Abstract
Background/Aims
Recently, patients with pancreatic cancer (PC) who underwent resection have exhibited improved survival outcomes, but comprehensive analysis is limited. We analyzed the trends of contributing factors.
Methods
Data of patients with resected PC were retrospectively collected from the Korean Health Insurance Review and Assessment Service (HIRA) database and separately at our institution. Cox regression analysis was conducted with the data from our institution a survival prediction score was calculated using the β coefficients.
Results
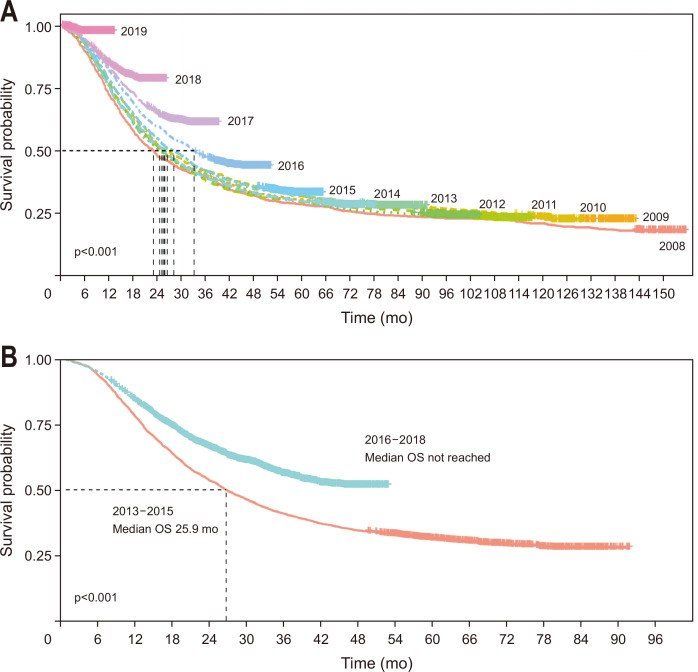
Comparison between the periods 2013–2015 (n=3,255) and 2016–2018 (n=3,698) revealed a difference in the median overall survival (25.9 months vs not reached, p<0.001) when analyzed with the HIRA database which was similar to our single-center data (2013–2015 [n=119] vs 2016–2018 [n=148], 20.9 months vs 32.2 months, p=0.003). Multivariable analyses revealed six factors significantly associated with better OS, and the scores were as follows age >70 years, 1; elevated carbohydrate antigen 19-9 at diagnosis, 1; R1 resection, 1; stage N1 and N2, 1 and 3, respectively; no adjuvant treatment, 2; FOLFIRINOX or gemcitabine plus nab-paclitaxel after recurrence, 4; and other chemotherapy or supportive care only after recurrence, 5. The rate of R0 resection (69.7% vs 80.4%), use of adjuvant treatment (63.0% vs 74.3%), and utilization of FOLFIRINOX or gemcitabine plus nab-paclitaxel (25.2% vs 47.3%) as palliative chemotherapeutic regimen, all increased between the two time periods, resulting in decreased total survival prediction score (mean 7.32 vs 6.18, p=0.004).
Conclusions
Strict selection of surgical candidates, more use of adjuvant treatment, and adoption of the latest combination regimens for palliative chemotherapy after recurrence were identified as factors of recent improvement.
Keywords: Pancreatic neoplasms, Adjuvant chemotherapy, Pancreatectomy, Prognosis, Palliative treatment
INTRODUCTION
Pancreatic cancer (PC) is the fourth leading cause of estimated cancer-related death in the United States, and the 5-year survival rate was approximately 11% in 2021.1 Surgical resection is the only curative treatment, and 15% to 20% of patients were reported as eligible for surgery at diagnosis.2 Several studies recently reported that survival in patients with PC who underwent curative resection had improved over time.3 Moreover, patients with PC diagnosed in the early stage had a remarkable improvement in mortality compared with those diagnosed in the advanced stage.4
The following management strategies are regarded as having led to better outcomes in patients with PC who underwent curative resection.5 First, the administration of neoadjuvant therapy (NAT), which has been reported to improve the survival rate in patients with borderline resectable PC (BRPC),6,7 is increasingly being utilized in such patients.8 Second, the proportion of patients who are treated with adjuvant therapy has increased.9 Moreover, a modified FOLFIRINOX (oxaliplatin, irinotecan, fluorouracil, and leucovorin) or gemcitabine plus capecitabine regimen is recommended and preferred as adjuvant therapy and has replaced other regimens.10-12 Third, combination regimens, such as FOLFIRINOX or gemcitabine plus nab-paclitaxel (GNP), have proven efficacy and are recommended as the first-line treatment after recurrence.13,14 Finally, centralization of pancreatic surgery in high-volume centers and intensive perioperative management are regarded as additional factors responsible for reducing perioperative mortality and complications.15,16
Despite the analysis of the role of each factor, integrative analyses are scarce. Thus, this study comprehensively analyzed those factors contributing to the survival of patients with resected PC and assessed changes in trends over time.
