Abstract
Aims
Influence of atrial fibrillation (AF) type on outcomes seen with catheter ablation vs. drug therapy is incompletely understood. This study assesses the impact of AF type on treatment outcomes in the Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation Trial (CABANA).
Methods and results
CABANA randomized 2204 patients ≥65 years old or <65 with at least one risk factor for stroke to catheter ablation or drug therapy. Of these, 946 (42.9%) had paroxysmal AF (PAF), 1042 (47.3%) had persistent AF (PersAF), and 215 (9.8%) had long-standing persistent AF (LSPAF) at baseline. The primary endpoint was a composite of death, disabling stroke, serious bleeding, or cardiac arrest. Symptoms were measured with the Mayo AF-Specific Symptom Inventory (MAFSI), and quality of life was measured with the Atrial Fibrillation Effect on Quality of Life (AFEQT). Comparisons are reported by intention to treat. Compared with drug therapy alone, catheter ablation produced a 19% relative risk reduction in the primary endpoint for PAF {adjusted hazard ratio [aHR]: 0.81 [95% confidence interval (CI): 0.50, 1.30]}, and a 17% relative reduction for PersAF (aHR: 0.83, 95% CI: 0.56, 1.22). For LSPAF, the ablation relative effect was a 7% reduction (aHR: 0.93, 95% CI: 0.36, 2.44). Ablation was more effective than drug therapy at reducing first AF recurrence in all AF types: by 51% for PAF (aHR: 0.49, 95% CI: 0.39, 0.62), by 47% for PersAF (aHR: 0.53, 95% CI: 0.43,0.65), and by 36% for LSPAF (aHR 0.64, 95% CI 0.41,1.00). Ablation was associated with greater improvement in symptoms, with the mean difference between groups in the MAFSI frequency score favouring ablation over 5 years of follow-up in all subgroups: PAF had a clinically significant −1.9-point difference (95% CI: −1.2 to −2.6); PersAF a −0.9 difference (95% CI: −0.2 to −1.6); LSPAF a clinically significant difference of −1.6 points (95% CI: −0.1 to −3.1). Ablation was also associated with greater improvement in quality of life in all subgroups, with the AFEQT overall score in PAF patients showing a clinically significant 5.3-point improvement (95% CI: 3.3 to 7.3) over drug therapy alone over 5 years of follow-up, PersAF a 1.7-point difference (95% CI: 0.0 to 3.7), and LSPAF a 3.1-point difference (95% CI: -1.6 to 7.8).
Conclusion
Prognostic treatment effects of catheter ablation compared with drug therapy on the primary and major secondary clinical endpoints did not differ consequentially by AF subtype. With regard to decreases in AF recurrence and improving quality of life, ablation was more effective than drug therapy in all three AF type subgroups.
ClinicalTrials.gov Identifier
Keywords: Paroxysmal atrial fibrillation, Persistent atrial fibrillation, Long-standing persistent atrial fibrillation, Antiarrhythmic drug therapy, Pulmonary vein isolation, Quality of life
Graphical Abstract
Graphical Abstract.
Primary endpoint outcomes and AF recurrence by AF type in the CABANA trial. Top panels show Kaplan–Meier curves for the CABANA primary endpoint (total mortality, disabling stroke, serious bleeding, or cardiac arrest) for patients with (A) paroxysmal, (B) persistent, or (C) long-standing persistent atrial fibrillation.
What’s new?
Atrial fibrillation type did not significantly alter the primary endpoint treatment effect size for catheter ablation relative to drug therapy.
Patients with atrial fibrillation should be made aware that ablation was significantly more effective than drug therapy for decreasing atrial fibrillation recurrence and improving quality of life in all atrial fibrillation types.
Introduction
Over the last 20 years, a consensus clinical taxonomy has evolved for atrial fibrillation (AF) that has strongly influenced both the direction of clinical research as well as decision making in clinical practice.1 Based on ad hoc criteria regarding the duration of AF episodes as well as the process needed to terminate the AF, patients with non-permanent AF are classified into one of three types or stages: paroxysmal AF (PAF), persistent AF (PersAF), or long-standing persistent AF (LSPAF). PAF refers to ‘early stage’ disease with a clinical pattern of self-terminating episodes and typically with a low total percentage of time spent in AF. The pattern of PAF is often associated with less atrial remodelling and fibrosis. PAF tends to be the most readily manageable form of the disease in terms of response to drug therapy and has the lowest associated risk of major cardiovascular events.2 PersAF represents an intermediate stage that lasts longer than 7 days and does not self-terminate within that period of time. LSPAF refers to the most advanced form of non-permanent AF. PersAF and LSPAF are generally expected to exhibit more extensive atrial remodelling and fibrosis, to be more resistant to rhythm control therapies, and to be associated with a higher risk of major adverse events.3
Underlying this classification system is the presumption that AF patients progress over time from less advanced form (PAF) to more advanced forms (PersAF and LSPAF) and that such progression is associated with worse outcomes. What is unknown is whether AF progression is the cause of increased event rates, or simply a risk marker for the primary causes, and whether the adverse prognosis associated with progression to a more advanced stage can be retarded or aborted with more effective rhythm control. In addition, it is unclear if there is a point in the natural history of AF at which effective rhythm control becomes less efficacious due to the extent of atrial myopathy and remodelling already present.
Catheter ablation studied in a randomized trial context offers an excellent tool to examine these questions since there is now considerable evidence that it offers substantially better rhythm control than conventional drug-based rhythm control.4 The Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial is the largest trial to date examining the effects of catheter ablation relative to drug therapy across the spectrum of AF. We previously reported that the response of the primary clinical endpoint to randomized treatment did not vary significantly by AF type.5 In addition, AF burden was reduced to a similar extent by ablation irrespective of baseline AF pattern.6 The present report provides a more in-depth examination of this pre-specified subgroup analysis from CABANA.
Methods
Trial design and patient population
The CABANA trial (ClinicalTrials.gov: NCT00911508) design and methods have been previously reported in detail.7 Briefly, the trial enrolled patients ≥65 years old or <65 with at least one risk factor for stroke (hypertension, heart failure, or history of stroke, diabetes, or other cardiac conditions) who had new onset or under-treated AF that warranted therapy. Patients who had a prior left atrial catheter ablation for AF or who had failed two or more antiarrhythmic drugs were excluded from the study. Written informed consent was obtained from all patients. Each site’s institutional review board or ethics committee approved the study. Eligible patients were randomized to percutaneous catheter ablation or to medical therapy with rate or rhythm control drugs.
Atrial fibrillation clinical phenotype definition
Atrial fibrillation type was defined using standard categories and was assigned by each enrolling site at trial entry.7 Paroxysmal AF was defined as episodes of the arrhythmia that terminate spontaneously within 7 days. Persistent AF included episodes that were sustained ≥7 days and were not self-terminating or that lasted fewer than 7 days but necessitated pharmacologic or electrical cardioversion. Long-standing persistent AF included episodes of continuous AF of >1 year.
