Abstract
Background: Whole-body vibration (WBV) is a commonly used physical exercise for disease prevention and rehabilitation. Recent studies indicated the beneficial mechanism of WBV may be associated with its anti-inflammatory potential, however, its regulatory roles on different inflammatory mediators remained controversial. The aim of this study was to perform a meta-analysis to re-confirm the effects of WBV exercise on various inflammatory factors. Methods: The PubMed, EMBASE and Cochrane Library databases were searched up to September 2023 to collect all articles comparing WBV with control (or post-pre trials). The effect size was expressed as the standardized mean difference (SMD) and 95% confidence intervals (CI). Results: A total of 31 eligible studies were included, including 14 pre-clinical and 17 clinical studies. The meta-analysis of pre-clinical studies showed that compared with the control group, WBV exercise could significantly reduce the level of IL-6 (SMD: -1.03, 95% CI: -1.93, -0.13), TNF-α (SMD: -1.36, 95% CI: -2.54, -0.17) (for disease subgroup), IL-1β (SMD: -2.20, 95% CI: -3.24, -1.15), IFN-γ (SMD: -1.91, 95% CI: -2.71, -1.12), IL-4 (SMD: -0.71, 95% CI: -1.39, -0.03) and IL-17 (SMD: -1.32, 95% CI: -2.05, -0.59) overall. Pooling of clinical studies revealed WBV exercise significantly reduced the level of TNF-α (WBV vs control: SMD: -1.11, 95% CI: -2.16, -0.06; post vs pre: SMD: -1.29, 95% CI: -1.91, -0.67), CRP (SMD: -3.59, 95% CI: -6.36, -0.82, P = 0.011) and enhanced the level of IL-10 (WBV vs control: SMD: 2.90, 95% CI: 1.10, 4.71; post vs pre: SMD: 1.75, 95% CI: 0.64, 2.87) and IL-6 (SMD: 0.91, 95% CI: 0.31, 1.52) (healthy subgroup). Conclusion: WBV may be an effective prevention and rehabilitation tool for inflammatory diseases.
Keywords: Whole-body vibration, inflammation, murine models, clinical trials, meta-analysis
Introduction
Physical exercise has been widely accepted as an important non-pharmacological strategy for prevention and rehabilitation treatment of several diseases [1]. However, the adherence of conventional aerobic and resistance exercise is often low (approximately 60%) in populations due to a lack of time, motivation, companionship, access to specialized facilities and poor physical conditions (such as fragility, reduced cognitive function and motor capacity) that leads to the difficulties to perform active exercise [2,3]. These disadvantages of conventional exercise indicate the requirement of alternative intervention approaches. Whole-body vibration (WBV) involves the exposure of the entire body to mechanical oscillations while the populations stand or sit on a vibration platform [4]. The intensity of vibrations transmitted to the populations can be regulated according to their frequency (5-60 Hz), amplitude (0.5-4 mm), acceleration (0.3 g-8 g) and durations of sessions (5-15 min per session) [4]. This kind of exercise can be assessed at home, in the local community or at rehabilitation units; requires little effort and motivation from the practitioner; needs a low exposure time and is suitable for individuals whom exercise is inconvenient. WBV is therefore suggested as a better alternative exercise. WBV had been applied for rehabilitation treatment of patients with knee osteoarthritis (OA) [5], cerebral palsy [6], metabolic syndrome [7], stroke [8], Parkinson [9] and prevention of falls and fractures in middle-aged and senior people [10,11], the improvement effects of which were confirmed in these meta-analysis studies.
Although the beneficial mechanism of physical exercise may be complex, it may be associated with its anti-inflammatory and immunomodulatory potential [12-15]. Khosravi et al. found exercise training significantly decreased pro-inflammatory markers in cancer survivors, especially C-reactive protein [CRP: standardized mean differences (SMD): -0.5, 95% confidence intervals (CI): -0.9, -0.06, P = 0.025] and tumor necrosis factor (TNF-α: SMD: -0.3, 95% CI: -0.5, -0.06, P = 0.004) [15]. Compared with pre-treatment, TNF-α levels were found to be significantly decreased in adult individuals with multiple sclerosis after regular exercise intervention (SMD: -0.51, 95% CI: -0.91, 0.11, P = 0.01) [13]. CRP was confirmed to be reduced in knee OA patients at 6-18 weeks after regular exercise therapy [14]. These findings from meta-analyses implied WBV, as an exercise model, may also function by changing the expression and secretion of inflammation-related mediators. This hypothesis had been identified in several pre-clinical and clinical studies. For example, Kerr et al. found WBV intervention significantly reduced the levels of interleukin (IL)-6, TNF-α and interferon-γ (IFN-γ) in both female and male stroke model mice [16]. Chen et al. reported WBV treatment inhibited the increase of the IL-1β and TNF-α in the brain sections of traumatic brain injury model mice [17]. Wang et al. demonstrated that regardless of low, middle or high frequency, WBV was effective in decreasing the expression of IL-1β in an early knee OA rat model [18]. Rodriguez-Miguelez et al. detected the TNF-α protein content was lowed, while IL-10 mRNA content and protein concentration increased in the WBV training group compared with the control elderly subjects [19]. Seefried et al. found compared with the baseline value, the level of CRP was significantly lower in maintenance hemodialysis patients after WBV intervention [20]. Oh et al. reported WBV exercise for patients with nonalcoholic fatty liver disease decreased the levels of TNF-α and CRP by 50.8% and 14.5%, respectively (P < 0.05) [21]. However, some studies showed no benefit of WBV on influencing the pro-inflammatory factors IL-1β, IL-6, IL-10, IFN-γ or TNF-α [22-25]. Even, Yu et al. found low-frequency vibration promoted the production of TNF-α to increase cartilage degeneration in knee OA [26]. These results suggested the anti-inflammatory mechanisms of WBV remained inconclusive.
Herein, this study was to perform a meta-analysis of all published studies to re-confirm the effects of WBV exercise on inflammatory factors in healthy or pathological model animals or human subjects. This study may provide a theoretical basis for guiding WBV training as a promising non-pharmacological rehabilitative and prevention method, particularly for inflammatory diseases.
Materials and methods
Search strategy
This meta-analysis was conducted following the 2020 Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. Three English electronic databases (PubMed, EMBASE and Cochrane Library) were searched from inception to September 2023. The search terms included: (“whole body vibration” OR “whole-body vibration” OR “vibration training” OR “vibration exercise” OR “vibration therapy”) AND (“inflammation” OR “inflammatory” OR “inflammatory biomarkers” OR “cytokines” OR “immune cells” OR “immunity”). The systematic reviews, meta-analyses and citation lists of the identified studies were also manually checked for potential eligible articles.
Inclusion and exclusion criteria
Two authors independently performed the literature selection and a third investigator was discussed when discrepancies occurred. Articles included had to meet the population, intervention, comparators, outcomes and study design (PICOS) criteria: (1) participants: murine or human subjects (healthy or pathological); (2) intervention: the experimental group underwent WBV exercise; (3) comparison: comparing with the control group that did not carry out WBV or comparing with the pre-treatment; (4) outcomes: the percentage of immune cells or the levels and expression of inflammatory markers [such as IL-1β, IL-4, IL-6, IL-8, IL-10, IL-17, TNF-α, IFN-γ, MCP-1 (monocyte chemoattractant protein-1), sTNFR1 (soluble TNF receptor 1) and sTNFR2 (soluble TNF receptor 2)] were reported; and (5) study design: randomized controlled trials (RCTs), non-RCTs or uncontrolled trials (post- vs pre-intervention).
Studies were excluded if they were: (1) duplicate publications; (2) secondary research studies such as case reports, systematic review, meta-analysis, conference abstracts, letters, expert comment or protocols; (3) outcomes of interest were not measured, only detected in one study or data (mean and standard deviation) were unavailable; (4) written in a language other than English; and (5) irrelevant topics.
Data extraction and quality assessment
The data were independently extracted by two reviewers and verified by the third author. The following relevant data were extracted from each eligible study and recorded in Microsoft Excel: the first author, year of publication, country, study design, participants (age, gender, disease condition), WBV intervention (machine, frequency, amplitude, acceleration, duration), sample size, outcome data and data assay method. The data in tables and texts were collected directly and the data in figures were estimated by the GetData Graph digitizer software (version 2.26; http://getdata-graph-digitizer.com/).
The methodological quality of the included studies was assessed by two reviewers independently, based on the modified Physiotherapy Evidence Database (PEDro) scale [27]. PEDro consisted 11 items as follows: (1) eligibility criteria, (2) random allocation, (3) concealed allocation, (4) baseline comparability, (5) masked participants, (6) masked therapists, (7) masked assessors, (8) adequate follow-up, (9) intention to treat analysis, (10) between-group comparison, and (11) point estimates and variability. PEDro scores ranged from 0 to 10 points, and studies with PEDro scores higher than 5 were considered to be of high quality.
Statistical analysis
All statistical analyses were conducted using STATA 13.0 (STATA Corporation, College Station, TX, USA). The effects of WBV interventions on each outcome were calculated as the SMD and 95% CIs. A negative effect size represented decreases in the levels of inflammatory markers after WBV exercise. The significance of the combined SMD was estimated by Z-test, with a p-value < 0.05 as the statistical threshold. The heterogeneity between studies was assessed using the χ2 test with Cochran Q and I2 statistic. P < 0.1 and I2 > 50% indicated the presence of a considerable risk of heterogeneity and thus, a random-effects model was selected for the pooled analysis; otherwise, the fixed-effects model was applied. Subgroup analysis was performed based on country (Asian or non-Asian), participants (healthy or diseases), WBV frequency (< 20 or > 20 Hz), WBV duration (< or > one week), assay method (PCR or others) and sample source (blood, synovial fluid or tissues). Publication bias was evaluated by Egger’s linear regression test. If publication bias existed (P < 0.05), the trim-and-fill method was then performed to correct the pooled estimate. The stability of the meta-analysis results was assessed by carrying out a leave-one-out sensitivity analysis (that is: one study was omitted each time and the effect size was then re-calculated).
Results
Search results
As shown in Figure 1, a total of 1647 records were initially identified through searching electronic databases. Of them, 1048 were duplicates and thus excluded. After reviewing the titles and abstracts, 555 studies were removed as they were systematic reviews (n = 17), case reports (n = 23), comments (n = 9), protocol (n = 8), without control (n = 4), and studies unrelated to our topic (n = 494, not WBV or not exploring inflammation mechanisms). The remaining 44 studies were downloaded for full reading, after which 13 of them were eliminated because of no WBV exercise (n = 2), without non-WBV control (n = 2), data unavailable (n = 5) and data only detected in single study (n = 4). Finally, 31 eligible studies were included in this meta-analysis, including 14 pre-clinical [16-18,22,23,28-36] and 17 clinical (9 experiment-control [19,24,25,37-42] that also contained post-pre test data and 8 post-pre [20,21,26,43-47]) studies.
Figure 1.
Flow diagram of included studies.
