Abstract
Introduction
Current topical treatments for psoriasis offer limited efficacy and are associated with long-term adverse effects in a subset of patients, highlighting the need for new therapeutic options. Cannabidiol (CBD), a non-psychoactive cannabinoid derived from Cannabis sativa L., has shown potential in reversing psoriasis pathology through its action on skin receptors in preclinical studies. Given the promising properties of CBD, transdermal patches containing this compound represent a novel approach to psoriasis treatment. However, comprehensive data on their efficacy and safety remain scarce.
Methods
We outline a randomized, double-blind, placebo-controlled trial to assess the efficacy and safety of CBD transdermal patches with minimal tetrahydrocannabinol (THC) in 60 patients with mild to moderate plaque-type psoriasis at a university hospital in Thailand (n = 60). This study aims to evaluate the changes in the local psoriasis severity index (LPSI), itch score via a visual analog scale, and occurrence of adverse events on day 0, 30, 60, and 90 of the study. Additionally, we will examine the alteration in the skin, gut, and oral microbiome in a subset of participants to explore potential correlations with treatment outcomes. The primary outcome will focus on the difference in LPSI scores at the end of the study period, employing an intention-to-treat analysis. Multivariate logistic regression will be used to identify baseline clinical and microbiological predictors of treatment response.
Conclusion
This study aims to investigate the efficacy and safety of CBD transdermal patches in alleviating the symptoms of psoriasis. The results of this study may highlight a novel topical treatment option that reduces suffering in patients with psoriasis. We also designed to provide a holistic evaluation by considering both clinical outcomes and the underlying biological mechanisms, including the interaction with the human microbiome. Through this trial, we aim to contribute valuable insights into personalized psoriasis management strategies.
Keywords: Cannabidiol, Cannabis, Microbiota, Psoriasis, Transdermal patch
Introduction
Psoriasis, a prevalent chronic immune-mediated systemic disease, imposes a significant physical, psychological, and social burden on affected individuals due to its disfiguring manifestations and associated comorbidities such as psoriatic arthritis, metabolic syndrome, inflammatory bowel disease, cardiovascular diseases, and psychiatric conditions [1–4]. Its prevalence varies globally, ranging from 0.27% to 11.4%, and is on an upward trend [5, 6].
The pathophysiology of psoriasis is complex, primarily driven by immune-mediated inflammation, with interleukin-17 (IL-17) and interleukin-23 (IL-23) playing pivotal roles in promoting uncontrolled keratinocyte proliferation and dysfunctional differentiation [1, 7]. Recent findings highlight a notable difference in the microbiota composition of psoriatic patients compared to healthy individuals, suggesting a significant role of the gut-skin axis in the disease’s pathogenesis through alteration in skin and gut microbiota [8–16].
Despite the availability of various treatments, many patients, particularly those with psoriasis vulgaris or plaque-type psoriasis, find conventional topical therapies either ineffective or burdened with adverse effects [7, 17–23]. This gap underscores the urgent need for novel therapeutic approaches.
Cannabidiol (CBD), a nonaddictive cannabinoid of Cannabis sativa L., has emerged as a promising candidate, demonstrated by preclinical studies to counteract psoriasis by modulating skin receptor activity, reducing keratinocyte proliferation, dampening inflammation, modifying Th1-Th2 balance, and inhibiting IL-17 [24–36]. However, clinical evidence supporting the use of CBD, especially in the form of transdermal patches, remains limited.
Therefore, our primary objective was to assess and compare the effectiveness of CBD transdermal patches with minimal THC content against placebo patches in alleviating the clinical severity of psoriasis, as measured by changes in the psoriasis area and severity index (PASI) scores from baseline to the study endpoint. Secondary objectives include (1) to evaluate the difference in itch relief provided by CBD patches with minimal THC versus placebo, utilizing itch scores based on a Visual Analog Scale (VAS). (2) To systematically document and analyze the incidence and nature of any adverse events associated with the use of CBD patches with minimal THC, ensuring a comprehensive safety profile. (3) To explore changes in the microbiome (encompassing gut, skin, and oral microbiota) among psoriasis patients treated with CBD patches with minimal THC. This includes examining the correlation between microbiome alterations and clinical outcomes, aiming to elucidate the potential role of microbiome dynamics in the therapeutic efficacy of CBD for psoriasis.
CBD and Its Role in Psoriasis Treatment
Research into cannabinoids has unveiled their complex interplay with the pathogenesis of psoriasis through various skin receptors, such as CB1, CB2, GPCR55, TRPV1, and PPAR γ receptors [24–29, 37]. CBD, a nonpsychoactive compound from Cannabis sativa, has demonstrated significant potential psoriasis management. Its efficacy, coupled with minimal addictive properties, positions CBD as a promising therapeutic agent. CBD achieves its effects by several mechanisms: inhibiting keratinocyte proliferation, reducing inflammation through the suppression of NF-kB and TNF-α activities, balancing Th1-Th2 immune responses, and modulating cytokine levels by inhibiting IL-17 and IFN-γ [24, 27, 28, 30, 37–42].
Additionally, cannabinoids impact CB1, CB2, and TRP channels located in cutaneous nerve fibers, mast cells, and keratinocytes, contributing to a reduction in itchiness [38, 43, 44], a common symptom in psoriasis patients. This antipruritic effect of CBD was supported by clinical trials in patients with atopic dermatitis [45, 46], where topical CBD application led to a notable decrease in itching.
The incorporation of CBD into dermatological products, including those aimed at treating psoriasis [34, 38] reflects its growing acceptance and observed benefits in skin care. Despite the relatively limited scope of clinical research focusing specifically on transdermal CBD applications for psoriasis, the existing evidence is promising. Studies have reported significant improvements, such as reductions in the PASI and local psoriasis severity index (LPSI) scores, following the use of CBD ointment [30, 34, 47]. These outcomes not only underscore the therapeutic potential of CBD in alleviating symptoms but also highlight the need for further investigation to fully understand its benefits and mechanisms of action in psoriasis treatment.
CBD Transdermal Patch: An Innovative Approach to Psoriasis Treatment
Transdermal patches represent a cornerstone in the evolution of drug delivery systems [48], offering a range of benefits over traditional administration routes. These patches excel due to their noninvasive application, absence of pain upon use, and their ability to bypass the first-pass metabolism, thereby ensuring a steady release of medication directly into the bloodstream. This method significantly reduces the risk of gastrointestinal side effects [48–50] and enhances patient compliance. Additionally, transdermal patches afford greater precision in medication dosing and minimize variability, advantages that are particularly pronounced when compared to other topical delivery forms like ointments [48].