Randomized treatment strategies
The catheter ablation procedures used in CABANA have been previously described.5,7 The basic ablation procedure was pulmonary vein isolation (PVI) using antral isolation, wide area circumferential ablation, or circular mapping catheter-guided techniques. Ancillary ablation procedures were performed at the discretion of the site investigator and included ablation of linear lesions, complex fractionated atrial electrograms, or ganglionated plexuses. Drug therapy included rate control or rhythm control medications with the protocol recommendation that rate control be used as initial management. In both treatment groups, the use of guideline-based anticoagulation was advocated but the use and duration of use were at the discretion of the site investigator.
Trial clinical outcomes
The primary endpoint was a composite of death, disabling stroke, serious bleeding, or cardiac arrest.7. Key secondary endpoints included total mortality, death or cardiovascular hospitalization, recurrence of AF, and quality of life (QOL) outcomes.
To collect information on recurrence of AF, CABANA used a proprietary electrocardiogram (ECG) monitoring system referred to as the ‘CABANA Box,’ as previously reported.6 The CABANA protocol pre-specified that the primary AF recurrence endpoints would be assessed in the subgroup who used the CABANA Box. Of the total 2204 trial enrolment, 803 patients were unable to use the CABANA Box, largely due to international regulatory restrictions. An additional 161 (65.8% of whom did not receive their randomized therapy during the trial) did not have post–90-day-blanking data following initiation of randomized study treatment, leaving an analysis cohort for the AF recurrence endpoint of 1240 patients. Atrial fibrillation recurrence was adjudicated by the CABANA ECG Core Laboratory and was defined as an episode of AF lasting ≥30 s and occurring after the 90-day-blanking period. AF recurrence was determined from all available CABANA Box data including symptom-driven recordings and 24 h auto-detect recordings.6
Quality of life data was collected by structured interview at baseline, 3, and 12 months after randomization, and annually thereafter, as described previously.8 The AF Effect on Quality of Life (AFEQT) and the Mayo AF-Specific Symptom Inventory (MAFSI) were pre-specified co-primary QOL endpoints in CABANA. The AFEQT is a 21-item instrument designed to assess AF-specific QOL in three domains: symptoms, daily activities, and treatment concerns. The summary score ranges from 100 (no AF-related disability) to 0 (complete AF-related disability), and a change of 5 points or more is considered a clinically significant change at the patient level. The 10-item MAFSI symptom checklist asks patients to rate the frequency of each of their symptoms over the past month. The frequency of symptoms is rated on a five-item Likert scale (0 = never, 4 = always). The MAFSI frequency summary score ranges from 0 (no AF symptoms) to 40 (all 10 symptoms constant), and the patient-level benchmark for interpretation of changes in the MAFSI frequency scale is ∼1.6 or more points.
Previously reported relevant CABANA trial results
The CABANA trial enrolled 2204 patients including 1042 with PersAF and 215 with LSPAF at baseline.5 Overall, randomization to ablation was associated with a 14% relative risk reduction (RRR) in the primary endpoint [hazard ratio (HR): 0.86; 95% confidence interval (CI): 0.65, 1.15; P = 0.30) and a 15% RRR in the key secondary endpoint of all-cause mortality (HR: 0.85; 95% CI: 0.60, 1.21; P = 0.38).5 These intention-to-treat results were interpreted as indeterminate given the inclusion of HR = 1 in the CIs. Both treatment-received and per-protocol comparisons showed larger treatment effects and greater precision. Secondary endpoints for death or cardiovascular hospitalization [HR: 0.83 (95% CI: 0.74, 0.93); P = 0.001], AF recurrence [HR: 0.52 (95% CI: 0.45, 0.60); P < 0.001], and QOL endpoints at 12 months all favoured the ablation strategy.5,8
As previously reported, the (unadjusted) intention-to-treat effect of ablation vs. drug therapy on the CABANA primary endpoint did not vary significantly as a function of baseline AF type: for PAF, HR: 0.82 (95% CI: 0.51, 1.31); for PersAF, HR: 0.87 (95% CI: 0.59, 1.28); for LSPAF, HR: 1.01 (95% CI: 0.39, 2.61) (interaction P-value = 0.93).5 AF burden reduction with ablation vs. drug therapy also did not vary according to baseline AF type out to 5 years following randomization.6
Statistical methods
Descriptive summary statistics reported include medians (25th, 75th percentiles) or means with standard deviations for continuous variables and counts (percentages) for categorical variables. Primary treatment comparisons were performed with treatment assigned at randomization (intention to treat). Treatment effects for ablation vs. drug therapy were estimated using a covariate-adjusted Cox proportional hazards model. Treatment effect sizes were summarized as adjusted hazard ratios (aHRs) with 95% CIs. Analyses were adjusted for the following baseline variables: age, sex, race/ethnicity, years since onset of AF, history of heart failure, structural heart disease, CHA2DS2-VASc score, history of coronary artery disease, and hypertension. Atrial fibrillation type and an interaction term, treatment group × AF type, were included in the model. The Wald statistic from the Cox model was used for formal significance testing.
Kaplan–Meier estimates were used to generate cumulative event rates when competing risk issues were not of concern, while cumulative incidence curves with adjustment for the competing risk of mortality were used for AF recurrence endpoints.
The QOL endpoints were analyzed with a repeated-measure mixed-effects model with baseline score and month 3, 12, 24, 36, 48, and 60 responses included as outcomes, and time, treatment group, and time x treatment group included as fixed effects.8 For each follow-up point, point estimates were generated for each treatment group, as well as adjusted treatment group mean differences (ablation score−drug score). Precision of estimates was assessed with 95% CIs. Missing QOL responses were not imputed because the mixed-effects model does not require either complete data on all patients or a uniform length of follow-up.
P-values, where provided, are intended as adjunctive interpretive aids reflecting the level of unexpectedness of observed effects under the assumption that the null hypothesis is true. No adjustments were made for multiple comparisons. All analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC).
