Abstract
CXCR4 is a chemokine receptor used by some strains of HIV-1 as an entry coreceptor in association with cell surface CD4 on human cells. In human immunodeficiency virus type 1 (HIV-1)-infected individuals, the appearance of viral isolates with a tropism for CXCR4 (T tropic) has been correlated with late disease progression. The presumed natural ligands for CXCR4 are SDF-1α and SDF-1β, which are proposed to play a role in blocking T-tropic HIV-1 cell entry. Here, we demonstrate that addition of an N-terminal methionine residue to SDF-1β (Met-SDF-1β) results in a dramatically enhanced functional activity compared to that of native SDF-1β. Equivalent concentrations of Met-SDF-1β are markedly more inhibitory for T-tropic HIV-1 replication than SDF-1β. A comparison of the biological activities of these two forms of SDF-1β reveals that Met-SDF-1β induces a more pronounced intracellular calcium flux yet binds with slightly lower affinity to CXCR4 than SDF-1β. Down-modulation of CXCR4 is similar after exposure of cells to either chemokine form for 2 h. However, after a 48-h incubation, the surface expression of CXCR4 is much lower for cells treated with Met-SDF-1β. The enhanced blocking of T-tropic HIV-1 by Met-SDF-1β appears to be related to prolonged CXCR4 down-modulation.
CXCR4 (CXC chemokine receptor 4; also called fusin or LESTR) was the first chemokine receptor identified as a necessary coreceptor for human immunodeficiency virus type 1 (HIV-1) cell entry (15). T-cell line-tropic (T-tropic) strains of HIV-1 were found to require CXCR4 in addition to CD4 to bind and enter target cells. The observations that monocyte-tropic (M-tropic) virus strain entry could be blocked by the chemokines macrophage inflammatory protein (MIP)-1α, MIP-1β, and RANTES (regulated an activation normal T cell expressed and secreted) (8) rapidly led to the identification of CCR5 (CC chemokine receptor 5) as another important coreceptor (1, 10, 14, 37). The ability of these CCR5 ligands to block M-tropic HIV-1 prompted a search for analogous CXCR4 ligands that can block T-tropic HIV-1. Stromal-cell-derived factor 1α and 1β (SDF-1α and -1β), which are splice variants of the same gene (31, 43, 47) differing by four additional amino acids at the C terminus of SDF-1β, were found to bind and signal through CXCR4 and block T-tropic virus entry (5, 32).
The precise mechanism(s) by which SDF-1 interferes with T-tropic HIV-1 cell entry is not known. Recent work has examined the biological activity of SDF-1, including chemotaxis, receptor binding, calcium mobilization, and down-modulation of CXCR4 (2, 9, 17, 18, 44). Several reports have shown that small molecules can block T-tropic strains in vitro (12, 19, 30, 40). Inhibition by SDF-1 or small molecules is thought to involve competitive binding to CXCR4, blocking binding of viral gp120 (12); the role of downstream events, such as receptor down-modulation or postreceptor signaling, remains to be fully elucidated. Two groups have reported that C-terminal-truncated CXCR4 supports T-tropic HIV-1 entry but is not down-modulated following exposure to SDF-1; without chemokine-mediated receptor down-modulation, SDF-1 does not efficiently block viral replication as measured by HIV-1 p24 protein production (2, 44). These results imply that down-modulation of CXCR4 or postreceptor signaling plays a crucial role in the antiviral activity of SDF-1.
In the present study we examine the activities of two forms of SDF-1: SDF-1β, beginning with the amino-terminal amino acid lysine, which is believed to represent the native amino-terminal sequence (6), and Met-SDF-1β, which has an added N-terminal methionine. We find significant functional differences between these two molecules. Our results indicate that receptor down-modulation is a crucial component in the inhibition of HIV-1 infection by SDF-1β.
MATERIALS AND METHODS
Cells.
The immortalized CD4+ cell lines T1 (38) and U937 (46) were used in these studies. Each of these lines is permissive for infection by HIV-1 IIIB, a strain which utilizes CXCR4 for entry (13). These cell lines were maintained in RPMI 1640 (Sigma, St. Louis, Mo.) supplemented with 20% heat-inactivated fetal calf serum (FCS), 10 mM HEPES, 2 mM glutamine, 100 U of penicillin/ml, and 10 μg of streptomycin/ml. In addition, primary CD4+ lymphocytes from HIV-1-seronegative donors were generated as previously described (49). In brief, freshly Ficoll-Hypaque gradient-purified peripheral blood mononuclear cells were stimulated with a CD3-CD8 bispecific monoclonal antibody (51), leading to stimulation of CD3+CD4+ cells and inhibition of CD3+CD8+ cells. After approximately 7 days, >98% of these cells coexpressed CD3 and CD4 (data not shown), at which time they were infected with HIV-1 or used for calcium flux experiments. These cells were grown and maintained in RPMI containing 10% FCS supplemented with 2 mM glutamine, 100 U of penicillin/ml, 10 μg of streptomycin/ml, and 50 U of interleukin 2/ml.
Preparation of Met-SDF-1β and SDF-1β proteins.
PCR was used with human SDF-1β cDNA as a template to construct two expression vectors for SDF-1β proteins. For Met-SDF-1β, DNA encoding the mature SDF-1β protein, beginning with the N-terminal lysine, was inserted into the Escherichia coli expression vector pAL781 (23), downstream of and in-frame with a vector-provided translation initiation codon, ATG. For SDF-1β, the expression and purification accessory sequence GroHEK was inserted between the initiation codon, ATG, and the mature SDF-1β sequence. GroHEK consists of a short N-terminal segment of E. coli GroE followed by a six-His sequence, a spacer, and an enterokinase recognition site (NH2-AAKDVKHHHHHHGSGSDDDDK) and has been shown to increase the expression yield in E. coli of several recombinant proteins (11a). The GroHEK enterokinase site was used for generating SDF-1β protein postpurification with the native amino terminus. All PCR-generated sequences were verified by DNA sequencing. Protein expression was carried out in the E. coli strain GI934 as described by Lu et al. (26). Following the induction period, polypeptide inclusion bodies containing either SDF-1β species were harvested from cell lysates by centrifugation, washed sequentially with buffers containing 1 M NaCl and 0.5% Triton X-100, solubilized in 6 M guanidine-HCl, and refolded by dialysis against pH 6.5 (Met-SDF-1β) or pH 5.5 (GroHEK-SDF-1β) buffers containing 15 mM sodium acetate, 15 mM sodium phosphate, 1 mM phenylmethylsulfonyl fluoride, and 1 mM para-aminobenzamidine. Refolded SDF-1β proteins were further purified from the clarified dialysate by ion-exchange chromatography on SP-650 and QAE-550 resins. Enterokinase cleavage (24) of GroHEK-SDF-1β was performed after the purified protein was dialyzed against phosphate-buffered saline (10 mM sodium phosphate, pH 7.3, and 150 mM NaCl).
