Abstract
Background and Aims
Tofacitinib is an oral Janus kinase inhibitor for the treatment of ulcerative colitis (UC). We report an integrated summary of tofacitinib safety from the completed global UC clinical program (9.2 years maximum tofacitinib exposure).
Methods
This analysis included patients receiving tofacitinib 5 or 10 mg twice daily (b.i.d.) from completed phase 2/3 placebo‐controlled studies, an open‐label, long‐term extension study and a randomized phase 3b/4 study. Proportions and incidence rates (IRs; unique patients with events/100 patient‐years [PY] of exposure) were evaluated for deaths and adverse events (AEs) of special interest (AESI).
Results
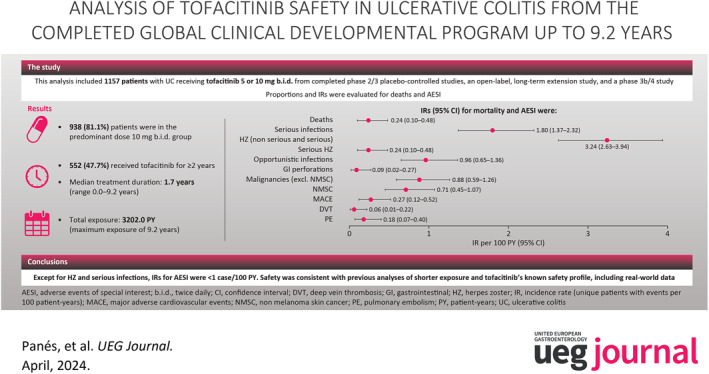
Overall, 1157 patients received ≥1 dose of tofacitinib 5 or 10 mg b.i.d.; 938 (81.1%) were in the predominant dose tofacitinib 10 mg b.i.d. group; 552 (47.7%) received tofacitinib for ≥2 years; total exposure: 3202.0 PY; 994 (85.9%) experienced AEs; 254 (22.0%) experienced serious AEs. Median treatment duration: 1.7 (range 0.0–9.2) years. IRs (95% CI) for combined tofacitinib doses: deaths 0.24 (0.10–0.48); serious infections (SIs) 1.80 (1.37–2.32); herpes zoster (HZ; non‐serious and serious) 3.24 (2.63–3.94); serious HZ 0.24 (0.10–0.48); opportunistic infections 0.96 (0.65–1.36); malignancies (excluding non‐melanoma skin cancer [NMSC]) 0.88 (0.59–1.26); NMSC 0.71 (0.45–1.07); major adverse cardiovascular events 0.27 (0.12–0.52); deep vein thrombosis 0.06 (0.01–0.22); pulmonary embolism 0.18 (0.07–0.40); and gastrointestinal perforations 0.09 (0.02–0.27).
Conclusions
Except for HZ and SIs, IRs for AESI were <1 case/100 PY. Safety was consistent with previous analyses of shorter exposure and tofacitinib's known safety profile, including real‐world data.
ClinicalTrials.gov
NCT00787202; NCT01465763; NCT01458951; NCT01458574; NCT01470612; NCT03281304.
Keywords: Janus kinase inhibitors, safety, tofacitinib, ulcerative colitis
Key summary.
Summarize the established knowledge on this subject.
Long‐term assessment of Janus kinase inhibitor safety in patients with ulcerative colitis (UC) is important to further assess adverse events of special interest (AESI) with long latency periods and/or that are rare.
Tofacitinib's safety in patients with moderately to severely active UC was consistent across two previous integrated analyses (≤7.8 years); with the exception of herpes zoster, the incidence rates (IRs; unique patients with events per 100 patient‐years [PY]) for AESI reported in the previous analysis were stable and generally consistent with the IRs reported for other UC treatments, including biological therapies.
What are the significant and/or new findings of this study?
We report an integrated summary of final tofacitinib safety data from the completed global clinical program (maximum exposure: 9.2 years; total exposure: 3202.0 PY).
Except for herpes zoster and serious infections, IRs for AESI were <1 per 100 PY.
These results demonstrate that the safety profile of tofacitinib in patients with UC remained stable over long periods of exposure (≤9.2 years) and was consistent with the known safety profile of tofacitinib, including real‐world data.
INTRODUCTION
Tofacitinib is an oral Janus kinase (JAK) inhibitor for the treatment of ulcerative colitis (UC). Safety and efficacy of tofacitinib in patients with moderately to severely active UC have previously been evaluated in three phase 2 and phase 3 induction studies, 1 , 2 a phase 3 maintenance study, 2 an open‐label, long‐term extension (OLE) study 3 and a phase 3b/4 study. 4 Tofacitinib's safety was consistent across two previous integrated analyses. 5 , 6
Long‐term assessment of JAK inhibitor safety in patients with UC is particularly important for adverse events (AEs) of special interest (AESI) with long latency periods, such as cardiovascular events and malignancies. 7 , 8 Here, we report an integrated summary of final tofacitinib safety data from the completed global clinical program, with a maximum exposure of 9.2 years (total exposure: 3202.0 patient‐years [PY]).
METHODS
Full details of the methodology, including cohort descriptions (Induction, Maintenance and Overall plus phase 3b/4 [2020]) and term definitions, have been reported previously. 5 , 6
Study designs and analysis cohort
Safety data were pooled from patients with UC who received placebo and/or tofacitinib 5 or 10 mg twice daily (b.i.d.) in six studies: four randomized, placebo‐controlled phase 2/3 induction and maintenance studies (NCT00787202; NCT01465763 [OCTAVE Induction 1]; NCT01458951 [OCTAVE Induction 2]; NCT01458574 [OCTAVE Sustain]), an OLE study (NCT01470612 [OCTAVE Open]) and a randomized phase 3b/4 study (NCT03281304 [RIVETING]). 1 , 2 , 3 , 4
A previously published safety analysis included interim data from the phase 3b/4 study. In comparison, the cohort reported here contained final data from the phase 3b/4 study (as of April 18, 2022; maximum tofacitinib exposure 9.2 years; hereafter defined as Overall Cohort) (Figure S1).