Study characteristics
The basic characteristics of the included studies are shown in Tables 1 and 2. These 14 pre-clinical studies [16-18,22,23,28-36] were published from 2011 to 2023. All of them were RCTs performed by authors of China (n = 8), Canada (n = 1), Iran (n = 1), Poland (n = 1), Thailand (n = 1) and USA (n = 2). WBV was set as a prevention tool for healthy mice/rats or a treatment approach for stroke, OA, obesity, osteoporosis, atherosclerosis, brain injury, ulcer and type 2 diabetes mellitus model mice/rats (Table 1). Nine clinical studies [19,24,25,37-42] were control trials, including 5 RCTs and 4 non-RCTs. They were published from 2012 to 2023. Their participants were obese, knee OA, chronic obstructive pulmonary disease (COPD), depressed adolescents, premenstrual syndrome (PMS) patients, healthy students and seniors enrolled from Italy (n = 2), Brazil (n = 2), USA (n = 1), Canada (n = 1), Germany (n = 1), Sweden (n = 1) and Egypt (n = 1) (Table 1). Eight studies published from 2014 to 2022 only compared the outcomes between after and before WBV treatment [20,21,26,43-47] (Table 2). Each one study evaluated the effects of WBV for patients with knee OA, interstitial lung disease, COPD, hemodialysis, fibromyalgia, nonalcoholic fatty liver disease; three studies reported the healthy or older humans. These post-pre studies were conducted in China, Germany (n = 2), Brazil (n = 1), USA (n = 1), Spain (n = 1), Brasil (n = 1) or Germany (n = 1) (Table 2). Inflammatory markers in blood (serum, plasma or peripheral-blood mononuclear cells), synovial fluid and various tissues were examined by PCR at mRNA levels, western blot, ELISA, enzyme-linked immunosorbent assay (ELISA), cytometric bead array (CBA), Bio-Plex assay and latex agglutination at protein levels (Table 1). All studies scored ≥ 5 on the PEDro scale, indicating all of them were of high quality (Table S1).
Table 1.
Basic characteristics of included articles that compared WBV vs non-WBV
| Author | Year | Country | Study design | Participants | No | Intervention | Control | Source | Lab method | Outcomes | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-clinical | Jiang D [28] | 2021 | China | RCT | Male aging mice (18-month old) | 12 | WBV in an LD-P vertical vibration machine (f: 15 Hz; am: 2 mm; ac: 0.68 g); for 30 min/day, 6 days/week for 12 weeks | No-WBV | Gastrocnemius muscles | WB | IL-6, MCP-1 |
| Kerr N [16] | 2022 | USA | RCT | Female and male tMCAO (stroke) model rats (10-13-month old) | 23 | WBV in a vibration device (Soloflex) (f: 40 Hz); for 30 min/day, 5 days/week for 30 days | No-WBV | Serum | Bio-Plex assay | IL-1β, IL-4, IL-6, IL-10, IL-17, IFN-γ, TNF-α | |
| Sun C [29] | 2015 | China | RCT | Male HFD (obesity), ND rats (8-week old) | 6 | WBV in an LD-P vertical vibration machine (Huanzhen Machinery Limited Company) (f: 25 Hz); for 30 min/day, 6 days/week for 8 weeks | No-WBV | Adipose | WB | IL-6, TNF-α | |
| Pawlak M [22] | 2013 | Poland | RCT | Adult male rats | 30 | WBV for 120 min in a vibration device (Power plate) (f: 50 Hz; am: 2.5 mm; ac: 4.79 g); for 120 min/day, 5 days/week for 3 and 6 months | No-WBV | Serum | ELISA | IL-1β, IL-6, IL-10 | |
| Wang H [30] | 2023 | China | RCT | Male knee OA model and sham mice (10-week old) | 24 | WBV in a vibration device (BodyGreen) (f: 10 Hz; am: 4 mm; ac: 0.73 g); for 20 min/day, 5 days/week for 4 weeks | No-WBV | Knee joint | PCR | IL-6, TNF-α | |
| Wang L [18] | 2020 | China | RCT | Male knee OA model rats (8-week old) | 40 | WBV in a customized magnetic levitation vibration platform (f: 10, 20, 40, 60 Hz; ac: 0.3 g); for 40 min/day, 5 days/week for 8 weeks | No-WBV | Distal femur | PCR, WB | IL-1β | |
| Tsai SH [31] | 2022 | China | RCT | Female OVX (osteoporosis) model mice (12-week old) | 15 | WBV in a vertically oscillating platform (BodyGreen) (f: 16 Hz; am: 2 mm; ac: 0.68 g); for 60 min/day, 5 days/week for 16 weeks | No-WBV | Splenocytes | ELISA | IL-4, IL-17, IFN-γ | |
| Wu H [32] | 2018 | China | RCT | ApoE-/- (atherosclerosis) model mice (8-week old) | 8 | WBV in an LD-P vertical vibration machine (Huanzhen Machinery Limited Company) (f: 15 Hz; am: 2 mm; ac: 0.68 g); for 30 min/day, 6 days/week for 12 weeks | No-WBV | Thoracic aorta | PCR, WB | IL-6 | |
| Naghii MR [33] | 2011 | Iran | RCT | Male heathy rats | 16 | WBV in a vibration device (f: 10-50 Hz; am: 2 mm; ac: 1-10 g); 15 min, 4 days/week in the first week; 45 min, 3 days/week until day 24; 60 min, 20 days until 8 weeks | No-WBV | Plasma | ELISA | IL-6, TNF-α | |
| McCann MR [34] | 2017 | Canada | RCT | Male healthy mice (10-week-old) | 18 | WBV in a vibration platform (f: 45 Hz; am: 74 µm; ac: 0.3 g); for 30 min/day, 5 days/week) for 2, 4 weeks, 8 weeks | No-WBV | Thoracic IVDs | PCR | IL-1β, IL-6, TNF-α | |
| Wano N [35] | 2021 | Thailand | RCT | Male pressure ulcer model mice (8-week old) | 32 | WBV in a vibration platform (f: 45 Hz; ac: 0.4 g); for 30 min/day, 5 days/week) for one and two weeks | No-WBV | Wound skin tissues | ELISA | TNF-α | |
| Chen T [17] | 2020 | China | RCT | Male TBI model mice (6-8 weeks) | WBV in a vibration machine (Deca Precision Measuring Instruments)(f: 30 Hz); twice per day for 20 days | No-WBV | Brain | ELISA | IL-1β, IL-6, IL-10, TNF-α | ||
| Chow SK [36] | 2019 | China | RCT | Female OVX (osteoporosis) model rats (9-month old) | 48 | WBV in a vibration machine (f: 35 Hz; ac: 0.3 g); for 20 min/d, 5 d/week for 1, 2, 4, 8 weeks | No-WBV | Serum | ELISA | IL-6, IL-10, TNF-α | |
| Weinheimer-Haus EM [23] | 2014 | USA | RCT | Male db/db (T2DM) model mice (12-16-week old) | 24 | WBV in a vibration platform (f: 45 Hz; am: 40 μm; ac: 0.4 g); for 30 min, 5 days/week for one and two weeks | No-WBV | Wound skin tissues | PCR, ELISA | IL-1β, IL-10, MCP-1, TNF-α | |
| Clinical | Bellia A [37] | 2014 | Italy | RCT | Obese patients | 29 | WBV in a dedicated platform (Nemes Perform) (f: 30 Hz; am: 2 mm); for 20 min/day, 3 days/week for 8 weeks | No-WBV | Plasma | Latex agglutination, Lincoplex ELISA | CRP, TNF-α |
| Simão AP [38] | 2012 | Brazil | RCT | Knee OA patients | 21 | WBV in a vibration device (Fitvibe) (f: 35-40 Hz; am: 4 mm; ac: 2.78-3.26 g); 3 days/week for 12 weeks | No-WBV | Plasma | ELISA | sTNFR1, sTNFR2 | |
| Neves CDC [24] | 2018 | Brazil | Non-RCT | COPD patients | 20 | WBV in a vibration device (Fitvibe) (f: 30-40 Hz; am: 2 mm; ac: 1.45-2.25 g); for 3 min, 3 days/week for 12 weeks | No-WBV | Plasma | CBA; ELISA | IL-6, IL-8, IFN-γ; sTNFR1, sTNFR2 | |
| Jawed Y [25] | 2020 | USA | Non-RCT | Healthy male humans | 14 | WBV in a vibration platform (Power Plate my 3) (f: 35 Hz; am: 4 mm); for 8 min | No-WBV | Plasma | ELISA | IL-6, IL-10, TNF-α | |
| Hazell TJ [39] | 2014 | Canada | Non-RCT | Healthy male students | 10 | WBV in a WAVE platform (Windsor) (f: 45 Hz; am: 2 mm); 15 min | No-WBV | Plasma | ELISA | IL-1β, IL-6, IL-10 | |
| Wunram H [40] | 2021 | Germany | Non-RCT | Depressed adolescents | 44 | WBV in a vibration platform (Galileo) (f: 20 Hz; am: 2 mm); for 30 min, 3-5 days/week for 6 weeks | No-WBV | Serum | ELISA, ECLIA | TNF-α, IL-6 | |
| Di Giminiani R [42] | 2020 | Italy | RCT | Healthy male sport science students | 40 | WBV for 24 min | No-WBV | Serum | ELISA | IL-6 | |
| Rodriguez-Miguelez P [19] | 2015 | Sweden | RCT | Seniors | 28 | WBV in a vibration platform (Fitvibe) (f: 20-35 Hz; am: 4 mm); for 0.5-1 min/day, 2 days/week for 8 weeks | No-WBV | PBMC | PCR, WB | IL-10, TNF-α | |
| Shehata MM [41] | 2023 | Egypt | RCT | PMS woman | 70 | WBV in a side-oscillating WBV platform (PS-CFM001) (f: 14 Hz; am: 1 mm); for 13 min, 3 days/week for 12 weeks | No-WBV | Blood | ELISA | CRP |
WBV, whole-body vibration; RCT, randomized controlled trials; tMCAO, transient middle-cerebral artery occlusion; HFD, high-fat diet; ND, normal diet; OA, osteoarthritis; OVX, ovariectomy; TBI, traumatic brain injury; T2DM, type 2 Diabetes Mellitus; COPD, chronic obstructive pulmonary disease; PMS, premenstrual syndrome; f, frequency; am, amplitude; ac, acceleration; IVD, intervertebral disc; PBMC, peripheral-blood mononuclear cells; PCR, polymerase chain reaction; WB, western blot; ELISA, enzyme-linked immunosorbent assay; CBA, cytometric bead array; IL, interleukin; CRP, C-reactive protein; TNF, tumor necrosis factor; IFN, interferon; MCP-1, monocyte chemoattractant protein-1; sTNFR1, soluble TNF receptor 1; sTNFR2, soluble TNF receptor 2.
Table 2.
