Abstract
Objectives
Persistent pulmonary hypertension (PPHT) of the newborn is a disorder of circulatory transition resulting in high pulmonary vascular resistance with extrapulmonary right-to-left shunts causing hypoxemia. In this study, our aim was to evaluate the risk factors, administered treatments, and mortality of patients followed in our neonatal intensive care unit (NICU) due to PPHT over the past six years.
Methods
Patients diagnosed with PPHT and followed in the NICU between January 2017 and November 2022 were included in the study. The sociodemographic characteristics, diagnoses that could lead to pulmonary hypertension, the presence of congenital anomalies, the duration of respiratory support treatment and hospital follow-up, treatments administered for PPHT, and mortality rates were evaluated.
Results
Out of 21 patients diagnosed with persistent pulmonary hypertension, 9 of them (42.9%) were male. The mean gestational age of the patients was 37.6±3.7 weeks, and their birth weight was 3006±819grams. The APGAR scores at 1 and 5 minutes were 4(2-7) and 6(3-8), respectively. Risk factors during the antenatal period included fetal distress (38.1%), oligohydramnios (23.8%), intrauterine growth restriction (23.8%), gestational diabetes (14.3%), preeclampsia (4.8%), and chorioamnionitis (4.8%). The median duration of invasive mechanical ventilation for cases requiring respiratory support was 20.1 days, while the median duration of non-invasive ventilation was 3.7 days. Patients with a diagnosis of persistent pulmonary hypertension were treated with inhaled nitric oxide (iNO) in 76.2% of cases, milrinone in 66.7% of cases, sildenafil in 52.4% of cases, and iloprost in 14.3% of cases. The length of hospital stay for patients was 38.4 days, and 9 (42.9%) patients died. The patients who died had severe PPHT along with fetal inflammatory response syndrome (FIRS), congenital heart disease, pulmonary hypoplasia, pneumothorax, hypoxic-ischemic encephalopathy (HIE), and congenital anomalies.
Conclusion
Persistent pulmonary hypertension, characterized by severe hypoxemia, is a neonatal emergency that necessitates early intervention, effective treatment of the underlying cause to prevent potential short-term and long-term morbidities and mortality. Effective treatment of the underlying cause in patients diagnosed with PPHT could reduce morbidity and mortality. It is inevitable to avoid the loss of patients with major abnormalities, severe comorbidities, and unpreventable organ dysfunctions.
Keywords: Inhaled nitric oxide, newborn, persistent pulmonary hypertension
Persistent pulmonary hypertension (PPHT) of the newborn is a pathology characterized by systemic hypoxemia, particularly more common in term and near-term infants. It occurs due to the continuation of postnatal fetal circulation because of high pulmonary vascular resistance, with right-to-left shunting in extrapulmonary shunts persisting in the absence of structural cardiac anomalies.[1, 2] Echocardiographic findings of PPHT include right ventricular hypertrophy, leftward deviation of the interventricular septum, tricuspid regurgitation, and right-to-left or bidirectional shunting in the patent foramen ovale (PFO) and patent ductus arteriosus (PDA).[3] Meconium aspiration syndrome (MAS), infection, and congenital diaphragmatic hernia, as well as antenatal exposure to cigarette smoke and nonsteroidal anti-inflammatory drugs, pose a risk for the development of PPHT. While PPHT commonly appears as a clinical symptom related to a specific group of diseases in newborns, it can also have an idiopathic origin.[1, 2] The incidence of term newborns varies between 0.43 and 6 per 1000 live births.[4] Newborns with PPHT are at risk for serious complications, including asphyxia, chronic lung disease, neurodevelopmental sequelae, and even death.[3] PPHT, which carries a high risk of mortality and morbidity, should be promptly detected using echocardiography, which is considered the gold standard, and treated promptly before complications develop.
In this single-center retrospective study, our aim was to evaluate the demographic characteristics of patients followed and treated for PPHT in our neonatal intensive care unit (NICU) over the past six years, along with risk factors, treatment modalities, and causes of mortality, in line with existing literature data.