Results
Study population
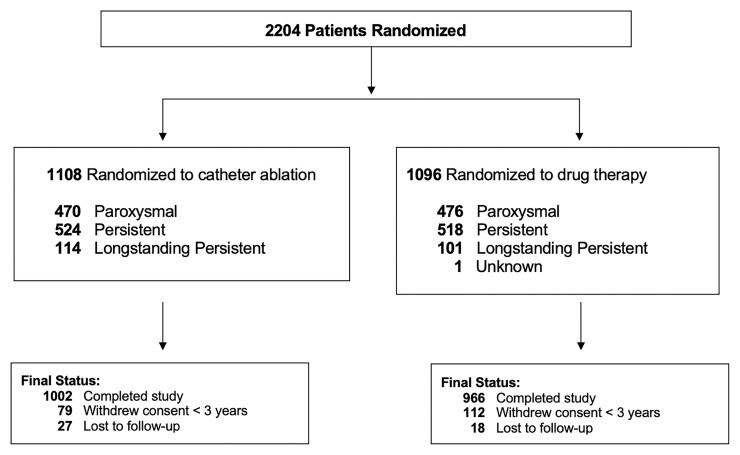
Of the 1108 patients randomized to the ablation group, 470 (42.4%) had PAF, 524 (47.3%) had PersAF, and 114 (10.3%) had LSPAF (Figure 1). Of the 1096 patients assigned to drug treatment, 476 (43.5%) had PAF, 518 (47.3%) had PersAF, and 101 (9.2%) had LSPAF. The AF subtype of one CABANA patient randomized to the drug group was unrecorded at baseline and that patient was not included in this analysis. Median duration of follow-up in CABANA was 48.5 months, with median duration of follow-up for PAF patients of 48.3 months, PersAF patients of 47.8 months, and LSPAF patients of 53.9 months (P = 0.001).
Figure 1.
Flow of patients in the CABANA trial by AF subtype. One patient had unknown atrial fibrillation type at baseline and was excluded from this analysis. Outcomes of patients who did not complete the study (i.e. withdrew consent or were lost to follow-up) were included to the point of consent withdrawal or final contact. Primary and key secondary endpoints were analyzed using time-to-event methodology; thus, all available follow-up information was utilized. For patients who did not complete the study and did not experience an outcome event, their time-to-event measure was censored at the last contact date. There was no imputation of outcome events. At the end of the trial, a publicly available death registry search was performed for patients enrolled in North America who were lost or withdrew from the trial.
Baseline characteristics
Baseline clinical characteristics and demographics were generally well balanced by treatment group across AF subtypes (Table 1). Comparing baseline characteristics across AF subtypes, pooling treatment groups, LSPAF patients were more often male (72.1 vs. 66.2% for PersAF and 57.1% for PAF) (see Supplementary material online, Table S1). New York Heart Association class II or greater heart failure symptoms were present in 26.2% of PAF, 41.6% of PersAF, and 47.4% of LSP. Median duration of AF was 2.9 years for LSPAF, 0.7 years for PersAF, and 1.1 year for PAF.
Table 1.
Baseline demographics and clinical characteristics by AF subtype
| Paroxysmal | Persistent | Long-standing persistent | ||||
|---|---|---|---|---|---|---|
| Baseline characteristics | Drug group N = 476 No. (%)a | Ablation group N = 470 No. (%)a | Drug group N = 518 No. (%)a | Ablation group N = 524 No. (%)a | Drug group N = 101 No. (%)a | Ablation group N = 114 No. (%)a |
| Age | ||||||
| Median (Q1, Q3) | 68 (63, 72) | 67 (62, 72) | 68 (62, 73) | 69 (63, 73) | 65 (61, 70) | 65 (60, 70) |
| <65 years old | 155 (32.6%) | 159 (33.8%) | 188 (36.3%) | 158 (30.2%) | 47 (46.5%) | 58 (50.9%) |
| 65–74 years old | 263 (55.3%) | 247 (52.6%) | 247 (47.7%) | 284 (54.2%) | 43 (42.6%) | 46 (40.4%) |
| ≥75 years old | 58 (12.2%) | 64 (13.6%) | 83 (16.0%) | 82 (15.6%) | 11 (10.9%) | 10 (8.8%) |
| Sex | ||||||
| Female | 212 (44.5%) | 194 (41.3%) | 166 (32.0%) | 186 (35.5%) | 27 (26.7%) | 33 (28.9%) |
| Male | 264 (55.5%) | 276 (58.7%) | 352 (68.0%) | 338 (64.5%) | 74 (73.3%) | 81 (71.1%) |
| Raceb | ||||||
| White | 435 (91.6%) | 433 (92.1%) | 480 (92.8%) | 480 (91.8%) | 92 (91.1%) | 105 (92.1%) |
| Black or African American | 17 (3.6%) | 12 (2.6%) | 18 (3.5%) | 23 (4.4%) | 3 (3.0%) | 4 (3.5%) |
| Otherc | 23 (4.8%) | 25 (5.3%) | 19 (3.7%) | 20 (3.8%) | 6 (5.9%) | 5 (4.4%) |
| Ethnicityb: Hispanic or Non-White | 53 (11.2%) | 51 (10.9%) | 47 (9.1%) | 47 (9.0%) | 12 (11.9%) | 15 (13.2%) |
| BMI (kg/m2) median (Q1, Q3) | 30 (26, 36) | 29 (26, 33) | 30 (26, 34) | 31 (27, 35) | 32 (28, 35) | 31 (27, 35) |
| AF severity (CCS Class)d | ||||||
| Class 0 | 46 (9.7%) | 43 (9.2%) | 65 (12.6%) | 53 (10.2%) | 7 (6.9%) | 9 (8.0%) |
| Class 1 | 74 (15.6%) | 79 (17.0%) | 76 (14.8%) | 67 (12.9%) | 23 (22.8%) | 20 (17.7%) |
| Class 2 | 146 (30.7%) | 158 (33.9%) | 169 (32.8%) | 151 (29.0%) | 38 (37.6%) | 41 (36.3%) |
| Class 3 | 178 (37.5%) | 157 (33.7%) | 177 (34.4%) | 206 (39.5%) | 27 (26.7%) | 38 (33.6%) |
| Class 4 | 31 (6.5%) | 29 (6.2%) | 28 (5.4%) | 44 (8.4%) | 6 (5.9%) | 5 (4.4%) |
| Heart function severity (NYHA Class)e | ||||||
| No CHF or Class I | 339 (71.4%) | 353 (76.2%) | 304 (59.3%) | 299 (57.5%) | 46 (45.5%) | 67 (58.8%) |
| Class II or greater | 136 (28.6%) | 110 (23.8%) | 209 (40.7%) | 221 (42.5%) | 55 (54.5%) | 47 (41.2%) |
| Medical history | ||||||
| Hypertension (>140/90 mmHg) | 377 (79.2) | 360 (76.6) | 431 (83.2) | 422 (80.5) | 92 (91.1) | 94 (82.5) |
| Baseline left ventricular hypertrophy | 120 (37.9%) | 139 (39.6%) | 157 (41.1%) | 153 (36.5%) | 51 (63.0%) | 42 (44.7%) |
| Hypertension or LVH | 386 (87.7%) | 376 (86.0%) | 447 (91.0%) | 449 (88.6%) | 94 (94.9%) | 99 (90.0%) |
