Abstract
A region in the carboxy terminus of the protein encoded by open reading frame 6 in early region 4 (E4orf6) of adenovirus type 5 was determined to be required for directing nuclear localization of the E1B 55-kDa protein and for efficient virus replication. A peptide encompassing this region, corresponding to amino acids 239 through 255 of the E4orf6 protein, was analyzed by circular dichroism spectroscopy. The peptide showed evidence of self-interaction and displayed the characteristic spectra of an amphipathic α helix in the helix-stabilizing solvent trifluoroethanol. Disrupting the integrity of this α helix in the E4orf6 protein by proline substitutions or by removing amino acids 241 through 250 abolished its ability to direct the E1B 55-kDa protein to the nucleus when both proteins were transiently expressed in HeLa cells. Expression of E4orf6 variants that failed to direct nuclear localization of the E1B 55-kDa protein failed to enhance replication of the E4 mutant virus, dl1014, whereas expression of the wild-type E4orf6 protein restored growth of dl1014 to near-wild-type levels. These results suggest that the E4orf6 protein contains an arginine-faced, amphipathic α helix that is critical for a functional interaction with the E1B 55-kDa protein in the cell and for the function of the E4orf6 protein during a lytic infection.
Open reading frame 6 of the E4 region (E4orf6) of the group C adenoviruses (Ad) encodes a multifunctional protein that promotes virus replication and acts as an oncoprotein (reviewed in reference 34). As part of a complex with the E1B 55-kDa protein, the E4orf6 protein promotes the export of late viral mRNA from the nucleus to the cytoplasm and concomitantly inhibits the transport of most host cell mRNA (4, 35, 46). In addition, the E4orf6 protein enhances viral DNA replication, late protein synthesis, and the stability of unprocessed late viral RNA in the nucleus (3, 4, 24). Furthermore, the E4orf6 protein, as well as the product of the E4 open reading frame 3 (E4orf3), stimulates the accumulation of viral mRNA containing the tripartite leader (38) and affects splicing patterns of late viral RNA (41, 42). Finally, the E4orf6 protein has recently been shown to act as an oncoprotein by binding the tumor suppressor p53 and inhibiting its interaction with the transcriptional initiation complex (10, 47).
Genetic analyses have demonstrated that the E4orf6 and E4orf3 proteins share partially redundant and overlapping functions during lytic infection. Ad mutants that fail to express the E4orf6 protein show a modest reduction in virus yield and plaquing efficiency compared to the wild-type virus (4, 24). By contrast, mutant Ads that lack the entire E4 region or fail to express the E4orf6 and E4orf3 proteins are severely restricted for growth (4, 24, 26). However, E4 deletion mutants that express the E4orf6 protein in the absence of all other E4-encoded proteins replicate to near-wild-type levels (1, 24). Replication of a complete E4 deletion virus could be restored by transfecting the mutant-virus-infected cells with a cDNA expressing the E4orf6 protein (32).
A portion of the E4orf6 protein associates with the E1B 55-kDa protein in the cell. Physical evidence for this complex derives from the ability of E1B 55-kDa-specific antibodies to indirectly immunoprecipitate the E4orf6 protein from Ad-infected cells (46, 48, 49), after coexpression of the proteins by transfection (21), and from cellular lysates reconstituted in vitro (49). Immunofluorescence and electron microscopy have demonstrated that the E4orf6–E1B 55-kDa protein complex localizes to the peripheries of the sites of viral transcription and RNA processing within the nucleus of Ad type 5 (Ad5)-infected cells (43). When transiently expressed, the E4orf6 protein localizes to the nucleus and directs the E1B 55-kDa protein to the nucleus. In the absence of the E4orf6 protein, the E1B 55-kDa protein is retained in the cytoplasm (21). In addition, expression of the E4orf6 protein relieved the apparent cytoplasmic retention of the E1B 55-kDa protein in primate cells but not in mouse or rat cells (21). Thus, a primate-specific cellular factor may be required for the E4orf6 protein-directed nuclear localization of the E1B 55-kDa protein. It has been proposed that the E4orf6–E1B 55-kDa protein complex modulates mRNA transport by sequestering a cellular factor required for mRNA export, thus inhibiting host mRNA export and directing this factor to the sites of viral transcription, where it is available for viral mRNA export (43). It is also possible that such a factor enables shuttling of the E4orf6–E1B 55-kDa protein complex as part of the mechanism of mRNA transport control (9). The identity of this hypothetical cellular factor is unknown, but recent studies have demonstrated that overexpression of a cellular protein related to hnRNP-U/SAF-A in Ad-infected cells overcomes the inhibition of host cytoplasmic mRNA accumulation, implicating this cellular protein as a potential factor used by the E4orf6–E1B 55-kDa protein complex (18).
The structural features of the Ad E4orf6–E1B 55-kDa protein interaction have been partially elucidated (48). The association of the E4orf6 protein with E1B 55-kDa proteins bearing small, in-frame insertions was measured by an immunoprecipitation assay. This analysis suggested that the integrity of two distinct segments of the E1B 55-kDa protein are required for association with the E4orf6 protein in vivo. The portions of the E1B 55-kDa protein important for the interaction with the E4orf6 protein map to amino acid 143 and the region between amino acids 262 and 326 (48). The investigators also showed that the amino terminus of the E4orf6 protein, expressed in bacteria as a translational fusion to glutathione S-transferase, could bind the E1B 55-kDa protein in a protein blot assay. This study provided additional evidence for an interaction between the amino terminus of the E4orf6 protein and the E1B 55-kDa protein by using 293 cells transfected with an E4orf6/7 cDNA. The E4orf6/7 protein is composed of the amino-terminal 58 residues of E4orf6 and 92 residues encoded by E4orf7. A portion of the E4orf6/7 protein expressed by transfection was indirectly recovered upon immunoprecipitation of the endogenous E1B 55-kDa protein, suggesting that the amino-terminal 58 residues of E4orf6 mediated binding between the two proteins. However, this result stands in contrast to prior results that failed to reveal an interaction between the E4orf6/7 protein and the E1B 55-kDa protein in Ad-infected cells (8, 24). The significance of an interaction between the E1B 55-kDa protein and the amino terminus of the E4orf6 protein is unclear at this time because the Ad E4orf6/7 mutant virus is essentially wild type in growth and the E4orf6/7 protein cannot replace the function of the E4orf6 protein during Ad infection.
Here we examine the structural elements of the E4orf6 protein required for the functional interaction with the E1B 55-kDa protein by analyzing intracellular localization of the viral proteins and virus replication. We show that a domain in the E4orf6 protein near the carboxy terminus is required for the nuclear colocalization of the E1B 55-kDa protein. This domain is an arginine-faced, amphipathic α helix, and the integrity of this domain in the E4orf6 protein is required for E1B 55-kDa protein nuclear colocalization. Disruption of the E4orf6 arginine-faced, amphipathic α helix not only abolishes E1B 55-kDa protein nuclear colocalization but also disrupts the function of the E4orf6 protein in an Ad infection. These findings suggest that the arginine-faced α helix near the carboxy terminus of the E4orf6 protein is essential for the function of the E4orf6 protein during a productive viral infection.
MATERIALS AND METHODS
Cell culture and viruses.
Cell culture media, cell culture supplements, and serum were obtained from Life Technologies (Gaithersburg, Md.) through the Tissue Culture Core Laboratory of the Comprehensive Cancer Center of Wake Forest University. HeLa (CCL2.2) and W162 cells (53) were maintained in Dulbecco modified Eagle’s minimal medium supplemented with 10% newborn calf serum as previously described (20).
dl309 served as the wild-type Ad5 used in these studies. dl309 lacks a portion of the E3 gene which has been shown to be dispensable for growth in culture (30). The E4 deletion virus, dl1014, was constructed by Bridge and Ketner and described previously (3). This virus is able to express only the orf4 protein from the E4 region. The wild-type virus, dl309, was propagated in 293 cells (22), and dl1014 was propagated in W162 cells (53). Virus stocks were prepared by sequential centrifugation through CsCl as described previously (20).
The recombinant vaccinia virus used to express the T7 RNA polymerase, vTF7.3, was created by Fuerst et al. (17). Expression of the E1B 55-kDa and E4orf6 genes from the T7 promoter was achieved as described previously (21). Briefly, cells were infected with vTF7.3 in reduced serum medium and transfected with 1 μg of plasmid DNA mixed with 3 μg of Lipofectin (Life Technologies) in accordance with the manufacturer’s recommendation. The cells were analyzed by immunofluorescence between 12 and 14 h postinfection-transfection.
Plasmids and site-directed mutagenesis.