Edman degradation performed on refolded Met-SDF-1β protein indicated that the N-terminal residue was indeed methionine, which is consistent with the known inefficiency of removal of N-terminal methionine residues by E. coli methionyl aminopeptidase when the penultimate N-terminal residue carries a bulky side chain (20). SDF-1β, with an N-terminal lysine, was generated after the removal of the 21-amino-acid tag, GroHEK, by enterokinase. Protein concentrations for purified proteins were determined by amino acid analysis and by their extinction coefficients at 280 nm: Met-SDF-1β, 9.0 × 103 liters/mol-cm, and SDF-1β, 8.7 × 103 liters/mol-cm. The endotoxin levels of the purified proteins were determined by Limulus amoebocyte lysate assay, and all purified protein was ≤20 endotoxin units/ml of protein. Wild-type human SDF-1α (with N-terminal lysine [data not shown]) was obtained from Peprotech (Rocky Hill, N.J.).
Viral inhibition assays. (i) HIV-1 stocks.
HIV-1 IIIB (T-tropic) was produced by fresh infection of T1 cells, and supernatant was harvested 4 to 5 days after infection. HIV-1 JR-CSF (M-tropic) (22) was produced by fresh infection of peripheral blood mononuclear cells after 3 days of phytohemagglutinin (PHA) stimulation (PHA blasts), followed by supernatant harvest 7 or 8 days after infection. Aliquots of virus stocks were cryopreserved at −80°C and thawed immediately before use. Viral titers were determined as previously described by limiting-dilution infection of C8166 cells (IIIB) (21) or PHA blasts (JR-CSF) (16).
(ii) HIV-1 inhibition assays.
CD4+ cells in log phase were resuspended in fresh medium, RPMI-20% FCS (T1 and U937) or RPMI-10% FCS with 50 U of interleukin 2 (primary CD4+ cells), at approximately 106/ml, and the appropriate chemokines were added to their final test concentrations for a 2-h incubation at 37°C. HIV-1 was then added at a multiplicity of infection (MOI; 50% tissue culture infectious doses per cell [TC1050]) of 10−2 for a 4-h incubation at 37°C. The cells were washed twice and plated in 24-well plates at 5 × 105/well in 2 ml of medium containing the appropriate chemokines at the indicated final concentrations. At 2- to 4-day intervals, 1 ml of supernatant was removed from each well for HIV-1 p24 antigen quantitation by enzyme-linked immunosorbent assay (DuPont, Boston, Mass.) and replaced with 1 ml of fresh medium supplemented with chemokines at the initial concentrations.
Calcium mobilization measurement.
Anti-CD3-activated human CD4+ cells or cells of the human monocyte-like line U937 were harvested from cultures in log phase, washed once in serum-free medium, and resuspended in loading medium at pH 7.1 (RPMI 1640 supplemented with 0.02% bovine serum albumin [BSA], 15 mM HEPES). T. 107 cells per ml was added 3 μl of a 1-μg/μl solution of the calcium dye Fluo-3 (Molecular Probes Inc., Eugene, Oreg.) dissolved in dimethyl sulfoxide (Sigma). The cells were mixed and cultured at 24°C for 20 to 30 min followed by two washes in loading medium and resuspended in phenol red-free RPMI 1640 supplemented with 0.02% BSA and penicillin-streptomycin. The cells were kept on ice until they were analyzed, at which time an aliquot was brought to room temperature. After a 40- to 60-s background reading, chemokine was added and the fluorescence cytometric data acquisition continued, using a FACScan flow cytometer (Becton Dickinson, San Jose, Calif.). The calcium mobilization data are expressed as flow cytometric histograms representing the fluorescence recorded during 40-s interval gates. Calcium studies on primary CD4+ lymphocytes and on the U937 cell line were repeated three times or more.
Chemokine receptor binding–125I-SDF-1α competition assays.
Because of the high level of nonspecific binding by some cell lines, we used cell line U937, which had the lowest background binding and gave the most reproducible binding data (data not shown). 125I-labeled SDF-1α (lactoperoxidase catalyzed iodine labeling) was supplied by DuPont, NEN (Boston, Mass.). The two forms of SDF-1β were compared for the ability to inhibit 125I-SDF-1α binding to U937 cells. The cells (5 × 105) in RPMI 1640 containing 1% BSA, 25 mM HEPES, and 0.1% sodium azide were added in the absence or presence of nonlabeled Met-SDF-1β or SDF-1β to 1-ml polypropylene tubes (Bio-Rad, Richmond, Calif.) along with 125I-SDF-1α. The samples (replicates of two to four) were placed on a shaker at 4°C for 120 min. The cell-associated 125I-SDF-1α was isolated by centrifugation of an aliquot of cells through 140 μl of 10% sucrose in RPMI 1640 with 25 mM HEPES. After the tubes were frozen in a dry ice-acetone bath, the tube tips were removed and the isotope concentration was determined with a gamma counter. Nonspecific binding was determined by the addition of 2 μM unlabeled SDF-1β. The data were analyzed, and Ki values were calculated using GraphPad Prism (GraphPad Software, Inc., San Diego, Calif.). The Kd values were determined with the Radlig program (Biosoft, Ferguson, Mo.) for nonlinear curve fitting of cold ligand inhibition.
Receptor-staining and down-modulation assays.