Basic characteristics of included articles that compared post-WBV vs pre-WBV
| Author | Year | Country | Participants | No | Intervention | Source | Lab method | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Yu PM [26] | 2021 | China | OA patients | 4 | WBV in a vibration device (Fitvibe) (f: 40 Hz; am: 2 mm); for 15 min/day, 5 days/week for 4 weeks | Synovial fluid | ELISA | TNF-α |
| Koczulla AR [43] | 2020 | Germany | ILD patients | 11 | WBV in a side-alternating vibration platform (Galileo) (f: increased from 6 to 26 Hz; am: 4-6 mm); for 6 min/day, 3 days/week for 8 weeks; for 9 min/day, 3 days/week for 4 weeks | Serum | NA | IL-6 |
| Koczulla AR [43] | 2020 | Germany | ILD patients | 16 | WBV in a side-alternating vibration platform (Galileo) (f: 5 Hz; am: 4-6 mm); for 6 min/day, 3 days/week for 4 weeks | Serum | NA | |
| Lage VKS [44] | 2018 | Brazil | COPD patients and healthy humans | 26 | WBV in a vibration device (Fitvibe) (f: 35 Hz; am: 2 mm); for 3 min/day, 5 days/week for 4 weeks | Plasma | CBA; ELISA | IL-6, IL-8, IL-10; sTNFR1, sTNFR2 |
| Sanni AA [47] | 2022 | USA | Healthy humans | 40 | WBV in a vibration platform (Power Plate Pro 5) (f: 30 Hz; am: 5, 2.5 mm; ac: 9 and 4.5 g); for 10 min | Plasma | ELISA | IL-6 |
| Cristi C [45] | 2014 | Spain | Older adults | 9 | WBV in a vibration device (Fitvibe) (f: 30-45 Hz; am: 2 mm; ac: 3.6-8.1 g); for 0.5-1 min/day, 3 days/week for 9 weeks | PBMCs | PCR, WB | CRP, IL-1β, IL-6, IL-10, TNF-α |
| Ribeiro VGC [46] | 2018 | Brasil | Fibromyalgia patients and healthy humans | 38 | WBV in a vibration device (Fitvibe) (f: 40 Hz; am: 4 mm; ac: 2-5 g); for 320 s | Plasma | ELISA | sTNFR1, sTNFR2 |
| Seefried L [20] | 2017 | Germany | Hemodialysis patients | 14 | WBV in a side-alternating vibration platform (Galileo) (f: 22 Hz); for 5 min in the first 4 weeks, 12.5 min during week 5-8 and 20 min in the last weeks 8-12 | Plasma | CRP | |
| Oh S [21] | 2019 | Japan | NAFLD patients | 25 | WBV in a vibration platform (Power Plate Pro-6) (f: 30-50 Hz); for 20 min, 2 days/week for 12 weeks | Plasma | ELISA | IL-6, TNF-α, CRP |
| Bellia A [37] | 2014 | Italy | Obese patients | 15 | WBV in a dedicated platform (Nemes Perform) (f: 30 Hz; am: 2 mm); for 20 min/day, 3 days/week for 8 weeks | Plasma | Latex agglutination, Lincoplex ELISA | CRP, TNF-α |
| Simão AP [38] | 2012 | Brazil | Knee OA patients | 11 | WBV in a vibration device (Fitvibe) (f: 35-40 Hz; am: 4 mm; ac: 2.78-3.26 g); 3 days/week for 12 weeks | Plasma | ELISA | sTNFR1, sTNFR2 |
| Neves CDC [24] | 2018 | Brazil | COPD patients | 10 | WBV in a vibration device (Fitvibe) (f: 30-40 Hz; am: 2 mm; ac: 1.45-2.25 g); for 3 min, 3 days/week for 12 weeks | Plasma | CBA; ELISA | IL-6, IL-8, IFN-γ; sTNFR1, sTNFR2 |
| Jawed Y [25] | 2020 | USA | Healthy male humans | 14 | WBV in a vibration platform (Power Plate my 3) (f: 35 Hz; am: 4 mm); for 8 min | Plasma | ELISA | IL-6, IL-10, TNF-α |
| Hazell TJ [39] | 2014 | Canada | Healthy male students | 10 | WBV in a WAVE platform (Windsor) (f: 45 Hz; am: 2 mm); 15 min | Plasma | ELISA | IL-1β, IL-6, IL-10 |
| Wunram H [40] | 2021 | Germany | Depressed adolescents | 21 | WBV in a vibration platform (Galileo) (f: 20 Hz; am: 2 mm); for 30 min, 3-5 days/week for 6 weeks | Serum | ELISA, ECLIA | TNF-α, IL-6 |
| Di Giminiani R [42] | 2020 | Italy | Healthy male sport science students | 20 | WBV for 24 min | Serum | ELISA | IL-6 |
| Rodriguez-Miguelez P [19] | 2015 | Sweden | Seniors | 16 | WBV in a vibration platform (Fitvibe) (f: 20-35 Hz; am: 4 mm); for 0.5-1 min/day, 2 days/week for 8 weeks | PBMC, serum | PCR, WB, ELISA, IT | IL-10, TNF-α, CRP |
| Shehata MM [41] | 2023 | Egypt | PMS woman | 35 | WBV in a side-oscillating WBV platform (PS-CFM001) (f: 14 Hz; am: 1 mm); for 13 min, 3 days/week for 12 weeks | Blood | ELISA | CRP |
WBV, whole-body vibration; OA, osteoarthritis; ILD, interstitial lung disease; COPD, chronic obstructive pulmonary disease; NAFLD, nonalcoholic fatty liver disease; PMS, premenstrual syndrome; f, frequency; am, amplitude; ac, acceleration; PBMC, peripheral-blood mononuclear cells; PCR, polymerase chain reaction; WB, western blot; ELISA, enzyme-linked immunosorbent assay; ECLIA, electrochemiluminescence immunoassay; CBA, cytometric bead array; IT, immunoturbidimetric; IL, interleukin; CRP, C-reactive protein; TNF, tumor necrosis factor; IFN, interferon; sTNFR1, soluble TNF receptor 1; sTNFR2, soluble TNF receptor 2.
Meta-analysis of pre-clinical studies (WBV vs non-WBV)
Ten studies with 21 datasets compared the level of IL-6 between WBV exercise group and the control group that did not receive WBV (Table S2). As there was significant heterogeneity among these studies (I2 = 80.9%, P < 0.001), a random-effects model was used. The pooled results revealed that compared with the control group, WBV exercise did not cause a significant change in the level of IL-6 (P = 0.144) (Table 3). However, the subgroup analysis showed WBV exercise could significantly reduce the level of IL-6 for the murine models of diseases (SMD: -1.03, 95% CI: -1.93, -0.13, P = 0.024) (Table 3).
Table 3.
Meta-analysis (pre-clinical studies: WBV vs non-WBV)
| Variables | No. | SMD | 95% CI | P E-value | I2 | P H-value | Model | Egger p | ||
|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 | Overall | 21 | -0.52 | -1.21, 0.18 | 0.144 | 80.9 | < 0.001 | R | 0.311 | |
| Country | Asian | 14 | -0.38 | -1.45, 0.69 | 0.489 | 85.4 | < 0.001 | R | ||
| Non-Asian | 7 | -0.71 | -1.41, -0.01 | 0.048 | 60.0 | 0.020 | R | |||
| Participants | Healthy | 9 | 0.16 | -0.88, 1.20 | 0.763 | 82.2 | < 0.001 | R | ||
| Diseases | 12 | -1.03 | -1.93, -0.13 | 0.024 | 77.8 | < 0.001 | R | |||
| WBV frequency | < 20 Hz | 5 | -2.10 | -4.90, 0.71 | 0.142 | 91.3 | < 0.001 | R | ||
| ≥ 20 Hz | 15 | -0.27 | -0.80, 0.25 | 0.304 | 57.4 | 0.003 | R | |||
| Other | 1 | 1.52 | 0.39, 2.65 | 0.008 | - | - | R | |||
| Duration | ≤ 1 week | 1 | 0.56 | -0.60, 1.72 | 0.343 | - | - | R | ||
| > 1 week | 20 | -0.58 | -1.31, 0.15 | 0.118 | 81.5 | < 0.001 | R | |||
| Sample source | Tissue | 12 | -0.82 | -2.04, 0.41 | 0.192 | 84.7 | < 0.001 | R | ||
| Blood | 9 | -0.15 | -0.84, 0.53 | 0.662 | 69.0 | 0.001 | R | |||
| Assay method | PCR | 6 | -0.71 | -2.46, 1.04 | 0.425 | 87.9 | < 0.001 | R | ||
| Other | 15 | -0.43 | -1.17, 0.31 | 0.253 | 77.3 | < 0.001 | R | |||
| IL-1β | Overall | 20 | -2.20 | -3.24, -1.15 | < 0.001 | 90.0 | < 0.001 | R | < 0.001 | |
| Country | Asian | 10 | -4.40 | -5.32, -3.47 | < 0.001 | 47.5 | 0.047 | F | ||
| Non-Asian | 10 | -0.13 | -0.97, 0.72 | 0.770 | 80.2 | < 0.001 | R | |||
| Participants | Healthy | 5 | 0.80 | -0.16, 1.77 | 0.103 | 70.6 | 0.009 | R | ||
| Diseases | 15 | -3.33 | -4.54, -2.12 | < 0.001 | 87.2 | < 0.001 | R | |||
| WBV frequency | < 20 Hz | 4 | -4.87 | -5.90, -3.83 | < 0.001 | 0.0 | 0.494 | F | ||
| ≥ 20 Hz | 16 | -1.47 | -2.49, -0.46 | 0.005 | 88.1 | < 0.001 | R | |||
| Duration | ≤ 1 week | 3 | -0.21 | -1.17, 0.75 | 0.673 | 59.3 | 0.086 | R | ||
| > 1 week | 17 | -2.63 | -3.92, -1.33 | < 0.001 | 90.7 | < 0.001 | R | |||
| Sample source | Tissue | 16 | -2.55 | -3.85, -1.25 | < 0.001 | 90.5 | < 0.001 | R | ||
| Blood | 4 | -0.99 | -2.65, 0.67 | 0.242 | 86.6 | < 0.001 | R | |||
| Assay method | PCR | 9 | -2.02 | -3.88, -0.16 | 0.033 | 91.9 | < 0.001 | R | ||
| Other | 11 | -2.39 | -3.70, -1.08 | < 0.001 | 89.0 | < 0.001 | R | |||
| IL-10 | Overall | 11 | 0.11 | -0.70, 0.92 | 0.788 | 78.0 | < 0.001 | R | 0.940 | |
| Country | Asian | 6 | -0.31 | -1.86, 1.24 | 0.695 | 83.5 | < 0.001 | R | ||
| Non-Asian | 5 | 0.45 | -0.44, 1.33 | 0.323 | 72.3 | 0.006 | R | |||
| Participants | Healthy | 2 | 0.52 | -0.12, 1.16 | 0.114 | 0.0 | 0.915 | F | ||
| Diseases | 9 | -0.00 | -1.07, 1.07 | 0.999 | 81.5 | < 0.001 | R | |||
| Duration | ≤ 1 week | 2 | -2.06 | -5.19, 1.08 | 0.199 | 88.1 | 0.004 | R | ||
| > 1 week | 9 | 0.52 | -0.24, 1.28 | 0.179 | 68.2 | 0.001 | R | |||
| Sample source | Tissue | 3 | 0.40 | -1.06, 1.86 | 0.595 | 65.5 | 0.055 | R | ||
| Blood | 8 | -0.02 | -1.05, 1.01 | 0.970 | 82.0 | < 0.001 | R | |||
| TNF-α | Overall | 20 | -0.82 | -1.68, 0.04 | 0.061 | 86.4 | < 0.001 | R | 0.646 | |
| Country | Asian | 13 | -0.66 | -1.93, 0.62 | 0.310 | 89.7 | < 0.001 | R | ||
| Non-Asian | 7 | -0.94 | -1.93, 0.05 | 0.063 | 74.1 | 0.001 | R | |||
| Participants | Healthy | 6 | 0.12 | -0.58, 0.83 | 0.720 | 44.2 | 0.111 | F | ||
| Diseases | 14 | -1.36 | -2.54, -0.17 | 0.025 | 88.8 | < 0.001 | R | |||
| WBV frequency | < 20 Hz | 2 | -1.56 | -3.53, 0.41 | 0.121 | 74.5 | 0.047 | R | ||
| ≥ 20 Hz | 17 | -0.76 | -1.78, 0.26 | 0.144 | 87.9 | < 0.001 | R | |||
| Other | 1 | 0.00 | -0.98, 0.98 | 1.000 | - | - | R | |||
| Duration | ≤ 1 week | 4 | -1.42 | -4.15, 1.32 | 0.310 | 93.9 | < 0.001 | R | ||
| > 1 week | 16 | -0.68 | -1.55, 0.19 | 0.126 | 82.9 | < 0.001 | R | |||
| Sample source | Tissue | 13 | -1.09 | -2.28, 0.10 | 0.073 | 87.5 | < 0.001 | R | ||
| Blood | 7 | -0.27 | -1.34, 0.80 | 0.622 | 79.8 | < 0.001 | R | |||
| Assay method | PCR | 7 | -0.61 | -1.38, 0.15 | 0.118 | 61.9 | 0.015 | R | ||
| Other | 13 | -0.84 | -2.19, 0.52 | 0.227 | 90.2 | < 0.001 | R | |||
| IFN-γ | Overall | 3 | -1.91 | -2.71, -1.12 | < 0.001 | 0.0 | 0.968 | F | 0.786 | |
| IL-4 | Overall | 3 | -0.71 | -1.39, -0.03 | 0.040 | 34.2 | 0.219 | F | 0.982 | |
| IL-17 | Overall | 3 | -1.32 | -2.05, -0.59 | < 0.001 | 33.9 | 0.220 | F | 0.250 | |
| MCP-1 | Overall | 2 | -0.97 | -11.74, 9.81 | 0.861 | 97.4 | < 0.001 | R | - | |
IL, interleukin; TNF, tumor necrosis factor; IFN, interferon; MCP-1, monocyte chemoattractant protein-1; SMD, standardized mean difference; CI, confidence interval; F, fixed-effects; R, random-effects; PH-value, significance for heterogeneity; PE-value, significance for effects.