Methods
We retrospectively evaluated digital records of our patients hospitalized in our NICU with the diagnosis of PPHT between 2017 and 2022 from the hospital digital database. When persistent cyanosis cannot be explained by underlying lung conditions and there is a difference of more than 10% between pre- and post-ductal oxygen saturation in pulse oximetry, or when arterial blood gas sampling shows a PaO2 below 100 mmHg despite administering 100% oxygen, or in cases where PaO2 is within the normal range and chest X-rays reveal either normal or reduced pulmonary blood flow without accompanying lung disease, consultation with a pediatric cardiologist is initiated. Subsequently, an echocardiographic assessment is conducted.[5]
In cases of individuals with normal structural cardiac anatomy undergoing echocardiographic examination for PPHT, right ventricular pressure (RVp) is assessed based on Doppler measurements of the tricuspid regurgitation jet flow. The severity of PPHT is determined by comparing the estimated RVp, calculated using the tricuspid regurgitation jet flow, with systemic blood pressure (BP). Mild PPHT is characterized by the estimated RVp being between half and three-quarters of systemic BP; moderate PPHT occurs when the estimated RVp is greater than three-quarters of systemic BP but lower than systemic BP; and severe PPHT is identified when the estimated RVp is higher than systemic BP. In cases of severe PPHT, a right-to-left shunt across the patent ductus arteriosus (PDA) and/or patent foramen ovale (PFO) was demonstrated.[6]
Among all of the patient records with the ICD-10 diagnosis codes of I-27.0 and I-27.2, we selected the patients with an echocardiographic diagnosis of severe PPHT. We recorded the sociodemographic data (gender, mean gestational age, birth weight, inborn-outborn) of these patients. The antenatal findings, perinatal risk factors (type of birth, APGAR scores at 1 and 5 minutes, intubation requirement), the presence of congenital anomalies, and accompanying diagnoses that could lead to PPHT (perinatal asphyxia, MAS, sepsis, bronchopulmonary dysplasia, diaphragmatic hernia, and congenital heart disease) were evaluated. The postnatal follow-up in the NICU was detailed in terms of respiratory support treatments (invasive and non-invasive ventilation, oxygen therapy days), oxygenation index (OI), treatments used for PPHT (inhaled nitric oxide (iNO), sildenafil, milrinone, and iloprost), inotropy requirements, length of hospital stay, and mortality of the patients with severe PPHT. Patients were divided into two groups according to their survival and evaluated for their demographic findings and respiratory support treatments.
The study received approval from our hospital's ethics committee on November 24, 2022, under approval number B.10.1.TKH.4.34.H.GP.0.01/368. The study complied with the principles of the Declaration of Helsinki.
Statistical Analysis
The patient data collected during the study was analyzed using IBM Statistical Package for the Social Sciences (SPSS) for Windows 23.0 (IBM Corp., Armonk, NY). Frequency and percentage were used to describe categorical data, while mean, standard deviation, median, minimum, and maximum were used as descriptive statistics for continuous data. Mann Whitney U test is used for the evaluation of the difference between the continuous variables of the groups, and the Chi square test is used for categorical data. A statistical significance level of p<0.05 was considered in the study.
Results
The number of live births in our hospital, which is a perinatology center, during the study period was 29711, and 6530 newborns were admitted to the NICU, who were 88% inborn. Severe PPHT was detected in 21 (0.32%) of the 6530 babies who were hospitalized in our NICU between January 2017 and December 2022. 19 out of these 21 patients were inborn, and 9 of them (42.9%) were male. The mean gestational age of the patients was 37.6±3.7 weeks, and their birth weight was 3006±819 grams. Only one of the patients was an immature infant with a gestational age of 23 weeks, and four of them were late preterm infants (Table 1).
Table 1.