| Diabetes (glucose ≥126 mg/dL) | 132 (27.7%) | 119 (25.3%) | 129 (24.9%) | 131 (25.0%) | 20 (19.8%) | 30 (26.3%) |
| CVA (prior) | 27 (5.7%) | 23 (4.9%) | 27 (5.2%) | 34 (6.5%) | 4 (4.0%) | 11 (9.6%) |
| Prior CVA or TIA | 51 (10.7%) | 43 (9.1%) | 45 (8.7%) | 59 (11.3%) | 7 (6.9%) | 15 (13.2%) |
| Thromboembolic events (peripheral) | 23 (4.8%) | 21 (4.5%) | 22 (4.2%) | 15 (2.9%) | 4 (4.0%) | 5 (4.4%) |
| Coronary artery disease | 94 (19.7%) | 80 (17.0%) | 102 (19.7%) | 115 (21.9%) | 20 (19.8%) | 13 (11.4%) |
| History of congestive heart failure | 54 (11.3%) | 48 (10.2%) | 92 (17.8%) | 104 (19.8%) | 17 (16.8%) | 22 (19.3%) |
| Sleep apnoea | 106 (22.3%) | 108 (23.0%) | 111 (21.4%) | 127 (24.2%) | 29 (28.7%) | 27 (23.7%) |
| Family history of atrial fibrillation | 62 (13.1%) | 51 (10.9%) | 52 (10.1%) | 62 (11.9%) | 8 (8.0%) | 17 (14.9%) |
| Left ventricular ejection fraction ≤35 | 6 (2.0%) | 4 (1.2%) | 24 (6.7%) | 29 (7.7%) | 1 (1.2%) | 5 (5.7%) |
| Co-morbidities | ||||||
| CHA2DS2-VASc scoref | ||||||
| Median (Q1, Q3) | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | 2 (2, 3) | 2 (1, 3) |
| 0–1 | 74 (15.5%) | 86 (18.3%) | 87 (16.8%) | 93 (17.7%) | 25 (24.8%) | 29 (25.4%) |
| 2 | 120 (25.2%) | 123 (26.2%) | 145 (28.0%) | 120 (22.9%) | 26 (25.7%) | 30 (26.3%) |
| 3 | 147 (30.9%) | 141 (30.0%) | 151 (29.2%) | 135 (25.8%) | 31 (30.7%) | 32 (28.1%) |
| 4 | 75 (15.8%) | 72 (15.3%) | 66 (12.7%) | 101 (19.3%) | 10 (9.9%) | 5 (4.4%) |
| ≥5 | 60 (12.6%) | 48 (10.2%) | 69 (13.3%) | 75 (14.3%) | 9 (8.9%) | 18 (15.8%) |
| Arrhythmia history | ||||||
| Years since onset of AF: Median (Q1, Q3) | 1.2 (0.3, 3.7) | 1.1 (0.3, 4.7) | 0.8 (0.3, 3.3) | 0.7 (0.2, 3.0) | 3.1 (1.2, 7.6) | 2.8 (1.5, 5.6) |
| Prior hospitalization for treatment of AF | 173 (36.3%) | 169 (36.0%) | 208 (40.2%) | 234 (44.7%) | 44 (43.6%) | 46 (40.4%) |
| Prior direct current cardioversion—AF | 94 (19.7%) | 73 (15.6%) | 273 (52.7%) | 290 (55.3%) | 44 (43.6%) | 35 (30.7%) |
| History of atrial flutter | 78 (16.5%) | 79 (17.2%) | 68 (13.3%) | 51 (9.9%) | 12 (12.1%) | 10 (9.0%) |
| Prior ablation for atrial flutter | 31 (6.5%) | 28 (6.0%) | 25 (4.8%) | 18 (3.4%) | 4 (4.0%) | 2 (1.8%) |
| Rhythm control therapyg | ||||||
| 1 Rhythm control drug | 223 (83.2%) | 188 (82.5%) | 198 (81.8%) | 173 (81.6%) | 31 (77.5%) | 37 (77.1%) |
| ≥ 2 Rhythm control drugs | 45 (16.8%) | 40 (17.5%) | 44 (18.2%) | 39 (18.4%) | 9 (22.5%) | 11 (22.9%) |
Unless otherwise noted AF, atrial fibrillation; AFL, atrial flutter; CCS, Canadian Cardiovascular Society; CHF, congestive heart failure; CVA, cerebral vascular accident; LVH, left ventricular hypertrophy; NYHA, New York Heart Association, Q1 and Q3, quartiles (25th and 75th percentiles); TIA, transient ischaemic attack.
Race/minority was determined by the site investigator in conjunction with the patient based on pre-defined categories as required by the National Institutes of Health (NIH) using NIH-specified categories.
Asian, American Indian/Alaskan Indian, Hawaiian or Other Pacific Islander and Multiracial.
On a scale of 0–4 in which 0 is the least severe and 4 is the most severe symptom of AF.
On a scale of I to IV in which I is the least severe and IV is the most severe symptom of heart failure.
On a scale of 0–9 in which 0 is the lowest risk of stroke and 9 is the highest risk of stroke.
Current or past use of rhythm control therapy reported at the time of enrolment.
Treatment characteristics by atrial fibrillation subtype in the ablation arm
The proportion of patients randomized to ablation who did not receive ablation did not differ by AF type (see Supplementary material online, Table S2). However, the use of rhythm control drugs in the post-blanking period was lowest in PAF (39.7%), intermediate in PersAF (46.5%), and highest in LSPAF (56.9%) (see Supplementary material online, Table S3).
Treatment characteristics by atrial fibrillation subtype in the drug therapy arm
Among patients randomized to drug therapy, at least one rhythm control drug in the post-blanking period was used in 90.7% of PAF, 85.1% of PersAF, and 51.0% of LSPAF (see Supplementary material online, Table S3). The most common rhythm control agent used was amiodarone in all three AF types (see Supplementary material online, Table S3).
In the drug therapy arm, the rate of crossover to ablation was 30.9% in PAF, 27.0% for PersAF, and 13.9% for LSPAF (P = 0.002) (see Supplementary material online, Table S2).
Adverse events
Adverse events were low across all AF subtypes in both treatment groups (see Supplementary material online, Tables S4A–4C). Predominant adverse events in the catheter ablation group included minor haematomas (2.3%) and pseudoaneurysms (1.1%) and in the drug therapy group, thyroid disorders (1.6%), and proarrhythmia (0.8%).
