Abstract
Aim
This meta-analysis's objective was to assess the effectiveness of ursodeoxycholic acid (UDCA) in the management of nonalcoholic fatty liver disease (NAFLD).
Methods
Electronic databases like PubMed, Embase, Scopus, and Cochrane Library were thoroughly looked for randomized controlled trials determining ursodeoxycholic acid's (UDCAs) effectiveness on the serum liver function tests in NAFLD patients. After screening, seven randomized controlled trials were incorporated overall. Utilizing a fixed effects model, quantitative data synthesis was performed in R version 4.3.1.
Results
The meta-analysis showed significant reductions in alanine transaminase (ALT) (p ≤ 0.0001), aspartate transaminase (p = 0.0009), and gamma-glutamyl transferase (GGT) (p ≤ 0.0001) after UDCA therapy. However, significant reductions in bilirubin (p = 0.6989) and alkaline phosphatase (ALP) (p = 0.1172) levels were not noted. Sensitivity analysis by removing the studies with some concerns of bias was successful in demonstrating a remarkable reduction in heterogeneity for aspartate transaminase and ALP, which was also observed while performing the subgroup analyses via dosage.
Conclusion
Ursodeoxycholic acid was beneficial in patients diagnosed with NAFLD as it significantly reduced aspartate transaminase, ALT and GGT levels. However, more randomized controlled trials are required to be conducted in the future to increase the certainty of the evident findings.
Clinical significance
This meta-analysis strengthens the evidence about the reductions in AST, ALT, and GGT levels observed with ursodeoxycholic acid therapy in NAFLD patients by pooling the data together from the latest RCTs thus proving its hepatoprotective effects which can be beneficial in preventing the associated complications.
How to cite this article
Patel VS, Mahmood SF, Bhatt KH, et al. Ursodeoxycholic Acid's Effectiveness in the Management of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Euroasian J Hepato-Gastroenterol 2024;14(1):92–98.
Keywords: Meta-analysis, Nonalcoholic fatty liver disease, Systematic review, Ursodeoxycholic acid
Introduction
Nonalcoholic fatty liver disease (NAFLD) is characterized by macrovesicular steatosis that affects at least 5% of hepatocytes, without any identifiable secondary cause. This condition encompasses a range of disorders that span from simple nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH), fibrosis, and cirrhosis.1 On a global scale, NAFLD has become the principal cause of chronic liver disease.2
Patients diagnosed with NAFLD exhibit an elevated likelihood of end-stage liver disease, hepatocellular carcinoma (HCC),3 and liver-related mortality.2 The presence of fibrosis is deemed a crucial indicator of unfavorable consequences in NAFLD as opposed to the histological characteristics of NASH.4
However, the involvement of the liver is merely a single element of the multifaceted manifestation of NAFLD. It is noteworthy that cardiovascular diseases are the number one cause of mortality among individuals with NAFLD. Liver-related mortality is only the third most prevalent cause of death.5
Ursodeoxycholic acid (UDCA) has proven to be known for being hepatoprotective in NAFLD. Ursodiol as UDCA is commonly known, is a bile acid that comprises 3% of the bile pool and possesses hydrophilic properties. Its efficacy in reducing cholestasis has been established in various studies.6 The hypothesized mechanism of action of ursodiol is it reduces the amount of hydrophobic bile acids in the hepatobiliary system, thereby mitigating the risk of hepatotoxicity. Additionally, it has been hypothesized that ursodiol exhibits immunomodulatory and antiapoptotic properties, rendering it a potential adjunctive therapy for acute or chronic graft vs host disease (GVHD) of the liver.7
Currently, the aforementioned agent is utilized for the dissolution of gallstones in specific patients.8Additionally, the use of UDCA has been established for the management of hepatobiliary disorders, including primary biliary cirrhosis (PBC).9. However, its effectiveness in the treatment of ailments such as hepatitis B and C virus infections or pediatric cholestasis, such as extrahepatic biliary atresia or primary sclerosis cholangitis, remains to be established.10–12
This meta-analysis has been structured with the purpose of addressing several key objectives.
It aims to conduct a rigorous systematic review of relevant studies that have investigated the use of UDCA in NAFLD's management.
It seeks to undertake a quantitative evaluation of the collected data from these studies to evaluate the impact of UDCA on bilirubin levels, liver enzymes, and other pertinent clinical endpoints.
It intends to explore potential sources of heterogeneity among the selected studies and to evaluate the accuracy of the data obtained.