Assessment of safety
The proportion of patients with AEs, serious AEs (SAEs) and deaths was calculated. AESI included: serious infections (SIs), herpes zoster (HZ), opportunistic infections (OIs), malignancies (excluding non‐melanoma skin cancer [NMSC]), major adverse cardiovascular events (MACE), deep vein thrombosis (DVT), pulmonary embolism (PE), and gastrointestinal perforations.
Statistical analysis
The Overall Cohort was categorized based on the predominant dose (PD) of tofacitinib. PD tofacitinib 5 and 10 mg b.i.d. were defined as an average total daily dose of tofacitinib <15 mg and ≥15 mg, respectively. Proportions and incidence rates (IRs; unique patients with events per 100 PY of exposure) with 95% confidence intervals (CI) were calculated for deaths and AESI in all cohorts using the exact Poisson method.
RESULTS
Patient demographics and disease characteristics
Patient demographics and disease characteristics have been reported previously and were generally similar across treatment groups (Table 1). 2 , 5 The Overall Cohort included 1157 patients. In total, 938 (81.1%) patients were in the PD tofacitinib 10 mg b.i.d. group, of whom 586/938 (62.5%) remained on a constant dose of tofacitinib 10 mg b.i.d (total exposure 928.8 PY). The remaining 219 patients were in the PD tofacitinib 5 mg b.i.d. group, of whom 13 (5.9%) remained on a constant 5 mg b.i.d dose (total exposure; 41.1 PY). Median treatment duration was 1.7 years (mean 2.8, range 0.0–9.2, total exposure 3202.0 PY); 552 (47.7%) patients received tofacitinib for ≥2 years.
TABLE 1.
Baseline demographics and disease characteristics in the tofacitinib UC clinical program by cohort.
| Induction Cohort (8 weeks) | Maintenance Cohort (52 weeks) | Overall plus phase 3b/4 [2020] Cohort (≤7.8 years) | Overall Cohort (≤9.2 years) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (N = 282; 44.8 PY) | Tofacitinib 10 mg b.i.d. (N = 938; 156.2 PY) | Placebo (N = 198; 100.4 PY) | Tofacitinib 5 mg b.i.d. (N = 198; 146.2 PY) | Tofacitinib 10 mg b.i.d. (N = 196; 154.3 PY) | PD tofacitinib 5 mg b.i.d. (N = 202; 783.1 PY) | PD tofacitinib 10 mg b.i.d. (N = 955; 2216.6 PY) | Tofacitinib all (N = 1157; 2999.7 PY) | PD tofacitinib 5 mg b.i.d. (N = 219; 902.3 PY) | PD tofacitinib 10 mg b.i.d. (N = 938; 2299.7 PY) | Tofacitinib all (N = 1157; 3202.0 PY) | |
| Age (years), mean (SD) a | 41.4 (14.4) | 41.3 (13.8) | 43.4 (14.0) | 41.9 (13.7) | 43.0 (14.4) | 44.5 (14.5) | 40.6 (13.7) | 41.3 (13.9) | 44.0 (14.4) | 40.6 (13.7) | 41.3 (13.9) |
| Total Mayo score, mean (SD) b , c | 8.9 (1.5) | 9.0 (1.5) | 3.3 (1.8) | 3.3 (1.8) | 3.4 (1.8) | 7.8 (2.5) | 8.8 (1.8) | 8.6 (2.0) | 7.9 (2.4) | 8.8 (1.8) | 8.6 (2.0) |
| Disease duration (years), mean (SD) b | 8.2 (6.8) | 8.2 (7.0) | 8.8 (7.5) | 8.3 (7.2) | 8.7 (7.0) | 8.3 (6.5) | 8.2 (7.1) | 8.2 (7.0) | 8.3 (6.6) | 8.2 (7.1) | 8.2 (7.0) |
| Prior TNFi failure, n (%) d | 124 (53.0) | 465 (51.4) | 89 (44.9) | 83 (41.9) | 92 (46.9) | 84 (41.6) | 499 (54.1) | 583 (51.9) | 93 (42.5) | 490 (54.1) | 583 (51.9) |
| Corticosteroid use at baseline, n (%) b | 127 (45.0) | 430 (45.8) | 100 (50.5) | 101 (51.0) | 86 (43.9) | 82 (40.6) | 441 (46.2) | 523 (45.2) | 89 (40.6) | 434 (46.3) | 523 (45.2) |
Note: Data for the Induction, Maintenance and Overall plus phase 3b/4 [2020] Cohorts have been reported previously. 5 , 6 Briefly, the Induction Cohort comprised patients who received placebo or tofacitinib 10 mg b.i.d. for 8 weeks in phase 2/3 induction studies 5 , 6 ; the Maintenance Cohort comprised patients who received placebo or tofacitinib 5 or 10 mg b.i.d. for 52 weeks in OCTAVE Sustain 5 , 6 ; and the Overall plus phase 3b/4 [2020] Cohort comprised patients who received ≥1 dose of tofacitinib 5 or 10 mg b.i.d. in any phase 2/3/OLE study plus a 6‐month interim analysis of the phase 3b/4 study (data cut‐off: 20 February 2020; tofacitinib exposure ≤7.8 years). 5 The Overall Cohort includes final data from the phase 3b/4 data as of April 18, 2022 (≤9.2 years of exposure). The phase 2/3 induction studies initially included groups that received tofacitinib 15 mg b.i.d.; however, further exploration of this dose was discontinued, and only those patients who received tofacitinib 15 mg b.i.d. induction treatment and subsequently received ≥1 dose of tofacitinib 5 or 10 mg b.i.d. in phase 3 maintenance or OLE studies were included in the Overall Cohort. 6 The decision to discontinue tofacitinib 15 mg b.i.d. was not based on new safety data or a new interpretation of existing safety data, it was based on feedback received from regulatory authorities for rheumatoid arthritis. 2 Exact Poisson distribution was used to calculate IR 95% CI.