Outcomes in atrial fibrillation types by intention-to-treat
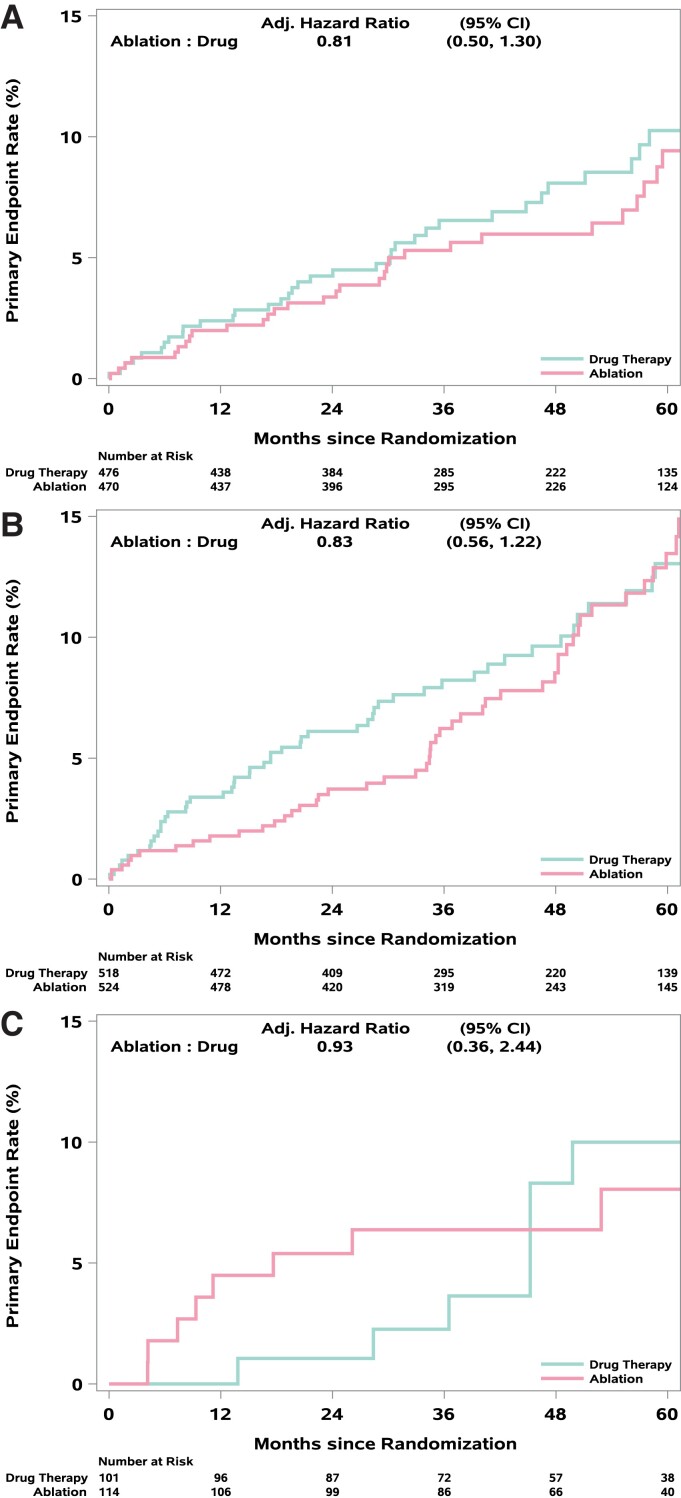
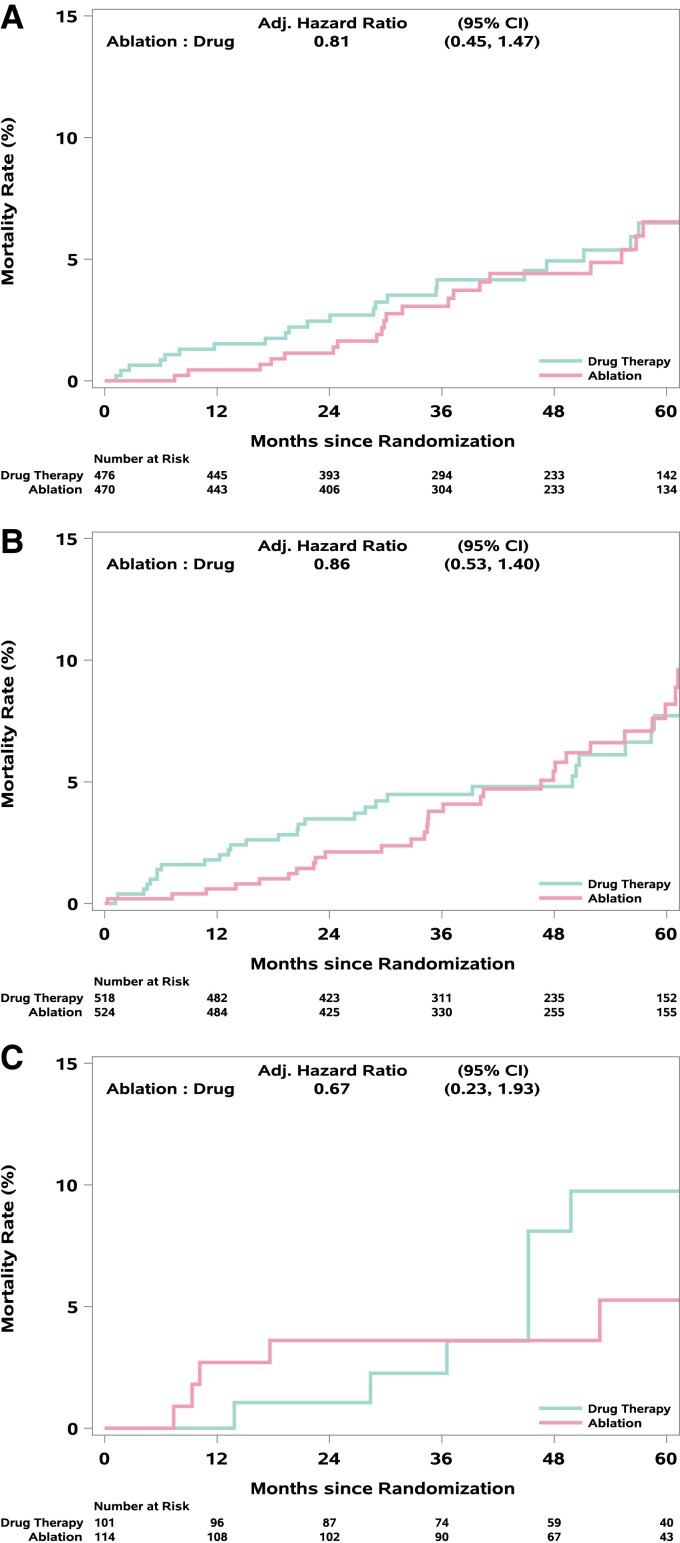
For the primary endpoint, the adjusted ablation to drug therapy HR was 0.81 (95% CI: 0.50, 1.30) for PAF, 0.83 (95% CI: 0.56, 1.22) for PersAF, and 0.93 (95% CI: 0.36, 2.44) for LSPAF (interaction treatment by AF type P-value = 0.96) (Table 2, Figure 2). The corresponding aHR estimates (95% CI) for all-cause mortality were 0.81 (0.45, 1.47), 0.86 (0.53, 1.40), and 0.67 (0.23, 1.93), respectively (interaction P-value = 0.91) (Table 2, Figure 3). Mortality rates at 12 months for ablation and drug therapy patients, respectively, were 0.4 and 1.5% for PAF, 0.6 and 1.8% for PersAF, and 2.7 and 0.0% for LSPAF (Figure 3). Corresponding rates at 60 months were 6.5 and 6.5% for PAF, 8.2 and 7.7% for PersAF, and 5.3 and 9.7% for LSPAF (Figure 3).