Despite these benefits, the development of CBD transdermal patches for therapeutic use faces specific challenges, chiefly due to the inherently lipophilic nature of cannabinoids. This characteristic complicates the formulation process, hindering effective skin absorption [50–52] and, consequently, the overall therapeutic efficacy. However, advancements in pharmaceutical technologies have identified medium-chain triglycerides (MCTs) as effective carriers for lipophilic drugs [53]. MCTs enhance the solubility and bioavailability of cannabinoids, facilitating their transdermal delivery. These carriers have been successfully employed in both in vitro and in vivo studies [54–57] to prepare cannabis-based formulations, demonstrating their potential to overcome the formulation challenges posed by the lipophilic properties of CBD.
The integration of MCTs into CBD transdermal patches signifies a promising advancement in psoriasis treatment, potentially maximizing the therapeutic benefits of CBD while mitigating the limitations associated with its delivery. This innovative approach could redefine the management of psoriasis, offering a more effective, user-friendly, and side-effect-free treatment option for patients.
Evaluating the Safety Profile of CBD
CBD, once administered, has a propensity to accumulate in organs with a rich blood supply, including the heart, brain, liver, and lungs [50]. Concerns regarding its safety have been raised, citing serious adverse events such as hepatocellular damage, pneumonia, cardiovascular disease, and potential impacts on fertility [58, 59]. Notably, these side effects have predominantly been observed in studies utilizing high dosages exceeding 200 mg/kg/day [60]. Common, less severe side effects associated with CBD use include appetite loss, diarrhea, drowsiness, and sedation [58, 59]. Moreover, prolonged usage has been linked to the accumulation of CBD in adipose tissue, which may lead to extended periods of lethargy and sedation [50].
The adverse effects of clinical trial CBD vary depending on the dose, duration, and dosage of administration. Oral CBD trials conducted for a period of 3 months found mild side effects such as reduced appetite, weight gain/loss, and tiredness when prescribed at doses less than 10 mg/kg/day. However, higher doses (10–20 mg/kg/d) were associated with a higher rate of serious adverse events, including elevated liver transaminase and respiratory infections [61–63]. However, it is crucial to differentiate the effects based on the method of CBD application. Topical application of CBD, such as through skin creams or transdermal patches, have demonstrated a considerably safer profile. Research in this domain typically reports fewer and less severe adverse events. Minor side effects, when they do occur, often include skin-related reactions such as erythema (redness), irritation or pain at the application site, changes in hair or skin color, somnolence (drowsiness), and diarrhea [31, 32, 64–69]. The duration of topical application studies is typically limited to around 3 months, except for open-label research that was extended from a double-blind, placebo-controlled, multicenter trial. The trial started with a daily administration of 390 mg of CBD, with the option to escalate the daily dosage to 585 mg and 780 mg, respectively [70]. A total of over 60 participants who completed the experiment for 18 months did not experience any abnormal liver tests whereas prolonged oral CBD administration increased hepatic transaminase levels [71, 72].
These findings suggest that topical CBD formulations may offer a safe alternative for therapeutic use, particularly for conditions like psoriasis, where direct skin application is feasible and beneficial. The differential safety profile associated with topical versus systemic CBD underscores the importance of selecting appropriate administration routes based on the therapeutic context and desired outcomes. Continued research and vigilant monitoring of adverse events are essential to fully understand and leverage the therapeutic potential of CBD while minimizing risks to patients.
Interplay between Gut, Skin, and Oral Microbiota in Psoriasis
Research has increasingly highlighted the critical role of microbiota in the pathogenesis of psoriasis, revealing marked alterations in microbial profiles across gut, skin, and oral ecosystems in affected individuals. Notably, psoriatic skin is characterized by significant shift in microbial biodiversity, including reduced α-diversity, which indicates a lower variety of bacterial species, and increased β-diversity, reflecting greater differences in microbial communities between psoriatic and healthy skin [8, 12, 73, 74]. These alterations suggest a profound dysbiosis that may contribute to the disease’s pathophysiology.
The gut-skin axis hypothesis further elaborate on this relationship, proposing that gut microbial dysbiosis in psoriasis patients triggers an inflammatory response, compromises intestinal barrier integrity, and facilitates the translocation of bacterial components and metabolites to the skin and systemic circulation [8, 11, 13, 15, 75, 76]. This process is believed to exacerbate psoriatic lesions. Supporting this, research has demonstrated a decline in the overall microbial diversity within the gut of psoriasis patients, alongside significant shifts in β-diversity, underscoring the interconnectedness of gut health and skin conditions [15, 16, 74, 76, 77].
Specific microbial taxa have been identified as differentiators between psoriatic and healthy states. In psoriatic skin, there is an increased prevalence of Streptococcus spp., Staphylococcus spp., and Corynebacterium spp., contrasted with a reduction in Staphylococcus epidermidis, Cutibacterium acnes, and Cutibacterium granulosum – bacteria typically associated with health skin [11, 12, 78]. Furthermore, the Firmicutes-to-Bacteroidetes ratio, a crucial indicator of microbial balance in the gut, is elevated in psoriasis patients, correlating with disease severity and associated comorbidities [8, 13–16]. Similarly, an increase in Ruminococcus gnavus and a decrease in beneficial bacteria such as Faecalibacterium prausnitzii and Akkermansia muciniphila have been observed, highlighting potential targets for therapeutic intervention [8–10, 13, 15, 16, 76, 79].
The role of the oral microbiome in psoriasis pathogenesis is less clear but noteworthy, particularly given the increased risk of periodontal disease in psoriatic patients, which shares underlying inflammatory mechanisms, including elevated Th-17 cells and IL-17 levels [80]. While the diversity of the oral microbiome in psoriasis patients does not significantly differ from that of healthy individuals [81–83], specific bacterial species show marked differences, suggesting a nuanced role in the disease’ systemic nature [77]. Together, mentioned findings underscore the complex interplay between various microbiota and psoriasis, offering insights into potential microbiome-centered therapeutic strategies and emphasizing the importance of a holistic approach to understanding and treating this multifaceted disease.
Methods
Study Medication
The intervention involves the use of CBD patches (referred to as C patches) and placebo control patches (referred to as P patches) for the study. Each C patch is formulated with 1.0 ± 0.1 mg of CBD, dissolved in a MCTs solvent, spread across a 2.0 ± 0.2 g yellow adhesive layer. The adhesive layer is then securely covered with aluminum foil to ensure stability and prevent degradation, as verified by certificate of analysis number KT060266. In contrast, the P patches are designed to mimic the C patches in appearance and packaging but contain only the 2.0 g yellow adhesive layer without CBD, also covered with aluminum foil, as confirmed by certificate of analysis number TDC 66-066. This design ensures the integrity of the double-blind aspect of the study by maintaining indistinguishability between the C and P patches, aside from their active ingredient content. Each patch in this research will have dimensions of 5 cm by 5 cm.