The plasmids carrying the E4orf6 and E1B 55-kDa genes were previously described (21). The cDNA encoding the E4orf6/7 protein was originally described by Freyer et al. (16). The coding region was subcloned from this construct by standard methods with PCR and placed under control of the T7 promoter in a pGEM plasmid (Promega, Madison, Wis.). The E4orf6 carboxy-terminal-truncation mutations were constructed in a pGEM plasmid (Promega) by removal of an appropriate restriction fragment encompassing the carboxy terminus of the E4orf6 protein. The mutations were confirmed by DNA sequencing.
Site-directed mutagenesis (27) was performed on the E4orf6 gene after transfer to the pAlter-1 plasmid (Promega). The leucine codon at amino acid 245 of the 294-amino-acid E4orf6 protein was changed to a proline codon (underlined) by using an oligonucleotide, 5′-CAAGGCGCCCTATGCTGCG-3′, to create the L245P E4orf6 variant. Another oligonucleotide, 5′-CGCTGCTGTGCCAGGCCTACAAGGCGCCCTA-3′, was used to create an E4orf6 variant (R241P) with a StuI restriction site at bp 720 of the E4orf6 coding region and a proline at amino acid 241 of the E4orf6 protein. The R241P E4orf6 variant cDNA was modified with another oligonucleotide, 5′-GCTGCGGGCGTCGCGAATCATCGCT-3′, that introduced an NruI restriction site at bp 750 of the E4orf6 coding region, replacing valine 250 with serine. The internal-deletion E4orf6 variant was made by removing the DNA fragment between the NruI and StuI sites of the R241P-V250S DNA. A proline residue was inserted between amino acids 255 and 256 of the E4orf6 protein by using an oligonucleotide, 5′-CGAATCATCGCTCCGGAGGAGACCACTG-3′, to create a BspEI site at bp 765 of the E4orf6 coding region. A proline was inserted between amino acids 235 and 236 of the E4orf6 protein (RC236RPC) by using an oligonucleotide that introduced a StuI restriction site at bp 708 of the E4orf6 coding region (5′-AAGTGAGATCAGGGTGAGGCCTTGCTGTGCCCGGAGG-3′). Finally, a proline residue was substituted at amino acid 239 by using an oligonucleotide, 5′-CAGGGTGCGCTGCTGTCCTAGGAGGACAAGGCGCC-3′, that created a AvrII site at bp 717 of the E4orf6 coding region. The mutations in the E4orf6 gene were identified by restriction analysis and confirmed by DNA sequencing.
Indirect immunofluorescence.
Indirect immunofluorescence and photomicroscopy of whole cells was conducted as previously described (43). Double-label immunofluorescence was performed with the mouse monoclonal antibody (MAb) 3 (38) specific for the amino terminus of the E4orf6 protein and the rat MAb 9C10 (Oncogene Science, Uniondale, N.Y. [54]) specific for the E1B 55-kDa protein. The secondary antibodies (Jackson ImmunoResearch, West Grove, Pa.) were multiple-label-qualified goat antibodies conjugated to dichlorotriazinylamino fluorescein and lissamine rhodamine sulfonyl chloride. Samples were examined with a Leitz Dialux 20 EB microscope fitted for epifluorescent illumination and photographed with TMax film (Eastman Kodak, Rochester, N.Y.) developed to an exposure index of 1600 ASA. Prints were prepared on high-contrast (no. 5) paper (Eastman Kodak), scanned at 300 dpi, and then cropped and assembled with Canvas 5.0 software (Deneba, Miami, Fla.) operating on a Macintosh microcomputer.
Protein structure.
Peptides (E4orf6 peptide [NH2-ARRTRRLMLRAVRIIAE-COOH] and L245P peptide [NH2-ARRTRRPMLRAVRIIAE-COOH]) were synthesized on an Applied Biosystems model 430A automated peptide synthesizer, and the amino and carboxy ends were left unblocked. Peptide concentrations were determined by quantitative amino acid analysis. Each peptide (200 μl) at a concentration (c) of 200 μM in 50 mM Na2PO4–150 mM NaCl (pH 7.0) in 0 to 80% trifluoroethanol (TFE) was dispensed in a stoppered optical cell with a path length (l) of 0.05 cm. The temperature of the sample was maintained at 25°C by circulation of water through a jacket surrounding the cell. Spectra were obtained on a Jasco 600 spectrophotometer by taking readings every 1 nm with a bandwidth of 1 nm. An average of four independent scans were baseline corrected and smoothed by using a third-order least-squared polynomial. Mean residue molar ellipticity ([θ]mean) in units of degrees times square centimeters per mole was calculated from the equation [θ]mean = [θ]observed × 1,000 × MRW/(10 × l × c). [θ]observed is the observed ellipticity in millidegrees, and MRW is the mean residue weight of the peptide. Structural and sequence analysis was performed with the assistance of the Wisconsin Package version 9.1 software (Genetics Computer Group, Madison, Wis.).
Complementation analysis and protein expression.
HeLa cells were infected with dl309 (30) or dl1014 (3) and simultaneously transfected with the E4orf6 variant cDNA constructs (see Fig. 7 for analysis). For these experiments, 4 × 105 cells in a 65-mm-diameter dish were exposed to 1 μg of plasmid DNA, 3 μg of Lipofectin, and 4 × 106 PFU of virus in a 2-ml volume of OptiMEM (Life Technologies). After 6 h at 37°C, the virus and plasmid mixture was replaced with normal growth medium and the cells were returned to normal growth conditions. After 48 h, the cells were collected, resuspended in urea sample buffer (7.5 M urea, 50 mM Tris [pH 6.8], 1% sodium dodecyl sulfate (SDS), 50 mM dithiothreitol, 5% 2-mercaptoethanol, 0.05% bromophenyl blue) and heated for 10 min at 65°C. The proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and electrophoretically transferred to nitrocellulose, and the E4orf6-related proteins were visualized on an E4orf6 Western blot with MAb 3 and chemiluminescence (Pierce, Rockford, Ill.). Replicate cultures of infected and transfected cells were harvested at 48 h postinfection to quantify virus yields. Detailed methods for Ad plaque assays have been described elsewhere (29). In brief, virus was harvested from HeLa cells by multiple cycles of freezing and thawing. The cell lysates were clarified by centrifugation and serially diluted for infection of W162 cells grown in six-well tissue culture dishes for plaque assays. After incubation with diluted lysates for 1 h, the W162 cells were overlaid with 0.7% SeaKem ME agarose (FMC BioProducts, Rockland, Maine) in Dulbecco modified Eagle’s minimal medium supplemented with 0.75% sodium bicarbonate and 4% newborn calf serum. The cells were overlaid with additional agarose in growth medium on the third and sixth day after infection. Plaques were visualized by staining with neutral red in an agarose overlay on the ninth day. Typically, data were collected from three dilutions in each series of dilutions. The virus yield was determined by linear regression and expressed as the number of plaques per milliliter of initial lysate. Replicate samples were compared with the two-tailed Student’s t test, where a probability of <0.05 was considered to represent a significant difference.
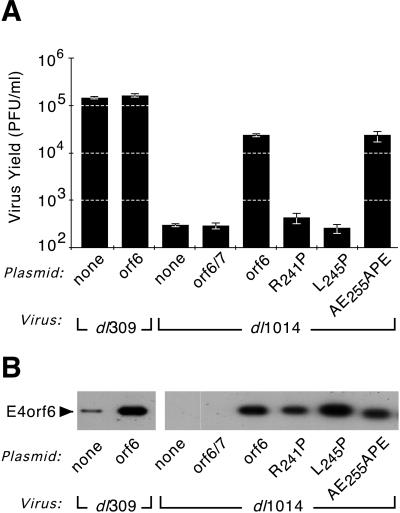
FIG. 7.
E4orf6 protein variants that retain the ability to direct the E1B 55-kDa protein to the nucleus rescue growth of an E4 deletion virus. HeLa cells were infected with the wild-type virus, dl309, or the E4 mutant virus lacking all but orf4, dl1014, at 10 PFU per cell and simultaneously transfected with cDNAs expressing the E4orf6-related constructs indicated at the left (Plasmid). The E4orf6-related proteins were expressed under the control of the major immediate-early promoter of CMV. (A) Virus was harvested after 48 h and quantified by plaque assay on the E4-complementing W162 cell line. The average amount of virus (expressed as PFU per milliliter) from three independent infections is shown with the standard deviations indicated. (B) In a parallel experiment, extracts of the infected and transfected cells were prepared at 48 h post-infection-transfection, and the proteins were separated by SDS-PAGE and transferred to a solid support. The E4orf6-related protein was visualized by immunoblotting with MAb 3 (38); the portion of the membrane containing the E4orf6 protein is shown.
