Abstract
Introduction
Coronavirus disease 2019 (COVID-19) encephalitis is characterized by viral entry into the brain, resulting in inflammation and a cascade of neuronal damage. Clinical manifestations include headaches, seizures, and movement disorders. A mortality rate of 20% and infrequent presentation make COVID-19 encephalitis a diagnostic challenge.
Case Presentation
We hereby present the case of a 55-year-old man with a history of diabetes mellitus (potential impact on COVID-19 severity discussed in the supplementary material) presenting with altered sensorium, swelling in the left eye, and involuntary jerky limb movements. Neurological examination revealed neck rigidity, myoclonic jerks, and an extensor plantar response. Brain magnetic resonance imaging (MRI) was performed, which revealed cortical enhancement in the bifrontal, temporal, and occipital lobes. Rapid progression of myoclonus, altered sensorium, and cortical enhancement on MRI suggested Creutzfeldt-Jacob disease. After a thorough workup, the diagnosis was COVID-19 encephalitis with rhino-orbital mucormycosis. The treatment regimen consisted of adequate glycemic control, remdesivir injection, intravenous and retroorbital liposomal amphotericin, and levetiracetam. The patient’s condition improved, and he was eventually discharged.
Conclusion
This case illustrates the uncommon presentation of COVID-19 with neurological involvement and emphasizes the value of history-taking, neuroimaging, and cerebrospinal fluid analysis. A high index of suspicion is critical for a prompt diagnosis and initiating therapy.
Keywords: COVID-19, Encephalitis, Creutzfeldt-Jacob disease
Introduction
In the wake of the coronavirus disease 2019 (COVID-19) pandemic, neurological manifestations of the causative virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), have garnered increased attention among healthcare professionals and researchers. COVID-19 can also present primarily with neurological signs, even in the absence of severe respiratory symptoms [1]. We present a unique case report of COVID-19-associated encephalitis exhibiting clinical and radiological characteristics initially suggestive of Creutzfeldt-Jakob disease (CJD). Identifying neurological illness linked to SARS-CoV-2 infection is critical for those who are asymptomatically infected. This case highlights the challenges in diagnosing COVID-19-related neurological complications and emphasizes the importance of comprehensive differential diagnosis in the context of a global health crisis [2]. This case report has been prepared following the CARE Checklist. A detailed description of how this case report adheres to the CARE Checklist is available as online supplementary material (for all online suppl. material, see https://doi.org/10.1159/000539741).
Case Presentation
The patient was a 55-year-old man with type 2 diabetes on irregular treatment and unvaccinated for COVID-19. Presented to the emergency department with a history of altered sensorium, left eye swelling for 5 days, and involuntary jerky movements of limbs for 3 days.
The patient’s family reported that he had visited his general practitioner for myalgia and fever a few weeks prior. He was prescribed amoxicillin and azithromycin and diagnosed with a lower respiratory tract infection. A few days later, he developed an altered sensorium, exhibited involuntary jerky movements throughout his body, and experienced swelling and closure of the left eye. The patient had no significant medical, family, or psychiatric histories. He was a nonsmoker and denied alcohol or recreational drug use. The patient was drowsy and responded only to painful stimuli, scoring E3V2M5 on the Glasgow Coma Scale. Blood pressure was 130/80 mm Hg, pulse rate was 87/min, and capillary blood sugar was 20.4 mmol/L.
His initial general physical examination revealed proptosis of the left eye. Also present were dryness of the tongue and myoclonic jerks. Neurological examination revealed bilateral extensor plantar responses and neck rigidity. Extraocular movements were restricted, with the possibility of 3,4,6 cranial nerve palsy, raising the suspicion of cavernous sinus involvement. All other systemic examinations were normal. The patient was suspected for COVID-19. Reverse transcription polymerase chain reaction (RT-PCR) testing was performed, which returned positive. The patient subsequently started on COVID-19 treatment (remdesivir and heparin).