Phase I: Irritation Test and Safety Assessment of Cannabis Transdermal Patch versus Placebo Patch
Phase I of our study is a carefully designed exploratory phase among 10 healthy volunteers aged between 18 and 60 years. The exclusion criteria for participation include individuals who are pregnant or breastfeeding, have a known allergy to medicinal cannabis or any components of the patch, suffer from severe liver or renal illnesses, have a history of schizophrenia or other major psychiatric conditions, exhibit addictive behaviors or a history of drug addiction (either recreational or prescription), as assessed through patient interviews and medical records, or have any infections or skin conditions on the inside of either arm.
Participants will be randomly assigned in a double-blind manner to apply a C patch on one forearm and a P patch on the opposite forearm. This randomization will be achieved using STATA version 15.0 software, employing a 1:1 ratio and block randomization method with blocks of two. The allocation will ensure an unbiased distribution, with five volunteers receiving the C patch on the right forearm and the P patch on the left, and the remaining five receiving the opposite arrangement.
Patches will be applied to the inner side of each forearm, positioned 6 cm above the elbow. Alcohol patch will be used to cleanse the skin prior to application; allow to dry for 5 min. Upon application, we will monitor for immediate allergic reaction or anaphylaxis for 30 min. Subsequent to this initial safety check, participants will be instructed on the correct application of the patch, identified only as “1” or “2” to maintain blinding, and instructed to wear the assigned patch for a duration of 6–10 h daily, by applying it before going to sleep and removing it upon waking up.
The evaluation of potential irritation or adverse skin reactions will be thorough and systematic, conducted by a physician on day 5, 10, 15, and 30. These assessments will include observing erythema and edema, skin atrophy, telangiectasia, and hypopigmentation, with severity graded on a scale from 0 (no reaction) to 3 (severe) (Table 1). Concurrently, participants will self-report itchiness and pain using a visual analog scale to provide subjective data on discomfort or irritation. Photographic documentation of the test areas will be taken on day 0 and at each subsequent visit to visually record any changes.
Table 1.
Phase I study evaluations including erythema and edema, skin atrophy, telangiectasia, and hypopigmentation
| Scoring | Erythema and swelling | Atrophy | Telangiectasia | Hypopigmentation |
|---|---|---|---|---|
| 0 | No reaction | No atrophy | No telangiectasia | No hypopigmentation |
| 1 | Mild erythema, no edema | Mild atrophy | Mild telangiectasia | Mild hypopigmentation |
| 2 | Marked erythema and mild edema | Moderate atrophy | Moderate telangiectasia | Moderate hypopigmentation |
| 3 | Marked erythema, edema and vesicle | Marked atrophy | Marked telangiectasia | Marked hypopigmentation |
All findings from Phase I will be meticulously reviewed by the Chulalongkorn University Faculty of Medicine Institutional Review Board before proceeding to Phase II, ensuring that the safety and well-being of participants are prioritized and that any significant concerns are addressed promptly.
Phase II Efficacy and Safety of Cannabis Transdermal Patch for Alleviating Psoriasis Symptoms
Study Design
Phase II is designed as a rigorous randomized, double-blind, placebo-controlled trial.
Participant Recruitment and Selection
The recruitment strategy encompasses a multi-faceted approach to ensure a diverse participant pool. Potential participants will be identified from the Division of Dermatology at King Chulalongkorn Memorial Hospital’s database. Additionally, recruitment efforts will be extended through direct encounters, telephone outreach, and digital campaigns, including advertisements on social media platforms such as Facebook.
Eligibility will be determined through a review of medical history, detailed physical examinations conducted by qualified physicians, and a careful assessment of medical records. All potential participants will undergo a screening process to ensure compliance with the inclusion and exclusion criteria meticulously defined for this study (Table 2). Written informed consent will be obtained from all participants ensuring adherence to the ethical guidelines as stipulated by the Declaration of Helsinki. The criteria for inclusion and exclusion will be detailed in Table 2, providing clear guidelines for participant selection.
Table 2.
Inclusion and exclusion criteria for study participation
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Procedures
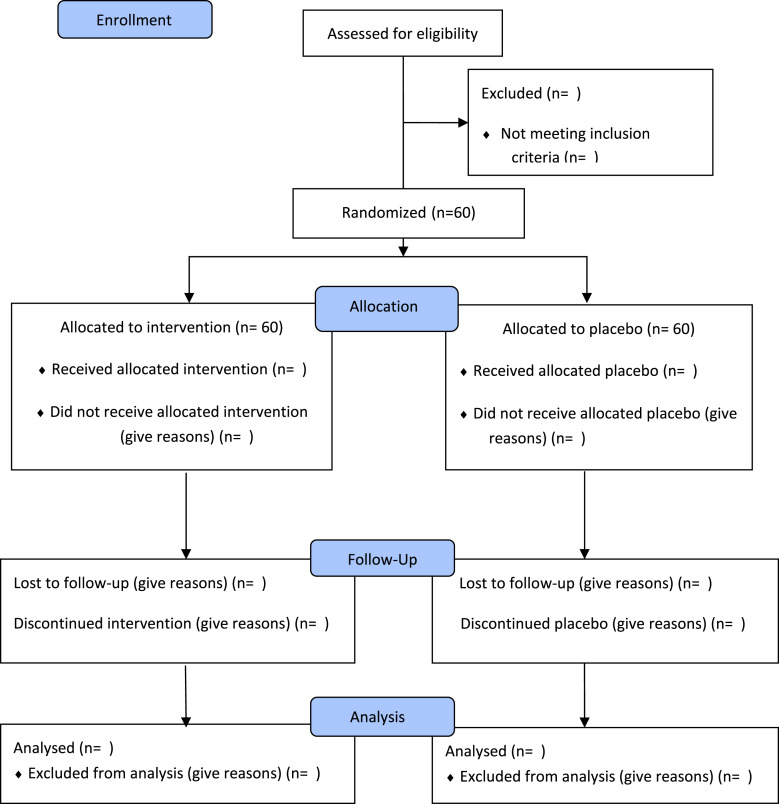
All participants who meet the eligibility criteria and provide their written consent are invited to respond to the baseline demographic and clinical characteristics. Each participant will have two psoriasis lesions with comparable size and severity selected by a dermatologist. The dermatologist will measure the distance in centimeters from a reference point to document the precise position of each plaque. The assignment of the intervention plaque or the control plaque will be done randomly by individuals who are not part of the study, in a 1:1 ratio. The placement of the two plaques is not necessarily limited to being on opposite sides of the body. However, if there are many possible locations for a lesion, priority will be given to the lesion that is located in the corresponding position on the other side of the body. An overview of the progress of the current trial is shown in the consolidated standards of reporting trials diagram (shown in Fig. 1). The participants are instructed to apply a P or C patch on selected plaques of psoriasis every day for 90 days and 6–10 h a day, covering a specific plaque without trimming it, by applying it before going to sleep and removing it upon waking up. The patients must refrain from applying any topical substances to the test region.
