Abstract
Objectives
International guidelines recommend resection of suspected localised renal cell carcinoma (RCC), with surgical series showing benign pathology in 30%. Non‐invasive diagnostic tests to differentiate benign from malignant tumours are an unmet need. Our objective was to determine diagnostic accuracy of imaging modalities for detecting cancer in T1 renal tumours.
Methods
A systematic review was performed for reports of diagnostic accuracy of any imaging test compared to a reference standard of histopathology for T1 renal masses, from inception until January 2023. Twenty‐seven publications (including 2277 tumours in 2044 participants) were included in the systematic review, and nine in the meta‐analysis.
Results
Forest plots of sensitivity and specificity were produced for CT (seven records, 1118 participants), contrast‐enhanced ultrasound (seven records, 197 participants), [99mTc]Tc‐sestamibi SPECT/CT (five records, 263 participants), MRI (three records, 220 participants), [18F]FDG PET (four records, 43 participants), [68Ga]Ga‐PSMA‐11 PET (one record, 27 participants) and [111In]In‐girentuximab SPECT/CT (one record, eight participants). Meta‐analysis returned summary estimates of sensitivity and specificity for [99mTc]Tc‐sestamibi SPECT/CT of 88.6% (95% CI 82.7%–92.6%) and 77.0% (95% CI 63.0%–86.9%) and for [18F]FDG PET 53.5% (95% CI 1.6%–98.8%) and 62.5% (95% CI 14.0%–94.5%), respectively. A comparison hierarchical summary receiver operating characteristic (HSROC) model did not converge. Meta‐analysis was not performed for other imaging due to different thresholds for test positivity.
Conclusion
The optimal imaging strategy for T1 renal masses is not clear. [99mTc]Tc‐sestamibi SPECT/CT is an emerging tool, but further studies are required to inform its role in clinical practice. The field would benefit from standardisation of diagnostic thresholds for CT, MRI and contrast‐enhanced ultrasound to facilitate future meta‐analyses.
Keywords: diagnostic accuracy, imaging, renal tumours
1. INTRODUCTION
Increasing use of cross‐sectional imaging has resulted in a rise in detection of incidental renal tumours. Current standard of care for T1 renal tumours, as defined by the Union for International Cancer Control, 1 is surgical resection. 2 However not all renal tumours are cancer, with up to 30% of partial nephrectomy specimens being benign. 3 Partial or radical nephrectomy represents overtreatment of benign renal tumours and can be avoided if the distinction is made accurately before surgery.
Despite high diagnostic accuracy of renal tumour biopsy, it has not been widely adopted due to concerns about bleeding, tumour seeding, non‐diagnostic samples, difficulties in accessing anatomically complex tumours and assessment of only localised areas within the tumour. 4 Diagnostic imaging therefore overcomes several important limitations of biopsy.
A recent descriptive review of novel imaging techniques for renal tumours concluded that [99mTc]Tc‐sestamibi SPECT/CT and radiolabelled girentuximab are the closest to clinical adoption. 5 However, the lack of quantitative analysis of diagnostic accuracy and how they compare to existing imaging techniques limits conclusions that can be drawn from the review.
In order to address the evidence gap, this systematic review was performed to determine and compare the diagnostic accuracy of various imaging modalities for detecting cancer in renal tumours.
2. METHODS
2.1. Protocol and registration
The protocol was developed according to PRISMA‐DTA 6 and principles outlined in the Cochrane Handbook for systematic reviews of diagnostic accuracy v2, 7 and prospectively registered with PROSPERO (CRD42022303473). Protocol deviations are summarised and justified in the protocol.
2.2. Eligibility criteria
Primary research articles evaluating the diagnostic accuracy of any imaging modality to characterise T1 renal tumours as malignant or benign as defined by a histopathological reference standard from surgery or biopsy were included. Prospective and retrospective studies were included. Studies that did not report sufficient diagnostic accuracy data, that is, the number of true and false positives and true and false negatives, were excluded. Studies that included participants with renal tumours of any stage were included if measures for T1 tumours could be extracted separately. Case–control studies were excluded as they are at high risk of bias. Full manuscripts and conference abstracts with sufficient information to meet the inclusion criteria were included.
2.3. Information sources
Comprehensive searches of electronic databases MEDLINE, EMBASE, Science Citation Index, The Cochrane Library, Clinicaltrials.gov and WHO trials register were performed from inception to 12 January 2023.
2.4. Search
Individual search strategies are detailed in Appendix S1. Due to the high number of texts during scoping searches (>40 000) we used a sensitivity‐maximising diagnostic filter to limit the results to a feasible number to review. 8 , 9 No language restrictions were applied.
Returned articles from each database were combined and duplicates removed using systematic review management software Covidence (available at covidence.org).
2.5. Study selection
Titles and abstracts were screened independently by two authors followed by full‐text screening in the same manner (JBF, VM, PI, VWSC, EZ or HW). Disagreements were discussed with a third author to reach consensus. Multiple publications from the same authors and institution with an overlapping recruitment period were managed by excluding the report with the smaller sample size. Reasons for exclusions were recorded. Hand searches of reference lists of included studies were performed to identify additional relevant literature. Non‐English language texts were translated to allow for screening and data extraction.
2.6. Data collection process
Data extraction was carried out independently by two authors from the research team (JBF, VM or PI) using a pre‐prepared and piloted form. Disagreements were reviewed and resolved by a third author (HW). Further information was sought from study authors where necessary.
2.7. Definitions for data extraction
The following data were extracted: study characteristics (authors, year of publication, institution, single or multi‐centre, country, language of publication, study period, study design, number of patients enrolled), patient characteristics (age, gender, ethnicity, number of tumours, lead tumour size, lead tumour volume), index test(s) (modality, manufacturer, model, specific settings, number of interpreters, presence of consensus interpretation, interpreter experience), reference standard(s) (modality, diagnostic criteria, number of interpreters, presence of consensus interpretation, interpreter experience), number of true positives, false positives, true negatives and false negatives. If data from multiple interpreters was presented, the results were averaged or the results from the authors' primary analysis were used. If results were reported at multiple thresholds, the diagnostic accuracy measures at each threshold were collected and the threshold used for the authors' primary analysis was used in our analysis. If studies explicitly stated that they had classified a malignant subtype of renal cell carcinoma (RCC) as benign due to indolent nature, we treated them as malignant in this review.
2.8. Risk of bias and applicability
Risk of bias and applicability concerns were assessed by two independent review authors (JBF, VM, PI) using the QUADAS‐2 tool and QUADAS‐C tools. 10 , 11 QUADAS‐2 and QUADAS‐C tools were customised to be relevant for this review (Appendix S2). Differences were resolved by a third author (HW).
2.9. Diagnostic accuracy measures
Sensitivity and specificity were reported as the principal measures of diagnostic accuracy. The unit of assessment was per lesion.
2.10. Synthesis of results and meta‐analysis
Study estimates of sensitivity and specificity were plotted on forest plots and receiver operating characteristic (ROC) space to explore between‐study variation in performance of each test. For imaging modalities with measures of diagnostic accuracy reported at the same threshold, bivariate analysis was attempted but convergence was not obtained. Therefore, univariate fixed‐effect model (determined by the model fit) was performed to calculate summary point estimates of sensitivity and specificity at that threshold. 12 Comparison of these tests was attempted using a hierarchical summary receiver operating characteristic (HSROC) model, but convergence was not obtained. For imaging modalities reported at different positive thresholds, meta‐analysis was not performed as the result is clinically uninterpretable. 7 When meta‐analysis was not performed, we reported the sensitivity and specificity with 95% confidence intervals from the individual studies, calculated with Review Manager version 5.4.1 (The Cochrane Collaboration, Software Update, Oxford, UK). Statistical analyses were performed with SAS v.9.4. The data and the code used for meta‐analysis are available from Appendix S3.
3. RESULTS
3.1. Study selection
The search identified 5350 unique records following removal of duplicates. Of these, 5065 were excluded on title and abstract screening. An additional 23 references were identified through scanning reference lists of the identified studies, related search function and citing reference search. Of the resulting 308 references, 281 were excluded following full‐text review, with reasons stated in Figure 1. Twenty‐seven studies including 2277 tumours in 2044 patients were included. Nine studies with 314 lesions in 306 participants were included in the meta‐analysis of diagnostic accuracy of [99mTc]Tc‐sestamibi SPECT/CT and [18F]FDG positron emission tomography (PET).