Investigation
An extensive blood workup was performed. Complete blood count (Table 1) indicated lymphocytopenia. Liver and renal function tests (Table 2) were normal. Human immunodeficiency virus and syphilis serology, erythrocyte sedimentation rate, and hepatitis B and C antibodies screening were normal.
Table 1.
Hemogram blood results
| Test | Result | Normal value |
|---|---|---|
| WBC | 5.00 × 109/L (N82, L15) | 4.0–11.0 × 109/L |
| Hemoglobulin | 110 g/L | 130–180 g/L |
| Platelets | 215,000/μL | 150,000–400,000/μL |
N, neutrophils; L, lymphocytes.
Table 2.
Results of the renal and liver function tests and inflammatory markers
| Test | Result | Normal values | Test | Result | Normal value |
|---|---|---|---|---|---|
| RBS | 20.4 mmol/L | <7.8 mmol/L | Sodium | 135 mEq/L | 136–145 mEq/L |
| HbA1c | 7.8% | <5.7% | COVID nasopharyngeal swab | Positive | |
| RT-PCR | |||||
| Urea | 48 mg/dL | 5–20 mg/dL | Ferritin | 800 ng/mL | 15–200 ng/mL |
| Creatinine | 0.7 mg/dL | 0.7–1.3 mg/dL | LDH | 350 U/L | 60–100U/L |
| Total bilirubin | 0.8 mg/dL | 0.3–1.2 mg/dL | CRP | 47 mg/L | 0.0–8.0 mg/L |
| Direct bilirubin | 0.2 mg/dL | 0–0.3 mg/dL | Urine ketone | Positive | |
| ALT | 35 U/L | 0–35 U/L | pH | 7.38 | 7.38–744 |
| SGPT | 38 U/L | 0–35 U/L | Serum bicarbonate | 20 mEq/L | 23–28 mEq/L |
RBS, random blood sugar; HbA1c, glycated hemoglobin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; RT-PCR, reverse transcriptase-polymerase chain reaction; LDH, lactate dehydrogenase; CRP, C-reactive protein.
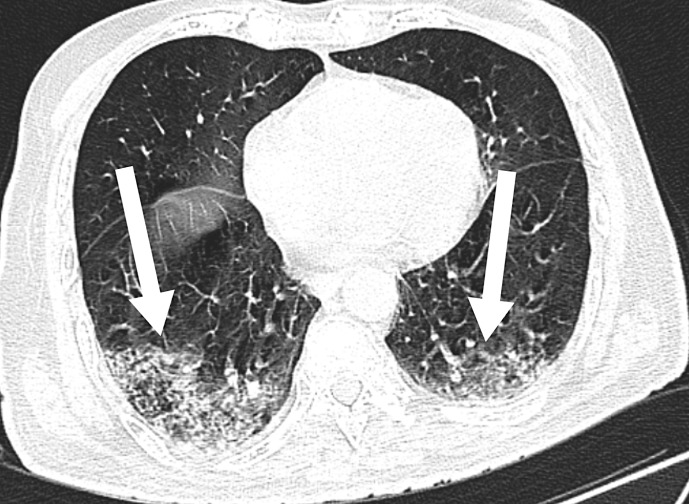
Chest computed tomography (Fig. 1) was performed to confirm lung involvement due to COVID-19. The scan showed bilateral ground-glass opacities with interlobular thickening, indicating COVID-19 Reporting and Data System category 5 with a severity score of 6/25.
Fig. 1.
Chest computed tomography showing bilateral ground-glass opacities.
