In a case–control study from the Vaccine Safety Datalink, no association was found between coronavirus disease 2019 (COVID-19) vaccination and stillbirth.
Abstract
OBJECTIVE:
Coronavirus disease 2019 (COVID-19) vaccination is recommended in pregnancy to reduce the risk of severe morbidity from COVID-19. However, vaccine hesitancy persists among pregnant people, with risk of stillbirth being a primary concern. Our objective was to examine the association between COVID-19 vaccination and stillbirth.
METHODS:
We performed a matched case–control study in the Vaccine Safety Datalink (VSD). Stillbirths and live births were selected from singleton pregnancies among persons aged 16–49 years with at least one prenatal, delivery, or postpartum visit at eight participating VSD sites. Stillbirths identified through diagnostic codes were adjudicated to confirm the outcome, date, and gestational age at fetal death. Confirmed antepartum stillbirths that occurred between February 14, 2021, and February 27, 2022, then were matched 1:3 to live births by pregnancy start date, VSD site, and maternal age at delivery. Associations among antepartum stillbirth and COVID-19 vaccination in pregnancy, vaccine manufacturer, number of vaccine doses received, and vaccination within 6 weeks before stillbirth (or index date in live births) were evaluated using conditional logistic regression.
RESULTS:
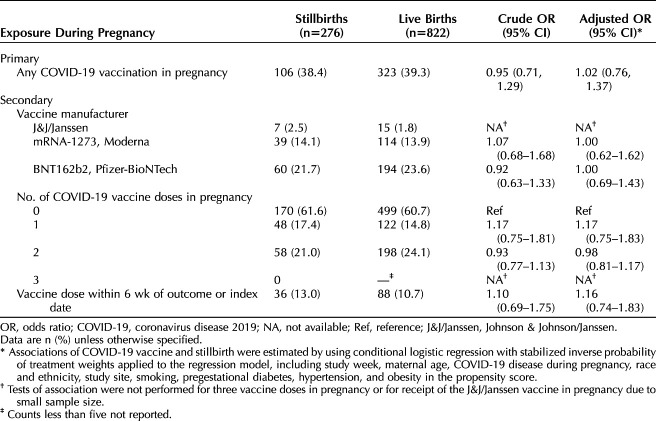
In the matched analysis of 276 confirmed antepartum stillbirths and 822 live births, we found no association between COVID-19 vaccination during pregnancy and stillbirth (38.4% stillbirths vs 39.3% live births in vaccinated individuals, adjusted odds ratio [aOR] 1.02, 95% CI, 0.76–1.37). Furthermore, no association between COVID-19 vaccination and stillbirth was detected by vaccine manufacturer (Moderna: aOR 1.00, 95% CI, 0.62–1.62; Pfizer-BioNTech: aOR 1.00, 95% CI, 0.69–1.43), number of vaccine doses received during pregnancy (1 vs 0: aOR 1.17, 95% CI, 0.75–1.83; 2 vs 0: aOR 0.98, 95% CI, 0.81–1.17), or COVID-19 vaccination within the 6 weeks before stillbirth or index date compared with no vaccination (aOR 1.16, 95% CI, 0.74–1.83).
CONCLUSION:
No association was found between COVID-19 vaccination and stillbirth. These findings further support recommendations for COVID-19 vaccination in pregnancy.
Coronavirus disease 2019 (COVID-19) in pregnancy confers an increased risk of hospitalization, admission to the intensive care unit, the need for mechanical ventilation, and adverse obstetric outcomes, including stillbirth.1–6 Vaccination remains the most important and effective tool for preventing hospitalizations and morbidity due to COVID-19, in both pregnant and nonpregnant populations,7–9 and is recommended in pregnancy by the Centers for Disease Control and Prevention (CDC)10 and the American College of Obstetricians and Gynecologists.11
Mounting evidence has demonstrated that COVID-19 vaccination in pregnancy provides both maternal and neonatal benefit, with reductions in severe maternal COVID-1912 and neonatal COVID-19 hospitalization.13–16 Despite benefits to the maternal-newborn dyad, vaccine hesitancy remains a public health challenge.11 Stillbirth (fetal death at 20 weeks of gestation or later) has been identified as an important outcome to include when evaluating the safety of vaccines in pregnancy and addressing vaccine hesitancy.17 Existing studies examining stillbirth and COVID-19 vaccination during pregnancy have been limited by small numbers of stillbirths and lack of stillbirth case adjudication to confirm the pregnancy outcome and date and gestational age at fetal death.18,19 More robust data are needed to examine the question of whether there is an association between COVID-19 vaccine exposure and stillbirth. The primary objective of this study was to compare the odds of COVID-19 vaccine exposure during pregnancy in pregnancies ending in stillbirth and those ending in live birth through a rigorous case–control study using the Vaccine Safety Datalink (VSD).