MATERIALS AND METHODS
1. Study population and design
1) National health insurance data
Approximately 97% of the Korean population (approximately 51 million people) is covered by the National Health Insurance Service, and the remaining 3% by the Medical Aid Program. The Korean Health Insurance Review and Assessment Service (HIRA) reviews all medical cost claims submitted for reimbursement to the National Health Insurance Service and Medical Aid Program in Korea. We acquired public data from the HIRA database and selected patients with PC who underwent curative surgical resection using claim information between January 1, 2008, and December 31, 2019. We defined PC as International Classification of Diseases, Tenth Revision code C25.x and code V193, a unique Korean code for national aid programs for critical and rare diseases. Procedural codes (Q7550, pancreatic tumor excision; Q7561, total pancreatectomy; Q7562, duodenal preserving pancreaticoduodenectomy; Q7563, pancreas body resection; Q7564, pancreas segmental resection; Q7565, distal pancreatectomy; Q7566, pancreas wedge resection; and Q7572, pylorus-preserving pancreaticoduodenectomy) regarding pancreatic surgery were collected from the HIRA database. Codes for chemotherapy regimens were also collected to exclude patients treated with preoperative chemotherapy. Overall survival (OS) with event or censoring was defined as the time from the diagnosis to death or the last follow-up (December 31, 2019). The date of diagnosis was defined as the first date on which a medical claim was made with the code C25.x. The date of death and censoring was defined as the date with the last medical claim in those with no more claims over the following 6 months and within 6 months, respectively.
2) Single-center data
To analyze the contributions of each clinical factor, we retrospectively reviewed electronic medical records of patients with PC who underwent curative upfront surgery in a single tertiary medical center between 2013 and 2018. Patients with the following conditions were included: (1) resectable PC or BRPC at the time of diagnosis by National Comprehensive Cancer Network guidelines; (2) pancreatectomy without NAT; (3) R0 or R1 resection of the surgical specimen; and (4) no history of other malignancy within the past 5 years. Patients with the following conditions were excluded: (1) locally advanced or metastatic PC at the time of diagnosis by National Comprehensive Cancer Network guidelines; (2) NAT before pancreatectomy; (3) macroscopic (R2) resection margin in the surgical specimen; and (4) cell types, including intraductal papillary mucinous neoplasm without invasive carcinoma or pancreatic neuroendocrine tumor/carcinoma. OS was defined as the time from the histological diagnosis of PC to death or last follow-up (February 28, 2022). Recurrence-free survival (RFS) was defined as the time from the surgery to evidence of recurrence by radiological evaluation or last follow-up (February 28, 2022). The 8th AJCC system was used for T and N staging.17
2. Clinical outcomes
The primary outcome was changes in clinical factors for OS. The secondary outcomes were changes in clinical factors for RFS and an assessment of the parameters that contributed to OS and PFS.
3. Statistical analysis
To compare the baseline characteristics and clinicopathologic factors of the participants between the two periods, chi-square and Fisher exact tests were used for categorical variables, and the Mann-Whitney U-test was used for continuous variables. OS and RFS were evaluated using the Kaplan-Meier method, and the difference in survival between the two periods was evaluated using the log-rank test. The Cox proportional hazard model was used to evaluate the association between time to event and risk factors of interest. A survival prediction score for OS and RFS was calculated using the β coefficients of clinical factors with a statistical significance of 0.05 based on multivariable Cox regression analyses.18 To examine the predictive ability of the model, the optimism-corrected c-index was calculated with 1,500 bootstrap replications.19 A c-index of >0.7 was considered to indicate a reasonable model.20 Data were analyzed using R (version 4.0.1; R Foundation for Statistical Computing, Vienna, Austria) and Rstudio (version 1.3.959; PBC, Boston, MA, USA) with R packages survAUC and survivalROC, respectively. All p-values were two-sided, and p<0.05 was considered statistically significant.
4. Ethics statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Seoul National University Bundang Hospital, Seongnam, Korea (IRB numbers: X-2101-661-907 and B-2204-749-103).
