta-analysis of historical data (16 publications including 476 patients who had mUM treated with monotherapy or combination systemic immunotherapy) was performed to establish an ORR benchmark (details are included in the Supplementary Appendix). Pooled rates across studies for ORR were calculated based on both a random-effects model and a fixed-effects model. Heterogeneity in effect size was formally examined using the Q statistic and the I2 statistic using Comprehensive Meta-Analysis software Version 2.2 (Biostat, Englewood, NJ). The pooled ORR estimate (a weighted mean of the observed ORR) was 5.5 % (95 % confidence interval [CI], 3.6–8.3 %). The study was powered to test whether the lower bound of the 95 % CI for ORR would exceed the upper bound (8.3 %) of the meta-analysis. The new sample size was estimated to provide a maximum width of the 95 % CI of ±11.3 % around the point estimate of ORR, assuming that ORR was 21 %.
Efficacy analyses involved all patients treated with melphalan/HDS during both the randomized and single-arm phases of the study. The primary efficacy end point, ORR, was determined for all the patients treated with melphalan/HDS. Unless specified otherwise, statistical testing used a two-sided test at the 0.05 significance level. Nominal p values were reported without control for study-wide type 1 error because analyses were exploratory. Demographic data were summarized using descriptive statistics. Summary statistics for continuous variables include mean, standard deviation, median, and range (minimum to maximum). Categorical variables are presented as frequency counts and percentages.
Time-to-event variables were summarized using Kaplan-Meier methods. For the calculation of time-to-event end points except for DOR, the start date was the patient eligibility date. Post hoc analysis was performed to compare OS by best overall response. Descriptive statistics were used for safety analysis in the safety population, which included all patients for whom a study treatment or procedure was attempted. No statistical testing was applied to the exploratory efficacy analyses. Analyses were performed using SAS/STAT software, Version 9.4 of the SAS System for Windows (SAS Institute Inc., Cary, NC).
Results
Patient Disposition and Baseline Characteristics
From February 2016 to October 2020, 102 patients were enrolled and assigned to the melphalan/HDS group (pooled from the randomized and non-randomized portions of the study). Of the 102 patients, 95 had at least one PHP procedure initiated (safety population), and 91 received treatment with melphalan/HDS (treated population).
More than one third (37.4 %) of the patients in the treated population completed the maximum of six cycles permitted per protocol. The primary reasons for discontinuation were disease progression (28.6 %) and AEs/serious AEs (18.7 %). At the time of the data cutoff (2 December 2022), the median follow-up period was 36.4 months, and 17.6 % of the treated patients were still being followed for survival. In the treated population, the median time from diagnosis of primary tumor to study entry was 39.3 months (range, 0.7–198.9 months), and the median time from diagnosis of metastatic disease to study entry was 5.5 months (range, 0.2–67.5 months) (Table 1). The liver metastasis stage distribution was 38.5 % for stage M1a mUM, 56.0 % for stage M1b mUM, and 5.5 % for stage M1c mUM.26 The majority of the patients (87.9 %) had an ECOG performance status score of 0 (Table 1). Of the treated population, 44 % had received prior therapy for mUM (Table 1).