METHODS
This study was approved by the institutional review boards of all participating sites and the CDC with a waiver of informed consent because this was a minimal risk observational study and was conducted consistent with federal law and CDC policy (see 45 C.F.R. part 46.114; 21 C.F.R. part 56.114).
We used an individually matched case–control study design to evaluate the association between COVID-19 vaccination and singleton antepartum stillbirth within the VSD population. The VSD, established in 1990, is a collaboration between the CDC and 13 large integrated health care organizations.20 With data on approximately 4% of the U.S. population, validated identification of pregnancies, the availability of detailed electronic health record (EHR) clinical data, and comprehensive vaccine data, the VSD provides a robust infrastructure for monitoring stillbirth after COVID-19 vaccination in pregnancy.21–23 Eight VSD sites contributed data to this study: the Colorado, Northwest, Northern California, Southern California, and Washington regions of Kaiser Permanente, HealthPartners, Marshfield Clinic, and Denver Health.
Pregnancies were identified using the validated dynamic pregnancy algorithm, which applies a hierarchical approach to pregnancy identification based on International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis and procedure codes. Additional data, including estimated date of delivery and last menstrual period, were obtained from the EHR for completed or ongoing pregnancies and were used to apply pregnancy start and end dates.24,25
Eligible individuals aged 16–49 years at pregnancy start with singleton pregnancies were included if the pregnant person had at least one documented prenatal, delivery, or postpartum care visit in a participating health care system. Individuals with multiple gestations were excluded due to higher risk of complications. Gestational age at delivery was extracted and confirmed, where possible, from the delivery encounter, infant record, or obstetric history, in that order, through review of the EHR. Individuals with pregnancies for which gestational age was not available through last menstrual period, estimated date of delivery, or birth records (for live births) or medical record review and adjudication (for stillbirths) were excluded. Live births that occurred before 22 weeks of gestation or with a birth weight of less than 500 g also were excluded, because these were considered incompatible with neonatal survival.
Using the dynamic pregnancy algorithm, we captured all potentially eligible pregnancies that ended in stillbirth (see Appendix 1, available online at http://links.lww.com/AOG/D704, for specific diagnosis codes) with pregnancy end dates during our planned study period of January 13, 2021, to February 28, 2022. January 13, 2021, was selected as the start date to allow for a minimum of 6 weeks for vaccine exposure to occur during pregnancy once the COVID-19 vaccine was widely available. Additional pregnancy- and outcome-related data, including ultrasound reports; prenatal and delivery records; and laboratory, pathology, genetic, and autopsy evaluation, were collected using manual medical record abstraction and entered into a standardized REDCap database.26,27 Stillbirths then were adjudicated by one of the obstetrician investigators. The goal of adjudication was to confirm the pregnancy outcome to be a stillbirth (antepartum intrauterine fetal death occurring at 20 weeks of gestation or later),22 and to determine the date and gestational age at fetal death. Pregnancies identified as ending in stillbirth by the dynamic pregnancy algorithm were excluded if an alternative pregnancy outcome was confirmed during the adjudication process; these included live birth, spontaneous abortion (for those with adjudicated gestational age of less than 20 weeks at delivery), pregnancy termination (including induction of labor for previable premature rupture of membranes or fetal anomalies), intrapartum death, and neonatal death.
Each confirmed stillbirth was matched 1:3, where possible, to eligible live births using greedy matching.28 Matching variables included maternal age within 3 years, pregnancy start date within 14 days, and VSD site.28 Matching by pregnancy start date allowed alignment of individuals in the case and control groups by pregnancy start date. To ensure a similar vaccination exposure window for those in the case and control groups, live births were censored as of the gestational age of the matched stillbirth case and an index date was assigned (ie, if the matched stillbirth occurred at 30 weeks of gestation, the date at which the live birth pregnancy reached 30 weeks of gestation was assigned as the index date).