RESULTS
1. Recent survival trends
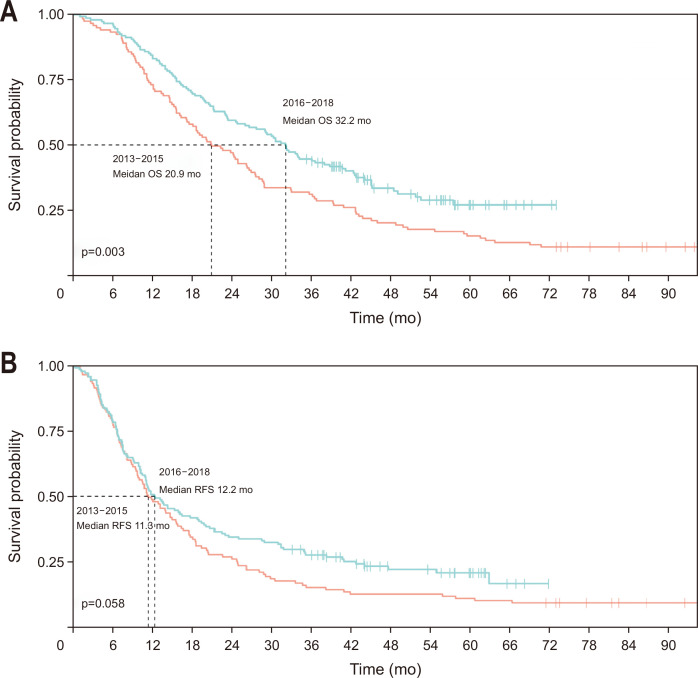
The OS of the 12,146 patients in the HIRA database has improved since 2016 (Fig. 1A). Stratifying the patients into two periods (2013–2015 [n=3,255] vs 2016–2018 [n=3,698]) revealed a significant difference in the median OS (25.9 months vs not reached, p<0.001) (Fig. 1B). Stratifying the 267 patients from the single-center data into the same two periods (2013–2015 [n=119] vs 2016–2018 [n=148]) also showed a significant difference in the median OS (20.9 months vs 32.2 months, p=0.003). The 1-, 3-, and 5-year OS rates were increased between the two periods (2013–2015 vs 2016–2018: 73.1% vs 83.1%, 31.1% vs 44.6%, and 15.1% vs 27.1%, respectively) (Fig. 2A). In contrast, the median RFS in the single-center data demonstrated a different tendency and was not statistically significantly different between the two periods (2013–2015: 11.3 months vs 12.2 months, p=0.058). The 1-, 3-, and 5-year RFS rates were increased between the two periods (2013–2015 vs 2016–2018: 47.9% vs 50.7%, 15.1% vs 27.6%, and 10.9% vs 20.8%, respectively) (Fig. 2B).
Fig. 1.
Kaplan-Meier survival curves. Overall survival (OS) of patients in the nationwide Health Insurance Review and Assessment Service database who had pancreatic cancer and underwent upfront surgery. (A) OS curve in terms of the year of diagnosis. (B) OS curve comparing two periods (2013–2015 vs 2016–2018).
Fig. 2.
Kaplan-Meier survival curves. Overall survival (OS) and recurrence-free survival (RFS) of patients in a single-center database who had pancreatic cancer and underwent upfront surgery. (A) OS curves comparing two periods (2013–2015 vs 2016–2018). The estimating 1-, 3-, and 5-year OS rates between the two periods (2013–2015 vs 2016–2018) were 73.1% (95% CI, 65.6% to 81.5%) versus 83.1% (95% CI, 77.3% to 89.4%), 31.1% (95% CI, 23.8% to 40.6%) versus 44.6% (95% CI, 37.3% to 53.4%), and 15.1% (95% CI, 9.9% to 23.2%) vs 27.1% (95% CI, 19.9% to 36.8%), respectively. (B) RFS curves comparing two periods (2013–2015 vs 2016–2018). The estimating 1-, 3-, and 5-year RFS rates between the two periods (2013–2015 vs 2016–2018) were 47.9% (95% CI, 39.7% to 57.8%) versus 50.7% (95% CI, 43.2% to 59.4%), 15.1% (95% CI, 9.9% to 23.2%) versus 27.6% (95% CI, 21.3% to 35.9%), and 10.9% (95% CI, 6.5% to 18.3%) versus 20.8% (95% CI, 14.8% to 29.2%), respectively.
2. Clinicopathologic characteristics in the single-center data
The median age of the patients was 68.7 years, and 141 were male (52.8%). Of the total 267 patients, 232 (86.9%) were diagnosed with resectable PC, and 161 (60.3%) underwent pancreaticoduodenectomy. The median serum carbohydrate antigen 19-9 (CA19-9) at diagnosis was 111 U/mL. Regarding surgical pathologic outcomes, 202 (75.7%) showed R0 resection. The T and N stages of the patients were as follows: 30 (11.2%) in T1, 168 (62.9%) in T2, and 69 (25.8%) in T3; 81 (30.3%) in N0, 114 (42.7%) in N1, and 72 (27.0%) in N2. Adenocarcinoma was noted in 223 (83.5%) cases, followed by intraductal papillary mucinous neoplasm associated with carcinoma in 22 (8.2%) and adenosquamous carcinoma in 18 (6.7%). A total of 185 patients (69.3%) received adjuvant treatment, and 203 experienced a recurrence (76.0%). Of these patients with recurrence, 100 (49.3%) were treated with palliative chemotherapy with FOLFIRINOX or GNP, 16 (7.9%) were treated with other regimens, and 87 (42.9%) were treated with supportive care only (Table 1).
Table 1.