FIGURE 1.
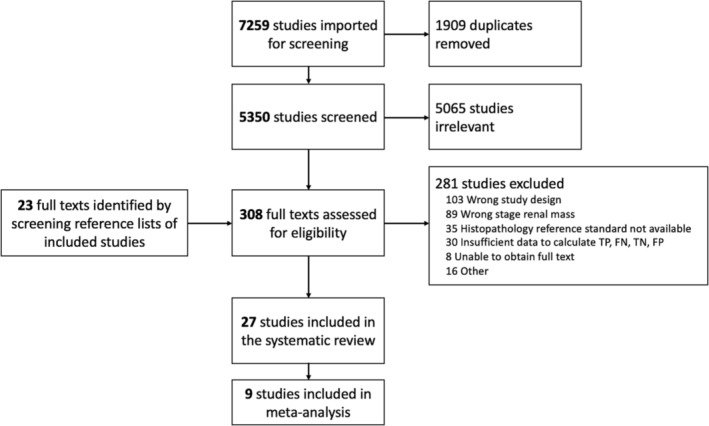
PRISMA flow diagram showing study selection process and reasons for exclusion from the meta‐analysis.
3.2. Study characteristics
Included studies reported the diagnostic accuracy of contrast‐enhanced computed tomography (CECT, seven studies), contrast‐enhanced ultrasound (CEUS, seven studies), [99mTc]Tc‐sestamibi SPECT/CT (five studies), magnetic resonance imaging (MRI, three studies), [18F]FDG PET (four studies), [68Ga]Ga‐PSMA‐11 PET (one study) and [111In]In‐girentuximab SPECT/CT (one study). Individual study characteristics are reported in Table 1.
TABLE 1.
Individual characteristics of included studies.
| Study information | Patient selection | Index test | Reference standard | Data for analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | Setting/country | Prospective or retrospective | Presentation | Sample size (participants) | Sample size (masses) | Females (%) | Average | Average tumour diameter (cm) | Tumour stage | Index test | Criteria for positive diagnosis | Histopathology reference standard | True positives | False positives | False negatives | True negatives |
| Grajo 2021 | University hospital, USA | Retrospective | Patients undergoing surgery for <7 cm tumours | 172 | 172 | 77 (45) | 62 | 3.7 | T1 | CECT | Aorta‐lesion attenuation difference >24 HU | Surgical | 79 | 13 | 27 | 14 |
| Li 2004 | Secondary or tertiary hospital, France | Retrospective | Patients undergoing evaluation for a solid renal tumour | 161 | 161 | 48 (30) | 66 | NR | T1 | CECT |
Hypervascular, heterogenous, enhancing mass that could be calcified/ necrotic/haemorrhagic |
Surgical | 145 | 9 | 0 | 7 |
| Millet 2011 | Secondary or tertiary hospital, France | Retrospective | Patients with <4 cm enhancing renal mass undergoing CT‐guided biopsy | 96 | 99 | 44 (46) | 65 | 2.3 | T1a | CECT | ‘Washout’ or ‘plateau’ enhancement pattern | Surgical/biopsy | 54 | 10 | 20 | 15 |
| Nassiri 2022 | University hospital, USA | Prospective | Patients undergoing surgery for renal mass <4 cm | 390 | 390 | NR | NR | NR | T1a | CECT | Point maximising product of sensitivity and specificity on ROC curve for a model including clinical and radiomic features | Surgical | 203 | 27 | 81 | 79 |
| Nishikawa 2015 | University hospital, Japan | Retrospective | Patients with renal masses <4 cm who underwent partial nephrectomy | 144 | 144 | 49 (34) | 61 | 2.4 | T1a | CECT | Early staining in the arterial phase and washout in the late phase | Surgical | 95 | 7 | 29 | 13 |
| Takebayashi 1999 | Secondary or tertiary hospital, Japan | Retrospective | Haemodialysis patients with suspected renal cancer who underwent nephrectomy | 23 | 222 | 6 (26) | 46 | NR | T1 | CECT | Renal mass with >10 HU enhancement but without fat density | Surgical | 23 | 2 | 1 | 196 |
| Lei 2012 | Secondary or tertiary hospital, China | Retrospective | Patients with renal tumours <3 cm who had CECT and CEUS | 132 | 132 | 59 (45) | 45 | 2.3 | T1a | CECT | Unclear | Surgical/biopsy | 102 | 7 | 17 | 6 |
| CEUS | 114 | 7 | 5 | 6 | ||||||||||||
| Atri 2015 | University hospital, Canada | Prospective | Adults with a <4 cm renal mass and biopsy/surgery diagnosis | 91 | 94 | 35 (38) | 62 | 2.7 | T1a | CEUS | Hypovascularity relative to the adjacent cortex in the arterial phase | Surgical/biopsy | 24 | 0 | 44 | 26 |
| Elbanna 2021 | University hospital, Canada | Retrospective | Patients with solid renal lesions who had CEUS and pathology diagnosis. Those with a definite solid enhancing lesion on CT/MR were excluded | 158 | 161 | 57 (36) | 59 | 2.3 | T1a | CEUS | Arterial‐phase enhancement less than renal cortex | Surgical/biopsy | 54 | 1 | 68 | 38 |
| Fu 2013 | University hospital, China | Unclear | Patients with a pathology‐proven renal lesion | 35 | 35 | 13 (37) | 45 | 3.5 | T1 | CEUS | Shear wave velocity >/= 2.355 m/s | Surgical/biopsy | 21 | 3 | 4 | 8 |
| Rowe 2013 (conference abstract) | Secondary or tertiary hospital, Canada | Unclear | Patients with solid renal masses undergoing surgery | 31 | 32 | 14 (45) | 65 | 3.1 | T1 | CEUS | Heterogeneous enhancement | Surgical | 15 | 1 | 9 | 7 |
| Eisenbrey 2015 | University hospital, USA | Prospective | Patients undergoing percutaneous cryoablation of an enhancing solid renal mass | 13 | 13 | 5 (38) | 71 | NR | T1a | CEUS | Early contrast washout | Biopsy | 8 | 1 | 1 | 3 |
| Sun 2020 | University hospital, China | Retrospective | Patients with renal tumours <3 cm | 37 | 37 | NR | 47 | 2.4 | T1a | CEUS | Not clear | Not stated | 22 | 1 | 0 | 14 |
| Sistani 2020 | Secondary or tertiary hospital, Canada | Retrospective | Patients who required further characterisation of a renal mass prior to treatment (at the discretion of the treating urologist) | 29 | 30 | 6 (21) | 60 | 3 | T1 | [99mTc]Tc‐MIBI | Absence of radiotracer uptake | Surgical/biopsy | 21 | 0 | 2 | 7 |
| Tzortzakakis 2017 | University hospital, Sweden | Prospective | Patients with solid T1 renal tumours eligible for surgery or biopsy with any renal function | 24 | 31 | NR | NR | NR | T1 | [99mTc]Tc‐MIBI SPECT/CT | Absence of radiotracer uptake | Surgical/biopsy | 13 | 2 | 4 | 12 |
| Viswambaram 2022 | University hospital, Australia | Prospective | Adult patients with solid small renal tumours ≥2 cm, or <2 cm if exophytic | 70 | 70 | 23 (33) | 63 | 3.4 | T1 | [99mTc]Tc‐MIBI SPECT/CT | No significant tracer uptake considered malignant | Surgical/biopsy | 49 | 4 | 6 | 11 |