It looks to provide a synthesized conclusion on the UDCA's significance in managing NAFLD and to address the consequences for the clinical approach and future research goals.
Materials and Methods
This systematic review and meta-analysis were carried out as per the PRISMA Guidelines 2020.13 Prior to the stage of data extraction, a protocol was registered on PROSPERO (registration number: CRD42023463029)
Data Sources and Search Strategy
Four of our reviewers independently searched through electronic databases PubMed Central, Embase, Scopus, and Cochrane Library to retrieve Randomized Controlled Trials (RCTs) published up to 25 June 2023. The search was confined to studies that were issued in English. The search was performed utilizing the following keywords: (Nonalcoholic Fatty Liver disease OR Nonalcoholic steatohepatitis) AND (Ursodeoxycholic acid OR Ursodiol) AND (Randomized Controlled Trial)
Study Selection Criteria
Clinical trials that met the criteria listed below were included:
Study design: Randomized Controlled Trials.
All RCT participants to be >18 years of age (population)
All patients should be diagnosed with NAFLD (population)
The intervention group received UDCA alone (intervention)
The control group that received placebo (Comparison)
Studies which measured outcomes in the form of alanine transaminase (ALT) and aspartate transaminase (AST), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), bilirubin not mandatory. (Outcome)
We excluded studies that were:
Non-human trials.
Non-English.
Including pregnant or <18-year-old patients.
Without any control group.
Unavailable in full-text form with only abstracts accessible.
Data Screening and Extraction
Three reviewers (VP, DJ, and MG) worked independently and thoroughly screened the titles, abstracts, and entire articles based on the inclusion/exclusion criteria. Any disagreement over a study's eligibility was settled by consensus. Data were retrieved and later tabulated, recording the following key information: First author, study design, year of publication, number of participants in total as well as the number of individually enrolled in the intervention vs control group, duration of intervention, the dosage of UDCA, geographical region where the RCT was conducted, age of patients, number of patients lost to follow-up in total, in addition to the ones lost in follow-up in intervention vs control group and serum levels of hepatic parameters in mean ± SD/SE (standard deviation/standard error) pre-and post-treatment.
Quality Assessment
Risk of bias assessment was independently conducted utilizing the Cochrane Collaboration's tool (RoB2 tool)14 and evaluated the subsequent domains: bias arising from the randomization process; bias due to deviations from intended intervention; bias due to missing outcome data; bias in measurement of the outcome; bias in selection of the reported result; and overall bias.
Statistical Analysis
We utilized R version 4.3.1 (Posit team (2023). RStudio: Integrated Development Environment for R. Posit Software, PBC, Boston, MA.) to analyze continuous variables in our study. When data was initially provided as mean ± SE, we transformed it into mean ± SD. The findings of our meta-analysis were presented as standardized mean difference (SMD)15 along with a 95% confidence interval (CI). A fixed-effect model was applied for analysis. Standardized mean difference values were not considered statistically significant when p > 0.05, whereas SMD values were considered statistically significant when p < 0.05. Additionally, we conducted I-square (I2) tests to assess statistical heterogeneity among the studies we analyzed. Statistical heterogeneity was considered substantial if I2 >60%, moderate if I2 30–60%, and low if <30%.16 The possible causes of heterogeneity were explored using sensitivity analysis, which was performed according to the risk of bias and by using a random-effects model when needed. To explore variations among the studies, subgroup analyses according to the intervention duration and dosage of UDCA were conducted.
Results
Study Selection Process and Study Characteristics
The study selection process is portrayed through the PRISMA flow diagram13 in Figure 1. The preliminary search yielded a total of 178 studies including 98 from PubMed, 22 from Embase, 33 from Scopus, and 25 from Cochrane Library. After removing 56 duplicate records, the remaining 122 records were reviewed for title and abstract. About 98 studies were excluded upon initial screening. Subsequently, after reading the entire text of each study, 14 more studies were excluded due to a different study design, insufficient data, and lack of a control group. Ultimately, 7 studies were incorporated into this meta-analysis.
Fig. 1.
