Abstract
Monocytes are precursors of tissue macrophages, which are major targets of human immunodeficiency virus type 1 (HIV-1) infection. Although few blood monocytes are infected, their resulting activation could play a key role in the pathogenesis of HIV disease by modulating their transendothelial migration and inducing the production of reactive oxygen species (ROS). ROS participate in chronic inflammation, HIV replication, and the apoptosis of immune system cells seen in HIV-infected subjects. Published data on monocyte activation are controversial, possibly because most studies have involved monocytes isolated from their blood environment by various procedures that may alter cell responses. We therefore used flow cytometry to study, in whole blood, the activation and redox status of monocytes from HIV-infected patients at different stages of the disease. We studied the expression of adhesion molecules, actin polymerization, and cellular levels of H2O2, Bcl-2, and thioredoxin. Basal H2O2 production correlated with viral load and was further enhanced by bacterial N-formyl peptides and endotoxin. The enhanced H2O2 production by monocytes from asymptomatic untreated patients with CD4+ cell counts above 500/μl was associated with a decrease in the levels of Bcl-2 and thioredoxin. In contrast, in patients with AIDS, Bcl-2 levels returned to normal and thioredoxin levels were higher than in healthy controls. Restoration of these antioxidant and antiapoptotic molecules might explain, at least in part, why monocyte numbers remain relatively stable throughout the disease. Alterations of adhesion molecule expression and increased actin polymerization could play a role in transendothelial migration of these activated monocytes.
Tissue macrophages are important targets of human immunodeficiency virus type 1 (HIV-1) infection and appear to play a key role in HIV disease progression (14). Macrophages are derived from blood monocytes, whose precise role in the pathogenesis of AIDS is controversial (36). Few blood monocytes are infected by HIV, but their activation plays a key role in their transendothelial migration to the tissues (34). In addition, activated monocytes, together with polymorphonuclear neutrophils, are a major source of reactive oxygen species (ROS) (2, 13), which are essential for bacterial killing (2). However, excessive ROS production not appropriately compensated by antioxidant molecules can lead to oxidative stress, which may also play an important role in pathogenesis of HIV infection through various mechanisms (4, 23). In particular, ROS synergize with proinflammatory cytokines, activating the NF-κB transcription factor and inducing HIV long terminal repeat transactivation in monocytic cell lines (22). ROS also potentiate the production by monocytes of proinflammatory cytokines (9, 17), which, in turn, can increase HIV replication (21). Furthermore, ROS participate in T-lymphocyte depletion by triggering programmed cell death (apoptosis) (7) and monocytes can also induce their own apoptosis by producing ROS (26, 45).
Regulation of ROS production by monocytes is dependent on an equilibrium between activation of NADPH oxidase (a multicomponent enzyme system which is the main source of ROS) and cellular levels of the different antioxidant molecules (39). Thioredoxin (Trx) is a small protein with thiol-reducing and radical-scavenging activities (19); Trx has also been reported to protect cells against anti-Fas antibody-induced apoptosis (30). The Trx-Trx peroxidase system, which is intimately involved in cell redox regulation, has recently been described as an endogenous regulator of apoptosis (46). Bcl-2 can protect cells against apoptosis triggered by various stimuli, including oxidative stress (18, 24, 25). However, the potential impact of ROS production by monocytes on their redox status in vivo, especially during HIV infection, is unknown.
Contradictory results on ROS production by monocytes in HIV infection have been reported. Several investigators have found a normal (15, 33, 37) or even enhanced (3, 44) response, whereas other have found diminished activities (10, 32, 40). These discrepancies could be due to the use of monocytes from patients at different stages of HIV disease and to the fact that most studies have involved monocytes isolated from their blood environment by various procedures, which may have different effects on surface receptor expression and thereby may alter cell responses (28). The use of whole blood thus seems more suitable for determining monocyte ROS production during disease progression. We have previously used this approach to show that neutrophils are activated during HIV infection (12).
The aim of this study was to analyze the activation and redox status of monocytes from patients at different stages of HIV infection, with the aim of clarifying the mechanisms of their detrimental effects on the course of the disease. For this purpose, we used flow cytometry analysis in whole blood to identify monocytes on the basis of their high CD14 expression and to measure (i) H2O2 production by monocytes, (ii) intracellular expression of Bcl-2 and Trx, and (iii) the activation status of monocytes in terms of adhesion molecule surface expression and actin polymerization, which appears to be involved in migration, receptor expression, and the oxidative burst (42).
MATERIALS AND METHODS
Reagents.
The reagents and sources were as follows: recombinant human tumor necrosis factor alpha (rhTNFα) (2 × 105 U/ml; Genzyme, Cambridge, Mass.), 2′,7′-dichlorofluorescin-diacetate (DCFH-DA) (Eastman Kodak, Rochester, N.Y.) lipopolysaccharide (LPS) endotoxin from Escherichia coli (O55:B5), N-formylmethionylleucylphenylalanine (fMLP), unlabelled phalloidin, fluorescein isothiocyanate (FITC)-conjugated phalloidin, l-α-lysophosphatidylcholine (Sigma Chemical Co., St. Louis, Mo.), phycoerythrin (PE)-conjugated monoclonal mouse anti-human CD14 antibody (PE-anti-CD14), FITC–anti-HLA-DR antibody, FITC-conjugated monoclonal mouse anti-human CD18 antibody and SimulTEST reagents (FITC-CD45 plus PE-CD14 [leukogate], FITC-γ1 plus PE-γ2 [control], FITC-CD3 plus PE-CD4, FITC-CD3 plus PE-CD8, FITC-CD3 plus PE-CD19, FITC-CD3 plus PE-CD16+CD56, FITC-CD8 plus PE-CD38, and FITC-CD8 plus PE–HLA-DR [Becton-Dickinson, Immunocytometry Systems, San Jose, Calif.]), FITC-conjugated monoclonal mouse anti-human CD11a antibody and FITC-conjugated monoclonal mouse anti-human CD11b antibody (Immunotech, Marseilles, France), FITC-conjugated monoclonal mouse anti-human Bcl-2 (Dako, Glostrup, Denmark), purified monoclonal mouse antibody to human Trx (provided by H. Masutani, Kyoto, Japan), purified monoclonal mouse antibody to human L-selectin (anti-LAM) (Coultronics, Hialeah, Fla.), FITC-goat anti-mouse immunoglobulins (FITC-GAM) (TEBU, Santa Cruz, Calif.), and fetal calf serum (Gibco Laboratories, Grand Island, N.Y.).