| Asi 2020 | University hospital, Turkey | Prospective | Adults with T1 renal masses undergoing surgery | 90 | 90 | 23 (26) | 55 | 4 | T1 | [99mTc]Tc‐MIBI SPECT/CT | Negative uptake | Surgical | 64 | 6 | 8 | 12 |
| Gorin 2016 | University hospital, USA | Prospective | Patients presenting for surgery for a solid, solitary T1 renal mass not concerning for a metastasis from alternate primary. eGFR >45 ml/min per 1.73 m2 | 50 | 50 | 13 (26) | 62 | 3 | T1 | [99mTc]Tc‐MIBI SPECT/CT | Radiotracer uptake | Surgical | 39 | 2 | 4 | 5 |
| Karyagar 2014 | University hospital, Turkey | Retrospective | Patients with suspected renal cancer who underwent FDG PET in the 2 weeks prior to surgery | 15 | 15 | NR | 55 | 4 | T1 | [18F]FDG PET | Obvious FDG uptake greater than renal parenchyma | Surgical | 1 | 0 | 12 | 2 |
| Kumar 2005 | Not reported | Retrospective | Patient with renal masses who underwent FDG PET. Glucose level <140 mg/dl | 4 | 4 | 2 (50) | 67 | 3.2 | T1 | [18F]FDG PET | Positive if FDG uptake was localised and its intensity was greater than the surrounding normal renal parenchyma | Not stated | 2 | 0 | 1 | 1 |
| Ozulker 2011 | University hospital, Turkey | Prospective | Patients with primary renal masses detected on CT/MR/US undergoing surgery | 13 | 13 | 8 (62) | 56 | 4.1 | T1 | [18F]FDG PET | FDG uptake greater than renal parenchyma, distinct from the collecting system | Surgical | 3 | 1 | 8 | 1 |
| Ramdave 2001 | Secondary or tertiary Hospital, Australia | Prospective | Patients with suspected localised primary renal cancer and tumour <7 cm and diagnostic pathology | 11 | 11 | 5 (45) | 68 | 4.5 | T1 | [18F]FDG PET | Increased intensity of FDG uptake in the lesion relative to comparable normal tissue | Surgical | 8 | 2 | 0 | 1 |
| Muselaers 2013 | University hospital, Netherlands | Prospective | Patients with incidental renal masses | 8 | 9 | 6 (75) | 63 | 4.8 | T1 | [111In]‐girentuximab SPECT/CT | Preferential uptake relative to renal parenchyma | Surgical | 7 | 0 | 0 | 2 |
| De Silva 2022 | University hospital, Australia | Retrospective | Patients with solid renal tumours and diagnostic pathology | 64 | 72 | 37 (58) | 66 | 4.1 | T1 | mpMRI | De Silva St George classification scheme (figure 1 in cited manuscript) | Surgical/biopsy | 49 | 3 | 2 | 18 |
| Johnson 2019 | University hospital, USA | Retrospective | Patient with cT1a renal tumour and mpMRI reporting ccLS and diagnostic pathology | 57 | 63 | 19 (33) | 62 | 2.7 | T1a | mpMRI | ccLS 4‐5 | Surgical/biopsy | 34 | 3 | 15 | 4 |
| Kay 2018 | University hospital, USA | Retrospective | Patients who underwent surgery for renal mass <4 cm with pre‐operative mpMRI | 99 | 99 | 47 (47) | 56 | 2.2 | T1a | mpMRI | A diagnostic algorithm (figure 10 in cited manuscript) | Surgical | 68 | 6 | 24 | 6 |
| Golan 2021 | Secondary or tertiary hospital, Israel | Prospective | Patients referred with a clinical stage 1 enhancing renal mass | 27 | 29 | 8 (30) | 66 | 3.7 | T1 | PSMA PET | All 68Ga‐PSMA‐11‐avid foci with higher uptake than adjacent renal parenchyma | Surgical/biopsy | 15 | 2 | 9 | 3 |
Abbreviations: [18F]FDG PET, [18F]fluorodeoxyglucose positron emission tomography; [99mTc]Tc‐MIBI SPECT/CT, [99mTc]Tc‐sestamibi single‐photon emission computed tomography/computed tomography; CECT, contrast‐enhanced computed tomography; CEUS, contrast‐enhanced ultrasound; mpMRI, multiparametric magnetic resonance imaging; NR, not reported; PSMA PET, [68Ga]Ga‐prostate‐specific membrane antigen‐11 positron emission tomography.
Participant demographics were as follows: mean age 59 years, 63% male, mean lesion size 3.2 cm, prevalence of renal malignancy 69% (IQR 50%–78%). For comparison, population level age‐specific incidence of kidney cancer is highest in >65 year olds, and 62% of kidney cancer cases occur in men. 13 Participant ethnicity was reported in three studies, all from the United States as follows: White participants 56%–84%, Black participants 6%–38%, Asian 6%–7% and Hispanic up to 16%. 14 , 15 , 16 For comparison, US census data reports population‐level ethnicity to be 76% White, 14% Black, 6% Asian and 4% mixed/other. 17 Hispanic origin, considered a distinct concept to race, is 19% (of any race). 17
The target condition was defined as any malignant lesion in 20 included studies, 16 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 and we were able to deduce diagnostic accuracy measures for the target condition in the remaining seven studies from the reported data, despite it not being the target condition. 14 , 15 , 37 , 38 , 39 , 40 , 41
Eight studies received non‐industry funding, 15 , 18 , 22 , 27 , 28 , 33 , 35 , 38 three studies were funded or part funded by industry, 14 , 19 , 41 11 studies did not report the source of funding, 23 , 25 , 26 , 29 , 30 , 31 , 32 , 36 , 37 , 39 , 40 and five stated no funding was received. 16 , 20 , 21 , 24 , 34
3.3. Risk of bias and applicability
Overall, there was a high or uncertain risk of bias for at least one domain in all included studies (Figure 2).
FIGURE 2.
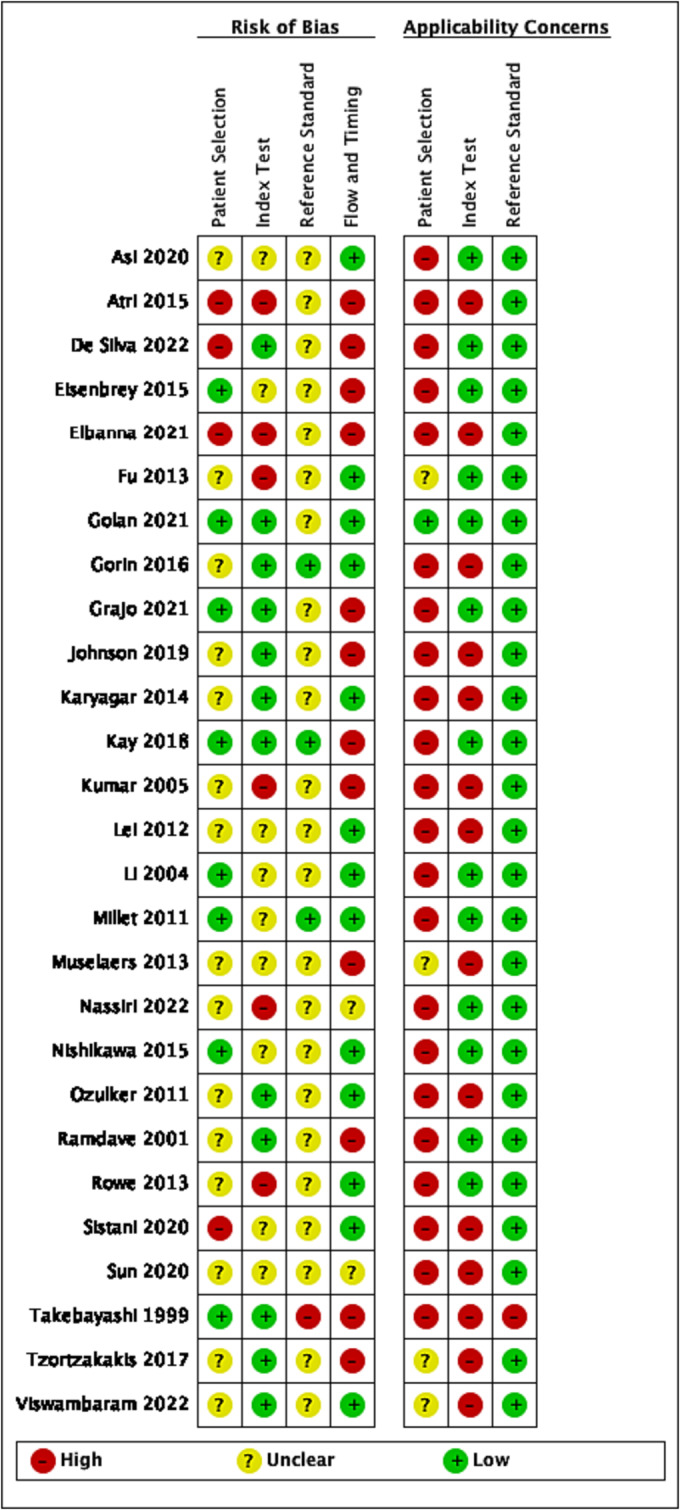
Risk of bias and applicability concerns summary: review authors' judgement about each domain for each included study.
