Abstract
Background: Coronavirus disease 2019 (COVID-19) affects different organ systems, including the skin. A retrospective analysis of skin manifestations in Chinese outpatient and inpatient settings is lacking. The study aims to analyze cutaneous manifestations in COVID-19 patients and the recurrence or aggravation of previous skin diseases. Materials and Methods: A retrospective cross-sectional study was conducted from November 2022 to July 2023 in a university hospital in eastern China. It involved reverse transcriptase polymerase chain reaction (RT-PCR)-positive COVID-19 patients, documenting various skin manifestations and the recurrence or aggravation of pre-existing skin conditions. The pattern of skin lesions and other variables were assessed. Results: The study included 303 patients, with 127 males and 176 females. Maculopapular rash was the predominant new cutaneous manifestation (54.92%), mainly in middle-aged individuals. Other findings included urticaria (16.39%), herpes zoster (11.89%), and herpes simplex (4.10%), vesicular rashes (2.46%), purpura (2.05%), erythema multiforme (1.64%), livedo reticularis (0.41%) and so on. Severe disease was associated with herpes zoster and livedo reticularis. Critical COVID-19 cases were linked to vesicular rashes, purpura, and erythema multiforme. The mean time for skin lesion emergence post-infection varied from 3 days for seborrheic dermatitis to 17.48 days for herpes zoster. Vasculitic manifestations correlated with elevated D-dimer levels. A total of 59 cases (19.47%) of recurrent or aggravated skin diseases were reported following infection with COVID-19, with dermatitis being the most common, followed by acne and folliculitis, psoriasis, urticaria, bullous pemphigoid, pemphigus, tinea corporis and androgenetic alopecia. Conclusion: The cutaneous phenotypes delineated in this study expand the dermatologic spectrum associated with COVID-19. Cutaneous manifestations may result from overactive immune responses, complement activation, and microvascular damage. Herpes zoster typically occurs in elderly COVID-19 patients with weaker immune systems or more severe diseases. Purpura and livedo reticularis, although rare, may indicate disease severity. It is possible to predict the course of COVID-19 with different severity through cutaneous manifestations. Recognizing these skin manifestations could aid in predicting COVID-19 severity and guide dermatologists in managing the pandemic response.
Keywords: COVID-19 infection, cutaneous manifestations, immune reaction
Introduction
In March 2020, the World Health Organization (WHO) declared the global COVID-19 outbreak a pandemic [1], and it still remains a global challenge. The main symptoms of COVID-19 include fever, fatigue, dry cough, anorexia, myalgia, etc. A part of severe patients may experience respiratory failure, multiple organ failure, and even death [1,2]. Subsequently, clinicians discovered that some COVID-19 patients may have cutaneous manifestations [2-6]. The prevalence of skin manifestations in COVID-19 patients varies greatly, from 0.2% to 20.4% [2,7-9]. The main manifestations of skin rashes were maculopapular rash, livedoid lesions, petechial lesions, urticaria, pernio-like lesion, and vesicular lesions, etc. [8,11-13]. More and more studies have reported that COVID-19 is associated with dermatological manifestations [14-16].
Several recent case reports and clinical series have described a variety of cutaneous manifestations associated with the infection to date; Nevertheless, several explanations have been proposed regarding the mechanisms that trigger the rash in COVID-19 patients, primarily the possibility that the virus can infect through an open wound or whether the cutaneous manifestations are related to immune responses or by a newly prescribed medication [17,18]. However, the pathogenesis of the cutaneous manifestations of COVID-19 is complex and not fully understood, further research is still needed.
In literature, many reviews and studies have defined the cutaneous findings of COVID-19 in hospitalized patients [19-21] or patient consultations which were seen via teledermatology and social media platforms. Nevertheless, no relevant reports from outpatient clinic in China have been published yet. This paper makes a retrospective analysis of cutaneous manifestations of COVID-19 outpatient and inpatient patients in a university hospital in eastern China. In this study, we aim to determine the prevalence and types of new onset cutaneous manifestations, as well as the recurrence or aggravation of pre-existing skin conditions in COVID-19 patients. Additionally, we seek to explore the association between the severity of COVID-19 and specific skin manifestations, identify any correlation between these manifestations and laboratory markers, and delineate the time course of skin lesion emergence following infection.
Materials and methods
Data collection
In this retrospective cross-sectional study, after ethical committee approval, the medical records of patients of both sexes, who tested positive for COVID-19 by reverse transcriptase-polymerase chain reaction (RTPCR) and were treated at The First Affiliated Hospital of Ningbo University, a university hospital in eastern China, between November 2022 and July 2023, were retrospectively reviewed.
A total of 303 outpatient and inpatient cases were included. Patients whose records contained any description of cutaneous-related complications were included in the sample. Patients include two types: patients with new onset of skin manifestations after COVID-19 infection and patients with recurrence or aggravation of previous skin diseases after COVID-19 infection. The following data were collected: age, sex, symptoms and signs of COVID-19, the severity of COVID-19, setting of care (outpatient, inpatient ward), past history, description of skin lesions, time of occurrence of skin lesions, the duration of disease, laboratory examinations.
Statistical analysis
The data is analyzed using SPSS Statistic 26.0 statistical software. Enumeration data is represented by frequency (N) and percentage (%), and metric data is represented by mean.
Ethical considerations
This study was approved by the Ethics Committee of the First Affiliated Hospital of Ningbo University (Ethical Number: 2023-R183A). All personal information was kept confidential.
Results
The study group included 303 patients with 127 (41.91%) males and 176 (58.09%) females (a mean age of 43.85 years and an age range of 1-89 years). 244 cases (80.53%) had a report of newly cutaneous manifestations when infected with COVID-19, including 100 males and 144 females. A total of 59 cases (19.47%) of recurrent or aggravated skin diseases were reported following infection with COVID-19, 27 of them males and 32 of them females.
Patients with new onset skin manifestations after COVID-19 infection (n=244)
Types of skin lesions
According to previous reports and the skin manifestations of patients in this paper, the dermatological patterns of COVID-19 can be classified as follows (Figure 1): maculopapular rash, urticarial and vesicular rashes, morbilliform, generalized pustular rash, erythema multiforme, herpes zoster, herpes simplex, purpura, livedo reticularis, folliculitis like lesions, pityriasis rosea, eye lesions, periungual lesions, cheilitis, scalp seborrheic dermatitis, alopecia areata, androgenetic alopecia, psoriasis, granuloma annulare, verruca vulgaris, pruritus, skin dryness and stinging.
Figure 1.