Table 2.
Clinical outcomes by atrial fibrillation subtype in the CABANA trial
| Adjusted hazard ratio (95% CI) ablation:drug | |
|---|---|
| Paroxysmal | |
| Primary endpointa | 0.81 (0.50, 1.30) |
| All-cause mortality | 0.81 (0.45, 1.47) |
| All-cause mortality or cardiovascular hospitalization | 0.67 (0.56, 0.81) |
| First AF recurrence after blanking periodb | 0.49 (0.39, 0.62) |
| Persistent | |
| Primary endpointa | 0.83 (0.56, 1.22) |
| All-cause mortality | 0.86 (0.53, 1.40) |
| All-cause mortality or cardiovascular hospitalization | 0.86 (0.74, 1.01) |
| First AF recurrence after blanking periodb | 0.53 (0.43, 0.65) |
| Long-standing persistent | |
| Primary endpointa | 0.93 (0.36, 2.44) |
| All-cause mortality | 0.67 (0.23, 1.93) |
| All-cause mortality or cardiovascular hospitalization | 1.64 (1.13, 2.40) |
| First AF recurrence after blanking periodb | 0.64 (0.41, 1.00) |
Composite of all-cause mortality, disabling stroke, serious bleeding, or cardiac arrest.
In patients who used the CABANA Box.
Figure 2.
(A–C) Kaplan–Meier plots for CABANA primary endpoint by AF type. Kaplan–Meier curves for the CABANA primary endpoint (total mortality, disabling stroke, serious bleeding, or cardiac arrest) for patients with (A) paroxysmal, (B) persistent, or (C) long-standing persistent atrial fibrillation.
Figure 3.
(A–C) Kaplan–Meier Plots for all-cause mortality by AF type. Kaplan–Meier curves for all-cause mortality for patients with (A) paroxysmal, (B) persistent, or (C) long-standing persistent atrial fibrillation.
For the composite endpoint of all-cause mortality or cardiovascular hospitalization (Table 2, Figure 4), the aHR was 0.67 (95% CI: 0.56, 0.81) for PAF, 0.86 (95% CI: 0.74, 1.01) for PersAF, and 1.64 (95% CI: 1.13, 2.40) for LSPAF (interaction P-value <0.001).
Figure 4.
(A–C) Kaplan–Meier plots for death or cardiovascular hospitalization by AF type. Kaplan–Meier curves for the combined endpoint of mortality or cardiovascular hospitalization for patients with (A) paroxysmal, (B) persistent, or (C) long-standing persistent atrial fibrillation.
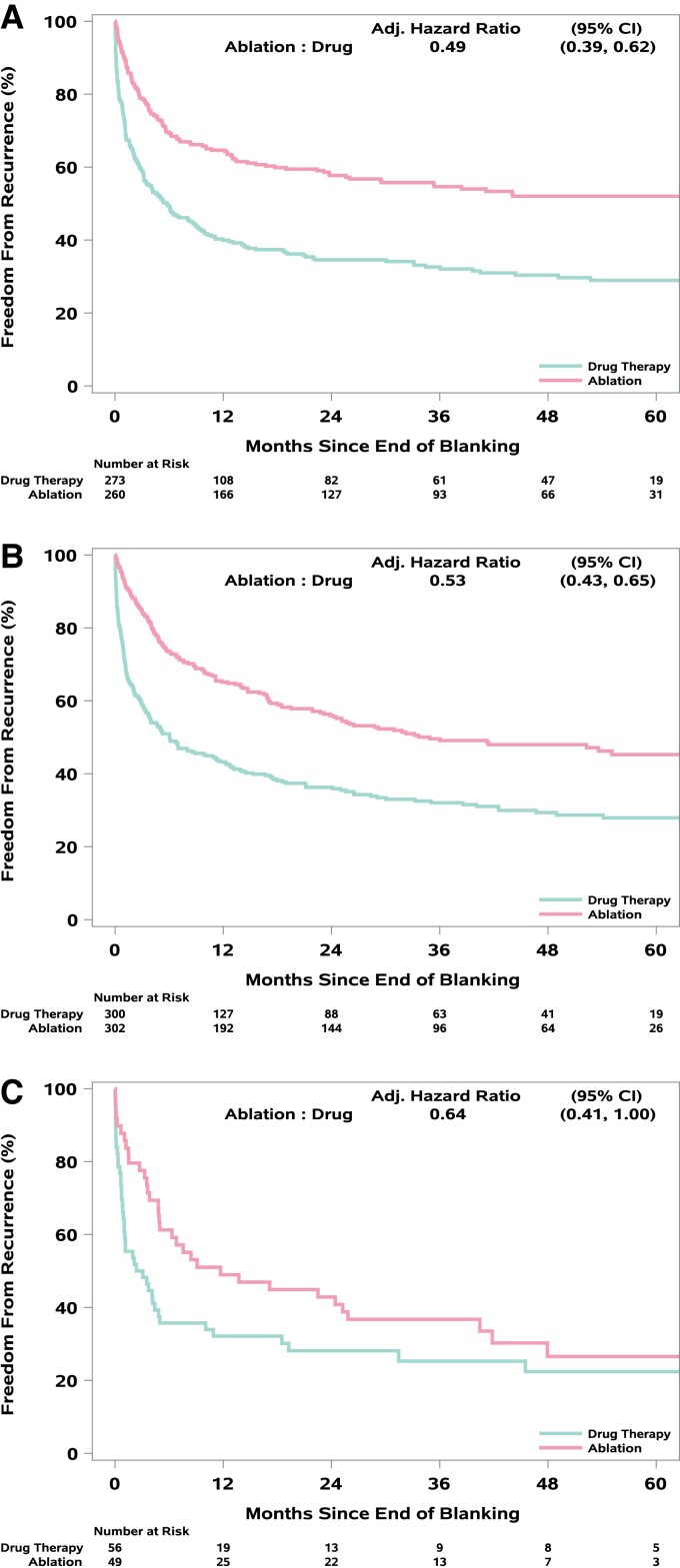
Time to first AF recurrence after the blanking period was consistently lengthened for ablation relative to drug therapy (in patients with CABANA Box recordings, n = 1240): aHR 0.49 (95% CI: 0.39, 0.62) for PAF, 0.53 (95% CI: 0.43, 0.65) for PersAF, and 0.64 (95% CI: 0.41, 1.00) for LSPAF (Table 2, Figure 5). At 12 months post-blanking period, 64.6% of ablation and 39.9% of drug arm patients remained free of AF in the PAF subgroup. Corresponding figures for PersAF were 65.1 and 43.2%, respectively, while the rates for LSPAF were 49.0 and 32.1%, respectively. Estimates of freedom from AF at 60 months were 52.0 and 28.9% for PAF, 45.3 and 27.9% for PersAF, and 26.6 and 22.4% for LSPAF (Figure 5).