Table 1.
Patient demographics, baseline characteristics, and exposure to study treatment for patients treated with melphalan/Hepatic Delivery System (treated population)
| Characteristic | Melphalan/HDS (n = 91) n (%) |
|---|---|
| Demographics and baseline characteristics | |
| Median age: years (range) | 61.0 (20–78) |
| Male sex | 44 (48.4) |
| Ethnicity | |
| Hispanic or Latino | 2 (2.2) |
| Non-Hispanic or Latino | 86 (94.5) |
| No response | 3 (3.3) |
| Race | |
| White | 86 (94.5) |
| Other | 2 (2.2) |
| No response | 3 (3.3) |
| Median time since primary diagnosis: months (range)a | 39.3 (0.7–198.9) |
| Median time since diagnosis of liver metastases: months (range)a | 5.5 (0.2–67.5) |
| Median time from primary diagnosis to metastasis to liver: months (range) | 33.0 (0.0–159.1) |
| ECOG performance status score | |
| 0 | 80 (87.9) |
| 1 | 9 (9.9) |
| Not recorded | 2 (2.2) |
| Elevated LDH | 32/86 (37.2) |
| Extent of liver involvement (%)b | |
| ≤25 | 72 (79.1) |
| 26−50 | 19 (20.9) |
| Largest hepatic lesionc,d | |
| ≤ 3 cm (stage M1a) | 35 (38.5) |
| 3.1 to 8 cm (stage M1b) | 51 (56.0) |
| ≥ 8.1 cm (stage M1c) | 5 (5.5) |
| Extrahepatic lesionsd | |
| Lung | 11/27 (40.7) |
| Lymph node | 5/27 (18.5) |
| Bonee | 4/27 (14.8) |
| Soft tissuef | 10/27 (37.0) |
| Brain | 0/27 (0.0) |
| Other visceralg | 6/27 (22.2) |
| Prior therapiesh | 40 (44.0) |
| Radiation | 10 (11.0) |
| Surgeryi | 13 (14.3) |
| Systemic | 23 (25.3) |
| Immune checkpoint inhibitor | 21 (23.1) |
| Chemotherapy | 3 (3.3) |
| SIRT | 1 (1.1) |
| TACE | 1 (1.1) |
| Targeted small molecule | 1 (1.1) |
| Exposure to study treatment | |
| No. of treatment cycles completed | |
| 1 | 7 (7.7) |
| 2 | 18 (19.8) |
| 3 | 11 (12.1) |
| 4 | 15 (16.5) |
| 5 | 6 (6.6) |
| 6 | 34 (37.4) |
HDS, hepatic delivery system; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; SIRT, Selective internal radiation therapy; TACE, transarterial chemoembolization
aMonths from diagnosis of either primary tumor or liver metastases to either randomization (double-arm phase) or eligibility (single-arm phase)
bAssessed by the investigator
cTumor staging per AJCC Cancer Staging Manual,7th edition
dBased on Independent Review Committee assessment
eIncludes spine, lumbar spine, pelvis, ribs, sacrum, and skull
fIncludes subcutaneous trunk and chest wall
gIncludes spleen and adrenal gland
hPatients with multiple therapies of a given type are counted once for that type
iIncludes only therapeutic surgeries/procedures and excludes non-therapeutic prior surgeries/procedures (e.g., biopsy). Each surgery/procedure was retrospectively classified as therapeutic or non-therapeutic
Efficacy
In the treated population, the primary end point, ORR, was 36.3 % (95 % CI, 26.44–47.01 %), as assessed by IRC (Table 2). The best overall response was a complete response (CR) for 7.7 %, a partial response (PR) for 28.6 %, and stable disease (SD) for 37.4 % of the patients (Table 2). In the treated population, DCR was 73.6 % (95 % CI, 63.35–82.31 %).
Table 2.
Clinical outcomes in patients treated with melphalan/Hepatic Delivery System (treated population, assessed by Independent Review Committee)
| Characteristic | Melphalan/HDS (n = 91) n (%) |
|---|---|
| Primary end point | |