Stock solutions of DCFH-DA (50 mM) and fMLP (10−2 M) were prepared in dimethyl sulfoxide and stored at −20°C. The solutions were diluted in phosphate-buffered saline (PBS; Pharmacia Fine Chemicals Uppsala, Sweden) immediately before use.
Subjects.
We studied 35 HIV-1-infected adults (9 females and 26 males; mean age, 38 ± 8 years). HIV seropositivity was detected by an enzyme-linked immunosorbent assay and confirmed by Western blotting. Patients with ongoing infections which might affect monocyte functions, particularly opportunistic infections, were excluded. HIV infection was classified according to the Centers for Disease Control and Prevention (CDC) criteria (8). Three groups of patients were studied: CDC class A (n = 10; asymptomatic, CD4+-cell counts, >500/μl; none of these patients were receiving any significant medication) (group 1), CDC class A (n = 9; asymptomatic or generalized lymphadenopathy) and CDC class B (n = 7; oral Candida albicans infection); CD4+-cell counts, <500/μl) (group 2), and CDC class C (n = 9; AIDS with documented opportunistic infection; CD4+-cell count, <500/μl) (group 3).
CD4+-cell counts were measured at the time of the study. All but one of the patients in groups 2 and 3 were receiving various nucleoside analogs, alone (zidovudine or lamivudine, n = 3) or combined (zidovudine plus lamivudine, zidovudine plus didanosine, zidovudine plus zalcitabine, stavudine plus didanosine, zidovudine plus lamivudine plus zalcitabine, n = 21). One patient received zidovudine and a protease inhibitor. Eighteen patients were receiving prophylaxis for opportunistic infections, consisting of aerosolized pentamidine with or without pyrimethamine in five cases, co-trimoxazole in six cases, and dapsone with or without pyrimethamine in seven cases. Four patients with AIDS were receiving prophylaxis for Mycobacterium avium complex infection, and two patients with AIDS were receiving secondary prophylaxis for cytomegalovirus retinitis. Blood samples were obtained during a routine visit. Fifteen HIV-seronegative members of the laboratory staff served as controls. Whole-blood samples were placed in an ice bath and transported immediately to the laboratory.
Quantification of viral load.
Blood was collected into sterile heparinate-treated vacuum tubes. The viral load in plasma was quantified by PCR amplification of viral RNA on duplicate samples collected on EDTA, as specified by the manufacturer (Amplicor HIV monitor; Roche). Viral RNA was quantified against an RNA quantification standard which was amplified simultaneously with each sample. Viral RNA was expressed as the number of RNA copies per milliliter. The detection limit was 200 copies/ml.
Assay of lymphocyte subsets.
Samples (100 μl) of fresh blood collected in EDTA tubes were mixed with 20 μl of the monoclonal reagent combination and incubated for 15 min in the dark at room temperature. Erythrocytes were lysed with fluorescence-activated cell sorter (FACS) lysing solution (Beckton Dickinson). After one wash in FACSflow buffer (400 × g for 5 min), leukocytes were resuspended in 1% paraformaldehyde–PBS. The samples were stored at 4°C and analyzed by flow cytometry within 24 h of fixation.
H2O2 production.
H2O2 production was measured by a flow-cytometric assay derived from the assay described by Bass et al. (5, 13). Fresh blood (1 ml) from healthy donors, collected onto preservative-free Liquemine (10 U/ml of blood), was preincubated for 15 min with 2′,7′-DCFH-DA (100 μM) in a 37°C water bath with gentle horizontal shaking. (DCFH-DA diffuses into cells and is hydrolyzed into 2′,7′-dichlorofluorescin [DCFH]. During the monocyte oxidative burst, nonfluorescent intracellular DCFH is oxidized into the highly fluorescent dichlorofluorescein [DCF] by H2O2.) The samples were then incubated with either rhTNF-α (100 U/ml) or LPS (5 μg/ml) diluted in PBS, or with PBS alone, at 37°C for 30 min. fMLP diluted in PBS (10−6 mol/liter [final concentration]), or a similar dilution of dimethyl sulfoxide in PBS, was added for 5 min at 37°C. These standard conditions of stimulation in whole blood were selected as previously described (13). The reaction was stopped, and samples were incubated with PE–anti-CD14 antibody for 30 min at 4°C. Erythrocytes were lysed with FACS lysing solution. After one wash (400 × g for 5 min) in PBS, leukocytes were suspended in 1% paraformaldehyde–PBS. The fixed samples were kept on ice until used for a flow-cytometric analysis on the same day. FACS lysing solution neither modified the amount of DCF generated nor increased the expression of activation markers such as CR3, as measured by flow cytometry (data not shown). Moreover, monocyte viability was not altered under our experimental conditions, as assessed in terms of propidium iodide exclusion by means of flow cytometry.