Cells from log-phase cultures (24 to 48 h after addition of fresh medium) were counted and adjusted to 1 × 105 to 2 × 105/ml by centrifugation and resuspension in the conditioned culture supernatant (i.e., fresh medium with fresh FCS was not added). Chemokine was added to the cells, followed by incubation at 37°C for the indicated periods of time. Controls included cells kept on ice with 0.1% sodium azide and cells cultured for 37°C in the absence of SDF-1β or other chemokine. For pulsing experiments, the cells were cultured with either form of SDF-1β or with medium for 6 or 12 h, centrifuged, either stained or washed again, resuspended in fresh medium, and returned to culture for the indicated time. At the end of the culture period, the cells were centrifuged and resuspended in Dulbecco’s phosphate-buffered saline (Gibco) containing 0.1% BSA, 0.1% sodium azide, and 10% aggregated rabbit serum (Biodesign, Kennebunk, Maine). The cells were divided into equal portions and incubated with isotype control antibody (immunoglobulin IgG2a or IgG2b) or with anti-CXCR4 monoclonal antibody (MAb), followed by washing and secondary incubation with phycoerythrin-conjugated goat anti-mouse F(ab′)2 (SouthernBiotechnology Associates, Birmingham, Ala.). For most experiments the anti-CXCR4 MAb 12G5 (PharMingen, San Diego, Calif.) was used; where indicated, we also used other anti-CXCR4 MAbs (R&D Systems, Minneapolis, Minn.). Samples were analyzed using a FACScan flow cytometer (Becton Dickinson). Fluorescence signals were collected by using logarithmic scales and are presented as the cytometric histogram or as the median fluorscence intensity (MFI) of the histogram using Cell Quest software (Becton Dickinson). With these values the ΔMFI is calculated where ΔMFI = anti-CXCR4 MFI − isotype control MFI. The percent decrease in MFI and percent decrease in CXCR4 were calculated identically as ([ΔMFI for cells cultured with chemokine]/[ΔMFI for cells cultured under control conditions] × 100).
RESULTS
Met-SDF-1β is a more potent inhibitor of HIV-1 than wild-type SDF-1β.
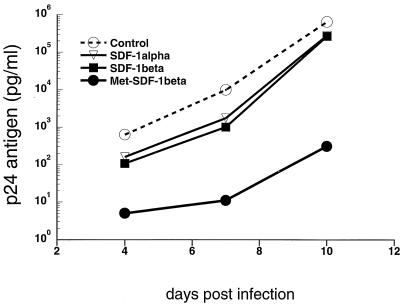
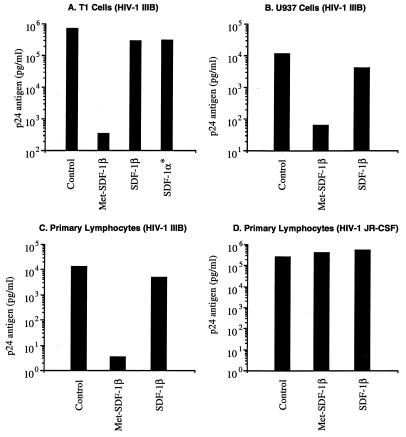
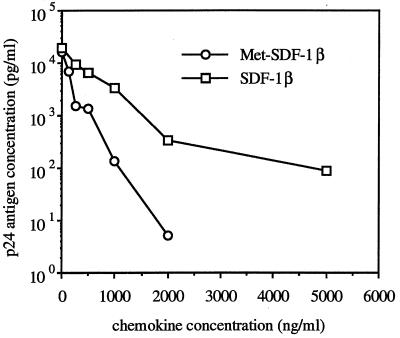
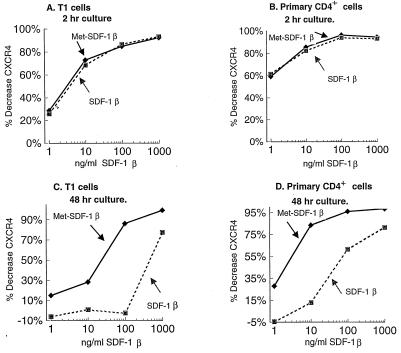
SDF-1α (and SDF-1β) has been shown to block T-tropic HIV-1 infection (2, 5, 9, 32, 44), and we confirmed these findings. Pretreatment and culture of acutely HIV-1 IIIB-infected CD4+ T1 cells with 1 μg of SDF-1β/ml or 1 μg of SDF-1α/ml resulted in approximately 90% inhibition at early time points and about 50% inhibition at the peak of infection (Fig. 1). Pretreatment and culture of acutely HIV-1 IIIB-infected transformed or primary CD4+ cell lines with 1 μg of SDF-1β/ml resulted in approximately 50% inhibition at the peak level of infection for all cell types tested (Fig. 2). Commercially available SDF-1α (Peprotech), which also begins with an N-terminal lysine, was tested and yielded similar levels of viral suppression (Fig. 1 and 2A), which is consistent with published results (5, 32). By contrast, Met-SDF-1β was markedly more inhibitory in the same culture system. At peak levels of viral infection Met-SDF-1β also gave much stronger inhibition for all cell types examined (Fig. 2A to C). Following pretreatment and culture with Met-SDF-1β, viral replication was consistently reduced by 100- to 1,000-fold in both transformed and primary CD4+ cells (Fig. 1 to 3). The inhibitory effects of both forms of SDF-1β were limited to T-tropic HIV-1; neither form of SDF-1β suppressed M-tropic HIV-1 replication in primary CD4+ cells (Fig. 2D). The functional difference between modified and unmodified SDF-1β was not due to cellular toxicity, as cell counts in cultures exposed to these chemokines were higher than those in untreated cultures (data not shown), indicating a protective effect. Titration of the two different forms of SDF-1β on primary CD4+ cells (Fig. 3) demonstrated that virus inhibition was dose-dependent and that Met-SDF-1β was more potent than SDF-1β over a broad range of concentrations. Of note, nearly complete virus suppression at 2,000 ng/ml was achieved with Met-SDF-1β, whereas SDF-1β was not fully suppressive even at 5,000 ng/ml. The data are representative of at least three experiments with primary CD4+ cells, comparing virus inhibition for the two forms of SDF-1β at different concentrations. As with other cell types, the inhibition at different time points was always greater with Met-SDF-1β than with SDF-1β.
FIG. 1.
Blocking of HIV-1 replication by SDF-1. T1 CD4+ cells were pretreated for 2 h with 1 μg of the indicated chemokine/ml, acutely infected with HIV-1 IIIB at an MOI of 10−2, and cultured in 2 ml of medium as described in the text. At 2- to 4-day intervals, 1 ml of medium was removed for quantitative HIV-1 p24 antigen determination and replaced with fresh medium containing 1 μg of the appropriate chemokine/ml. HIV-1 p24 antigen concentrations were determined at the indicated days of culture.
FIG. 2.