Characteristics, ventilation durations and risk factors of patients with pulmonary hypertension
| Characteristics | n=21 |
|---|---|
| Gender (male/female) (n) | 9/12 |
| Gestational age (wk) (mean±SD) | 37.6±3.7 |
| Birth weight (g) (mean±SD) | 3006±819 |
| Cesarean section, n (%) | 15 (71.4) |
| 5th minute APGAR score, median (lower-upper limit) | 6 (3-8) |
| Required intubation at birth, n(%) | 17 (81) |
| Inborn babies, n (%) | 19 (90.5) |
| Hospital stay (day), (mean±SD) | 38.4±44.9 |
| Mortality, n (%) | 9 (42.9) |
| Ventilation duration median (lower-upper limit) | |
| Noninvasive (day) | 3 (1-14) |
| Invasive (day) | 11 (1-187) |
| High frequency ventilation (day) | 5,5 (1-37) |
| Oxygen therapy duration, day, median(lower-upper limit) | 18 (1-187) |
| Risk factors, n (%) | |
| Fetal distress | 8 (38.1) |
| Meconium Aspiration Syndrome | 6 (28.6) |
| Congenital Diaphragmatic hernia | 6 (28.6) |
| Hypoxic Ischemic Encephalopathy | 6 (28.6) |
| Oligohydramnios | 5 (23.8) |
| Intrauterine growth restriction | 5 (23.8) |
| Gestational diabetes | 3 (14.3) |
| Preeclampsia | 1 (4.8) |
| Chorioamnionitis | 1 (4.8) |
Risk factors for PPHT during the antenatal period included fetal distress (38.1%), congenital diaphragmatic hernia (CDH) (%28.6), oligohydramnios (23.8%), intrauterine growth restriction (IUGR) (23.8%), gestational diabetes (14.3%), preeclampsia (4.8%), and chorioamnionitis (4.8%) (Table 1). Out of the six patients (28.6%) diagnosed with hypoxic-ischemic encephalopathy (HIE) and subsequently diagnosed with PPHT during therapeutic hypothermia, four of them had MAS, while two were diagnosed with intrauterine growth restriction (IUGR). Out of the five premature patients, two had diaphragmatic hernia, while the others had FIRS, congenital heart disease, and congenital anomalies.
The APGAR scores at 1 and 5 minutes were 4 (2-7) and 6 (3-8), respectively. Seventeen patients (81%) required intubation at birth. The median duration of invasive mechanical ventilation was 11 (1-187) days, while the duration of high-frequency ventilation (HFOV) was 5.5 (1-37) days, non-invasive ventilation was 3 (1-14) days, and oxygen therapy was 18 (1-187) days.
Inhaled nitric oxide was used in 76.2% of the cases, milrinone in 66.7%, sildenafil in 52.4%, and iloprost in 14.3% of the cases. The ratios of combined drug therapies used for PPHT are indicated in Figure 1. All patients required inotropic support for the treatment of hypotension. Ten patients received both HFO and surfactant, as well as iNO. Among patients who received all three treatments, four died. The median length of hospital stay was 38.4 days. Nine (42.9%) patients died, and seven of them were term babies. The deceased cases had diagnoses of pulmonary hypoplasia, CDH, pneumothorax, polycystic kidney disease, HIE, congenital heart disease, FIRS, Scimitar syndrome, and DiGeorge syndrome, in addition to PPHT. The oxygenation index (OI) of these patients on the day of death was calculated at 25 (17-33). Five of the patients died on their 1st day of life. The non-invasive ventilation, nitric oxide use days, and hospitalization duration were significantly less than the patients who survived (p<0.05 for each) (Table 2).
Figure 1.
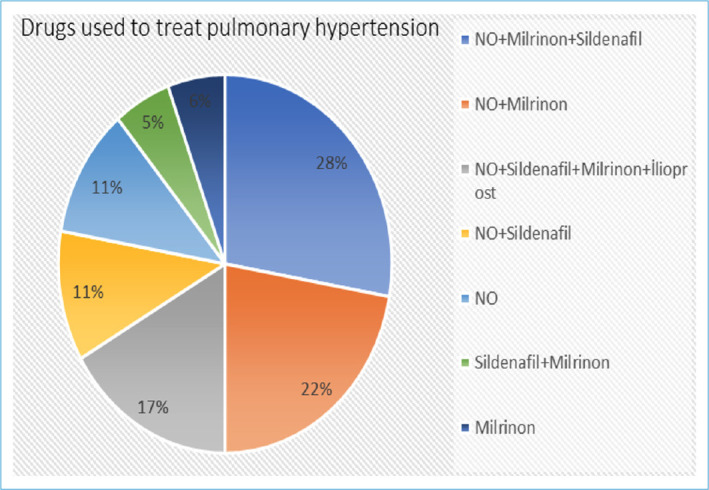
Drugs used to treat pulmonary hypertension.