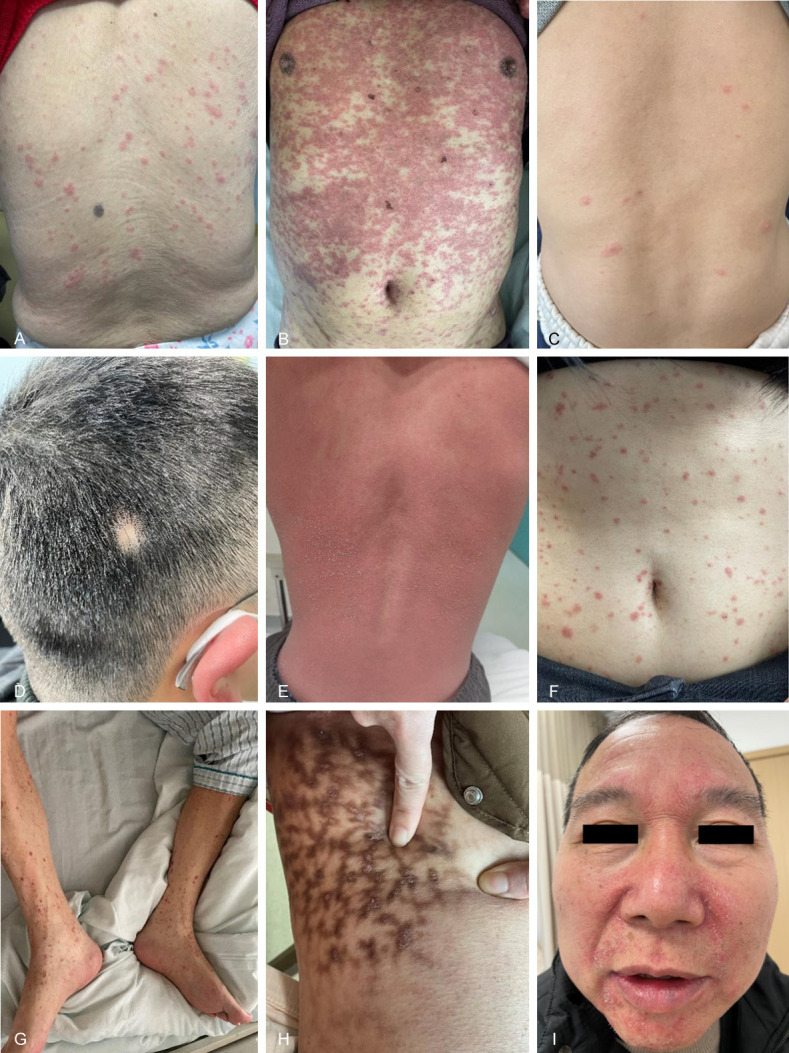
Cutaneous manifestations of COVID-19 patients: (A) Maculopapular rash, (B) Erythema multiforme, (C) Vesicular eruption, (D) Alopecia areata, (E) Generalized pustular rash, (F) Acute guttate psoriasis, (G) Purpura, (H) Livedo reticularis combined with psoriasis, (I) Seborrheic dermatitis.
Among the 244 patients with new onset cutaneous manifestations, maculopapular rash was the most common findings (54.92%), followed by urticaria (16.39%, 2 cases with combined maculopapular rash), herpes zoster (11.89%, one case of combined maculopapular rash), herpes simplex (4.10%, 3 cases of combined maculopapular rash and 2 cases of cheilitis). The respective proportion of other cutaneous manifestations were less than 2.5% (Table 1). There were 6 cases (2.46%) of vesicular rashes (including 2 cases of chickenpox like, 2 cases of skin infection, 1 case of bullous pemphigoid, and 1 case of pemphigus), 6 cases of facial erythema and papules (all manifested as maculopapular rashes, one case with eyelid redness and swelling, and one case with herpes simplex), 5 cases (2.05%) of purpura (2 cases of combined maculopapular rash and one of erythema multiforme), 4 cases (1.64%) of folliculitis like skin lesions (2 cases of folliculitis, 1 case of acne, 1 case of rosacea), 4 cases of pityriasis rosea, 4 cases of erythema multiforme (2 cases combined with maculopapular rash and 1 case combined with purpura), 4 cases of morbilliform (2 cases accompanied by maculopapular rash), 3 cases (1.23%) of cheilitis (2 cases combined with herpes simplex and 1 case combined with facial erythema and papules), 3 cases of eye lesions (3 cases of eye redness and swelling combined with macular papules like lesions, of which 2 cases combined with conjunctival congestion), 2 cases (0.82%) of periungual lesions (1 case of paronychia, 1 case of nail desquamation accompanied by maculopapular rash), 2 cases of psoriasis (2 cases accompanied by maculopapular rash), 2 cases of skin dryness and stinging. There was only one case of scalp seborrheic dermatitis (accompanied by maculopapular rash), generalized pustular rash (accompanied by maculopapular rash), granuloma annulare, alopecia areata, livedo reticularis, verruca vulgaris and pruritus, respectively (Table 1).
Table 1.
Summary of characteristics of the patients with new onset skin manifestations after COVID-19 infection
| Types of skin lesions | Age, mean (years) | Number of cases (n=244, 100%) | Average days for the appearance of skin lesions | COVID-19 course (days) | Severity of COVID-19 | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mild | Moderate | Severe | Critical | Asymptomatic | |||||
| Maculopapular rash | 47.97 | 134 (54.92%) | 8.27 | 11.08 | 105 (78.36%) | 21 (15.67%) | 3 (2.24%) | 4 (2.99%) | 1 (0.75%) |
| Herpes simplex | 40.44 | 10 (4.10%) | 9.60 | 7.60 | 10 (100%) | 0 | 0 | 0 | 0 |
| Urticaria | 28.83 | 40 (16.39%) | 9.70 | 7.68 | 37 (92.5%) | 3 (7.5%) | 0 | 0 | 0 |
| Herpes zoster | 57.59 | 29 (11.89%) | 17.48 | 8.45 | 24 (82.76%) | 4 (13.79%) | 1 (3.45%) | 0 | 0 |
| Vesicular rashes | 50.4 | 6 (2.46%) | 9.67 | 14.67 | 3 (50%) | 2 (33.33%) | 0 | 1 (16.67%) | 0 |
| Purpura | 53.2 | 5 (2.05%) | 8.20 | 14.20 | 3 (60%) | 1 (20%) | 0 | 1 (20%) | 0 |
| Morbilliform | 47.00 | 4 (1.64%) | 7.25 | 11.00 | 3 (75%) | 1 (25%) | 0 | 0 | 0 |
| Cheilitis | 47.00 | 3 (1.23%) | 4.67 | 9.67 | 2 (66.67%) | 1 (33.33%) | 0 | 0 | 0 |
| Pityriasis rosea | 25.00 | 4 (1.64%) | 9.25 | 7.25 | 3 (75%) | 1 (25%) | 0 | 0 | 0 |
| Generalized pustular rash | 18.00 | 1 (0.41%) | 4.00 | 8.00 | 1 (100%) | 0 | 0 | 0 | 0 |
| Erythema multiforme | 30.00 | 4 (1.64%) | 5.00 | 13.00 | 3 (75%) | 0 | 0 | 1 (25%) | 0 |
| Androgenetic alopecia | 44.00 | 1 (0.41%) | 14.00 | 8.00 | 1 (100%) | 0 | 0 | 0 | 0 |
| Alopecia areata | 37.67 | 6 (2.46%) | 10.60 | 12.00 | 6 (100%) | 0 | 0 | 0 | 0 |
| Periungual lesions | 50.50 | 2 (0.82%) | 17.0 | 14.50 | 1 (50%) | 1 (50%) | 0 | 0 | 0 |
| Eye lesions | 50.00 | 3 (1.23%) | 8.33 | 11.00 | 3 (100%) | 0 | 0 | 0 | 0 |
| Psoriasis | 36.00 | 2 (0.82%) | 14.00 | 9.00 | 2 (100%) | 0 | 0 | 0 | 0 |
| Seborrheic dermatitis | 26.00 | 1 (0.41%) | 3.00 | 7.00 | 1 (100%) | 0 | 0 | 0 | 0 |
| Livedo reticularis | 45.00 | 1 (0.41%) | 7.00 | 18.00 | 0 | 0 | 1 (100%) | 0 | 0 |
| Folliculitis like lesions | 37.75 | 4 (1.64%) | 8.00 | 9.00 | 3 (75%) | 1 (25%) | 0 | 0 | 0 |
| Granuloma annulare | 40.00 | 1 (0.41%) | 7.00 | 8.00 | 1 (100%) | 0 | 0 | 0 | 0 |
| Skin dryness, stinging | 34.00 | 2 (0.82%) | 6.00 | 8.50 | 2 (100%) | 0 | 0 | 0 | 0 |
| Pruritus | 89.00 | 1 (0.41%) | 5.00 | 8.00 | 1 (100%) | 0 | 0 | 0 | 0 |
| Verruca vulgaris | 26.00 | 1 (0.41%) | 15.00 | 10.00 | 1 (100%) | 0 | 0 | 0 | 0 |
The mean age of patients with different types of skin lesions
The mean age of patients with cutaneous manifestations was 43.85 years (ranging from 1 to 89 years). Urticaria, generalized pustular rash, pityriasis rosea, seborrheic dermatitis, verruca vulgaris were more common in younger patients (mean age <30 years). Maculopapular rash, morbilliform, erythema multiforme, herpes simplex, livedo reticularis, folliculitis like lesions, cheilitis, alopecia areata, androgenetic alopecia, psoriasis, granuloma annulare, skin dryness and stinging were more common in the middle-aged patients (mean age 30-50 years). Herpes zoster, vesicular rashes, purpura, eye lesions, periungual lesions, pruritus were more common in the middle-aged and elderly (mean age >50 years) (Table 1).