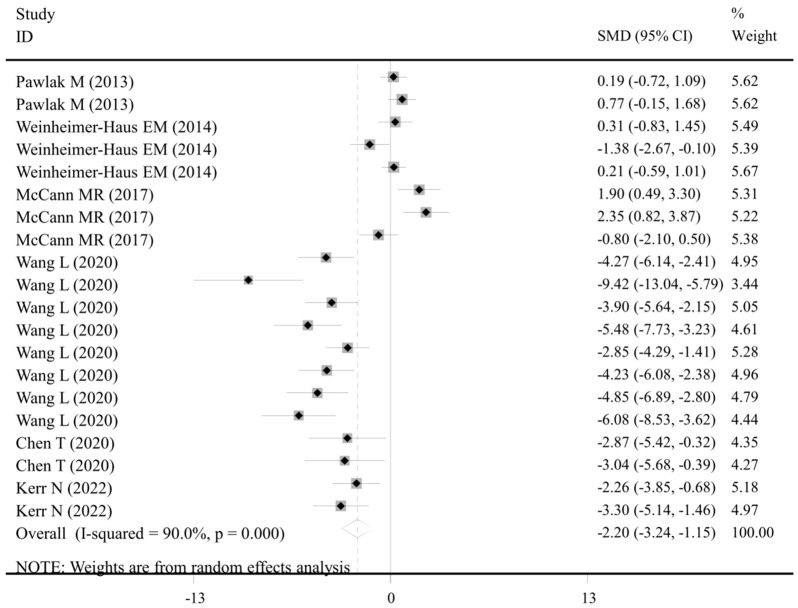
Six studies with 20 datasets recorded the level of IL-1β in the WBV exercise group and the control group that did not receive WBV (Table S2). Under the random-effects model (I2 = 90.0%, P < 0.001), the pooled results showed that compared with the control group, WBV exercise significantly inhibited the level of IL-1β (SMD: -2.20, 95% CI: -3.24, -1.15, P < 0.001) (Table 3; Figure 2). The subgroup analysis confirmed that WBV frequency and IL-6 assay methods did not influence the inhibition effect of WBV exercise on IL-6 (all still significant), but long-term WBV exercise (> one week) may be more effective for disease control (Table 3).
Figure 2.
Forest plot of the effects of WBV exercise on interleukin-1β levels. Data were from pre-clinical studies comparing WBV with controls (overall). WBV, whole-body vibration; SMD, standardized mean difference; CI, confidence interval.
Five studies with 11 datasets measured the level of IL-10 in the WBV and non-WBV groups (Table S2). Under the random-effects model (I2 = 78.0%, P < 0.001), the combined results showed no significant difference in the level of IL-10 between two groups (P = 0.788) (Table 3). The subgroup analysis also confirmed that WBV exercise had no effect on IL-10 (P > 0.05) (Table 3).
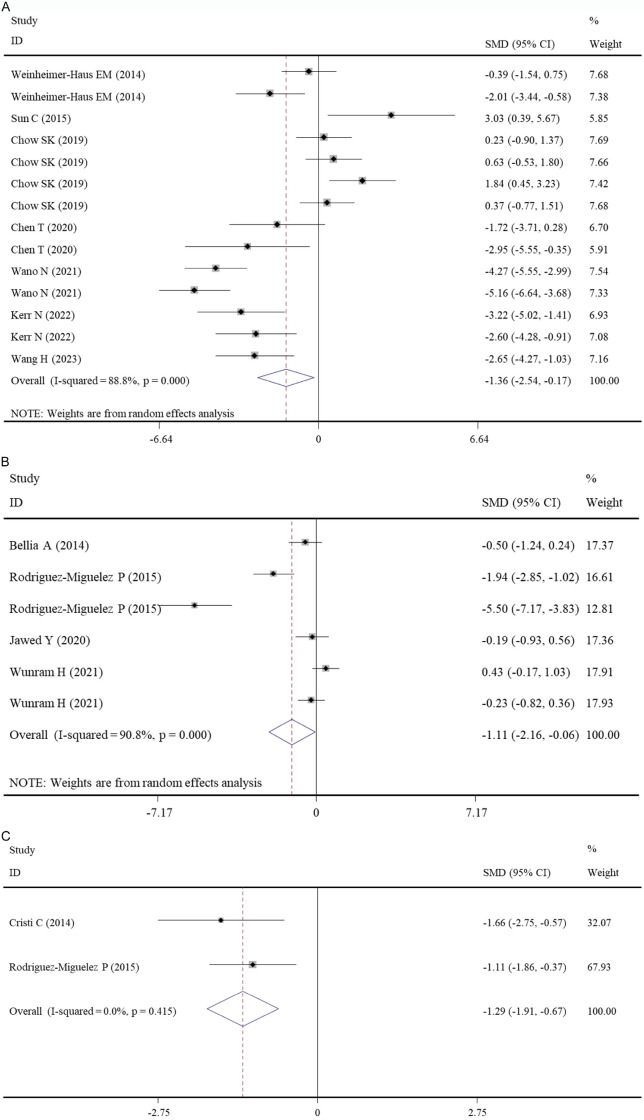
Nine studies with 20 datasets examined the level of TNF-α in murine which underwent WBV or not (Table S2). The meta-analysis with a random-effects model (I2 = 86.4%, P < 0.001) showed WBV exercise only induced a borderline statistically significant improvement in TNF-α (SMD: -0.82, 95% CI: -1.68, 0.04, P = 0.061) (Table 3). The subgroup analysis found that WBV exercise may be particularly suitable for disease models to reduce their TNF-α levels (SMD: -1.36, 95% CI: -2.54, -0.17, P = 0.025) (Table 3; Figure 3A).
Figure 3.
Forest plot of the effects of WBV exercise on tumor necrosis factor-α levels. A. Data from pre-clinical studies comparing WBV exercise with controls (subgroup, disease models); B. Data from clinical studies comparing WBV exercise with control (overall); C. Data from clinical studies comparing post- with pre-WBV exercise (subgroup, PCR assay). WBV, whole-body vibration; SMD, standardized mean difference; CI, confidence interval.
Two studies provided the data of IFN-γ, IL-4, IL-17 and MCP-1 in two groups. The pooled results concluded that compared with the control group, WBV exercise significantly suppressed the level of IFN-γ (SMD: -1.91, 95% CI: -2.71, -1.12, P < 0.001), IL-4 (SMD: -0.71, 95% CI: -1.39, -0.03, P = 0.04), IL-17 (SMD: -1.32, 95% CI: -2.05, -0.59, P < 0.001), but had not effect on MCP-1 (P > 0.05) (Table 3). Subgroup analysis was not performed for them because of too few articles included.
Meta-analysis of clinical studies (WBV vs non-WBV)
Five studies with 10 datasets investigated the differences of IL-6 in people undergoing WBV exercise or not (Table S2). Findings from the random-effect model (I2 = 64.2%, P = 0.003) showed that the level of IL-6 was not statistically different between these two groups (P = 0.751). This non-significant result was also demonstrated in all subgroups (P > 0.05) (Table 4).
Table 4.