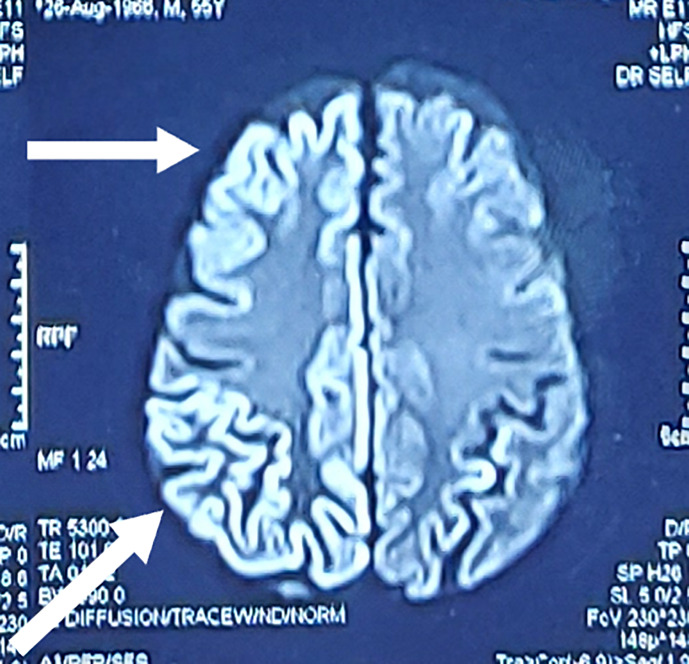
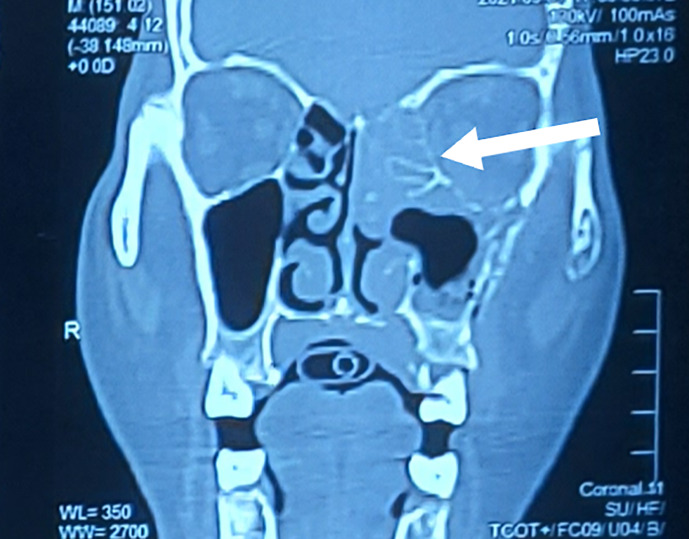
Head computed tomography revealed no acute intracranial abnormalities and eliminated the possibility of stroke. Electrocardiography showed a normal sinus rhythm. Since patient had orbital swelling and altered sensorium, brain and orbit magnetic resonance imaging (MRI) (Fig. 2) showed substantial bilateral cortical hyperintensity involving the bilateral frontal, temporal, bilateral occipital, and right parietal regions, suggesting the possibility of encephalitis, as well as thickening of the left superior orbital fissure soft tissue. Hyperintensity and bone erosion were also observed in the left maxillary and ethmoid sinuses (Fig. 3), suggesting invasive fungal sinusitis with cavernous sinus involvement, in addition to encephalitis. The cortical enhancement and myoclonus raised suspicion for CJD.
Fig. 2.
Brain magnetic resonance imaging showing cortical enhancement in the frontal, parietal, temporal, and occipital areas.
Fig. 3.
Computed tomography of the paranasal sinus showing involvement of the left maxillary and ethmoid sinuses.
Electroencephalography revealed generalized slowing and no periodic lateralized epileptiform discharges. A lumbar puncture was performed to draw a sample of cerebrospinal fluid (CSF), which was then sent for analysis. The CSF analysis (Table 3) revealed glucose 4.9 mmol/L, proteins 40 mg/dL, and 90% lymphocytic pleocytosis. CSF was positive for COVID-19 on RT-PCR. However, CSF was negative for culture and encephalitis panel.
Table 3.