Distribution of skin lesions
Trunk, lower limb, and upper limb were the main involved regions. The involvement of groin, scalp, nail and buttock were rare. The maculopapular lesions, urticaria, vesicular rashes, morbilliform, pityriasis rosea, generalized pustular rash, erythema multiforme, psoriasis, skin dryness, stinging and pruritus were more common in trunk, lower limbs and upper limbs. Labial mucosa involvement mainly occurs in maculopapular lesions, herpes simplex, herpes zoster and cheilitis. Face involvement was more common in maculopapular rash, urticaria, herpes simplex, vesicular rashes, purpura, morbilliform, cheilitis, erythema multiforme, folliculitis like lesions. Eye lesions can also be accompanied by maculopapular rash, herpes simplex and herpes zoster. Vulva and groin involvement were more common in maculopapular rash, herpes zoster and granuloma annulare. Two patients with nail lesions. Maculopapular lesions, herpes simplex, herpes zoster, vesicular rashes, seborrheic dermatitis, androgenetic alopecia, alopecia areata all have scalp involvement (Table 2).
Table 2.
Distribution of skin lesions in patients with new onset skin manifestations after COVID-19 infection
| Types of skin lesions | Skin lesions location | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Upper limbs | Lower limbs | Trunk | Lip | Face | Vulva | Eye | Groin | Scalp | Nail | Buttock | |
| Maculopapular rash (n=134) | 57 (42.54%) | 70 (52.24%) | 73 (54.48%) | 4 (2.99%) | 28 (20.90%) | 11 (8.21%) | 6 (4.48%) | 3 (2.24%) | 4 (2.99%) | 0 | 0 |
| Herpes simplex (n=10) | 1 (10%) | 1 (10%) | 3 (30%) | 7 (70%) | 8 (80%) | 0 | 1 (10%) | 0 | 1 (10%) | 0 | 1 (10%) |
| Urticaria (n=40) | 37 (92.5%) | 36 (90%) | 40 (100%) | 0 | 5 (12.5%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Herpes zoster (n=29) | 0 | 1 (3.45%) | 17 (58.62%) | 2 (6.90%) | 8 (27.59%) | 2 (6.90%) | 4 (13.79%) | 1 (3.45%) | 1 (13.79%) | 0 | 0 |
| Vesicular rashes (n=6) | 2 (33.33%) | 5 (83.33%) | 3 (50%) | 0 | 2 (33.33%) | 0 | 0 | 0 | 1 (16.67%) | 0 | 0 |
| Purpura (n=5) | 1 (20%) | 5 (100%) | 2 (40%) | 0 | 1 (20%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Morbilliform (n=4) | 3 (75%) | 4 (100%) | 4 (100%) | 0 | 1 (25%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Cheilitis (n=3) | 0 | 0 | 0 | 3 (100%) | 2 (66.67%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Pityriasis rosea (n=4) | 3 (75%) | 3 (75%) | 4 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Generalized pustular rash (n=1) | 1 (100%) | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Erythema multiforme (n=4) | 4 (100%) | 4 (100%) | 3 (75%) | 0 | 3 (75%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Androgenetic alopecia (n=1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 0 | 0 |
| Alopecia areata (n=6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 (100%) | 0 | 0 |
| Periungual lesions (n=2) | 0 | 1 (50%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (100%) | 0 |
| Eye lesions (n=3) | 0 | 0 | 0 | 0 | 1 (33.33%) | 0 | 3 (100%) | 0 | 0 | 0 | 0 |
| Psoriasis (n=2) | 1 (50%) | 2 (100%) | 1 (50%) | 0 | 0 | 0 | 0 | 0 | 1 (50%) | 0 | 0 |
| Seborrheic dermatitis (n=1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 0 | 0 |
| Livedo reticularis (n=1) | 0 | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Folliculitis like lesions (n=4) | 0 | 0 | 0 | 0 | 3 (75%) | 0 | 0 | 0 | 1 (25%) | 0 | 0 |
| Granuloma annulare (n=1) | 0 | 0 | 1 (100%) | 0 | 0 | 0 | 0 | 1 (100%) | 0 | 0 | 0 |
| Skin dryness, stinging (n=2) | 2 (100%) | 2 (100%) | 2 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pruritus (n=1) | 1 (100%) | 1 (100%) | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Verruca vulgaris (n=1) | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Time of appearance of skin lesions after infection with COVID-19
Table 1 presents the average time at onset of skin lesions after infection with COVID-19. Skin manifestations with the longest mean time (17.48 days) of appearance of skin lesions is herpes zoster. Seborrheic dermatitis is the skin lesions with the shortest mean time (3 days). Skin manifestations with the mean time of emergence of skin lesions less than 7 days include facial erythematous papules, cheilitis, erythema multiforme, seborrheic dermatitis, generalized pustular eruption, livedo reticularis, dry and tingling, pruritus; with an average time of 7-14 days include maculopapular rash, urticaria, herpes simplex, granuloma annulare, alopecia areata, vesicular lesions, purpura, folliculitis like lesions, pityriasis rosea, eye lesions, psoriasis, morbilliform, androgenetic alopecia; with an average time longer than 14 days include herpes zoster, perionychial lesions, verruca vulgaris.
Severity and symptoms of COVID-19
COVID-19 patients were classified into mild, moderate, severe and critical based on their severity according to the WHO COVID-19 Scale [22]. The severity and symptoms of COVID-19 with different types of skin lesions were shown in Tables 1 and 3. The majority of patients with skin manifestations were mild, which may be related to the fact that most patients included were outpatients (n=212, 86.89%). Only one case of maculopapular rash presented as asymptomatic. The types of skin lesions with moderate COVID-19 include maculopapular rash, urticaria, herpes zoster, vesicular rashes, purpura, morbilliform, cheilitis, pityriasis rosea, periungual lesions and folliculitis like lesions; skin lesions with severe COVID-19 include maculopapular rash, herpes zoster and livedo reticularis; skin manifestations with critical COVID-19 include maculopapular rash, vesicular rashes, purpura and erythema multiforme.
Table 3.