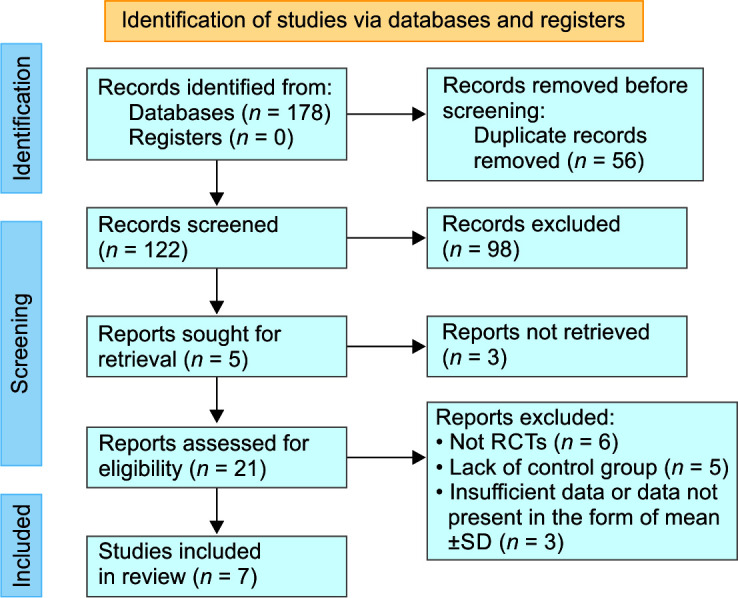
PRISMA flow diagram demonstrating study selection process
Encompassing 760 patients in all from the seven selected RCTs, consisting of 379 in the intervention and 381 participants in the placebo groups. Out of which 94 patients were lost to follow-up or were not able to complete the trials for various reasons. The study characteristics of the seven selected RCTs are presented in Table 1 and Supplementary Table 1. This meta-analysis comprises studies from all around the globe, spanning across France, Germany, Greece, United States, Canada, Iran, Egypt, Brazil and Mexico.17–23 Included trials have treatment duration ranging from 45 days to 2 years. The total daily dose of UDCA varied from 5–10 to 28–35 mg/kg/day across the included studies.
Table 1.
Study characteristics
| Author; year | Country | Duration | Dosage | Treatment | No. of participants at the start of the trial | No. of participants that completed the trial |
|---|---|---|---|---|---|---|
| Ratziu et al., 201117 | France | 12 months | 28–35 mg/kg/d | UDCA | 60 | 55 |
| Placebo | 66 | 61 | ||||
| Leuschner et al., 201018 | Germany, Greece | 18 months | 23–28 mg/kg/d | UDCA | 95 | 78 |
| 23–28 mg/kg | Placebo | 91 | 82 | |||
| Lindor et al., 200419 | United States, Canada | 2 years | 13–15 mg/kg/d | UDCA | 78 | 55 |
| Placebo | 86 | 64 | ||||
| Mojtahedi et al., 202320 | Iran | 3 months | 5–10 mg/kg/d | UDCA | 30 | 30 |
| Placebo | 30 | 30 | ||||
| Elhini et al., 202221 | Egypt | 6 months | 5–10 mg/kg/d | UDCA | 87 | 80 |
| Placebo | 80 | 80 | ||||
| Santos et al., 200322 | Brazil | 3 months | 10 mg/kg/d | UDCA | 15 | 14 |
| Placebo | 15 | 14 | ||||
| Méndez-Sánchez et al., 200423 | Mexico | 1.5 months | 13–17 mg/kg/d | UDCA | 14 | 12 |
| Placebo | 13 | 11 |
Risk of Bias Assessment
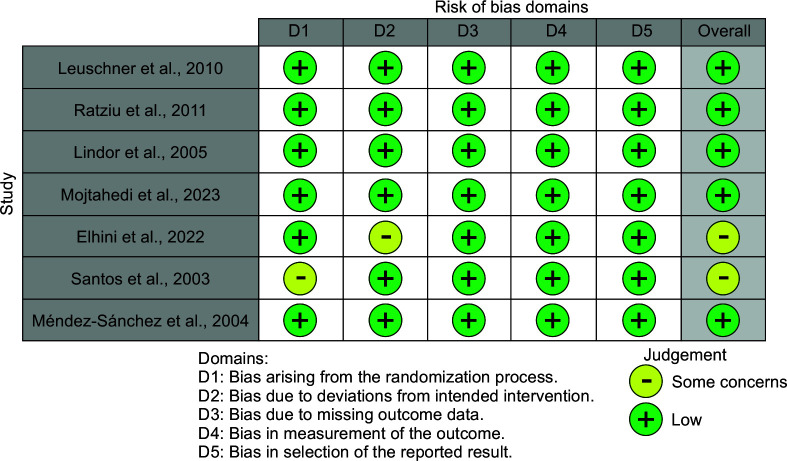
The Cochrane Collaboration Risk of Bias tool14 was utilized for the evaluation of all potential sources of bias. According to the Cochrane Statement of Risk of Bias,14 each domain was deemed low, with some concerns or a high risk of bias. 7 studies17–23 were evaluated out of which five studies were of low concern17–20,23 and two were deemed with some concerns21,22 (Fig. 2).