Baseline Characteristics
| Characteristic | Total (n=267) |
|---|---|
| Age, yr | 68.7 (61.6–74.5) |
| Sex | |
| Male | 141 (52.8) |
| Female | 126 (47.2) |
| Serum CA19-9 at diagnosis, U/mL | 111 (31–335) |
| Resectability | |
| RPC | 232 (86.9) |
| BRPC | 35 (13.1) |
| Operation type | |
| PD | 161 (60.3) |
| Non-PD | 106 (39.7) |
| Pathologic information | |
| Surgical margins | |
| R0 | 202 (75.7) |
| R1 | 65 (24.3) |
| T stage* | |
| T1 | 30 (11.2) |
| T2 | 168 (62.9) |
| T3 | 69 (25.8) |
| N stage* | |
| N0 | 81 (30.3) |
| N1 | 114 (42.7) |
| N2 | 72 (27.0) |
| Angiolymphatic invasion | 169 (63.3) |
| Perineural invasion | 239 (89.5) |
| Pathologic type | |
| Adenocarcinoma | 223 (83.5) |
| IPMN associated with carcinoma | 22 (8.2) |
| Adenosquamous carcinoma | 18 (6.7) |
| Others | 4 (1.6) |
| Adjuvant treatment | 185 (69.3) |
| Palliative chemotherapy after recurrence | |
| No recurrence | 64 (24.0) |
| FOLFIRINOX or GNP | 100 (37.5) |
| Neither FOLFIRINOX nor GNP | 16 (6.0) |
| Supportive care only | 87 (32.6) |
Data are presented as median (interquartile range) or number (%).
CA19-9, carbohydrate antigen 19-9; RPC, resectable pancreatic cancer; BRPC, borderline resectable pancreatic cancer; PD, pancreaticoduodenectomy; IPMN, intraductal papillary mucinous neoplasm; FOLFIRINOX, combination of 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin; GNP, gemcitabine plus nab-paclitaxel.
*T and N stages were assessed based on the 8th edition of American Joint Committee on Cancer staging system.
3. Clinical factors affecting survival outcomes and survival prediction scores
In the multivariable Cox regression analysis in single-center data, an age ≤70 years old, R0 resection, lower N stage, adjuvant treatment, and no recurrence followed by palliative chemotherapy with FOLFIRINOX or GNP after recurrence were significantly associated with a better OS (Table 2, Supplementary Table 1). The survival prediction scores for OS were calculated using the β coefficients, and the weighted scores were calculated by dividing each β coefficient by the smallest β coefficient (0.325) and rounded to the nearest integer. The weighted scores were as follows: >70 years old, 1; elevated serum CA19-9 at diagnosis, 1; R1 resection, 1; N1, 1; N2, 3; no adjuvant treatment, 2; FOLFIRINOX or GNP after recurrence, 4; other chemotherapy or supportive care after recurrence, 5. The c-index of this model was 0.723. The total score was calculated as indicated in Formula 1 shown below:
Table 2.
Multivariate Cox Regression Analysis for Overall Survival
| Clinical value | No. of patients |
Multivariate analysis | Survival prediction score† | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | β coefficient | Score‡ | |||
| Age | ||||||
| ≤70 yr (Ref) | 144 | 0 | ||||
| >70 yr | 123 | 1.57 (1.17–2.12) | 0.003 | 0.455 | 1 | |
| Serum CA19-9 at diagnosis | ||||||
| <37 U/mL (Ref) | 79 | 0 | ||||
| ≥37 U/mL | 188 | 1.50 (1.07–2.11) | 0.019 | 0.404 | 1 | |
| Resectability | ||||||
| RPC (Ref) | 232 | |||||
| BRPC | 35 | 1.25 (0.84–1.85) | 0.274 | |||
| Surgical margins | ||||||
| R0 (Ref) | 202 | 0 | ||||
| R1 | 65 | 1.41 (1.02–1.95) | 0.039 | 0.419 | 1 | |
| T stage* | ||||||
| T1 (Ref) | 30 | |||||
| T2 | 168 | 1.06 (0.57–1.96) | 0.858 | |||
| T3 | 69 | 1.44 (0.75–2.76) | 0.277 | |||
| N stage† | ||||||
| N0 (Ref) | 81 | 0 | ||||
| N1 | 114 | 1.36 (0.92–2.02) | 0.126 | 0.325§ | 1 | |
| N2 | 72 | 2.35 (1.50–3.70) | <0.001 | 0.953 | 3 | |
| Angiolymphatic invasion | ||||||
| No (Ref) | 98 | |||||
| Yes | 169 | 1.14 (0.81–1.61) | 0.443 | |||
| Perineural invasion | ||||||
| No (Ref) | 28 | |||||
| Yes | 239 | 0.96 (0.54–1.72) | 0.894 | |||
| Adjuvant treatment | ||||||
| Yes (Ref) | 185 | 0 | ||||
| No | 82 | 1.69 (1.21–2.35) | 0.002 | 0.629 | 2 | |
| Palliative chemotherapy after recurrence | ||||||
| No recurrence (Ref) | 64 | 0 | ||||
| FOLFIRNOX or GNP | 100 | 2.78 (1.63–4.76) | <0.001 | 1.150 | 4 | |
| Neither FOLFIRINOX nor GNP | 16 | 4.79 (2.33–9.88) | <0.001 | 1.620 | 5 | |
| Supportive care only | 87 | 4.96 (2.89–8.52) | <0.001 | 1.690 | 5 | |
HR, hazard ratio; CI, confidence interval; CA19-9, carbohydrate antigen 19-9; RPC, resectable pancreatic cancer; BRPC, borderline resectable pancreatic cancer; FOLFIRINOX, combination of 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin; GNP, gemcitabine plus nab-paclitaxel.