The symptoms of patients with new onset skin manifestations after COVID-19 infection
| Types of skin lesions | Symptoms of COVID-19 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| T≥38.5°C | T<38.5°C | Cough | Sore throat | Nasal congestion, runny nose | Decreased olfactory function | Chest tightness | Palpitation | Diarrhea | Vomit | Headache | Shortness of breath | Syncope | Body aches | Poor appetite | |
| Maculopapular rash (n=134) | 31 (23.13) | 103 (76.87) | 131 (97.76) | 130 (97.01) | 30 (22.39) | 2 (1.49%) | 2 (1.49%) | 1 (0.75%) | 3 (2.24%) | 1 (0.75%) | 30 (22.39%) | 6 (4.48%) | 0 | 0 | 28 (20.90%) |
| Herpes simplex (n=10) | 2 (20%) | 8 (80%) | 10 (100%) | 10 (100%) | 4 (40%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Urticaria (n=40) | 4 (10%) | 36 (90%) | 40 (100%) | 39 (97.5%) | 12 (30%) | 0 | 1 (2.5%) | 0 | 1 (2.5%) | 0 | 1 (2.5%) | 1 (2.5%) | 1 (2.5%) | 0 | 0 |
| Herpes zoster (n=29) | 8 (27.59%) | 21 (72.41%) | 26 (89.66%) | 26 (89.66%) | 8 (27.59%) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.45%) | 0 | 1 (3.45%) | 0 |
| Vesicular rashes (n=6) | 1 (16.67%) | 5 (83.33%) | 6 (100%) | 6 (100%) | 2 (33.33%) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (16.67%) | 0 | 0 | 0 |
| Purpura (n=5) | 1 (20%) | 4 (80%) | 3 (60%) | 1 (20%) | 4 (80%) | 0 | 0 | 0 | 1 (20%) | 0 | 0 | 1 (20%) | 0 | 0 | 1 (20%) |
| Morbilliform (n=4) | 3 (75%) | 1 (25%) | 4 (100%) | 4 (100%) | 2 (50%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cheilitis (n=3) | 2 (66.67%) | 1 (33.33%) | 3 (100%) | 3 (100%) | 1 (33.33%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.33%) | 0 |
| Pityriasis rosea (n=4) | 1 (25%) | 3 (75%) | 4 (100%) | 4 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Generalized pustular rash (n=1) | 0 | 1 (100%) | 1 (100%) | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Erythema multiforme (n=4) | 2 (50%) | 2 (50%) | 4 (100%) | 4 (100%) | 1 (25%) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (25%) | 0 | 0 | 0 |
| Androgenetic alopecia (n=1) | 0 | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alopecia areata (n=6) | 1 (16.67%) | 5 (83.33%) | 6 (100%) | 6 (100%) | 2 (33.33%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Periungual lesions (n=2) | 1 (50%) | 1 (50%) | 1 (50%) | 1 (50%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eye lesions (n=3) | 0 | 3 (100%) | 3 (100%) | 3 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Psoriasis (n=2) | 1 (50%) | 1 (50%) | 2 (100%) | 2 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Seborrheic dermatitis (n=1) | 1 (100%) | 0 | 1 (100%) | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Livedo reticularis (n=1) | 0 | 1 (100%) | 1 (100%) | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 0 | 0 | 0 |
| Folliculitis like lesions (n=4) | 0 | 4 (100%) | 1 (100%) | 1 (100%) | 2 (50%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Granuloma annulare (n=1) | 0 | 1 (100%) | 1 (100%) | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Skin dryness, stinging (n=2) | 0 | 2 (100%) | 2 (100%) | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pruritus (n=1) | 0 | 1 (100%) | 1 (100%) | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Verruca vulgaris (n=1) | 0 | 1 (100%) | 1 (100%) | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Among all types of skin lesions, fever (temperature <38.5°C), cough, sore throat, nasal congestion and runny nose were the cardinal symptom. Syncope only occurs in patients with urticaria. Shortness of breath only occurs in severe and critical patients. Pruritus was the most prominent cutaneous symptom particularly in maculopapular rash and pruritus. Pain was the most frequent symptom in herpes zoster, herpes simplex, vesicular rashes.
Characteristics of hospitalized patients
Among 32 inpatient patients, one was mild (3.13%), 22 were moderate (68.75%), 5 were severe (15.63%), 4 were critically severe (12.5%). Among them, there were 23 cases of maculopapular lesions (1 case combined with purpura, erythema multiforme and 1 case with perionychial lesions), 2 cases of purpura, 1 case of erythema multiforme, 1 case of urticaria, 2 cases of herpes zoster, 2 cases of vesicular lesions, 1 case of cheilitis, 1 case of perionychial lesions, 1 case of folliculitis like lesions and 1 case of livedo reticularis.
Among patients with maculopapular rashes, one was mild, 16 were moderate, 3 were severe, and 3 were critically severe; among purpura patients, one was moderate and one was critical type; one patient with erythema multiforme was critical; one patient with urticaria was moderate; among patients with herpes zoster, there was one case of the moderate type and one case of the severe type; among patients with vesicular rashes, there was one severe and one moderate; one patient with cheilitis was of the moderate type; one patient with perionychial lesions was of the moderate type; one patient with folliculitis like lesions was moderate; one patient with livedo reticularis was of severe type.
COVID-19 course
The course of COVID-19 with different types of skin lesions were shown in Table 1. Livedo reticularis had the longest mean course of COVID-19 (18 days) and seborrheic dermatitis had the shortest mean course (7 days). Skin manifestations with the mean course less than 8 days include urticaria, herpes simplex, pityriasis rosea, facial erythematous papules and seborrheic dermatitis. Skin lesions with an average course of 8-14 days include maculopapular rash, herpes zoster, alopecia areata, folliculitis like lesions, cheilitis, eye lesion, erythema multiforme, psoriasis, morbilliform, generalized pustular eruption, androgenetic alopecia, granuloma annulare, verruca vulgaris, pruritus, skin dryness and tingling. Cutaneous manifestations with an average course longer than 14 days include vesicular rashes, purpura, perionychial lesions and livedo reticularis.
Laboratory examination
Increased hypersensitive C-reactive protein, leukocytosis and elevated D-dimer were the main laboratory findings in most of the cases. Other laboratory findings include decreased albumin, elevated eosinophils, elevated procalcitonin, decreased hemoglobin, decreased blood potassium, increased creatinine, etc. Vasculitic manifestations (purpura, livedo reticularis) were correlated with elevated D-dimer values (Table 4).
Table 4.
Laboratory findings in patients with new onset skin manifestations after COVID-19 infection
| Cases underwent laboratory examinations | Laboratory Findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Leukocytosis | CRP increase | Albumin decrease | Eosinophils increas | Procalcitonin increase | Hemoglobin decrease | D-dimer increase | Blood potassium decrease | Creatinine increase | |
| Maculopapular rash (n=36) | 17 (47.22%) | 23 (63.89%) | 12 (33.33%) | 1 (2.78%) | 6 (16.67%) | 8 (22.22%) | 13 (36.11%) | 9 (25%) | 4 (11.11%) |
| Herpes simplex (n=1) | 0 | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Urticaria (n=4) | 3 (75%) | 3 (75%) | 2 (50%) | 0 | 0 | 1 (25%) | 1 (25%) | 1 (25%) | 0 |
| Herpes zoster (n=6) | 2 (33.33%) | 4 (66.67%) | 0 | 0 | 1 (16.67%) | 1 (16.67%) | 1 (16.67%) | 1 (3.45%) | 0 |
| Vesicular rashes (n=6) | 4 (66.67%) | 3 (50%) | 4 (66.67%) | 0 | 1 (16.67%) | 2 (33.33%) | 3 (50%) | 0 | 1 (16.67%) |
| Purpura (n=5) | 1 (20%) | 1 (20%) | 1 (20%) | 0 | 1 (20%) | 1 (20%) | 3 (60%) | 0 | 1 (20%) |
| Morbilliform (n=2) | 0 | 1 (50%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pityriasis rosea (n=1) | 1 (100%) | 0 | 1 (100%) | 0 | 0 | 1 (100%) | 1 (100%) | 1 (100%) | 0 |
| Generalized pustular rash (n=1) | 0 | 1 (100%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Erythema multiforme (n=2) | 1 (50%) | 1 (50%) | 0 | 0 | 0 | 0 | 1 (50%) | 0 | 0 |
| Periungual lesions (n=1) | 0 | 1 (100%) | 1 (100%) | 0 | 0 | 1 (100%) | 1 (100%) | 1 (100%) | 0 |
| Psoriasis (n=2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Livedo reticularis (n=1) | 1 (100%) | 1 (100%) | 1 (100%) | 0 | 0 | 0 | 1 (100%) | 0 | 0 |
| Folliculitis like lesions (n=1) | 0 | 1 (100%) | 0 | 0 | 0 | 1 (100%) | 0 | 1 (100%) | 0 |
Laboratory examinations was not performed in cases of alopecia areata, androgenetic alopecia, seborrheic dermatitis, granuloma annulare, cheilitis, eye lesions, skin dryness, stinging, pruritus and verruca vulgaris.