Eleven included studies were prospective, 14 , 15 , 16 , 18 , 19 , 23 , 32 , 33 , 36 , 38 , 41 14 were retrospective, 20 , 21 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 35 , 37 , 39 , 40 and two studies were not clear. 22 , 34 All studies were single centre. There was one fully paired retrospective comparative study of CEUS versus CECT, 28 and all others were cross sectional diagnostic accuracy studies of a single index test.
3.3.1. Participant selection
Patient selection was heterogeneous across studies, with the majority of participants included based on management strategy, including partial nephrectomy, 31 nephrectomy, 40 any surgical resection, 15 , 16 , 18 , 24 , 25 , 26 , 29 , 32 , 34 ablation 14 or patients who underwent CT guided biopsy. 30 Other patients were selected on the basis of having a histological diagnosis from any of the following as part of standard clinical care: biopsy, fine needle aspiration or surgery. 19 , 20 , 22 , 27 , 28 , 32 , 33 , 36 , 37 , 38 , 39 One study included patients referred for CEUS when CT, MR or US was indeterminate. 21 Two small studies included all‐comers, 23 , 41 and in one study, the criteria for case selection were unclear. 35
We considered surgical‐only populations to have high applicability concerns. Surgical patients are likely to be younger and fitter than surveillance populations, 42 reflected in the study population of this review being younger on average than population‐level data for kidney cancer. Younger patients are more likely to have benign tumours, 43 reflected in the high proportion of benign tumours in this review, and cause applicability concerns for the wider population of patients with renal masses.
Thirteen studies restricted eligibility to patients with solid tumours, 14 , 15 , 19 , 20 , 21 , 24 , 29 , 30 , 31 , 34 , 36 , 38 , 41 seven included both solid and cystic tumours, 18 , 26 , 27 , 32 , 39 , 40 and eight did not report if included lesions were solid, cystic or a mixture. 16 , 22 , 23 , 25 , 28 , 33 , 35 , 37
3.3.2. Index test
Criteria for a positive CEUS and MRI tests were at different thresholds in each study, or the threshold was not reported. For CECT, contrast enhancement was generally included in the description of a positive test, with 40 or without 29 , 30 , 31 a defined increase in Hounsfield units between pre and post contrast phases. Alternative criteria were also described 16 , 24 or the threshold not defined. 28
All five studies reporting diagnostic accuracy of [99mTc]Tc‐sestamibi SPECT/CT used the same threshold of absent radiotracer uptake in the tumour to signify malignancy. 15 , 18 , 36 , 38 , 39 [99mTc]Tc‐sestamibi SPECT/CT images were reported by two clinicians in collaboration to reach consensus, limiting the applicability to clinical practice where most diagnostic imaging is reported by a single clinician.
Four small studies, each with 4–15 participants reported the diagnostic accuracy of [18F]FDG PET 25 , 27 , 32 , 33 with a common positive threshold of FDG uptake in the tumour greater than the surrounding renal parenchyma.
3.3.3. Reference standard
Generally, there was poor reporting of reference standard conduct and therefore unclear risk of bias. However, where histology was performed as part of standard care, we deemed applicability concerns to be low in all but one study that described pathologic diagnosis made solely on morphology, 40 when the addition of immunohistochemistry is a minimum standard. Diagnostic criteria used to identify the target condition were not reported for 23 studies, 14 , 16 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 27 , 28 , 29 , 32 , 33 , 34 , 35 , 36 , 38 , 39 , 40 , 41 one study reported International Society of Urological Pathology guidelines, 15 and four studies reported the World Health Organisation classification system 2004 26 , 30 , 31 and 2016 editions. 37 Ten studies stated that the reference test was interpreted without knowledge of the results of the index test. 15 , 16 , 19 , 20 , 21 , 22 , 24 , 25 , 26 , 30
3.3.4. Flow and timing
Studies were deemed at high risk of bias if some participants were excluded from the analysis. 14 , 20 , 21 , 24 , 26 , 27 , 33 , 37 , 38 , 40 , 41
3.3.5. Risk of bias in the comparison
For the single study that included a direct comparison of CEUS versus CECT, 28 risk of bias in the comparison was unclear for patient selection, conduct or interpretation of the index test, conduct or interpretation of the reference standard and at low risk of bias in the comparison for flow and timing.
3.4. Results of individual imaging modalities
Forest plots of estimates of sensitivity and specificity along with the 95% confidence intervals for each included study are presented in Figure 3.
FIGURE 3.
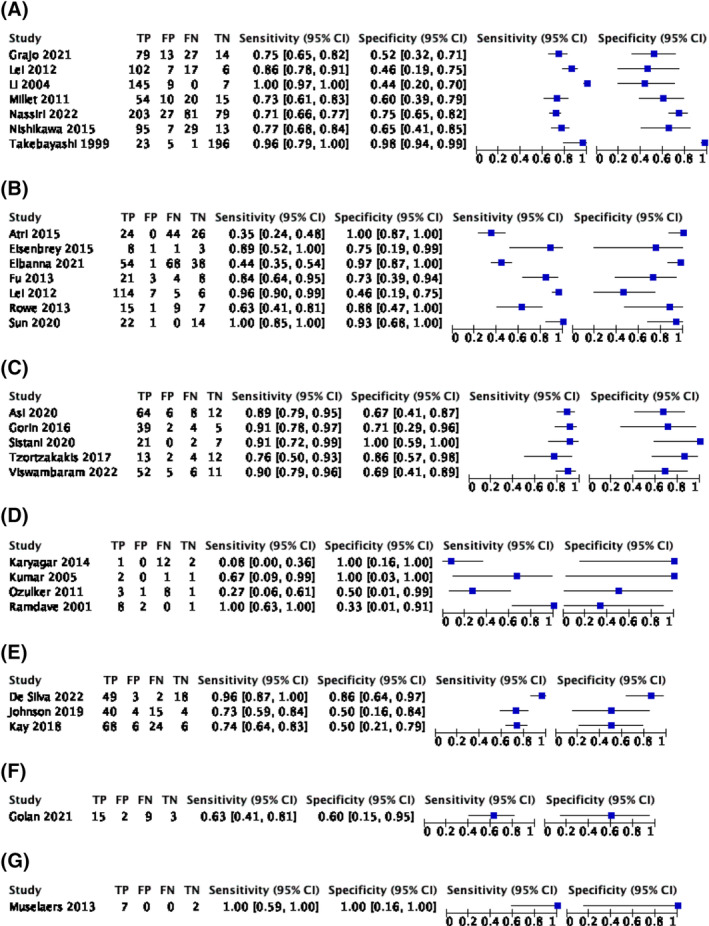
Forest plot of estimates of sensitivity and specificity of (A) contrast‐enhanced computed tomography, (B) contrast‐enhanced ultrasound, (C) [99mTc]Tc‐sestamibi SPECT/CT, (D) [18F]FDG PE, (E) multiparametric magnetic resonance imaging, (F) [68Ga]Ga‐PSMA‐11 PET and (G) [111In]In‐girentuximab SPECT/CT for the diagnosis of tumour malignancy. CI, confidence interval; FN, false negatives; FP, false positives; TN, true negatives; TP, true positives.