RESULTS
The carboxy terminus of the E4orf6 protein is required for E1B 55-kDa protein nuclear colocalization.
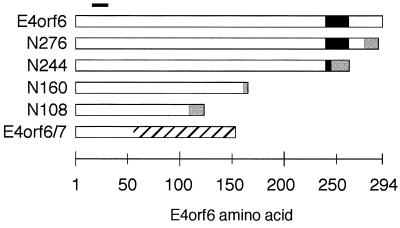
In Ad-infected cells, the E4orf6 protein directs the E1B 55-kDa protein to the peripheries of the viral transcription centers within the nucleus (43). Although the E1B 55-kDa protein is restricted to the cytoplasm when transiently expressed, it is directed to the nucleus when coexpressed with the E4orf6 protein (21). To determine if a discrete region of the E4orf6 protein is required for the nuclear localization of the E1B 55-kDa protein, a series of carboxy-terminal-truncation variants was created in E4orf6 cDNA. The E4orf6 sequences of these truncation variants are represented in Fig. 1, as well as the amino acids derived from vector sequences that are expressed in these variants, the E4orf7 sequence for the E4orf6/7 splice variant, and the MAb 3 (38) antibody recognition site, which is preserved in each of these constructs. These truncation variants were coexpressed with the E1B 55-kDa protein in HeLa cells with the vaccinia virus/T7 RNA polymerase infection-transfection expression system (17). The E4orf6 truncation variants (Fig. 2, left column) and the E1B 55-kDa protein (Fig. 2, center column) were visualized by indirect immunofluorescence. The nucleus was visualized by staining DNA with DAPI (4′,6-diamidino-2-phenylindole) (Fig. 2, right column). Unless otherwise noted, typical staining patterns are represented in Fig. 2 and 6.
FIG. 1.
Schematic representation of the carboxy-terminal-truncation variants of the E4orf6 protein and E4orf6/7 protein. The 294-residue E4orf6 protein is represented by the bar at the top of the figure. The solid portion of the bar represents the postulated extent of the arginine-faced, amphipathic α helix described in this report. Truncation variants are named according to the number of E4orf6-derived amino acids included in the variant, where the open bar represents E4orf6 amino acids. Amino acids encoded by vector sequences are represented by shading. Amino acids encoded by E4orf7 are indicated by cross-hatching. The solid black line over residues 16 through 22 indicates the epitope recognized by the E4orf6-specific antibody, MAb 3.
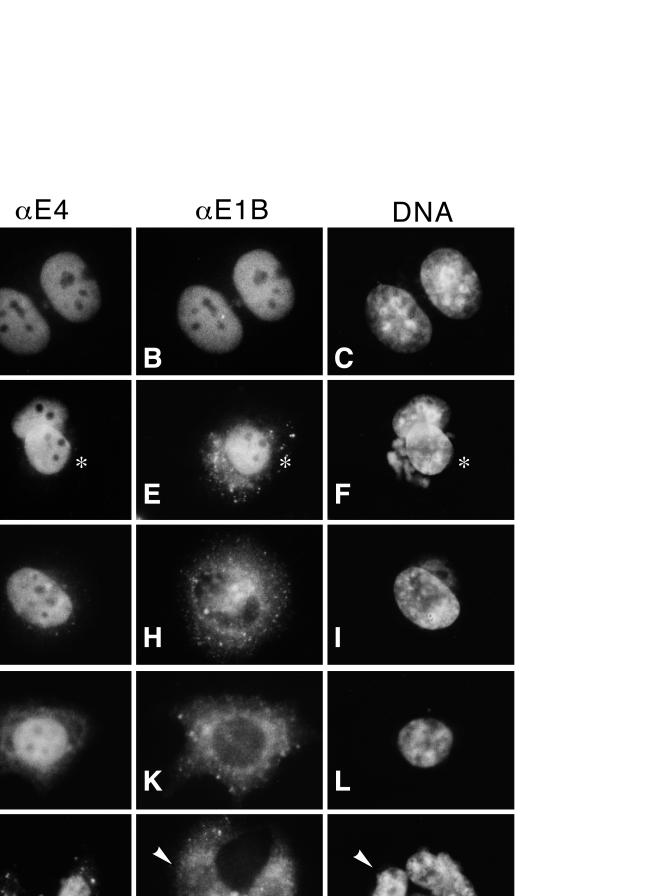
FIG. 2.
A region near the carboxy terminus of the E4orf6 protein is required to direct the E1B 55-kDa protein to the nucleus. HeLa cells were infected with the recombinant vaccinia virus vTF7.3 to establish expression of the T7 RNA polymerase and then transfected with cDNA under control of the T7 promoter encoding an E4orf6-related protein (indicated on the left) and the E1B 55-kDa protein. The Ad proteins were visualized by double-label immunofluorescence at 12 to 14 h posttransfection, and representative cells are shown except as noted in the text. E4orf6 proteins were visualized with the mouse MAb 3 (left column; αE4), E1B 55-kDa protein was visualized with the rat MAb 9C10 (center column; αE1B), and DNA was visualized with DAPI (right column; DNA). The asterisks in panels D, E, and F identify cells that express both the N276 E4orf6 variant and the E1B 55-kDa protein; the other cell in each field expresses only the N276 protein. The arrowheads in panels M, N, and O identify cells displaying the typical localization seen in approximately 90% of cells expressing the N108 E4orf6 protein.
FIG. 6.
Disruption of the predicted arginine-faced, amphipathic α helix in the E4orf6 protein abolishes its ability to direct nuclear localization of the E1B 55-kDa protein. Coexpression of the E1B 55-kDa protein and the indicated E4orf6 protein variants (described in Table 1) was established, and the localization of the Ad proteins was determined as described in the legend to Fig. 2. E4orf6 proteins were visualized with the mouse MAb 3 (left column; αE4), E1B 55-kDa protein was visualized with the rat MAb 9C10 (center column; αE1B), and DNA was visualized with DAPI (right column; DNA). Representative cells are shown for all constructs analyzed.
As previously reported (21), the E4orf6 protein localizes to the nucleus (Fig. 2A) and directs the E1B 55-kDa protein into the nucleus (Fig. 2B). In the absence of the E4orf6 protein, the E1B 55-kDa protein was restricted to the cytoplasm (reference 21 and data not shown). Staining for the wild-type viral proteins appears coincident in the nucleus and was excluded from the nucleoli (Fig. 2A and B). Similar results were obtained when the N276 E4orf6 truncation variant was coexpressed with the E1B 55-kDa protein (Fig. 2D and E). In both cells expressing the N276 variant seen in Fig. 2D, staining for the N276 variant appears indistinguishable from the wild-type E4orf6 staining pattern. Only one of the two cells seen in Fig. 2D also expressed the E1B 55-kDa protein; in this cell, most of the E1B 55-kDa protein appeared to localize to the nucleus (Fig. 2E).
Although the N276 E4orf6 truncation variant retained the ability to direct the E1B 55-kDa protein to the nucleus, this effect was not uniformly observed in all cells. Rather, in most of the cells expressing the N276 variant, a fraction of the total E1B 55-kDa protein remained in the cytoplasm. Thus, the N276 E4orf6 truncation variant retained the ability to direct the E1B 55-kDa protein to the nucleus, although less effectively than the wild-type E4orf6 protein (compare Fig. 2B and E). The E4orf6-specific staining pattern in the cell expressing the E1B 55-kDa protein (Fig. 2D) is the same as that seen in the cell expressing the N276 protein alone. This suggests that at the level of resolution afforded by immunofluorescence microscopy, the E1B 55-kDa protein does not affect localization of the N276 protein. In a similar manner, the localization of the other E4orf6 variants examined in this study was not affected by E1B 55-kDa protein expression.