Determination of intracellular expression of Bcl-2 and Trx molecules.
Whole blood (100 μl) was incubated with PE-anti-CD14 for 30 min at 4°C. Erythrocytes were lysed with FACS lysing solution. Leukocytes were washed twice with PBS containing 2% fetal calf serum. Paraformaldehyde (0.25%) was then added while vortexing, and the samples were incubated in the dark for 15 min at room temperature. After one wash with PBS, the leukocytes were incubated with ice-cold PBS–70% methanol in the dark for 60 min at 4°C to permeabilize the cell membranes. After one wash in PBS, the samples were incubated with FITC–anti-Bcl-2 and anti-Trx antibodies for 30 min at 4°C. To study Trx expression, samples were then incubated with FITC-conjugated goat-anti mouse immunoglobulin G. After one wash with ice-cold PBS-fetal calf serum, the cells were resuspended in 1% paraformaldehyde–PBS and kept on ice until used for flow-cytometric analysis. Nonspecific antibody binding was determined on cells incubated with the same concentration of an irrelevant antibody of the same isotype.
Determination of adhesion molecule expression at the monocyte surface.
Whole blood was either kept on ice or incubated at 37°C with rhTNF-α (100 U/ml), LPS (5 μg/ml) or control solutions for 5 min. Expression of adhesion molecules at the monocyte surface was studied as previously described (13). To study CD11b and CD18 molecule expression on CD14high cells, samples (100 μl) were incubated at 4°C for 45 min with the following monoclonal reagent combinations: FITC-anti-CD11b plus PE-anti-CD14 and FITC-anti-CD18 plus PE-anti-CD14. To study L-selectin expression on CD14high cells, samples were incubated with nonconjugated anti-LAM antibody for 30 min at 4°C, washed with ice-cold PBS, and then incubated at 4°C for 30 min with FITC-GAM; after one wash in ice-cold PBS, samples were incubated with PE-anti-CD14 at 4°C for 30 min. Erythrocytes were lysed with FACS lysing solution, and leukocytes were resuspended in 1% paraformaldehyde–PBS and kept on ice until used for flow cytometry. Nonspecific antibody binding was determined on cells incubated with the same concentration of an irrelevant antibody of the same isotype.
F-actin content of monocytes.
Whole blood was either kept on ice or incubated at 37°C with either rhTNF-α (100 U/ml) or LPS (5 μg/ml) diluted in PBS, or with PBS alone, for 1 min. After incubation with anti-CD14 antibody, leukocytes (obtained after erythrocyte lysis) were fixed with 1% paraformaldehyde–PBS and the F-actin content was measured in a flow-cytometric assay as previously described (13). The cell suspension (100 μl) was incubated for 30 min at 0°C in 100 μl of 8% paraformaldehyde and 200 μl of l-α-lysophosphatidylcholine per ml in PBS, without or with 1 mM unlabeled phalloidin to measure the nonspecific binding of FITC-phalloidin. FITC-phalloidin (20 μM) was then added to the suspension, and incubation was continued for 30 min at 0°C. After one wash in PBS, the cells were resuspended in 1% paraformaldehyde–PBS.
Flow cytometry.
We used a FACScan (Becton-Dickinson, Immunocytometry Systems) with a 15-mW, 488-nm argon laser. Anti-CD14 antibody was used to identify the monocyte population, which appeared on the PE-CD14 antibody fluorescence histogram as highly fluorescent CD14+ cells (CD14high cells). CD14 expression was the same in HIV-infected patients and in controls. A total of 104 CD14high cells were counted per sample, and fluorescence pulses were amplified by 4-decade logarithmic amplifiers. The green fluorescence of DCF, FITC-phalloidin, FITC-anti-CD11a, FITC-anti-CD11b, FITC-anti-CD18, FITC–anti-Bcl-2, and FITC-GAM was recorded from 515 to 545 nm, and the orange fluorescence of PE-anti-CD14 was recorded from 563 to 607 nm. In all cases, experiments with unstained cells were run and photomultiplier settings were adjusted so that the unstained cell population appeared in the lower-left-hand corner of the fluorescence display. Single-cell controls were used to optimize signal compensation. All the results were obtained with a constant photomultiplier gain. The data were analyzed with LYSIS II software (Becton-Dickinson), and the mean fluorescence intensity was used to quantify the responses. The effect of agonists on the monocyte oxidative burst was calculated by using a stimulation index, defined as the ratio of the mean fluorescence intensity of stimulated cells to that of unstimulated cells.
Statistical analysis.
All results are expressed as means ± standard errors of the mean (SEM). The group means were compared by using analysis of variance followed by a multiple comparison of means by Fisher’s least-significant-difference procedure. P ≤ 0.05 was considered significant. Correlations were identified with the Spearman rank correlation coefficient (ρ).
RESULTS
Characteristics of the study population.