| Objective response rate: % (95 % CI)a | 36.3 (26.44–47.01) |
| No. of patients who achieved objective response | 33 |
| Best overall responseb,c | |
| Complete response | 7 (7.7) |
| Partial response | 26 (28.6) |
| Stable disease | 34 (37.4) |
| Progressive disease | 23 (25.3) |
| Not evaluable | 1 (1.1) |
| Secondary end points | |
| Median duration of response in responders: months (95 % CI)d | 14.0 (8.31–17.74); n = 33 |
| Disease control rate: % (95 % CI)a | 73.6 (63.35–82.31) |
| No. of patients who achieved disease control | 67 |
| Median progression-free survival: months (95 % CI)d | 9.0 (6.34–11.56) |
| Progression-free survival at 6 months: % (95 % CI)d | 65 (54–74) |
| Progression-free survival at 1 year: % (95 % CI)d | 38 (27–48) |
| Median overall survival: months (95 % CI)d | 20.5 (16.79–25.26) |
| Overall survival at 1 year: % (95 % CI)d | 80 (70–87) |
| Overall survival at 2 years: % (95 % CI)d | 43 (32–53) |
| Exploratory end points | |
| Median time to objective response: months (95 % CI)d | 3.3 (2.86–5.59); n = 33 |
| Median hepatic progression-free survival: months (95 % CI)d | 13.9 (9.30–16.66) |
| Hepatic objective response rate: % (95 % CI)a | 41.8 (31.50–52.57); n = 38 |
HDS, hepatic delivery system; CI, confidence interval
aPatients without at least 1 post-baseline response assessment were designated as non-responders
bBest overall response per Independent Review Committee (Response Evaluation Criteria in Solid Tumors v1.1) from the date of randomization/eligibility until disease progression
cFor complete response or partial response, confirmation was required by repeat assessment ≥4 weeks after initial documentation. To qualify as stable disease, the image must have been taken at least 9 weeks after start of therapy
dKaplan-Meier estimate
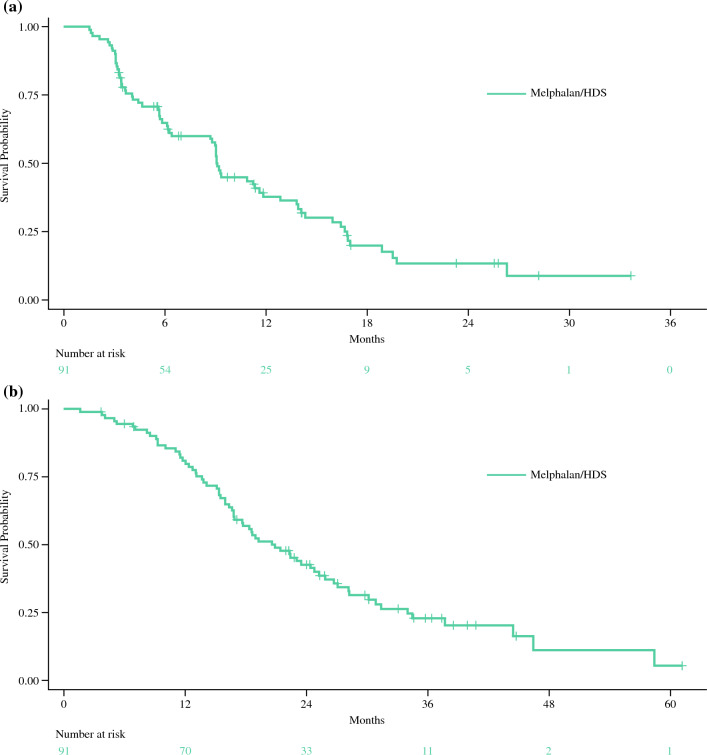
Responders had a median DOR of 14.0 months (95 % CI, 8.31–17.74 months; Table 2). The mPFS was 9 months (95 % CI, 6.34–11.56 months; Table 2; Fig. 1a). At the 2 December 2022 data cutoff, the median OS in the treated population was 20.5 months (95 % CI, 16.79–25.26 months), with 80 % of the patients surviving at least 1 year and 43 % of the patients surviving at least 2 years (Table 2; Fig. 1b). The median time to objective response and hepatic PFS were respectively 3.3 months (95 % CI, 2.86–5.59 months) and 13.9 months (95 % CI, 9.30–16.66 months). The hepatic ORR was 41.8 % (95 % CI, 31.50–52.57 %; Table 2).
Fig. 1.
Kaplan-Meier plots of a progression-free survival and b overall survival in patients treated with melphalan/Hepatic Delivery System (HDS) (treated population: assessed by Independent Review Committee)
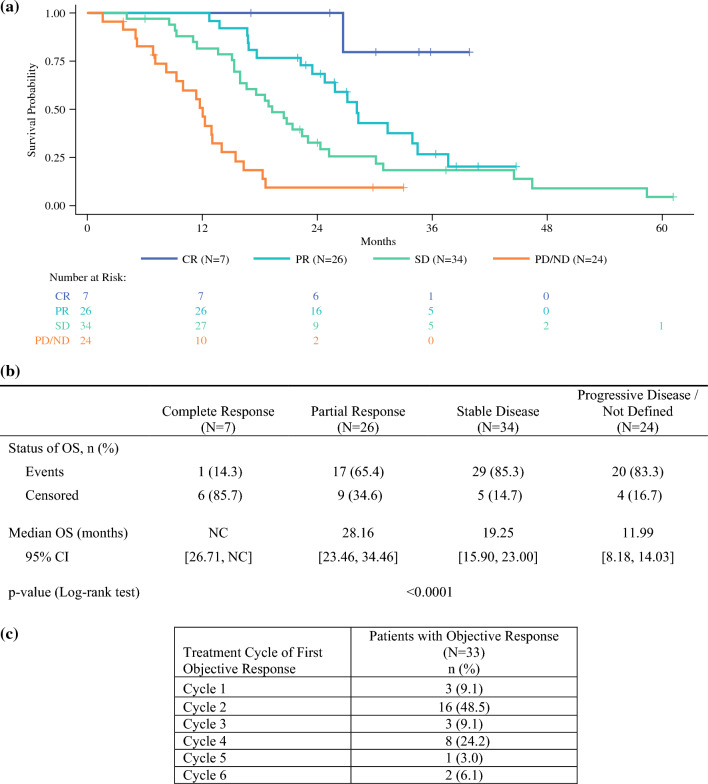
The results from a post hoc analysis of the relationship between tumor response and survival demonstrated a statistically significant difference (P < 0.0001) between the patients who had a best overall response of PR, SD, and progressive disease (PD), with respective median OS values of 28.2 months (95 % CI, 23.46–34.46 months), 19.3 months (95 % CI, 15.90–23.00 months), and 12.0 months (95 % CI, 8.18–14.03 months). The median OS could not be calculated for the patients with CR because only one event was observed before the data cutoff (Fig. 2a and b). The analysis of objective response by PHP treatment cycle showed that more than 50 % of all objective responses began after the first or second treatment cycle (Fig. 2c).
Fig. 2.