Figure 5.
(A–C) Cumulative incidence plots for freedom from AF recurrence by AF type. Cumulative incidence curves for freedom from AF recurrence, adjusted for the competing risk of death, for patients with (A) paroxysmal, (B) persistent, or (C) long-standing persistent atrial fibrillation among CABANA patients who received randomized treatment and used the CABANA ECG recording system in the post-blanking period.
Quality of life outcomes
In PAF, ablation reduced the frequency of symptoms of AF as measured by the MAFSI frequency score more than drug therapy throughout 5 years of follow-up (see Supplementary material online, Table S5, Supplementary material online, Figure S1), with a clinically significant −1.9-point difference (95% CI: −1.2 to −2.6) between treatment groups when averaged across all follow-up time intervals (see Supplementary material online, Figure S1). The AFEQT overall score also showed ablation in PAF patients had a clinically significant 5.3-point improvement in AF-related QOL over drug therapy alone (95% CI: 3.3 to 7.3) averaged over all follow-up time intervals (see Supplementary material online, Table S6, Supplementary material online, Figure S2).
In patients with PersAF, the ablation arm had smaller mean improvements in AF symptoms (MAFSI overall mean difference −0.9, 95% CI: −0.2 to −1.6) and in AF-related QOL (1.7, 95% CI: 0.0 to 3.7) (see Supplementary material online, Tables S7 and S8, Supplementary material online, Figures S3 and S4).
In LSPAF, MAFSI AF symptom frequency averaged over all time intervals out to 60 months, results favoured ablation with a mean difference of −1.6 points (95% CI: −0.1 to −3.1) (see Supplementary material online, Table S9, Supplementary material online, Figure S5). For AF QOL as measured by the AFEQT in the LSPAF subtype, the mean treatment effect was 3.1 (95% CI: −1.6 to 7.8) (see Supplementary material online, Table S10, Supplementary material online, Figure S6).
Discussion
Three findings from the present report merit special attention. First, the prognostic effects of ablation relative to drug therapy in CABANA were consistent across AF type, although CIs were too wide to exclude a null effect in any subgroup. Most prior randomized trials of catheter ablation have included only a segment of the non-permanent AF population, typically PAF, and have not been large enough and with long enough follow-up to examine effects on prognosis.9 Second, the relative effectiveness of ablation for preventing AF recurrence was similar across AF type, although absolute rates of freedom from recurrence after ablation were highest in PAF (55% at 5 years) and lowest in LSPAF (28% at 5 years). Finally, the effects of ablation on AF-related QOL were comparable across AF types.
The contemporary relevance of atrial fibrillation type
Early interest in classifying AF phenotypes was stimulated by findings that AF was often initiated by ectopic atrial beats originating in the pulmonary veins, and catheter ablation at these initiation sites appeared to eliminate the resulting ‘paroxysms of atrial fibrillation’ in some patients.10 Subsequent work showed that ablation was much more effective when used in PAF compared with PersAF.11 Small clinical trials confirmed enhanced freedom from AF recurrence with ablation in PAF.12–15 A few small trials also suggested improved rhythm control with ablation in ‘chronic’ AF16 or PersAF.17
Classification of clinical AF into 3 types as employed in the present analysis has been used over the past two decades in multiple AF clinical practice guidelines in the US, Canada, and Europe to frame treatment recommendations.1,18 As noted above, AF rhythm management clinical trials have often defined eligibility for enrolment based on AF type. In addition, clinicians routinely use these descriptors in practice to communicate their understanding of current disease severity/risk. Similar to many clinically derived staging systems, AF type is based on Ad hoc divisions of select AF features, specifically duration and method of termination. The data required for this classification are typically obtained opportunistically from relatively short ECG recordings, often made in response to concerning symptoms. Recent work using implantable cardiac monitors or other implanted cardiac devices suggests that AF type based on such incomplete ECG recording data may substantially misclassify patients.19,20 In addition, a growing body of work suggests that other features of AF, particularly, maximum AF duration or AF burden, may better characterize AF prognosis and response to therapy.20 Nonetheless, AF type connects with a large body of literature covering contemporary prognosis and response to therapy, which makes it of continued clinical relevance.
Prognostic effects of catheter ablation
One of the most concerning aspects of AF is its strong association with long term increased rates of mortality in cohort and population studies. This relationship raises two important questions. Most important for patients and clinicians is the issue of whether AF causes the increased mortality rates in some way or is instead simply a risk marker. Related to that issue is the question of whether the increased mortality risks can be attenuated or eliminated by therapeutically addressing the AF state. CABANA was designed in part to directly examine these questions.7 In intention-to-treat comparisons, the ablation strategy had an indeterminate effect on the primary composite event rate relative to drug therapy, and results with all-cause mortality were similar.5 When examined according to AF type (Table 2, Figures 2A–2C), the ablation: drug hazard ratio for the primary endpoint was similar for PAF and PersAF (0.81 and 0.83, respectively) and somewhat larger for LSPAF (0.93) with all having relatively wide CIs that included no effect (i.e. HR = 1). For mortality alone, relative treatment effect sizes were comparable with the results in LSPAF complicated by low event numbers and crossing survival curves.
Freedom from atrial fibrillation recurrence
We previously reported results from CABANA on AF burden by AF type.6 At baseline, PAF patients spent about 20% of recording time in AF, whereas PersAF and LSPAF together spent almost 70% of recording time in AF. Both subgroups showed significantly greater reduction in AF occurrence with ablation out to 5 years. In the PAF group, AF burden was generally 2–4% at each follow-up Holter recording for the ablation arm and 6–10% for the drug therapy arm. For the PersAF/LSPAF combined group, follow-up burden in the ablation arm varied between 9 and 21% and in the drug arm between 18 and 36%. In the present report, we describe the time to first recurrent AF starting from the end of the blanking period by AF type. The average treatment effect showed AF recurrence was reduced by about 50% by ablation for all three AF types although absolute freedom from AF at 5 years with ablation was highest for PAF (52.0%), intermediate for PersAF (45.3%), and worst for LSPAF (26.6%). Results at 1 year for the PAF subgroup are very similar to recently reported 1-year results from the EARLY-AF trial on both a relative and absolute scale.9
Quality of life outcomes
In the present analysis, patients in all three AF types showed both improved AF-related symptoms (MAFSI) and improved AF-related quality of life (AFEQT) out to 5 years. Baseline MAFSI scores and AFEQT scores were similar across the three AF types. Thus, the AF type classification does not appear strongly related to the factors that determine symptom status in the CABANA cohort. The scores for the ablation arm patients also did not differ across the AF type subgroups in follow-up. Therefore, the primary QOL effects of the absolute treatment difference over time seems to be related to how much the drug therapy arm improved. The largest treatment differences were seen at about 1 year with some later attenuation due to further improvements in the drug arm without any evidence of worsening in the ablation arm.
