Abstract
Background/Aims
Endoscopic papillectomy (EP) is increasingly used as an alternative to surgery for managing benign ampullary neoplasms. However, post-EP resection margins are often positive or indeterminate, and there is no consensus on the management of ampullary adenomas with positive or indeterminate margins after EP. This study was designed to compare the long-term outcomes between resected margin-negative (RMN) and resected margin-positive/indeterminate (RMPI) groups and to identify factors associated with clinical outcomes.
Methods
This retrospective analysis included patients with ampullary adenoma without evidence of adenocarcinoma who underwent EP between 2004 and 2016. The RMN and RMPI groups were compared for recurrence rates and recurrence-free duration during a mean follow-up duration of 71.7±39.8 months. Factors related to clinical outcomes were identified using multivariate analysis.
Results
Of the 129 patients who underwent EP, 82 were in the RMN group and 47 were in the RMPI group. The RMPI group exhibited a higher recurrence rate compared to the RMN group (14.6% vs 34.0%, p=0.019). However, the recurrence-free duration was not significantly different between the groups (34.7±32.6 months vs 36.2±27.4 months, p=0.900). Endoscopic treatment successfully managed recurrence in both groups (75% vs 75%). Submucosal injection was a significant risk factor for residual lesions (hazard ratio, 4.11; p=0.009) and recurrence (hazard ratio, 2.57; p=0.021).
Conclusions
Although ampullary adenomas with positive or indeterminate margins after EP showed a higher rate of recurrence at long-term follow-up, endoscopic treatment was effective with favorable long-term outcomes. Submucosal injection prior to resection was associated with increased risk of recurrence and residual lesions.
Keywords: Endoscopic papillectomy, Ampulla of Vater, Adenoma, Prognosis
INTRODUCTION
Ampulla of Vater (AOV) tumors are relatively rare diseases,1 and among the tumors arising from the AOV, ampullary adenomas are the most common type.2 Due to their malignant potential, resection is recommended for ampullary adenomas.3 In the past, surgical resection such as pancreaticoduodenectomy and local surgical resection were the preferred treatment methods for ampullary neoplasms. However, surgical treatment is associated with high morbidity and mortality rates.4-6 In recent years, endoscopic papillectomy (EP) has emerged as an effective and less invasive alternative treatment for ampullary adenomas.7-9 Despite the clear advantages of EP over surgery, such as less invasiveness, there are some limitations with EP. Recurrence rates have been reported to be as high as 33%,10,11 and optimal papillectomy techniques and strategies for managing recurrences need to be determined.
The resection margins of ampullary tumors after EP are often positive or indeterminate due to the complex anatomy of the ampulla, making endoscopic resection difficult and leading to incomplete resection, local recurrence, and interval cancer. Moreover, evaluating the margins of resected ampullary adenoma specimens pathologically is challenging due to the burning effect of EP.12 Although previous studies have demonstrated the feasibility of EP for ampullary adenomas, they have been limited by short follow-up durations of 6 months to 3 years.8,13,14 Consequently, the long-term outcomes of ampullary adenomas according to resected margin status and proper management of resected margin-positive or indeterminate cases remain unclear. It is also controversial whether additional surgery is necessary for all patients with adenoma displaying a positive or indeterminate margin. Lastly, there is no consensus on the management of ampullary adenomas with positive or indeterminate margins after EP. Therefore, this study aimed to compare the long-term outcomes of the resected margin-negative (RMN) group and resected margin-positive/indeterminate (RMPI) group and identify the risk factors of local recurrence and residual lesions.
MATERIALS AND METHODS
1. Patients
Patients with ampullary adenoma who underwent EP at Asan Medical Center (Seoul, South Korea) between March 2004 and March 2016 were retrospectively reviewed. The inclusion criteria were patients who (1) had ampullary adenoma diagnosed with adenoma in resected specimens, (2) had macroscopic complete resection of the ampullary adenoma, and (3) had follow-up data for more than 2 years. Patients with other tumor types on the final pathologic result or insufficient follow-up duration (<2 years) were excluded. The patients were categorized into the RMN group and the RMPI group. The Institutional Review Board of Asan Medical Center approved this study (IRB number: 2023-0028). The informed consent was waived.