3.4.1. CECT
Seven studies including 1118 patients with 1320 renal lesions reported estimates of sensitivity and specificity for CEUS to detect malignancy in T1 renal tumours ranging from 71% to 100% and 44% to 98%, respectively (Figure 3A). One study was an outlier in forest plots and ROC space, 40 likely due to the study population of 23 participants with end‐stage renal failure with 222 renal lesions, mostly uncomplicated renal cysts, thus overestimating measures of diagnostic accuracy. Another study reported diagnostic accuracy of a model including clinical and radiomic data (i.e. artificial intelligence‐guided data characterisation) from CT and was therefore not comparable. 16 The remaining studies used different thresholds to define a positive test, so meta‐analysis was not performed. 12
3.4.2. CEUS
Seven studies including 197 patients with 504 renal lesions reported estimates of sensitivity and specificity for CEUS to detect malignancy in T1 renal tumours ranging from 35% to 100% and 0% to 100% (Figure 3B). These studies used different thresholds to define a positive test, so meta‐analysis was not performed. 12
3.4.3. [99mTc]Tc‐sestamibi SPECT/CT
Five studies including 271 renal lesions in 263 patients reported estimates of sensitivity and specificity for [99mTc]Tc‐sestamibi SPECT/CT to detect malignancy in T1 renal tumours (Figure 3C). All included studies reported measures of diagnostic accuracy at the same positive threshold that was radiotracer uptake in the tumour less than the surrounding renal parenchyma. Meta‐analysis using a univariate fixed‐effect regression model because of sparse data and determined by best model fit returned summary estimates of sensitivity and specificity for [99mTc]Tc‐sestamibi SPECT/CT to detect malignancy of 88.6% (95% CI 82.7%–92.6%) and 77.0% (95% CI 63.0%–86.9%), respectively (Figure 4).
FIGURE 4.
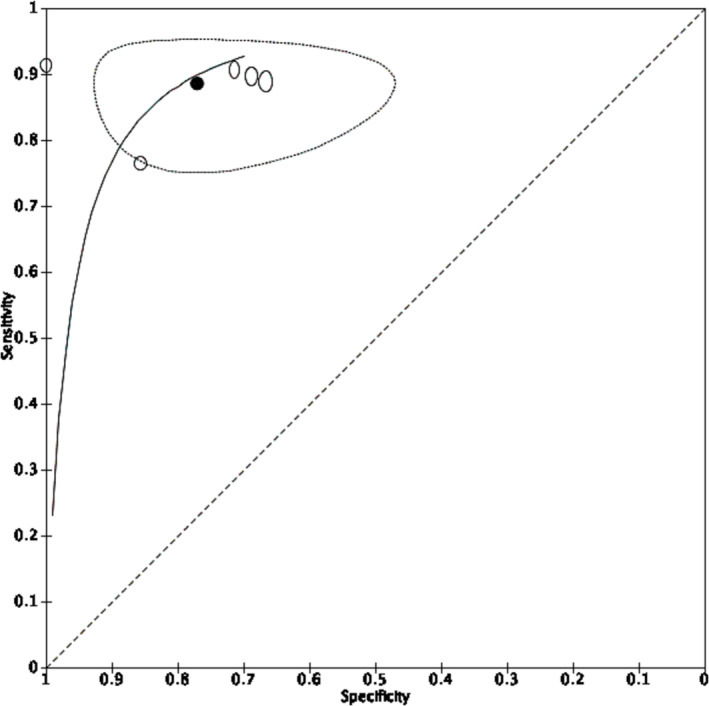
Summary receiver operating characteristic curve of five included studies reporting the diagnostic accuracy of [99mTc]Tc‐sestamibi SPECT/CT to detect malignancy in patients presenting with T1 renal tumours. ○ = estimate from individual study ● = summary estimate = 95% confidence region. Summary estimates of sensitivity and specificity to detect cancer are 88.6% (95% CI 82.7%–92.6%) and 77.0% (95% CI 63.0%–86.9%), respectively.
3.4.4. [18F]FDG PET
Four studies including 43 patients with 43 lesions reported estimates of sensitivity and specificity for [18F]FDG PET/CT to detect malignancy in T1 renal tumours (Figure 3D). All included studies reported measures of diagnostic accuracy at the same positive threshold of radiotracer uptake in the tumour relative to the surrounding renal parenchyma. Meta‐analysis using univariate mixed‐effects regression model because of sparse data and determined by best model fit returned summary estimates of sensitivity and specificity for [18F]FDG PET to detect malignancy of 53.5% (95% CI 1.6%–98.8%) and 62.5% (95% CI 14.0%–94.5%), respectively (Figure 5). An HSROC model to compare diagnostic accuracy of [18F]FDG PET/CT with [99mTc]Tc‐sestamibi SPECT/CT was attempted but did not converge.
FIGURE 5.
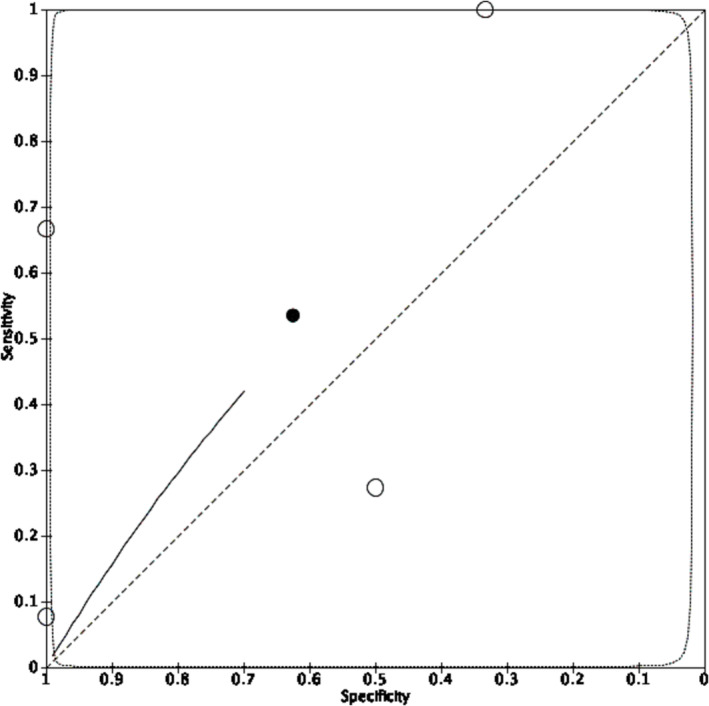
Summary receiver operating characteristic curve of four included studies reporting the diagnostic accuracy of [18F]FDG PET to detect malignancy in patients presenting with T1 renal tumours. ○ = estimate from individual study ● = summary estimate = 95% confidence region. Summary estimates of sensitivity and specificity for [18F]FDG PET to detect cancer are 53.5% (95% CI 1.6%–98.8%) and 62.5% (95% CI 14.0%–94.5%), respectively.
3.4.5. Multiparametric magnetic resonance imaging
Three studies including 220 patients with 234 renal lesions reported estimates of sensitivity and specificity for MRI to detect malignancy in T1 renal tumours ranging from 73% to 96% and 50% to 86%, respectively (Figure 3E). Different thresholds were used to define a positive test, so meta‐analysis was not performed. 12
3.4.6. [68Ga]Ga‐PSMA‐11 PET
One study including 27 patients with 29 renal lesions reported estimates of sensitivity and specificity for [68Ga]Ga‐PSMA‐11 PET to detect malignancy in T1 renal tumours of 63% (95% CI 41%–81%) and 60% (95% CI 15%–95%), respectively (Figure 3F).
3.4.7. [111In]In‐girentuximab SPECT/CT
One study including eight patients with nine renal lesions reported estimates of sensitivity and specificity for [111In]ln‐girentuximab SPECT/CT to detect malignancy in T1 renal tumours of 100% (95% CI 59%–100%) and 100% (95% CI 16%–100%), respectively (Figure 3G).
4. DISCUSSION
4.1. Principal findings
This is the first systematic review and meta‐analysis of all imaging modalities for the detection of malignancy in T1 renal tumours. We included 27 studies involving 2277 tumours in 2044 participants evaluating the diagnostic accuracy of CECT, CEUS, [99mTc]Tc‐sestamibi SPECT/CT, mpMRI, [18F]FDG PET, [68Ga]Ga‐PSMA‐11 PET and [111In]ln‐girentuximab SPECT/CT.