Blocking of HIV-1 replication by SDF-1. CD4+ cells were pretreated for 2 h with 1 μg of the indicated chemokine/ml, acutely infected with HIV-1 IIIB or JR-CSF at an MOI of 10−2 TCID50/cell, and cultured in 2 ml of medium as described in the text. At 2- to 4-day intervals, 1 ml of medium was removed for quantitative HIV-1 p24 antigen determination and replaced with fresh medium containing 1 μg of the appropriate chemokine/ml. The peak levels of p24 are shown, approximately 7 to 10 days after infection, for each cell type examined. The relationship between inhibition by SDF-1 and Met-SDF-1 was seen at all time points. The asterisk denotes commercially acquired SDF-1α (Peprotech). The data are representative of three or more experiments for each cell type.
FIG. 3.
Titration of HIV-1 inhibition by Met-SDF-1β versus wild-type SDF-1β. Primary CD4+ cells were pretreated for 2 h with the indicated concentrations of each form of SDF-1β, infected with HIV-1 IIIB, and cultured with the indicated concentration of chemokine as for Fig. 1. HIV-1 p24 antigen concentrations were done at 3-day intervals; values for day 6 are shown.
Met-SDF-1β has a lower binding affinity than SDF-1β for CXCR4.
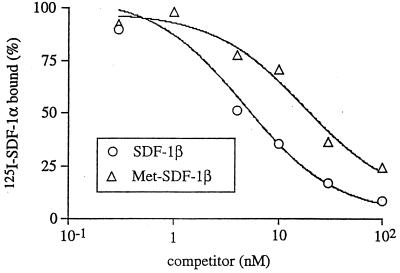
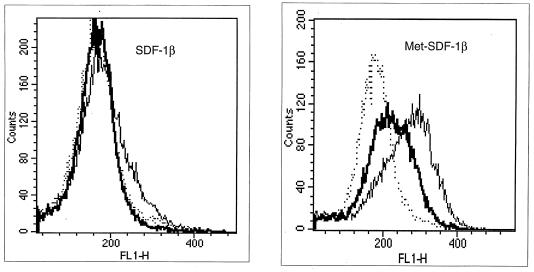
To investigate the role of receptor binding in the differential HIV-1 inhibition by the two forms of SDF-1β, we compared their affinities for CXCR4. Unlabeled SDF-1β and Met-SDF-1β were each tested for the ability to inhibit binding of 125I-SDF-1α to U937 cells. Using a Kd of 3 nM for 125I-SDF-1α (9), the calculated Ki for SDF-1β was 4 nM (34 ng/ml) and the Ki for Met-SDF-1β was 15 nM (129 ng/ml) (Fig. 4). Using an updated EBDA program (28), the Kd for SDF-1β was found to be 2.8 nM while the binding for Met-SDF-1β was found to be 12.4 nM (not shown). These data indicate that the greater inhibition by Met-SDF-1β was not due to enhanced affinity for the CXCR4 receptor.
FIG. 4.
Competitive binding assay of Met-SDF-1β versus wild-type SDF-1β. Binding of 125I-SDF-1α, added at 0.3 nM, to U937 cells was determined in the presence of serial concentrations of unlabeled SDF-1β or Met SDF-1β, as described in Materials and Methods. After a 2-h incubation, the cell-bound 125I-SDF-1 was isolated and counted. The plot shows the percentage of 125I-SDF-1α bound as a function of cold SDF-1β or Met-SDF-1β added.
Met-SDF-1β is more potent at inducing calcium mobilization than SDF-1β.
The abilities of both forms of SDF-1β to signal via CXCR4 were evaluated in calcium mobilization assays. U937 cells exposed to Met-SDF-1β exhibited a much higher level of calcium mobilization than SDF-1β (Fig. 5). Furthermore, the response to Met-SDF-1β appeared to yield a higher level of cytoplasmic calcium for a longer time. Cells treated with SDF-1β returned to baseline fluorescence within 60 s after the addition of chemokine, whereas cells treated with Met-SDF-1β still showed significant fluorescence 100 s after chemokine was added. A similar difference in calcium mobilization was seen for primary CD4+ cells, where Met-SDF-1β also induced a prolonged calcium mobilization (data not shown). The highest level of calcium mobilization was found with levels of SDF-1β added at 1 to 2 μg/ml for both primary CD4+ cells and the U937 cell line (data not shown).
FIG. 5.
Calcium mobilization induced by Met-SDF-1β versus wild-type SDF-1β. U937 cells were loaded with Fluo-3, and calcium mobilization was determined by flow cytometry. Gates, each 40 s in duration, were drawn starting at zero time (R1; dashed line), 60 s (R2; thin solid line), and 100 s (R3; thick solid line). The chemokines SDF-1β (final concentration, 2 μg/ml) and Met-SDF-1β (final concentration, 1 μg/ml) were added at approximately 45 s. The data are representative of three or more experiments for U937 and primary CD4+ T lymphocytes.
Down-modulation of CXCR4 expression by Met-SDF-1β is prolonged.
To evaluate the role of CXCR4 down-modulation, we studied the density of CXCR4 expression after exposure of cells to either form of SDF-1β. Both forms induced a dramatic decrease in cell surface CXCR4 after a short-term incubation (Fig. 6A and B). The down-modulations of CXCR4 following a 2-h culture were similar for Met-SDF-1β and unmodified SDF-1β, and this was observed in both immortalized (Fig. 6A and data not shown) and primary (Fig. 6B) CD4+ cells over a broad range of concentrations. Significant receptor down-modulation was noted even at low concentrations (1 to 10 ng/ml), reaching a maximum effect at 100 to 1,000 ng/ml (12 to 120 nM).
FIG. 6.
CXCR4 expression following culture with Met-SDF-1β versus wild-type SDF-1β. T1 cells (A and C) and primary CD4+ lymphocytes (B and D) were incubated with the indicated concentrations of Met-SDF-1β (⧫), SDF-1β (■), or medium without added chemokine. The expression level of CXCR4 was determined with anti-CXCR4 MAb (12G5) and flow cytometry following 2 h (A and B) or 48 h (C and D) of culture. The percent decrease of CXCR4 was calculated as (ΔMFI for cells cultured with chemokine)/(ΔMFI for control cells) × 100, as described in Materials and Methods. Similar results were obtained in multiple experiments (n > 3).
