Abstract
The development of human T-lymphotropic virus type 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) is closely associated with the activation of T cells which are HTLV-1 specific but may cross-react with neural antigens (Ags). Immature dendritic cells (DCs), differentiated from normal donor monocytes by using recombinant granulocyte-macrophage colony-stimulating factor and recombinant interleukin-4, were pulsed with HTLV-1 in vitro. The pulsed DCs contained HTLV-1 proviral DNA and expressed HTLV-1 Gag Ag on their surface 6 days after infection. The DCs matured by lipopolysaccharides stimulated autologous CD4+ T cells and CD8+ T cells in a viral dose-dependent manner. However, the proliferation level of CD4+ T cells was five- to sixfold higher than that of CD8+ T cells. In contrast to virus-infected DCs, DCs pulsed with heat-inactivated virions activated only CD4+ T cells. To clarify the role of DCs in HAM/TSP development, monocytes from patients were cultured for 4 days in the presence of the cytokines. The expression of CD86 Ag on DCs was higher and that of CD1a Ag was more down-regulated than in DCs generated from normal monocytes. DCs from two of five patients expressed HTLV-1 Gag Ag. Furthermore, both CD4+ and CD8+ T cells from the patients were greatly stimulated by contact with autologous DCs pulsed with inactivated viral Ag as well as HTLV-1-infected DCs. These results suggest that DCs are susceptible to HTLV-1 infection and that their cognate interaction with T cells may contribute to the development of HAM/TSP.
Human T-lymphotropic virus type 1 (HTLV-1) is the causative agent of HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (16). HAM/TSP is thought to be an autoimmune disease induced by activated autoreactive T cells (8). T lymphocytes obtained from HAM/TSP patients produce interleukin-2 (IL-2) in vivo and proliferate spontaneously in vitro without any additional stimuli or cytokines (24). Although HTLV-1 Tax protein induces IL-2 production and IL-2 receptor expression on the surface of HTLV-1-infected T cells, which may lead to the proliferation of the virus-infected T cells by an autocrine mechanism, the major cellular components of in vitro proliferating T cells seem to be T cells which are uninfected and interacting with HTLV-1-infected antigen-presenting cells (APCs). The most characteristic feature of peripheral blood mononuclear cells (PBMCs) of HAM/TSP patients is the spontaneous proliferation of T lymphocytes (SPL) that are largely suppressed by the addition of monoclonal antibodies (MAbs) to major histocompatibility complex (MHC) class II, CD86, and CD58 antigens (Ags) (14a), and the SPL levels reflect disease activity of HAM/TSP. Previously we reported that HTLV-1-immortalized CD4+ T cells directly stimulate CD4+ but not CD8+ T cells (22). In the active stage of HAM/TSP, both CD4+ and CD8+ T cells accumulate in lesions of the spinal cord; however, in the chronic state of the disease, CD8+ T cells are predominantly found there (23).
Dendritic cells (DCs) are the most potent APCs and so can stimulate not only naive CD4+ T cells but also CD8+ T cells. The DCs located in the blood and periphery are functionally immature in that they are effective in the uptake and processing of Ag but not in stimulating lymphocytes. After exposure to inflammatory cytokines and bacterial products or interaction with activated T cells through their surface CD40 molecules, DCs undergo a process of maturation and become able to present Ags for priming and stimulating T cells (15, 18).
In addition to HTLV-1-infected CD4+ T cells, some DCs obtained from the peripheral blood of HAM/TSP patients have been found to be infected with HTLV-1 (12). These observations strongly suggest that HTLV-1-infected DCs play a crucial role in the production of autoreactive T cells in HAM/TSP patients. In this study, we found that DCs differentiated from peripheral monocytes from healthy donors as well as HAM/TSP patients by using recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF) and rIL-4 were infected with HTLV-1 in vitro and subsequently matured stimulated autologous CD4+ and CD8+ T cells without forming a syncytium.
MATERIALS AND METHODS
Study subjects and cell preparation.
PBMCs were donated under informed consent by five patients diagnosed with HAM/TSP at the Kagoshima University Hospital and by six uninfected healthy donors. The PBMCs were isolated from heparinized blood by using Ficoll-Paque (Pharmacia, Uppsala, Sweden) and cryopreserved in liquid nitrogen as described by Katahira et al. (10). The DCs were prepared from peripheral monocytes as described previously (13). In brief, 106 freshly isolated or cryopreserved PBMCs per ml were plated in a six-well flat-bottom tissue culture plate and cultured for 3 h. Non-plastic-adherent cells were washed out by prewarmed medium, and the remaining adherent monocytes were cultured with 1% autologous plasma for 5 days in the presence of 50 ng of rGM-CSF (donated by Kirin Brewery Co., Tokyo, Japan) and of 10 ng of rIL-4 (Genzyme, Cambridge, Mass.) per ml. rGM-CSF and rIL-4 were supplied every 2 days. On day 5 of cell culture, immature DCs were pulsed with either live or heat (56°C, 30 min)-inactivated HTLV-1 Ags. Maturation of DCs was performed by addition of 10 ng of lipopolysaccharide (LPS) from Escherichia coli O111:B4 (Difco Laboratories, Detroit, Mich.) per ml for 24 h, and matured DCs were assessed for APC functions after treatment with 50 μg of mitomycin C per ml. When DCs were generated from monocytes from HAM/TSP patient PBMCs, the PBMCs were depleted of CD2+ T cells in advance by using immunomagnetic beads coated with anti-CD2 MAb (Dynabeads 450; Dynal, Oslo, Norway), and subsequently plastic adherent monocytes were obtained. The concentration of heterodimeric IL-12 (p70) in culture supernatant fluids of Ag-pulsed DCs was measured by using an enzyme immunoassay kit (Immunotech, Westbrook, Mass.).