Post hoc analysis. a Kaplan-Meier plot of overall survival by best overall response. b Overall survival by best overall response and c first occurrence of objective response by treatment cycle in patients treated with the melphalan/Hepatic Delivery System (treated population, assessed by Independent Review Committee). CI, confidence interval; CR, complete response; NC, not calculable; ND, not defined; OS, overall survival; PD, progressive disease; PR, partial response; SD, stable disease
Safety
Among the 95 patients assessed for safety after treatment with melphalan/HDS (including attempted treatments), all the patients reported at least one treatment-emergent AE (TEAE) (Table 3). The most common TEAEs (any grade) were thrombocytopenia (65.3 %), anemia (63.2 %), nausea (56.8 %), fatigue (53.7 %), leukopenia (46.3 %), neutropenia (34.7 %), vomiting (34.7 %), and alanine aminotransferase (ALT) increased (31.6 %) (Table 3). Grades 3 and 4 TEAEs were reported in 81.1 % of the patients including grade 3 or 4 thrombocytopenia (54.7 %), leukopenia (33.7 %), anemia (32.6 %), and neutropenia (29.5 %) (Table 3). The most common serious TEAEs reported were thrombocytopenia (15.8 %) and neutropenia (10.5 %) (Table 3). Three patients died during the study. The causes of death were cardiac arrest, acute hepatic failure, and bacterial peritonitis, occurring at 43, 62, and 64 days, respectively, after the last study treatment. None of the three deaths were considered related to study treatment, device, or procedure. Treatment was discontinued for 17.9 % of the patients due to TEAEs (Table 3).
Table 3.
Treatment-emergent adverse events in patients treated with melphalan/Hepatic Delivery System (safety population)a
| Parameters | Melphalan/HDS (n = 95) n (%) |
|
|---|---|---|
| Any TEAE leading to discontinuation of study treatment | 17 (17.9) | |
| Any TEAE leading to dose reduction of study treatment | 13 (13.7) | |
| Death | 3 (3.2) | |
| Any grade | Grade 3/4 | |
|---|---|---|
| Any TEAE | 95 (100.0) | 77 (81.1) |
| Thrombocytopeniab | 62 (65.3) | 52 (54.7) |
| Anemiac | 60 (63.2) | 31 (32.6) |
| Nausea | 54 (56.8) | 0 (0.0) |
| Fatigue | 51 (53.7) | 0 (0.0) |
| Leukopeniad | 44 (46.3) | 32 (33.7) |
| Neutropeniae | 33 (34.7) | 28 (29.5) |
| Vomiting | 33 (34.7) | 0 (0.0) |
| ALT increased | 30 (31.6) | 3 (3.2) |
| INR increased | 29 (30.5) | 8 (8.4) |
| Activated PTT prolonged | 27 (28.4) | 8 (8.4) |
| AST increased | 27 (28.4) | 4 (4.2) |
| Blood alkaline phosphatase increased | 26 (27.4) | 2 (2.1) |
| Back pain | 25 (26.3) | 1 (1.1) |
| Dyspnea | 22 (23.2) | 2 (2.1) |
| Abdominal pain upper | 21 (22.1) | 1 (1.1) |
| Headache | 18 (18.9) | 0 (0.0) |
| Abdominal pain | 16 (16.8) | 0 (0.0) |
| Contusion | 16 (16.8) | 0 (0.0) |
| Diarrhea | 15 (15.8) | 1 (1.1) |
| Decreased appetite | 15 (15.8) | 0 (0.0) |
| Pyrexia | 15 (15.8) | 0 (0.0) |
| Cough | 14 (14.7) | 0 (0.0) |
| Hypocalcemia | 12 (12.6) | 3 (3.2) |
| Troponin I increased | 12 (12.6) | 2 (2.1) |
| Asthenia | 12 (12.6) | 0 (0.0) |
| Hypotension | 11 (11.6) | 3 (3.2) |
| Lethargy | 11 (11.6) | 0 (0.0) |
| Blood bilirubin increased | 10 (10.5) | 3 (3.2) |
| Groin pain | 10 (10.5) | 0 (0.0) |
| Pain in extremity | 10 (10.5) | 0 (0.0) |
| Hypophosphatemia | 9 (9.5) | 7 (7.4) |
| Febrile neutropenia | 8 (8.4) | 7 (7.4) |
| Any serious TEAE | 43 (45.3) | 38 (40.0) |
| Thrombocytopeniab | 15 (15.8) | 15 (15.8) |
| Neutropeniae | 10 (10.5) | 10 (10.5) |
| Febrile neutropenia | 7 (7.4) | 6 (6.3) |
| Leukopeniad | 5 (5.3) | 5 (5.3) |
HDS, hepatic delivery system; TEAE, treatment-emergent adverse event; ALT, alanine aminotransferase; INR, international normalized ratio; PTT, partial thromboplastin time; AST, aspartate aminotransferase
aTEAEs reported in at least 10 % of patients (any grade) or in at least 5 % of patients (grade 3/4 and serious TEAEs) treated with melphalan/Hepatic Delivery System
bThrombocytopenia includes thrombocytopenia and platelet count decreased
cAnemia includes anemia, febrile bone marrow aplasia, anemia, normochromic normocytic anemia, and red blood cell count decreased
dLeukopenia includes leukopenia, lymphocyte count decreased, lymphopenia, and white blood cell count decreased
eNeutropenia includes neutropenia and neutrophil count decreased
Peri-procedural TEAEs that occurred in 20 % or more of the patients were anemia (56.0 %), thrombocytopenia (50.5 %), nausea (41.8 %), international normalized ratio (INR) increased (30.8 %), vomiting (29.7 %), prolonged activated partial thromboplastin time (PTT) (28.6 %), fatigue (23.1 %), aspartate aminotransferase (AST) increased (23.1 %), and ALT increased (22.0 %) (Table 4).