COVID-19 patients with recurrence or aggravation of previous skin diseases
A total of 59 cases of recurrent or aggravated skin diseases were included after infection with COVID-19, 27 of them males and 32 of them females. The characteristics of COVID-19 patients with recurrence or aggravation of previous skin diseases were shown in Table 5. Among the 59 patients, dermatitis was the most common findings (22/59), followed by acne and folliculitis (14/59), psoriasis (12/59), urticaria (5/59), bullous pemphigoid (3/59), pemphigus (1/59), tinea corporis (1/59) and androgenetic alopecia (1/59).
Table 5.
Summary of characteristics of COVID-19 patients with recurrence or aggravation of previous skin diseases
| Recurrence or aggravation of previous skin diseases (n=59) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Psoriasis (n=12) | Acne and folliculitis (n=14) | Urticaria (n=5) | Dermatitis (n=22) | Bullous pemphigoid (n=3) | Pemphigus (n=1) | Tinea corporis (n=1) | Androgenetic alopecia (n=1) | |
| Skin lesions location | ||||||||
| Upper limbs | 11 (91.67%) | 0 | 4 (80%) | 15 (68.18%) | 3 (100%) | 1 (100%) | 0 | 0 |
| Lower limbs | 12 (100%) | 0 | 4 (80%) | 13 (59.09%) | 2 (66.67%) | 1 (100%) | 1 (100%) | 0 |
| Trunk | 11 (91.67%) | 7 (50%) | 5 (100%) | 8 (36.36%) | 3 (100%) | 1 (100%) | 0 | 0 |
| Lip | 0 | 0 | 0 | 0 | 0 | 1 (100%) | 0 | 0 |
| Face | 1 (8.33%) | 12 (85.71%) | 0 | 5 (22.73%) | 0 | 0 | 0 | 0 |
| Vulva | 0 | 0 | 0 | 1 (4.55%) | 0 | 0 | 0 | 0 |
| Scalp | 5 (41.67%) | 1 (7.14%) | 0 | 2 (9.09%) | 0 | 0 | 0 | 1 (100%) |
| Time of recurrence or aggravation of skin lesions (days) | ||||||||
| Average time | 6.58 | 6.07 | 10.2 | 8.32 | 2.67 | 2 | 2 | 10 |
| COVID-19 Course (days) | ||||||||
| Average course | 8.92 | 7.29 | 6.6 | 11.05 | 17.75 | 10 | 9 | 10 |
| Severity of COVID-19 | ||||||||
| Mild | 11 (91.67%) | 13 (92.86%) | 5 (100%) | 19 (86.36%) | 0 | 1 (100%) | 1 (100%) | 1 (100%) |
| Moderate | 1 (8.33%) | 0 | 0 | 2 (3.93%) | 2 (66.67%) | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Critical | 0 | 0 | 0 | 0 | 1 (33.33%) | 0 | 0 | 0 |
| Asymptomatic | 0 | 1 (7.14%) | 0 | 1 (4.55%) | 0 | 0 | 0 | 0 |
| Symptoms of COVID-19 | ||||||||
| T≥38.5°C | 4 (33.33%) | 0 | 1 (20%) | 5 (22.73%) | 3 (100%) | 0 | 0 | 0 |
| T<38.5°C | 8 (66.67%) | 14 (100%) | 4 (80%) | 17 (77.27%) | 0 | 1 (100%) | 1 (100%) | 1 (100%) |
| Cough | 11 (91.67%) | 12 (85.71%) | 4 (80%) | 16 (72.73%) | 2 | 1 (100%) | 1 (100%) | 0 |
| Sore throat | 11 (91.67%) | 13 (92.86%) | 4 (80%) | 17 (77.27%) | 2 (66.67%) | 1 (100%) | 1 (100%) | 0 |
| Nasal congestion runny nose | 3 (25%) | 7 (50%) | 1 (20%) | 3 (13.64%) | 2 (66.67%) | 0 | 0 | 0 |
| Shortness of breath | 0 | 0 | 0 | 0 | 1 (33.33%) | 0 | 0 | 0 |
| Body aches | 0 | 0 | 1 (20%) | 0 | 1 (33.33%) | 0 | 0 | 0 |
| Laboratory examination | ||||||||
| Leukocytosis | 2 (16.67%) | 1 (7.14%) | 1 (20%) | 3 (13.64%) | 3 (100%) | 1 (100%) | 0 | 0 |
| CRP increase | 1 (8.33%) | 1 (7.14%) | 1 (20%) | 1 (4.55%) | 2 (66.67%) | 1 (100%) | 0 | 0 |
| Albumin decrease | 0 | 0 | 0 | 0 | 3 (100%) | 0 | 0 | 0 |
| D-dimer increase | 0 | 0 | 0 | 0 | 1 (33.33%) | 1 (100%) | 0 | 0 |
| Creatinine increase | 0 | 0 | 0 | 0 | 1 (33.33%) | 0 | 0 | 0 |
| Blood potassium decrease | 0 | 0 | 0 | 0 | 1 (33.33%) | 1 (100%) | 0 | 0 |
Trunk, lower limb, and upper limb were the main involved regions. The involvement of lip and vulva were rare. Labial mucosa involvement only occurs in pemphigus, vulva involvement only occurs in dermatitis. Face involvement was more common in psoriasis, acne, folliculitis and dermatitis. Scalp involvement was more common in psoriasis, acne, folliculitis, dermatitis and androgenetic alopecia.
Urticaria is the skin disease with the longest mean time (10.2 days) for recurrence or aggravation after COVID-19 infection. Skin diseases with the shortest mean time (2 days) for aggravation is pemphigus and tinea corporis. Skin manifestations with the mean time of recurrence or aggravation less than 7 days include psoriasis, acne, folliculitis, bullous pemphigoid, pemphigus and tinea corporis; with an average time of 7-14 days include urticaria, dermatitis and androgenetic alopecia.
As for course of COVID-19, bullous pemphigoid had the longest mean course (17.75 days) and urticaria had the shortest mean course (6.6 days). Skin manifestations with the mean course less than 8 days include urticaria and acne and folliculitis. Skin lesions with an average course of 8-14 days include psoriasis, dermatitis, pemphigus, tinea corporis and androgenetic alopecia. Skin diseases with an average course longer than 14 days include bullous pemphigoid.
Most patients with recurrent or aggravated skin diseases were mild, except bullous pemphigoid. The types of skin lesions with moderate COVID-19 include psoriasis, dermatitis and bullous pemphigoid; skin lesions with asymptomatic COVID-19 include acne, folliculitis and dermatitis. Only once case of critical COVID-19 presented as bullous pemphigoid.
Among all types of skin disease, fever, cough, sore throat, nasal congestion and runny nose were the cardinal symptom. Shortness of breath only occurs in patients with bullous pemphigoid. Body aches occurs in patients with urticaria and bullous pemphigoid.
Increased hypersensitive C-reactive protein, leukocytosis were the main laboratory findings in most of the cases. Elevated D-dimer was found in bullous pemphigoid and pemphigus. Other laboratory findings include decreased albumin, decreased blood potassium, increased creatinine, etc.