Meta-analysis (clinical studies: WBV vs non-WBV)
| Variables | No. | SMD | 95% CI | P E-value | I2 | P H-value | Model | Egger p | ||
|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 | Overall | 10 | 0.06 | -0.32, 0.45 | 0.751 | 64.2 | 0.003 | R | 0.118 | |
| Participants | Healthy | 7 | -0.08 | -0.54, 0.38 | 0.736 | 63.1 | 0.013 | R | ||
| Diseases | 3 | 0.38 | -0.24, 1.00 | 0.229 | 59.2 | 0.086 | R | |||
| WBV frequency | ≥ 20 Hz | 7 | 0.03 | -0.37, 0.44 | 0.877 | 49.9 | 0.063 | F | ||
| NA | 3 | 0.16 | -0.79, 1.10 | 0.746 | 84.8 | 0.001 | R | |||
| Duration | ≤ 1 week | 3 | 0.38 | -0.24, 1.00 | 0.229 | 59.2 | 0.086 | R | ||
| > 1 week | 7 | -0.08 | -0.54, 0.38 | 0.736 | 63.1 | 0.013 | R | |||
| IL-10 | Overall | 6 | 2.90 | 1.10, 4.71 | 0.002 | 93.7 | < 0.001 | R | 0.002 | |
| Duration | ≤ 1 week | 4 | 0.65 | 0.22, 1.09 | 0.003 | 0.0 | 0.525 | F | ||
| > 1 week | 2 | 10.19 | 2.16, 18.22 | 0.013 | 92.4 | < 0.001 | R | |||
| Assay method | PCR | 5 | 1.97 | 0.36, 3.57 | 0.017 | 91.6 | < 0.001 | R | ||
| Other | 1 | 6.29 | 4.42, 8.16 | < 0.001 | - | - | R | |||
| TNF-α | Overall | 6 | -1.11 | -2.16, -0.06 | 0.038 | 90.8 | < 0.001 | R | 0.003 | |
| Participants | Healthy | 3 | -2.43 | -4.90, 0.04 | 0.054 | 94.2 | < 0.001 | R | ||
| Diseases | 3 | -0.07 | -0.61, 0.47 | 0.793 | 52.6 | 0.121 | R | |||
| Duration | ≤ 1 week | 1 | -0.19 | -0.93, 0.56 | 0.625 | - | - | R | ||
| > 1 week | 5 | -1.35 | -2.64, -0.06 | 0.041 | 92.6 | < 0.001 | R | |||
| Assay method | PCR | 1 | -1.94 | -2.85, -1.02 | < 0.001 | - | - | R | ||
| Other | 5 | -0.93 | -2.05, 0.18 | 0.101 | 90.8 | < 0.001 | R | |||
| CRP | Overall | 2 | -5.08 | -14.82, 4.66 | 0.307 | 99.0 | < 0.001 | R | - | |
| sTNFR1 | Overall | 2 | 0.94 | -1.22, 3.10 | 0.393 | 89.5 | < 0.001 | R | - | |
| sTNFR2 | Overall | 2 | 0.22 | -0.69, 1.14 | 0.630 | 53.2 | 0.144 | R | - | |
| IL-1β | Overall | 3 | -0.04 | -0.55, 0.47 | 0.879 | 0.0 | 0.984 | F | 0.063 | |
IL, interleukin; TNF, tumor necrosis factor; CRP, C-reactive protein; sTNFR1, soluble TNF receptor 1; sTNFR2, soluble TNF receptor 2; SMD, standardized mean difference; CI, confidence interval; F, fixed-effects; R, random-effects; PH-value, significance for heterogeneity; PE-value, significance for effects.
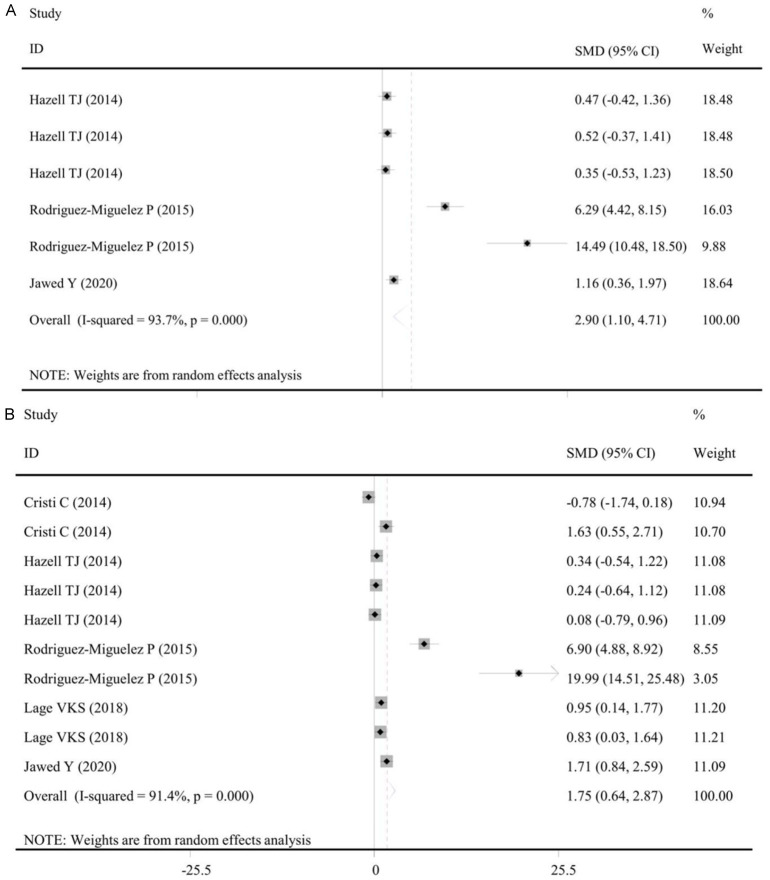
Three studies with 6 datasets measured the level of IL-10 in the WBV and non-WBV groups (Table S2). Under the random-effects model (I2 = 93.7%, P < 0.001), the pooled results showed WBV exercise significantly enhanced the level of IL-10 compared with controls (SMD: 2.90, 95% CI: 1.10, 4.71, P = 0.002) (Table 4; Figure 4A), which also confirmed in all subgroups (Table 4).
Figure 4.
Forest plot of the effects of WBV exercise on interleukin-10 levels. A. Data from clinical studies comparing WBV exercise with control (overall); B. Data from clinical studies comparing post- with pre-WBV exercise (overall). WBV, whole-body vibration; SMD, standardized mean difference; CI, confidence interval.
Four studies with 6 datasets examined the level of TNF-α in individuals which underwent WBV or not (Table S2). The meta-analysis with a random-effects model (I2 = 86.4%, P < 0.001) showed in comparison with control, WBV exercise significantly reduced the level of TNF-α (SMD: -1.11, 95% CI: -2.16, -0.06, P = 0.038) (Table 4; Figure 3B). The subgroup analysis found that WBV exercise may be more effective when it lasted for more than one week (P < 0.05) (Table 3).
Two and three datasets respectively analyzed the data of CRP, sTNFR1, sTNFR2, and IL-1β between two groups. The meta-analysis identified no statistically significant differences in these four inflammatory mediators between two groups (P > 0.05) (Table 4). Subgroup analysis was not performed for them because of too few articles included.
Meta-analysis of clinical studies (post-WBV vs pre-WBV)
Ten studies with 25 datasets compared the level of IL-6 in populations before and after WBV exercise (Table S2). Surprisingly, the pooled analysis showed that WBV exercise increased the level of IL-6 compared with pre-treatment (SMD: 0.91, 95% CI: 0.31, 1.52, P = 0.003) (Table 5). This significant result was also demonstrated in several subgroups (Non-Asian country, healthy participants, WBV frequency ≥ 20 Hz, duration ≤ 1 week and assay by PCR, WB and ELISA; P < 0.05) (Table 5).
Table 5.
Meta-analysis (clinical studies: post-WBV vs pre-WBV)
| Variables | No. | SMD | 95% CI | P E-value | I2 | P H-value | Model | Egger p | ||
|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 | Overall | 25 | 0.91 | 0.31, 1.52 | 0.003 | 93.2 | < 0.001 | R | < 0.001 | |
| Country | Asian | 1 | -0.40 | -0.96, 0.16 | 0.163 | - | - | R | ||
| Non-Asian | 24 | 0.97 | 0.34, 1.61 | 0.003 | 93.3 | < 0.001 | R | |||
| Participants | Healthy | 18 | 1.33 | 0.47, 2.20 | 0.002 | 94.6 | < 0.001 | R | ||
| Diseases | 7 | -0.12 | -0.42, 0.18 | 0.428 | 24.9 | 0.239 | F | |||
| WBV frequency | < 20 Hz | 1 | -0.29 | -0.98, 0.41 | 0.421 | - | - | R | ||
| ≥ 20 Hz | 20 | 0.75 | 0.11, 1.40 | 0.022 | 92.6 | < 0.001 | R | |||
| NA | 4 | 2.09 | -0.31, 4.49 | 0.088 | 96.5 | < 0.001 | R | |||
| Duration | ≤ 1 week | 17 | 1.40 | 0.52, 2.28 | 0.002 | 94.7 | < 0.001 | R | ||
| > 1 week | 8 | -0.10 | -0.52, 0.32 | 0.641 | 60.2 | 0.014 | R | |||
| Assay method | PCR | 1 | 1.36 | 0.33, 2.40 | 0.010 | - | - | R | ||
| Other | 22 | 1.00 | 0.32, 1.68 | 0.004 | 93.9 | < 0.001 | R | |||
| NA | 2 | -0.19 | -0.73, 0.34 | 0.480 | 0.0 | 0.681 | F | |||
| IL-1β | Overall | 5 | 0.39 | -0.23, 1.01 | 0.216 | 54.4 | 0.067 | R | 0.021 | |
| Duration | ≤ 1 week | 3 | 0.13 | -0.38, 0.64 | 0.616 | 0.0 | 0.713 | F | ||
| > 1 week | 2 | 0.90 | -0.92, 2.71 | 0.332 | 83.9 | 0.013 | R | |||
| Assay method | PCR | 1 | 1.85 | 0.73, 2.97 | 0.001 | - | - | F | ||
| Other | 4 | 0.10 | -0.35, 0.55 | 0.661 | 0.0 | 0.865 | F | |||
| CRP | Overall | 8 | -1.81 | -3.97, 0.35 | 0.100 | 97.6 | < 0.001 | R | 0.286 | |
| Country | Asian | 1 | -1.53 | -2.16, -0.89 | < 0.001 | - | - | R | ||
| Non-Asian | 7 | -1.87 | -4.55, 0.81 | 0.172 | 97.9 | < 0.001 | R | |||
| Participants | Healthy | 3 | 1.15 | -1.75, 4.06 | 0.437 | 95.2 | < 0.001 | R | ||
| Diseases | 5 | -3.59 | -6.36, -0.82 | 0.011 | 98.0 | < 0.001 | R | |||
| WBV frequency | < 20 Hz | 2 | -8.40 | -9.45, -7.35 | < 0.001 | 0.0 | 1.000 | F | ||
| ≥ 20 Hz | 6 | 0.29 | -1.09, 1.66 | 0.683 | 93.6 | < 0.001 | R | |||
| Assay method | PCR | 1 | 1.85 | 0.73, 2.98 | 0.001 | - | - | R | ||
| Other | 6 | -2.69 | -5.56, 0.20 | 0.068 | 98.1 | < 0.001 | R | |||
| NA | 1 | -0.36 | -1.10, 0.39 | 0.350 | - | - | R | |||
| IL-10 | Overall | 10 | 1.75 | 0.64, 2.87 | 0.002 | 91.4 | < 0.001 | R | 0.001 | |
| Participants | Healthy | 9 | 1.95 | 0.67, 3.23 | 0.003 | 92.3 | < 0.001 | |||
| Diseases | 1 | 0.95 | 0.14, 1.77 | 0.022 | - | - | ||||
| Duration | ≤ 1 week | 6 | 0.70 | 0.23, 1.18 | 0.004 | 45.6 | 0.102 | R | ||
| > 1 week | 4 | 5.87 | 1.55, 10.20 | 0.008 | 96.8 | < 0.001 | R | |||
| Assay method | PCR | 2 | 3.01 | -4.52, 10.53 | 0.433 | 97.8 | < 0.001 | R | ||
| Other | 8 | 1.36 | 0.35, 2.38 | 0.009 | 88.1 | < 0.001 | R | |||
| TNF-α | Overall | 11 | -0.02 | -1.31, 1.27 | 0.974 | 95.4 | < 0.001 | R | 0.898 | |
| Country | Asian | 2 | -0.37 | -9.37, 8.63 | 0.936 | 97.2 | < 0.001 | R | ||
| Non-Asian | 9 | 0.16 | -1.01, 1.32 | 0.791 | 94.1 | < 0.001 | R | |||
| Participants | Healthy | 7 | -0.62 | -2.84, 1.61 | 0.588 | 96.9 | < 0.001 | R | ||
| Diseases | 4 | 0.61 | -0.23, 1.45 | 0.157 | 76.0 | 0.006 | R | |||
| Duration | ≤ 1 week | 1 | -0.19 | -1.58, 1.19 | < 0.001 | - | - | R | ||
| > 1 week | 10 | 1.65 | 0.78, 2.52 | 0.786 | 95.5 | < 0.001 | R | |||
| Sample source | Synovial fluid | 1 | 4.32 | 1.51, 7.12 | 0.003 | - | - | R | ||
| Blood | 9 | -0.33 | -1.65, 0.98 | 0.619 | 95.7 | < 0.001 | R | |||
| Assay method | PCR | 2 | -1.29 | -1.91, -0.67 | < 0.001 | 0.0 | 0.415 | F | ||
| Other | 9 | -0.84 | -2.19, 0.52 | 0.696 | 96.0 | < 0.001 | R | |||
| sTNFR1 | Overall | 6 | -0.04 | -1.94, 1.86 | 0.969 | 95.9 | < 0.001 | R | 0.888 | |
| Participants | Healthy | 2 | 2.51 | -1.69, 6.70 | 0.242 | 96.9 | < 0.001 | R | ||
| Diseases | 4 | -1.28 | -3.23, 0.76 | 0.217 | 94.6 | < 0.001 | R | |||
| Duration | ≤ 1 week | 4 | 0.22 | -2.64, 3.07 | 0.882 | 97.2 | < 0.001 | R | ||
| > 1 week | 2 | -0.53 | -2.85, 1.78 | 0.650 | 91.6 | 0.001 | R | |||
| sTNFR2 | Overall | 6 | 0.22 | -1.31, 1.74 | 0.781 | 94.4 | < 0.001 | R | 0.210 | |
| Participants | Healthy | 2 | -0.86 | -2.83, 1.10 | 0.388 | 92.3 | < 0.001 | R | ||
| Diseases | 4 | 0.79 | -1.46, 3.03 | 0.491 | 95.4 | < 0.001 | R | |||
| Duration | ≤ 1 week | 4 | 0.22 | -2.64, 3.07 | 0.882 | 97.2 | < 0.001 | R | ||
| > 1 week | 2 | -0.53 | -2.85, 1.78 | 0.650 | 91.6 | 0.001 | R | |||
| IL-8 | Overall | 3 | -0.05 | -0.51, 0.42 | 0.850 | 0.0 | 0.378 | F | 0.951 | |
IL, interleukin; TNF, tumor necrosis factor; CRP, C-reactive protein; sTNFR1, soluble TNF receptor 1; sTNFR2, soluble TNF receptor 2; SMD, standardized mean difference; CI, confidence interval; F, fixed-effects; R, random-effects; PH-value, significance for heterogeneity; PE-value, significance for effects.