Concentration of cell-free HTLV-1 virions and detection of HTLV-1 infection.
HTLV-1 infection usually occurs by cell-to-cell contact of target cells with virus-infected cells. Since HTLV-1-infected CD4+ T cells and HTLV-1-producing cell line MT-2, a generous gift from I. Miyoshi, Kochi Medical School, have APC function as described previously (22), the elimination of the virus-infected T cells from the DC culture populations is required to assess the APC function of virus-infected DCs. However, this is difficult because of the high resistance of virus-infected T cells to mitomycin treatment. Therefore, we collected the culture supernatant of MT-2 cells, concentrated the HTLV-1 virions by using a BMM hollow fiber (Asahi Chemical Industry Co., Ltd.) as reported earlier (14), and used them as the source of virus. For detection of HTLV-1 proviral DNA, DNA was prepared from CD4+ T cells or DCs by using a DNA extractor kit (Wako Pure Chemical Industries, Ltd., Osaka, Japan). The DNA was amplified by PCR using a primer pair corresponding to nucleotides 1729 to 1750 and 1942 to 1964 of the HTLV-1 gag region. When no band was detectable in the PCR products, nested PCR was conducted by using a primer pair corresponding to nucleotides 1756 to 1777 and 1919 to 1940. The HTLV-1 proviral DNA was detected as a 185-bp band. The 50% cell culture infective dose (CCID50) of concentrated cell-free HTLV-1 was determined by using CD4+ T cells in the presence of 2 μg of phytohemagglutinin (Difco) and 100 U of rIL-2 (TGP-3; Takeda Chemical Industries, Osaka, Japan) per ml, and the DCs were infected with 20 CCID50 of HTLV-1. Infection of DC with HTLV-1 was determined by PCR analysis and by cytometry analysis (FACScan; Becton Dickinson Immunocytometry Systems, San Jose, Calif.) for surface expression of HTLV-1 Gag and HTLV-1 Env proteins.
Analysis of cell surface Ag.
The expression of cell surface Ag on the DCs was determined by using a FACScan (Becton Dickinson). Live DCs (5 × 103) were gated and analyzed. To eliminate dead cells from analysis, propidium iodide (Sigma Chemical Co., St. Louis, Mo.) was used. We used fluorescein isothiocyanate-conjugated MAb against HLA-DR (L243; Becton Dickinson) and HLA-ABC (G46-2.6), CD40 (5C3; PharMingen, San Diego, Calif.), phycoerythrin-labeled MAb to CD54 (HA58), CD58 (L304.4); (Becton Dickinson), CD86 (IT2.2), CD11c (B-ly6); (PharMingen), and CD83 (HB15a; Immunotech), and purified murine MAb to CD1a (NA1/34; Serotec Ltd., Oxford, England), HTLV-1 Gag (GIN14), and HTLV-1 Env (F10) (generously provided by Y. Tanaka, Department of Hygiene, Kitazato University), followed by fluorescein isothiocyanate-labeled goat F (ab′)2 anti-mouse immunoglobulins (Igs) (Tagoimmunologicals, Camarillo, Calif.). The optimal concentrations of MAbs were determined in advance. DCs in an apoptotic process were determined by staining with Annexin-V (Genzyme).
Proliferation assay.
Autologous MLR was conducted by using DCs as the stimulators and unseparated T cells and purified CD4+ T cells or CD8+ T cells as the responder population. DCs unpulsed, pulsed with heat-inactivated HTLV-1 Ags, or infected with HTLV-1 were treated with mitomycin C and plated to give a DC responder ratio of 1:20 or 1:40. Proliferation of stimulator cells was confirmed to be completely blocked by mitomycin C. The responder T cells were purified as follows. Freshly thawed PBMCs were depleted of MHC class II+ cells by using magnetic beads coated with MAb to MHC class II Ag (Dynabeads 450; Dynal) and further treated with beads coated with either CD4 or CD8 MAb to select T cells negatively. The purity of CD4+ T cells or CD8+ T cells was more than 98%, and contamination of the purified population by HLA-DR-positive cells was less than 2%. The indicated number of responder cells (105/well unless otherwise specified) were plated in 96-well round-bottom tissue culture plates. The cell proliferation during the last 16 h of the 5- to 6-day culture in the presence of 10% heat-inactivated human serum (a generous gift of Kagoshima Red Cross Blood Center) was quantified by incubating the cells with [3H]thymidine at 1 μCi/well. The results are expressed as the mean difference in counts per minute obtained from triplicate cultures.