Fig. 2.
Risk of bias assessment according to Cochrane guidelines
Meta-analysis
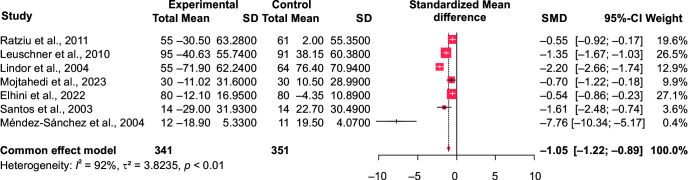
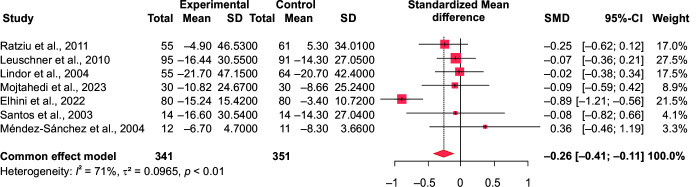
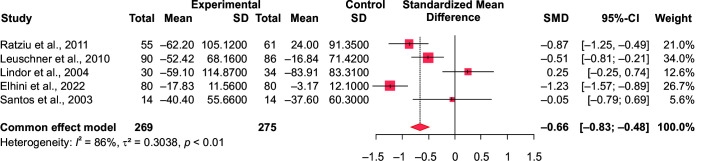
In comparison to the control group (n = 351), our meta-analysis showed a significant effect of ursodeoxycholic acid in reducing the serum alanine aminotransferase (ALT) (n = 341; SMD = –1.05 at 95% CI [–1.22 to –0.89], p < 0.0001, I2 = 91.9% and aspartate aminotransferase (AST) levels in the intervention group SMD = –0.26, 95% CI [–0.41 to –0.11], p = 0.0009, I2 = 70.7%) (Figs 3 and 4). Similarly, its impact on the gamma-glutamyl transferase (GGT) levels was significant too (SMD = –0.66, 95% CI [–0.83 to –0.48], p ≤ 0.0001). (Fig. 5). However, ALP and total bilirubin in the experimental groups were not found to be significant [SMD = –0.14, 95% CI (–0.32 to 0.04), p = 0.12; and SMD = 0.05, 95% CI (–0.19 to 0.28), p = 0.7, respectively]. There was a high heterogeneity noted which is often biased when the number of included studies is small.24
Fig. 3.
Forest plot comparing the ALT values of intervention (UDCA) and control group
Fig. 4.
Forest plot comparing the AST values of intervention (UDCA) and control group
Fig. 5.
Forest plot comparing the GGT values of intervention (UDCA) and control group
Subgroup Analyses
Subgroup analyses stratified by the duration of intervention (>6 months17–19 and ≤6 months)20–23 and dosage of the regimen (>20 mg/kg/day17,18 and ≤20 mg/kg/day)19–23 were performed. The results of which are portrayed in Supplementary Table 2. The results indicated that a treatment duration of >6 months did not show a reduction in ALT, AST, ALP, and bilirubin levels. However, there was a significant reduction in ALT, AST, ALP, GGT, Bilirubin levels when the duration of intervention was less than 6 months. Furthermore, subgroup analysis by dosage regimen showed no significant reductions for any hepatic parameters other than ALT and GGT. However, GGT values always showed significant reductions no matter how it was subgrouped. Additionally, a substantial decrease in heterogeneity was observed when grouping the studies for AST and ALP parameters.