The exposure of interest was the receipt of at least one COVID-19 vaccine (primary series or booster dose) between the pregnancy start date and the date of fetal death or the equivalent index date for live births. COVID-19 vaccine administration information was obtained from each site’s EHR, medical and pharmacy claims, and through the bidirectional exchange with state or regional immunization information systems.23 For individuals in both the case and control groups, vaccines were further classified based on the manufacturer (Moderna, Pfizer-BioNTech, or Johnson & Johnson/Janssen), the number of doses received during pregnancy (not inclusive of prepregnancy doses), and receipt of the COVID-19 vaccine within 6 weeks of the stillbirth or index date.
We determined a priori important confounders associated with stillbirth and with propensity to be vaccinated during pregnancy.29 Potential confounders included maternal age, VSD site, race and ethnicity, the number of prenatal visits before index date, and the presence of comorbidities associated with increased propensity to receive a COVID-19 vaccine during pregnancy or with increased risk of fetal death. The presence of comorbidities was defined as having one or more inpatient or two or more outpatient diagnoses (ICD-10-CM diagnosis codes are shown in Appendix 1, http://links.lww.com/AOG/D704) for the period 3 years before pregnancy (and from March 2020 for COVID-19) through the stillbirth or index date. The presence of obesity in the 6 months before pregnancy or the first trimester, if preconception data were not available, was identified using both ICD-10-CM codes and a body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) of 30 or higher. Tobacco use from 3 months before the stillbirth or index date was captured from EHR data and supplemented by diagnosis codes. Other comorbidities—classified as binary variables and identified through ICD-10-CM codes—included cancer, preexisting cardiovascular disease, pregestational diabetes, chronic hypertension, pulmonary disease, alcohol use, substance use, and systemic lupus erythematosus. Diagnoses of medically attended COVID-19 during pregnancy were ascertained using ICD-10-CM diagnosis codes.
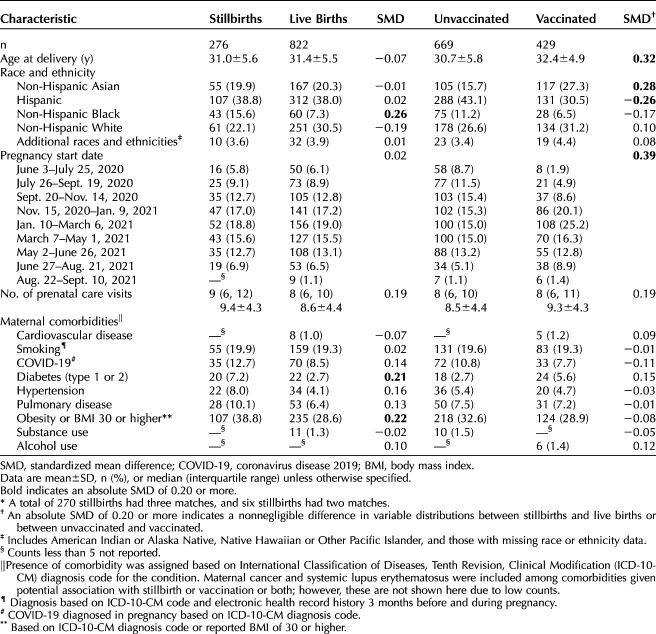
Baseline characteristics were described for individuals with pregnancies that ended in stillbirth compared with live birth and by COVID-19 vaccination status using means and SDs, or frequency distributions, as appropriate. Differences between exposed and unexposed, and stillbirths and live births, were noted using standardized mean difference (SMD). An SMD of 0.20 or more or −0.20 or less between COVID-19 vaccine exposed and unexposed indicated a potential confounder. To address potential confounding, we constructed propensity scores for vaccination that used a generalized additive model with binomial distribution. Propensity scores were developed for each contrast: receipt of a COVID-19 vaccine during pregnancy, vaccine manufacturer, first or second dose of the vaccine received during pregnancy, and receipt of the vaccine within the 6 weeks before stillbirth or index date. The following covariates were modeled as main effects: COVID-19 during pregnancy, race and ethnicity, study site, smoking, pregestational diabetes, chronic hypertension, and obesity. Age and study week were included as cubic splines. Stabilized inverse probability of treatment weights (SIPTW) were calculated. Standardized mean difference was used to evaluate whether covariates were balanced after applying SIPTW.