Table 2.
Comparison of patients according to the outcome
| Characteristics | Exitus n=9 | Survived n=12 | p |
|---|---|---|---|
| Gender (male/female) (n) | 3/6 | 6/6 | >0.05 |
| Gestational age (wk) (mean±SD) | 36.6±5.2 | 38.3±2.0 | >0.05 |
| Birth weight (g) (mean±SD) | 2706±997 | 3232±607 | >0.05 |
| Cesarean section, n (%) | 5 (55.6) | 10 (83.3) | >0.05 |
| 5th minute APGAR score, | |||
| median (lower-upper limit) | 6 (3-7) | 6 (4-8) | >0.05 |
| Required intubation at birth, n (%) | 8(88.9) | 9(75) | >0.05 |
| Inborn babies, n (%) | 8 (88.9) | 11 (91.6) | >0.05 |
| Ventilation duration, (day) median, (lower-upper limit) | |||
| Noninvasive (day) | 0.5 (0-1) | 3.5 (1-14) | 0.04 |
| Invasive (day) | 1 (1-187) | 11.5 (5-27) | >0.05 |
| High frequency ventilation (day) | 1 (1-37) | 8 (2-20) | >0.05 |
| Oxygen therapy duration, day, median (lower-upper limit) | 1 (1-187) | 21 (10-33) | >0.05 |
| Nitric oxide duration, days, median (lower-upper limit) | 1 (1-14) | 7 (2-11) | 0.02 |
| Sildenafil duration, days, median (lower-upper limit) | 7 (1-46) | 14 (2-36) | >0.05 |
| Hospital stay (day), median (lower-upper limit) | 1 (0.3-187) | 36 (21-121) | 0.02 |
Discussion
Persistent pulmonary hypertension is a high-mortality condition affecting preterm and term infants, characterized by a delayed reduction in pulmonary vascular resistance postnatally, resulting in pulmonary arterial hypertension and hypoxemia.[7] The incidence of the disease, which constitutes 2-10% of admissions to the NICU, is 0.43-6 per 1000 live births overall and 5.4 per 1000 live births in late preterm infants. In our hospital, during the six years covering our study, there were 29711 live births, and 6530 newborns were admitted to the NICU. Persistent pulmonary hypertension is associated with a mortality rate ranging from 4% to 33%.[4, 5, 8] During the six years, out of 6,530 infants admitted to the NICU in our hospital, 21 (0.32%) were diagnosed with severe PPHT, and 9 (42.9%) died.
Although PPHT may be the result of several divergent neonatal disorders, adverse intrauterine stimuli such as chronic hypoxia and hypertension can lead to the pathophysiology. Perinatal risk factors for PPHT include pulmonary parenchymal disease, such as meconium aspiration or pneumonia, which clearly increases the likelihood that infants will develop PPHT.[9] Our patients’ risk factors for PPHT included fetal distress, HIE, CDH, MAS, oligohydramnios, IUGR, gestational diabetes, preeclampsia, and chorioamnionitis. In a study conducted in our country, the most common etiological factor for PPHT among 50 infants with identified PPHT was congenital pneumonia in term infants (65%), while in preterm infants, respiratory distress syndrome (100%) was the predominant etiological factor. In both groups, perinatal asphyxia was identified as the second most common etiological factor.[10]
The diagnosis of RDS was not present in our cases, as we do not perform echocardiography on very low birth weight infants on their 1st day of life and as the presence of high pulmonary pressures is physiologic.