2. EP procedure
All procedures were performed by gastroenterologists (D.W.S., T.J.S., and D.O.) experienced in endoscopic retrograde cholangiopancreatography. Prophylactic antibiotics were given to all patients, and conscious sedation was achieved with midazolam or propofol and meperidine. The entire endoscopic procedure was performed using a standard side-view duodenoscope (TJF-260V; Olympus, Tokyo, Japan). Tumor resection was performed using a polypectomy snare (Captivator snare; Boston Scientific, Marlborough, MA, USA) and a blended current (Endocut-Q mode, effect 3, duration 2, interval 5) from the electrosurgical unit (VIO 300D; ERBE Elektromedizin GmbH, Tübingen, Germany). Patients with ampullary tumor were first assessed by a duodenoscope with endoscopic biopsies for histological confirmation of tumor. After exclusion of AOV cancer, to examine its characteristics and resectability, laboratory tests and computed tomography were also undertaken. In case where significant intraductal extension (>10 mm) was suspected, endoscopic ultrasound or magnetic resonance cholangiopancreatography was performed to assess extent and depth of tumor. The techniques of EP in our institution and endoscopic images of the resection surface are shown in Fig. 1 and Supplementary Video 1. The tumor was grasped from the oral to the anal side of the major papilla. Submucosal injection using normal saline mixed with epinephrine was performed at the discretion of the endoscopist. Resection was performed in the en bloc fashion when feasible. In cases where en bloc resection was impossible due to large tumor size or snaring failure, piecemeal resection and/or argon plasma coagulation (APC) was performed. Prophylactic pancreatic or biliary stents were inserted at the discretion of the attending endoscopist.
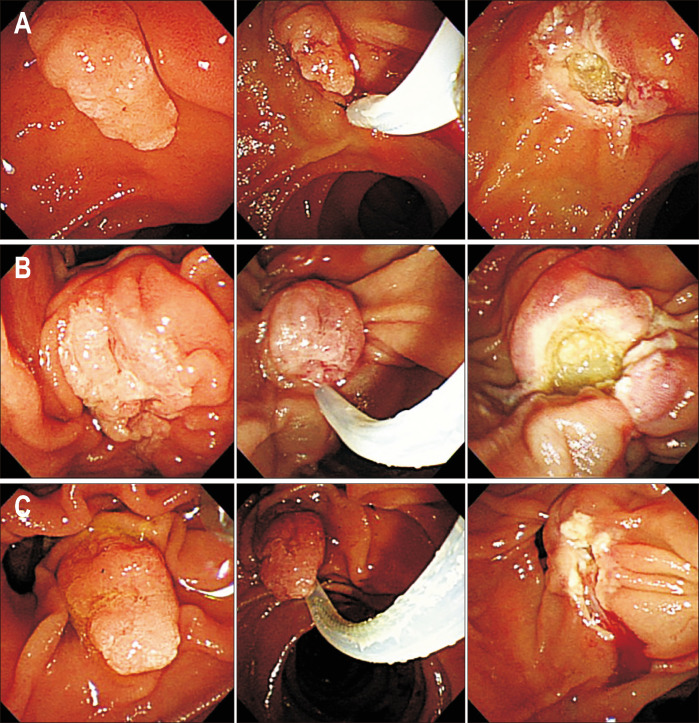
Fig. 1.
Endoscopic papillectomy: (A) a negative resection margin, (B) a positive resection margin, and (C) an indeterminate resection margin.
3. Histopathologic evaluation of resected specimens
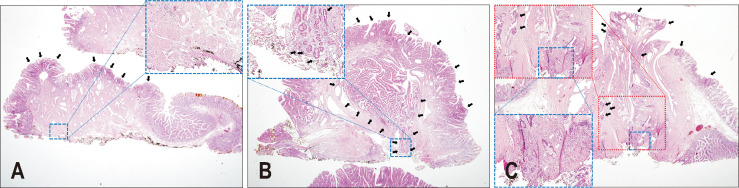
One experienced pathologist (S.M.H.), who specializes in pancreatobiliary pathology, re-examined the resected EP specimens to analyze the following histologic features: tumor size, growth pattern (tubular, villotubular, and villous), degree of dysplasia, and resection margin status. The degree of dysplasia was classified as low- or high-grade based on structural complexity, nuclear stratification, and nuclear atypia. High-grade dysplasia was classified when dysplastic cells displayed an increased degree of structural complexity, nuclear stratification, and nuclear atypia. The lateral and deep resection margins of the pathological stump were assessed and classified as positive, negative, or indeterminate. Representative histologic images are depicted in Fig. 2. A negative margin referred to cases in which no dysplastic cells were present on any of the lateral or deep margins (Fig. 2A); conversely, a positive margin referred to cases in which any grade dysplastic cells were present on any of the lateral or deep margins (Fig. 2B). Indeterminate resection margin referred to cases in which the involvement of the margin could not be because the presence of dysplastic cells could not be evaluated, such as with nuclear elongation caused by electric coagulation artifact (Fig. 2C).
Fig. 2.
Representative histologic images of tubular adenomas with resection marginal status of endoscopic papillectomy specimens (hematoxylin and eosin stain, ×10). Tubular adenoma with (A) a negative margin. Dysplastic cells (black arrows) are on the duodenal mucosal surface. Blue inset: medium-power magnification (×40) of the deep resection margin (black ink). No dysplastic cells were present on the margin. Tubular adenoma with (B) a positive margin. Dysplastic cells (black arrows) were continuously present on the duodenal mucosal surface, intra-ampulla, and deep resection margin (black ink). Blue inset: medium-power magnification (×40) of the deep resection margin (black ink). Tubular adenoma with (C) an indeterminate resection margin. Dysplastic cells (black arrows) were discontinuously present on the duodenal mucosal surface and intra-ampulla. Similar atypical cells with nuclear elongation were present on the deep resection margin (black ink). However, thermal artifacts (white arrows) led to nuclear pseudo-elongation, which prohibited differentiating with true dysplastic cells on the resection margin. Red inset: medium-power magnification (×40) of the deep resection margin (black ink). Blue inset: high-power magnification (×100) of the deep resection margin in the red inset (black ink).