Fig. 1.
CONSORT flow diagram. Flowchart of phases of Efficacy and Safety of Cannabis Transdermal Patch for Alleviating Psoriasis Symptoms: A Randomized Controlled Trial (CanPatch).
Adherence
Face-to-face reminder sessions are conducted at initial product dispensing and three subsequent visits. This session reviews the application of transdermal patches, including scheduling, storage, and missing dosage solutions. At each follow-up appointment, unused patches and used patches’ packages are tallied and documented.
Randomization
Two similar psoriasis lesions of each patient are randomly assigned to the intervention group or control group by block randomization with a ratio 1: 1 with blocks of 4 by STATA version 15.0 (StataCorp. 2017. Stata Statistical Software: Release 15; StataCorp LLC, College Station, TX, USA).
Blinding
After double-blind randomization, individuals not involved in the study translate the randomization code into instructions on which patches should be used on which lesion. They place the instructions in sealed envelopes that are then delivered to patients randomly by a physician. Each lesion is instructed in the same type of patch every day. The front of an opaque sealed envelope is the study ID and the date the envelope will be opened. Therefore, patients and physicians are blinded. Allocation concealment is ensured, as the service will not release the randomization code until data collection has been completed. A code might be broken in extraordinary cases where knowledge of the actual therapy is vital for future patient care.
Trial Sites
This study was conducted at Division of Dermatology, Department of Medicine, King Chulalongkorn Memorial Hospital, Bangkok, Thailand.
Biological Sample Collection for Genetic Analysis
Biological sample collection includes 1 mL of saliva and 1 mL of feces collected by participants in separate containers prepared by researchers and skin samples from three regions, two lesional skin samples, and one sample of nonlesional skin collected by skin taping by physicians. The five containers per individual containing nucleic acid preservation buffer will be transported to the laboratory in 30 min at room temperature or as soon as possible at 4°C. Upon arrival at the laboratory of the Faculty of Medicine, Chulalongkorn University, the sample will be extracted by Quick-DNA™ H M W MagBead Kit (Cat. No. D6060, Zymo Research) and then amplified by the PCR method. The PCR amplification products will be purified by AMPure XP (Beckman Coulter) and the quantity will be measured by NanoPhotometer® C40 (Implen, USA). After that, DNA will be combined with the solution for the Ligation Sequencing Kit (LSK-109, Oxford Nanopore Technologies) to perform DNA sequencing using the Nanopore MinION sequencing system. All samples will be recorded and barcoded with a unique storage ID.
Data Collection and Management
Data collection in this study is based on personal characteristics, clinical evaluations, and self-reported measures. Baseline demographic and clinical characteristics include age (years), sex, weight, body mass index, familial history of psoriasis, duration of psoriasis, comorbidities (diabetes mellitus, hypertension, dyslipidemia, obesity, metabolic syndrome, cardiovascular disease, psoriatic arthritis, inflammatory bowel disease, psychiatric disorders, among others) and concurrent therapies for systemic psoriasis. The LPSI, self-reported itching on the visual analog scale, reported adverse events, and microbial profile are collected at the time points below.
All investigators will have access to the final trial data set. All documents will be safely stored in the Skin Unit Research Facilities for Academic and Clinical Excellence (SURFACE), Division of Dermatology, Department of Medicine, Faculty of Medicine, Chulalongkorn University, for 10 years. No identifiable data will be recorded, and all documents will be recorded with the research identification number.
Outcome Measures
Evaluations are collected at four time points: 0th, 30th, 60th and 90th day of the study. Fifty percent of the participants are chosen at random to have their saliva, feces, and skin samples collected if they provide written consent regarding the storage and future testing of biological materials. An overview of all the assessment points and results is shown in Table 3.
Table 3.
SPIRIT schedule for enrolment, interventions, and assessments
| Enrolment | Allocation | Intervention | Close-out | |||
|---|---|---|---|---|---|---|
| Timepoint | 0 | 0 | 30th | 60th | 90th | 120th |
| Enrolment | ||||||
| Eligibility screen | X | |||||
| Written informed consent | X | |||||
| Collect demographics and baseline clinical data | X | |||||
| Collect a biological sample | O | O | ||||
| Allocation | X | |||||
| Interventions | ||||||
| Intervention group |

|
|||||
| Control group |

|
|||||
| Assessments | ||||||
| LPSI | X | X | X | X | ||
| Reported adverse events | X | X | X | |||
| Demographics and baseline clinical data | X | |||||
| Microbial profile | O | |||||
O, 50% of the participants were randomly chosen to collect saliva, feces, and skin samples.
Primary Outcome Measure: LPSI
Each lesion will be assessed with a modified psoriasis area severity index called the LPSI. The LPSI is the sum of the following symptoms evaluated by the physician: erythema (redness), induration (thickness) and desquamation (scaling infiltration). Each score was classified as follows: 0 = no symptoms, 1 = slight symptoms, 2 = moderate symptoms, 3 = marked symptoms, 4 = very marked symptoms [84]. P.A., a dermatology professor, conducts all LPSI evaluations for this study.
Secondary Outcome Measures
The Itch Score by the Visual Analog Scale
Another secondary outcome measure is the mean or median itch score on the visual analog scale for the lesion treated with C patch versus P patch at each visit. The visual analog scale consists of a line 10 cm long, with verbal anchors at either end. The patient places a mark on the line corresponding to the patient's intensity rating. VAS is one of the most commonly used methods for assessing the severity of pruritus, as it provides an easy, rapid and valid estimate of the itch [85–87]. Participants will be asked to indicate the point within 3 min of this study.
The Alpha-Diversity, Beta-Diversity, and Relative Abundance of Microbiota
Biological samples, including saliva, feces, and skin, will be analyzed for microbial alteration in the form of microbial diversity. The diversity of microbes will be defined as the proportion and abundance distribution of different types of organism. The abundance distribution includes alpha diversity, which is an abundance of different bacterial taxa in a single sample, and beta diversity, which is microbial diversity in different samples. Relative abundance is the ratio of the absolute abundance of a taxon to the total absolute abundance of all taxa in a unit volume of an ecosystem [8, 88, 89]. The median of changes in alpha-diversity and beta-diversity from the start to day 90 of both C and P groups is examined.
Reporting of Adverse Events
Participants are encouraged to contact research staff if they have concerns about mental or physical health decline. Upon the occurrence of an adverse event, the patient would receive treatment for the adverse event at King Chulalongkorn Memorial Hospital for free.
Discontinuation and Withdrawal
Patients who experience major adverse effects after enrolling in the study, participate in less than 80% of the research period, or are unwilling to continue participating in the trial will be excluded. However, the data already collected and the reason for the cessation of study participation may be included in the final report.