To suppress the proliferation of T cells stimulated by autologous Ag-pulsed DCs, we used the purified MAbs L243 (anti-HLA-DR), IT2.1 (anti-CD86), HA58 (anti-CD54), TS2/9.1.4.3 (anti-CD58), and TRAP1 (anti-CD40L; PharMingen). The MAbs were purified from the culture supernatant or ascitic fluid by using 40% saturated ammonium sulfate and caprylic acid (Sigma). The purity of the MAb was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and only a single band was observed. The optimal concentration of MAb for suppression was determined in advance. Percent suppression was calculated as 100 × [1 − (mean Δcpm of cultures with MAb/mean Δcpm of cultures without Ab)].
RESULTS
In vitro infection of DCs with HTLV-1.
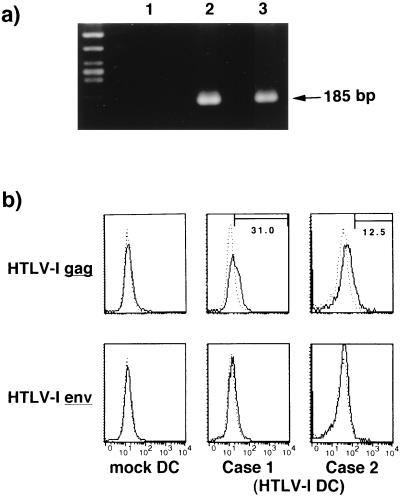
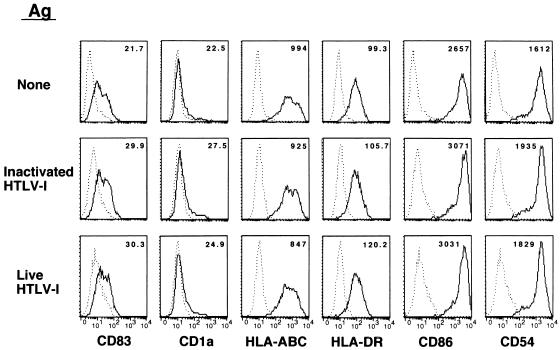
We used a cell-free viral source to eliminate the influence of HTLV-1-infected T cells for assessment of the APC function of DCs. Immature DCs were pulsed with 20 CCID50 of HTLV-1. After 6 days of culture in the presence of rGM-CSF and rIL-4, the DCs were found to be infected with HTLV-1 (Fig. 1). HTLV-1-pulsed DCs contained proviral DNA detectable as a faint signal amplified by PCR of both HTLV-1 gag and tax regions (not shown) and as a strong gag band by nested PCR (Fig. 1a). The DCs expressed HTLV-1 Gag Ag on their surface (Fig. 1b), but expression of HTLV-1 Env Ag was low, probably because of poor sensitivity of the MAb (Fig. 1b). In contrast, DCs pulsed with heat-inactivated virus did not express HTLV-1 Gag Ag. The immature DCs pulsed with live HTLV-1 and subsequently stimulated with LPS were phenotypically compared with those unpulsed or pulsed with heat-inactivated HTLV-1 Ags. However, there was no apparent difference among them in the expression of HLA-DR, CD54, CD83, CD86, and CD1a Ags (Fig. 2) or CD58 Ag (not shown), suggesting that they were similarly matured. Also, there was no obvious increase in the number of Annexin-V-positive DCs or syncytium-forming cells in the virus-infected populations (not shown). We measured the concentration of IL-12 heterodimer (p70) in the supernatant of virus-infected LPS-matured DCs and found no significant increase in their production of IL-12 (not shown).
FIG. 1.
(a) Detection of HTLV-1 proviral DNA by nested PCR. Immature DCs were exposed to live HTLV-1 virions for 6 days in vitro. A genomic DNA was extracted from mock- or HTLV-1-infected DCs and amplified by PCR. Lane 1, mock-infected DCs; lanes 2 and 3, HTLV-1-infected DCs. (b) Expression of HTLV-1 Ags on virus-infected DCs. Immature DCs were pulsed with live HTLV-1 for 6 days and examined for HTLV-1 Gag and Env Ag expression by FACScan. –––, control MAb; ———, indicated MAb. The number in each panel represents percent positive cells.
FIG. 2.