*T and N stages were assessed based on the 8th edition of American Joint Committee on Cancer staging system; †Harrell’s c-index by bootstrap validation (resampling, n=1,500)=0.723; ‡The weighted scores were calculated by dividing each β coefficient by the smallest §β coefficient (0.325) and rounding to the nearest integer.
Formula 1. Survival prediction score of OS.
Total score for OS=(1×>70 year old) + (1×elevated serum CA19-9 at diagnosis) + (1×R1 resection) + (1×N1 or 3×N2 stage) + (2×no adjuvant treatment) + (4×[FOLFIRINOX or GNP after recurrence] or 5×[other chemotherapy or supportive care after recurrence])
The median OS progressively decreased with higher total survival prediction scores for OS (Supplementary Fig. 1A).
R0 resection, lower T and N stage, no angiolymphatic invasion, and adjuvant treatment were significantly associated with better RFS in multivariate analyses (Supplementary Tables 2 and 3). The survival prediction score for RFS was calculated using the above methods. The survival prediction scores for RFS were calculated using the β coefficients, and the weighted scores were calculated by dividing each β coefficient by the smallest β coefficient (0.477) and rounded to the nearest integer. The weighted scores were as follows: R1 resection, 1; T1, 1; T2, 2; N1, 1; N2, 2; angiolymphatic invasion, 1; and no adjuvant treatment, 1. The c-index of this model was 0.709. The total score was calculated as indicated in Formula 2 shown below:
Formula 2. Survival prediction score for RFS.
Total score for RFS= (1×R1 resection) + (1×N1 or 2×N2 stage) + (1×T2 or 2×T3) + (1×angiolymphatic invasion) + (1×no adjuvant treatment)
The RFS progressively decreased with higher total survival prediction scores for RFS (Supplementary Fig. 1B).
4. Changes in clinicopathologic factors between two periods
The following clinicopathologic factors were increased between the two periods (2013–2015 vs 2016–2018); resectable PC, R0 surgical margin, lower T stage, adjuvant treatment, and palliative chemotherapy with FOLFIRINOX or GNP. The 90-day mortality (1.7% vs 1.4%, p>0.999) did not differ between the two periods. The median length of hospital stay after surgery was significantly different between the two periods (12 days vs 8 days, p<0.001) (Table 3).
Table 3.
Changes in Clinicopathologic Factors between Two Periods
| Clinical value | 2013–2015 (n=119) | 2016–2018 (n=148) | Total (n=267) | p-value |
|---|---|---|---|---|
| Age | 0.965 | |||
| ≤70 yr | 64 (53.8) | 80 (54.1) | 144 (53.9) | |
| >70 yr | 55 (46.2) | 68 (45.9) | 123 (46.1) | |
| Serum CA19-9 at diagnosis | 0.955 | |||
| <37 U/mL | 35 (29.4) | 44 (29.7) | 79 (29.6) | |
| ≥37 U/mL | 84 (70.6) | 104 (70.3) | 148 (70.4) | |
| Resectability | <0.001 | |||
| RPC | 93 (78.2) | 139 (93.9) | 232 (86.9) | |
| BRPC | 26 (21.8) | 9 (6.1) | 35 (13.1) | |
| Surgical margins | 0.044 | |||
| R0 | 83 (69.7) | 119 (80.4) | 202 (75.7) | |
| R1 | 36 (30.3) | 29 (19.6) | 65 (24.3) | |
| T stage* | 0.047 | |||
| T0 | 10 (8.4) | 20 (13.5) | 30 (11.2) | |
| T1 | 70 (58.8) | 98 (66.2) | 168 (62.9) | |
| T2 | 39 (32.8) | 30 (20.3) | 69 (25.8) | |
| N stage* | 0.161 | |||
| N0 | 37 (31.1) | 44 (29.7) | 81 (30.3) | |
| N1 | 44 (37.0) | 70 (47.3) | 114 (42.7) | |
| N2 | 38 (31.9) | 34 (23.0) | 72 (27.0) | |
| Angiolymphatic invasion | 0.736 | |||
| Yes | 74 (62.2) | 95 (64.2) | 169 (63.3) | |
| No | 45 (37.8) | 53 (35.8) | 98 (36.7) | |
| Adjuvant treatment | 0.047 | |||
| Yes | 75 (63.0) | 110 (74.3) | 185 (69.3) | |
| No | 44 (37.0) | 38 (27.5) | 82 (30.7) | |
| Palliative chemotherapy after recurrence | <0.001 | |||
| No recurrence | 23 (19.3) | 41 (27.7) | 64 (24.0) | |
| FOLFIRINOX | 22 (18.5) | 48 (32.4) | 70 (26.2) | |
| GNP | 8 (6.7) | 22 (14.9) | 30 (11.2) | |
| Neither FOLFIRINOX nor GNP | 13 (10.9) | 3 (2.0) | 16 (6.0) | |
| Supportive care only | 53 (44.5) | 34 (23.0) | 87 (32.6) | |
| Follow-up period, mo | 20.9 (11.3–42.7) | 32.2 (15.6–44.5) | 26.6 (13.9–44.0) | 0.055 |
| Length of hospital stay after surgery | 12.0 (9.5–17.0) | 8.0 (7.0–11.0) | 10.0 (8.0–14.0) | <0.001 |
| 90-day mortality rate after surgery | 2 (1.7) | 2 (1.4) | 4 (1.5) | 1.000 |
Data are presented as number (%) or median (interquartile range).