Meta‐analysis of studies evaluating [99mTc]Tc‐sestamibi SPECT/CT showed summary estimates of sensitivity and specificity to detect malignancy of 88.6% (95% CI 82.7–92.6) and 77.0% (95% CI 63.0%–86.9%) respectively. Four small, statistically heterogeneous studies evaluating [18F]FDG PET had summary estimates of sensitivity and specificity of 53.5% (95% CI 1.6‐98.8%) and 62.5% (95% CI 14.0‐94.5%).
Meta‐analyses for CECT, CEUS and MRI were not appropriate because studies adopted different thresholds to define a positive test and it is not clinically meaningful. The single study that directly compared CEUS with CECT was not of sufficiently high methodological quality to warrant further discussion of the results. The field would benefit from the reporting of diagnostic accuracy at standardised, pre‐specified thresholds, which could be guided by existing literature.
4.2. Findings in the context of existing evidence
Previous meta‐analyses have reported diagnostic performance of CEUS versus CECT and/or MRI for renal tumours. 44 , 45 In the event of different positive thresholds across studies, an HSROC model is recommended to produce a summary curve rather than point estimates for sensitivity and specificity, 7 which was not the statistical approach adopted in either review. 44 , 45 Further, these reviews chose to include imaging follow‐up as a reference standard. While a period of initial surveillance provides helpful information on the trajectory of a renal lesion, growth rate does not differentiate benign from malignant disease as many cancers remain stable in size 46 and benign tumours can exhibit growth. 47 In our own review, we excluded studies where the reference test included imaging surveillance.
There have been two previously published systematic reviews of [99mTc]Tc‐sestamibi SPECT/CT by Wilson et al. in 2020 and Basile et al. in 2023. 48 , 49 These reviews reported higher estimates of sensitivity (90%–91%) and specificity (86%) than our own, albeit with overlapping confidence intervals. Several new studies have been published since the former, and the latter is limited by inclusion of case–control studies that are at high risk of bias, excluding histological subtypes other than RCC, oncocytoma or angiomyolipoma and classifying hybrid oncocytic/chromophobe tumours (HOCT) as benign. While misclassifying HOCT as benign is likely of little clinical consequence given their indolent nature, the World Health Organisation defines them as malignant and has recently included them in the emerging entity of ‘low‐grade oncocytic tumours’ (LOT). 50 Furthermore, both reviews differed from our own by including tumours of all T stages and therefore had a higher proportion of malignant histology (78%–83% vs. 69%). The ability to differentiate benign from malignant tumours is most relevant in the T1 setting where clinicians report higher willingness to manage benign tumours conservatively. 51 Further prospective studies of [99mTc]Tc‐sestamibi SPECT/CT are awaited, 52 and work evaluating its role as an replacement test for biopsy, add‐on test, or triage test is needed.
A MRI‐based ‘clear cell likelihood score’ has shown pooled estimates of sensitivity and specificity of 80% (95% CI 75%–85%) and 74% (95% CI 65%–81%) to detect clear cell RCC in a systematic review and meta‐analysis of six studies including 825 T1a renal masses. 53 Additionally, [89Zr]DFO‐girentuximab PET/CT has been reported in a conference abstract to have sensitivity of 86% [80%, 90%] and 87% [79%, 92%], also for detecting clear cell RCC with the full manuscript awaited. 54 These studies were not included in our review as it was not possible to extract diagnostic accuracy data for benign versus malignant lesions. Clinically, these tests may have a triage role supporting active treatment for patients with a positive test for clear cell RCC; however, patients with a negative test would still require further diagnostics.
Radiomics has received growing interest, including in the setting of renal tumours. 5 , 55 Advanced computing may allow extraction of quantitative spatial information from medical imaging to detect differences imperceptible to the human eye. Only one manuscript including radiomics from CT was of sufficient quality for inclusion in this review and reported area under the curve of 0.77 (95% CI 0.69–0.85) for a model including radiomics and clinical factors. 16 No comparison was made with radiologist reporting of imaging.
4.3. Limitations
We applied diagnostic filters in our search strategy to limit the returned texts to a feasible number to screen. The filters used have a sensitivity of 98.6% for MEDLINE 9 and 100% for Embase, 8 so the risk of having omitted relevant studies is low.
A limitation of our review is that most participants underwent surgical resection or diagnostic biopsy, due to our inclusion criteria necessitating a histopathological reference standard. In doing so, we limit the applicability of our results to patients on surveillance without histopathological diagnosis.
Eighty‐four studies were excluded from our review because they included all stages of renal tumour and it was not possible to extract diagnostic accuracy data for T1 tumours alone. We advocate future diagnostic accuracy studies reporting measures of diagnostic accuracy for each tumour stage to facilitate future reviews.
We chose per‐lesion rather than per‐participant analysis as information at the level of the lesion is important for clinical decision making. For example, if a patient had multiple synchronous renal lesions—some malignant and others benign—then urologists would favour treating the malignant tumours, and not the benign ones in an effort to preserve renal function. However, this approach assumes independence of the lesions in a single participant, and therefore, measures of diagnostic accuracy are likely overestimated for studies that included participants with multiple lesions. 56
4.4. Deviations from the protocol
We revised our original protocol from including only T1a to all T1 renal tumours due to sparse data for T1a lesions alone. The protocol change was registered with PROSPERO. T1a renal tumours have the highest prevalence of benign histology when compared to tumours of greater size and T stage, 43 and extended eligibility to larger tumours has likely resulted in a higher prevalence of malignant histology, although the mean size of included tumours was 3.2 cm. For all imaging modalities, it is conceivable that the diagnostic accuracy increases with increasing tumour size due to both resolution limits and less signal contamination in the tumour volume from normal surrounding renal parenchyma.
5. CONCLUSIONS
Imaging‐based diagnostics for risk stratifying renal tumours is an unmet need. Currently, the optimal imaging strategy to characterise T1 renal tumours is not clear because of heterogeneity and sparse data as well as a lack of direct comparisons. [99mTc]Tc‐sestamibi SPECT/CT is an emerging tool, but further studies are required to inform its role in clinical practice. We advocate future diagnostic accuracy studies reporting performance at each tumour stage and standardisation of the diagnostic threshold used to consider CT, MRI and CEUS positive for cancer.
AUTHOR CONTRIBUTIONS
The study was conceived and designed by HW, ME, MGBT and KG. The protocol was developed and published (HW, JBF, VWSC, RH, VK, ME, MGBT, KG). Study screening and data extraction was performed by HW, JBF, VM, PI, VWSC and EZ. Analysis was performed by HW with support from KG. The manuscript was written by HW, with revisions from JBF, RH, VK, MGBT and KG. All co‐authors approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
HW receives salary support from The Urology Foundation, Pan London Cancer Alliance (Royal Marsden Partners, North Central London Cancer Alliance, North East London Cancer Alliance, South East London Cancer Alliance and the NIHR BRCs) and the Wellcome/EPSRC Centre for Interventional and Surgical Sciences. The promotions and salaries of KG are dependent upon the publishing of research protocols and findings. Other authors have no relevant interests to declare.
Supporting information
Appendix S1. Search Strategies.
Appendix S2. Customised QUADAS‐2 and QUADAS‐C tools.
Appendix S3. Statistical code.
Data S1. Supporting Information.
ACKNOWLEDGEMENTS
None.