Two studies with 5 datasets measured the level of IL-1β in populations before and after WBV exercise (Table S2). The pooled results showed WBV exercise did not have effects on the level of IL-1β (P = 0.216) (Table 5), which also confirmed in subgroup analyses (Table 5).
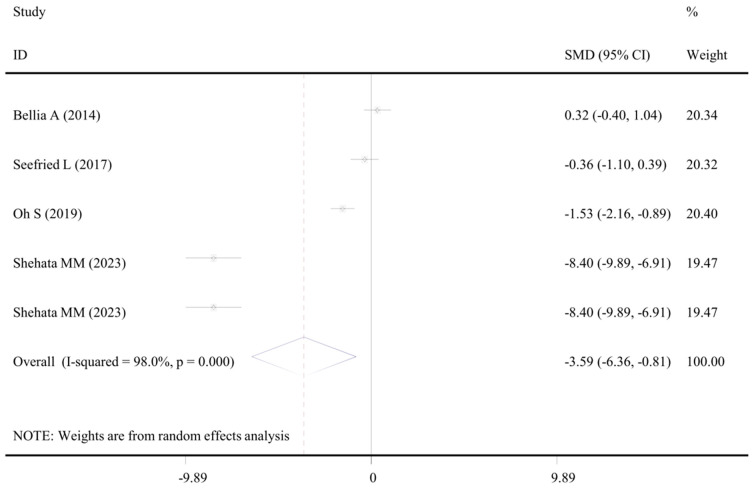
Four studies with 6 datasets examined the level of CRP in populations before and after WBV exercise (Table S2). The overall meta-analysis did not detect a significant difference (P = 0.100), but the subgroup analysis indicated compared with the baseline value, the level of CRP in populations with diseases was significantly decreased after WBV exercise (SMD: -3.59, 95% CI: -6.36, -0.82, P = 0.011) (Table 5; Figure 5).
Figure 5.
Forest plot of the effects of WBV exercise on C-reactive protein levels. Data were from clinical studies comparing post- with pre-WBV exercise (disease subgroup). WBV, whole-body vibration; SMD, standardized mean difference; CI, confidence interval.
Five studies with 10 datasets recorded the level of IL-10 in populations before and after WBV exercise (Table S2). Under the random-effects model (I2 = 91.4%, P < 0.001), the pooled results showed the level of IL-10 in the populations undergoing WBV exercise was significantly higher than that before treatment (SMD: 1.75, 95% CI: 0.64, 2.87, P = 0.002) (Table 5; Figure 4B), which also confirmed in all subgroups (Table 5). This significant result was also demonstrated in several subgroups (Non-Asian country, healthy participants, WBV frequency ≥ 20 Hz, duration ≤ 1 week and assay by PCR, WB and ELISA; P < 0.05) (Table 5).
Seven studies with 11 datasets examined the level of TNF-α in individuals before and after WBV exercise (Table S2). Although the overall meta-analysis indicated the level of TNF-α was not significantly changed following WBV exercise (P > 0.05) (Table 5), the subgroup analysis found that WBV exercise may reduce the expression of TNF-α at mRNA level (SMD: -1.29, 95% CI: -1.91, -0.67, P < 0.001) (Table 5; Figure 3C).
Four studies with 6 datasets investigated the difference of sTNFR1 and sTNFR2 between before and after WBV exercise. Both of the overall and subgroup meta-analyses did not identify statistically significant differences in these two indicators between before and after WBV exercise (P > 0.05) (Table 5).
Two studies with 3 datasets analyzed the change of IL-8 before and after WBV exercise. The meta-analysis identified the level of IL-8 was not significantly altered after WBV exercise (P = 0.850) (Table 5).
Publication bias and sensitivity analyses
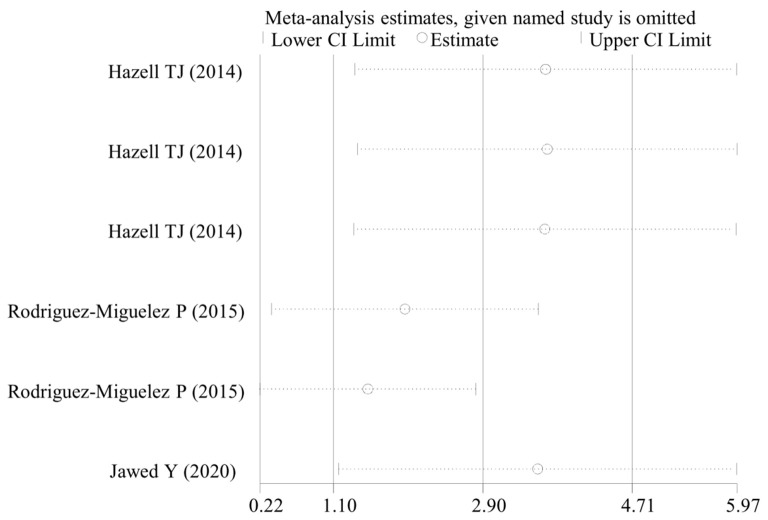
Egger’s test was performed to determine the potential publication bias. The results showed that there were significant publication bias in the analysis of IL-1β with the pre-clinical studies (P < 0.001), IL-10 (P = 0.002), TNF-α (P = 0.003) with the WBV vs non-WBV clinical studies, IL-6 (P < 0.001), IL-1β (P = 0.021) and IL-10 (P = 0.001) with the post-WBV vs pre-WBV clinical studies. After filling the missing data using the trim-and-fill method to adjust publication bias, the SMD of IL-1β and TNF-α were not changed; the SMD for IL-6 (0.74, 95% CI: 0.11, 1.37) was slightly changed, but the result remained significant (P = 0.02); the level of IL-10 (WBV vs non-WBV: SMD: 1.52, 95% CI: 3.61, -0.56, P = 0.152; post- vs pre-WBV: SMD: 0.63, 95% CI: -0.74, 2.00, P = 0.370) was found not to be significantly increased. These findings indicated the caution to use IL-10 changes to explain the function mechanisms of WBV exercise. However, the sensitivity analyses demonstrated our results on IL-10 were stable (Figure 6).
Figure 6.
Sensitivity analysis for IL-10. CI, confidence interval.
Discussion
Although a recent study had linked the inflammatory biomarker responses and WBV [48], this only preliminarily reviewed four individual clinical studies. To the best of our knowledge, our study was the first meta-analysis of all published pre-clinical (14) and clinical (17) articles to comprehensively investigate the effects of WBV exercise on the levels of various inflammatory mediators. The significant results from our study can be summarized in the following five points: 1) meta-analysis of pre-clinical studies showed compared with controls, WBV exercise significantly reduced IL-1β, IFN-γ, IL-4 and IL-17 levels in murine models; 2) meta-analysis of pre-clinical and clinical studies showed in comparison with controls or pre-intervention, WBV exercise had significant effects on inhibiting TNF-α levels; 3) meta-analysis of clinical studies showed relative to controls or pre-intervention, WBV exercise enhanced IL-10 levels; 4) IL-6 was found to be decreased in disease murine models by WBV exercise compared with control, while was observed to be increased from pre- to post-WBV intervention; and 5) WBV exercise resulted in significant decreases in CRP for patients from pre- to post-intervention.
TNF-α was reported to be located in the upstream of the cytokine cascade [49]. TNF-α activated mitogen-activated protein kinase, p38, and nuclear factor kappa B signaling pathways and then induced the expression of other inflammatory mediators, such as IL-1β, IL-6, IL-8, CRP and MCP-1 [50-52]. Thus, up-regulation of TNF-α was a very critical step contributing to the development of various inflammation-related diseases, including stroke [53], obesity [54], OA [55], depression [56] and NAFLD [57]. Inactivating or blocking TNF-α was considered as the main target in the treatment of these inflammation-related diseases [58]. In line with this hypothesis, several studies found WBV lowered the levels of TNF-α as well as its downstream (IL-1β and CRP) and alleviated the symptoms [21,37]. This hypothesis was also confirmed in our meta-analysis of both pre-clinical and clinical studies and previous studies that focused on other exercise interventions [13,15].