In contrast, there was a marked difference between the two forms of SDF-1β after a 48-h incubation. T1 cells demonstrated a complete recovery of CXCR4 expression with exposure to 1 to 100 ng of SDF-1β/ml (Fig. 6C). Although the primary CD4+ cells were more sensitive to the chemokine-induced down-modulatory effects, near-complete recovery was seen at 1 to 10 ng of SDF-1β/ml (Fig. 6D). For cells treated with Met-SDF-1β, however, reexpression of surface CXCR4 was far more impaired. At the higher concentrations of Met-SDF-1β, CXCR4 down-modulation remained nearly complete after 48 h (Fig. 6C and D). Even at lower concentrations of Met-SDF-1β, CXCR4 expression remained depressed, particularly in primary CD4 cells (Fig. 6D). At all concentrations tested, at 48 h, cell surface CXCR4 levels were much lower after Met-SDF-1β treatment than after SDF-1β treatment.
These differences were not explained by altered detection of CXCR4. Cells treated with either form of SDF-1β in the presence of 0.1% sodium azide at 4°C demonstrated equivalent anti-CXCR4 antibody staining (not shown), in agreement with a previously published report (44).
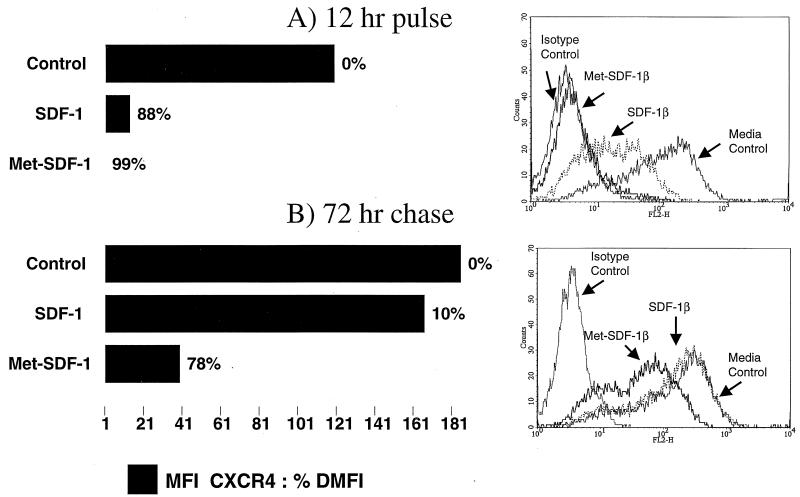
To determine if the level of down-modulation was dependent upon the continuous presence of SDF-1β in culture, we incubated primary CD4+ cells in the absence or presence of either form of chemokine at 1 μg/ml for 12 h, washed the cells twice, and returned them to culture without added chemokine for an additional 72 h. Staining these cells clearly demonstrated that the surface expression of CXCR4 was markedly reduced compared to that of the control following the initial 12-h pulse with either SDF-1β or Met-SDF-1β (Fig. 7). However, following the 72-h chase without added chemokine, the level of CXCR4 returned to control levels for cells pulsed with SDF-1β (Fig. 7). In contrast, cells exposed to Met-SDF-1β demonstrated continued down-modulation of CXCR4 (∼80%) after the 72-h chase (Fig. 7). Similar receptor recovery data was observed with a 6-h pulse and a 48-h chase (data not shown). In summary, reexpression of CXCR4 following treatment with Met-SDF-1β is significantly delayed compared to that after treatment with the wild-type SDF-1β.
FIG. 7.
CXCR4 expression following a 12-h incubation and a 72-h chase; comparison of culture with Met-SDF-1β versus wild-type SDF-1β. Primary CD4+ lymphocytes were incubated with 1 μg of SDF-1β/ml, 1 μg of Met-SDF-1β/ml, or medium without added chemokine. The CXCR4 receptor level was determined for these three experimental groups following the first 12 h of culture (A) and following a 12-h culture, a wash, and a further 72-h culture in the absence of additional chemokine (B). Cells were stained with the anti-CXCR4 MAb 12G5 (see Materials and Methods for details), and the data were expressed as the ΔMFI, bar graph, and the percentage decrease in MFI compared to control cells shown as the % DMFI to the right of each bar graph. The histograms for the 12-h pulse (top) and the 72-h chase (bottom) are shown to the right of the corresponding bar graph. Isotype Control, cells cultured with medium only or chemokine and stained with isotype control; Media Control, cells cultured with medium only and stained with 12G5; SDF-1β, cells cultured with 1 μg of SDF-1β/ml and stained with 12G5; Met-SDF-1β, cells cultured with 1 μg of Met-SDF-1β/ml and stained with 12G5. Log fluorescense is shown on the x axis, and the cell number is shown on the y axis.
DISCUSSION
The discovery that chemokine receptors are required for HIV-1 envelope (gp120) to mediate infection of CD4+ cells (1, 4, 5, 7, 8, 11, 14, 15, 32, 48, 53) has ushered in a new era of investigation of HIV-1 pathogenesis. The pathogenic importance of these receptors has been demonstrated clearly in observations of individuals with a deletion mutation in CCR5; HIV-1-infected individuals heterozygous for the Δ32-CCR5 mutation appear to have delayed disease progression, and individuals homozygous for the mutation are relatively resistant to infection (10, 25, 36, 39). The finding that the chemokine ligands of CCR5 and CXCR4 are capable of blocking the use of these receptors by HIV-1 in vitro has suggested a possible role for chemokines as inhibitors of viral replication in vivo. Indirect evidence includes the observation that CD8+ cells from some HIV-1-infected individuals who are asymptomatic for an extended period produce higher levels of RANTES, MIP-1α, and MIP-1β (54) than noninfected controls. In addition, a mutation has been identified in the gene for SDF-1β which appears to delay disease progression (50).
The molecular mechanism for blocking by SDF-1 of T-tropic HIV-1 infection was initially thought to involve simple competition between chemokine and virus for binding to chemokine receptors. Peptide (30) and small-molecule (12, 40) antagonists of CXCR4 were found to inhibit viral replication selectively without measurable signaling through CXCR4, suggesting that binding alone was sufficient. In addition, Crump et al. studied N-terminal-truncated forms of SDF-1 and found a correlation between chemokine affinity for CXCR4 and HIV-1 inhibitory activity (9). These studies were interpreted to indicate that competition for binding to CXCR4 was the key factor in blocking of viral infection by receptor agonists or antagonists.