4. Follow-up
All patients who underwent EP underwent follow-up endoscopy at 1 month, 3 months, 6 months, and subsequently every 6 months or yearly for at least 5 years. The follow-up examinations included blood tests, duodenoscopy with surveillance biopsy, and computed tomography scan. Computed tomography scans were performed at 3, 6, and 12 months after EP and repeated annually thereafter. In cases where residual or recurrent tumors were identified during follow-up duodenoscopy, endoscopic biopsies were performed. Additional treatment, such as surgical resection or endoscopic treatment, was considered when the biopsies showed residual or recurrent tumors.
5. Definitions and outcome measurements
Definitions of remission and recurrence varied among previous studies.7,8,10,15-17 Based on previous studies, the following definitions were used. Remission was defined as no residual lesion confirmed by endoscopy with biopsy ≥6 months after the initial EP. Residual adenoma was defined as the endoscopic evidence of adenomatous tissue (macroscopic or microscopic) during endoscopic follow-up within 6 months after the initial EP. Recurrence was defined as detecting adenomatous tissue at the resection site after achieving remission. The recurrence-free duration was defined as the time interval between remission and recurrence. The following outcome parameters were evaluated: recurrence rate, recurrence-free duration, time to recurrence, cancer development, and potential risk factors.
6. Statistical analysis
Statistical analyses were performed using R software version 3.3.4 (The R Foundation for Statistical Computing, Vienna, Austria). For intergroup comparison, the chi-square test or Fisher exact test was used for categorical variables, and the Student t-test was used for continuous variables. Cumulative recurrence rate was depicted using the Kaplan-Meier analysis. Univariate and multivariate analyses of potential risk factors for residual lesions and recurrence were performed using the Cox proportional hazards analysis. Multivariate analyses included factors with p<0.20 in the univariate analysis. A two-sided p-value of <0.05 was considered statistically significant.
RESULTS
1. Patient characteristics
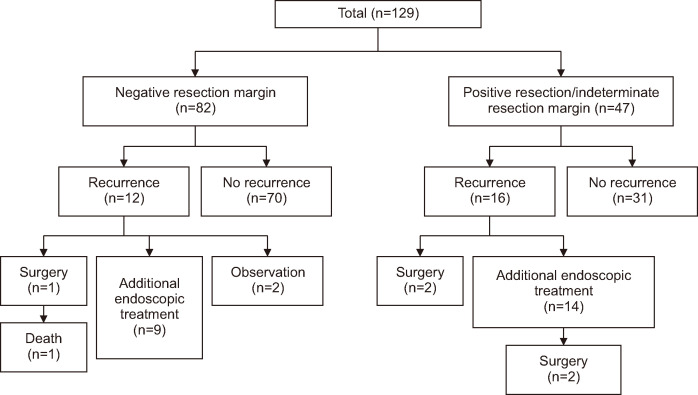
A total of 129 patients were included in this study. Patient demographics are shown in Table 1. Of these patients, 82 were included in the RMN group, and 47 were included in the RMPI group (Fig. 3). The mean age of the patients was 56.2±10.8 years, and there were no significant differences in demographics between the two groups (RMN and RMPI group).
Table 1.
Patient Demographics and Procedural Characteristics
| Characteristic | Negative (n=82) | Positive/indeterminate (n=47) | Total (n=129) | p-value |
|---|---|---|---|---|
| Patient demographics | ||||
| Male sex | 58 (70.7) | 29 (61.7) | 87 (67.4) | 0.391 |
| Age, yr | 56.6±10.5 | 55.6±11.3 | 56.2±10.8 | 0.581 |
| FAP | 3 (3.7) | 1 (2.1) | 4 (3.1) | >0.999 |
| Procedural characteristics | ||||
| Ampulla | 0.415 | |||
| Major | 79 (96.3) | 47 (100) | 126 (97.7) | |
| Major and minor | 2 (2.4) | 0 | 2 (1.6) | |
| Minor | 1 (1.2) | 0 | 1 (0.8) | |
| Tumor size (pathologic specimen), cm | 1.5±0.8 | 1.4±0.5 | 1.5±0.7 | 0.353 |
| Growth pattern | 0.688 | |||
| Tubular adenoma | 69 (84.1) | 37 (78.7) | 106 (82.2) | |
| Villotubular adenoma | 10 (12.2) | 7 (14.9) | 17 (13.2) | |
| Villous adenoma | 3 (3.7) | 3 (6.4) | 6 (4.7) | |
| Histologic grade | 0.536 | |||
| High-grade dysplasia | 19 (23.2) | 14 (29.8) | 33 (25.6) | |
| Low-grade dysplasia | 63 (76.8) | 33 (70.2) | 96 (74.4) | |
| Type of resection | 0.814 | |||
| En bloc | 74 (90.2) | 41 (87.2) | 115 (89.1) | |
| Piecemeal | 8 (9.8) | 6 (12.8) | 14 (10.9) | |
| Intraductal extension | 2 (2.4) | 1 (2.1) | 3 (2.3) | >0.999 |
| Submucosal injection | 32 (39.0) | 21 (44.7) | 53 (41.1) | 0.658 |
| Additional ablation (APC) | 36 (43.9) | 19 (40.4) | 55 (42.6) | 0.842 |
| Pancreatic stenting | 46 (56.1) | 30 (63.8) | 76 (58.9) | 0.501 |
| Biliary stenting | 28 (34.1) | 20 (42.6) | 48 (37.2) | 0.446 |
Data are presented as number (%) or mean±SD.