Discussion
After 3 years from the beginning of COVID-19 pandemic, the world is still facing a crisis. In the previous literature, approximately half of the patients are asymptomatic leading to uncontrolled viral transmission [3,23-26]. COVID-19-related cutaneous manifestations may aid clinicians in early diagnosis, before the development of other symptoms, and may also be used to identify complications that need to be treated [3].
The present study describes a spectrum of cutaneous presentations associated with COVID-19 infection including inflammatory lesions, vascular lesions, etc. Maculopapular rash and urticaria were the most common skin manifestations in patients with COVID-19 in our studies and other reports [3,10,15,20,27-29]. The pathogenesis of urticaria like lesions can be explained by complement activation caused by antigen-antibody complexes and subsequent degranulation of mast cells [3]. HerreroMoyano et al. hypothesized that maculopapular rashes could be caused by a cytokine storm produced by a hyperactive immune system against the virus [30]. Some of the previous research demonstrated maculopapular rash and urticaria-like lesions could occurred simultaneously with other symptoms [3,10]. While our studies had noticed a later mean onset of maculopapular rash and urticaria-like lesions after infection with COVID-19 in our populations (average latency times of 8.27/9.7 days). These suggests that for patients with suspected COVID-19, paying close attention to their skin manifestations may be helpful for early diagnosis. These also tells us that testing for COVID-19 should not be ignored when a patient presents with a cutaneous phenotype, and treatment of COVID-19 infections may be able to alleviate the cutaneous manifestations.
According to reports from different countries, COVID-19 vesicular eruption occurs in 3.77%-15% of adult patients [2,31,32]. These types of skin manifestations were typically seen in middle-aged patients with moderate to severe disease [10,12,32,33]. In our study, the median time from COVID-19 infection to vesicular eruption was 9.67 days. The trunk of the body, extremities were commonly affected in this type of rash, which were consistent with the description in the literature [10,27,33,34]. An overactive immune system resulting in a “cytokine storm” in the skin could be the pathophysiology of vesicular lesions [35]. There is also a possibility that SARS-CoV-2 is directly cytopathic to endothelium dermal vessels, resulting in the formation of vesicles [36].
In our study, herpes zoster typically occurs in elderly COVID-19 patients with weaker immune systems or more severe diseases. The median time from COVID-19 infection to herpes zoster was 17.48 days. According to a retrospective cohort study, adults over 50 years of age with COVID-19 have a 15% greater risk of reactivating herpes zoster than controls without COVID-19 [37]. This reminds us that for elder patients with more severe conditions, it is necessary to pay attention to their cutaneous manifestations in order to take corresponding treatment measures in a timely manner. A mechanism for the increase in herpes zoster associated with COVID-19 is not fully understood. Possible explanation is that the strong immunity to SARS-CoV-2 impairs the immune system’s ability to monitor varicella zoster [12].
The emergence of purpura and livedo reticularis is relatively rare, but their appearance may be related to the severity and course of the disease. Livedo reticularis and purpura had the longer mean course (18 days and 14.2 days respectively). In our studies, 20% of the purpura patients were critical and 100% of the livedo reticularis was severe. It is reported that purpuric lesions and livedo reticularis are more frequent in middle-aged and elderly patients with severe COVID-19 infections [8,34], which is corresponding to our results. It has been proposed that purpura skin lesions result from a pauci-inflammatory thrombogenic vasculopathy [2]. The relationship between hypercoagulability and COVID-19 infections may contribute to livedo reticularis pathogenesis, confirmed by the presence of higher D-dimer and fibrin degradation product levels in patients with severe COVID-19 and livedoid lesions [38]. With a mortality rate of 10%, livedoid lesions rank highest among all cutaneous manifestations [10]. For patients with these skin manifestations, it is necessary to monitor their condition progression closely and adjust treatment plans promptly.
In previous studies, chilblain-like lesions typically occur later in the course of the infection and last longer (for about a week or two on average) and usually appear in younger asymptomatic patients or with mild COVID-19 disease [3,6,10,39,40], while there were no chilblain-like lesions patients in our study. Pathogenesis behind chilblain could involve virus induced type I interferonopathy, thrombosis/coagulopathy, and vasculitis caused by endothelial damage are proposed to be the main factors in the pathogenesis of the chilblain-like lesions [21,41].
A bidirectional relationship exists between psoriasis and infection, certain infections can trigger psoriasis and psoriasis is associated with an increased risk of serious infections [42]. In our study, there were two patients with new onset of psoriasis following COVID-19 infection and 12 patients with recurrence or aggravation of psoriasis after COVID-19 infection. Xiaoyu Gu et al. suggested that COVID-19 susceptibility was associated with genetic predisposition to psoriasis in a Mendelian randomization study in the Journal of American Association of Dermatology [43].
Additionally, COVID-19 may play a role in various immune-related dermatological manifestations. Alopecia areata also appeared in our study is thought to be a dermatologic condition of COVID-19, with cases most often appearing 1-2 months following infection [44]. COVID-19 is associated with alopecia as one of its possible sequelae. A follow-up study of 63 COVID-19 patients found 24.1% to have alopecia [45]. There is still no clear understanding of the mechanisms involved in the development of alopecia resulting from COVID-19 [12].
Other skin manifestations observed in COVID-19 patients in our study include pytiriasis rosea, generalized pustular rash, erythema multiforme, morbilliform, herpes simplex, folliculitis like lesions, eye lesions, periungual lesions, cheilitis, seborrheic dermatitis, androgenetic alopecia, psoriasis, granuloma annulare, verruca vulgaris, pruritus, skin dryness and stinging. Other studies have also reported the following conditions: sebopsoriasis, Covid arm [46], lichen planus [47], painful ulcers on the hard palate and tongue, all fingernails onychopathy, diffuse pruritic pustular eruption, symmetrical drug-related intertriginous and flexural exanthema-like erythematous rash, pruriginous and painful subcutaneous nodular lesions, eruptive angiomas [2].
Disease severity was mild for majority of the dermatologic manifestations, which may be related to the fact that most patients included were outpatients. In our work, skin manifestations with critical COVID-19 include maculopapular rash, vesicular rashes, purpura and erythema multiforme. Skin lesions with severe COVID-19 include maculopapular rash, herpes zoster and livedo reticularis. This suggests that for patients with severe COVID-19, paying close attention to whether they have the above skin manifestations may be helpful for early diagnosis.
Fever and cough were frequent concomitant symptoms, while syncopal episodes only occurred with urticaria. Shortness of breath only occurs in severe and critical patients. The highest proportion of patients with a body temperature ≥38.5°C were morbilliform.
The mean time of emergence of skin lesions after infection with COVID-19 ranged from 3 days for seborrheic dermatitis to 17.48 days for herpes zoster. The shortest COVID-19 course was seen with seborrheic dermatitis, the longest disease course was seen livedo reticularis. It is possible to predict the course of COVID-19 with different severity through skin manifestations.
A total of 59 cases of recurrent or aggravated skin diseases were included after infection with COVID-19. Among these patients, dermatitis was the most common findings, followed by acne and folliculitis, psoriasis, urticaria, bullous pemphigoid, pemphigus, tinea corporis and androgenetic alopecia. The worsening of above diseases may be related to the patient’s immune status or withdrawal of medications required for protopathy.
Laboratory findings included elevated CRP, leukocytosis, hypoalbuminemia, hyperlipidemia, neutrophilia, increased procalcitonin, anemia, elevated D-dimer, hypokalemia, and increased creatinine in variable proportions across the rash subtypes. These findings suggest that COVID-19 related skin eruptions may be accompanied by systemic inflammation, coagulation abnormalities, etc. Vasculitic manifestations (purpura, livedo reticularis) were correlated with disease severity and D-dimer values.