Abbreviations: AE, adverse event; AESI, adverse events of special interest; b.i.d., twice daily; CI, confidence interval; DVT, deep vein thrombosis; GI, gastrointestinal; HZ, herpes zoster; IR, incidence rate (unique patients with events per 100 patient‐years of exposure); MACE, major adverse cardiovascular events; N, number of patients treated in the treatment group; n, number of unique patients with a particular adverse event; NMSC, non‐melanoma skin cancer; OI, opportunistic infection; OLE, open‐label; long‐term extension; PD, predominant dose; PE, pulmonary embolism; PY, patient‐years; SD, standard deviation; SI, serious infection; TNFi, tumor necrosis factor inhibitor; UC, ulcerative colitis; VTE, venous thromboembolic event.
Data are from screening of phase 3 induction studies (OCTAVE Induction 1&2; NCT01465763 and NCT01458951, respectively) for the Induction and Maintenance Cohorts, and from Day 1 (start of active treatment in the tofacitinib UC clinical program) for the Overall plus phase 3b/4 [2020] and Overall Cohorts.
Data are from baseline of the phase 3 maintenance study (OCTAVE Sustain) for the Maintenance Cohort, and from Day 1 (start of active treatment in the tofacitinib UC clinical program) for the Overall Cohort.
For the Overall plus phase 3b/4 [2020] Cohort, N = 953 and N = 1155 for the PD tofacitinib 10 mg b.i.d. and tofacitinib all groups, respectively; and for the Overall Cohort, N = 936 and N = 1155 for the PD tofacitinib 10 mg b.i.d. and tofacitinib all groups, respectively.
Data are from baseline of OCTAVE Induction 1&2; for the Overall plus phase 3b/4 [2020] Cohort, N = 922 and N = 1124 for the PD tofacitinib 10 mg b.i.d. and tofacitinib all groups, respectively; for the Overall Cohort, N = 905 and N = 1124 for the PD tofacitinib 10 mg b.i.d. and tofacitinib all groups, respectively (excludes phase 2).
AEs, SAEs and deaths in the Overall Cohort
In total, 994/1157 (85.9%) patients experienced AEs and 254/1157 (22.0%) experienced SAEs (Table S1).
Eight deaths were reported (IR [95% CI], 0.24 [0.10–0.48]; one case each of cardiac arrest [patient also had malignant lung cancer], aortic dissection, hepatic angiosarcoma, acute myeloid leukemia, PE [patient also had cholangiocarcinoma metastasized to the peritoneum], malignant melanoma, metastatic adenocarcinoma and COVID‐19 pneumonia/respiratory failure), all in patients in the PD tofacitinib 10 mg b.i.d. group (Table S1; Figure 1a).
FIGURE 1.
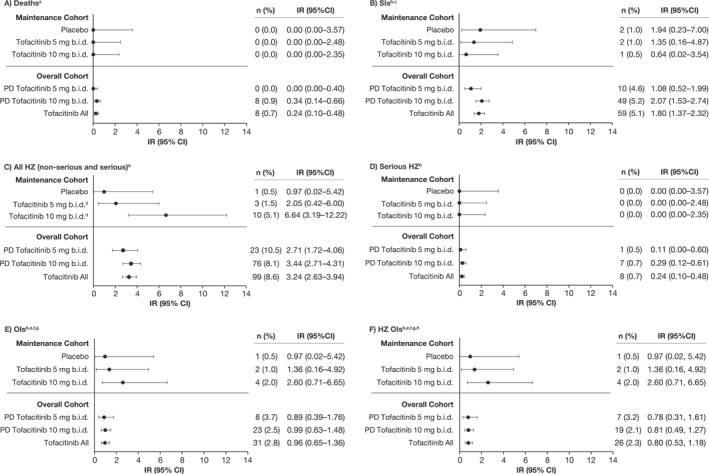
IRs (unique patients with events per 100 PY of exposure) for (a) deaths, (b) SIs, (c) all HZ (non‐serious and serious), (d) serious HZ, (e) OIs, and (f) HZ OIs in the tofacitinib UC clinical program, by cohort. Data for the Maintenance Cohort have been reported previously. 5 , 6 Briefly, the Maintenance Cohort comprised patients who received placebo or tofacitinib 5 or 10 mg b.i.d. for 52 weeks in OCTAVE Sustain 5 , 6 ; and the Overall Cohort comprised patients who received ≥1 dose of tofacitinib 5 or 10 mg b.i.d. in any phase 2/3/OLE study plus final data from the phase 3b/4 data as of 18 April 2022 (≤9.2 years of exposure). Exact Poisson distribution was used to calculate IR 95% CI. AE, adverse event; b.i.d., twice daily; CI, confidence interval; HZ, herpes zoster; IR, incidence rate (unique patients with events per 100 PY of exposure); N, number of patients treated in the treatment group; n, number of unique patients with a particular adverse event; OI, opportunistic infection; OLE, open‐label, long‐term extension; PD, predominant dose; SI, serious infection; PY, patient‐years; UC, ulcerative colitis. aFor the Maintenance Cohorts, events that occurred >28 days after the last dose of the study drug were excluded; for the Overall Cohort, events outside the 28‐day risk period were included. bEvents that occurred >28 days after the last dose of the study drug were excluded. cDefined as any infection AE that required hospitalization or parenteral antimicrobials or met other criteria that required the infection to be classified as a serious AE. dIRs of HZ in the Maintenance Cohort were numerically higher with tofacitinib 5 mg b.i.d. versus placebo and statistically higher with tofacitinib 10 mg b.i.d. versus placebo. eData are from baseline of OCTAVE Induction 1&2; for the Overall Cohort, N = 905 and N = 1124 for the PD tofacitinib 10 mg b.i.d. and tofacitinib all groups, respectively (excludes phase 2). fAdjudicated events. gExcludes tuberculosis and HZ with two adjacent dermatomes. hDisseminated or multidermatomal HZ events were considered to be HZ OIs.