Associations of COVID-19 vaccine and stillbirth were evaluated using conditional logistic regression with receipt of a COVID-19 vaccine during pregnancy as the dependent variable in the model. The SIPTW were applied to the regression model and the Efron30 method was used for treating ties. Associations were reported as odds ratios (ORs) with 95% CIs. The odds of vaccination before the date of fetal death or index date for live births were compared between individuals in the case and control groups. Analyses were also performed for COVID-19 vaccination received within 6 weeks of stillbirth or index date, vaccine manufacturer, and number of vaccine doses received during pregnancy compared with no receipt of a COVID-19 vaccine during pregnancy.
Statistical significance was set at P=.05. This study was anticipated to have 80% power to detect an OR of 1.5 based on 400 confirmed stillbirths and a 1:3 match ratio of stillbirths to live births, assuming an exposure to COVID-19 vaccine of 26%, 0.1 correlation of COVID-19 vaccine with other covariates, and an alpha value of 0.05. However, fewer than anticipated stillbirths were confirmed, and vaccine uptake was higher than anticipated, at 39%. A post hoc power analysis, based on the final stillbirth case number and observed proportion with COVID-19 vaccine exposure, determined that the study had 80% power to detect an OR of 1.52. Power was estimated using PAS software, testing for the odds ratio in a matched case–control design. The analytic approach followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guidelines for case–control studies.
RESULTS
We identified 465 pregnancies with a stillbirth diagnosis. After adjudication, 276 (59.4%) stillbirths were confirmed through adjudication and were able to be matched to a live birth control with final outcome dates (dates when adjudicated stillbirths actually occurred within the study period) of February 14, 2021, to February 27, 2022. The outcomes of the other pregnancies determined through adjudication to not be stillbirths are shown in Appendix 2, available online at http://links.lww.com/AOG/D704; the two most common reasons for exclusion were pregnancy ending in termination rather than stillbirth (ie, for previable premature rupture of membranes, 60/465 [12.9%]) or in spontaneous abortion before 20 weeks of gestation (54/465 [11.6%]). Stillbirths were matched to 822 singleton live births: six stillbirths were matched to two live births, 270 stillbirths were matched to three live births, and two stillbirths were excluded due to inability to match to live births. The gestational age at stillbirth ranged from 20 to 43 weeks, with a median gestational age of 31 weeks (interquartile range 25–37 weeks). Individuals with pregnancies that ended in stillbirth were more likely than those with pregnancies ending in live birth to be non-Hispanic Black (15.6% of stillbirths vs 7.3% of live births; SMD 0.26) and to have comorbidities such as diabetes (7.2% of stillbirths vs 2.7% of live births; SMD 0.21) and obesity (38.8% of stillbirths vs 28.6% of live births; SMD 0.22) (Table 1). When comparing vaccinated and unvaccinated pregnant individuals, vaccinated individuals were older (mean maternal age in vaccinated individuals: 32.4 years vs 30.7 years in unvaccinated individuals; SMD 0.32), more likely to identify as Asian (27.3% of vaccinated individuals vs 15.7% of unvaccinated individuals; SMD 0.28), and less likely to identify as Hispanic (30.5% of vaccinated individuals vs 43.1% of unvaccinated individuals; SMD −0.26) than unvaccinated individuals.
Table 1.