The N244 E4orf6 truncation variant localized to the nucleus in a manner identical to that of the wild-type E4orf6 protein (Fig. 2G) but failed to direct the E1B 55-kDa protein to the nucleus (Fig. 2H). In N244-expressing cells, the localization of the E1B 55-kDa protein was identical to that of cells expressing the E1B 55-kDa protein alone. In contrast to the N244 or N276 truncation variants, a fraction of the total N160 protein was observed in the cytoplasm (Fig. 2J). In addition, the N160 truncation variant, like the N244 variant, failed to direct the E1B 55-kDa protein to the nucleus (Fig. 2K) in cells expressing both proteins. Successively larger carboxy-terminal deletions of the E4orf6 protein gave rise to proteins that partitioned increasingly in the cytoplasm. This is illustrated by the localization of the N108 variant in Fig. 2M. Most of the E4orf6-related protein in these cells accumulated in brightly staining bodies in the cytoplasm and nucleus, possibly as aggregates of malfolded protein. In the 10% of cells containing the N108 variant in the nucleus, the staining pattern did not resemble the wild-type E4orf6 staining pattern because nucleolar exclusion of the protein was not evident. The E1B 55-kDa protein failed to localize to the nucleus in all cells expressing the N108 variant (Fig. 2N). The E4orf6/7 protein, composed of the first 58 residues of the E4orf6 protein and 92 residues of the E4orf7 protein, localized predominantly to the nucleus and was excluded from the nucleoli (Fig. 2P). The localization of the E4orf6/7 protein was most similar to that of the N160 truncation variant because a fraction of the total E4orf6/7 protein expressed in cells was found in the cytoplasm (Fig. 2P). Moreover, the E4orf6/7 protein failed to direct the E1B 55-kDa protein to the nucleus (Fig. 2Q).
The inability of the E4orf6 variants to direct nuclear localization of the E1B 55-kDa protein was not due to reduced levels of these proteins. Lysates derived from parallel cultures of cells expressing the E4orf6 truncation variants analyzed in Fig. 2 were analyzed by an immunoblot to determine the steady-state level of the E4orf6-related proteins (Fig. 3). With the exception of the E4orf6/7 protein, the levels of all protein variants seen in Fig. 3 were comparable to or exceeded the level of the wild-type E4orf6 protein. The N108 variant (Fig. 3, lane 5) appeared to be expressed to levels significantly greater than any of the other E4orf6 variants. This amount of protein was not anticipated on the basis of the weak immunofluorescence intensity seen in Fig. 2M. Perhaps the MAb 3 epitope is less accessible in aggregates of N108 within the cell prepared for immunofluorescence. Finally, the apparent mobilities of the E4orf6 variant proteins shown in Fig. 3 agree with the predicted values, although the aberrant electrophoretic mobility of E4orf6-related proteins is evident in Fig. 3. Note that the wild-type E4orf6 protein (Fig. 3, lane 1) migrates with an apparent molecular mass of 29 kDa (49), although the protein has a predicted molecular mass of 34 kDa. In a similar manner, the E4orf6/7 protein (Fig. 3, lane 6) migrates at an apparent molecular mass of 20 kDa (8), although it has a predicted molecular mass of 17 kDa.
FIG. 3.
Carboxy-terminal truncation variants of the E4orf6 protein are expressed to comparable levels in HeLa cells. The E1B 55-kDa protein and E4orf6 variant proteins depicted in Fig. 1 were expressed by the vaccinia virus/T7 RNA polymerase system as described in the legend to Fig. 2. Total cell protein was isolated 14 h post-infection-transfection, separated by SDS-PAGE, and transferred to a solid support. The E4orf6-related protein was visualized by immunoblotting with MAb 3 (38). The approximate positions of molecular mass standards are indicated in kilodaltons on the left.
From these results it seemed likely that the region between amino acids 244 and 276 of the E4orf6 protein was important for E1B 55-kDa protein nuclear localization. Therefore the primary structure and potential secondary structure of this region were closely examined. The region is predominantly composed of arginine and hydrophobic residues. Both the empirical secondary structure algorithm of Chou and Fasman and the theoretical algorithm of Garnier, Osguthorpe, and Robson predict that an α helix exists between amino acids 244 and 260 (Fig. 4A) (6, 19). However, the Chou and Fasman secondary-structure prediction algorithm predicts that this α helix begins at amino acid 239. A hydrophilicity (33) plot predicts that this region is flanked by two hydrophilic, surface-exposed regions (Fig. 4A). In addition, the region has a significant hydrophobic moment at approximately 100° of rotation between adjacent residues from amino acids 239 through 253 of the E4orf6 protein (Fig. 4B), corresponding to the writhe of an α helix (45). Since this region is predicted to exist as an α helix, a helical-wheel diagram of this region was constructed (Fig. 4C). Strikingly, this structure appears as an amphipathic α helix consisting of a hydrophobic face and a hydrophilic face that is almost exclusively composed of arginine residues. Furthermore, an Eisenberg plot of this region yields maximum values for the mean hydrophobic moment (μH = 0.58) and the mean hydrophobicity (H = 0.023) that are characteristic of amphipathic α helix that lies at a boundary between the hydrophobic core of the protein and a surface-exposed face of the protein (12, 13). From these considerations, it seemed possible that such a striking feature in the E4orf6 polypeptide may govern the nuclear localization of the E1B 55-kDa protein.
FIG. 4.
The region of the E4orf6 protein required to direct E1B 55-kDa protein to the nucleus is predicted to contain an arginine-faced, amphipathic α helix. (A) The E4orf6 protein between amino acids 194 and 294 of the E4orf6 protein was analyzed for hydrophilicity by the method of Kyte and Doolittle (33), for surface probability by the method of Emini et al. (14), and for predicted α helices by the methods of Chou and Fasman (6) and Garnier et al. (19). Regions of striking hydrophilicity and high surface probability are highlighted by shading. The hatched region indicates predicted α-helical segments that are analyzed further below. (B) The hydrophobic moment of the E4orf6 protein between amino acids 225 and 260 is shown for all possible angles of rotation. Contour lines are plotted for values of 0.35 and 0.45 as determined by the method of Finer-Moore and Stroud (15) with a window of 11 amino acids. The dashed lines flank the region of the plot corresponding to the angle of rotation associated with an α helix (100° ± 5°). (C) A helical-wheel presentation of amino acids 239 through 255 of E4orf6 depicts an amphipathic structure dominated by arginine residues on one face and hydrophobic residues on the other face. Charged amino acids are indicated by the charge at neutral pH, and hydrophobic amino acids are boxed.
An arginine-faced, amphipathic α helix exists within the E4orf6 protein.
To test if the amino acids of the predicted E4orf6 α-helical region can adopt an α-helical secondary structure, a synthetic peptide corresponding to amino acids 239 to 255 of the E4orf6 protein was prepared and tested for secondary structure by circular dichroism (CD) spectroscopy. CD and proton magnetic resonance studies of peptides derived from proteins of known secondary structure show that the conformational preference of the peptides correlates well with the secondary structures of the respective regions in the proteins (11). In near-physiological conditions, the CD spectrum of the E4orf6 peptide resembles spectra correlated with the atypical conformation (Fig. 5A), a conformation previously identified as random coil, consisting of a large negative band around 200 nm and a peak or shoulder with a small negative value at 220 nm (23). This is the expected behavior for a peptide composed of over 50% hydrophobic residues (9 of 17) in an aqueous environment. TFE, an organic polar solvent, stabilizes α-helical structure by strengthening intermolecular hydrogen bonding and fostering peptide-peptide dimerization of amphipathic peptides (28, 39). Although TFE-induced multimerization of the E4orf6 peptide (data not shown), the spectra of the peptide in 30% or less TFE were not characteristic of any known secondary structures (Fig. 5A). However, the E4orf6 peptide spectra obtained in 30% or less TFE intersect at an isobestic point at 209 nm, which is indicative of a biphasic secondary-structure transition. By contrast, in greater than 30% TFE, the CD spectra of the E4orf6 peptide resembled the spectra of an α helix containing a positive peak around 190 to 195 nm and two negative peaks at 208 and 222 nm (Fig. 5A) (23, 25). In addition, the E4orf6 peptide spectra in 30% or greater TFE cross at an isobestic point at 201 nm that is indicative of a second biphasic transition (Fig. 5A). Although the magnitudes of the minima at 208 and 222 nm exceed the theoretical values for a monomeric α-helical peptide (5, 25), recent studies have demonstrated that multimers of amphipathic peptides produce CD spectra with minima at 222 and 208 nm comparable to those observed for the E4orf6 peptide in Fig. 5A (44, 52).
FIG. 5.
Peptides corresponding to the predicted arginine-faced, amphipathic α helix of the E4orf6 protein exhibit α-helical spectra when analyzed by CD spectroscopy in the presence of TFE. CD spectra of the E4orf6 peptide (A) and L245P peptide (B) at a concentration of 200 μM in 50 mM Na2PO4–150 mM NaCl (pH 7.0) at 25°C in various concentrations of TFE. The measured CD values were converted to mean residue molar ellipticities, [θ]mean, and plotted as a function of the incident wavelength. The arrowheads above the x axis identify the minima associated with an α-helical spectrum. (C) Changes in [θ]mean measured at 222 nm in increasing concentrations of TFE reveal a triphasic transition in the secondary structure of the E4orf6 peptide. The value for [θ]mean measured at 222 nm is plotted on the y axis as a function of the percent TFE on the x axis.