CA19-9, carbohydrate antigen 19-9; RPC, resectable pancreatic cancer; BRPC, borderline pancreatic cancer; FOLFIRINOX, combination of 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin; GNP, gemcitabine with nab-paclitaxel regimen.
*T and N stages were assessed based on the 8th edition of American Joint Committee on Cancer staging system.
5. Changes in survival prediction score between the two periods
The total survival prediction score for OS showed a significant decrease between 2013–2015 and 2016–2018 (mean: 7.32 vs 6.18, p=0.004) (Table 4). Of the six factor scores for OS, there was a significant decrease in the surgical margin status score (mean: 0.30 vs 0.20, p=0.044), adjuvant treatment score (mean: 0.74 vs 0.51, p=0.047), and palliative chemotherapy after recurrence score (mean: 3.78 vs 3.14, p<0.001) between 2013–2015 and 2016–2018. Similarly, the total survival prediction score for RFS significantly decreased between the two periods (mean: 3.55 vs 3.09, p=0.028) (Supplementary Table 4). Of the five factor scores for RFS, there was a significant decrease in surgical margin status score (mean: 0.30 vs 0.20, p=0.044), T stage score (mean: 1.24 vs 1.07, p=0.015), and adjuvant treatment score (mean: 0.37 vs 0.26, p=0.047).
Table 4.
Changes in the Score of the Survival Prediction Score for Overall Survival between Two Periods
| Survival prediction score | 2013–2015 (n=119) | 2016–2018 (n=148) | Total (n=267) | p-value |
|---|---|---|---|---|
| Age group | 0.46±0.50 | 0.46±0.50 | 0.46±0.50 | 0.965 |
| Serum CA19-9 at diagnosis | 0.71±0.46 | 0.70±0.46 | 0.70±0.46 | 0.955 |
| Surgical margins | 0.30±0.46 | 0.20±0.40 | 0.24±0.43 | 0.044 |
| N stage* | 1.33±1.22 | 1.16±1.09 | 1.24±1.15 | 0.431 |
| Adjuvant treatment | 0.74±1.91 | 0.51±0.88 | 0.61±0.92 | 0.047 |
| Palliative chemotherapy after recurrence | 3.78±1.91 | 3.14±1.99 | 3.43±1.98 | <0.001 |
| Total score | 7.32±2.95 | 6.18±3.08 | 6.69±3.07 | 0.004 |
Data are presented as mean±SD.
CA19-9, carbohydrate antigen 19-9.
*N stage was assessed based on the 8th edition of American Joint Committee on Cancer staging system.
DISCUSSION
We analyzed the difference in survival in patients with PC who underwent upfront surgery between 2013–2015 and 2016–2018 and determined which recent changes in clinical management were responsible. Our study data showed improved survival over time at the national level and a similar trend in a single-center dataset. Six clinical factors (age, serum CA19-9 at diagnosis, resection margin, nodal stages, adjuvant treatment, and recurrence with palliative chemotherapy) affected survival to varying degrees. Of them, the difference in strict selection of surgical candidates, more use of adjuvant treatment, and recent combination palliative chemotherapy after recurrence were considered to contribute to the recent improvement.
The 2023 National Comprehensive Cancer Network guidelines consider imaging findings, elevated serum CA19-9, large primary tumors, large regional lymph nodes, excessive weight loss, and extreme pain as high-risk features.21 These factors affect the selection of appropriate patients for upfront surgery. Since recent studies suggested that NAT leads to better survival in patients with BRPC based on imaging criteria,6,7 some studies reported that the ratio of upfront surgeries has decreased in patients with BRPC.8 Similarly, our study revealed that the proportion of BRPC decreased, while R0 resection increased between the two periods, and these factors were regarded to contribute to the recent improvement in RFS and OS.22,23 Moreover, serum CA19-9 at diagnosis is associated with nodal involvement and margin status positivity after surgery.24 However, our results revealed that the level of serum CA19-9 at diagnosis did not differ between the two periods. In terms of tumor size, stage T3 (>4 cm) was decreased over the periods in our study. It has been reported that tumor size is a reliable predictor of early recurrence, and our study showed similar results.25 However, T stage was not significant in multivariate OS analysis but only in univariate OS analysis. This finding was compatible with the previous study which reported the limitation of the current T-stage protocol.26 Since the ESPAC-1 trial, adjuvant treatment after curative resection has been the standard of care for decades.27 In the Netherlands national cohort, the adjuvant chemotherapy ratio gradually increased (2001–2004 vs 2005–2008 vs 2009–2012 vs 2013–2016; 6.8% vs 21.1% vs 49.5% vs 56.2%).28 A recent cohort study in China reported similar trends (2012–2015 vs 2016–2017 vs 2018–2019; 41.2% vs 55.3% vs 58.1%),9 which were compatible with our results (2013–2015 vs 2016–2018; 63.0% vs 74.3%). Regarding the adjuvant treatment regimen, most patients in the present study received gemcitabine adjuvant chemotherapy. Only 14 patients received the gemcitabine plus capecitabine regimen as an adjuvant treatment because it was approved in 2017.12 Furthermore, no patient received the modified FOLFIRINOX regimen as it was approved in Korea in 2019.11
In the present study, patients treated with FOLFIRINOX or GNP after recurrence exhibited better survival than those treated with other regimens or supportive care only, and the proportion of these patients increased. This finding is consistent with the longer median survival in the control group (gemcitabine group) in the recent PRODIGE 24 and APACT trials (median OS: 35.5 and 37.7 months) than in patients treated with gemcitabine in the CONKO-1 trial (median OS: 20.2 months).10,29,30 Based on the findings of our study and recent studies, recent palliative combination chemotherapy may also contribute to improved survival in patients with recurrence.