Selected Demographic Characteristics of Individuals by Pregnancy Outcome and Vaccination Status in the Vaccine Safety Datalink, February 14, 2021–February 27, 2022*
In the primary analysis, 38.4% of individuals with pregnancies ending in stillbirth and 39.3% of those with matched pregnancies ending in live birth were exposed to at least one COVID-19 vaccination during pregnancy (Table 2). There was no significant association between stillbirth and receipt of COVID-19 vaccination in pregnancy after applying SIPTW (adjusted odds ratio [aOR] 1.02, 95% CI, 0.76–1.37). In secondary analyses, we found no association between stillbirth and receipt of a COVID-19 vaccine when comparing by vaccine manufacturer (Moderna vs none: aOR 1.00, 95% CI, 0.62–1.62; Pfizer-BioNTech vs none: aOR 0.99, 95% CI, 0.69–1.43), nor between stillbirth and receipt of one COVID-19 vaccine dose compared with none (aOR 1.17, 95% CI, 0.75–1.83) or two COVID-19 vaccine doses compared with none (aOR 0.98, 95% CI, 0.81–1.17). The Johnson & Johnson/Janssen vaccine was administered too infrequently to generate meaningful product-specific analyses. There was no increase in the odds of receipt of the COVID-19 vaccine within the 6 weeks before the stillbirth or index date, as compared with no vaccination during pregnancy (aOR 1.16, 95% CI, 0.74–1.83).
Table 2.
Matched Case–Control Analysis of Stillbirths Compared With Live Births After Maternal Coronavirus Disease 2019 (COVID-19) Vaccination During Pregnancy in the Vaccine Safety Datalink, February 14, 2021–February 27, 2022, Using Conditional Logistic Regression With Stabilized Inverse Probability of Treatment Weights
DISCUSSION
This robust case–control study did not detect an association between stillbirth and receipt of COVID-19 vaccination during pregnancy. Furthermore, there was no association by vaccine manufacturer, number of COVID-19 vaccine doses received during pregnancy, or receipt of the vaccine within the 6 weeks before stillbirth. These results should provide reassurance to pregnant individuals and health care professionals that COVID-19 vaccines may be administered during pregnancy without increasing the risk of pregnancy loss at or beyond 20 weeks of gestation. These results complement the existing body of evidence that COVID-19 vaccination does not increase the risk of pregnancy loss within the first 20 weeks of gestation (ie, spontaneous abortion), based on evidence from the same VSD source population and time period31,32 and other studies.33,34
Although pregnant persons were not included in the initial-phase trials of the COVID-19 vaccines, other postmarketing cohort studies have not detected any associations between COVID-19 vaccination and stillbirth.1,35,36 One of the largest published studies examining stillbirth as a primary outcome combined data from the Pregnancy Register in Sweden and the Medical Birth Registry of Norway.36 The authors of that cohort study noted a similar risk of stillbirth between individuals who were and were not vaccinated during pregnancy (adjusted hazard ratio 0.86, 95% CI, 0.63–1.17), but also acknowledged the low proportion of pregnant individuals who received COVID-19 vaccination during pregnancy (18%). Furthermore, case record review and adjudication were not performed, potentially resulting in stillbirth misclassification and overestimation or underestimation of the true incidence of stillbirth in both groups. Although most prior studies have demonstrated a neutral association between COVID-19 vaccination and stillbirth, one published retrospective cohort study from Australia did suggest reduced odds of stillbirth in vaccinated individuals.1 However, these results should be interpreted with caution, because these analyses did not account for immortal time bias, where individuals with shorter pregnancies (ie, those resulting in stillbirth) have less opportunity to be vaccinated.37
Our study had several strengths and advantages over previous studies. Stillbirths were clinically reviewed and adjudicated by obstetrician investigators, reducing outcome misclassification. Although our study did not reach the projected target for stillbirths, we included a larger number of stillbirths than in previous studies, which allowed for secondary analyses by vaccine manufacturer and number of vaccine doses in pregnancy that were not previously possible. Additionally, by censoring live birth pregnancies at the gestational age at which the matched stillbirth occurred, we reduced the potential for immortal time bias. Comprehensive COVID-19 vaccination data were ascertained through the VSD, which is often not possible in other U.S. studies. Methodologically, the case–control design was well-suited to the study of stillbirth given its rare occurrence. As in any case–control study, there is a potential for bias if the comparison or control population systematically differs from the case population. We limited this bias by applying similar inclusion and exclusion criteria before matching and using a single, well-described source population from the VSD.