A second peptide, corresponding to the sequence of the E4orf6 protein between residues 239 and 255 with leucine 245 of the E4orf6 protein changed to proline, L245P, was synthesized and tested for secondary structure by CD spectroscopy. Proline was introduced into the E4orf6 peptide because its rigid-ringed backbone cannot conform to the phi and psi angles required for an α helix (37). Thus, the introduction of a proline residue in the E4orf6 peptide should disrupt its α-helical secondary structure. In near-physiological buffer conditions, the spectra of the L245P peptide resembled those of an atypical structure (Fig. 5B). The addition of TFE also induced multimerization of the L245P peptide (data not shown), and like the E4orf6 peptide, the L245P peptide exhibited an atypical conformation in less than 30% TFE (Fig. 5B). By contrast, in the presence of 30% or more TFE, the CD spectra of the L245P peptide resembled spectra of an α-helical peptide. In addition, all of the L245P peptide spectra cross at an isobestic point at 204 nm, which is indicative of an atypical-to-α-helical two-phase transition. A similar biphasic transition from atypical to α-helical secondary structure has been reported for the 26-residue melittin peptide upon dimerization (52).
The multiphasic transition of the E4orf6 peptide and the biphasic transition of the L245P peptide in increasing TFE concentrations are more apparent upon plotting the [θ]mean at 222 nm versus TFE concentration (Fig. 5C). The curves obtained from the E4orf6 peptide exhibit two saturation points that may reflect a triphasic transition, whereas the L245P peptide does not achieve saturation even in 80% TFE. As expected, the proline residue in the L245P peptide encumbers α-helix formation. Indeed, the helical content of both peptides in greater than 30% TFE is directly proportional to the [θ]mean at 222 nm. Therefore, although both peptides are able to adopt an α-helical conformation in greater than 30% TFE, the E4orf6 peptide has a greater helical content than the L245P peptide.
The integrity of an arginine-faced, amphipathic α helix is required for E1B 55-kDa protein nuclear localization.
To test whether the arginine-faced, amphipathic α helix of the E4orf6 protein is important for the nuclear localization of the E1B 55-kDa protein, a small in-frame deletion and proline replacement or insertion mutations were introduced into this region (Table 1). These E4orf6 variants were then tested for their ability to direct the E1B 55-kDa protein to the nucleus. The E4orf6 variants were coexpressed with the E1B 55-kDa protein by the vaccinia virus/T7 RNA polymerase infection-transfection system (17), and the localization of the E4orf6 variants and the E1B 55-kDa protein was determined by indirect immunofluorescence. The variant bearing the internal deletion, Δ241–250, localized to the nucleus to the same extent as the wild-type E4orf6 protein (Fig. 6D) but failed to direct the E1B 55-kDa protein to the nucleus (Fig. 6E), thus supporting the importance of this region of the E4orf6 protein for nuclear localization of the E1B 55-kDa protein.
TABLE 1.
E4orf6 variants bearing mutations in the vicinity of the arginine faced, amphipathic α helixa
| E4orf6 variant | Amino acid sequence from 236 through 255 |
|---|---|
| Wild type | NH2-R CCARRTRRLMLRAVRIIA E-COOH |
| Δ241–250b | NH2-R CCAR----------RIIA E-COOH |
| RC236RPCc | NH2-RPCCARRTRRLMLRAVRIIA E-COOH |
| A239Pc | NH2-R CCPRRTRRLMLRAVRIIA E-COOH |
| R241Pc | NH2-R CCARPTRRLMLRAVRIIA E-COOH |
| L245Pc | NH2-R CCARRTRRPMLRAVRIIA E-COOH |
| AE255APEc | NH2-R CCARRTRRLMLRAVRIIAPE-COOH |
Amino acids 236 through 255 of the E4orf6 protein are shown in the single-letter amino acid code, and the designation of the appropriate construct is indicated.
Removal of amino acids 241 through 250 is indicated by dashes.
The replacement or insertion of proline is indicated by the boldfaced P.
Proline residues were introduced at the beginning, middle, and end of the predicted α helix to potentially disrupt the α helix. These E4orf6 variants were coexpressed with the E1B 55-kDa protein, and the localization of each protein was determined. The R241P variant localized to the nucleus to the same extent as the wild-type E4orf6 protein (compare Fig. 6A and G). However, the R241P variant failed to direct the nuclear localization of the E1B 55-kDa protein (Fig. 6H). Although the introduction of proline at position 241 replaced the charged arginine residue with a neutral amino acid, when arginine 241 was replaced with leucine, this variant directed E1B 55-kDa protein nuclear localization as well as the wild-type E4orf6 protein (data not shown). Therefore, it seems likely that the introduction of proline at position 241, rather than the loss of the charged arginine, disrupted the function of the E4orf6 protein. It should be noted that when the R241P variant protein was coexpressed with the E1B 55-kDa protein under control of the cytomegalovirus (CMV) major immediate-early promoter, rather than by the vaccinia virus/T7 RNA polymerase system, a portion of the E1B 55-kDa protein localized to the nucleus in a small fraction of cells (6 of 86) expressing both proteins. This suggested that this variant may retain some ability to interact with the E1B 55-kDa protein (data not shown). The basis for the difference in behavior between the proteins expressed by these two systems is not known. However, the limited ability of the R241P protein to direct nuclear localization of the E1B 55-kDa protein may give rise to the partial function of this mutant protein that was measured in the experiment described below (see Fig. 7).
The L245P variant, like the R241P variant, also localized to the nucleus (Fig. 6G) and failed to direct the nuclear localization of the E1B 55-kDa protein (Fig. 6H). By contrast, the AE255APE variant acted like the wild-type E4orf6 protein with respect to the localization of both E4orf6 protein (Fig. 6M) and the E1B 55-kDa (Fig. 6N) protein. Unlike the R241P variant, both the L245P and AE255APE variants expressed from the CMV immediate-early promoter affected the E1B 55-kDa protein in a manner identical to that seen with the vaccinia virus expression system. Additional variant proteins containing a proline replacement, A239P, and a proline insertion, RC236RPC, were unable to direct nuclear localization of the E1B 55-kDa protein, although these variants were also localized to the nucleus (data not shown). These results are consistent with the suggestion that the arginine-faced, amphipathic α helix of the E4orf6 protein is required for its ability to direct the E1B 55-kDa protein to the nucleus because this property can be abolished by disrupting the integrity of this α helix with proline residues.
Disrupting the ability of the E4orf6 protein to direct nuclear localization of the E1B 55-kDa protein abolishes E4orf6 function during a lytic Ad infection.
Introduction of a proline residue into the predicted arginine-faced, amphipathic α helix of the E4orf6 protein not only reduced the α-helical content of this region (Fig. 5) but also disrupted nuclear colocalization of the E1B 55-kDa protein (Fig. 6). To determine if the E4orf6 variants containing proline mutations could complement growth of the E4 mutant virus, dl1014, which expresses only the E4orf4 protein (32), HeLa cells were infected with dl1014 and simultaneously transfected with cDNAs expressing the E4orf6 proline variants. Forty-eight hours after infection, the amount of virus in these cells was quantified by a plaque assay with an E4-complementing cell line, W162 (53).
Cells infected with a phenotypically wild-type virus, dl309, produced the same amount of virus whether transfected with the E4orf6 cDNA or untransfected (Fig. 7A). Therefore, ectopic expression of the E4orf6 protein did not alter virus production. As expected, cells infected with dl1014 produced nearly 1,000-fold less virus than dl309-infected cells (Fig. 7A). However, cells infected with dl1014 and simultaneously transfected with an E4orf6 cDNA produced 200-fold more virus than untransfected dl1014-infected cells (Fig. 7A). This yield corresponds to a fivefold reduction compared to wild-type dl309-infected cells. The infected and transfected cells were evaluated by immunofluorescence for expression of an early viral protein, the E1B 55-kDa protein, and the E4orf6-related protein. Essentially all of the cells expressed the E1B 55-kDa protein and therefore were infected with the virus. However, only 20 to 30% of the cells expressed the E4orf6-related protein (data not shown). Thus, the limited transfection efficiency may account for most, if not all of the decreased virus yield from dl1014-infected cells transfected with the wild-type E4orf6 cDNA. By contrast, expression of the E4orf6/7 protein in dl1014-infected cells failed to affect growth of the mutant virus. These cells produced statistically the same amount of virus as untransfected dl1014-infected cells (Fig. 7A), indicating that the E4orf6/7 protein cannot supply E4orf6 function during Ad infection.