Sample Size Calculation
The median LPSI score for transdermal CBD and placebo use was compared for primary outcome in this study. The calculated sample size per group is 54 people with the following parameters: power = 0.90, α = 0.05, effect size of 0.68 (based on a study by Ben et al. [90] using the LPSI score as an outcome of the efficacy of psoriasis improvement), and allocation ratio of 1:1. However, it is possible that 10% of the participants will withdraw from the study [91]. The researchers determine that the total sample size is 60 individuals.
Statistical Methods
This is a parallel group randomized trial with a control arm. Data will be reported and presented in accordance with the revised CONSORT statement [92]. Statistical analysis will be performed using STATA version 15.0 (StataCorp. 2017. Stata Statistical Software: Release 15; StataCorp LLC.) on an intention-to-treat basis. All exploratory tests will be two-tailed, with alpha = 0.05. Baseline demographics will be documented and summarized for both the total population and each patch group. Categorical data will be expressed as a number and depending on which is more appropriate, continuous data will be reported as mean +/− standard deviation or median +/− interquartile range. Because the primary outcome or LPSI score is an ordinal composite score, nonparametric comparison approaches are used. The Mann-Whitney rank sum test will be used to compare the LPSI scores of the control and treatment groups on day 90 and at subsequent visits. Missing data for the primary outcome, the LPSI score at 90 days of follow-up, will be imputed using a number of approaches, including regression and multiple imputation, as part of a sensitivity analysis. The Wilcoxon signed-rank test will be used to compare the LPSI scores obtained before and after intervention. The graph will provide the median and interquartile range of LPSI scores for each visit, providing a picture of the trend in psoriasis severity. The Mann-Whitney rank sum test or unpaired t test, depending on the data distribution, will be used to compare the itch scores of the control and treated groups at each visit. If the data have a normal distribution, the paired t test will be used to compare the itch score recorded before and after intervention. However, if the data do not follow a normal distribution, the Wilcoxon signed ranks test will be used. A multivariate logistic regression analysis will be done on the baseline clinical parameters and the LPSI score at day 90. The adverse events will be portrayed as a number of events in the table. There are no scheduled interim statistical analyses.
Sequencing and Microbiome Data Analysis
The taxonomic classification will be assigned for the V3/V4 16S region. All taxonomic classifications will be implemented within QIIME2. Bacterial diversity is determined by alpha diversity and beta diversity, which will be calculated by the QIIME 2 pipeline. Alpha diversity will be analyzed using Shannon’s diversity and Simpson's diversity. Beta diversity will be analyzed by Bray-Curtis dissimilarity. The Mann-Whitney rank sum test will be used to compare changes in alpha and beta diversity from day 0 to day 90 between the control and treatment groups. The Wilcoxon signed rank test will be used to compare alpha or beta diversity data collected prior to and following treatments. A multivariate logistic regression analysis will be performed to examine the relationship between alpha and beta diversity change and the LPSI score on day 90.
Trial Status and Timeline
The research will be advertised from June 2024 to August 2024. Subsequently, subject recruitment will occur from August 2024 to September 2024. The duration of the intervention will be between September 2024 and November 2024. Data analysis will be conducted from November 2024 to February 2025. The presentation of the data and the preparation of the manuscript will be completed by March 2025.
Discussion
This study will be the first randomized controlled study to assess the efficacy and safety of CBD-containing transdermal patches with minimal THC in patients with mild to moderate plaque-type psoriasis. It will also be the first to investigate the correlation of the baseline and alteration of the microbial profile and the efficacy and safety of CBD with minimal THC patches, which can help with customized patches, in addition to age, sex, body mass index, and genetic variables. If the intervention shows significant positive results, the promise of minimal THC transdermal CBD patches as an alternative topical therapy option for psoriasis sufferers will be emphasized, and additional studies on a larger scale may be conducted on this issue. Further research could examine (i) the efficacy and cost-effectiveness compared to the standard of care, (ii) barriers to implementation (e.g., social stigma, cost of administration, and legalization policies), (iii) The extended-term safety of maintaining the usage of CBD transdermal patch therapy.
The strengths of this study include (i) a double-blind randomized design, (ii) a review of the aspects of the patients and physicians of the psoriasis symptoms, (iii) transdermal patches that could better control the amount of CBD than the previous form of transdermal CBD administration, and (iv) investigation of personalized factors that include the microbial profile and demographic and clinical characteristics. Certain characteristics or the initial microbiome profile may be linked to a more favorable or less favorable treatment response. This information may potentially be used to determine the appropriate dosage of a CBD transdermal patch in the future. However, this study also has several limitations including (i) recruiting and providing intervention at a single site and in an academic medical center setting that could limit generalizability and (ii) the subjective nature of the itch score, which includes individual variation. (iii) In phase II study, two selected psoriasis regions may be in close proximity to one another or vastly separated, and CBD absorption may also differ based on the skin parameters of the sites [50]. The study results will be released to the participating physicians, patients, and the general medical community. For reproducible research, we will transfer a collection of entirely anonymized data to a suitable data archive for sharing purposes. Any protocol amendment will be approved by the Institutional Review Board of Chulalongkorn University and the Thai Clinical Trials Registry prior to implementation.
Statement of Ethics
The Institutional Review Board of the Faculty of Medicine, Chulalongkorn University (MDCU IRB No. 0194/66 COA No. 1232/2023) approved the study on September 19, 2023. The trial was registered in the Thai Clinical Trials Registry under the number 20220518004. Written informed consent will be obtained from all participants, ensuring adherence to the ethical guidelines as stipulated by the Declaration of Helsinki.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The study has received financial support from the Thai Traditional Medical Knowledge Fund (No. 1/2567). The funder has no role in the design, data collection, data analysis, or reporting of the study.
Author Contributions
P.S., P.A., and K.P. conceived of the study. P.S., P.A., T.O., P.C., and S.P. collected data. P.S. and K.P. performed formal analysis. K.P. and N.H. acquired funding. P.S., P.A., T.O., P.C., and S.P. perform investigation. P.S., P.A., P.P., K.P., and N.H. designed methodology. P.A., K.P., and N.H. administered the project, provided resource, and supervised. PP, PC, and SP provided software. P.A,. P.P., T.O., P.C., S.P., and N.H. validated and visualized. P.S. and K.P. drafted the manuscript. All authors contributed to the refinement of the study protocol and approved the final manuscript.
Funding Statement
The study has received financial support from the Thai Traditional Medical Knowledge Fund (No. 1/2567). The funder has no role in the design, data collection, data analysis, or reporting of the study.
Data Availability Statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants but are available from the corresponding author (K.P.) upon reasonable request.