Limitations to this case–control study should be noted. Small effects of COVID-19 vaccination on stillbirth may not have been detected due to the use of a defined study period and restricted sample size. Furthermore, the study was powered to the primary outcome, but the subanalyses (ie, by COVID-19 vaccination received within 6 weeks of stillbirth or index date, vaccine manufacturer, and number of vaccine doses received during pregnancy) may have been underpowered to detect a difference. As a retrospective observational study, we had incomplete ascertainment of possible confounders, such as prior pregnancy history (ie, history of prior stillbirth and adverse pregnancy outcomes, and parity). It was not feasible to conduct medical record reviews for all live births in the control group, nor are these data always reliably available through EHR review. Thus, we included only covariates that could be identified in automated data files. It should be acknowledged that results obtained from the VSD may not be generalizable to the general pregnant population, because the VSD includes an insured population receiving health care. Finally, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was not included as a covariate due to the high likelihood of incomplete ascertainment of this outcome given the increased use of home COVID-19 antigen testing during the latter part of the study. However, a COVID-19 diagnosis was included as a comorbidity.
Vaccine hesitancy has resulted in lower vaccine acceptance and uptake among pregnant people than in the general population.38,39 The results of this robust case–control study can be used to provide reassurance to both pregnant patients and health care professionals that COVID-19 vaccination in pregnancy is not associated with an increased risk of pregnancy loss after 20 weeks of gestation.
Footnotes
This work was supported by the Centers for Disease Control and Prevention (Contract 200-2012-53526). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Mentioning a product or company name is for identification purposes only and does not constitute an endorsement by the CDC.
Financial Disclosure Gabriela Vazquez-Benitez reports AbbVie and Sanofi Pasteur provided research-related funds unrelated to this work to her institution. Sangini Sheth's institution received payment from the NIH, and they received payment from Merck and ACOG. Malini DeSilva's institution received payments from Westat, Inc., and the Minnesota Department of Health. Darios Getahun's institution received payment from the NIH, Garfield Memorial Fund, and Hologic, Inc. Nicola Klein's institution received research support from Pfizer. Money was paid to her institution from Merck and Sanofi Pasteur. Joshua Williams has served as a content reviewer for Immunize 4 Good. Heather Lipkind subcontracted via Health Partners as a consultant for the Vaccine Safety Datalink. Money was paid to her institution from PCORI and Pfizer (external DMSV for COVID-19 vaccination). The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/D705.
REFERENCES
- 1.Hui L, Marzan MB, Rolnik DL, Potenza S, Pritchard N, Said JM, et al. Reductions in stillbirths and preterm birth in COVID-19-vaccinated women: a multicenter cohort study of vaccination uptake and perinatal outcomes. Am J Obstet Gynecol 2023;228:585.e1–16. doi: 10.1016/j.ajog.2022.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes BL, Sandoval GJ, Metz TD, Clifton RG, Grobman WA, Saade GR, et al. First- or second-trimester SARS-CoV-2 infection and subsequent pregnancy outcomes. Am J Obstet Gynecol 2023;228:226.e1–9. doi: 10.1016/j.ajog.2022.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metz TD, Clifton RG, Hughes BL, Sandoval GJ, Grobman WA, Saade GR, et al. Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA 2022;327:748–59. doi: 10.1001/jama.2022.1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith ER, Oakley E, Grandner GW, Ferguson K, Farooq F, Afshar Y, et al. Adverse maternal, fetal, and newborn outcomes among pregnant women with SARS-CoV-2 infection: an individual participant data meta-analysis. BMJ Glob Health 2023;8:e009495. doi: 10.1136/bmjgh-2022-009495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr 2021;175:817–26. doi: 10.1001/jamapediatrics.2021.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeSisto CL, Wallace B, Simeone RM, Polen K, Ko JY, Meaney-Delman D, et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization—United States, March 2020-September 2021. MMWR Morb Mortal Wkly Rep 2021;70:1640–5. doi: 10.15585/mmwr.mm7047e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagan N, Barda N, Biron-Shental T, Makov-Assif M, Key C, Kohane IS, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med 2021;27:1693–5. doi: 10.1038/s41591-021-01490-8 [DOI] [PubMed] [Google Scholar]