The HIV-infected patients were classified as follows: asymptomatic untreated patients with normal CD4+-cell counts (group 1), asymptomatic and symptomatic non-AIDS patients with CD4+-cell counts below 500/μl (group 2), and AIDS patients (group 3). Group 2 and 3 patients were receiving nucleoside analogs (see Materials and Methods). As shown in Table 1, the monocyte count was significantly increased in group 1 and was normal in groups 2 and 3. Lymphocyte counts were significantly decreased in groups 2 and 3 relative to the healthy controls. CD8+-cell counts were significantly increased in group 1 relative to the healthy controls. The percentage of CD8+ lymphocytes that expressed CD38 or HLA-DR antigens was significantly increased in all the patient groups relative to the healthy controls and was inversely proportional to the CD4+-cell count, as previously reported (27) (ρ = 0.7 [P = 0.0001] and ρ = 0.6 [P = 0.0001] for the percentage of CD8/CD38 and CD8/HLA-DR, respectively). The viral load was significantly higher in group 3 than in the other HIV-infected patients and was lower in group 2 than in group 1, no doubt because group 2 patients were taking antiretrovirus therapy.
TABLE 1.
Characteristics of the study population
| Characteristic | Value (mean ± SEM) ind:
|
|||
|---|---|---|---|---|
| Controls | Group 1 | Group 2 | Group 3 | |
| Monocyte counta | 377 ± 31 | 554 ± 80* | 446 ± 50 | 372 ± 75 |
| Lymphocyte counta | 2,339 ± 190 | 2,669 ± 194 | 1,313 ± 152*† | 1,139 ± 381*† |
| CD4+-cell counta | 836 ± 76 | 749 ± 100 | 244 ± 41*† | 90 ± 27*† |
| CD8+-cell counta | 803 ± 96 | 1,378 ± 190 | 765 ± 112 | 727 ± 303† |
| CD8/CD38+b | 22.5 ± 2.2 | 38.4 ± 3.9* | 41.4 ± 2.7* | 57.6 ± 3.3*‡ |
| CD8/HLA-DRb | 9.1 ± 2.1 | 23.6 ± 2.8* | 27.4 ± 2.1* | 34.4 ± 5.4* |
| Viral RNA (103)c | 77.1 ± 30.7 | 28.3 ± 14.2 | 229.6 ± 80.1° | |
Values are given per microliter.
CD8+ cells that were CD38 positive or HLA-DR positive, expressed as a percentage of all lymphoid cells.
Viral RNA was measured in plasma by PCR and is expressed in 103 copies per milliliter.
*, significantly different from control values (P < 0.05); °, significantly different from groups 1 and 2 (P < 0.05); †, significantly different from group 1 (P < 0.05); ‡, significantly different from group 2 (P < 0.05).
H2O2 production by monocytes in whole blood from HIV-infected patients correlates with viral load.
As shown in Table 2, spontaneous H2O2 production was significantly higher with unstimulated monocytes from groups 1 and 3 than from the healthy controls, with monocytes from the controls expressing low background fluorescence. Spontaneous H2O2 production by monocytes from group 2 patients was lower than in groups 1 and 3. Individual spontaneous H2O2 production by monocytes from HIV-infected patients correlated with the viral load (Tables 1 and 2; ρ = 0.6, P = 0.0004), suggesting that these monocytes were activated in the patient’s circulation in a direct relationship to HIV infection.
TABLE 2.
H2O2 production by monocytes from HIV-infected patients and control subjectsa
| Stimulus | H2O2 production (mean ± SEM) byb:
|
|||
|---|---|---|---|---|
| Controls | Group 1 | Group 2 | Group 3 | |
| PBS | 27.7 ± 2.2 | 52.1 ± 10.3* | 32.4 ± 3.8° | 53.2 ± 10.5* |
| fMLP | 41.0 ± 0.4 | 91.6 ± 24.9* | 54.3 ± 3.4† | 77.1 ± 14.8* |
| LPS | 99.8 ± 9.9 | 158.9 ± 24.3* | 153.6 ± 18.0* | 182.6 ± 24.1* |
| LPS + fMLP | 233.8 ± 25.6 | 227.4 ± 45.2 | 292.1 ± 35.3 | 276.0 ± 45.7 |
After preincubation with DCFH-DA (100 μmol/liter) for 15 min at 37°C, whole blood was pretreated with PBS or LPS (5 μg/ml) for 30 min and then with PBS or fMLP at 10−6 mol/liter for 5 min. Anti-CD14 antibody was used to identify the monocyte population. The mean fluorescence intensity of DCF was recorded as described in Materials and Methods.
*, significantly different from control values (P < 0.05); °, significantly different from groups 1 and 3 (P < 0.05); †, significantly different from group 1 (P < 0.05).
The bacterial N-formyl peptide, fMLP (when present at 10−6 mol/liter for 5 min), increased H2O2 production by monocytes from HIV-infected patients, and the increase was significant in groups 1 and 3 relative to the healthy controls. LPS, a more powerful stimulus of the monocyte oxidative burst, significantly increased H2O2 production in the three groups of patients. It is noteworthy that optimal stimulation of monocytes under priming conditions with LPS followed by stimulation with fMLP induced strong H2O2 production in both the HIV-infected patients and the control subjects, indicating a normal maximal capacity of monocytes from HIV-infected patients to produce H2O2. Similar data were obtained after pretreatment with TNF-α followed by fMLP stimulation (data not shown).
In sum, monocytes from HIV-infected patients spontaneously produced H2O2 to a degree that correlated with the viral load. In addition, H2O2 production was further increased in response to bacterial stimuli added separately, and maximal ROS production under conditions of optimal stimulation was normal.
Intracellular levels of Bcl-2 and Trx in monocytes undergo a biphasic evolution during the disease progression.