Some studies have analyzed survival trends and their contributing factors.31,32 These investigations presented each factor’s change and its degree of risk separately but did not perform a comprehensive, integrative analysis of all factors. Furthermore, despite the role of palliative chemotherapy as an essential factor in OS, it was often neglected. Thus, our study comprehensively analyzed the changes in factors contributing to improving survival, including palliative chemotherapy after recurrence. Then, an intuitive survival prediction scoring model was developed, and the survival contribution of each factor was quantitatively evaluated.
This study has several limitations. First, the HIRA data did not include detailed clinicopathologic information. Therefore, we could not compare the clinicopathologic changes in this nationwide database. Second, the follow-up period was not sufficient to estimate OS in the HIRA dataset from 2016 to 2018, and the median OS in this dataset was not reached. In addition, the median OS in the HIRA data may have been overestimated owing to the operational definitions instead of actual data. However, the comparison of two datasets was possible because it was analyzed by the log-rank test which eliminated the censored data, and the difference of OS in the HIRA data was compatible with that in single-center data. Consequently, very similar survival trends were identified in the two datasets in our study (HIRA database and data from a single center). Third, a retrospective study using single-center tertiary data may show selection bias due to an absence of external validation. However, we attempted to compensate for this limitation using bootstrap resampling methods. Fourth, none of the patients in the single-center dataset received modified FOLFIRINOX as an adjuvant treatment. Therefore, we could not observe the effect of a recent adjuvant chemotherapy regimen, and further study is warranted to reflect recent changes.
Regarding the survival trends in patients with resected PC, we presented the recent improvement over time at the national level and suggested weighting several clinical factors to predict OS and RFS. Additionally, we showed the changes in clinicopathologic parameters and risk scores over time. Our study could provide insight into the management strategies of early-stage PC.
ACKNOWLEDGEMENTS
This study used the Korean Health Insurance Review and Assessment Service (HIRA) research data (M20210105918) made by the HIRA. The views expressed are those of the author(s) and not necessarily those of the HIRA and the Ministry of Health and Welfare.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study concept and design: J.H.J., J.K. Data acquisition: K.J., J.S.L., J.C.L., J.W.K., Y.S.Y., J.H.H., H.S.H. Data analysis and interpretation: S.H.W. Drafting of the manuscript: J.H.J. Critical revision of the manuscript for important intellectual content: J.H.H. Statistical analysis: S.H.W. Administrative, technical, or material support; study supervision: J.K. Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Supplementary materials can be accessed at https://doi.org/10.5009/gnl230303.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Park W, Chawla A, O'Reilly EM. Pancreatic cancer: a review. JAMA. 2021;326:851–862. doi: 10.1001/jama.2021.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aaltonen P, Carpén O, Mustonen H, et al. Long-term nationwide trends in the treatment of and outcomes among pancreatic cancer patients. Eur J Surg Oncol. 2022;48:1087–1092. doi: 10.1016/j.ejso.2021.11.116. [DOI] [PubMed] [Google Scholar]
- 4.Blackford AL, Canto MI, Klein AP, Hruban RH, Goggins M. Recent trends in the incidence and survival of stage 1A pancreatic cancer: a surveillance, epidemiology, and end results analysis. J Natl Cancer Inst. 2020;112:1162–1169. doi: 10.1093/jnci/djaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strobel O, Neoptolemos J, Jäger D, Büchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16:11–26. doi: 10.1038/s41571-018-0112-1. [DOI] [PubMed] [Google Scholar]
- 6.Murphy JE, Wo JY, Ryan DP, et al. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2018;4:963–969. doi: 10.1001/jamaoncol.2018.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Versteijne E, van Dam JL, Suker M, et al. neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the Dutch randomized PREOPANC trial. J Clin Oncol. 2022;40:1220–1230. doi: 10.1200/JCO.21.02233. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhari VA, Mitra A, Gupta V, et al. Neoadjuvant therapy in borderline resectable pancreatic cancer: outcomes in the era of changing practices and evolving evidence. Surgery. 2022;171:1388–1395. doi: 10.1016/j.surg.2021.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Zhang G, Li B, Yin X, et al. Systemic therapy and perioperative management improve the prognosis of pancreatic ductal adenocarcinoma: a retrospective cohort study of 2000 consecutive cases. Int J Surg. 2022;104:106786. doi: 10.1016/j.ijsu.2022.106786. [DOI] [PubMed] [Google Scholar]