The E4orf6 proline variants that failed to direct the E1B 55-kDa protein to the nucleus largely failed to complement growth of dl1014. E4orf6-R241P expression in dl1014-infected cells increased virus yields twofold over those of untransfected or L245P-transfected cells (Fig. 7A). This slight, albeit significant (P < 0.05), increase may reflect the limited ability of the E4orf6-R241P protein to direct E1B 55-kDa protein nuclear localization in a small number of cells when expressed from the CMV immediate-early promoter. Expression of the E4orf6-L245P protein completely failed to rescue growth of dl1014. Cells infected with dl1014 and transfected with a cDNA encoding E4orf6-L245P protein produced the same amount of virus as untransfected dl1014-infected cells (Fig. 7A). By contrast, expression of the E4orf6-AE255APE variant in dl1014-infected cells complemented virus growth to the same extent that the wild-type E4orf6 cDNA complemented virus growth (Fig. 7A). The amount of viral DNA synthesized in the simultaneously infected and transfected cells was measured by hybridization analysis and was found to reflect the yield of virus (data not shown). Thus, the severity of the defect measured for the proline variants R241P and L245P suggests that the arginine-faced, amphipathic α helix may be important for the multiple roles of the E4orf6 protein, including viral DNA synthesis, viral mRNA transport, and the stability of nuclear viral mRNA.
In a parallel experiment, levels of the E4orf6 proteins were measured to determine if aberrant expression of the E4orf6-related proteins could account for decreased virus yields. A portion of the cells used to determine virus yields were used to determine E4orf6 expression levels by E4orf6 immunoblotting. Expression of the E4orf6 protein from a plasmid in dl309-infected cells increased E4orf6 expression 10-fold compared to that of untransfected dl309-infected cells (Fig. 7B). As expected, neither the E4orf6/7-transfected nor untransfected dl1014-infected cells expressed a wild-type E4orf6 protein. dl1014-infected cells that were transfected with cDNAs encoding the E4orf6-related proteins contained approximately the same amount of E4orf6-related protein (Fig. 7B). Expression of the E4orf6 variant was greatest in cells expressing the E4orf6-L245P protein, yet this mutation was the most defective in its ability to rescue dl1014. From this analysis, it seems unlikely that aberrant expression levels of the E4orf6 proline variants could account for differences in virus yields.
DISCUSSION
In this study, we identified a structural element of the E4orf6 protein required for its functional interaction with the E1B 55-kDa protein in the cell and demonstrated that this element is required for productive Ad infection. This element is an arginine-faced, amphipathic α helix that is essential for E1B 55-kDa protein nuclear colocalization. E4orf6 protein variants lacking or containing disruptions within this arginine-faced, amphipathic α helix failed to direct the E1B 55-kDa protein to the nucleus when the proteins were transiently expressed. Furthermore, E4orf6 variants that could not direct the nuclear colocalization of the E1B 55-kDa protein also failed to supply E4orf6 function in cells infected with an E4 deletion virus. The severity of the defect of these E4orf6 variants during an Ad infection suggests that the arginine-faced, amphipathic α helix is crucial for the multiple functions of the E4orf6 protein.
A region within the carboxy terminus of the E4orf6 protein is required for the nuclear colocalization of the E1B 55-kDa protein. By contrast, a recent study of Rubenwolf et al. suggests that a region within the amino-terminal 58 amino acids of the E4orf6 protein mediates E4orf6–E1B 55-kDa protein binding in vitro (48). In this study, carboxy-terminal E4orf6 protein fragments failed to bind the E1B 55-kDa protein whereas amino-terminal E4orf6 protein fragments, including the E4orf6/7 protein, bound the E1B 55-kDa protein in vitro. These investigators also were able to indirectly immunoprecipitate the E4orf6/7 protein with E1B 55-kDa protein-specific antibodies after expressing the E4orf6/7 protein by transfection in 293 cells. However, these same investigators, as well as others, failed to detect an interaction between the E4orf6/7 protein and the E1B 55-kDa protein during Ad infection (8, 24, 48). The reason for these discrepancies is not known but may be due to the different experimental approaches used to measure the E4orf6–E1B 55-kDa protein interaction. Further support for the significance of the carboxy terminus of E4orf6 in E1B 55-kDa protein nuclear localization can be derived from an unpublished observation from our laboratory. An E4orf6 protein variant lacking the first 58 amino acids failed to localize to the nucleus and failed to promote E1B 55-kDa protein nuclear colocalization; however, the addition of a nuclear localization signal from the large T antigen of simian virus 40 to this variant promoted the nuclear localization of the protein and concurrently restored its ability to direct the E1B 55-kDa protein to the nucleus. Although we cannot exclude the possibility that the amino terminus of the E4orf6 protein is involved in binding the E1B 55-kDa protein, the results presented in this study suggest that the carboxy terminus of the E4orf6 protein is essential for E1B 55-kDa protein nuclear localization.
The region of the E4orf6 protein required for the nuclear localization of the E1B 55-kDa protein is an arginine-faced, amphipathic α helix. A synthetic peptide corresponding to this region adopted an α-helical structure as measured by CD spectroscopy. Although the shapes of the L245P peptide CD spectra resembled the shapes of the spectra for the wild-type E4orf6 peptide in the presence of TFE, the magnitudes of the minima of the L245P peptide at 208 and 222 nm are less than that of the wild-type E4orf6 peptide. The behavior of the L245P peptide suggests that the L245P protein may retain some α-helical structure in this region. Nonetheless, this mutant protein was unable to promote the nuclear localization of the E1B 55-kDa protein. Similarly, E4orf6 protein variants containing proline residues at amino acids 239 and 241 also failed to direct the E1B 55-kDa protein to the nucleus. However, an E4orf6 protein variant encoding proline after amino acid 255 promoted the nuclear localization of the E1B 55-kDa protein as well as the wild-type E4orf6 protein. These results are consistent with the possibility that an α helix extends from amino acids 239 through 245, but not beyond amino acid 255. A hydrophobic-moment plot of this region reveals that an α helix between amino acids 239 and 253 would exhibit a strong hydrophobic moment (Fig. 4B). This observation leads us to propose the existence of an α helix through residue 253. In addition, amino acid 251 of the E4orf6 protein is an arginine. Therefore, if the arginine face of this α helix is critical for E4orf6 function, it seems likely that the α helix would include arginine 251. Since amino acids 252 and 253 of this region are α-helix-favoring isoleucine residues, it also seems likely that these residues would be part of the arginine-faced amphipathic α helix. Because the proline-containing variants, RC236RPC and A239P, were defective at directing nuclear localization of the E1B 55-kDa protein, we cannot definitively identify the amino terminus of the arginine-faced, amphipathic α helix. However, it seems reasonable that amino acid 239 is the amino-terminal residue of this α helix, since amino acid 239 is an α-helix-favoring alanine residue and amino acids 237 and 238 are non-α-helix-promoting cysteine residues. From these considerations, we suggest that an arginine-faced amphipathic α helix exists between amino acids 239 and 253 and that this α helix lies at an interface between the hydrophobic core of the protein and a solvent-exposed face.
Most amphipathic α helices that have been described are membrane associated and frequently contribute to the aqueous pore of a protein channel through a membrane (reference 51 and references therein). However, an arginine-rich, amphipathic α helix that mediates protein-protein interaction has been identified in the human immunodeficiency virus (HIV) Nef protein (2). The Nef protein is dispensable for virus growth in vitro but is a key factor in the pathogenesis of HIV (31, 36). In HIV-infected cells, the Nef protein modulates the activity of kinases involved in T-cell activation pathways; the arginine-rich, amphipathic α helix in the amino-terminal region of Nef is required for Nef binding to an uncharacterized serine kinase and the Lck tyrosine kinase (2, 7, 50). To our knowledge, the Nef and E4orf6 proteins are the only two examples of proteins that use an arginine-rich amphipathic α helix to mediate protein-protein interaction.
Previous studies in our laboratory suggest that the interaction between the E4orf6 protein and the E1B 55-kDa protein in vivo is indirect and that a primate-specific cellular factor is required for the E4orf6-mediated nuclear localization of the E1B 55-kDa protein. Perhaps the arginine-faced, amphipathic α helix of the E4orf6 protein interacts with such a cellular factor. Additional support for the existence of an interaction between this α helix and unknown cellular factors derives from unpublished observations on the long-term expression of the E4orf6 protein and the L245P protein. We found that expression of the wild-type E4orf6 protein in HeLa or 293 cells could not be sustained for greater than 4 weeks, whereas expression of the L245P protein could be maintained indefinitely (42a). Perhaps both the Nef and E4orf6 viral proteins recruit cellular factors to modulate host activities through an arginine-rich, amphipathic α helix.