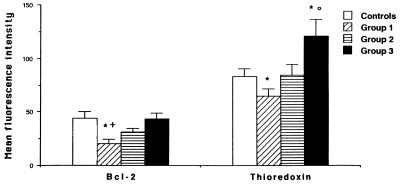
Since Trx and Bcl-2 have been implicated in redox regulation and programmed cell death, we measured their basal expression in monocytes from HIV-infected patients. As shown in Fig. 1, intracellular expression of Bcl-2 and Trx was significantly lower in monocytes from HIV-infected patients (group 1) than from control subjects. This decrease was not observed in groups 2 and 3; in fact, Trx expression was significantly higher in group 3 than in the control subjects.
FIG. 1.
Basal expression of Trx and Bcl-2 in monocytes from HIV-1-infected patients. After cell permeabilization with methanol, whole-blood samples were incubated with anti-Bcl-2 or anti-Trx antibodies. The mean fluorescence intensity of anti-Bcl-2 and anti-Trx antibodies was recorded as described in Materials and Methods, and the mean fluorescence intensity of the isotypic control was subtracted. Values are means ± SEM. ∗, significantly different from control values (P < 0.05); ○, significantly different from groups 1 and 2 (P < 0.05); +, significantly different from group 3 (P < 0.05).
Expression of activation markers involved in monocyte migration.
Modulation of the expression of adhesion molecules at the monocyte surface is one of the first steps of transendothelial migration. As shown in Table 3, the mean fluorescence intensity generated by anti-L-selectin antibody binding to unstimulated monocytes was significantly lower in all the groups of patients than in the healthy control subjects, while CD11b and CD18 expression was significantly higher in all the groups of patients than in the controls. These results reflected basal activation of monocytes from all the HIV-infected patients.
TABLE 3.
L-selectin and CD11b/CD18 expression at the surface of resting monocytes from HIV-infected patients and healthy controlsa
| Patient group | Mean fluorescence intensity ± SEMb for:
|
||
|---|---|---|---|
| L-selectin | CD11b | CD18 | |
| Controls | 86.4 ± 7.7 | 30.7 ± 2.1 | 30.7 ± 1.8 |
| Group 1 | 50.9 ± 6.6* | 45.6 ± 3.8* | 43.5 ± 4.0* |
| Group 2 | 64.3 ± 5.0* | 46.2 ± 3.1* | 41.9 ± 2.9* |
| Group 3 | 65.8 ± 7.7* | 56.8 ± 5.2* | 44.7 ± 3.5* |
Whole-blood samples maintained at 4°C were incubated with anti-LAM1.3, anti-CD11b, or anti-CD18 antibodies for 30 min at 4°C, and the mean fluorescence intensity was recorded as described in Materials and Methods. Values obtained with an irrelevant antibody of the same isotype were subtracted.
*, significantly different from control values (P < 0.05).
After stimulation with LPS (5 μg/ml) or rhTNF-α (100 U/ml) for 30 min at 37°C, a comparable alteration of adhesion molecule expression was observed in the healthy control subjects and HIV-infected patients: L-selectin was no longer detectable, and CD11b expression increased strongly, reflecting normal shedding of L-selectin and normal maximal CD11b expression in HIV-infected patients (data not shown). Similar results were observed for CD11a expression at the monocyte surface.
In parallel, actin polymerization involved in monocyte migration was determined as the F-actin content in monocytes from HIV-infected patients. The mean fluorescence intensity of FITC-phalloidin binding to unstimulated monocytes was significantly higher in the HIV-infected patients (38.5 ± 3.5, 43.7 ± 2.4, and 39.8 ± 3.6 in groups 1, 2, and 3, respectively; 29.5 ± 2.8 in control subjects [P < 0.05]). There was no significant increase in fluorescence intensity with the stage of HIV disease. Similar results were observed after a 30-min incubation at 37°C. After stimulation with LPS (5 μg/ml) or TNF (100 U/ml), FITC-phalloidin binding was similar in the patients and controls, showing that maximal F-actin polymerization was unaffected by HIV infection (data not shown).
DISCUSSION
In this report, we provide evidence that HIV infection induces an activation state of circulating monocytes associated with alteration of their redox status. Indeed, monocytes from HIV-infected patients spontaneously produce increased amounts of H2O2, to a degree that correlates with the viral load. They also have an increased capacity to produce H2O2 in response to suboptimal stimulation by bacterial products. Monocyte activation was demonstrated by reduced L-selectin expression, increased CD11b/CD18 expression, and increased actin polymerization. These alterations were constant throughout the disease, while variations of H2O2 production and Bcl-2 and Trx levels suggest a tight in vivo regulation of the redox status of monocytes from HIV-infected patients. These results could explain, at least in part, why monocyte numbers remain relatively stable throughout the disease.
There have been conflicting reports on the oxidative burst potential of monocytes isolated from HIV-infected patients in response to various stimuli (3, 10, 15, 32, 33, 37, 40, 44). These discrepancies could, at least in part, be due to methodological differences, particularly monocyte isolation procedures. We therefore studied H2O2 production by monocytes in their blood environment. It is noteworthy that under optimal conditions of stimulation (priming with LPS or rhTNF-α followed by stimulation with fMLP), no difference in H2O2 production by monocytes from the patients and healthy controls was observed, demonstrating normal maximal ROS production. This is in keeping with data reported by Meyer and Nielsen (31) demonstrating that the monocyte oxidative burst in response to fMLP after priming with granulocyte-macrophage colony-stimulating factor and gamma interferon does not differ in HIV-infected patients and healthy control subjects. Our results therefore suggest that the higher susceptibility to bacterial infections of HIV-infected patients cannot be attributed to a reduced capacity of monocytes to generate ROS.