Table 4.
Peri-procedural treatment-emergent adverse events in patients treated with melphalan/Hepatic Delivery System (treated population)a
| Parameters | Melphalan/HDS (n = 91) n (%) |
|
|---|---|---|
| Any grade | Grade 3/4 | |
| Any peri-procedural TEAE | 80 (87.9) | 51 (56.0) |
| Anemiab | 51 (56.0) | 27 (29.7) |
| Thrombocytopeniac | 46 (50.5) | 35 (38.5) |
| Nausea | 38 (41.8) | 0 (0.0) |
| INR increased | 28 (30.8) | 8 (8.8) |
| Vomiting | 27 (29.7) | 0 (0.0) |
| Activated PTT prolonged | 26 (28.6) | 8 (8.8) |
| AST increased | 21 (23.1) | 3 (3.3) |
| Fatigue | 21 (23.1) | 0 (0.0) |
| ALT increased | 20 (22.0) | 1 (1.1) |
| Leukopeniad | 17 (18.7) | 12 (13.2) |
| Back pain | 15 (16.5) | 1 (1.1) |
| Hypocalcemia | 12 (13.2) | 3 (3.3) |
| Troponin I increased | 12 (13.2) | 2 (2.2) |
| Contusion | 10 (11.0) | 0 (0.0) |
| Pyrexia | 10 (11.0) | 0 (0.0) |
| Hypophosphatemia | 8 (8.8) | 7 (7.7) |
| Any serious TEAE | 20 (22.0) | 16 (17.6) |
| Thrombocytopeniac | 5 (5.5) | 5 (5.5) |
HDS, hepatic delivery system; TEAE, treatment-emergent adverse event; INR, international normalized ratio; PTT, partial thromboplastin time; AST, aspartate aminotransferase; ALT, alanine aminotransferase
aPeri-procedural TEAEs reported in at least 10 % of patients (any grade) or in at least 5 % of patients (grade 3/4 and serious TEAEs) in patients treated with melphalan/Hepatic Delivery System. TEAEs with onset from the procedure date to the earlier of discharge from hospital or day 3 were considered peri-procedural
bAnemia includes anemia, febrile bone marrow aplasia, anemia, normochromic normocytic anemia, and red blood cell count decreased
cThrombocytopenia includes thrombocytopenia and platelet count decreased
dLeukopenia includes leukopenia, lymphocyte count decreased, lymphopenia, and white blood cell count decreased
Discussion
Metastatic uveal melanoma remains a difficult-to-treat disease with limited therapeutic options. Tebentafusp, a bispecific immunotherapeutic agent, is indicated for HLA-A*02:01-positive adult patients with unresectable mUM27 and represents a treatment option for approximately 45 % of mUM patients who are HLA-A*02:01-positive.5 The HEPZATO KIT, recently approved by the FDA based on the results from the FOCUS study, is the only FDA-approved liver-directed treatment for patients with mUM and is not limited by tumor genotype, thus offering broad utility for this indication.
The FOCUS study evaluated PHP using the drug/device combination of melphalan/HDS for treatment of patients with unresectable mUM. This approach enables locoregional delivery of a high melphalan dose to the liver and minimizes systemic exposure and melphalan-related AEs with the use of active filters to remove excess melphalan after liver perfusion.
The study population was heterogeneous and included patients with hepatic-only disease and those with hepatic and limited extrahepatic disease, patients with up to 50 % of liver tumor involvement (79.1 % of patients had ≤25 % liver tumor burden), and both previously treated (44.0 %) and treatment-naïve (56.0 %) patients. The diverse study population together with operational performance at 23 study centers enabled a robust evaluation of the efficacy and safety of melphalan/HDS in patients with unresectable mUM.