Limitations
Several caveats should be considered in the interpretation of this study. First, AF phenotype assigned with routinely available data at the time of trial enrolment is imprecise and some misclassification is likely. Second, CABANA was powered for a test of the size of the treatment effect using the entire enrolled cohort. Subgroup treatment comparisons therefore lack precision and are subject to the well-known perils of subgroup analysis. This point is particularly noteworthy for interpreting treatment results in the LSPAF subtype given its relatively small number of patients. Comparisons of effect size estimates as well as absolute event rate differences can provide useful insights even when 95% CIs do not exclude a null treatment effect (i.e. HR = 1). Third, while intention-to-treat comparisons are the only formal comparison method that preserves the protection of randomization, crossovers may have biased the mean treatment effect towards the null. Previous analyses showed larger statistically significant treatment effect estimates in CABANA using ‘as-treated’ and ‘per-protocol’ definitions of treatment assignment.5 Finally, treatment decisions in the ablation arm beyond the requirement for PVI were left to the discretion of each site’s investigative team, and we are unable to determine how much these choices altered patient outcomes across the AF type spectrum.
Conclusion
For patients enrolled in CABANA, AF type did not significantly alter the primary or major secondary clinical endpoint treatment effect sizes for catheter ablation relative to drug therapy. For decreases in AF recurrence and for improving QOL, ablation was more effective than drug therapy in all three AF type subgroups.
Supplementary Material
Acknowledgements
The authors are indebted to the investigators at the CABANA sites and to the patients who participated and made this study possible.
Contributor Information
Kristi H Monahan, Mayo Clinic, 1216 2nd St. SW, Rochester, MN 55902, USA.
T Jared Bunch, Intermountain Health Care, University of Utah, Salt Lake City, UT 84132, USA.
Daniel B Mark, Duke Clinical Research Institute, Duke University, Durham, NC 27701, USA.
Jeanne E Poole, University of Washington Medical Center, University of Washington, Seattle, WA 98195, USA.
Tristram D Bahnson, Duke Clinical Research Institute, Duke University, Durham, NC 27701, USA.
Hussein R Al-Khalidi, Duke Clinical Research Institute, Duke University, Durham, NC 27701, USA.
Adam P Silverstein, Duke Clinical Research Institute, Duke University, Durham, NC 27701, USA.
Melanie R Daniels, Duke Clinical Research Institute, Duke University, Durham, NC 27701, USA.
Kerry L Lee, Duke Clinical Research Institute, Duke University, Durham, NC 27701, USA.
Douglas L Packer, Mayo Clinic, 1216 2nd St. SW, Rochester, MN 55902, USA.
Supplementary material
Supplementary material is available at Europace online.
Funding
This work was supported by the National Institutes of Health grants U01HL089709, U01HL089786, U01HL089907, and U01HL089645; St Jude Medical Foundation and Corporation; Biosense Webster Inc.; Medtronic Inc.; and Boston Scientific Corporation.
Data Availability
CABANA is an NIH/NHLBI sponsored trial, and the trial data sets will be made public via the NIH BioLINCC website.
References
- 1. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 2. Ganesan AN, Chew DP, Hartshorne T, Selvanayagam JB, Aylward PE, Sanders P, et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J 2016;37:1591–1602. [DOI] [PubMed] [Google Scholar]
- 3. Lau DH, Linz D, Schotten U, Mahajan R, Sanders P, Kalman JM. Pathophysiology of paroxysmal and persistent atrial fibrillation: rotors, foci and fibrosis. Heart Lung Circ 2017;26:887–893. [DOI] [PubMed] [Google Scholar]
- 4. Parameswaran R, Al-Kaisey AM, Kalman JM. Catheter ablation for atrial fibrillation: current indications and evolving technologies. Nat Rev Cardiol 2021;18:210–225. [DOI] [PubMed] [Google Scholar]
- 5. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poole JE, Bahnson TD, Monahan KH, Johnson G, Rostami H, Silverstein AP, et al. Recurrence of atrial fibrillation after catheter ablation or antiarrhythmic drug therapy in the CABANA trial. J Am Coll Cardiol 2020;75:3105–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Moretz K, et al. Catheter ablation versus antiarrhythmic drug therapy for atrial fibrillation (CABANA) trial: study rationale and design. Am Heart J 2018;199:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne J, et al. Cryoablation or drug therapy for tnitial treatment of atrial fibrillation. N Engl J Med 2021;384:305–315. [DOI] [PubMed] [Google Scholar]
- 10. Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 11. Oral H, Knight BP, Tada H, Ozaydin M, Chugh A, Hassan S, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation 2002;105:1077–1081. [DOI] [PubMed] [Google Scholar]
- 12. Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M, Saliba W, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005;293:2634–2640. [DOI] [PubMed] [Google Scholar]
- 13. Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333–340. [DOI] [PubMed] [Google Scholar]
- 14. Morillo CA, Verma A, Connolly SJ, Kuck KH, Nair GM, Champagne J, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA 2014;311:692–700. [DOI] [PubMed] [Google Scholar]
- 15. Nielsen J C, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med 2012;367:1587–1595. [DOI] [PubMed] [Google Scholar]
- 16. Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F Jr, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med 2006;354:934–941. [DOI] [PubMed] [Google Scholar]
- 17. Stabile G, Bertaglia E, Senatore G, De Simone A, Zoppo F, Donnici G, et al. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation For The Cure Of Atrial Fibrillation Study). Eur Heart J 2006;27:216–221. [DOI] [PubMed] [Google Scholar]
- 18. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. 2012 HRS/EHRA/ECAS Expert Consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528–606. [DOI] [PubMed] [Google Scholar]
- 19. Charitos EI, Purerfellner H, Glotzer TV, Ziegler PD. Clinical classifications of atrial fibrillation poorly reflect its temporal persistence: insights from 1,195 patients continuously monitored with implantable devices. J Am Coll Cardiol 2014;63:2840–2848. [DOI] [PubMed] [Google Scholar]
- 20. Steinberg BA, Li Z, O'Brien EC, Pritchard J, Chew DS, Bunch TJ, et al. Atrial fibrillation burden and heart failure: data from 39,710 individuals with cardiac implanted electronic devices. Heart Rhythm 2021;18:709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
CABANA is an NIH/NHLBI sponsored trial, and the trial data sets will be made public via the NIH BioLINCC website.