Publication Bias
Visual assessment of funnel plots revealed potential publication bias for ALT, AST, and ALP, as an asymmetry was noted. Although an evident symmetry was observed in the funnel plots of GGT and bilirubin. However, owing to the quantity of studies in this meta-analysis being small, the results can be considered inconclusive.25
Discussion
Nonalcoholic fatty liver disease is a chronic condition that affects roughly a quarter of adults globally.26 With such an incidence rate, our purpose is to study the possible advantages of UDCA therapy for individuals with NAFLD. This meta-analysis probed the effects of UDCA therapy, evaluating its impact on five key parameters: ALT, AST, GGT, ALP, and bilirubin. Elevated AST and ALT values are indicators of hepatocellular injury and indicate hepatic cell membrane damage.27 Gamma-glutamyl transferase is found in both liver and biliary epithelial cells and has been shown to be a definite indicator of hepatobiliary disorders. Meanwhile, ALP levels might indicate liver illness or bone development difficulties.28 Bilirubin is a byproduct of hemoglobin breakdown, and its elevated concentration often parallels hepatocyte injury, resulting in jaundice.29
Nonalcoholic fatty liver disease is often associated with hypertension, hyperlipidemia, type 2 diabetes, visceral obesity, and insulin resistance.30 These complications often result in the emergence of new long-term health issues, adversely affecting patients' overall quality of life. Ursodeoxycholic acid is a bile acid that is formed during metabolism by gut bacteria, has been proven to be an effective non-surgical approach for treating cholesterol gallstones and PBC.31 A recent study has demonstrated a significant decline in total cholesterol levels among patients, especially in those with PBC, after UDCA therapy.32 With its remarkable potential for treating chronic liver diseases, such as PBC33–35 and NASH,18,36,37 UDCA is now being considered a viable therapy option. UDCA has a number of therapeutic benefits, including the ability to prevent cell death, reduce TNF-α (Tumor necrosis factor) levels in the circulation, alleviate endoplasmic reticulum stress, and enhance the liver's insulin sensitivity. These characteristics imply that UDCA may be useful in treating NASH.38 It has been discovered that administering UDCA to PBC patients decreases the levels of liver damage markers in their blood. This advantageous impact is thought to be due to UDCA's ability to protect liver cells, prevent cell death, and combat oxidative stress, making it a good immune system regulator.39 Another potential benefit is that UDCA therapy lowers inflammation by protecting liver cells from necrosis,40 thereby lowering the local inflammatory response. UDCA is thought to minimize oxidative stress in liver cells by boosting the amounts of protective chemicals like glutathione and thiol-containing proteins like metallothionein.41 Furthermore, it may inhibit liver cell death by minimizing mitochondrial membrane depolarization and decreasing the generation of dangerous reactive oxygen species.42 Furthermore, UDCA has anti-inflammatory properties in the liver by decreasing NF-β (nuclear factor)-dependent transcription via glucocorticoid receptor activation.43
Ursodeoxycholic acid is usually well tolerated and has a low toxic level. The only recorded adverse effect is diarrhea, which is estimated to affect fewer than 5% of individuals.44–46 It is recommended to take ursodeoxycholic acid with meals to improve absorption since it stimulates the gallbladder to release bile acids.47
In this meta-analysis, we observed statistically significant findings for ALT, AST, and GGT after performing our analysis using a fixed-effect model. This implies that the administration of UDCA to the experimental group had an impact, leading to reduction in ALT, AST, and GGT levels in comparison with the control group. However, it's essential to take into account that these changes come with a degree of high heterogeneity. Conversely, things differ for ALP and bilirubin. In our study, the changes found for these two parameters turned out to be statistically insignificant.
When we performed a subgroup analysis based on the dose and duration of treatment, we found that the dose of UDCA had no significant influence on AST and ALP results. In other words, the dose of UDCA did not influence its effectiveness in reducing these liver markers. The findings of our subgroup analysis based on treatment duration were intriguing. We observed that treatment durations of more than 6 months had insignificant results, but those of less than 6 months had significant outcomes. This demonstrates that the duration of treatment has minimal influence on how UDCA affects liver parameters. Or this could just be a consequence of the subgrouping of studies and not necessarily indicate anything. It should be noted, however, that our study encountered difficulties owing to the high heterogeneity in treatment duration. This might be a drawback of our study, and as a consequence, we cannot say with conviction that treatment duration has no influence on UDCA's efficacy in lowering hepatic markers.
It is crucial to highlight that our meta-analysis does have some limitations. The levels of ALT, AST, and GGT were significantly reduced, although there was also a lot of heterogeneity. This degree of heterogeneity is frequently seen when the total number of research taken into consideration is limited.24 So, we decided to do a sensitivity analysis to overcome this constraint. We selectively eliminated two studies from our analysis due to their significant potential for bias. What is remarkable is that after these studies were eliminated, we saw a considerable improvement. The heterogeneity in the AST and ALP values improved and reached zero. Additionally, we discovered that upon grouping the studies by dosage, for AST and ALP, there was an improvement in heterogeneity.