Incidence of AESI in the Overall Cohort
In total, SIs were reported in 59 patients (IR [95% CI], 1.80 [1.37–2.32]), 10 in the PD tofacitinib 5 mg b.i.d. group and 49 in the PD tofacitinib 10 mg b.i.d. group (IRs [95% CI], 1.08 [0.52–1.99] and 2.07 [1.53–2.74], respectively) (Table S1; Figure 1b).
HZ events were reported in 99 patients (IR [95% CI], 3.24 [2.63–3.94]), 23 in the PD tofacitinib 5 mg b.i.d. group and 76 in the PD tofacitinib 10 mg b.i.d. group (IRs [95% CI], 2.71 [1.72–4.06] and 3.44 [2.71–4.31], respectively) (Table S1; Figure 1c). In total, 7/1157 (0.6%) patients had recurrent HZ events. The overall IR for HZ OIs was 0.80 (95% CI 0.53–1.18).
Malignancies (excluding NMSC) were reported in 29 patients (IR [95% CI], 0.88 [0.59–1.26]), five in the PD tofacitinib 5 mg b.i.d. group and 24 in the PD tofacitinib 10 mg b.i.d. group (IRs [95% CI], 0.54 [0.18–1.26] and 1.01 [0.65–1.51], respectively) (Table S1; Figure 2a). Malignancy events (excluding NMSC) included: one case each of acute myeloid leukemia, Bowen's disease, Epstein‐Barr virus–associated lymphoma, essential thrombocythemia, hepatic angiosarcoma, leiomyosarcoma, esophageal adenocarcinoma, penile dysplasia, renal cell carcinoma, vulvar cancer, prostate cancer; two cases each of cervical dysplasia, cholangiocarcinoma, diffuse large B‐cell lymphoma, lung cancer, malignant melanoma; three cases of breast cancer; and five cases of colorectal cancer.
FIGURE 2.
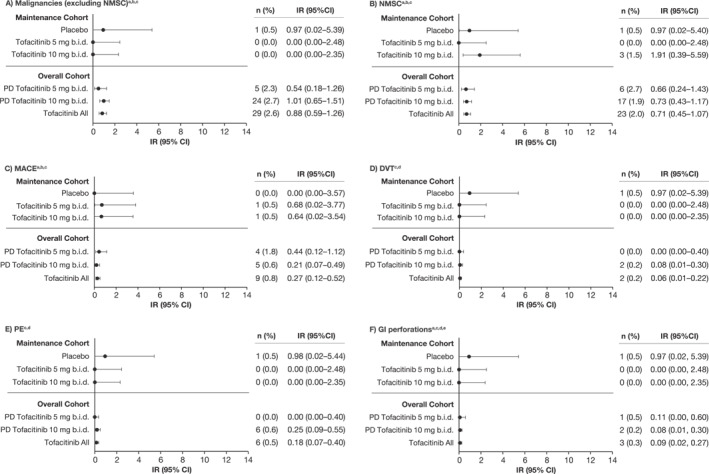
IRs (unique patients with events per 100 PY of exposure) for (a) malignancies (excluding NMSC), (b) NMSC, (c) MACE, (d) DVT, (e) PE, and (f) GI perforations in the tofacitinib UC clinical program, by cohort. Data for the Maintenance Cohort have been reported previously. 5 , 6 Briefly, the Maintenance Cohort comprised patients who received placebo or tofacitinib 5 or 10 mg b.i.d. for 52 weeks in OCTAVE Sustain 5 , 6 ; and the Overall Cohort comprised patients who received ≥1 dose of tofacitinib 5 or 10 mg b.i.d. in any phase 2/3/OLE study plus final data from the phase 3b/4 data as of 18 April 2022 (≤9.2 years of exposure). Exact Poisson distribution was used to calculate IR 95% CI. b.i.d., twice daily; CI, confidence interval; DVT, deep vein thrombosis; GI, gastrointestinal; IR, incidence rate (unique patients with events per 100 PY of exposure); MACE, major adverse cardiovascular events; N, number of patients treated in the treatment group; n, number of unique patients with a particular adverse event; NMSC, non‐melanoma skin cancer; OLE, open‐label, long‐term extension; PD, predominant dose; PE, pulmonary embolism; PY, patient‐years; UC, ulcerative colitis.a for the Overall Cohort, N = 905 and N = 1124 for the PD tofacitinib 10 mg b.i.d. and tofacitinib all groups, respectively (excludes phase 2). bFor the Maintenance Cohort, events that occurred >28 days after the last dose of the study drug were excluded; for the Overall Cohort, events outside the 28‐day risk period were included. cAdjudicated events. dEvents that occurred >28 days after the last dose of the study drug were excluded. eGI perforation excludes Preferred Terms of pilonidal cyst, perirectal abscess, rectal abscess, anal abscess, perineal abscess and any Preferred Terms containing the term fistula.