- 10.Conroy T, Castan F, Lopez A, et al. Five-year outcomes of FOLFIRINOX vs gemcitabine as adjuvant therapy for pancreatic cancer: a randomized clinical trial. JAMA Oncol. 2022;8:1571–1578. doi: 10.1001/jamaoncol.2022.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 12.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 13.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 14.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:2128–2137. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latenstein AEJ, Mackay TM, van der Geest LGM, et al. Effect of centralization and regionalization of pancreatic surgery on resection rates and survival. Br J Surg. 2021;108:826–833. doi: 10.1093/bjs/znaa146. [DOI] [PubMed] [Google Scholar]
- 17.van Roessel S, Kasumova GG, Verheij J, et al. International validation of the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system in patients with resected pancreatic cancer. JAMA Surg. 2018;153:e183617. doi: 10.1001/jamasurg.2018.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan LM, Massaro JM, D'Agostino RB., Sr Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23:1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 19.Alonzo TA. Clinical prediction models: a practical approach to development, validation, and updating: by Ewout W. Steyerberg. Am J Epidemiol. 2009;170:528. doi: 10.1093/aje/kwp129. [DOI] [Google Scholar]
- 20.Wolbers M, Koller MT, Witteman JC, Steyerberg EW. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. 2009;20:555–561. doi: 10.1097/EDE.0b013e3181a39056. [DOI] [PubMed] [Google Scholar]
- 21.National Comprehensive Cancer Network (NCCN), author NCCN Guidelines Version 2.2023 Pancreatic Adenocarcinoma [Internet] NCCN; Plymouth Meeting: c2023. [cited 2023 Aug 3]. Available from: https://www.nccn.org/search-result?indexCatalogue=nccn-search-index&searchQuery=Pancreatic%20Adenocarcinoma . [Google Scholar]
- 22.Versteijne E, Suker M, Groothuis K, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J Clin Oncol. 2020;38:1763–1773. doi: 10.1200/JCO.19.02274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machairas N, Raptis DA, Velázquez PS, et al. the impact of neoadjuvant treatment on survival in patients undergoing pancreatoduodenectomy with concomitant portomesenteric venous resection: an international multicenter analysis. Ann Surg. 2021;274:721–728. doi: 10.1097/SLA.0000000000005132. [DOI] [PubMed] [Google Scholar]
- 24.Coppola A, La Vaccara V, Farolfi T, et al. Role of CA 19.9 in the management of resectable pancreatic cancer: state of the art and future perspectives. Biomedicines. 2022;10:2091. doi: 10.3390/biomedicines10092091.6f3d58e6c0b249b68f74e53ee5f0fdc3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saka B, Balci S, Basturk O, et al. Pancreatic ductal adenocarcinoma is spread to the peripancreatic soft tissue in the majority of resected cases, rendering the AJCC T-stage protocol (7th edition) inapplicable and insignificant: a size-based staging system (pt1: ≤2, pt2: >2-≤4, pt3: >4 cm) is more valid and clinically relevant. Ann Surg Oncol. 2016;23:2010–2018. doi: 10.1245/s10434-016-5093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe G, Ushida Y, Oba A, et al. Impact of tumor size on the outcomes of patients with resectable distal pancreatic cancer: lessons learned from a series of 158 radical resections. Ann Surg Oncol. 2022;29:378–388. doi: 10.1245/s10434-021-10560-7. [DOI] [PubMed] [Google Scholar]
- 27.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 28.Latenstein AEJ, van der Geest LGM, Bonsing BA, et al. Nationwide trends in incidence, treatment and survival of pancreatic ductal adenocarcinoma. Eur J Cancer. 2020;125:83–93. doi: 10.1016/j.ejca.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Tempero MA, Pelzer U, O'Reilly EM, et al. Adjuvant nab-paclitaxel + gemcitabine in resected pancreatic ductal adenocarcinoma: results from a randomized, open-label, phase iii trial. J Clin Oncol. 2023;41:2007–2019. doi: 10.1200/JCO.22.01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 31.Hank T, Hinz U, Reiner T, et al. A pretreatment prognostic score to stratify survival in pancreatic cancer. Ann Surg. 2022;276:e914–e922. doi: 10.1097/SLA.0000000000004845. [DOI] [PubMed] [Google Scholar]
- 32.Habib JR, Kinny-Köster B, Bou-Samra P, et al. Surgical decision-making in pancreatic ductal adenocarcinoma: modeling prognosis following pancreatectomy in the era of induction and neoadjuvant chemotherapy. Ann Surg. 2023;277:151–158. doi: 10.1097/SLA.0000000000004915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.