In contrast, our data strongly suggest that down-modulation of chemokine receptors is required for efficient inhibition of cell entry by CXCR4-utilizing strains of HIV-1. Although Met-SDF-1β binds CXCR4 with lower affinity on the U937 cell line than the naturally-occurring form of SDF-1β, we find that the modified SDF-1β molecule is orders of magnitude more potent in suppressing viral replication for U937 and T-lymphocyte targets (Fig. 1 to 3). Amara et al. used a C-terminal-truncated CXCR4, which supported HIV-1 entry but did not signal or down-modulate in response to SDF-1. Blocking of HIV-1 by SDF-1 was impaired in this system, suggesting that competition between the chemokine and gp120 for binding to the chemokine receptor gives only weak or partial inhibition (2). Signoret et al. further verified endocytosis-mediated down-modulation of CXCR4 as important in the antiviral activity of SDF-1 (44). It is possible that the two forms of SDF-1β used in this study bind to CD4+ lymphocytes with the same affinity. However, the high nonspecific binding of SDF-1 to most cell lines (9) would also preclude a direct binding measurement for the primary CD4+ T lymphocytes used in this study.
Met-SDF-1β was also found to induce a more profound and prolonged intracellular calcium mobilization than SDF-1β. The relationship of this effect to viral inhibition is unclear, but signaling has been found by others to be required for virus entry or chemokine blocking. We found that neither Met-SDF-1β nor SDF-1β affected replication of an M-tropic strain of HIV-1, suggesting that the differential inhibitory effects of these chemokines are exerted at the level of virus entry.
Following a 48-h culture, we observed a sustained decrease in CXCR4 expression for cells cultured with less than 1 μg of Met-SDF-1β/ml, while cells cultured with less than 1 μg of SDF-1β/ml either fully regained receptor expression or exhibited only a slight decrease in receptor expression. The 48-h culture with 1 μg of Met-SDF-1β/ml gave the largest decrease in CXCR4 expression, with levels at or approaching those with isotype control staining. The majority of receptor staining was also lost for cells cultured with 1 μg of SDF-1β/ml, resulting in a decrease for receptor staining to 25 to 30% of that found for control cells (Fig. 5). This small difference in receptor staining was found to correlate with a near-total HIV-1 inhibition for cells cultured with 1 μg of Met-SDF-1β/ml, while 2 to 3 log units less inhibition was found for cells cultured with the unmodified form of SDF-1β (Fig. 1 to 3).
Fluorescence staining of cells gives a relative measurement, and although there is general agreement that 700 to 1,000 receptors per cell are required for detection by fluorescence staining (41), a direct correlation between the decrease in MFI and cell surface density is not possible under the conditions used here (41). Recent work has demonstrated that at normal lymphocyte-like levels of CD4, only about 2,000 CCR5 receptors are required for maximal infection by HIV-1 and infection will still occur even with as few as 700 receptors (34). These results, along with the work described here, indicate that cellular infection requires only a small number of receptors; to achieve complete inhibition in the presence of high levels of CD4 requires complete or near-complete down-modulation of CXCR4. Thus, the percent change in receptor expression is not important; rather, the number of receptors that remain on the cell surface will most likely determine the level of inhibition (or infection) for HIV-1.
The decreases in CXCR4 immunofluorescence staining following short-term cultures of 2 h with either form of SDF-1β were similar, with each approaching 100% at the 1-μg/ml concentration. After a 48-h exposure to chemokines, down-modulation was significantly greater for Met-SDF-1β-exposed cells. This difference was noted even when the cells were cultured briefly with chemokine followed by removal and a further 3-day culture without chemokine (Fig. 7). The functional property of Met-SDF-1β that correlates with increased HIV-1 inhibition appears to be its ability to cause prolonged receptor down-modulation even following removal of the chemokine from the culture.
The central role of chemokine receptor down-modulation in achieving the most effective antiviral effect is also supported by recent studies of modified forms of RANTES and their interactions with CCR5. An N-terminal-modified RANTES analogue, Met-RANTES, was found to retain M-tropic HIV-1-inhibitory activity while not inducing a signal through CCR5 (3), suggesting that postreceptor signaling is not required to block infection. An analogue similar to Met-RANTES, with an aminooxypentane (AOP) moiety added to the N terminus of RANTES (AOP-RANTES), was found to have enhanced HIV-1-blocking activity (45). AOP-RANTES had higher affinity for CCR5 while lacking signaling capability, indicating that it might be a better competitive inhibitor for viral binding than wild-type RANTES. A follow-up study clarified the mechanism, however, by demonstrating that AOP-RANTES down-modulates CCR5 and interferes with the recycling of this receptor (27). Primary lymphocytes exposed to this chemokine analogue were found to have prolonged down-modulation of CCR5 due to retention of receptor in endosomes. This impairment of cell surface CCR5 reexpression was suggested to be the mechanism for the enhanced antiviral activity of AOP-RANTES.
Natural modification by CD26, a dipeptidyl peptidase, may play a role in inactivating chemokines able to inhibit HIV-1. CD26 can remove two amino acids from the N terminus of proteins with a proline or alanine at the penultimate position (29), and it has been shown to cleave RANTES, SDF-1, and certain other chemokines (33, 35, 42). Whereas truncation of RANTES by CD26 does not reduce its ability to block M-tropic HIV-1 infection (33, 35), CD26 cleavage of SDF-1 abrogates its HIV-1-inhibitory activity (42), in agreement with previous studies examining N-terminal-truncated SDF-1 (9). The dependence of CD26 activity on a penultimate proline or alanine suggests that Met-SDF-1β would not be a substrate, as shown for other XXP peptides (peptides with proline in the third position) (52). The cell lines used in our study readily stained with anti-CD26-specific antibodies (data not shown); however, comparison of our data with that of Shioda et al. (42) suggests that the enhanced HIV-1 inhibition by Met-SDF-1β is not due solely to its resistance to degradation by CD26. In the study by Shioda et al. (42), addition of a CD26 inhibitor gave only a modest increase in HIV-1 inhibition by SDF-1α, dissimilar to the striking enhancement of HIV-1 inhibition observed in the current study with Met-SDF-1β.
In summary, we show for the first time that a modified form of SDF-1, Met-SDF-1β, is a markedly more efficient inhibitor of T-tropic HIV-1 than wild-type SDF-1β. Compared to SDF-1β, Met-SDF-1β has lower affinity for CXCR4, triggers a more potent calcium mobilization, and causes prolonged down-modulation of its receptor, CXCR4. As detailed above, the binding and signaling properties are different from those of similar N-terminal-modified RANTES analogues. The data presented here demonstrate a near-total inhibition of T-tropic HIV-1 replication by an SDF-1 analogue. This inhibition correlates with an enhanced level of CXCR4 down-modulation, suggesting a near-complete inhibition of virus entry.