NMSCs were reported in 23 patients (IR [95% CI], 0.71 [0.45–1.07]), six in the PD tofacitinib 5 mg b.i.d. group and 17 in the PD tofacitinib 10 mg b.i.d. group (IRs [95% CI], 0.66 [0.24–1.43] and 0.73 [0.43–1.17], respectively) (Table S1; Figure 2b). There were 17 patients with basal cell carcinomas and 11 with squamous cell carcinomas.
MACE were reported in nine patients (IR [95% CI], 0.27 [0.12–0.52]), four in the PD tofacitinib 5 mg b.i.d. group and five in the PD tofacitinib 10 mg b.i.d. group (IRs [95% CI], 0.44 [0.12–1.12] and 0.21 [0.07–0.49], respectively) (Table S1; Figure 2c). These events included one case each of hemorrhagic stroke, aortic dissection, cardiac arrest, acute coronary syndrome, acute myocardial infarction, cerebellar hemorrhage and myocardial infarction, and two cases of a cerebrovascular accident.
The venous thromboembolic (VTE) event IR was 0.24 (95% CI 0.10–0.48). DVT events were reported in two patients (IR [95% CI], 0.06 [0.01–0.22]; one event was assessed as related to the study drug by the investigator) and PE events were reported in six patients (IR [95% CI], 0.18 [0.07–0.40]) 5 ; all were in the PD tofacitinib 10 mg b.i.d. group (Table S1; Figure 2d,e). Of the six patients with PE events, four had a history of risk factors and two did not. Both patients without a notable history had low disease activity prior to the PE event (partial Mayo scores prior to event: 1 and 0, respectively).
DISCUSSION
This integrated tofacitinib safety analysis of the completed global clinical program included data with a maximum exposure of 9.2 years (total exposure: 3202.0 PY) and represents the longest follow‐up of randomized patients with UC treated with an approved or investigational therapy to date. Longer follow‐ups are important to accurately evaluate AEs with long latency periods and/or that are rare.
The Overall Cohort included an additional 202.3 PY of exposure versus the previously reported Overall plus phase 3b/4 [2020] Cohort, and AESI IRs were consistent between the two cohorts. 5
The HZ IR was 3.24 (95% CI 2.63–3.94). In comparison, a lower HZ IR of 0.5 (95% CI 0.1–1.9) was reported in a Canadian real‐world study (REMIT‐UC) of 334 tofacitinib‐treated patients with UC. Authors hypothesized this was mainly due to protective effects of HZ vaccination given the high proportion (198/230 [86.1%]) of vaccinated patients in their study. 9 There were potentially more Asian patients in this analysis than REMIT‐UC; increased HZ rates were reported in Asian versus non‐Asian patients. 10 In a study of claims data from the United States (US) by Curtis and colleagues, in which key clinical trial‐like criteria similar to those used in the phase 3 OCTAVE clinical trials were applied to create a population‐based UC trial‐like cohort treated with tumor necrosis factor inhibitors (TNFi), a numerically lower HZ IR of 1.77 (95% CI 1.34–2.29) was reported. 11
In the current analysis, malignancies were reported in 29 patients (excluding NMSC) (IR [95% CI], 0.88 [0.59–1.26]). Real‐world studies have observed few or no malignancies in tofacitinib‐treated patients with UC, 12 , 13 , 14 likely attributable to their relatively short follow‐up times. In comparison, the malignancy IR in REMIT‐UC (total exposure 375 PY) was 0.8 (95% CI 0.2–2.3), 9 consistent with the malignancy IR observed in this analysis. The NMSC IR in this analysis (0.71 [95% CI 0.45–1.07]) was higher than that observed in a larger 3‐year real‐world study of 408 tofacitinib‐treated patients in Spain by Chaparro and colleagues (0.47 [95% CI 0.11–1.87]). 15 In the population‐based UC TNFi‐treated cohort analyzed by Curtis and colleagues, a numerically lower malignancies (excluding NMSC) IR (0.63 [95% CI 0.43–0.90]) and numerically higher NMSC IR (1.69 [95% CI 1.35–2.10]) were reported versus this study. 11
MACE was reported in nine patients, and the MACE IR was 0.27 (95% CI 0.12–0.52). This was consistent with the IR reported by Chaparro and colleagues (IR [95% CI], 0.23 [0.03–1.66]). 15 No real‐world studies of patients with UC have been conducted using the definition of MACE utilized here. In a real‐world study of patients with moderate to severe inflammatory bowel disease in the US, IR per 1000 PY (95% CI) of stroke and acute myocardial infarction were 4.63 (3.36–6.21) and 2.91 (1.94–4.21), respectively. 16 In addition, the MACE IR (0.51 [95% CI 0.31–0.79]) reported by Curtis and colleagues in a population‐based UC TNFi‐treated cohort was numerically higher than that reported here. 11 Of note, these studies were conducted in patients in the US, where approximately half of the population between 2017 and 2020 had some form of cardiovascular disease. 17 The majority of patients who experienced a MACE in the OCTAVE clinical program had an intermediate–high baseline 10‐year risk of atherosclerotic cardiovascular disease. 18 Similar results were reported in tofacitinib‐treated patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. 19
Two DVT and six PE events were reported (IRs [95% CI], 0.06 [0.01–0.22] and 0.18 [0.07–0.40], respectively; VTE IR [95% CI], 0.24 [0.10–0.48]). In real‐world studies, the proportion of patients experiencing VTE events ranged from 0% to 5% 9 , 12 , 13 , 14 , 20 , 21 , 22 , 23 and IRs (1.1 and 1.3) were numerically higher than those reported here. 9 , 13 In addition, IRs for DVT (1.41 [95% CI 1.00–1.93]) and PE (0.54 [95% CI 0.30–0.89]) were numerically higher in the population‐based UC TNFi‐treated cohort analyzed by Curtis and colleagues versus those reported here. 11 It is possible that patients in a real‐world setting are more refractory to treatment and have less disease control than patients in clinical trial cohorts. In addition, VTE events in this analysis were reviewed by an adjudication committee, which is expected to result in a more robust confirmation of diagnosis than is generally performed in analyses of real‐world data. In comparison, the IRs in this analysis were consistent with those observed for the general UC population (DVT IR 0.07–0.30 and PE IR 0.04–0.20, respectively). 24 , 25 , 26
AESI IRs were generally similar across treatment groups, including placebo during Induction and Maintenance, with the exception of HZ as previously reported. 5 Dose dependency of IRs in the two Overall Cohorts could not be fully evaluated, as discussed previously alongside further limitations of the analysis. 5 As discussed above, a limitation of contextualization of these results with data from real‐world studies is that some AESIs are likely to be under reported in real‐world studies due to less robust confirmation and/or identification versus clinical trials.