Warren H, Fanshawe JB, Mok V, Iyer P, Chan VW‐S, Hesketh R, et al. Imaging modalities for characterising T1 renal tumours: A systematic review and meta‐analysis of diagnostic accuracy. BJUI Compass. 2024;5(7):636–650. 10.1002/bco2.355
REFERENCES
- 1. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population‐based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. [DOI] [PubMed] [Google Scholar]
- 2. Ljungberg B, Albiges L, Abu‐Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European Association of Urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022;82:399–410. [DOI] [PubMed] [Google Scholar]
- 3. Kim JH, Li S, Khandwala Y, Chung KJ, Park HK, Chung BI. Association of prevalence of benign pathologic findings after partial nephrectomy with preoperative imaging patterns in the United States from 2007 to 2014. JAMA Surg. 2019;154(3):225–231. 10.1001/jamasurg.2018.4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marconi L, Dabestani S, Lam TB, Hofmann F, Stewart F, Norrie J, et al. Systematic review and meta‐analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol [Internet]. 2016;69(4):660–673. 10.1016/j.eururo.2015.07.072 [DOI] [PubMed] [Google Scholar]
- 5. Roussel E, Capitanio U, Kutikov A, Oosterwijk E, Pedrosa I, Rowe SP, et al. Novel imaging methods for renal mass characterization: a collaborative review. Eur Urol. 2022;81:476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McInnes M, Moher D, Thombs B, McGrath T, Bossuyt P. Preferred reporting items for a systematic review and meta‐analysis of diagnostic test accuracy studies. The PRISMA‐DTA statement. Jama. 2018;319(4):388–396. 10.1001/jama.2017.19163 [DOI] [PubMed] [Google Scholar]
- 7. Deeks J, Bossuyt P, Leeflang M, Takwoingi Y. Cochrane handbook for systematic reviews of diagnostic test accuracy. Version 2.0. [DOI] [PMC free article] [PubMed]
- 8. Wilczynski NL, Haynes RB, Team H. EMBASE search strategies for identifying methodologically sound diagnostic studies for use by clinicians and researchers. BMC Med. 2005;3:7. 10.1186/1741-7015-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haynes B, Wilczynski NL. Optimal search strategies for retrieving scientifically strong studies of diagnosis from Medline: analytical survey. Bmj. 2004;328(7447):1040. 10.1136/bmj.38068.557998.EE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 11. Ang B, Mallett S, Takwoingi Y, Davenport CF, Hyde CJ, Whiting PF, et al. QUADAS‐C: a tool for assessing risk of bias in comparative diagnostic accuracy studies. Ann Intern Med. 2021;174(11):1592–1599. 10.7326/M21-2234 [DOI] [PubMed] [Google Scholar]
- 12. Takwoingi Y, Guo B, Riley RD, Deeks JJ. Performance of methods for meta‐analysis of diagnostic test accuracy with few studies or sparse data. Stat Methods Med Res. 2017;26(4):1896–1911. 10.1177/0962280215592269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cancer Research UK . Kidney cancer incidence statistics [Internet]. Available at: https://www.cancerresearchuk.org/health‐professional/cancer‐statistics/statistics‐by‐cancer‐type/kidney‐cancer/incidence?_ga=2.25678974.257405545.1609191159‐651160254.1609191159
- 14. Eisenbrey JR, Shaw CM, Lyshchik A, Machado P, Lallas CD, Trabulsi EJ, et al. Contrast‐enhanced subharmonic and harmonic ultrasound of renal masses undergoing percutaneous cryoablation. Acad Radiol. 2015;22(7):820–826. 10.1016/j.acra.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 15. Gorin MA, Rowe SP, Baras AS, Solnes LB, Ball MW, Pierorazio PM, et al. Prospective evaluation of 99mtc‐sestamibi SPECT/CT for the diagnosis of renal oncocytomas and hybrid oncocytic/chromophobe tumors. J Urol [Internet]. 2016;195(4 SUPPL 1):e1031. [Google Scholar]
- 16. Nassiri N, Maas M, Cacciamani G, Varghese B, Hwang D, Lei X, et al. A radiomic‐based machine learning algorithm to reliably differentiate benign renal masses from renal cell carcinoma. Eur Urol Focus. 2022;8(4):988–994. 10.1016/j.euf.2021.09.004 [DOI] [PubMed] [Google Scholar]
- 17. United States census bureau [Internet]. 2023. Available at: https://www.census.gov/quickfacts/fact/dashboard/US/RHI125222#RHI125222
- 18. Asi T, Tuncali MÇ, Tuncel M, Alkanat NEİ, Hazir B, Kösemehmetoğlu K, et al. The role of Tc‐99m MIBI scintigraphy in clinical T1 renal mass assessment: does it have a real benefit? Urol Oncol. 2020;38(12):937.e11–937.e17. 10.1016/j.urolonc.2020.07.018 [DOI] [PubMed] [Google Scholar]
- 19. Atri M, Tabatabaeifar L, Jang HJ, Finelli A, Moshonov H, Jewett M. Accuracy of contrast‐enhanced us for differentiating benign from malignant solid small renal masses. Radiology. 2015;276(3):900–908. 10.1148/radiol.2015140907 [DOI] [PubMed] [Google Scholar]
- 20. de Silva S, Lockhart KR, Aslan P, Nash P, Hutton A, Malouf D, et al. Differentiation of renal masses with multi‐parametric MRI: the de Silva St George classification scheme. BMC Urol. 2022;22(1). 10.1186/s12894-022-01082-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elbanna KY, Jang HJ, Kim TK, Khalili K, Guimaraes LS, Atri M. The added value of contrast‐enhanced ultrasound in evaluation of indeterminate small solid renal masses and risk stratification of cystic renal lesions. Eur Radiol. 2021;31(11):8468–8477. [DOI] [PubMed] [Google Scholar]
- 22. Hua FN, Yang B, Ping WS, Lei L, Jie ZS. Acoustic radiation force impulse imaging in differential diagnosis of renal tumours. Chin J Med Imag Technol. 2013;29(4):621–624. [Google Scholar]
- 23. Golan S, Aviv T, Groshar D, Yakimov M, Zohar Y, Prokocimer Y, et al. Dynamic 68Ga‐PSMA‐11 PET/CT for the primary evaluation of localized renal mass: a prospective study. J Nucl Med. 2021;62(6):773–778. 10.2967/jnumed.120.251272 [DOI] [PubMed] [Google Scholar]
- 24. Grajo JR, Batra NV, Bozorgmehri S, Magnelli LL, Pavlinec J, O'Malley P, et al. Validation of aorta‐lesion‐attenuation difference on preoperative contrast‐enhanced computed tomography scan to differentiate between malignant and benign oncocytic renal tumors. Abdom Radiol (NY). 2021;46(7):3269–3279. [DOI] [PubMed] [Google Scholar]
- 25. Karyagar S, Koc ZP, Karyagar SS, Ozulker T, Topal C, Karaguzel E, et al. Dual time F‐18 FDG PET/CT imaging in the diagnosis of renal cell cancer. Curr Med Imaging Rev. 2014;10(2):134–139. [Google Scholar]
- 26. Kay FU, Canvasser NE, Xi Y, Pinho DF, Costa DN, De Leon AD, et al. Diagnostic performance and interreader agreement of a standardized MR imaging approach in the prediction of small renal mass histology. Radiology. 2018;287(2):543–553. 10.1148/radiol.2018171557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumar R, Chauhan A, Lakhani P, Xiu Y, Zhuang H, Alavi A. 2‐deoxy‐2‐[F‐18]fluoro‐D‐glucose‐positron emission tomography in characterization of solid renal masses. Mol Imaging Biol. 2005;7(6):431–439. 10.1007/s11307-005-0026-z [DOI] [PubMed] [Google Scholar]
- 28. Lei L, Fu NH, Yang B, Wei SP. Comparative analysis of contrast‐enhanced ultrasound and contrast‐enhanced CT in diagnosis of small renal cell carcinoma. Chin J Med Imag Technol. 2012;28(4):760–764. [Google Scholar]
- 29. Li G, Cuilleron M, Gentil‐Perret A, Tostain J. Characteristics of image‐detected solid renal masses: implication for optimal treatment. Int J Urol. 2004;11(2):63–67. [DOI] [PubMed] [Google Scholar]
- 30. Millet I, Doyon FC, Hoa D, Thuret R, Merigeaud S, Serre I, et al. Characterization of small solid renal lesions: can benign and malignant tumors be differentiated with CT? AJR am J Roentgenol. 2011;197(4):887–896. [DOI] [PubMed] [Google Scholar]