Expression of various molecules on DCs pulsed with HTLV-1. Immature DCs were differentiated from normal healthy donor monocytes, pulsed with 20 CCID50 of live HTLV-1 or equivalent dose of heat-inactivated virions, and matured by LPS (10 ng/ml). –––, control MAb; ———, indicated MAb. The number in each panel represents the mean fluorescence intensity.
Cellular interaction between HTLV-1-infected DCs and T cells.
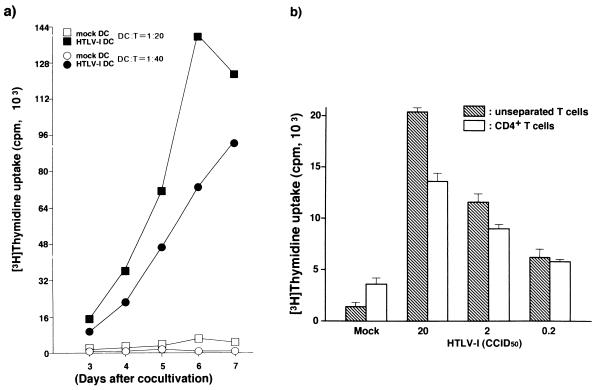
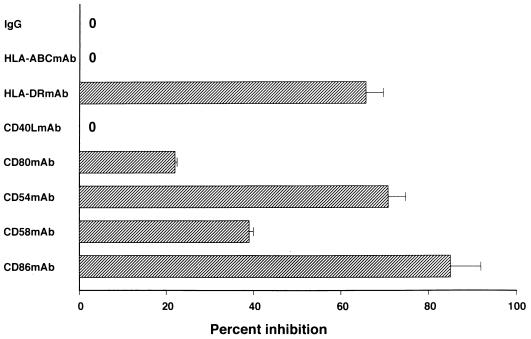
The APC function of mature HTLV-1-infected DCs was assessed by autologous MLR. The DCs infected with HTLV-1 in the immature state and subsequently matured with LPS for 24 h stimulated T cells in a time-dependent fashion at DC:T-cell ratios of 1:20 and 1:40 (Fig. 3a). The virus-infected DCs activated both unseparated and CD4+ T cells in a manner depending on the viral dose with which the immature DCs were pulsed (Fig. 3b). However, the virus-infected DCs could not transmit virus to CD4+ T cells or make them immortalize (not shown). The molecules associated with the interaction of viral Ag-bearing DCs and T cells were determined by using MAbs to Ags (Fig. 4). When MAbs to HLA-DR, CD86, or CD54 Ag were added to the culture at 10 μg/ml, the T-cell proliferation induced by the DCs was suppressed by more than 60%. Around 40% of the T-cell proliferation was inhibited by anti-CD58 MAb; however, little suppression of them was induced by MAbs to CD40L and to HTLV-1 Gag and Env. These findings suggest that the two cell components mainly interact in a cognate fashion through the MHC and costimulatory and adhesion molecules.
FIG. 3.
(a) Time-dependent T-cell proliferation stimulated by HTLV-1-infected DCs. Mature HTLV-1-infected DCs were generated by exposure of immature DCs to 20 CCID50 of HTLV-1 and cocultured with unseparated T cells (105/well) at a DC:T-cell ratio of 1:20 or 1:40 in triplicate. Mock-infected DCs were used as a negative control. (b) Ag dose-dependent proliferation of T cells. Immature DCs were pulsed with various doses of live HTLV-1, and unseparated or CD4+ T cells (5 × 104/well) were cocultured with HTLV-1- or mock-infected DCs at a DC:T-cell ratio of 1:20 in triplicate.
FIG. 4.
Inhibitory effect of various MAbs on DC-CD4 interaction. HTLV-1-infected mature DCs and CD4+ T cells (105/well) were cocultured for 6 days in the presence or absence of MAbs (10 μg/ml) at a DC:CD4+ T-cell ratio of 1:20. The proliferation of T cells in the absence of test MAb was 96,514 ± 1,783 cpm (mean ± standard deviation of triplicate assays). A mixture of normal mouse IgG subclasses (IgG1, Ig2a, Ig2b, and Ig3) was used as control antibody. A representative of three independent experiments is shown.
Influence of viral infection on the APC function of DCs.