FAP, familial adenomatous polyposis; APC, argon plasma coagulation.
Fig. 3.
Flowchart of study patients.
2. Procedural outcomes
Procedural characteristics are summarized in Table 1. En bloc resection was achieved in 115 patients (89.1%) and piecemeal resection was performed in 14 patients (10.9%). Prophylactic pancreatic duct stents were placed in 76 patients (58.9%) and biliary stents were placed in 48 patients (37.2%). Submucosal injection prior to resection was performed in 53 patients (41.1%). Additional ablation was performed in 55 patients (42.6%) for residual lesions or intra-procedural bleeding at the time of initial EP. The two groups (RMN and RMPI group) did not show significant differences in the rates of submucosal injection prior to resection (39.0% vs 44.7%, p=0.658), en bloc resection (90.2% vs 87.2%, p=0.81), prophylactic pancreatic stent placement (56.1% vs 63.8%, p=0.501), and biliary stent placement (34.1% vs 42.6%, p=0.446). The rate of additional ablation was also similar between the two groups (43.9% vs 40.4%, p=0.842). Regarding histopathological findings, there were no significant differences in the mean tumor size of the pathologic specimen (1.5±0.8 cm vs 1.4±0.5 cm, p=0.353), histologic grade, or final pathology between the two groups.
3. Clinical outcomes
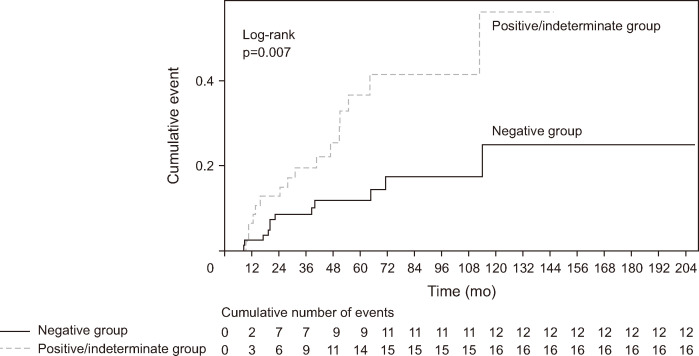
The clinical outcomes of the patients are presented in Table 2. The mean follow-up duration did not significantly differ between the RMN group and the RMPI group (72.7±41.3 months vs 69.9±37.6 months, p=0.700). The RMPI group showed a higher recurrence rate compared with the RMN group (14.6% [12/82] vs 34% [16/47], p=0.019). However, the mean interval to recurrence did not significantly differ between the two groups (34.7±32.6 months vs 36.2±27.4 months, p=0.900). The recurrence-free duration (34.7±32.6 months vs 36.2±27.4 months, p=0.900), residual lesion rate (8.5% vs 19.1%, p=0.138), and the total number of procedures required (1.3±1.0 vs 1.7±1.6, p=0.121) did not significantly differ between the two groups as well. Recurrent tumors were successfully managed endoscopically in both groups (75% [9/12] vs 75% [12/16], p>0.999). The Kaplan-Meier analysis of the cumulative incidence of recurrence after EP showed significant differences between the two groups (p=0.007 by log-rank test) (Fig. 4).
Table 2.
Follow-up Data
| Variable | Negative (n=82) | Positive/indeterminate (n=47) | Total (n=129) | p-value |
|---|---|---|---|---|
| Follow-up duration, mo | 72.7±41.3 | 69.9±37.6 | 71.7±39.8 | 0.700 |
| Residual lesion | 7 (8.5) | 9 (19.1) | 16 (12.4) | 0.138 |
| Time to notice residual lesion, mo | 1.4±0.4 | 2.7±1.3 | 2.1±1.2 | 0.020 |
| Recurrence | 12 (14.6) | 16 (34.0) | 28 (21.7) | 0.019 |
| Time to recurrence, mo | 34.7±32.6 | 36.2±27.4 | 35.5±29.2 | 0.900 |
| No. of total procedures required | 1.3±1.0 | 1.7±1.6 | 1.5±1.2 | 0.121 |
| Cancer development | 1 (1.2) | 0 | 1 (0.8) | >0.999 |
| Death | 1 (1.2) | 0 | 1 (0.8) | >0.999 |
Data are presented as mean±SD or number (%).