References
- 1. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker J. Psoriasis. Lancet. 2021;397(10281):1301–15. [DOI] [PubMed] [Google Scholar]
- 2. Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(2):205–12. [DOI] [PubMed] [Google Scholar]
- 3. Naldi L, Mercuri SR. Epidemiology of comorbidities in psoriasis. Dermatol Ther. 2010;23(2):114–8. [DOI] [PubMed] [Google Scholar]
- 4. Oliveira MFSP., Rocha BO, Duarte GV. Psoriasis: classical and emerging comorbidities. Bras Dermatol. 2015;90(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iskandar IYK, Parisi R, Griffiths CEM, Ashcroft DM; Global Psoriasis Atlas . Systematic review examining changes over time and variation in the incidence and prevalence of psoriasis by age and gender. Br J Dermatol. 2021;184(2):243–58. [DOI] [PubMed] [Google Scholar]
- 6. Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6):1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olejniczak-Staruch I, Ciążyńska M, Sobolewska-Sztychny D, Narbutt J, Skibińska M, Lesiak A. Alterations of the skin and gut microbiome in psoriasis and psoriatic arthritis. Int J Mol Sci. 2021;22(8):3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen G, Chen ZM, Fan XY, Jin YL, Li X, Wu SR, et al. Gut-brain-skin Axis in psoriasis: a review. Dermatol Ther. 2021;11(1):25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen L, Li J, Zhu W, Kuang Y, Liu T, Zhang W, et al. Skin and gut microbiome in psoriasis: gaining insight into the pathophysiology of it and finding novel therapeutic strategies. Front Microbiol. 2020;11:589726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Francesco MA, Caruso A. The gut microbiome in psoriasis and crohn’s disease: is its perturbation a common denominator for their pathogenesis? Vaccines. 2022;10(2):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferček I, Lugović-Mihić L, Tambić-Andrašević A, Ćesić D, Grginić AG, Bešlić I, et al. Features of the skin microbiota in common inflammatory skin diseases. Life. 2021;11(9):962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sikora M, Stec A, Chrabaszcz M, Knot A, Waskiel-Burnat A, Rakowska A, et al. Gut microbiome in psoriasis: an updated review. Pathogens. 2020;9(6):463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kierasińska M, Donskow-Łysoniewska K. Both the microbiome and the macrobiome can influence immune responsiveness in psoriasis. Cent Eur J Immunol. 2021;46(4):502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Polak K, Bergler-Czop B, Szczepanek M, Wojciechowska K, Frątczak A, Kiss N. Psoriasis and gut microbiome-current state of art. Int J Mol Sci. 2021;22(9):4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Colucci R, Moretti S. Implication of human bacterial gut microbiota on immune-mediated and autoimmune dermatological diseases and their comorbidities: a narrative review. Dermatol Ther. 2021;11(2):363–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Le Roux E, Frow H. “Diagnosis and management of mild to moderate psoriasis”. Prescriber. 2020;31(7–8):9–17. [Google Scholar]
- 18. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–60. [DOI] [PubMed] [Google Scholar]
- 19. Krueger GG, Feldman SR, Camisa C, Duvic M, Elder JT, Gottlieb AB, et al. Two considerations for patients with psoriasis and their clinicians: what defines mild, moderate, and severe psoriasis? What constitutes a clinically significant improvement when treating psoriasis? J Am Acad Dermatol. 2000;43(2 Pt 1):281–5. [DOI] [PubMed] [Google Scholar]
- 20. Papp KA, Gniadecki R, Beecker J, Dutz J, Gooderham MJ, Hong CH, et al. Psoriasis prevalence and severity by expert elicitation. Dermatol Ther. 2021;11(3):1053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiricozzi A, Pimpinelli N, Ricceri F, Bagnoni G, Bartoli L, Bellini M, et al. Treatment of psoriasis with topical agents: recommendations from a tuscany consensus. Dermatol Ther. 2017;30(6):e12549. [DOI] [PubMed] [Google Scholar]
- 22. Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63(4):278–85. [PMC free article] [PubMed] [Google Scholar]
- 23. Kessler TR. Treating patients with moderate-to-severe psoriasis vulgaris. JAAPA. 2022;35(3):28–35. [DOI] [PubMed] [Google Scholar]
- 24. Baswan SM, Klosner AE, Glynn K, Rajgopal A, Malik K, Yim S, et al. Therapeutic potential of cannabidiol (CBD) for skin health and disorders. Clin Cosmet Investig Dermatol. 2020;13:927–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Río CD, Millán E, García V, Appendino G, DeMesa J, Muñoz E. The endocannabinoid system of the skin. A potential approach for the treatment of skin disorders. Biochem Pharmacol. 2018;157:122–33. [DOI] [PubMed] [Google Scholar]
- 26. Wilkinson JD, Williamson EM. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J Dermatol Sci. 2007;45(2):87–92. [DOI] [PubMed] [Google Scholar]
- 27. Ramot Y, Sugawara K, Zákány N, Tóth BI, Bíró T, Paus R. A novel control of human keratin expression: cannabinoid receptor 1-mediated signaling down-regulates the expression of keratins K6 and K16 in human keratinocytes in vitro and in situ. PeerJ. 2013;1:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Norooznezhad AH, Norooznezhad F. Cannabinoids: possible agents for treatment of psoriasis via suppression of angiogenesis and inflammation. Med Hypotheses. 2017;99:15–8. [DOI] [PubMed] [Google Scholar]
- 29. Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M. Cannabinoids as novel anti-inflammatory drugs. Future Med Chem. 2009;1(7):1333–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palmieri B, Laurino C, Vadalà M. A therapeutic effect of CBD-enriched ointment in inflammatory skin diseases and cutaneous scars. Clin Ter. 2019;170(2):e93–9. [DOI] [PubMed] [Google Scholar]
- 31. Tijani AO, Thakur D, Mishra D, Frempong D, Chukwunyere UI, Puri A. Delivering therapeutic cannabinoids via skin: current state and future perspectives. J Control Release. 2021;334:427–51. [DOI] [PubMed] [Google Scholar]
- 32. Mahmood F, Lim MM, Kirchhof MG. A survey of topical cannabis use in Canada. J Cutan Med Surg. 2022;26(2):156–61. [DOI] [PubMed] [Google Scholar]
- 33. Yeroushalmi S, Nelson K, Sparks A, Friedman A. Perceptions and recommendation behaviors of dermatologists for medical cannabis: a pilot survey. Complement Ther Med. 2020;55:102552. [DOI] [PubMed] [Google Scholar]