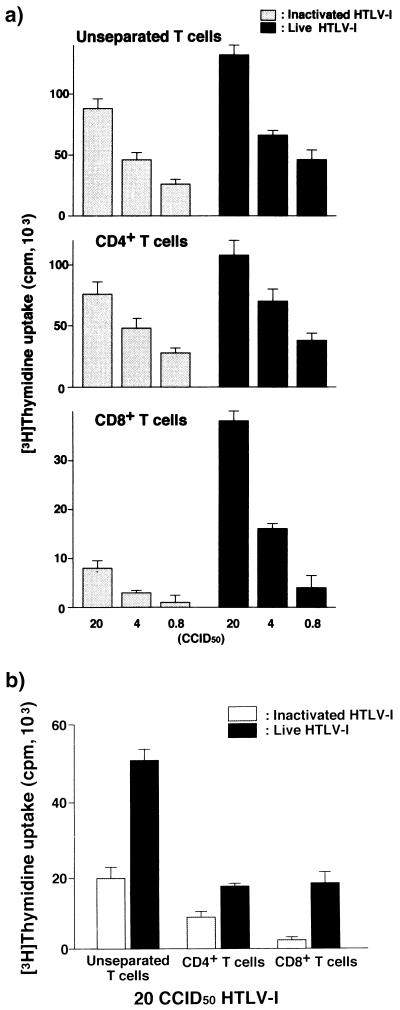
It is known that immature DCs take up exogenous Ags through the mannose receptor on their surface, by macropinocytosis, and by other mechanisms (20). We determined the functional influence of viral infection on DCs by comparing the APC function of DCs infected with live virus with the APC function of those primed with heat-inactivated Ags (Fig. 5). Both DCs stimulated unseparated and purified CD4+ T cells, although the T-cell proliferation induced by DCs with heat-inactivated virus was about 60% of that stimulated by virus-infected DCs (Fig. 5a). However, there was a marked difference in the ability to stimulate CD8+ T cells between the two DCs: only virus-infected DCs stimulated them in a viral dose-dependent manner (Fig. 5a). Comparing the magnitude of proliferation of CD4+ T cells and CD8+ T cells induced by HTLV-1-infected DCs, we found the former to be four- to fivefold higher than latter. In the next experiment, mature DCs were prepared in advance and subsequently pulsed with inactivated virions or live virus. While infected DCs stimulated CD4+ T cells and CD8+ T cells equally, DCs pulsed with inactivated Ags no longer stimulated CD8+ T cells (Fig. 5b). Immature DCs pulsed with either inactivated or live virus for 48 h but not exposed to any maturation factor did not stimulate T cells as did mature DCs (not shown).
FIG. 5.
Comparison of T-cell-stimulating activities of HTLV-1-infected DCs and inactivated virion-pulsed DCs. (a) Immature DCs were either infected with various doses of HTLV-1 or pulsed with an equivalent dose of heat-inactivated virions, subsequently matured by LPS, and cocultured with autologous unseparated, CD4+ and CD8+ T cells (105/well) for 5 days at a DC:T-cell ratio of 1:20. (b) LPS-matured DCs were exposed to live or inactivated virus for 4 days and cocultured with various T cells (105/well) for 5 days at a DC:T-cell ratio of 1:20. A representative result of three independent experiments is shown. Each experiment was performed in triplicate.
Phenotypic and functional features of DCs from HAM/TSP patients.
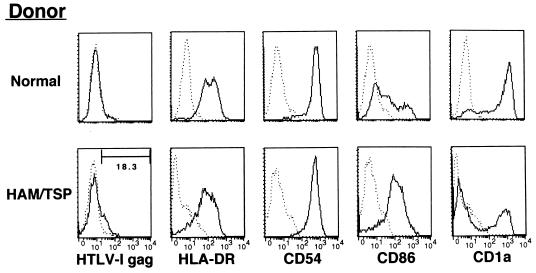
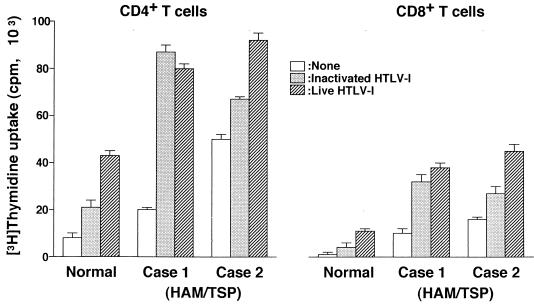
To determine whether DCs play an important role in T-cell proliferation in HAM/TSP patients, we cultured monocytes from the patients after depletion of CD2+ T cells for 4 days in the presence of rGM-CSF and rIL-4. Immature DCs obtained from two of five HAM/TSP patients expressed HTLV-1 Gag Ag on their surface without any exogenous in vitro exposure to the virus (Fig. 6). Furthermore, all DCs from HAM/TSP patients examined more strongly expressed CD86 Ag and were down-regulated in CD1a expression compared with those from uninfected donors (Fig. 6). DCs pulsed with inactivated Ag or infected in vitro equally and greatly stimulated autologous CD4+ T cells and CD8+ T cells (Fig. 7). Although the proliferation of CD4+ T cells was higher than that of CD8+ T cells, the level of both T cells in HAM/TSP patients was obviously higher than that of T cells from uninfected donors. Furthermore, HAM/TSP patient DCs expressing HTLV-1 Gag Ag prior to in vitro viral exposure (Fig. 7, case 2) induced higher T-cell proliferation than those lacking expression of the Ag (normal and case 1).
FIG. 6.