The cutaneous phenotypes delineated in this study expand the dermatologic spectrum associated with COVID-19. By early recognizing skin lesions suggestive of COVID-19, dermatologists could play a crucial role in responding to the SARS-CoV-2 pandemic, particularly in the case of asymptomatic infections where this recognition could direct toward an early diagnosis of infection that certainly leads to a better prognosis. COVID-19 infections may be characterized by skin manifestations, which may be poorly recognized due to a lack of dermatological consultations during the pandemic. It is possible to predict the course of COVID-19 with different severity through cutaneous manifestations. For this reason, we consider collections of all cutaneous manifestations useful so that colleagues can recognize them. In COVID-19 patients, the mere presence of skin manifestations is not indicative of disease severity, and it is also highly dependent on the type of lesions. The chilblain-like and the vascular lesions represent the extremes of a spectrum that ranges from the chilblain-like to the vascular, a patient’s prognosis worsens as the severity of the disease increases. Herpes zoster typically occurs in elderly COVID-19 patients with weaker immune systems or more severe diseases. The emergence of purpura and livedo reticularis is relatively rare, but their appearance may be related to the severity and course of the disease. Those with vascular lesions should also be considered as high-priority patients for further medical care. Further investigations are warranted to elucidate the etiopathogenic mechanisms underlying this diverse range of skin manifestations in COVID-19 infection.
Among the limitations are the modest number of samples and the lack of histopathological correlations. We need to focus on longitudinal observations of immune profiling, and clinicopathologic correlations to advance our understanding of COVID-19 pathogenesis through the skin. Second, some factors are as follows: skin manifestations caused by COVID-19 protective measures, cutaneous adverse drug reaction and chance occurrence were not excluded. Another limitation was the absence of data about the COVID-19 patients without skin manifestations. It is imperative to conduct future cohort studies in which patients with and without skin manifestations are compared, as well as other factors related to the disease. A study of this kind can contribute to a better understanding of the prognostic significance of the cutaneous manifestations for COVID-19 patients.
Acknowledgements
This work was supported by Medical Health Science and Technology Project of Zhejiang Province (Grant number: 2022PY022).
Disclosure of conflict of interest
None.
References
- 1.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martora F, Villani A, Fabbrocini G, Battista T. COVID-19 and cutaneous manifestations: a review of the published literature. J Cosmet Dermatol. 2023;22:4–10. doi: 10.1111/jocd.15477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jamshidi P, Hajikhani B, Mirsaeidi M, Vahidnezhad H, Dadashi M, Nasiri MJ. Skin manifestations in COVID-19 patients: are they indicators for disease severity? A systematic review. Front Med (Lausanne) 2021;8:634208. doi: 10.3389/fmed.2021.634208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giavedoni P, Podlipnik S, Pericàs JM, Fuertes de Vega I, García-Herrera A, Alós L, Carrera C, Andreu-Febrer C, Sanz-Beltran J, Riquelme-Mc Loughlin C, Riera-Monroig J, Combalia A, Bosch-Amate X, Morgado-Carrasco D, Pigem R, Toll-Abelló A, Martí-Martí I, Rizo-Potau D, Serra-García L, Alamon-Reig F, Iranzo P, Almuedo-Riera A, Muñoz J, Puig S, Mascaró JM Jr. Skin manifestations in COVID-19: prevalence and relationship with disease severity. J Clin Med. 2020;9:3261. doi: 10.3390/jcm9103261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estébanez A, Pérez-Santiago L, Silva E, Guillen-Climent S, García-Vázquez A, Ramón MD. Cutaneous manifestations in COVID-19: a new contribution. J Eur Acad Dermatol Venereol. 2020;34:e250–e251. doi: 10.1111/jdv.16474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212–e213. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 7.Carrascosa JM, Morillas V, Bielsa I, Munera-Campos M. Cutaneous manifestations in the context of SARS-CoV-2 infection (COVID-19) Actas Dermosifiliogr (Engl Ed) 2020;111:734–742. doi: 10.1016/j.ad.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Wen W, Mu Z, Du X, Han X. Prevalence of cutaneous manifestations in COVID-19: a meta-analysis. J Dermatol. 2023;50:622–636. doi: 10.1111/1346-8138.16672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galván Casas C, Català A, Carretero Hernández G, Rodríguez-Jiménez P, Fernández-Nieto D, Rodríguez-Villa Lario A, Navarro Fernández I, Ruiz-Villaverde R, Falkenhain-López D, Llamas Velasco M, García-Gavín J, Baniandrés O, González-Cruz C, Morillas-Lahuerta V, Cubiró X, Figueras Nart I, Selda-Enriquez G, Romaní J, Fustà-Novell X, Melian-Olivera A, Roncero Riesco M, Burgos-Blasco P, Sola Ortigosa J, Feito Rodriguez M, García-Doval I. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71–7. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al Ali A, Al-Shidhani S, Al-Balushi F, Alhinai M, Al-Azri AR, Al Lawati SAL, Al Ghailani F, Al Riyami R. Cutaneous manifestations of COVID-19: an experience from Oman. Cureus. 2021;13:e16667. doi: 10.7759/cureus.16667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakashima C, Kato M, Otsuka A. Cutaneous manifestations of COVID-19 and COVID-19 vaccination. J Dermatol. 2023;50:280–289. doi: 10.1111/1346-8138.16651. [DOI] [PubMed] [Google Scholar]
- 13.Zupin L, Moltrasio C, Tricarico PM, Del Vecchio C, Fontana F, Marzano AV, Crovella S. Paraviral cutaneous manifestations associated to SARS-CoV-2 Omicron variant. Infect Dis (Lond) 2023;55:181–188. doi: 10.1080/23744235.2022.2153913. [DOI] [PubMed] [Google Scholar]
- 14.Unterluggauer L, Pospischil I, Krall C, Saluzzo S, Kimeswenger S, Karolyi M, Wenisch C, Lamprecht B, Guenova E, Winkler S, Viczenczova C, Bergthaler A, Weninger W, Hoetzenecker W, Stary G. Cutaneous manifestations of SARS-CoV-2: a two center, prospective, case-controlled study. J Am Acad Dermatol. 2021;85:202–4. doi: 10.1016/j.jaad.2021.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thuangtong R, Angkasekwinai N, Leeyaphan C, Triwongwaranat D, Thanomkitti K, Munprom K, Kulthanan K. Patient recovery from COVID-19 infections: follow-up of hair, nail, and cutaneous manifestations. Biomed Res Int. 2021;2021:5595016. doi: 10.1155/2021/5595016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goyal PK, Mohammed TO, Mahmoud A, Zaidi AJ, Nguyen CV. COVID-19 infection leading to acute pustular dermatoses. Arch Dermatol Res. 2023;315:685–697. doi: 10.1007/s00403-022-02450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavanagh G, Wambier CG. Reply to: “Personal protective equipment recommendations based on COVID-19 route of transmission”. J Am Acad Dermatol. 2020;83:e47. doi: 10.1016/j.jaad.2020.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta MK, Lipner SR. Personal protective equipment recommendations based on COVID-19 route of transmission. J Am Acad Dermatol. 2020;83:e45–e46. doi: 10.1016/j.jaad.2020.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dupont L, Duquia RP, Pizutti GW, Nunes FB, Branchini G, Mosquera ESB, Bonamigo RR. Cutaneous manifestations in patients with COVID-19 treated at a University Hospital in Southern Brazil. Cureus. 2022;14:e31566. doi: 10.7759/cureus.31566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar P, Radha G, Muthukrishnan M, Chandrasekaran B, Subbiah P, Raman J. Cutaneous manifestations associated with COVID-19 infection in a COVID-designated hospital in North Chennai - A descriptive cross-sectional study. Indian Dermatol Online J. 2022;14:67–71. doi: 10.4103/idoj.idoj_279_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Özen T, Kahraman FC, Öcal S, Ovalı HF. Skin, mucosa and nail findings in hospitalized pediatric patients with Coronavirus disease-2019 (COVID-19) An Bras Dermatol. 2023;98:208–215. doi: 10.1016/j.abd.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Living Guidance for Clinical Management of COVID-19. Version 23 November 2021. Available online: https://apps.who.int/iris/bitstream/handle/10665/349321/WHO-2019-nCoV-clinical-2021.2-eng.pdf (accessed on 7 July 2022)