- 34. Martins AM, Gomes AL, Vilas Boas I, Marto J, Ribeiro HM. Cannabis-based products for the treatment of skin inflammatory diseases: a timely review. Pharmaceuticals. 2022;15(2):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tammaro A, Magri F, Chello C, Giordano D, Parisella FR, De Marco G, et al. A successful topical treatment for cutaneous inflammatory diseases:an additional or alternative therapy to topical steroids. Eur Ann Allergy Clin Immunol. 2019;51(3):129–30. [DOI] [PubMed] [Google Scholar]
- 36. Yeroushalmi S, Nemirovsky D, Mamlouk M, Feldman D, Nelson K, Sparks A, et al. Consumer perspectives on and utilization of medical cannabis to treat dermatologic conditions. J Drugs Dermatol. 2022;21(1):31–6. [DOI] [PubMed] [Google Scholar]
- 37. Sheriff T, Lin MJ, Dubin D, Khorasani H. The potential role of cannabinoids in dermatology. J Dermatolog Treat. 2020;31(8):839–45. [DOI] [PubMed] [Google Scholar]
- 38. Jhawar N, Schoenberg E, Wang JV, Saedi N. The growing trend of cannabidiol in skincare products. Clin Dermatol. 2019;37(3):279–81. [DOI] [PubMed] [Google Scholar]
- 39. Martinelli G, Magnavacca A, Fumagalli M, DellʼAgli M, Piazza S, Sangiovanni E. Cannabis sativa and skin health: dissecting the role of phytocannabinoids. Planta Med. 2022;88(7):492–506. [DOI] [PubMed] [Google Scholar]
- 40. Tóth KF, Ádám D, Bíró T, Oláh A. Cannabinoid signaling in the skin: therapeutic potential of the “C(ut)annabinoid” system. Molecules. 2019;24(5):918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Osafo N, Yeboah OK, Antwi AO. Endocannabinoid system and its modulation of brain, gut, joint and skin inflammation. Mol Biol Rep. 2021;48(4):3665–80. [DOI] [PubMed] [Google Scholar]
- 42. Namazi MR. Cannabinoids, loratadine and allopurinol as novel additions to the antipsoriatic ammunition. J Eur Acad Dermatol Venereol. 2005;19(3):319–22. [DOI] [PubMed] [Google Scholar]
- 43. Avila C, Massick S, Kaffenberger BH, Kwatra SG, Bechtel M. Cannabinoids for the treatment of chronic pruritus: a review. J Am Acad Dermatol. 2020;82(5):1205–12. [DOI] [PubMed] [Google Scholar]
- 44. Makhakhe L. Topical cannabidiol (CBD) in skin pathology: a comprehensive review and prospects for new therapeutic opportunities. S Afr Fam Pract. 2022;64(1):e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maghfour J, Rietcheck HR, Rundle CW, Runion TM, Jafri ZA, Dercon S, et al. An observational study of the application of a topical cannabinoid gel on sensitive dry skin. J Drugs Dermatol. 2020;19(12):1204–8. [DOI] [PubMed] [Google Scholar]
- 46. Maghfour J, Rundle CW, Rietcheck HR, Dercon S, Lio P, Mamo A, et al. Assessing the effects of topical cannabidiol in patients with atopic dermatitis. Dermatol Online J. 2021;27(2). [PubMed] [Google Scholar]
- 47. Puaratanaarunkon T, Sittisaksomjai S, Sivapornpan N, Pongcharoen P, Chakkavittumrong P, Ingkaninan K, et al. Topical cannabidiol-based treatment for psoriasis: a dual-centre randomized placebo-controlled study. J Eur Acad Dermatol Venereol. 2022;36(9):e718–20. [DOI] [PubMed] [Google Scholar]
- 48. Pastore MN, Kalia YN, Horstmann M, Roberts MS. Transdermal patches: history, development and pharmacology. Br J Pharmacol. 2015;172(9):2179–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Paudel KS, Milewski M, Swadley CL, Brogden NK, Ghosh P, Stinchcomb AL. Challenges and opportunities in dermal/transdermal delivery. Ther Deliv. 2010;1(1):109–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84(11):2477–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Junaid MSA, Tijani AO, Puri A, Banga AK. In vitro percutaneous absorption studies of cannabidiol using human skin: exploring the effect of drug concentration, chemical enhancers, and essential oils. Int J Pharm. 2022;616:121540. [DOI] [PubMed] [Google Scholar]
- 52. Tanner T, Marks R. Delivering drugs by the transdermal route: review and comment. Skin Res Technol. 2008;14(3):249–60. [DOI] [PubMed] [Google Scholar]
- 53. Ramella A, Roda G, Pavlovic R, Cas MD, Casagni E, Mosconi G, et al. Impact of lipid sources on quality traits of medical cannabis-based oil preparations. Molecules. 2020;25(13):2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stolar O, Hazan A, Vissoker RE, Kishk IA, Barchel D, Lezinger M, et al. Medical cannabis for the treatment of comorbid symptoms in children with autism spectrum disorder: an interim analysis of biochemical safety. Front Pharmacol. 2022;13:977484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dahlgren MK, Lambros AM, Smith RT, Sagar KA, El-Abboud C, Gruber SA. Clinical and cognitive improvement following full-spectrum, high-cannabidiol treatment for anxiety: open-label data from a two-stage, phase 2 clinical trial. Commun Med. 2022;2(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tonoyan L, Babu D, Reiz B, Le T, Siraki AG. Heating of consumer cannabis oils can lead to free radical initiated degradation, causing CBD and THC depletion. Free Radic Biol Med. 2022;192:77–83. [DOI] [PubMed] [Google Scholar]
- 57. Feng W, Qin C, Cipolla E, Lee JB, Zgair A, Chu Y, et al. Inclusion of medium-chain triglyceride in lipid-based formulation of cannabidiol facilitates micellar solubilization in vitro, but in vivo performance remains superior with pure sesame oil vehicle. Pharmaceutics. 2021;13(9):1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chesney E, Oliver D, Green A, Sovi S, Wilson J, Englund A, et al. Adverse effects of cannabidiol: a systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology. 2020;45(11):1799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Meissner H, Cascella M. Cannabidiol (CBD). StatPearls. Treasure island (FL): StatPearls publishing copyright © 2022 StatPearls Publishing LLC.; 2022. [Google Scholar]
- 60. Huestis MA, Solimini R, Pichini S, Pacifici R, Carlier J, Busardò FP. Cannabidiol adverse effects and toxicity. Curr Neuropharmacol. 2019;17(10):974–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Madeo G, Kapoor A, Giorgetti R, Busardò FP, Carlier J. Update on cannabidiol clinical toxicity and adverse effects: a systematic review. Curr Neuropharmacol. 2023;21(11):2323–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Anciones C, Gil-Nagel A. Adverse effects of cannabinoids. Epileptic Disord. 2020;22(S1):29–32. [DOI] [PubMed] [Google Scholar]
- 63. Liu S, He Z, Li J. Long-term efficacy and adverse effects of cannabidiol in adjuvant treatment of drug-resistant epilepsy: a systematic review and meta-analysis. Ther Adv Neurol Disord. 2023;16:17562864231207755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maghfour J, Rietcheck H, Szeto MD, Rundle CW, Sivesind TE, Dellavalle RP, et al. Tolerability profile of topical cannabidiol and palmitoylethanolamide: a compilation of single-centre randomized evaluator-blinded clinical and in vitro studies in normal skin. Clin Exp Dermatol. 2021;46(8):1518–29. [DOI] [PubMed] [Google Scholar]