Expression of various molecules on DCs generated from uninfected donors and HAM/TSP patients. DCs were generated by using rGM-CSF and rIL-4 from peripheral monocytes for 4 days. Monocytes were purified from PBMCs depleted of CD2+ T cells. The number in the panel for HTLV-1 gag staining of HAM/TSP patient DCs represents the percent positive cells. –––, control MAb; ———, indicated MAb.
FIG. 7.
Autologous T-cell proliferation by DCs from HAM/TSP patients. DCs were generated from peripheral monocytes obtained from an uninfected donor and HAM/TSP patients. Plastic-adherent monocytes were obtained from the population of CD2+ T cells depleted in advance by using beads. CD4+ or CD8+ T cells (5 × 104/well) were stimulated with 2.5 × 103 autologous HTLV-1-infected, inactivated Ag-pulsed or unpulsed DCs per well for 5 days in triplicate. Mature DCs from HAM/TSP patient 2 expressed HTLV-1 Ag on the surface without any exposure to HTLV-1 Ag in vitro and those from patient 1 did not.
DISCUSSION
Human DCs are known to be susceptible to infection with various viruses, including measles virus, human immunodeficiency virus type 1 (HIV-1), and influenza virus (2–4, 6, 7). This report presents the evidence that HTLV-1 is another virus able to infect DCs in vitro. In one respect, measles virus and HIV-1 are similar in the ability to infect DCs (1): they induce apoptosis and syncytium formation in DCs; once infected, DCs, in turn, markedly reduce the proliferative response of T cells to autologous as well as allogenic stimuli. DCs required only a small amount of the virus to be infected, and the virus-infected DCs may directly contribute to viral transmission to T cells, resulting in the high level of death of CD4+ T cells. In contrast, influenza virus very efficiently infects DCs in a nontoxic fashion since the expression of viral protein on the DC surface is sustained for at least 2 days while their viability is retained (2). The influenza virus-infected DCs induce a considerably higher proliferative response of CD8+ T cells than of CD4+ T cells. Live virus and heat-inactivated virus induce equally strong cytotoxic T-lymphocyte (CTL) responses in virus-infected DCs. In these respects, HTLV-1 resembles influenza virus and markedly contrasts with measles virus or HIV-1 in that HTLV-1-infected DCs survived more than 6 days without showing any cytopathic effect, such as apoptotic cell death, and stimulated T cells without inducing their apoptosis or syncytium formation. The HTLV-1-infected DCs and uninfected CD4+ T cells cognately interacted through MHC, adhesion, and costimulating molecules. However, in contrast with influenza virus, HTLV-1-infected or viral Ag-pulsed DCs preferentially stimulated CD4+ T cells. The proliferation of CD4+ T cells was around fivefold greater than that of CD8+ T cells. Furthermore, the proliferation of CD8+ T cells from healthy donors required the infection of DCs with HTLV-1 since little CD8+ T-cell proliferation was induced by DCs exposed to heat-inactivated virus. However, the virus-infected DCs could not transmit virus to T cells or make them immortalize (not shown). This point is in line with the report by Zucker-Franklin et al. that clearly indicates the lack of productive HTLV-1 infections by DCs (25). MT-2 cells are known to produce defective virus particles as well as replication-competent virus. The preferential activation of CD4+ T cells might be associated with this type of virus.
HTLV-1 infection causes two different diseases, HAM/TSP and adult T-cell leukemia (ATL). HAM/TSP could be categorized as an autoimmune disease in that it is manifested by autoreactive T cells which are activated by contact with HTLV-1-infected APCs (22). Both CD4+ and CD8+ T cells play an important role in the induction of disease. In the active phase of the disease, both CD4+ and CD8+ T cells accumulate in the spinal cord lesions and CD8+ T cells are predominantly found there in the chronic phase (23). Patients with HAM/TSP alternatively repeat active and inactive states, and disease activity is reflected by the extension of SPL. In the absence of HTLV-1-infected activated CD4+ T cells, monocytes from HAM/TSP patients phenotypically and functionally produced more mature DCs after a short period of culture in the presence of the cytokines, which results in directed differentiation into immature DCs (19, 21). Both CD4+ T cells and CD8+ T cells responded by proliferating strongly in autologous DCs pulsed with inactivated virions as well as those infected with HTLV-1. Although we could not obtain definitive data indicating viral infection to HAM/TSP patient DCs by PCR due to a possible contamination of a small number of virus-infected T cells, the mature DCs from some patients carried viral Ags detectable by FACScan without any apparent exposure to HTLV-1 in vitro, and they stimulated T cells to significantly higher levels than DCs bearing undetectable viral Ags. These