- 23.Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the achilles’ heel of current strategies to control Covid-19. N Engl J Med. 2020;382:2158–2160. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, Taylor J, Spicer K, Bardossy AC, Oakley LP, Tanwar S, Dyal JW, Harney J, Chisty Z, Bell JM, Methner M, Paul P, Carlson CM, McLaughlin HP, Thornburg N, Tong S, Tamin A, Tao Y, Uehara A, Harcourt J, Clark S, Brostrom-Smith C, Page LC, Kay M, Lewis J, Montgomery P, Stone ND, Clark TA, Honein MA, Duchin JS, Jernigan JA Public Health-Seattle and King County and CDC COVID-19 Investigation Team. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu S, Lin J, Zhang Z, Xiao L, Jiang Z, Chen J, Hu C, Luo S. Alert for non-respiratory symptoms of coronavirus disease 2019 (COVID-19) patients in epidemic period: a case report of familial cluster with three asymptomatic COVID-19 patients. J Med Virol. 2021;93:518–521. doi: 10.1002/jmv.25776. [DOI] [PubMed] [Google Scholar]
- 26.Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395:1418–1420. doi: 10.1016/S0140-6736(20)30917-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Askin O, Altunkalem RN, Altinisik DD, Uzuncakmak TK, Tursen U, Kutlubay Z. Cutaneous manifestations in hospitalized patients diagnosed as COVID-19. Dermatol Ther. 2020;33:e13896. doi: 10.1111/dth.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atzori L, Perla S, Atzori MG, Ferreli C, Rongioletti F. Cutaneous drug eruptions associated with COVID-19 therapy. JAAD Int. 2020;1:73–76. doi: 10.1016/j.jdin.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terzi K, Kesici S, Özsürekci Y, Bayrakci B. Periorbital erythema is a common cutaneous manifestation in COVID-19. Clin Exp Dermatol. 2021;46:1316–1317. doi: 10.1111/ced.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herrero-Moyano M, Capusan TM, Andreu-Barasoain M, Alcántara-González J, Ruano-Del Salado M, Sánchez-Largo Uceda ME, Calzado-Villarreal L, Pérez-González Y. A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema. J Eur Acad Dermatol Venereol. 2020;34:e460–e464. doi: 10.1111/jdv.16631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Masson A, Bouaziz JD, Sulimovic L, Cassius C, Jachiet M, Ionescu MA, Rybojad M, Bagot M, Duong TA SNDV (French National Union of Dermatologists-Venereologists) Chilblains is a common cutaneous finding during the COVID-19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83:667–670. doi: 10.1016/j.jaad.2020.04.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marzano AV, Genovese G, Fabbrocini G, Pigatto P, Monfrecola G, Piraccini BM, Veraldi S, Rubegni P, Cusini M, Caputo V, Rongioletti F, Berti E, Calzavara-Pinton P. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83:280–285. doi: 10.1016/j.jaad.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez-Nieto D, Ortega-Quijano D, Jimenez-Cauhe J, Burgos-Blasco P, de Perosanz-Lobo D, Suarez-Valle A, Cortes-Cuevas JL, Carretero I, Garcia-Del Real C, Fernandez-Guarino M. Clinical and histological characterization of vesicular COVID-19 rashes: a prospective study in a tertiary care hospital. Clin Exp Dermatol. 2020;45:872–875. doi: 10.1111/ced.14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Giorgi V, Recalcati S, Jia Z, Chong W, Ding R, Deng Y, Scarfi F, Venturi F, Trane L, Gori A, Silvestri F, Gao XH, Lotti T. Cutaneous manifestations related to coronavirus disease 2019 (COVID-19): a prospective study from China and Italy. J Am Acad Dermatol. 2020;83:674–675. doi: 10.1016/j.jaad.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Nieto D, Ortega-Quijano D, Segurado-Miravalles G, Pindado-Ortega C, Prieto-Barrios M, Jimenez-Cauhe J. Comment on: cutaneous manifestations in COVID-19: a first perspective. Safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol. 2020;34:e252–e254. doi: 10.1111/jdv.16470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Criado PR, Abdalla BMZ, De Assis IC, Van Blarcum de Graaff Mello C, Caputo GC, Vieira IC. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? Revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745–756. doi: 10.1007/s00011-020-01370-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhavsar A, Lonnet G, Wang C, Chatzikonstantinidou K, Parikh R, Brabant Y, Servotte N, Shi M, Widenmaier R, Aris E. Increased risk of herpes zoster in adults ≥50 years old diagnosed with COVID-19 in the United States. Open Forum Infect Dis. 2022;9:ofac118. doi: 10.1093/ofid/ofac118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freeman EE, McMahon DE, Lipoff JB, Rosenbach M, Kovarik C, Takeshita J, French LE, Thiers BH, Hruza GJ, Fox LP American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83:486–492. doi: 10.1016/j.jaad.2020.05.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morey-Olive M, Espiau M, Mercadal-Hally M, Lera-Carballo E, Garcia-Patos V. Cutaneous manifestations in the current pandemic of coronavirus infection disease (COVID 2019) An Pediatr (Engl Ed) 2020;92:374–375. doi: 10.1016/j.anpede.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouaziz JD, Duong TA, Jachiet M, Velter C, Lestang P, Cassius C, Arsouze A, Domergue Than Trong E, Bagot M, Begon E, Sulimovic L, Rybojad M. Vascular skin symptoms in COVID-19: a french observational study. J Eur Acad Dermatol Venereol. 2020;34:e451–e452. doi: 10.1111/jdv.16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rademaker M, Agnew K, Anagnostou N, Andrews M, Armour K, Baker C, Foley P, Gebauer K, Gupta M, Marshman G, Rubel D, Sullivan J, Wong LC. Psoriasis and infection. A clinical practice narrative. Australas J Dermatol. 2019;60:91–98. doi: 10.1111/ajd.12895. [DOI] [PubMed] [Google Scholar]
- 43.Gu X, Chen X, Shen M. Association of psoriasis with risk of COVID-19: a 2-sample Mendelian randomization study. J Am Acad Dermatol. 2022;87:715–717. doi: 10.1016/j.jaad.2022.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christensen RE, Jafferany M. Association between alopecia areata and COVID-19: a systematic review. JAAD Int. 2022;7:57–61. doi: 10.1016/j.jdin.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyazato Y, Morioka S, Tsuzuki S, Akashi M, Osanai Y, Tanaka K, Terada M, Suzuki M, Kutsuna S, Saito S, Hayakawa K, Ohmagari N. Prolonged and late-onset symptoms of coronavirus disease 2019. Open Forum Infect Dis. 2020;7:ofaa507. doi: 10.1093/ofid/ofaa507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Picone V, Martora F, Fabbrocini G, Marano L. “Covid arm”: abnormal side effect after Moderna COVID-19 vaccine. Dermatol Ther. 2022;35:e15197. doi: 10.1111/dth.15197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Picone V, Fabbrocini G, Martora L, Martora F. A case of new-onset Lichen Planus after COVID-19 vaccination. Dermatol Ther (Heidelb) 2022;12:801–805. doi: 10.1007/s13555-022-00689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