In accordance with the results of Trial et al. (44), we found increased basal H2O2 production by whole-blood monocytes from HIV-infected patients in the later stages of the infection (group 3) relative to that in the healthy control subjects. We also extended this observation to asymptomatic untreated patients with CD4+-cell counts greater than 500/μl (group 1). However, in the asymptomatic and symptomatic non-AIDS patients undergoing treatment (group 2), in whom the viral load in plasma was the lowest, basal H2O2 production was not significantly higher than in the control subjects. These data suggest that circulating-monocyte activation is directly related to the viral load in plasma. This was further supported by a positive correlation between individual viral load and monocyte H2O2 production.
The oxidative response to fMLP and LPS by monocytes from HIV-infected patients was increased, suggesting that the background oxidative stress generated by monocytes from these patients could be enhanced by intercurrent infections. In particular, increased H2O2 production after fMLP stimulation points to in vivo priming by cytokines, as in other clinical settings such as bacterial infections (6). This increased ROS production by monocytes could participate in oxidative injury, which has been implicated in the pathophysiology of HIV infection (9, 17, 22, 23).
Trx and Bcl-2 protect cells against apoptosis induced by oxidative stress (18, 24, 25, 46). We therefore determined the cellular level of these antioxidant and antiapoptotic compounds in parallel with H2O2 production by monocytes from HIV-infected patients. Our results demonstrate a significant reduction in Trx and Bcl-2 levels in monocytes from asymptomatic untreated HIV-infected patients. In contrast, in the patients with AIDS, Bcl-2 levels returned to normal and Trx levels were significantly increased. These results suggest that the expression of Trx and Bcl-2 may be dually regulated in circulating monocytes from HIV-infected patients: in the early stages of the disease, a decrease could result from degradation of these molecules in protecting cells against ROS-induced oxidative stress. This downregulation might be compensated for in the later stages of the disease by upregulation at the transcriptional level, as suggested by our recent in vitro findings (1). Several investigators have also reported that Trx expression is induced by a variety of stressors, including mitogens, X-ray and UV irradiation, hydrogen peroxide, and virus infection (38). In particular, Masutani et al. (29) reported that oxidative stress induced Trx promoter transactivation. These mechanisms could be aimed at compensating for the oxidative stress associated with the viral load. In contrast to lymphocytes, no reduction in monocyte numbers was observed in blood throughout the disease. These differences between monocytes and lymphocytes during HIV disease suggest that monocyte viability may be tightly regulated by antiapoptotic and antioxidant molecules such as Trx and Bcl-2, whose upregulation could limit the rate of ROS production and apoptosis in the later stages of the disease.
The increased ROS production correlated with viral load, suggesting that monocyte activation might be directly related to stimulation by HIV. We confirmed the decrease in L-selectin expression and the increase in CD11b/CD18 in the later stages of infection (11, 35, 44). We also extended this observation to patients with CD4 cell counts above 500/μl. No significant difference was found between the patient groups, and hence no correlation with viral load was found. A similar pattern of increase in F-actin content, whatever the stage of the disease, was also observed in the HIV-infected patients. Modulation of adhesion molecules and cytoskeleton components secondary to monocyte activation may play a key role in transendothelial migration of the monocytes to the tissues. A major mechanism by which HIV-infected monocytes penetrate through the blood-brain barrier is through upregulation of adhesion molecules on endothelial cells, mediated by monocyte activation (34). Moreover, a recent study showed that macrophage infiltration of the brain was a better correlate of clinical dementia than was the number of virus-infected cells (16). Therefore, monocyte activation, clearly demonstrated here, rather than HIV-1 infection itself, could play a key role in the neuropathogenesis of HIV-1 encephalitis and dementia.
In conclusion, we found that whole-blood monocytes from HIV-infected patients spontaneously produced H2O2, to a degree that correlated with viral load and could participate in the overall oxidative injury in these patients. This increased ROS production was associated with changes in the expression of the antiapoptotic and antioxidant compounds Bcl-2 and Trx during the course of the disease. This modulation could be attributed to dual regulation by oxidative stress and viral load and could explain, at least in part, the relatively stable number of monocytes during the course of the disease. In addition, changes in adhesion molecule expression and increased actin polymerization could play a role in transendothelial migration of these activated monocytes.
ACKNOWLEDGMENTS
S. Pillet and M. H. Prevost contributed equally to this work.
This work was supported by a grant from ANRS.