In conclusion, IRs for AESI were consistent with prior safety analyses (≤7.8 years) and the known safety profile of tofacitinib, including real‐world data.
AUTHOR CONTRIBUTIONS
Julian Panés: Conceptualization (equal); visualization (equal); writing – original draft (equal); writing – review & editing (equal). Geert R. D’Haens: Conceptualization (equal); visualization (equal); writing – original draft (equal); writing – review & editing (equal). Bruce E. Sands: Conceptualization (equal); visualization (equal); writing – original draft (equal); writing – review & editing (equal). Siew C. Ng: Conceptualization (equal); visualization (equal); writing – original draft (equal); writing – review & editing (equal). Nervin Lawendy: Conceptualization (equal); visualization (equal); writing – original draft (equal); writing – review & editing (equal). Nicole Kulisek: Conceptualization (equal); visualization (equal); writing – original draft (equal); writing – review & editing (equal). Xiang Guo: Conceptualization (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review & editing (equal). Joseph Wu: Conceptualization (equal); formal analysis (lead); validation (equal); visualization (equal); writing – original draft (equal); writing – review & editing (equal). Ivana Vranic: Conceptualization (equal); visualization (equal); writing – original draft (equal); writing – review & editing (equal). Remo Panaccione: Conceptualization (equal); visualization (equal); writing – original draft (equal); writing – review & editing (equal). Séverine Vermeire: Conceptualization (equal); visualization (equal); writing – original draft (equal); writing – review & editing (equal). All authors approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
Julian Panés has received grants and/or research support from AbbVie and Pfizer Inc, is a member of the speakers' bureau for Abbott, Ferring, Janssen, Pfizer Inc and Takeda; has been an advisory board member for AbbVie, Arena, Athos, Atomwise, Boehringer Ingelheim, Celgene, Celsius, Celltrion, Ferring, Galapagos, Genentech/Roche, GlaxoSmithKline, Janssen, Mirum, Morphic, Nestle, Origo, Pandion, Pfizer Inc, Progenity, Prometheus, Protagonist, Revolo, Robarts, Sanofi, Takeda, Theravance and Wasserman; and has developed educational presentations for AbbVie, Janssen, Pfizer Inc, Roche and Takeda. Geert R. D’Haens has received speaker fees from AbbVie, Boehringer Ingelheim, Celltrion, Johnson & Johnson, Pfizer Inc, Takeda and Tillotts; and has been an advisory board member for AbbVie, Alimentiv, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Cosmo, Eli Lilly, Galapagos, GlaxoSmithKline, Johnson & Johnson, Takeda, Pfizer Inc, Polpharma, Prometheus, Tillotts and Ventyx. Bruce E. Sands has acted as a consultant for, or received speaker fees from, AbbVie, Abivax, Alimentiv, Amgen, Arena Pharmaceuticals, Artugen Therapeutics, AstraZeneca, Boehringer Ingelheim, Boston Pharmaceuticals, Calibr, Celgene, Celltrion, ClostraBio, Equillium, Enthera, Evommune, Fresenius Kabi, Galapagos, Genentech (Roche), Gilead Sciences, GlaxoSmithKline, Gossamer Bio, Index Pharmaceuticals, Innovation Pharmaceuticals, Inotrem, Kaleido, Kallyope, Eli Lilly, Merck, Morphic Therapeutics, MRM Health, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Q32 Bio, Sun Pharma, Surrozen, Target RWE, Teva, TLL Pharmaceutical and Ventyx Biosciences; has received research grants, consulting and/or speaking fees or other support from Bristol Myers Squibb, Janssen, Pfizer Inc, Takeda and Theravance Biopharma; and is a shareholder of Ventyx Biopharma. Siew C. Ng has received grants and/or research support from AbbVie, Ferring, Janssen and Olympus; has received speaker fees from AbbVie, Ferring, Janssen, Menarini, Pfizer Inc, Takeda and Tillotts; has been an advisory board member for AbbVie, Ferring, Janssen and Pfizer Inc; holds a Directorship with Microbiota I Center; and is the scientific co‐founder and a shareholder of GenieBiome Limited. Nervin Lawendy, Nicole Kulisek, Xiang Guo, Joseph Wu and Ivana Vranic are employees and shareholders of Pfizer Inc. Remo Panaccione has received grants and/or research support from AbbVie, Janssen, Pfizer Inc and Takeda; and has acted as a consultant for and/or received speaker fees from Abbott, AbbVie, Alimentiv, Amgen, Arena, AstraZeneca, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Cosmos, Eisai, Elan, Eli Lily, Ferring, Galapagos, Fresenius Kabi, Genentech, Gilead Sciences, GlaxoSmithKline, JAMP Bio, Janssen, Merck, Mylan, Novartis, Oppilan Pharma, Organon, Pandion, Pendopharm, Pfizer Inc, Progenity, Prometheus, Protagonist, Roche, Sandoz, Satisfai Health, Shire, Sublimity, Takeda, Theravance, Trellus, Viatris and Ventyx. Séverine Vermeire has received grants from AbbVie, Galapagos, MSD, Pfizer Inc and Takeda; has acted as a consultant for AbbVie, AbolerIS Pharma, Alimentiv, Arena, AstraZeneca, Avaxia, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, CVasThera, Dr Falk Pharma, Eli Lilly, Ferring, Galapagos, Genentech/Roche, Gilead Sciences, Hospira, IMIDomics, Janssen, Johnson & Johnson, Materia Prima, MiroBio, Morphic, MRM Health, MSD, Mundipharma, Pfizer Inc, Pro Digest, Progenity, Prometheus, Robarts, Second Genome, Shire, Surrozen, Takeda, Theravance, Tillotts and Zealand; and has received speaker fees from AbbVie, Dr Falk Pharma, Ferring, Hospira, MSD, Takeda and Tillotts.