A recent report from Dobbelstein et al. suggests that the arginine-faced, amphipathic α helix of the E4orf6 protein acts as a nuclear retention signal (9). These investigators failed to observe shuttling of the wild-type E4orf6 protein between nuclei within a heterokaryon formed of HeLa and mouse cells, whereas an E4orf6 variant containing a glutamic acid in place of arginine 248 was observed to shuttle. However, in previous studies, we found that the wild-type E4orf6 protein does shuttle between nuclei within heterokaryons formed of HeLa or rat cells alone (21) or within heterokaryons of HeLa and rat cells (unpublished data). The reason for these differences is unclear but may reflect the use of different cell lines or different experimental conditions. Although we did not directly measure shuttling of the E4orf6 variants in the experiments reported here, we failed to detect a difference between the intracellular distribution of any E4orf6 proteins bearing mutations in the arginine-faced α helix and the wild-type E4orf6 protein. However, carboxy-terminal truncations that removed the α helix as well as 80 or more amino acids on the amino-terminal side of the α helix created proteins that partitioned more strongly in the cytoplasm than the wild-type E4orf6 protein. These results suggest that sequences within the E4orf6 protein indeed affect the extent to which the protein partitions between the nucleus and cytoplasm; however, these sequences appear to be amino terminal to the α helix identified in this report.
Expression of the E4orf6 protein restored growth of the E4 deletion virus, dl1014, to near-wild-type levels (Fig. 7). Two previously published observations led us to anticipate this result. Ketner et al. (32) demonstrated that ectopically expressed E4orf6 protein restored growth of a larger E4 deletion mutant. Similarly, E4 mutant viruses that express only the E4orf6 protein grow to near-wild-type levels (1, 3, 26). Strikingly, the L245P E4orf6 variant, which failed to direct nuclear localization of the E1B 55-kDa protein (Fig. 6), completely failed to enhance replication of dl1014 (Fig. 7). By contrast, expression of the R241P variant provided a slight but significant (P < 0.05) enhancement to dl1014 growth over untransfected or L245P-transfected cells. Unlike the L245P variant, the R241P variant displayed a limited ability to direct nuclear localization of the E1B 55-kDa protein when expressed from the CMV immediate-early promoter (data not shown). The reason for this limited ability is not clear, but it may, for example, be indicative of a truncated, arginine-faced, amphipathic α helix. Nonetheless, this result reinforces the correlation between the ability of the E4orf6 protein to direct the E1B 55-kDa protein to the nucleus and the ability of the E4orf6 protein to function during Ad lytic growth.
In conclusion, we have demonstrated that an arginine-faced α helix near the carboxy terminus of the E4orf6 protein is essential for the E4orf6 protein to direct nuclear localization of the E1B 55-kDa protein. Furthermore, we suggest that the arginine-faced α helix is critical for the function of the E4orf6 protein during a productive viral infection. These findings are consistent with the possibility that in the absence of the E4orf3 protein, the E4orf6 protein can enhance virus growth only through its interaction with the E1B 55-kDa protein. Alternatively, the arginine-faced, amphipathic α helix of the E4orf6 protein may be important for the multiple functions of the E4orf6 protein during an Ad infection. Perhaps the basic face of the amphipathic α helix mediates interactions with multiple cellular factors in a manner similar to that described for the Nef protein of HIV. The identity of such factors should provide valuable insight into the means by which the E4orf6 protein can mediate mRNA export, promote viral mRNA accumulation, modulate viral mRNA splicing, enhance viral DNA synthesis and act as an oncoprotein.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI35589 from the National Institute of Allergy and Infectious Disease. J.S.O. was supported in part by NIH Training Grant T32 AI07401 to the Department of Microbiology and Immunology. Tissue culture reagents and services were provided by the Tissue Culture Core Laboratory and oligonucleotide synthesis was performed by the DNA Synthesis Core Laboratory, both at the Comprehensive Cancer Center of Wake Forest University supported in part by NIH grant CA12197. Peptide synthesis and amino acid analysis were performed in the Protein Analysis Core Laboratory of the Comprehensive Cancer Center of Wake Forest University, supported in part by NIH grants CA-12197 and RR-04869, as well as by a grant from the North Carolina Biotechnology Center.
We gratefully acknowledge Gary Ketner (Johns Hopkins University) for the dl1014 virus and W162 cells and Tom Shenk (Princeton University) for the dl309 virus. We also thank John Parks for the use of the Jasco 600 CD spectrophotometer. We acknowledge Mark Lively and Mark Morris for assistance with peptide synthesis and analysis and Roy Hantgan for assistance with protein structure analysis. Felicia Goodrum, Doug Lyles, and Griff Parks provided valuable advice on the work in progress and on manuscript preparation.
REFERENCES
- 1.Armentano D, Sookdeo C C, Hehir K M, Gregory R J, St. George J A, Prince G A, Wadsworth S C, Smith A E. Characterization of an adenovirus gene transfer vector containing an E4 deletion. Hum Gene Ther. 1995;6:1343–1353. doi: 10.1089/hum.1995.6.10-1343. [DOI] [PubMed] [Google Scholar]
- 2.Baur A S, Saas G, Laffert B, Willbold D, Cheng-Mayer C, Peterlin B M. The N-terminus of Nef from HIV-1/SIV associates with a protein complex containing Lck and a serine kinase. Immunity. 1997;6:283–291. doi: 10.1016/s1074-7613(00)80331-3. [DOI] [PubMed] [Google Scholar]
- 3.Bridge E, Ketner G. Redundant control of adenovirus late gene expression by early region 4. J Virol. 1989;63:631–638. doi: 10.1128/jvi.63.2.631-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bridge E, Ketner G. Interaction of adenoviral E4 and E1B products in late gene expression. Virology. 1990;174:345–353. doi: 10.1016/0042-6822(90)90088-9. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y H, Yang J T, Chau K H. Determination of the alpha-helix and beta-form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974;13:3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- 6.Chou P Y, Fasman G D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- 7.Collette Y, Duartre H, Benziane A, Ramos-Morales F, Benarous R, Harris M, Olive D. Physical and functional interaction of Nef with Lck. J Biol Chem. 1996;271:6333–6341. doi: 10.1074/jbc.271.11.6333. [DOI] [PubMed] [Google Scholar]
- 8.Cutt J R, Shenk T, Hearing P. Analysis of adenovirus early region 4-encoded polypeptides synthesized in productively infected cells. J Virol. 1987;61:543–552. doi: 10.1128/jvi.61.2.543-552.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobbelstein M, Roth J, Taylor-Kimberly W, Levine A, Shenk T. Nuclear export of the E1B 55-kDa and E4 34-kDa adenoviral oncoproteins mediated by a rev-like signal sequence. EMBO J. 1997;16:4276–4284. doi: 10.1093/emboj/16.14.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobner T, Horikoshi N, Rubenwolf S, Shenk T. Blockage by adenovirus E4orf6 of transcriptional activation by the p53 tumor suppressor. Science. 1996;272:1470–1473. doi: 10.1126/science.272.5267.1470. [DOI] [PubMed] [Google Scholar]
- 11.Dyson H J, Merutka G, Waltho J P, Lerner R A, Wright P E. Folding of peptide fragments comprising the complete sequence of proteins. Models for initiation of protein folding. I. Myohemerythrin. J Mol Biol. 1992;226:795–817. doi: 10.1016/0022-2836(92)90633-u. [DOI] [PubMed] [Google Scholar]
- 12.Eisenberg D, Schwartz E, Komaromy M, Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984;179:125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg D, Weiss R M, Terwilliger T C, Wilcox W. Hydrophobic moments and protein structure. Faraday Symp Chem Soc. 1982;17:109–120. [Google Scholar]