A few meta-analyses have been already conducted on this topic.48,49 The addition of two more recent studies, Mojtahedi et al.20 and Elhini et al.,21 both published within the past 2 years, distinguishes our study. Their inclusion has significantly improved the overall results. These studies show that UDCA can reduce ALT levels while simultaneously also improving AST and GGT parameters.
Conclusion
After comprehensively combining the latest data from multiple studies on UDCA's effectiveness on liver markers in NAFLD patients, we concluded that UDCA was beneficial in not only effectively reducing the ALT levels but also the AST and GGT levels, which as far as we know has not previously been successfully established in other meta-analysis. However, more RCTs are required to increase the certainty of results, especially regarding the duration of UDCA and if longer duration means much more effective reductions in liver function parameters.
Clinical Significance
In the context of NAFLD management, the profound reductions observed in lowering ALT, AST, and GGT levels with UDCA therapy advocate its role as a promising adjunctive therapy in NAFLD patients. While lifestyle changes are the traditional treatment for NAFLD, they have their own limitations that can be addressed using UDCA as an adjuvant. Which owing to its antioxidant properties has been proven favorable in the management of dyslipidemia and in decreasing the risk of atherosclerotic cardiovascular disease in NAFLD patients.
Orcid
Vaibhavi S Patel https://orcid.org/0009-0005-8618-9856
Safa F Mahmood https://orcid.org/0009-0001-8347-2075
Kunal H Bhatt https://orcid.org/0009-0006-7872-6532
Richisha M Khemkar https://orcid.org/0000-0003-3370-1156
Devanshi R Jariwala https://orcid.org/0009-0004-8896-9605
Bilal Harris https://orcid.org/0009-0002-8526-9211
Mirna M George https://orcid.org/0009-0002-7056-0954
Reuel A Kurudamannil https://orcid.org/0000-0002-7189-7535
Onyekachi E Anyagwa https://orcid.org/0000-0002-2991-9833
Rajeeka S Tak https://orcid.org/0000-0002-3240-6932
Maha Kassem https://orcid.org/0000-0001-5899-1175
Supplementary Materials
All the supplementary materials are available online on the website of www.EJOHG.com.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Maurice J, Manousou P. Non-alcoholic fatty liver disease. Clinical Medicine (London, England) 2018;18(3):245–250. doi: 10.7861/clinmedicine.18-3-245. 29858436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3.Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology. 2018;155(6):1828–1837.e2. doi: 10.1053/j.gastro.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389–397.e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65(5):1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofmann AF. Pharmacology of ursodeoxycholic acid, an enterohepatic drug. Scand J Gastroenterol Suppl. 1994;204:1–15. doi: 10.3109/00365529409103618. [DOI] [PubMed] [Google Scholar]
- 7.Floreani A, Mangini C. Primary biliary cholangitis: Old and novel therapy. Eur J Intern Med. 2018;47:1–5. doi: 10.1016/j.ejim.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Di Ciaula A, Wang DQ, Wang HH, et al. Targets for current pharmacologic therapy in cholesterol gallstone disease. Gastroenterol Clin North Am. 2010;39(2):245–264. doi: 10.1016/j.gtc.2010.02.005. viii-ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin RA, Kowalski TE, Khandelwal M, et al. Ursodiol for hepatobiliary disorders. Ann Intern Med. 1994;121(3):207–218. doi: 10.7326/0003-4819-121-3-199408010-00009. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Liu J, Gluud C. Bile acids for viral hepatitis. Cochrane Database Syst Rev. 2007;2007(4):CD003181. doi: 10.1002/14651858.CD003181.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotb MA. Review of historical cohort: ursodeoxycholic acid in extrahepatic biliary atresia. J Pediatr Surg. 2008;43(7):1321–1327. doi: 10.1016/j.jpedsurg.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 12.Feldstein AE, Perrault J, El-Youssif M, et al. Primary sclerosing cholangitis in children: A long-term follow-up study. Hepatology. 2003;38(1):210–217. doi: 10.1053/jhep.2003.50289. [DOI] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JPT, Savović J, Page MJ, et al. Cochrane; 2023. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). [Google Scholar]