Fig. 4.
Kaplan-Meier analysis of cumulative recurrence of ampulla of Vater adenoma after endoscopic papillectomy according to margin status.
In the RMN group, local recurrence occurred in 12 of 82 (14.6%) patients during follow-up. Among the recurrent cases, 11 cases were diagnosed as adenoma and one was diagnosed as adenocarcinoma. Of these 12 patients, eight patients with recurrent adenoma underwent additional endoscopic treatment using APC or snare resection. In the case of adenocarcinoma, the patient was diagnosed with AOV cancer at 71.3 months after initial EP and subsequently underwent pylorus-preserving pancreaticoduodenectomy, but died from multiple hepatic and lung metastases 4 months after the surgery.
In the RMPI group, local recurrence occurred in 16 of 47 (34.0%) patients during follow-up. All recurrent tumors were diagnosed as adenomas. Of these 16 patients, 14 patients underwent additional endoscopic treatment using APC or snare resection, and two patients with high-grade dysplasia underwent pylorus-preserving pancreaticoduodenectomy due to malignant potential of the lesions. Among 14 cases with additional endoscopic treatment, two patients had subsequent surgery because the tumor could not be completely removed with endoscopic treatment.
4. Risk factors for residual lesion and local recurrence
Risk factors for residual lesion and local recurrence were analyzed using the Cox regression analysis, and the results are presented in Tables 3 and 4, respectively. Submucosal injection was the only factor significantly related to residual lesion in both univariate and multivariate analysis (hazard ratio [HR], 4.11; p=0.009). Multivariable analysis showed that high-grade dysplasia (HR, 2.74; p=0.011), intraductal extension (HR, 10.34; p=0.004), and submucosal injection (HR, 2.57; p=0.021) were significant risk factors for local recurrence.
Table 3.
Univariate and Multivariate Analyses of Risk Factors for Residual Lesion
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Male sex | 0.88 (0.30–2.55) | 0.815 | |||
| Age | 1.03 (0.99–1.09) | 0.169 | 1.03 (0.98–1.08) | 0.304 | |
| Familial adenomatous polyposis | 1.63 (0.21–12.54) | 0.639 | |||
| Tumor size >10 mm | 7.38 (0.97–55.95) | 0.053 | 5.63 (0.73–43.60) | 0.098 | |
| Villous or villotubular growth pattern (vs tubular adenoma) | 0.73 (0.17–3.23) | 0.680 | |||
| High histologic grade | 1.51 (0.34–6.73) | 0.586 | |||
| Piecemeal resection (vs en bloc) | 3.78 (1.29–11.09) | 0.016 | 2.33 (0.75–7.19) | 0.142 | |
| Submucosal injection | 4.11 (1.42–11.92) | 0.009 | 3.12 (1.03–9.40) | 0.043 | |
| Additional ablation | 2.36 (0.85–6.51) | 0.097 | 1.70 (0.59–4.91) | 0.324 | |
HR, hazard ratio; CI, confidence interval.
Table 4.
Univariate and Multivariate Analyses of Risk Factors for Recurrence
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Male sex | 0.83 (0.38–1.79) | 0.626 | |||
| Age | 1.00 (0.97–1.03) | 0.932 | |||
| Familial adenomatous polyposis | 1.76 (0.41–7.57) | 0.447 | |||
| Tumor size >10 mm | 1.04 (0.47–2.30) | 0.930 | |||
| Villous or villotubular growth pattern (vs tubular adenoma) | 1.95 (0.86–4.45) | 0.111 | 2.33 (0.99–5.44) | 0.050 | |
| High histologic grade | 2.34 (1.09–5.01) | 0.029 | 2.74 (1.26–5.96) | 0.011 | |
| Piecemeal resection (vs en bloc) | 1.44 (0.50–4.16) | 0.500 | |||
| Intraductal extension | 13.06 (2.90–58.86) | <0.001 | 10.34 (2.10–50.88) | 0.004 | |
| Submucosal injection | 2.32 (1.10–4.93) | 0.029 | 2.57 (1.16–5.72) | 0.021 | |
| Additional ablation | 1.03 (0.50–2.19) | 0.931 | |||
HR, hazard ratio; CI, confidence interval
DISCUSSION
EP has been reported as a safe and effective procedure for treating ampullary adenoma and has been accepted as an alternative to surgery.7-9 Pathologic evaluation of the resected margin of the tumor is crucial because the treatment strategy varies depending on the margin status. However, pathological evaluation of resected margins can be difficult due to the burning effect caused by the use of blended electrical current.12 It is still controversial whether additional surgery should be performed for all patients with adenoma with a positive or indeterminate margin. Although a few studies have reported long-term outcomes of cases with a positive or indeterminate margin after EP, there is still no consensus about the management of ampullary adenomas that display positive or indeterminate margins after EP.13,18 In this study, we investigated the clinical outcome of cases with resected margin-positive or indeterminate margin after EP.