- 8.Goldshtein I, Nevo D, Steinberg DM, Rotem RS, Gorfine M, Chodick G, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA 2021;326:728–35. doi: 10.1001/jama.2021.11035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrag SJ, Verani JR, Dixon BE, Page JM, Butterfield KA, Gaglani M, et al. Estimation of COVID-19 mRNA vaccine effectiveness against medically attended COVID-19 in pregnancy during periods of delta and omicron variant predominance in the United States. JAMA Netw Open 2022;5:e2233273. doi: 10.1001/jamanetworkopen.2022.33273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. COVID-19 vaccines while pregnant or breastfeeding. Accessed October 16, 2023. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html#:∼:text=Studies%20including%20hundreds%20of%20thousands,risks%20of%20vaccination%20during%20pregnancy [Google Scholar]
- 11.American College of Obstetricians and Gynecologists. COVID-19 vaccination considerations for obstetric–gynecologic care. Accessed October 16, 2023. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care [Google Scholar]
- 12.Morgan JA, Biggio JR, Jr., Martin JK, Mussarat N, Elmayan A, Chawla HK, et al. Pregnancy outcomes in patients after completion of the mRNA coronavirus disease 2019 (COVID-19) vaccination series compared with unvaccinated patients. Obstet Gynecol 2023;141:555–62. doi: 10.1097/aog.0000000000005072 [DOI] [PubMed] [Google Scholar]
- 13.Halasa NB, Olson SM, Staat MA, Newhams MM, Price AM, Boom JA, et al. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged <6 months — 17 States, July 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022;71:264–70. doi: 10.15585/mmwr.mm7107e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halasa NB, Olson SM, Staat MA, Newhams MM, Price AM, Pannaraj PS, et al. Maternal vaccination and risk of hospitalization for Covid-19 among infants. N Engl J Med 2022;387:109–19. doi: 10.1056/NEJMoa2204399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashani-Ligumsky L, Lopian M, Cohen R, Senderovich H, Czeiger S, Halperin A, et al. Titers of SARS CoV-2 antibodies in cord blood of neonates whose mothers contracted SARS CoV-2 (COVID-19) during pregnancy and in those whose mothers were vaccinated with mRNA to SARS CoV-2 during pregnancy. J Perinatol 2021;41:2621–4. doi: 10.1038/s41372-021-01216-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simeone RM, Zambrano LD, Halasa NB, Fleming-Dutra KE, Newhams MM, Wu MJ, et al. Effectiveness of maternal mRNA COVID-19 vaccination during pregnancy against COVID-19-associated hospitalizations in infants aged <6 months during SARS-CoV-2 Omicron predominance — 20 states, March 9, 2022-May 31, 2023. MMWR Morb Mortal Wkly Rep 2023;72:1057–64. doi: 10.15585/mmwr.mm7239a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tavares Da Silva F, Gonik B, McMillan M, Keech C, Dellicour S, Bhange S, et al. Stillbirth: case definition and guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine 2016;34:6057–68. doi: 10.1016/j.vaccine.2016.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, Swift M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM 2021;3:100467. doi: 10.1016/j.ajogmf.2021.100467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trostle ME, Limaye MA, Avtushka V, Lighter JL, Penfield CA, Roman AS. COVID-19 vaccination in pregnancy: early experience from a single institution. Am J Obstet Gynecol MFM 2021;3:100464. doi: 10.1016/j.ajogmf.2021.100464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baggs J, Gee J, Lewis E, Fowler G, Benson P, Lieu T, et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics 2011;127(suppl 1):S45–53. doi: 10.1542/peds.2010-1722H [DOI] [PubMed] [Google Scholar]