In asphyxiated patients, lung diseases (such as MAS and pulmonary hemorrhage), acidosis, sepsis, hypotension, and impaired cardiac function are associated with PPHT. The mortality rate increases when PPHT occurs in conjunction with HIE. PPHT has been reported at a rate of 6-25% in newborns with HIE who were enrolled in clinical studies of head and whole-body cooling, which is higher than the rate in the general population.[11, 12] Shankaran et al.[13], who investigated the relationship between the degree of hypothermia and PPHT, demonstrated that when patients were cooled to 33.5 and 32oC for 72 or 120 hours, the need for iNO and extracorporeal membrane oxygenation (ECMO) increased in the group cooled to 32oC. They also demonstrated that the mild hypothermia used in HIE treatment was not associated with PPHT. Six of our patients (four with MAS and two with IUGR) received therapeutic hypothermia for HIE, and their body temperatures were maintained within the range of 33.5-34.5oC.
Congenital diaphragmatic hernia contributes to neonatal morbidity and mortality worldwide. PPHT is a key component of CDH pathophysiology and a critical consideration for management and therapeutic options. Clinical assessment of individual phenotypes may help guide personalized management strategies, including the effective use of pulmonary vasodilators and ECMO.[14] Despite meaningful advances in neonatal intensive care, the mortality rate in infants with CDH is approximately 60%.[9] Among our patients, six of them had the antenatal diagnosis of CDH, were intubated right after birth in the delivery room, followed with HFOV support, and received iNO, sildenafil, and iloprost in addition to milrinone. Unfortunately, 50% died besides these support therapies.
In PPHT, the goal is to address acidosis, hypoxemia, hemodynamics, and the treatment of underlying causes while achieving selective pulmonary vasodilation. The use of pulmonary vasodilators following the implementation of lung protective ventilation strategies is the main point of management.[5]
Inhaled nitric oxide remains the only United States Food and Drug Administration (FDA) approved pulmonary vasodilator therapy for late preterm and term infants with PPHT.[7] Sixteen of our patients received iNO. Approximately 30% of cases do not respond to iNO and require ECMO. Combining HFOV with iNO significantly improves oxygenation, reduces the need for ECMO, and decreases mortality.[2, 8, 15] In our study, we found that 14 of our patients received iNO in combination with HFOV. Patients with severe pulmonary hypertension who had a fatal outcome experienced significantly shorter hospitalization durations, which were also correlated with the duration of receiving NO. Similarly, due to the severity of pulmonary hypertension leading to a fatal outcome, these cases necessitated invasive mechanical ventilation, and their durations of noninvasive mechanical ventilation were found to be significantly shorter.
There is evidence of surfactant deficiency in lung problems that cause secondary PPHT. Improved ventilation after surfactant treatment facilitates the distribution of iNO in the pulmonary circulation. It is essential to remember that surfactant and HFO can be employed as rescue treatments in PPHT.[15,16] Gonzalez et al.[15] demonstrated that in moderate to severe PPHT, the early and combined use of surfactant with iNO when compared to standard iNO treatment, results in better oxygenation and faster recovery and prevents the progression to severe PPHT. This approach also reduces the need for ECMO and lowers the risk of death. In our study, ten patients received both HFO and surfactant, in addition to iNO. Among patients who received all three treatments, four died.
While medical advancements have significantly improved survival in PPHT, their cost, technical challenges, and the inability to provide a cure in every case have prompted research into alternative treatment options, particularly in units with limited resources. Research has been conducted on other therapeutic options such as phosphodiesterase inhibitors, prostanoids, endothelin antagonists, and magnesium sulfate. In the literature, the combination of iNO with sildenafil has been extensively studied. In situations where NO therapy is not applicable or a response to treatment is not achieved, alternative treatments such as sildenafil, milrinone, inhaled prostacyclin, endothelin receptor antagonists, and magnesium sulfate can be employed.[10]
Imam et al.[17] aimed to assess the efficacy of milrinone versus sildenafil as available alternative therapeutics in treating PPHT and found that both milrinone and sildenafil are effective and well-tolerated in neonates with PPHN, particularly when iNO and ECMO are not available. Milrinone is superior to sildenafil in improving oxygenation without lowering blood pressure parameters. Uslu et al.[18] compared magnesium sulfate (MgSO4) and sildenafil in the treatment of PPHT. They found that the OI decreased more rapidly with sildenafil, and the ventilation duration was longer in the MgSO4 group due to its sedative and muscle relaxant effects. Additionally, inotropic support requirements were higher in the MgSO4 group for similar reasons. The study concluded that sildenafil was a more effective treatment compared to MgSO4.[18] We administered sildenafil, iNO, and iloprost treatments at rates of 52.4%, 76.2%, and 14.3%, respectively, to lower pulmonary pressure. However, MgSO4 has not been used for PPHT treatment in these patients, most probably due to their persistent severe systemic hypotension. Sildenafil was administered in combination with other treatments in our patient cohort. The most commonly used combinations were sildenafil and milrinone together (28%), followed by sildenafil alone (5%), and milrinone alone (6%). We believe that the use of combination therapies increases the chances of success.