differences may relate to the extension of SPL. Furthermore, DCs have been reported to be infected with virus detectable by in situ hybridization in vivo (12). These results seem to suggest that CD4+ and CD8+ T cells are primed with HTLV-1 Ags and that DCs play an important pathogenic role in the activation of T cells in HAM/TSP. The proliferation level of CD4+ T cells induced by DCs appears to depend on the concentration of Ag pulsing the DCs. In HAM/TSP patients, the frequency of CD4+ T cells infected with HTLV-1 is higher than that in ATL patients and asymptomatic carriers (11). Studies in which a large number of patients are enrolled are required; however, it may be possible that DCs can be easily infected with HTLV-1 in HAM/TSP patients, as seen in two patients who had DCs bearing HTLV-1 Ags on the surface in vivo. Particularly in the active state, HTLV-1-infected T cells are effectively produced, which may lead to generation of HTLV-1 Ag-bearing DCs, result in the activation of CD4+ T cells. However, the activated CD4+ T cells are a good target of HTLV-1 infection and contribute to the production of newly infected T cells as reported previously (22). Otherwise, they might be killed by a process of activation-induced cell death as found in the HIV-1 system. In either process, the virus-specific CD4+ T cells will be selectively lost. Extracellular HTLV-1 Tax protein is found in sera of HAM/TSP patients and other HTLV-1-infected or noninfected individuals (5, 17, 26). The presence of a higher concentration of the protein might preferentially activate CD4+ T cells, as DCs primed with heat-inactivated virions poorly stimulated CD8+ T cells in the absence of help by CD4+ T cells. This immunological situation may, again, lead to production of virus-infected cells. Therefore, the high Ag load period should be shortened and the Ag-bearing DCs should be eliminated by CTL or other factors. In this respect, the clonal frequency of a CD8+ CTL precursor specific for HTLV-1 Ags is reported to be extremely high in HAM/TSP patients (9), and in this study, the patient CD8+ T cells were found to respond strongly to DCs bearing HTLV-1 Ags in the absence of CD4+ T cells. Therefore, the HAM/TSP patient CD8+ T cells should be primed with viral Ags in vivo, and the immunologically competent and viral Ag-bearing DCs should certainly be present in HAM/TSP patients as ascertained in this study (Fig. 6). DCs pulsed at the immature phase and subsequently matured contribute to production of memory CD8+ T cells and in controlling the number of virus-infected CD4+ T cells.
The development of ATL is thought to be closely associated with the loss of CD8+ T cells in number as well as in function. DCs obtained from tumor-bearing animals showed dysfunction in T-cell stimulation. Therefore, HTLV-1-infected DCs might be a good therapeutic and preventive tool against ATL.
In conclusion, we found in this study evidence that DCs were infected with HTLV-1 in vitro and stimulated both CD4+ and CD8+ T cells. The viral Ag-bearing DCs seem to play a major pathogenic role in HAM/TSP.
ACKNOWLEDGMENTS
We acknowledge the contribution of N. Makino to preparation of the manuscript and thank M. L. Robbins for reviewing the English.
This work was supported in part by a Grant-in-Aid for a Second-Term Comprehensive 10-year Strategy for Cancer Control from the Ministry of Health and Welfare of Japan.
REFERENCES
- 1.Bhardwaj N. Interactions of viruses with dendritic cells: a double-edged sword. J Exp Med. 1997;186:795–799. doi: 10.1084/jem.186.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhardwaj N, Bender A, Gonzalez N, Bui L K, Garrett M C, Steinman R M. Influenza virus-infected dendritic cells stimulate strong proliferative and cytolytic responses from human CD8+ T cells. J Clin Investig. 1994;94:797–807. doi: 10.1172/JCI117399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron P U, Fredenthal P S, Barker J M, Gezelter S, Inaba K, Steinman R M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science (Washington, DC) 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 4.Cameron P U, Pope M, Gezelter S, Steinman R M. Infection and apoptotic cell death of CD4+ T cells during an immune response to HIV-1 pulsed dedritic cells. AIDS Res Hum Retroviruses. 1994;10:61–71. doi: 10.1089/aid.1994.10.61. [DOI] [PubMed] [Google Scholar]
- 5.Dhib-Jalbut S, Hoffman P M, Yamabe T, Sun D, Xia J, Eisenberg H, Bergey G, Ruscetti F W. Extracellular human T-cell lymphotropic virus type I Tax protein induces cytokine production in adult human microglial cells. Ann Neurol. 1994;36:787–790. doi: 10.1002/ana.410360516. [DOI] [PubMed] [Google Scholar]