- 21.Kharbanda EO, Vazquez-Benitez G, Lipkind HS, Sheth SS, Zhu J, Naleway AL, et al. Risk of spontaneous abortion after inadvertent human papillomavirus vaccination in pregnancy. Obstet Gynecol 2018;132:35–44. doi: 10.1097/AOG.0000000000002694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panagiotakopoulos L, McCarthy NL, Tepper NK, Kharbanda EO, Lipkind HS, Vazquez-Benitez G, et al. Evaluating the association of stillbirths after maternal vaccination in the Vaccine Safety Datalink. Obstet Gynecol 2020;136:1086–94. doi: 10.1097/AOG.0000000000004166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groom HC, Crane B, Naleway AL, Weintraub E, Daley MF, Wain K, et al. Monitoring vaccine safety using the Vaccine Safety Datalink: assessing capacity to integrate data from Immunization Information systems. Vaccine 2022;40:752–6. doi: 10.1016/j.vaccine.2021.12.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naleway AL, Gold R, Kurosky S, Riedlinger K, Henninger ML, Nordin JD, et al. Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink. Vaccine 2013;31:2898–903. doi: 10.1016/j.vaccine.2013.03.069 [DOI] [PubMed] [Google Scholar]
- 25.Naleway AL, Crane B, Irving SA, Bachman D, Vesco KK, Daley MF, et al. Vaccine Safety Datalink infrastructure enhancements for evaluating the safety of maternal vaccination. Ther Adv Drug Saf 2021;12:20420986211021233. doi: 10.1177/20420986211021233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergstralh EJ, Kosanke JL, Jacobsen SJ. Software for optimal matching in observational studies. Epidemiology 1996;7:331–2. [PubMed] [Google Scholar]
- 29.Management of stillbirth. Obstetric Care Consensus No. 10. American College of Obstetricians and Gynecologists. Obstet Gynecol 2020;135:e110–32. doi: 10.1097/AOG.0000000000003719 [DOI] [PubMed] [Google Scholar]
- 30.Efron B. Logistic regression, survival analysis, and the Kaplan-Meier curve. J Am Stat Assoc 1988;83:414–25. doi: 10.1080/01621459.1988.10478612 [DOI] [Google Scholar]
- 31.Kharbanda EO, Haapala J, DeSilva M, Vazquez-Benitez G, Vesco KK, Naleway AL, et al. Spontaneous abortion following COVID-19 vaccination during pregnancy. JAMA 2021;326:1629–31. doi: 10.1001/jama.2021.15494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kharbanda EO, Haapala J, Lipkind HS, DeSilva MB, Zhu J, Vesco KK, et al. COVID-19 booster vaccination in early pregnancy and surveillance for spontaneous abortion. JAMA Netw Open 2023;6:e2314350. doi: 10.1001/jamanetworkopen.2023.14350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magnus MC, Gjessing HK, Eide HN, Wilcox AJ, Fell DB, Haberg SE. Covid-19 vaccination during pregnancy and first-trimester miscarriage. N Engl J Med 2021;385:2008–10. doi: 10.1056/NEJMc2114466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calvert C, Carruthers J, Denny C, Donaghy J, Hillman S, Hopcroft LEM, et al. A population-based matched cohort study of early pregnancy outcomes following COVID-19 vaccination and SARS-CoV-2 infection. Nat Commun 2022;13:6124. doi: 10.1038/s41467-022-33937-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fell DB, Dimanlig-Cruz S, Regan AK, Haberg SE, Gravel CA, Oakley L, et al. Risk of preterm birth, small for gestational age at birth, and stillbirth after covid-19 vaccination during pregnancy: population based retrospective cohort study. BMJ 2022;378:e071416. doi: 10.1136/bmj-2022-071416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magnus MC, Ortqvist AK, Dahlqwist E, Ljung R, Skar F, Oakley L, et al. Association of SARS-CoV-2 vaccination during pregnancy with pregnancy outcomes. JAMA 2022;327:1469–77. doi: 10.1001/jama.2022.3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Regan AK, Fell DB, Wise LA, Vazquez-Benitez G, Haberg SE, Ogar C, et al. Challenges & opportunities for the epidemiological evaluation of the effects of COVID-19 vaccination on reproduction and pregnancy. Vaccine 2023;41:5931–5. doi: 10.1016/j.vaccine.2023.08.032 [DOI] [PubMed] [Google Scholar]
- 38.Rawal S, Tackett RL, Stone RH, Young HN. COVID-19 vaccination among pregnant people in the United States: a systematic review. Am J Obstet Gynecol MFM 2022;4:100616. doi: 10.1016/j.ajogmf.2022.100616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Razzaghi H, Meghani M, Pingali C, Crane B, Naleway A, Weintraub E, et al. COVID-19 vaccination coverage among pregnant women during pregnancy—eight integrated health care organizations, United States, December 14, 2020-May 8, 2021. MMWR Morb Mortal Wkly Rep 2021;70:895–9. doi: 10.15585/mmwr.mm7024e2 [DOI] [PMC free article] [PubMed] [Google Scholar]