A common side effect of drugs used in PPHT treatment is systemic hypotension, necessitating close monitoring of blood pressure to prevent shock and hypoperfusion.[3, 7, 8, 15, 18] All of our cases required inotropic support.
Conclusion
In this study, evaluating cases diagnosed with severe pulmonary hypertension in the NICU, a critical patient group, we found that factors such as MAS, asphyxia, polycystic kidney disease, sepsis, and CDH were associated with mortality in cases that resulted in fatality due to pulmonary hypertension. Therefore, we believe that effective treatment of the underlying cause in patients diagnosed with PPHT could reduce morbidity and mortality.
Footnotes
Please cite this article as ”Sahin O, Gok NR, Colak D, Oner T, Guran O, Yavanoglu Atay F, et al. Risk Factors and Mortality in Newborns with Persistent Pulmonary Hypertension: A Six-Year Single-Center Experience. Med Bull Sisli Etfal Hosp 2024;58(2):165–170”.
Disclosures
Ethics Committee Approval
The study received approval from Umraniye Training and Research Hospital Ethics committee on November 24, 2022, under approval number B.10.1.TKH.4.34.H.GP.0.01/368.
Funding Source
There are no funding sources.
Peer-review
Externally peer-reviewed.
Conflict of Interest
There are no conflicts of interest for each autors.
Financial Support
The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Authorship Contributions
Concept – O.S., N.R.G., D.C., T.O., O.G., F.Y.A., I.M.A.; Design – O.S., N.R.G., D.C., T.O., O.G., F.Y.A., I.M.A.; Supervision – O.S., N.R.G., D.C., T.O., O.G., F.Y.A., I.M.A.; Data collection &/ or processing – O.S., N.R.G., D.C.; Analysis and/or interpretation – O.S., N.R.G., O.G., T.O., F.Y.A., I.M.A.; Literature search – O.S., I.M.A., N.R.G., D.C., O.G., F.Y.A.; Writing O.S., I.M.A.; Critical review – O.S., I.M.A., N.R.G., O.G., D.C., T.O., F.Y.A.
Use of AI for Writing Assistance
None declared.