- 6.Fugier-Vivier I, Servet-Delprat C, Rivailler P, Rissoan M-C, Liu Y-J, Rabourdin-Combe C. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186:813–823. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grosjean I, Caux C, Bella C, Berger I, Wild F, Banchereau J, Kaiserlian D. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J Exp Med. 1997;186:801–812. doi: 10.1084/jem.186.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollsberg P, Hafler D A. Pathogenesis of diseases induced by human lymphotropic virus type I infection. N Engl J Med. 1993;328:1173–1182. doi: 10.1056/NEJM199304223281608. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson S, Shida H, McFarlin D E, Fauci A S, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature (London) 1990;348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 10.Katahira Y, Yashiki S, Fujiyoshi T, Nomura K, Tara M, Tomita M, Setoyama M, Kanzaki T, Shida H, Sonoda S. In vitro induction of cytotoxic T lymphocytes against HTLV-I-infected T-cells from adult T-cell leukemia patients, asymptomatic HTLV-I carriers and seronegative healthy donors. Jpn J Cancer Res. 1995;86:21–27. doi: 10.1111/j.1349-7006.1995.tb02983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubota R, Fujiyoshi T, Izumo S, Yashiki S, Maruyama I, Osame M, Sonoda S. Fluctuation of HTLV-I proviral DNA in peripheral blood mononuclear cells of HTLV-I-associated myelopathy. J Neuroimmunol. 1993;42:147–154. doi: 10.1016/0165-5728(93)90004-i. [DOI] [PubMed] [Google Scholar]
- 12.Macatonia S E, Cruickshank J K, Rudge P, Knight S C. Dendritic cells from patients with tropical spastic paraparesis are infected with HTLV-I and stimulate autologous lymphocyte proliferation. AIDS Res Hum Retroviruses. 1992;8:1699–1706. doi: 10.1089/aid.1992.8.1699. [DOI] [PubMed] [Google Scholar]
- 13.Makino M, Baba M. Establishment of a cryopreservation method of human peripheral blood mononuclear cells for efficient production of dendritic cells. Scand J Immunol. 1997;45:618–622. doi: 10.1046/j.1365-3083.1997.d01-441.x. [DOI] [PubMed] [Google Scholar]
- 14.Makino M, Ishikawa G, Yamaguchi K, Okada Y, Watanabe K, Sasaki-Iwaki Y, Manabe S, Honda M, Komuro K. Concentration of live retrovirus with a regenerated cellulose hollow fiber, BMM. Arch Virol. 1994;139:87–96. doi: 10.1007/BF01309456. [DOI] [PubMed] [Google Scholar]
- 14a.Makino M, Azuma M, Wakamatsu S, Suruga Y, Izumo S, Yokoyama M M, Baba M. Marked suppression of T cells by a benzothiophene derivative in patients with human T-lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis. Clin Diagn Lab Immunol. 1999;6:316–322. doi: 10.1128/cdli.6.3.316-322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Doherty U, Steinman R M, Peng M, Cameron P U, Gezelter S, Kopeloff I, Swiggard W J, Pope M, Bhardwaj N. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J Exp Med. 1993;178:1067–1078. doi: 10.1084/jem.178.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;i:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 17.Reddy T R, Li X, Jones Y, Ellisman M H, Ching G Y, Liem R K H, Wong-Staal F. Specific interaction of HTLV tax protein and a human type IV neuronal intermediate filament protein. Proc Natl Acad Sci USA. 1998;95:702–707. doi: 10.1073/pnas.95.2.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roake J A, Rao A S, Morris P J, Larsen C P, Hankins D F, Austyn J M. Dendritic cell loss from non-lymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J Exp Med. 1995;181:2237–2247. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch P O, Steinman R M, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate mocromolucules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor a. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takamoto T, Makino M, Azuma M, Kanzaki T, Baba M, Sonoda S. HTLV-I-infected T cells activate autologous CD4+ T cells susceptible to HTLV-I infection in a costimulatory molecule-dependent fashion. Eur J Immunol. 1997;27:1427–1432. doi: 10.1002/eji.1830270620. [DOI] [PubMed] [Google Scholar]
- 23.Umehara F, Izumo S, Nakagawa M, Ronquillo A T, Takahashi K, Matsumuro K, Sato E, Osame M. Immunocytochemical analysis of the cellular infiltrate in the spinal cord lesions in HTLV-I-associated myelopathy. J Neuropathol Exp Neurol. 1993;52:424–430. doi: 10.1097/00005072-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Usuku K, Sonoda S, Osame M, Yashiki S, Takahashi K, Matsumoto M, Sawada T, Tsuji K, Tara M, Igata A. HLA haplotype-linked high immune responsiveness against HTLV-I in HTLV-I-associated myelopathy: Comparison with adult T-cell leukemia/lymphoma. Ann Neurol. 1988;23:S143–S150. doi: 10.1002/ana.410230733. [DOI] [PubMed] [Google Scholar]
- 25.Zucker-Franklin D, Fraig M, Grusky G. Interaction of human immunodeficiency virus type 1, human T-cell leukemia/lymphoma virus type I (HTLV-I), and HTLV-II with in vitro-generated dendritic cells. Clin Diagn Lab Immunol. 1995;2:343–348. doi: 10.1128/cdli.2.3.343-348.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zucker-Franklin D, Gorman J G. HTLV-I provirus in seronegative healthy blood donors. Lancet. 1997;349:999. doi: 10.1016/S0140-6736(05)62896-6. [DOI] [PubMed] [Google Scholar]