- 14.Emini E A, Hughes J V, Perlow D S, Boger J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J Virol. 1985;55:836–839. doi: 10.1128/jvi.55.3.836-839.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finer-Moore J, Stroud R M. Amphipathic analysis and possible formation of the ion channel in an acetylcholine receptor. Proc Natl Acad Sci USA. 1984;81:155–159. doi: 10.1073/pnas.81.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freyer G A, Katoh Y, Roberts R J. Characterization of the major mRNAs from adenovirus 2 early region 4 by cDNA cloning and sequencing. Nucleic Acids Res. 1984;12:3503–3519. doi: 10.1093/nar/12.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuerst T R, Niles E G, Studier F W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabler S, Schütt H, Groitl P, Wolf H, Shenk T, Dobner T. E1B 55-kilodalton-associated protein: a cellular protein with RNA-binding activity implicated in nucleocytoplasmic transport of adenovirus and cellular mRNAs. J Virol. 1998;72:7960–7971. doi: 10.1128/jvi.72.10.7960-7971.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garnier J, Osguthorpe D J, Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978;120:97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- 20.Goodrum F D, Ornelles D A. The early region 1B 55-kilodalton oncoprotein of adenovirus relieves growth restrictions imposed on viral replication by the cell cycle. J Virol. 1997;71:548–561. doi: 10.1128/jvi.71.1.548-561.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodrum F D, Shenk T, Ornelles D A. Adenovirus early region 4 34-kilodalton protein directs the nuclear localization of the early region 1B 55-kilodalton protein in primate cells. J Virol. 1996;70:6323–6335. doi: 10.1128/jvi.70.9.6323-6335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham F L, Smiley J, Russell W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–72. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 23.Greenfield N, Fasman G D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969;8:4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- 24.Halbert D N, Cutt J R, Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol. 1985;56:250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holtzwarth G, Doty P. The ultraviolet circular dichroism of polypeptides. J Am Chem Soc. 1965;87:218–228. doi: 10.1021/ja01080a015. [DOI] [PubMed] [Google Scholar]
- 26.Huang M M, Hearing P. Adenovirus early region 4 encodes two gene products with redundant effects in lytic infection. J Virol. 1989;63:2605–2615. doi: 10.1128/jvi.63.6.2605-2615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutchison C A, III, Phillips S, Edgell M H, Gillam S, Jahnke P, Smith M. Mutagenesis at a specific position in a DNA sequence. J Biol Chem. 1978;253:6551–6560. [PubMed] [Google Scholar]
- 28.Ishii Y. The local and global unfolding of coiled-coil tropomyosin. Eur J Biochem. 1994;221:705–712. doi: 10.1111/j.1432-1033.1994.tb18783.x. [DOI] [PubMed] [Google Scholar]
- 29.Jones N, Shenk T. Isolation of deletion and substitution mutants of adenovirus type 5. Cell. 1978;13:181–186. doi: 10.1016/0092-8674(78)90148-4. [DOI] [PubMed] [Google Scholar]
- 30.Jones N, Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979;17:683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- 31.Kestler H W, III, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 32.Ketner G, Bridge E, Virtanen A, Hemström C, Pettersson U. Complementation of adenovirus E4 mutants by transient expression of E4 cDNA and deletion plasmids. Nucleic Acids Res. 1989;17:3037–3048. doi: 10.1093/nar/17.8.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 34.Leppard K N. E4 gene function in adenovirus, adenovirus vector, and adeno-associated virus infections. J Gen Virol. 1997;78:2131–2138. doi: 10.1099/0022-1317-78-9-2131. [DOI] [PubMed] [Google Scholar]
- 35.Leppard K N, Shenk T. The adenovirus E1B 55 kd protein influences mRNA transport via an intranuclear effect on RNA metabolism. EMBO J. 1989;8:2329–2336. doi: 10.1002/j.1460-2075.1989.tb08360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luria S, Chambers I, Berg P. Expression of the type 1 human immunodeficiency virus Nef protein in T cells prevents antigen receptor-mediated induction of interleukin 2 mRNA. Proc Natl Acad Sci USA. 1991;88:5326–5330. doi: 10.1073/pnas.88.12.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacArthur M W, Thorton J M. Influence of proline residues on protein conformation. J Mol Biol. 1991;218:397–412. doi: 10.1016/0022-2836(91)90721-h. [DOI] [PubMed] [Google Scholar]
- 38.Marton M J, Baim S B, Ornelles D A, Shenk T. The adenovirus E4 17-kilodalton protein complexes with the cellular transcription factor E2F, altering its DNA-binding properties and stimulating E1A-independent accumulation of E2 mRNA. J Virol. 1990;64:2345–2359. doi: 10.1128/jvi.64.5.2345-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson J W, Kallenbach N R. Stabilization of the ribonuclease S-peptide alpha-helix by trifluoroethanol. Proteins. 1986;1:211–217. doi: 10.1002/prot.340010303. [DOI] [PubMed] [Google Scholar]
- 40.Nordqvist K, Akusjärvi G. Adenovirus early region 4 stimulates mRNA accumulation via 5′ introns. Proc Natl Acad Sci USA. 1990;87:9543–9547. doi: 10.1073/pnas.87.24.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nordqvist K, Öhman K, Akusjärvi G. Human adenovirus encodes two proteins which have opposite effects on accumulation of alternatively spliced mRNAs. Mol Cell Biol. 1994;14:437–445. doi: 10.1128/mcb.14.1.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Öhman K, Nordqvist K, Akusjärvi G. Two adenovirus proteins with redundant activities in virus growth facilitates tripartite leader mRNA accumulation. Virology. 1993;194:50–58. doi: 10.1006/viro.1993.1234. [DOI] [PubMed] [Google Scholar]
- 42a.Orlando, J. S., and D. A. Ornelles. Unpublished data.
- 43.Ornelles D A, Shenk T. Localization of the adenovirus early region 1B 55-kilodalton protein during lytic infection: association with nuclear viral inclusions requires the early region 4 34-kilodalton protein. J Virol. 1991;65:424–429. doi: 10.1128/jvi.65.1.424-429.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park K, Perczel A, Fasman G D. Differentiation between transmembrane helices and peripheral helices by the deconvolution of circular dichroism spectra of membrane proteins. Protein Sci. 1992;1:1032–1049. doi: 10.1002/pro.5560010809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pauling L, Corey R. Atomic coordinates and structure factors for two helical configurations of polypeptide chains. Proc Natl Acad Sci USA. 1951;37:235–240. doi: 10.1073/pnas.37.5.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pilder S, Moore M, Logan J, Shenk T. The adenovirus E1B-55K transforming polypeptide modulates transport or cytoplasmic stabilization of viral and host cell mRNAs. Mol Cell Biol. 1986;6:470–476. doi: 10.1128/mcb.6.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Querido E, Marcellus R C, Lai A, Charbonneau R, Teodoro J G, Ketner G, Branton P E. Regulation of p53 levels by the E1B 55-kilodalton protein and E4orf6 in adenovirus-infected cells. J Virol. 1997;71:3788–3798. doi: 10.1128/jvi.71.5.3788-3798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubenwolf S, Schütt H, Nevels M, Wolf H, Dobner T. Structural analysis of the adenovirus type 5 E1B 55-kilodalton-E4orf6 protein complex. J Virol. 1997;71:1115–1123. doi: 10.1128/jvi.71.2.1115-1123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarnow P, Hearing P, Anderson C W, Halbert D N, Shenk T, Levine A J. Adenovirus early region 1B 58,000-dalton tumor antigen is physically associated with an early region 4 25,000-dalton protein in productively infected cells. J Virol. 1984;49:692–700. doi: 10.1128/jvi.49.3.692-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sawai E T, Baur A S, Peterlin B M, Levy J A, Cheng-Mayer C. A highly conserved region and membrane targeting of Nef from primate immunodeficiency viruses are required for association with a cellular serine kinase activity. J Biol Chem. 1995;270:15307–15314. doi: 10.1074/jbc.270.25.15307. [DOI] [PubMed] [Google Scholar]
- 51.Segrest J P, Garber D W, Brouillette C G, Harvey S C, Anantharamaiah G M. The amphipathic alpha helix: a multifunctional structural motif in plasma apolipoproteins. Adv Protein Chem. 1994;45:303–369. doi: 10.1016/s0065-3233(08)60643-9. [DOI] [PubMed] [Google Scholar]
- 52.Takei J, Remenyi A, Clake A R, Dempsey C E. Self-association of disulfide-dimerized melittin analogues. Biochemistry. 1998;37:5699–5708. doi: 10.1021/bi9729007. [DOI] [PubMed] [Google Scholar]
- 53.Weinberg D H, Ketner G. A cell line that supports the growth of a defective early region 4 deletion mutant of human adenovirus type 2. Proc Natl Acad Sci USA. 1983;80:5383–5386. doi: 10.1073/pnas.80.17.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zantema A, Fransen J A, Davis O A, Ramaekers F C, Vooijs G P, DeLeys B, van der Eb A J. Localization of the E1B proteins of adenovirus 5 in transformed cells, as revealed by interaction with monoclonal antibodies. Virology. 1985;142:44–58. doi: 10.1016/0042-6822(85)90421-0. [DOI] [PubMed] [Google Scholar]