The primary end point of the study was met by a wide margin. With an ORR of 36.3 %, as assessed by IRC, the lower bound of the 95 % CI for ORR (26.4 %) was well above the upper bound from the benchmark meta-analysis (8.3 %). Efficacy of PHP was consistent for patients with both hepatic-only and hepatic and extrahepatic disease, including ORRs of 37.5 % and 33.3 %, respectively, and mPFS periods of 9.3 and 6.2 months, respectively. These results compare favorably with the overall ORR of 11.5 % and mPFS of 1.5 months for hepatic-only disease versus 3.7 months for hepatic and extrahepatic disease reported in treatment-naïve mUM patients receiving nivolumab plus ipilimumab.11
The efficacy of melphalan/HDS against extrahepatic lesions may be, at least in part, explained by residual melphalan systemic exposure, estimated at 14 % of the administered dose. In the FOCUS study, mOS also was consistent in patients with hepatic-only disease (20.8 months) and hepatic and extrahepatic disease (18.9 months). This consistency contrasts with mOS results seen with nivolumab plus ipilimumab treatment, which suggest shorter survival in patients with hepatic-only mUM at a median of 9.2 versus 15.5 months for patients with hepatic and extrahepatic disease.11
The mOS of 20.5 months compares favorably with results from meta-analyses of survival rates across various treatments, including chemotherapy, systemic ICI, and liver-directed therapy, which range from 10.2 to 12.8 months across all therapies.2,3 In the IMCgp100-202 study5, the mOS was 21.7 months in the tebentafusp arm and 16 months in the control arm. The mPFS of 9.0 months also compares favorably with the mPFS of 3.3 months from a meta-analysis of various treatments3 and with the mPFS from the IMCgp100-202 study of 3.3 months in the tebentafusp group and 2.9 months in the control group.
Comparisons with results from other clinical studies evaluating liver-directed therapies in mUM patients are difficult given markedly different patient populations (e.g., exclusion of patients with extrahepatic disease and other methodologic differences). The EORTC 18021 study of treatment-naïve patients compared efficacy and safety of fotamustine administered intravenously or via hepatic intra-arterial (HIA) infusion. In the HIA arm, mOS was 14.6 months, mPFS was 4.5 months, and ORR was 10.5 %.28 In a double-blind, randomized phase 2 study, mOS was 21.5 months, mPFS was 10.4 months, and ORR was 21.2 %.29
The safety profile of melphalan/HDS in the FOCUS study was characterized mainly by hematologic toxicity due to systemic exposure to residual melphalan. Melphalan/HDS patients receive high doses of melphalan locoregionally (up to 220 mg per treatment), and the perfusion system filters remove up to 86 % of the administered melphalan dose.30 In the FOCUS study, as expected with the resultant level of systemic melphalan exposure, a majority of the patients experienced severe myelosuppression. The observed safety profile was consistent with previous experience at these exposure levels.31,32
The hematologic AEs were generally transient in nature, with nadirs at the end of the second week after each treatment, and were treated mostly on an outpatient basis with observation. Tolerability of melphalan/HDS was good, as evidenced by a median of four completed treatment cycles and nearly 40 % of the patients completing the planned six treatment cycles as per study protocol. Analysis of safety data by PHP treatment cycle did not indicate cumulative toxicity. Analysis of efficacy by treatment cycle indicated that more than 50 % of the objective tumor responses had onset after the first or second treatment cycle. No new safety signals for melphalan/HDS were reported.
An area of ongoing investigation is the combination of melphalan/HDS treatment with nivolumab and ipilimumab in mUM patients. Recently published results from a small phase 1b clinical study show a good safety profile for this combination as well as an intriguing early activity signal (DCR 100 %, ORR 86 %).33 The rationale for this combination regimen is based on the ability of melphalan/HDS to enhance antigen presentation by killing cancer cells, resulting in immunomodulation, whereas anti–CTLA-4 and anti–PD-1/PD/L1 antibodies enhance immune responses to weak tumor antigens and activation of tumor-reactive immune cells.
Melphalan/HDS is a promising liver-directed treatment option for unresectable mUM. Additional clinical studies of other tumor types with hepatic metastases and combinations with immunotherapy are needed to further explore the full clinical potential of this novel treatment approach.
Conclusions
The FOCUS study provides robust evidence of the clinical benefit of melphalan/HDS for patients with unresectable mUM. This therapy offers a potential treatment option for patients with this rare indication, which is associated with a poor prognosis and has limited treatment options. Overall, the results demonstrated a favorable benefit-risk profile of melphalan/HDS for this patient population.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgment
We thank the patients, their families and caregivers, and the study teams at the participating sites. The authors acknowledge Seth Maxwell and Angela Wharton, who are employees of Delcath Systems, Inc., for their involvement in this study, including contributions to data analysis. All the authors critically reviewed the manuscript and approved the final version before submission, including the authorship list. Editorial support was provided by Preetinder Kaur of Certara under the direction of the authors following Good Publication Practice guidelines (Ann Intern Med. 2022;175:1298–1304) and was funded by Delcath Systems Inc., New York, USA.