ACKNOWLEDGMENTS
We acknowledge the following: for technical help, Genetics Institute DNA Synthesis and DNA Sequencing Groups, Robert Gassaway, Stephanie Cook, Jenifer Thomas, Richard Zollner, and Jacob Cutter; for discussion, Max Follettie, Pat Gage, Steve Clark, Wei Wei Huang, and Jim Hoxie.
This work was paid for in part by NIH grants to Bruce Walker, Otto Yang, and Michelle Dziejman (K08AI01413-01, RO1AI43203-01, and AI097160-02).
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: A RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Amara A, Gall S L, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier J-L, Arenza-Seisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1 alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arenzana-Seisdedos F, Virelizier J, Rousset D, Clark-Lewis I, Loetscher P, Moser B, Baggiolini M. HIV blocked by chemokine antagonist. Nature. 1996;383:400. doi: 10.1038/383400a0. [DOI] [PubMed] [Google Scholar]
- 4.Bates P. Chemokine receptors and HIV-1: an attractive pair? Cell. 1996;86:1–3. doi: 10.1016/s0092-8674(00)80070-7. [DOI] [PubMed] [Google Scholar]
- 5.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–832. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 6.Bleul C C, Fuhlbrigge R C, Casasnovas J M, Aiuti A, Springer T A. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 8.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 9.Crump M P, Gong J-H, Loetscher P, Rajarathnam K, Amara A, Arenzana-Seisdedos F, Virelizier J-L, Baggiolini M, Sykes B D, Clark-Lewis I. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 1997;16:6996–7007. doi: 10.1093/emboj/16.23.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, Allikmets R, Goedert J J, Buchbinder S P, Vittinghoff E, Gomperts E, Donfield S, Vlahov D, Kaslow R, Saah A, Rinaldo C, Detels R, Study H G D, Study M A C, Study M H C, Cohort S F C, Study A, O’Brien S J. Genetic Restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 11.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 11a.DiBlasio-Smith, E. A., and J. McCoy. Unpublished data.
- 12.Doranz B, Grovit-Ferbasi K, Sharron M, Mao S, Goetz M, Daar E, Doms R, O’Brien W. A small molecule inhibitor directed against chemokine receptor CXCR4 prevents its use as an HIV-1 coreceptor. J Exp Med. 1997;186:1395–1400. doi: 10.1084/jem.186.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CCR5, CCR3 and CCR2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 14.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 16.Gartner S, Popovic M. Virus isolation and production. In: Aldovini A, Walker B D, editors. Techniques in HIV research. New York, N.Y: Stockton Press; 1990. pp. 53–66. [Google Scholar]
- 17.Haribabu B, Richardson R M, Fisher I, Sozzani S, Peiper S C, Horuk R, Ali H, Snyderman R. Regulation of human chemokine receptors CXCR4. J Biol Chem. 1997;272:28726–28731. doi: 10.1074/jbc.272.45.28726. [DOI] [PubMed] [Google Scholar]
- 18.Hasselgesser J, Liang M, Hoxie J, Greenberg M, Brass L F, Orsini M J, Taub D, Horuk R. Identification and characterization of the CXCR4 chemokine receptor in human T cell lines: ligand binding, biological activity, and HIV-1 infectivity. J Immunol. 1998;160:877–883. [PubMed] [Google Scholar]
- 19.Heveker N, Montes M, Germeroth L, Amara A, Trautmann A, Alizon M, Schneider-Mergener J. Dissociation of the signalling and antiviral properties of SDF-1-derived small peptides. Curr Biol. 1998;8:369–376. doi: 10.1016/s0960-9822(98)70155-1. [DOI] [PubMed] [Google Scholar]
- 20.Hirel P, Schmitter J, Dessen P, Fayat G, Blanquet S. Extent of N-terminal methionine excision from Escherichia coli proteins is governed by the side-chain length of the penultimate amino acid. Proc Natl Acad Sci USA. 1989;86:8247–8251. doi: 10.1073/pnas.86.21.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson V A, Walker B D. HIV-infected cell fusion assay. In: Aldovini A, Walker B D, editors. Techniques in HIV research. New York, N.Y: Stockton Press; 1990. pp. 92–94. [Google Scholar]
- 22.Koyanagi Y, Miles S, Mitsuyasu R T, Merrill J E, Vinters H V, Chen I S Y. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 23.LaVallie E, DiBlasio E, Kovacic S, Grant K, Schendel P F, McCoy J M. A thioredoxin gene fusion expression system that circumvents inclusion body formation in the E. coli cytoplasm. Bio/Technology. 1993;11:187–193. doi: 10.1038/nbt0293-187. [DOI] [PubMed] [Google Scholar]
- 24.LaVallie E R, Rehemtulla A, Racie L A, DiBlasio E, Ferenz C, Grant K, Light A, McCoy J. Cloning and functional expression of a cDNA encoding the catalytic subunit of bovine enterokinase. J Biol Chem. 1993;268:23311–23317. [PubMed] [Google Scholar]
- 25.Liu R, Paxton W, Choe S, Ceradini D, Martin S, Horuk R, MacDonald M, Stuhlmann H, Koup R, Landau N. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 26.Lu Z, DiBlasio-Smith E A, Grant K L, Warne N W, La Vallie E, Collins-Racie L, Follettie M, Williamson W, McCoy J. Histidine-patch thioredoxins; mutant forms of thioredoxin with metal chelating affinity that provide for convenient purifications of thioredoxin fusion proteins. J Biol Chem. 1996;271:5059–5065. [PubMed] [Google Scholar]
- 27.Mack M, Luckow B, Nelson P J, Cihak J, Simmons G, Clapham P R, Signoret N, Marsh M, Stangassinger M, Borlat F, Wells T N C, Schlondorff D, Proudfoot A E I. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J Exp Med. 1998;187:1215–1224. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McPherson G. A practical computer based approach to the analysis of radioligand binding experiments. Comput Programs Biomed. 1983;17:107–114. doi: 10.1016/0010-468x(83)90031-4. [DOI] [PubMed] [Google Scholar]
- 29.Mentlein R. Proline residues in the maturation and degradation of peptide hormones and neuropeptides. FEBS Lett. 1988;234:251–256. doi: 10.1016/0014-5793(88)80092-9. [DOI] [PubMed] [Google Scholar]