The recurrence rate after EP was higher in the RMPI group compared to the RMN group (14.6% vs 34.0%, p=0.019). The recurrence rate in our study was comparable to those reported in previous studies (10% to 33%).10,11 While a median follow-up duration of less than 3 years has been used in many reports,8,13,14 patients in our study underwent post-EP surveillance for a mean of 71.7±39.8 months, which allowed this study to provide some insight into the natural history and progression of ampullary adenomas. We found that the recurrence-free duration was not significantly different between the RMN and RMPI groups (34.7 months vs 36.2 months, p=0.900). Some studies have proposed a post-EP surveillance duration of at least 2 years,9,19 and we found that regardless of the resected margin, recurrence occurred even after 30 months in both groups. Current guidelines recommend long-term monitoring of patients after EP for at least 5 years,20 and our results support the idea that surveillance for at least 5 years after EP is beneficial.
During the long-term follow-up of the 82 patients with a negative margin, 11 patients experienced adenoma recurrence, and one patient had a recurrent tumor with ampullary adenocarcinoma and underwent additional surgery but eventually died due to multiple metastases a few months after the surgery. The remaining 11 patients, including two with high-grade dysplasia, did not receive additional surgery after EP and were managed either endoscopically or through careful observation. Among the 47 patients with a positive/indeterminate margin, 16 patients had adenoma recurrence during long-term follow-up. Two of the 16 cases with high-grade dysplasia underwent surgery when recurrence occurred, and two of the 16 cases underwent additional surgery due to persistent adenoma despite additional endoscopic management. The remaining 12 patients did not undergo additional surgery after EP, but were successfully managed endoscopically. In this study, 75% of the recurrent tumors (RMN 75% [9/12], RMPI 75% [12/16]) were successfully treated with repeated endoscopic mucosal resection or APC, which are reliable and feasible methods for treating local recurrent ampullary tumors arising after EP.13,21 In addition, there have been a few reports of radiofrequency ablation therapy for intraductal extension of AOV adenoma.22-25 The indication for endoscopic therapy for AOV adenomas has been expanding. Pancreaticoduodenectomy and local surgical resection have been considered the treatment of choice for ampullary neoplasms, but they may be excessively invasive for benign neoplasms and associated with relatively high mortality and morbidity rates.4-6 Therefore, additional surgery may not be necessary for all patients with adenoma with a positive or indeterminate margin.
It can be challenging to make a pathological diagnosis of the tissue stump and differentiate a residual lesion from a recurrent lesion in clinical practice based on endoscopic findings. In this study, residual lesions were detected even after histological complete resection (RMN 8.5% vs RMPI 19.1%, p=0.138), indicating that evaluating the margins of resected ampullary tumors after EP is sometimes difficult. Furthermore, there is a diagnostic challenge for pathologists when it comes to ampullary biopsies, as various artifacts such as inflammation, bile exposure, stones, stents, and sphincterotomy can induce histologic changes.26 There is a lack of well-defined diagnostic criteria for distinguishing dysplasia from reactive change.
Several risk factors for residual or recurrent adenoma have been suggested, including familial adenomatous polyposis, intraductal extension, and piecemeal resection.27-29 In this study, intraductal extension, high-grade dysplasia, and submucosal injection were risk factors for recurrent adenoma, while piecemeal resection was not. Some researchers recommended performing submucosal injection of diluted epinephrine prior to resection to reduce the risk of perforation and bleeding.30,31 However, the complexity of the ampulla structure makes the benefit of submucosal injection uncertain. Other studies have shown that submucosal injection is related to a higher risk of residual tumors and shorter recurrence-free survival without the advantage of achieving complete resection or reducing post-EP adverse events.32,33 The European Society of Gastrointestinal Endoscopy guidelines and recent expert consensus on EP recommend submucosal injection only in cases of laterally spreading lesions.20,34 In this study, submucosal injection was a significant risk factor for both residual and recurrent adenoma in multivariate analysis.
The present study has several limitations that need to be addressed. First, it was a retrospective and single-center study. Therefore, the results may not be generalizable to different populations or clinical settings. A larger-scale prospective study is needed to confirm our findings. Second, although EP was performed by experienced endoscopists in our institution, the endoscopic technique used may not be the standard of practice for other endoscopists. Despite these limitations, this study is valuable in that it is the first to include patients with more than 5 years of follow-up, providing valuable insights into the long-term outcomes of EP for ampullary adenomas.
In conclusion, although cases with a positive or indeterminate margin after EP showed a higher rate of recurrence at long-term follow-up compared to negative margin cases, we found that endoscopic treatment can be an effective and safe option with favorable long-term outcomes. Based on our results, endoscopic treatment may be considered as the first-line treatment for cases of recurrence, taking into account the risk of perioperative surgical morbidity and mortality associated with more invasive surgical procedures.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study conception and design: D.W.S. Data analysis and interpretation: J.L., D.O., T.J.S., D.H.P., S.M.H., S.K.L. Drafting of the manuscript: J.L., D.O. Critical revision of the manuscript for important intellectual content: D.W.S. Statistical analysis: J.L. Study supervision: D.W.S. Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Supplementary materials can be accessed at https://doi.org/10.5009/gnl230451.