- 31. Nishikawa M, Miyake H, Kitajima K, Takahashi S, Sugimura K, Fujisawa M. Preoperative differentiation between benign and malignant renal masses smaller than 4 cm treated with partial nephrectomy. Int J Clin Oncol. 2015;20(1):150–155. [DOI] [PubMed] [Google Scholar]
- 32. Özülker T, Özülker F, Özbek E, Özpaçaci T. A prospective diagnostic accuracy study of F‐18 fluorodeoxyglucose‐positron emission tomography/computed tomography in the evaluation of indeterminate renal masses. Nucl Med Commun. 2011;32:265–272. 10.1097/MNM.0b013e3283442e3b [DOI] [PubMed] [Google Scholar]
- 33. Ramdave S, Thomas GW, Berlangieri SU, Bolton DM, Davis I, Danguy HT, et al. Clinical role of F‐18 fluorodeoxyglucose positron emission tomography for detection and management of renal cell carcinoma. J Urol. 2001;166(3):825–830. [PubMed] [Google Scholar]
- 34. Rowe NE, Bird J, Romagnoli C, Luke PP. 2021 contrast‐enhanced ultrasound of solid renal masses: non‐invasive discrimination between renal cell carcinoma and benign renal tumors. J Urol. 2013;189(4S). [Google Scholar]
- 35. Sun D, Lu Q, Wei C, Li Y, Zheng Y, Hu B. Differential diagnosis of <3 cm renal tumors by ultrasonography: a rapid, quantitative, elastography self‐corrected contrast‐enhanced ultrasound imaging mode beyond screening. Br J Radiol. 2020;93(1112):20190974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Viswambaram P, Picardo A, Hohnen A, Pham K, Macdonald W, Hayne D, et al. Technetium‐99 m‐sestamibi single‐photon emission computerised tomography (CT)/CT in the prediction of malignant versus benign small renal masses. BJU Int. 2022. 10.1111/bju.15737 [DOI] [PubMed] [Google Scholar]
- 37. Johnson BA, Kim S, Steinberg RL, de Leon AD, Pedrosa I, Cadeddu JA. Diagnostic performance of prospectively assigned clear cell likelihood scores (ccLS) in small renal masses at multiparametric magnetic resonance imaging. Urol Oncol. 2019;37(12):941–946. 10.1016/j.urolonc.2019.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tzortzakakis A, Gustafsson O, Karlsson M, Ekström‐Ehn L, Ghaffarpour R, Axelsson R. Visual evaluation and differentiation of renal oncocytomas from renal cell carcinomas by means of 99mTc‐sestamibi SPECT/CT. EJNMMI Res. 2017;7(1). 10.1186/s13550-017-0278-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sistani G, Bjazevic J, Kassam Z, Romsa J, Pautler S. The value of 99mTc‐sestamibi single‐photon emission computed tomography‐computed tomography in the evaluation and risk stratification of renal masses. Can Urol Assoc J. 2021;15(6):197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takebayashi S, Hidai H, Chiba T, Takagi H, Koike S, Matsubara S. Using helical CT to evaluate renal cell carcinoma in patients undergoing hemodialysis: value of early enhanced images. AJR am J Roentgenol [Internet]. 1999;172(2):429–433. [DOI] [PubMed] [Google Scholar]
- 41. Muselaers CHJ, Boerman OC, Oosterwijk E, Langenhuijsen JF, Oyen WJG, Mulders PFA. Indium‐111‐labeled girentuximab ImmunoSPECT as a diagnostic tool in clear cell renal cell carcinoma. Eur Urol [Internet]. 2013;63(6):1101–1106. [DOI] [PubMed] [Google Scholar]
- 42. Xing M, Kokabi N, Zhang D, Ludwig JM, Kim HS. Comparative effectiveness of thermal ablation, surgical resection, and active surveillance for T1a renal cell carcinoma: a surveillance, epidemiology, and end results (SEER)–Medicare‐linked population study. Radiology. 2018;288(1):81–90. 10.1148/radiol.2018171407 [DOI] [PubMed] [Google Scholar]
- 43. Fernando A, Fowler S, Brien TO. Nephron‐sparing surgery across a nation – outcomes from the British Association of Urological Surgeons 2012 national partial nephrectomy audit. BJU Int. 2016;117:874–882. 10.1111/bju.13353 [DOI] [PubMed] [Google Scholar]
- 44. Furrer MA, Spycher SCJ, Büttiker SM, Gross T, Bosshard P, Thalmann GN, et al. Comparison of the diagnostic performance of contrast‐enhanced ultrasound with that of contrast‐enhanced computed tomography and contrast‐enhanced magnetic resonance imaging in the evaluation of renal masses: a systematic review and meta‐analysis. Eur Urol Oncol. 2020;3(4):464–473. 10.1016/j.euo.2019.08.013 [DOI] [PubMed] [Google Scholar]
- 45. Zhang F, Li R, Li G, Jin L, Shi Q, Du L. Value of contrast‐enhanced ultrasound in the diagnosis of renal cancer and in comparison with contrast‐enhanced computed tomography: a meta‐analysis. J Ultrasound Med. 2019;38(4):903–914. 10.1002/jum.14769 [DOI] [PubMed] [Google Scholar]
- 46. Jewett MAS, Mattar K, Basiuk J, Morash CG, Pautler SE, Siemens DR, et al. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol. 2011;60(1):39–44. 10.1016/j.eururo.2011.03.030 [DOI] [PubMed] [Google Scholar]
- 47. Neves J, Varley R, Agnesi S, Withington J, Rodrigues FB, Warren H, et al. Growth and renal function dynamics of renal oncocytomas on active surveillance: a retrospective cohort analysis. BJU Int. 2021;128(6):722–727. 10.1111/bju.15499 [DOI] [PubMed] [Google Scholar]
- 48. Wilson MP, Katlariwala P, Murad MH, Abele J, Mcinnes MDF, Low G. Diagnostic accuracy of 99mTc ‐ sestamibi SPECT/CT for detecting renal oncocytomas and other benign renal lesions: a systematic review and meta‐analysis. Abdom Radiol (NY). 2020;45(8):2532–2541. 10.1007/s00261-020-02469-8 [DOI] [PubMed] [Google Scholar]
- 49. Basile G, Fallara G, Verri P, Uleri A, Chiti A, Gianolli L, et al. The role of 99mTc‐sestamibi single‐photon emission computed tomography/computed tomography in the diagnostic pathway for renal masses: a systematic review and meta‐analysis. Eur Urol. 2023. [DOI] [PubMed] [Google Scholar]
- 50. Moch H, Amin MB, Berney DM, Compérat EM, Gill AJ, Hartmann A, et al. The 2022 World Health Organization classification of tumours of the urinary system and male genital organs—part a: renal, penile, and testicular tumours. Eur Urol. 2022. PMID: Available at: https://linkinghub.elsevier.com/retrieve/pii/S0302283822024678 [DOI] [PubMed] [Google Scholar]
- 51. Warren H, Neves JB, Tran MGB. Renal oncocytoma: landscape of diagnosis and management. BJU Int. 2021;128(6):685–687. 10.1111/bju.15496 [DOI] [PubMed] [Google Scholar]
- 52. Warren H, Wagner T, Gorin MA, Rowe S, Holman BF, Pencharz D, et al. Protocol for a MULTI‐Centre feasibility study to assess the use of 99m Tc‐sestaMIBI SPECT/CT in the diagnosis of kidney tumours (MULTI‐MIBI study). BMJ Open. 2023;13(1):e067496. 10.1136/bmjopen-2022-067496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tian J, Teng F, Xu H, Zhang D, Chi Y, Zhang H. Systematic review and meta‐analysis of multiparametric MRI clear cell likelihood scores for classification of small renal masses. Front Oncol. 2022;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shuch BM, Pantuck AJ, Bernhard JC, Morris MA, Master VA, Scott AM, et al. Results from phase 3 study of 89Zr‐DFO‐girentuximab for PET/CT imaging of clear cell renal cell carcinoma (ZIRCON). J Clin Oncol. 2023;41(6 supplement):LBA602. [Google Scholar]
- 55. Mühlbauer J, Egen L, Kowalewski KF, Grilli M, Walach MT, Westhoff N, et al. Radiomics in renal cell carcinoma—a systematic review and meta‐analysis. Cancers (Basel). 2021;13:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zwinderman AH, Glas AS, Bossuyt PM, Florie J, Bipat S, Stoker J. Statistical models for quantifying diagnostic accuracy with multiple lesions per patient. Biostatistics. 2008. Jul;9(3):513–522. 10.1093/biostatistics/kxm052 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Search Strategies.
Appendix S2. Customised QUADAS‐2 and QUADAS‐C tools.
Appendix S3. Statistical code.
Data S1. Supporting Information.