ETHICS APPROVAL
All studies were conducted in accordance with the Declaration of Helsinki, International Council for Harmonisation Guidelines for Good Clinical Practice and local regulations. Participating institutions provided Institutional Review Board approval prior to participation.
PATIENT CONSENT STATEMENT
All patients provided written informed consent.
CLINICAL TRIAL REGISTRATION
All included trials were registered as required as described in the respective original publications.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
The authors would like to thank the patients, investigators and study teams who were involved in the tofacitinib UC clinical program. This study was sponsored by Pfizer. Medical writing support, under the direction of the authors, was provided by Sarah Leneghan, PhD, CMC Connect, a division of IPG Health Medical Communications, and was funded by Pfizer, New York, NY, USA, in accordance with Good Publication Practice (GPP 2022) guidelines (Ann Intern Med. 2022;175:1298–1304).
Panés J, D’Haens GR, Sands BE, Ng SC, Lawendy N, Kulisek N, et al. Analysis of tofacitinib safety in ulcerative colitis from the completed global clinical developmental program up to 9.2 years of drug exposure. United European Gastroenterol J. 2024;12(6):793–801. 10.1002/ueg2.12584
DATA AVAILABILITY STATEMENT
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de‐identified participant data. See https://www.pfizer.com/science/clinical‐trials/trial‐data‐and‐results for more information.
REFERENCES
- 1. Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367(7):616–624. 10.1056/nejmoa1112168 [DOI] [PubMed] [Google Scholar]
- 2. Sandborn WJ, Su C, Sands BE, D'Haens GR, Vermeire S, Schreiber S, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723–1736. 10.1056/nejmoa1606910 [DOI] [PubMed] [Google Scholar]
- 3. Sandborn WJ, Lawendy N, Danese S, Su C, Loftus EV Jr, Hart A, et al. Safety and efficacy of tofacitinib for treatment of ulcerative colitis: final analysis of OCTAVE Open, an open‐label, long‐term extension study with up to 7.0 years of treatment. Aliment Pharmacol Ther. 2022;55(4):464–478. 10.1111/apt.16712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vermeire S, Su C, Lawendy N, Kobayashi T, Sandborn WJ, Rubin DT, et al. Outcomes of tofacitinib dose reduction in patients with ulcerative colitis in stable remission from the randomised RIVETING trial. J Crohns Colitis. 2021;15(7):1130–1141. 10.1093/ecco-jcc/jjaa249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sandborn WJ, D'Haens GR, Sands BE, Pannaccione R, Ng SC, Lawendy N, et al. Tofacitinib for the treatment of ulcerative colitis: an integrated summary of up to 7.8 years of safety data from the global clinical programme. J Crohns Colitis. 2023;17(3):338–351. 10.1093/ecco-jcc/jjac141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sandborn WJ, Panes J, D'Haens GR, Sands BE, Su C, Moscariello M, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol. 2019;17(8):1541–1550. 10.1016/j.cgh.2018.11.035 [DOI] [PubMed] [Google Scholar]
- 7. Faye AS, Holmer AK, Axelrad JE. Cancer in inflammatory bowel disease. Gastroenterol Clin North Am. 2022;51(3):649–666. 10.1016/j.gtc.2022.05.003 [DOI] [PubMed] [Google Scholar]
- 8. Bunu D‐M, Timofte C‐E, Ciocoiu M, Floria M, Tarniceriu C‐C, Barboi O‐B, et al. Cardiovascular manifestations of inflammatory bowel disease: pathogenesis, diagnosis, and preventive strategies. Gastroenterol Res Pract. 2019;2019:3012509. 10.1155/2019/3012509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ma C, Panaccione R, Xiao Y, Khandelwal Y, Murthy SK, Wong ECL, et al. REMIT‐UC: real‐world effectiveness and safety of tofacitinib for moderate‐to‐severely active ulcerative colitis: a Canadian IBD Research Consortium multicenter national cohort study. Am J Gastroenterol. 2023;118(5):861–871. 10.14309/ajg.0000000000002129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Winthrop KL, Melmed GY, Vermeire S, Long MD, Chan G, Pedersen RD, et al. Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis. 2018;24(10):2258–2265. 10.1093/ibd/izy131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Curtis JR, Regueiro M, Yun H, Su C, DiBonaventura M, Lawendy N, et al. Tofacitinib treatment safety in moderate to severe ulcerative colitis: comparison of observational population cohort data from the IBM MarketScan® administrative claims database with tofacitinib trial data. Inflamm Bowel Dis. 2021;27(9):1394–1408. 10.1093/ibd/izaa289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Honap S, Chee D, Chapman TP, Patel M, Kent AJ, Ray S, et al. Real‐world effectiveness of tofacitinib for moderate to severe ulcerative colitis: a multicentre UK experience. J Crohns Colitis. 2020;14(10):1385–1393. 10.1093/ecco-jcc/jjaa075 [DOI] [PubMed] [Google Scholar]