- 65. Xu DH, Cullen BD, Tang M, Fang Y. The effectiveness of topical cannabidiol oil in symptomatic relief of peripheral neuropathy of the lower extremities. Curr Pharm Biotechnol. 2020;21(5):390–402. [DOI] [PubMed] [Google Scholar]
- 66. Maida V, Shi RB, Fazzari FGT, Zomparelli L. Topical cannabis-based medicines: a novel paradigm and treatment for non-uremic calciphylaxis leg ulcers: an open label trial. Int Wound J. 2020;17(5):1508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yin HY, Hadjokas N, Mirchia K, Swan R, Alpert S. Commercial cannabinoid oil-induced stevens-johnson syndrome. Case Rep Ophthalmol Med. 2020;2020:6760272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Scheffer IE, Hulihan J, Messenheimer J, Ali S, Keenan N, Griesser J, et al. Safety and tolerability of transdermal cannabidiol gel in children with developmental and epileptic encephalopathies: a nonrandomized controlled trial. JAMA Netw Open. 2021;4(9):e2123930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Heussler H, Cohen J, Silove N, Tich N, Bonn-Miller MO, Du W, et al. A phase 1/2, open-label assessment of the safety, tolerability, and efficacy of transdermal cannabidiol (ZYN002) for the treatment of pediatric fragile X syndrome. J Neurodev Disord. 2019;11(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. O'Brien TJ, Berkovic SF, French JA, Messenheimer JA, Sebree TB, Bonn-Miller MO, et al. Adjunctive transdermal cannabidiol for adults with focal epilepsy: a randomized clinical trial. JAMA Netw Open. 2022;5(7):e2220189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Park YD, Linder DF, Pope J, Flamini JR, Moretz K, Diamond MP, et al. Long-term efficacy and safety of cannabidiol (CBD) in children with treatment-resistant epilepsy: results from a state-based expanded access program. Epilepsy Behav. 2020;112:107474. [DOI] [PubMed] [Google Scholar]
- 72. Szaflarski JP, Devinsky O, Lopez M, Park YD, Zentil PP, Patel AD, et al. Long-term efficacy and safety of cannabidiol in patients with treatment-resistant epilepsies: four-year results from the expanded access program. Epilepsia. 2023;64(3):619–29. [DOI] [PubMed] [Google Scholar]
- 73. Habeebuddin M, Karnati RK, Shiroorkar PN, Nagaraja S, Asdaq SMB, Khalid Anwer M, et al. Topical probiotics: more than a skin deep. Pharmaceutics. 2022;14(3):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Damiani G, Bragazzi NL, McCormick TS, Pigatto PDM, Leone S, Pacifico A, et al. Gut microbiota and nutrient interactions with skin in psoriasis: a comprehensive review of animal and human studies. World J Clin Cases. 2020;8(6):1002–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Le ST, Toussi A, Maverakis N, Marusina AI, Barton VR, Merleev AA, et al. The cutaneous and intestinal microbiome in psoriatic disease. Clin Immunol. 2020;218:108537. [DOI] [PubMed] [Google Scholar]
- 76. Sinha S, Lin G, Ferenczi K. The skin microbiome and the gut-skin axis. Clin Dermatol. 2021;39(5):829–39. [DOI] [PubMed] [Google Scholar]
- 77. Todberg T, Kaiser H, Zachariae C, Egeberg A, Halling AS, Skov L. Characterization of the oral and gut microbiota in patients with psoriatic diseases: a systematic review. Acta Derm Venereol. 2021;101(7):adv00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Carmona-Cruz S, Orozco-Covarrubias L, Sáez-de-Ocariz M. The human skin microbiome in selected cutaneous diseases. Front Cell Infect Microbiol. 2022;12:834135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Eppinga H, Sperna Weiland CJ, Thio HB, van der Woude CJ, Nijsten TEC, Peppelenbosch MP, et al. Similar depletion of protective Faecalibacterium prausnitzii in psoriasis and inflammatory bowel disease, but not in hidradenitis suppurativa. J Crohns Colitis. 2016;10(9):1067–75. [DOI] [PubMed] [Google Scholar]
- 80. Zorba M, Melidou A, Patsatsi A, Ioannou E, Kolokotronis A. The possible role of oral microbiome in autoimmunity. Int J Womens Dermatol. 2020;6(5):357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Curtis MA, Diaz PI, Van Dyke TE. The role of the microbiota in periodontal disease. Periodontol 2000. 2020;83(1):14–25. [DOI] [PubMed] [Google Scholar]
- 82. Costalonga M, Herzberg MC. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol Lett. 2014;162(2 Pt A):22–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vieira Colombo AP, Magalhães CB, Hartenbach FA, Martins do Souto R, Maciel da Silva-Boghossian C. Periodontal-disease-associated biofilm: a reservoir for pathogens of medical importance. Microb Pathog. 2016;94:27–34. [DOI] [PubMed] [Google Scholar]
- 84. Weinstabl A, Hoff-Lesch S, Merk HF, von Felbert V. Prospective randomized study on the efficacy of blue light in the treatment of psoriasis vulgaris. Dermatol. 2011;223(3):251–9. [DOI] [PubMed] [Google Scholar]
- 85. Reich A, Heisig M, Phan NQ, Taneda K, Takamori K, Takeuchi S, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol. 2012;92(5):497–501. [DOI] [PubMed] [Google Scholar]
- 86. Lazaridou A, Elbaridi N, Edwards RR, Berde CB. Chapter 5: pain assessment. In: Benzon HT, Raja SN, Liu SS, Fishman SM, Cohen SP, editors. Essentials of pain medicine. 4th ed.Elsevier; 2018. p. 39–46.e1. [Google Scholar]
- 87. Phan NQ, Blome C, Fritz F, Gerss J, Reich A, Ebata T, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol. 2012;92(5):502–7. [DOI] [PubMed] [Google Scholar]
- 88. Human Microbiome Project Consortium . Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lin H, Peddada SD. Analysis of microbial compositions: a review of normalization and differential abundance analysis. NPJ Biofilms Microbiomes. 2020;6(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bende B, Kui R, Németh A, Borsos M, Tóbiás Z, Erős G, et al. A randomized controlled trial with a medical device containing sodium hyaluronate and nicotinic acid to increase the efficacy of ultraviolet phototherapy in psoriasis. Dermatol Ther. 2020;10(4):651–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wang X, Ji X. Sample size estimation in clinical research: from randomized controlled trials to observational studies. Chest. 2020;158(1s):S12–20. [DOI] [PubMed] [Google Scholar]
- 92. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother. 2010;1(2):100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants but are available from the corresponding author (K.P.) upon reasonable request.