- 30.Murakami T, Nakajima T, Koyanagi Y, Tachibana K, Fujii N, Tamamura H, Yoshida N, Waki M, Matsumoto A, Yoshie O, Kishimoto T, Yamamoto N, Nagasawa T. A small molecule CXCR4 inhibitor that blocks T cell line-tropic HIV-1 infection. J Exp Med. 1997;186:1389–1393. doi: 10.1084/jem.186.8.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci USA. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J, Arenzana-Seisdedos F, Schwartz O, Heard J, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 33.Oravecz T, Pall M, Roderiquez G, Gorrell M, Ditto M, Nguyen N Y, Boykins R, Unsworth E, Norcross M A. Regulation of the receptor specificity and function of the chemokine RANTES (regulated on activation, normal T cell expressed and secreted) by dipeptidyl peptidase IV (CD26)-mediated cleavage. J Exp Med. 1997;186:1865–1872. doi: 10.1084/jem.186.11.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Platt E J, Wehrly K, Duhmann S E, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Proost P, De Meester I, Schols D, Struyf S, Lambeir A-M, Wuyts A, Opdenakker G, De Clercq E, Scharpe S, Van Damme J. Amino-terminal truncation of chemokines by CD26/dipeptidyl-peptidase IV. J Biol Chem. 1998;273:7222–7227. doi: 10.1074/jbc.273.13.7222. [DOI] [PubMed] [Google Scholar]
- 36.Rana S, Besson G, Cook D G, Rucker J, Smyth R J, Yi Y, Turner J D, Guo H H, Du J G, Peiper S C, Lavi E, Samson M, Libert F, Liesnard C, Vassart G, Doms R W, Parmentier M, Collman R G. Role of CCR5 in infection of primary macrophages and lymphocytes by macrophage-tropic strains of human immunodeficiency virus: resistance to patient-derived and prototype isolates resulting from the delta ccr5 mutation. J Virol. 1997;71:3219–3227. doi: 10.1128/jvi.71.4.3219-3227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rucker J, Samson M, Doranz B J, Libert F, Berson J F, Yi Y, Smyth R J, Collman R G, Broder C C, Vassart G, Doms R W, Parmentier M. Regions in β-chemokine receptors CCR5 and CCR2b that determine HIV-1 cofactor specificity. Cell. 1996;87:437–446. doi: 10.1016/s0092-8674(00)81364-1. [DOI] [PubMed] [Google Scholar]
- 38.Salter R D, Cresswell P. Impaired assembly and transport of HLA-A and B antigens in a mutant TxB cell hybrid. EMBO J. 1986;5:943–949. doi: 10.1002/j.1460-2075.1986.tb04307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi J, Smyth R J, Collman R G, Doms R W, Vassart G, Parmentier M. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 40.Schols D, Struyf S, Van Damme J, Este J A, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med. 1997;186:1383–1388. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shapiro H M. Practical flow cytometry. 3rd ed. New York, N.Y: Wily-Liss, Inc.; 1995. p. 302. [Google Scholar]
- 42.Shioda T, Kato H, Ohnishi Y, Tashiro K, Ikegawa M, Nakayama E E, Hu H, Kato A, Sakai Y, Liu H, Honjo T, Nomoto A, Iwamoto A, Morimoto C, Nagai Y. Anti-HIV-1 and chemotactic activities of human stromal cell-derived factor 1α (SDF-1α) and SDF-1β are abolished by CD26/dipeptidyl peptidase IV-mediated cleavage. Proc Natl Acad Sci USA. 1998;95:6331–6336. doi: 10.1073/pnas.95.11.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirozu M, Nakano T, Inazawa J, Tashiro K, Tada H, Shinogara T, Honjo T. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF-1) gene. Genomics. 1995;28:495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- 44.Signoret N, Oldridge J, Pelchen-Matthews A, Klasse P J, Tran T, Brass L F, Rosenkilde M M, Schwartz T W, Holmes W, Dallas W, Luther M A, Wells T N C, Hoxie J A, Marsh M. Phorbol esters and SDF-1 induce rapid endocytosis and down-modulation of the chemokine receptor CXCR4. J Cell Biol. 1997;139:651–664. doi: 10.1083/jcb.139.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simmons G, Clapham P R, Picard L, Offord R E, Rosenkilde M M, Schwartz T W, Buser R, Wells T N C, Proudfoot A E I. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 46.Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U937) Int J Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 47.Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Signal sequence trap: a cloning strategy for secreted proteins and type 1 membrane proteins. Science. 1993;261:600–603. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- 48.Weiss R A. HIV receptors and the pathogenesis of AIDS. Science. 1996;272:1886–1887. doi: 10.1126/science.272.5270.1885. [DOI] [PubMed] [Google Scholar]
- 49.Wilson C C, Wong J T, Girard D, Merril D P, Dynan M, An D, Kalams S A, Johnson R P, Hirsch M S, D’Aquila R T, Walker B D. Ex vivo expansion of CD4+ lymphocytes from HIV-1 infected persons in the presence of combination antiretroviral therapy. J Infect Dis. 1995;172:88–96. doi: 10.1093/infdis/172.1.88. [DOI] [PubMed] [Google Scholar]
- 50.Winkler C, Modi W, Smith M W, Nelson G W, Wu X, Carrington M, Dean M, Honjo T, Tashiro K, Yabe D, Buchbinder S, Vittinghoff E, Goedert J J, O’Brien T R, Willoughby A, Gomperts E, Vlahov D, Phair J, O’Brien S J. Genetic restriction of AIDS pathogenesis by an SDF-1 chemokine gene variant. Science. 1998;279:389–392. doi: 10.1126/science.279.5349.389. [DOI] [PubMed] [Google Scholar]
- 51.Wong J K, Hezareh M, Gunthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 52.Wrenger S, Reinhold D, Hoffmann T, Kraft M, Frank R, Faust J, Neubert K, Ansorge S. The N-terminal X-X-Pro sequence of HIV-Tat protein is important for the inhibition of dipeptidyl peptidase IV (DP IV/CD26) and the suppression of mitogen-induced proliferation of human T cells. FEBS Lett. 1996;383:145–149. doi: 10.1016/0014-5793(96)00221-9. [DOI] [PubMed] [Google Scholar]
- 53.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 54.Zagury D, Lachgar A, Chams V, Fall L, Bernard J, Zagury J-F, Bizzini B, Gringeri A, Santagostino E, Rappaport J, Feldman M, O’Brien S, Burny A, Gallo R. C-C chemokines, pivotal in protection against HIV type 1 infection. Proc Natl Acad Sci USA. 1998;95:3857–3861. doi: 10.1073/pnas.95.7.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]