IL-6 was analyzed in both pre-clinical and clinical studies. It decreased in pre-clinical RCT, but no change in clinical control studies and even increased in post-pre-clinical studies. These differences may be attributed to two aspects: 1) most of participants in clinical studies were healthy. IL-6 was also proved to stimulate growth and proliferation of normal cells [59]. Thus, the increase in IL-6 levels by WBV may be a protective inflammatory pathway to prevent the development of apoptotic diseases; 2) only one session of WBV exercise (< 30 min) was performed in several clinical studies [25,39], while multiple bouts of WBV exercise [22,28,29,32] were applied in animal studies. Thus, the non-significant change in IL-6 levels may be due to the low effectiveness of short-term WBV exercise.
The present meta-analysis has some limitations. First, the number of included studies was relative small for several inflammatory mediators, particularly IL-1β of clinical trials which may be the reasons to lead to a significant decrease by WBV in animal studies, but not in clinical trials. Although IFN-γ, IL-4 and IL-17 were found to be significantly decreased by WBV, only three datasets were included and thus the conclusion remained dubious. The effects of WBV on immune cells (e.g. Treg [60], lymphocytes [61]) could not be analyzed because only one study reported them. Second, the study heterogeneity existed for analysis of most of indicators and could not be removed by the subgroup analysis, which may also influence the reliability of our conclusion. Third, some data were extracted with the GetData Digitizer, which may be slightly different from the actual data. Thus, more experiments (particularly RCT clinical trials) should be performed to confirm the anti-inflammatory effects of WBV exercise.
Conclusion
This present meta-analysis based on the available pre-clinic and clinical evidence suggests that WBV exercise may mainly function by reducing the levels of pro-inflammatory IL-1β, TNF-α, CRP and enhancing the level of anti-inflammatory IL-10. It regulation roles on IL-6 may be different for healthy populations and patients.
Acknowledgements
This study was supported by the program of Shanghai Science and Technology Committee (Grant Nos. 22Y11912100, 20S31905600) and Geriatric Rehabilitation-Nursing Innovation Center Project of Shanghai Sanda University (No. 2023-14).
Disclosure of conflict of interest
None.
Table S1
Table S2
References
- 1.Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25(Suppl 3):1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 2.Lin B, Zhang Z, Mei Y, Liu L, Ping Z. The influential factors of adherence to physical activity and exercise among community-dwelling stroke survivors: a path analysis. J Clin Nurs. 2022;31:2632–2643. doi: 10.1111/jocn.16091. [DOI] [PubMed] [Google Scholar]
- 3.O’Neil-Pirozzi TM, Cattaneo G, Solana-Sánchez J, Gomes-Osman J, Pascual-Leone A. The importance of motivation to older adult physical and cognitive exercise program development, initiation, and adherence. Front Aging. 2022;3:773944. doi: 10.3389/fragi.2022.773944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martínez F, Rubio JA, Ramos DJ, Esteban P, Mendizábal S, Jiménez F. Effects of 6-week whole body vibration training on the reflex response of the ankle muscles: a randomized controlled trial. Int J Sports Phys Ther. 2013;8:15–24. [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu CG, Chui CS, Chow SKH, Cheung WH, Wong RMY. Effects of whole-body vibration therapy on knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. J Rehabil Med. 2022;54:jrm00266. doi: 10.2340/jrm.v54.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saquetto M, Carvalho V, Silva C, Conceição C, Gomes-Neto M. The effects of whole body vibration on mobility and balance in children with cerebral palsy: a systematic review with meta-analysis. J Musculoskelet Neuronal Interact. 2015;15:137–144. [PMC free article] [PubMed] [Google Scholar]
- 7.Coelho-Oliveira AC, Monteiro-Oliveira BB, Gonçalves de Oliveira R, Reis-Silva A, Ferreira-Souza LF, Lacerda ACR, Mendonça VA, Sartorio A, Taiar R, Bernardo-Filho M, Sá-Caputo D. Evidence of use of whole-body vibration in individuals with metabolic syndrome: a systematic review and meta-analysis. Int J Environ Res Public Health. 2023;20:3765. doi: 10.3390/ijerph20043765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park YJ, Park SW, Lee HS. Comparison of the effectiveness of whole body vibration in stroke patients: a meta-analysis. Biomed Res Int. 2018;2018:5083634. doi: 10.1155/2018/5083634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arenales Arauz YL, Ahuja G, Kamsma YPT, Kortholt A, van der Zee EA, van Heuvelen MJG. Potential of whole-body vibration in Parkinson’s disease: a systematic review and meta-analysis of human and animal studies. Biology (Basel) 2022;11:1238. doi: 10.3390/biology11081238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jepsen DB, Thomsen K, Hansen S, Jørgensen NR, Masud T, Ryg J. Effect of whole-body vibration exercise in preventing falls and fractures: a systematic review and meta-analysis. BMJ Open. 2017;7:e018342. doi: 10.1136/bmjopen-2017-018342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma C, Liu A, Sun M, Zhu H, Wu H. Effect of whole-body vibration on reduction of bone loss and fall prevention in postmenopausal women: a meta-analysis and systematic review. J Orthop Surg Res. 2016;11:24. doi: 10.1186/s13018-016-0357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salimans L, Liberman K, Njemini R, Kortekaas Krohn I, Gutermuth J, Bautmans I. The effect of resistance exercise on the immune cell function in humans: a systematic review. Exp Gerontol. 2022;164:111822. doi: 10.1016/j.exger.2022.111822. [DOI] [PubMed] [Google Scholar]
- 13.Shobeiri P, Seyedmirzaei H, Karimi N, Rashidi F, Teixeira AL, Brand S, Sadeghi-Bahmani D, Rezaei N. IL-6 and TNF-α responses to acute and regular exercise in adult individuals with multiple sclerosis (MS): a systematic review and meta-analysis. Eur J Med Res. 2022;27:185. doi: 10.1186/s40001-022-00814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puts S, Liberman K, Leysen L, Forti L, Muyldermans E, Vaes P, Nijs J, Beckwée D, Bautmans I. Exercise-induced effects on inflammatory markers and brain-derived neurotrophic factor in patients with knee osteoarthritis. A systematic review with meta-analysis. Exerc Immunol Rev. 2023;29:22–53. [PubMed] [Google Scholar]
- 15.Khosravi N, Stoner L, Farajivafa V, Hanson ED. Exercise training, circulating cytokine levels and immune function in cancer survivors: a meta-analysis. Brain Behav Immun. 2019;81:92–104. doi: 10.1016/j.bbi.2019.08.187. [DOI] [PubMed] [Google Scholar]
- 16.Kerr N, Sanchez J, Moreno WJ, Furones-Alonso OE, Dietrich WD, Bramlett HM, Raval AP. Post-stroke low-frequency whole-body vibration improves cognition in middle-aged rats of both sexes. Front Aging Neurosci. 2022;14:942717. doi: 10.3389/fnagi.2022.942717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen T, Liu WB, Ren X, Li YF, Li W, Hang CH, Wang YH. Whole body vibration attenuates brain damage and neuroinflammation following experimental traumatic brain injury. Front Cell Dev Biol. 2022;10:847859. doi: 10.3389/fcell.2022.847859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Wang Z, Liu Q, Su J, Wang T, Li T. Effect of whole body vibration on HIF-2α expression in SD rats with early knee osteoarthritis. J Bone Miner Metab. 2020;38:491–500. doi: 10.1007/s00774-020-01092-3. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Miguelez P, Fernandez-Gonzalo R, Collado PS, Almar M, Martinez-Florez S, de Paz JA, González-Gallego J, Cuevas MJ. Whole-body vibration improves the anti-inflammatory status in elderly subjects through toll-like receptor 2 and 4 signaling pathways. Mech Ageing Dev. 2015;150:12–19. doi: 10.1016/j.mad.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Seefried L, Genest F, Luksche N, Schneider M, Fazeli G, Brandl M, Bahner U, A Heidland A. Efficacy and safety of whole body vibration in maintenance hemodialysis patients - a pilot study. J Musculoskelet Neuronal Interact. 2017;17:268–274. [PMC free article] [PubMed] [Google Scholar]
- 21.Oh S, Oshida N, Someya N, Maruyama T, Isobe T, Okamoto Y, Kim T, Kim B, Shoda J. Whole-body vibration for patients with nonalcoholic fatty liver disease: a 6-month prospective study. Physiol Rep. 2019;7:e14062. doi: 10.14814/phy2.14062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pawlak M, Kaczmarek D, Nowak A, Krutki P. Low-volume whole-body vibration lasting 3 or 6 months does not affect biomarkers in blood serum of rats. Acta Physiol Hung. 2013;100:48–53. doi: 10.1556/APhysiol.99.2012.003. [DOI] [PubMed] [Google Scholar]
- 23.Weinheimer-Haus EM, Judex S, Ennis WJ, Koh TJ. Low-intensity vibration improves angiogenesis and wound healing in diabetic mice. PLoS One. 2014;9:e91355. doi: 10.1371/journal.pone.0091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neves CDC, Lacerda ACR, Lage VKS, Soares AA, Chaves MGA, Lima LP, Silva TJ, Vieira ÉLM, Teixeira AL, Leite HR, Matos MA, Mendonça VA. Whole body vibration training increases physical measures and quality of life without altering inflammatory-oxidative biomarkers in patients with moderate COPD. J Appl Physiol (1985) 2018;125:520–528. doi: 10.1152/japplphysiol.01037.2017. [DOI] [PubMed] [Google Scholar]
- 25.Jawed Y, Beli E, March K, Kaleth A, Loghmani MT. Whole-body vibration training increases stem/progenitor cell circulation levels and may attenuate inflammation. Mil Med. 2020;185(Suppl 1):404–412. doi: 10.1093/milmed/usz247. [DOI] [PubMed] [Google Scholar]
- 26.Yu PM, Lin Y, Zhang C, Wang HM, Wei Q, Zhu SY, Wei QC, Wang ZG, Pan HX, Huang RD, He CQ. Low-frequency vibration promotes tumor necrosis factor-α production to increase cartilage degeneration in knee osteoarthritis. Cartilage. 2021;13:1398S–1406S. doi: 10.1177/1947603520931178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–721. [PubMed] [Google Scholar]
- 28.Jiang D, Liu C, Chen Y, Xing X, Zheng D, Guo Z, Lin S. Metabolomics study of whole-body vibration on lipid metabolism of skeletal muscle in aging mice. Int J Sports Med. 2021;42:464–477. doi: 10.1055/a-1268-8458. [DOI] [PubMed] [Google Scholar]