Disclosure
Dr. Jonathan Zager–global principal investigator in the FOCUS phase 3 study and serves on Delcath Systems Medical Advisory Board. Education and Training grant through Delcath. Dr. Marlana Orloff–Advisory Board: Delcath, Replimune. Consultant: Immunocore. Steering Committee: Ideaya. Speaker: Immunocore. Dr. David Eschelman–Received <$5K consulting fees from Delcath. Dr. Evan Glazer–My institution was a site for the clinical trial. I received no direct payment. Delcath paid for the clinical trial to my institution. Dr. J. Howard–I was paid as a consultant to Delcath as a proctor for this trial to ensure new institutions adhered to protocol. Prof. Erika Richtig–Medical University of Graz received payments for conducting the Phase 3 study. Dr. Sebastian Ochsenreither–Advice: Immunocore, Delcath, Janssen. Speakers’ bureau: Immunocore. Dr. Georgia Beasley–Clinical trial funding paid to institution from Replimune, checkmate pharmaceuticals, Philogen, Delcath. Advisory board BMS 10.2023. Dr. Anja Gesierich–University Hospital Würzburg received payments for conducting the Phase 3 study. Professor Reinhard Dummer–intermittent, project focused consulting and/or advisory relationships with Novartis, Merck Sharp & Dhome (MSD), Bristol-Myers Squibb (BMS), Roche, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, Catalym, Second Genome, Regeneron, Alligator, T3 Pharma, MaxiVAX SA, Pfizer, Simcere and touchIME outside the submitted work. Dr. Ana Arance–Consulting or advisory role: Pierre- Fabre, Novartis, Roche, BMS, MSD, Biontech. Speakers’ Bureau: Pierre- Fabre, Novartis, Roche, BMS, MSD. Travel, Accommodations, Expenses: BMS, MSD. Mr. Stephen Fenwick–Liverpool University Hospitals NHS Trust received support for conducting this Phase 3 study from the trial sponsor. Dr. Joseph Sacco–I have been a paid member of advisory boards for Delcath, Replimune and Immunocore. I have received grant funding from Astra Zeneca and BMS (paid to institution). I have had travel and conference attendance paid for by MSD, BMS and Replimune. Dr. Matthew Wheater–Institutional (University Hospital Southampton) funding for conducting a commercial trial. Personal funding for participation in advisory board for Delcath.
Footnotes
The original online version of this article was revised due to a retrospective Open Access order.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/18/2024
A Correction to this paper has been published: 10.1245/s10434-024-15886-6
References
- 1.Carvajal RD, Sacco JJ, Jager MJ, et al. Advances in the clinical management of uveal melanoma. Nat Rev Clin Oncol. 2023;20:99–115. doi: 10.1038/s41571-022-00714-1. [DOI] [PubMed] [Google Scholar]
- 2.Rantala ES, Hernberg M, Kivelä TT. Overall survival after treatment for metastatic uveal melanoma: a systematic review and meta-analysis. Melanoma Res. 2019;29:561–568. doi: 10.1097/CMR.0000000000000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khoja L, Atenafu EG, Suciu S, et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: an international rare cancers initiative (IRCI) ocular melanoma study. Ann Oncol. 2019;30:1370–1380. doi: 10.1093/annonc/mdz176. [DOI] [PubMed] [Google Scholar]
- 4.Lane AM, Kim IK, Gragoudas ES. Survival rates in patients after treatment for metastasis from uveal melanoma. JAMA Ophthalmol. 2018;136:981–986. doi: 10.1001/jamaophthalmol.2018.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan P, Hassel JC, Rutkowski P, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2021;385:1196–1206. doi: 10.1056/NEJMoa2103485. [DOI] [PubMed] [Google Scholar]
- 6.Koch EA, Petzold A, Wessely A, et al. Immune checkpoint blockade for metastatic uveal melanoma: patterns of response and survival according to the presence of hepatic and extrahepatic metastasis. Cancers Basel. 2021;13:3359. doi: 10.3390/cancers13133359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mignard C, Huvier AD, Gillibert A, et al. Efficacy of immunotherapy in patients with metastatic mucosal or uveal melanoma. J Oncol. 2018;2018:1908065. doi: 10.1155/2018/1908065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Najjar YG, Navrazhina K, Ding F, et al. Ipilimumab plus nivolumab for patients with metastatic uveal melanoma: a multicenter, retrospective study. J Immunother Cancer. 2020;8:e000331. doi: 10.1136/jitc-2019-000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Algazi AP, Tsai KK, Shoushtari AN, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer. 2016;122:3344–3353. doi: 10.1002/cncr.30258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelster MS, Gruschkus SK, Bassett R, et al. Nivolumab and ipilimumab in metastatic uveal melanoma: results from a single-arm phase II study. J Clin Oncol. 2021;39:599–607. doi: 10.1200/JCO.20.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piulats JM, Espinosa E, De La Merino CL, et al. Nivolumab plus ipilimumab for treatment-naïve metastatic uveal melanoma: an open-label, multicenter, phase II trial by the Spanish Multidisciplinary Melanoma Group (GEM-1402) J Clin Oncol. 2021;39:586–598. doi: 10.1200/JCO.20.00550. [DOI] [PubMed] [Google Scholar]
- 12.Pham JP, On L, Ardolino L, et al. Efficacy of immune checkpoint inhibition in metastatic uveal melanoma: a systematic review and meta-analysis. Melanoma Res. 2023;33:316–325. doi: 10.1097/CMR.0000000000000900. [DOI] [PubMed] [Google Scholar]
- 13.Moy CS, Albert DM, Diener-West M, et al. Cause-specific mortality coding: methods in the Collaborative Ocular Melanoma Study COMS report no. 14. Control Clin Trials. 2001;22:248–262. doi: 10.1016/S0197-2456(01)00113-1. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network (NCCN 2022). NCCN clinical practice guidelines in oncology for melanoma: uveal. Version 2.2022. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1488.