REFERENCES
- 1.Rosenberg J, Welch JP, Pyrtek LJ, Walker M, Trowbridge P. Benign villous adenomas of the ampulla of Vater. Cancer. 1986;58:1563–1568. doi: 10.1002/1097-0142(19861001)58:7<1563::AID-CNCR2820580730>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 2.Kang SH, Kim KH, Kim TN, et al. Therapeutic outcomes of endoscopic papillectomy for ampullary neoplasms: retrospective analysis of a multicenter study. BMC Gastroenterol. 2017;17:69. doi: 10.1186/s12876-017-0626-5.8d1729c4817441d99bb4ee2ab41e6e4f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stolte M, Pscherer C. Adenoma-carcinoma sequence in the papilla of Vater. Scand J Gastroenterol. 1996;31:376–382. doi: 10.3109/00365529609006414. [DOI] [PubMed] [Google Scholar]
- 4.Tran TC, Vitale GC. Ampullary tumors: endoscopic versus operative management. Surg Innov. 2004;11:255–263. doi: 10.1177/155335060401100409. [DOI] [PubMed] [Google Scholar]
- 5.Song J, Liu H, Li Z, Yang C, Sun Y, Wang C. Long-term prognosis of surgical treatment for early ampullary cancers and implications for local ampullectomy. BMC Surg. 2015;15:32. doi: 10.1186/s12893-015-0019-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H, Park JY, Kwon W, Heo JS, Choi DW, Choi SH. Transduodenal ampullectomy for the treatment of early-stage ampulla of Vater cancer. World J Surg. 2016;40:967–973. doi: 10.1007/s00268-015-3316-x. [DOI] [PubMed] [Google Scholar]
- 7.Li SL, Li W, Yin J, Wang ZK. Endoscopic papillectomy for ampullary adenomatous lesions: a literature review. World J Gastrointest Oncol. 2021;13:1466–1474. doi: 10.4251/wjgo.v13.i10.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S, Wang Z, Cai F, et al. New experience of endoscopic papillectomy for ampullary neoplasms. Surg Endosc. 2019;33:612–619. doi: 10.1007/s00464-018-6577-2. [DOI] [PubMed] [Google Scholar]
- 9.Cheng CL, Sherman S, Fogel EL, et al. Endoscopic snare papillectomy for tumors of the duodenal papillae. Gastrointest Endosc. 2004;60:757–764. doi: 10.1016/S0016-5107(04)02029-2. [DOI] [PubMed] [Google Scholar]
- 10.Lee R, Huelsen A, Gupta S, Hourigan LF. Endoscopic ampullectomy for non-invasive ampullary lesions: a single-center 10-year retrospective cohort study. Surg Endosc. 2021;35:684–692. doi: 10.1007/s00464-020-07433-7. [DOI] [PubMed] [Google Scholar]
- 11.Chathadi KV, Khashab MA, et al. ASGE Standards of Practice Committee, author. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2015;82:773–781. doi: 10.1016/j.gie.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Ito K, Fujita N, Noda Y, et al. Impact of technical modification of endoscopic papillectomy for ampullary neoplasm on the occurrence of complications. Dig Endosc. 2012;24:30–35. doi: 10.1111/j.1443-1661.2011.01161.x. [DOI] [PubMed] [Google Scholar]
- 13.Sakai A, Tsujimae M, Masuda A, et al. Clinical outcomes of ampullary neoplasms in resected margin positive or uncertain cases after endoscopic papillectomy. World J Gastroenterol. 2019;25:1387–1397. doi: 10.3748/wjg.v25.i11.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamao T, Isomoto H, Kohno S, et al. Endoscopic snare papillectomy with biliary and pancreatic stent placement for tumors of the major duodenal papilla. Surg Endosc. 2010;24:119–124. doi: 10.1007/s00464-009-0538-8. [DOI] [PubMed] [Google Scholar]
- 15.Tringali A, Valerii G, Boškoski I, et al. Endoscopic snare papillectomy for adenoma of the ampulla of Vater: long-term results in 135 consecutive patients. Dig Liver Dis. 2020;52:1033–1038. doi: 10.1016/j.dld.2020.05.029. [DOI] [PubMed] [Google Scholar]
- 16.Sahar N, Krishnamoorthi R, Kozarek RA, et al. Long-term outcomes of endoscopic papillectomy for ampullary adenomas. Dig Dis Sci. 2020;65:260–268. doi: 10.1007/s10620-019-05812-2. [DOI] [PubMed] [Google Scholar]
- 17.Kawashima H, Ohno E, Ishikawa T, et al. Endoscopic papillectomy for ampullary adenoma and early adenocarcinoma: analysis of factors related to treatment outcome and long-term prognosis. Dig Endosc. 2021;33:858–869. doi: 10.1111/den.13881. [DOI] [PubMed] [Google Scholar]
- 18.Muro S, Kato H, Matsumi A, et al. The long-term outcomes of endoscopic papillectomy and management of cases of incomplete resection: a single-center study. J Gastrointest Surg. 2021;25:1247–1252. doi: 10.1007/s11605-020-04532-7. [DOI] [PubMed] [Google Scholar]