- 15.Higgins JPT, Li T, Deeks JJ (editors) Cochrane; 2023. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). [Google Scholar]
- 16.Deeks JJ, Higgins JPT, Altman DG (editors) Cochrane; 2023. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). [Google Scholar]
- 17.Ratziu V, de Ledinghen V, Oberti F, et al. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54(5):1011–1019. doi: 10.1016/j.jhep.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Leuschner UF, Lindenthal B, Herrmann G, et al. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: A double-blind, randomized, placebo-controlled trial. Hepatology (Baltimore, Md.) 2010;52(2):472–479. doi: 10.1002/hep.23727. [DOI] [PubMed] [Google Scholar]
- 19.Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39(3):770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 20.University A, Mojtahedi K, Joukar F, et al. (n.d.). Bull Pharm Sci Assiut University. 2023;46(1):347–360. [Google Scholar]
- 21.Elhini SH, Wahsh EA, Elberry AA, et al. The impact of an SGLT2 Inhibitor versus ursodeoxycholic acid on liver steatosis in diabetic patients. Pharmaceuticals (basel) 2022;15(12):1516. doi: 10.3390/ph15121516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos VN, Lanzoni VP, Szejnfeld J, et al. A randomized double-blind study of the short-time treatment of obese patients with nonalcoholic fatty liver disease with ursodeoxycholic acid. Braz J Med Biol Res. 2003;36(6):723–729. doi: 10.1590/s0100-879x2003000600007. [DOI] [PubMed] [Google Scholar]
- 23.Méndez-Sánchez N, González V, Chávez-Tapia N, et al. Weight reduction and ursodeoxycholic acid in subjects with nonalcoholic fatty liver disease. A double-blind, placebo-controlled trial. Ann Hepatol. 2004;3(3):108–112. 15505596 [PubMed] [Google Scholar]
- 24.von Hippel PT. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med Res Methodol. 2015;15:35. doi: 10.1186/s12874-015-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 26.Araújo AR, Rosso N, Bedogni G, et al. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int. 2018;38(Suppl 1):47–151. doi: 10.1111/liv.13643. [DOI] [PubMed] [Google Scholar]
- 27.Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000;342(17):1266–1271. doi: 10.1056/NEJM200004273421707. [DOI] [PubMed] [Google Scholar]
- 28.Siller AF, Whyte MP. Alkaline phosphatase: Discovery and naming of our favorite enzyme. J Bone Miner Res. 2018;33(2):362–364. doi: 10.1002/jbmr.3225. [DOI] [PubMed] [Google Scholar]
- 29.Beckingham IJ, Ryder SD. ABC of diseases of liver, pancreas, and biliary system. Investigation of liver and biliary disease. BMJ. 2001;322(7277):33–36. doi: 10.1136/bmj.322.7277.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiang Z, Chen YP, Ma KF, et al. The role of ursodeoxycholic acid in non-alcoholic steatohepatitis: A systematic review. Medscape. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishizaki K, Imada T, Tsurufuji M. Hepatoprotective bile acid ‘ursodeoxycholic acid (UDCA)' Property and difference as bile acids. Hepatol Res. 2005;33(2):174–177. doi: 10.1016/j.hepres.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 32.Simental-Mendía LE, Simental-Mendía M, Sánchez-García A, et al. Impact of ursodeoxycholic acid on circulating lipid concentrations: A systematic review and meta-analysis of randomized placebo-controlled trials. Lipids Health Dis. 2019;18(1):88. doi: 10.1186/s12944-019-1041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parés A, Caballería L, Rodés J, et al. Long-term effects of ursodeoxycholic acid in primary biliary cirrhosis: Results of a double-blind controlled multicentric trial. UDCA-Cooperative Group from the Spanish Association for the Study of the Liver. J Hepatol. 2000;32(4):561–566. doi: 10.1016/s0168-8278(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 34.Poupon RE, Eschwège E, Poupon R. Ursodeoxycholic acid for the treatment of primary biliary cirrhosis. Interim analysis of a double-blind multicentre randomized trial. The UDCA-PBC Study Group. J Hepatol. 1990;11(1):16–21. doi: 10.1016/0168-8278(90)90265-s. [DOI] [PubMed] [Google Scholar]