References
- 1.Sivaslı E, Yurdakök M, Karagöz T, Korkmaz A, Yiğit Ş, Tekinalp G. Inhaled iloprost to treat neonatal pulmonary hypertension. Çocuk Sağ Hast Derg [Article in Turkish] 2005;48:142–6. [Google Scholar]
- 2.Özkan H, Köksal N, Çetinkaya M. Persistan pulmoner hipertansiyon. Güncel Pediatri [Article in Turkish] 2006;3:105–8. [Google Scholar]
- 3.Pedersen J, Hedegaard ER, Simonsen U, Krüger M, Infanger M, Grimm D. Current and future treatments for persistent pulmonary hypertension in the newborn. Basic Clin Pharmacol Toxicol. 2018;123:392–406. doi: 10.1111/bcpt.13051. [DOI] [PubMed] [Google Scholar]
- 4.Çakır U, Tayman C, Büyüktiryaki M, Yakut Hİ. Persistent pulmonary hypertension of the newborn. J Gynecol Obstetr Neonatol [Article in Turkish] 2017;14:182–6. [Google Scholar]
- 5.Mandell E, Kinsella JP, Abman SH. Persistent pulmonary hypertension of the newborn. Pediatr Pulmonol. 2021;56:661–9. doi: 10.1002/ppul.25073. [DOI] [PubMed] [Google Scholar]
- 6.Arun Özer E, Demirel G, Tüzün F. Türk Neonatoloji Derneği Term Yenidoğanda Solunum Sıkıntısı Tanı, Tedavi ve Korunma Rehberi. Ankara: Güneş Kitabevleri; 2021:38–56. [Google Scholar]
- 7.Dilli D. Neonatal persistan pulmoner hipertansiyonda vazodilatör tedavi. In: Tekin AN, editor. Neonatolojide Gri Alanlar. 1st ed. Ankara: Türkiye Klinikleri; 2023. pp. 29–38. [Google Scholar]
- 8.Abdelkreem E, Mahmoud SM, Aboelez MO, Abd El Aal M. Nebulized magnesium sulfate for treatment of persistent pulmonary hypertension of newborn: a pilot randomized controlled trial. Indian J Pediatr. 2021;88:771–7. doi: 10.1007/s12098-020-03643-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delaney C, Cornfield DN. Risk Factors for persistent pulmonary hypertension of the newborn. Pulm Circ. 2012;2:15–20. doi: 10.4103/2045-8932.94818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaşa B, Dincer E, Babayiğit A, Yılmaz Semerci A, Memur Ş, Sağlam Ö, et al. Risk factors for persistent pulmonary hypertension of the newborn and determining the clinical prognosis. J Child [Article in Turkish] 2022;22:110–6. doi: 10.26650/jchild.2022.1113770. [DOI] [Google Scholar]
- 11.Lakshminrusimha S, Shankaran S, Laptook A, McDonald S, Keszler M, Van Meurs K, et al. Pulmonary hypertension associated with hypoxic-ischemic encephalopathy—antecedent characteristics and comorbiditie. J Pediatr. 2018;196:45–51. doi: 10.1016/j.jpeds.2017.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uslu S, Dursun M, Bülbül A. Meconium aspiration syndrome (MAS) Sisli Etfal Hastan Tip Bul [Article in Turkish] 2015;49:85–95. [Google Scholar]
- 13.Shankaran S, Laptook AR, Pappas A, McDonald SA, Das A, Tyson JE, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. JAMA. 2014;312:2629–39. doi: 10.1001/jama.2014.16058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhombal S, Patel N. Diagnosis & management of pulmonary hypertension in congenital diaphragmatic hernia. Semin Fetal Noanatal Med. 2022;27:101383. doi: 10.1016/j.siny.2022.101383. [DOI] [PubMed] [Google Scholar]
- 15.González A, Bancalari A, Osorio W, Luco M, González A, Pérez H, et al. Early use of combined exogenous surfactant and inhaled nitric oxide reduces treatment failure in persistent pulmonary hypertension of the newborn: a randomized controlled trial. J Perinatol. 2021;41:32–8. doi: 10.1038/s41372-020-00777-x. [DOI] [PubMed] [Google Scholar]
- 16.Lipkin PH, Davidson D, Spivak L, Straube R, Rhines J, Chang CT. Neurodevelopmental and medical outcomes of persistent pulmonary hypertension in term newborns treated with nitric oxide. J Pediatr. 2002;140:306–10. doi: 10.1067/mpd.2002.122730. [DOI] [PubMed] [Google Scholar]
- 17.Imam SS, El-Farrash RA, Taha AS, Saleh GA. Milrinone versus sildenafil in treatment of neonatal persistent pulmonary hypertension: a randomized control trial. J Cardiovasc Pharmacol. 2022;80:746–52. doi: 10.1097/FJC.0000000000001332. [DOI] [PubMed] [Google Scholar]
- 18.Uslu S, Kumtepe S, Bulbul A, Comert S, Bolat F, Nuhoglu A. A comparison of magnesium sulphate and sildenafil in the treatment of the newborns with persistent pulmonary hypertension: a randomized controlled trial. J Trop Pediatr. 2011;57:245–50. doi: 10.1093/tropej/fmq091. [DOI] [PubMed] [Google Scholar]