Results of the CSF analysis
| Test | Result | Normal values |
|---|---|---|
| Cell count | 20 cells/mm3 (90% lymphocytes) | 0–5 cells/mm3 |
| Glucose | 4.9 mmol/L | 2.2–4.4 mmol/L |
| Protein | 40 mg/dL | 15–60 mg/dL |
| Gram stain | No organism is seen | |
| Culture | No growth | |
| Herpes simplex virus I and II DNA PCR | Negative | |
| Varicella zoster virus DNA PCR | Negative | |
| COVID-19 PCR | Positive | |
| Autoimmune panel | Negative | |
| Encephalitis panel | Negative |
Treatment
In view of the findings, the patient was diagnosed with COVID-19 encephalitis with rhino-orbital cerebral mucormycosis. The patient was administered with remdesivir injection and started on ceftriaxone, vancomycin, and dexamethasone, along with intravenous fluids. Since the patient had ketosis, intravenous insulin was administered as a continuous infusion [3]. Levetiracetam and sodium valproate tablets were administered to manage myoclonic jerks [4]. Injectable liposomal amphotericin and retroorbital amphotericin were administered for orbital mucormycosis.
Endoscopic debridement of the left maxillary and ethmoid sinuses was performed to remove infective foci. On repeat brain MRI with contrast, hyperintensity decreased compared with the earlier scans. The patient responded favorably to treatment and regained consciousness, with mild cognitive dysfunction. However, the visual defect in the left eye persisted, with visual acuity limited to hand gestures.
Outcomes
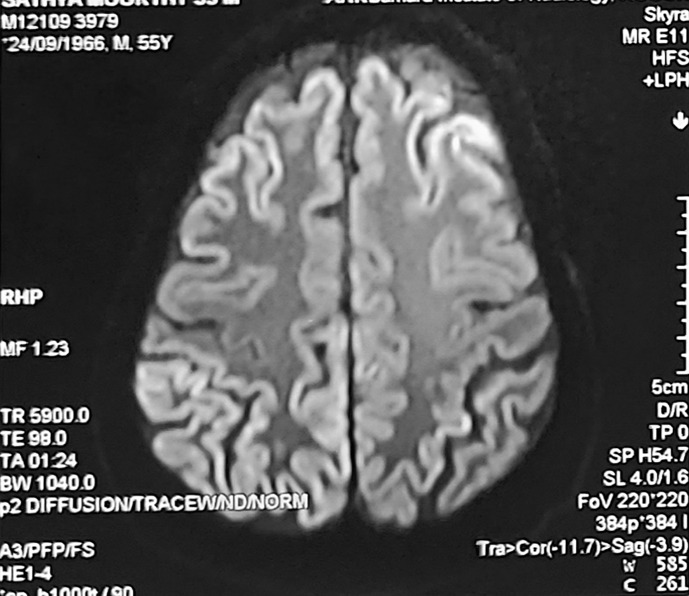
On follow-up visits, the patient was able to perform his routine activities despite some residual defects in vision. Follow-up brain MRI (Fig. 4) showed improvement in cortical enhancement, which correlates clinically.
Fig. 4.
Follow-up MRI showing improvement in cortical enhancement, which correlates clinically.
Discussion
CJD is a rare neurological condition characterized by a rapid disease progression and poor prognosis. Given comparable clinical conditions that can be exhibited, COVID-19 with neurological complications can be misdiagnosed as CJD, which could result in ineffective therapy and unfavorable consequences. The exact mechanism by which COVID-19 induces encephalitis remains unknown [5]. However, SARS-CoV-2 may actually harm brain cells or even induce an autoimmune attack on the brain. Rarely, the clinical signs, neuroimaging results, and CSF profile of COVID-19-associated encephalitis may resemble those of CJD, which would make differential diagnosis challenging. The presence of a positive 14-3-3 protein in the CSF is one of the main characteristics of CJD [6]. However, COVID-19-associated encephalitis can also induce the increased expression of this protein. Therefore, a positive 14-3-3 protein result alone is insufficient for a CJD diagnosis. A real-time quaking-induced conversion (RT-QuIC) assay is a crucial diagnostic procedure for CJD [7]. The CSF analysis is thus performed to look for aberrant prion proteins. However, in early-stage CJD, the RT-QulC assay can also return negative. Therefore, a negative RT-QulC assay does not rule out CJD.