- 13. Deepak P, Alayo QA, Khatiwada A, Lin B, Fenster M, Dimopoulos C, et al. Safety of tofacitinib in a real‐world cohort of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2021;19(8):1592–1601.e1593. 10.1016/j.cgh.2020.06.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chaparro M, Garre A, Mesonero F, Rodríguez C, Barreiro‐de Acosta M, Martínez‐Cadilla J, et al. Tofacitinib in ulcerative colitis: real‐world evidence from the ENEIDA registry. J Crohns Colitis. 2021;15(1):35–42. 10.1093/ecco-jcc/jjaa145 [DOI] [PubMed] [Google Scholar]
- 15. Chaparro M, Acosta D, Rodríguez C, Mesonero F, Vicuña M, Barreiro‐de Acosta M, et al. Real‐world evidence of tofacinitib in ulcerative colitis: short‐term and long‐term effectiveness and safety. Am J Gastroenterol. 2023;118(7):1237–1247. 10.14309/ajg.0000000000002145 [DOI] [PubMed] [Google Scholar]
- 16. McAuliffe ME, Lanes S, Leach T, Parikh A, Faich G, Porter J, et al. Occurrence of adverse events among patients with inflammatory bowel disease in the HealthCore Integrated Research Database. Curr Med Res Opin. 2015;31(9):1655–1664. 10.1185/03007995.2015.1065242 [DOI] [PubMed] [Google Scholar]
- 17. American Heart Association . 2024 heart disease and stroke statistics update fact sheet: at‐a‐glance. 2024. https://www.heart.org/‐/media/PHD‐Files‐2/Science‐News/2/2024‐Heart‐and‐Stroke‐Stat‐Update/2024‐Statistics‐At‐A‐Glance‐final_2024.pdf. Accessed 13 February 2024.
- 18. Schreiber S, Rubin DT, Ng SC, Peyrin‐Biroulet L, Danese S, Modesto I, et al. Major adverse cardiovascular events by baseline cardiovascular risk in patients with ulcerative colitis treated with tofacitinib: data from the OCTAVE clinical programme. J Crohns Colitis. 2023;17(11):1761–1770. 10.1093/ecco-jcc/jjad104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kristensen LE, Strober B, Poddubnyy D, Leung Y‐Y, Jo H, Kwok K, et al. Association between baseline cardiovascular risk and incidence rates of major adverse cardiovascular events and malignancies in patients with psoriatic arthritis and psoriasis receiving tofacitinib. Ther Adv Musculoskelet Dis. 2023;15:1759720X221149965. 10.1177/1759720x221149965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Biemans VBC, Sleutjes JAM, de Vries AC, Bodelier AGL, Dijkstra G, Oldenburg B, et al. Tofacitinib for ulcerative colitis: results of the prospective Dutch Initiative on Crohn and Colitis (ICC) registry. Aliment Pharmacol Ther. 2020;51(9):880–888. 10.1111/apt.15689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seo GH, Jung SH. The comparative risk of serious adverse events with tofacitinib and TNF inhibitors in patients with ulcerative colitis: the Korean experience as revealed by a national database. J Korean Med Sci 2022;37(16):e123. 10.3346/jkms.2022.37.e123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kochar BD, Cheng D, Cai T, Ananthakrishnan AN. Comparative risk of thrombotic and cardiovascular events with tofacitinib and anti‐TNF agents in patients with inflammatory bowel diseases. Dig Dis Sci. 2022;67(11):5206–5212. 10.1007/s10620-022-07404-z [DOI] [PubMed] [Google Scholar]
- 23. Straatmijer T, van Schaik FDM, Bodelier AGL, Visschedijk M, de Vries AC, Ponsioen CY, et al. Effectiveness and safety of tofacitinib for ulcerative colitis: two‐year results of the ICC registry. Aliment Pharmacol Ther. 2023;57(1):117–126. 10.1111/apt.17248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kappelman MD, Horvath‐Puho E, Sandler RS, Rubin DT, Ullman TA, Pedersen L, et al. Thromboembolic risk among Danish children and adults with inflammatory bowel diseases: a population‐based nationwide study. Gut. 2011;60(7):937–943. 10.1136/gut.2010.228585 [DOI] [PubMed] [Google Scholar]
- 25. Bernstein CN, Blanchard JF, Houston DS, Wajda A. The incidence of deep venous thrombosis and pulmonary embolism among patients with inflammatory bowel disease: a population‐based cohort study. Thromb Haemost. 2001;85(3):430–434. 10.1055/s-0037-1615600 [DOI] [PubMed] [Google Scholar]
- 26. Weng M‐T, Park SH, Matsuoka K, Tung C‐C, Lee JY, Chang C‐H, et al. Incidence and risk factor analysis of thromboembolic events in East Asian patients with inflammatory bowel disease, a multinational collaborative study. Inflamm Bowel Dis. 2018;24(8):1791–1800. 10.1093/ibd/izy058 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de‐identified participant data. See https://www.pfizer.com/science/clinical‐trials/trial‐data‐and‐results for more information.