- 35.Poupon RE, Balkau B, Eschwège E, et al. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N Engl J Med. 1991;324(22):1548–1554. doi: 10.1056/NEJM199105303242204. [DOI] [PubMed] [Google Scholar]
- 36.Gianturco V, Troisi G, Bellomo A, et al. Impact of combined therapy with alpha-lipoic and ursodeoxycolic acid on nonalcoholic fatty liver disease: Double-blind, randomized clinical trial of efficacy and safety. Hepatol Int. 2013;7(2):570–576. doi: 10.1007/s12072-012-9387-y. [DOI] [PubMed] [Google Scholar]
- 37.Laurin J, Lindor KD, Crippin JS, et al. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: A pilot study. Hepatology. 1996;23(6):1464–1467. doi: 10.1002/hep.510230624. [DOI] [PubMed] [Google Scholar]
- 38.Kotb MA. Molecular mechanisms of ursodeoxycholic acid toxicity & side effects: Ursodeoxycholic acid freezes regeneration & induces hibernation mode. Int J Mol Sci. 2012;13(7):8882–8914. doi: 10.3390/ijms13078882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishizaki K, Iwaki T, Kinoshita S, et al. Ursodeoxycholic acid protects concanavalin A-induced mouse liver injury through inhibition of intrahepatic tumor necrosis factor-alpha and macrophage inflammatory protein-2 production. Eur J Pharmacol. 2008;578(1):57–64. doi: 10.1016/j.ejphar.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 40.Heuman DM, Pandak WM, Hylemon PB, et al. Conjugates of ursodeoxycholate protect against cytotoxicity of more hydrophobic bile salts: in vitro studies in rat hepatocytes and human erythrocytes. Hepatology. 1991;14(5):920–926. doi: 10.1002/hep.1840140527. [DOI] [PubMed] [Google Scholar]
- 41.Mitsuyoshi H, Nakashima T, Sumida Y, et al. Ursodeoxycholic acid protects hepatocytes against oxidative injury via induction of antioxidants. Biochem Biophys Res Commun. 1999;263(2):537–542. doi: 10.1006/bbrc.1999.1403. [DOI] [PubMed] [Google Scholar]
- 42.Rodrigues CM, Ma X, Linehan-Stieers C, et al. Ursodeoxycholic acid prevents cytochrome c release in apoptosis by inhibiting mitochondrial membrane depolarization and channel formation. Cell Death Differ. 1999;6(9):842–854. doi: 10.1038/sj.cdd.4400560. [DOI] [PubMed] [Google Scholar]
- 43.Miura T, Ouchida R, Yoshikawa N, et al. Functional modulation of the glucocorticoid receptor and suppression of NF-kappaB-dependent transcription by ursodeoxycholic acid. J Biol Chem. 2001;276(50):47371–47378. doi: 10.1074/jbc.M107098200. [DOI] [PubMed] [Google Scholar]
- 44.Poupon RE, Chrétien Y, Poupon R, et al. Serum bile acids in primary biliary cirrhosis: Effect of ursodeoxycholic acid therapy. Hepatology. 1993;17(4):599–604. doi: 10.1002/hep.1840170412. [DOI] [PubMed] [Google Scholar]
- 45.Calmus Y, Gane P, Rouger P, et al. Hepatic expression of class I and class II major histocompatibility complex molecules in primary biliary cirrhosis: Effect of ursodeoxycholic acid. Hepatology. 1990;11(1):12–15. doi: 10.1002/hep.1840110104. [DOI] [PubMed] [Google Scholar]
- 46.Stiehl A, Rudolph G, Sauer P, et al. Efficacy of ursodeoxycholic acid treatment and endoscopic dilation of major duct stenoses in primary sclerosing cholangitis. An 8-year prospective study. J Hepatol. 1997;26(3):560–566. doi: 10.1016/s0168-8278(97)80421-7. [DOI] [PubMed] [Google Scholar]
- 47.Saksena S, Tandon RK. Ursodeoxycholic acid in the treatment of liver diseases. Postgrad Med J. 1997;73(856):75–80. doi: 10.1136/pgmj.73.856.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin X, Mai M, He T, et al. Efficiency of ursodeoxycholic acid for the treatment of nonalcoholic steatohepatitis: A systematic review and meta-analysis. Expert Rev Gastroenterol Hepatol. 2022;16(6):537–545. doi: 10.1080/17474124.2022.2083605. [DOI] [PubMed] [Google Scholar]
- 49.Zhang W, Tang Y, Huang J, et al. Efficacy of ursodeoxycholic acid in nonalcoholic fatty liver disease: An updated meta-analysis of randomized controlled trials. Asia Pac J Clin Nutr. 2020;29(4):696–705. doi: 10.6133/apjcn.202012_29(4).0004. [DOI] [PubMed] [Google Scholar]