- 29.Sun C, Zeng R, Cao G, Song Z, Zhang Y, Liu C. Vibration training triggers brown adipocyte relative protein expression in rat white adipose tissue. Biomed Res Int. 2015;2015:919401. doi: 10.1155/2015/919401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Zhang C, Zhu S, Gao C, Gao Q, Huang R, Liu S, Wei X, Zhang H, Wei Q, He C. Low-frequency whole-body vibration can enhance cartilage degradation with slight changes in subchondral bone in mice with knee osteoarthritis and does not have any morphologic effect on normal joints. PLoS One. 2023;18:e0270074. doi: 10.1371/journal.pone.0270074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai SH, Tseng YH, Chiou WF, Chen SM, Chung Y, Wei WC, Huang WC. The effects of whole-body vibration exercise combined with an isocaloric high-fructose diet on osteoporosis and immunomodulation in ovariectomized mice. Front Nutr. 2022;9:915483. doi: 10.3389/fnut.2022.915483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu H, Zhang Y, Yang X, Li X, Shao Z, Zhou Z, Li Y, Pan S, Liu C. Whole body vibration retards progression of atherosclerosis via insulin-like growth factor 1 in apolipoprotein E-deficient mice. Biomed Res Int. 2018;2018:4934861. doi: 10.1155/2018/4934861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naghii MR, Hedayati M. Whole body vibration as a safe exercise training method induces no impaired alterations on rat plasma antioxidant biomarkers. Acta Physiol Hung. 2013;100:321–328. doi: 10.1556/APhysiol.100.2013.009. [DOI] [PubMed] [Google Scholar]
- 34.McCann MR, Veras MA, Yeung C, Lalli G, Patel P, Leitch KM, Holdsworth DW, Dixon SJ, Séguin CA. Whole-body vibration of mice induces progressive degeneration of intervertebral discs associated with increased expression of Il-1β and multiple matrix degrading enzymes. Osteoarthritis Cartilage. 2017;25:779–789. doi: 10.1016/j.joca.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Wano N, Sanguanrungsirikul S, Keelawat S, Somboonwong J. The effects of whole-body vibration on wound healing in a mouse pressure ulcer model. Heliyon. 2021;7:e06893. doi: 10.1016/j.heliyon.2021.e06893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chow SK, Chim YN, Wang J, Zhang N, Wong RM, Tang N, Leung KS, Cheung WH. Vibration treatment modulates macrophage polarisation and enhances early inflammatory response in oestrogen-deficient osteoporotic-fracture healing. Eur Cell Mater. 2019;38:228–245. doi: 10.22203/eCM.v038a16. [DOI] [PubMed] [Google Scholar]
- 37.Bellia A, Sallì M, Lombardo M, D’Adamo M, Guglielmi V, Tirabasso C, Giordani L, Federici M, Lauro D, Foti C, Sbraccia P. Effects of whole body vibration plus diet on insulin-resistance in middle-aged obese subjects. Int J Sports Med. 2014;35:511–516. doi: 10.1055/s-0033-1354358. [DOI] [PubMed] [Google Scholar]
- 38.Simão AP, Avelar NC, Tossige-Gomes R, Neves CD, Mendonça VA, Miranda AS, Teixeira MM, Teixeira AL, Andrade AP, Coimbra CC, Lacerda AC. Functional performance and inflammatory cytokines after squat exercises and whole-body vibration in elderly individuals with knee osteoarthritis. Arch Phys Med Rehabil. 2012;93:1692–700. doi: 10.1016/j.apmr.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 39.Hazell TJ, Olver TD, Hamilton CD, Lemon PW. Addition of synchronous whole-body vibration to body mass resistive exercise causes little or no effects on muscle damage and inflammation. J Strength Cond Res. 2014;28:53–60. doi: 10.1519/JSC.0b013e318296484f. [DOI] [PubMed] [Google Scholar]
- 40.Wunram HL, Oberste M, Hamacher S, Neufang S, Grote N, Krischer MK, Bloch W, Schönau E, Bender S, Fricke O. Immunological effects of an add-on physical exercise therapy in depressed adolescents and its interplay with depression severity. Int J Environ Res Public Health. 2021;18:6527. doi: 10.3390/ijerph18126527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shehata MMA, Maged AM, Kotb A, Ogila AI, Lasheen Y, Salah N, Mohsen RA, Fouad M, Abd-Elazeim AS. Whole-body vibration versus supervised aerobic exercise on hormonal parameters and inflammatory status in women with premenstrual syndrome: a randomized controlled trial. Int J Gynaecol Obstet. 2023;162:493–501. doi: 10.1002/ijgo.14737. [DOI] [PubMed] [Google Scholar]
- 42.Di Giminiani R, Rucci N, Capuano L, Ponzetti M, Aielli F, Tihanyi J. Individualized whole-body vibration: neuromuscular, biochemical, muscle damage and inflammatory acute responses. Dose Response. 2020;18:1559325820931262. doi: 10.1177/1559325820931262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koczulla AR, Boeselt T, Koelpin J, Kaufhold F, Veith M, Nell C, Jarosch I, Spielmanns M, Alter P, Kähler C, Greulich T, Vogelmeier CF, Glöckl R, Schneeberger T, Kenn K, Kahn NC, Herth FJF, Kreuter M. Effects of vibration training in interstitial lung diseases: a randomized controlled trial. Respiration. 2020;99:658–666. doi: 10.1159/000508977. [DOI] [PubMed] [Google Scholar]
- 44.Lage VKS, Lacerda ACR, Neves CDC, Chaves MGA, Soares AA, Lima LP, Martins JB, Matos MA, Vieira ÉLM, Teixeira AL, Leite HR, Oliveira VC, Mendonça VA. Acute effects of whole-body vibration on inflammatory markers in people with chronic obstructive pulmonary disease: a pilot study. Rehabil Res Pract. 2018;2018:5480214. doi: 10.1155/2018/5480214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cristi C, Collado PS, Márquez S, Garatachea N, Cuevas MJ. Whole-body vibration training increases physical fitness measures without alteration of inflammatory markers in older adults. Eur J Sport Sci. 2014;14:611–619. doi: 10.1080/17461391.2013.858370. [DOI] [PubMed] [Google Scholar]
- 46.Ribeiro VGC, Mendonça VA, Souza ALC, Fonseca SF, Camargos ACR, Lage VKS, Neves CDC, Santos JM, Teixeira LAC, Vieira ELM, Teixeira Junior AL, Mezêncio B, Fernandes JSC, Leite HR, Poortmans JR, Lacerda ACR. Inflammatory biomarkers responses after acute whole body vibration in fibromyalgia. Braz J Med Biol Res. 2018;51:e6775. doi: 10.1590/1414-431X20176775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanni AA, Blanks AM, Derella CC, Horsager C, Crandall RH, Looney J, Sanchez S, Norland K, Ye B, Thomas J, Wang X, Harris RA. The effects of whole-body vibration amplitude on glucose metabolism, inflammation, and skeletal muscle oxygenation. Physiol Rep. 2022;10:e15208. doi: 10.14814/phy2.15208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreira-Marconi E, Teixeira-Silva Y, Meirelles AG, Melo-Oliveira MES, Santos ACG, Reis-Silva A, Paineiras-Domingos LL, Seixas A, Dionello CDF, Sá-Caputo DDC, Bernardo-Filho M. Inflammatory biomarker responses to whole-body vibration in subjects with different clinical status: a systematic review. Int J Environ Res Public Health. 2022;19:14853. doi: 10.3390/ijerph192214853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vanden Berghe W, Vermeulen L, De Wilde G, De Bosscher K, Boone E, Haegeman G. Signal transduction by tumor necrosis factor and gene regulation of the inflammatory cytokine interleukin-6. Biochem Pharmacol. 2000;60:1185–1195. doi: 10.1016/s0006-2952(00)00412-3. [DOI] [PubMed] [Google Scholar]
- 50.Grund EM, Kagan D, Tran CA, Zeitvogel A, Starzinski-Powitz A, Nataraja S, Palmer SS. Tumor necrosis factor-alpha regulates inflammatory and mesenchymal responses via mitogen-activated protein kinase kinase, p38, and nuclear factor kappaB in human endometriotic epithelial cells. Mol Pharmacol. 2008;73:1394–1404. doi: 10.1124/mol.107.042176. [DOI] [PubMed] [Google Scholar]
- 51.Bass VL, Wong VC, Bullock ME, Gaudet S, Miller-Jensen K. TNF stimulation primarily modulates transcriptional burst size of NF-κB-regulated genes. Mol Syst Biol. 2021;17:e10127. doi: 10.15252/msb.202010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han Y, He T, Huang DR, Pardo CA, Ransohoff RM. TNF-alpha mediates SDF-1 alpha-induced NF-kappa B activation and cytotoxic effects in primary astrocytes. J Clin Invest. 2001;108:425–435. doi: 10.1172/JCI12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xue Y, Zeng X, Tu WJ, Zhao J. Tumor necrosis factor-α: the next marker of stroke. Dis Markers. 2022;2022:2395269. doi: 10.1155/2022/2395269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tzanavari T, Giannogonas P, Karalis KP. TNF-alpha and obesity. Curr Dir Autoimmun. 2010;11:145–56. doi: 10.1159/000289203. [DOI] [PubMed] [Google Scholar]
- 55.Liu S, Cao C, Zhang Y, Liu G, Ren W, Ye Y, Sun T. PI3K/Akt inhibitor partly decreases TNF-α-induced activation of fibroblast-like synoviocytes in osteoarthritis. J Orthop Surg Res. 2019;14:425. doi: 10.1186/s13018-019-1394-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma K, Zhang H, Baloch Z. Pathogenetic and therapeutic applications of tumor necrosis factor-α (TNF-α) in major depressive disorder: a systematic review. Int J Mol Sci. 2016;17:733. doi: 10.3390/ijms17050733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ezquerro S, Mocha F, Frühbeck G, Guzmán-Ruiz R, Valentí V, Mugueta C, Becerril S, Catalán V, Gómez-Ambrosi J, Silva C, Salvador J, Colina I, Malagón MM, Rodríguez A. Ghrelin reduces TNF-α-induced human hepatocyte apoptosis, autophagy, and pyroptosis: role in obesity-associated NAFLD. J Clin Endocrinol Metab. 2019;104:21–37. doi: 10.1210/jc.2018-01171. [DOI] [PubMed] [Google Scholar]
- 58.Soldatos A, Toro C, Hoffmann P, Romeo T, Deuitch N, Brofferio A, Aksentijevich I, Kastner DL, Ombrello AK. TNF-blockade for primary stroke prevention in adenosine deaminase 2 deficiency: a case series. Neurol Neuroimmunol Neuroinflamm. 2023;10:e200073. doi: 10.1212/NXI.0000000000200073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iglesias M, Plowman GD, Woodworth CD. Interleukin-6 and interleukin-6 soluble receptor regulate proliferation of normal, human papillomavirus-immortalized, and carcinoma-derived cervical cells in vitro. Am J Pathol. 1995;146:944–52. [PMC free article] [PubMed] [Google Scholar]
- 60.Yin H, Berdel HO, Moore D, Davis F, Liu J, Mozaffari M, Yu JC, Baban B. Whole body vibration therapy: a novel potential treatment for type 2 diabetes mellitus. Springerplus. 2015;4:578. doi: 10.1186/s40064-015-1373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song N, Liu X, Feng Q, Xu M, Lan X, Li M, Liu R, Li C, Dong T, Wang D, Liu S. Whole body vibration triggers a change in the mutual shaping state of intestinal microbiota and body’s immunity. Front Bioeng Biotechnol. 2019;7:377. doi: 10.3389/fbioe.2019.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.