- 15.Prescribing information for the HEPZATO KIT Hepatic Delivery System. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/201848s000lbl.pdf.
- 16.Bethlehem MS, Katsarelias D, Bagge RO. Meta-analysis of isolated hepatic perfusion and percutaneous hepatic perfusion as a treatment for uveal melanoma liver metastases. Cancers Basel. 2021;13:4726. doi: 10.3390/cancers13184726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagge RO, Nelson A, Shafazand A, et al. Isolated hepatic perfusion with melphalan for patients with isolated uveal melanoma liver metastases: a multicenter, randomized, open-label, phase III trial (the SCANDIUM trial) J Clin Oncol. 2023;41:3042–3050. doi: 10.1200/JCO.22.01705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Modi S, Gibson T, Vigneswaran G, et al. Chemosaturation with percutaneous hepatic perfusion of melphalan for metastatic uveal melanoma. Melanoma Res. 2022;32:103–111. doi: 10.1097/CMR.0000000000000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meijer TS, Burgmans MC, de Leede EM, et al. Percutaneous hepatic perfusion with melphalan in patients with unresectable ocular melanoma metastases confined to the liver: a prospective phase II study. Ann Surg Oncol. 2021;28:1130–1141. doi: 10.1245/s10434-020-08741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgmans MC, de Leede EM, Martini CH, Kapiteijn E, Vahrmeijer AL, van Erkel AR. Percutaneous isolated hepatic perfusion for the treatment of unresectable liver malignancies. Cardiovasc Intervent Radiol. 2016;39:801–814. doi: 10.1007/s00270-015-1276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Artzner C, Mossakowski O, Hefferman G, et al. Chemosaturation with percutaneous hepatic perfusion of melphalan for liver-dominant metastatic uveal melanoma: a single center experience. Cancer Imaging. 2019;19:31. doi: 10.1186/s40644-019-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewald CLA, Warnke MM, Brüning R, et al. Percutaneous hepatic perfusion (PHP) with melphalan in liver-dominant metastatic uveal melanoma: the German experience. Cancers Basel. 2022;14:118. doi: 10.3390/cancers14010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Published 28 May 2009 (v4.03: 14 June 2010). U.S. Department of Health and Human Services: National Institutes of Health, National Cancer Institute. Available at https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf.
- 26.Edge SB, Byrd DR, Compton CC, et al. (eds). Malignant melanoma of the uvea. In AJCC Cancer Staging Manual. 7th ed. Springer: New York, 2010, pp 547–60.
- 27.KIMMTRAK (tebentafusp-tebn) USPI. Immunocore. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761228s000lbl.pdf.
- 28.Leyvraz S, Piperno-Neumann S, Suciu S, et al. Hepatic intra-arterial versus intravenous fotemustine in patients with liver metastases from uveal melanoma (EORTC 18021): a multicentric randomized trial. Ann Oncol. 2014;25:742–746. doi: 10.1093/annonc/mdt585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valsecchi ME, Terai M, Eschelman DJ, et al. Double-blinded, randomized phase II study using embolization with or without granulocyte-macrophage colony-stimulating factor in uveal melanoma with hepatic metastases. J Vasc Interv Radiol. 2015;26:523–32.e2. doi: 10.1016/j.jvir.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Leede EM, Burgmans MC, Meijer TS, et al. Prospective clinical and pharmacological evaluation of the Delcath system’s second-generation (GEN2) hemofiltration system in patients undergoing percutaneous hepatic perfusion with melphalan. Cardiovasc Intervent Radiol. 2017;40:1196–1205. doi: 10.1007/s00270-017-1630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong TML, Samim M, Kapiteijn E, et al. Predictive parameters in patients undergoing percutaneous hepatic perfusion with melphalan for unresectable liver metastases from uveal melanoma: a retrospective pooled analysis. Cardiovasc Intervent Radiol. 2022;45:1304–1313. doi: 10.1007/s00270-022-03225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meijer TS, Burgmans MC, Fiocco M, et al. Safety of percutaneous hepatic perfusion with melphalan in patients with unresectable liver metastases from ocular melanoma using the Delcath System’s second-generation hemofiltration system: a prospective non-randomized phase II trial. Cardiovasc Intervent Radiol. 2019;42:841–852. doi: 10.1007/s00270-019-02177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong TML, Burgmans MC, Speetjens FM, et al. Combining melphalan percutaneous hepatic perfusion with ipilimumab plus nivolumab in advanced uveal melanoma: first safety and efficacy data from the phase Ib part of the Chopin trial. Cardiovasc Intervent Radiol. 2023;46:350–359. doi: 10.1007/s00270-022-03338-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.