REFERENCES
- 1.Aillet F, Masutani H, Elbim C, Raoul H, Chêne L, Nugeyre M T, Paya C, Barré-Sinoussi F, Gougerot-Pocidalo M A, Israel N. Human immunodeficiency virus induces a dual regulation of Bcl-2, resulting in a persistent infection of CD4+ T- or monocytic cell lines. J Virol. 1998;72:9698–9705. doi: 10.1128/jvi.72.12.9698-9705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babior B M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984;64:959–966. [PubMed] [Google Scholar]
- 3.Bandres J C, Trial J, Musher D M, Rossen R D. Increased phagocytosis and generation of reactive oxygen products by neutrophils and monocytes of men with stage 1 human immunodeficiency virus infection. J Infect Dis. 1993;168:75–83. doi: 10.1093/infdis/168.1.75. [DOI] [PubMed] [Google Scholar]
- 4.Baruchel S, Wainberg M A. The role of oxidative stress in disease progression in individuals infected by the human immunodeficiency virus. J Leukoc Biol. 1992;52:111–114. doi: 10.1002/jlb.52.1.111. [DOI] [PubMed] [Google Scholar]
- 5.Bass D A, Parce J W, Dechatelet L R, Szejda P, Seeds M C, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983;130:1910–1917. [PubMed] [Google Scholar]
- 6.Bass D A, Olbrantz P, Szeda P, Seeds M C, McCall C E. Subpopulations of neutrophils with increased oxidative product formation in blood of patients with infection. J Immunol. 1986;136:860–866. [PubMed] [Google Scholar]
- 7.Buttke T, Sandstrom P A. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994;15:7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morbid Mortal Weekly Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 9.Chaudri G, Clark I A. Reactive oxygen species facilitate the in vitro and in vivo lipopolysaccharide-induced release of tumor necrosis factor. J Immunol. 1989;143:1290–1297. [PubMed] [Google Scholar]
- 10.Chen T P, Roberts R L, Wu K G, Ank B J, Stiehm E R. Decreased superoxide anion and hydrogen peroxide production by neutrophils and monocytes in human immunodeficiency virus-infected children and adults. Pediatr Res. 1993;34:544–550. doi: 10.1203/00006450-199310000-00032. [DOI] [PubMed] [Google Scholar]
- 11.Desroches C V, Rigal D. Leukocyte function-associated antigen-1 expression on peripheral blood mononuclear cell subsets in HIV-1 seropositive patients. Clin Immunol Immunopathol. 1990;56:159–168. doi: 10.1016/0090-1229(90)90138-g. [DOI] [PubMed] [Google Scholar]
- 12.Elbim C, Prevot M H, Bouscarat F, Franzini E, Chollet-Martin S, Hakim J, Gougerot-Pocidalo M A. PMN from HIV-infected patients show enhanced activation, diminished fMLP-induced L-selectin shedding and an impaired oxidative burst after cytokine priming. Blood. 1994;84:2759–2766. [PubMed] [Google Scholar]
- 13.Elbim C, Hakim J, Gougerot-Pocidalo M A. Heterogeneity in Lewis x and sialyl-Lewis x antigen expression on monocytes in whole blood. Relation to stimulus-induced oxidative burst. Am J Pathol. 1998;152:1081–1090. [PMC free article] [PubMed] [Google Scholar]
- 14.Fauci A M. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988;239:617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- 15.Flo R W, Naess A, Nilsen A, Harthug S, Solberg C O. A longitudinal study of phagocyte function in HIV-infected patients. AIDS. 1994;8:771–777. doi: 10.1097/00002030-199406000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Glass J D, Fedor H, Wesselingh S L, McArthur J C. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 17.Gougerot-Pocidalo M A, Roche Y, Fay M, Perianin A, Bailly S. Oxidative injury amplifies interleukin-1 activity produced by human monocytes. Int J Immunopharmacol. 1989;11:961–969. doi: 10.1016/0192-0561(89)90119-7. [DOI] [PubMed] [Google Scholar]
- 18.Hockenberry D M, Oltvai Z N, Yin X M, Milliman C L, Korsmeyer S J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 19.Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- 20.Homsy T, Meyer M, Tateno M, Clarkson S, Levy J A. The Fc and not CD4 receptor mediates antibody enhancement of HIV infection in human cells. Science. 1988;244:1357–1360. doi: 10.1126/science.2786647. [DOI] [PubMed] [Google Scholar]
- 21.Israel N, Hazan U, Alcami J, Munnier F, Arenzana Seidedos F, Bachelerie F, Israel A, Virelizier J L. Tumor necrosis factor stimulates transcription of HIV-1 in human T lymphocytes, independently and synergistically with mitogens. J Immunol. 1989;143:3956–3960. [PubMed] [Google Scholar]
- 22.Israel N, Gougerot-Pocidalo M A, Aillet F, Virelizier J L. Redox status of cells influences constitutive or induced NF-κB translocation and HIV long terminal repeat activity in human T and monocytic cell lines. J Immunol. 1992;149:3386–3393. [PubMed] [Google Scholar]
- 23.Israel N, Gougerot-Pocidalo M A. Oxidative stress in human immunodeficiency virus infection. Cell Mol Life Sci. 1997;53:864–870. doi: 10.1007/s000180050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korsmeyer S J, Yin X M, Oltvai Z N, Veis-Novack D J, Linette G P. Reactive oxygen species and the regulation of cell death by the Bcl-2 gene family. Biochim Biophys Acta. 1995;1271:63–66. doi: 10.1016/0925-4439(95)00011-r. [DOI] [PubMed] [Google Scholar]