Given the difficulty in differentiating between COVID-19-associated encephalitis and CJD, the symptoms and indicators of both diseases should be considered in individuals with rapidly worsening neurological symptoms [8]. Patients with probable COVID-19-related encephalitis need to be actively monitored for CJD symptoms such as myoclonus, ataxia, and rapidly worsening dementia. To establish a diagnosis of CJD, further testing, such as RT-QulC assay, should be performed if these symptoms intensify. Early detection and treatment of COVID-19-associated encephalitis are crucial, and the cornerstone of treatment for this disease is immunotherapy [9]. However, further research is required to determine the most suitable course of action for this newly discovered syndrome.
Conclusion
The presentation mimicking CJD emphasizes the importance of maintaining a broad differential diagnosis, and it adds to the growing knowledge of COVID-19’s diverse neurological presentations. The role of a comprehensive clinical workup, including detailed history taking, neuroimaging, and CSF analysis, is crucial for an accurate diagnosis. A high index of suspicion for COVID-19 encephalitis is essential for prompt diagnosis and initiation of appropriate therapy, potentially improving the outcome of the disease.
Patient Perspective
In the early days of my battle with COVID-19, the world seemed like a blur of uncertainty. I never imagined that my journey to recovery would not only test my physical strength, but also challenge my spirit. During this tumultuous time, I found myself in the capable hands of the encephalitis team, who became my beacon of hope. The days spent in isolation were lonely and daunting, as I grappled with the consequences of COVID-19 that manifested as encephalitis. The team of dedicated healthcare professionals transformed my despair into determination. Their unwavering support, expertise, and compassion guided me through thein darkest moments.
Every step of my recovery felt like a triumph, from relearning basic skills to regaining my independence. The encephalitis team’s commitment to my well-being was evident in every therapy session, every encouraging word, and every reassuring smile.
Today, as I stand on the other side of this challenging journey, I am profoundly grateful to my encephalitis team. Their tireless efforts and belief in my recovery were instrumental in helping me regain my health. My journey may have begun with uncertainty, but it continues with newfound strength, resilience, and gratitude to the remarkable individuals who made it possible. Thank you, from the bottom of my heart.
Informed Consent
I hereby grant my informed consent for publication of my medical case report in Case Reports in Neurology. I understand that my identity will be kept confidential and only essential medical information will be disclosed. I am aware that the publication of this case will contribute to medical knowledge and may be accessible to a wide audience. I have had the opportunity to ask questions and seek clarification regarding the publication process. I willingly authorize the publication of my case report and understand that I may withdraw this consent at any time before publication.
Statement of Ethics
This retrospective review of patient data did not require ethical approval in accordance with local/national guidelines. Informed written consent was obtained from next-of-kin for publication of the details of the medical case and any accompanying image.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was not supported by any sponsor or funder.
Author Contributions
Naveenkumar Nallathambi and Shriganesh P Naidu: corresponding authors; Yogesh S and Balamanikandan P: supervision. Adithyan C: participated in writing of the manuscript. Navvin S: provided and cared for study patient. Hariharan Seshadri: technical editing of the manuscript. Mohanapriya N: collected data. Suriya Prakash: critically reviewed the study proposal.
Funding Statement
This study was not supported by any sponsor or funder.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.
Supplementary Material.
Supplementary Material.
References
- 1. Pilotto A, Odolini S, Masciocchi S, Comelli A, Volonghi I, Gazzina S, et al. Steroid-responsive encephalitis in coronavirus disease 2019. Ann Neurol. 2020;88(2):423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fisher RS, Cross JH, D’Souza C, French JA, Haut SR, Higurashi N, et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia. 2017;58(4):531–42. [DOI] [PubMed] [Google Scholar]
- 5. Bridwell R, Long B, Gottlieb M. Neurologic complications of COVID19. Am J Emerg Med. 2020;38(7):1549.e3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zerr I, Kallenberg K, Summers DM, Romero C, Taratuto A, Heinemann U, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain. 2009;132(Pt 10):2659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Geschwind MD. Prion diseases. Continuum Lifelong Learn Neurol. 2015;21(6 Neuroinfectious Disease):1612–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARSCoV-2). J Med Virol. 2020;92(7):699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.