- 19.Ahn DW, Ryu JK, Kim J, et al. Endoscopic papillectomy for benign ampullary neoplasms: how can treatment outcome be predicted? Gut Liver. 2013;7:239–245. doi: 10.5009/gnl.2013.7.2.239.1196ea380a2a4386a14b9747aa95f018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanbiervliet G, Strijker M, Arvanitakis M, et al. Endoscopic management of ampullary tumors: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53:429–448. doi: 10.1055/a-1397-3198. [DOI] [PubMed] [Google Scholar]
- 21.Nam K, Song TJ, Kim RE, et al. Usefulness of argon plasma coagulation ablation subsequent to endoscopic snare papillectomy for ampullary adenoma. Dig Endosc. 2018;30:485–492. doi: 10.1111/den.13008. [DOI] [PubMed] [Google Scholar]
- 22.Rustagi T, Irani S, Reddy DN, et al. Radiofrequency ablation for intraductal extension of ampullary neoplasms. Gastrointest Endosc. 2017;86:170–176. doi: 10.1016/j.gie.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Tringali A, Matteo MV, Orlandini B, et al. Radiofrequency ablation for intraductal extension of ampullary adenomatous lesions: proposal for a standardized protocol. Endosc Int Open. 2021;9:E749–E755. doi: 10.1055/a-1387-7880.2a6168db5714486592cb67bf7b899eed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi YH, Yoon SB, Chang JH, Lee IS. The safety of radiofrequency ablation using a novel temperature-controlled probe for the treatment of residual intraductal lesions after endoscopic papillectomy. Gut Liver. 2021;15:307–314. doi: 10.5009/gnl20043.7523d87dfa464b22b26c8f374bf4b62f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho SH, Oh D, Song TJ, et al. Long-term outcomes of endoscopic intraductal radiofrequency ablation for ampullary adenoma with intraductal extension after endoscopic snare papillectomy. Gut Liver. 2023;17:638–646. doi: 10.5009/gnl220201.80d6289b404c4381b544b3b1473e4786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellizzi AM, Kahaleh M, Stelow EB. The assessment of specimens procured by endoscopic ampullectomy. Am J Clin Pathol. 2009;132:506–513. doi: 10.1309/AJCPUZWJ8WA2IHBG. [DOI] [PubMed] [Google Scholar]
- 27.Ridtitid W, Tan D, Schmidt SE, et al. Endoscopic papillectomy: risk factors for incomplete resection and recurrence during long-term follow-up. Gastrointest Endosc. 2014;79:289–296. doi: 10.1016/j.gie.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma T, Jang EJ, Zukerberg LR, et al. Recurrences are common after endoscopic ampullectomy for adenoma in the familial adenomatous polyposis (FAP) syndrome. Surg Endosc. 2014;28:2349–2356. doi: 10.1007/s00464-014-3467-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bohnacker S, Seitz U, Nguyen D, et al. Endoscopic resection of benign tumors of the duodenal papilla without and with intraductal growth. Gastrointest Endosc. 2005;62:551–560. doi: 10.1016/j.gie.2005.04.053. [DOI] [PubMed] [Google Scholar]
- 30.Monson JR, Donohue JH, McEntee GP, et al. Radical resection for carcinoma of the ampulla of Vater. Arch Surg. 1991;126:353–357. doi: 10.1001/archsurg.1991.01410270099016. [DOI] [PubMed] [Google Scholar]
- 31.Desilets DJ, Dy RM, Ku PM, et al. Endoscopic management of tumors of the major duodenal papilla: refined techniques to improve outcome and avoid complications. Gastrointest Endosc. 2001;54:202–208. doi: 10.1067/mge.2001.116564. [DOI] [PubMed] [Google Scholar]
- 32.Chung KH, Lee SH, Choi JH, et al. Effect of submucosal injection in endoscopic papillectomy of ampullary tumor: propensity-score matching analysis. United European Gastroenterol J. 2018;6:576–585. doi: 10.1177/2050640617745459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyun JJ, Lee TH, Park JS, et al. A prospective multicenter study of submucosal injection to improve endoscopic snare papillectomy for ampullary adenoma. Gastrointest Endosc. 2017;85:746–755. doi: 10.1016/j.gie.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Fritzsche JA, Fockens P, Barthet M, et al. Expert consensus on endoscopic papillectomy using a Delphi process. Gastrointest Endosc. 2021;94:760–773. doi: 10.1016/j.gie.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.