- 25.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 26.Laochumroonvorapong P, Paul S, Elkon K B, Kaplan G. H2O2 induces monocyte apoptosis and reduces viability of Mycobacterium avium-M. intracellulare within cultured human monocytes. Infect Immun. 1996;64:452–459. doi: 10.1128/iai.64.2.452-459.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levacher M, Hulstaert F, Tallet S, Ullery S, Pocidalo J J, Bach B A. The significance of activation markers on CD8 lymphocytes in human immunodeficiency syndrome: staging and prognostic value. Clin Exp Immunol. 1992;90:376–382. doi: 10.1111/j.1365-2249.1992.tb05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macey M G, McCarthy D A, Vordermeier S, Newland A C, Brown K A. Effects of cell purification methods on CD11b and L-selectin expression as well as the adherence and activation of leucocytes. J Immunol Methods. 1995;181:211–219. doi: 10.1016/0022-1759(95)00003-s. [DOI] [PubMed] [Google Scholar]
- 29.Masutani H, Hirota K, Sasada T, Ueda-Taniguchi Y, Taniguchi Y, Sono H, Yodoi J. Transactivation of an inducible anti-oxidative stress protein, human thioredoxin by HTLV-1 Tax. Immunol Lett. 1996;54:67–71. doi: 10.1016/s0165-2478(96)02651-x. [DOI] [PubMed] [Google Scholar]
- 30.Matsuda M, Masutani H, Nakamura H, Miyajima S, Yamauchi A, Yonehara S, Uchida A, Irimajiri K, Horiuchi A, Yodoi J. Protective activity of adult T cell leukemia derived factor (ADF) against tumor necrosis factor-dependent cytotoxicity on U937 cells. J Immunol. 1991;147:3837–3841. [PubMed] [Google Scholar]
- 31.Meyer C N, Nielsen H. Priming of neutrophil and monocyte activation in human deficiency virus infection. Comparison of granulocyte colony-stimulating factor, granulocyte-macrophage colony stimulating factor and interferon gamma. APMIS. 1996;104:640–646. doi: 10.1111/j.1699-0463.1996.tb04924.x. [DOI] [PubMed] [Google Scholar]
- 32.Muller F, Rollag H, Froland S S. Reduced oxidative burst responses in monocytes and monocyte-derived macrophages from HIV-infected subjects. Clin Exp Immunol. 1990;82:10–15. doi: 10.1111/j.1365-2249.1990.tb05396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen H, Kharazmi A, Faber V. Blood monocyte and neutrophil functions in the acquired immune deficiency syndrome. Scand J Immunol. 1986;24:291–296. doi: 10.1111/j.1365-3083.1986.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 34.Nottet H S L M, Perdisky Y, Sasseville V G, Nukuna A N, Bock P, Zhai Q H, Sharer L R, McComb R D, Swindells S, Soderland C, Gendelman H E. Mechanisms for transendothelial migration of HIV-1-infected monocytes into brain. J Immunol. 1996;156:1284–1295. [PubMed] [Google Scholar]
- 35.Palmer S, Hamblin A S. Increased CD11/CD18 expression on the peripheral blood leukocytes of patients with HIV disease: relationship to disease severity. Clin Exp Immunol. 1993;93:344–349. doi: 10.1111/j.1365-2249.1993.tb08183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perno C F, Crowe S M, Kornbluth R S. A continuing enigma: the role of cells of macrophage lineage in the development of HIV disease. J Leukoc Biol. 1997;62:1–3. doi: 10.1002/jlb.62.1.1. [DOI] [PubMed] [Google Scholar]
- 37.Poli G, Bottazzi B, Acero R, Bersani L, Rossi V, Introna M, Lazzarin A, Galli M, Mantovani A. Monocyte function in intravenous drug abusers with lymphadenopathy syndrome and in patients with acquired immunodeficiency syndrome: selective impairment of chemotaxis. Clin Exp Immunol. 1985;62:136–142. [PMC free article] [PubMed] [Google Scholar]
- 38.Sachi Y, Masutani H, Tada K, Okamoto T, Takigama M, Yodoi J. Induction of ADF/TRX by oxidative stress in keratinocytes and lymphoid cells. Immunol Lett. 1995;44:189–193. doi: 10.1016/0165-2478(95)00213-o. [DOI] [PubMed] [Google Scholar]
- 39.Seres T, Ravichandran V, Moriguchi T, Rokutan K, Thomas J A, Johnston R B. Protein S-thiolation and dethiolation during the respiratory burst in human monocytes. A reversible post-translational modification with potential for buffering the effects of oxidant stress. J Immunol. 1996;156:1973–1980. [PubMed] [Google Scholar]
- 40.Spear G T, Kessler H A, Rothberg L, Phair J, Landay A L. Decreased oxidative burst activity of monocytes from asymptomatic HIV-infected individuals. Clin Immunol Immunopathol. 1990;54:184–191. doi: 10.1016/0090-1229(90)90080-a. [DOI] [PubMed] [Google Scholar]
- 41.Spear G T, Ou C, Kessler H A, Moore J L, Schochetman G, Landay A L. Analysis of lymphocytes, monocytes, and neutrophils from human immunodeficiency virus (HIV)-infected persons for HIV DNA. J Infect Dis. 1990;162:1239–1244. doi: 10.1093/infdis/162.6.1239. [DOI] [PubMed] [Google Scholar]
- 42.Stossel T P. The machinery of blood cell movements. Blood. 1994;84:367–379. [PubMed] [Google Scholar]
- 43.Takeda A, Tuazon C, Ennis F A. Antibody-enhanced infection by HIV-1 via Fc receptor-mediated entry. Science. 1988;242:580–583. doi: 10.1126/science.2972065. [DOI] [PubMed] [Google Scholar]
- 44.Trial J, Birdsall H H, Hallum J A, Crane M L, Rodriguez-Barradas M C, de Jong A L, Krishnan B, Lacke C E, Figdor C G, Rossen R D. Phenotypic and functional changes in peripheral blood monocytes during progression of human immunodeficiency virus infection. J Clin Invest. 1995;95:1690–1701. doi: 10.1172/JCI117845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Um H D, Orenstein J M, Wahl S M. Fas mediates apoptosis in human monocytes by a reactive oxygen intermediate dependent pathway. J Immunol. 1996;156:3469–3477. [PubMed] [Google Scholar]
- 46.Zhang P, Liu B, Kang S W, Seo M S, Rhee S G, Obeid L M. Thioredoxin peroxidase is a novel inhibitor of apoptosis with a mechanism distinct from that of Bcl-2. J Biol Chem. 1997;272:30615–30618. doi: 10.1074/jbc.272.49.30615. [DOI] [PubMed] [Google Scholar]